Abstract
Minaçu virus was isolated from Ochlerotatus scapularis (Diptera: Culicidae) in Minaçu, Goiás State, Brazil, in 1996. In attempting characterization of virus serological (hemagluttination inhibition, HI; indirect immunofluorescence assay, IFA), physicochemical [test for deoxycholate acid (DCA) sensitivity; polyacrylamide gel electrophoresis (PAGE)] tests and ultrastructural studies were made. Virus was also assayed in suckling mice after intracerebral inoculation of 0.02 ml and in VERO and C6/36 cells with 0.1 ml of viral suspension containing 105 LD50/ml. Inoculated and control systems were observed daily. Every 24 h, one control and two inoculated animals were killed for tissue testing, including histopathological changes by haematoxylin and eosin (HE)-stained sections, which were semi-quantified. Research into viral antigen in the tissues of mice [central nervous system (CNS), liver, heart, lungs, spleen and kidneys] was carried out by the immunohistochemical technique using the peroxidase system. The virus only replicated in VERO cells, with antigen positive by IFA. Positive complement fixation tests were only obtained using antiserum of Minaçu virus. Minaçu virus is DCA resistant; haemagglutinating activity was negative. By electronic microscopy non-enveloped virus particles were 75 nm in diameter. PAGE analysis showed Minaçu virus genome profile with 10 RNA segments. Infected, non-killed animals died 7 days after inoculation. Tissue lesions were observed in all organs, except the lungs. Intense lesions were observed in the CNS and the heart, where neurone and cardiocyte necroses, respectively, were noted. The liver, spleen and kidneys had moderate tissue changes. Viral antigens were more abundant in the CNS and the heart, and absent in the lungs. In conclusion, Minaçu virus belongs to the family Reoviridae, genus Orbivirus.
Keywords: antigen detection, immunohistochemistry, Minaçu virus, Orbivirus, pathology, Reoviridae, ultrastructure
The Amazon Forest is one of the largest sources of arbovirus fauna in the world. This is not only due to its favourable climate, but mainly to the wide diversity of its fauna composed of a large variety of haematophagous arthropods and wild animals that are the key elements for the maintenance of arboviruses (Travassos da Rosa et al. 1997).
In Brazil, at least 210 different types of arboviruses have been isolated; 195 of them were first identified in the Brazilian Amazon region, and several of these viruses have not been found in other regions (Pinheiro et al. 1996; Vasconcelos et al. 1998).
Although detailed studies of some of these viruses have led to taxonomical definitions, several other arboviruses remain without taxonomic classification.
Processes leading to clinical symptoms and pathological changes are not yet sufficiently known for most arboviruses. Assays have shown that some of them multiply first in the dermal dendritic cells and in lymph nodes near the area bitten by the infected arthropod. The viral agent is carried by lymph-transporting vessels reaching the bloodstream, disseminating the virus throughout the host' organs and tissues. After multiplying in these localities, the viruses are thrown into the bloodstream again. At this point, viraemia occurs, which coincides with the period of fever in human beings. In some cases, infection stops at this point and the patient can recover completely and uneventfully. On the other hand, other infections may progress and, depending on the case, more severe exanthematic, neurological or haemorrhagic conditions result (Vasconcelos et al. 1992).
Thirty-four of the 195 types of arboviruses and other viruses infecting vertebrates, already isolated and identified in the Amazon Region, are known to be pathogenic for human beings (Travassos da Rosa et al. 1997). The pathogenesis of several other arboviruses, however, lacks detailed study. Several of these poorly known viruses might be found to cause diseases in human beings and other animals in future, thus requiring additional public health policies. In this article, we indicate the taxonomic position of Minaçu virus and the tissue changes caused in experimentally infected Swiss suckling mice.
Materials and methods
Viral strain
The Minaçu virus, strain BeAR 548794 used in this assay was isolated in November 1996, from pooled mosquito species Ochlerotatus scapularis (Diptera: Culicidae) captured in Minaçu, State of Goiás, Brazil.
Viral susceptibility in cell cultures
C6/36 and VERO cell lineages were used for Minaçu virus cultivation. Viral growth was attempted by infection of 25 cm2 flasks containing C6/36 or VERO monolayers by 1:10 infected brain macerate. Uninfected flasks were kept as controls. The infected and the non-infected control flasks were observed daily, to detect cytopathic effects (CPE). Ten days after inoculation, or when approximately 75% of the cells showed CPE, specimens were collected for immunofluorescent assay (IFA) and for virus stocks, as described previously (Beaty et al. 1989).
Serological procedures
Antigen and hyper-immune serum preparation
Viral antigen was prepared, by the sucrose-acetone method, from Minaçu virus-infected mice brains, and used to detect the haemagglutinating activity of the virus and the results for viral antigen detection by complement fixation (CF) tests, using previously published protocols (Clarke & Casals 1958; Beaty et al. 1989). Minaçu virus hyper-immune serum was obtained from infected adult mice (5–6 weeks). The immunization programme consisted of four weekly intraperitoneal injections of Minaçu virus-infected mice brain suspension (1:10 in 0.85% NaCl solution). The hyper-immune serum was obtained from blood taken by intracardiac puncture, at least 7 days after the last immunization injection (Beaty et al. 1989).
