Abstract
This was a study on the oxidative stress due to ischaemia (I) and reperfusion (R) in skeletal muscle tissue. Using a tourniquet, groups of rats were submitted to ischaemia for 4 h, followed by different reperfusion periods. The animals were divided in four groups: control; 4 h of ischaemia (IR); 4 h of ischaemia plus 1 h reperfusion (IR-1 h); 4 h of ischaemia plus 24 h reperfusion (IR-24 h); and 4 h of ischaemia plus 72 h reperfusion (IR-72 h). At the end of the procedures, samples of soleus muscle were collected and frozen in n-hexane at −70 °C. Cryostat sections were submitted to haematoxylin–eosin, succinate dehydrogenase (SDH) and nicotinamide adenine dinucleotide-tetrazolium reductase (NADH-TR) stains. An additional muscle sample was processed for electron microscopy. No alterations were found in control animals. IR group showed fibres had normal aspect besides some round, acidophilic and hypertrophic fibres. There were several fibres with angular outlines and smaller diameters in this group compared with control group. NADH-TR/SDH reaction was moderately intense in most fibres. In some fibres, cytoplasm showed areas without activity and other fibres had very intense reactivity. IR-1 h group showed oedema hypercontracted fibres with disorganized myofibrils, mitochondria with focal lesions and dilated sarcoplasmic reticulum. NADH-TR/SDH reaction was moderate to weak. IR-24 h showed intense inflammatory infiltrate in the endomysium and perimysium. NADH-TR/SDH reaction was similar to IR-1 h. IR-72 h showed necrotic fibres, areas with inflammatory infiltrate, reduced muscle fibres at different stages of necrosis and phagocytosis, and many small round and basophilic fibres characterizing a regeneration process. NADH-TR/SDH reaction was weak to negative. Our results suggest that ischaemia and the subsequent 1-, 24- and 72-h reperfusions induced progressive histological damage. Although progressive, it may be reversible because there were ultrastructural signs of recovery after 72-h reperfusion. This recovery could in part be due to the low oxidative stress identified by the morphological and histochemical analysis.
Keywords: ischaemia, morphology, muscle, rat, reperfusion, soleus
Skeletal muscular tissue is particularly resistant to lesions, being able to bear long periods of ischaemia (Miller & Welch 1949; Hughes 1957; Thompsom et al. 1970; Blaisdell et al. 1978; Matsen et al. 1980; Mubarak & Hargens 1983; Bushell et al. 2002). However, experimental reports show morphological alterations in skeletal muscle after different periods of ischaemia (Fishback & Fishback 1932; Le Gros Clark & Blomfield 1945; Harmam 1947; Copenhaver et al. 1956; Dahlback & Rais 1966; Karpatti et al. 1974; Sardinha 1994). In the morphological study of skeletal musculature, several ischaemia and reperfusion experimental models have been described: canine gracilis muscle (Kuzon et al. 1986), rat cremaster muscle (Suval et al. 1987), canine hind paw muscle (Diana & Laughlin 1974), rat isolated hind paw muscle (Beyersdorf et al. 1989; Sexton et al. 1990) and the tourniquet model applied to the source of rat hind paw (Strock & Majno 1969; Zimiani et al. 2005). Morphological muscle studies in animals submitted to 5-h ischaemia did not show irreversible cellular lesions (Dahlback & Rais 1966; Harris et al. 1986). However, 2 h after ischaemia, it is already possible to identify, with histochemical analysis, small muscular lesions which become severe according to the ischaemia duration (Makitie & Teravainen 1977).
Although literature reports few histological alterations in muscular tissue at the initial stage of ischaemia, they do show severe alterations after circulation re-establishment (Malan & Tatoni 1963; Parks & Granger 1986; Perry & Wadhwa 1988; Korthuis et al. 1989; Haimovici 1990; Wang et al. 2005).
Several factors contribute to the induction of muscular lesions after ischaemia and reperfusion; they include muscle type, duration of ischaemia/reperfusion and procedure to induce the insults. The present study aimed to describe histochemical and morphological alterations induced by ischaemia and different periods of reperfusion in rat skeletal muscle tissue.
