Abstract
Purpose
To determine whether key associated features of hyperandrogenic anovulation (HA) in predominately Caribbean-Hispanic (CH) adolescent girls can be combined to improve the early diagnosis of HA.
Methods
Unselected observational sample of females aged 12 to 21 years, (mean 17.5±2.4 yrs), (64% CH, 28% African-American (AA)). 120 subjects provided a menstrual history, had a physical examination, and a follicular phase fasting blood drawn for LH, FSH, testosterone (T), sex hormone binding globulin (SHBG), 17-OH progesterone (17-OHP), androstenedione (Δ4A), glucose and insulin. We prospectively categorized subjects into four groups: G I (n=42) had normal menses and normal physical exam; G II (n=41) had normal menses and abnormal physical exam, ie. signs indicating possible hyperandrogenism and/or insulin resistance, including at least one of obesity, hirsutism, acne, or acanthosis nigricans (AN); G III (n=15) had abnormal menses and normal physical exam, and G IV (n=22) had HA with BOTH abnormal menses and abnormal physical exam, i.e. girls most likely to develop polycystic ovary syndrome. Hormonal levels and additional clinical and physical characteristics of interest were compared among the 4 groups.
Results
Group IV subjects had significantly higher waist circumference measurements, independent of overweight status, than all other groups. As hypothesized, Group IV subjects had significantly higher androgen levels and significantly lower SHBG levels than all other groups. FAI, SHBG, and waist circumference had the highest diagnostic accuracy for predicting Group IV status (i.e. HA phenotype).
Conclusions
Markers of insulin resistance and hyperandrogenemia, including waist circumference, FAI, and SHBG best associate with irregular menstrual cycles and the HA phenotype in ethnic minority adolescent girls.
Keywords: Hyperandrogenic anovulation, PCOS, Overweight, Adolescents, Hyperandrogenemia
INTRODUCTION
Although hyperandrogenic anovulation (HA) begins at or before puberty, diagnosing the condition in adolescents is not straight forward. It would be useful to identify HA early in life as girls with this menstrual disorder are at risk for adult polycystic ovary syndrome (PCOS) and its complications. Both the NIH and the Rotterdam criteria assist in the diagnosis of PCOS in adults, (1, 2) however, both have limitations for diagnosis in adolescents. The relatively short duration of exposure to elevated androgens in peri-menarchal girls is believed to be less likely to lead to hirsutism and other clinical signs of androgen excess than is characteristic of adults with PCOS. Evaluation of ovarian morphology by transvaginal ultrasound is often not possible in adolescents. Infertility is rarely diagnosed in adolescents. In our clinical experience with ethnic minority adolescents, HA is underdiagnosed. We believe this is because such adolescents often lack the classic clinical criteria for HA or PCOS including hirsutism or acne. Since obesity per se is associated with insulin resistance and increased androgen production by the ovaries,(3) we reasoned that it might serve as a potential early clinical predictor of HA.
Relative to non-Hispanic white women, Caribbean-Hispanic (CH) ethnicity and PCOS independently and additively decrease insulin action in adult women.(4) In a sequential, clinical, convenience sample of predominantly CH and African American adolescent girls, we categorized a proposed clinical spectrum of HA (see definition, page 5, under Clinical Definitions) using the traditional markers of menstrual irregularity, hirsutism, and acne and adding physical markers of insulin resistance, obesity and acanthosis nigricans. We then tested the relationships among our clinical categories and hormonal levels as well as additional physical characteristics of interest, clitoral size and waist circumference. We hypothesized that both serum androgen levels and anthropometric markers of insulin resistance would be strongly associated with menstrual irregularity and would thus assist in the early detection of HA. We also assessed the accuracy of these hormonal and physical measures for detecting HA.
METHODS
Subjects
Between July 2001 and June 2004, female patients aged 12 to 21 years were consecutively approached in the waiting rooms of the general pediatric outpatient clinics, the emergency department (ED), and on the adolescent inpatient floor at the Children’s Hospital at Montefiore. The study was approved by the Institutional Review Board of Montefiore Medical Center. We obtained informed consent from subjects and their parents.
We excluded premenarchal girls younger than age 15. We included premenarchal girls aged 15 or older with secondary sexual characteristics to be sure not to miss girls with HA with late menarche. We excluded adolescents with serious chronic illnesses, those who were taking hormonally active or anticonvulsant medications, as well as girls who had been pregnant within the past six months. Girls who had previously taken hormonal contraceptives were included only if they had resumed spontaneous menses.
Of 444 girls approached, 88 refused to participate and 198 were excluded (132 were taking hormones (mostly contraceptives), 29 were recently pregnant, 33 had a serious chronic illness, and four were premenarchal). Of the 158 eligible subjects, 120 completed the entire protocol.
Four per cent of the 120 subjects were recruited from the inpatient floor, 76% from the clinics, and 20% from the ED. Half of the subjects were being seen for routine health evaluations and 40% for a health problem, e.g. injury, infection. Only 10% had a chief complaint related to the menstrual cycle. This clinical sample represented the population of adolescents seen at the Children’s Hospital at Montefiore and did not reflect a specialized endocrine sample.
Measures
Questionnaire
Upon recruitment, all subjects completed a 36-item multiple-choice questionnaire. Specific questions used for this analysis related to age at menarche, the number of menstrual periods over the preceding year, and a history of menstrual cycle abnormality over the preceding year.
Clinical Definitions
Unlike adult women, normal cycle length for adolescents ranges from 21 to 42 days with more cycle length variability and a median cycle length of 34 days.(5,6) Since inquiring about the number of days between periods can be confusing for adolescents, cycle patterns were determined by asking subjects to recall the total number of menstrual periods over the preceding year.
We defined normal menstrual cycles when subjects reported having between 10 and 12 periods over the past year. Secondary amenorrhea was defined as the absence of menses for six months or longer in a subject who previously had menstrual periods.(7) We defined oligomenorrhea when subjects reported nine or fewer menses over the past year.
