Abstract
In recent years, avian systematics has been characterized by a diminished reliance on morphological cladistics of modern taxa, intensive palaeornithogical research stimulated by new discoveries and an inundation by analyses based on DNA sequences. Unfortunately, in contrast to significant insights into basal origins, the broad picture of neornithine phylogeny remains largely unresolved. Morphological studies have emphasized characters of use in palaeontological contexts. Molecular studies, following disillusionment with the pioneering, but non-cladistic, work of Sibley and Ahlquist, have differed markedly from each other and from morphological works in both methods and findings. Consequently, at the turn of the millennium, points of robust agreement among schools concerning higher-order neornithine phylogeny have been limited to the two basalmost and several mid-level, primary groups. This paper describes a phylogenetic (cladistic) analysis of 150 taxa of Neornithes, including exemplars from all non-passeriform families, and subordinal representatives of Passeriformes. Thirty-five outgroup taxa encompassing Crocodylia, predominately theropod Dinosauria, and selected Mesozoic birds were used to root the trees. Based on study of specimens and the literature, 2954 morphological characters were defined; these characters have been described in a companion work, approximately one-third of which were multistate (i.e. comprised at least three states), and states within more than one-half of these multistate characters were ordered for analysis. Complete heuristic searches using 10 000 random-addition replicates recovered a total solution set of 97 well-resolved, most-parsimonious trees (MPTs). The set of MPTs was confirmed by an expanded heuristic search based on 10 000 random-addition replicates and a full ratchet-augmented exploration to ascertain global optima. A strict consensus tree of MPTs included only six trichotomies, i.e. nodes differing topologically among MPTs. Bootstrapping (based on 10 000 replicates) percentages and ratchet-minimized support (Bremer) indices indicated most nodes to be robust. Several fossil Neornithes (e.g. Dinornithiformes, Aepyornithiformes) were placed within the ingroup a posteriori either through unconstrained, heursitic searches based on the complete matrix augmented by these taxa separately or using backbone-constraints. Analysis confirmed the topology among outgroup Theropoda and achieved robust resolution at virtually all levels of the Neornithes. Findings included monophyly of the palaeognathous birds, comprising the sister taxa Tinamiformes and ratites, respectively, and the Anseriformes and Galliformes as monophyletic sister-groups, together forming the sister-group to other Neornithes exclusive of the Palaeognathae (Neoaves). Noteworthy inferences include: (i) the sister-group to remaining Neoaves comprises a diversity of marine and wading birds; (ii) Podicipedidae are the sister-group of Gaviidae, and not closely related to the Phoenicopteridae, as recently suggested; (iii) the traditional Pelecaniformes, including the shoebill (Balaeniceps rex) as sister-taxon to other members, are monophyletic; (iv) traditional Ciconiiformes are monophyletic; (v) Strigiformes and Falconiformes are sister-groups; (vi) Cathartidae is the sister-group of the remaining Falconiformes; (vii) Ralliformes (Rallidae and Heliornithidae) are the sister-group to the monophyletic Charadriiformes, with the traditionally composed Gruiformes and Turniciformes (Turnicidae and Mesitornithidae) sequentially paraphyletic to the entire foregoing clade; (viii) Opisthocomus hoazin is the sister-taxon to the Cuculiformes (including the Musophagidae); (ix) traditional Caprimulgiformes are monophyletic and the sister-group of the Apodiformes; (x) Trogoniformes are the sister-group of Coliiformes; (xi) Coraciiformes, Piciformes and Passeriformes are mutually monophyletic and closely related; and (xii) the Galbulae are retained within the Piciformes. Unresolved portions of the Neornithes (nodes having more than one most-parsimonious solution) comprised three parts of the tree: (a) several interfamilial nodes within the Charadriiformes; (b) a trichotomy comprising the (i) Psittaciformes, (ii) Columbiformes and (iii) Trogonomorphae (Trogoniformes, Coliiformes) + Passerimorphae (Coraciiformes, Piciformes, Passeriformes); and (c) a trichotomy comprising the Coraciiformes, Piciformes and Passeriformes. The remaining polytomies were among outgroups, although several of the highest-order nodes were only marginally supported; however, the majority of nodes were resolved and met or surpassed conventional standards of support. Quantitative comparisons with alternative hypotheses, examination of highly supportive and diagnostic characters for higher taxa, correspondences with prior studies, complementarity and philosophical differences with palaeontological phylogenetics, promises and challenges of palaeogeography and calibration of evolutionary rates of birds, and classes of promising evidence and future directions of study are reviewed. Homology, as applied to avian examples of apparent homologues, is considered in terms of recent theory, and a revised annotated classification of higher-order taxa of Neornithes and other closely related Theropoda is proposed. © 2007 The Linnean Society of London, Zoological Journal of the Linnean Society, 2007, 149, 1–95.
Keywords: Aves, cladistics, classification, convergence, homology, morphology, ontogeny, palaeontology, phylogenetics, Neornithes, taxonomy
INTRODUCTION
‘But as far as the problem of the relationship of the orders of birds is concerned, so many distinguished investigators have labored in this field in vain, that little hope is left for spectacular break-throughs.’ (Stresemann, 1959: 277)
‘It must be remembered that the basic avian structure was determined at an early stage in the evolutionary history of birds because of the rigorous limitations placed upon a flying vertebrate. Consequently, adaptations in the birds have been along lines that are not always indicated by the details of anatomy, a fact that makes these vertebrates highly interesting to the student of recent animals but difficult subjects for the palaeontologist.’ (Colbert, 1980: 187)
Maturation of avian phylogenetics
Confines of tradition
The opening quotation from Colbert (1980) clearly articulates a fundamental assumption of functional constraint under which many avian systematists laboured for more than a century (Wyles et al., 1983). Apparently retarded rates of morphological and molecular change (Primmer & Ellegren, 1998; Stanley & Harrison, 1999) strongly influenced evolutionary theory as applied to birds, e.g. prompting assessment of phylogenetic principles for morphologically ‘uniform’ groups (Bock, 1963a). This duality – higher-order diversity defying phylogenetic inference and study of morphological variation lacking unified phylogenetic focus – was influential during the last century.
Avian systematics has followed a general tri-phasic pattern: (i) a descriptive period – epitomized by the landmark works by Huxley (1867), Fürbringer (1888) and Gadow (1892, 1893), in which early classifications of the period were based solely on anatomical evidence and distinctly informal in nature (Seebohm, 1888, 1889, 1890a, b, c, 1895; Clark, 1901); (ii) a comparative (multitaxic) period – typically confined to single skeletal elements, articulations, limbs or organ systems (e.g. Bock, 1959, 1960a, b; Cracraft, 1968; Ames, 1971); and (iii) a phylogenetic period – the primary literature considered herein.
Important advances in avian systematics have been typified by studies focused on key extant taxa – e.g. Balaeniceps rex (Cottam, 1957) and Pedionomus torquatus (Olson & Steadman, 1981) – or promising aspects of anatomy – e.g. appendicular myology (Garrod, 1873a, 1874) – as well as a few broad surveys of modern taxa (Cracraft, 1986; Cracraft & Mindell, 1989). Regardless of method, however, scale of avian phylogenetics seldom exceeded single orders prior to 1990, when palaeontological finds revived such broad systematic endeavours. From the earliest years of avian systematics, ornithologists were attracted to taxa posing confusing combinations of characters, and a few systematists showed an uncanny recognition of taxa that were key to problems concerning larger groups (Table 1).
Table 1.
Selected references concerning neognathous Neornithes qualifying as perennial problems of higher-order (supra-ordinal) systematics (see Sibley & Ahlquist, 1972, 1981, 1990)
Percy Roycroft Lowe (British Museum), despite an idiosyncratic view of ontogeny in evolution (Livezey, 1995a) and pre-Hennigian concepts of phylogenetic reconstruction, undertook early and under-appreciated attempts to resolve the phylogenetic positions of problematic avian groups. Early works by Lowe emphasized the vexing Charadriiformes and allied Gruiformes (Lowe, 1922, 1923, 1924, 1925, 1931a, b), the ratites (Lowe, 1928, 1930, 1942, 1944a), ‘primitive’ characters of Sphenisciformes (Lowe, 1933), characters of Archaeopteryx possibly germane to an alliance between birds and dinosaurs (Lowe, 1935, 1944b), the perplexingly apomorphic Apodiformes (Lowe, 1939), and preliminary diagnoses for Cuculiformes (Lowe, 1943), Piciformes (Lowe, 1946) and Coraciiformes (Lowe, 1948). Intermittently during the same period, Lowe also considered possible relationships among ratites and some non-avian Theropoda, e.g. Struthiomimus and Ornitholestes, although he was hampered by the prevailing confusion between synapomorphy and symplesiomorphy and their respective implications for phylogeny (Lowe, 1928, 1930, 1935, 1942, 1944a, b). René Verheyen (Institut Royale Belgique) authored approximately 35 papers during 1950–60 that centred on problems of avian systematics by means of semi-quantitative methods (e.g. Verheyen, 1956a, b, 1960a, b, 1961). The work by Verheyen, however, was deemed idiosyncratic and largely ignored (Sibley & Ahlquist 1990).
Sibley & Ahlquist (1972, 1987, 1990) chronicled avian systematics since the late 18th century. Raikow (1985a) reviewed the philosophical underpinnings of avian systematics in recent decades, and clarified for the time the fundamental differences among various systematic schools. Avian systematics in the late 20th century has been marked by a trough in morphological phylogenetics (Fautin & Watling, 1999; Jenner, 2004a) and a concomitant peak in molecular systematics. The pessimism expressed by Sibley & Ahlquist (1990) regarding the phylogenetic potential of morphological characters, however, contrasts with surveys of the contributions of both (Patterson, Williams & Humphries, 1993). Bledsoe & Raikow (1990) concluded that considerable congruence existed among reconstructions based on DNA–DNA hybridization, sequence-based analyses, and comparative morphology. In a survey of the history of avian molecular systematics, Meyer & Zardoya (2003) recounted discrepancies between reconstructions of basal lineages based on mtDNA and nuclear genes. As discourse among schools increased, it was evident that the familiar demons of avian systematics haunted both morphological and molecular practices: differential selection and adaptation, convergence, extinction of lineages, challenges of homology and alignment, and heterogeneity of evolutionary rates and branch-lengths.
Palaeontological contributions
Fossils essentially are amenable only to morphological study, with the exception of a few, fortunate recoveries of ‘ancient DNA’ (Cooper et al., 1992, 2001; Austin, Smith & Thomas, 1997; Cooper, 1997; Sorenson et al., 1999; Paxinos et al., 2002), and typically provide only substandard anatomical material or incomplete specimens. Some of the most intense conflicts among avian systematists stemmed either from a commitment to phenetics or the idiosyncracies of palaeornithological perspectives (e.g. Cracraft, 1979, 1980, 1981; Olson, 1982). Influential for avian systematics was the view that avian fossils are both fragile and correspondingly rare (Olson, 1985), despite compendia indicative of extensive taxonomic diversity (Brodkorb, 1963, 1964, 1967, 1971a, b, 1978). Deficiencies in the fossil record (Olson, 1985) and challenges of homology (e.g. Sereno, 2001), however, did not diminish a reliance on new fossils to resolve the broad outlines of avian evolution (Feduccia, 1980, 1995, 1996).
Palaeontological contributions have been confounded by speculative evolutionary scenarios that extend beyond the underlying systematics (Feduccia, 1973, 1977c, 1995, 1996, 2003). The purported issue of ‘fossil mosaics’ (Eldredge, 1989) – a predictable consequence of heterogeneity in evolutionary rates among characters – further exacerbated the interpretation of evolutionary change (Livezey, 1997a). Martin (1983: 291) concluded that during the 150 years of avian palaeontology, ‘… a major burden for palaeornithologists has been a lack of comparative skeletons of recent birds’, and that the ‘other major problem is the incompleteness of most avian fossils.’ With the latter we agree, but the former is less a problem of availability than the result of under-utilization, a factor worsened by the rush to a molecular era.
Ethological and parasitological phylogenetics
Behavioural characters are only infrequently used in formal cladistic analyses (e.g. Hughes, 1996; Lee et al., 1996; Kennedy et al., 1996, 2000; Slikas, 1998; Birdsley, 2002), or precursors thereof (Van Tets, 1965). Complete designs have not been attempted for lack of comparable data for species of interest (Wimberger & de Queiroz, 1996), and some are limited to assessments a posteriori for phylogenetic signal (Winkler & Sheldon, 1993; Lee, Feinstein & Cracraft, 1997; McCracken & Sheldon, 1997). Phylogeneticists have come to consider selected ethological traits – notably displays of courtship – worthy of phylogenetic interpretation (Delacour & Mayr, 1945; Johnsgard, 1961; Archibald, 1976; Paterson, Wallis & Gray, 1995). Patterns of interspecific hybridization have perhaps the longest history of study, notably among Anseriformes (Sibley, 1957; Johnsgard, 1960, 1963; Scherer & Hilsberg, 1982). Eventually, interfertility was recognized to be plesiomorphic and comparatively conservative (Prager & Wilson, 1975), and therefore interspecific hybridization to be uniformative with respect to phylogenetics (Cohen et al., 1997; Braun & Brumfield 1998; Andersson, 1999). Similarly, phylogenetics of ectoparasites has been explored only infrequently in phylogenetic reconstructions of birds (Paterson, Gray & Wallis, 1993; Paterson & Gray, 1996; Page et al., 1998; Johnson et al., 2002; Storer, 2002; Smith, Page & Johnson, 2004; Banks, Palma & Paterson, 2006). Consequently, the two primary sources of phylogenetic signal for birds during the 20th century have been morphological variation and molecular (increasingly DNA sequence) data.
Molecular phylogenetics
Following an implicit rejection of DNA hybridization on the grounds of its phenetic nature and woefully incomplete distance matrices, molecular systematics focused on the cladistics of parsimony or increasingly explored the probabilistics of maximum-likelihood and Bayesian methods. Phylogenetic analyses based solely on mitochondrial genes de jour (e.g. cyt b, 12S) initially were accorded considerable validity (Sraml et al., 1996; Mindell, Sorenson & Dimcheff, 1998; Johnson & Sorenson, 1998, 1999; McCracken et al., 1999), but these works effectively were trumped by those based on entire mitochondrial genomes (Paton, Haddrath & Baker, 2002) or including nuclear genes, with few exceptions (García-Moreno, Sorenson & Mindell, 2003). Similarly, explorations of very limited numbers of genes (Templeton, 1983; Groth & Barrowclough, 1999; Paton et al., 2003; Chubb, 2004a, b; Fain & Houde, 2004) were eclipsed by expanded analyses of nuclear data with diversified taxonomic samples (Hughes & Baker, 1999; Donne-Goussé, Laudet & Hänni, 2002; Sorenson et al., 2003). This progression of analytical refinements and expanded taxonomic representation, despite the continued challenges discovered in each (e.g. Cotton & Page, 2002), is likely to continue and perhaps accelerate with the implementation of studies based on ‘total evidence’ (Huelsenbeck, Bull & Cunningham, 1996; Baker, Yu & DeSalle, 1998; Ballard et al., 1998; Bininda-Emonds, Gittleman & Steel, 2002; Cracraft et al., 2004).
Current status of avian phylogenetics
‘… the currently accepted arrangement of birds in no way reflects the probable evolutionary history of the class…. The arrangement used here is predicated mainly on the assumptions that birds originated on land rather than in the water, and that highly specialized waterbirds are more derived than less specialized ones…. a consensus has emerged that birds originated, if not in trees, certainly on land. Therefore, we should look for the most primitive taxa among the land birds.’ (Olson, 1985: 83, 84)
‘If one had to summarize the current state of knowledge, the most pessimistic view would see the neoavian tree as a “comb,” with little or no resolution among most traditional families and orders.’ (Cracraft et al., 2004: 475)
‘Perhaps the greatest unsolved problem in avian systematics is the evolutionary relationships among modern higher-level taxa.’ (James, 2005: 1052)
Harrison et al. (2004: 974) concluded: ‘It is almost an offense against birds that the deep mammalian tree is virtually resolved … whilst there are still major uncertainties about many aspects of the avian evolutionary tree.’ In support of this sentiment, the authors cited fundamental discordance among phylogenetic inferences for birds based on mitochondrial and nuclear genomes, an assessment at odds with a contemporary review by García-Moreno et al. (2003). Discussion of morphological efforts by Harrison et al. (2004) was limited to the uncertainties raised by Cracraft (1981, 2001) but verified increasingly by analyses (Cracraft, 1982a, 1986, 1988; Cracraft & Mindell, 1989; Cracraft et al., 2004; Mayr, 2005a). Reconstructions of the higher-order relationships of birds based on morphological characters, in turn, have been depauperate in both characters and taxa and seldom genuinely cladistic (e.g. Cracraft, 1986, 1988, 2001; Cracraft & Clarke, 2001; Mayr & Clarke, 2003).
Regardless of the taxonomic group considered, however, the sobering truth is that the goal of phylogenetics is extremely ambitious and without easy or uniformly reliable means of accomplishment. It is beyond debate that the conceptual framework of morphological cladistics (Hennig, 1966) and ever-increasing computational power has led to significant progress. Nevertheless, it is also clear that many phylogenetic problems have proven resistant to all attempts at solution and seem destined to controversy. Also, phylogenetic endeavours are replete with disagreements in method (both for reconstruction and for evaluation of estimates) and types of evidence considered most reliable. Currently, the tendency is to consider molecular reconstructions as representing the future of avian phylogenetics, and that it is simply a matter of time, perhaps less than a decade, before a global consensus is achieved within the systematic community (Barrowclough, 1992; Livezey & Zusi, 2001; Stanley & Cracraft, 2002).
Deficiencies in taxa or characters typically render comparisons among investigations problematic (Bledsoe & Raikow, 1990), and attempts to reconcile the phylogenetic evidence for Aves substantiate this generality (Cracraft & Mindell, 1989; Mayr, Manegold & Johansson, 2003, 2004a; Dyke & Van Tuinen, 2004; Griffiths et al., 2004). Indicative of disappointing progress in mid- and lower-order avian phylogenetics is the conclusion that basal (higher-order) nodes may be irresolvable or accurate approximations of genuine, explosive radiations (Poe & Chubb, 2004). While demonstrably true of analyses confined to few characters or limited taxonomically (Kumazawa & Nishida, 1995), a single decade of uninspiring inference is insufficient to judge solution to be beyond hope.
The current status of molecular resolution of deepest neornithine nodes, however, serves to underline the likelihood that many genes provide inadequate phylogenetic signal for the problems at hand, a deficiency exacerbated by basal polarities necessarily based on closest extant relatives that are unfortunately comparatively distantly related, e.g. Crocodylia and Testudines (Larhammar & Milner, 1989; Iwabe et al., 2004). The fact that ‘nearest’ outgroup(s) for molecular analysis need to be be extant has had unfortunate implications for rooting, in that for Neornithes these outgroups are comparatively distantly related and may converge on ‘white noise’ as indicators of avian polarities, especially for rapidly evolving mitochondrial data.
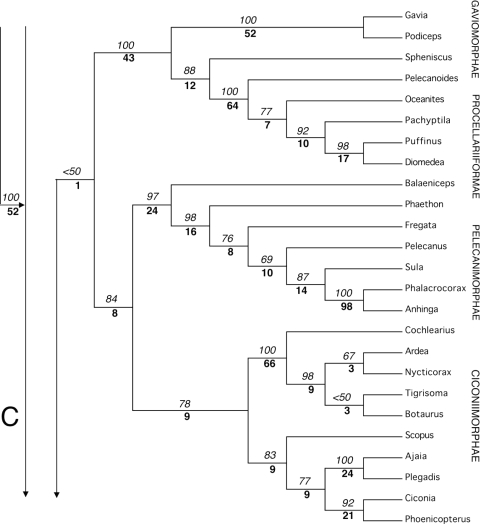
Like morphological estimates, a number of potential pitfalls (rooting aside) afflict molecular reconstructions, e.g. serial homoplasy by misalignment, distortions related to silent substitutions, unrealistic treatment of ‘gaps’, and unequal evolutionary rates over extended intervals of geological time and among lineages. Furthermore, disagreement persists if not expands regarding methodological preferences – e.g. classes of data employed, protocols for alignment (i.e. diagnosis of serial homology), choice among reconstructive methods, and assessment of resolution and support (Felsenstein, 2004). Until substantial agreement concerning methods is attained and accurate synergism among molecular and morphological methods secured, the field will remain vulnerable to methodological bias and a tolerance for poorly supported hypotheses of phylogeny, in which even the best-supported works disagree significantly (see Figs 4–9).
Figure 4.
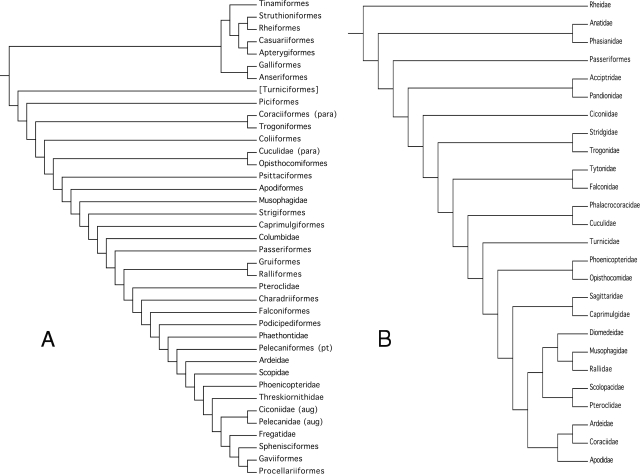
Molecular phylogenetic trees proposed in previous studies (see Fig. 1 for details), IV. A, Sibley & Ahlquist (1990: figs 354–356), simplified to orders, wherein parenthetical ‘para’ indicates paraphyly of sampled members, and ‘aug’ indicates unconventional content; B, Mindell et al. (1997).
Figure 9.
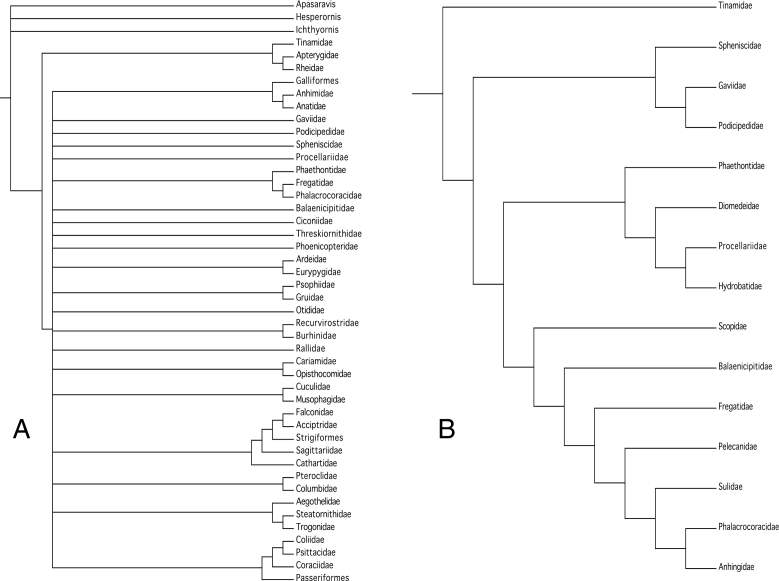
Molecular phylogenetic trees proposed in previous studies (see Fig. 1 for details), IX. A, Pereira & Baker (2006a); B, Slack et al. (2006a).
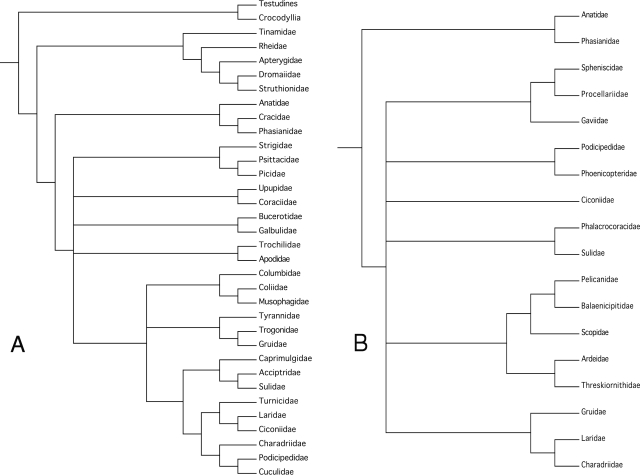
Figure 2.
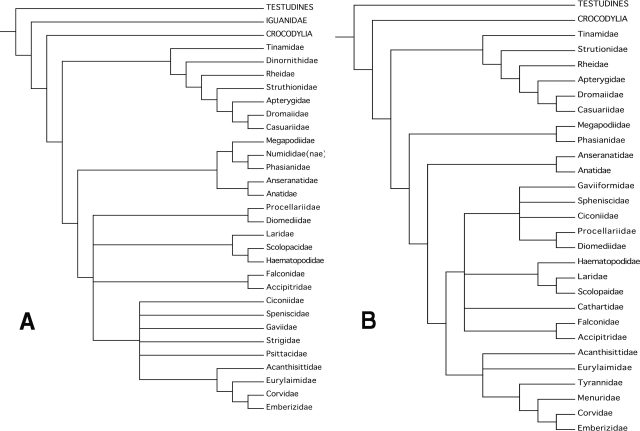
Morphological phylogenetic trees proposed in previous studies (see Fig. 1 for details), II. A, Mayr & Clarke (2003); B, Bourdon et al. (2005).
Figure 5.
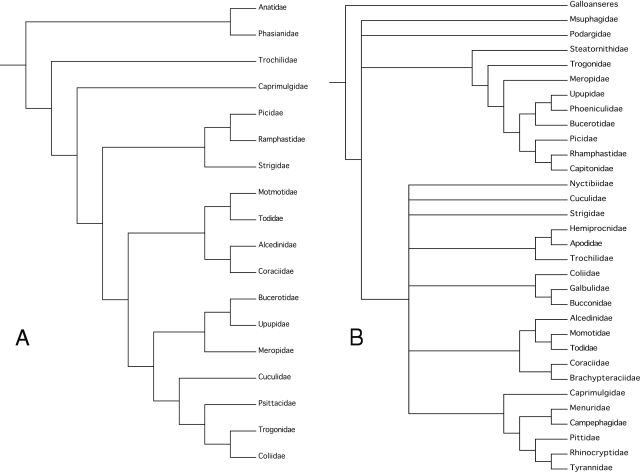
Molecular phylogenetic trees proposed in previous studies (see Fig. 1 for details), V. A, Espinosa de los Monteros (2000); B, Johansson et al. (2001).
Goals and objectives
The primary purpose of this paper is to present a morphologically based phylogenetic hypothesis of higher-order relationships of Neornithes. A compendium of characters is provided within the companion work (Livezey & Zusi, 2006), including a bibliographic synthesis, annotations of prior uses of synonymous and related characters, and a compact disc of the data matrix for refinement and augmentation. The secondary objective of this work is to provide a cladistic alternative to the molecular phenetics of Sibley & Ahlquist (1990), at least for non-passeriform families, and to serve as a framework for lower-level studies of included families. An earlier paper on philosophical and methodogical issues (Livezey & Zusi, 2001), despite an explicit disclaimer to the contrary, frequently has been cited as a phylogenetic hypothesis appropriate for comparison with works considered complete by their authors, even regarding positions of individual taxa (e.g. Cracraft et al., 2003). We began the present study with the opinion that the phylogenetic signal encoded within avian anatomy is, with adequate study of both definitive and ontogenetic variation of an adequate sample of modern lineages, more than sufficient for the reconstruction of the higher-order phylogeny of Neornithes. We remain at least as optimistic concerning this goal.
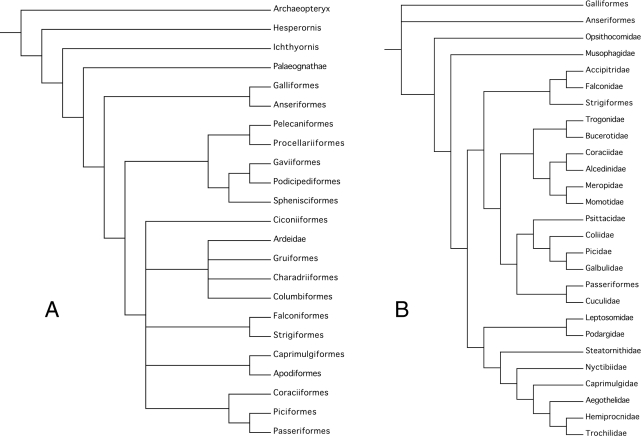
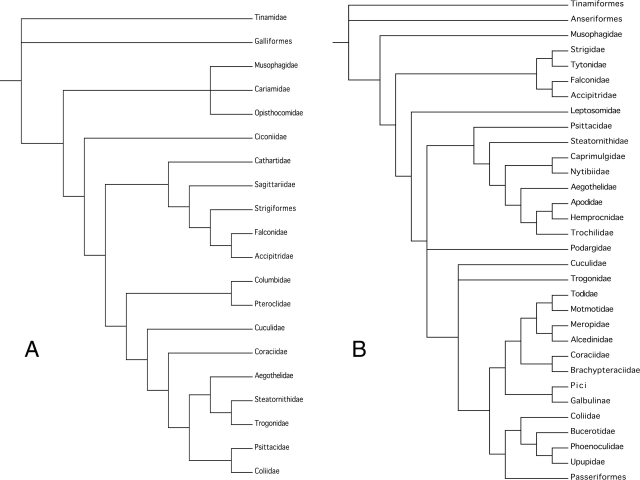
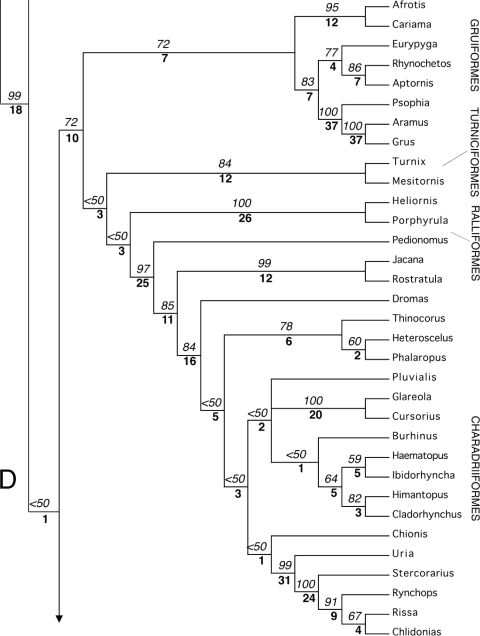
The present phylogenetic hypothesis is intended to serve both as a baseline estimate and ‘scaffold’ for finer-scale reconstructions of terminal clades (i.e. families), as attempts at broad reconstructions of the phylogeny of Neornithes to date have been limited, at the very least, in taxonomic representation (e.g. Slack et al., 2006b) or discredited methods of inference (Sibley & Ahlquist, 1990). We also sought to provide robust nodes supplemental to the few phylogenetic hypothesis currently employed for calibrations of age based on fossils (e.g. Dyke & Van Tuinen, 2004; Pereira & Baker, 2006a) or their surrogates (Van Tuinen, Stidham & Hadly, 2006). Integration of these data with a rich matrix of DNA-sequence data (Cracraft et al., 2004) is planned to explore the power of ‘total evidence’ to recover both higher-level and lower-level avian phylogeny. Perhaps most importantly for the facilitation of future analyses, be these morphological or molecular, is the identification of sister-groups (optimal outgroups) for purposes of rooting analyses of single orders or families. The comparatively sparse representation of taxa in the present analysis reflected logistical limits, but was considered adequate for achieving the stated objectives. Findings herein principally were compared with modern higher-order reconstructions (e.g. Mindell et al., 1997; Mayr & Clarke, 2003; Mayr et al., 2003), the most critical of which are summarized graphically here (Figs 1–9). Works of narrower scope are considered where issues of familial monophyly persist, with emphasis on truly phylogenetic works as opposed to eclectic or phenetic assessments (Raikow, 1985a).
Figure 1.
Morphological phylogenetic trees proposed in previous studies, I. A, Cracraft (1988); B, Mayr et al. (2003). Some trees were subjected to topologically neutral modifications of taxa to facilitate comparisons (also Figs 2–9). See corresponding papers for analytical methods and topological statistics.
METHODS
Included taxa
Taxonomic sampling and exemplars
Taxonomic diversity generally represents a much greater logistical burden than diversity of characters in phylogenetic analyses, and challenges imposed by taxa can be exacerbated by unfortunate sampling (Maddison & Maddison, 1992; Graybeal, 1998; Swofford, 2002; Felsenstein, 2004). However, it has been demonstrated that density of taxonomic sampling for the ingroup varies directly with expected accuracy, support and resolution of resultant trees (Lecointre et al., 1993), although the importance of taxonomic density appears to be greatest for sequence data (especially with respect to long-branch attraction). Expectations of resolution and accuracy that are related to richness of morphological characters, unlike for sequence data (Lecointre et al., 1994), have not been subjected to numerical assessment, but logically are significantly related. The importance of monophyly of the groups represented by exemplars prompted the citation, where available, of analyses germane to the monophyly and content of taxonomic families represented here by exemplars.