Complement fixation test
Complement fixation tests were carried out using the protocol described by Beaty et al. (1989). Briefly, dilutions in twofold series (1:8–1:16 for antigen sources, and 1:8–1:64 for antibodies) were made by using two units of guinea-pig complement. The Minaçu virus antigen was used as a positive control when tested with the Minaçu virus hyper-immune serum (anti-Minaçu virus serum). CF tests were made against hyper-immune serum of grouped and ungrouped and unassigned arboviruses isolated in Brazil and in other countries (Table 1). After 16 h of incubation at 4 °C, the reaction was revealed using haemolysin (anti-sheep) and sensitized sheep erythrocytes; reading was conducted according to the observed haemolytic percentage, and the positive titres were recorded as the highest dilution showing a haemolytic percentage of up to 25%.
Table 1.
Hyperimmune serum samples for several viruses tested against antigens of Minaçu virus (Be AR 548794) by CF tests, with negative result
| Family | Genus | Antigenic group | Hyper-immune sera for viruses |
|---|---|---|---|
| Togaviridae | Alphavirus | Group A | Group A, Mayaro, VEE, EEE, WEE, Mucambo, Pixuna, Trocara, Aura, Una, Chikungunya |
| Unassigned | Triniti | ||
| Flaviviridae | Flavivirus | Group B | Group B, YF, SLE, Ilheus, DEN-1, DEN-2, DEN-3, DEN-4, Cacipacore, Bussuquara, Rocio, Uganda S, Kokobera, Wesselbron, Zika |
| Bunyaviridae | Bunyavirus | Anopheles A | Group Anopheles A, Lukuni, Arumateua, Caraipe, Tacaiuma, Trombetas, Tucurui |
| Bunyamwera | Group Bunyawera, Wyeomyia, Maguari, Cache Valley, Kairi, Iaco, Macauã, Sorocaba, Taiassuí, Tucunduba, Xingu, Northway | ||
| Group C | Group C, Caraparu, Apeu, Itaqui, Murutucu, Nepuyo, Oriboca, Caraparu-like | ||
| California | Melao, Guaroa, Serra do Navio, Keystone, Trivittatus, Snowshoe Hare, Jamestown Can | ||
| Capim | Group Capim, Bush Bush, Acara, Benevides, Benfica, Capim, Guajara, Moriche | ||
| Gamboa | Gamboa | ||
| Guama | Group Guama, Mirim, Guaratuba, Ananindeua, Bimiti, Catu, Guama, Moju, Timboteua | ||
| Turlock | Turlock | ||
| Simbu | Manzanilla, Oropouche, Utinga | ||
| Tentative species | Mojui dos Campos | ||
| Phlebovirus | Phlebotomus | Group Phlebotomus, Icoaraci, Itaituba, Alenquer, Ambe, Anhanga, Ariquemes, Belterra, Bujaru, Candiru,Itaporanga, Jacunda, Joa, Morumbi, Munguba, Oriximina, Pacui, Serra Norte, Tapara, Turuna, Uriurana, Urucuri, Arumowot, Gabek Forest | |
| Unassigned | Ungrouped | Belem, Para, Santarem, Pacora, Witwatersrand | |
| Reoviridae | Coltivirus | Colorado Tick Fever | Colorado Tick Fever |
| Orbivirus | Kemerovo | Chenuda | |
| Bluetongue | Bluetongue | ||
| Changuinola | Group Changuinola, Acatinga, Acurene, Almerim, Altamira, Anapu, araçai, Aratau, Aruana, Arawete, Assurinis, Bacajai, Bacuri, Balbina, Barcarena, Caninde, Canoal, Catete, Coari, Cupixi, Gorotire, Gurupi, Iopaca, Ipixaia, Irituia, Iruana, Itaboca, Jamanxi, Jandia, Jari, Jatuarana, Jutai, Kararao, Malgaço, Monte Dourado, Ourem, Pacaja, Parakana, Paranati, Parauapebas, Paru, Pependana, Pindobai, Piratuba, Purus, Saraca, Serra sul, Surubim, Tapirope, Tekupeu, Timbizal, Tocantins, Tocaxa, Tuere, Tumucumaque, Uatuma, Uxituba, Xaraira, Xiwanga | ||
| Corriparta | Acado, Jacareacanga | ||
| EHD | EHD-NJ, EHD-Alberta, EHD-BT-Orbi | ||
| Eubenangee | Eubenangee | ||
| Ieri | Ieri | ||
| Palyam | Abadina, Bunyip Creek, CSIRO 11, D'Aguilar, Marrakai | ||
| Wallal | Wallal | ||
| Tentative species | Codajas, Tembe, Itupiranga | ||
| Rhabdoviridae | Vesiculovirus | Vesicular stomatitis | Cocal, Piry, Carajas, Maraba |
| Tentative species | Aruac, Inhangapi, Xiburema, Jurona, Kwatta, | ||
| Unassigned | Ungrouped | Chaco, Mosqueiro, Timbo, Sena Madureira, Marco, Cuiaba | |
| Bracorhabdovirus | Curionopolis, Itacaiunas | ||
| Arenaviridae | Arenavirus | Tacaribe (LCM) | Amapari, Flexal |
| Orthomyxoviridae | Thogotovirus | Thogotovirus | Araguari |
| Herpesviridae | Herpesvirus | Unassigned | Parixa, Agua Preta |
| Paramyxoviridae | Paramyxovirus | Ungrouped | Mapuera |
| Poxviridae | Unassigned | Ungrouped | Cotia-like |
| Unassigned | Unassigned | Ungrouped | Itupiranga, Matucare, Estero Real, Papura, Juruaca, Iriri |
Immunofluorescent assay
Immunofluorescent assay was carried out using VERO and C6/36 cells infected with the Minaçu virus, following a technique previously described (Tesh 1979).
