Abstract
In the present study, we examined whether exposing rats to a high-dose regimen of manganese chloride (Mn) during the postnatal period would depress presynaptic dopamine functioning and alter nonassociative and associative behaviors. To this end, rats were given oral supplements of Mn (750 μg/day) on postnatal days (PD) 1–21. On PD 90, dopamine transporter (DAT) immunoreactivity and [3H]dopamine uptake were assayed in the striatum and nucleus accumbens, while in vivo microdialysis was used to measure dopamine efflux in the same brain regions. The effects of postnatal Mn exposure on nigrostriatal functioning were evaluated by assessing rotorod performance and amphetamine-induced stereotypy in adulthood. In terms of associative processes, both cocaine-induced conditioned place preference (CPP) and sucrose-reinforced operant responding were examined. Results showed that postnatal Mn exposure caused persistent declines in DAT protein expression and [3H]dopamine uptake in the striatum and nucleus accumbens, as well as long-term reductions in striatal dopamine efflux. Rotorod performance did not differ according to exposure condition, however Mn-exposed rats did exhibit substantially more amphetamine-induced stereotypy than vehicle controls. Mn exposure did not alter performance on any aspect of the CPP task (preference, extinction, or reinstatement testing), nor did Mn affect progressive ratio responding (a measure of motivation). Interestingly, acquisition of a fixed ratio task was impaired in Mn-exposed rats, suggesting a deficit in procedural learning. In sum, these results indicate that postnatal Mn exposure causes persistent declines in various indices of presynaptic dopaminergic functioning. Mn-induced alterations in striatal functioning may have long-term impact on associative and nonassociative behavior.
Keywords: dopamine transporter immunoreactivity, [3H]dopamine uptake, in vivo microdialysis, conditioned place preference, progressive ratio, stereotypy
Introduction
Manganese chloride (Mn) is a complex trace mineral that in high amounts causes neurotoxic effects in humans, with children appearing to be particularly at risk (Mena et al., 1967; Cawte, 1985; Pal et al., 1999; Tran et al., 2002a,b; Kostial et al., 2005; Fitsanakis et al., 2006a). Humans exposed to excess Mn for prolonged periods often exhibit extrapyramidal motor impairments similar to Parkinson's Disease (Calne et al., 1994; Mergler, 1999; Aschner, 2000), as well as cognitive deficits and psychiatric disturbances (Mergler et al., 1994; Aschner, 2000). Although Mn impacts multiple neurotransmitter systems (Fitsanakis et al., 2006b), the complex symptomology characteristic of Mn toxicity suggests that both the mesocorticolimbic and nigrostriatal dopamine systems might be affected. Consistent with this hypothesis, MRI studies indicate that Mn toxicity is associated with excessive Mn accumulation in the basal ganglia as well as other cortical and subcortical structures (Dietz et al., 2001; Joseph et al., 2005; Klos et al., 2006; Uchino et al., 2007).
Studies using rats and nonhuman primates provide evidence that Mn exposure alters dopaminergic functioning at presynaptic terminals. For example, exposing monkeys to Mn reduces striatal dopamine content and alters dopamine release characteristics (Bird et al., 1984; Guilarte et al., 2006). In rats, infusion of Mn during microdialysis reduces K+-stimulated dopamine efflux in the striatum (Vidal et al., 2005), while chronic Mn exposure causes long-term reductions in striatal dopamine levels (Gianutosos and Murray, 1982; Komura and Sakamoto, 1992; Ingersoll et al., 1995; Tran et al., 2002a,b; Aschner et al., 2005). Changes to membrane-associated proteins are also a component of Mn toxicity, because prolonged Mn exposure substantially reduces dopamine transporter (DAT) binding sites in rat and monkey striatum (Eriksson et al., 1992; Reichel et al., 2006). Curiously, studies examining DAT functioning have provided mixed results, with Mn exposure reported to either increase or decrease [3H]-dopamine uptake in rat striatum depending on experimental conditions (Lai et al., 1982; Leung et al., 1982).
Despite the various neuronal changes resulting from Mn toxicity, there is remarkably little evidence that Mn exposure disrupts the unlearned and learned behaviors of rats and nonhuman primates. For example, cynomolgus macaques show only subtle motoric deficits and near normal working memory and problem solving abilities after long-term Mn sulfate treatment (Guilarte et al., 2006; Schneider et al., 2006). Likewise, infant rhesus monkeys fed soy formula containing Mn chloride performed no differently than controls on complex learning tasks (e.g., delayed nonmatch to sample, continuous performance test, etc.) (Golub et al., 2005). In adult rats, unlearned locomotor responding is often disrupted by perturbations of the nigrostriatal and mesocorticolimbic dopamine systems, therefore it is surprising that Mn has been reported to increase (Nachtman et al., 1986; Calabresi et al., 2001), decrease (Ingersoll et al., 1995; Talavera et al., 1999), or have no effect (Dorman et al., 2000; Reichel et al., 2006) on spontaneous locomotor activity. Interestingly, Mn-exposed rats show reduced locomotor responsiveness when challenged with cocaine or amphetamine (indirect dopamine agonists), suggesting that subtle motoric deficits can be unmasked by pharmacological challenge (Leung et al., 1982; Reichel et al., 2006). In the only studies examining whether Mn exposure disrupts learned behaviors, Tran and colleagues found that Mn- and vehicle-treated rats performed similarly on passive avoidance and burrowing detour tasks (Tran et al., 2002b), while Mn-treated rats exhibited deficits on a shock avoidance task (Tran et al., 2002a).
The purpose of the present study was to further examine whether postnatal Mn exposure has long-term effects on presynaptic dopamine functioning and behavior. Using [3H]GBR 12935, we previously showed that exposing rats to Mn (750 μg) on PD 1–21 reduced the number of striatal DAT binding sites when measured in adulthood (Reichel et al., 2006). One of the goals of the present study was to extend these findings and determine whether postnatal exposure to a high dose of Mn would attenuate DAT protein expression, [3H]dopamine uptake, and dopamine efflux in the striatum and nucleus accumbens of adult rats. A second goal of this study was to determine whether postnatal Mn exposure would affect neuronal systems underlying associative and nonassociative behavior. The conditioned place preference (CPP) paradigm was used because development of a place preference requires the formation of Pavlovian associations between the physical characteristics of the drug-paired chamber (conditioned stimulus) and cocaine (unconditioned stimulus) (Tzschentke, 1998). Dopaminergic projections to the nucleus accumbens must be intact and functional for normal CPP performance (Baker et al., 1998; Sellings and Clarke, 2003). In addition, separate groups of rats were trained on a sucrose-reinforced operant task that included both fixed ratio (FR) and progressive ratio schedules. These operant procedures require the formation of stimulus-response associations (Mackintosh, 1974) and are sensitive to dopamine depletion in the nucleus accumbens (Aberman et al., 1998; Hamill et al., 1999) and striatum (Featherstone and McDonald, 2004a; Faure et al., 2005). The integrity of nigrostriatal functioning was also assessed by testing vehicle- and Mn-exposed rats on the accelerating rotorod task and in automated activity chambers. In the latter experiment, rats were pretreated with saline or amphetamine (2 or 4 mg/kg) because psychostimulant-induced motor stereotypies are known to be mediated by the striatum (Jaber et al., 1995; Rebec et al., 1997; Canales and Graybiel, 2000).
It was hypothesized that postnatal exposure to a high-dose regimen of Mn (750 μg/day) would attenuate cocaine-induced CPP, impair acquisition of the FR tasks, lower the ‘break point’ (i.e., reduce motivation) on the progressive ratio task, impair rotorod performance, and lessen the intensity of amphetamine-induced motor stereotypies. A high-dose Mn regimen was used because similar Mn schedules are purported to approximately mimic human exposure conditions that cause acute neurotoxicity (Dorman et al., 2000) and possibly result in long-term neural and behavioral deficits (Tran et al., 2002a,b). In regard to dose, it is noteworthy that rats are considerably less sensitive to Mn than are humans or other primates (Aschner et al., 2005).
Experimental Procedures
Animals
Subjects were 155 male and 32 female rats of Sprague-Dawley descent (Charles River, Hollister, CA, USA), born and raised at California State University, San Bernardino (CSUSB). Litters were culled to ten pups on PD 1. Rats were weaned on PD 24 and group housed with littermates. Rats had free access to standard rodent chow (Harlan-Tekland #8604) and water. The colony room was maintained at 21–23°C and kept under a 12-h light/dark cycle. Subjects were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Drugs and chemicals
Mn chloride, (−)-cocaine hydrochloride, D-amphetamine sulfate salt, dopamine, and nomifensine maleate salt were purchased from Sigma (St. Louis, MO, USA). Cocaine and amphetamine were dissolved in saline and injected intraperitoneally (IP) at a volume of 1 ml/kg. [3H]Dopamine (specific activity = 45 Ci/mM) was purchased from GE Healthcare (Piscataway, NJ, USA).
In vivo Mn administration
On PD 1–21, rats were given daily oral supplements (via micropipette) of 0 or 750 μg Mn dissolved in 25 μl of a 10% sucrose solution. Since a constant amount of Mn was delivered each day, rats received more Mn, relative to body weight, during the neonatal period than at later ages.
Rotorod apparatus
Motor function was assessed on a computer-controlled accelerating rotorod (Rotamex-5, Columbus Instruments, Columbus, OH, USA) with 9.5 cm wide channels and a 7 cm (dia.) rod. The rotorod was equipped with a photobeam array for automatically determining latency until the rat fell off the rod.
Locomotor activity apparatus
Locomotion was assessed in activity monitoring chambers (25.5 × 25.5 × 41 cm), consisting of acrylic walls, a plastic floor, and an open top (Coulbourn Instruments, Allentown, PA, USA). Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to determine distance traveled (a measure of locomotor activity) and repetitive motor movements (a measure of stereotypy). The position of each rat was determined every 100 msec.
CPP apparatus
Conditioning and testing were done in rectangular wooden chambers that had three compartments arranged in a truncated T-shape. The two large end compartments (37 × 30 × 45 cm) were adjacent to each other and separated by a removable partition. A third smaller compartment (the placement chamber; 18 × 18 × 45 cm) was located at the side of the junction between the two larger compartments and was connected to them by a removable partition (when this partition was open rats could enter either end compartments from the placement chamber). The color, flooring, and odor of each compartment varied. One end compartment had white walls, wire mesh flooring, and pine bedding, while the other end compartment had black walls, metal rod flooring, and cedar bedding. The small side compartment had gray walls and a solid wood floor.