We sought to maximize richness of characters and represent higher-order taxa within logistic limits that: (i) represented (sub)familial diversity among non-passeriform Neornithes; (ii) provided special insights into interfamilial groups (Livezey, 1997a, 1998a); (iii) were suitably represented by essential specimens; and (iv) included taxa of special interest to avian systematics. Neornithine families were represented by one or more exemplars deemed in most cases to reflect at least a ‘basal’ member (i.e. candidate sister-taxon of other members) of the taxon in question. This method is not without difficulties, as concerns persist regarding the use of exemplars as terminal surrogates for higher-order taxa (Bininda-Emonds, Bryant & Russell, 1998), notably where polymorphism is involved (Yeates, 1995; Simmons, 2001) or monophyly of terminals represented by single exemplars is in question. Also, limitations on specimens of specialized preparations impose critical deficiences on resultant data matrices, an abiding concern of anatomical collections of birds (Livezey & Zusi, 2001; Livezey, 2003a). Relatively strong support for monophyly of most clades alleviated concerns regarding taxonomic sampling, especially given the number of morphological characters employed. However, use of minimal numbers of exemplars justifies caution in the diagnostics given for diverse orders and families herein (Table 2).
Table 2.
Median branch lengths (L) subtending clade identified by taxon among MPTs (values in brackets pertain to polytomies), respective Bremer (support) indices (B) for clades (i.e. non-terminal taxa in analysis), and apomorphies both unambiguous (i.e. invariant for set of MPTs) and diagnostic (CI = 1.00) or supportive (0.50 ≤ CI < 1.00) for corresponding taxa (Appendix 1). Characters (numbered) and states (lettered in italics) identify terminal condition of transformation attributed to internode in question; characters, states and provenance of features were described by Livezey & Zusi (2006)
| Taxon | L | B | Diagnostic apomorphies | Supportive apomorphies |
|---|---|---|---|---|
| Aves | [82] | 12 | 1b, 214b, 1518b, 1912b, 1987d, 2218c | 338b, 708b, 789a, 1329b, 1312c, 1510a, 2438b, 2446b |
| Ornithurae | 124 | 2 | – | 1470b |
| Eoaves | 139 | 13 | 418c, 515b, 1280b, 1297b, 1474b, 2108b, 2212c | 1333d, 1452b, 1701c, 2227c, 2440c, 2446d |
| Neornithes | 98 | 11 | 22c | 221b, 1586a, 1687a, 1688a, 1690a, 1819d, 2134b, 2383a |
| Palaeognathae | 106 | 13 | 540b, 631b, 656b, 659b, 1750b, 2029b, 2436c, 2945b | 1330b, 1523a, 2028b, 2133c |
| Crypturi* | 102 | – | 924b | 1361b, 1453b, 1635b, 1645b, 1844b, 2351b, 2497a |
| Ratitae† | 241 | 50 | 129a, 250b, 474a, 523b, 547b, 555b, 765b, 767a, 901b, 923b, 926b, 958b, 1046b, 1051b, 1263b, 1341b, 1537b, 1554b, 1564c, 1861a, 2022b, 2045b, 2165b, 2184b, 2512b, 2721b, 2757c, 2794b, 2798b, 2867b | 476b, 506b, 600a, 927b, 1008e, 1019b, 1041a, 1053b, 1098f, 1122b, 1258a, 1333e, 1336c, 1337c, 1346c, 1346b, 1353a, 1364c, 1371c, 1450a, 1497b, 1509b, 1548b, 1694b, 1707a, 1709a, 1744a, 1747c, 1756d, 1766b, 1773b, 1924b, 1998a, 2015d, 2167a, 2479b, 2522b, 2547b, 2568b, 2717b, 2769b, 2808b, 2811a, 2821a, 2868a |
| Casuariimorphae | 88 | 31 | 352b, 1120b, 1121b, 1167b, 1170b, 1390b, 2306b | 413c, 930b, 952b, 962b, 1156b, 1789b, 1844b, 1355b, 2667b, 2949b |
| Struthionimorphae | 100 | 44 | 107b, 1065b, 1154b, 1169b, 1784b, 1896b, 2002b, 2302b, 2326b, 2398c, 2795b, 2824b | 1371c, 1551b, 1568b, 1756c |
| Neognathae | 142 | 52 | 213b, 523c, 579b, 601b, 1096b, 1106b, 1487c, 1809b, 1953b, 2068b, 2108c, 2209b, 2216b, 2217b | 2e, 4c, 109b, 112c, 468b, 583c, 600d, 731d, 1497d, 1633c, 1789b, 2294b |
| Galloanserimorphae | 82 | 18 | 117b, 513b, 546b, 601c, 698b, 723b, 2073b, 2855b, 2915b | – |
| Galliformes | 137 | 86 | 542b, 625b, 1077b, 1109b, 1247b, 1257b, 1362b, 1657b, 1906b, 2693b, 2859b, 2907b | 109d, 378b, 524b, 600c, 750b, 1175c, 1196b, 1330b, 1792c, 2146a |
| Anseriformes | 97 | 41 | 95b, 278b, 2052b, 2073c, 2148b, 2454b, 2724b, 2747b, 2913b | 422b, 1333b, 2497a |
| Neoaves | 81 | 18 | 1280c, 2502b, 2586b, 2893b, 2895b, 2896b, 2900d | 480c, 517c, 600e, 1721b |
| Natatores | 51 | 1 | – | – |
| Pygopodo-tubinares | 104 | 43 | 195c, 1432b, 2076b, 2413a | – |
| Gaviomorphae | 95 | 52 | 534c, 538b, 748b, 2117b, 2147b, 2249b, 2256b | 927b, 946c, 1514b, 1766b, 1924b, 2077b, 2089d, 2287b, 2362b, 2402a |
| Gaviiformes | 125 | – | 457b, 1407b, 1532b, 1893b, 2002c, 2320b, 2331b, 2411b, 2644b, 2694b, 2882b, 2886b | 1193b, 1820b, 2133b, 2322b, 2349c |
| Podicipediformes | 121 | – | 823b, 2304b, 2435c, 2642b, 2658b, 2771b, 2784b | 164b, 1008d, 1657b, 2054d, 2056b, 2429b |
| Procellariimorphae | 66 | 12 | 196b, 722b, 1347b, 2404b, 2630b, 2744b, 2933b | 2225c, 2356b, 2729d |
| Sphenisciformes | 225 | – | 448b, 534b, 910b, 933b, 1422b, 1424b, 1495b, 1517b, 1530b, 1541b, 1542b, 1544b, 1556b, 1571b, 1736b, 1749c, 1751b, 2293c, 2366b, 2528b, 2543b, 2546b, 2720b, 2790b, 2791b, 2870b | 385e, 994b, 1302b, 1353c, 1364d, 1443b, 1451c, 1516c, 1580c, 1581b, 1694b, 1707c, 1733a, 1755c, 1756c, 1820b, 2004b, 2133b, 2446c, 2522b, 2563b, 2584b, 2588b, 2610c, 2810c, 2812d, 2868a |
| Procellariiformes | 33 | 64 | 1305b, 1306b, 2847b | 2819a |
| Stegano-grallatores | 61 | 8 | – | 1138b |
| Pelecanimorphae | 102 | 24 | 720b, 1241b, 2107b, 2751b | 24a, 372b, 1144b, 1540d, 1999b, 2089b, 2729b |
| Balaenicipitiformes | 158 | – | 257b, 288b, 293b, 433b | 153b, 286b, 304b, 566b, 740b, 762b, 769c, 780c, 1309b, 1514b, 2346b |
| Pelecaniformes | 111 | 16 | 335b, 889b, 1832b, 2573b | 35b, 48b, 147b, 946d, 2181b, 2351b, 2388b, 2406b, 2548b |
| Ciconiimorphae | 55 | 9 | 1543b | 1138c, 2179b, 2830b |
| Ciconiiformes | 58 | 9 | – | 2420b |
| Ardeiformes | 106 | 66 | 116b, 175b, 754b, 2097b, 2391b, 2396b, 2458b, 2800b, 2834b, 2836b, 2851b | 35b, 147b, 529b, 535b, 831b, 1238c, 2028b, 2330b, 2388b |
| Terrestrornithes‡ | 42 | 1 | – | – |
| Charadriimorphae | 45 | 10 | – | 2575b |
| Gruiformes | 40 | 7 | 2111b | 2146a, 2197b |
| Charadriiformes | 30 | 25 | – | 2462b |
| Dendrornithes§ | 54 | 4 | – | – |
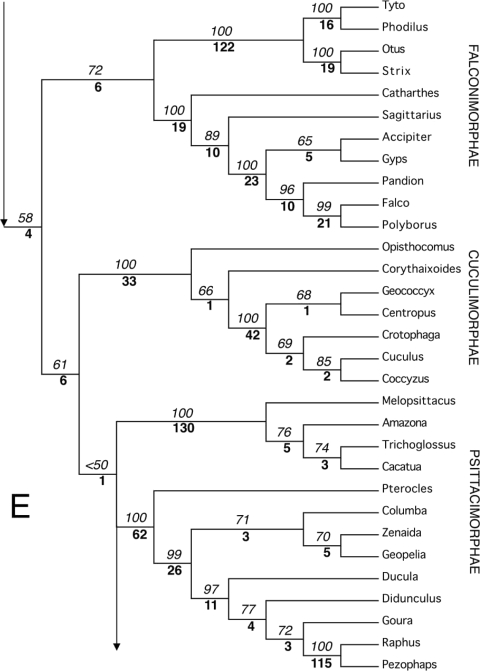
| Falconimorphae | 62 | 6 | 2916b | 2431b |
| Falconiformes | 83 | 19 | 120d, 1857b, 1938b, 2343b, 2428b | 1153c, 2133b, 2854b, 2899b |
| Strigiformes | 172 | 122 | 13b, 154b, 174b, 193b, 548b, 1549b, 1714b, 2072b, 2200a, 2286b, 2300b | 128c, 249b, 1540c, 1569c, 1779b, 1822b, 2407b, 2412b, 2583c, 2602a, 2694b, 2710d, 2743b |
| Anomalogonates¶ | 56 | 6 | – | – |
| Cuculimorphae | 185 | 33 | 1572b | 1007b, 1336b, 1614b, 1651d, 1658c, 1866b, 2634c, 2849a |
| Opisthocomiformes | 131 | – | 959b, 1064b, 2857b | 385e, 566b, 850e, 1063b, 1140b, 1329d, 1651e, 1658d, 2091b, 2510b, 2575b, 2710d, 2845b |
| Cuculi | 85 | 42 | 937b, 2034b, 2046b, 2061b, 2200c, 2334e | 1122b, 2498b |
| Psittacimorphae | 52 | 1 | – | – |
| Psittaciformes | 177 | 130 | 118b, 246b, 354b, 410b, 429b, 593b, 605c, 650b, 679b, 703b, 761b, 1060b, 1210b, 2334d, 2405d, 2490b, 2703b, 2712b, 2832b, 2876c, 2878b, 2884b | 772b, 2028b, 2127b, 2133d, 2203b, 2491b, 2498b, 2673b, 2710c, 2849e, 2854c, 2935b, 2941b |
| Columbiformes | 106 | 62 | 1723b, 2119b, 2557b, 2846b | 351b, 1175b, 1307b, 1356b, 1369b, 1417c, 2036b, 2575b, 2710d, 2722b |
| Incessores** | 101 | 2 | – | – |
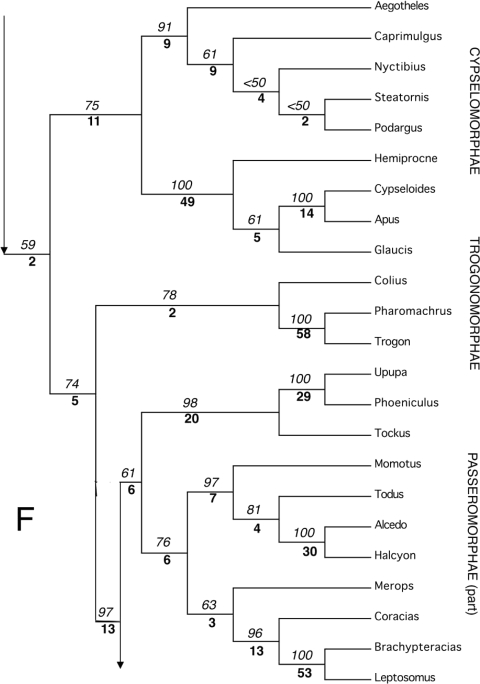
| Cypselomorphae | 70 | 11 | 2786b | 1365b, 2903b |
| Apodiformes | 97 | 49 | 1125b, 1375b, 1416b, 1455b, 1465b, 1466b, 2449c, 2661b | 38b, 1346b, 2466a, 2549c, 2674b |
| Caprimulgiformes | 77 | 9 | 280b, 1271b, 1979b, 2198c, 2921b | 128c, 230b, 249c, 450b, 1999b, 2583c, 2903d, 2933b |
| Trogones†† | 38 | 2 | – | – |
| Trogonomorphae | 43 | 2 | 1919b | – |
| Trogoniformes | 83 | 58 | 450b, 935b, 1720b, 2333b | 244b, 531b, 2100b, 2593c, 2613b |
| Coliiformes | 108 | – | 512b, 1032b, 2338b, 2367b | 398b, 2036b, 2127b, 2673b, 2702b |
| Passerimorphae‡‡ | [62] | 13 | 1807b, 2590b | 718b, 1453b |
| Coraciiformes | 51 | 6 | 2360b | 2723b |
| Piciformes | 58 | 9 | 2334g, 2335b | 1300b, 1709c, 1981b, 2498d |
| Passeriformes | 96 | 55 | 159b, 1228b, 1463b, 1895c, 2669b, 2687b, 2874b, 2877b, 2890b, 2891b | 1127b, 1540e, 1559b, 2789c |
Redundant with taxon of next-lower rank – Dromaeomorphae – by hierarchical classification, and equivalent to apomorphies of terminal taxon Tinamiformes. Other example are (i) Subcohort Galloanseres comprising solely the Superorder Galloanserimorphae; and (ii) Superorder Casuariimorphae comprising solely the Order Casuariiformes.
Pertains to estimate exclusive of two extinct members (Dinornithiformes, Aepyornithiformes); see Methods.
Redundant with taxon of next-lower rank – Telmatorae – and therefore latter was not tabulated.
Redundant with taxon of next-higher rank – Raptores – and therefore latter was not tabulated.
Redundant with taxon of next-lower rank – Coccygae – and therefore latter was not tabulated.
Musophagidae.
Redundant with taxon of next-lower rank – Cypselomorphae – and therefore latter was not tabulated.
Redundant with taxon of next-lower rank – Trogonomorphae – and therefore latter was not tabulated.
Redundant with taxon of next-higher rank – Pico-clamatores – and therefore latter was not tabulated.
Crocodylia and non-avian theropod Dinosauria served as ‘ultra-deep’ and primary outgroups, respectively, to root Neornithes (Maddison, Donoghue & Maddison, 1984; Janke & Arnason, 1997), but the inclusion of most published characters in placing these taxa (Benton & Clark, 1988; Evans, 1988; Benton, 1999; Cao et al., 2000; Brochu, 2001; Brochu & Norell, 2001) chronicled the acquisition of avian characters during the Mesozoic (Carroll, 1997). The recent extension of avian roots, both by newly discovered avialian taxa and confirmation of early roots among non-avian theropods, circumvented difficulties of establishing basal polarities for Neornithes based on inadequate diversity of Mesozoic relatives or (for narrower reconstructions) or dubious reliance on the problematic Palaeognathae, notably caused by the complex of apomorphy and plesiomorphy of ratites relative to the Tinaniformes (Bertelli, Giannini & Goloboff, 2002). These outgroups optimized rooting by the hierarchy of information afforded by multiple (nested) outgroups (Barriel & Tassy, 1998; Lyons-Weiler, Hoelzer & Tausch, 1998) and avoided the analytical problems implicit with hypothetical ancestors (Bryant, 1997, 2001).
Four comparatively distant outgroups were sampled for estimating deep polarities – non-Archosauromorpha (informative states of comparable characters at the approximate origin of the archosaurian clade), Crocodylomorpha (i.e. non-dinosaurian Archosauria), Ornithischia (i.e. non-saurischian Dinosauria) and Sauropodomorpha (modalities of non-theropod Saurischia). Among non-avian Theropoda, Herrerasaurus served as the most informative of the generic outgroups (Sereno, 1994; Sereno & Novas, 1994). Groupings among outgroups (i.e. among non-avian taxa) were of only secondary interest, however, whereas establishment of a realiable root for the Neornithes was the principal priority.
Indeterminate and redundant contributions of some outgroup taxa with respect to the primary objective of this analysis, as well as excessive proportions of missing data recognized upon completion of the data matrix, prompted limited pruning and merging of taxa (primarily outgroups) for analysis: (a) taxa pruned –Euparkeria, Syntarsus, Eoraptor, Saurornitholestes, ‘Caenognathidae’, Microvenator, Citipati, Chironestes, Ornitholestes, Segnosaurus, Avimimus, Sinornithosaurus, Microraptor, Erlicosaurus, Shuvuuia, Jehelornis, Gobipteryx, Patagopteryx; Diatrymiformes, Dromornithiformes (Rich, 1979, 1980; Murray & Megirian, 1998; Murray & Vickers-Rich, 2004), Sylviornis (Poplin & Mourer-Chauviré, 1985; Mourer-Chauviré & Balouet, 2005); (b) taxa merged: {Allosaurus, Sinraptor}∼ Allosauroidea; {Tyrannosaurus, Albertosaurus}∼ Tyrannosauridae; {Sinovenator, Sinornithoides, Troodon}∼Troodontidae; {‘Enantiornithidae’, Iberomesornis, Cathayornis, Concornis, Neuquenornis, Eoalulavis, Protopteryx}∼Enantiornithes; {Mononykus, Patagonykus, Alvarezsaurus}∼Alvarezsauroidea.
Two subfossil taxa – Aepyornithiformes and Dinornithiformes – for which character states were only marginally recovered, were excluded for the primary global search, and provisionally placed by means of two different protocols. This measure was taken because simple analysis of these imperfectly known, broadly similar, large ratites led to an apparently artefactual couplet –‘long-branch’ distortions exacerbated by missing data (Wiens, 2005) – as sister-group of other ratites exclusive of Apterygidae. First, each was analysed in the absence of the other in a global, unconstrained analysis. Second, each was separately placed by means of heuristic searches in which the primary tree was used as a backbone constraint. The Dinornithiformes were scored as two families (Dinornithidae and Emeidae) as approximated by Cracraft (1976a, b) and Worthy and Holdaway (2002) during character analyses, but analysed as a single, merged taxon in light of their virtually identical scores. Accordingly, the ‘trimmed-merged’ data matrix provided in digital form by Livezey & Zusi (2006) comprised 150 ingroup taxa and 35 outgroups.
Phylogenetic analysis
General philosophy
Most standard methodological issues were detailed in the foregoing companion work (Livezey & Zusi, 2006), including the analytical perspectives that serve to justify the delimitation of characters and states, ordering of states, and related options requisite to preparation of characters for analysis. Noteworthy is a principal reliance on the literature for many characters of non-avian Theropoda. In the present installment, the foregoing characters were subjected to phylogenetic analysis sensu morphological cladistics (Kluge & Wolf, 1993) coupled with the criterion of parsimony of character evolution as implied by the resultant phylogenetic hypothesis (Eldredge & Cracraft, 1980; Wiley, 1981; Brady, 1982; Farris, 1982; Felsenstein, 1983, 2004; Semple & Steel, 2003). In light of the practical and theoretical implications of adopting the parsimony criterion (Felsenstein, 1983, 2004), alternative methods were not practical for this analysis because of missing data (Felsenstein, 1979; Kluge, 1997a, b, 1999) – e.g. optimizations of morphological characters on branching models under selected models of stochastic change (Huelsenbeck, Nielsen & Bollback, 2003) or maximum-likelihood analysis (Lewis, 2001). Global parsimony – i.e. minimal total for character-state changes required by final tree (i.e. ‘shortness’) – served as the criterion of optimality for trees recovered through searches (Sober, 1982, 2005). The data matrix was not revised iteratively conditional on outcomes of analysis, nor was ordering of characters conditional on such runs. Instead, the entire data matrix summarized herein was completed prior to the analytical phase, thereby maintaining a partition between (i) definition of characters and states, coding of taxa, and issues of weighting and ordering, and (ii) phylogenetic analysis.
Characters and states
Unfortunate logistic limitations, not oversight or philosophical considerations, prevented the inclusion of character descriptions with the present analytical work. Although a reflection of our unexpected success in defining 2954 morphological characters relevant to the project (Livezey & Zusi, 2006), it precluded the familiar juxtaposition of descriptive material with analytical inferences. We anticipate that this inconvenience will be ameliorated by the coordinated publication of the descriptive atlas of characters and digitally recorded data matrix (Livezey & Zusi, 2006), to be made available virtually at cost. We strongly recommend that those interested in the present work procure a copy of its sister publication, as it is through examination of both that meaningful improvements will be made.
Where mutually exclusive states of a single character were diagnosable (Stevens, 2000), a single multistate character was defined (Mishler, 1994, 2005). Where two or more included states are observed for a single taxon, a coding of polymorphism was used and analysed specifically as given (i.e. not as uncertainty). The expanse of time reflected by the scale of the analysis also is expected to be associated with the number of multistate characters recognized (Lipscomb, 1992; Steel & Penny, 2005), i.e. scale of time and taxonomic divergences may be expected to be related directly to scale in evolution of form (Grant & Kluge, 2004). Multistate characters encode features manifesting comparatively great evolutionary change and may include greater potential phylogenetic signal, and states thereof will be optimized at multiple internodes (Simmons, Reeves & Davis, 2004). Unless otherwise indicated, characters were analysed as unordered.
Ordering can impose significant constraints on solution sets (Hauser & Presch, 1991; Forey & Kitching, 2000), and was used only where determined to be defensibly realistic, e.g. naturally ordinated (Livezey & Zusi, 2006). For example, multistate characters of forms ‘small, medium, large’, ‘absent, miniscule, prominent’, courses of passage of types ‘depressio, sulcus, arcus, tuba’, and junctura of types ‘articulatio, sutura, synostosis’ were considered naturally ordered, counter-evidence lacking. Fundamentally, ordering of states within a character was fundamental to definition and differentiation of characters, basic to the delimitation of states, and represented an extension of parsimony by inclusion of information on linear likelihoods in coding schemes. Such reasoning precluded meaningful use of arbitrary analytical variants such as treating all characters or partitions thereof as unordered. Hypotheses of transformation were sufficiently simple to obviate reliance on step-matrices (Ree & Donoghue, 1998), linear ordering being the sole condition imposed. Different numbers of states among characters can impose different levels of influence simply by different numbers of state changes among characters (James, 2004), but we considered such differential influence to be realistic and justified as it encoded diverse richness of evolutionary change instead of arbitrarily imposing uniformity on contributions of signal. Therefore, no attempt was made to counter-weight multistate characters. Moreover, no method of explicit weighting – a priori (Neff, 1986; Wheeler, 1986; Sharkey, 1989) or successive (Farris, 1969) – was employed in this analysis, although some perceive weighting effects to be implicit by other means (Haszprunar, 1998).
In this work, characters qualifying as autapomorphies at this analytical scale (i.e. apomorphic state limited to single included terminal taxon) were included in all analyses because most served as synapomorphies of the higher-order groups represented by respective exemplars, and many were included in previous publications as diagnostic of the clades represented by exemplars. In addition, such characters are intended to serve others performing lower-level analyses subsequently using some or all of the present matrix. Although autapomorphies did not serve to group taxa at this scale, the limited number detected here were retained because our interests not only included delimitation of clades but also were intended to provide a reasonable representation of evolutionary rates both among internodes and among terminal branches, of interest in many studies of evolutionary rates (e.g. Omland, 1997a, b). Also, autapomorphic differences (deriving from both unique changes or homoplasy) are critical to long-standing issues of perceived (phenetic) distinction and evolutionary divergence among taxa of debated relationships. Furthermore, such characters do not bias support indices such as bootstrapping (Harshman, 1994a), and by definition do not influence topologies. Also, a small minority of characters manifesting two or more states in original codings (included in the matrix to permit alternative taxonomic treatments) were rendered invariant by merging and pruning of taxa as detailed herein; this treatment was considered simpler than outright manipulation of the matrix analysed. The primary parameter of logistical concern where parsimony is the criterion of optimization is the number of taxa (Kim, 1998), a dimension that in the present work was favourably countered by number of characters.
Included characters manifested a range of homoplasy (Sanderson & Donoghue, 1989). However, the number of morphological characters employed here exceeded the domain for which meaningful comparison with other works is feasible (Swofford, 1991; Sanderson & Donoghue, 1989, 1996) and evaluation of a suite of related issues – e.g. rates of evolution, notions of relative ‘reliability’ of different data types, patterns of homoplasy (Faith, 1989; Sanderson, 1991), and Markovian informativeness (Shpak & Churchill, 2000) – was not logistically feasible.
Search for optimal solution
The character matrix was constructed using MACCLADE (Maddison & Maddison, 1992; Prendini, 2003), and analyses were performed on a Macintosh G5 2.5-GHz dual-processor computer. Primary phylogenetic analyses were performed using PAUP* version 4.0b10 (Swofford, 2002). Given the size of the data set and the corresponding universe of possible trees delimited (Felsenstein, 1978), we undertook a thorough exploration of the tree space to circumscribe the optimal solution set, i.e. the set of maximally parsimonious trees (MPTs), summarized graphically by a strict consensus tree of this set.
The set of MPT(s) (min [total length] = 19 553) recovered during heuristic searches in PAUP (MULPARS, TBR, random-addition of taxa, 10 000 random starting trees, MAXTREES = 20 000) was confirmed by a full ratchet-analysis (Goloboff, 1999; Nixon, 1999; Müller, 2004, 2005), including five random-addition cycles of 200 ratchet iterations each; the ratchet analyses, employed to escape local suboptima, recovered 97 trees across 1000 topological islands. Choice of optimizations (DELTRAN vs. ACCTRAN) was ineffectual, and neither DOLLO nor IRREVERSIBLE options were used. Of particular relevance to this comparatively large analysis were recent discussions of: (i) efficient means for finding solutions for large data sets (Maddison, 1991; Page, 1993; Rice, Donoghue & Olmstead, 1997; Quicke, Taylor & Provis, 2001; Salter, 2001), (ii) effects of missing data (Wilkinson, 1995, 2003; Wiens, 2003) and (iii) analytical relevance of branch lengths (Maddison, 1993; Lyons-Weiler & Hoelzer, 1997; Farris, Källersjö & De Laet, 2001; Norell & Wheeler, 2003; Wilkinson, LaPointe & Gower, 2003).
Summary statistics used here were: total length, L; consistency index, CI (Klassen, Mooi & Kicke, 1991; Kim 1996; Källersjö, Albert & Farris, 1999); retention index, RI (Farris, 1989); rescaled consistency index, RC; and skewness index (g1) based on 105 topologies randomly generated from the same data matrix (Huelsenbeck, 1991; Källersjöet al., 1992). Despite its popularity, the CI is negatively correlated with number of both taxa and characters analysed (Archie, 1989; Sanderson & Donoghue, 1989), making meaningful comparisons of indices across scales of analysis and classes of characters is difficult. Characters manifesting homoplasy can impose structural resolution and thereby result in smaller solution sets of MPTs (Källersjö, Albert & Farris, 1999). The set of equally parsimonious topologies (i.e. solutions differing only in optimization of characters on branches of trees of identical topology or solutions differing in branching structure but of equal length) were summarized using a strict consensus tree. Summary statistics for strict consensus trees were component information, Nelson–Platnick term and total information, and Mickevich consensus information.
Support for individual clades was measured by two statistics (Mort et al., 2000; Wilkinson et al., 2003): (i) percentages of 10 000 bootstrapped replicates in which the node was conserved (Felsenstein, 1985; Sanderson, 1995), indices considered informative even if assumptions concerning precision and absence of bias are unrealistic (Felsenstein & Kishino, 1993; Hillis & Bull, 1993); and (ii) Bremer (support) indices, the estimated minimal number of additional steps required wherein the given node, by inverse constraint, is not conserved (Bremer, 1994, 1997). The latter were estimated using PRAP (Müller, 2004, 2005), metrics similar to the PC-compatible algorithms of Goloboff (1999) and Nixon (1999). Ratchet methods were used in order to find the minimum Bremer index by avoidance of entrapment in local optima (Maddison, 1991). For the Bremer (support) indices, 20 ratchet replicates per node were used (Müller 2004, 2005). The popular alternative protocol, TREEROT, was not employed because its primary asset –‘partitioned’ support indices – were not a priority here and (most importantly) the algorithm lacks the ratchet (Sorenson, 1999).
Comparisons with other trees
Tests of alternative hypotheses proposed by other authors were equivalent to local penalties, i.e. minimal differences in total length imposed by the alternative hypothesis, while other aspects of the MPT (exclusive of pruning of taxa essential for comparability) were conserved (Kluge, 1997a, b). These estimates were made by simple transfer of branch(es) within the consensus cladogram using MACCLADE (Maddison & Maddison, 1992), which holds other topological groupings constant. This procedure differs from searches constrained only to the grouping of interest, typically performed using ancillary searches under inverse constraints, as in protocols for estimation of Bremer (support) indices.
Critical concepts and terminology
‘It is possible[50% likelihood] that – 1) a distant relationship exists between Apteryx and a tinamou-galliform assemblage; … (5) the diurnal birds of prey may be allied to the owls through the Falconidae … It is improbable[formerly widely believed, since discredited] that – 1) a close relationship exists between Rhea and the tinamous; … (3) Pandion deserves familial status in the Falconiformes …’ (Sibley & Ahlquist, 1972: 241), emphasis added.
‘… the mousebirds, or colies, [i] have no close living relatives, … [ii] they are the only survivors of an ancient divergence … Their [iii] closest living relatives are probably …’ (Sibley & Ahlquist, 1990: 363)
Before considering specific findings in the present study, a clarification of critical terms is essential. The first of the foregoing quotes comprises four statements of perceived probability that either make no objective sense or are self-contradictory by conventional standards. Also, the second quote contains three conclusions (i–iii) for a single group based on a single data set that are: either mutually contradictory (i and iii), or of undetermined meaning (ii vs. either i or iii). In cladistic terms, ‘most closely’ implies ‘closely’ in that hierarchy defines relative relationships. Sister-groups are by definition the ‘most closely related’ of any taxa compared. For example, in cladistic terms, an assumption of monophyly of life on earth implies that every taxon has a close relative and/or closest relative, regardless of extinctions. In other words, degree of relatedness is relative: all lineages have a closest relative and therefore a close relative. Sister-groups need not meet some standard of similarity or absolute antiquity of divergence to qualify. However, under an expectation of at least a limited correlation between evolutionary change in morphology with time – neither ‘clock-like’ nor wildly heterogeneous and completely disassociated – sister-taxa can be expected to share degrees of similarity broadly related to time since divergence, such that recency of divergence between sister-taxa tends to be associated with similarity, and antiquity of such divergence to be associated with dissimilarity.
RESULTS
Minimal-length trees or MPTs
The search for MPTs recovered 97 trees of minimal length (19 553 steps) under standard ordering of multistate characters and rooting by outgroup taxa as given (see Methods). This solution set (2.04 × 1011 rearrangements assessed) had the following summary statistics: CI = 0.2432; RC = 0.1664; RI = 0.6842; and skewness, g1|105 = −0.4258.
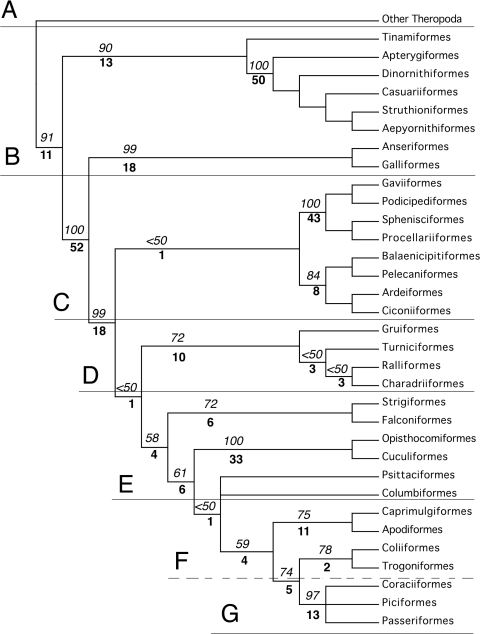
A strict consensus tree of the MPTs (Figs 10–18) was completely resolved for the Neornithes with the exception of six polytomies (mostly trichotomies, some nested, discussed below), uncertainties sufficiently limited so as to obviate a majority-rule consensus tree for the primary solutions set, or to delimit ambiguity where one or more ‘rogue taxa’ may be influential (Sumrall, Brochu & Merck, 2001). The strict consensus tree for the 97 MPTs shared the following summary statistics: (i) component information, 173; (ii) Nelson–Platnick term information, 4367; (iii) Nelson–Platnick total information, 4540; and (iv) Mickevich consensus information, 0.168.
Figure 10.
Ordinal-level strict consensus tree for orders of Neornithes based on 2954 morphological characters, indicating delimitations of segments detailed in Figures 12–18.
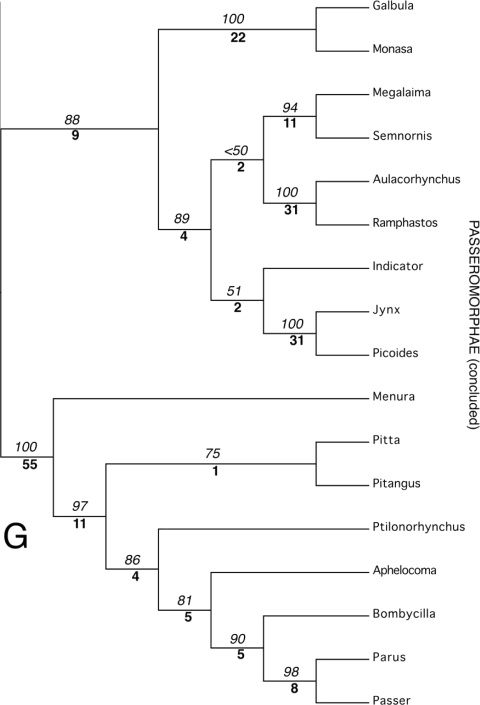
Figure 18.