Virus titration
Suckling mice (2–3 days old) were inoculated intracerebrally with 0.02 ml of 10-fold serial dilutions (10−1 to 10−8) of Minaçu virus suspension prepared in buffered PBS-0.75% bovine albumin solution with antibiotics (penicillin = 100 U/ml; streptomycin = 100 μg/ml). The viral titre was calculated following the method developed by Reed and Muench (1938), and expressed as DL50/0.02 ml.
Sensitivity to deoxycholate acid (DCA)
Deoxycolate acid is a lipid solvent and is used to recognize whether a virus has or does not have an envelope (which is composed principally of lipids). The DCA sensitivity test was conducted following Theiler' (1957) method. Briefly, 1:5 suspension of the stock virus in suckling mice brains diluted in buffered PBS-0.75% bovine albumin solution and antibiotics was centrifuged at 6708 g at 4 °C for 1 h to obtain more clean purified suspension. Equivalent volumes of the DCA (1:500) and DCA-free suspension (control without DCA) were mixed and incubated for 1 h at 37°C. Serial 10-fold dilutions (10−1 to 10−8) were inoculated (0.02 ml) by the intracerebral route in newborn mice (2–3 days old). Titres were also calculated using the Reed and Muench (1938) method.
Electron microscopy
Negative staining (NS)
Supernatants of infected and uninfected (negative control) C6/36 and VERO cells culture were used after the second and fourth viral passages respectively. A drop of cell culture supernatant free of cell debris was added to a copper 400 mesh grid and coated with formvar plastic film reinforced by a thin coat layer and processed as previously described (Hsiung et al. 1979; Barth 1984).
Electron immunomicroscopy
Supernatants of VERO cells infected with Minaçu virus showing 75% CPE and negative control (non-infected cells) were centrifuged at 11 269 g. for 15 min, at 4 °C. The supernatants were diluted in the hyper-immune serum for Minaçu virus at 1:250 and 1:500 (e.g. one part of supernatant to 249 or 499 parts of serum). Samples were homogenized, stirred for 2 and 12 h at 4 °C, respectively, and then centrifuged at 11269 g for 30 min at 4 °C. The pellet was placed on copper 400 mesh grids. Finally, NS analysis was performed by transmission electron microscopy (TEM).
Ultra-thin sections
VERO cells infected with Minaçu virus, as well as negative control (non-infected cells) were used for ultra-thin section procedures. The material was collected at intervals of 12, 24, 36, 48, 60, 72, 84, 96 and 108 h postinoculation (p.i.), and processed following a technique previously published (Luft 1961; Reynolds 1963; Karnovsky 1965; Hepler 1980).
Histological examination
For histopathological study, suckling mice were inoculated by the intracerebral route with 0.02 ml of a 1:10 suspension of brains from mice infected with Minaçu virus containing approximately 2 × 10−5 LD50, as previously described (Beaty et al. 1989). At daily intervals, after inoculation, one normal (non-inoculated control) and two infected mice were anaesthetized and the tissues (brain, lungs, heart, liver, spleen and kidneys) taken for histological examination and fixed immediately in 10% buffered formalin solution. After 24 h, the samples were transferred to 70% ethanol for storage. Tissue samples were processed and embedded in paraffin wax for sectioning, sections were stained by haematoxylin and eosin (HE), and were examined by light microscopy as described elsewhere (Prophet et al. 1992; Michalany 1998). Histopathological analysis was carried out following a 0–3 scale to semi-quantify histological events, where: 0 = absent; 1 =minimal; 2 = moderate; and 3 = intense. Each organ was examined for the presence of necrosis, apoptosis, inflammatory infiltrate, degeneration and haemorrhage (Xiao et al. 2001).
Immunohistochemical assays
Antigens of Minaçu virus were detected by the immunohistochemical technique using the enzyme peroxidase, following the procedures described by Hsu et al. (1981) and Xiao et al. (2001). Briefly, the procedures were as follows: Sections of paraffin-embedded tissues were deparaffinized with xylene and processed for viral antigen detection. Specifically for Minaçu virus, hyper-immune anti-Minaçu serum diluted to 1:800 was added and the slides were incubated at 4 °C for 18 h, and washed in PBS. The secondary antibody, LSAB system (Dako, Carpinteria, CA, USA), 1 μl streptavidin, 1 μl biotin diluted in 1000 μl PBS, was then applied with peroxidase. Afterwards, slides were immersed in 3.3′ diaminobenzidin (Dako). Sections were counterstained in water with haematoxylin 1:1, washed in running water, ammoniac water, and in distilled water; sections were mounted with Entelan (Merck, Darmstadt, Germany) and examined by light microscopy.
RNA extraction
RNA extraction was carried out by the phenol-chloroform method, following the manufacturer' instructions. Samples were analysed by the one-dimensional 5% polyacrylamide gel electrophoresis (PAGE) method for 60 min at 100 V (Laemmli 1970). The gel was processed in a NaOH 10 m solution, formaldehyde at 37% and demineralized water, until the bands of the control (Irituia virus) appeared and reached the desired parameters. At this point, the solution was removed and replaced by an acetic acid solution at 5%, to stop the reaction.
