Abstract
Interferon-gamma (IFNγ) is a central component of the complex cytokine and inflammatory response that contributes to liver cell injury in hepatitis. We report that in the primary hepatocyte IFNγ synergizes with the mechanistically distinct apoptotic stimuli CD95, tumour necrosis factor-alpha (TNFα) and UV-irradiation. For the first time in primary hepatocytes, we show that IFNγ-mediated apoptotic signalling requires the cell surface interaction of CD95 and its ligand, and we demonstrate that IFNγ induces soluble CD95 ligand release from hepatocyte monolayers. Utilizing c-myc phosphorothioate antisense fragments, we suppresses hepatocyte apoptosis induced by IFNγ. In summary, we identify apoptotic pathways that contribute to IFNγ-mediated cell death. The hepatocellular response to IFNγ signalling can be modulated by cytokines and by the interruption of CD95 interaction with its ligand. We present evidence to suggest that c-myc contributes to IFNγ signalling.
Keywords: antisense, apoptosis, c-myc, CD95, CD95L, hepatocyte, interferon gamma, primary culture, tumour necrosis factor-alpha, UV-irradiation
Interferon-gamma (IFNγ) has been identified as an important mediator of inflammatory liver cell injury and death in both human disease and animal models of hepatitis (Burke et al. 1996; Mizuhara et al. 1996). Persistent hepatic inflammation results in cycles of hepatocyte death and regeneration, hepatic fibrosis and a predisposition to hepatocellular tumour formation. In vitro, IFNγ-induces apoptosis of primary murine hepatocytes (Kano et al. 1997). While multiple factors modulate this response, the underlying molecular mechanisms remain elusive.
Death receptors are members of the tumour necrosis factor (TNF) receptor super-family and include TNFR1, CD95, the TRAIL receptors DR4 and DR5 and DR6. All are characterized by a cytoplasmic death domain that interacts with adapter molecules resulting in activation of signalling and ultimately effector caspases that mediate apoptosis (reviewed Ashkenazi & Dixit 1998). Death receptor induced apoptosis has been implicated in the pathogenesis of inflammatory liver disease. CD95 induces hepatocyte apoptosis in vitro (Ni et al. 1994; Rouquet et al. 1996) and in vivo resulting in fulminant hepatic failure (Ogasawara et al. 1993). TNFα has been implicated as an effector of liver injury in animal models of hepatitis (Gantner et al. 1995a,b). In vivo, an interaction of this complex cytokine network effects liver cell injury and repair.
We have previously shown that IFNγ induces primary hepatocyte apoptosis in the context of serum deprivation and that specific growth factors suppress the apoptotic response (McCullough et al. 2006). Both findings are characteristic of c-myc-induced apoptosis (Harrington et al. 1994). C-myc sensitizes cells to mechanistically different stimuli including serum deprivation, hypoxia, IFNγ, death receptors CD95 and TNFα and genotoxic agents (Evan et al. 1992; Harrington et al. 1994). IFNγ has been shown to sensitize multiple cell lineages to cytokine and death receptor-mediated apoptosis (Harrington et al. 1994; Klefstrom et al. 2002; Ricci et al. 2004). Furthermore, effective killing by c-Myc requires the cell surface interaction of CD95 and CD95L (Hueber et al. 1997).
We hypothesized that, in the primary hepatocyte, IFNγ induces apoptosis via a myc-mediated mechanism. To test this hypothesis, we assessed whether, in our system, IFNγ-mediated apoptosis exhibits similar characteristics to c-myc-induced cell death. We show that IFNγ synergizes with mechanistically distinct apoptotic stimuli. We show that effective induction of apoptosis by IFNγ requires the cell surface interaction of CD95 and its ligand, and that IFNγ induces sCD95 ligand release from primary hepatocyte monolayers. Finally, we show that c-myc phosphorothioate antisense fragments attenuate hepatocyte apoptosis in response to IFNγ. We offer a hypothesis that there is a selective advantage conferred by associating the immunomodulatory IFNγ pathway with the oncogene c-myc in hepatocytes.