Methods
Twenty-five adult male Wistar rats, weighing 250–300 g, were divided into five groups of five animals each: control (C); 4 h of ischaemia (I); 4 h of ischaemia plus 1 h of reperfusion (IR-1 h), 4 h of ischaemia plus 24 h of reperfusion (IR-24 h); and 4 h of ischaemia plus 72 h of reperfusion (IR-72 h). Animals were obtained from the Animal House of the Biological Sciences Center at the Universidade Estadual de Londrina where they were bred. They were transferred to the Animal House of Department of Pathological Sciences and kept in polyethylene cages with a stainless steel cover, under controlled temperature and ventilation and a 12-h photoperiod. Animals had free access to water and commercial food during the course of the experimental procedures. The experimental protocol was approved by the Ethics Committee in Animal Experimentation of Botucatu School of Medicine UNESP-Botucatu Campus.
To induce ischaemia, an Esmarch bandage was used in the left hind limb (Concannon et al. 1992) and a rubber tourniquet was applied to the limb root. After 4 h, the tourniquet was removed and the limb reperfused for 0, 1, 24 or 72 h. At the end of protocol, the ischaemic/reperfused soleus muscles were excised from animals killed under pentobarbital sodium (200 mg/kg body weight). After collection, the muscles were frozen in n-hexane at −70 °C cooled in liquid nitrogen for 2 min. Several series of histological sections (10 μm) were made using a cryostat microtome at −20 °C, and were stained with haematoxylin–eosin; others were submitted to nicotinamide adenine dinucleotide-tetrazolium reductase (NADH-TR) and succinate dehydrogenase (SDH) (Dubowitz & Brooke 1973; Loughin 1992), to evaluate the muscle metabolic pattern. Other muscle fragments were immersed in 2.5% glutaraldehyde in 0.1 M, pH 7.4 phosphate buffer, processed and submitted to ultrastructural analysis.
Results
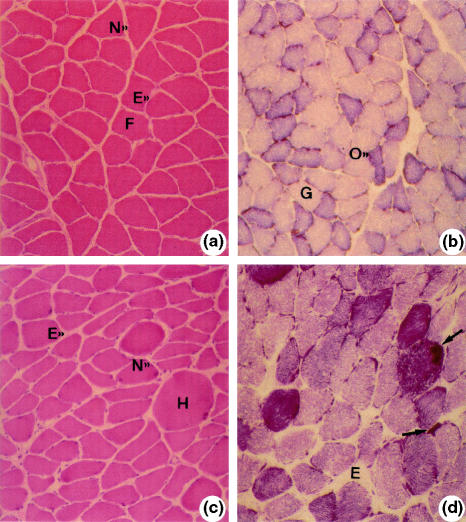
Analysis of control group showed polygonal or round fibres and peripheral nuclei. The fibres were grouped in normal pattern fascicles (Figure 1a). Analysis of NADH-TR/SDH (oxidative metabolism) pattern revealed fibres with intense, moderate and weak reactivity (Figure 1b).
Figure 1.
Soleus muscle: a and b (control). Normal muscle fibres (F). Peripheral nucleus (N). Endomysium (E). Haematoxylin–eosin (HE) 40×; b, oxidative (O) and glycolytic fibres (G). SDH 40×; c (ischaemia of 4 h). Round hypertrophic fibre (H). Nucleus (N). Endomysium (E). HE 40×; d (ischaemia of 4 h). Irregular distribution of oxidative metabolism enzymes: formazan rough granulations (arrows). Oedema (E), NADH-TR, 40×.
After 4 h of ischaemia (I), fibres had normal aspect besides round acidophilic fibres, some had hypertrophic aspect, and there were several fibres with angular outlines and smaller diameters compared with controls (Figure 1c). NADH-TR/SDH reactions were moderately reactive in most fibres. In some fibres, cytoplasm showed areas with no activity and in other areas with amorphous formazan aggregates – especially in the peripheral region. Other fibres had very intense reactivity, with reaction products distributed in amorphous aggregates and/or strongly stained cytoplasm regions (Figure 1d).