Operant apparatus
Rats were tested in standard operant conditioning chambers (Coulbourn Instruments, Allentown, PA, USA), measuring 29 × 26 × 33 cm (L × W × H). Active and inactive levers were located on the front wall 10 cm above the chamber floor on either side of a food aperture positioned 2 cm above the floor. Each chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise. Chamber operation was controlled by an IBM compatible computer interfaced with a data collection program.
DAT immunoreactivity procedure
On PD 90, male rats (N = 16) were killed by rapid decapitation and accumbal sections were dissected bilaterally using a brain matrix and punch, while striatal sections were removed free hand. Striatal and accumbal samples were homogenized in a lysis buffer (20 mM Tris (pH 8.0) containing 137 mM NaCl, 10% glycerol, 1% Nonidet P-40, 0.5 mM sodium orthovanadate, 1 mM PMSF and protease inhibitor cocktail) and centrifuged twice for 20 min at 14 000 g (4°C). Protein concentrations were determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA) based on the method of Bradford (1976), using bovine serum albumin as a standard. Homogenates were frozen at −80°C until assay.
On the day of assay, striatal and accumbal tissue homogenates (50 μg/protein) were mixed with laemmli loading buffer, incubated at room temperature for 1 hr, and loaded on 10% polyacrylamide gels. Gels were electrophoresed at 175 V for 3.5 hr. Proteins were transferred to a PVDF membrane (Immun-Blot, Bio-Rad Laboratories) and blocked for 2 hr in a solution of 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween-20. Blots were incubated overnight at room temperature with the primary antibody for DAT (SC-14002, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:300. Blots were washed eight times (5 min each) in wash buffer (Tris-buffered saline, with 0.1% Tween-20) and then incubated in goat anti-rabbit horseradish peroxidase-linked IgG (1:10 000; Pierce Biotechnology, Rockford, IL, USA) for 2 hr at room temperature. Following this incubation, membranes were washed eight times (5 min each) in wash buffer and then incubated for 4 min in peroxidase-chemiluminescence substrate (Super Signal West, Pierce Biotechnology). Immunoreactive bands were visualized using film based autoradiography and quantified using a computer assisted densitometer (model GS-700, Bio-Rad Laboratories). Protein loading and transfer were controlled by stripping (Restore™, Pierce Biotechnology), reblocking, and then reprobing the membranes with a monoclonal antibody to glyceraldehydes 3-phosphate dehydrogenase (GAPDH; Imgenex, San Diego, CA, USA) at a dilution of 1:20 000. Each sample was assayed in duplicate and matched controls were run on each gel.
[3H]Dopamine uptake assay
[3H]Dopamine uptake assays were conducted using a previously published protocol (Zhu et al., 2004). On PD 90, male rats (N = 16) were killed by rapid decapitation and the nucleus accumbens and striatum were dissected in the manner just described. Striatal and accumbal samples were immediately homogenized in 20 vols of ice-cold 0.32 M sucrose solution with a hand-held Teflon pestle homogenizer. Homogenates were centrifuged for 10 min at 1,000×g (4°C) and the resulting supernatant was poured off and stored on ice. The pellet was resuspended in 0.32 M sucrose and centrifuged for 10 min at 1,000×g (4°C). The combined supernatants were then centrifuged for 30 min at 17,000×g (4°C). Pellets were resuspended in 20 volumes (i.e., 20 μl of buffer per mg of tissue) of ice-cold oxygenated assay buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline, and 0.1 mM l-ascorbic acid, pH 7.4).
Synaptosomes (20–40 μg protein) were preincubated in assay buffer for 10 min at 37°C. To determine [3H]dopamine uptake, six concentrations (25–500 nM) of cold and hot dopamine (10 nM) were used. Nonspecific [3H]dopamine uptake was determined in the presence of 10 μM nomifensine. Total assay volume was 1 ml. After the addition of dopamine, incubation continued for 5 min at 37°C and was terminated by inclusion of 3 ml of ice-cold assay buffer followed by immediate filtration through Whatman GF/B glass fiber filters (presoaked with 0.1 % polyethyleneimine). Filters were washed three times with 3 ml ice-cold buffer using a Brandel cell harvester (model M-24, Gaithersburg, MD, USA). Radioactivity was determined by liquid scintillation spectrometry (model LS6500, Beckman Coulter, Fullerton, CA, USA). Protein concentrations were determined using the Bio-Rad Protein Assay as described above.
In vivo microdialysis
Male rats (striatum: N = 16; nucleus accumbens: N = 12) were anesthetized with 55 mg/kg (IP) sodium pentobarbital (Sigma) and a dialysis guide cannula (model CMA/11, CMA, Solna, Sweden) equipped with a dummy probe was surgically implanted in the left striatum (AP = +0.2; ML = +3.2; DV = −5.0 mm) or left nucleus accumbens shell (AP = +1.7; ML = +0.9; DV = −6.0 mm) using coordinates from the rat brain atlas of Paxinos and Watson (1998). After a 1 day recovery period, rats were placed in a clear cylindrical (25.5 cm in diameter) testing chamber (Instech, Plymouth Meeting, MA, USA) and habituated to the tether system for 1 hr. On the following day, tubing from the microdialysis probe (CMA/11) was attached to a dual-channel quartz-lined swivel (model 375/D/22QM, Instech) mounted on a counterbalanced arm with tether. Using a microinfusion pump (model CMA/102, CMA), artificial cerebrospinal fluid (145 mM NaCl, 2.7 mM KCl, 1.4 CaCl2, 1.0 MgCl2, 2.0 Na2HPO4, pH = 7.4) was constantly perfused through the probe at a rate of 1.5 μl/min. The length of the permeable portion of the membrane was 3.0 mm for the striatum and 2.0 mm for the nucleus accumbens. Following a 3 hr “wash-out” period, dialysate samples were collected continuously in 250-μl polyethylene vials (containing 10 μl of 0.1 N perchloric acid) every 20 min during an 80-min baseline period. Following baseline, rats were injected with cocaine (20 mg/kg, IP) and samples were collected every 20 min for 160 min.
Dialysate samples were analyzed for dopamine using HPLC with electrochemical detection. Samples were tested using a 717plus autosampler (Waters, Milford, MA, USA), an MD-150 column (ESA, Chelmsford, MA, USA) and a Coulochem II electrochemical detector (triple detector electrode system, ESA). The mobile phase (75 mM NaH2PO4·H2O, 1.4 mM OSA, 10 μM EDTA, 10% acetonitrile; pH 3.1 using H3PO4) was delivered via a Waters 1525 binary HPLC pump at a flow rate of 0.5 ml/min. Levels of detected dopamine were expressed as pg/10 μl dialysate.
Following testing, rats received an overdose of sodium pentobarbital prior to being rapidly perfused with a 4% paraformaldehyde solution. Brains were sectioned coronally (60 μm) using a cryostat and tissue was stained with thionin for verification of probe placement. Data from animals with inappropriate probe placements were excluded from later statistical analysis.
Rotorod procedure
On PD 90, vehicle- and Mn-exposed male rats (N = 48) were individually habituated to a stationary rod for 3 min after which they were confined to a holding cage. After 10 min, the same rat was placed on a rotating rod that increased in speed from 2 to 20 revolutions per minute (RPM) over a 100 s period (i.e., an increase of 2 RPM every 10 s). Upon falling, the rat was returned to the holding cage for 5 min. The procedure of placing the rat on the rotating rod was repeated twice, with a 5 min interval between each trial. Data recorded were peak RPM attained and latency until falling. Scores were averaged across the three testing trials.
Stereotypy and locomotor activity
On PD 91, vehicle- and Mn-exposed male rats (these are the same 48 rats used in the rotorod experiment) were injected with saline or amphetamine (2 or 4 mg/kg, IP). Rats were immediately placed in activity chambers where the duration of repetitive motor movements and distance traveled (cm) were assessed for 150 min. Repetitive motor movements were defined as the total number of repetitive coordinate changes on the X-Y axes that occurred within 2 s (three back and forth movements were required before the behavior qualified as a repetitive motor movement).
In addition, rats were videotaped and behavioral intensity scores (a measure of stereotypy) were later determined by an observer blind to treatment conditions. For this measure, rats were assessed every 10 min (for a 2-min period) using a behavioral intensity scale developed by Creese and Iversen (1973). This scale uses the following rating system: 0 = asleep or inactive, 1 = normal exploratory activity (no repetitive behaviors), 2 = discontinuous activity with prominent (repetitive) sniffing or rearing, 3 = continuous activity with repetitive sniffing and rearing in a fixed path, 4 = continuous activity with repetitive sniffing and rearing in the same location, 5 = continuous activity with repetitive chewing or gnawing in a fixed path, 6 = continuous activity with repetitive chewing or gnawing in the same location.
Place preference conditioning
Female rats and mice often show a more robust place preference than males (Russo et al., 2003; Balda et al., 2006), therefore the ability of Mn to differentially affect the CPP performance of male and female rats was assessed during a 20-day CPP procedure. The CPP experiment consisted of one preconditioning day, eight conditioning days, one preference test day, eight extinction days, one extinction test day, and one reinstatement test day. On PD 90, male (N = 31) and female (N = 32) rats were individually set in the gray placement chamber of the CPP apparatus. After rats entered either the black or white compartment, the partition isolating the placement chamber was lowered and rats were allowed 15 min access to the black and white compartments. Conditioning lasted for eight days (PD 91–98) and consisted of alternating daily placements in the black and white compartments. On the first day of conditioning, half of the rats were injected with cocaine (0, 10, or 20 mg/kg, IP) and restricted to the white compartment, while the other half were injected with saline and restricted to the black compartment. Conditions were reversed on the following day (e.g., rats previously given saline in the black compartment were injected with 0, 10, or 20 mg/kg cocaine and placed in the white compartment). On the preference test day (PD 99), rats were left uninjected and given free access to the black and white compartments for 15 min. Extinction occurred across the following eight days (PD 100–107) and consisted of daily injections of saline followed by alternating daily placements in the black and white compartments for 30 min. On the extinction test day (PD 108), rats were set in the placement chamber and allowed 15 min access to the black and white compartments. On the reinstatement test day (PD 109), all rats received a priming injection of 20 mg/kg cocaine and were again allowed 15 min access to the black and white compartments.
