Abstract
Recombinant Eimeria antigen (rEA) has been shown to have potent anticancer and antiviral activity in respective mouse disease models, presumably through robust immune stimulation that occurs via TLR11, a pattern recognition receptor that recognizes profilin-like proteins expressed on apicomplexan protozoans. Comparable immunostimulatory activity in other species has yet to be demonstrated. Since rEA is known to be highly effective in treating Punta Toro virus (PTV) infection in mice, its ability to elicit protective immunity in the hamster PTV infection model was investigated. rEA was given alone, or in combination with IL-18 or IL-2, and virally challenged hamsters were observed for mortality. Cytokine transcript profiles for IL-12p40, IL-21, IFN-γ and TNF-α were assessed to evaluate the induction of these inflammatory mediators known to be induced in mice following exposure to rEA. A dose of 100 μg of rEA, given once 4 h prior to viral challenge, and a second time on day 3 of the infection, was found to be the most effective prophylactic therapy protecting 60% of treated hamsters from mortality, compared to only 5–10% observed in animals receiving placebo. Increased expression of IFN-γ and IL-12p40 was evident following treatment with rEA. The data suggest that rEA does induce host antiviral responses in hamsters that result in significant protection from death, although determining the most appropriate dose for intervention in other species, including humans, will likely be challenging.
Keywords: Punta Toro virus, Phlebovirus, Antiviral, Immune modulator, Profilin
1. Introduction
While searching for proteins with anticancer activity, a potent modulator of the innate immune system was identified from a protozoan, Eimeria spp. [1]. A recombinant form of the protein, rEA, induces inflammatory mediators including interleukin (IL)-12, monocyte chemoattractant protein (MCP)-1, IL-6, tissue necrosis factor (TNF)-α, and interferon (IFN)-γ. In addition, rEA has demonstrated anticancer activity in mice and humans [2], as well as antiviral activity in mice [3, 4].
Punta Toro virus (PTV), family Bunyaviridae, genus phlebovirus, readily produces significant hepatic disease in mice and hamsters that resembles severe disease caused by the related human and livestock pathogen, Rift Valley fever virus [5–7]. Previously, the immunostimulatory and antiviral effects of rEA were explored in the mouse and hamster PTV infection models. Single or multi-dose treatments with nanogram quantities of rEA were shown to be highly protective in mice, whereas no protective effect was seen in hamsters at dosages equivalent to 100times the lowest effective dose in mice [3]. Based on these findings and other reports, the evidence suggested that the mechanism by which rEA is recognized in mice differs from that of the hamster system [8–10]. It is conceivable, however, that the ineffectiveness of rEA treatment in the hamster model may also be due to the difference in disease pathogenesis and severity compared to the mouse model.
In this study, treatment of PTV disease in hamsters with considerably higher doses of rEA was explored. Moreover, rEA treatment was combined with several proinflammatory cytokines to investigate whether such an approach could improve or enhance efficacy since the possibility has been previously explored in mice with some success [4]. Since IL-12 and IFN-γ are among the most prominent cytokines elicited in mice following exposure to rEA [1, 3], we also investigated whether these and other cytokines were induced in hamsters at the transcriptional level.
2. Materials and methods
2.1. Animals
Female 7 – 8 week-old golden Syrian hamsters were obtained from Charles River Laboratories (Wilmington, MA) and acclimated for 2 – 3 days prior to use. Hamsters ranged from 100–115 g at the time of initial treatment. Animal procedures complied with USDA guidelines and were approved by the Utah State University Institutional Animal Care and Use Committee.
2.2. Virus
PTV, Adames strain, was obtained from Dr. Dominique Pifat of the U. S. Army Medical Research Institute for Infectious Diseases, Ft. Detrick (Frederick, MD). The virus used in these studies was from a stock prepared following 4 passages of the original virus through LLC-MK2 monkey kidney cells (American Type Culture Collection, Manassas, VA) and one passage through hamsters. Virus stocks were prepared from pooled liver homogenates and diluted in sterile minimal essential medium for inoculation.