Detailed segment of strict consensus tree of all MPTs recovered in present study. Part G. Neornithes: Piciformes, and Passeriformes. Nodes are labelled above by percentages of bootstrapped replicates in which node was retained (italics), and below by Bremer support indices (bold type).
Outgroup taxa: Mesozoic roots of Aves
Non-Neornithine Aves
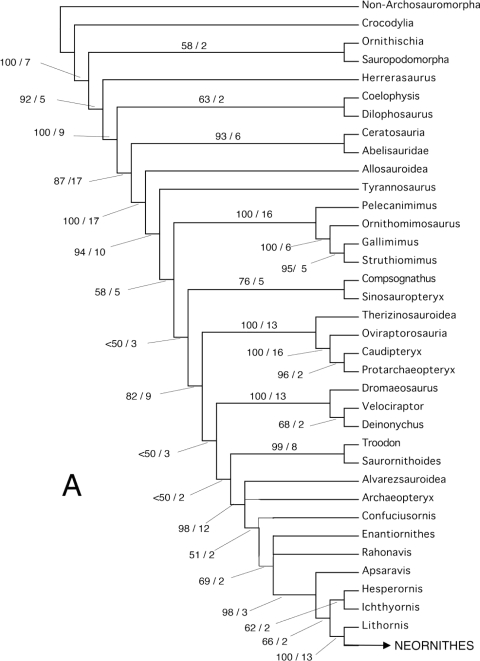
In light of the growing consensus regarding fossil lineages of the Mesozoic and widely employed characters thereof, broad agreement between our findings and those of others treating pre-neornithine birds was not unexpected. Relationships among outgroup taxa in this analysis generally were consistent with recent analyses (Martin, 1983; Witmer, 1991; Holtz, 1998; Padian & Chiappe, 1998; Clarke & Chiappe, 2001; Chiappe, 2001, 2002; Clarke & Norell, 2002, 2004; Clark, Norell & Makovicky, 2002; Chiappe & Dyke, 2002; Maryanska, Osmólska & Wolsan, 2002; Pisani et al., 2002; Snively, Russell & Powell, 2004; Mayr, Pohl & Peters, 2005; Zhou & Zhang, 2005).
Critical for empirical rooting of ingroup taxa, as opposed to hypothetical ancestors or other synthetic means of proposing polarities, this congruence lends credence to assessments of polarities of characters at the most basal of neornithine nodes (e.g. the divergence of neognathous from palaeognathous taxa). Crocodylians fell as predicted among the basal Archosauria (Larhammar & Milner, 1989; Hedges, 1994). Principal exceptions from a growing consensus of palaeontologists were reversed positions or irresolution within two pairs (Fig. 12): (i) Troodontoidea (Troodon and Saurornithoides) and Dromaeosauroidea; and (ii) Rahonavis and Apsaravis, the latter couplet being equally parsimonious whether paraphyletic to other taxa or as sister taxa. Details of positions among outgroups are of secondary interest here, but it is noteworthy that the few instances of incongruence with other studies were associated with exceptionally poorly supported nodes or polytomies in the present work (Fig. 12). It is likely that the generally lower support indices among pre-Neornithes reflect missing key taxa and poor preservation of those coded.
Figure 12.
Detailed segments of strict consensus tree of all MPTs recovered in present study. Part A. Outgroup (non-neornithine) taxa. Nodes are labelled by percentages of bootstrapped replicates in which node was retained (numerator), and below by Bremer support indices (denominator).
Neornithes
‘… it is probable that the majority of living genera [of birds] were in existence by the end of the Tertiary… . Most, perhaps all, of the [modern] orders of birds had become established by the end of the Eocene.’ (Brodkorb, 1971a: 42)
‘The phylogenetic position of Palaeogene birds thus indicates that diversification of the crown-groups of modern avian “families” did not take place before the Oligocene, irrespective of their relative position within Neornithes (crown-group birds).’Mayr (2005a: 515)
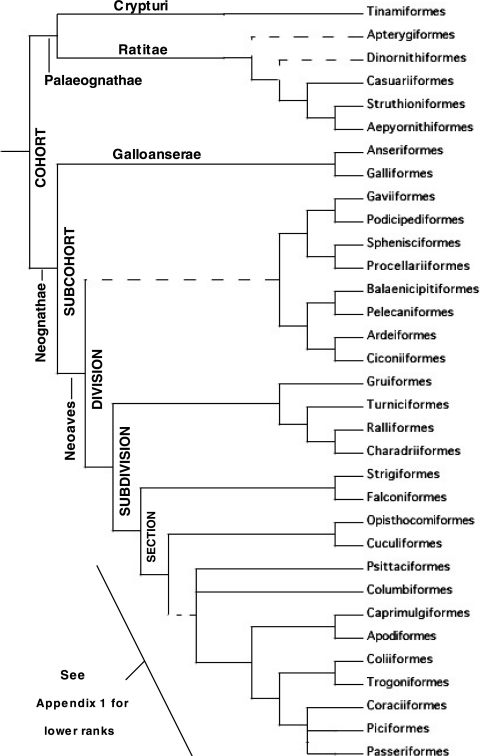
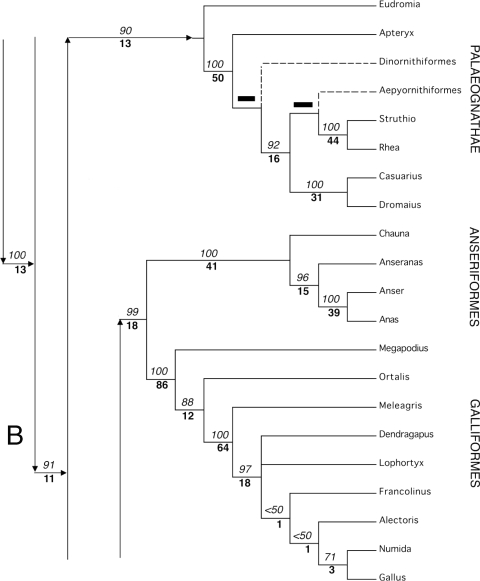
Strong support for monophyly of the Neornithes (Table 2; Figs 10, 11) was conferred. Notable, however, in the present reconstruction was its poor congruence with the ‘tapestry’ depicted by Sibley & Ahlquist (1990), in which only three higher-order taxa – their Ratitae, Galloanserae and Procellarioidea, and monophyly of one contentious order (Caprimulgiformes) – were in significant agreement with corresponding clades in the present analysis. Points of disagreement, however, were abundant and included much of the topological (diagrammatic) structure in the two works, and notably included the following groupings depicted by Sibley & Ahlquist (1990: fig. 4A): (i) monophyly of {Ratitae, Galloanserae}; (ii) provisional, exceptionally basal placement of Turnix; (iii) very basal positions and interposition of Piciformes, Coraciiformes, Coliiformes, Trogoniformes and Passeriformes; (iv) multiple discrepancies associated with hypotheses of polyphyly of Pelecaniformes and Ciconiiformes, and (v) inclusion of some Gaviiformes, Podicipediformes, Sphenisciformes and Falconiformes among these groups. Topological dichotomies that hierarchically group modern orders of Neornithes were sought (Fig. 10), and these formed the ordinal basis for a higher-order classification (Appendix 1).
Figure 11.
Simplified summary tree for uppermost, supraordinal ranks of avian classification. Dashed internodes correspond to marginally supported clades. For complete classification, see Appendix 1.
In the following, descriptions of findings, statistics of support, etc., were presented in figures, and reference to these was employed in place of repetition of metrics in the text. Consequently, readers are directed to the appropriate figures and tables where narratives refer to robustness, support and relative parsimony of alternative hypotheses.
Modern palaeognathous birds
This analysis revealed the relationships among the palaeognathous birds to be exceptionally resolved, well supported, virtually unambiguous, empirically rich, markedly traditional, and supported by an unprecedented sample of outgroups. The ratites or flightless modern palaeognathous birds have been the subject of more anatomical and molecular study than any other avian group, an important motivation for which concerned diagnoses of plesiomorphic and apomorphic morphological characters in a group widely recognized to represent an early branch among Neornithes but for which useful outgroups were lacking (Balouet, 1984; Zusi, 1993). Basal polarities of characters of plesiomorphic condition among modern and closely related fossil palaeognathous taxa (Houde & Olson, 1981; Houde, 1988; Leonard et al., 2005) awaited resolution by means of the most primitive Aves, many recovered only recently (Appendix 1).
Taxonomically orientated anatomical studies, emphasizing ratites or more inclusive in scope, ensued during the 19th and 20th centuries (Fürbringer, 1888; Feduccia, 1980; Houde & Haubold, 1987), and investigations of phylogenetic emphasis were among the earliest for Neornithes (Verheyen, 1960a; Sibley & Ahlquist, 1972; Cracraft, 1974a; Wattel, Stapel & de Jong, 1988). In some cases, inference of the primary grade of divergences of palaeognathous, galloanserine and other neognathous taxa aided in the recovery of historical patterns and broad outlines of phylogeny of palaeognathous taxa, patterns that were to prove beyond the limits of mtDNA for resolution (Härlid, Janke & Arnason, 1997, 1998).
Most prior studies regardless of method – notably excepting early works conceptually confined by the dated biogeographical paradigm of static continents (Briggs, 2003) or phenetic perspectives on affinities (McDowell, 1948; de Beer, 1956; Storer, 1960a, 1971a, b; Sibley & Frelin 1972) – have hypothesized that the palaeognathous birds are the sister-group of other Neornithes, the Tinamiformes are the sister-order of the ratites among palaeognathous taxa (Caspers, Wattel & de Jong, 1994; de Kloet & de Kloet, 2003), and accordingly the ratites are monophyletic (Bock, 1963b; Prager et al., 1976; Stapel et al., 1984; Bock & Bühler, 1988; Härlid et al., 1997; Lee et al., 1997; Van Tuinen, Sibley & Hedges, 1998; Dyke, 2001a; Dyke & Van Tuinen, 2004; Slack et al., 2006a, b). These findings counter early disputes based in part on biogeography, isolated interpretations of fossils (Houde & Olson, 1981), speculations regarding heterochrony (Feduccia, 1985) and (subsequently admitted) analytical anomalies (Härlid & Arnason, 1998). Notable in the last of the foregoing categories was the initial inference of a sister-relationship between a neognathous group comprising the Galliformes and Anseriformes and the palaeognathous birds by Sibley & Ahlquist (1990), a topology rendering at the outset the polyphyly of neognathous taxa; subsequently these authors depicted the neognathous birds as monophyletic.
Monophyly of the Tinamiformes was supported by the molecular analyses by Paton et al. (2002) and Harrison et al. (2004), but minimal taxonomic sampling diminished the generality of these inferences. Sister-group relationships of palaeognathous orders – Struthioniformes and Rheiformes, and Dromaiiformes and Casuariiformes – were supported strongly here (Fig. 13) and elsewhere (Lee, Feinstein & Cracraft, 1997; Leonard, Dyke & Van Tuinen, 2005). A minority of earlier findings (Figs 7A, 8B) provided weak evidence of paraphyly of the Struthioniformes and Rheiformes with respect to a sister-grouping of Dromaiiformes and Casuariiformes and also provided weak support for the Apterygiformes as as sister-group to the latter (Van Tuinen, Sibley & Hedges, 2000; Cooper et al., 1992, 2001; Paton et al., 2002; Harrison et al., 2004). Despite support indices suggestive of robustness in several of the molecular works, questions regarding Bayesian bootstrap values (Simmons, Pickett & Miya, 2004) justify caution in such assessments.
Figure 13.
Detailed segment of strict consensus tree of all MPTs recovered in present study. Part B. Neornithes: Palaeognathae and Galloanserae. Nodes are labelled above by percentages of bootstrapped replicates in which node was retained (italics), and below by Bremer support indices (bold type).
Figure 7.
Molecular phylogenetic trees proposed in previous studies (see Fig. 1 for details), VII. A, Paton et al. (2002); B, Sorenson et al. (2003).
Figure 8.
Molecular phylogenetic trees proposed in previous studies (see Fig. 1 for details), VIII. A, Chubb (2004a); B, Harrison et al. (2004).
The Apterygiformes, herein placed as sister-group to all other ratites (Fig. 13), have been inferred to occupy a marked diversity of positions in prior studies (Cracraft, 1974a, 2001; Lee et al., 1997; Cooper et al., 2001; Haddrath & Baker, 2001; Paton et al., 2002; Harrison et al., 2004). Also, the position of the Apterygiformes relative to the extinct Dinornithiformes varied (Vickers-Rich et al., 1995). The Apterygiformes are the most speciose and genetically subdivided of extant orders of ratites (Baker et al., 1995; Burbridge et al., 2003), but are significantly less diverse than the formerly sympatric Dinornithiformes.
The position of the Dinornithiformes also remains a point of controversy, in part because of missing data for this extinct, diverse group; monophyly and relationships among members have been confirmed (Baker et al., 2005). Cracraft (1974a, 2001) considered the Dinornithiformes to be the sister-group of the Apterygiformes, contrary to Cooper et al. (1992, 2001), Van Tuinen et al. (1998, 2000), Haddrath & Baker (2001) and the present provisional inferences. In most respects, the topologies for ratites inferred by Lee et al. (1997) and Dyke & Van Tuinen (2004: fig. 4) most closely approximated that inferred here (Fig. 13).
Missing data for two orders of ratites – Dinornithiformes and Aepyornithiformes – proved analytically problematic if included unconditionally with extant ratites. Unrestricted analysis of these extinct, moderately related, highly divergent, sparsely coded lineages resulted in a suspicious placement of these two orders as sister taxa. The large numbers of missing data in the two extinct lineages, many lacking in both taxa, prompted two alternative analyses to be performed. Global searches of Dinornithiformes (excluding the poorly known Aepyornithiformes) and placements within the MPT as backbone-constraint placed the moas to be the sister-group of other ratites exclusive of Apterygiformes (Fig. 13), contrary to a sister-relationship between these New Zealand endemics as advocated by Cracraft (1974a, 2001). By backbone-constraints or exclusion of the Dinornithiformes, the Aepyornithiformes were placed as the sister group of the clade comprising Struthionidae and Rheidae (Fig. 13).
Galliformes and Anseriformes: land and water fowl
Interordinal relationships
The sister-group relationship between the Galliformes and Anseriformes, reaffirmed here (Fig. 13), was inferred previously by Cracraft (1981, 1988), Cracraft & Mindell (1989), and substantiated thoroughly using morphological (Dzerzhinsky, 1995; Caspers et al., 1997; Livezey, 1997a, 1998a; Cracraft & Clarke, 2001; Dyke, 2003; Mayr & Clarke, 2003) and molecular data (Bleiweiss et al., 1994, 1995; Groth & Barrowclough, 1999; Van Tuinen et al., 2000, 2001; Cracraft, 2001; Prychitko & Moore, 2003; Chubb, 2004a; Harrison et al., 2004; Simon et al., 2004; Smith, Li & Zhijian, 2005). However, marginally supported counter-proposals persist (Ericson, 1996, 1997; Ericson, Parsons & Johansson, 1998; Bourdon, 2005).
Anseriformes
Within the waterfowl (Anseriformes), sequential sister-group relationships of the Anhimidae, Anseranatidae and Anatidae, respectively, was previously demonstrated by Livezey (1997a) and confirmed here (Fig. 13). Monophyly of the morphologically diverse and speciose Anatidae, including the true geese (Anserinae) and typical ducks (Anatinae), is essentially beyond dispute (Livezey, 1986). There exist departures from this arrangement by a minority of workers (Olson & Feduccia, 1980a; Sraml et al., 1996), but this topology has been substantiated using diverse evidence (Livezey, 1986, 1997a; Quinn, 1992; Donne-Gousséet al., 2002). The historical hypothesis placing the Phoenicopteridae within the Anseriformes (Table 1) was among the early casualties of formal phylogenetics (Livezey, 1997a, 1998a).
Galliformes
The pioneering myological works by Hudson, Lanzillotti & Edwards (1959) and Hudson & Lanzillotti (1964) provided early hints concerning relationships of Galliformes, but unfortunately these surveys were not cladistic and followed Peters (1934) in considering unique Opisthocomus as an aberrant galliform. Studies of galliform fossils continue to be phenetic in approach (Mourer-Chauviré, 2000; Göhlich & Mourer-Chauviré, 2005). Fortunately, this pattern is likely to change with the increasingly common phylogenetic analyses of galliforms (Dyke, Gulas & Crowe, 2003) and an improved fossil record (Mayr & Weidig, 2004; Mayr, 2005a).
In the present work, relationships of two families within the Galliformes – Megapodiidae (Birks & Edwards, 2002) and Cracidae (Pereira & Baker, 2004; Grau et al., 2005) as mutually monophyletic, sequential sister-groups to all remaining galliforms – agree with placements by other investigators (Prager & Wilson, 1976; Cracraft et al., 2004). Some workers (Hudson et al., 1966), however, suggested a sister-group relationship between the two families (superfamily Cracoidea), as opposed to placement as successive sister-groups (paraphyletic) to other galliforms (Fig. 13).
The robust placement of Meleagrididae as sister-group to the Phasianidae sensu lato in the present work (Fig. 13) opposes inclusion of the family among the enormous complement of other galliforms (reviewed by Sibley & Ahlquist, 1990). The present finding also differs with the indeterminate placement of this distinctive group from most galliforms by Dyke et al. (2003). Dyke et al. (2003: fig. 3) depicted the Megapodiidae and Cracidae as basal, successive sister-groups to the diverse and speciose ‘Phasianoidea’; the latter group included Numida and Acryllium (Numidinae) as members of a polytomous assemblage immediately basal to Meleagris, Agriocharus, Tetraonidae, and a clade comprising 39 taxa of other galliforms inviting taxonomic subdivision. Most of the large-bodied genera of phasianoids (e.g. Gallus, Phasianus) and the ‘Old World quail and partridges’ were among a large, basal polytomy of the ‘phasianoids’ exclusive of the guineafowl (Numidinae). Some of the nodes within this large group, including those resolving Meleagridae and Tetraonidae relative to megapodiid and cracid galliforms, were not sustained by Dyke et al. (2003: fig. 3) in a strict consensus of 1700 MPTs based on 102 characters. Also, the tree inferred here (Fig. 13) departed from those recovered using molecular data (Dimcheff, 2002; Dimcheff, Drovetski & Mindell, 2002).
Figure 3.
Morphological phylogenetic trees proposed in previous studies (see Fig. 1 for details), III. A, Mayr (2005b); B, Mayr (2005f: fig. 9), excluding fossils Prefica and Paraprefica.
The vast majority of galliform taxa are members of a morphologically conservative group (Holman, 1961), many formerly included among the Perdicidae or Odontophoridae (Sibley & Ahlquist, 1990). These taxa also posed problems of resolution in the present work (Fig. 13), and nodes among these taxa were sufficiently weak as to permit alternative local topologies (i.e. a terminal polytomy). Armstrong, Braun & Kimball (2001) found that mitochondrial and nuclear DNA similarly resolved groupings within a sparse but broad sample of Galliformes. Basal nodes of the latter taxa are broadly consistent with some higher-order topologies (Prager & Wilson, 1976; Helm-Bychowski & Wilson, 1986; Crowe et al., 1992; Kimball et al., 1999; Gutiérrez, Barrowclough & Groth, 2000; Lucchini et al., 2001; Dimcheff et al., 2002; Pereira, Baker & Wajntal, 2002). The single exception among this group (based on included genera) is the strongly supported sister-group relationship between Gallus (Phasianidae) and Numida (Numidinae). The Numidinae were inferred to be the sister-group of the Phasianidae by Kimball et al. (1999) and Pereira & Baker (2006a).
Marine assemblage
A diversity of mutually distinctive groups of aquatic birds have been the focus of much early speculation regarding the potentially misleading effects of similarities of locomotion leading to morphological convergence. Most evocative of these speculations concerned the Gaviiformes and Podicipediformes (e.g. Stolpe, 1935; Storer, 1956, 1960b), foot-propelled diving specialists that prompted arguments based on phenetics, assumptions of ancestral status for fossils, simplistic proposals of evolutionary trends and (most fundamentally) a failure to meet conventional standards of phylogenetic inference. These shortcomings notwithstanding, such proposals from this era gave rise to a general and uncritical acceptance of rampant convergence uniquely afflicting morphological characters, claims that persist to the present day.
Various alliances among the Gaviiformes, Podicipediformes and Procellariiformes were suggested by Mayr & Amadon (1951), and proved consistent with myological data analysed by McKitrick (1991a, b) and molecular patterns recovered by Watanabe et al. (2006). A relationship between the Gaviidae and Charadriiformes was considered plausible by Storer (1956). Without explanation, however, Storer (1971b) listed the loons and grebes together immediately following the Charadriiformes, in apparent contradiction to his previous opinion. Foreshadowing a natural radiation of marine birds, Ho et al. (1976) inferred a comparatively close relationship of the Sphenisciformes with other primarily marine orders, and fossil evidence for loons – of only marginal quality, optimistic appraisals by Olson (1992a) and Mayr (2004a) notwithstanding – suggests an early origin at least for the Gaviiformes. A phylogenetic alliance among the Sphenisciformes, Procellariiformes, Gaviiformes and Podicipediformes was substantiated as well by Cracraft (1982a), and this was indicated by Nunn & Stanley (1998) and Slack et al. (2006a) on molecular grounds.
The comparatively robust skeletal elements of penguins predispose them to fossil preservation, and recently recovered remains hold promise for stratigraphic chronology (Slack et al., 2006b). The clade of basal marine taxa inferred herein evolved myriad modes of foraging (Storer, 1971a): (i) Gaviiformes and Podicipediformes being extremely specialized foot-propelled diving birds; (ii) Sphenisciformes and Pelecanoididae (Procellariiformes) being wing-propelled diving birds, submarine ‘flight’ of the former rendering members aerially flightless (Livezey, 1989a); and (iii) Procellariiformes, comprising hover-foraging Oceanitidae and other families combining wind-powered gliding and plunge-diving (Del Hoyo, Elliott & Sardgatal, 1992). Some fossil groups remain of uncertain ordinal affinity – e.g. the wing-propelled Plotopteridae (Olson & Hasegawa, 1979, 1996; Olson, 1980; Goedert, 1988; Goedert & Cornish, 2002; Mayr, 2004b) – and did not merit analysis herein, where states for cranial characters are critical but specimens are woefully incomplete. Early descriptions suggested the inclusion of the Plotopteridae among Pelecaniformes is competitive with an alternative relationship to Sphenisciformes for which pectoral similarities were emphasized (Mayr, 2004b). Dissent regarding the ordinal relationships of the Plotopteridae is consistent, to a point, with the interordinal relationships of the Pelecaniformes and Sphenisciformes inferred herein (Fig. 14).
Figure 14.
Detailed segment of strict consensus tree of all MPTs recovered in present study. Part C. Neornithes: nodes are labelled above by percentages of bootstrapped replicates in which node was retained (italics), and below by Bremer support indices (bold type).
Monophyly of the Sphenisciformes seldom has been doubted, and resolution of relationships among modern and fossil species was achieved (Ksepka, Bertelli & Giannini, 2006), but the position of this distinctive marine group remains a long-standing controversy. This duality of distinct synapomorphy and symplesiomorphy underlies a number of classificatory problems of Aves, in which marked distinction of groups tends to confound comparisons with other groups. Of the alternatives proposed, an affinity with the Procellariiformes has received broadest support, both in the present analysis (Fig. 14) and elsewhere (Cracraft, 1981, 1986, 1988).
Despite agreement with the inferences by Cracraft (1982a), it is predictable that strong confirmation of a sister-group relationship between the Gaviiformes and Podicipediformes (Fig. 14) herein will engender concerns of artefactual pairing by convergence (Storer, 1956, 1971a, 2000, 2002). Storer (2002: 16) felt that the non-phylogenetic work by Stolpe (1935) ‘… demonstrated that the similarities among the loons, grebes, … resulted from convergent evolution …’ The inclusion of the Mesozoic Hesperornithiformes with modern Gaviiformes and Podicipediformes by Cracraft (1982a), a finding not supported here (Figs 10, 14), was the inference subjected to greatest criticism. Obvious similarities of form and life history have prompted exceptional attention to differences between the two orders (e.g. Sibley & Ahlquist, 1972: table 1), tallies without benefit of polarities or phylogenetics. In many cases, these rationalizations are undermined with respect to functional comparisons, e.g. the Gaviidae employ feet for primary propulsion but also use their wings (Olson, 1985), and members of the two orders also differ in the movements typical of the pelvic limb (Storer, 1956). Pairing of the Gaviiformes with the Podicipediformes as sister-groups has been championed by Cracraft (1982a, 1988), a proposal not without opposition (e.g. Storer, 1956, 1960b, 1971a; Sibley & Ahlquist, 1972, 1990). Additional support for this ordinal pairing has been reported ( Cracraft & Mindell, 1989; Bourdon, Boya & Iarochène, 2005), but most other analyses excluded one or both of these key orders, rendering comparisons among such works regarding these orders impossible.
Without a consensus regarding a relationship between the Podicipedidae and Gaviidae, the former have been the subject of several extraordinary proposals, based on relatively weak evidence or mere speculation. Olson (1985: 168), under the subheading ‘Family Incertae Sedis Podicipedidae’, stated: ‘In looking beyond their obvious specializations for diving, I cannot see that the grebes (Podicipedidae) would be out of place in the Gruiformes.’ A more precise proposal for the latter is a possible affinity on myological grounds with the gruiforms Rhynochetos and Eurypyga (Zusi & Storer, 1969). An apparent variant of this speculation was a possible relationship with the Heliornithidae and the closely related Rallidae (Beddard, 1893; Olson, 1985; Houde, 1994). Also, a tenuous alliance between the Podicipedidae and Cuculidae was depicted by Van Tuinen et al. (2000), but subsequent works have failed to support this grouping. Another position recently inferred for the Podicipediformes relates to the Phoenicopteridae (Van Tuinen et al., 2001; Mayr, 2004c), a proposal considered further below.
In most respects, inferences herein regarding the Procellariiformes were among the least contentious for the marine assemblage, whether in comparison with traditional (Kuroda, 1954) or modern reconstructions (Nunn & Stanley, 1998; Kennedy & Page, 2002; Watanabe et al., 2006). A moderate departure from traditional arrangements is the finding herein of the Diomedeidae (albatrosses) as comparatively derived, with other Procellariiformes paraphyletic to the typical Procellariidae (Austin, 1996; Gómez-Díaz et al., 2006) and Diomedeidae (Nunn et al., 1996).
Pelecans and allies: totipalmate birds
The totipalmate or pelecaniform birds, as traditionally defined, remain a higher-order group of extraordinary controversy, but in reality the suite of unifying characters, stressed by Beddard (1898), has been expanded for decades beyond the totipalmy cited as sole uniting anatomical character for the order by Sibley & Ahlquist (1972). Polyphyly of the order was inferred subsequently by Sibley & Ahlquist (1990) and Hedges & Sibley (1994). The status of the Pelecaniformes has been debated since the core assemblage was included in widely recognized classifications (Mayr & Amadon, 1951; Wetmore, 1930, 1960), and points of controversy include those of monophyly, content and interordinal position, as empirically derived from metric (Verheyen, 1960b), neontological (Cracraft, 1985), palaeontological (Bourdon, 2005; Bourdon et al., 2005) and molecular perspectives (Siegel-Causey, 1997; Farris et al., 1999).
The exceptional heterogeneity of traditionally included families – e.g. frigatebirds, gannets and pelicans – render questions of membership especially problematic. Perhaps most intriguing of the debated memberships is that of the shoebill or Balaeniceps (Reinhardt, 1860, 1862; Cottam, 1957; Feduccia, 1977a; Mayr, 2003a). Purportedly intermediate features of ‘stork-like’ and ‘pelican-like’ forms (Van Tuinen et al., 2001; Bourdon et al., 2005) have extended to proposals of pelecaniform affinity of the hammerkop (Scopidae). In agreement with the present analysis, the consensus of available phylogenetic works places the distinct Phaethontidae as sister-group to other pelecaniforms exclusive of Balaeniceps (Mayr & Clarke, 2003), with an alternative position hypothesized for the Phaethontidae as an exceptional plesiomorph allied to some pelecaniforms and the Procellariiformes (Bourdon et al., 2005). The present study also resolved Balaeniceps as sister-group to the clade comprising Phaethontidae and other (traditional) Pelecaniformes. Scopus was not inferred here to be closely related to the Pelecaniformes (Fig. 14), contraMayr (2003a).
Relationships among traditional Pelecaniformes (excluding Balaeniceps), inferred cladistically by Cracraft (1985: figs 6, 7), agreed with the inferences presented herein (Figs 10, 14), whereas comparisons between the studies with respect to the orders Sphenisciformes, Gaviiformes, Podicipediformes and Procellariiformes were not possible. Sibley & Ahlquist (1990) proposed a ‘four-fold’ polyphyly of Pelecaniformes among the most notable departures of their analysis from contemporary arrangements, whereas several other traditional elements were conserved in their scheme. Hedges & Sibley (1994), based on an analysis impoverished in both data and taxa, also suggested polyphyly of taxa traditionally considered pelecaniform in a work remonstrated by Farris et al. (1999). Syntheses by Van Tets (1965) and Siegel-Causey (1997: fig. 6.3) reaffirmed ordinal monophyly (exclusive of Phaethontidae) using morpho-ethological data, whereas molecular reconstructions violated ordinal monophyly by topologically variable inclusions of the Diomedeidae, Procellariidae and Cathartidae (Siegel-Causey, 1997: fig. 6.2). One minor departure from tradition by Sibley & Ahlquist (1990) was a terminal triad in which the Phalacrocoracidae were placed as sister-group to the Anhingidae and Sulidae.
Figure 6.
Molecular phylogenetic trees proposed in previous studies (see Fig. 1 for details), VI. A, Van Tuinen et al. (2000); B, Van Tuinen et al. (2001).
Kennedy & Spencer (2004) weakly confirmed monophyly of the traditionally constituted order, in part by use of appropriate outgroups but despite heterogeneous taxonomic sampling of ingroup families. Three weakly resolved departures by Kennedy & Spencer (2004) from the hypothesis inferred herein (Fig. 14) were: (i) reversal of the positions of the Phaethontidae relative to the Fregatidae + Pelecanidae; (ii) a sister-relationship between the Pelecanidae and Phaethontidae; and (iii) paraphyly of Phalacrocoracidae and Anhingidae to the Sulidae.
The Phalacrocoracidae and Anhingidae – families long considered closely related and strikingly similar in external and skeletal aspects (Siegel-Causey, 1988) – have been subjected to unexpected hypotheses of relationship. A series of related papers (Kennedy, Spencer & Gray, 1996; Kennedy, Gray & Spencer, 2000; Kennedy & Spencer, 2000, 2004; Kennedy et al., 2005), based on limited taxonomic representation of pelecaniform families and unconventional analytical methods, mustered mtDNA sequences and behavioural data that favoured paraphyly of these two families to the Sulidae, also inferred phenetically by Sibley & Ahlquist (1990). Based on the present analysis (Table 3), however, a sister-group relationship between Phalacrocoracidae and Anhingidae is strongly favoured.
Table 3.
