Abstract
We analyzed point-prevalence data from 35 recent studies of human populations in which Plasmodium falciparum and one other Plasmodium species were the reported causes of malaria infections. For the P. falciparum–Plasmodium vivax pair, higher overall prevalence in a human population is associated with fewer mixed-species infections than expected on the basis of the product of individual species prevalences. This is not true for P. falciparum–Plasmodium malariae.
Our current understanding of parasite communities rests on several decades of fruitful studies of helminths (Noble, 1960; Schad, 1963; Kennedy, 1975; Scott and Gibbs, 1986; Stock and Holmes, 1988; Esch et al., 1990; Fernandez and Esch, 1991; Booth and Bundy, 1992; Kennedy and Bush, 1992). Many of the ecological and evolutionary insights from these pioneering studies could be tested among parasitic protists as well, building from analyses of relatively static, simple patterns among a few congeners toward those of dynamic interactions among multiple genera. Among helminths, for instance, Dobson (1985, 1990; Dobson and Roberts, 1994) has emphasized that aggregated intraspecific distributions may diminish interspecific competition, and Lotz and Font (1991, 1994; Lotz et al., 1995) have stressed that species rarity and recruitment may modulate patterns of interspecific association.
Four species of Plasmodium cause human malaria, P. falciparum, P. malariae, P. ovale, and P. vivax. Sympatric combinations of these species occur in human populations and within infected individuals. Various phenomena associated with species co-occurrence have been studied as such for more than a century, e.g., Thayer and Hewetson (1895), since an era in which the very existence of more than one species was subject to debate. Biogeographic anomalies such as the absence of P. vivax and presence of P. ovale in West Africa and the spotty worldwide distribution of P. malariae have periodically attracted intense interest (see Molineaux, 1988), as have the frequencies of species co-occurrence within individuals and the possible correlates of mixed-species infections. This paper focusses on the latter 2 topics, particularly in those human populations in which recent reports suggest that only P. falciparum and 1 other Plasmodium species co-occur.
In a massive survey of Plasmodium species prevalence in humans, Knowles and White (1930) recorded mixed-species infections at various frequencies at various sites on all 6 inhabited continents and noted that particular species combinations were often seasonal. They complained that mixed-species infections were often underreported, and demonstrated the phenomenon in a test of microscopists. Cohen’s (1973) analysis of the epidemiological literature concluded that deficits in the reported prevalence of mixed-species infections were not wholly artifactual, but common, often statistically significant phenomena with a previously unsuspected biological basis in heterologous immunity. His hypothesis was later extended to encompass the seasonal species patterns and surplus prevalence of mixed-species infections detected during the Garki project (Molineaux and Gramiccia, 1980; Molineaux et al., 1980). Richie’s analysis (1988) argued that species coexistence was made possible only through antigenic divergence and hence the avoidance of heterologous immunity. He suggested that competitive interspecific suppression in simultaneous infections might be balanced by some form of reciprocal successional facilitation.
Unfortunately, few recent epidemiological reports examine mixed-species malaria infections; the studies by Fox and Strickland in Pakistan (1989) and Rosenberg et al. in Thailand (1990) are unique in reporting and discussing their findings that mixed P. falciparum–P. vivax infections occur at the levels expected and at levels far less than expected, respectively. The frequency with which unsuspected P. vivax infections emerge when protected patients clear their P. falciparum infections, in a variety of locations (Looareesuwan et al., 1987; Takagi et al., 1988; Nguyen and Keystone, 1989; Schuurkamp, 1992), hints that many mixed-species infections still escape detection. Though it is conceivable that this phenomenon derives solely from P. vivax hypnozoites, molecular-level detection methods produce wildly disproportionate increases in the reported prevalence of mixed-species infections (Barker et al., 1989; Relf et al., 1990; Brown et al., 1992; Arai et al., 1994) and may do so with several combinations of species; in studies comparing polymerase chain reaction (PCR) techniques to microscopy, for example, Snounou, Pinheiro et al. (1993) found that in Guinea Bissau mixed-species cases accounted for roughly half of a 142% increase and in Thailand (Snounou, Viriyakosol et al., 1993) three-quarters of a 22% increase in infections detected.
Although P. ovale was seldom recognized as a distinct etiologic entity until the 1960s, reports of human populations in which just 1 or 2 Plasmodium species caused malaria became typical only as the global malaria-eradication campaigns of that era subsided. Accordingly, our predecessors based their analyses almost exclusively on studies in which 3 Plasmodium species were reported present. As we hypothesize that some conclusions about mixed-species Plasmodium infections may vary with the number or identity of the species involved, or both, we defer our examination of similar polysympatric circumstances and questions of heterologous immunity to a later paper.
Here we address questions of spatial and temporal heterogeneity in the prevalence of dual infections. Cohen (1973) briefly examined geographic heterogeneity and seasonal variation in the contexts of “parasite rate,” i.e., overall prevalence, and “ecology,” respectively, and concluded that both factors (and both contexts) were at most minor influences. Richie (1988) considered both factors, at least implicitly, and concluded that no general relationships could obtain because “nearly every imaginable prevalence pattern has been found by one survey or another … there are geographic differences in the way in which human malaria species interact and … these interactions may even change from year to year in a given locale.” In this paper we analyze recent epidemiological data to determine if distinct prevalence patterns exist and, if so, if their influence is as weak as Cohen suggested and as confined to particular points in space and time as Richie proposed.
MATERIALS AND METHODS
We surveyed English- and French-language epidemiological reports published from 1984 to 1995, inclusive, and available in Countway or Mayr libraries at Harvard University; thus, the epidemiological information we used was more recent than that used in Richie’s (1988) or Cohen’s (1973) analyses of mixed-species Plasmodium infections. Many recent studies report results only for P. falciparum even if other species were also present; several reports contain unresolvable arithmetic inconsistencies. We analyzed only studies from which we could obtain complete, exact contingency tables, i.e., those that include figures for an “uninfected“ category, and that in some manner explicitly report the presence or absence of multiple species and of mixed-species infections, and if present explicitly distinguish the numbers and species involved. In 2-species cases, however, we assumed the obvious interpretation of “mixed infections.” We excluded studies that detected only antibodies and those that do not fully identify the period, place, and methods of sample collection. When the same data appeared in more than 1 eligible report, we analyzed only the more comprehensive set. Given a choice between data gathered in mass surveys and data gathered by other methods in the same community during the same period, we used only the mass-survey data. For the several studies that used molecular-level detection techniques as well as microscopy, we analyzed only the microscopy results.
Our analytical objectives paralleled those of our predecessors, and our analytical methods paralleled those of Cohen (1973) in particular. Our truncated chronological range and restriction to complete, exact data produced several advantages. Because the data were not censored, we could base our comparisons on significance tests with categorical values, rather than on confidence limits on parameter estimates (see Cohen, 1971). We could more readily identify studies in which repeated sampling might have occurred. We could assume that P. ovale and P. vivax had been properly distinguished. The clear disadvantage of our approach was that the information we compiled was too limited to allow analyses of many variables considered in Richie’s verbal, or Cohen’s statistical, or both, analyses; for instance, we found little recent data that relate mixed-species infection prevalence to spleen size.