We defined hyperandrogenism when subjects had clinical evidence of androgen excess, including hirsutism or acne. We defined insulin resistance when subjects had clinical evidence of hyperinsulinemia, including overweight and acanthosis nigricans. We defined hyperandrogenic anovulation (HA) when subjects had clinical evidence of hyperandrogenism and/or insulin resistance and symptoms of anovulation, namely secondary amenorrhea or oligomenorrhea. The rationale for including physical signs of insulin resistance as well as hyperandrogenism in the clinical definition of HA was that adolescent girls with elevated androgen and/or insulin levels, that might predestine them to overt HA in the future, might not have physical evidence of hyperandrogenism at the time of the study.
Physical Examination
One of three cross-trained investigators performed a standardized physical examination on each subject at the time of recruitment. Height and weight were recorded and the BMI calculated. We used the Centers for Disease Control and Prevention (CDC) definition of “overweight”, for children aged 2 to 19 years, as a BMI ≥ 95th percentile for age and sex.(8–11) In accordance with the Anthropometric Standardization Reference Manual guidelines, waist circumference was measured to the nearest centimeter.. (12) Hirsutism was defined by a Ferriman Gallwey score (FGS) of ≥ 8. (13) Acne severity was graded according to the number of papulopustular lesions. We recorded acne only if subjects had 10 or more lesions. (14, 15) Clitoral size was defined as the length of the clitoral glans multiplied by the width to obtain a clitoral index and was measured using calipers. (16) High interobserver agreement was demonstrated for clitoral index measurements repeated on 21 subjects by two of the investigators (correlation coefficient of 0.89, 95% CI 0.74–0.96). Finally, the presence and location of acanthosis nigricans was recorded.
Laboratory Studies
After an overnight fast, subjects returned between 8:00 am and 10:00 am in the early follicular phase (day 2–10) of their next menstrual cycle for blood drawing. Oligomenorrheic or amenorrheic subjects were asked to complete blood-drawing on the day of recruitment, if willing and fasting. Serum was tested for LH, FSH, testosterone (T), sex hormone binding globulin (SHBG), 17-OH progesterone (17-OHP), androstenedione (Δ4A), glucose and insulin. Free Androgen Index (FAI) was calculated from testosterone and SHBG values [FAI= T(nmol/L) × 100/SHBG(nmol/L)]. LH and FSH were measured in duplicate using a solid-phase, two-site fluoroimmunometric assay (DELFIA, Perkin Elmer; Gaithersburg, MD: sensitivity 0.05 U/L and 0.05 U/L, intra-assay coefficient of variation (CV) 2.9% and 3.2%, and inter-assay CV 5.5% and 8.7%, respectively). (17, 18) Testosterone was measured in duplicate using time-resolved fluoroimmunoassay (DELFIA: sensitivity 0.3nmol/L intra-assay CV was 22.8% at a mean of 0.2 nmol/L and 16.9% at a mean of 3.71 nmol/L and inter-assay CVs were 10.3% and 17.7% at the same levels). A second testosterone assay system was also used to confirm results (DSL, Webster, TX) and had similar parameters. SHBG and 17-OHP were assayed using commercially available reagents from Diagnostic Products Corporation (DPC) (Los Angeles, CA: SHBG sensitivity 0.5nmol/L, intra-assay CV 4.3% and inter-assay CV 8.6%). (19, 20) Androstenedione was measured in duplicate using the Coat-a-Count assay from DPC (sensitivity 0.140 nmol/L, intra-assay CV 5.7 % and inter-assay CV 8.4%). Serum glucose was analyzed by the Olympus AU400 Chemistry Autoanalyzer using the Olympus Glucose Kit. Serum insulin was measured by radioimmunoassay in the Hormone Assay Laboratory of the Einstein Diabetes Research and Training Center. (21)
Subjects with amenorrhea or oligomenorrhea all had thyroid function studies and prolactin levels measured and all were normal. Subjects with physical examination findings suggestive of virilization (including male-pattern balding, breast atrophy, and deep voice), as well as those with androgen levels exceeding assay upper limit norms (T, Δ4A, or fasting 17-OHP level exceeding 200ng/dL, 500 ng/dl, or 400 ng/dl, respectively) underwent additional evaluation to rule out adrenal hyperplasia or tumors. Only one subject, a girl with normal menstrual cycles and physical examination, had a 17-OHP level > 400 ng/dL, and an ACTH-stimulation test was normal. Eleven subjects had a T level persistently > 200 ng/dL or a Δ4A persistently > 500 ng/dL. Of these, six had hyperandrogenic anovulation, based upon a benign history of hyperandrogenism and/or insulin resistance, abnormal menstrual cycles, and no abnormal mass findings on pelvic/adrenal ultrasound and five subjects were lost to follow-up, all of whom had normal menstrual cycles and normal physical exam.
Categorization of the Clinical Spectrum of Hyperandrogenic Anovulation (HA) in Adolescents
We prospectively developed a method of categorizing the expected clinical spectrum of HA and then applied this categorization to our subjects. The first clinical Group I, defined as “normal”, consisted of subjects with normal menstrual cycles and no physical signs of hyperandrogenism and/or insulin resistance. Group II subjects had normal menstrual cycles but had physical signs of hyperandrogenism and/or insulin resistance, including at least one of: overweight, hirsuitism, acne, or acanthosis nigricans. Group III subjects had abnormal menstrual cycles, either secondary amenorrhea or oligomenorrhea, but no physical signs of hyperandrogenism and/or insulin resistance. Subjects in Group IV had BOTH abnormal menstrual cycles and physical signs of hyperandrogenism and/or insulin resistance. Group IV was categorized by us as most likely to have HA and to develop polycystic ovary syndrome as defined by adult criteria.