Alternative topological inferences and minimal differences in tree length (additional steps) relative to placements in MPTs (Figs 11–17), conditional on other topological alterations being prohibited (optimizations of characters thereon permitted). Higher-order taxa correspond to classification proposed in Appendix 1
| Taxon | Alternative hypothesis* | Δ length | References |
|---|---|---|---|
| Palaeognathae | ∪ Galloanseromorphae | 54 | Sibley & Ahlquist (1990) |
| Ratitae (global)† | Δ topology | 31 | Cracraft (1974a) |
| Ratitae (local)† | Δ topology | 63 | Cracraft (1974a) |
| Δ topology‡ | 17 | Cooper et al. (2001) | |
| Δ topology‡ | 13 | Haddrath & Baker (2001) | |
| Galloanserimorphae | Polyphyly§ | [19] | Bourdon et al. (2005) |
| Galliformes | Polyphyly | 90 | Dyke et al. (2003) |
| Megapodiidae | ∪ Cracidae | 10 | Dyke et al. (2003) |
| Meleagrididae | ∪ Phasianidae | 20 | Dyke et al. (2003) |
| Anseriformes | Δ familial topology | 54 | Olson & Feduccia (1980a); Livezey (1997a) |
| Anhimae | ∪∨⊂ Galliformes | 41 | Olson & Feduccia (1980a); Livezey (1997a) |
| Gaviomorphae | ∪ Charadriomorphae | 72 | Storer (1956); Olson (1985) |
| Podicipediformes | ∪ Phoenicopteridae | 146 | Mayr & Clarke (2003); Mayr (2004a) |
| ∪ Charadriomorphae | 54 | Storer (1956) | |
| ∪ Eurypygidae | 182 | Zusi & Storer (1969) | |
| ∪ Ralliformes | 159 | Olson (1985); Houde (1994) | |
| Pelecaniformes | Δ topology | 344 | Kennedy & Spencer (2004: fig. 1B) |
| Sulae | Δ topology | 125 | Kennedy et al. (2005: fig. 8) |
| Balaenicepitidae | ¬∪ Pelecaniformes | 30 | Cracraft (1985); Mayr (2003a) |
| Scopidae | ∪∨⊂ Pelecaniformes | 23 | Mayr (2003a) |
| Threskiornithidae | ∪∨⊂ Charadriiformes | 174 | Olson (1978) |
| Ardeidae | ⊂ (Turnices ∪ Eurypygae) | 75 | Olson (1978) |
| Phoenicopteridae | ∪ Anseriformes | 107 | Feduccia (1976, 1977b); Hagey et al. (1990) |
| ∪ Cladorhynchini | 154 | Olson & Feduccia (1980b) | |
| Gruiformes (traditional) | Monophyly¶ | 11 | Livezey (1998b) |
| Charadriiformes | Δ topology | 60 | Strauch (1978) fideChu (1995: fig. 1) |
| Δ topology | 106 | Sibley & Ahlquist (1990) fidePaton et al. (2003) | |
| Δ topology | 82 | Chu (1995: fig. 8), excluding Ibidorhyncha | |
| Mesitornithidae | ∪ Cuculiformes | 107 | Mayr & Ericson (2004) |
| Strigiformes | ∪ Caprimulgiformes | 43 | Hoff (1966) |
| Cathartidae | ∪∨⊂ Ciconiiformes | 112 | Ligon (1967); Rea (1983); Avise et al. (1994a) |
| Opisthocomidae | ∪∨⊂ Galliformes | 120 | Hudson et al. (1959); Hudson & Lanzillotti (1964) |
| ∪ Cuculiformes** | 22 | Avise et al. (1994b); Hughes & Baker (1999) | |
| Caprimulgiformes | Polyphyly | 31 | Mayr (2002a, b) |
| ∪ Cypselomorphae | 42 | Mayr (2002a, 2003c, 2004d, 2005f, g) | |
| Aegothelidae | ∪ Apodiformes | 31 | Mayr (2002a, 2003c, 2004d, 2005f, g) |
| Steatornithidae | ∪ Trogoniformes | 102 | Mayr (2003b) |
| Hemiprocnidae | ∪ Apodidae, monophyly | 5 | Sibley & Ahlquist (1990: fig. 361) |
| Apodidae | ∪ Passeri (Hirundinidae) | 193 | Shufeldt (1889b); Van Tuinen (2002) |
| Galbulae | ∪∨⊂ Coraciiformes | 20 | Olson (1983a) |
| Coraciiformes | Δ topology, ∈ Trogoniformes | 199 | Lowe (1946); Maurer & Raikow (1981) |
| Coracii | Δ topology | 64 | Cracraft (1971b) |
| Menura | ∪∨⊂ Passeri | 11 | Irestedt et al. (2001); Barker et al. (2002) |
Set-symbolism coopted for concise statement of phylogenetic hypotheses, as follows: ∪, sister-group (disjoint) union; ⊂, included as subclade; ∈, included as a member taxon; ∨, or; Δ, change in; ¬, not (negation of predicate argument).
Local optima for Aepyornithiformes and Dinornithiformes (as bi-ordinal sister-group to ratites exclusive of Apterygiformes) and global optima (former as sister-group to Struthionidae and Rheidae, latter as sister-group to ratites exclusive of Apterygiformes).
Comparisons excluded effects due to differences in outgroup taxa, as well as tentatively placed Aepyornithiformes.
Doubtful comparability given differences in taxonomic samples between studies.
Corresponds to that proposed by Livezey (1998b), exclusive of Pedionomidae and fossil gruiforms (Cracraft 1969, 1971a, 1973a).
Alternative hypothesis compared sister-grouping with Cuculiformes exclusive of Musophagidae.
Storks, herons and allies
‘Wading birds’, as delimited here, comprise the typically long-legged, long-necked storks and herons, and exclude the morphologically reminiscent cranes and allies (Gruiformes) and the potentially allied shorebirds (Charadriiformes). Highest-order nodes resolved in the present study defined a primary division of (i) ‘herons’ from (ii) ‘storks’ and allies as sister-groups (Fig. 14). Among the ‘storks’, Scopus is the sister-group to other members, the latter comprising clades partitioning the (i) ibises and spoonbills, and (ii) flamingos and typical storks. Within the ‘herons’, the only notable finding is the placement of Cochlearius as sister-group to other herons (Fig. 14), an inference consistent with traditional classifications (e.g. Wetmore, 1960) and earlier findings (Cracraft, 1967a; Sheldon, Jones & McCracken, 2000).
Shufeldt (1901b) suggested affinities between the Phoenicopteridae (flamingos) and both the Anseriformes (waterfowl) and the Ciconiiformes (storks and traditional allies). Olson (1978) questioned the monophyly of the traditional Ciconiiformes on phenetic grounds, suggested charadriiform affinities of Phoenicopteridae and Threskiornithidae, and expressed uncertainty regarding the ordinal placement of the herons (Ardeidae). Van Tuinen et al. (2001), based on conventional molecular estimates and the phenetics of DNA–DNA hybridization, found no support for monophyly of the Ciconiiformes in an analysis including representatives from several other traditional groups. Molecular reconstructions by Slikas (1997), however, confirmed monophyly of the morphologically diverse, ‘true’ storks (Scopus and Balaeniceps not sampled), groupings that also were afforded significant ethological support (Slikas, 1998).
It has been hypothesized in recent years that the Phoenicopteridae may be the sister-group of the grebes (Podicipediformes), a proposal supported by tenuous molecular (Van Tuinen et al., 2001) and morphological evidence (Mayr & Clarke, 2003; Mayr, 2004c; but see Storer, 2006). Given the variable viewpoints expressed regarding the Phoenicopteridae as well (Gadow, 1877; Shufeldt, 1889a; Feduccia, 1976, 1977a), this couplet offered the hope of dispensing with two challenging taxonomic placements by means of a single union, a circumstance not uncommonly an artefact of long-branch attraction (Philippe et al., 2005). Both of these autapomorphic taxa have been subjected to classificatory confusion for more than a century (e.g. Weldon, 1883; Shufeldt, 1901b; Jenkin, 1957), with affinities of the flamingos considered plausible between either the Ciconiiformes or the Anseriformes. Despite robust support for the more traditional position in the present analysis (Tables 2, 3; Figs 10, 14) and the minimal evidence presented by others for the proposal of the Podicipediformes, the latter hypothesis merits examination on the grounds of its superficial implausibility and the marked rearrangements of higher-order avian relationships it would imply. Supplementary morphological support for a sister-group relationship between grebes and flamingos marshalled by Mayr & Clarke (2003), however, required the exclusion (in a second analysis) of the loons – heretofore the global sister-group of the grebes – to sustain the grouping in question. Both exclusion of the Gaviiformes and narrow sampling of characters and taxa with which the Phoenicopteriformes were evaluated by Mayr (2004c) weakened the resultant inferences regarding the relationships of flamingos.
Chubb (2004a: 148) recovered 50% and 78% bootstrap support for this taxonomic couplet in analyses of different partitions of the ZENK gene, and joined Van Tuinen et al. (2001) in the speculation that: ‘… because both grebes and flamingos are highly derived morphologically and adapted to unique aquatic niches, their potential evolutionary alliance has previously gone unnoticed.’ Unfortunately, this rationalization is vulnerable to criticism because: (i) modifications for foot-propelled diving of grebes are comparable with those of several other groups of Neornithes – e.g. some Anatidae (Oxyurini, Mergini), Gaviidae, Phalacrocoracidae and Anhingidae; and (ii) the ‘unnoticed alliance’ between grebes and flamingos recognized by Chubb (2004a) instead was countered by a number of apomorphies in each genus that are shared with other taxa – e.g. Podiceps with Gavia, Phoenicopterus with (other) Ciconiiformes. The present data set (Livezey & Zusi, 2006) supports the rejection of this novel proposal involving the grebes and flamingos (Table 3; Fig. 14), and suggests that the taxonomic proposal for the couplet by Sangster (2005) is premature.
Cranes, rails, shorebirds and allies
The remaining long-legged, statuesque denizens of early successional, often wet habitats, together with the true shorebirds, compose the sister-group of remaining neornithine taxa (Fig. 15). These families, typically included within the traditional Charadriiformes and Gruiformes, have a long, perhaps unequalled history of debate in the ornithological literature (reviewed by Sibley & Ahlquist, 1972, 1990; Livezey, 1998b). Primary points of controversy concern the monophyly of the Gruiformes, and relationships between the taxa traditionally referred to the Gruiformes and the Charadriiformes; the latter order is known for especially great diversity in structure of the skull (Kozlova, 1961).
Figure 15.
Detailed segment of strict consensus tree of all MPTs recovered in present study. Part D. Neornithes: Gruiformes and Charadriiformes. Nodes are labelled above by percentages of bootstrapped replicates in which node was retained (italics), and below by Bremer support indices (bold type).
Gruiformes and allies
In an analysis of phylogeny and flightlessness of the Rallidae (Livezey, 1998b, 2003b), the traditionally delimited Gruiformes appeared to be monophyletic when analysed with only limited outgroups. However, in the more extensive sampling of higher-order groups of the present analysis (Fig. 15), this assemblage was resolved to be paraphyletic to the Charadriiformes. Most families included among the Gruiformes have been the subject of comparatively intense debate with respect to taxonomic position, e.g. Sibley, Ahlquist & DeBenedictus (1993) prepared an addendum for the Rallidae and allied families, and Houde (1994) revealed the difficulties of resolving the phylogenetic position of the Heliornithidae within the order. Nonetheless, the order contributed to early perceptions of southern-hemispheric origins of many non-passeriform birds (Cracraft, 1982b).
In the present work, most families formerly included among the Gruiformes were inferred to be monophyletic (Fig. 15), forming a single clade within which a primary bifurcation established the first of two subclades comprising the Otididae (Pitra et al., 2002) and Cariamidae (Livezey, 1998b). The second of the primary gruiform clades, and sister-group of the foregoing clade, comprised the sister-groups of (i) Eurypygae (i.e. Eurypygidae, Rhynochetidae and Aptornithidae as sequential sister-groups) and (ii) the nominate suborder Grues (i.e. Psophiidae, Aramidae and Gruidae as sequential sister-groups). New information on the Eocene fossil Eogrus (Cracraft, 1969; Clarke et al., 2005a) is consistent with monophyly of the Gruidae inferred by other means (Krawjewski & King, 1996). With the exception of an alternative position hypothesized for the subfossil Aptornithidae (Livezey, 1994; Houde et al., 1997), arrangements of these ordinally defining families have engendered only limited dissent (Mitchell, 1915; Livezey, 1994, 1998b).
Several families formerly included within the Gruiformes by Livezey (1998b), as detailed above, were inferred herein to be members of the sister-group of the Gruiformes, and specifically were resolved as two sequential sister-groups of the Charadriiformes (Fig. 15). Several of these have attracted an inordinate interest pertaining to phylogenetic position, diversity of form, intraordinal membership (e.g. Turnicidae) and manifestation of morphological intermediacy of others – e.g. Pedionomidae and Otididae (Gadow, 1891a; Bock & McEvey, 1969; Olson & Steadman, 1981). The present analysis provisionally placed the Turnicidae and Mesitornithidae as sister-taxa and the first of the two sequential sister-taxa (taxa paraphyletic) to the Charadriiformes (Fig. 15). Rotthowe & Starck (1998) agreed with both the present analysis and that by Livezey (1998b) regarding an affinity between the Turnicidae and Gruiformes, but Mayr & Ericson (2004) proposed a close relationship between the Mesitornithidae and Cuculiformes. The remaining sequential sister-group (lineage in this grade) comprised the Rallidae and its sister-group Heliornithidae (Fig. 15), a close relationship inferred both by Houde (1994) and Livezey (1998b), among others.
Charadriiformes
The preceeding clades subtended a clade herein interpreted as comprising the Charadriiformes. The true shorebirds, as resolved here (Fig. 15), comprise families of comparatively obvious ordinal affinity and great apomorphy, and generally accepted as monophyletic (Strauch, 1978; Björklund, 1994; Chu, 1994, 1995; Moum et al., 1994; Moum, Arnason & Arnason, 2002; Friesen, Baker & Piatt, 1996; Thomas, Wills & Székely, 2004a; Bridge, Jones & Baker, 2005). Relationships among several major groups of charadriiform birds have been inferred (e.g. Thomas, Wills & Székely, 2004b); however, the systematics of the group remains markedly controversial (Strauch, 1985; Christian, Christidis & Schodde, 1992; Paton et al., 2002; Ericson et al., 2003a; Van Tuinen, Waterhouse & Dyke, 2004; Paton & Baker, 2006; Pereira & Baker, 2006b).
The present analysis established the monophyly of the Charadriiformes, of which the Pedionomidae constituted the sister-group to other members (Fig. 15). The latter finding represents a slight departure from the marginal inclusion of Pedionomus among Gruiformes and affinities of the genus with the charadriiform Jacanidae (Whittingham, Sheldon & Emlen, 2000) and Rostratulidae inferred by Livezey (1998b), and is consistent with the inferences by Olson and Steadman (1981) and Ericson (1997). Within the Charadriiformes, Pedionomus is the sister-group to: (i) the bifamilial couplet comprising the Jacanidae and Rostratulidae, (ii) the monotypic Dromadidae and (iii) a clade comprising Thinocoridae and the sister-families Scolopacidae (e.g. Heteroscelus) and Phalaropodidae (Fig. 15); and (iv) a terminal clade comprising two major subclades and multiple, only partially dichotomously resolved families (Fig. 15). These broad groupings bear notable similarities with the suborders defined by Lowe (1931a).
The remaining clade of the Charadriiformes comprises two major subclades, both of which are weakened by three marginally robust, defining nodes (Fig. 15). The first comprises in turn three lineages or subclades: (i) the Charadriidae; (ii) the sister-groups Cursorinae and Glareolinae (collectively constituting the Galreolidae); and (iii) a clade comprising the Burhinidae and its sister-group comprising two bifurcate clades, the Haematopodidae (united exclusively with monotypic Ibidorhynchus), and the Recurvirostridae (united exclusively with monotypic Cladorhynchus). The other major, pectinate subclade within the Charadiformes comprises, respectively, the sequential sister-groups Chionididae, Alcidae, Stercorariidae, Rynchopidae and Laridae (Fig. 15).
Birds of prey–diurnal and nocturnal
Raptors or birds of prey – comprising the diurnal Falconiformes and (principally) nocturnal Strigiformes – share a primary reliance on carnivory, by scavenging or capture of prey and associated functional commonalities. The sister-relationship of these raptorial orders inferred herein (Figs 10, 16) and by Mayr et al. (2003) has been the subject of suspicion based on phenetic tallies of differences (Gadow, 1893; Beddard, 1898) and speculations concerning convergences and raptorial specializations (Sibley & Ahlquist, 1972; Cracraft, 1981). However, these orders differ in many respects and manifest substantial diversity within orders, conditions as suggestive of comparatively ancient divergence of sister-groups sharing general raptorial lifestyles and independent (order- and family-specific) morphological refinements. This clade is first in a sequence of four – the birds of prey, Opisthocomus, Cuculiformes, Psittaciformes and Columbiformes – that are sequential sister-groups of remaining Neornithes. Although all of these orders were robust with respect to individual monophyly, the four highest-order branches supporting these orders were not (Figs 10, 15–17), rendering the branching sequence provisional.
Figure 16.
Detailed segment of strict consensus tree of all MPTs recovered in present study. Part E. Neornithes: Falconiformes, Strigiformes, Cuculiformes and Psittaciformes. Nodes are labelled above by percentages of bootstrapped replicates in which node was retained (italics), and below by Bremer support indices (bold type).
Figure 17.
Detailed segment of strict consensus tree of all MPTs recovered in present study. Part F. Neornithes: Columbiformes, Caprimulgiformes, Apodiformes, Coliiformes, Trogoniformes and Coraciiformes. Nodes are labelled above by percentages of bootstrapped replicates in which node was retained (italics), and below by Bremer support indices (bold type).
In addition to suspicions of convergence, several concerns may be seen as opposing the phylogeny inferred herein: (i) an alternative interordinal hypothesis that presumes the Strigiformes to be most closely related to the non-raptorial but similarly nocturnal Caprimulgiformes; (ii) an hypothesis that holds the New World vultures (Cathartidae) to be more closely related to the Ciconiidae than to typical birds of prey; and (iii) several counterproposals concerning certain families and genera of Falconiformes, notably positions of the terrestrially specialized secretary-bird (Sagittarius serpentarius), the piscivorous ospreys (Pandion haliaetus), and the distinctive Falconidae relative to other diurnal raptors.
Falconiformes
In the present analysis, however, Cathartidae was resolved as the sister-group of other Falconiformes – an inference considered ‘probable’ by Sibley & Ahlquist (1972) – and the Sagittariidae was sister-group of the order exclusive of the Cathartidae. The Falconiformes, exclusive of the foregoing two families, comprised a pair of sister-clades: (i) the Accipitridae, including Old World vultures (e.g. Gyps), and (ii) a clade comprising the Pandionidae and its sister-group the Falconidae, the latter including the caracaras (Fig. 16).
Jollie (1976, 1977a, b, c) comparatively surveyed morphological characters of the Falconiformes in a monograph largely limited to anatomical phenetics and influenced by suspicions of functional convergence. Exclusive of primarily syringeal evidence (Griffiths, 1994), the only phylogenetic study of diurnal raptors based on morphological characters remains that by Holdaway (1994). Unfortunately, most studies treat most families within the Falconiformes (as construed herein) in only limited capacitiy or secondary focus, e.g. as outgroups for the Falconidae (Griffiths, 1994, 1999; Haring et al., 2001; Griffiths et al., 2004), or in treatments of other phylogenetic issues within the Accipitridae (Seibold & Helbig, 1996; Helbig et al., 2005; Lerner & Mindell, 2005). Cytotaxonomy appears to possess signal, especially in the comparatively intensively studied Falconiformes, but even phenetic groupings of cytotaxonomy have defied interpretation (Ansari & Kaul, 1986). The recent sequence-based phylogeny proposed for the diurnal birds of prey (Lerner & Mindell, 2005) emphasized species-level relationships within the Accipitridae, and Fain & Houde (2004) failed to resolve relationships among the diurnal raptors. Lerner & Mindell (2005) differed from the present analysis in the placement of Pandion as more closely related to the Accipitridae than to the Falconidae or Phalcobaeninae. A sister-group relationship between Cathartidae and other Falconiformes, as inferred herein (Fig. 16), was recovered by Mayr & Clarke (2003), although the latter differed regarding the Strigiformes, Accipitridae and Falconidae.
Ligon (1967) tallied characters suggestive of a phenetic ‘affinity’ between the Cathartidae and Ciconiiformes. Evidently derived from studies by Gadow (1893), Beddard (1898) and Jollie (1953, 1976, 1977a, b, c), works that included Pelecaniformes and Procellariiformes as alternative candidates, the work by Ligon (1967) was a comparison of favoured features solely between the Cathartidae and selected representatives of Ardeidae, Ciconiidae and Accipitridae. Ligon (1967) did not consider polarities or include a formal analysis based on a broad array of characters, and most of the phenetic differences are not convincingly distinct; many features were cast in terms of antiquated typology (Cracraft et al., 2003; Zusi & Livezey, 2006), such as the ‘palatal types’ of Huxley (1867). Nevertheless, this hypothesis found a receptive audience (Cracraft, 1972a; Cracraft & Rich, 1972; König, 1982; Rea, 1983; Emslie, 1988; Seibold & Helbig, 1995; Slikas, 1997; Lerner & Mindell, 2005), and it arguably is more popular than it is empirically robust.
Seibold & Helbig (1995) concluded that limited mtDNA sequence data supported a close relationship between the Cathartidae and storks. Subsequent analyses of the data used by Seibold & Helbig (1995) – revised and augmented by Hackett et al. (1995) and Avise & Nelson (1995) – largely were not comparable because of methodological differences. Avise, Nelson & Sibley (1994a) and Wink (1995) compiled weak molecular evidence to test the hypothesis, the results of which were equivocally consistent with the hypothesis of Ligon (1967). Analyses including these taxa during the following decade (Figs 1–10) failed to support the exclusion of the Cathartidae from Falconiformes sensu stricto, or associate the family with the Ciconiiformes.
Strigiformes
The other substantive debate regarding birds of prey concerns the relative support for a sister-group relationship between: (i) diurnal and nocturnal raptors, or (ii) the similarly noctural Strigiformes and Caprimulgiformes (Hoff, 1966; Sibley & Ahlquist, 1972; Randi et al., 1991; Wink & Heidrich, 1999). The current analysis strongly confirmed a sister-group relationship between the Strigiformes and the Falconiformes (Fig. 16), a union also supported by Cracraft (1988), Mayr & Clarke (2003) and Mayr et al. (2003). Recent molecular studies have placed the Strigiformes tenuously with a striking diversity of taxa, including the Psittacidae, Picidae and Rhamphastidae (Espinosa de los Monteros, 2000; Van Tuinen et al., 2000). Fossils that exhibit generalized raptorial characters or those of both Strigiformes and Falconiformes also have been described (Mayr, 2000a, b, 2005b; Mayr & Daniels, 2001).
With respect to familial relationships within the Strigiformes, the present analysis reaffirmed a basal bifurcation between barn-owls (Tytonidae) and typical owls (Strigidae), with the former including Phodilus (Fig. 16). Phodilus (bay owl) has been considered of variable intermediacy to both strigiform families (Table 1), but most recent molecular data bearing on Phodilus (G. Barrowclough, pers. comm.) are consistent with the present findings (Fig. 16).
Hoatzin, cuckoos, pigeons, parrots and allies
This group of medium-sized landbirds approximates part of the ‘Anomalogonatae’ of Garrod (1874) and Beddard (1898), largely synonymous with the earlier branches within the ‘higher landbird assemblage’ of Olson (1985). Clades informally included in this grade of modern landbirds are characterized by mutually exclusive apomorphies rendering many of the groups among the most readily recognized of birds. Members of this subterminal grade of avian orders have been the subject of numerous studies, but nonetheless a clear consensus regarding their interordinal affinities has failed to emerge (Bleiweiss, Kirsch & Lapointe, 1994; Bleiweiss, Kirsch & Shafi, 1995; Johansson et al., 2001). As noted previously, these taxa –Opisthocomus, Cuculiformes, Psittaciformes and Columbiformes – traditionally were accorded ordinal rank, and were resolved here as a grade in which defining nodes achieved only marginal support. Accordingly, this series of clades conservatively can be considered to compose a tri-ordinal grade or corresponding polytomy that bridges the Cuculiformes with the Caprimulgiformes and Apodiformes. The latter ambiguity primarily relates to the failure to resolve the order of branching of the Psittaciformes relative to the Columbiformes (Fig. 17). Nevertheless, the orders branching from this grade were each strongly supported.
The Opisthocomidae – solely comprising the unusual hoatzin (Opisthocomus hoazin) – has been allied with Tinamidae, Galliformes, Cuculiformes, Columbidae, Pteroclidae, Rallidae, Otididae and Coliidae, among other higher-order groups (Table 1). Recent attempts to resolve the uncertainty of position of this monotypic lineage by molecular means have proven largely unsuccessful, principally by mutual contradiction or ambiquity of findings (Avise, Nelson & Sibley, 1994b; Hedges et al., 1995; Marceliano, 1996; Sorenson et al., 2003), and also because of contaminated sequence data (Avise & Nelson, 1995; Hackett et al., 1995). A growing number of works are at least consistent with an affinity between Opisthocomus and the Cuculidae (Sibley & Ahlquist, 1972, 1990; Hughes & Baker, 1999), despite disputes regarding method and differences in taxonomic sampling. In the present analysis, Opisthocomus was placed as the sister-group of the Cuculiformes, the latter weakly including the Musophagidae (Veron, 1999) as sister-group to the Cuculidae (Table 2; Fig. 16).
Uncertainties of phylogenetic position and superficial plesiomorphy of Opisthocomus led some (e.g. Feduccia, 1980, 1996; Olson, 1985) to suggest that the taxon derives from the ‘roots’ of Neornithes. This proposal is consistent with a perception that the species descended from uniquely primitive ancestry, a view exemplified by its description as a ‘reptilian’ bird by Parker (1891), its use as the only neornithine explicitly figured with Archaeopteryx or non-avian Theropoda (Brodkorb, 1971a; Feduccia, Lingham-Soliar & Hinchliffe, 2005: fig. 26), and the much-publicised retention and use of weakly functional ungues alulares in the genus prior to fledging (Shufeldt, 1918). In actuality, such ‘wing claws’ are retained by members of many modern avian orders in variably vestigial states (Livezey & Zusi, 2006). Accordingly, morphological and molecular evidence for the purported plesiomorphy of Opisthocomus is ambiguous at best: most studies place the genus as closely related to the Cuculiformes (Hughes & Baker, 1999; present study), whereas a few analyses suggest a more distant relationship (Mayr et al., 2003; Mayr, 2005b).
Various other studies, most with only marginal taxonomic sampling, have inferred a sister-group relationship between Opisthocomus and the Cariamidae (Mayr & Clarke, 2003; Mayr, 2005c) or inclusion within an eclectic assemblage defying plausible explanation in light of other findings (Fain & Houde, 2004). The unique alimentary features of Opisthocomus, notably refinements for herbivorous or ruminant digestion (Dominguez-Bello, Ruiz & Michelangeli, 1993; Kornegay, Schilling & Wilson, 1994), are of little phylogenetic significance as they are autapomorphic among Neornithes. However, the lysozymes associated with fermentation by Kornegay et al. (1994, 2003) suggest Opisthocomus to be more similar to Columba than Gallus.
Phylogenetic studies of the Cuculidae per se are surprisingly few, but include taxonomically inclusive attempts at morphological and ethological insights (Seibel, 1988; Hughes, 1996, 2000; Posso & Donatelli, 2001) as well as a molecular exploration (Sorenson & Payne, 2003). Berger (1960) compiled characters distinguishing the Cuclidae from the Musophagidae, many of which show homoplasy at wider scales of comparison. The molecular study by Johnson et al. (2000), the primary focus of which were the Malagasy couas, resulted in a topology within the family broadly similar to that inferred herein, differences in sampling notwithstanding (Fig. 16).
Pigeons and sandgrouse
The Columbidae traditionally are recognized as monophyletic, whereas the interordinal position of the Columbiformes remains a primary point of dispute. The incompletely resolved position inferred here (Fig. 17): (i) compares reasonably well with the semi-speculative tree by Cracraft (1988); (ii) accords acceptably with the poorly resolved reconstructions by Mayr & Clarke (2003), Mayr et al. (2003) and Mayr (2005c); and (iii) is only weakly congruent with the placements by Van Tuinen et al. (2000) and Fain & Houde (2004). The fossil record of the Columbidae from the Palaeogene is poor, and described as non-existent by Mayr (2005a). Sampling of the Columbidae was comparatively intense in the present study so as to affirm the monophyly of such a diverse family and to expand the thoroughness of placements of the extinct ‘raphids’Raphus and Pezophaps (Livezey, 1993).
The present analysis indicated monophyly of flightless Raphus cucullatus and Pezophaps solitaria, one of the principal hypotheses proposed for the ‘raphids’ (Livezey, 1993). Goura and Didunculus, historically speculated to be sister-genera, were placed as paraphyletic to the raphids (Fig. 17). These inferences general agree with those by Shapiro et al. (2002: fig. 1) and Johnson & Clayton (2000a, b), and revealed generic partitions within the Columbidae in considerable agreement with the present work. The Pteroclidae (sand-grouse) have been the topic of study for more than a century (Gadow, 1882; Shufeldt, 1901c; Stegmann, 1957, 1959; Fjeldså, 1976). The pteroclids were placed herein as the sister-group of the Columbidae – a view favoured by the majority over an hypothesized alliance with the Charadriiformes (Sibley & Ahlquist, 1972, 1990).
Parrots and allies
The primary mystery of this unique order is its interordinal position, a debate clearly manifested by the myriad groupings inferred for it in phylogenetic works during the last two decades. Monophyly of the Psittaciformes, not amenable to testing with the few exemplars included here, has been assumed (Smith, 1975) or affirmed by diverse morphological (Sibley & Ahlquist, 1972, 1990) and molecular means (Ovenden et al., 1987; Christidis et al., 1991; Leeton et al., 1994; Miyaki et al., 1998; Eberhard, Wright & Bermingham, 2001; Eberhard & Bermingham, 2001, 2004; Groombridge et al., 2004; Russello & Amato, 2004; de Kloet & de Kloet, 2005; Ribas et al., 2005; Tavares et al., 2006). Higher-order relationships are less clear, and the order has been allied with: (i) groups comprising sufficient diversities of neognathous taxa as to establish little progress (Sibley & Ahlquist, 1990; Fain & Houde, 2004); (ii) Trogonidae and/or Coliidae (Espinosa de los Monteros, 2000; Mayr, 2000b, 2005d, e; Mayr & Clarke, 2003); (iii) Picidae (Van Tuinen et al., 2000); (iv) Coliidae and some Pici (Mayr et al., 2003); and (v) Strigidae (Harrison et al., 2004). Although the present analysis provides no single, well-supported and precise position for the order, the evidence compiled herein is consonant with a (perhaps deep) relationship between the Columbiformes and Psittaciformes. This interordinal union was inferred by Burton (1974) in a study of Didunculus, and also confirmed by Sibley & Ahlquist (1972: 241) and Cracraft (1981).
Goatsuckers, swifts and hummingbirds, mousebirds and trogons
Overview
This heterogeneous group of moderate to small landbirds of controversial relationships – approximately synonymous with the ‘Coccygomorphae’ (Huxley, 1867), the Anomalogonatae (Garrod, 1874; Beddard, 1898), or part of the ‘ “higher” landbird assemblage’ (Olson, 1985) – includes some of the most specialized and distinctive of modern birds. Unlike the foregoing groups, most higher-order nodes within this assemblage – i.e. those structuring relationships among orders – are robustly resolved (Fig. 17). Essential findings herein were monophyly of the traditional Caprimulgiformes (including Aegothelidae), and monophyly of its sister-group Apodiformes. The Apodiformes comprised the crested-swifts (Hemiprocnidae) as sister-group to a clade comprising the mutually monophyletic typical swifts (Apodidae) and hummingbirds (Trochilidae).
Caprimulgiformes
A minority of works failed to recover monophyly of the Caprimulgiformes, either by unresolved polytomy (Johansson et al., 2001), variably constituted paraphyly to Trogonidae or Apodiformes (Mayr & Clarke, 2003; Mayr et al., 2003; Mayr, 2005f; Barrowclough, Groth & Mertz, 2006), or uniquely proposed alternative alliances with the Accipitridae and Sulidae (Van Tuinen et al., 2000), Sagittariidae (Mindell et al., 1997), or Mesitornithidae (Fain & Houde, 2004). The present phylogeny affirms monophyly of the traditional Caprimulgiformes, although recent analyses suggest that the present study may not have been adequate to capture all ‘family-level’ variation in the order by omission of Batrachostomus and Eurostopodus (Sibley et al., 1988; Mariaux & Braun, 1996). The moderately distant relationship between the nocturnal Caprimulgiformes and Strigiformes – considered closely related by some (Sibley et al., 1988) – was favoured herein, a finding consistent with the hypothesis that at least the ocular refinements for nocturnality in these two orders are not homologous (Fidler, Kuhn & Gwinner, 2004). In this context it is noteworthy that another aspect of colour vision shows low consistency with avian phylogeny (Ödeen & Håstad, 2003). Support for groups within the Caprimulgiformes in the present work was marginal at best, and for practical purposes might be considered to be a polytomy of the included families. Palaeontological proposals suggest that fossil members of the Caprimulgiformes (and certain other groups) currently endemic to the southern hemisphere previously extended to the Palearctic (Olson, 1987; Mayr, 1999a, b, 2002a, b, 2005b, f).
Fidler et al. (2004) also presented equivocal evidence that the owlet-frogmouths (Aegothelidae) are not members of the Caprimulgiformes, as traditionally classified, a proposal augmented by some morphological evidence (Mayr 2002a, b) and DNA sequences (Barrowclough et al., 2006). The latter studies led to marginally supported transfers of the Aegothelidae – herein inferred to be the sister-family of other Caprimulgiformes – to an alternative position as sister-group of the Apodiformes, a reasonably economical concession from global parsimony using the present data set (Table 3). Although the position of the Aegothelidae remains uncertain, mtDNA sequence data are consistent with the monophyly of this family (Dumbacher, Thane & Fleischer, 2003). A complete picture of caprimulgiform phylogeny must await comprehensive integration of putative fossil members (Olson, 1987; Mayr, 1999a, 2002a, b, 2005b, f).
The oilbird (Steatornis caripensis) – a nocturnal, cavernicolous frugivore – is one of the most challenging of avian genera with respect to phylogenetic position, irrespective of method. Recent analyses have differed regarding even the ordinal placement of this taxon, traditionally assigned to a monotypic family (Garrod, 1873b; Mariaux & Braun, 1996; Livezey & Zusi, 2001; Mayr, 2003b; Barrowclough et al., 2006). The present work tentatively resolved Steatornis to be a highly apomorphic member of the Caprimulgiformes (Fig. 17).
Apodiformes
This order is monophyletic and, as traditionally construed, comprises the highly derived crested-swifts, swifts, swiftlets and hummingbirds. The interfamilial relationships inferred here (Fig. 17) – Hemiprocnidae (crested-swifts) as sister-group to a clade comprising the mutually monophyletic Apodidae (swifts) and Trochilidae (hummingbirds) – have received growing support from other works (e.g. Chubb, 2004b) in tabling the largely antiquated contention that the hummingbirds were closely related to the Passeriformes and related variants of this hypothesis (Table 1). Departures from the present hypothesis included that of monophyly of the Hemiprocnidae and Apodidae (Chubb, 2004b). The molecular phenetics of Sibley & Ahlquist (1990), including where re-analysed (Harshman, 1994b) or augmented (Bleiweiss et al., 1994, 1995), also differed by resolving the Trochilidae as phenetic ‘sister-group’ to all other Apodiformes, prompting the former to be ordinally distinguished as Trochiliformes. The speciose hummingbirds (Trochilidae) achieved phylogenetic diversity in concert with the related apodids (Mayr, 2003c, 2004d, 2005g), a radiation second only to passeriforms in scale (Bleiweiss, Kirsch & Matheus, 1997; Bleiweiss, 1998a, b, c; García-Moreno et al., 2006), and evolved a diversity of concomitant apomorphies, some of which overcame unique locomotory challenges (e.g. Altshuler, Dudley & Ellington, 2004). Phylogenetic study of the swifts (Apodidae) by palaeontological (Mayr, 2001a, 2003c, 2005g) and molecular means (Dumbacher et al., 2003; Chubb, 2004b; Thomassen et al., 2005), resolved a fundamental dichotomy between taxa possessing capacities for echolocation (Thomassen et al., 2003).