Operant conditioning
Starting on PD 86, male rats (N = 16) were food deprived and eventually maintained at 85–90% of their initial body weight. Upon initially reaching 90% body weight, rats were placed in operant chambers where they were allowed to lever press for a sucrose pellet on a fixed ratio 1 (FR 1) schedule. Rats that did not reach criterion in five days were hand-shaped using successive approximations. After reaching criterion on the FR 1 schedule, each rat was individually advanced to an FR 3 schedule and then an FR 10 schedule. In all cases, two consecutive days with a minimum of 50 responses on the active lever were required before a rat was advanced to the next schedule.
After completion of FR 10 training, rats were switched to a progressive ratio schedule where the number of responses necessary to receive the sucrose pellet increased exponentially through the following series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 693, 737, 901. Each progressive ratio session terminated when the rat failed to complete the ratio for a particular reinforcer within 20 min from delivery of the previous reinforcer. Rats received eight consecutive daily sessions of progressive ratio training. The number of days necessary to reach criterion (FR training only), the number of reinforcements received, the number of responses made on the active and inactive levers, and the last ratio successfully completed were recorded. ‘Break point’ was defined as the number of reinforcements received because the last ratio completed has the disadvantage of not being a linear measure (Richardson and Roberts, 1996).
Data analysis
Litter effects were controlled through both experimental design and statistical procedures (for a review, see Zorrilla, 1997). In most experiments no more than one subject per litter was assigned to a particular group. In cases where this procedure was not possible (e.g., the rotorod experiment), a single litter mean was calculated from multiple littermates assigned to the same group (Holson and Pearce, 1992; Zorrilla, 1997). In the behavioral and neurochemistry experiments litter was used, whenever possible, as the unit of analysis for statistical purposes (Zorrilla, 1997). With this statistical model each litter, rather than each rat, is treated as an independent observation (i.e., a within analysis using one value/condition/litter). Within-subjects statistical procedures were not used when analyzing CPP data because sex was included as a variable and individual litters did not contain enough subjects to provide one subject per group.
For the DAT immunoreactivity experiment, samples were assayed in duplicate and normalized to GAPDH levels before statistical analysis using a paired t test (Mn group) for each brain region. For the [3H]DA uptake experiment, maximal velocity (Vmax) and affinity (Km) were determined using GraphPad Prism software (version 4.0; GraphPad Software, San Diego, CA, USA) and subsequently analyzed using a paired t test (Mn group). In the microdialysis experiment, data were analyzed using separate two-way (Mn group × Time block) within-subjects analyses of variance (ANOVA) for each brain region. Because DA release had not peaked 20 min after cocaine injection, the first time block was not included in the statistical analyses.
Behavioral data from the rotorod experiment were analyzed using paired t tests (Mn group); whereas, stereotypy (behavioral intensity and repetitive motor movements) and locomotor activity data were analyzed using separate three-way (Mn group × Amphetamine condition × Time block) within-subjects ANOVAs. In the CPP experiment, preconditioning data were analyzed using a three-way (Mn group × Sex × Compartment) mixed model ANOVA. Preference, extinction, and reinstatement data (i.e., amount of time spent in the drug-paired compartment) were analyzed using separate three-way (Mn group × Cocaine condition × Sex) between-subjects ANOVAs. In the operant experiment, progressive ratio data (i.e., number of reinforcements received and number of responses made on the active lever) were analyzed using two-way (Mn group × Day) within-subjects ANOVAs, whereas FR 1 data (i.e., days to reach criterion) were analyzed using a paired t test (Mn group) and a χ2 test incorporating Yates' correction for continuity (Bruning and Kintz, 1997). Scores on the criterion variable ranged from 2 to 5, because rats were hand-shaped if the criterion was not attained in five days. With the exception of the CPP experiment, which included female rats, body weight data from all experiments were combined for statistical and presentation purposes. Postnatal weights were analyzed using a two-way (Mn group × Age) repeated measures ANOVA, while adult weights were analyzed with an independent t-test (Mn group). When required, significant higher order interactions were further analyzed using one- or two-way ANOVAs. Newman-Keuls tests and paired t tests were used for making post hoc comparisons [P<0.05].
Results
Body weight
Although a trend was apparent, Mn exposure did not significantly affect body weights on PD 1–21 (Table 1) [P>0.05]. When measured in adulthood, mean body weights of the vehicle (x̄ = 497 g, SEM = ±5.3) and Mn (x̄ = 500 g, SEM = ±5.3) groups did not differ [P>0.05].
Table 1.
Mean (±SEM) body weight (g) of rats exposed to vehicle or manganese (Mn) on PD 1–21
| Treatment | Postnatal Day (PD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PD 1 | PD 3 | PD 5 | PD 7 | PD 9 | PD 11 | PD 13 | PD 15 | PD17 | PD 19 | PD 21 | |
| Vehicle | 7.9 ±.1 | 10.7 ±.2 | 14.4 ±.2 | 18.6 ±.4 | 23.1 ±.4 | 27.8 ±.6 | 32.7 ±.6 | 37.7 ±.6 | 42.9 ±.7 | 49.0 ±.9 | 58.0 ±1.0 |
| 750 μg Mn | 7.8 ±.2 | 10.2 ±.2 | 13.6 ±.3 | 17.8 ±.4 | 22.3 ±.4 | 26.6 ±.4 | 31.1 ±.7 | 36.2 ±.5 | 41.2 ±.6 | 47.4 ±.8 | 57.2 ±.9 |
DAT immunoreactivity
Postnatal Mn exposure produced a persistent decline (18%) in striatal DAT protein expression when measured on PD 90 (Fig. 1) [Mn effect, t(7)=3.26, P<0.05]. Similar changes were observed in the nucleus accumbens, because DAT protein expression of the Mn group was reduced by 19% relative to control values [Mn effect, t(7)=2.43, P<0.05].
Fig. 1.
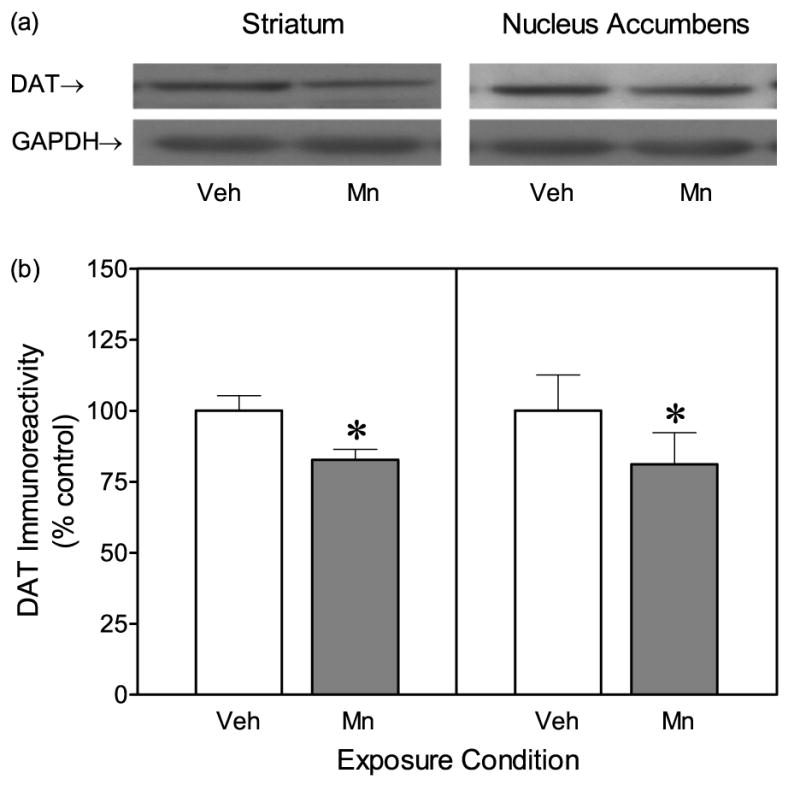
Dopamine transporter (DAT) protein expression in the striatum and nucleus accumbens of adult male rats (n = 8 per group). Rats had been exposed to vehicle or 750 μg manganese (Mn) on PD 1–21. (a) Representative immunoblots of total DAT immunoreactivity (GAPDH was used for normalizing sample loading). (b) Mean (±SEM) densitometry values of DAT immunoreactivity expressed as percent of vehicle controls. *Significantly different from vehicle controls (P<0.05).
[3H]Dopamine uptake assay
Exposing rats to Mn during the postnatal period caused a 30.3% reduction in striatal [3H]dopamine uptake (i.e., Vmax) when compared to vehicle controls (Table 2) [Mn effect, t(7)=3.16, P<0.05]. Mn exposure also produced a long-term decrease (16.8%) in [3H]dopamine uptake in the nucleus accumbens [Mn effect, t(6)=2.68, P<0.05]. Km for [3H]dopamine uptake was elevated (i.e., affinity declined) in Mn-exposed rats relative to vehicle controls, but differences in affinity only reached statistical significance in the striatum [Mn effect, t(7)=2.43, P<0.05].
Table 2.
Maximal velocity (Vmax) and affinity (Km) of [3H]DA uptake in striatal and accumbal synaptosomes on PD 90.
| Exposure Condition | Striatum (n = 8) | Nucleus Accumbens (n = 7) | ||
|---|---|---|---|---|
| Vmax | Km | Vmax | Km | |
| Vehicle | 27.20 (±2.5) | 103.48 (±8.1) | 25.73 (±1.7) | 119.15 (±15.7) |
| 750 μg Mn | 18.97 (±2.8)* | 157.10 (±27.6)* | 21.43 (±1.2)* | 150.86 (±18.1) |
Male rats were exposed to vehicle or 750 μg manganese (Mn) on PD 1–21. Mean (±SEM) Vmax values are presented in pmol/min/mg protein, while mean (±SEM) Km values are presented in nM.
Denotes significantly different from rats exposed to vehicle (P<0.05).