DATA ANALYSIS
To determine the association of hormonal levels and additional physical characteristics with our prospective clinical groupings of subjects, serum T, Δ4A, SHBG, LH, LH:FSH ratios, glucose, and glucose:insulin ratios as well as clitoral size and waist circumference were compared among Groups I to IV, using ANOVA and post-hoc analysis with the Scheffe test. Non-parametric analyses using the Kruskal-Wallis test and Mann-Whitney test were performed if the assumptions of ANOVA were violated. Bivariate correlations were performed to evaluate the relationships between the various hormonal and physical characteristics. To assess the accuracy of select hormonal tests and additional physical characteristics for distinguishing Group IV subjects (i.e. diagnosing HA by our clinical categorization), Receiving Operating Characteristic (ROC) analysis was performed. ROC curves were generated for these tests, using clinical categorization [Group I (normal) versus Group IV (HA)] as the outcome variable. The tests were compared by calculating the area under the curve and inflection points of curves were sought. The 95% confidence interval for each test and sensitivity and specificity were calculated. To assess the effect of including physical signs of insulin resistance, overweight and/or acanthosis nigricans, in our clinical categorization of HA, we conducted additional analyses, including removing all subjects with overweight and/or acanthosis nigricans. All statistical analyses were conducted with SPSS 13.0 for Windows.
RESULTS
Demographic Characteristics of the Sample
Of 120 subjects, 20% were between the ages of 12 and 15 years, 33% were between the ages of 15 and 18 years, and 47% were between the ages of 18 and 21 years. Mean age was 17.5 ± 2.4 years. Overall, 19% were overweight (BMI≥ 95th percentile). Ten to 12 menstrual cycles per year were reported by 62% and nine subjects reported between 13 and 17 cycles per year and thus remained within the normal cycle length ranges of 21 to 42 days. Thirty-seven subjects (31%) had nine or fewer menstrual cycles per year; 11/37 had secondary amenorrhea and one was aged 15 and premenarcheal. Acanthosis nigricans was documented in 33% of the total sample, acne in 15% and hirsutism in 15%.
Demographic Characteristics by Category
Of the 120 subjects, we designated 42 as Group I, 41 as Group II, 15 as Group III, and 22 subjects as Group IV. Thus 22/120 (18%) of this non-endocrine sample of primarily ethnic minority adolescents fulfilled our prospective clinical diagnostic criteria for hyperandrogenic anovulation (HA). (Table 1a)
Table 1.
| Table 1a. Characteristics of Subjects by Category | |||||
|---|---|---|---|---|---|
| Normal | Abnormal physical exam | Abnormal cycles | Abnormal physical exam & abnormal cycles | ||
| Group I (N=42) | Group II (N=41) | Group III (N=15) | Group IV (N=22) | P value | |
| Demographic Characteristics | |||||
| Age, mean (SD), yrs | 17.4 (2.47) | 17.4 (2.48) | 17.0 (1.68) | 18.0 (2.46) | 0.660 |
| Ethnic origin No. (%)
Hispanic Black White Other |
23 (54.8) 14 (33.3) 3 (7.1) 2 (4.8) |
25 (61.0) 11 (26.8) 4 (9.8) 1 (2.4) |
9 (60.0) 4 (26.7) 1 (6.7) 1 (6.7) |
16(72.7) 4 (18.2) 0(0) 2 (9.1) |
0.5811 0.6391 0.5291 0.6981 |
| Age at Menarche, median, yrs | 11.0 | 12.0 | 13.0 | 11.5 | <0.012 |
| Gynecologic Age, median, yrs | 5.7 | 6.7 | 3.9 | 6.5 | 0.0342 |
| Physical Exam Findings | |||||
| Body Mass Index, mean (SD) kg/m2 | 23.1 (3.19) | 27.6 (9.35) | 22.4 (2.98) | 32.2 (7.36) | <0.013 |
| Overweight (≥95th % ile for age) (%) | - | 32 | - | 50 | 0.1541 |
| Waist Circumference, mean (SD), cm | 70.2 (6.49) | 77.7 (14.14) | 71.2 (7.42) | 89.4 (15.86) | <0.014 |
| Ferriman Gallwey Score, Median | 2 | 2 | 2 | 5.5 | <0.015, * |
| Clitoral Index, Median, mm2 | 18 | 26 | 21 | 35 | <0.016, * |
| Hirsutism (%) | - | 24 | - | 36 | 0.3161 |
| Acanthosis Nigricans (%) | - | 54 | - | 77 | 0.0661 |
| Acne (%) | - | 27 | - | 32 | 0.6761 |
| Table 1b. Characteristics by Category: Analysis Restricted to Subjects without Overweight and/or Acanthosis Nigricans* | |||||
| Normal | Abnormal physical exam | Abnormal cycles | Abnormal physical exam & abnormal cycles | ||
| Subjects with BMI<95th %ile (n=96)* | Group I (N=42) | Group II (N=28) | Group III (N=15) | Group IV (N=11) | P value (ANOVA) |
| Body Mass Index, mean (SD) kg/m2 | 23.1 (3.2) | 23.2 (3.9) | 22.4 (3.0) | 26.9 (2.7) | <0.01a |
| Waist Circumference, mean (SD), cm | 70.2 (6.5) | 71.7 (8.2) | 71.2 (7.4) | 79.0 (8.6) | <0.01b |
| Ferriman Gallwey Score, Median | 2 | 2 | 2 | 5 | 0.03c |
| Clitoral Index, Median, mm2 | 18 | 24 | 21 | 30 | 0.07d |
| Subjects without Acanthosis Nigricans (n=81)* | Group I (N=42) | Group II (N=19) | Group III (N=15) | Group IV (N=5) | P value (ANOVA) |
| Body Mass Index, mean (SD), kg/m2 | 23.1 (3.2) | 24.6 (5.8) | 22.4 (3.0) | 27.9 (3.1) | 0.03e |
| Waist Circumference, mean (SD), cm | 70.2 (6.5) | 74.1 (11.1) | 71.2 (7.4) | 79.9 (10.7) | 0.05 |
| Ferriman Gallwey Score, Median | 2 | 3 | 2 | 2 | 0.38d |
| Clitoral Index, Median, mm2 | 18 | 25 | 21 | 15 | 0.23d |
| Subjects with BMI<95th %ile and no Acanthosis Nigricans (n=75)* | Group I (N=42) | Group II (N=14) | Group III (N=15) | Group IV (N=4) | P value (ANOVA) |
| Body Mass Index, mean (SD), kg/m2 | 23.1 (3.2) | 21.9 (3.9) | 22.4 (3.0) | 27.6 (3.4) | 0.02f |
| Waist Circumference, mean (SD), cm | 70.2 (6.5) | 68.8 (6.7) | 71.2 (7.4) | 80.6 (12.2) | 0.03g |
| Ferriman Gallwey Score, Median | 2 | 3 | 2 | 2 | 0.30d |
| Clitoral Index, Median, mm2 | 18 | 25 | 21 | 20 | 0.27d |
SD – standard deviation
p<0.05 for significantly positive trend from group I to IV by Kendall’s Tau_b and Spearman’s rho.