Coliiformes and Trogoniformes
Modest support was afforded herein to a sister-group relationship between the Trogoniformes and Coliiformes (Fig. 17). Both orders have proven challenging to place among other avian orders (Garrod, 1876; Forbes, 1881), but monophyly of these orders has not be questioned (Espinosa de los Monteros, 1998, 2000). The present analysis confirms earlier anatomical (Verheyen, 1956b) and molecular analyses (Espinosa de los Monteros, 1998, 2000), in which this couplet also was inferred in turn to be closely related to Coraciiformes (Berman & Raikow, 1982). Johansson & Ericson (2004) conceded that appropriate outgroups for rooting analyses of these orders was problematic, a problem that led Moyle (2005) to employ a suite of outgroups. Among the more notable expansions of palaeodistributional limits have pertained to the Trogoniformes (Mayr, 1998a, 2001b) and Coliiformes (Mayr, 2000b, 2001a), although diagnostic evidence was poor by general neontological standards.
This couplet raises the possibility of artefactual grouping by way of long-branch attraction (Lyons-Weiler & Hoelzer, 1997; Wilson, 1999; Bergsten, 2005). Examination of ranges of terminal branch lengths compiled in the MPTs –Colius (84–133) and Trogonidae (10–17), with the branch subtending clade having a range of lengths 36–97 – suggests that the Trogonidae are not obviously vulnerable to an artefactual grouping. This judgement is supported further by the inclusion of a number of multistate, supportive characters (Wägele, 1996).
Coraciiform, piciform, and passeriform birds
Overview
Long recognized as a speciose, diverse and widespread group, historical disagreements pertaining to these orders have turned on familial memberships (e.g. Trogonidae) and delimitation of orders within the assemblage (Lowe, 1948; Wetmore, 1960; Sibley & Ahlquist, 1972: 241). Predictably, some subgroups manifested intermediate suites of characters and have proven least tractable (Burton 1984: fig. 32); the latter have been addressed most pointedly, perhaps, in palaeontological diagnoses (Ballmann, 1979; Mayr 1998b, c; Mayr & Daniels, 2001). Also, where data are less numerous, a common alternative to monophyly of the Coraciiformes or Piciformes is resolution of the two as sequential sister-groups (paraphyletic) to the Passeriformes. Although monophyly of the tri-ordinal assemblage was substantiated here (Figs 17, 18), the analysis revealed several alternative arrangements among the three orders, represented by the polytomy in the strict consensus tree of MPTs (Figs 17, 18).
Coraciiformes
Monophyly of the families traditionally included within the Coraciiformes has been a point of disagreement for almost a century (Murie, 1872b, c, 1873; Lowe, 1948; Sibley & Ahlquist, 1972, 1990), and persists as a palaeontological challenge (Mayr, 2000d, 2005h, i; Mayr & Mourer-Chauviré, 2003; Mayr et al., 2003). This state of affairs has been prolonged by poor representation of the order in many recent family-level, multi-ordinal analyses (e.g. Espinosa de los Monteros, 2000; Van Tuinen et al., 2000), with notable exceptions including the analyses by Johansson et al. (2001) and Kirchman et al. (2001). Taxonomically narrow analyses include morphological works by Cracraft (1971b), Burton (1974) and Maurer & Raikow (1981), and the molecular phenetics of Sibley & Ahlquist (1990) and Bleiweiss et al. (1994).
The Coraciiformes were found herein to be a monophyletic member of a trichotomy that included the Piciformes and Passeriformes (Figs 10, 11, 17, 18), but the magnitude of support for monophyly of the Coraciiformes was only moderate, and generally was exceeded by that for included nodes. The ordinal work by Maurer & Raikow (1981) proved most relevant in this context, but conclusions of the two analyses differed considerably. Evidently, restriction of the outgroups and characters included in the analysis by Maurer & Raikow (1981) resulted in contradictory findings symptomatic of diminished signal, e.g. inversions of taxa within the ingroup and inclusion of Trogonidae within the ingroup.
The very distinctive hornbills (Bucerotidae), together with a sister-group comprising the Upupidae and Phoeniculidae, were situated as the sister-group of other coraciiforms (Fig. 18). This group was considered a separate order allied to other Coraciiformes by Burton (1984) and Kemp (1995). Manegold (2005), however, inferred the Coraciiformes to be polyphyletic and comprising: (i) the ‘Bucerotes’ (Upupidae, Phoeniculidae and Bucerotidae) as sister-group to the mutually monophyletic Piciformes (including Galbulae) and Passeriformes; (ii) the ‘Alcediniformes’ (all other members of the traditional order not included elsewhere); and (iii) Leptosomus, excluded from the Coraciiformes and of indeterminate ordinal relationship.
The remaining members fell into two sister-groups: one of these comprised the Motmotidae and its sister-group comprising the Todidae and Alcedinidae, the Todidae of uncontested monophyly (Overton & Rhoads, 2004), and the Alcedinidae monophyletic but perhaps comprising two or more distinct subgroups (Fry, 1980; Marks & Willard, 2005). The remaining member of this pair of sister-groups comprised the sequential sister-groups of Meropidae, Coraciidae, Brachypteraciidae and Leptosomatidae (Fig. 17). The Motmotidae were inferred herein to be the sister-group of a clade comprising the Todidae and Alcedinidae (Moyle, 2006). However, some ‘intermediacy’ of morphological and molecular characters of the tody-motmot (Hylomanes) and the Todidae suggests possible paraphyly of the Motmotidae (as traditionally constituted) or the Todidae (Overton & Rhoads, 2004). The Meropidae (bee-eaters), of established monophyly (Fry, 1984; Burt, 2004), were inferred here to be the sister-group to remaining Coraciiformes (Fig. 17), the latter known in the vernacular as ‘rollers’. As detailed above, morphological assessments of the memberships and positions of these families differ significantly (Manegold, 2005).
Piciformes
The Galbulidae and Bucconidae were inferred herein to be sister-groups, and together as forming the sister-group of other Piciformes. The remaining Piciformes in this analysis comprised two sister-groups (Fig. 18), each of which comprised two, provisionally monophyletic families: (i) Capitonidae (Moyle, 2004) and Rhamphastidae (Eberhard & Bermingham, 2001; Wechstein, 2005); and (ii) Indicatoridae and Picidae (Prychitko & Moore, 1997; DeFilippis & Moore, 2000; Weibel & Moore, 2002a, b). Support for neither of the latter clades was strong, approximating only 50% bootstrap support (Fig. 18). This arrangement is consistent with much of the classification proposed by Burton (1984). One point of current debate is the possible paraphyly of the Capitonidae, in which member taxa represent successive sister-groups to the (monophyletic) Ramphastidae (e.g. Prum, 1988; Sibley et al., 1988; Lanyon & Hall, 1994; Barker & Lanyon, 2000). Unfortunately, despite comparative richness of the record, fossil members of these groups have provided few insights into the phylogeny of modern piciforms (Mayr, 2001c, d, 2004e, 2005h, i).
Monophyly of the Piciformes, most often challenged regarding membership of the Galbulae, has been controversial – e.g. Olson (1983a), Raikow & Cracraft (1983), Lanyon & Zink (1987), Johansson & Ericson (2003) – despite comparatively detailed anatomical study (Burton, 1974) and related phylogenetic analyses (Simpson & Cracraft, 1981; Swierczewski & Raikow, 1981; Avise & Aquadro, 1987; Manegold, 2005). Most attempts to reconstruct the phylogenetics of the order have been variably inclusive with respect to included families and limited to molecular evidence (Webb & Moore, 2005; Wechstein, 2005; Benz, Robbins & Peterson, 2006), and resultant findings posed no serious contradictions to the inferences made here.
Passeriformes
The Passeriformes are a dominant evolutionary component of the global avifauna, and the phylogeny of the order has figured prominently in terminological disputes regarding faunal ‘radiations’ (Barker et al., 2004), ‘key innovations’ of evolutionary change (Raikow & Bledsoe, 2000; Olson, 2001) and ‘evolutionary success’ (Raikow, 1988). Current consensus by avian systematists holds the Passeriformes to be one of the most recently differentiated and apomorphic of lineages of modern birds, with a growing body of evidence for Gondwanan genesis (Ericson et al., 2002a). However, analyses limited to the mitochondrial genome (Moore & deFilippis, 1997), the early mainstay of sequence analyses (Kessler & Avise, 1985; Ast et al., 1997; Braun & Kimball, 2002), resulted in several studies in the placement of Passeriformes as the sister-group of most or all other Neoaves (Mindell et al., 1997, 1999), a topological shift of exceptional magnitude and enormous evolutionary implications. This finding, mirrored by the phenetics depicted by Sibley & Ahlquist (1990) and very recent analyses based principally on mitogenomics (Pereira & Baker, 2006b; Slack et al., 2006b), since has been attributed (Cracraft et al., 2003, 2004) in subsequent works to (unavoidable) reliance on most closely related but nevertheless distant outgroups – e.g. Crocodylia, Testudines – which probably serve as unreliable sources of information on avian polarities. This circumstance, compounded by weak taxonomic sampling or shortcomings of mitochondrial data for reconstruction of deep nodes (e.g. Mindell et al., 1996; Tsaousis et al., 2005), necessitate caution in corresponding inferences. Fortunately, with respect to genomes analysed, principal differences reduce to rotations of three or four variably nested nodes (Johnson, 2001: fig. 3).
As for monophyly and composition of the Passeriformes (Beecher, 1953; Mayr, 1958; Olson, 1971; Feduccia, 1973, 1977c; Brom, 1990), the present analysis was necessarily limited to selected genera and families of this enormous group, as were the few previous morphologically based phylogenies of the group (Raikow, 1982, 1994a, b). A number of passeriform subgroups, mostly at comparatively low taxonomic levels, appear to have undergone cladogenesis sufficiently recently to reflect vicariance related to recent glaciations and current continental patterns (Edwards & Wilson, 1990), but controversy regarding this tempo persists (Klicka & Zink, 1997; Johnson & Cicero, 2004; Zink & Klicka, 2006). The meagre palaeontological evidence available indicates an origin of the Passeriformes to be no later than the early Eocene (Boles, 1995, 1997; Barker et al., 2004; Mayr & Manegold, 2004).
The present analysis substantiated the interordinal position and monophyly of representatives of major subgroups of the Passeriformes (Fig. 18). Within the narrow taxonomic sample analysed herein (cf. Sibley, 1974; Cracraft, 1992a; Helm-Bychowski & Cracraft, 1993; Nunn & Cracraft, 1996; Barker et al., 2002; Irestedt et al., 2002), Menura was resolved as the sister-group of other members of the order, i.e. a member of the non-passerines (Fig. 18). Menura typically is situated crownward of basal Acanthisitta (but see Gadow, 1893; Ames, 1971) and included among the basal oscine passerines (Sibley & Ahlquist, 1970; Sibley, 1974; but see Ericson et al., 2002a, b, 2003b, 2006). This minimally exemplified and questionably resolved subgroup was inferred to be the sister-group of remaining passeriforms, first followed by a poorly represented grade of suboscine passerines (Tyrannides) – Tyrannidae and Pittidae (Prum, 1993). The Tyrannides in turn subtends the oscine passerines (Passerides), within which two major subgroups (Ericson et al., 2006) were sparsely represented but arranged in accord with current consensus (Fig. 18). Barker et al. (2002) inferred the Ptilonorhynchidae (Stonor, 1938; Cracraft, 1992a; Nunn & Cracraft, 1996; Cracraft & Feinstein, 2000; Johansson et al., 2001) to be the sister-group of the remaining passeriform taxa. Among other Passerides, the single exemplar of the Corvida (Aphelocoma) was the sister-group of the three representatives of the Passerida –Bombycilla, Parus and Passer (Ericson et al., 2000; Ericson & Johansson, 2003). With the exception of the position of Menura in the present analysis, the broad subdivisions inferred here agree with the majority of other recent works (Edwards, Arctander & Wilson, 1991; Irestedt et al., 2001; Ericson et al., 2003b; Cracraft et al., 2004; Spicer & Dunipace, 2004) and are consistent with palaeogeographical evidence for an Australasian origin for the oscines (Boles, 1995, 1997; Barker et al., 2004).
Branch lengths and evolutionary change
Morphological characters employed in cladistic analyses tend to be held to unrealistic standards, and to serve as sources of insights (not expected of molecular characters) beyond mere inference of phylogenetic relationships. For example, in some circles there is an expectation that, in addition to resolving phylogenetic relationships of multiple taxa, apomorphies supportive of nodes should make obvious functional sense (e.g. debates regarding aquatic lineages and possible convergences) and permit interpretation resembling lists of (semi)diagnostic characters for nested series of taxa. In some cases, particularly where taxonomic scale is low and a functional focus pertains, such patterns and trends are discernible. However, with increasing taxonomic scale, these are in the minority, and like DNA sequence data, such diagnostic transparency and functional interpretation is seldom attainable. Many subtle features possessed of phylogenetic signal may be structural artefacts of functionally neutral details of anatomy, historical accidents that prove variably reliable through the process of evolutionary modification with descent.
Nevertheless, quantification of evolutionary change is critical to estimates of rates and correlation of change among characters and related evolutionary topics (e.g. Omland, 1997a, b; Nunn & Stanley, 1998), and exploration of this aspect of reconstructions is intended to pre-empt misplaced expectations or distorted perspectives. The tempo and mode of morphological evolution and cladogenesis have held the interests of systematists for decades (Simpson, 1944; Cracraft, 1984), pre-dating the advent of molecular methods or assumptions made for them (e.g. uniform or ‘clock-like’ evolutionary rates). Antiquity of lineages provides opportunity, other parameters being equal, for increased expectation of evolutionary changes (probabilistic, not deterministic expectation), and where such lineages comprise only modest numbers of members – i.e. limited evolutionary opportunities for departures from uniformity or reversals within a given lineage – such change also tends to lead to comparatively direct diagnosticity of terminal lineages. Intuitive relevance of origins, ages, longevities of lineages and expectations of evolutionary divergence notwithstanding, these topics have been underserved by newly acquired empirical evidence. Van Tuinen et al. (2006: table 2) listed 14 avian families construed to show molecular variation significantly lower than that expected on the basis of current taxonomic status. Given present findings, however, this issue appears illusory, with virtually all taxa in question having early origins, including the Anhimidae (Anseriformes), Podicipedidae and Spheniscidae, three being members of the Pelecaniformes, and the remaining examples members of either the Ciconiiformes, the Gruiformes or the Charadriiformes.
Unlike molecular evolution, no strict assumptions or dependence on constant or uniform rates of change have been made for morphological characters. In the present analysis, branch lengths varied substantially depending on specific optimizations, and therefore comparisons of lengths, like the internodes in trees, were not restricted to unambiguous changes. Instead, central tendencies of branch lengths of MPTs were quantified by median lengths, and variation among optimizations by standard deviations and ranges of lengths recovered. For Neornithes, numbers of changes optimized as autapomorphies averaged 41 (SD = 33, range 4–186). By contrast, for single lineages, maximal lengths of terminal branches were: 186 for Spheniscus, 132 for Phoenicopterus and 131 for Mesitornis. Minimal lengths of terminal branches were: 4 for Francolinus and Alectoris. At higher phylogenetic scales (interordinal and interfamimlial), branch lengths had the following summary statistics: mean = 36, SD = 33, range 5–133. In general, then, terminal branch lengths were approximately 10% greater than those of the branches subtending them (i.e. deeper internodes). A pattern of short internodes has been inferred previously (Cracraft et al., 2004), but the attribution of cause to realities of evolutionary intervals vs. diminished power of resolution remains contentious.
A survey of the minimal branch lengths included in the MPT revealed that branches among higher-order nodes were extraordinarily similar to associated terminal branches (latter being those subtending individual taxa) in means and variances of branch lengths. However, comparative numbers of the more critical diagnostic and supportive characters within the Neornithes revealed that character-based definitions of highest-order clades (corresponding to the most ancient of synapomorphies) were disappointingly low, whereas those for superorders and orders (Appendix 2) were comparatively robust and included suites of diagnostic character-states (Table 2). However, the correspondence among ‘raw’ branch lengths, statistics of nodal support and numbers of ‘diagnostic’ apomorphies generally was poor (Table 2), in agreement with the findings of Farris et al. (2001) and Wilkinson (2003).
DISCUSSION
Broad comparisons with prior studies
‘Survival of the fittest will decide which of the many competing theories [of avian phylogeny] will prevail. Only one can survive. Each revisor attempts to shorten the struggle by acting as a selective factor.’ (Stresemann, 1959: 269)
‘Where the root of the Neoaves goes, however, is highly uncertain and seems likely to remain a very difficult problem.’ (Stanley & Cracraft, 2002: 39)
Perspectives and findings
In the published record of phylogenetics, it has become virtually customary simply to generate phylogenetic hypotheses of varying consonance with little or no consideration of factors underlying divergent inferences (Figs 1–9). This tradition has led to a false sense of congruence among studies, especially among molecular systematists. We consider that it is incumbent upon authors to consider the points of disagreement as well as the most plausible underlying philosophical and empirical reasons for the differences. A reasonable degree of detail in such deliberations inevitably will include points of contention and opinion, and we hope that these will challenge the current ambience of consensus and invite constructive debate of these important issues. At the same time, however, it is logistically unfeasible that large-scale studies (e.g. the present work) be held to standards of character descriptions and illustrations in analytical works that are logistically realistic in more common, small-scale works. For example, in the present study, a conservative estimate of character-states eligible for illustration would approach 7000. Nonetheless, access to underlying data for all studies should be made practically available by alternative means, and include formal descriptions of characters as analysed, and essential figures and references to critical descriptive works (e.g. Livezey & Zusi, 2006).
Deep tradition and the ‘tapestry’
Broad affinities of long standing between avian orders – traditionally only implied to variable degrees by adjacency in linear classifications (Clark, 1901; Wetmore, 1930, 1960; Mayr & Amadon, 1951) – that were not supported by the present analysis were: (i) Galliformes as closely allied with Falconiformes; (ii) Gaviiformes, Podicipediformes and Sphenisciformes placed as the most basal of ‘Carinatae’; and (iii) a truly basal position of Opisthocomus among Neornithes. Although confidence in the ‘tapestry’ (e.g. Monroe, 1989) diminished markedly within a few years of publication, the proposals by Sibley & Ahlquist (1990) were ‘rewoven’ by Harshman (1994b), ‘dusted off’ by Mooers & Cotgreave (1994), and continue to be cited for justification and design of sequence-based analyses (e.g. Fain & Houde, 2004). Limited reverence for the tome by Sibley and Ahlquist (1990) lingers, most conspicuously in the non-systematic literature (e.g. Del Hoyo, Elliott & Sardgatal, 2001), principally because of its taxonomic scale and molecular basis (e.g. Chubb, 2004b; Fain & Houde, 2004).
Given the controversy and contradictory nature of the era, it is appropriate to compare our findings with the groups delimited by Sibley & Ahlquist (1990), bearing in mind that the present phylogenetic analysis is of limited comparability with the phenetics of DNA–DNA hybridization. First, despite the unprecedented number of taxa analysed, the earlier work was invalidated shortly after its appearance because of problems stemming from phenetic methodology, sparsity of the distance matrix, absence of a root and irreducibility of data-type, some deficiencies having been identified prior to its release (Cracraft, 1987, 1992b; Houde, 1987; Sarich, Schmid & Marks, 1989; Barrowclough, 1992; Lanyon, 1992; Mindell, 1992). Simplification of the reconstruction by Sibley & Ahlquist (1990: figs 354–356) to ordinal terminal taxa (Fig. 4) reveals the diagram to be continuously pectinate throughout most of the neognathous birds, and largely reflects ‘chaining’ of least dissimilar elements, an artefact common to some agglomerative algorithms. Cracraft et al. (2004) considered current knowledge of avian phylogeny to be of comparable irresolution.
A most peculiar aspect of the ‘tapestry’ is the reversal of mid-basal and apical higher-taxa – e.g. Piciformes and Passeriformes as sister-groups to the ‘Ciconiiformes’ (sensuSibley & Ahlquist, 1990) and allies – a finding countered by the vast majority of other analyses (Cracraft & Mindell, 1989; Johansson et al., 2001; Braun & Kimball, 2002; Edwards et al., 2002; Paton et al., 2002; Mayr & Clarke, 2003; Mayr et al., 2003; Prychitko & Moore, 2003; Dyke & Van Tuinen, 2004; Harrison et al., 2004; Poe & Chubb, 2004). This phenetic artefact undoubtedly contributed to the poor congruence of the present phylogenetic hypothesis with that by Sibley & Ahlquist (1990), in which only four higher-order groups – their Ratitae, Galloanserae and Procellarioidea, and monophyly of one currently contentious order (Caprimulgiformes) – showed broad agreement in both works. The present analysis strongly countered the polyphyly inferred by Sibley and Ahlquist (1990) for the Pelecaniformes and Columbiformes, and differed as well regarding paraphyly of the Coraciiformes and Cuculiformes, the alternative positions of Galliformes + Anseriformes, and the provisional placement of the Turniciformes (Fig. 4).
Sibley & Ahlquist (1972: 240–241) listed 34 summary inferences entitled ‘Probabilities and Possibilities’, presented under four levels of perceived likelihood. Of the conclusions listed, agreement (with minor qualifications) with the present analysis was achieved for: all eight (100%) of the ‘highly probable’ conclusions; seven of ten (70%) deemed ‘probable’; four of ten (40%) considered ‘possible’; and only two of six statements (33%) classified as ‘improbable’, essentially logical negations of views included among the ‘highly probable’.
Contemporary studies
Comparisons among most phylogenetic hypotheses are compromised by differential taxonomic sampling and nodes afforded only tenuous support. The present phylogenetic hypothesis, depicted to ordinal scale (Figs 10, 11), approximated the tree depicted by Cracraft (1988) most closely of prior works, issues of comparability notwithstanding. The present analysis, almost 20 years subsequent to that by Cracraft (1988), represents a return to the broad outlines of the latter, seminal work. Given the different scales of the two analyses in terms of taxa and characters, however, it is unreasonable to assume similarities to be the result of reliance on ‘the same characters’.
An increasing proportion of all studies confirm positions and monophyly of Palaeognathae, Galloanserimorphae and major subclades thereof. However, most molecular studies (e.g. Van Tuinen et al., 2000, 2001; Paton et al., 2002; Van Tuinen, 2002; Chubb, 2004a), as well as analyses based on combined data (Dyke & Van Tuinen, 2004), differed significantly with parts of the present hypothesis, especially those pertaining to the Pelecaniformes, Ciconiiformes, Podicipedidae, Opisthocomiformes, Cathartidae, Caprimulgiformes and Coraciiformes (Figs 12–18). There was considerable disagreement among recent molecular studies alone (e.g. Espinosa de los Monteros, 2000; Johansson et al., 2001; Poe & Chubb, 2004), regardless of data analysed (Philippe et al., 1996; Graur & Li, 2000), which reveals contrasts only between morphological and molecular inferences to be oversimplifications of modern study (e.g. Braun & Brumfield, 1998; Van Tuinen, 2002).
Comparisons with the limited number of other analyses (Figs 1–3) were virtually uniformative because palaeontological works have tended to emphasize narrow taxonomic groups considered likely to accommodate newly described or controversial taxa, and also to limit characters to those scoreable for the taxon or fossil of interest (e.g. Clarke et al., 2005b), with some exceptions (e.g. Mayr & Clarke, 2003). Several provisional and ongoing reconstructions by Cracraft et al. (2004) were not considered here. A survey of comparable cladistic studies of morphological or molecular bases (cf. Cracraft, 2001; Mayr & Clarke, 2003; Cracraft et al., 2004; Fain & Houde, 2004; Clarke et al., 2005b) revealed that the present analysis achieved considerable agreement with most of the latter studies concerning the widely supported (com)positions of the Palaeognathae and Galloanserimorphae, and an allied clade dominated by marine and wading birds (Figs 10–18).
Adjudication of success
It is to be hoped that diverse approaches will converge empirically toward common analytical standards (Lake, 1997) and a solution for which acceptance is widespread and merited. However, there are no standards of accuracy against which phylogenetic analyses of natural lineages can be calibrated (i.e. known histories), and therefore the assessment of progress is elusive. Hypothetico-deductive empiricism may reveal critical characteristics of scientific hypotheses, but cannot provide ‘proof’ of a hypothesis (Helfenbein & DeSalle, 2005).
Given that proof of hypotheses or certain recognition of the single, true phylogeny is unattainable, the strongest support for a specific reconstruction (beyond intrinsic robustness) lies in common elements shared by other analyses – empirical (not popular) consensus. Such studies are most potent where performed independently using new data. Likelihood of correctness of molecular and morphological reconstructions cannot be judged a priori, especially across all classes of investigation. Such assessments are conditional on individual cases, and decisions based on consistency with prior analyses, degree of resolution (assuming bifurcations are the primary cladogenetic mode), size and diversity of data on which the hypothesis was based, and analytical properties of included characters. The relevance of statistics internal to single trees – e.g. robustness of nodes and consistency indices – to the likelihood of global accuracy is undecided (Benton, 2001).
Consequently, an important element of phylogenetic study is comparison of findings with the estimates of other investigators, especially comparisons of those aspects of trees that withstand variations in method or data base. However, against which topology or topologies does one compare specific findings? This quandary especially afflicts those disposed to a dichotomous view of morphological and molecular estimates of history. Provision of a sample of trees (Figs 1–9) was intended, in part, to emphasize the dilemma that faces investigators wishing to evaluate hypotheses comparatively. It appears that until some kind of genuine consensus is achieved, systematists are compelled to pit their findings against a plethora of other, marginally comparable works.
Molecular systematics: competitor or collaborator?
At present, molecular systematics is characterized both by the coexistence of general (if not unbridled) optimism (Van Tuinen, 2002) and by profound doubts regarding resolution of substantial segments of neornithine phylogeny (Poe & Chubb, 2004). Yet the current dominance of avian systematics by molecular methods is sufficiently profound as to lead some to consider palaeontology to be the sole justification for a continued role for morphology in systematics or to question its value altogether (e.g. Stevens, 2000; Scotland, Olmstead & Bennett, 2003; Jenner, 2004a, b). Nevertheless, historical signal from genes and their morphological products offer a potentially fruitful synergy (Jenner 2004a: 340), one that exceeds the use of morphology for placements of fossils.
An unfortunate pattern has emerged in molecular circles, however, in which perennial problems of avian systematics (Table 1) are attributed to the relative impotence or unreliability of morphological clues to phylogeny (e.g. Monroe, 1989; Sibley & Ahlquist, 1990; Givnish & Sytsma, 1997a, b; Sorenson et al., 1999; Paton et al., 2003; Paton & Baker, 2006), or as justification for merely mapping morphological characters a posteriori onto molecular trees (e.g. Gittleman et al., 1996; Slikas, 1997; McCracken et al., 1999; Van Tuinen, 2002; Huelsenbeck et al., 2003). Therefore, it would be negligent to forego this opportunity to counter this perception explicitly (e.g. Shafer, Clark & Kraus, 1991; Hillis & Wiens, 2000; Marques & Gnaspini, 2001). We do not intend an assault on molecular methodology, but seek to refute persistent prejudices that afflict morphological phylogenetics (cf. Smith, 1998; Baker & Gatesy, 2002), to underline the distinctness between ease of application and reliability in phylogenetic methods, and to encourage objectivity in assessment of findings.
Perhaps the deficiency attributed most widely to morphological phylogenetics stems from suspicions of morphological convergence, concerns seldom empirically substantiated and to which molecular methods are widely assumed to be immune (Lockhart et al., 1994; Goldring & Dean, 1998; Lee, 1997, 1999; Sorenson et al., 1999; Yang & Bielawski, 2000). To date, assumptions of morphological convergence principally are made where convenient and are seldom reversed, with few exceptions (e.g. McCracken et al., 1999; McCracken & Sorenson, 2005). However, verification of convergence in molecular data (Holmquist, Pearl & Jukes, 1983; Kornegay et al., 1994; Philippe et al., 1996) is increasingly frequent. For morphology, we hope that intuitive claims of convergence will be supplanted by phylogenetically framed analyses of refined morphological and functional data (Raikow, 1985b), especially those pertinent to the: pectoral limb (Middleton & Gatesy, 2000; Burness, Chardine & Darveau, 2005); pelvic limb (Gatesy, 1991; Gatesy & Biewiener, 1991; McKitrick, 1993; Patak & Baldwin, 1998; Carrano & Biewener, 1999; Abourachid, 2000, 2001; Abourachid & Renous, 2000; Hutchinson, 2001a, b, 2002; Zeffer & Norberg, 2003; Zeffer et al., 2003; Fujita, 2004); skull and associated musculature (Müller, 1961a, b, 1963; Weber, 1990, 1993; Zusi & Livezey, 2000; Bout, 1997; Meekangvan et al., 2006); and general body form (Nudds & Rayner, 2006; Bybee, Lee & Lamm, 2006).
Studies based both on molecular and morphological phylogenetics (Figs 1–9) manifest substantial disagreement both within and between schools (Patterson et al., 1993), and remain comparable in resolution and support, with disputes often conjectural in nature. Both classes of data present substantial challenges of homology (further below), and those that face molecular systematists (Wheeler et al., 1995; Philippe et al., 1996; Phillips et al., 2000; Jenner, 2004a, b; Wiens, 2004) are remarkably similar to those afflicting morphological phylogeneticists. Problems of homology in molecular applications, principally related to ‘gaps’, indels, and their implications for serial homology and sequence alignment (Redelings & Suchard, 2005), include: bias in substitution and codons (Collins, Wimberger & Naylor, 1994; Kreitman & Antezana, 2000); concerted evolution (Drouin & Moniz de Sá, 1995; Eberhard et al., 2001); pseudogenes (Nielsen & Arctander, 2001); silent substitutions and undetected heterogeneity in rates of substitution (Wakeley, 1994; Simon et al., 1996); selectively constrained evolutionary rates of repetitive DNA families (Chen et al., 1991); homoplasy indirectly related to the four-state sampling universe of nucleotides (Wägele, 1995, 1996); and independence of molecular ‘characters’ (Zardoya et al., 1998; Graur & Li, 2000; Felsenstein, 2004).
Similarly, subjectivities of sampling and analysis beset both morphologists and molecular systematists, including: sampling of genes (Zardoya & Meyer, 1996; Moore & deFilippis, 1997; Pollock et al., 2002) and taxa (Bergsten, 2005); comparative weighting (García-Moreno, 2004); branch support (Felsenstein & Kishino, 1993; Suzuki, Glazko & Nei, 2002; Alfaro, Zoller & Lutzoni, 2003); and model selection (Mort et al., 2000; Buckley, Simon & Chambers, 2001; Huelsenbeck et al., 2002; Simmons et al., 2004; Lee & Hugall, 2005; Pickett & Randle, 2005; Pickett et al., 2005; Steel & Pickett, 2006). In addition, the critical distinction between ‘gene trees’ and ‘species trees’, which can differ significantly (Page & Charleston, 1997; Berglund-Sonnhammer et al., 2006), may be overlooked or ignored (Pamilo & Nei, 1988; Doyle, 1992, 1996; Moore, 1995; Maddison, 1997; Page & Charleston, 1997; Thornton, 2002; Geeta, 2003).
Despite these considerable challenges, molecular systematics clearly holds great potential for resolution of many problems of avian systematics, particularly in the Passeriformes. An informal survey of the passeriform literature since 1990 revealed studies of diverse taxonomic scales: 11 subordinal, five superfamilial, 34 (sub)familial, 55 generic and 24 (super)specific. This considerable success notwithstanding, largely unexplored is the potential of enterprises jointly including molecular characters of sequence and higher-order genomic structure (Kadi et al., 1993; Delport, Ferguson & Bloomer, 2002; Prychitko & Moore, 2003; Slack et al., 2003; de Kloet & de Kloet, 2003, 2005; Edwards, Jennings & Shedlock, 2005), the latter ignored at considerable peril (Winnepennincks & Backeljau, 1996). Together with morphological data of fossil and modern taxa, such molecular diversity appears to be essential for progress at many scales of avian phylogeny (Graybeal, 1994; Edwards et al., 2002; Harrison et al., 2004; Simon et al., 2004).
Appeal of the novel and unexpected
Apparent departures from taxonomic groups supported throughout much of the cladistic or molecular eras have been frequent during recent years (Cracraft et al., 2003, 2004). Fain & Houde (2004: 2570) proposed the entertainment of a number of counter-intuitive and weakly supported groupings in their analysis, in the spirit of freeing systematists from being ‘… guided by preconceptions of relationships.’ The latter appeal for objectivity is unquestionably laudable, but the fact that the proposed groups were merely novel does not constitute affirmation of any kind. Similarly, Pons, Hassanin & Crochet (2005: 686) stated that their study: ‘… identifies for the first time some sister relationships that had never been suggested before.’[emphasis added]. Although many traditionally recognized higher-order groups deserve formal analysis, novelty of resultant proposals is irrelevant to these endeavours. Realization of this potential primarily turns on two issues of modern systematics – rigorous and nomenclaturally transparent analyses that bridge subdisciplines (beyond the recent penchant to use fossils in molecular phylogenies for estimates of evolutionary rates), and empirically justified views and integration of morphological evidence in an era of increasing reliance on molecular inference.