Methods
Hepatocyte isolation and culture
Primary hepatocytes from adult male C3H mice 8–12 weeks were isolated by a retrograde two-step perfusion procedure and purified by centrifugation through Percoll (Amersham Pharmacia Biotech, Little Chalfont, UK). The hepatocytes were plated onto fibronectin coated 2-well chamber slides (Life Technologies, Paisley, UK) in modified Dulbecco's Modified Eagle's Medium/Nutrient Mixture F12 Ham (DMEM/F12; Life Technologies) containing 4 mM l-glutamine (Life Technologies), insulin, transferrin, sodium selenite media supplement (ITS) (Sigma, Poole, UK), 0.04 μg/ml dexamethasone and 50 mg/ml gentamicin (Life Technologies). Cells were plated at a density of 0.2 × 105/cm2. After 3 h, media was replaced with modified Chee's media (Sigma) containing l-glutamine, ITS, dexamethasone and gentamicin as above and, where stated, 2% FCS and 50 ng/ml EGF (Sigma). IFNγ (Gibco, Paisley, UK) was added at 100 U/ml unless otherwise stated. Phosphorothioate c-myc antisense oligonucleotides, that targets the initiation codon (AUG) and the subsequent four codons of the c-myc gene, a non-sense control and fluorescent oligonucleotides (Calbiochem, San Diego, CA, USA) were added at to culture media at a final concentration of 30 μM. Cells were cultured in a humid 5% CO2/95% air atmosphere at 37 °C. Experimental culture media was replaced every 24 h. Values shown are mean ± standard error of the mean (SEM). Statistics were performed using a Mann–Whitney U-test on graphpad Instat Software (GraphPad, San Diego, CA, USA).
Feulgen's staining for apoptosis
Apoptosis is a morphological form of cell death, thus direct light microscopy remains the gold standard for distinguishing apoptotic and necrotic cells. At indicated time points hepatocyte monolayers were fixed in Boum's fixative (85% methanol, 5% glacial acetic acid, 10% of 40% formalin solution) at 4 °C overnight. Following denaturation in 5 M HCl for 45 min at room temperature, slides were stained with Schiff's reagent (Sigma) for 1 h and counterstained with 0.1% light green (Merck, Lutterworth, UK) before application of cedarwood oil (Sigma) and a glass coverslip. A minimum of 500 cells were counted and results expressed as a percentage of total cells.
sCD95L ELISA
Quantification of soluble murine CD95 ligand was performed using the Quantikine Murine Fas Ligand Immunoassay Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturers instructions. Culture media supernatants were collected at 24-h intervals for a total of 72 h and stored at −20 °C until required. Supernatants from UV-irradiated primary hepatocytes were assayed as a control for non-specific apoptotic effects. Optical density of each well was determined using a plate reader at 450 nm.
c-Myc antisense
The phosphorothioate c-myc antisense sequence, 5′-CACGTTGAGGGGCAT 3-′, and a non-sense control sequence, 5′-AGTGGCGGAGACTCT 3-′ (Calbiochem) were utilized at 30 μM. A fluorescein-labelled c-myc antisense fragment (Calbiochem) was used to demonstrate adequate cellular uptake, visualized under a fluorescent microscope (Zeiss, Welwyn Garden City, UK) and image analysis performed using Metamorph software (Molecular Devices, Downingtown, PA, USA).
Results
IFNγ synergizes with diverse apoptotic stimuli in the hepatocyte
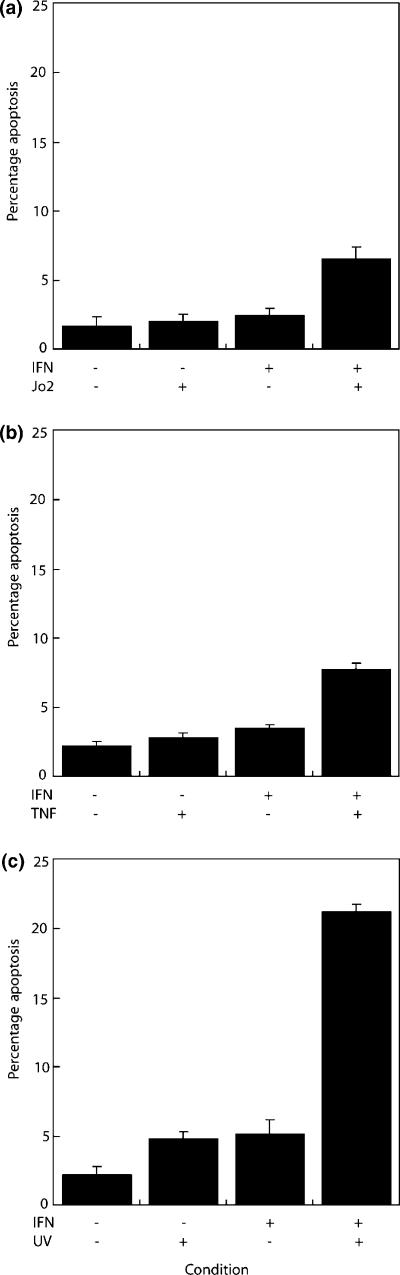
Interferon-gamma induces primary hepatocyte apoptosis in the context of serum deprivation (McCullough et al. 2006). We hypothesized that, consistent with characteristics of c-myc induced apoptosis, IFNγ synergizes with diverse apoptotic stimuli in the primary hepatocyte. Activation of CD95-signalling, induces liver cell apoptosis in vitro and in vivo (Ogasawara et al. 1993; Ni et al. 1994). Primary hepatocytes were cultured in serum-free media in the presence or absence of IFNγ and the anti-CD95 antibody, Jo-2 (Figure 1) for 24 h. At 100 ng/ml, the Jo-2 antibody induced significantly higher levels of hepatocyte apoptosis when co-cultured with IFNγ compared with controls (6.5 ± 0.9 vs. 2.0 ± 0.5) (P = 0.0217).