2.3. Test materials
rEA was produced as previously described [1, 3]. Recombinant human IL-2, trade name PROLEUKIN®, was obtained from Chiron Corporation (Emeryville, CA). Murine IL-18 was purchased from R & D Systems (Minneapolis, MN). rEA and cytokines were prepared for injection in vehicle consisting of 0.1% BSA in PBS. The specified rEA and cytokine dosages were delivered in a single intraperitoneal (i.p) 0.5 ml injection on days 0 and 3 of the infection. In several experiments, the mismatched double-stranded RNA, poly(I:C12U), trade name Ampligen®, was used as an immune modulatory positive control. poly(I:C12U), was kindly provided by HEMISPHERx Biopharma (Philadelphia, PA) and diluted in sterile injection-grade saline just prior to administration.
2.4. In vivo efficacy studies
Hamsters were weighed on the morning of infection and grouped so that the average hamster weight per group across the entire experiment varied by less than 5 grams. Animals, 10 per group, were treated i.p. on day 0 (4 h pre-virus inoculation) and day 3 with the indicated doses of rEA, IL-18, IL-2, or combinations thereof. A placebo group consisting of 20 animals was included in each experiment and received vehicle injections. In several experiments, poly(I:C12U) was given as a single 100 μg i.p. injection 4 h before challenge. For all challenge studies, hamsters were inoculated by subcutaneous injection of ~50 plaque-forming units (PFU) of PTV. Animals were held 21 days for observation.
2.5. Quantitative RT-PCR analysis of hamster cytokine induction by rEA
Hamsters were treated with a single 100 μg i.p. injection of rEA and spleen tissue was harvested for RNA purification at various times following exposure. Spleens were homogenized using a Tissue Tearor™ (Biospec Products, Bartlesville, OK) and total RNA was prepared using RNeasy reagents from Qiagen (Valencia, CA) following the procedure for tissue samples. All samples were treated with DNase (Turbo DNA-free™, Ambion, Austin, TX) to remove any contaminating genomic material prior to quantitative (q)RT-PCR analysis. The Superscript III Platinum® One-Step Quantitative RT-PCR System from Invitrogen (Carlesbad, CA) was used for all analyses following the recommended protocols. Primers and probe sequences for IFN-γ, TNF-α, IL-12p40, IL-21 and the housekeeping normalization gene, γ-actin, are shown in Table 1.
Table 1.
Primer sets and probes for gene expression analysis in hamsters
| Gene | Forward primer sequence | Reverse primer sequence | Probe sequence (amplicon size) |
|---|---|---|---|
| IFN-γ | 5′-TGT TGC TCT GCC TCA CTC AGG-3′ | 5′-AAG ACG AGG TCC CCT CCA TTC-3′ | 5′-(6FAM)TGG CTG CTA CTG CCA GGG CAC ACT C-(TAMRA)-3′ (130 bp) |
| TNF-α | 5′-TGA GCC ATC GTG CCA ATG-3′ | 5′-AGC CCG TCT GCT GGT ATC AC-3′ | 5′-(6FAM)-CGG CAT GTC TCT CAA AGA CAA CCA G-(TAMRA)-3′ (79 bp) |
| IL-12p40 | 5′-AAT GCG AGG CAG CAA ATT ACT C-3′ | 5′-CTG CTC TTG ACG TTG AAC TTC AAG-3′ | 5′-(6FAM)-CCT GCT GGT GGC TGA CTG CAA TCA-(TAMRA)-3′ (88 bp) |
| IL-21 | 5′-GGA CAG TGG CCC ATA AAA CAA G-3′ | 5′-TTC AAC ACT GTC TAT AAG ATG ACG AAG TC-3′ | 5′-(6FAM)-CAA GGG CCA GAT CGC CTC CTG ATT-(TAMRA)-3′ (80 bp) |
| γ-actin | 5′-ACA GAG AGA AGA TGA CGC AGA TAA TG-3′ | 5′-GCC TGA ATG GCC ACG TAC A-3′ | 5′-(TET)-TTG AAA CCT TCA ACA CCC CAG CC-(TAMRA)-3′ (70 bp) |
2.6. Statistical analysis
The log-rank test was used for comprehensive survival analysis using JMP statistical software (SAS, Cary, North Carolina).