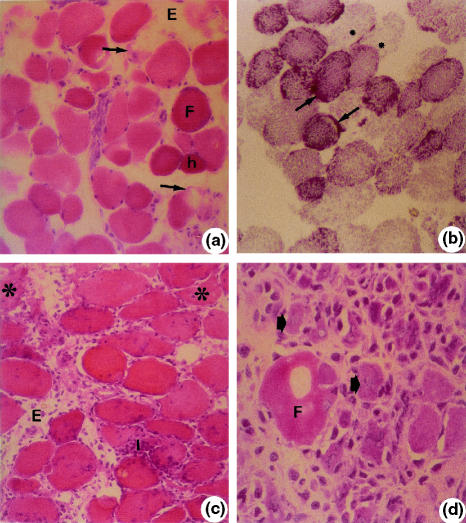
After 4 h of ischaemia and 1 h of reperfusion (IR-1 h), muscular tissue showed oedema. Most fibres had varying degrees of hypercontraction with round outlines, some with areas of no cytoplasmic material and others in advanced necrosis (Figure 2a). In this group, NADH-TR/SDH reactions were moderate to weak in most fibres. Some fibres showed very intense reactions with clusters or amorphous formazan aggregates as well as some areas with no oxidative activity (Figure 2b).
Figure 2.
Soleus muscle. a (IR-1 h). Round (F). Hypercontracted fibres (h). Fibre with myofibril disorganization (arrows). Oedema (E). Haematoxylin–eosin (HE) 40×; b (IR-1 h). Round fibres with irregular distribution of oxidative metabolism enzymes (granulations) (arrows). Lack of enzyme activity (*). NADH-TR 40×; c (IR-24 h). Intense inflammatory infiltrate (I). Oedema (E). Necrotic fibres (*). HE, 40×, d (IR-72 h). Necrotic fibre (F). Small basophilic fibres with central nucleus (arrows). HE, 100×.
After 4 h of ischaemia and 24 h of reperfusion (IR-24 h), muscles had more pronounced morphological alterations with less edematous tissue, intense inflammatory infiltrate, fibres with round outlines with high variation in diameters; some were also hyperstained. Other fibres had areas with no cytoplasmic material and other areas in necrosis (Figure 2c). NADH-TR/SDH reactions were similar to that observed in 4 h of ischaemia and 1 h of reperfusion.
After 4 h of ischaemia and 72 h of reperfusion (IR-72 h), muscle fibres were in necrosis, there was a less pronounced inflammatory infiltrate in the connective tissue, and several fibres had basophilic cytoplasm and central nucleus (Figure 2d). Reactivity to NADH-TR/SDH reactions was weak (data not shown).
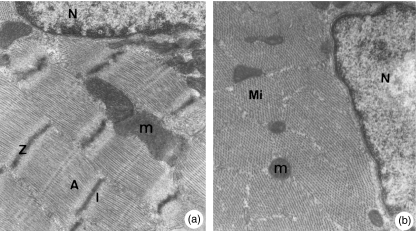
Ultrastructural analysis from control group showed muscle fibre with normal aspects (Figure 3a,b). After 4 h of ischaemia (I), muscle fibres presented normal myofibrils, but there were mitochondria with condensation in cristae and others in oedema with decreased number of cristae (Figure 4a). Animals submitted to IR-1 h showed hypercontracted myofibrils and clusters of mitochondria in the fibre peripheral region. The mitochondria had disorganized cristae concentrically arranged or forming electron-dense aggregates. In the fibre peripheral region, there were areas with no myofibrils and swelling sarcoplasmatic reticulum (Figure 4b). Animals submitted to IR-24 h showed highly disorganized myofibrils and mitochondria of different diameters and round outlines containing electron-dense granules. Also some mitochondria showed areas with no cristae and others had areas with electron-dense cristae aggregates (Figure 4c).
Figure 3.
a, b. Soleus muscle (control). Muscle fibres with normal aspects. Nucleus (N). Mitochondria (m). Myofibrils showing sarcomers with Z, A and I lines. a: 21,000× and b: 27,000×.
Figure 4.