Results
Cell culture
Minaçu virus grew only in VERO cells. Cultures showed CPE on day 4 when at least 75% of cells showed CPE, and were tested by IFA using the hyper-immune serum anti-Minaçu virus which showed viral antigens in the cytoplasm. Minaçu virus did not replicate in C6/36 nor were the IFA positive using C6/36 cell culture.
Haemagglutinin antigen activity
The antigen (sucrose-acetone) prepared from Minaçu virus-infected mouse showed negative results between pH 6.0 and 7.0, indicating the absence of haemagglutinin protein for goose erythrocytes.
CF tests
Complement fixation tests conducted with the antigens prepared from mouse brain infected with Minaçu virus showed no reaction against the tested immune serum (Table 1). Reaction only occurred when the viral antigen was tested against its respective homologous serum. The reciprocal titre of Minaçu virus antigen and its homologous immune serum was 4096/2048.
Sensitivity to DCA
The titre (DL50/0.02 ml) of Minaçu virus without DCA, in suckling mice was 5.4. After treatment with DCA, the titre was 5.3, indicating virus resistance to the substance and suggesting the absence of an envelope in Minaçu virus particles.
Negative staining
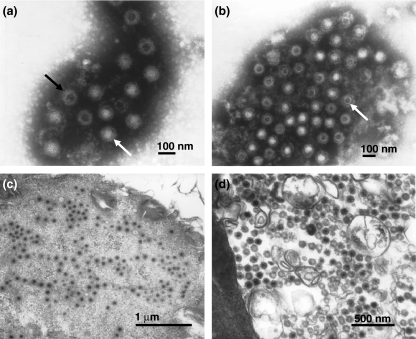
Only VERO cell culture showed the presence of spherical viral particles, approximately 75 mm in diameter (Figure 1a). Viral particles were not observed in C6/36 cells.
Figure 1.
(a) Negative staining of Minaçu virus, in fluid of VERO cells (5 days p.i.), showing complete (white arrow) and incomplete (black arrow) viral particles (114,000×). (b) Electronic immunomicroscopy of Minaçu virus and homologous serum showing viral particles (arrow) surrounded by a dense antibody halo at the dilution 1:500 (90,000×). (c, d) Electronic micrographs of Minaçu virus in ultra-thin sections of VERO cells (4 days p.i.). Note the viral particles within the cell cytoplasm (60,000×) (c) and the absence of a viral envelope (80,000×) (d).
Electron immunomicroscopy
Antigen-antibody reaction in the VERO supernatant cell infected with Minaçu virus (5 days p.i.), and its respective homologous immune serum, is shown in Figure 3b. Several groupings of spherical viral particles, surrounded by a dense antibody halo were observed in the dilution 1:500 and 1:250 (Figure 1b), differing from the negative control VERO cells.
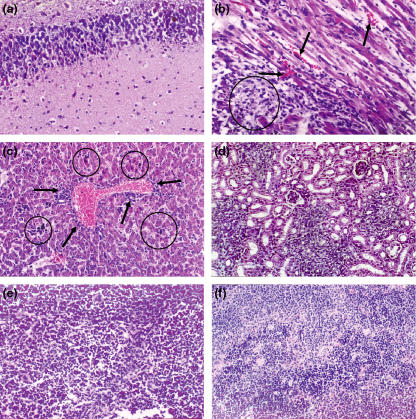
Figure 3.
Histological changes in the brain, heart, liver and kidneys of suckling mice intracerebrally infected with the Minaçu virus, 6 days post-infection. (a) Brain with extensive area of lithic necrosis −200×; (b) heart with necrotic areas (circle), micro-haemorrhages (arrows) and intense oedema −400×; (c) liver with focal areas of cellular necrosis, and acidophilic corpuscles (circles). Note the perivascular inflammatory infiltrate in the portal space (arrows) −200×; (d) kidney with slightly tumefied renal tubules and slightly congested interstitial area −200×; (e) spleen showing cellular necrosis in red pulp – 200×; (f) spleen with cellular necrosis and haematic infiltration – 200×.
Ultra-thin sections
Sections of processed blocks of C6/36 cell cultures infected with Minaçu virus showed no viral particle at any of the time intervals p.i. Viral particles were observed in cell cytoplasm after day 4 p.i., in sectioned blocks of infected VERO cell cultures. The viral particles were 75 nm in diameter and no viral envelope was detected (Figure 1d). Viral particles were not found in control cells and in C6/36 cells.
Analysis of viral RNA
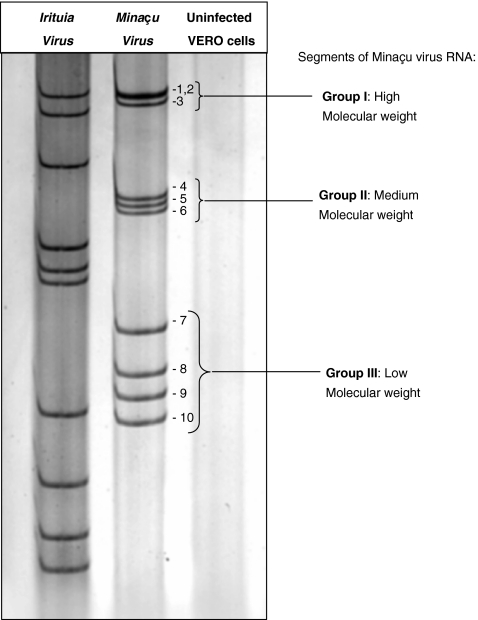
Analysis of the electrophoretic RNA profile of Minaçu virus was made with supernatants of infected VERO cell cultures during the second and third passages. The cells were collected after 75% CPE. A 5% PAGE produced segregation of 10 RNA bands, divided into three groups – group I: three bands with higher molecular weight; group II: three bands with intermediary molecular weight; group III: four bands with lower molecular weight (Figure 2).