Among studies that report exactly 2 Plasmodium species and meet our criteria, only 2 analyze their findings with respect to mixed-species infections (Fox and Strickland, 1989; Rosenberg et al., 1990), so we could not perform a meta-analysis in the literal sense, e.g., Hedges and Olkin (1985); we did not pool or partition studies or their reported results as such, e.g., Friedenreich (1993), except in grouping studies by the number and identities of the Plasmodium species involved. Instead, we extracted categorical point-prevalence data from each report to construct contingency tables, each of which contained counts of the presence and absence within individuals of each of the Plasmodium species reported present in the human population. Several tables contained 1 or more 0 entries; with a single 0 entry in a table, we added 0.5 to each cell to estimate the test statistic (Pagano and Gauvreau, 1993); given 2 zero entries in a table (as occurred in just under 5% of tables; see below), we eliminated it from further analysis. We tested each table against the standard null hypothesis of species sampling independence, i.e., that the dual-infection prevalence is the product of the individual species prevalences. Because none of the marginal totals was fixed, we used the G-test, with Williams’ correction (Sokal and Rohlf, 1981). Following Cohen (1973), we counted a table for which P < 0.01 significantly different from that expected.
Our predecessors examined hypotheses about spatial and temporal heterogeneity at the level of specific sites, seasons, and/or years. We hypothesized further that conclusions about prevalence patterns of mixed-species infections might vary with the particular spatial and temporal scales of analysis. We considered our basic, lowest-level sampling unit (level A) to be a single village sampled during a period lasting no longer than 1 mo, our most-aggregated, top-level sampling unit (level D) to be multiple villages sampled over over a period of multiple months, and the intermediate units to be multiple villages sampled within 1 mo (level B) and/or 1 village sampled over multiple mo (level C). Because species prevalence data from each village each month might constitute a contingency table (albeit usually a tacit one), a single epidemiological report might produce only 1 contingency table, several, or many. Because we had no basis for making assumptions about any underlying distribution(s) of prevalences in the studies, we used the nonparametic Mann–Whitney test for distributional differences (Sokal and Rohlf, 1981).
RESULTS
Table I summarizes general information about Plasmodium species occurrence. At the level of human populations, recent authors have detected 8 of the 15 possible species combinations, including the co-occurrence of all 4 species in populations in Madagascar and New Guinea. Among the missing combinations, P. malariae and P. vivax were most recently reported to co-occur without P. falciparum in a remote region of Peru (Sulzer et al., 1975). The lack of the Duffy antigen/chemokine receptor in most West African populations excludes P. vivax. At the level of human individuals, recent authors have detected 11 of the 15 possible species combinations. The 4 missing combinations are those that include the simultaneous presence of P. ovale and P. vivax. The only reported instance of co-occurrence of all 4 species in an individual relied on PCR-based detection techniques (Snounou, Viriyakosol et al., 1993).
TABLE I.
A summary of recent reports of species occurrence in human populations and individuals.*
| Plasmodium spp. | F | M | O | V | FM | FO | FV | MO | MV | OV | FMO | FMV | FOV | MOV | FMOV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human population | X | X | X | X | X | X | X | X | |||||||
| Human individual | X | X | X | X | X | X | X | X | X | X | X |
X indicates the existence of at least one published report. F, M, 0, V indicate the occurrence (sole or combined) of P. falciparum, P. malariae, P. ovale, and P. vivax.
Thirty-five reports fulfill our criteria and report the prevalence of exactly 2 Plasmodium species. Seven studies from Africa report the co-occurrence of P. falciparum and P. malariae, and 2 of P. falciparum and P. ovale. Nineteen studies from south Asia, 5 from southeast Asia, and 2 from South America report the co-occurrence of P. falciparum and P. vivax. From these 35 reports, we extracted 219 contingency tables, eliminated 10 that indicated the presence of only 1 species, i.e. with double zeroes (see above), sorted the remaining tables according to the particular species pair and spatial-temporal sampling unit involved, then tested each for departures from species sampling independence. Table II presents the results; 91% of tables contained sample sizes greater than 100. Statistically significant departures from independence appeared in 112 tables overall (54%). We considered the 4 P. falciparum–P. ovale tables too few to include in further analyses. In all but 1 of the 107 significant P. falciparum–P. vivax tables, the observed number of mixed-species infections was less than that expected. The converse held in all 5 significant P. falciparum–P. malariae tables. Among the 192 P. falciparum–P. vivax tables, the ratio of significant to nonsignificant tables shifted with the level of analysis, from 1:1 at level A, to 4:3 at B, 3:2 at C, and 2:1 at D. This did not appear to be true of the 13 tables pairing P. falciparum and P. malariae. We judged these sufficient for further analysis only at level A, however.
TABLE II.
A summary of 209 contingency tables from 35 reports.*
| Significant tables at level |
Nonsignificant tables at level |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Reports with | A | B | C | D | A | B | C | D | |
| F, M | A single table | 4 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 |
| F, O | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| F, V | 14 | 1 | 1 | 0 | 1 | 5 | 0 | 2 | 4 | |
| F, M | Multiple tables | 3 | 3 | 1 | 1 | 0 | 3 | 1 | 0 | 0 |
| F, O | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | |
| F, V | 12 | 31 | 47 | 13 | 13 | 28 | 36 | 7 | 3 | |
| F, M | Total | 7 | 3 | 1 | 1 | 0 | 6 | 2 | 0 | 0 |
| F, O | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | |
| F, V | 26 | 32 | 48 | 13 | 14 | 33 | 36 | 9 | 7 | |
Abbreviations as in Table I; levels of analysis and significance are explained in the text. References:(1) Adak et al., 1994; (2) Ansari et al., 1986; (3) Arai et al., 1994; (4) Barker et al., 1989; (5) Choudhury et al., 1987; (6) de Arruda et al., 1986; (7) Dossou-Yovo et al., 1994; (8) Dutta and Bhattacharyya, 1990; (9, 10) Dutta et al., 1991, 1994; (11) Ejezie et al., 1991; (12) Gazin et al., 1987; (13) Hedman et al., 1986; (14) Laserson et al., 1994; (15, 16) Malhotra et al., 1985a, 1985b; (17) Mathur et al., 1992; (18) Nalin et al., 1985; (19, 20) Nosten et al., 1987, 1991; (21) Prasad and Sharma, 1990; (22) Raccurt et al., 1993; (23) Ray et al., 1994; (24) Roche et al., 1991; (25) Rosenberg et al., 1990; (26) Schapira and da Costa, 1988; (27) Sharma et al., 1985; (28–30) Singh et al., 1988, 1989, 1995; (31) Soro et al., 1989; (32, 33) Strickland et al., 1987, 1988; (34) Warsame et al., 1989; (35) Yadav et al., 1993.