Morphological homology–ontogeny, function and phylogeny
Insights from avian phylogeny
Hope for success lies in a pluralistic approach to evidence (Cracraft et al., 2004). This goal, in turn, is conditional on the surrender of prejudice and a common concept of homology. Ornithological systematics is replete with assumptions, assertions and inferences concerning homology and its role in the recognition of characters and evolutionary patterns (Freudenstein, 2005). In practice, variously defined ‘sameness’ is the basis for pre-analytical (a priori) assessments of homology in phylogenetics (Wake, 1999), but resultant phylogenies provide the historical framework within which homology is confirmed a posteriori (Haszprunar, 1998). However, non-historical criteria have been attached to the concept of homology virtually since its theoretical origins, of which ontogeny and function were perhaps the most common. Accordingly, alternative perceptions have influenced avian phylogenetics virtually throughout its history, particularly regarding homology and paedomorphic characters, homoplasy and convergence, the concept of Grundplans (e.g. Weber, 1990), and implications of ontogeny and genetics for homology of characters.
Phylogenetics and homology
Homology is synapomorphy at some phylogenetic level (Nelson, 1994), and is defined a priori as ‘similarity due to common descent’ (West-Eberhard, 2003: 485). Hall (2003) equated homology with identity (despite change) made evident by phylogeny: homology, reversals, rudimentia, vestigia, atavisms and parallelisms. Considerations of parallelism and convergence for Aves involve aspects of cranial structures (Starck, 1969) among outgroups (Carroll, 1988, 1997; Unwin, 1993; Brochu, 2001). Examples of atavism are few, but include the recurrence of a plesiomorphic pelvic muscle among Paradiesidae (Raikow, Borecky & Berman, 1980). Strong examples of morphological parallelism in birds involve the evolutionary loss of flight by flightless Rallidae (Livezey, 2003b).
Similarity and homology
Homology is conditional on essential, potentially mutable ‘sameness’ of a character manifesting continuity of descent within a phylogenetic hypothesis, whereas common function and ontogeny are not conditions thereof (Hall, 1994, 2003; Wake, 1999). Müller & Newman (1999) advocated secondary qualities of generation, integration and autonomy of a structure for the status of homology to be conferred, nuances herein considered components of the essential ‘sameness’, if considered at all. Variants of characters recognized in a phylogenetic context (putative homologues) and manifesting modification with descent – affected by any of a number of mechanisms of evolutionary change (selection, drift, mutation, ontogenetic deviation) – are treated as ‘states’ of a given character here.
Ontogeny and homology
The ontogenetic mechanisms that produce homologous states of a character are of considerable evolutionary interest and may prove critical in particular cases of diagnosis (Wagner, 1989), but do not qualify as criteria of homology of terminal features per se (Cracraft, 1967b; Hall, 2003). Genetics of ontogeny, however, can provide unique insights into the bases of likely homologues, e.g. odontogenesis and the edentuly of modern birds (Chen et al., 2000; Mitsiadis, Caton & Cobourne, 2006).
A synthetic view of homology holds that respective developmental stages of members of lineages, interpreted hierarchically within a phylogenetic framework, are each potential homologues capable of partitioned evolutionary patterns (Abouheif, 1997). Thus, homologues are defined within each developmental stage of each character (Hall, 2003), e.g., genes, developmental processes and stages thereof. However, judgements of homology based on ontogenetic processes are mistaken extensions of identity of descent across quasi-autonomous developmental modules (Rieppel, 1992, 1994; Wagner, 1994; Santini & Stellwag, 2002; Arthur, 2004). Traditional assertions that homologues must share genetic foundations represent similar overextensions of historical identity (Hall, 2003). Variants, including asymmetry, of a terminal character evolved during phylogenetic descent by means of developmental change are homologues of the given character, and variation in the ontogenetic mechanisms behind evolution of the character are not necessarily evidence of non-homology of the resultant states (Hall, 1994; Cooke, 2004). For practical considerations, predefinitive homologues are problematic for fossil birds as useful fossil embryos are rare (Elzanowski, 1981; Norell et al., 1994).
Developmental sequences include potentially distinct components such as developmental cascades, changes in timing (heterochrony) and position (heterotopy), and frame shifts (Hall, 1984; McKinney et al., 1990; Schulmeister & Wheeler, 2004). Where ontogenetic mechanisms per se are potential characters, the concept of modularity of development (Minelli, 1998; Raff & Raff, 2000) implies a delimitation of ontogenetic processes as characters in themselves. Of recent concern is the digital frame-shift within the digiti manus avium (Wagner & Gauthier, 1999), which counters the former embryological hypothesis of Hinchliffe (1985) that is still advocated by Burke & Feduccia (1997) and Feduccia (1999). Subsequent study has implicated Hox genes in such shifts (Chiu et al., 2000; Vargas & Fallon, 2005a, b), although the proposal is not without controversy (Galis, Kundrát & Metz, 2005). The modularity of development permits the view of the hypothesis of Wagner & Gauthier (1999) as but one characterization of several plausible candidates based on embryological principles (Galis, van Alphen & Metz, 2002; Hamrick, 2002; Welten et al., 2005).
Several other instances are variably conspicuous cases of heterochrony (McKinney et al., 1990; Klingenberg, 1998; Livezey, 2003b) – e.g. shifts in general developmental trajectories of Megapodidae (Starck & Sutter, 2000) and that of the avian furcula (Hall, 2001). Perceptions regarding the diagnostic relevance of anatomical position with respect to homology vary (Zelditch & Fink, 1996), e.g. the partly positional argument of the ‘rostro-parasphenoid’ process as distinct from the traditionally defined processus basipterygoideus (Weber, 1993), typological paradigms (Richardson, Minelli & Coates, 1999), and the role of function (Elzanowski, 1977). Patterns imposed by altered proximodistal developmental axes of appendages (Richardson, Jeffrey & Tabin, 2004) or action of regulatory (e.g. Hox) genes (Galis, 1999; Telford, 2000) increasingly are recognized in changes among transitional and terminal (definitive) developmental homologues. To date, most references to heterochrony have emphasized paedomorphic characters, i.e. variants of homologues typical of juveniles of plesiomorphic relatives (Livezey, 1995a, 2003b; Fink, 1988), and include more than simple alteration of growth rates (Starck & Sutter, 2000). Instead of undermining homology, such instances of heterochrony provide potentially novel synapomorphies among paedomorphs (Cracraft, 1981; Raff et al., 1990).
The law of Von Baer (Gould, 1977) – the biogenetic law – postulated that the order of developmental stages in an individual reflects the phylogenetic series of increasingly apomorphic states found in that lineage. In some cases, this series approximates the evolutionary changes leading to the terminal state (Gould, 1977), and may provide possible insights into polarities and transformation series (Kraus, 1978; Alberch, 1985; Shubin, 1994; Jeffrey et al., 2002; Grant & Kluge, 2004; Schulmeister & Wheeler, 2004) – i.e. states consistent with the ‘ontogenetic criterion’ (Alberch, 1985; Meier, 1997; Mabee, 2000). Avian candidates for this criterion include the angulus coracoscapularis and mutliple unions of elements or anlagen of the definitive avian shoulder girdle (Livezey & Zusi, 2006).
Function, homology and convergence
Cladistic (parsimony) analysis often is charged with a disregard for functional implications and convergence of character states, causing systematists to mistake similar but independently derived features among distantly related taxa as homologous. Convergence without demonstrated phylogenetic influence, as well as naive historical examples – e.g. purported affinities of swifts and swallows, tabled decades ago (e.g. Shufeldt 1889b; Lowe, 1939; contraVan Tuinen, 2002) – do not merit consideration here. Bock (1967, 1979) and Homberger (1980) considered function to be a critical criterion of homology, the independent study of which being required prior to inclusion of the structure in question in a phylogenetic analysis. Notable examples of this paradigm concern specializations of the feeding apparatus of Coraciiformes (Rawal & Bhatt, 1974) and Picidae (Bock, 1999), or cranial refinements among Charadriidae (Kozlova, 1961). Hypotheses of homology between features require a phylogenetic framework, and mere similarity of function in two potential homologues fails to demonstrate or exclude homology or convergence.
Convergence frequently is invoked in the context of adaptation (Coddington, 1994) and, at least in ornithological tradition, by superficial comparisons of the structures among distantly related lineages that share function, e.g. pelvic limbs of pursuit divers (cormorants, mergansers, loons), forelimbs of wing-propelled divers (alcids, diving petrels, penguins), or bills of piscivores (herons, anhingas, kingfishers). It is notable, however, that phylogenetic relationships among these examples were not obfuscated herein by these analogous similiarities in light of the totality of characters analysed, and that the purported instances of convergence were limited to a minority of phylogenetically analysed characters.
Given that character-states are homologous variants of a particular character defined a priori by critical similarity and a posteriori by continuity of descent, considerations of function, although of evolutionary interest, are not directly germaine to homology or its diagnosis (Lauder, 1994). Function, and its possible relationship to form, constitute but one potential precondition of convergence – one component of homoplasy (Hall, 2003). Examples of homologues cited independently of ontogeny in birds include: the processus basipterygoideus (Elzanowski, 1977) and modifications for dorsoventral movements of the carpus (Vazquez, 1992). Given that homology, and therefore homoplasy, are diagnosed reliably only within a phylogenetic context, non-hierarchical assessments of homoplasy (Faith, 1989; Zeffer et al., 2003) offer few if any insights.
Simpson (1944) discussed the differentiation of convergence from parallelism in closely related taxa under the term ‘parallel evolution’, and Bock (1963a) described it in the context of ‘evolutionary homodynamy’. Attribution of taxonomic groupings to convergence is conditional on: (i) the case for homology and plausible selection effecting changes (Fusco, 2001); (ii) reliability of phylogenetic analyses indicating disjunction of the disputed groups (Sommer, 1999); and (iii) the independence of phylogenetic reconstructions from sources of potential bias (Lee & Doughty, 1997). Two avian examples follow that might be taken by some to exemplify cases of ‘convergence’ of morphological characters leading to erroneous phylogenetic groups, exercises critical to assessing perceptions relative to empirical evidence (Wiens et al., 2003).
Ratites. We found strong support for monophyly of ratites, a finding in agreement with current consensus. The prior hypothesis of polyphyly was confounded by a perspective of static continents, convergence (Cracraft, 1974a) and a phenetic emphasis on differences (McDowell, 1948; Starck, 1955; Lang, 1956; Romer, 1968; Storer, 1971a). Synapomorphies of ratites not reasonably related to flightlessness or giantism fail as cladistic support for polyphyly. Finally, advocates of convergence fail to propose an empirically supported, plausible alternative hypothesis of relationship(s) consistent with the morphological (and molecular) data.
In our analysis, no support was found to ally any ratites to other non-ratite taxa; by contrast, 75 characters were ‘diagnostic’ or ‘highly supportive’ of monophyly of ratites (Table 2). Of these, 30 referred to the pectoral girdle or wing. If these 30 characters are accepted to be homologous as coded, they would lend support to the monophyly of ratites and (by parsimony) their shared flightlessness. On the other hand, if analysis had indicated that these same characters were optimized parsimoniously as non-homologous (but not coded a priori as such, absent evidence), the inference would be consistent with the possibility that flightlessness evolved in parallel more than once within a palaeognathous clade. Neither the present phylogeny nor alternative scenarios provide conclusive evidence for hypotheses concerning relative sequence(s) in the evolution of flightlessness in ratites; evolutionary trends of this kind are best explored through optimizations of character-suites a posteriori on the phylogenetic hypothesis (Fig. 13).
Candidates for parallelism of potential phylogenetic influence include the synostosis scapulocoracoideum of ratites and its marked similarity with those of non-avian Theropoda (Feduccia, 1986), and convergent enlargement of the angulus coracoscapularis of flightless Neornithes (Livezey & Humphrey, 1986; Livezey, 1988, 1989a, b, c, 1990, 1992a, b, 1993, 1995a), especially in the ratites and Rallidae (Livezey, 2003b). Diagnosis of the scapulocoracoideum as atavism would hinge on the phylogenetic history of the feature. A similar challenge attends classification of other pectoral changes among ratites as plesiomorphy, synapomorphy, parallelism or convergence.
Grebes and loons. We inferred the loons and grebes to be sister taxa, with no comparable support for positioning either taxon more strongly elsewhere (Table 3). Of the 17 characters diagnostic or highly supportive of this relationship (Table 2), 11 are from the pelvic girdle and limb (Livezey & Zusi, 2006). Those who considered these taxa to be only distantly related typically espoused a certainty that the similarities of the hindlimb and pelvis were misleading convergences associated with foot-propelled diving (Storer, 1956: 426; Storer, 1971a: 5). Suspected convergence is not supported by the differences in the hindlimbs of loons and grebes in that such are at least as parsimoniously interpreted to be: (i) symplesiomorphies differentially lost or modified in the lineages following divergence; or (ii) autapomorphies acquired independently following divergence of the orders. Neither has been shown to be parsimoniously synapomorphic with one or more other avian orders (Fig. 14). It is noteworthy that proponents of an alliance between the grebes and flamingos are tolerant of multiple dissimilarities between the groups (Chubb, 2004a). Whatever the scenario, the support index for this couplet of orders (Table 2; Fig. 14) significantly counters a convergent history for these characters, and an alliance with either the Charadriomorphae or the Phoenicopteridae entailed substantial sacrifices in parsimony (Table 3).
Palaeornithology: contrasting perspectives, common goals
Contrasts of ends and means
Until recently the fossil record for birds was marginalized with respect to formal phylogenetics, with most fossil taxa being fragmentary representatives or close relatives of modern groups. A spate of newly discovered fossils from the late Mesozoic has clarified greatly the theropod roots of birds. Despite consensus concerning the phylogenetic implications of new Mesozoic fossils and a number of shared goals, neontological and palaeontological schools often work at cross purposes. A former obstruction to unified analysis was a tradition of speculative evolutionary scenarios with strong palaeontological underpinnings, notably concerning evolutionary transitions and diversification (Olson, 1985; Feduccia, 1995, 2003; Chatterjee, 1997; Kardong & Zweers, 1997; Zweers & Vanden Berge, 1997a, b; Zweers et al., 1997; Bleiweiss, 1998c; Feduccia et al., 2005) and avifaunal ‘assemblages’ (Brodkorb, 1971a, 1976; Olson, 1985), that served as surrogates for ecological data not available for fossil lineages and past eras. A significant convergence in cladistic methods notwithstanding, it remains an unfortunate impediment that goals, expectations, nomenclature and assumptions of avian palaeontologists and neontologists (Cracraft, 1972b, 1974b, 1978, 1979, 1980) exist in largely parallel circles and have failed to realize a commonality of professional purpose. The most serious analytical challenges posed by avian fossils derive from missing data (Kearney, 2002; Kearney & Clark, 2003), which may affect the characters admitted for analysis.
Nomenclatural divergence, analytical corollaries
Issues of strict taxonomy aside, philosophical differences between the subdisciplines also involve long-standing perceptions of the diagnosibility of direct ancestry (e.g. Brodkorb, 1976; Olson, 1976). Palaeontological viewpoints regarding ancestral status of fossils also hold implications for nomenclature of fossil lineages (e.g. ‘stem-groups’) in phylogenetics (Benton, 2000), analytical validity of ‘ghost lineages’ (Norell, 1992), and the evolutionary significance of fossil ‘mosaics’ (Norell & Clarke, 2001; Dyke & Van Tuinen, 2004). A neontological perspective, however, considers fossils to differ from modern representatives solely by extinct status and quality of preservation, with many modern lineages representing more informative plesiomorphs of extant clades than any fossil member – e.g. anseriforms Anhimidae, Anseranas vs. fossil Presbyornis (Livezey, 1997a). Where adequately preserved for phylogenetic placement, fossils also may provide an estimate of minimal age of the group it represents, but this estimate is imprecise and subject to bias. Ironically, a misunderstanding of such estimation contributed to early arguments concerning ‘temporal incongruence’ and against a theropod origin for birds (e.g. Brochu & Norell, 2000, 2001).
Although the definitions of ‘stem’ and ‘crown’ groups are relatively simple (Meier & Richter, 1992), the former sharing conceptual roots with earlier terms of assumed or possible direct ancestry such as ‘plesions’ (Wiley, 1981), it seems that these designations carry important nomenclatural implications (Benton, 2000) and may impede the integration and interpretation of fossil and modern taxa by identical means. Where ancillary assumptions regarding local polarities and implications of ‘stem-group’ members are made in analyses based on narrow samples of taxa (e.g. Bourdon, 2005; Bourdon et al., 2005) or characters (e.g. Mayr, 2002a, 2003a, b, c, 2004e, 2005i; Mayr & Clarke, 2003; Mayr et al., 2003; Mayr & Ericson, 2004), or if the fossil material is of marginal quality (e.g. Mayr, 2002c, 2004e, 2005f), the differences between neontological and palaeontological schools can be substantial. In many contexts, it appears virtually inescapable that ‘stem-group’ status implicitly conserves the notion of possible or likely ancestry relative to the corresponding crown-group, and thereby suggests an evolutionary role beyond mere cladistic position (e.g. successive sister-groups).
Moreover, inclusion of a fossil in a ‘stem-group’ (Mayr, 2002c, 2005d) can lead to alternative analytical protocols, e.g. speculations of local polarities and substitution of hypothesized instead of observable character states to lend support to trees including mulitple fossils (e.g. Mayr, 2002c, 2004f, 2006a). The comparatively well known Pseudasturidae – formerly assigned to the Family Quercypsittacidae (Psittaciformes) by Mourer-Chauviré (1992) – were judged to combine ‘intermediacy’ in a number of characters purportedly diagnostic of psittaciforms with similarities to the ‘Galbulae’ (Piciformes), and were referred to the ordinal ‘stem-group’ of Psittaciformes by Mayr (2002c). Informal hypotheses of polarities in analyses of fossil birds – e.g. by Mayr (2005i: characters 5 and 12), Mayr et al. (2003: characters 1, 6, 11, 29, 35 and 71), and Mayr & Ericson (2004: character 55) – evidently intended to impose ‘local’ initial states in a particular context and often asserted in character descriptions (Mayr, 2002c), are innocuous if these are inferred from direct analysis rather than imposed based on preconceptions regarding a taxon. Evidently, however, in some cases states observed for given terminal taxa are replaced by states purportedly representative of ‘stem-group’ members (i.e. states hypothesized to be predecessors to those of taxa included in the corresponding ‘crown group’). Examples of the latter – confirmation of which requires character descriptions and the data matrix – include characters 5, 9, 18, 26, 30 and 68 of Mayr et al. (2003).
A related tradition of avian palaeontology is the imposition of intuitive trends that exceed the strict empirical content of available fossil material. For example, newly discovered fossils – notably a ‘stem-group hummingbird’ (Eurotrochilus inexpectatus) from the early Oligocene of Germany of purportedly modern grade – motivated Mayr (2003c, 2004d, 2005a, g) to enter the debate concerning the relationships of the Apodiformes. These efforts included a critique (Mayr, 2001f) of a description of a fossil taxon by Dyke (2001c), the attribution by Mayr (2004d) (based on limited taxonomic comparisons) of morphological specializations both for hovering flight and for nectarivory to Eurotrochilus, and speculations on the earliest evidence of avian nectarivory and the coevolution of certain bird-pollinated angiosperms in the New World. Given the oversimplification of distributions of characters, especially within the Apodiformes (Cohn, 1968; Karhu, 1992, 1999, 2001), and the wider controversy based on molecular data (Dumbacher et al., 2003; Thomassen et al., 2003, 2005; Chubb, 2004b), it is unfortunate that the characters included by Mayr (2001f, 2003c, 2004d, 2005g) totalled from 25 to 98, and failed to provide a synthesis of all relevant characters was not provided for relevant taxa prior to speculating regarding graded specializations and coevolutionary trends in the early Cenozoic.
Plesiomorph or interordinal ‘intermediate’?
Perhaps the most prevalent idiosyncracy of palaeontological perspectives is the reputed importance of fossils as a source of phylogenetic ‘bridges’ between extant, comparatively divergent lineages (Mayr, 2006b). However, neither the published record nor phylogenetic theory supports this notion, and the role of interordinal ‘linking’ lineages is at least as often revealed by extant taxa (Livezey, 1997a). The taxonomic history of Presbyornis illustrates the potential that such expectations may hold for phylogenetic placements of fossils with respect to modern higher-order groups.
Wetmore (1926) originally described Presbyornis from a single element from the Eocene of western North America as a charadriiform, but later (with abundant additional material) it was asserted to be a ‘transitional’ shorebird and indicative of a close relationship between Charadriiformes and Anseriformes (Olson & Feduccia, 1980a), the intuitive methods employed in the latter being criticized by Raikow (1981). More than a decade later and based on direct cladistic analysis of both Presbyornis and modern taxa, the genus was determined to be a plesiomorphic anatoid (Ericson, 1997; Livezey, 1997a). Subsequently, Presbyornis (and synonyms) has been the genus of choice for referral of fossils from the Eocene of England (Harrison & Walker, 1976a), Eocene of Mongolia (Kurochkin, 1988), Palaeocene of eastern North America (Olson, 1994; Ericson, 1997), Cretaceous of Antarctica (Noriega & Tambussi, 1995), late Palaeocene of North America (Benson, 1999), and Cretaceous of Mongolia (Kurochkin, Dyke & Karhu, 2002).
An examination of the material upon which these referrals were made raises reasonable doubts as to diagnostic reliability, and reveals the role of the comparatively well represented fossil Presbyornis as a palaeontological ‘strange attractor’ for other, variably preserved fossils of uncertain affinities. As the referrals of fossils to the early Anseriformes escalated, purported allies of Presbyornis also increased in number and morphological diversity: Olson (1994) reported a ‘giant’Presbyornis from the Palaeocene of eastern North America, Alvarenga (1999) referred a fossil from the mid-Tertiary of Brazil to the Anhimidae; Olson (1999b) phenetically allied Anatalavis from the London Clay to the modern Australian endemic Anseranatidae, a placement disputed by Dyke (2001b); Mourer-Chauviréet al. (2004) allied Anserpica from the Oligocene of Europe to the same family; and Clarke et al. (2005b) likened Vegavis (Cretaceous of Antarctica) to Presbyornis and referred the genus to the Anatoidea by a nested series of analyses of published data sets, by a method similar to that of supertrees.
The saga of Presbyornis also extended to the interordinal realm of fossil referrals, and provided insights into the alliance formerly alleged between Presbyornis and Phoenicopteridae by way of the poorly understood Juncitarsus (Olson & Feduccia, 1980a, b; Ericson, 1999), and thereby the subsequently proposed relationship between Phoenicopteridae and Podicipedidae. In addition, Cheneval & Escuillié (1992) cited similarities between grebes and the flamingo-like Palaelodidae in the pelvic appendage – the very class of characters considered by many of these authors to be prone to convergence and therefore unreliable in uniting grebes with loons.
Nevertheless, Mayr (2004c: 140) considered the sister-group relationship between grebes and flamingos to be ‘… one of the best supported higher-level clades within modern birds.’Mayr (2005a: 523) then suggested that the intermediacy of two skeletal features between Juncitarsus (Eocene of Wyoming) and the Palaelodidae (Oligocene of Europe), fossils traditionally allied to the Phoenicopteridae, ‘… provides a morphological link between Phoenicopteriformes and Podicipediformes.’ As for the early inferences made for Presbyornis, to which Juncitarsus and phoenicopterids were compared (Ericson, 1999), misclassification of fossils can lead to significant errors where informal phenetics and exceptional treatment of fossils are involved (Livezey, 1997a), problems not correctable by adoption of empirically depauperate taxonomic nomenclatures (e.g. ‘stems’ and ‘crowns’) and contradictory views on the phylogenetic roles of fossil taxa.
Fossil neornithes: preservation and opportunities
Referrals, old and new
Despite the foregoing critique, well-preserved fossils can provide important insights into avian evolution, especially the Mesozoic origins of the group, and many potentially important fossils currently have yet to be described (J. A. Clarke, pers. comm.) and are beyond the scope of the present work. Unfortunately, a majority of fossil Neornithes, both of Mesozoic (Hope, 2002) and Cenozoic age (Brodkorb, 1963, 1964, 1967, 1971b), were named based on material not permitting meaningful inclusion in a formal cladistic analysis of modern scale. Moreover, classifications of many of these taxa were made phenetically, and with a marked tendency to refer new taxa to the modern taxon perceived to be most similar (Livezey & Martin, 1988; Livezey, 1997a, 2003c). Fortunately, increased use of cladistic analyses makes it likely that such records, especially those spanning the late Mesozoic and early Cenozoic, will provide an increasingly refined palaeontological dimension to avian phylogenetics.
Given the limitations of direct diagnosis (Table 2) and the phenetics of seeking the best neornithine group in which to place a fossil (Livezey & Martin, 1988; Livezey, 1997a), what is the recommended means for evaluation of a new fossil with respect to the present data set? Two paths seem most informative at present: (i) unconstrained analysis of the present data set, appended with the codings for the fossil taxon, however incomplete (within reasonable limits of informativeness); or (ii) analysis of the fossil taxon under a backbone-constraint for modern lineages (e.g. Figs 13–18). The latter probably will prove optimal in those cases where missing data are especially numerous or where even higher-order affinities are indiscernible, and especially where both circumstances pertain. Taxonomic groups of greatest diversity and quality of preservation hold the greatest potential for such insights, and these merit special emphasis here, especially those broadly consistent with groupings inferred here and for groups having few modern members.
Diversity, aquatic and terrestrial
Fossils have been referred, although not all by phylogenetic means, to all modern families of the Pelecaniformes: Phaethontidae (Harrison & Walker, 1976b; Olson, 1983b, 1985; Mayr & Smith, 2002), Fregatidae (Olson, 1977), Fregatidae or Sulidae (Olson & Matsuoka, 2005), Sulidae (Olson & Rasmussen, 2001; Mayr, 2002d; Stucchi & Urbina, 2004), Pelecanidae (Olson, 1999a), Phalacrocoracidae (Mayr, 2001c) and Anhingidae (Alvarenga, 1995; Alvarenga & Guilherme, 2003; Mourer-Chauviréet al., 2004). In addition, the controversially referred Plotopteridae (Mayr, 2004b) have increased in palaeodiversity (Olson & Hasegawa, 1979, 1996; Olson, 1980; Goedert, 1988). Less well justified is the putative membership of a group of widespread, fossil, pseudo-denticulate birds – Odontopterygiformes (Owen, 1873; Howard, 1957; Goedert, 1989; Averianov et al., 1991; Zusi & Warheit, 1992; González-Barba et al., 2002) – for which a modest analysis mustered marginal support as an alternative sister-group to the Anseriformes (Bourdon, 2005).
The Coliiformes, Trogoniformes, Coraciiformes and Piciformes merit renewed examination as these groups (e.g. Bucconidae sensu lato, including Primobucconidae), as well as specimens of uncertain affinity (Olson, 1992b), also have received multiple new fossil referrals (Harrison, 1982a; Mayr, 2000c) – including Trogoniformes (Mourer-Chauviré, 1980; Mayr, 1998a, 1999a, 2001b, 2003b), Coliiformes (Olson & Houde, 1989; Houde & Olson, 1992; Mayr & Peters, 1998; Mayr, 2000b, 2001a, 2005d, e; Dyke & Waterhouse, 2000; Kristoffersen, 2001; Mayr & Mourer-Chauviré, 2004), Coraciiformes (Olson, 1976, 1992b; Mourer-Chauviré, 1985; Mayr & Mourer-Chauviré, 2000; Mayr, Mourer-Chauviré & Weidig, 2004b) and Piciformes (Mayr, 2001d, 2005h, i). Broadly delimited zygodactyl taxa (Feduccia & Martin, 1976; Mayr, 1998c, 2001e, 2004e, 2005h, i) complete the apparent palaeodiversity of ‘higher’ landbirds (Fig. 18), and contrasts with modern passeriform dominance (Manegold, Mayr & Mourer-Chauviré, 2004).
The Psittaciformes, at least the modern members of which are anatomically distinctive, have attracted a number of newly described fossils, some of which obscure this distinctness (Mayr, 2002c), and thus the order has undergone pronounced extensions of its palaeodistributional limits (Harrison, 1982b; Mourer-Chauviré, 1992; Mayr & Daniels, 1998; Stidham, 1998; Dyke & Mayr, 1999; Brochu & Norell, 2000; Dyke & Cooper, 2000; Mayr, 2001g, 2002c; Mayr & Göhlich, 2004; James, 2005). The uniquely apomorphic form of the crania of some taxa in this order is so extreme (Smith, 1975) as to pose challenges of comparability, and many modern members also manifest distinctly modified pectoral girdles and apomorphic pelvic skeletons (Smith, 1975; Livezey & Zusi, 2006). However, some fragments controversially referred to this clade are of potential relevance to the origins of modern orders and the K–T boundary (Stidham, 1998 vs. Dyke & Mayr, 1999), and merit reassessment.
Spatiochronological dimensions of phylogenetics
Preservation and inferred distribution
A traditional referral of issues of ‘deep time’ to palaeontology (Brochu et al., 2004) evidently reflects, in part, the rapidity with which fossil evidence was conjoined with modern phylogenetics for the calibration of geological time with phylogenetic hypotheses. Palaeocalibration of ages provided by fossil records in combination with models of molecular phylogenetics predictably turns on taxonomic groups possessed of rich, accurately aged fossils and reliable phylogenies.
These cross-disciplinary works progressed (perhaps too) rapidly toward attempts at global treatments of Neornithes that were influenced by undue inclusion of fossils of unreliable identity and age. In addition, the early spate of efforts favoured classes of models (e.g. Markovian) that facilitate minimization or ‘smoothing’ of discrepancies between calibrations and branching patterns as opposed to realistic incorporation of heterogeneous evolutionary rates (Sheldon et al., 2000; Brochu & Norell, 2001; Van Tuinen & Hedges, 2001; Dyke, 2003; Pol et al., 2004; Van Tuinen & Dyke, 2004; Van Tuinen et al., 2006). The latter, often underappreciated, reality reflects the likelihood of preservation and a negative skewness of such records expected to be inversely correlated with body size and related heteroscedasticity that is directly correlated with geological age. These palaeontological issues are confounded by unrealistic assumptions of molecular trees and models in which the fossil data are incorporated. Not surprisingly, informativeness of such exercises to date has been limited – i.e. modern orders have been inferred to have very early origins (Pereira & Baker, 2006a: table 1; Van Tuinen et al., 2006: tables 1, 2). Nevertheless, a phylogenetic hypothesis of high support and resolution (Figs 10–18) is an essential starting point – one, however, conditional on independent testing and augmentation. Another precondition of success, aside from well-documented fossil records (e.g. Clarke et al., 2003), is use of realistic assumptions regarding molecular evolution where calibration of ages of divergence events is among the objectives (Pereira & Baker, 2006a).
Calibration of time
Failure to verify the existence of a molecular ‘clock’ notwithstanding (García-Moreno, 2004), an endeavour of particular interest regards bringing to bear the calibration of geological time – the ‘time axis’ of Benton (1996) – through phylogenetically placed fossil taxa, thereby estimating a minimal age of corresponding nodes in a phylogeny and recavering the temporal pattern of avian diversification (Hedges et al., 1996; Mindell et al., 1996; Miyaki et al., 1998; Cooper & Penny, 1997; Kumar & Hedges, 1998; Sepkowski, 1999; Waddell et al., 1999; Cracraft, 2001). Direct use of stratigraphic data for inference of trees by means of parsimony or ‘stratocladistics’ has been criticized on several methodological grounds, and is especially inappropriate for the sparse avian fossil record (Fisher, 1992; Huelsenbeck & Rannala, 2000).
Well-supported phylogenies for molecular models are critical for extrapolations of evolutionary rates from fossil-based point-estimates of geological age (Marshall, 1990; Springer, 1995; Arbogast et al., 2002; Broham et al., 2002; Smith & Peterson, 2002; Broham, 2003; Brochu, Sumrall & Theodor, 2004; Van Tuinen & Hedges, 2004). Disagreements among calibrations to date are consistent with evidence for significant variation among rates of evolution (Thorne, Kishno & Painter, 1998; Johnson & Cicero, 2004; Cicero & Johnson, 2006; Zink & Klicka, 2006), the effects of outgroups (Waddell et al., 1999), initial estimates of which (e.g. Shields & Wilson, 1987) continue to be used. Other problems stem from the limited suitability of stratigraphic data in phylogenetic contexts (Huelsenbeck & Rannala, 2000), and effects of topological aspects of trees (Pol et al., 2004). The continued controversy concerning the position of the Passeriformes relative to other Neoaves (especially Fig. 10) – considered by many to reflect effects of outgroup and relative evolutionary rates of mtDNA – presents a critical issue for attempts at calibrations more precise than Mesozoic vs. Cenozoic origins (Stanley & Cracraft, 2002; Cracraft et al., 2004; Pereira & Baker, 2006a, b; Slack et al., 2006a).
Accordingly, the prospect of using currently available palaeontological data to calibrate evolutionary rates is disconcerting, regardless of the phylogenetic framework conjoined, principally because of a paucity of fossils that are reliably classified and of precise age (Hope, 2002; Livezey, 2003c). However, the existence of avian lineages in the late Mesozoic has been substantiated directly by palaeontological evidence (Olson, 1992a; Dalla Vecchia & Chiappe, 2002; Grellet-Tinner & Norell, 2002; Schweitzer et al., 2002). For example, the estimated origin of megapodiid galliforms in the Cretaceous (Pereira & Baker, 2006b) agrees well with estimates for the comparably ancient Anseriformes.