Chi-square
Kruskal-Wallis
ANOVA, Post-hoc Scheffe (group I vs. II, p=0.03, group I vs. IV, group III vs. IV, p<0.01)
ANOVA, Post-hoc Scheffe (group I vs. II, p=0.04, group I vs. IV, group II vs IV, group III vs. IV, p<0.01)
Kruskal-Wallis, Mann-Whitney (group IV vs groups I, II, III, p<0.05)
Kruskal-Wallis, Mann-Whitney (group I vs II, group III vs IV, p<0.03, group I vs IV, p<0.01,)
SD – standard deviation,
Of the 120 subjects, 6 were overweight, 21 had acanthosis nigricans, and 18 were both overweight and had acanthosis nigricans
ANOVA, Post-hoc Scheffe (group I vs. IV, p=0.01, group II vs. IV,p=0.02, group III vs. IV, p=0.01).
ANOVA, Post-hoc Scheffe (group I vs. IV, p<0.01, group II vs. IV,p=0.05, group III vs. IV, p=0.07).
Kruskal-Wallis, Mann-Whitney (group I vs II p=0.05, Group I vs IV, p<0.01, group III vs IV, p=0.07)
Kruskal-Wallis.
ANOVA, Post-hoc Scheffe (group I vs. IV, p=0.08, group II vs. IV,p=0.06).
ANOVA, Post-hoc Scheffe (group I vs. IV, p=0.08, group II vs. IV,p=0.03, group III vs. IV, p=0.05).
ANOVA, Post-hoc Scheffe (group I vs. IV, p=0.05, group II vs. IV,p=0.04).
There were no significant differences in the mean age and ethnicity among the four groups. Median age at menarche and gynecologic age (age at recruitment - age at menarche) differed significantly among the four groups in a manner that was not congruent with clinical grouping. These differences persisted after removing subjects with overweight or acanthosis nigricans from the analysis.
Physical Characteristics by Category
Subjects in Group IV had significantly higher median waist circumference and FGS measures than the other groups. (Table 1a) When we removed from the analysis all overweight subjects and/or all subjects with acanthosis nigricans (Table 1b), differences in BMI and waist circumference persisted among the four groups with Group IV subjects having significantly higher BMI and waist circumference than all other groups. Subjects in Group IV without obesity or acanthosis nigricans but who fulfilled our prospective diagnostic criteria for HA because of hirsutism or acne were still significantly heavier with higher waist circumferences than subjects in the other groups.
Hormonal Comparisons by Category
i. Androgen Comparisons
T, SHBG, FAI, Δ4A, LH and LH:FSH ratios all differed significantly among the four groups. (Table 2) Group IV subjects had higher FAI and lower SHBG than all other groups. There was a significant positive trend in T, FAI, Δ4A, and LH levels, and LH:FSH ratios, and a significant negative trend in SHBG levels from Group I to Group IV.
Table 2.
Comparison of Androgen Levels, Glucose, and Glucose: Insulin Ratios Among the Four Groups
| Normal | Abnormal physical exam | Abnormal Cycles | Abnormal physical exam& abnormal cycles | ||
|---|---|---|---|---|---|
| Group I (N=42*) | Group II (N=41*) | Group III (N=15*) | Group IV (N=22*) | P value (ANOVA) | |
| T, mean, ng/dL (SD) | 65.6 (43.5) | 62.0 (28.4) | 77.6 (70.4) | 99.3(71.2) | <0.051,** |
| SHBG, mean, ug/dL (SD) | 1.8 (0.7) | 1.5 (0.8) | 1.5 (0.8) | 0.8 (0.3) | <0.012,*** |
| FAI, mean (SD) | 4.3 (4.0) | 5.8 (5.0) | 6.2 (5.3) | 17.3 (24.7) | <0.013,** |
| Δ 4 A, mean, ng/dL (SD) | 294.0 (124.0) | 311.6 (114.1) | 343.7 (177.4) | 446.5 (187.1) | <0.014, ** |
| LH, mean, IU/L (SD) | 6.2 (8.2) | 4.8 (3.0) | 9.3 (6.4) | 9.2 (4.8) | <0.015, ** |
| LH:FSH ratio (SD) | 1.2 (1.0) | 1.1 (1.1) | 2.8 (4.8) | 1.9 (1.1) | <0.016, ** |
| Fasting glucose, median (range) (mg/dL) | 84.0 (55–99) | 84.0(55–101) | 85.0(58–91) | 83.5 (70–101) | 0.979 |
| GIR, median (range) | 6.6 (2.4–21) | 4.7 (1.4–17.8) | 5.4 (3.8–22.8) | 3.9 (1.2–21.5) | <0.017,*** |
SD – standard deviation GIR – Glucose: Insulin Ratio
for SHBG, Δ4A, LH, and FSH, Ns are 41, 41, 15, and 21 for GI, GII, GIII, and GIV, respectively, as the androgen levels in 2 samples were not done; for T and FAI, N is 36, 36, 14, and 19 for GI, GII, GIII, and GIV, respectively, as testosterone levels in 15 samples were not done; for glucose and insulin levels all 120 subjects were tested.
p<0.05 for significantly positive trend from group I to IV by Kendall’s Tau_b and Spearman’s rho.
p<0.01 for significantly negative trend from group I to IV by Kendall’s Tau_b and Spearman’s rho.