In vivo microdialysis
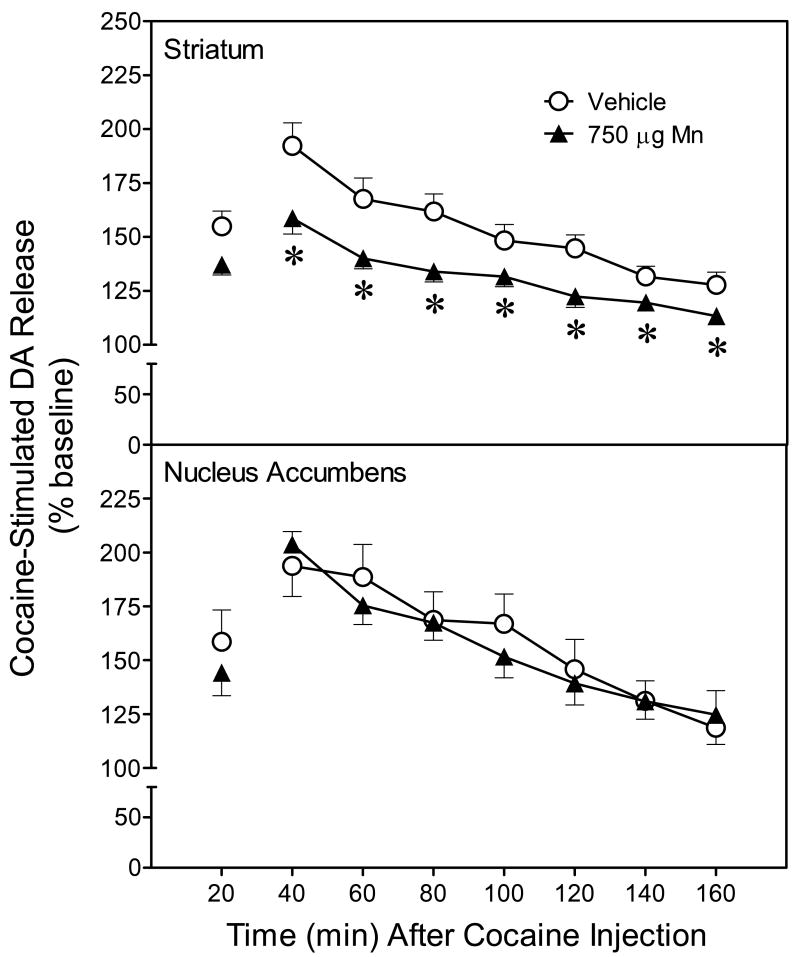
Postnatal Mn exposure did not alter basal dopamine levels (collected across the final four 20-min baseline periods) in either the striatum or nucleus accumbens (Table 3). After cocaine administration, striatal dopamine efflux was elevated in both the vehicle and Mn groups, but the percent increase was relatively smaller in Mn-treated animals (upper graph, Fig. 2) [Mn main effect, F(1, 7)=14.26, P<0.05]. More specifically, cocaine-stimulated dopamine release was significantly reduced in the striatum of Mn-exposed rats, relative to control rats, from 40 min to 160 min post-injection [Mn × Time interaction, F(6, 42)=3.38, P<0.05]. Cocaine also increased dopamine efflux in the nucleus accumbens, but this effect did not vary according to treatment group (Fig. 2, lower graph) [P>0.05].
Table 3.
Basal dopamine levels (pg/10 μl dialysate) in the striatum (n = 8) and nucleus accumbens (n = 6) on PD 90.
| Exposure Condition | Striatum | Nucleus Accumbens |
|---|---|---|
| Vehicle | 10.67 (±0.3) | 4.04 (±0.3) |
| 750 μg Mn | 11.57 (±0.4) | 4.04 (±0.3) |
Extracellular dopamine levels were determined using in vivo microdialysis in freely moving rats. Values represent mean (±SEM) dopamine levels during the final four 20-min baseline periods. Male rats were exposed to vehicle or 750 μg manganese (Mn) on PD 1–21.
Fig. 2.
Mean (±SEM) extracellular dopamine levels in the striatum (upper graph) and nucleus accumbens (lower graph) following administration of 20 mg/kg cocaine. Dopamine efflux was measured using in vivo microdialysis in freely moving adult male rats (n = 6–8 per group). Data are expressed as percent of baseline dopamine levels. Rats had been exposed to vehicle or 750 μg manganese (Mn) on PD 1–21. *Significantly different from vehicle controls (P<0.05).
Rotorod
Early Mn exposure did not affect performance on the rotorod, because mean latencies to fall off the rod and peak RPM attained did not vary according to exposure group (Table 4) [P>0.05].
Table 4.
Mean (±SEM) rotorod performance of vehicle and manganese (Mn) exposed rats on PD 90.
| Exposure Condition | Latency to Fall | Peak RPM Attained |
|---|---|---|
| Vehicle | 40.04 s (±3.9) | 8.98 (±0.8) |
| 750 μg Mn | 43.18 s (±4.1) | 9.55 (±0.7) |
Male rats were exposed to vehicle or 750 μg Mn on PD 1–21.
Stereotypy and locomotor activity
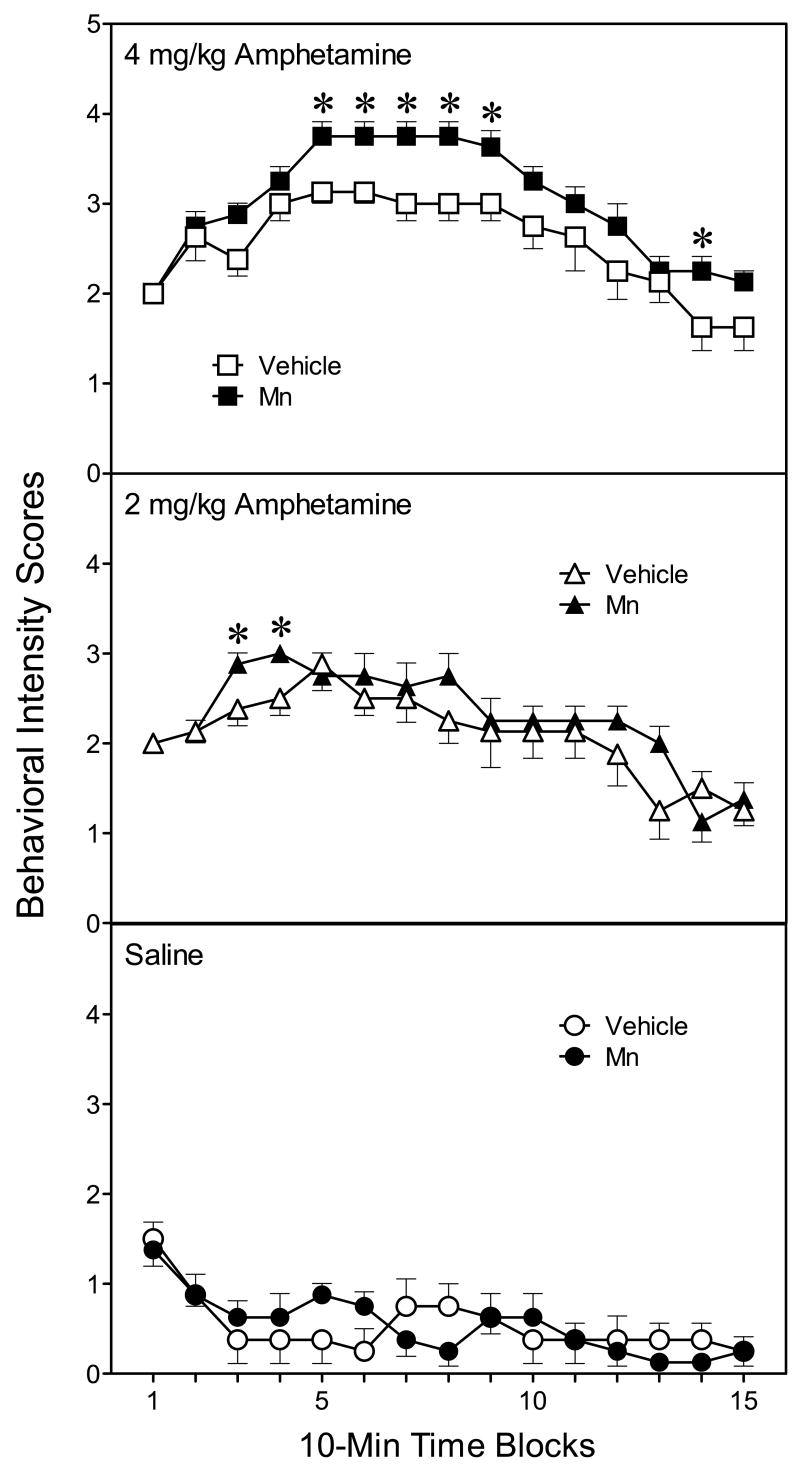
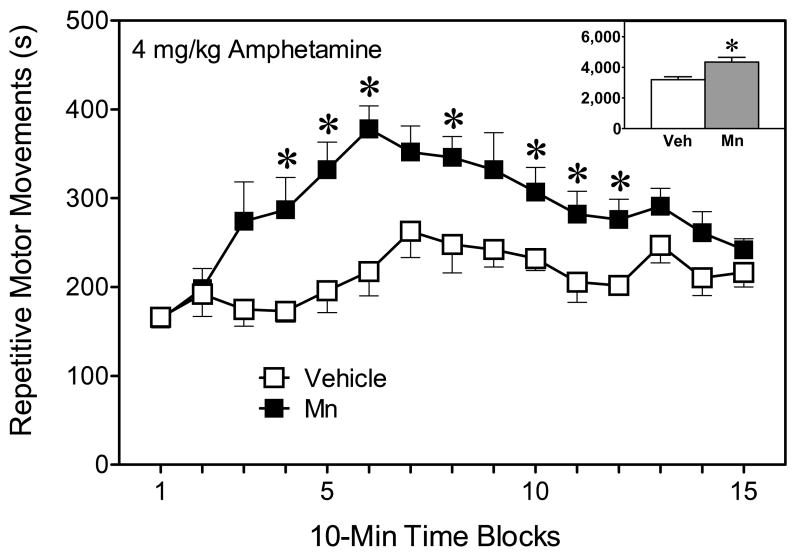
In terms of stereotypy, rats receiving 4 mg/kg amphetamine evidenced significantly greater behavioral intensity scores than rats injected with 2 mg/kg amphetamine, while saline-treated rats scored at basal levels (Fig. 3) [Amphetamine main effect, F(2, 14)=41.86, P<0.001]. The effects of amphetamine on behavioral intensity scores differed depending on exposure condition. Thus, Mn-exposed rats injected with 4 mg/kg amphetamine exhibited enhanced behavioral intensity scores on time blocks 5–9 and 14 when compared to vehicle-exposed rats given the same dose of amphetamine (upper graph, Fig. 3) [Mn × Amphetamine × Time block interaction, F(28, 196)=1.57, P<0.05]. The lower dose of amphetamine (2 mg/kg) also induced greater behavioral intensity scores in Mn-exposed rats than vehicle controls, but only on time blocks 3 and 4 (middle graph, Fig. 3). Analysis of repetitive motor movements (another measure of stereotypy) produced a similar pattern of results, with the exception that Mn-induced differences were only observed after treatment with 4 mg/kg amphetamine [Mn × Amphetamine × Time block interaction, F(28, 196)=1.78, P<0.05]. Specifically, Mn-exposed rats injected with 4 mg/kg amphetamine exhibited longer durations of repetitive motor movements than vehicle-exposed rats given the same dose of amphetamine (Fig. 4) [Mn main effect, F(1, 7)=12.07, P<0.01]. This effect varied according to time block, with Mn-exposed rats showing more repetitive motor movements than vehicle-exposed rats on time blocks 4–6, 8, and 10–12 [Mn × Time block interaction, F(14, 98)=2.79, P<0.01].