Soleus muscle. a (ischaemia of 4 h). Myofibrils (Mi). Normal mitochondria (m). Edematous mitochondria with reduced cristae (*). 21,000×. b (IR-1 h). Disorganized myofibrils (Mi). Mitochondria with electron-dense material clusters in their interior (m). Fibre area with no myofibrils (*). Swelling vesicles (V). 27,000×. c (IR-24 h). Disorganized myofibrils (Mi). Electron-dense material clusters in mitochondria (m). Mitochondria with reduced cristae (*). 14,500×. d (IR-72 h). Multinucleated muscle fibre in regeneration. Nuclei (N), mitochondria (arrows). Contractile myofilaments (arrow head), 7250×.
Animals submitted to IR-72 h showed fibres in regeneration with several central nuclei, loose chromatin and evident nucleolus. The sarcoplasma showed myofibrils in organization, mitochondria and areas without myofibrils (Figure 4d).
Discussion
It is well recognized that ischaemia followed by reperfusion (IR) in skeletal muscle represents an important clinical problem in many vascular diseases and musculoskeletal trauma. The significant mortality and morbidity can be due to compartment syndrome, rhabdomyolysis, renal failure, limb loss, systemic inflammatory syndrome and respiratory and mesenteric injuries. It has been emphasized the importance of ischaemia duration as a progressive increase of ultrastructural lesions takes place between 2- and 7-h ischaemia insult in skeletal muscular tissues (Harris et al. 1986). Regarding reperfusion injury, several reports have showed that a period over 2 h of reperfusion is enough to establish the muscular lesion (Strock & Majno 1969; Santavirta et al. 1979; Long et al. 1989; Moreno 1991; Sternbergh & Adelman 1992; Appell et al. 1997; Cruz et al. 1997).
In the current study, we showed that the morphological injury severity induced by ischaemia of 4 h increased progressively according to perfusion duration. A slow twitch muscle with aerobic metabolism, soleus, was selected (Bigard et al. 1998) because it is considered a muscle more sensitive to ischaemia when compared with the fast twitch muscle, such as gastrocnemius (Karpatti et al. 1974; Jennische et al. 1979). In fact, gastrocnemius has both oxidative and glycolytic metabolism and it is composed by a heterogeneous mixture of type 1 and type 2 muscle fibres. On the other hand, soleus predominantly has type 1 muscle fibres (slow twitch) with an oxidative metabolism and therefore more sensitive to changes of oxygen providing, when compared with gastrocnemius.
After 4 h of ischaemia (I), the muscle showed round hyaline and hypertrophic fibres. These findings are in accordance with other studies with similar ischaemia periods (Karpatti et al. 1974; Jennische et al. 1979; Labbe et al. 1987; Lindsay et al. 1990).
The structural changes identified in IR-1 h group were previously also described in tibialis anterior muscle of rats. The authors reported loss of structural integrity, areas of hypercontraction at light microscopy and also dilated sarcoplasmatic reticulum and disruption of myofibril structure at electron-microscopic examination (Bushell et al. 2002).
The intense inflammatory infiltrate and disruption of myofibril structure identified in IR-24 h group are in agreement with another study that subjected skeletal muscle of mice to a similar model (Wiersema et al. 2000). Necrosis areas identified in animal muscles from IR-24 h were also described after 48 h of reperfusion in rat thigh flap submitted to 4-h ischaemia (Marian et al. 2005).
The morphological alterations of IR-72 h group corresponded to a remarkable regeneration. In fact, both histological (several fibres with basophilic cytoplasm and central nucleus and some multinucleated fibres) and ultrastructural examinations (fibres in regeneration with several central nuclei, loose chromatin and evident nucleolus; the sarcoplasma showed myofibrils in organization, mitochondria and areas without myofibrils) characterize fibres in regeneration process. Our results are in accordance with other investigators that described areas with inflammatory infiltrate, phagocytosis and other regeneration characteristics in skeletal muscle rodents submitted to IR-72 h (Harralson et al. 2005) and IR-192 h (Wiersema et al. 2000). Other study using soleus and gastrocnemius submitted to lower ischaemia period (2 h) also showed necrotic fibres with phagocytosis and some regeneration after 18 h of reperfusion (Karpatti et al. 1974).