3. Results
3.1. Escalation of rEA dose for prophylaxis of PTV infection
Doses of up to 1 μg of rEA, which is equivalent to 100-times the lowest effective dose in mice on a per weight basis, have previously been tested in the hamster PTV infection with no evidence of success [3]. To examine whether a higher dose of rEA could have a beneficial impact on survival outcome, we evaluated a dose 10-fold greater than previously tested. As shown in Figure 1, the 10-μg dose failed to significantly improve the outcome or extend survival time. Interestingly, the 1 μg treatment group showed a slight hint of protection as 4 of the 10 animals survived the challenge (Figure 1). Although statistical analysis employing the Fisher’s exact test to assess increases in total survivors indicated low level significance (P = 0.0312), by log-rank analysis significance was not achieved. The 0.1-μg dose completely failed to protect animals from death or extend survival. Treatment with the poly(I:C12U) positive control resulted in highly significant protection of challenged hamsters, although 100% survival was not observed.
Figure 1.
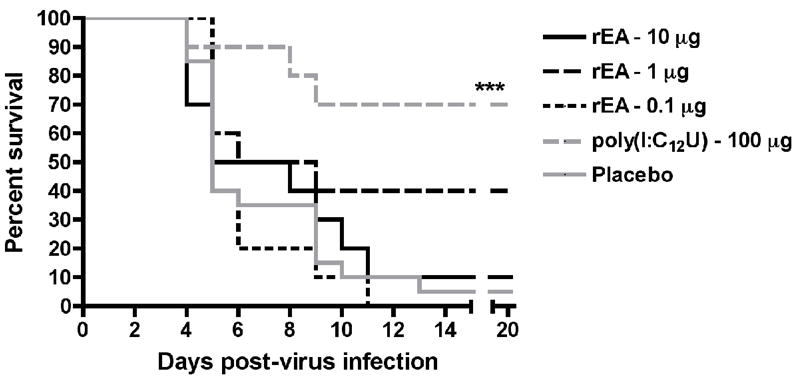
Effect of rEA prophylaxis on survival outcome in hamsters challenged with PTV. Hamsters (n = 10/group) were treated i.p. with rEA (10, 1 or 0.1 μg) 4 h prior to infectious challenge with PTV and on day 3 of the infection. A group of 10 animals was treated i.p. with a single dose of poly(I:C12U) (100 μg) 4 h prior to virus challenge and served as the positive control. ***P < 0.01 compared to 0.1% BSA/PBS placebo-treated hamsters (n = 20) by log-rank analysis.
3.2. High dose rEA and combined IL-18 treatment reduces mortality from PTV infection
Since there appeared to be some protective effect in the initial experiment with the 1 μg rEA treatment, a follow-up study was conducted that included 1-, 10- and 100-μg doses in order to confirm the lack of activity seen at the 10-μg dose and assess whether a higher quantity of rEA could provide protection. Further, in an effort to explore possible synergistic activity of certain proinflammatory cytokines with rEA, we also tested the combination of IL-18 with rEA. Remarkably, similar results were seen with the 1- and 10-μg rEA montherapeutic treatments, as the former again protected 40% of challenged hamsters, while the latter was confirmed to be ineffective (Figure 2A). Considering these results, the significant protection afforded by the 100-μg rEA treatment dose was surprising. Despite the lack of efficacy with the 10-μg dose of rEA, when combined with 1 μg of IL-18, 40% survival was observed with extended survival times, resulting in a statistically significant level of protection (Figure 2B). IL-18 by itself failed to protect any hamsters from PTV challenge as 100% of the animals died by day 7 of the infection.
Figure 2.