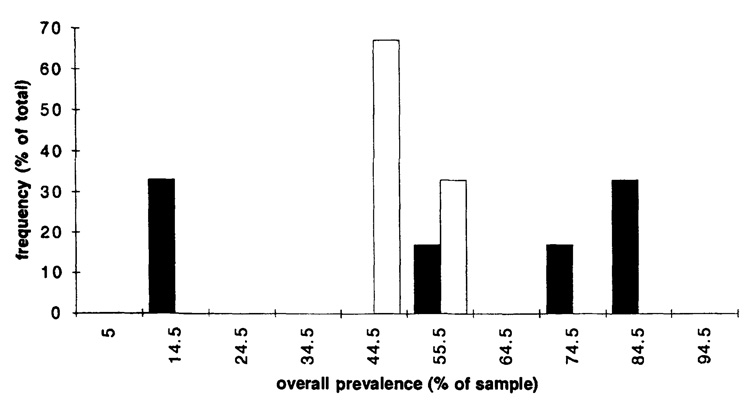
The deficit in mixed-species infections appears to be associated with overall prevalence of infection. Figure 1 and Figure 2 show distributional relationships between prevalence and G-test results for contingency tables within each sampling-unit group. The corresponding Mann–Whitney test results are: for the P. falciparum–P. vivax pair, for levels A and B, P < 0.0001, for level C, 0.0001 < P < 0.0005, for level D, 0.001 < P < 0.005; for P. falciparum–P. malariae, for level A (Fig. 2), P > 0.1. G-tests of the hypothesis that for P. falciparum–P. vivax an overall prevalence of 35% distinguishes nonsignificant from significant tables yield P < 0.0001 for all levels except D, for which 0.01 < P < 0.025; for D, 0.005 < P < 0.01 for a division at 30% prevalence. Transferring tables across rows or columns to attain P > 0.01 in these G-tests requires transfers equal to at least 28% of a marginal total for level A, 25% for B, 42% for C, and 9% for D. In sum, for the P. falciparum–P. vivax pair, this collection of tables shows a strong association between higher overall prevalences of infection and observed frequencies of mixed-species infection that are less than the product of individual species prevalences. This relationship does not appear to hold for P. falciparum–P. malariae, whereas for P. falciparum–P. vivax, it holds across 4 spatial-temporal levels of analysis.
FIGURE 1.
Relationships between overall prevalence of infection (horizontal axis) and frequency (vertical axis) of tables that show significant (solid bars) or nonsignificant (hollow bars) differences in mixed-species infections from the expected values for Plasmodium falciparum-Plasmodium vivax. Parts a–d show the results for levels of analysis A, B, C, and D, respectively (see text). The numbers on the horizontal axis represent midpoints of percentage prevalence intervals, e.g., 14.5 represents the interval 10–19%; numbers on the vertical axis represent percentages of the total number of tables within the relevant group (significant or nonsignificant) at that level.
FIGURE 2.
Relationships between overall prevalence of infection (horizontal axis) and frequency (vertical axis) of tables that show significant (solid bars) or nonsignificant (hollow bars) differences in mixed-species infections from the expected values for Plasmodium falciparum-Plasmodium malariae for level of analysis A (see text). Conventions are as in Figure 1.
A long-standing conservative rule of thumb with respect to contingency tables is that each cell count should be 5 or greater; values less than 5 may lead to too-frequent rejection of the null hypothesis (Sokal and Rohlf, 1981). Therefore, we used G and Mann–Whitney tests to check our results against the possibility of biased relationships between G-statistic significance and either the table sample sizes or the distributions of table entries with values less than 5 (and particularly zeroes). For P < 0.01 we found only 2 suggestions of such biases; in testing the categories 0–4 versus >4 for level A, we found significant deficits of entries in the significant 0–4 and nonsignificant >4 cells; in testing the categories 0 versus >0 for level B, we found significant deficits of entries in the significant 0 and nonsignificant >0 cells. In both cases, these biases would work against the principal patterns presented here.
Fox and Strickland (1989) noted that their conclusions relied on a prior demonstration by Fox et al. (1987) that the overall and species-specific prevalences found in surveys of village clinic attendees sufficiently mimicked those in community surveys. Because 16 of the P. falciparum–P. vivax studies used here acquired data either through clinics or with other techniques that collect blood only in cases of self-reported recent fevers, neither of which are random surveys of the population, we were concerned that the higher overall prevalence in these studies might bias our findings. To test for this possibility, we divided the contingency tables within each sampling unit into 2 subgroups, e.g., a clinical subgroup and a survey subgroup. The mean overall prevalence in the clinical subgroup was higher than the survey subgroup by 26% for level A, 14% for B, 29% for C, and 74% for D. We used the Mann–Whitney test to examine distributional differences between the constituent significant and nonsignificant categories within each subgroup, between the 2 significant categories across the subgroups, and between the 2 nonsignificant categories across the subgroups. All of the across-subgroup tests produced P-values greater than 0.025, and all but 1 of the within-subgroup tests produced P-values less than 0.01, with the sole discrepancy P = 0.025 for the comparison of significant and nonsignificant categories within the clinical subgroup for level D. That is, the association between higher overall prevalence and deficit of mixed-species infection holds for both subgroups.
Cohen’s (1973) analysis considered overall prevalence as tied solely to geography and considered seasonality only as 1 of 4 elements of site “ecology.” Richie (1988) considered variation in prevalence patterns over time at a given site. Few of the reports cited here allow further resolution of spatial, temporal, and age-related factors influencing overall and mixed-species infection prevalence; among those few we found no clear pattern, although data within each such report do support the prevalence-deficit relationship suggested above.
DISCUSSION
The key result of our analysis is that in human populations in which only P. falciparum and P. vivax are reported to cause malaria, high overall prevalence of infection is associated with significant deficits of dual infections. This association appears to be stronger and more general than suggested by our predecessors, whose analyses were based largely on studies in which 3 Plasmodium species were reported present. The lack of similar results for the P. falciparum–P. malariae pair and the lack of sensitivity to sample size argue that our result is not simply a byproduct of increases in microscopist workloads. Further-more, this association is apparently not completely explained by any of the additional factors we considered here, i.e., clinical status, site, month, year, or spatial-temporal scale of analysis.
Knowles and White (1930) recorded 15 instances that would meet our 2-species criteria, but only the report with the highest overall prevalence (73%) produces a contingency table significantly different from that expected; it shows a deficit of dual P. falciparum–P. vivax infections. Richie (1988) cites the unusual P. malariae–P. vivax report noted above, which yields tables with high overall prevalence (64–86%), but no significant departures from expected values. The only other 2-species report he cites shows 70% overall prevalence and a significant deficit of dual P. falciparum–P. vivax infections. The sole 2-species report used in Cohen’s analysis (1973) involves P. falciparum and P. malariae; it shows a 3% overall prevalence and a significant deficit of dual infections. His section on “parasite rate,” however, includes 3-species results that suggest that some overall prevalence between 30% and 47% might distinguish tables that are nonsignificant from those that are significant by his statistic “s” (see his table 1 and table 2). The remainder of his results address ecological factors, age groups, and spleen sizes; with a few exceptions (see especially table 5, from Peters and Standfast [1957]), they support the notion of a dividing line between 30 and 35% overall prevalence. Our results appear to be consistent with this reinterpretation.