Post-hoc Scheffe (group II vs. IV, p=0.08):
Post-hoc Scheffe (group I vs. IV, p<0.01, group II vs. IV, p<0.01, group III vs IV, p<0.05)
Post-hoc Scheffe (group I vs. IV, II vs IV, III vs IV, p<0.01)
Post-hoc Scheffe (group I vs. IV, p<0.01, group II vs. IV, p<0.01)
Post-hoc Scheffe (group II vs. IV, p<0.06)
Post-hoc Scheffe (group II vs. III, p=0.04)
Kruskal-Wallis, Mann-Whitney (group I vs II, I vs IV, III vs IV, p<0.01)
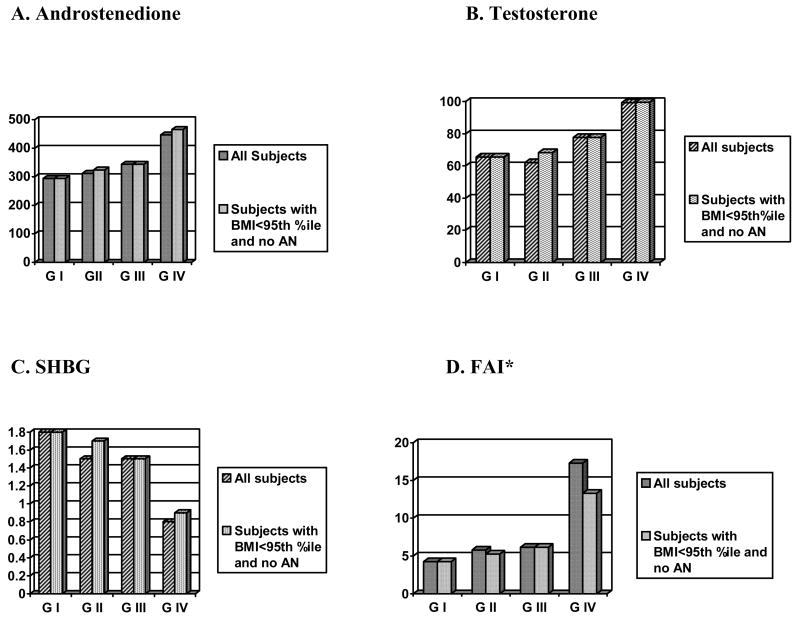
To assess the sensitivity of our hormonal levels, we re-analyzed the data after removing all overweight subjects, and the differences among the androgens persisted. When we removed all subjects with acanthosis nigricans or all subjects with hirsutism (analysis not shown), the significant differences persisted only in SHBG and FAI. When we removed subjects with both acanthosis nigricans and overweight, the significant differences persisted only in FAI; while not statistically significant, the T and Δ4A increased and the SHBG decreased with increasing group number (I to IV). (Figure 1)
Figure 1. Androgen Comparisons Among Groups Excluding Subjects with Both Overweight (BMI>95th %ile) and Acanthosis Nigricans (AN).
* For FAI: ANOVA (p<0.05)/Post-hoc Scheffe, Group I vs IV, (p<0.05), II vs IV, (p=0.09)
All subjects: For SHBG, Δ4A - Ns are 41, 41, 15, and 21 for GI, GII, GIII, and GIV, respectively; for T and FAI - N is 36, 36, 14, and 19 for GI, GII, GIII, and GIV, respectively. Subjects with BMI<95th % ile and no AN: For SHBG, Δ4A - Ns are 41, 14, 15, and 4 for GI, GII, GIII, and GIV, respectively; for T and FAI, N is 36, 12, 14, and 3 for GI, GII, GIII, and GIV, respectively.
ii. Differences by Ethnicity
Of the 120 subjects, 73 (61%) were Caribbean-Hispanic (CH) and 33 (28%) were African-American (AA). To test if the differences in androgen levels found in the entire sample (n=120) differed in CH subjects, median T, SHBG, FAI, Δ4A, and LH levels were compared among Groups I through IV for only the CH subjects using non-parametric analyses. Except for T (p=0.13), the statistical outcomes remained the same in the CH group (p<0.01 for SHBG; p<0.01 for FAI; p=0.037 for Δ4A; and p=0.013 for LH.) The trends from Group I to IV for Hispanic subjects were consistent with those seen for the entire sample.
iii. Glucose and Insulin Comparisons
Of the 120 subjects, only 3 had a borderline impaired fasting glucose level of 101 mg/dL (>100mg/dL) and the median glucose levels of subjects did not differ among the four groups (Table 2). As expected, the glucose:insulin ratios (GIRs), however, were different among the four groups with a significant negative trend from Group I to IV. Group I subjects (normals) had higher median GIR than subjects in Groups II and IV. When we removed all overweight subjects and/or all subjects with acanthosis nigricans from the analysis, the differences in the GIRs no longer persisted among the four groups.
Bivariate Correlations
In the total sample of 120 subjects, FAI and Δ4A were significantly positively associated with BMI, waist circumference, FGS, and clitoral index (all p<0.01 and p<0.05, respectively) and SHBG was significantly negatively associated with the same physical characteristics (all p<0.01). When overweight subjects were removed, the association between all physical characteristics and FAI persisted (p<0.01); the association of Δ4A with FGS was no longer significant and the associations of SHBG with BMI and clitoral index were also no longer significant. We also found a significant positive association between BMI and clitoral index, FGS, and waist circumference (all p<0.01).