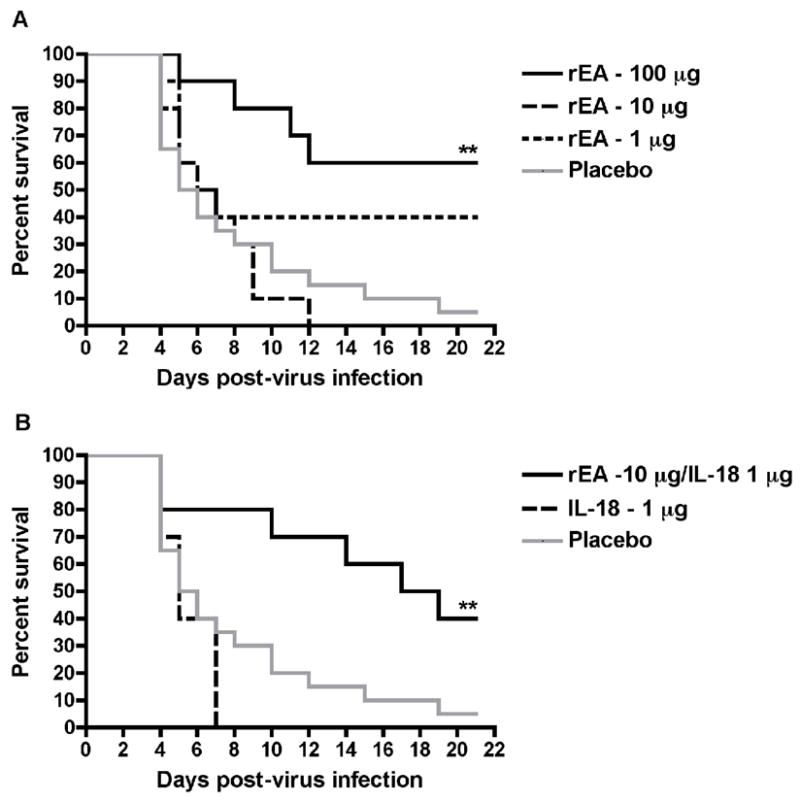
Survival of PTV-infected hamsters treated i.p. with rEA, IL-18, or both in combination. Hamsters (n = 10/group) were dosed i.p. with various doses of rEA (A), 1 μg of IL-18 or a combination of 10 μg rEA with 1 μg IL-18 (B). The survival curves for this experiment are presented in two graphs with the placebo data included in both for ease of visualization. **P < 0.01 compared to 0.1% BSA/PBS placebo-treated hamsters (n = 20) by log-rank analysis.
3.3. Evaluation of higher-dose rEA alone and in combination with IL-18 and IL-2
Having seen the greatest level of protection with rEA at the 100-μg dose, we next evaluated a 320 μg dose. As shown in Figure 3, the higher dose of rEA did significantly improve survival outcome, but this was primarily due to considerable delay in time of death of the majority of the animals compared to the placebo group, wherein the hamsters died from the PTV challenge more quickly than in the first two studies. Since IL-18 was effective with the 10-μg dose of rEA, it was hypothesized that increasing the dose of rEA to the more effective 100-μg amount would improve efficacy. This combination also weakly, yet significantly, protected infected hamsters similar to that observed with the 320-μg dose of rEA administered alone (Figure 3). More impressive was the combination of IL-2 (12 μg) with rEA (100 μg), which protected 60% of the animals from a highly lethal inoculum of PTV (Figure 3).
Figure 3.
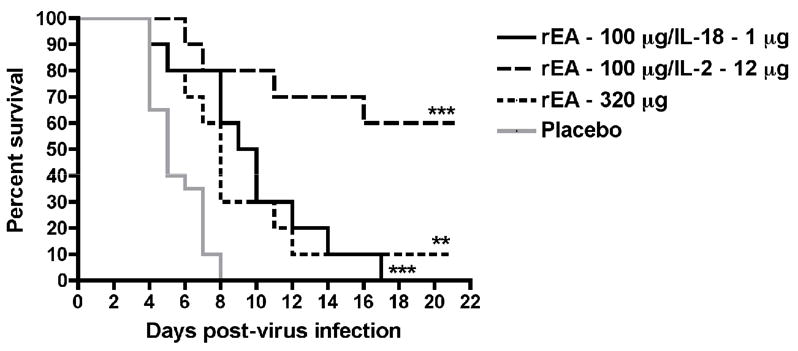
Survival of PTV-infected hamsters treated i.p. with rEA alone or in combination with IL-18 or IL-2. Groups of hamsters (n = 10) received a 320-μg dose of rEA or a 100-μg dose of rEA administered in combination with IL-18 (1 μg) or IL-2 (12 μg). The first treatment was initiated 4 h pre-virus challenge with the second dose administered on day 3 of the infection. **P < 0.01; ***P < 0.001 compared to placebo-treated hamsters (n = 20) by log-rank analysis.