Figure 2.
Electrophoretic RNA profile of Irituia and Minaçu viruses, and of non-infected VERO cellular culture (negative control) processed in a 5% polyacrylamide gel. Note the segregation of 10 bands of RNA for the Minaçu virus, as observed for the Irituia virus, which belongs to the family Reoviridae, genus Orbivirus. Note that the bands have different placements, suggesting different molecular weights.
Histological examination
Suckling mice were intracerebrally inoculated with 105 LD50/ml. Signs of disease were not observed in inoculated or in control animals 1 day after inoculation and no significant histological changes occurred in animal viscera of the control group during all the assay. Inoculated animals killed 2 days post-inoculation (dpi) presented brain changes. Focal encephalitis with vascular congestion, micro-haemorrhage and rare necrosis cell foci in the cortex area (Figure 3a) were observed in the brain. In animals killed 3 and 4 dpi, the brain showed a moderate encephalitic lesion with oedema, hyperplasia of the vascular endothelium associated with congestion, and neurones with apoptotic and necrotic aspects, with retracted and acidophilic cytoplasm and nuclear cariorrexis. At 5 dpi, the brain showed cortex areas with large number of retracted and acidophilic neurones (apoptotic), accompanied by espongiotic aspects, alterations of neural tissue (oedema), hyperplasia of the vascular endothelium and strong congestion. There was a high intensification of these features on day 6.
In animals killed 1 and 2 dpi the white pulp spleen showed hypoplasia and intense reduction in red pulp erythrocyte numbers. In animals killed 3 and 4 dpi, spleen changes persisted and in infected animals killed 5 dpi we observed cellular proliferation in the spleen, with structuring of lymphoid follicles; the red pulp was well seen due to its slight congestion. At 6 dpi, the white pulp with well-built lymph nodal follicles was noted in the spleen and the red pulp was slightly congested.
The heart muscle showed small, focal myocardial changes which was followed by a slight oedema at 2, 3 and 4 dpi. At 6 dpi an unexpected severe myocarditis occurred with oedema and several necrotic foci associated with a mononuclear inflammatory infiltrate and focal haemorrhage (Figure 3b). The liver alterations were characterized by hepatocytic necrosis, sometimes with a moderate mononuclear inflammatory reaction, and rare apoptotic corpuscles with moderate reaction of portal tracts. In infected animals at 5 dpi, several foci of hepatocyte necrosis were found in the liver, without lobule preference and in different sizes; it was always associated with mononuclear inflammatory reactions in the acini and portal tract. Isolated apoptotic corpuscles were seen in the hepatic parenchyma. On day 6, the hepatic alterations were more intense (Figure 3c). Kidneys showed well-preserved glomeruli, but renal tubules were slightly tumefied, and a moderate interstitial congestion was observed (Figure 3d). Lesions in all organs intensified on day 7 p.i., when all inoculated animals died.
Animals inoculated via the intraperitoneal and subcutaneous routes showed similar tissue alteration. Pancreas and lung were examined but no alteration was observed in them.
Immunohistochemical examination
Results obtained by HE staining were highlighted by the antigenic detection using immunohistochemistry. Immunostaining intensified daily, until animal death on day 7 p.i.; as excepted, immunoreaction occurred predominantly within the cytoplasm of parenchyma cells, as brown granular deposits (Figure 4a,b). Immunostaining with viral antigens occurred in several brain structures, from base nuclei to the cortex, in neurones and in glia cells.
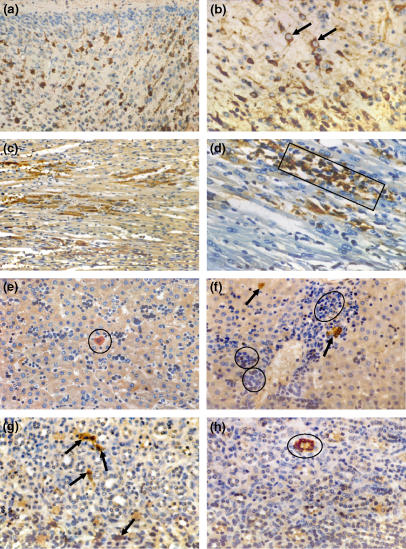
Figure 4.
Immunohistochemical assays showing antigenic staining of brain, heart, liver, and kidney sections of suckling mice intracerebrally experimentally infected with Minaçu virus, by using a specific anti-Minaçu antibody. (a) Cerebral cortex with neuronal bodies and stained axons. Note extensive antigenic expression on neurones, 3 days p.i. −200×; (b) same region, higher magnification. Note details of granulated cytoplasm stained with viral antigen (arrows), 3 days p.i. −400×; (c) low-power view of cardiac tissue with cells stained for viral antigens in the myocardium, 5 days p.i. −200×; (d) amplified view showing the group of cardiac cells intensely stained with the viral antigen (rectangle), 6 days p.i. −400×; (e) section of liver tissue with mononuclear inflammatory infiltrate, 2 days p.i. Note immunostaining hepatocytes (circle) −400×; (f) liver, area near portal space. Note perivascular inflammatory infiltrate (circles), residues of necrotic hepatocytes and immunoassay hepatocytes with viral antigen (arrows), 5 days p.i. −400×; (g) kidney section showing several immunostaining cells of the renal stroma (arrows), 6 days p.i. −400×; (h) kidney section with slight inflammatory process and cells of renal tubule immunostaining (circle), 6 days p.i. −400×.