Fig. 3.
Mean (±SEM) behavioral intensity scores of male rats (n = 8 per group) injected with saline or amphetamine (2 or 4 mg/kg) on PD 91. Rats had been exposed to vehicle or 750 μg manganese (Mn) on PD 1–21. Behavioral intensity scores were based on a rating scale where values ranged from 0 (asleep or inactive) to 6 (continuous activity with repetitive chewing or gnawing in the same location). *Significantly different from rats exposed to vehicle and challenged with the identical dose of amphetamine.
Fig. 4.
Mean (±SEM) repetitive motor movements of male rats (n = 8 per group) injected with 4 mg/kg amphetamine on PD 91 (these are the same rats as described in Fig. 3). Rats had been exposed to vehicle or 750 μg manganese (Mn) on PD 1–21. *Significantly different from rats exposed to vehicle.
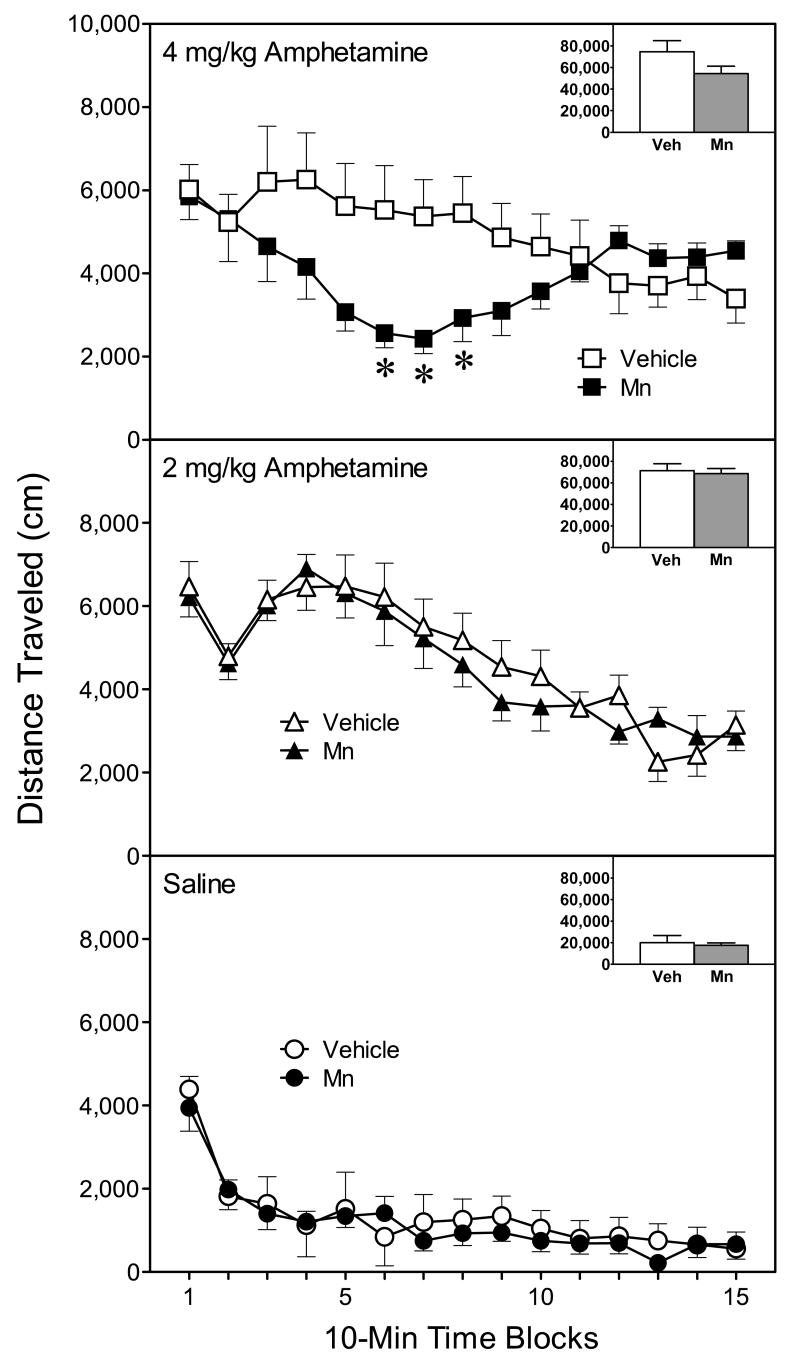
In terms of locomotor activity, rats injected with amphetamine (2 or 4 mg/kg) had greater distance traveled scores than saline-treated rats (Fig. 5) [Amphetamine main effect, F(2, 14)=41.86, P<0.001]. The effects of the amphetamine variable differed according to Mn condition and time block. Specifically, Mn-exposed rats treated with 4 mg/kg amphetamine exhibited significantly less locomotor activity than vehicle-exposed rats injected with the same dose of amphetamine (Fig 5, upper graph) [Mn × Amphetamine × Time block interaction, F(28, 196)=2.61, P<0.001]. The latter effect reached statistical significance on time blocks 6–8. In contrast, the distance traveled scores of vehicle- and Mn-exposed rats were not differentially affected by either saline or 2 mg/kg amphetamine (Fig. 5, lower and middle graphs).
Fig. 5.
Mean (±SEM) distance traveled of male rats (n = 8 per group) injected with saline or amphetamine (2 or 4 mg/kg) on PD 91 (these are the same rats as described in Fig. 3). Rats had been exposed to vehicle or 750 μg manganese (Mn) on PD 1–21. *Significantly different from rats exposed to vehicle and challenged with the identical dose of amphetamine.
Cocaine-induced place preference conditioning
On the preconditioning day, rats showed a consistent preference for the black compartment (x̄ = 539.8 s, SEM = ±11.0) versus the white compartment (Mn: x̄ = 360.2 s, SEM = ±11.0) [Compartment main effect, F(1, 59)=66.03, P<0.05]: an effect that did not differ according to Mn exposure condition or sex. Because of this compartment preference, rats were uniformly conditioned with cocaine on the originally nonpreferred (white) side.
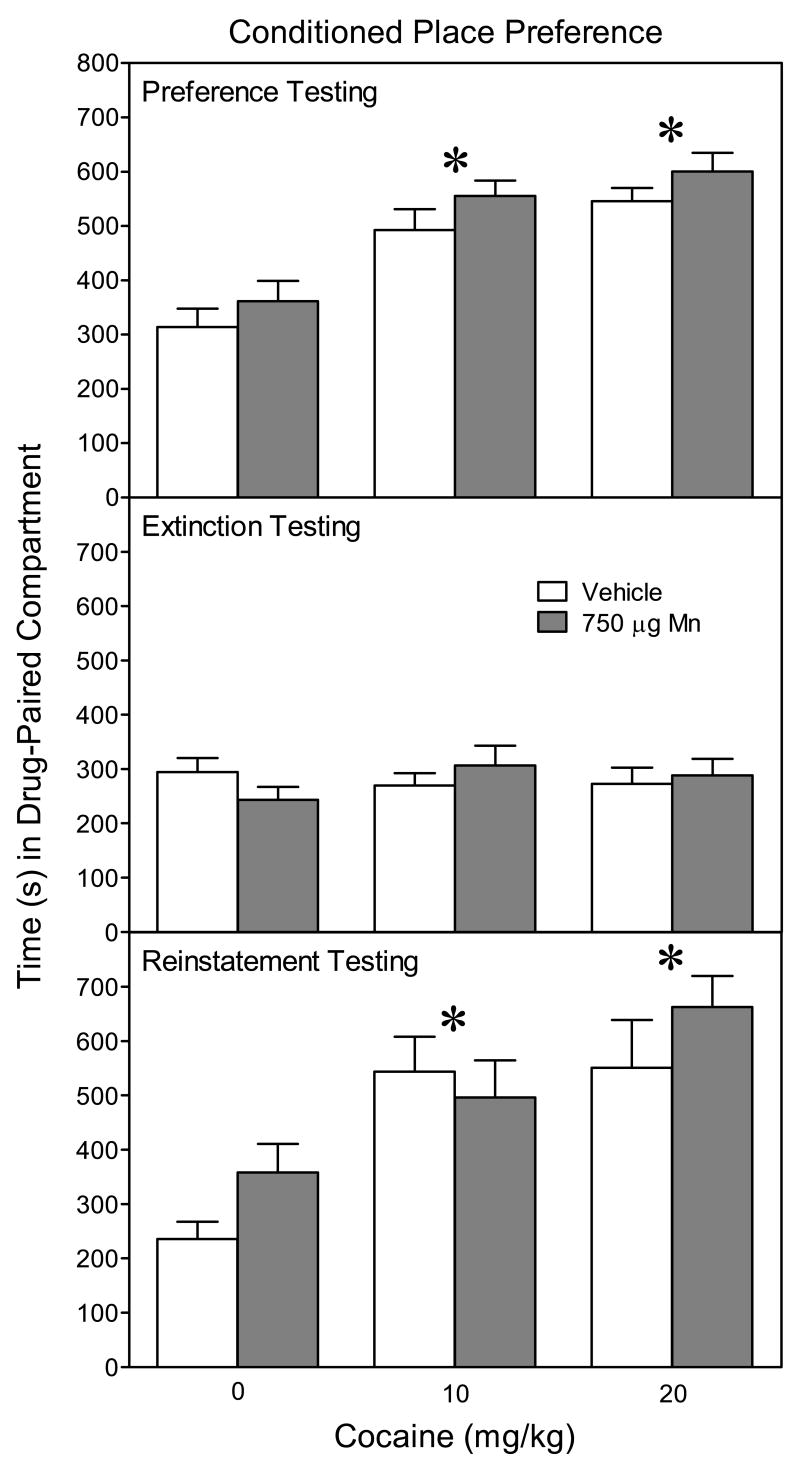
On the preference test day, rats that received cocaine showed a robust preference for the drug-paired (white) compartment (Fig. 6, upper graph). Specifically, place conditioning with 10 or 20 mg/kg cocaine caused an increase in time spent on the drug-paired side relative to saline controls [Cocaine main effect, F(2, 51)=27.38, P<0.05]. After 8 extinction days, the place preference for the cocaine-paired compartment disappeared, because rats conditioned with cocaine or saline spent similar amounts of time in the white compartment (Fig. 6, middle graph). During reinstatement testing, a priming dose of cocaine (20 mg/kg) reinstated the place preference of rats originally conditioned with either 10 or 20 mg/kg cocaine (Fig. 6, lower graph) [Cocaine main effect, F(2, 51)=12.25, P<0.05]. There was no evidence that Mn caused any alterations in the acquisition, extinction, or reinstatement of cocaine CPP. Place preference performance also did not differ according to sex, with the exception that on the extinction test day male rats (x̄ = 307.3 s, SEM = ±14.1) spent more time in the white compartment than did female rats (x̄ = 252.6 s, SEM = ±16.7) [Sex main effect, F(1, 51)=5.74, P<0.05].