Accuracy of Select Hormonal Tests for Diagnosing Hyperandrogenic Anovulation (HA) Using our Clinical Categorization
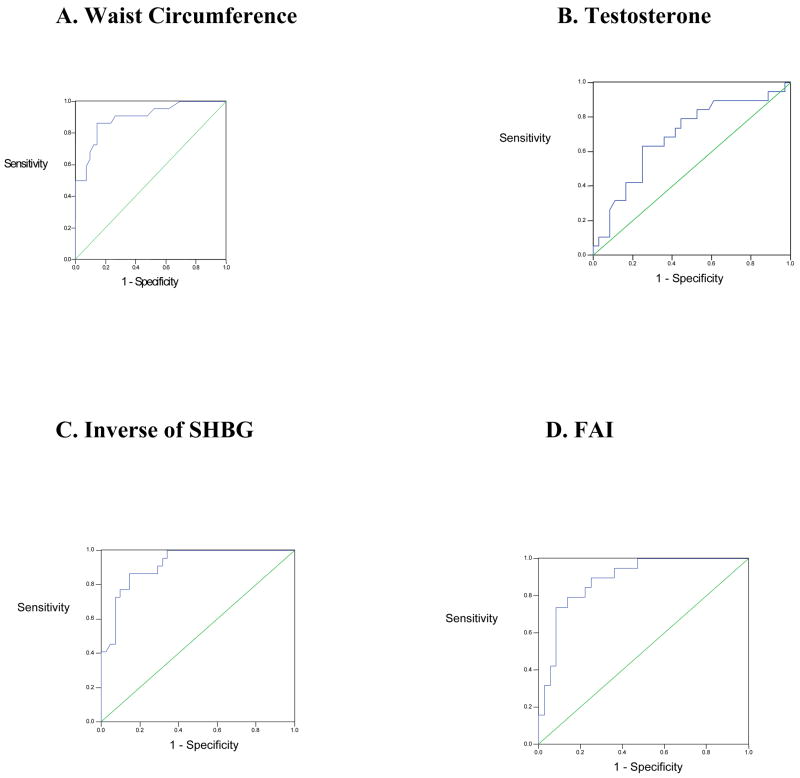
FAI and 1/SHBG had the highest accuracy of all of the hormone tests. Using ROC curve analysis, we found that if SHBG was less than 1.2 ug/dL and FAI exceeded 6.0, there was a 92% and 89% likelihood, respectively, that a randomly selected case would receive a higher and more suspicious rating of HA than a randomly selected normal case. (Table 3, Figure 2)
Table 3.
Receiving Operating Characteristics (ROC) Curve Analysis to Evaluate the Diagnostic Accuracy of Select Biochemical Tests and Clinical Markers for the Diagnosis of Hyperandrogenic Anovulation
| Biochemical Test or Clinical Marker | Area under the ROC curve | 95% Confidence Interval | Inflection Point of Greatest Sensitivity and Specificity (sensitivity, specificity) |
|---|---|---|---|
| Testosterone | 0.689 | 0.686–0.923 | 73.4 (0.632, 0.750) |
| 1/SHBG | 0.917 | 0.852–0.983 | 1.2 (0.864, 0.854) |
| FAI | 0.887 | 0.867–0.991 | 6.0 (0.842, 0.778) |
| Δ 4A | 0.743 | 0.602–0.884 | 339 (0.667, 0.805) |
| LH | 0.763 | 0.630–0.896 | 6.2 (0.727, 0.707) |
| LH: FSH Ratio | 0.748 | 0.610–0.887 | 1.2 (0.818, 0.293) |
| 1/GIR | 0.776 | 0.639–0.913 | 5.3 (0.818, 0.690) |
| Clitoral Index | 0.782 | 0.659–0.905 | 24.5 (0.714, 0.707) |
| Age at Menarche | 0.583 | 0.425–0.741 | 11.5 (0.545, 0.548) |
| Waist Circumference | 0.897 | 0.815–0.979 | 75.25 (0.864, 0.857) |
| Waist Circumference* | 0.824 | 0.717–0.931 | 77.75 (0.786, 0.714) |
| BMI* | 0.839 | 0.739–0.939 | 27.0 (0.786, 0.730) |
ROC curves were generated using redefined physical exam criteria with overweight and acanthosis nigricans removed from prospective physical exam criteria.
Figure 2. Summary ROC curves for Waist Circumference, Testosterone, SHBG, and FAI.
* Diagonal is reference line
Accuracy of Select Physical Characteristics for Diagnosing Hyperandrogenic Anovulation (HA)
Waist circumference had the highest diagnostic accuracy of the physical characteristics that we tested, with a waist circumference of 75.25 cm or greater indicating a 90% likelihood that a randomly selected case would receive a higher and more suspicious rating of HA than a randomly selected normal case. (Table 3, Figure 2) The accuracies for age at menarche and clitoral index were low. When we removed overweight and acanthosis nigricans from our prospective physical exam criteria, ROC curve analysis indicated that both waist circumference and BMI still had reasonably high accuracy for HA (82%). However, the accuracy was lower (sensitivity 78.6%, specificity 71.4%) for a waist circumference of 77.75 cm than when overweight and acanthosis nigricans are included in the criteria. (Table 3)
DISCUSSION
We found a strong relationship between body size and shape (high BMI and waist circumference), hyperandrogenemia, and irregular menses in this non-endocrine clinical sample of predominantly Caribbean-Hispanic and African-American adolescent girls. Our findings concur with others who have associated hyperandrogenemia with obesity in adults (22) and in peripubertal children (23). We also found that FAI, SHBG, and waist circumference are key predictors of hyperandrogenic anovulation (HA) in our sample of adolescents.
Obesity and elevated serum androgens have independent, deleterious effects on glucose tolerance and androgen production, which may also be synergistic. (24–27) Obesity begets insulin resistance, the associated hyperinsulinemia suppresses hepatic production of SHBG, and in turn free plasma testosterone becomes elevated.(28–30) Women with PCOS have insulin resistance that is more severe than can be accounted for by obesity alone (25, 31, 32) and this leads to even greater reductions in SHBG and higher androgens. In turn insulin may drive androgen secretion in PCOS, thus causing a feed-forward mechanism promoting hyperandrogenemia.
In our adolescent sample, obesity associated strongly with features of HA, suggesting that the mechanism for the effects of obesity on excessive androgen production in adolescents is similar to that seen in adult women with PCOS. BMI, waist circumference, and androgen levels were significantly greater and SHBG was significantly less in subjects who met our definition of HA compared to all others, independent of overweight status. These findings raise the possibility that high BMI should be considered as one of the physical abnormalities of HA in adolescent girls. Although comparison of individual groups indicates that androgen and SHBG levels differed between subjects in the three groups (Groups I, II, and III) that did not meet our designation for a clinical diagnosis of HA, these differences were not significant. Further, the diagnostic accuracy of SHBG, FAI and waist circumference at predicting differences between Group I and Group II and between Group I and Group III were low for all variables (results not shown). The differences between androgen and SHBG levels in subjects with at least one physical sign of hyperandrogenism and/or insulin resistance and abnormal menstrual cycles (Group IV) versus all others, however, were significant. Separately, each of these physical signs may not be associated with elevated androgen levels in adolescents, yet a combination of at least one physical sign and irregular menses (as seen in Group IV subjects) is significantly associated with increased androgen and decreased SHBG levels, relative to normals. The role of body size and shape in diagnosing HA in ethnic minority adolescents is highlighted by our finding that subjects with acne and/or hirsutism and menstrual abnormalities had elevated BMI and waist circumference, even when they did not meet CDC criteria for overweight. These subjects also had significantly higher androgen levels and lower SHBG levels relative to normals.