Because prevalence describes frequencies of associations, it connotes a pattern of more specific biological interactions, for instance, at the levels of immunity, pathogenicity, or transmission. We do not know what processes generate the pattern reported here, but these seem to us the most critical areas for prospective empirical studies.
For instance, several older studies (Thomson, 1934; Wilson, 1936) mention the simultaneous presence of gametocytes of 2 (or more) species in dual infections; Shute (1946) remarked that gametocytes of both species were typically numerous in dual P. falciparum–P. vivax malariotherapy cases. Graves et al. (1988) noted that the presence of P. falciparum gametocytes appeared to reduce the infectivity of P. vivax, but not P. malariae, gametocytes when either was present concurrently. The studies we cite provide virtually no information regarding the transmission of mixed-species infections.
Thayer and Hewetson (1895) compared frequencies of inpatient admission and outpatient treatment to gauge the relative severities of P. falciparum and P. vivax infections; by this measure, mixed infections with the 2 putative species were more severe than infections with either alone. Unfortunately, few subsequent reports have examined the clinical implications of mixed-species malaria infections. One recent hospital study seems to support the notion that dual P. falciparum–P. vivax infection increases severity (Gopinathan and Subramanian, 1982, 1986) and another that there is no difference in clinical symptoms (Kremsner et al., 1989). Some of the data we extracted from a recent study of diagnostic criteria (Genton et al., 1994) suggest the possibility that mixed-species infections had a slightly beneficial effect, perhaps mediated by a higher prevalence of P. malariae; if so, this might fit the hypothesis of Black et al. (1994) that the incidence of fever in malaria and the presence of dual P. falciparum–P. malariae infection are inversely related.
Knowles and White (1930), Cohen (1988), and Richie (1973) each warned that the detection of prevalence involves densities and durations of infection as well as actual frequencies. Rosenberg et al. (1990) noted that in the Thai study, as at Garki, P. falciparum density was higher in dual than in single infections. In dual infections in the Pakistani study, the 2 species most often occurred at high or low densities simultaneously, which for Fox and Strickland (1989) argued against hypotheses of interspecific suppression (Mayne and Young, 1938; Molineaux et al., 1980). But again, these 2 reports are unique in the recent literature and, to the best of our knowledge, the most detailed information available about mixed-species infection dynamics in individual hosts remains malariotherapy charts. Figure 3 implies that even were all malaria infections mixed-species, a sampling of the intertwined patterns of dynamic infra- and suprapopulations could produce virtually any pattern of point-prevalence data. We suggest that in this sense some of the apparent deficits in mixed-species infections may represent both an artifact of method and a fact of parasite biology, rather than simply one or the other.
FIGURE 3.
An infection-history curve from the malariotherapy treatment of a neurosyphilis patient, case 220–1085 (Boyd and Kitchen, 1937). The vertical axis measures log 10 parasitized-erythrocyte (PE) density (per cc, from microscopy-based estimates); the horizontal axis counts days. The time course of Plasmodium falciparum asexual parasitemia (solid line) and gametocytemia (dashed line) and Plasmodium vivax asexual parasitemia (dotted line) is shown following inoculation of the patient (on day 0) by simultaneous feeding of 1 cage of Anopheles stephensi infectious for P. falciparum and another infectious for P. vivax (each infected for an unspecified period). The estimated detection threshold was 10 PE per cc (hence log 10 PE = 1).
We expect that molecular-level detection methods will encourage empirical studies of these multilayered patterns and the biological processes that beget them, and that from these we will learn much more about the association of high overall prevalences with deficits of dual infections. Despite our relatively cautious, coarse-grained analytical approach, like our predecessors we remain wary of the many methodological variables that may confound results within a single field study as well as comparisons between studies. Accordingly, we hope that our work will not be mistaken as definitive, but will instead stimulate a program of prospective studies, sufficiently well planned and well executed to incorporate such factors as parasitemias and gametocytemias. Gametocytes embody a critical immunity-mediated link between parasite dynamics at the individual-host and host-population levels and are clearly among the plausible but underappreciated currencies of Plasmodium species interactions. Given that the volume of blood processed in detecting a human infection has typically been less than that in a mosquito bloodmeal, the role of Plasmodium recruitment processes in mixed-species phenomena seems particularly opaque and intriguing.
TABLE III.
Data from 209 contingency tables.*
| SPP | LVL | SAMP | PRV | SIG | REF | SPP | LVL | SAMP | PRV | SIG | REF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F, M | A | 195 | 17 | N | 34 | F, V | B | 170 | 24 | N | 29 |