Fig. 6.
Mean (±SEM) time spent in the drug-paired compartment on the preference test day (upper graph), extinction test day (middle graph), and reinstatement test day (lower graph). Male (n = 4–6 per group) and female (n = 5–6 per group) rats were given alternating daily injections of cocaine (0, 10, or 20 mg/kg) and saline from PD 91–98. Rats had been exposed to vehicle or 750 μg manganese (Mn) on PD 1–21. *Significantly different from rats conditioned with 0 mg/kg cocaine (P<0.05).
Sucrose-reinforced operant responding
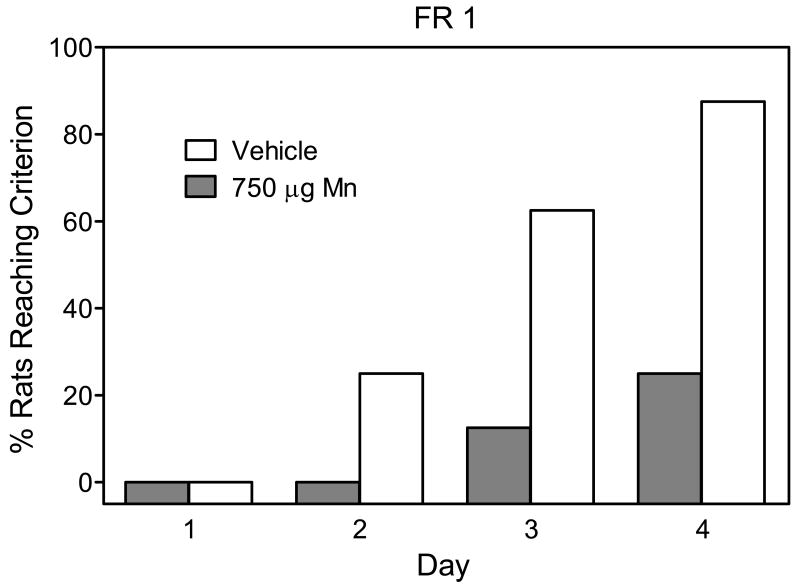
Postnatal Mn exposure significantly impaired acquisition of the FR 1 task (Fig.7). Specifically, 7 out of 8 vehicle-exposed rats reached criterion on the FR 1 task within five days, while only 2 out of 8 Mn-exposed rats reached criterion [Yates' corrected χ2=4.06, P<0.05]. Even with an upper ceiling of five days (i.e., rats not reaching criterion were eventually hand-shaped), vehicle controls (x̄ = 3.2 days, SEM = ±0.37) acquired the FR task in significantly fewer days than Mn-exposed rats (x̄ = 4.6 days, SEM = ±0.26) [Mn effect, t(7)=2.43, P<0.05]. After acquiring the FR 1 task, all rats from both groups took the minimal number of days (two in each case) to reach criterion on the FR 3 and FR 10 schedules (data not shown).
Fig. 7.
Percent of male rats (n = 8 per group) that reached criterion on the fixed ratio 1 (FR 1) task according to day. Successful completion of the criterion was defined as making a minimum of 50 responses on the active lever for two consecutive days. Rats had been exposed to vehicle or 750 μg manganese (Mn) on PD 1–21.
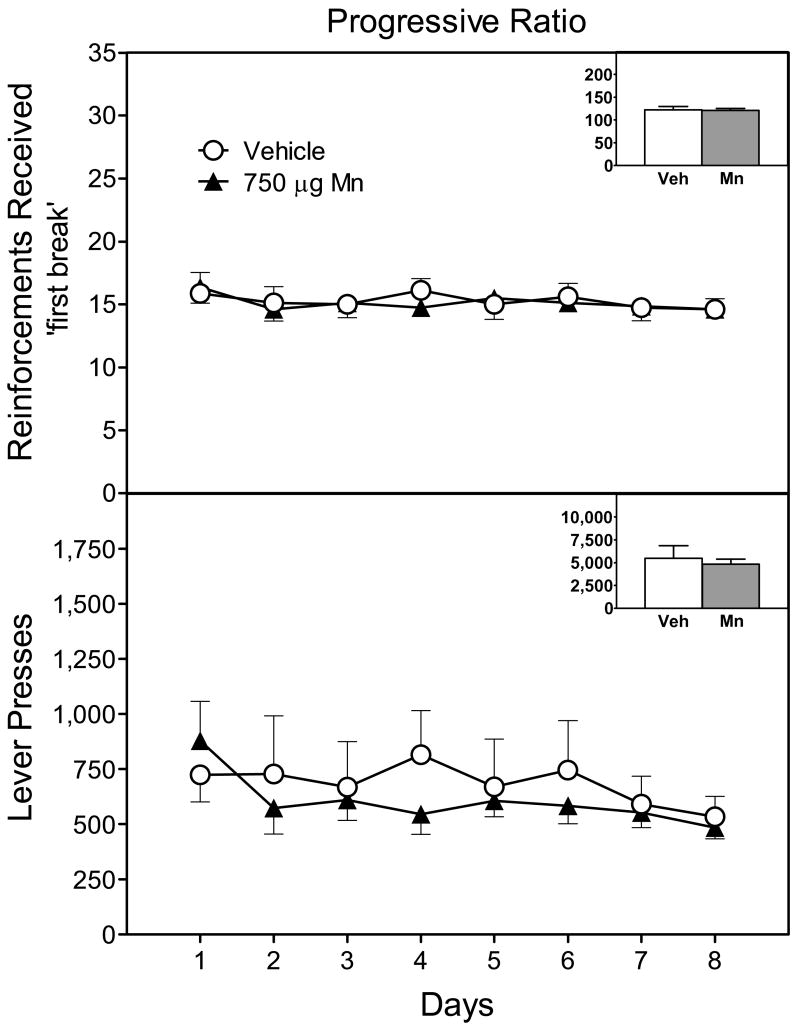
Performance on the progressive ratio schedule was not altered by pretreatment condition, as the break point (number of reinforced responses) for the vehicle- and Mn-exposed rats did not differ (Fig. 8, upper graph) [P>0.05]. Total number of lever presses during progressive ratio training was also unaffected by Mn exposure on PD 1–21 (Fig. 8, lower graph) [P>0.05].
Fig. 8.
Mean (±SEM) number of reinforcements received (upper graph) and lever presses (lower graph) on a progressive ratio task (n = 8 male rats per group). Rats had been exposed to vehicle or 750 μg manganese (Mn) on PD 1–21. The insets show mean number of reinforcements received and lever presses collapsed across days 1–8.
Discussion
The present results show that exposing rats to a high dose of Mn (750 μg) on PD 1–21 causes a long-term decrement in presynaptic dopaminergic functioning that persists into adulthood. Mn-induced reductions in DAT protein expression, while modest, were apparent in both the striatum and nucleus accumbens. The latter finding is consistent with previous work from our laboratory showing that DAT binding site density (Bmax) in the striatum declined after postnatal Mn exposure (Reichel et al., 2006). Experimental manipulations do not always affect DAT binding sites and protein expression in an identical manner (see Zhu et al., 2005; Shepard et al., 2006), but it now appears that Mn toxicity causes a general reduction in DAT that is detectable using either immunoblotting or radioligand techniques.
In addition to reducing DAT protein expression, postnatal Mn exposure caused long-term changes in the kinetic parameters of [3H]dopamine uptake. Specifically, the maximal velocity (Vmax) of [3H]dopamine uptake in the striatum and nucleus accumbens was substantially lower in Mn-exposed rats than vehicle controls. Past research was equivocal about whether Mn exposure affects [3H]dopamine uptake, with the same group of researchers alternately reporting that Mn increases (Leung et al., 1982) and decreases (Lai et al., 1982) the synaptosomal uptake of dopamine. Consistent with Lai et al. (1982), the present data show that postnatal Mn exposure causes a prolonged reduction in [3H]dopamine uptake. This particular outcome was anticipated because manipulations (in this case Mn exposure) that decrease DAT protein expression or DAT binding sites should also cause a concomitant reduction in [3H]dopamine uptake (Chagkutip et al., 2003; Zhu et al., 2005). Besides altering Vmax, Km was elevated in Mn-treated rats, indicating that Mn exposure decreased the affinity of [3H]dopamine for DAT in the striatum. This pattern of results (i.e., decreased Vmax and increased Km) also occurs after acrylamide exposure and is typical of a neurotoxic response (LoPachin et al., 2006). Interestingly, adding Mn sulfate to striatal synaptosomal preparations decreases [3H]dopamine uptake as well as DAT binding site density (Bmax) (Chen et al., 2006). Although the latter results are similar to those described in the present study, it is uncertain whether acute and subchronic Mn treatment alters dopaminergic functioning via the same mechanisms. For example, our results cannot be due to acutely elevated Mn levels at the time of testing, because rats exposed to Mn during the postnatal period do not have increased levels of serum or brain Mn in adulthood (Reichel et al., 2006).
To determine whether Mn exposure has long-term effects on dopamine release characteristics, in vivo microdialysis was used to measure dopamine efflux in the striatum and nucleus accumbens of freely moving rats on PD 90. Results showed that basal dopamine release did not differ according to treatment condition, but that responsiveness to cocaine (20 mg/kg) was blunted in rats previously exposed to Mn. More specifically, cocaine-induced dopamine efflux was reduced in the striatum of Mn-treated rats relative to vehicle controls, while no differences in dopamine efflux were observed in the nucleus accumbens. In this regard, it is noteworthy that Mn caused greater reductions of [3H]dopamine uptake in the striatum than the nucleus accumbens, thus suggesting that the mesocorticolimbic pathway is less sensitive to the toxic effects of Mn than the nigrostriatal pathway. This possible regional difference in toxicity is not due to disparities in Mn accumulation, which is approximately equal in the striatum and nucleus accumbens (Kontur and Fechter, 1988). Alterations in presynaptic dopamine functioning were also not due to elevated levels of Mn at the time of testing, because we previously showed, using the identical Mn administration procedure (750 μg/day on PD 1–21), that striatal Mn accumulation was significantly enhanced on PD 14 and PD 21, but not on PD 90 (Reichel et al., 2006).