In the heart, immunostaining of antigens in myocardial cells was observed as early as 36 h after the mice were inoculated. Interestingly, immunostaining of antigens was not followed by visible changes in histopathology in sections stained by HE until day 3 p.i. It seems that the functioning organ compensates itself. Immunostaining was still slight or moderate at that point. On day 6 and day 7 p.i., however, lesions were exuberant and showed intense cellular immunostaining; viral antigens could be seen in the cardiac muscle, in necrotic areas containing stained macrophages and cardiocytes (Figure 4c,d).
In the liver, immunostaining was observed in preserved, isolated and/or grouped hepatocytes, and particularly in damaged cells in several foci of parenchyma necrosis. By contrast, antigens were not found in the vascular endothelium, in biliar canaliculi or in the interstitial tissues (Figure 4e,f).
Our findings regarding the kidneys were surprising. HE showed slight tissue changes whereas IHC examination showed that both stroma cells and renal tubular cells had viral antigens, as shown by immunostaining; the antigens were more easily identified after day 6 p.i. (Figure 4g,h).
Discussion
Viral inoculation in continuous cell lines showed that Minaçu virus infected and produced CPE in VERO cells; C6/36 cells were resistant as all cultures were negative by IFA, suggesting that viral replication does not occur in C6/36 cells. In fact, only infected VERO cell cultures showed granular cytoplasmatic fluorescence. The sucrose-acetone extracted antigen produced from brains of infected suckling mice showed no haemagglutinin antigen activity, meaning that this virus has not produced a haemagglutinin antigen.
Viral antigen and hyper-immune serum titre specific to Minaçu virus produced in mice showed high titres in CF tests, and there was no serological reaction between the Minaçu virus antigen and immune sera prepared for viruses of several antigenic groups or against polyvalent immune sera of tested groups (Table 1).
Throughout the identification process, Minaçu virus was initially tested against immune sera of already classified arboviruses isolated in the Brazilian Amazon Region. Within these groups, arboviruses isolated from O. scapularis were selected at a preliminary stage, because some of the main arboviruses identified in the region were isolated from this arthropod species, and some of them have been associated with highly important human pathogens, such as Ilheus virus, Mayaro virus, Saint Louis encephalitis virus, among others (Karabatsos 1985). All 195 viruses previously isolated in the Amazon were tested and the results were negative, as mentioned previously.
Sensitivity test to DCA showed that the Minaçu virus is resistant to the solvent, indicating absence of the viral envelope (Theiler 1957). Reoviridae is the only known virus family that includes several arboviruses without an envelope. The result of this test, therefore, pointed to a preliminary placing of the virus in the Reoviridae family. According to the classification of arboviruses based on physicochemical properties, members of this family do not have viral envelope and are, therefore, resistant to DCA (Van Regenmortel et al. 2000).
In support of this suggestion we made an ultrastructural study to confirm the DCA test results. Viral particles were visible by TEM. Ultrastructural studies of the Minaçu virus, using the NS technique, showed that viral particles were spherical, approximately 75 mm in diameter, with projecting spikes (Figure 1a).
For demonstrating antigen-antibody reactions, we used the immunomicroscopy technique with specific antibodies to Minaçu virus. Results were positive for the specific hyper-immune serum prepared from Minaçu virus and showed the formation of a dense antibody halo of specific virus proteins around the virus particles (Figure 1b). Ultra-thin sections of processed blocks with Minaçu virus showed viral particles only within the cellular cytoplasm (Figure 1c). Almost no viral particles were found in the extracellular areas and particularly no viral envelopes were found in the particles (Figure 1d). Results from studies of the fine structure have supported results obtained by the DCA test and confirm that the Minaçu virus particles have no envelope.
These results reinforce our assumptions and practically define Minaçu as a new arbovirus within the family Reoviridae. Viruses belonging to this family have particles of 60–80 nm in diameter, are not enveloped, replicate in the cellular cytoplasm, and have some serotypes which contain polypeptide (λ2) with projections from the core to the external surface of the virion (Schiff & Fields 1990), as observed for the virus under study.
Viruses belonging to the family Reoviridae contain RNA genome segmented in 9–12 bands and are classified in nine genera: Orthoreovirus (Reovirus), Orbivirus, Rotavirus, Coltivirus, Aquareovirus, Cypovirus, Phytoreovirus, Fijivirus and Oryzavirus. The last four infect only insects and plants (Van Regenmortel et al. 2000). In attempting to classify Minaçu virus into a genus belonging to this family, we made an analysis of the RNA eletrophoretic profile of the virus by the PAGE method. Results showed that the viral strain under study has 10 genomic segments (Figure 2). These results indicate the classification of the Minaçu virus in the family Reoviridae and, based on this electrophoretic profile, the virus belongs to the genus Orbivurus. Within the Reoviridae, viruses belonging to the genus Orbivurus have a 10-segment RNA genome, and have been isolated from haematophagous insects and wild vertebrates (Van Regenmortel et al. 2000).