Reports have shown a relationship among insults of IR, alterations in tissue oxygen metabolism and cellular changes (Santavirta et al. 1979; Carvalho et al. 1997; Troitzsch et al. 2005; Zimiani et al. 2005). Impairment between free radicals and antioxidant production lead to oxidative stress. This mechanism is often related to the cell degeneration process. In the current study, we did identify a pronounced inflammatory infiltrate (IR-1 h, IR-24 h), which could stimulate free radical production and therefore the oxidative stress. In addition, it was observed a progressive morphological and histochemical degeneration from IR-1 h until IR-24 h. The severity degree was accompanied by decrease of oxidative metabolism (NADH-TR/SDH), showing moderate (I, IR-1 h, IR-24 h) to weak (IR-72 h) reactivity. The change of oxidative metabolism was specially observed between IR-24 h and IR-72 h, indicating an oxidative stress reduction. This statement is supported by fibres in regeneration process identified morphologically at 72nd hour of reperfusion. Besides the less pronounced inflammatory infiltrate, we also observed weak reactivity by histochemical analyses at IR-72 h. These conditions may represent an environment of low oxidative stress. Previous studies have shown that oxidative stress leads to progressive cell changes, but the antioxidant supplementation can attenuate this process (Marchetti et al. 1999; McCord et al. 2000). Metabolism and IR injury also have been studied in skeletal muscles by biochemical analysis and no alterations were observed when the evaluation occurs immediately after 4 h of ischaemia period (Korthuis et al. 1985), but changes appear after 2 h of reperfusion (Troitzsch et al. 2005), suggesting a progressive metabolism alteration.
In our study, we used an aerobic/oxidative muscle. After 4 h of ischaemia and 1 h of reperfusion, the soleus muscle showed interstitial oedema and round fibres. In contrast, other authors described pronounced lesions in mouse soleus muscle after 90 min of ischaemia and 1 h of reperfusion, these were: intense oedema in the interstitial space, large number of necrotic fibres, rupture of capillaries and endothelial oedema (Appell et al. 1997). On the other hand, it was not identified morphological alterations in canine gracilis muscle after 3 h of ischaemia and 2 h of reperfusion, suggesting that resistance to the ischaemia (Cruz et al. 1997) is probably related to its metabolism type (glycolytic).
We identified in the IR-24 h group, morphological lesions represented by intense inflammatory infiltrate in the endomysium and perimysium, and pronounced necrosis intense inflammatory infiltrate. It is in accordance with Hirose et al. (1997) that also identified severe lesions in the gastrocnemius muscle using a 4 h of ischaemia and 24 h of reperfusion model. Racz et al. (1997) found similar morphological alterations in the soleus muscle after 2- and 24-h reperfusion using 1 and 2 h of ischaemia. In the IR-72 h group, in spite of the presence of some muscle fibres in degeneration, many muscular fibres in regeneration were identified. It was reported that muscles submitted to short periods of ischaemia (1 h) regenerated faster than those submitted to longer (2 h) periods (Racz et al. 1997). The authors suggested that this fact may be associated to the soleus phenotypic characteristics. Our results showed a progressive increase in lesions in soleus muscle after 4 h of ischaemia and 1, 24 and 72 h reperfusion. However, these results seem to be influenced by muscle type, ischaemia and reperfusion periods. Similar to results in the literature, our study showed that the soleus muscle was sensitive to ischaemia and reperfusion, but it revealed remarkable regenerative characteristics after 72 h of reperfusion. Our results suggest that ischaemia and the subsequent reperfusion for 1, 24 and 72 h induced progressive morphological damage that may not be irreversible due to signs of muscle regeneration identified at 72nd hour. This regeneration can be partly due to the low oxidative stress identified by morphological and histochemical assessment.
Acknowledgments
We thank to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support and Sueli Cruz Michelin and Pedro Dionísio for technical assistance. This work is part of the PhD thesis presented to UNESP – Botucatu in 2002.