When considered together, our results show that postnatal Mn exposure causes persistent declines in DAT protein expression and [3H]dopamine uptake in the striatum and nucleus accumbens, as well as long-term reductions in striatal dopamine efflux and DAT binding sites (Reichel et al., 2006). The most obvious explanation is that these changes in presynaptic functioning are caused by Mn-induced neurotoxicity affecting dopamine terminals. General support for this explanation is provided by a large number of in vivo and in vitro studies showing that Mn is toxic to dopamine-containing cells of the basal ganglia (for reviews, see Hirata, 2002; Takeda, 2003; Erikson et al., 2007). Other classes of neurons (e.g., glutamatergic and GABAergic) in the basal ganglia also exhibit a neurotoxic response to Mn (Fitsanakis et al., 2006b), therefore loss of neuronal inputs might be secondarily responsible for some of the perturbations in presynaptic dopamine functioning. Most notably, Mn readily accumulates in the globus pallidus (Fitsanakis et al., 2006b) and there are extensive direct and indirect connections between this structure and the striatum and nucleus accumbens (Nakano, 2000). Finally, DAT protein expression is normally regulated by a variety of kinases (Vaughan et al., 1997; Carvelli et al., 2002; Morón et al., 2003), thus it cannot be excluded that early Mn exposure might cause long-term changes in regulatory proteins that modulate DAT expression and, indirectly, alter dopamine uptake and efflux. The persistent increase in striatal Km values (Table 2) is consistent with the idea that Mn exposure alters the functioning of remaining transporters, while also decreasing DAT protein expression and [3H]GBR 12935 binding sites (Reichel et al., 2006).
In addition to directly measuring various components of presynaptic dopamine functioning, we assessed the long-term impact of postnatal Mn exposure on associative and nonassociative behavior. Interestingly, vehicle- and Mn-exposed rats performed similarly on the accelerating rotorod task, which is sensitive to major disruptions of nigrostriatal functioning (Wagner and Walsh, 1987; Meshul et al., 2000; Kawasaki et al., 2007). These results suggest that early Mn exposure was not responsible for any gross motor disturbances in adulthood. More subtle motoric effects were apparent, however, because Mn-exposed rats were more sensitive to the stereotypy-inducing effects of amphetamine. At a transitional dose of amphetamine (2 mg/kg), stereotypy was about equally evident in the vehicle and Mn groups; however, a higher dose of amphetamine (4 mg/kg) preferentially induced stereotypy in Mn-exposed rats (i.e., behavioral intensity scores were elevated and repetitive motor movements increased). The locomotor activity data is consistent with these findings because Mn-exposed rats exhibited a progressive decline in locomotion that re-emerged toward the end of the 150-min testing session (see Fig. 5). In normal rats this pattern of results is often observed after high doses of amphetamine, because: (a) locomotor activity decreases as more intense stereotyped behaviors are preferentially expressed, and (b) locomotor activity frequently re-emerges during a post-stereotypy phase (Segal and Kuczenski, 1987; Kuczenski and Segal, 1989; Rebec et al., 1997). Taken together, these unexpected results (we hypothesized that Mn would reduce stereotypy) suggest that early Mn exposure induces long-term neural changes that increase the sensitivity of the nigrostriatal system to amphetamine. The nature of these neural changes is uncertain, but it may involve an increase in dopamine receptor sensitivity or alterations in a second messenger signaling pathway. Lastly, it remains possible that Mn may impact stereotypy through actions in a different brain region, but the striatum remains the most likely locus because it is the primary structure responsible for motor stereotypies (Jaber et al., 1995; Rebec et al., 1997; Canales and Graybiel, 2000).
In terms of associative behavior, we assessed the long-term impact of postnatal Mn exposure on drug- and sucrose-reinforced appetitive learning. In the CPP task, both doses of cocaine (10 and 20 mg/kg) induced place preference conditioning and reinstated CPP after a prolonged extinction phase. Importantly, postnatal Mn exposure did not alter performance on any aspect of the CPP task (i.e., place conditioning, extinction, or reinstatement testing). Various brain regions and neural networks combine to mediate CPP (for reviews, see Bardo, 1998; McBride et al., 1999), with the mesocorticolimbic dopamine pathway being particularly important for reward (Baker et al., 1998; Sellings and Clarke, 2003). Because the nucleus accumbens is a critical target region for the mesocorticolimbic pathway, we hypothesized that Mn-induced alterations in accumbal functioning would disrupt CPP performance. Results indicated, however, that the magnitude of Mn-induced toxicity in the nucleus accumbens, and other relevant brain regions, was apparently insufficient to impair performance on the CPP task. Additionally, CPP performance has been reported to vary according to sex, with female rats and mice showing more robust place preferences than males (Russo et al., 2003; Balda et al., 2006; but see Crawford et al., 1995; Campbell and Spear, 1999). For this reason we included both sexes in the CPP experiment but found that (a) Mn did not differentially affect place preference conditioning of male and female rats, and (b) preference testing and reinstatement performance did not differ according to sex (perhaps due to insufficient n). The only exception was that male rats spent more time in the nonpreferred (white) compartment on the extinction test day than female rats. This effect was not due to sex-related differences in the strength of Pavlovian drug-environment associations, because saline-treated male rats spent more time in the white compartment than saline-treated females.
Postnatal Mn exposure did alter sucrose-reinforced operant responding in adulthood. Specifically, Mn-treated rats took longer than controls to reach criterion on the FR 1 schedule. On subsequent schedules (FR 3, FR 10, and progressive ratio), Mn- and vehicle-exposed rats performed similarly. The poor performance of the Mn group on the FR 1 task may reflect an associative deficit, because the CPP data suggest that reward processes were unaffected by Mn exposure, while performance on the progressive ratio schedule indicated that Mn-treated rats did not have motivational or motoric deficits. Both the striatum and nucleus accumbens are important for operant responding, with dopamine transmission in the striatum being necessary for acquisition of procedural tasks and habit formation (Robbins et al., 1990; Featherstone and McDonald, 2004a; Yin et al., 2004; Faure et al., 2005), whereas accumbal dopamine release is important for sustained goal-directed behaviors (for reviews, see Salamone and Correa, 2002; Salamone et al., 2003). 6-Hydroxydopamine (6-OHDA) lesions of the nucleus accumbens disrupt performance on high, but not low, FR schedules (Aberman and Salamone, 1999), suggesting that Mn-induced changes in accumbal functioning were not responsible for the performance decrement on our FR 1 task. Instead, the striatum is suspect, since 6-OHDA and quinolinic acid lesions of the dorsolateral striatum disrupt acquisition of even simple operant tasks (Robbins et al., 1990; Featherstone and McDonald, 2004a, 2005). Because only FR 1 responding was affected in the present study, the most parsimonious explanation is that Mn-induced alterations in striatal functioning may have caused a modest impairment in stimulus-response learning. Consistent with this conclusion, Featherstone and McDonald (2004b) reported that striatal lesions did not disrupt performance on a food-reinforced CPP task, while the same rats previously exhibited deficits in operant responding.
In summary, exposing rats to a high dose of Mn caused long-term changes in presynaptic dopamine functioning, which included declines in DAT protein expression, [3H]dopamine uptake, and dopamine efflux. Postnatal Mn exposure also caused an increased sensitivity to the stereotypy-inducing effects of amphetamine. CPP and progressive ratio performance was unaffected by Mn toxicity, although acquisition of a FR 1 task was impaired in Mn-exposed rats. These associative and nonassociative effects may be due to Mn-induced alterations in striatal dopaminergic functioning
Acknowledgments
We thank Linda Dwoskin for her technical assistance with the [3H]dopamine uptake assay. This work was partially supported by a grant (GM073842) from the National Institutes of Health.