The intracerebrally infected suckling mice used to evaluate pathological changes showed that the infection is pantropic and presents neurotropism associated with viscerotropism. Histopathological changes caused by the virus were intensified day by day, post-inoculation, especially in the brain, although other organs such as the spleen, liver and heart were also affected. On day 3 and day 4 p.i., necrotic foci appeared and a few apoptotic bodies were observed, especially in the brain. Moderate brain necrosis with great cellular retraction in the hippocampus and cerebral cortex were observed, whereas changes observed in the spleen remained the same. Hepatic lesions became evident and may be mainly due to tissue necrosis or the direct action of the virus on the tissue.
Signs of spleen regeneration by the structuring of lymphoid follicles were seen at 5 dpi, together with a well-defined red pulp with slight congestion. At this stage, the liver had signs of intense damage, shown by foci of hepatocyte necrosis of different sizes. There was intense portal mononuclear inflammation, although with isolated, apoptotic bodies (Figure 3c). Brain lesions were more extensive, with several cortex areas with neuronal death, oedema and congestion (Figure 3a). The first signs of heart damage appeared on this day.
Surprisingly, at the final experimental period (6–7 dpi), cardiac conditions became severe due to a fulminating myocarditis. Slight tissue changes in the kidneys were also observed. These lesions, particularly the heart lesions, call for attention because the organs seemed to have only a small oedema in the first days of infection. Myocardial lesions were characterized by intense oedema and several necrotic areas with mononuclear inflammatory infiltrate and focal haemorrhage (Figure 3b). During this period, animals died, showing severe encephalitic damage, particularly at the base nucleus of the cortex, and the cerebellum.
According to Tyler and Fields (1990), viruses belonging to the family Reoviridae have tropism for several tissues and cells, seemingly depending on the inoculation route. The viruses then spread throughout the host organism, reaching tissues and cells for which they have tropism. Assays conducted with mice and other animal models have shown that members of the family Reoviridae belonging to several antigenic groups, particularly in the genus Orbivirus, cause morphological lesions in organs such as the brain, heart, lungs, liver, pancreas and muscles, among other tissues (Table 2) (Karabatsos 1985).
Table 2.
Anatomo-pathological changes in experimental assays with arboviruses belonging to the family Reoviridae, genus Orbivirus, in some animal models (adapted from Karabatsos 1985; Tyler & Fields 1990)
| Virus | Animal | Route of inoculation | Affected organ | Lesion type |
|---|---|---|---|---|
| Chenuda | Suckling mice | IC, SC | CNS | Congestion and oedema, paralysis |
| Blood vessels | Perivascular infiltrate, haemorrhage | |||
| Lymphatic ganglions | Degeneration | |||
| Kemerovo | Suckling mice | IC, SC | Brain | Encephalitis with inflammatory lesions, Lymphocytic meningitis, |
| Rhesus | Epiderm | |||
| Brain, kidney, intestine, lungs and metanefron | Focal necroses, infiltrated with plasmacyte and macrophage | |||
| Bluetongue | Goat | IC, IP, SC | Buccal, nasal and intestinal mucoses | Inflammatory lesion |
| Goat in fetal stage | Inflammatory lesion | |||
| Cattle | Brain | Necrosis | ||
| Suckling mice | Oral, teats and udder mucoses | Encephalitis | ||
| Brain | ||||
| Irituia | Suckling mice | IC | Brain | Encephalitis |
| Epizootic haemorrhagic disease | Deer | IC, IP | Blood cells | Disorder of the maturation mechanism |
| All organs (liver, heart and kidneys) and tissues | Generalized haemorrhages | |||
| Ieri | Suckling mice | IC | Brain | Encephalitis |
| Minaçu (Be AR 548794) | Suckling mice | IC, IP, SC | Brain | Necrosis, retracted and acidophilic neurones, cellular rarefaction, apoptotic bodies, severe congestion and oedema; |
| Spleen | White pulp with well-developed lymphoid follicles and slightly congested red pulp | |||
| Liver | Hepatocytic necrosis with isolated apoptotic corpuscles | |||
| Heart | Intense myocarditis with several necrotic and haemorrhagic focuses | |||
| Kidneys | Slightly tumefied tubules and slightly congested interstitial areas |
IC, intracerebral; IP, intraperitoneal; SC, subcutaneous.
Lesions caused by Minaçu virus in suckling mice were similar to those caused by the Colorado tick fever virus, also a Reoviridae, although belonging to the genus Coltivirus. According to histopathological studies (Table 2), and as described by Karabatsos (1985), tissue lesions for all other viruses belonging to the family Reoviridae are more moderate or limited compared with the lesions caused by the Minaçu virus. In fact, the destructive capacity of the virus is impressive, because it mainly affects the brain, spleen, liver and heart. Minaçu virus can be used as an experimental infection model for Orbivirus pathogenesis with neuro and viscerotropisms.
The histopathological changes described following our experiment with suckling mice inoculated with Minaçu virus support the few other results available in the literature concerning tissue changes in experimental infections caused by arboviruses belonging to the family Reoviridae, genus Orbivirus (Karabatsos 1985; Tyler & Fields 1990).
Results of the IHC tissue examination of mouse organs reinforce the observations obtained by HE, and were useful for staining cells with viral antigens. Minaçu virus antigens were found in the CNS (brain) (Figure 4a,b) and in several viscera. Indeed, several cells also showed viral antigens in the heart, liver and kidneys (Figure 4). The virus may therefore be able to reproduce in these organs.