Special attention relates to the oldest fossil record for a member of the Neornithes, increasingly with respect to hypotheses of descent relative to massive faunal upheavals following the K–T boundary (Feduccia, 1977c, 1995; Olson & Feduccia, 1980a; Olson & Parris, 1987; Paton et al., 2002, 2003). Despite considerable effort, few points of agreement among phylogenetic calibration of rates and fossil records have been achieved (Benton, 1999, 2001; Dyke & Mayr, 1999; Van Tuinen & Hedges, 2001). In part, disagreements reflect variable reliances on assumpitions of ‘clock-like’ molecular evolution (Helm-Bychowski & Wilson, 1986; Van Tuinen & Hedges, 2001; Van Tuinen & Hadly, 2004). The unrealistic assumption of ‘clock-like’ molecular change (Brochu et al., 2004) has led to diverse means of ‘correction’ or analytical adjustments (Mooers & Harvey, 1994; Sanderson, 1997; Mindell et al., 1998; Bleiweiss, 1998c; Ho et al., 2005), increased sampling of fossils (Springer, 1995; Smith & Peterson, 2002; García-Moreno, 2004; Pereira & Baker, 2006a, b), incorporation of multiple ‘clocks’ (Van Tuinen & Dyke, 2004) and relaxation of estimators through Bayesian methods (Yang & Rannala, 2006).
For example, Mayr (2002c) stated that the earliest passeriform is no older than the early Oligocene, whereas Cracraft et al. (2004) inferred the order to have originated prior to the K–T boundary, a discrepancy of magnitude likely to weaken associated calibrations. Recent attempts to bracket times of avian cladogenesis by Dyke & Van Tuinen (2004: fig. 3) based on the few widely accepted higher-order relationships necessarily encompassed relatively few major lineages of birds, whereas a priority accorded expanded taxonomic samples led Van Tuinen et al. (2006) to accept calibrations based on many fossils classified from the literature, relationships derived from the phenetics of DNA hybridization, and a null model incorporating questionable assumptions concerning molecular evolution (Pereira & Baker, 2006b) and a basal polytomy for Neoaves.
Palaeobiogeography and the spatial dimension
There is considerable optimism bestowed upon fossil taxa for the reconstruction of historical biogeography (Olson, 1985; Carroll, 1997). Southern-hemispheric patterns interpreted in terms of tectonic fragmentation and movements are manifested in the literature of avian systematics (Glenny, 1954; Cracraft, 1973b, 1975, 1976c, 1982c; Hedges et al., 1996; de Kloet & de Kloet, 2005). Realistic reconstructions of historical biogeography require effects of vicariance events within continents – e.g. mountainous uplifts or glaciation – as a secondary class of abiotic antecedants of phylogenetic diversification (Ploeger, 1968; Cracraft, 1982c, d).
Of greater empirical substance for Aves, perhaps, are inferences of historical vicariance, notably those fortified by robust phylogenetic analyses and showing congruent geographical patterns. Most important of these for birds is the recurrent pattern of southern origins among many lineages, collectively suggestive of a critical role for Gondwana in early avian origins and diversification and most strikingly coincident with the K–T boundary (Cracraft, 2001). Patterns consistent with southern genesis are especially compelling in light of a biased tendency for migratory habit to counter northern–southern hemispheric patterns relative to those of eastern–western hemispheres (Böhning-Gaese, González-Guzmán & Brown, 1998). Taxa for which circumstantial evidence of this kind is consistent with southern-hemispheric origins (Cracraft, 1973b), include: Ratitae (Cracraft, 1974a; Haddrath & Baker, 2001), Anseriformes (Livezey, 1986, 1997a, 1998a), Galliformes (Dyke et al., 2003), Sphenisciformes (Cracraft, 1988; Cracraft & Mindell, 1989; Harrison et al., 2004), Gruiformes (Cracraft, 1973a, 1982b; Livezey, 1998b), Psittaciformes (Cracraft, 1988; Cracraft & Mindell, 1989; Miyaki et al., 1998; de Kloet & de Kloet, 2005), Trochilidae (Bleiweiss, 1998d) and suboscine Passeriformes (Ericson, Johansson & Parsons, 2000; Irestedt et al., 2001, 2002; Ericson et al., 2002a, b, 2003b; Barker et al., 2002, 2004; Edwards & Boles, 2002; Yuri & Mindell, 2002; Ericson & Johansson, 2003; Chubb, 2004b).
The Palaearctic understandably dominated palaeogeographical hypotheses in the early 20th century, especially for fossils from the late Cenozoic (Ploeger, 1968). The prevalence of terrestrial groups during the Palaeogene considered ‘basal’ to the Passeriformes (i.e. branching from the lineage culminating in the Passeriformes and its sister-group) prompted Mayr (2005a) to suggest that the former taxa may have occupied ‘passeriform’ niches prior to the Oligocene. This hypothesis should be amenable to testing by morphological comparisons but is contingent on the resolution of debated dates of origin of the Passeriformes (Boles, 1995, 1997; Cracraft et al., 2004; Mayr & Manegold, 2004). Among the avian clades most frequently cited with respect to adaptive radiation, key innovation, ontogenetic underpinnings and sheer diversity – phenomena of prime interest (Starck, 1969; Smith, 1994) – are the Apodiformes and Passeriformes. Accordingly, the Apodiformes (especially the Trochilidae) attracted substantial anatomical (Cohn, 1968; Karhu, 1992, 1999, 2001) and phylogenetic study (Dyke, 2001c; Mayr, 2001f, 2003c, 2004d, 2005a, g; Thomassen et al., 2003, 2005; Chubb, 2004b). The Passeriformes, however, hold a position of unique diversity – comprising 60% of extant Aves (Cracraft et al., 2004) and unmatched global distribution (Fitzpatrick, 1988; Kochmer & Wagner, 1988), evolutionary success (Raikow, 1986, 1988; Vermeij, 1988) and adaptation (Baum & Larson, 1991).
Evolutionary radiations and the K-T controversy
Such palaeogeographical patterns have shed light on the theory of ‘adaptive radiation’ (Gould & Eldredge, 1977; Eldredge & Cracraft, 1980; Levinton, 1988; Eldredge, 1989; Valentine, 1990; Jablonski, 2000; Schluter, 2000), ‘explosive radiation’ (Feduccia, 1980, 1995, 1996, 2003; Sheehan & Fastovsky, 1992; Cooper & Penny, 1997; Kardong & Zweers, 1997; Cooper & Fortey, 1998), and ‘mass extinction’ (Jablonski, 2005) of Aves around the K–T boundary. Other biogeographical hypotheses of significance relate cladogenetic patterns and faunal diversity to tectonic movements (Hedges et al., 1996; Craw, Grehan & Heads, 1999; Humphries & Parenti, 1999), and trans-Gondwanan dispersal (Cracraft, 1973b, 1975, 1976c, 1982b, c, 2001).
In particular, the Charadriiformes have been the focus of substantial, speculative scenarios regarding a special evolutionary role involving multiple avian groups and major extinctions. The notion that ‘shorebirds’ are fundamental to an understanding of avian evolution across the K–T boundary (Olson & Feduccia, 1980a, b) is no longer considered promising, and was based in part on a preconception of Charadriiformes as phenotypic intermediates bridging higher-order avian groups (Zweers & Vanden Berge, 1997a, b; Zweers, Vanden Berge & Berkhoudt, 1997; Dyke et al., 2002; Paton et al., 2002).
Quantitative estimation of rates of evolutionary change (Rodriguez-Trelles, Tarrio & Ayala, 2002) – given robust phylogenies (Marshall, 1990) and adequate fossil records (Sepkowski, 1999) – have fostered more detailed hypotheses of phylogenetic bottlenecks and ‘explosive’ radiation near the K–T boundary (Feduccia, 1995, 2003; but see Stanley & Cracraft, 2002). However, there is growing evidence, at least based on Bayesian analyses of data largely or entirely from the mitochondrial genome, that most or all neornithine orders date from the late Cretaceous (Grellet-Tinner & Norell, 2002; Schweitzer et al., 2002; Dalla Vecchia & Chiappe, 2002; Pereira & Baker, 2006b; Slack et al., 2006a, b; Van Tuinen et al., 2006). If accurate, despite the vulnerability of such data to suboptimal rooting, this record undermines early anticipations of K–T boundary effects in modern orders and an evolutionary timespan in which major divergences of neornithine lineages would extend through the early and middle Cenozoic. Expectations for avian fossils of such antiquity are correspondingly conservative, and although fossils of such age potentially offer new calibration points for early avian lineages, there is diminished hope for points of calibration bearing on the relative antiquity of modern (super)orders of birds or precise molecular estimates of associated evolutionary rates characteristic of phylogenetic lineages.
Priorities for future investigation
Current points of irresolution
Based on the present analysis (Figs 1–8) and other studies during recent decades (Figs 10–18), the area of primary ignorance for avian phylogenetics is the heretofore refractory groupings within the Neoaves, with principal problems being the highest-level nodes (notably the position of the Passeriformes within this group) and the comparatively routine but significant work of phylogeny within orders and families (Fig. 11; Appendix 1). Optimism remains justified, however, with new genes and molecular signal of scale higher than simple sequences and indels under exploration (e.g. retroposons). Reliance solely on a single scale of homology, e.g. the indels upon which Fain & Houde (2004) proposed largely hemisperically concordant ‘Metaves’ and ‘Coronaves’, is herein inferred to be nomina nuda (Appendix 1). This fixation on single-scale molecular analyses justifiably led Harshman et al. (2006: 42) to ask: ‘Can four million bases [nucleotides] resolve the [avian] tree?’ Fortunately, there are a number of anatomical fields of study that remain virtually untouched in modern phylogenetic contexts (Livezey & Zusi, 2001, 2006), a circumstance of hope in light of the palaeontological discoveries that will necessitate refinement of these and refined anatomical complexes coded (e.g. os palatinum of Archaeopteryx; Mayr et al., 2005; Zusi & Livezey, 2006).
Several episodes of avian phylogeny were incompletely resolved in the present analysis (Figs 12–18), and will require significantly intensified sampling of taxa to solve:
Positions of Aepyornithiformes and Dinornithiformes relative to extant ratites (Fig. 13).
Resolution of genera within the Phasianoidea (Fig. 13).
Resolution of the positions of the Psittaciformes and Columbiformes (Figs 16, 17).
Determination of the relationships among several poorly resolved nodes involving the traditional Charadriiformes and Gruiformes, within the ‘central’ Charadriiformes (Fig. 15), for which alternative proposals continue to appear (Simmons et al., 2004; Van Tuinen et al., 2004; Paton & Baker, 2006; Pereira & Baker, 2006a), a task likely to require inclusion of rich suites of such integumentary characters as the natal integument for reconstruction of deeper nodes, and aspects of the definitive externum for resolution of shallower nodes (Jehl, 1968, 1971; Livezey, 1991, 1995b, c, d, 1996a, b, c, 1997c).
Confirmation of relationships of the families of Caprimulgiformes (Fig. 17).
Resolution of the trichotomy among the Coraciiformes, Piciformes and Passeriformes (Figs 17, 18), and resolution of subordinal and familial phylogeny within the Passeriformes, and affirming the position of the Passeriformes relative to other Neoaves.
Make available an empirically grounded platform for finer-scale analyses of single orders or families of Neornithes as an alternative to the classical literature or the ‘tapestry’ by Sibley & Ahlquist (1990), with priority accorded to comparatively old, multifamilial orders (e.g. Galliformes, Procellariiformes) or traditionally challenging groups (e.g. Pelecanimorphae).
Phylogenetic integration of well-preserved fossils into the phylogeny, both serving as additional taxa for resolution or revision of groups and as points of calibration of (minimal) ages of lineages of which these are members.
An especially rewarding class of study awaits optimization of life-historical attributes at the present phylogenetic scale, attributes such as sexual dimorphism, parental care and reproductive parameters (Wyles, Kunkel & Wilson, 1983; Winkler & Sheldon, 1993; Wesolowski, 1994; Wimberger & de Queiroz, 1996; Figuerola, 1999; Geffen & Yom-Tov, 2001; Tullberg, Ah-King & Temrin, 2002; Roulin, 2004; Pereira & Baker, 2005; Ekman & Ericson, 2006) as a starting point for more detailed studies in evolutionary biology. This area of study can advance only with: (i) use of well-resolved, robustly supported phylogenies, often not feasible (e.g. Cubo, 2003); and (ii) refinement of methods for optimization a posteriori of attributes, including where phylogenies include polytomies (Saunders, Smith & Campbell, 1984; Temrin & Sillén-Tullberg, 1994, 1995; Omland, 1997a, b; Ligon, 1999; Richardson et al., 1999).
An unfortunate aspect of such methods has been revealed by a number of optimizations that relied on the phenetics of Sibley & Ahlquist (1990), ostensibly as it was the only hypothesis of adequate taxonomic breadth for the desired survey (e.g. Van Tuinen et al., 2006). Attributes so assessed include body mass (Maurer, 1998), wing length (McCall, Nee & Harvey, 1998) and correlates of flightlessness (Cubo & Arthur, 2001). Most such published surveys have recovered significant patterns in selected morphological attributes despite the virtually universal view that the quasi-phylogeny that was used is unreliable. This incongruity indicates that apparent significance of optimizations is essentially meaningless, but more importantly provided a fortuitous insight that statistically significant patterns can emerge from inaccurate phylogenetic hypotheses and that it may be prudent to adopt more conservative critical values for tests of this nature. Until more discriminating methods are available, significance in this context should not be assessed against a null model of random change but instead against randomized evolution with varied descent or reserved for comparisons between phylogenies.
Broadened phylogenetic horizons
Philippe & Laurent (1998) entitled their paper with a challenge of undisputed cogency: ‘How good are deep phylogenetic trees?’ An expansion of phylogenetics of the Theropoda and Dinosauria is well underway, however, and will be central to a robust foundation for avian phylogeny, including significant implications for ‘global’ homology and anatomical nomenclature. This exploration should lead to phylogenetic hypotheses among Vertebrata of increasing scale, especially in light of character analyses already accomplished for non-avian Tetrapoda (Benton & Clark, 1988; Evans, 1988; Nielsen, 1995; Zardoya & Meyer, 1996; Laurin & Reisz, 1997; Philippe & Laurent, 1998; Zardoya et al., 1998; Xia, Xie & Kjer, 2003; Suzuki, Laskowski & Lee, 2004; Hill, 2005). Similar explorations among deep roots by molecular and morphological means also hold promise for the phylogenetic resolution of an expanded ‘super-clade’ of Reptilia and allied Tetrapoda (Benton, 1990; Graybeal, 1994; Kumazawa & Nishida, 1995; Mindell et al., 1999; Ruta, Coates & Quicke, 2003), including (sub)fossil taxa to the degree permitted by remains (Handt et al., 1994; Taylor, 1996) and logistic limits on life-historical data available for fossil taxa. In contrast to issues of quality of the fossil record (Wagner, 2000a) and limits on signal recoverable from fossils (Wagner, 2000b), potential for neontological study remains underexplored, especially that involving soft-tissue anatomical systems (Wägele, 1995).
In light of the evident attraction of probabilistic reconstructions, phylogenetics may benefit most from an expansion of Bayesian methods to address problems of incomplete data (Gelman & Xiao-Li, 2004), robustness of estimates (Insua & Ruggeri, 2000) and refined optimization, including (quasi-)likelihood methods, both parametric and non-parametric (Heyde, 1997; Beiko et al., 2006; Anisimova & Gascuel, 2006). In both major classes of probabilistic models, renewed attention is justified to the analytical properties of branching processes (Harris, 1963; Athreya & Jagers, 1997; Kimmel & Axelrod, 2002; Haccou, Jagers & Vatutin, 2005), for which statistical methods have been elaborated only recently. In a problem of this unprecedented scale, it is critical for modern systematists to exploit a diversity of sources of data as a means to effect even-handed assessments of historical pattern.
An overview of the literature (Figs 1–10) reveals that much remains to be accomplished in avian phylogenetics. Significant advances principally lie in studies of great taxonomic scale and diverse support that target nodes of ordinal and higher taxonomic scales of Neoaves, in conjunction with a solution of the persistent disputes among morphological, mitogenomic and nuclear findings. In combination with incorporation of additional, evolutionarily conservative characters of soft anatomy (Oliveira et al., 2004) and karyotypes (Shetty, Griffin & Graves, 1999; Burt, 2002), the methods of ‘total-evidence’ analyses hold promise for phylogenetic scales and calibration of ages previously not feasible (Stanley & Cracraft, 2002; Baker & Gatesy, 2002; Cracraft et al., 2004; Yang & Rannala, 2006), a potential not without early tests (e.g. Kennedy & Page, 2002) and new methodological challenges (Baker et al., 1998; Ballard et al., 1998; Bang, Schultz & DeSalle, 2002; Bininda-Emonds et al., 2002).
Acknowledgments
This research was supported by National Science Foundation (NSF) grant BSR-9396249 to Livezey (PI/PD), NSF grant DEB-9815248 to B.C.L. (co-PI/PD) and R.L.Z. (co-PI), NSF grant TOL-0228604 to the Tree-of-Life group for the phylogeny of Theropoda (Livezey, co-PI), and National Museum of Natural History grant RI-85337000 to R.L.Z. We thank the following individuals for granting and facilitating access to specimens or for special preparation of specimens in their institutions: G. Graves, S. Olson, R. Banks, J. Dean and F. Grady at National Museum of Natural History, Smithsonian Institution, Washington, DC; S. Rogers at Carnegie Museum of Natural History, Pittsburgh, PA; J. Cracraft, G. Barrowclough and M. Norell at American Museum of Natural History, New York, NY; R. Payne and J. Hinshaw at University of Michigan, Museum of Zoology, Ann Arbor, MI; J. V. Remsen at Museum of Natural Science, Louisiana State University, Baton Rouge, LA; P. Currie at Royal Tyrrell Museum of Palaeontology, Drumheller, AB, Canada; S. Chapman and A. Milner at British Museum (Natural History), London, UK; R. Prys-Jones and J. Cooper at Natural History Museum, Tring, UK; E. Pasquet, C. Lefèvre, D. Gouget and M. Véran at Muséum National d’Histoire Naturelle, Paris, France; D. Unwin at Humboldt-Universität, Berlin, Germany; G. Mayr at Forschungsinstitut Senckenberg, Frankfurt, Germany; H. Mayr at Bayerische Staatssammlung für Paläontologie und Geology, Munich, Germany; G. Viohl at Jura-Museums, Eichstätt, Germany; J. Sanz and F. Ortega at Universidad Autonóma de Madrid, Madrid, Spain; W. Longmore and L. Christidis at Museum Victoria, Melbourne, Australia; P. Murray at Museum of Central Australia, Alice Springs, Australia; A. Tennyson and S. Bartle at National Museum of Natural History (Te Papa Tongarewa), Wellington, New Zealand; and P. Scofield and H. Schlumpf at Canterbury Museum, Christchurch Univeristy, Christchurch, New Zealand. We are grateful to M. C. McKitrick for granting access to unpublished myological data on Charadriiformes, P. Murray for access to unpublished material on Dromornithidae, and S. Olson for unpublished notes on Steatornis; K. Müller for his generous assistance with use of PRAP on our data both to confirm lengths of MPTs and to estimate minimal Bremer (support) indices; G. F. Barrowclough and J. Cracraft for sharing molecular insights; A. Knox and J. Wible for editorial input and encouragement, B. DeWalt and P. Trunzo for administration of financial resources; and A. Campbell and T. Harper for graphical aid. Finally, we appreciate reviews of the manuscript by J. A. Clarke and an anonymous reviewer.
APPENDIX 1
The following proposal for a higher-order classification of Class Aves is intended to encode natural groups as recovered in the foregoing phylogenetic analysis of the companion morphological data (Livezey & Zusi, 2006). Procedures of phylogenetic classification followed Wiley (1981) and Cracraft (1974b, 1978). We avoided the current divergence between rank-free Phylocode and traditional Linnean formats, as well as the palaeontological penchant for ‘stem’ and ‘crown’ groups. The four principles considered here were: (i) hierarchical grouping by phylogenetic relationship; (ii) preference to familiar, available taxa; (iii) preference given to names based on included type genera, where all other considerations are equal; and (iv) coordination of taxonomic ranks by similar emendation of names. Higher-order group names were chosen to conform most closely with others published comparatively recently and conformal with several conventions: (i) incertae sedis (indicative of unconfirmed monophyly and/or content); and (ii) sedis mutabilis, where a taxon comprises three or more members of equal rank (i.e. lineages in polytomy). Among Neornithes, the sequencing convention (Wiley, 1981) was used only for ordinal ranks for some taxa traditionally considered to be Gruiformes.
The comparatively simplified phylogeny upon which this classification is based is depicted in Figure 11. Exemplary taxa (often nominate genera) actually coded and analysed are shown explicitly in trees (Figs 13–18), and the higher-order taxa (mostly families) that correspond to the exemplars appear in the following classification. Families included in higher taxa are limited largely to those represented by exemplars analysed, e.g. two subfamilies of Anatidae as opposed to all recognized by Livezey (1997b). This convention is most notable with respect to the exceptionally diverse and minimally represented Passeriformes and embracing superorder. However, inclusion of comparatively recently recognized family group names within two orders of Superorder Psittacimorphae (Psittaciformes and Columbiformes), not represented among exemplars, was intended to counter under-representation of non-passeriform clades as well as to accommodate uncertainty of phylogenetic placement of exemplary genera with respect to recognized (sub)families.
No protocol for derivation of taxa of higher rank has been codified; a recent attempt was that by Sibley et al. (1988, 1990). The proposal made here is but one of many alternatives, including 150 years of provisional classifications. Although the ‘sequence convention’ might be applied to the very highest taxonomic ranks, we elected to retain distinct, dichotomous taxa to draw attention to these highest-order ranks within Neornithes; this permits hierarchical clarity, but it also results in some redundancy of higher-order names (Table 2). However, the convention was applied to names for some ranks listed prior to the Neornithes – parvclasses, sections, etc. (cf. Ratitae). We chose to use historical names over proliferation of new (semi)synonyms to preserve taxonomic history and despite the fact that this perpetuated some names of variably different content and inappropriate etymology and diagnosis (as understood by the original authors of these taxa). In some cases, acceptable taxa for some highest-order ranks were not found, and in these few instances new taxa were proposed, e.g. Terrestrornithes, and hyphenates of several others.
Among several frequently cited, 19th-century authors of higher-order taxa, two –Rafinesque (1815) and Leach (1820) – were disqualified following the adjudication of most modern systematists (Bock, 1994). We avoided group names of strongly militaristic overtones, e.g. the ‘brigades’ and ‘legions’ of Gadow (1893). We found the compendia by Lambrecht (1933), Wetmore (1930, 1960), Mayr (1958), Storer (1960a, 1971b), Brodkorb (1963, 1964, 1967, 1971b, 1978), Sibley et al. (1988, 1990) and Sibley & Ahlquist (1972, 1990) to be critical for ascertainment of taxonomic authorships. Many taxa named by Stresemann (1959) or delimited by Verheyen (1961) also were adopted. Full citations of references for higher-order taxa were not included herein for the sake of brevity. For taxa of rank greater than ordinal, we adopted, where possible, the system of suffixes proposed by Sibley et al. (1988, 1990) and Sibley & Ahlquist (1990). Given the dubious comparability of taxonomic ranks of higher-order names, we elected to forego annotation of taxa of supraordinal rank with the conventional specification of ‘new rank’ in this proposal.
The higher-order names given in bold type reflect inferred groupings, and although beyond the ranks of pervue by the ICZN, we provide diagnostic and supportive characters for these taxa (Table 2; Livezey & Zusi, 2006), whether new or conserved from historical works. The latter basis was preferred for naming higher-order groups, with inexactitude of content conceded as in such names used by Clarke & Norell (2002). For example, the content of an established name (e.g. Ornithurae) implicitly is defined herein (i.e. sensu present study). Taxonomy bearing on outgroup taxa (i.e. those preceeding Neornithes) are considered especially tentative. A minor point of contention is the position of Lithornis (Houde, 1988), a relative to palaeognathous Neornithes, inferred to be the sister-group of Tinamidae by Clarke & Norell (2002) and Clarke (2004), but inferred to be the sister-group of Neornithes by Clarke & Chiappe (2001), Leonard et al. (2005) and the present analysis (Fig. 12). Should a sister-group relationship between Lithornis and modern palaeognathous taxa be favoured, Panpalaeognathae Gauthier and de Queiroz, 2001 is available for the clade comprising both groups. Use of the traditional, higher-order taxon Carinatae Merrem, 1813, was precluded by provisional monophyly of Hesperornis and Ichthyornis in the present study (see also Rees & Lindgren, 2005), avoiding as well the implication of the name with respect to secondary obsolesence or loss of the carina sterni among Neornithes (Livezey, 2003a).
Supraordinal names proposed herein were intended to follow the convention of seniority of taxa and (to a lesser degree) included type family, as is typical of lower-scale taxa. Three important higher-order synonyms are: NeoavesSibley et al., 1988, senior to PlethornithesGroth & Barrowclough, 1999 (availability questionable in present context), and distinct from EoavesSibley et al., 1988. There are also, two taxa –‘Cracrafti’ and ‘Conglomerati’– informally proposed as alternatives by Slack et al. (2006a). Unused herein is the potentially useful higher-order name Euornithes Sereno, 1999. Gaviomorphae replaced Colymbimorphae (Gadow, 1893) by revision of the former type genus Colymbus. We also replaced the senior, unfamiliar Dypsporomorphae Ogilvie-Grant, 1898, with the more familiar derivation Pelecanimorphae. Similar reasoning led to the suppression of Aetomorphae (Huxley, 1864) by Falconimorphae (Seebohm, 1890), the latter derived from an included ordinal taxon, but junior to the less representative alternative of Strigimorphae (Wagler, 1830). Uniform emendation of superorders was not imposed herein for non-Neornithes. The importance of dichotomy among higher-order names of comparable rank for comparability with the phylogenetic tree resulted in redundancy of supraordinal names for some clades, e.g. Subdivision Dendrornithes comprises a single Section Raptores, which in turn comprises a single Superorder Raptoromorphae. Antiquity of historical, higher-order taxa often resulted in minor differences in content – e.g. AnomalogonatesGarrod, 1874 optimally should exclude the Cuculiformes for consistency with the myological diagnosis implied by the name.
Further study will probably subdivide Superorder Passerimorphae so as to comprise the Superorder CoracomorphaeHuxley, 1867 and Superorder Passerimorphae (Linnaeus, 1758), the latter to comprise Piciformes and Passeriformes (cf. Manegold, 2005). Within the Passeriformes, the most suspicious anomaly in the present analysis was that of Menura; broader samples may justify its transfer to the Passerida, and thereby the first subordinal taxon instead may comprise the Acanthisittidae (Barker et al., 2002, 2004; Ericson et al., 2002a, b), representatives of which were not available for analysis here.
Principally because of limitations on available specimens, delegation of ordinal rank to the extinct elephant-birds (Aepyornithiformes) was favoured marginally over inclusion at lower rank within the Struthioniformes. A detailed classification of Order Anseriformes, including fossil taxa, was presented by Livezey (1997b), and a preliminary classification of the traditionally delimited Gruiformes, significantly revised by the present analysis relative to that proposed by Livezey (1998b), which tentatively recognized monophyly of the traditional order. (Sub)fossil taxa are plausible candidates for inclusion as sequential sister-groups of Galloanseromorphae (Diatrymidae, Gastornithidae and Dromornithidae) or membership within the Galliformes (Sylviornithidae) or Anseriformes (e.g. Mourer-Chauviré & Balouet, 2005) and are included based on published description (e.g. Cracraft, 1968; Livezey, 1997a) and cursory examinations. Material essential for rigorous diagnosis is rare or lacking, but we considered provisional hypotheses to indicate groupings likely but as yet undemonstrated by formal analysis preferable in such cases to no inference presented at all.