References
- Appell HJ, Duarte JA, Gloser S, et al. Administration of tourniquet. II. Prevention of postischemic oxidative stress can reduce muscle edema. Arch. Orthop. Trauma Surg. 1997;116:101–105. [PubMed] [Google Scholar]
- Beyersdorf F, Matheis G, Kruger S, et al. Avoiding reperfusion injury after limb revascularization: experimental observations and recommendations for clinical application. J. Vasc. Surg. 1989;6:757–766. [PubMed] [Google Scholar]
- Bigard AX, Boehm E, Veksler V, Mateo P, Anflous K, Ventura-Clapier R. Muscle unloading induces slow to fast transitions in myofibrillar but not mitochondrial properties. Relevance to skeletal muscle abnormalities in heart failure. J. Mol. Cell. Cardiol. 1998;30:2391–2401. doi: 10.1006/jmcc.1998.0798. [DOI] [PubMed] [Google Scholar]
- Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84:822–834. [PubMed] [Google Scholar]
- Bushell AJ, Klenerman L, Davies H, Grierson I, McArdle A, Jackson MJ. Ischaemic preconditioning of skeletal muscle 2. Investigation of the potential mechanisms involved. J. Bone Joint Surg. 2002;84:1189–1193. doi: 10.1302/0301-620x.84b8.9362. [DOI] [PubMed] [Google Scholar]
- Carvalho AJ, McKee NH, Green HJ. Metabolic and contractile responses of fast and slow twitch rat skeletal muscles to ischemia and reperfusion. Plast. Reconstr. Surg. 1997;99:163–171. doi: 10.1097/00006534-199701000-00025. [DOI] [PubMed] [Google Scholar]
- Concannon MJ, Kester CG, Welsh CF, Puckett CL. Patterns of free radical production after tourniquet ischemia: implications for the hand surgeon. Plast. Reconstr. Surg. 1992;89:846–852. doi: 10.1097/00006534-199205000-00012. [DOI] [PubMed] [Google Scholar]
- Copenhaver WM, Moore DH, Ruska H. Electron microscopic and histochemical observations of muscle degeneration after tourniquet. J. Biophys. Biochem. Cytol. 1956;2:755–763. doi: 10.1083/jcb.2.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz CAT, Massuda CA, Cherri J, Piccinato CE. Metabolic alterations of skeletal muscle during ischemia and reperfusion. J. Cardiovasc. Surg. 1997;38:473–477. [PubMed] [Google Scholar]
- Dahlback L, Rais O. Morphological changes in striated muscle following ischemia: immediate postischemic phase. Acta Chir. Scand. 1966;131:430–440. [PubMed] [Google Scholar]
- Diana JN, Laughlin MH. Effect of ischemia on capillary pressure and equivalent pore radius in capillaries of the isolated dog hind limb. Circ. Res. 1974;35:77–101. doi: 10.1161/01.res.35.1.77. [DOI] [PubMed] [Google Scholar]
- Dubowitz V, Brooke MH. Muscle Biopsy: A Modern Approach. London: W.B. Saunders; 1973. p. 475. [Google Scholar]
- Fishback DK, Fishback HR. Studies of experimental muscle degeneration. I. Factors in the production of muscle degeneration. Am. J. Pathol. 1932;8:193–214. [PMC free article] [PubMed] [Google Scholar]
- Haimovici H. Ischemia–reperfusion syndrome of skeletal muscle. J. Cardivasc. Surg. 1990;31:318–319. [PubMed] [Google Scholar]
- Harmam JW. A histological study of skeletal muscle in acute ischemia. Am. J. Pathol. 1947;23:551–565. [PMC free article] [PubMed] [Google Scholar]
- Harralson T, Grossi FV, Quan EE, et al. Ischemic preconditioning of skeletal muscle: duration of late-phase protection. Ann. Plast. Surg. 2005;55:216–222. doi: 10.1097/01.sap.0000164578.85395.c7. [DOI] [PubMed] [Google Scholar]
- Harris K, Walker PM, Mickle DAG, et al. Metabolic response of skeletal muscle to ischemia. Am. J. Physiol. 1986;250:213–220. doi: 10.1152/ajpheart.1986.250.2.H213. [DOI] [PubMed] [Google Scholar]