| 798 | 18 | 22 | 893 | 24 | 29 | ||||||
| 151 | 52 | 12 | 2,011 | 25 | 32 | ||||||
| 150 | 75 | 13 | 257 | 27 | 29 | ||||||
| 146 | 81 | 13 | 1,631 | 29 | 27 | ||||||
| 124 | 82 | 7 | 784 | 31 | 27 | ||||||
| 600 | 43 | S* | 31 | 1,685 | 33 | 27 | |||||
| 338 | 46 | 7 | 251 | 35 | 29 | ||||||
| 666 | 58 | 31 | 648 | 36 | 27 | ||||||
| B | 489 | 53 | N | 11 | 96 | 36 | 28 | ||||
| 296 | 78 | 13 | 295 | 43 | 29 | ||||||
| 1,266 | 51 | S* | 31 | 629 | 45 | 29 | |||||
| C | 462 | 55 | S* | 7 | 226 | 63 | 28 | ||||
| F, O | A | 189 | 58 | N | 26 | 1,694 | 15 | S | 29 | ||
| C | 1,130 | 27 | N | 24 | 3,684 | 19 | 29 | ||||
| 185 | 61 | 24 | 2,957 | 20 | 29 | ||||||
| D | 1,315 | 31 | N | 24 | 1,948 | 20 | 29 | ||||
| F, V | A | 251 | 5 | N | 32 | 3,980 | 20 | 29 | |||
| 418 | 10 | 18 | 2,871 | 21 | 29 | ||||||
| 1,178 | 11 | 32 | 3,563 | 22 | 29 | ||||||
| 1,076 | 12 | 32 | 791 | 23 | 27 | ||||||
| 98 | 13 | 3 | 6,586 | 23 | 29 | ||||||
| 442 | 14 | 32 | 2,323 | 24 | 29 | ||||||
| 377 | 16 | 19 | 1,020 | 25 | 29 | ||||||
| 587 | 17 | 32 | 2,677 | 25 | 29 | ||||||
| 407 | 18 | 32 | 3,196 | 33 | 27 | ||||||
| 58 | 19 | 16 | 3,877 | 34 | 27 | ||||||
| 194 | 20 | 32 | 1,139 | 35 | 27 | ||||||
| 912 | 22 | 32 | 1,997 | 35 | 27 | ||||||
| 22 | 23 | 16 | 1,014 | 36 | 27 | ||||||
| 864 | 24 | 32 | 826 | 36 | 27 | ||||||
| 39 | 26 | 16 | 466 | 36 | 29 | ||||||
| 15 | 27 | 21 | 521 | 38 | 29 | ||||||
| 52 | 27 | 16 | 395 | 38 | 29 | ||||||
| 504 | 31 | 16 | 510 | 40 | 27 | ||||||
| 885 | 32 | 15 | 458 | 43 | 28 | ||||||
| 266 | 34 | 16 | 474 | 43 | 29 | ||||||
| 333 | 37 | 32 | 553 | 44 | 28 | ||||||
| 87 | 38 | 16 | 723 | 45 | 29 | ||||||
| 321 | 41 | 15 | 156 | 47 | 28 | ||||||
| 207 | 43 | 16 | 281 | 47 | 28 | ||||||
| 637 | 47 | 16 | 2,002 | 48 | 27 | ||||||
| 61 | 51 | 16 | 691 | 48 | 27 | ||||||
| 120 | 51 | 10 | 389 | 48 | 29 | ||||||
| 1,698 | 53 | 16 | 452 | 48 | 29 | ||||||
| 446 | 56 | 16 | 653 | 49 | 2 | ||||||
| 244 | 60 | 16 | 864 | 50 | 29 | ||||||
| 23 | 61 | 16 | 603 | 50 | 29 | ||||||
| 70 | 61 | 2 | 251 | 52 | 27 | ||||||
| 112 | 71 | 15 | 202 | 54 | 28 | ||||||
| 930 | 16 | S* | 32 | 1,423 | 55 | 27 | |||||
| 131 | 37 | S | 2 | 605 | 56 | 27 | |||||
| 179 | 40 | 32 | 298 | 59 | 29 | ||||||
| 1,263 | 41 | 16 | 754 | 61 | 17 | ||||||
| 200 | 43 | 2 | 928 | 61 | 29 | ||||||
| 1,819 | 44 | 16 | 696 | 64 | 29 | ||||||
| 155 | 45 | 2 | 637 | 68 | 29 | ||||||
| 123 | 46 | 16 | 362 | 70 | 28 | ||||||
| 914 | 47 | 16 | 274 | 74 | 28 | ||||||
| 263 | 47 | 16 | 944 | 74 | 28 | ||||||
| 1,974 | 49 | 16 | 311 | 76 | 2 | ||||||
| 331 | 51 | 15 | C | 3,166 | 15 | N | 32 | ||||
| 168 | 51 | 16 | 831 | 17 | 30 | ||||||
| 456 | 51 | 16 | 382 | 18 | 6 | ||||||
| 1,025 | 53 | 16 | 2,381 | 19 | 32 | ||||||
| 2,277 | 56 | 16 | 339 | 20 | 6 | ||||||
| 1,226 | 56 | 16 | 1,182 | 22 | 32 | ||||||
| 1,360 | 57 | 16 | 1,342 | 29 | 23 | ||||||
| 3,313 | 59 | 16 | 322 | 31 | 6 | ||||||
| 188 | 60 | 1 | 258 | 33 | 6 | ||||||
| 83 | 60 | 15 | 826 | 25 | S | 4 | |||||
| 401 | 65 | 16 | 524 | 36 | 32 | ||||||
| 588 | 65 | 16 | 571 | 40 | 4 | ||||||
| 738 | 70 | 15 | 157 | 43 | 6 | ||||||
| 124 | 70 | 15 | 1,860 | 44 | 25 | ||||||
| 705 | 71 | 15 | 96 | 48 | 6 | ||||||
| 167 | 71 | 2 | 225 | 50 | 2 | ||||||
| 93 | 83 | 2 | 21,715 | 52 | 16 | ||||||
| 61 | 84 | 2 | 261 | 52 | 2 | ||||||
| 149 | 85 | 15 | 2,017 | 53 | 25 | ||||||
| 397 | 86 | 15 | 224 | 56 | 2 | ||||||
| 209 | 90 | 15 | 4,471 | 59 | 15 | ||||||
| B | 1,065 | 1 | N | 29 | 254 | 73 | 2 | ||||
| 746 | 1 | 29 | D | 13,245 | 5 | N | 5 | ||||
| 755 | 2 | 29 | 612 | 13 | 9 | ||||||
| 1,896 | 4 | 29 | 529 | 16 | 9 | ||||||
| 101 | 5 | 29 | 7,353 | 18 | 32 | ||||||
| 54 | 7 | 29 | 2,891 | 27 | 33 | ||||||
| 184 | 8 | 29 | 190 | 27 | 8 | ||||||
| 1,110 | 8 | 29 | 48 | 48 | 14 | ||||||
| 1,545 | 9 | 29 | 55,121 | 17 | S | 29 | |||||
| 3,714 | 10 | 29 | 1,554 | 28 | 6 | ||||||
| 1,900 | 10 | 29 | 1,435 | 29 | 9 | ||||||
| 1,272 | 10 | 29 | 1,397 | 31 | 4 | ||||||
| 2,646 | 10 | 29 | 25,515 | 35 | 27 | ||||||
| 2,168 | 12 | 29 | 1,358 | 37 | 20 | ||||||
| 2,699 | 13 | 32 | 7,309 | 46 | 35 | ||||||
| 2,108 | 14 | 29 | 9,897 | 46 | 29 | ||||||
| 753 | 14 | 27 | 15,473 | 53 | 35 | ||||||
| 796 | 17 | 27 | 8,200 | 55 | 35 | ||||||
| 2,643 | 17 | 32 | 12,209 | 57 | 35 | ||||||
| 714 | 18 | 27 | 964 | 58 | 2 | ||||||
| 150 | 20 | 29 | 2,510 | 59 | 28 | ||||||
| 182 | 23 | 27 | 1,042 | 59 | 28 | ||||||
| 309 | 23 | 29 |
Abbreviations: species, SPP; level of analysis, LVL; sample size, SAMP; overall prevalence, PRV (%); and statistical significance, SIG; where S indicates a table with an observed prevalence of mixed-species infection < the product of individual-species prevalences, S* the converse, and N a table with mixed-species prevalence equal to that product. Other abbreviations and reference (REF) numbers as in Table II.
ACKNOWLEDGMENTS
We gratefully acknowledge the support of the Maurice Pechet Foundation and the contributions of A.O. Bush, C. Cavanaugh, L. Chapman, K. Fischer, H. Kamowitz, D. Lieberman, C. Lindell, S. Sacks, K. Victor, 2 anonymous reviewers and the Countway and Mayr libraries at Harvard University. Some of this material is based upon preparatory work supported under a National Sciences Foundation Graduate Fellowship awarded to Ellis McKenzie.