Subclass Avialae Gauthier, 1986
[Infraclass Alvarezsauria (Bonaparte, 1991)]
Infraclass Aves Linnaeus, 1758
Parvclass Palaeoaves; new name
Superorder ArchaeornithesGadow, 1893
Order Archaeopterygiformes Fürbringer, 1888
Family Archaeopterygidae Huxley, 1872
Order Confuciusornithiformes (Chiappe et al., 1999)
Family Confuciusornithidae Hou et al., 1995
Superorder Euenantiornithes Walker, 1981; incertae sedis
Order Rahonaviformes; new name
Family Rahonavidae; new name
Order Apsaraviformes; new name
Family Apsaravidae; new name
Parvclass Ornithurae Haeckel, 1866
Superorder Odontoholomorphae (Stejneger, 1885)
Order Hesperornithiformes (Fürbringer, 1888)
Family Hesperornithidae Marsh, 1872
Order Ichthyornithiformes (Marsh, 1873)
Family Ichthyornithidae (Marsh, 1873)
Parvclass EoavesSibley et al., 1988; incertae sedis
Order Lithornithiformes Houde, 1988
Family Lithornithidae Houde, 1988
Parvclass NeornithesGadow, 1893
Cohort Palaeognathae Pycraft, 1900
Subcohort Crypturi Goodchild, 1891
Superorder Dromaeomorphae (Huxley, 1867)
Order Tinamiformes (Huxley, 1872)
Family Tinamidae Gray, 1840
Subcohort Ratitae Merrem, 1813
[Superorder Apterygimorphae; incertae sedis]
Order Apterygiformes (Haeckel, 1866)
Family Apterygidae Gray, 1840
Order Dinornithiformes (Gadow, 1893)
[Family Anomalopterygidae (Archey, 1941)]
[Family Dinornithidae (Owen, 1843)]
Superorder Casuariimorphae; new taxon
Order Casuariiformes (Forbes, 1884)
Family Casuariidae Kaup, 1847
Family Dromaiidae Richmond, 1908
Superorder Struthionimorphae; new taxon
Order Aepyornithiformes (Newton, 1884)
Family Aepyornithidae Bonaparte, 1853
Order Struthioniformes (Latham, 1790)
Family Struthionidae Vigors, 1825
Family Rheidae (Bonaparte, 1853)
Cohort Neognathae Pycraft, 1900
Subcohort GalloanseraeSibley & Ahlquist, 1990
Superorder Galloanserimorphae (Sibley et al., 1988)
Order Galliformes (Temminck, 1820)
Suborder Craci Sibley et al., 1988; incertae sedis
Superfamily Megapodioidea (Lesson, 1831)
Family Megapodiidae Lesson, 1831
[Family Sylviornithidae Mourer-Chauviré & Balouet, 2005]
Superfamily Cracoidea (Vigors, 1825)
Family Cracidae Vigors, 1825
Suborder Phasiani (Vigors, 1825)
Superfamily Meleagridoidea (Gray, 1840)
Family Meleagrididae Gray, 1840
Superfamily Phasianoidea (Vigors, 1825); sedis mutabilis
Family Phasianidae (Vigors, 1825); sedis mutabilis
(Sub)Family Tetraonidae Vigors, 1825
Subfamily Perdicinae (Bonaparte, 1838)
Subfamily Odontophorinae Gould, 1844
Subfamily Phasianinae Vigors, 1825
Subfamily Numidinae Reichenbach, 1850
[Order Dromornithiformes Fürbringer, 1888]
Family Dromornithidae Vigors, 1825
[Order Diatrymiformes (Shufeldt, 1913)]
Family Diatrymidae Shufeldt, 1913
Order Anseriformes (Wagler, 1831)
Suborder Anhimae Wetmore & Miller, 1926
Family Anhimidae Stejneger, 1885
Suborder Anseres Wagler, 1831
Superfamily Anseranatoidea (Sclater, 1880)
Family Anseranatidae Sclater, 1880
Superfamily Anatoidea (Vigors, 1825)
[Family Presbyornithidae Wetmore, 1926]
Family Anatidae (Vigors, 1825)
Subfamily Anserinae Vigors, 1825
Subfamily Anatinae (Vigors, 1825)
Subcohort NeoavesSibley et al., 1988
Division Natatores Baird, 1858
Subdivision Pygopodo-tubinares; new taxon
Superorder Gaviomorphae; new taxon
Order Gaviiformes Wetmore & Miller, 1926
Family Gaviidae Allen, 1897
Order Podicipediformes (Fürbringer, 1888)
Family Podicipedidae Bonaparte, 1831
Superorder Procellariimorphae (Fürbringer, 1888)
Order Sphenisciformes Sharpe, 1891
Family Spheniscidae Bonaparte, 1831
Order Procellariiformes Fürbringer, 1888
Suborder Pelecanoidi (Gray, 1871)
Family Pelecanoididae Gray, 1871
Suborder Procellarae (Gadow, 1893)
Superfamily Oceanitoidea (Huxley, 1868)
Family Oceanitidae Forbes, 1882
Superfamily Procellarioidea (Fürbringer, 1888)
Family Procellariidae Vigors, 1825
Subfamily Procellariinae (Vigors, 1825)
Subfamily Pachyptilinae (Oliver, 1930)
Family Diomedeidae Gray, 1840
Subdivision Stegano-grallatores; new taxon
Superorder PelecanimorphaeHuxley, 1867
[Order Odontopterygiformes (Spulski, 1910)]
Family Odontopterygidae Lambrecht, 1933
Order Balaenicipitiformes (Sclater, 1924)
Suborder Balaenicipites (Sclater, 1924)
Family Balaenicipitidae (Sclater, 1924)
Order Pelecaniformes Sharpe, 1891
Suborder Phaethontes (Sharpe, 1891)
Family Phaethontidae Brandt, 1831
Suborder Steganopodes (Chandler, 1916)
Infraorder Fregatides (Sharpe, 1891)
Superfamily Fregatoidea (Garrod, 1874)
Family Fregatidae Garrod, 1874
Infraorder Pelecanides (Sharpe, 1891)
Parvorder Pelecanida (Sharpe, 1891); new rank
Family Pelecanidae Vigors, 1825
Parvorder Sulida (Reichenbach, 1849); new rank
Superfamily Suloidea (Reichenbach, 1849)
Family Sulidae Reichenbach, 1849
Superfamily Phalacrocoracoidea (Bonaparte, 1854)
Family Phalacrocoracidae (Bonaparte, 1854)
Family Anhingidae Ridgway, 1887
Superorder Ciconiimorphae (Garrod, 1874)
Order Ciconiiformes Garrod, 1874
Suborder Scopi (Bonaparte, 1853)
Family Scopidae (Bonaparte, 1853)
Suborder Ciconiae (Bonaparte, 1874)
Superfamily Ciconioidea (Sundevall, 1836)
Family Ciconiidae Sundevall, 1836
Family Phoenicopteridae Bonaparte, 1838
Superfamily Threskiornithoidea (Richmond, 1917)
Family Threskiornithidae Richmond, 1917
Family Plataleidae (Bonaparte, 1838)
Order Ardeiformes (Wagler, 1831)
Family Cochleariidae Ridgway, 1887
Family Ardeidae Vigors, 1825
Subfamily Botaurinae Bock, 1956
Tribe Botaurini (Bock, 1956)
Tribe Tigriornithini Bock, 1956
Subfamily Ardeinae (Vigors, 1825)
Tribe Nycticoracini Bock, 1956
Tribe Ardeini Bock, 1956
Division Terrestrornithes; new taxon
Subdivision Telmatorae (Lowe, 1931)
Superorder CharadriimorphaeHuxley, 1867
Order Gruiformes (Bonaparte, 1854)
Suborder Cariamae (Wagler, 1830)
Infraorder Otides Sibley et al., 1988
Family Otididae Gray, 1840
Infraorder Cariamides (Fürbringer, 1888)
Superfamily Cariamoidea (Gray, 1853); sedis mutabilis
[Family Bathornithidae Wetmore, 1933]
Family Cariamidae Bonaparte, 1853
[Family Phorusrhacidae (Ameghino, 1899)]
Suborder Eurypygae (Fürbringer, 1888)
Infraorder Eurypygides Sibley et al., 1988
Family Eurypygidae Selby, 1840
Infraorder Rhynochetides Sharpe, 1891
Family Rhynochetidae Newton, 1868
Family Aptornithidae Bonaparte, 1856
Suborder Grues Bonaparte, 1854
Superfamily Psophioidea (Bonaparte, 1831)
Family Psophiidae Bonaparte, 1831
Superfamily Gruoidea (Vigors, 1825)
Family Aramidae Bonaparte, 1854
Family Gruidae Vigors, 1825
Order Turniciformes (Huxley, 1868); incertae sedis
Family Turnicidae (Gray, 1840)
Family Mesitornithidae Wetmore, 1960
Order Ralliformes (Reichenbach, 1854)
Family Heliornithidae Gray, 1841
Family Rallidae (Reichenbach, 1854)
Order Charadriiformes (Fürbringer, 1888)
Suborder Pedionomae (Gadow, 1893)
Family Pedionomidae Gadow, 1893
Suborder Parrae (Gadow, 1893)
Family Jacanidae Stejneger, 1885
Family Rostratulidae Ridgway, 1919
Suborder Limicolae (Beddard, 1898)
Infraorder Dromaides (Sharpe, 1891)
Family Dromadidae Gray, 1840
Infraorder Scolopacides (Strauch, 1978)
Superfamily Thinocoroidea (Gray, 1845)
Family Thinocoridae (Gray, 1845)
Superfamily Scolopacoidea (Vigors, 1825)
Family Scolopacidae Vigors, 1825
Family Phalaropodidae Bonaparte, 1831
Infraorder Charadriides (Huxley, 1867); incertae sedis
Superfamily Charadrioidea (Vigors, 1825)
Family Charadriidae Vigors, 1825
Superfamily Glareoloidea (Brehm, 1831)
Family Glareolidae Brehm, 1831
Subfamily Glareolinae Brehm, 1831
Subfamily Cursoriinae Gray, 1840
Superfamily Burhinoidea (Mathews, 1912)
Family Burhinidae Mathews, 1912
Superfamily Haematopoidea (Bonaparte, 1838)
Family Haematopidae Bonaparte, 1838
Subfamily Haematopodinae (Bonaparte, 1838)
Subfamily Ibidorhynchinae Bonaparte, 1856
Family Recurvirostridae (Bonaparte, 1831)
Subfamily Recurvirostrinae Bonaparte, 1831
Subfamily Himantopodinae Reichenbach, 1849
Tribe Himantopodini Sibley et al., 1988
Tribe Cladorhynchini; new taxon
Suborder Lari Sharpe, 1891; incertae sedis
Infraorder Chionidides Sharpe, 1891
Family Chionididae Lesson, 1828
Infraorder Alcides (Sharpe, 1891)
Family Alcidae (Vigors, 1825)
Infraorder Larides (Sharpe, 1891)
Superfamily Laroidea (Bonaparte, 1831)
Family Stercorariidae Gray, 1870
Family Laridae (Bonaparte, 1831)
Subfamily Larinae Bonaparte, 1831
Subfamily Sterninae Bonaparte, 1838
Superfamily Rynchopoidea (Bonaparte, 1838)
Family Rynchopidae (Bonaparte, 1838)
Subdivision Dendrornithes (Verheyen, 1961)
Section Raptores Baird, 1858
Superorder Falconimorphae (Seebohm, 1890)
Order Falconiformes Seebohm, 1890
[Suborder Teratornithi (Miller, 1909)]
Suborder Cathartae (Coues, 1824)
Family Cathartidae (Lafresnaye, 1839)
Suborder Accipitres (Vieillot, 1816)
Infraorder Serpentariides (Seebohm, 1890)
Family Sagittariidae Finsch & Hartlaub, 1870
Infraorder Falconides (Sharpe, 1874)
Superfamily Falconoidea (Vigors, 1824)
Family Falconidae Vigors, 1824
Subfamily Falconinae Vigors, 1824
Subfamily Polyborinae Lafresnaye, 1839
Family Pandionidae Sclater & Salvin, 1873
Superfamily Accipitroidea (Vieillot, 1816)
Family Accipitridae (Vieillot, 1816)
Subfamily Accipitrinae (Vieillot, 1816)
Subfamily Gypaetinae (Vieillot, 1816)
Order Strigiformes (Wagler, 1830)
Family Tytonidae (Mathews, 1912)
Subfamily Tytoninae Mathews, 1912
Subfamily Phodilinae Beddard, 1898
Family Strigidae (Gray, 1840)
Section AnomalogonatesGarrod, 1874
Subsection CoccygesHuxley, 1867; incertae sedis
Superorder CuculimorphaeSibley et al., 1988
Order Opisthocomiformes (L’Herminer, 1837)
Family Opisthocomidae Swainson, 1837
Order Cuculiformes (Wagler, 1830)
Suborder Musophagi Seebohm, 1890
Family Musophagidae Bonaparte, 1831
Suborder Cuculi Wagler, 1830
Family Cuculidae Vigors, 1825; sedis mutabilis
Subfamily Neomorphinae Shelley, 1891
Subfamily Centropodinae Horsfield, 1823
Subfamily Crotophaginae Swainson, 1837
Subfamily Cuculinae (Vigors, 1825)
Subfamily Phaenicophacinae (Horsfield, 1822)
Superorder Psittacimorphae (Huxley, 1867); incertae sedis
Order Psittaciformes (Wagler, 1830); sedis mutabilis
Family Nestoridae (Bonaparte, 1850)
Family Psittacidae (Illiger, 1811)
Family Cacatuidae Gray, 1840
Family Loriinidae Selby, 1836
Order Columbiformes (Garrod, 1874)
Suborder Pterocletes (Boucard, 1876)
Family Pteroclidae Bonaparte, 1831
Suborder Columbae (Latham, 1790)
Family Columbidae (Illiger, 1811)
Subfamily Columbinae (Illiger, 1811)
Subfamily Didunculinae Gray, 1848
Subfamily Gourinae Gray, 1840
Family Raphidae Wetmore, 1930
Subsection Incessores Baird, 1858
Superorder CypselomorphaeHuxley, 1867
Order Caprimulgiformes (Ridgway, 1891)
Suborder Aegotheli Sibley et al., 1988
Family Aegothelidae (Bonaparte, 1853)
Suborder Caprimulgi Ridgway, 1881; sedis mutabilis
Family Caprimulgidae Vigors, 1825
Family Nyctibiidae (Chenu & Des Murs, 1851)
Family Podargidae (Gray, 1840)
Family Steatornithidae (Gray, 1846)
Order Apodiformes Peters, 1940
Suborder Hemiprocni; new taxon
Family Hemiprocnidae Oberholser, 1906
Suborder Apodi (Peters, 1940)
Family Apodidae (Hartert, 1897)
Subfamily Cypselinae Bonaparte, 1838
Subfamily Apodinae Hartert, 1897
Family Trochilidae Vigors, 1825
Subsection Trogones; new name
Superorder Trogonomorphae; new taxon
[Order Sandcoleiformes Houde & Olson, 1992]
Family Sandcoleidae Houde & Olson, 1992
Order Coliiformes (Murie, 1872)
Family Coliidae (Swainson, 1836)
Order Trogoniformes Wetmore & Miller, 1926
Family Trogonidae Lesson, 1828
Subsection Pico-clamatores; new name
Superorder PasserimorphaeSibley et al., 1988; sedis mutabilis
Order Coraciiformes Forbes, 1884
Suborder Bucerotes Fürbringer, 1888
Infraorder Upupides (Seebohm, 1890)
Family Upupidae Bonaparte, 1831
Family Phoeniculidae Sclater, 1924
Infraorder Bucerotides (Fürbringer, 1888)
Family Bucerotidae (Gray, 1847)
Suborder Halcyones (Forbes, 1884)
Superfamily Motmotoidea (Gray, 1840)
Family Motmotidae Gray, 1840
Superfamily Alcedinoidea (Stejneger, 1885)
Family Todidae Vigors, 1825
Family Alcedinidae (Bonaparte, 1831)
Subfamily Alcedininae Bonaparte, 1831
Subfamily Halcyoninae (Vigors, 1825)
Suborder Coracii (Forbes, 1884)
Infraorder Meropides (Fürbringer, 1888)
Family Meropidae Vigors, 1825
Infraorder Coraciides (Wetmore & Miller, 1926)
Superfamily Coracioidea (Vigors, 1825)
Family Coraciidae Vigors, 1825
Superfamily Leptosomatoidea (Bonaparte, 1850)
Family Leptosomatidae Bonaparte, 1850
Family Brachypteraciidae (Sharpe, 1892)
Order Piciformes (Meyer & Wolf, 1810)
Suborder Galbulae (Fürbringer, 1888)
Family Galbulidae Bonaparte, 1831
Family Bucconidae Boie, 1826
Suborder Pici (Meyer & Wolf, 1810)
Superfamily Capitonoidea (Bonaparte, 1840)
Family Capitonidae Bonaparte, 1840
Family Rhamphastidae Vigors, 1825
Superfamily Picoidea (Vigors, 1825)
Family Indicatoridae Swainson, 1837
Family Picidae Vigors, 1825
Subfamily Jynginae Bonaparte, 1838
Subfamily Picinae Bonaparte, 1838
Order Passeriformes (Linnaeus, 1758)
Suborder Menurae (Sharpe, 1891)
Family Menuridae (Lesson, 1828)
Suborder Passeres Linnaeus, 1758
Infraorder Tyrannides Sibley et al., 1988
Family Tyrannidae Vigors, 1825
Family Pittidae Swainson, 1831
Infraorder Passerides (Linnaeus, 1758)
Parvorder Corvida Sibley et al., 1988
Family Ptilinorhynchidae Gray, 1841
Family Corvidae Vigors, 1825
Parvorder Passerida Sibley et al., 1988
Family Bombycillidae Swainson, 1831
Family Paridae Vigors, 1825
Family Passeridae Illiger, 1811
REFERENCES
- Abouheif E. Developmental genetics and homology: a hierarchical approach. Trends in Ecology and Evolution. 1997;12:405–408. doi: 10.1016/s0169-5347(97)01125-7. [DOI] [PubMed] [Google Scholar]
- Abourachid A. Bipedal locomotion in birds: the importance of functional parameters in terrestrial adaptation in Anatidae. Canadian Journal of Zoology. 2000;78:1994–1998. [Google Scholar]
- Abourachid A. Kinematic parameters of terrestrial locomotion in cursorial (ratites), swimming (ducks), and striding birds (quail and guinea fowl) Comparative Biochemistry and Physiology (Part A) 2001;131:113–119. doi: 10.1016/s1095-6433(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Abourachid A, Renous S. Bipedal locomotion in ratites (Paleognatiform [sic]): examples of cursorial birds. Ibis. 2000;142:538–549. [Google Scholar]
- Alberch P. Problems with the interpretation of developmental sequences. Systematic Zoology. 1985;34:46–58. [Google Scholar]
- Alfaro ME, Zoller S, Lutzoni F. Bayes or bootstrap? A simulation study comparing the performance of Bayesian Markov chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Molecular Biology and Evolution. 2003;20:255–266. doi: 10.1093/molbev/msg028. [DOI] [PubMed] [Google Scholar]
- Altshuler DL, Dudley R, Ellington CP. Aerodynamic forces of revolving hummingbird wings and wing models. Journal of Zoology (London) 2004;264:327–332. [Google Scholar]
- Alvarenga HMF. A large and probably flightless anhinga from the Miocene of Chile. Courier Forschungsinstitut Senckenberg. 1995;181:149–162. [Google Scholar]
- Alvarenga HMF. A fossil screamer (Anseriformes: Anhimidae) from the middle Tertiary of southeastern Brazil. Smithsonian Contributions to Paleobiology. 1999;89:223–230. [Google Scholar]
- Alvarenga HMF, Guilherme E. The anhingas (Aves: Anhingidae) from the upper Tertiary (Miocene-Pliocene) of southwestern Amazonia. Journal of Vertebrate Paleontology. 2003;23:614–621. [Google Scholar]
- Ames PL. The morphology of the syrinx in passerine birds. Peabody Museum of Natural History Bulletin. 1971;37:1–194. [Google Scholar]
- Andersson M. Hybridization and skua phylogeny. Proceedings of the Royal Society of London (Series B) 1999;266:1579–1585. [Google Scholar]
- Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Systematic Biology. 2006;55:539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- Ansari HA, Kaul D. Cytotaxonomic study in the order Falconiformes (Aves) Zoologica Scripta. 1986;15:351–356. [Google Scholar]
- Arbogast BS, Edwards SV, Wakeley J, Beerli P, Slowinski JB. Estimating divergence times from molecular data on phylogenetic and population genetic time scales. Annual Review of Ecology and Systematics. 2002;33:707–740. [Google Scholar]
- Archibald GW. Crane taxonomy as revealed by the unison call. In: Lewis JC, editor. Proceedings of the National Crane Workshop, I. Stillwater, OK: Oklahoma State University; 1976. pp. 225–251. [Google Scholar]
- Archie JW. Homoplasy excess ratios: new indices for measuring levels of homoplasy in phylogenetic systematics and a critique of the consistency index. Systematic Zoology. 1989;38:253–269. [Google Scholar]
- Armstrong MH, Braun EL, Kimball RT. Phylogenetic utility of avian ovomucoid intron G: a comparison of nuclear and mitochondrial phylogenies in Galliformes. Auk. 2001;118:799–804. [Google Scholar]
- Arthur W. Biased embryos and evolution. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- Ast JC, Dimcheff D, Sorenson MD, Mindell D. Phylogenetic analysis of total mitochondrial DNA in birds. Cladistics. 1997;13:162. [Google Scholar]
- Athreya KB, Jagers P, editors. Classical and modern branching processes. New York: Springer; 1997. [Google Scholar]
- Austin JJ. Molecular phylogenetics of Puffinus shearwaters: preliminary evidence from mitochondrial cytochrome b gene sequences. Molecular Phylogenetics and Evolution. 1996;6:77–88. doi: 10.1006/mpev.1996.0060. [DOI] [PubMed] [Google Scholar]
- Austin JJ, Smith AB, Thomas RH. Paleontology in a molecular world: the search for authentic ancient DNA. Trends in Ecology and Evolution. 1997;12:303–306. doi: 10.1016/S0169-5347(97)01102-6. [DOI] [PubMed] [Google Scholar]
- Averianov AO, Pantekyev AV, Potapova OR, Nessov LA. Bony-toothed birds (Aves: Pelecaniformes: Odontopterygia) from the late Paleocene and Eocene of the western margin of ancient Asia. Proceedings of the Zoological Institute of the USSR Academy of Science. 1991;239:3–12. [Google Scholar]
- Avise JC, Aquadro CF. Malate dyhydrogenase isoenzymes provide a phylogenetic marker for the Piciformes (woodpeckers and allies) Auk. 1987;104:324–328. [Google Scholar]
- Avise JC, Nelson WS, et al. (1995)] Molecular Phylogenetics and Evolution. 1995;4:319. doi: 10.1006/mpev.1994.1019. Reply [to Hackett. [DOI] [PubMed] [Google Scholar]
- Avise JC, Nelson WS, Sibley CG. DNA sequence support for a close phylogenetic relationship between some storks and New World vultures. Proceedings of the National Academy of Science USA. 1994a;91:5173–5177. doi: 10.1073/pnas.91.11.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avise JC, Nelson WS, Sibley CG. Why one-kilobase sequences from mitochondrial DNA fail to solve the hoatzin phylogenetic enigma. Molecular Phylogenetics and Evolution. 1994b;3:175–184. doi: 10.1006/mpev.1994.1019. [DOI] [PubMed] [Google Scholar]
- Baker AJ, Daugherty CH, Colbourne R, McLennan JL. Flightless brown kiwis of New Zealand possess extremely subdivided population structure and cryptic species like small mammals. Proceedings of the National Academy of Science USA. 1995;92:8254–8258. doi: 10.1073/pnas.92.18.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RH, Yu X, DeSalle R. Assessing the relative contribution of molecular and morphological characters in simultaneous-analysis trees. Molecular Phylogenetics and Evolution. 1998;9:427–436. doi: 10.1006/mpev.1998.0519. [DOI] [PubMed] [Google Scholar]
- Baker RH, Gatesy J. Is morphology still relevant? In: DeSalle R, Giribet G, Wheeler W, editors. Molecular systematics and evolution: theory and practice. Basel: Birkhäuser-Verlag; 2002. pp. 163–174. [Google Scholar]
- Baker AJ, Huymen LJ, Haddrath O, Millar CD, Lumbert DM. Reconstucting the tempo and mode of evolution in an extinct clade of birds with ancient DNA: the giant moas of New Zealand. Proceedings of the National Academy of Science USA. 2005;102:8257–8262. doi: 10.1073/pnas.0409435102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard JWO, Thayer MK, Newton AF, Jr, Grismer ER. Data sets, partitions, and characters: philosophies and procedures for analyzing multiple data sets. Systematic Biology. 1998;47:367–396. doi: 10.1080/106351598260770. [DOI] [PubMed] [Google Scholar]
- Ballmann P. Fossile Glareolidae aus dem Miozän des Nördlinger Ries (Aves: Charadriiformes) Bonner Zoologische Beiträge. 1979;30:51–101. [Google Scholar]
- Balouet J-C. Les Paleognathes (Aves) sont-ils primitifs? Bulletin de la Société Zoologique de France. 1984;108:648–653. [Google Scholar]
- Bang R, Schultz TR, DeSalle R. Development, homology and systematics. In: DeSalle R, Giribet G, Wheeler W, editors. Molecular systematics and evolution: theory and practice. Basel: Birkhäuser Verlag; 2002. pp. 175–186. [Google Scholar]
- Banks JC, Palma RL, Paterson AM. Cophylogenetic relationships between penguins and their chewing lice. Journal of Evolutionary Biology. 2006;19:156–166. doi: 10.1111/j.1420-9101.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- Barker FK, Barrowclough GF, Groth JG. A phylogenetic hypothesis for passerine birds: taxonomic and biogeographic implications of an analysis of nuclear DNA sequence data. Proceedings of the Royal Society of London (Series B) 2002;269:295–308. doi: 10.1098/rspb.2001.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker FK, Cibois A, Schlikler P, Feinstein J, Cracraft J. Phylogeny and diversification of the largest avian radiation. Proceedings of the National Academy of Science USA. 2004;101:11040–11045. doi: 10.1073/pnas.0401892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker FK, Lanyon SM. The impact of parsimony weighting schemes on inferred relationships among toucans and neotropical barbets (Aves: Piciformes) Molecular Phylogenetics and Evolution. 2000;15:215–234. doi: 10.1006/mpev.2000.0752. [DOI] [PubMed] [Google Scholar]
- Barnikol HA. Vergleichend anatomische und taxonomisch phylogenetische Studien am Kopf der Opisthocomiformes. Musophagidae, Galli, Columbae und Cuculi. Ein Beitrag zum Opisthocomus-Problem. Zoologische Jahrbücher (Abteilung für Systematik) 1953;81:487–526. [Google Scholar]
- Barriel V, Tassy P. Rooting with multiple outgroups: consensus versus parsimony. Cladistics. 1998;14:193–200. doi: 10.1111/j.1096-0031.1998.tb00332.x. [DOI] [PubMed] [Google Scholar]
- Barrowclough GF. Biochemical studies of the higher level systematics of birds. Bulletin of the British Ornithologists’ Club. 1992;112(Suppl.):39–52. [Google Scholar]
- Barrowclough GF, Groth JG, Mertz LA. The RAG-1 exon in the avian order Caprimulgiformes: phylogeny, heterozygosity, and base composition. Molecular Phylogenetics and Evolution. 2006;41:238–248. doi: 10.1016/j.ympev.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Bartlett AD. On the affinities of Balæniceps. Proceedings of the Zoological Society of London. 1861;1861:131–181. [Google Scholar]
- Bartlett AD. Note on the habits and affinities of the kagu (Rhinochetus jubatus) Proceedings of the Zoological Society of London. 1862;1862:218–219. [Google Scholar]
- Bartlett E. Remarks on the affinities of Mesites. Proceedings of the Zoological Society of London. 1877;1877:292–293. [Google Scholar]
- Baum DA, Larson A. Adaptation reviewed: a phylogenetic methodology for studying character macroevolution. Systematic Zoology. 1991;40:1–18. [Google Scholar]
- Beddard FE. A contribution to the anatomy of Scopus umbretta. Proceedings of the Zoological Society of London. 1884;1884:543–553. [Google Scholar]
- Beddard FE. On the syrinx and other points in the anatomy of the Caprimulgidæ. Proceedings of the Zoological Society of London. 1886;1886:147–153. [Google Scholar]
- Beddard FE. On certain points in the visceral anatomy of Balæniceps rex, bearing upon its affinities. Proceedings of the Zoological Society of London. 1888;1888:284–290. [Google Scholar]
- Beddard FE. Contributions to the anatomy of the hoatzin (Opisthocomus cristatus) with particular reference to the structure of the wing in the young. Ibis. 1889;31:283–293. [Google Scholar]
- Beddard FE. On Phodilus badius, with remarks on its systematic position. Ibis. 1890;32:293–304. [Google Scholar]
- Beddard FE. Contributions to the anatomy of the kagu (Rhinochetus jubatus) Proceedings of the Zoological Society of London. 1891;1891:9–21. [Google Scholar]
- Beddard FE. On the osteology, pterylosis, and muscular anatomy of the American fin-foot (Heliornis surinamensis) Ibis. 1893;35:30–40. [Google Scholar]
- Beddard FE. Notes upon the anatomy of Phaethon. Proceedings of the Zoological Society of London. 1897;1897:288–295. [Google Scholar]
- Beddard FE. The structure and classification of birds. London: Longmans and Green; 1898. [Google Scholar]
- Beecher WJ. A phylogeny of the oscines. Auk. 1953;79:270–333. [Google Scholar]
- Beiko RG, Keith JM, Harlow TJ, Ragan MA. Searching for converence in phylogenetic Markov chain Monte Carlo. Systematic Biology. 2006;55:553–565. doi: 10.1080/10635150600812544. [DOI] [PubMed] [Google Scholar]
- Benson RD. Presbyornis isoni and other late Paleocene birds from North Dakota. Smithsonian Contributions to Paleobiology. 1999;89:253–259. [Google Scholar]
- Benton MJ. Phylogeny of major tetrapod groups: morphological data and divergence dates. Journal of Molecular Evolution. 1990;30:409–424. doi: 10.1007/BF02101113. [DOI] [PubMed] [Google Scholar]
- Benton MJ. Testing the time axis of phylogenies. In: Harvey PH, Leigh Brown AJ, Maynard Smith J, Nee S, editors. New uses for new phylogenies. Oxford, UK: Oxford University Press; 1996. pp. 217–233. [Google Scholar]
- Benton MJ. Early origins of modern birds and mammals: molecules vs. morphology. Bioessays. 1999;21:314–321. doi: 10.1002/(SICI)1521-1878(199912)22:1<1043::AID-BIES8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Benton MJ. Stems, nodes, crown clades, and rank-free lists: is Linnaeus dead? Biological Reviews (Cambridge) 2000;75:633–648. doi: 10.1111/j.1469-185x.2000.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Benton MJ. Finding the tree of life: matching phylogenetic trees to the fossil record through the 20th century. Proceedings of the Royal Society of London (Series B) 2001;268:2123–2130. doi: 10.1098/rspb.2001.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton MJ, Clark JM. Archosaur phylogeny and the relationships of the Crocodylia. In: Benton MJ, editor. The phylogeny and classification of the tetrapods, amphibians, reptiles, birds. Vol. 1. Oxford, UK: Systematics Association; 1988. pp. 295–338. [Google Scholar]
- Benz BW, Robbins MB, Peterson AT. Evolutionary history of woodpeckers and allies (Aves: Picidae): placing key taxa on the phylogenetic tree. Molecular Phylogenetics and Evolution. 2006;40:389–399. doi: 10.1016/j.ympev.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Berger AJ. Some anatomical characters of the Cuculidae and the Musophagidae. Wilson Bulletin. 1960;72:60–103. [Google Scholar]
- Berglund-Sonnhammer A-C, Steffansson P, Betts MJ, Liberles DA. Optimal gene trees from sequences and species trees using a soft interpretation of parsimony. Journal of Molecular Evolution. 2006;63:240–250. doi: 10.1007/s00239-005-0096-1. [DOI] [PubMed] [Google Scholar]
- Bergsten J. A review of long-branch attraction. Cladistics. 2005;21:163–193. doi: 10.1111/j.1096-0031.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- Berman SL, Raikow RJ. The hind limb musculature of the mousebirds (Coliiformes) Auk. 1982;99:41–57. [Google Scholar]
- Bertelli S, Giannini NP, Goloboff PA. A phylogeny of the tinamous (Aves: Palaeognathiformes) based on integumentary characters. Systematic Biology. 2002;51:959–979. doi: 10.1080/10635150290102492. [DOI] [PubMed] [Google Scholar]
- Bininda-Emonds ORP, Bryant HN, Russell AP. Supraspecific taxa as terminals in cladistic analysis: implicit assumptions of monophyly and a comparison of methods. Biological Journal of the Linnean Society. 1998;64:101–133. [Google Scholar]
- Bininda-Emonds ORP, Gittleman JL, Steel MA. The (super) tree of life: procedures, problems, and prospects. Annual Review of Ecology and Systematics. 2002;33:265–289. [Google Scholar]
- Birdsley JS. Phylogeny of the tyrant flycatchers (Tyrannidae) based on morphology and behavior. Auk. 2002;119:715–734. [Google Scholar]
- Birks SM, Edwards SV. A phylogeny of the megapodes (Aves: Megapodiidae) based on nuclear and mitochondrial DNA sequences. Molecular Phylogenetics and Evolution. 2002;23:408–421. doi: 10.1016/s1055-7903(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Björklund M. Phylogenetic relationships among Charadriiformes: reanalysis of previous data. Auk. 1994;111:825–832. [Google Scholar]
- Bledsoe AH, Raikow RJ. A quantitative assessment of congruence between molecular and nonmolecular estimates of phylogeny. Journal of Molecular Evolution. 1990;30:247–259. [Google Scholar]
- Bleiweiss R. Relative-rate tests and biological causes of molecular evolution in hummingbirds. Molecular Biology and Evolution. 1998a;15:481–491. [Google Scholar]
- Bleiweiss R. Phylogeny, body mass, and genetic consequences of lek-mating behavior in hummingbirds. Molecular Biology and Evolution. 1998b;15:492–498. [Google Scholar]
- Bleiweiss R. Fossil gap analysis supports early Tertiary origin of trophically diverse avian orders. Geology. 1998c;26:323–326. [Google Scholar]
- Bleiweiss R. Origin of hummingbird faunas. Biological Journal of the Linnean Society. 1998d;65:77–97. [Google Scholar]
- Bleiweiss R, Kirsch JAW, Lapointe FJ. DNA–DNA hybridization-based phylogeny for ‘higher’ nonpasserines: reevaluating a key part of the avian family tree. Molecular Phylogenetics and Evolution. 1994;3:248–255. doi: 10.1006/mpev.1994.1027. [DOI] [PubMed] [Google Scholar]
- Bleiweiss R, Kirsch JAW, Matheus JC. DNA hybridization evidence for the principal lineages of hummingbirds (Aves: Trochilidae) Molecular Biology and Evolution. 1997;14:325–343. doi: 10.1093/oxfordjournals.molbev.a025767. [DOI] [PubMed] [Google Scholar]
- Bleiweiss R, Kirsch JAW, Shafi N. Confirmation of a portion of the Sibley–Ahlquist ‘tapestry’. Auk. 1995;112:87–97. [Google Scholar]
- Bock WJ. Preadaptation and multiple evolutionary pathways. Evolution. 1959;13:194–211. [Google Scholar]
- Bock WJ. Secondary articulation of the avian mandible. Auk. 1960a;77:19–55. [Google Scholar]
- Bock WJ. The palatine process of the premaxilla in the Passeres. Bulletin of the Museum of Comparative Zoology. 1960b;122:361–488. [Google Scholar]
- Bock WJ. Evolution and phylogeny in morphologically uniform groups. American Naturalist. 1963a;97:265–285. [Google Scholar]
- Bock WJ. The cranial evidence for ratite affinities. In: Sibley CG, editor. Proceedings XIII International Ornithological Congress. Washington, DC: American Ornithologists’ Union; 1963b. pp. 39–54. [Google Scholar]
- Bock WJ. The use of adaptive characters in avian classification. In: Snow DW, editor. Proceedings XIV International Ornithological Congress. Oxford, UK: Blackwell Scientific Publications; 1967. pp. 61–74. [Google Scholar]
- Bock WJ. The homology concept: its philosophical foundation and practical methodology. Zoologische Beiträge (Neue Folge) 1979;32:327–353. [Google Scholar]
- Bock WJ. History and nomenclature of avian family-group names. Bulletin of the American Museum of Natural History. 1994;222:1–281. [Google Scholar]
- Bock WJ. Functional and evolutionary morphology of woodpeckers. Ostrich. 1999;70:23–31. [Google Scholar]
- Bock WJ, Bühler P. The evolution and biogeographical history of the palaeognathous birds. In: van den Elzen R, Schuchmann K-L, Schmidt-Koenig K, editors. Current topics in avian biology. Bonn, Germany: Deutsche Ornithologische-Geselshaft; 1988. pp. 31–36. [Google Scholar]
- Bock WJ, McEvey A. Osteology of Pedionomus torquatus (Aves: Pedionomidae) Proceedings of the Royal Society of Victoria (New Series) 1969;82:187–232. [Google Scholar]
- Böhm M. Über den Bau des jugendlichen Schädels von Balaeniceps rex nebst Bemerkungen über dessen systematische Stellung und über das Gaumenskelett der Vögel. Zeitschrift für Morphologie und Oekologie der Tiere. 1930;17:677–718. [Google Scholar]
- Böhning-Gaese K, González-Guzmán LI, Brown JH. Constraints on dispersal and the evolution of the avifauna of the Northern Hemisphere. Evolutionary Ecology. 1998;12:767–783. [Google Scholar]
- Böker H. Flugvermögen und Kropf bei Opisthocomus cristatus und Stringops[sic]Habroptilus. Gegenbaurs Morphologisches Jahrbuch. 1929;63:152–207. [Google Scholar]
- Boles WE. The world’s oldest songbird (Aves: Passeriformes) Nature. 1995;374:321–322. [Google Scholar]
- Boles WE. Fossil songbirds (Passeriformes) from the early Eocene of Australia. Emu. 1997;97:43–50. [Google Scholar]
- Bourdon E. Osteological evidence for sister group relationship between pseudo-toothed birds (Aves: Odontopterygiformes) and waterfowls [sic] (Anseriformes) Naturwissenschaften. 2005;92:586–591. doi: 10.1007/s00114-005-0047-0. [DOI] [PubMed] [Google Scholar]
- Bourdon E, Boya B, Iarochène M. Earliest African neornithine bird: a new species of Prophaethontidae (Aves) from the Paleocene of Morocco. Journal of Vertebrate Paleontology. 2005;25:157–170. [Google Scholar]
- Bout RG. Postures of the avian craniocervical column. Journal of Morphology. 1997;231:287–295. doi: 10.1002/(SICI)1097-4687(199703)231:3<287::AID-JMOR7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Brady RH. Parsimony, hierarchy, and biological implications. In: Platnick NI, Funk VA, editors. Advances in cladistics. Vol. 2. New York: Columbia University Press; 1982. pp. 49–60. [Google Scholar]
- