Abstract
Inflammation has evolved as a protective response to insult or injury, it's a primordial response that eliminates or neutralises foreign organisms or material, the resolution of inflammation encompasses the endogenous anti-inflammatory mechanisms that protect us against excessive tissue injury and promote the restoration of tissue structure and function. In fact, our well being and survival depends upon its efficiency and carefully-balanced control. In general, the innate inflammatory response initiates within minutes and, if all is well, resolves within hours. In contrast, chronic inflammation persists for weeks, months or even years. Here, we are going to discuss the key endogenous checkpoints necessary for mounting an effective yet limited inflammatory response and the crucial biochemical pathways necessary to prevent its persistence.
Keywords: acute inflammationn, chronic inflammation, eccosanoids, macrophage, resolution, signalling
The Inflammatory Response (Celsus 90 AD)
Acute inflammation is characterized by leucocyte recruitment from the circulation, classically defined by the initial trafficking of polymorphonuclear granulocytes followed by monocytes which differentiate locally into macrophages (Majno 1975). Invariably, this response is triggered by tissue mast cells and resident macrophages, whose degranulation and activation sequentially release a battery of inflammatory mediators, including bioactive amines (Histamine and 5-HT), cytokines, chemokines as well as lipid mediators that collectively recruit and activate inflammatory cells as well as bring about oedema formation. While this system has an enormous capacity for synergy and redundancy, over the years it has served as the stable basis for the development of anti-inflammatory drug discovery, typified by the development of non-steroidal anti-inflammatory inhibitors of eicosanoid synthesis beginning in the 1960s to more recent times with the inhibition of the actions of tumour necrosis factor-α (TNFα). These early days of inflammation research that focused on elucidating the nature of soluble pro-inflammatory mediators have now given way to the view that inflammation is far more complex and sophisticated than originally appreciated. This is illustrated by the production of both pro- and anti-inflammatory eicosanoids which have important roles in orchestrating the inflammatory response (Lawrence et al. 2002). Such clear diversity is not the preserve of lipid mediators but it extends to cytokines, chemokines and the expression of both activating receptors and inhibitory receptors by inflammatory cells. On this theme of biological diversity, recent evidence suggests that alternative pattern recognition receptors of the scavenger receptor and C-type lectin families may play equally important roles in the recognition of microbes and the regulation of the host-inflammatory response. Thus, the C-type lectin, Dectin-1 (Brown & Gordon 2005), was recently shown to act in concert with the TLR-2 to activate macrophages exposed to β-glucans from the yeast candida albicans (Gantner et al. 2003). A number of these receptors also recognize endogenous inflammatory ligands including the scavenger receptors SR-A and CD36, both of which have been described to mediate the phagocytosis of apoptotic cells leading to a downregulation of macrophage activation (Savill et al. 1990; Ren et al. 1995; Savill et al. 2003). Thus, many of the factors that drive inflammation also double up in bringing about its resolution.
Signalling pathways in inflammation
The inflammatory response is characterized by coordinated activation of various signalling pathways that regulate expression of both pro- and anti-inflammatory mediators in resident tissue cells and recruited leucocytes. Currently, most of our knowledge of signalling in inflammation is gained from studying members of interleukin 1 (IL-1) and TNF receptor families and the Toll-like microbial pattern recognition receptors (TLRs) which in fact belong to the IL-1R family. IL-1 and TNFα represent the archetypal pro-inflammatory cytokines which are rapidly released upon tissue injury or infection. TLRs recognize microbial molecular patterns; hence, the term pattern recognition receptor (PRR). Therefore, TLRs represent a germline encoded non-self recognition system that is hard-wired to trigger inflammation. However, there is some suggestion that endogenous ligands may trigger TLRs during tissue injury and certain disease states which may act to promote inflammation in the absence of infection (Karin et al. 2006). Although structurally different, these receptors use similar signal transduction mechanisms. Receptor engagement results in recruitment of adaptor proteins, that possess either Toll-IL-1 receptor (TIR) domains in the case of TLRs and IL-1R or death domains (DD) in the case of the TNFR family, linked to the regulation of cell survival (Muppidi et al. 2004). Once recruited, these adaptors recruit further signalling proteins that belong to the TRAF family (Arch et al. 1998; Baud & Karin 2001) and various protein kinases, including IRAK1 and 4 in the case of TIR signalling (Suzuki et al. 2002) and RIP kinases in the case of TNFR signalling (Kelliher et al. 1998; Wertz et al. 2004). These molecules activate several effector pathways, the most important of which lead to activation of Mitogen Activated Protein Kinases (MAPK) (Chang & Karin 2001; Kyriakis & Avruch 2001), including JNK (Karin & Gallagher 2005) and p38 MAPK (Zarubin & Han 2005), as well as IκB kinases (IKK) (Ghosh & Karin 2002). The MAPKs lead to direct and indirect phosphorylation and activation of various transcription factors, especially those that belong to the bZIP family: AP-1 (Karin 1995) and CREB (Park et al. 2005), which bind to the promoters of pro-inflammatory genes. MAPKs also regulate pro-inflammatory gene expression through post-transcriptional mechanisms such as mRNA turnover, mRNA transport and translation (Chen et al. 1998; Dean et al. 2004; Winzen et al. 2004). The IKKs, which form a complex composed of two catalytic subunits-IKKα and IKKβ and a regulatory subunit IKKγ/NEMO, are responsible for activation of the NF-κB transcription factor (Ghosh & Karin 2002), which has emerged as a central regulator of inflammatory and immune responses (Li & Verma 2002; Bonizzi & Karin 2004). Target genes for the IKK and MAPK pathways include IL-1 and TNFα, generating a feed-forward mechanism to amplify the inflammatory response. The pro-inflammatory cytokines IL-6, IL-12 and type I interferons (IFNs), which are also target genes for IKK and MAPK regulation, signal via receptor associated tyrosine kinases (RTKs) that belong to the JAK group, whose activation results in phosphorylation and nuclear translocation of STAT transcription factors (O'Shea et al. 2002). Engagement of cytokine receptors as well as TLRs, can also lead to activation of phosphoinositide-3-kinases (PI3K), which in turn activate other proteins kinases such as AKT (Martin et al. 2003). Collectively, these proteins kinases coordinate the expression of a large number of pro-inflammatory mediators to initiate and maintain the inflammatory response.
Inhibition of pro-inflammatory signalling
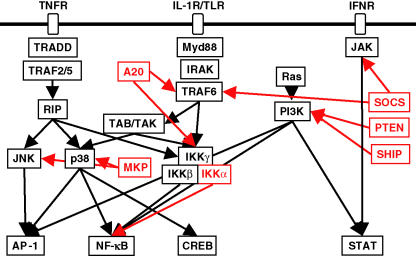
All of the intracellular signalling pathways described above, which contribute to the onset of innate immunity and inflammation are also subject to negative regulation. PI3K signalling is inhibited by the PTEN phosphatase that belongs to the Protein Tyrosin Phosphatase (PTP) family; some of its other members, for instance SHIP, SHP1/2 and CD45, are responsible for negative regulation of TK signalling (Neel et al. 2003). MAPK kinase phosphatases (MKPs), which also belong to the PTP family, control the duration of MAPK activation as recently shown for TNFα-mediated JNK activation (Kamata et al. 2005). Inducible suppressors of cytokine signalling (SOCS), which function as ubiquitin ligases, are responsible for the negative feedback control of JAK–STAT signalling (Alexander & Hilton 2004). A20 is another inducible ubiquitin ligase, which functions as a negative feedback regulator of TLR and TNFR signalling to IKK and NF-κB. A20 is also a direct target gene for the NF-κB pathway constituting a negative feedback loop for NF-κB activation (Boone et al. 2004). Recently, a new pathway for negative regulation of IKK/NF-κB was described from observations made in mice that harbour a variant of IKKα, IKKαAA, that cannot be activated by upstream regulators. Although IKKαAA mice do not develop ‘spontaneous’ inflammation, they develop an exaggerated inflammatory response when challenged with bacteria or fungal cell wall particles (Lawrence et al. 2005). These studies established that while IKKβ catalytic activity is important for the activation of NF-κB through phosphorylation of endogenous inhibitory (IκB) proteins (Maeda et al. 2003), IKKα is required for termination of NF-κB activation through phosphorylation of the transcription factors RelA (p65) and c-Rel (Lawrence et al. 2005). IKK-mediated phosphorylation results in polyubiquitination of the target protein, leading to its accelerated degradation via the 26S proteasome. However, while IκB degradation is essential for NF-κB activation and nuclear translocation, the accelerated degradation of nuclear Rel proteins via IKKα-mediated phosphorylation, is important for controlling the duration of NF-κB activation. The evolution of two catalytic sub-units in the IKK complex with opposing, yet complimentary, activity therefore ensures rapid and transient activation of NF-κB (Figure 1).
Figure 1.
Schematic illustration of the co-ordinated activation of pro-inflammatory signalling pathways by TLR ligands and the pro-inflammatory cytokines TNFα, IL-1 and IFN. Adaptor molecules (MyD88, TRADD and TRAF) and receptor associated kinases (RIP, IRAK and JAK) couple to downstream kinase cascades (MAPK; JNK, p38; TAB/TAK, IKK) which regulate the activation of transcription factors (AP-1, NF-κB, CREB and STAT) and the expression of pro-inflammatory genes. A number of negative regulatory mechanisms (shown in red) limit the activation of specific signalling pathways; SOCS targets JAK/STAT and TLR signalling; PTEN and SHIP phosphatases block PI3K; A20 and IKKα negatively regulate the NF-κB pathway; the MAPK phosphatase MKP limits activation of JNK and p38.
As discussed below, pro-inflammatory signalling pathways have the capacity to induce the parallel expression of anti-inflammatory mediators, such as IL-10. Recent studies reveal that the signalling pathway used by TLRs to activate expression of pro- and anti-inflammatory cytokines diverges at the level of the adaptor proteins TRAF3 and TRAF6, such that TRAF3 is critical for induction of IL-10 expression and in its absence, expression of the TRAF6-dependent pro-inflammatory cytokines IL-6 and IL-12 is dramatically increased (Häcker et al. 2006). The balance between the TRAF3- and TRAF6-generated signals may therefore play an important role in controlling the inflammatory response and its perturbation may interfere with the proper resolution of inflammation.
A deficiency in any one of these negative regulators may result in either ‘spontaneous’ chronic inflammation, perhaps reflecting host cell activation by PAMPs present in endogenous microflora, or an exaggerated inflammatory response to insult or injury that culminates in severe inflammation and damage to the host. Although all of these negative regulatory mechanisms affect different signalling pathways, genetic studies in mice have shown that even the absence of one negative regulator is sufficient to result in serious inflammatory disorders. Undoubtedly, aberrations in such negative regulatory pathways will be found to contribute to the development of chronic inflammatory diseases.
Resolving acute inflammation
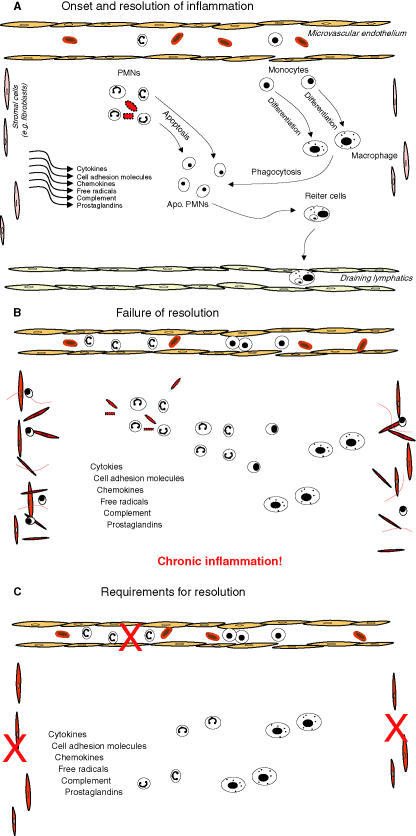
The receptors and signalling pathways that initiate and promote the inflammatory response have become increasingly well characterized; however, relatively a little is known about how acute inflammation resolves to prevent chronic inflammatory diseases. We have already discussed the intracellular checkpoints that limit the activation of inflammatory cells either directly in response to infection or tissue injury or through paracrine activation by pro-inflammatory cytokines. If we were to define the fundamental requirements for the successful resolution of inflammation it would become increasingly clear that the most simple but absolutely critical determinant for the inflammatory response to switch off is the neutralization and elimination of the injurious agents that initiated it in the first place. Failure to achieve this first step will invariably lead to chronic inflammation with the nature of the agent in question almost certainly dictating the aetiology of the developing chronic immune response. For example, chronic granulomatous disease is characterized by severe, protracted and often fatal infection, which results from a failure of the phagocytic NADPH oxidase enzyme system to produce superoxide and kill invading infections leading to a predisposition to recurrent bacterial and fungal infections and the development of inflammatory granulomas (Goldblatt & Thrasher 2000). Successfully dispensing with the inciting stimulus will signal a cessation to pro-inflammatory mediator synthesis (eicosanoids, chemokines, cytokines, cell adhesion molecules, etc.) and lead to their catabolism. This would halt further leucocyte recruitment and oedema formation. These are probably the very earliest determinants for the resolution of acute inflammation, the outcome of which signals the next stage of cell clearance. The clearance phase of resolution, be it innate [polymorphonuclear leucocyte (PMN) or eosinophil driven] or adaptive (lymphocyte mediated), also has a number of mutually dependent steps (Figure 2). The clearance routes available to inflammatory leucocytes include systemic recirculation (Hughes et al. 1997) or local death of influxed PMNs, eosinophils or lymphocytes followed by their phagocytosis by recruited monocyte-derived macrophages (Heasman et al. 2003). Once phagocytosis is complete, macrophages can leave the inflamed site by lymphatic drainage (Bellingan et al. 2002) with evidence that a small population may die locally by apoptosis (Gilroy et al. 2003). If all of these pathways are strictly followed then acute inflammation will resolve without causing excessive tissue damage and give little opportunity for the development of chronic, non-resolving inflammation. As with the onset phase of the acute inflammatory response, which is driven by a cohort of well-described endogenous factors, the resolution phase of the response is also highly coordinated and under the tight control of what may be called ‘pro-resolution’ factors. In contrast to onset, however, these resolution phase factors are less well-described.
Figure 2.
Illustration of the cellular kinetics and sequential release of mediators during the evolution of the inflammatory response from onset to resolution. (A) Inflammation causes the immediate and sequential release of signalling factors including chemokines, cytokines, eicosanoids, etc. that bring leucocytes (PMNs, eosinophils) from the microvasculature to the site of inflammation to neutralize the injurious agent. After leucocyte trafficking, peripheral blood monocytes accumulate at the inflammatory site and differentiate locally in to larger more granular phagocytosing macrophages. This is well-understood sequence of events, the inhibition of which has provided the rationale for the development of inflammatory agents. Once the inflammatory cells have neutralized the injurious agent they must be disposed of in a controlled and effective manner. Apoptotic PMNs or eosinophils are phagocytosed by macrophages, which in turn are cleared from the site of inflammation either by dying locally or by programmed cell death or by clearing to the draining lymphatics, which in the case of the inflamed peritoneum, for instance, are the parathymic lymph nodes. (B) Given a favourable genetic predisposition, failure of acute inflammation to resolve adequately could result in a predisposition to chronic inflammation, collateral tissue injury or auto-immunity typified by the accumulation of inflammatory leucocytes fibrosis and auto-antibodies to endogenous cellular and tissue antigens. (C) Thus, for the effective resolution of acute inflammation we need to curtail further influx of inflammatory leucocytes (PMNs, eosinophils, etc.), signal monocytes/macrophages to phagocytose effete leucocytes and clear all these cells form the site of injury once the inflammatory stimulus posses no further threat. One final but very important aspect of resolution is that the pro-inflammatory phenotype of the stromal cells within the injured tissue, revert back to its prior physiological state as there is evidence that chronic inflammation may be perpetuated by stromal cells attaining a phenotype that retains pro-inflammatory leucocytes at sites of inflammation longer than is necessary inhibiting their efficient clearance. For instance, it has been suggested that the interaction of stromal cells taken from site of chronic inflammation has an enhanced ability to activate leucocytes contributing to the longevity of the response.
Early ‘Stop’ signals
Inflammation is a reaction of the microcirculation that is characterized by the movement of serum proteins and leucocytes from the blood to the extravascular tissue with PMNs or eosinophils predominating at the early onset phase giving way to phagocytosing macrophages leading to resolution. One well-described event in the transition towards resolution is the replacement of PMNs or eosinophils by monocytes and phagocytosing macrophages. However, until recently our understanding of the signals that control this cell profile switch was unclear. Studies addressing this issue of leucocyte infiltration in peritoneal inflammation have suggested that the interaction between IL-6 and its soluble receptor, sIL-6R, forms one of the major determinants of this switch from PMNs to monocytes (Hurst et al. 2001; McLoughlin et al. 2003). It was shown that sIL-6R, produced by the infiltrating PMNs, forms a complex with IL-6 which, in turn, directly modulates CC and CXC chemokine expression. Thus, CXC chemokine synthesis, induced by IL-1 and TNFα, was suppressed, whereas the CC chemokine CCL2 (MCP-1) was promoted. This chemokine shift suppresses further neutrophil recruitment in favour of sustained mononuclear cell influx. In addition to chemokines, the eicosanoids also orchestrate the early transition to resolution in acute inflammation. Transcellular metabolism of arachidonic acid by lipoxygenase/lipoxygenase interaction pathways gives rise to the lipoxin (LX) family of eicosanoid metabolites (Serhan 2002). LXs display selective actions on leucocytes that include inhibition of PMN chemotaxis (Levy et al. 2001), PMN adhesion to and transmigration through endothelial cells (Papayianni et al. 1996), as well as PMN-mediated increases in vascular leakage (Serhan et al. 1999). It is unclear at this point whether there is any cross-talk between the LXs and IL-6/sIL-6R complex signalling in the control of leucocyte profile switching. Nonetheless, it seems that when acute inflammation needs to resolve the IL-6/sIL-6R, chemokines and LXs representing some of the earliest signals that control the switch from very early PMNs to monocyte/macrophage.
The transition to resolution
Once PMNs and eosinophils have done their job and their help is no longer needed, what happens next? At this juncture, it must be borne in mind that these are a formidable cell lineage and if left unchecked could do untold damage to an already inflamed site. After all, these cells are designed to combat infection by releasing hydrolytic and proteolytic enzymes as well as generating reactive oxygen species. Therefore, PMNs and eosinophils must be disposed of in a controlled and effective manner. To oversee this, nature has come up with an ingenious way of defusing such potentially explosive cells called programmed cell death or apoptosis. Apoptosis of inflammatory cells is a physiological process for the non-phlogistic removal of cells. During apoptosis cells maintain an intact membrane and, therefore, do not release their potentially histotoxic agents. Necrosis of inflammatory leucocytes, on the other hand, involves a loss of membrane integrity leading to the release of potentially toxic intracellular contents (Heasman et al. 2003). Moreover, apoptotic cells express a repertoire of surface molecules that allow their recognition and phagocytosis by macrophages (Fadok et al. 2001). Despite stating above that once the injurious agent has been neutralized PMNs and eosinophils are redundant, in fact, the way in which these cells die helps the resolution process enormously. Recognition of these apoptotic cells by macrophages does not liberate pro-inflammatory agents from the macrophages themselves but can release anti-inflammatory signals such as IL-10 and TGFβ (Huynh et al. 2002). Thus, not only is apoptosis a non-inflammatory way of disposing of cells, but this method has the added advantage of conferring upon macrophages an anti-inflammatory phenotype conducive to resolution. It is important to note that if not recognized and disposed of apoptotic cells will eventually undergo secondary necrosis releasing damaging intracellular contents and amplifying the inflammatory response. Therefore, increasing the rate of apoptosis, as a potentially anti-inflammatory strategy, must be matched by a mechanism that upregulates macrophage phagocytic clearance capacity (Ward et al. 1999). Thus, the removal process might also be susceptible to selective modulation by pharmacological agents for therapeutic gain.
As mentioned above, the strategy of enhancing leucocyte apoptosis must also be paralleled with enhancing their phagocytosis by macrophages and other non-professional phagocytes. On this theme, there are an increasing number of factors that aid the phagocytic clearance of apoptotic granulocytes. Ligation of the matrix receptor CD44, for instance, results in the rapid and specific internalization of apoptotic PMN (McCutcheon et al. 1998). Besides, controlling PMN trafficking, LXs also stimulate monocyte chemotaxis and adherence. Certainly, this may seem dangerous for inflammation as too many monocyte-derived macrophages can be a bad thing, but these LX-chemoattracted macrophages accelerate resolution by enhancing phagocytosis of apoptotic PMNs in a non-phlogistic manner (Godson et al. 2000). In addition to a role in granulocyte apoptosis, glucocorticoids facilitate the phagocytic response. It was recently found that exposure of peripheral blood monocytes to glucocorticoids during the first 24 h of the 5-day culture period induced a highly phagocytic monocyte-derived macrophage phenotype (Giles et al. 2001). Functional and morphological homogeneity was matched by cell surface phenotype, including specific induction of expression of the haemoglobin scavenger receptor, CD163 following glucocorticoid treatment. A potentially pro-resolution role for CD163 was demonstrated recently in both in vitro and in vivo models of resolving inflammation (Philippidis et al. 2004). Here, these authors showed that human peripheral blood monocyte-derived macrophages either in culture medium or in resolving phase cantharidin-induced skin blisters express CD163. These authors also found elevated levels of CD163 on circulating monocytes in cardiac surgical patients during the resolution phase of the systemic inflammatory response to cardiopulmonary bypass surgery. In each case, binding of the hemoglobin–haptoglobin complex to CD163-bearing cells elicited potent IL-10 secretion, which in turn enhanced hemeoxygenase 1, widely shown to have anti-inflammatory and tissue protective properties. Such induction of hemeoxygenase 1 was observed in vivo 24–48 h after the onset of cardiopulmonary bypass surgery. This is coincident with the observations of Willis and colleagues who showed that hemeoxygenase 1 was expressed during and essential for the resolution (24–48 h phase) of a rat carrageenin-induced pleurisy (Willis et al. 1996). Thus, apoptosis and the phagocytosis of apoptotic cells are crucial to the resolution process, failure of which may predispose, as mentioned earlier, to chronic inflammation and possibly autoimmunity. This has been proposed in the case of systemic lupus erythematosus; an autoimmune syndrome that is associated with the presence of autoantibodies to endogenous antigens exposed on dead or dying cells, which failed to be cleared and disposed of. Studies in mice, for instance, have established a role for the complement receptor C1q on macrophages in the development of this disease. C1q was discovered to be important for the phagocytic clearance of apoptotic cells; in the absence of this receptor mice developed a lupus like syndrome (Botto et al. 1998). The persistence of apoptotic cells and necrotic bodies led to the development of an inappropriate immune response to endogenous antigens. Evidence has also been established in human SLE patients for an association between C1q deficiency and disease (Walport et al. 1998). The phagocytosis of apoptotic cells has been suggested to play an important role in the negative regulation of macrophage activation; apoptotic leucocytes may well fit into the category of endogenous anti-inflammatory mediators; therefore, the mechanisms of apoptosis and the clearance of apoptotic cells may be critical in the development of chronic inflammation.
Unblocking the drains!
The role of the lymphatic system in the context of the resolution of acute innate inflammation is shamefully understudied given its essential function in draining inflammatory mediators and effete leucocyte away from the inflamed site (Bellingan et al. 1996). We have already discussed the importance of PMN clearance to the resolution of acute inflammation, but it is equally important that phagocytosing inflammatory macrophages are cleared away from the inflamed site to prevent local macrophage-induced tissue damage and potentially granuloma tissue damage and the development of chronic inflammation. However, despite the need to understand the endogenous control of macrophage clearance during acute inflammatory resolution, a little is known about this field. There is increasing evidence that macrophage clearance from an inflamed site is a highly regulated event. Using an experimental model of acute resolving peritonitis, it was shown that macrophages adhere specifically to mesothelium overlying draining lymphatics and that their emigration rate is regulated by the state of macrophage activation (Bellingan et al. 1996, 2002) providing the first evidence that macrophage emigration from the inflamed site is controlled by adhesion molecule regulation of macrophage–mesothelial interactions. This report highlights the importance of adhesion molecules controlling clearance of inflammatory macrophages into the draining lymphatic circulation, thus highlighting new pathways in the resolution of acute inflammation.
Soluble mediators of resolution: opportunities for drug discovery
Returning to eicosanoids, PGD2, a metabolite of the action of hematopoietic prostaglandin D2 synthase on COX-derived PGH2, has emerged recently as an eicosanoid with both pro- as well as anti-inflammatory properties. PGD2 undergoes dehydration in vivo and in vitro to yield biologically active PGs of the J2 series, including PGJ2, Δ12,14-PGJ2 and 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2). In addition to being a high-affinity natural ligand for anti-inflammatory peroxisome proliferators-activated receptor gamma (PPARγ), 15d-PGJ2 also exerts its effects through PPARγ-dependent as well as -independent mechanisms to suppress pro-inflammatory signalling pathways and the expression of genes that drive the inflammatory response. 15d-PGJ2 also preferentially inhibits monocyte rather than PMN trafficking through the differential regulation of cell-adhesion molecule and chemokine expression (Gilroy et al. 2004). We have shown that COX 2-derived PGD2 metabolites contribute to the resolution of acute inflammation (pleuritis) through the preferential synthesis of PGD2 and 15d-PGJ2 (Gilroy et al. 1999), which, along with the alternative DNA-binding p50–p50 homodimers complexes of NF-κB (Lawrence et al. 2001) bring about resolution by inducing leucocyte apoptosis (Gilroy et al. 2003). Recently, we extended these studies to examining the role of PGD2 metabolites in the resolution of adaptive immunity and lymphocyte function (Proc Natl Acad Sci USA, in press). Indeed, there is an increasing body of evidence detailing the differential effects of PGD2 metabolites on leucocyte apoptosis as well as the signalling pathways involved (Rossi et al. 2000; Ward et al. 2002). In addition to the well-known eicosanoids, there is a new generation of lipid mediators showing real promise as endogenous anti-inflammatories. Resolvins and docosatrienes are fatty acid metabolites of the COX/lipoxygenase pathways, where the omega-3 fatty-acid constituents of fish oils docosahexaenoic acid and eicosapentaneoic acid are the substrates and not arachidonic acid. These resolvins and docosatrienes were identified in inflammatory exudates during the resolving phase of acute inflammation and shown to be potent inhibitors of PMN transendothelial migration, microglial-cell cytokine expression and to ameliorate experimental models of dermal inflammation and leucocyte accumulation in peritonitis at nanogram doses (Serhan 2004). These studies on resolvin metabolism are uncovering surprising new avenues in anti-inflammation research, putting fatty acid metabolites right at the forefront of potential drug therapy. These studies are also challenging the existing dogma that not all eicosanoids are detrimental to inflammation and are putting a balanced view of their role in pathophysiology. To add fuel to this notion, a recent and very surprising paper has shown that eicosanoids of the LXs family, described above, are orally active in models of acute inflammation (Bannenberg et al. 2004).
Conclusions
In conclusion, it is clear the inflammatory response has a number of built-in checkpoint controls that limit the duration and magnitude of acute inflammation. Defects in these endogenous anti-inflammatory pathways will undoubtedly predispose to the development of chronic inflammatory diseases. A further understanding and analysis of these pathways in the pathogenesis of chronic inflammation is required. This will allow the pursuit of therapeutic strategies to correct possible defects in these feedback control systems or manipulate these pathways to suppress inflammation. However, it is equally clear that the resolution of inflammation is driven by a complex set of pro-resolution mediators that regulate specific cellular events required to clear inflammatory cells from the site of injury or infection and restore tissue homeostasis. These endogenous anti-inflammatory and pro-resolution mechanisms are clearly intimately linked; however, the true goal in the treatment of chronic inflammatory diseases must be to inhibit persistent inflammation and restore tissue function. To achieve this goal, we must improve our understanding of the resolution of inflammation and identify possible approaches to promote this process in combination with anti-inflammatory therapy. Perhaps the, as yet unidentified, anti-resolution factors that may prevent the proper resolution of inflammation would represent appropriate targets to achieve this goal.
References
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- Arch RH, Gedrich RW, Thompson CB. Tumor necrosis factor receptor-associated factors (TRAFs) – a family of adapter proteins that regulates life and death. Genes. Dev. 1998;12:2821–2830. doi: 10.1101/gad.12.18.2821. [DOI] [PubMed] [Google Scholar]
- Bannenberg G, Moussignac RL, Gronert K, et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br. J. Pharmacol. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J. Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- Bellingan GJ, Xu P, Cooksley H, et al. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J. Exp. Med. 2002;196:1515–1521. doi: 10.1084/jem.20011794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Boone DL, Turer EE, Lee EG, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- Botto M, Dell'Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Brown GD, Gordon S. Immune recognition of fungal beta-glucans. Cell Microbiol. 2005;7:471–479. doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chen CY, Del Gatto-Konczak F, Wu Z, Karin M. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science. 1998;280:1945–1949. doi: 10.1126/science.280.5371.1945. [DOI] [PubMed] [Google Scholar]
- Dean JL, Sully G, Clark AR, Saklatvala J. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell Signal. 2004;16:1113–1121. doi: 10.1016/j.cellsig.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J. Clin. Invest. 2001;108:957–962. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl.):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Giles KM, Ross K, Rossi AG, Hotchin NA, Haslett C, Dransfield I. Glucocorticoid augmentation of macrophage capacity for phagocytosis of apoptotic cells is associated with reduced p130Cas expression, loss of paxillin/pyk2 phosphorylation, and high levels of active Rac. J. Immunol. 2001;167:976–986. doi: 10.4049/jimmunol.167.2.976. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, McMaster S, Sawatzky DA, Willoughby DA, Lawrence T. Inducible cyclooxygenase-derived 15-deoxy(Delta)12–14PGJ2 brings about acute inflammatory resolution in rat pleurisy by inducing neutrophil and macrophage apoptosis. Faseb. J. 2003;17:2269–2271. doi: 10.1096/fj.02-1162fje. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug. Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- Goldblatt D, Thrasher AJ. Chronic granulomatous disease. Clin. Exp. Immunol. 2000;122:1–9. doi: 10.1046/j.1365-2249.2000.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker H, Redecke V, Blagoev B, et al. Specificity in Toll-like receptor signaling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- Heasman SJ, Giles KM, Ward C, Rossi AG, Haslett C, Dransfield I. Glucocorticoid-mediated regulation of granulocyte apoptosis and macrophage phagocytosis of apoptotic cells: implications for the resolution of inflammation. J. Endocrinol. 2003;178:29–36. doi: 10.1677/joe.0.1780029. [DOI] [PubMed] [Google Scholar]
- Hughes J, Johnson RJ, Mooney A, Hugo C, Gordon K, Savill J. Neutrophil fate in experimental glomerular capillary injury in the rat. Emigration exceeds in situ clearance by apoptosis. Am. J. Pathol. 1997;150:223–234. [PMC free article] [PubMed] [Google Scholar]
- Hurst SM, Wilkinson TS, McLoughlin RM, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat. Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003;19:725–737. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- Majno G. The Healing Hand: Man and Wound in the Ancient World. Cambridge, MA: Harvard University Press; 1975. [Google Scholar]
- Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J. Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- McCutcheon JC, Hart SP, Canning M, Ross K, Humphries MJ, Dransfield I. Regulation of macrophage phagocytosis of apoptotic neutrophils by adhesion to fibronectin. J. Leukoc. Biol. 1998;64:600–607. doi: 10.1002/jlb.64.5.600. [DOI] [PubMed] [Google Scholar]
- McLoughlin RM, Witowski J, Robson RL, et al. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J. Clin. Invest. 2003;112:598–607. doi: 10.1172/JCI17129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21:461–465. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The ’Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl.):S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- Papayianni A, Serhan CN, Brady HR. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J. Immunol. 1996;156:2264–2272. [PubMed] [Google Scholar]
- Park JM, Greten FR, Wong A, et al. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis-CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ. Res. 2004;94:119–126. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J. Exp. Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Kapahi P, Natoli G, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Savill J, Gregory C, Haslett C. Cell biology. Eat me or die. Science. 2003;302:1516–1517. doi: 10.1126/science.1092533. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: an update and role in anti-inflammation and pro-resolution. Prostaglandins Other Lipid. Mediat. 2002;68–69:433–455. doi: 10.1016/s0090-6980(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem. Cell. Biol. 2004;122:305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Takano T, Clish CB, Gronert K, Petasis N. Aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogs inhibit neutrophil-mediated changes in vascular permeability. Adv. Exp. Med. Biol. 1999;469:287–293. doi: 10.1007/978-1-4615-4793-8_42. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Duncan GS, et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- Walport MJ, Davies KA, Botto M. C1q and systemic lupus erythematosus. Immunobiology. 1998;199:265–285. doi: 10.1016/S0171-2985(98)80032-6. [DOI] [PubMed] [Google Scholar]
- Ward C, Dransfield I, Chilvers ER, Haslett C, Rossi AG. Pharmacological manipulation of granulocyte apoptosis: potential therapeutic targets. Trends Pharmacol. Sci. 1999;20:503–509. doi: 10.1016/s0165-6147(99)01391-7. [DOI] [PubMed] [Google Scholar]
- Ward C, Dransfield I, Murray J, Farrow SN, Haslett C, Rossi AG. Prostaglandin D2 and its metabolites induce caspase-dependent granulocyte apoptosis that is mediated via inhibition of I kappa B alpha degradation using a peroxisome proliferator-activated receptor-gamma-independent mechanism. J. Immunol. 2002;168:6232–6243. doi: 10.4049/jimmunol.168.12.6232. [DOI] [PubMed] [Google Scholar]
- Wertz I E, O'Rourke KM, Zhou H, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat. Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- Winzen R, Gowrishankar G, Bollig F, Redich N, Resch K, Holtmann H. Distinct domains of AU-rich elements exert different functions in mRNA destabilization and stabilization by p38 mitogen-activated protein kinase or HuR. Mol. Cell. Biol. 2004;24:4835–4847. doi: 10.1128/MCB.24.11.4835-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell. Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]