LITERATURE CITED
- Adak T, Batra CP, Mittal PK, Sharma VP. Epidemiological study of malaria outbreak in a hotel construction site of Delhi. Indian Journal of Malariology. 1994;31:126–131. [PubMed] [Google Scholar]
- Ansari MA, Sharma VP, Razdan RK, Batra CP. Malaria situation in Meerut district villages (U.P.) Indian Journal of Malariology. 1986;23:147–150. [PubMed] [Google Scholar]
- Arai M, Mizukoshi C, Kubochi F, Kakutani T, Wataya Y. Detection of Plasmodium falciparum in human blood by a nested polymerase chain reaction. American Journal of Tropical Medicine and Hygiene. 1994;51:617–626. doi: 10.4269/ajtmh.1994.51.617. [DOI] [PubMed] [Google Scholar]
- Barker RH, Jr, Suebsaeng L, Rooney W, Wirth DF. Detection of Plasmodium falciparum infection in human patients: A comparison of the DNA probe method to microscopic diagnosis. American Journal of Tropical Medicine and Hygiene. 1989;41:266–272. [PubMed] [Google Scholar]
- Black J, Hommel M, Snounou G, Pinder M. Mixed infections with Plasmodium falciparum and P. malariae and fever in malaria. Lancet. 1994;i:1095. doi: 10.1016/s0140-6736(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Booth M, Bundy DAP. Comparative prevalences of Ascaris lumbricoides, Trichuris trichiura and hookworm infections and the prospects for combined control. Parasitology. 1992;105:151–157. doi: 10.1017/s0031182000073807. [DOI] [PubMed] [Google Scholar]
- Boyd MF, Kitchen SF. Simultaneous inoculation with Plasmodium falciparum and Plasmodium vivax. American Journal of Tropical Medicine. 1937;17:855–861. [Google Scholar]
- Brown AE, Kain KC, Pipithkul J, Webster HK. Demonstration by the polymerase chain reaction of mixed Plasmodium falciparum and P. vivax infections undetected by conventional microscopy. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992;86:609–612. doi: 10.1016/0035-9203(92)90147-5. [DOI] [PubMed] [Google Scholar]
- Choudhury DS, Sharma VP, Bhalla SC, Aggarwal SS, Das SK. Malaria prevalence in patients attending primary health centres in ten districts of Uttar Pradesh. Indian Journal of Malariology. 1987;24:79–83. [PubMed] [Google Scholar]
- Cohen JE. Estimation and interaction in a censored 2 × 2 × 2 contingency table. Biometrics. 1971;27:379–386. [PubMed] [Google Scholar]
- Cohen JE. Heterologous immunity in human malaria. Quarterly Review of Biology. 1973;48:467–489. doi: 10.1086/407705. [DOI] [PubMed] [Google Scholar]
- de Arruda M, Carvalho MB, Nussenzweig RS, Maracic M, Ferreira AW, Cochrane AH. Potential vectors of malaria and their different susceptibility to Plasmodium falciparum and Plasmodium vivax in northern Brazil identified by immunoassay. American Journal of Tropical Medicine and Hygiene. 1986;35:873–881. doi: 10.4269/ajtmh.1986.35.873. [DOI] [PubMed] [Google Scholar]
- Dobson AP. The population dynamics of competition between parasites. Parasitology. 1985;91:317–347. doi: 10.1017/s0031182000057401. [DOI] [PubMed] [Google Scholar]
- Dobson AP. Models for multi-species parasite-host communities. In: Esch GW, Bush AO, Aho JM, editors. Parasite communities: Patterns and processes. London, U.K.: Chapman and Hall; 1990. pp. 261–288. [Google Scholar]
- Dobson AP, Roberts M. The population dynamics of parasitic helminth communities. Parasitology. 1994;109:s97–s108. doi: 10.1017/s0031182000085115. [DOI] [PubMed] [Google Scholar]
- Dossou-Yovo J, Ouattara A, Doannio JMC, Riviere F, Chauvancy G, Meunier JY. Aspects du paludisme dans un village de savane humide de Cote d’Ivoire. Medecine Tropicale. 1994;54:331–336. [PubMed] [Google Scholar]
- Dutta P, Bhattacharyya DR. Malaria survey in some parts of Namsang circle of Tirap district, Arunachal Pradesh. Journal of Communicable Diseases. 1990;22:92–97. [PubMed] [Google Scholar]
- Dutta P, Bhattacharyya DR, Dutta LP. Epidemiological observations on malaria in some parts of Tengakhat PHC, Dibrugarh district, Assam. Indian Journal of Malariology. 1991;28:121–128. [PubMed] [Google Scholar]
- Dutta P, Bhattacharyya DR, Khan SA, Sharma CK, Goswami BK. Some observations on malaria in Boko PHC of Kamrup district, Assam. Journal of Communicable Diseases. 1994;26:52–55. [PubMed] [Google Scholar]
- Ejezie GC, Ezedinachi ENU, Usanga EA, Gemade EII, Ikpatt NW, Alaribe AAA. Malaria and its treatment in rural villages of Aboh Mbaise, Imo state, Nigeria. Acta Tropica. 1991;48:17–24. doi: 10.1016/0001-706x(90)90061-4. [DOI] [PubMed] [Google Scholar]
- Esch GW, Bush AO, Aho JM. Parasite communities: Patterns and processes. London, U.K.: Chapman and Hall; 1990. 335 pp. [Google Scholar]
- Fernandez J, Esch GW. Guild structure of larval trem-atodes in the snail Helisoma anceps: Patterns and processes at the individual host level. Journal of Parasitology. 1991;77:528–539. [PubMed] [Google Scholar]
- Fox E, Strickland GT. The interrelationship of Plasmodium falciparum and P. vivax in the Punjab. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1989;83:471–473. doi: 10.1016/0035-9203(89)90251-4. [DOI] [PubMed] [Google Scholar]
- Fox E, Strickland GT, Sarwar M, Shamim M, Zafar-Latif A, Khaliq AA. Reliable assessment of malaria prevalence through village clinics. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81:115–117. doi: 10.1016/0035-9203(87)90300-2. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM. Methods for pooled analyses of epidemiologic studies. Epidemiology. 1993;4:295–302. doi: 10.1097/00001648-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Gazin P, Boillot F, Ouedraogo JB, Carnevale P, Ambroise-Thomas P. Effectiveness of single dose treatment with chloroquine of malaria in West Africa and measurement of chloroquine urinary excretion. Annales de la Societe Belge de Medecine Tropicale. 1987;67:329–334. [PubMed] [Google Scholar]
- Genton B, Smith T, Baea K, Narara A, Al-Yaman F, Beck H-P, Hii J, Alpers M. Malaria: How useful are clinical criteria for improving the diagnosis in a highly endemic area? Transactions of the Royal Society of Tropical Medicine and Hygiene. 1994;88:537–541. doi: 10.1016/0035-9203(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Gopinathan VP, Subramanian AR. Pernicious syndromes in Plasmodium infections. Medical Journal of Australia. 1982;2:568–572. [PubMed] [Google Scholar]