We observed relatively high serum testosterone levels in our subjects, compared to the normal ranges provided by the assay manufacturers. To investigate further, we measured testosterone levels using three different assay systems, with similar findings in all three. The most likely explanation for our testosterone data is that current assay technology does not have adequate resolving power at the lower limits of assay sensitivity.(33) However, our testosterone measurements were of sufficient accuracy and precision to support our Group IV definition of HA. It may be problematic in clinical practice to attempt to diagnose testosterone excess using commercial assays. Since the dominant predictors in our Group IV definition of HA were SHBG and FAI, which relies more on SHBG than on total testosterone, our results suggest that in populations similar to ours, SHBG and insulin sensitivity may be more useful than testosterone in predicting HA and the future development PCOS.
Our ROC curve findings support the use of FAI and SHBG as key predictors of hyperandrogenic anovulation (HA) in our population. The high accuracy of FAI is in agreement with the 2003 Rotterdam Consensus Group’s preferred method for assessing hyperandrogenemia. (34–36) While there is little consensus regarding the diagnostic utility of depressed SHBG levels, Santoro et al. demonstrated, that SHBG is strongly inversely associated with a metabolic syndrome phenotype and increased bioavailable androgens.(3) Our findings that SHBG and GIR progressively decreased from subjects in Group I (normals) to Group IV (HA), supports the presence of insulin resistance and the need for a clinical evaluation for metabolic syndrome in adolescent girls with HA. The fact that GIR differences no longer persisted when subjects with overweight and/or acanthosis nigricans were removed, yet FAI differences did, indicates that factors other than obesity and insulin resistance also influence androgen production.
Waist circumference, a simple measurement to obtain, was strongly associated with insulin resistance, elevated androgens, and irregular menses in adolescent girls. However, the waist circumference cut-point of >88 cm--the standard criteria by which the metabolic syndrome is diagnosed in adult women, (37)--may not be appropriate for adolescents. When the cut-point is lowered to 75.25 cm in our sample, the sensitivity and specificity are 86.4% and 85.7%, respectively. Our finding that both greater BMI and waist circumference were predictive of hyperandrogenic anovulation (HA) even when overweight subjects were excluded supports our notion that a high BMI should trigger an evaluation for HA in ethnic minority adolescent girls with irregular menses even when they do not have other physical signs of hyperandrogenism. Thus, inclusion of weight, BMI, and waist circumference may be important for early detection of HA in minority adolescents. Early diagnosis and treatment may prevent adult PCOS and its serious long-term consequences. Although obesity and acanthosis nigricans have been strongly associated,(38) our findings did not support inclusion of acanthosis nigricans as one of the physical abnormalities of HA. We also did not find clitoral size to be a useful predictor of HA.
The major limitation of our study is that we used a convenience, clinical sample of adolescents, and not a random, population-based sample. Although we avoided specialized endocrine patients, and thereby assured that our sample was less likely to be biased in favor of subjects with HA, our results may not be generalizable to all adolescents. They may serve, however, to further the discussion that addresses the conundrum of diagnosing and appropriately treating HA and preventing its future consequences in adolescent girls.
In summary, we have performed an analysis of a simple clinical categorization scheme for the detection of hyperandrogenic anovulation (HA) in ethnic minority adolescent girls. Our findings support the concept that including measures of body size and shape can increase confidence in a clinical diagnosis of HA. The strong relationship we observed between body size and shape, SHBG, and hyperandrogenemia in our predominantly Caribbean-Hispanic adolescents indicates that further attention should be paid to these clinical variables as more specific diagnostic adjuncts for hyperandrogenic anovulation than previously acknowledged.
Acknowledgments
NICHHD: Grant number: #R03 HD40821-01
This research was also supported in part by the NIH: K24:HD041978 (PI Nanette Santoro), the Diabetes Research and Training Center grant (P01 DK20541), and the General Clinical Research Center grant (M01-RR12248) awarded to the Albert Einstein College of Medicine This research was also supported in part by the William T. Grant Foundation
Dr. Harry Shamoon and the General Clinical Research Center staff – data collection and lab assays
Goli Adel-lab assays
Lucy Gordon, MD- data collection
Rosa Sapadin, RN – data collection
Footnotes
Conflict of Interest: J.R., P.M., H.W.C., and S.M.C. have nothing to declare. N.S. consults for Ferring, Wyeth and Pfizer and received lecture fees from Berlex, Wyeth, Serono and Pfizer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zawadski JK, Dunaif A. Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rational Approach. In: Dunaif A, Givens JR, Haseltine F, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. pp. 377–84. [Google Scholar]
- 2.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Santoro N, Torrens J, Crawford S, et al. Correlates of circulating androgens in mid-life women: the study of women’s health across the nation. J Clin Endocrinol Metab. 2005;90(8):4836–45. doi: 10.1210/jc.2004-2063. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A, Sorbara L, Delson R, et al. Ethnicity and polycystic ovary syndrome are associated with independent and additive decreases in insulin action in Caribbean-Hispanic women. Diabetes. 1993;42(10):1462–8. doi: 10.2337/diab.42.10.1462. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Task Force on Adolescent Reproductive Health. World Health Organization multicenter study on menstrual and ovulatory patterns in adolescent girls. II. Longitudinal study of menstrual patterns in the early postmenarcheal period, duration of bleeding episodes and menstrual cycles. World Health Organization Task Force on Adolescent Reproductive Health. J Adolesc Health Care. 1986;7(4):236–44. [PubMed] [Google Scholar]