- Hirose J, Yamaga M, Ide J, Tanoue M, Takagi K. Reduced ischemia–reperfusion injury in muscle. Acta Orthop. Scand. 1997;68:369–373. doi: 10.3109/17453679708996179. [DOI] [PubMed] [Google Scholar]
- Hughes CW. Arterial repair during the Korean war. Ann. Surg. 1957;147:555–561. [PMC free article] [PubMed] [Google Scholar]
- Jennische E, Amundson B, Haljamae H. Metabolic responses in feline red and white skeletal muscle to shock and ischemia. Acta Physiol. Scand. 1979;106:39–45. doi: 10.1111/j.1748-1716.1979.tb06367.x. [DOI] [PubMed] [Google Scholar]
- Karpatti G, Carpenter S, Melmed C, Eisen AA. Experimental ischemic myopathy. J. Neurol. Sci. 1974;23:129–161. doi: 10.1016/0022-510x(74)90148-8. [DOI] [PubMed] [Google Scholar]
- Korthuis RJ, Granger DN, Towmsley MI, Taylor AK. The role oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ. Res. 1985;57:599–609. doi: 10.1161/01.res.57.4.599. [DOI] [PubMed] [Google Scholar]
- Korthuis RJ, Smith JK, Carden DL. Hypoxic reperfusion attenuates postischemic microvascular injury. Am. J. Physiol. 1989;256:315–319. doi: 10.1152/ajpheart.1989.256.1.H315. [DOI] [PubMed] [Google Scholar]
- Kuzon W, Walker P, Mickle D, Harris K, Pynn B, Mckee N. An isolated skeletal muscle model suitable for acute ischemia and reperfusion injury. J. Surg. Res. 1986;41:28–37. doi: 10.1016/0022-4804(86)90004-1. [DOI] [PubMed] [Google Scholar]
- Labbe R, Lindsay T, Walker PM. The extent and distribution of skeletal muscle necrosis after graded periods of complete ischemia. J. Vasc. Surg. 1987;6:152–157. doi: 10.1067/mva.1987.avs0060152. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark WE, Blomfield LB. The efficiency of intramuscular anastomoses with observations on the regeneration of devascularized muscle. J. Anat. 1945;79:15–32. [PMC free article] [PubMed] [Google Scholar]
- Lindsay TJ, Liauw S, Romaschin AD, Walker PM. The effect of ischemia/reperfusion on adenine nucleotide metabolism and xanthine oxidase production in skeletal muscle. J. Vasc. Surg. 1990;12:8–15. doi: 10.1067/mva.1990.19946. [DOI] [PubMed] [Google Scholar]
- Long JW, Jr, Laster JL, Stevens RP, Silver WP, Silver D. Contractile and metabolic function following an ischemia–reperfusion injury in skeletal muscle: influence of oxygen free radical scavengers. Microcirc. Endothelium Lymphatics. 1989;5:351–363. [PubMed] [Google Scholar]
- Loughin M. Muscle Biopsy: A Laboratory Investigation. London: Butterworth-Heinemann; 1992. p. 241. [Google Scholar]
- McCord JM. The evolution of free radicals and oxidative stress. Am. J. Med. 2000;108:652–659. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- Makitie J, Teravainen H. Histochemical studies of striated muscle after temporary ischemia in the rat. Acta Neuropathol. 1977;37:247–253. doi: 10.1007/BF00692055. [DOI] [PubMed] [Google Scholar]
- Malan E, Tatoni G. Physio- and anatomopathology of acute ischemia of the extremities. J. Cardiovasc. Surg. 1963;4:2–10. [PubMed] [Google Scholar]
- Marchetti G, Lodola E, Licciardello L, Colombo A. Use of N-acetylcysteine in the management of coronary artery diseases. Cardiologia. 1999;44:633–637. [PubMed] [Google Scholar]
- Marian CF, Jiga LP, Ionac M. Ischemic preconditioning of free muscle flaps: an experimental study. Microsurgery. 2005;25:524–531. doi: 10.1002/micr.20158. [DOI] [PubMed] [Google Scholar]
- Matsen FA, Winquist RA, Krugmire RB. Diagnoses and management of compartmental syndromes. J. Bone Joint Surg. Am. 1980;62:286–291. [PubMed] [Google Scholar]
- Miller HH, Welch CS. Quantitative studies on the time factor in arterial injuries. Am. Surg. 1949;130:428–438. [PMC free article] [PubMed] [Google Scholar]
- Moreno JB. Efeito do alfa-tocoferol na atenuação das lesões de isquemia–reperfusão em membro posterior de rato. Dissertação. Botucatu: Faculdade de Medicina, Universidade Estaudal Paulista; 1991. [Google Scholar]
- Mubarak SJ, Hargens AR. Acute compartment syndromes. Surg. Clin. North Am. 1983;63:539–569. doi: 10.1016/s0039-6109(16)43030-6. [DOI] [PubMed] [Google Scholar]
- Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am. J. Physiol. 1986;250:749–753. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- Perry MA, Wadhwa SS. Gradual reintroduction of oxygen reduces reperfusion injury in cat stomach. Am. j. Physiol. Gastrointest. Liver physiol. 1988;254:366–312. doi: 10.1152/ajpgi.1988.254.3.G366. [DOI] [PubMed] [Google Scholar]
- Racz IB, Illyes G, Sarkadi L, Hamar J. The functional and morphological damage of ischemic reperfused skeletal muscle. Eur. Surg. Res. 1997;29:254–263. doi: 10.1159/000129531. [DOI] [PubMed] [Google Scholar]
- Santavirta S, Luoma A, Arstila AU. Morphological and biochemical changes in rabbit striated muscle of after experimental tourniquet ischemia. Res. Exp. Med. 1979;174:245–251. doi: 10.1007/BF01851416. [DOI] [PubMed] [Google Scholar]
- Sardinha WE. Isquemia e reperfusão da musculatura esquelética em ratos. Inibição das lesões lipoperoxidativas mediadas por radicais livres pela desferroxamina. Tese. São Paulo: Escola Paulista de Medicina; 1994. [Google Scholar]
- Sexton WJ, Kortius RJ, Laughin MH. Ischemia–reperfusion isolated rat hindquarters. J. Appl. Physiol. 1990;68:387–392. doi: 10.1152/jappl.1990.68.1.387. [DOI] [PubMed] [Google Scholar]
- Sternbergh WC, Adelman B. Skeletal muscle fiber type does not predict sensitivity to postischemic damage. J. Surg. Res. 1992;53:535–541. doi: 10.1016/0022-4804(92)90103-7. [DOI] [PubMed] [Google Scholar]
- Strock PE, Majno G. Vascular responses to experimental tourniquet ischemia. Surg. Gynecol. Obstet. 1969;129:309–318. [PubMed] [Google Scholar]
- Suval WD, Duram WN, Boriá MP, Hobson RW, Berendsen PB, Ritter AB. Microvascular transport and endothelial cell alterations preceding skeletal muscle damage in ischemia and reperfusion injury. Am. J. Surg. 1987;154:211–218. doi: 10.1016/0002-9610(87)90181-4. [DOI] [PubMed] [Google Scholar]
- Thompsom JE, Singler L, Raut PS, Austin DJ, Patman RD. Arterial embolectomy: a 20 years experience with 163 cases. Surgery. 1970;67:212–220. [PubMed] [Google Scholar]
- Troitzsch D, Vogt S, Abdul-Khaliq H, Moosdorf R. Muscle tissue oxygen tension and oxidative metabolism during ischemia and reperfusion. J. Surg. Res. 2005;128:9–14. doi: 10.1016/j.jss.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Fang XH, Stephenson LL, Bajnosa RC, Khiabani KT, Zamboni WA. Microcirulatory effects of melatonin in rat skelatal muscle after prolonged ischemia. J. Pineal. Res. 2005;39:57–65. doi: 10.1111/j.1600-079X.2005.00215.x. [DOI] [PubMed] [Google Scholar]
- Wiersema AM, Oyen WJ, Dirksen R, Verhofstad AA, Corstens FH, van der Vliet JA. Early assessment of skeletal muscle damage after ischaemia–reperfusion injury using Tc-99 m-glucarate. Cardiovasc. Surg. 2000;8:186–191. doi: 10.1016/s0967-2109(00)00007-7. [DOI] [PubMed] [Google Scholar]
- Zimiani K, Guarnier FA, Miranda HC, Watanabe MA, Cecchini R. Nitric oxide mediated oxidative stress injury in rat skeletal muscle subjected to ischemia/reperfusion as evaluated by chemiluminescence. Nitric Oxide. 2005;13:196–203. doi: 10.1016/j.niox.2005.07.002. [DOI] [PubMed] [Google Scholar]