3.4. High dose rEA is more effective alone than in combination with IL-2
Based on our findings from the studies leading up to this point, we next evaluated the combination of 100 μg of rEA with several doses of IL-2. When rEA was administered alone, it was again effective in protecting 60% of challenged hamsters from death due to PTV infection (Figure 4A). Despite the previous result demonstrating dramatic protection offered by the combined effects of rEA (100 μg) with 12 μg of IL-2 (Figure 3), this and other IL-2 dose combinations explored did not produced significant protection compared to animals receiving placebo treatments (Figure 4B). Notably, the survival curves for all combinations and the positive control treatment, poly(I:C12U), were slightly better than that for the placebo group. When IL-2 doses were administered alone, only the 38-μg dose appeared to shift the survival curve favorably (Figure 4A). Nevertheless, the combination of this dose with 100 μg of rEA was unremarkable.
Figure 4.
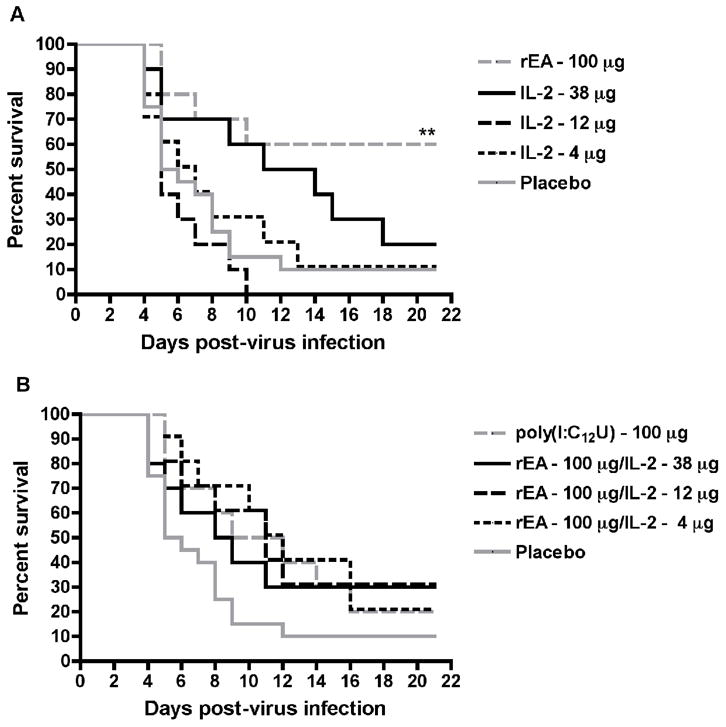
Effect of rEA and varying combinations of IL-2 on survival outcome in hamsters challenged with PTV. Groups of hamsters (n = 10) were dosed i.p. with rEA (100 μg) and IL-2 (38, 12 or 4 μg) alone (A) or in combination (B), 4 h prior to infectious challenge with PTV and on day 3 of the infection. A group of 10 animals was treated with a single i.p. dose of poly(I:C12U) (100 μg) 4 h prior to infectious challenge and served as the positive control. The survival curves for this experiment are presented in two graphs with the placebo data included in both for ease of visualization. **P < 0.01 compared to 0.1% BSA/PBS placebo-treated hamsters (n = 20) by log-rank analysis.
3.5. Treatment with rEA induces transcription of IFN-γand IL-12p40
We next evaluated the expression of various proinflammatory cytokines following i.p. treatment with the 100-μg dose of rEA, which was found to be optimal for protecting hamsters from mortality due to PTV infection. As shown in Figure 5, clear temporal induction of IFN-γ and IL-12p40 was evident, with the most pronounced increase in expression being observed with IFN-γ 3–5 h after exposure. On the other hand, the kinetics of induction was different for IL-12p40, which had highest expression levels 2 hours after treatment. Although there appeared to be some TNF-α transcript induction, particularly at the 2-h time point, it was relatively weak compared to that seen for IFN-γ and IL-12p40 (Figure 5). Induction of IL-21 message was also relatively low with no clear pattern. Of the genes examined, rEA most dramatically induced splenic expression of IFN-γ and IL-12p40, with the latter peaking several hours after exposure, while the former had greatest expression at the 5 h sampling time.
Figure 5.