Abbreviations
- ANOVA
Analysis of variance
- CPP
conditioned place preference
- DAT
dopamine transporter
- EDTA
ethylenediaminetetraacetic acid
- FR
fixed ratio
- GAPDH
glyceraldehydes 3-phosphate dehydrogenase
- HEPES
2-hydroxyethyl-piperazine butane sulfonic acid
- 6-OHDA
6-hydroxydopamine
- IP
intraperitoneal
- Mn
manganese chloride
- PD
postnatal day
- RPM
revolutions per minute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2-3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Aschner M. Manganese: brain transport and emerging research needs. Environ Health Perspect. 2000;108 3:429–432. doi: 10.1289/ehp.00108s3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Baker DA, Fuchs RA, Specio SE, Khroyan TV, Neisewander JL. Effects of intraaccumbens administration of SCH-23390 on cocaine-induced locomotion and conditioned place preference. Synapse. 1998;30:181–193. doi: 10.1002/(SICI)1098-2396(199810)30:2<181::AID-SYN8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51:341–349. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Bird ED, Anton AH, Bullock B. The effect of manganese inhalation on basal ganglia dopamine concentrations in rhesus monkey. Neurotoxicology. 1984;5:59–65. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruning JL, Kintz BL. Computational handbook of statistics. 4th. New York: Allyn & Bacon; 1997. [Google Scholar]
- Calabresi P, Ammassari-Teule M, Gubellini P, Sancesario G, Morello M, Centonze D, Marfia GA, Saulle E, Passino E, Picconi B, Bernardi G. A synaptic mechanism underlying the behavioral abnormalities induced by manganese intoxication. Neurobiol Dis. 2001;8:419–432. doi: 10.1006/nbdi.2000.0379. [DOI] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic Parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Campbell J, Spear LP. Effects of early handling on amphetamine-induced locomotor activation and conditioned place preference in the adult rat. Psychopharmacology. 1999;143:183–189. doi: 10.1007/s002130050934. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Carvelli L, Morón JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeb-Lundberg LM, Merrill G, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002;81:859–869. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- Cawte J. Psychiatric sequelae of manganese exposure in the adult, foetal and neonatal nervous systems. Aust N Z J Psychiatry. 1985;19:211–217. doi: 10.3109/00048678509158825. [DOI] [PubMed] [Google Scholar]
- Chagkutip J, Vaughan RA, Govitrapong P, Ebadi M. 1-Methyl-4-phenylpyridinium-induced down-regulation of dopamine transporter function correlates with a reduction in dopamine transporter cell surface expression. Biochem Biophys Res Commun. 2003;311:49–54. doi: 10.1016/j.bbrc.2003.09.155. [DOI] [PubMed] [Google Scholar]
- Chen MK, Lee JS, McGlothan JL, Furukawa E, Adams RJ, Alexander M, Wong DF, Guilarte TR. Acute administration alters dopamine transporter levels in the non-human primate striatum. Neurotoxicology. 2006;27:229–236. doi: 10.1016/j.neuro.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology. 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. Blockage of amphetamine-induced motor stimulation and stereotypy in the adult rat following neonatal treatment with 6-hydroxydopamine. Brain Res. 1973;55:369–382. doi: 10.1016/0006-8993(73)90302-8. [DOI] [PubMed] [Google Scholar]
- Dietz MC, Ihrig A, Wrazidlo W, Bader M, Jansen O, Triebig G. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ Res. 2001;85:37–40. doi: 10.1006/enrs.2000.4068. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Vitarella D, Byerly FL, Goetz J, Miller R. Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure. J Appl Toxicol. 2000;20:179–187. doi: 10.1002/(sici)1099-1263(200005/06)20:3<179::aid-jat631>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Thompson K, Aschner J, Aschner M. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther. 2007;113:369–377. doi: 10.1016/j.pharmthera.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson H, Gillberg PG, Aquilonius SM, Hedström KG, Heilbronn E. Receptor alterations in manganese intoxicated monkeys. Arch Toxicol. 1992;66:359–364. doi: 10.1007/BF01973632. [DOI] [PubMed] [Google Scholar]
- Faure A, Haberland U, Condé F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Dorsal striatum and stimulus-response learning: lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a simple discrimination task. Behav Brain Res. 2004a;150:15–23. doi: 10.1016/S0166-4328(03)00218-3. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Dorsal striatum and stimulus-response learning: lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a stimulus-response-based instrumental discrimination task, while sparing conditioned place preference learning. Neuroscience. 2004b;124:23–31. doi: 10.1016/j.neuroscience.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Lesions of the dorsolateral striatum impair the acquisition of a simplified stimulus-response dependent conditional discrimination task. Neuroscience. 2005;136:387–395. doi: 10.1016/j.neuroscience.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Avison MJ, Gore JC, Aschner JL, Aschner M. The use of magnetic resonance imaging (MRI) in the study of manganese neurotoxicity. Neurotoxicology. 2006a;27:798–806. doi: 10.1016/j.neuro.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Au C, Erikson KM, Aschner M. The effects of manganese on glutamate, dopamine and gamma-aminobutyric acid regulation. Neurochem Int. 2006b;48:426–433. doi: 10.1016/j.neuint.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Gianutosos G, Murray MT. Alterations in brain dopamine and GABA following inorganic or organic manganese administration. Neurotoxicology. 1982;3:75–82. [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula, soy formula, or soy formula with added manganese. Neurotoxicol Teratol. 2005;27:615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Hamill S, Trevitt JT, Nowend KL, Carlson BB, Salamone JD. Nucleus accumbens dopamine depletions and time-constrained progressive ratio performance: effects of different ratio requirements. Pharmacol Biochem Behav. 1999;64:21–27. doi: 10.1016/s0091-3057(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Hirata Y. Manganese-induced apoptosis in PC12 cells. Neurotox Teratol. 2002;24:639–653. doi: 10.1016/s0892-0362(02)00215-5. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Ingersoll RT, Montgomery EB, Jr, Aposhian HV. Central nervous system toxicity of manganese. I. Inhibition of spontaneous motor activity in rats after intrathecal administration of manganese chloride. Fund Appl Toxicol. 1995;27:106–113. doi: 10.1006/faat.1995.1113. [DOI] [PubMed] [Google Scholar]
- Jaber M, Cador M, Dumartin B, Normand E, Stinus L, Bloch B. Acute and chronic amphetamine treatments differently regulate neuropeptide messenger RNA levels and Fos immunoreactivity in rat striatal neurons. Neuroscience. 1995;65:1041–1050. doi: 10.1016/0306-4522(94)00537-f. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Ishihara K, Ago Y, Baba A, Matsuda T. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a radical scavenger, prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in the substantia nigra but not the striatum. J Pharmacol Exp Ther. 2007;322:274–281. doi: 10.1124/jpet.106.119206. [DOI] [PubMed] [Google Scholar]
- Klos KJ, Chandler M, Kumar N, Ahlskog JE, Josephs KA. Neuropsychological profiles of manganese neurotoxicity. Eur J Neurol. 2006;13:1139–1141. doi: 10.1111/j.1468-1331.2006.01407.x. [DOI] [PubMed] [Google Scholar]
- Komura J, Sakamoto M. Effects of manganese forms on biogenic amines in the brain and behavioral alterations in the mouse: long-term oral administration of several manganese compounds. Environ Res. 1992;57:34–44. doi: 10.1016/s0013-9351(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Kontur JP, Fechter LD. Brain regional manganese levels and monoamine metabolism in manganese-treated neonatal rats. Neurotoxicol Teratol. 1988;10:295–303. doi: 10.1016/0892-0362(88)90031-1. [DOI] [PubMed] [Google Scholar]
- Kostial K, Blanuša M, Piasek M. Regulation of manganese accumulation in perinatally exposed rat pups. J Appl Toxicol. 2005;25:89–93. doi: 10.1002/jat.1039. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JC, Leung TK, Guest JF, Davison AN, Lim L. The effects of chronic manganese chloride treatment expressed as age-dependent, transient changes in rat brain synaptosomal uptake of amines. J Neurochem. 1982;38:844–847. doi: 10.1111/j.1471-4159.1982.tb08709.x. [DOI] [PubMed] [Google Scholar]
- Leung TK, Lai JC, Tricklebank M, Davison AN, Lim L. Chronic manganese treatment of rats alters synaptosomal uptake of dopamine and the behavioural response to amphetamine administration. J Neurochem. 1982;39:1496–1499. doi: 10.1111/j.1471-4159.1982.tb12599.x. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Barber DS, He D, Das S. Acrylamide inhibits dopamine uptake in rat striatal synaptic vesicles. Toxicol Sci. 2006;89:224–234. doi: 10.1093/toxsci/kfj005. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. New York: Academic Press; 1974. [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Mena I, Marin O, Fuenzalida S, Cotzias GC. Chronic manganese poisoning. Clinical picture and manganese turnover. Neurology. 1967;17:128–136. doi: 10.1212/wnl.17.2.128. [DOI] [PubMed] [Google Scholar]
- Mergler D. Neurotoxic effects of low level exposure to manganese in human populations. Environ Res. 1999;80:99–102. doi: 10.1006/enrs.1998.3902. [DOI] [PubMed] [Google Scholar]
- Mergler D, Huel G, Bowler R, Iregren A, Belanger S, Baldwin M, Tardit R, Smargiassi A, Martin L. Nervous system dysfunction among workers with long-term exposure to manganese. Environ Res. 1994;64:151–180. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Cogen JP, Cheng HW, Moore C, Krentz L, McNeill TH. Alterations in rat striatal glutamate synapses following a lesion of the cortico- and/or nigrostriatal pathway. Exp Neurol. 2000;165:191–206. doi: 10.1006/exnr.2000.7467. [DOI] [PubMed] [Google Scholar]
- Morón JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtman JP, Tubben RE, Commissaris RL. Behavioral effects of chronic manganese administration in rats: locomotor activity studies. Neurobehav Toxicol Teratol. 1986;5:711–715. [PubMed] [Google Scholar]
- Nakano K. Neural circuits and topographic organization of the basal ganglia and related regions. Brain Dev. 2000;22:S5–S16. doi: 10.1016/s0387-7604(00)00139-x. [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Rebec GV, White IM, Puotz JK. Responses of neurons in dorsal striatum during amphetamine-induced focused stereotypy. Psychopharmacology. 1997;130:343–351. doi: 10.1007/s002130050249. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Wacan JJ, Farley CM, Stanley BJ, Crawford CA, McDougall SA. Postnatal manganese exposure attenuates cocaine-induced locomotor activity and reduces dopamine transporters in adult male rats. Neurotox Teratol. 2006;28:323–332. doi: 10.1016/j.ntt.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Meth. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Giardini V, Jones GH, Reading P, Sahakian BJ. Effects of dopamine depletion from the caudate-putamen and nucleus accumbens septi on the acquisition and performance of a conditional discrimination task. Behav Brain Res. 1990;38:243–261. doi: 10.1016/0166-4328(90)90179-i. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118:222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Behavioral and neurochemical characteristics of stimulant-induced augmentation. Psychopharmacol Bull. 1987;23:417–424. [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Chuang DT, Shaham Y, Morales M. Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacology. 2006;185:505–513. doi: 10.1007/s00213-006-0316-4. [DOI] [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Talavera EJ, Arcaya JL, Giraldoth D, Suarez J, Bonilla E. Decrease in spontaneous motor activity and in brain lipid peroxidation in manganese and melatonin treated mice. Neurochem Res. 1999;24:705–708. doi: 10.1023/a:1021064711866. [DOI] [PubMed] [Google Scholar]
- Tran TT, Chowanadisai W, Crinella FM, Chicz-DeMet A, Lönnerdal B. Effect of high dietary manganese intake of neonatal rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status. Neurotoxicology. 2002a;23:635–643. doi: 10.1016/s0161-813x(02)00091-8. [DOI] [PubMed] [Google Scholar]
- Tran TT, Chowanadisai W, Lönnerdal B, Le L, Parker M, Chicz-DeMet A, Crinella FM. Effects of neonatal dietary manganese exposure on brain dopamine levels and neurocognitive functions. Neurotoxiology. 2002b;23:645–651. doi: 10.1016/s0161-813x(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Uchino A, Noguchi T, Nomiyama K, Takase Y, Nakazono T, Nojiri J, Kudo S. Manganese accumulation in the brain: MR imaging. Neuroradiology. 2007;49:715–720. doi: 10.1007/s00234-007-0243-z. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem. 1997;272:15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- Vidal L, Alfonso M, Campos F, Faro LR, Cervantes RC, Durán R. Effects of manganese on extracellular levels of dopamine in rat striatum: an analysis in vivo by brain microdialysis. Neurochem Res. 2005;30:1147–1154. doi: 10.1007/s11064-005-7775-6. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Walsh SL. Increased sensitivity of mice to tremorogenic agents following MPP+ Psychopharmacology. 1987;92:470–472. doi: 10.1007/BF00176480. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]