Figure 1.
Interferon-gamma (IFNγ) synergizes with CD95, tumour necrosis factor-alpha (TNFα) and UV-irradiation to induce hepatocyte apoptosis. Hepatocyte monolayers were cultured in serum-depleted media, in the presence or absence of IFNγ and 100 ng/ml of the anti-CD95 antibody, Jo-2 (A), 100 U/ml of TNFα (B), or following 50 J/M2 UV-irradiation (C).
TNFα has been implicated as an effector of liver injury in animal models of hepatitis (Gantner et al. 1995a,b). In vitro, IFNγ and TNFα act synergistically to induce apoptosis in hepatocytes (Shinagawa et al. 1991; Sasagawa et al. 2000). We confirm that at 24 h, IFNγ and TNFα induce significant levels of apoptosis compared with controls (7.8 ± 0.5%vs. 2.2 ± 0.4%) (P < 0.05). Thus, IFNγ synergizes with CD95 and TNFα death receptor induced apoptosis in the primary hepatocyte.
UV-irradiation induces apoptosis in primary hepatocyte cultures (Schrenk et al. 2004). Significantly increased levels of apoptosis are observed in hepatocytes exposed to 50 J/M2 UV-irradiation co-cultured with IFNγ at 24 h (21.3 ± 0.5 vs. 2.8 ± 0.6%) (P = 0.0048), compared with untreated cells. This suggests that a synergistic response can be observed between IFNγ and mechanistically distinct apoptotic stimuli.
The interaction of CD95/ CD95L is required for IFNγ mediated apoptotic response
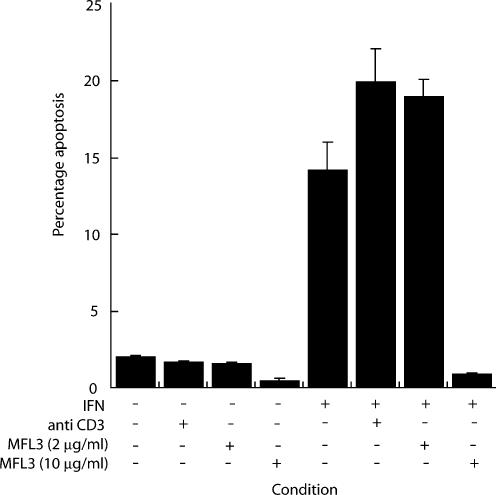
Effective killing by c-Myc requires the cell surface interaction of CD95 and CD95L(Hueber et al. 1997). To test our hypothesis that IFNγ-mediated apoptosis exhibits similar characteristics, we utilized the anti CD95L antibody, MFL-3, that interrupts the cell surface interaction of CD95 and its ligand, CD95L. Anti-CD3 was used as a class matched control antibody. Apoptosis was observed at 72 h (13.8 ± 2.1%) in IFNγ treated cells. At 10 μg/ml the anti-CD95L antibody MFL-3 completely inhibited IFNγ-induced hepatocyte apoptosis (P = 0.02) (Figure 2). Neither MFL-3 nor anti-CD3 antibody alone had any effect over control conditions. Thus, IFNγ-induced hepatocyte apoptosis requires the cell surface interaction of CD95 and its ligand CD95L, in vitro.
Figure 2.
Anti-CD95 ligand antibody abrogates interferon-gamma (IFNγ)-induced apoptosis in primary murine hepatocytes. Monolayers were cultured in serum-depleted media with or without IFNγ (100 U/ml). Cells were co-incubated with anti CD95L antibody, MFL-3 at 2 μg/ml or 10 μg/ml or a class controlled antibody, anti-CD3 at 10 μg/ml, for 72 h. The anti-CD95L antibody, MFL-3, completely abrogates the hepatocyte apoptotic response to IFNγ.
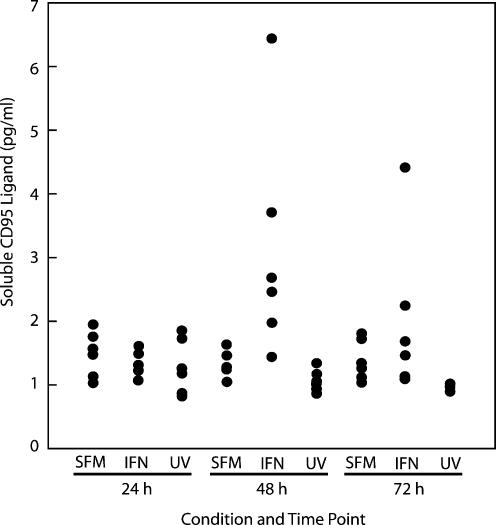
In normal liver, hepatocytes express CD95 but not CD95L (Roskams et al. 2000). Enhanced expression of CD95 and CD95L is observed in inflammatory liver disease and IFNγ transgenic mouse liver (Okamoto et al. 1998; Nakae et al. 2001). We hypothesized that, in our system, autocrine and paracrine activation of the CD95 pathway occurred via production of soluble CD95L by the hepatocyte. To test this, cells were cultures in serum-depleted media in the presence or absence of IFNγ (100 U/ml) for 72 h. UV-irradiated cells were used as an apoptotic control. Media at 24-h intervals and soluble CD95L detected using a CD95L ELISA. A significant increase in soluble CD95L production was detected at 24 and 48 h in hepatocytes treated with IFNγ (P < 0.05). No such increase was detected in UV-induced hepatocyte apoptosis, suggesting that soluble CD95L release is a specific IFNγ-related cell death response (Figure 3).
Figure 3.
Interferon-gamma (IFNγ)-induces the release of soluble CD95L in primary hepatocyte cultures. Apoptosis was induced in hepatocyte monolayers with IFNγ or UV-irradiation as previously described. Supernatant media was collected from six monolayers at 24 h intervals and stored at −70 °C. Samples were analysed using a commercially available soluble CD95 ligand ELISA. Significant induction of soluble Fas ligand is detected in supernatants of IFNγ-treated cells at 24–48 h (P < 0.05). This is an effect specific to IFNγ-mediated apoptosis. Data presented here represent hepatocytes from one perfusion, with supernatants isolated from six monolayers for each condition.
c-Myc antisense fragments attenuate the hepatocyte response to IFNγ
Having demonstrated that IFNγ-mediated apoptosis exhibits similar characteristics to c-myc induced death, we sought to test the hypothesis that c-myc was central to the hepatocyte response to IFNγ. We utilized phosphorothioate modified antisense fragments to interrupt gene function. Fluorescein-labelled c-myc antisense phosphorothioate oligonucleotides, added to culture media at a concentration of 30 μM, accumulated in the cell. Fragments were first detected at 1 h and remained at 24 h (data not shown). In all subsequent experiments antisense strands were reapplied every 24 h.
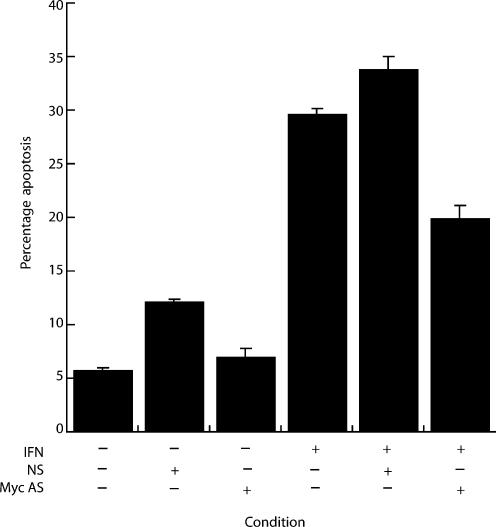
A phosphorothioate-modified c-myc antisense strand, at 30 μM, decreased the levels of IFNγ-induced apoptosis by 32.8% (19.9 ± 1.3%vs. 29.6 ± 0.6%) at 72 h (Figure 4). The non-sense fragments were associated with a small increase in apoptotic rates of IFNγ treated cells (33.8 ± 1.2%vs. 29.6 ± 0.6%). This suggests that, at least in part, c-myc mediates the primary hepatocyte response to IFNγ.
Figure 4.
Phosphorothioate c-myc antisense oligonucleotides partially attenuate interferon-gamma (IFNγ)-mediated hepatocyte apoptosis. Hepatocytes were cultured, as described, in the presence or absence of IFNγ (100 U/ml) with either an antisense phosphorothioate modified c-myc antisense strand (Myc AS) or a control, non-sense (NS) fragment, all at a final concentration of 30 μM in modified Chee's medium. Apoptosis was assessed morphologically.
Discussion
We demonstrate that IFNγ acts synergistically with apoptosis induced by UV-irradiation and the death receptors CD95 and TNFαR, in the primary hepatocyte. We show that effective induction of apoptosis in response to IFNγ requires the cell surface interaction of CD95 with its ligand. In our system, soluble CD95 ligand is released from hepatocyte monolayers treated with IFNγ. Apoptosis induced by UV-irradiation is not associated with soluble CD95 release, suggesting that this is an IFNγ-specific effect. Thus, IFNγ-induced apoptosis in the primary hepatocyte exhibits similar characteristics to apoptosis induced by c-myc overexpression including apoptosis induction in association with serum deprivation (McCullough et al. 2006), synergy with mechanistically distinct apoptotic stimuli, and the requirement of CD95/CD95L interaction. C-myc phosphorothioate fragments, used previously to disrupt c-myc gene function, partially abrogate IFNγ-induced hepatocyte apoptosis, supporting our hypothesis that c-myc, in part, mediates apoptosis in our system.
We show that IFNγ synergizes with apoptosis induced the CD95 and TNFα death receptor pathways, both of which have been implicated as effectors of liver cell injury in vivo (Ogasawara et al. 1993) and in vitro (Shinagawa et al. 1991; Ni et al. 1994; Rouquet et al. 1996). The molecular pathogenesis of this observation may include IFNγ modulation of death receptor expression (Shin et al. 2001a,b), caspase upregulation (O'Connell et al. 2000; Kim et al. 2002) and down-regulation of anti-apoptotic proteins (Varela et al. 2001). Likely multiple pathways act in concert and that tissue and species variations exist.
UV-irradiation is known to induce apoptosis in primary hepatocytes (Schrenk et al. 2004). While much of the dissection of the effector pathway of UV-mediated apoptosis has been performed in keratinocytes, IFNγ- and UV-induced apoptotic pathways may interact at several points. UV-irradiation induces ligand-independent clustering of CD95 (Aragane et al. 1998) and Bax-induced release of mitochondrial cytochrome c and activation of caspase 9 (Esposti 2002; Degli Esposti & Dive 2003), both of which may be downstream events following IFNγ-receptor activation.
We show that CD95 interaction with its ligand is required for effective IFNγ-induced apoptosis in the primary hepatocyte (Figure 3). These results are compatible with IFNγ acting upstream of the cell surface interaction of CD95/CD95L. Our findings that exogenous ligation of CD95 receptors augments IFNγ-apoptosis (Figure 1) suggest that in the presence of IFNγ alone, the CD95 autocrine loop is not maximally activated, but that a low level of CD95 autocrine stimulation occurs in the resting hepatocyte in our culture system. Our findings are compatible with the evolution of higher eukaryotic apoptotic mechanisms around the evolutionarily more primitive death receptors.
Hepatocytes constitutively express CD95 at low levels, which is increased in some pathological states such as chronic active hepatitis and acute liver failure (Galle et al. 1995; Roskams et al. 2000). Soluble CD95 ligand (sCD95L) is generated by alternative splicing of CD95L mRNA, or by proteolytic cleavage of the membrane bound form. There is limited data available on the production of sCD95L by hepatocytes. Soluble CD95L can induce hepatocyte apoptosis in animal models and is significantly elevated in serum from patients with acute hepatic failure (Nakae et al. 2001; Tagami et al. 2003). We demonstrate that sCD95L is detectable in supernatants of primary hepatocyte cultures exposed to IFNγ at 24–48 h. No such increase in seen in supernatants from UV-induced apoptotic hepatocytes, indicating that sCD95L release is a specific response to IFNγ. This is the first report of IFNγ-induced sCD95L production in primary hepatocyte cultures. Although we assume that sCD95L is produce by the hepatocytes, we cannot exclude the possibility that contaminant Kupfer cells or lymphocytes are the source of sCD95L in our model, although we believe this is unlikely. Firstly, daily microscopic inspection on monolayers failed to identify contaminant cells and secondly hepatocytes were maintained in arginine-free media to limit the survival of non-parenchymal cells.
The timing of sCD95L production is noteworthy. Ligand detection is maximal between 24 and 48 h, although hepatocyte apoptosis is not detected until 72 h (McCullough et al. 2006). CD95 induced hepatocyte apoptosis, in the presence of a co-stimulant such as a translation or protein kinase inhibitor, is detectable at 4 h and maximal by 24–30 h (Ni et al. 1994; Rouquet et al. 1996). Soluble CD95L is known to interact with extracellular matrix proteins, and specifically binds fibronectin (Aoki et al. 2001), the substratum in our cell culture model. The retention of sCD95L on the fibronectin layer may conceivably contribute to concentration of the ligand in the cellular microenvironment and subsequently achieving the threshold of CD95 signalling, thus in part explaining the delay between sCD95L production and the triggering of apoptosis.
Isolation and culture of primary hepatocytes is associated with c-myc induction comparable with that observed in mitogen treated cells (Kost & Michalopoulos 1990). This may be an adaptive response to culture conditions, secondary to connective tissue-bound growth factor release during the enzymatic digestion of the extracellular matrix (Bashkin et al. 1989) or a result of increased portal flow during liver perfusion (Isomura et al. 1993). Nonetheless, hepatocyte primary culture offers a model of c-myc induction. C-myc has been widely studied and loss of function achieved using antisense fragments (Heikkila et al. 1987). We utilized a commercially available antisense fragment that targets the initiation codon and is effective in many systems (Heikkila et al. 1987). This fragment decreased IFNγ-induced apoptosis by approximately 30% compared with controls in our study, suggesting a role for c-myc in IFNγ-induced hepatocyte apoptosis. The lack of complete attenuation of the apoptotic response suggests either that c-myc contributes partially to apoptosis in this system or a lack of complete suppression of c-myc expression by the oligonucleotide fragments.
Interferon-gamma is integral to the host defence against viral infection and is a key regulator of inflammation. Inhibition of cell cycle prevents viral replication and apoptosis facilitates the clearing of virally infected cells. Increasing evidence suggests that IFNγ-mediated cell cycle suppression and apoptosis induction may function in tumour suppression (Chawla-Sarkar et al. 2003). We hypothesize that IFNγ acts as a rheostat, sensitizing the hepatocyte to apoptotic stimuli, in part via c-myc. Chronic inflammatory states are associated with tumorigenesis, possibly a result of persistent cytokine signals promoting the survival of cells harbouring gene defects. Hepatocytes containing damaged DNA or dsRNA show an increased susceptibility to IFNγ (Kalai et al. 2002). Tumorigenic cells frequently show deregulation of c-myc expression. Such cells, in the context of persistent hepatic inflammation, may be more susceptible to IFN-mediated apoptosis. This provides the organism with an elegant protective mechanism in which mediation of the inflammatory state by IFNγ, which predisposes to tumorigenesis, is linked with a tumour suppressive potentiation of apoptosis in the liver. The further studies that are required to test this hypothesis may provide insight into the evolution of apoptotic mechanisms in hepatocytes and may contribute toward novel therapeutic strategies in the treatment of chronic hepatitis and tumour formation.
Acknowledgments
CM was supported by the Gordon Fellowship from the Faculty of Medicine, University of Edinburgh and by the Scottish Hospitals Endowment Research Trust (SHERT).
References
- Aoki K, Kurooka M, Chen JJ, Petryniak J, Nabel EG, Nabel GJ. Extracellular matrix interacts with soluble CD95L: retention and enhancement of cytotoxicity. Nat. Immunol. 2001;2:333–337. doi: 10.1038/86336. [DOI] [PubMed] [Google Scholar]
- Aragane Y, Kulms D, Metze D, et al. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J. Cell. Biol. 1998;140:171–182. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Bashkin P, Doctrow S, Klagsbrun M, Svahn CM, Folkman J, Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989;28:1737–1743. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- Burke GW, Cirocco R, Viciana A, et al. Early graft loss secondary to massive hemorrhagic necrosis following orthotopic liver transplantation. Evidence for cytokine-mediated univisceral Shwartzman reaction. Transplantation. 1996;61:1370–1376. doi: 10.1097/00007890-199605150-00015. [DOI] [PubMed] [Google Scholar]
- Chawla-Sarkar M, Lindner DJ, Liu YF, et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M, Dive C. Mitochondrial membrane permeabilisation by Bax/Bak. Biochem. Biophys. Res. Commun. 2003;304:455–461. doi: 10.1016/s0006-291x(03)00617-x. [DOI] [PubMed] [Google Scholar]
- Esposti MD. The roles of bid. Apoptosis. 2002;7:433–440. doi: 10.1023/a:1020035124855. [DOI] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Galle PR, Hofmann WJ, Walczak H, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J. Exp. Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner F, Leist M, Jilg S, Germann PG, Freudenberg MA, Tiegs G. Tumor necrosis factor-induced hepatic DNA fragmentation as an early marker of T cell-dependent liver injury in mice. Gastroenterology. 1995a;109:166–176. doi: 10.1016/0016-5085(95)90282-1. [DOI] [PubMed] [Google Scholar]
- Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology. 1995b;21:190–198. doi: 10.1016/0270-9139(95)90428-x. [DOI] [PubMed] [Google Scholar]
- Harrington EA, Bennett MR, Fanidi A, Evan GI. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO. J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila R, Schwab G, Wickstrom E, et al. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. Nature. 1987;328:445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- Hueber AO, Zornig M, Lyon D, Suda T, Nagata S, Evan GI. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science. 1997;278:1305–1309. doi: 10.1126/science.278.5341.1305. [DOI] [PubMed] [Google Scholar]
- Isomura H, Sawada N, Nakajima Y, et al. Increase in portal flow induces c-myc expression in isolated perfused rat liver. J. Cell. Physiol. 1993;154:329–332. doi: 10.1002/jcp.1041540216. [DOI] [PubMed] [Google Scholar]
- Kalai M, Van Loo G, Vanden Berghe T, et al. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ. 2002;9:981–994. doi: 10.1038/sj.cdd.4401051. [DOI] [PubMed] [Google Scholar]
- Kano A, Watanabe Y, Takeda N, Aizawa S, Akaike T. Analysis of IFN-gamma-induced cell cycle arrest and cell death in hepatocytes. J Biochem (Tokyo) 1997;121:677–683. doi: 10.1093/oxfordjournals.jbchem.a021639. [DOI] [PubMed] [Google Scholar]
- Kim KB, Choi YH, Kim IK, et al. Potentiation of Fas- and TRAIL-mediated apoptosis by IFN-gamma in A549 lung epithelial cells: enhancement of caspase-8 expression through IFN-response element. Cytokine. 2002;20:283–288. doi: 10.1006/cyto.2003.2008. [DOI] [PubMed] [Google Scholar]
- Klefstrom J, Verschuren EW, Evan G. c-Myc augments the apoptotic activity of cytosolic death receptor signaling proteins by engaging the mitochondrial apoptotic pathway. J. Biol. Chem. 2002;277:43224–43232. doi: 10.1074/jbc.M206967200. [DOI] [PubMed] [Google Scholar]
- Kost DP, Michalopoulos GK. Effect of epidermal growth factor on the expression of protooncogenes c-myc and c-Ha-ras in short-term primary hepatocyte culture. J. Cell. Physiol. 1990;144:122–127. doi: 10.1002/jcp.1041440116. [DOI] [PubMed] [Google Scholar]
- McCullough CT, Tura BJ, Harrison DJ. Growth factor attenuation of IFNgamma-mediated hepatocyte apoptosis requires p21waf-1. Int. J. Exp. Pathol. 2006;87:275–281. doi: 10.1111/j.1365-2613.2006.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuhara H, Uno M, Seki N, et al. Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology. 1996;23:1608–1615. doi: 10.1053/jhep.1996.v23.pm0008675184. [DOI] [PubMed] [Google Scholar]
- Nakae H, Narita K, Endo S. Soluble Fas and soluble Fas ligand levels in patients with acute hepatic failure. J. Crit. Care. 2001;16:59–63. doi: 10.1053/jcrc.2001.25470. [DOI] [PubMed] [Google Scholar]
- Ni R, Tomita Y, Matsuda K, et al. Fas-mediated apoptosis in primary cultured mouse hepatocytes. Exp. Cell. Res. 1994;215:332–337. doi: 10.1006/excr.1994.1349. [DOI] [PubMed] [Google Scholar]
- O'Connell J, Bennett MW, Nally K, O'Sullivan GC, Collins JK, Shanahan F. Interferon-gamma sensitizes colonic epithelial cell lines to physiological and therapeutic inducers of colonocyte apoptosis. J. Cell. Physiol. 2000;185:331–338. doi: 10.1002/1097-4652(200012)185:3<331::AID-JCP3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Yamakawa T, Yamamura K, Hino O. Induction of Fas ligand and Fas antigen mRNA expressions in interferon-gamma transgenic mouse liver. Jpn. J. Pharmacol. 1998;78:233–235. doi: 10.1254/jjp.78.233. [DOI] [PubMed] [Google Scholar]
- Ricci MS, Jin Z, Dews M, et al. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol. Cell. Biol. 2004;24:8541–8555. doi: 10.1128/MCB.24.19.8541-8555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskams T, Libbrecht L, Van Damme B, Desmet V. Fas and Fas ligand: strong co-expression in human hepatocytes surrounding hepatocellular carcinoma; can cancer induce suicide in peritumoural cells? J. Pathol. 2000;191:150–153. doi: 10.1002/(SICI)1096-9896(200006)191:2<150::AID-PATH612>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Rouquet N, Carlier K, Briand P, Wiels J, Joulin V. Multiple pathways of Fas-induced apoptosis in primary culture of hepatocytes. Biochem. Biophys. Res. Commun. 1996;229:27–35. doi: 10.1006/bbrc.1996.1753. [DOI] [PubMed] [Google Scholar]
- Sasagawa T, Hlaing M, Akaike T. Synergistic induction of apoptosis in murine hepatoma Hepa1-6 cells by IFN-gamma and TNF-alpha. Biochem. Biophys. Res. Commun. 2000;272:674–680. doi: 10.1006/bbrc.2000.2835. [DOI] [PubMed] [Google Scholar]
- Schrenk D, Schmitz HJ, Bohnenberger S, Wagner B, Worner W. Tumor promoters as inhibitors of apoptosis in rat hepatocytes. Toxicol. Lett. 2004;149:43–50. doi: 10.1016/j.toxlet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shin EC, Ahn JM, Kim CH, et al. IFN-gamma induces cell death in human hepatoma cells through a TRAIL/death receptor-mediated apoptotic pathway. Int. J. Cancer. 2001a;93:262–268. doi: 10.1002/ijc.1310. [DOI] [PubMed] [Google Scholar]
- Shin EC, Shin WC, Choi Y, Kim H, Park JH, Kim SJ. Effect of interferon-gamma on the susceptibility to Fas (CD95/APO-1)-mediated cell death in human hepatoma cells. Cancer. Immunol. Immunother. 2001b;50:23–30. doi: 10.1007/s002620000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa T, Yoshioka K, Kakumu S, et al. Apoptosis in cultured rat hepatocytes: the effects of tumour necrosis factor alpha and interferon gamma. J. Pathol. 1991;165:247–253. doi: 10.1002/path.1711650309. [DOI] [PubMed] [Google Scholar]
- Tagami A, Ohnishi H, Hughes RD. Increased serum soluble Fas in patients with acute liver failure due to paracetamol overdose. Hepatogastroenterology. 2003;50:742–745. [PubMed] [Google Scholar]
- Varela N, Munoz-Pinedo C, Ruiz-Ruiz C, Robledo G, Pedroso M, Lopez-Rivas A. Interferon-gamma sensitizes human myeloid leukemia cells to death receptor-mediated apoptosis by a pleiotropic mechanism. J. Biol. Chem. 2001;276:17779–17787. doi: 10.1074/jbc.M100815200. [DOI] [PubMed] [Google Scholar]