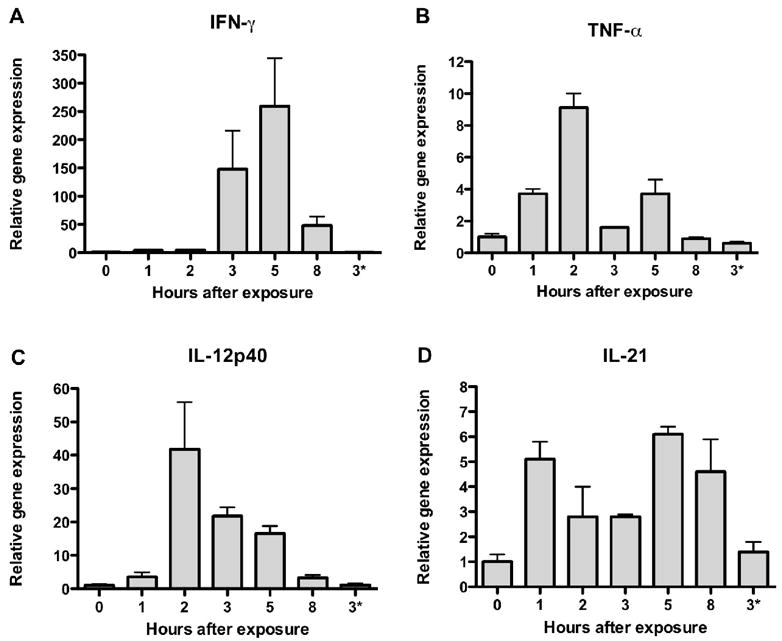
Induction of inflammatory cytokines following exposure to rEA. Individual hamsters were treated i.p. with a single dose of 100 μg of rEA and spleens were harvested at 0, 1, 2, 3, 5 and 8 h after treatment. Spleens were homogenized and total RNA was isolated for qRT-PCR analysis. Relative splenic transcript levels for IFN-γ (A), TNF-α (B), IL-12p40 (C) and IL-21 (D) were determined and values were normalized to γ-actin gene expression. Data are expressed as relative gene expression compared to the time 0 baseline value set as 1. 3*; gene expression from hamster treated with vehicle alone with RNA collection at 3 h.
4. Discussion
We have previously reported on the efficacy of rEA for the treatment of PTV infection in mice, with no apparent protective effect in the hamster infection model [3]. In mice, TLR11 recognizes the rEA profilin-like protein and other closely related apicomplexan protozoan profilins [11]. This recognition leads to a profound induction of IL-12, a cytokine which drives the IFN-γ response. Although the mouse efficacy data are impressive, humans lack a functional TLR11 gene [9], and therefore, the mouse model is not the best system to investigate the activity of rEA as a potential prophylaxis or therapeutic for human use. Considering the fact that mice are the only known animal species known to express a fully functional TLR11 [9], it is unlikely that hamsters possess a functional copy of the gene. For this reason, further studies in the hamster model were pursued as hamsters are more likely to respond to rEA in manner more relevant to the human condition.
The efficacy data reported here suggest that although significant protection can be afforded by prophylactic intervention with rEA, optimal dosing in the PTV hamster infection model is quite challenging. In two independent experiments, a dose of 1 μg of rEA protected 40% of challenged hamsters (Figures 1 and 2). Although just under the level of significance by log-rank analysis, application of the Fisher’s exact test when only considering the outcome of survival or death did result in significant protection compared to animals receiving placebo (P = 0.0312). Surprisingly, treatments with 10 μg of rEA were completely ineffective, whereas a 100-μg dose was most effective. The optimal efficacy of the 100-μg rEA dose had to be verified (Figure 4), as treatment with 320 μg of the drug offered only weak protection (Figure 3). The 320 μg quantity of rEA may have some toxicity associated with it. Since we did not assess this treatment on uninfected hamsters, we can’t rule out this possibility.