- Gopinathan VP, Subramanian AR. Vivax and falciparum malaria seen at an Indian service hospital. Journal of Tropical Medicine and Hygiene. 1986;89:51–55. [PubMed] [Google Scholar]
- Graves PM, Burkot TR, Carter R, Cattani JA, Lagog M, Parker J, Brabin BJ, Gibson FD, Bradley DJ, Alpers MP. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua New Guinea. Parasitology. 1988;96:251–263. doi: 10.1017/s003118200005825x. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, Florida: Academic Press; 1985. 369 pp. [Google Scholar]
- Hedman P, Rombo L, Bjorkman A, Brohult J, Kihamia CM, Potter J, Stenbeck J. Sensitivity in vivo of Plasmodium falciparum to chloroquine and pyrimethamine/sulfadoxine in a coastal area of Tanzania. Annals of Tropical Medicine and Parasitology. 1986;80:7–11. doi: 10.1080/00034983.1986.11811979. [DOI] [PubMed] [Google Scholar]
- Kennedy CR. Ecological animal parasitology. Oxford, U.K.: Blackwell Scientific Publishers; 1975. 163 pp. [Google Scholar]
- Kennedy CR, Bush AO. Species richness in helminth communities: The importance of multiple congeners. Parasitology. 1992;104:189–197. doi: 10.1017/s0031182000060935. [DOI] [PubMed] [Google Scholar]
- Knowles R, White RS. Indian Medical Research Memoirs, No. 18. Calcutta, India: 1930. Studies in the parasitology of malaria; 436 pp. [Google Scholar]
- Kremsner PG, Zotter GM, Graninger W, Rocha RM, Bienzle U, Feldmeier H. Clindamycin is effective against Plasmodium falciparum but not against P. vivax in mixed infections. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1989;83:332–333. doi: 10.1016/0035-9203(89)90491-4. [DOI] [PubMed] [Google Scholar]
- Laserson KE, Petralanda I, Hamlin DM, Almera R, Fuentes M, Carrasquel A, Barker RH., Jr Use of the polymerase chain reaction to directly detect malaria parasites in blood samples from the Venezuelan Amazon. American Journal of Tropical Medicine and Hygiene. 1994;50:169–180. doi: 10.4269/ajtmh.1994.50.169. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S, White NJ, Chittamas S, Bunnag D, Harinasuta T. High rate of Plasmodium vivax relapse following treatment of falciparum malaria in Thailand. Lancet. 1987;ii:1052–1054. doi: 10.1016/s0140-6736(87)91479-6. [DOI] [PubMed] [Google Scholar]
- Lotz JM, Bush AO, Font WF. Recruitment-driven, spatially discontinuous communities: A null model for transferred patterns in target communities of intestinal helminths. Journal of Parasitology. 1995;81:12–24. [PubMed] [Google Scholar]
- Lotz JM, Font WF. The role of positive and negative associations in the organization of communities of intestinal helminthes of bats. Parasitology. 1991;103:127–138. doi: 10.1017/s0031182000059370. [DOI] [PubMed] [Google Scholar]
- Lotz JM, Font WF. Excess positive associations in communities of intestinal helminths of bats: A refined null hypothesis and a test of the facilitation hypothesis. Journal of Parasitology. 1994;80:398–413. [PubMed] [Google Scholar]
- Malhotra MS, Shukla RP, Sharma VP. Studies on the incidence of malaria in Gadarpur town of Terai, distt. Nainital, U.P. Indian Journal of Malariology. 1985a;22:57–60. [PubMed] [Google Scholar]
- Malhotra MS, Shukla RP, Sharma VP. A three year report of the malaria clinic in Haldwani, district Nainital, U.P. Indian Journal of Malariology. 1985b;22:123–126. [PubMed] [Google Scholar]
- Mathur KK, Harpalani G, Kalra NL, Murthy GGK, Narasimham MVVL. Epidemic of malaria in Barmer district (Thar desert) of Rajasthan during 1990. Indian Journal of Malariology. 1992;29:1–10. [PubMed] [Google Scholar]
- Mayne B, Young MD. Antagonism between species of malaria parasites in induced mixed infections. Public Health Reports. 1938;53:1289–1291. [Google Scholar]
- Molineaux L. The epidemiology of human malaria as an explanation of its distribution, including some implications for its control. In: Wernsdorfer WH, McGregor I, editors. Malaria. Edinburgh, U.K.: Churchill Livingstone; 1988. pp. 913–998. [Google Scholar]
- Molineaux L, Gramiccia G. Geneva, Switzerland: World Health Organization; The Garki Project. 1980
- Molineaux L, Storey J, Cohen JE, Thomas A. A longitudinal study of human malaria in the West African savanna in the absence of control measures: Relationships between different Plasmodium species, in particular P. falciparum and P. malariae. American Journal of Tropical Medicine and Hygiene. 1980;29:725–737. doi: 10.4269/ajtmh.1980.29.725. [DOI] [PubMed] [Google Scholar]
- Nalin DR, Mahood F, Rathor H, Muttalib A, Sakai R, Chowdhry MA, Safdar G, ul Haq I, Munir M, Suleiman M, Bashir M, Mujtaba SM. A point survey of periurban and urban malaria in Karachi. Journal of Tropical Medicine and Hygiene. 1985;88:7–15. [PubMed] [Google Scholar]
- Nguyen CD, Keystone JS. Mixed falciparum and vivax malaria in Canadian travellers. Canadian Medical Association Journal. 1989;141:101–104. [PMC free article] [PubMed] [Google Scholar]
- Noble ER. Fishes and their parasite-mix as objects for ecological studies. Ecology. 1960;41:593–596. [Google Scholar]
- Nosten F, Imvithaya S, Vincenti M, Delmas G, Lebihan G, Hausler B, White N. Malaria on the Thai-Burmese border: Treatment of 5192 patients with mefloquine-sulfadoxine-pyrimethamine. Bulletin of the World Health Organization. 1987;65:891–896. [PMC free article] [PubMed] [Google Scholar]
- Nosten F, ter Kuile F, Maelankirri L, Decludt B, White NJ. Malaria during pregnancy in an area of unstable endemicity. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1991;85:425–429. doi: 10.1016/0035-9203(91)90205-d. [DOI] [PubMed] [Google Scholar]
- Pagano M, Gauvreau K. Principles of biostatistics. Belmont, California: Duxbury Press; 1993. 524 pp. [Google Scholar]
- Peters W, Standfast H. Report on a malaria survey in the Sepik district. Medical Journal of Australia. 1957;1:861–868. doi: 10.5694/j.1326-5377.1957.tb59970.x. [DOI] [PubMed] [Google Scholar]
- Prasad RN, Sharma SN. Outbreak of malaria in Banda PHC of district Shahjahanpur (U.P.) Indian Journal of Malariology. 1990;27:47–50. [PubMed] [Google Scholar]