The large quantity of viral antigens found in the brain and heart of infected animals may lead to inaccurate diagnoses of death from encephalitis or myocarditis. Myocarditis is not commonly diagnosed among viruses of the genus Orbivirus, family Reoviridae (Karabatsos 1985; Tyler & Fields 1990). The complexity of the cardiac lesions caused by the Minaçu virus gives this organism the status of a new model for myocarditis caused by orbiviruses.
Acknowledgments
The authors thank Prof. Ralph Lainson for his invaluable comments and suggestions. They are also grateful to Dr Amélia Travassos da Rosa, Basílio Silva Buna, Geraldo Mendes da Silva and Valter Campos for technical assistance. This study was supported by CAPES (LCM), IEC/SVS/MS and CNPq (process 302770/02-0).
References
- Barth OM. Estudos sobre a contrastação negativa de suspensões virais. Rev. Bras. Biol. 1984;44:71–80. [PubMed] [Google Scholar]
- Beaty BJ, Calisher CH, Shope RE. Arboviruses. In: Schmidt NJ, Emmons EW, editors. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. Washington: American Public Health Association; 1989. pp. 797–855. [Google Scholar]
- Clarke DH, Casals J. Technique for hemagglutination and hemagglutination inhibition with arthropod-borne viruses. Am. J. Trop. Med. Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Hepler PK. Membranes in the mitotic apparatus of Barley cells. J. Cell Biol. 1980;86:490–499. doi: 10.1083/jcb.86.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung GD, Fong CKY, August MJ. The use of electron microscopy for diagnosis of virus infections: an overview. Prog. Med. Virol. 1979;25:133–159. [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Karabatsos N. International Catalogue of Arboviruses, including certain other Viruses of Vertebrates. 3. San Antonio, TX: American Society of Tropical Medicine and Hygiene; 1985. p. 1141. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutenaldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 1965;27:137. [Google Scholar]
- Laemmli UK. Cleavages of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luft JH. Improvements in epoxy resin embedding methods. J. Biophys. Biochem. Cytol. 1961;2:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalany J. Técnica histológica em anatomia patológica: com instruções para cirurgião, enfermeira e citotécnico. 3. Brazil: EPU: São Paulo; 1998. p. 295. [Google Scholar]
- Pinheiro FP, Travassos da Rosa APA, Vasconcelos PFC. Arboviroses. In: Veronesi R, Focaccia R, editors. Tratado de Infectologia. São Paulo: Atheneu; 1996. pp. 169–180. [Google Scholar]
- Prophet EB, Mills B, Arrington JB, Sobin LH. Laboratory Methods in Histotechnology. Washington: American Registry of Pathology; 1992. p. 80. [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty per cent end-points. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Reynolds ES. The use of lead citrate and high pH as an electron opaque stain in electron microscopy. J. Cell Biol. 1963;17:208–213. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff LA, Fields BN. Reoviruses and their replication. In: Fields BN, Knipe DM, editors. Virology. New York, USA: Raven Press; 1990. pp. 1275–1306. [Google Scholar]
- Tesh RB. A method for the isolation and identification of dengue viruses, using mosquito cell cultures. Am. J. Trop. Med. Hyg. 1979;28:1053–1059. doi: 10.4269/ajtmh.1979.28.1053. [DOI] [PubMed] [Google Scholar]
- Theiler M. Action of sodium deoxycholate on arthropod-borne viruses. Proc. Soc. Exp. Biol. Med. 1957;96:380–382. doi: 10.3181/00379727-96-23483. [DOI] [PubMed] [Google Scholar]
- Travassos da Rosa APA, Travassos da Rosa JFS, Pinheiro FP, Vasconcelos PFC. Arboviroses. In: Leão RNQ, editor. Doenças Infecciosas e Parasitárias – Enfoque Amazônico. Belém: CEJUP, UEPA, Instituto Evandro Chagas; 1997. pp. 207–225. [Google Scholar]
- Tyler KL, Fields BN. Reoviruses. In: Fields BN, Knipe DM, editors. Virology. Vol. 2. New York, USA: Raven Press; 1990. pp. 1307–1328. [Google Scholar]
- Van Regenmortel MHV, Fauquet CM, Bishop DHL, et al. Virus Taxonomy. VII report of the ICTV. San Diego, CA: Academic Press; 2000. p. 1167. [Google Scholar]
- Vasconcelos PFC, Travassos da Rosa APA, Dégallier N, Pinheiro FP, Travassos da Rosa JFS. Clinical and ecoepidemiological situation of human arboviruses in Brazilian Amazon. Braz. J. Adv. Sci. 1992;44:117–124. [Google Scholar]
- Vasconcelos PFC, Travassos da Rosa APA, Pinheiro FP, et al. Arboviruses pathogenic for man in Brazil. In: Travassos da Rosa APA, Vasconcelos PFC, Travassos da Rosa JFS, editors. An Overview of Arbovirology in Brazil and Neighbouring Countries. Belém: Instituto Evandro Chagas; 1998. pp. 72–99. [Google Scholar]
- Xiao SY, Guzman H, Zhang H, Travassos da Rosa APA, Tesh RB. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg. Infect. Dis. 2001;7:714–721. doi: 10.3201/eid0704.010420. [DOI] [PMC free article] [PubMed] [Google Scholar]