Further complicating matters, the lack of dose-responsiveness seen with rEA was also seen with the combination studies with IL-18 and IL-2. IL-18 was chosen based on evidence that it may synergize with the IL-12 induced by rEA to maximize the production of IFN-γ [12]. IL-2 was selected based on its ability to expand T cell populations as well as inducing IFN-γ and TNF-α[13, 14]. In addition, the combination of rEA and IL-2 has demonstrated enhanced anti-tumor activity in hamsters (Rosenberg et al., unpublished experiments). In the initial study wherein IL-18 use was explored, it was tested in combination with the 10-μg dose of rEA, providing limited, yet significant protection. Given independently, survival outcome following treatments with IL-18 or the 10-μg dose of rEA was worse than observed with the placebo treatment. Despite the apparent synergistic effect seen, when the dose of the rEA in the combination was increased to 100 μg, only limited protection was afforded. Arguably, the precipitous death curve associated with the experiment suggests that in this particular study, the virus inoculum was more potent, thus reducing the antiviral effects in the context of survival, with the most apparent effects being seen in the extended survival times. Nevertheless, within the same experiment highly remarkable efficacy was seen with the combination of 100 μg of rEA and 12 μg of IL-2. The latter however, could not be verified. The combination studies also suffered from the fact that the cytokines tested were not of hamster origin since hamster-specific cytokines are not commercially available, raising the question that the human and mouse cytokines used likely lack functionality in the hamster system. For these reasons, additional studies were not pursued at this juncture.
Given that it has historically provided significant protection against PTV infection in the hamster model [3], it was rather surprising that poly(I:C12U) was relatively ineffective in the final experiment. Although efficacy in the context of survival was not apparent, the poly(I:C12U)-treated hamsters that died from the infection survived an average of 3 days longer than the placebo-treated animals. Presumably, the increase in infectious virus inoculated, a 6-fold increase in PFU from that previously reported [3], likely contributed to the reduced efficacy of the immunoprotective effect induced by poly(I:C12U). In addition, in the hamster model, PTV has been shown to delay the type I IFN response, which appears to be an important determinant of pathogenesis [15]. Evidence that the type I IFN response in mice is antagonized by a similar process remains to be elucidated. The enhanced susceptibility of hamsters to PTV, compared to mice, may result as a consequence of the disruption of the antiviral program through inhibition of type I IFN, which is exploited by a number of other pathogenic viruses [16, 17]. Thus, it is not surprising that PTV infection in hamsters may be more difficult to treat than infection in mice.
In mice, TLR11 senses the rEA protein evoking a robust IL-12 and IFN-γ release that is readily detectable systemically [3, 10, 11]. Due to the dearth of reagents for evaluating cytokine induction in the hamster system, we were limited to examining the expression of proinflammatory factors at the transcriptional level. Despite the clear induction of IFN-γ and IL-12p40 in hamsters treated with rEA, it is difficult to quantitatively compare the gene expression findings from spleen tissue to those of serum cytokine protein levels. Nonetheless, we were able to ascertain that these genes were induced in hamsters, consistent with previous findings in mice [1, 3]. Moreover, moderate increase in IFN-γ and IL-12 production has been seen in phase I clinical trials with cancer patients [2]. Unfortunately, unlike in mice, the tools to accurately measure type I IFN (IFN-α/β) in hamsters at the protein or even RNA levels are not available. Notably however, type I IFN induction through rEA treatment was not seen in mice [3].
In vitro studies have shown that the ED50 (effective dose, 50% of maximum response) in mice is 0.009 ng/mL, whereas the ED50 for hamsters is 16 ng/mL (B. Rosenberg, unpublished experiments). The difference between the mouse and hamster responses has presented significant challenges in determining the correct doses for use the both cancer and viral research. In mice, a single i.p. injection of 1 ng of rEA protected 100% of challenged animals, despite evidence of waning protection at the level of inhibition of viral replication and liver disease at this lowest tested dose [3]. Considering our results in the hamster model, it is certainly possible that doses in the range of 20-160 μg of rEA may improve upon the protection elicited by the 100-μg dose. It is also unclear as to the mechanism by which the protective effect of rEA fluctuates from weak at the 1-μg dose, to nonexistent at 10 μg, to optimal at 100 μg. This may be the subject of future study. Importantly, significant prophylaxis against PTV challenge in hamsters was shown to be induced by rEA treatment. Complete protection however, as seen with other antivirals that target viral replication [18], has not been achievable. Finding the most effective dose and treatment regimen appears to be the most daunting challenge facing the development of rEA as an intervention for viral infections.
Acknowledgments
We thank Dr. Heather Greenstone for critical review of the manuscript. We also thank Erik Ostler, Devin Jackson and Lee Audd for technical support.