- Raccurt CP, Bourianne C, Lambert MT, Tribouley J, Mandji O, Amadou A, Bouloumie J, Ripert C. Indices paludometriques, ecologie larvaire et activite trophique des Anopheles a Djohong (Adamaoua, Cameroun) en saison des pluies. Medecine Tropicale. 1993;53:355–361. [PubMed] [Google Scholar]
- Ray P, Ansari MA, Sharma YD. Plasmodium vivax: Immune responses in a cross-section of the population in the Delhi area of India. American Journal of Tropical Medicine and Hygiene. 1994;51:436–443. [PubMed] [Google Scholar]
- Relf WA, Boreham RE, Tapchaisri P, Khusmith S, Healey A, Upcroft P, Tharavanij S, Kidson C. Diagnosis of Plasmodium vivax malaria using a specific deoxyribonucleic acid probe. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1990;84:630–634. doi: 10.1016/0035-9203(90)90128-2. [DOI] [PubMed] [Google Scholar]
- Richie TL. Interactions between malaria parasites infecting the same vertebrate host. Parasitology. 1988;96:607–639. doi: 10.1017/s0031182000080227. [DOI] [PubMed] [Google Scholar]
- Roche J, de Diego JA, Penin P, Santos M, del Rey J. An epidemiological study of malaria in Bioko and Annobon islands (Equatorial Guinea) Annals of Tropical Medicine and Parasitology. 1991;85:477–487. doi: 10.1080/00034983.1991.11812597. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Andre RG, Ngampatom S, Hatz C, Burge R. A stable, oligosymptomatic malaria focus in Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1990;84:14–21. doi: 10.1016/0035-9203(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Schad GA. Niche diversification in a parasitic species flock. Nature. 1963;198:404–406. [Google Scholar]
- Schapira A, da Costa F. Studies on malaria prophylaxis with chlorproguanil or chloroquine in Mozambique. Central African Journal of Medicine. 1988;34:44–49. [PubMed] [Google Scholar]
- Schuurkamp GJT. Ph.D. thesis. Port Moresby: University of Papua New Guinea; 1992. The epidemiology of malaria and filariasis in the Ok Tedi region of Western province, Papua New Guinea; 341 pp. [Google Scholar]
- Scott ME, Gibbs HC. Long-term population dynamics of pinworms (Syphacia obvelata and Aspiculuris tetraptera) in mice. Journal of Parasitology. 1986;72:652–662. [PubMed] [Google Scholar]
- Sharma VP, Uprety HC, Srivastava PK, Chandrahas RK. Studies on malaria transmission in hutments of Delhi. Indian Journal of Malariology. 1985;22:77–84. [PubMed] [Google Scholar]
- Shute PG. Latency and long-term relapses in benign tertian malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1946;40:189–200. doi: 10.1016/0035-9203(46)90056-9. [DOI] [PubMed] [Google Scholar]
- Singh N, Sharma VP, Shukla MM, Chand G. Malaria outbreak in Kundam block, district Jabalpur (M.P.) Indian Journal of Malariology. 1988;25:41–49. [PubMed] [Google Scholar]
- Singh N, Sharma VP, Mishra AK, Singh OP. Bio-environmental control of malaria in a tribal area of Mandla district, Madhya Pradesh, India. Indian Journal of Malariology. 1989;26:103–120. [PubMed] [Google Scholar]
- Singh N, Shukla MM, Srivastava R, Sharma VP. Prevalence of malaria among pregnant and non-pregnant women of district Jabalpur, Madhya Pradesh. Indian Journal of Malariology. 1995;32:6–13. [PubMed] [Google Scholar]
- Snounou G, Pinheiro L, Goncalves A, Fonseca L, Dias F, Brown KN, do Rosario VE. The importance of sensitive detection of malaria parasites in the human and insect hosts in epidemiological studies, as shown by the analysis of field samples from Guinea Bissau. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87:649–653. doi: 10.1016/0035-9203(93)90274-t. [DOI] [PubMed] [Google Scholar]
- Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Molecular and Biochemical Parasitology. 1993;58:283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York: W. H. Freeman Co., New York; 1981. 859 pp. [Google Scholar]
- Soro B, Davis CE, Peyron FM, Yao F, Saki ZM, Nguyen-Dinh P, Breman JG. Formulation of malaria treatment policy for children in Cote d’Ivoire as chloroquine resistant Plasmodium falciparum spreads into West Africa. Annals of Tropical Medicine and Parasitology. 1989;83:101–106. doi: 10.1080/00034983.1989.11812317. [DOI] [PubMed] [Google Scholar]
- Stock TM, Holmes JC. Functional relationships and microhabitat distributions of enteric helminths of grebes (Podicipedidae): The evidence for interactive communities. Journal of Parasitology. 1988;74:214–227. [PubMed] [Google Scholar]
- Strickland GT, Fox E, Hadi H. Malaria and splenomegaly in the Punjab. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988;82:667–670. doi: 10.1016/0035-9203(88)90188-5. [DOI] [PubMed] [Google Scholar]
- Strickland GT, Zafir-Latif A, Fox E, Khaliq AA, Chowdhry MA. Endemic malaria in four villages of the Pakistani province of Punjab. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81:36–41. doi: 10.1016/0035-9203(87)90274-4. [DOI] [PubMed] [Google Scholar]
- Sulzer AJ, Cantella R, Colichon A, Gleason NN, Walls KW. A focus of hyperendemic Plasmodium malariae–P. vivax with no P. falciparum in a primitive population in the Peruvian Amazon jungle. Bulletin of the World Health Organization. 1975;52:273–278. [PMC free article] [PubMed] [Google Scholar]
- Takagi T, Kano S, Masuda G, Suzuki M. Submicroscopic double infection of Plasmodium falciparum in P. vivax malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988;82:384. doi: 10.1016/0035-9203(88)90127-7. [DOI] [PubMed] [Google Scholar]
- Thayer WS, Hewetson J. The malarial fevers of Baltimore. Baltimore, Maryland: The Johns Hopkins Press; 1895. 218 pp. [Google Scholar]
- Thomson JG. Malaria in Nyasaland. Proceedings of the Royal Society of Medicine. 1934;28:391–404. [PMC free article] [PubMed] [Google Scholar]
- Warsame M, Perlmann H, Ali S, Hagi H, Farah S, Lebbad M, Bjorkman A. The seroreactivity against Pfl55 (RESA) antigen in villagers from a mesoendemic area in Somalia. Tropical Medicine and Parasitology. 1989;40:412–414. [PubMed] [Google Scholar]
- Wilson DB. Rural hyper-endemic malaria in Tanganyika territory. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1936;29:583–619. doi: 10.1016/0035-9203(62)90050-0. [DOI] [PubMed] [Google Scholar]
- Yadav RN, Tiwari SN, Tyagi PK, Kulshrestha AK, Prakash A. Malaria in Shankargarh PHC, Allahabad district (U.P.): a clinical report. Indian Journal of Malariology. 1993;30:9–16. [PubMed] [Google Scholar]