- 6.Treloar AE, Boynton RE, Behn BG, et al. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12(1 Pt 2):77–126. [PubMed] [Google Scholar]
- 7.Wentz AC. Amenorrhea: Evaluation and Treatment. In: Jones HW III, Wentz AC, Burnett LS, editors. Novak’s Textbook of Gynecology. 11. Baltimore, MD: Williams and Wilkins; 1988. p. 351. [Google Scholar]
- 8.Flegal KM, Ogden CL, Wei R, et al. Prevalence of overweight in US children: comparison of US growth charts from the Centers for Disease Control and Prevention with other reference values for body mass index. Am J Clin Nutr. 2001;73(6):1086–93. doi: 10.1093/ajcn/73.6.1086. [DOI] [PubMed] [Google Scholar]
- 9.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 11.Troiano RP, Flegal KM. Overweight children and adolescents: description, epidemiology, and demographics. Pediatrics. 1998;101(3 Pt 2):497–504. [PubMed] [Google Scholar]
- 12.Lohman TG, Roche AF, Martorell R, editors. Human Kinetic Books. Champaign; Illinois: 1988. Anthropometric Standardization Reference Manual, abridged edition; pp. 44–46. [Google Scholar]
- 13.Hatch R, Rosenfeld R, Kim MH, et al. Hirsutism: Implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815. doi: 10.1016/0002-9378(81)90746-8. [DOI] [PubMed] [Google Scholar]
- 14.Wishart JM. An open study of triphasil and diane 50 in the treatment of acne. australas J Dermatol. 1991;32:51–4. doi: 10.1111/j.1440-0960.1991.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 15.Redmond GPOW, Lippman JS, et al. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: A randomized, placebo-controlled trial. Obstet Gynecol. 1997;89(4):615–22. doi: 10.1016/S0029-7844(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 16.Verkauf BS, Von Thron J, O’Brien WF. Clitoral size in normal women. Obstet Gynecol. 1992;80(1):41–4. [PubMed] [Google Scholar]
- 17.Santoro N, Banwell T, Tortoriello D, et al. Effects of aging and gonadal failure on the hypothalamic-pituitary axis in women. Am Jo Obstet Gynecol. 1998;178:732–41. doi: 10.1016/s0002-9378(98)70483-1. [DOI] [PubMed] [Google Scholar]
- 18.Santoro N, Isaac B, Neal-Perry G, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88:5502–9. doi: 10.1210/jc.2002-021839. [DOI] [PubMed] [Google Scholar]
- 19.Mushayandebvu T, Castracance VD, Gimpel T, et al. Evidence for diminished midcycle ovarian androgen production in older reproductive aged women. Fertil Steril. 1996;65:721–2. doi: 10.1016/s0015-0282(16)58203-x. [DOI] [PubMed] [Google Scholar]
- 20.Klein RS, Lo Y, Santoro N, et al. Androgen levels in older men who have or who are at risk of acquiring HIV infection. Clin Infect Dis. 2005;41:1794–803. doi: 10.1086/498311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sotsky MJ, Shilo S, Shamoon H. Regulation of Counterregulatory Hormone Secretion in Man during Hypoglycemia and Exercise. J Clin Endocrinol Metab. 1989;68:9–12. doi: 10.1210/jcem-68-1-9. [DOI] [PubMed] [Google Scholar]
- 22.Korhonen S, Hippelainen M, Vanhala M, et al. The androgenic sex hormone profile is an essential feature of metabolic syndrome in premenopausal women: a controlled community-based study. Fertil Steril. 2003;79(6):1327–34. doi: 10.1016/s0015-0282(03)00347-9. [DOI] [PubMed] [Google Scholar]
- 23.McCartney CR, Prendergast KA, Chhabra S, et al. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2005-1852. [DOI] [PubMed] [Google Scholar]
- 24.Nestler JE, Clone J, Blackard WG. The central role of obesity (hyperinsulinemia) in the pathogenesis of polycystic ovary syndrome. Am J Obstet Gynecol. 1989;161(5):1095–7. doi: 10.1016/0002-9378(89)90640-6. [DOI] [PubMed] [Google Scholar]
- 25.Dunaif A, Segal KR, Futterweit W, et al. Profound peripheral insulin resistance, independent of obesity, in the polycystic ovary syndrome. Diabetes. 1989;38:1165–74. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 26.Sozen I, Arici A. Hyperinsulinism and its interaction with hyperandrogenism in polycystic ovary syndrome. Obstet Gynecol Surv. 2000;55(5):321–8. doi: 10.1097/00006254-200005000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2005 doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 28.Dunaif A, Graf M, Mandeli J, et al. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987;65(3):499–507. doi: 10.1210/jcem-65-3-499. [DOI] [PubMed] [Google Scholar]
- 29.Barbieri RL, Makris A, Randall RW, et al. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab. 1986;62(5):904–10. doi: 10.1210/jcem-62-5-904. [DOI] [PubMed] [Google Scholar]
- 30.Nestler JE, Jakubowicz DJ, de Vargas AF, et al. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83(6):2001–5. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 31.Chang RJ, Nakamura RM, Judd HL, et al. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983;57(2):356–9. doi: 10.1210/jcem-57-2-356. [DOI] [PubMed] [Google Scholar]
- 32.Morales AJ, Laughlin GA, Butzow T, et al. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab. 1996;81(8):2854–64. doi: 10.1210/jcem.81.8.8768842. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Catlin DH, Demers LM, et al. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89(2):534–43. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- 34.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 35.Cibula D, Hill M, Starka L. The best correlation of the new index of hyperandrogensim with the grade of increased body hair. Eur J Endocrinol. 2000;143:405–8. doi: 10.1530/eje.0.1430405. [DOI] [PubMed] [Google Scholar]
- 36.Imani B, Eijkemans MJ, de Jong FH, et al. Free androgen index and leptin are the most prominent endocrine predictors of ovarian response during clomiphene citrate induction of ovulation in normogonadotropicc oligoamenorrheic infertility. J Clin Endocrinol Metab. 2000;85(2):676–82. doi: 10.1210/jcem.85.2.6356. [DOI] [PubMed] [Google Scholar]
- 37.Alexander CM, Landsman PB, Teutsch SM, et al. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52(5):1210–4. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 38.Hirschler V, Aranda C, Oneto A, et al. Is acanthosis nigricans a marker of insulin resistance in obese children? Diabetes Care. 2002;25(12):2353. doi: 10.2337/diacare.25.12.2353. [DOI] [PubMed] [Google Scholar]