This work was supported in part by contract NO1-AI-15435 from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenberg B, Juckett DA, Aylsworth CF, Dimitrov NV, Ho SC, Judge JW, et al. Protein from intestinal eimeria protozoan stimulates il-12 release from dendritic cells, exhibits antitumor properties in vivo and is correlated with low intestinal tumorigenicity. Int J Cancer. 2005;114:756–765. doi: 10.1002/ijc.20801. [DOI] [PubMed] [Google Scholar]
- 2.Rader JS, Aylsworth CF, Juckett DA, Mutch DG, Powell MA, Lippmann L, et al. Phase i study and preliminary pharmacology of the novel innate immune modulator rbbx-01 in gynecological cancers. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-07-4250. In press. [DOI] [PubMed] [Google Scholar]
- 3.Gowen BB, Smee DF, Wong MH, Judge JW, Jung KH, Bailey KW, et al. Recombinant eimeria protozoan protein elicits resistance to acute phlebovirus infection in mice but not hamsters. Antimicrob Agents Chemother. 2006;50:2023–2029. doi: 10.1128/AAC.01473-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Julander JG, Judge JW, Olsen AL, Rosenberg B, Schafer K, Sidwell RW. Prophylactic treatment with recombinant eimeria protein, alone or in combination with an agonist cocktail, protects mice from banzi virus infection. Antiviral Res. 2007;75:14–19. doi: 10.1016/j.antiviral.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson GW, Jr, Slayter MV, Hall W, Peters CJ. Pathogenesis of a phleboviral infection (punta toro virus) in golden syrian hamsters. Arch Virol. 1990;114:203–212. doi: 10.1007/BF01310749. [DOI] [PubMed] [Google Scholar]
- 6.Fisher AF, Tesh RB, Tonry J, Guzman H, Liu D, Xiao SY. Induction of severe disease in hamsters by two sandfly fever group viruses, punta toro and gabek forest (phlebovirus, bunyaviridae), similar to that caused by rift valley fever virus. Am J Trop Med Hyg. 2003;69:269–276. [PubMed] [Google Scholar]
- 7.Pifat DY, Smith JF. Punta toro virus infection of c57bl/6j mice: A model for phlebovirus-induced disease. Microb Pathog. 1987;3:409–422. doi: 10.1016/0882-4010(87)90011-8. [DOI] [PubMed] [Google Scholar]
- 8.Lauw FN, Caffrey DR, Golenbock DT. Of mice and man: Tlr11 (finally) finds profilin. Trends Immunol. 2005;26:509–511. doi: 10.1016/j.it.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, et al. The evolution of vertebrate toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, et al. Tlr11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 11.Yarovinsky F, Sher A. Toll-like receptor recognition of toxoplasma gondii. Int J Parasitol. 2006 doi: 10.1016/j.ijpara.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, et al. Interferon-gamma-inducing factor enhances t helper 1 cytokine production by stimulated human t cells: Synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 13.Ortaldo JR, Mason AT, Gerard JP, Henderson LE, Farrar W, Hopkins RF, 3rd, et al. Effects of natural and recombinant il 2 on regulation of ifn gamma production and natural killer activity: Lack of involvement of the tac antigen for these immunoregulatory effects. J Immunol. 1984;133:779–783. [PubMed] [Google Scholar]
- 14.Toribio ML, Alonso JM, Barcena A, Gutierrez JC, de la Hera A, Marcos MA, et al. Human t-cell precursors: Involvement of the il-2 pathway in the generation of mature t cells. Immunol Rev. 1988;104:55–79. doi: 10.1111/j.1600-065x.1988.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 15.Perrone LA, Narayanan K, Worthy M, Peters CJ. The s segment of punta toro virus (bunyaviridae, phlebovirus) is a major determinant of lethality in the syrian hamster and codes for a type i interferon antagonist. J Virol. 2007;81:884–892. doi: 10.1128/JVI.01074-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basler CF. Interferon antagonists encoded by emerging rna viruses; in Palese P (ed Modulation of host gene expression and innate immunity by viruses. Dordrecht: Springer; 2005. pp. 197–220. [Google Scholar]
- 17.Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: A lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 18.Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, et al. In vitro and in vivo activities of t-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007;51:3168–3176. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


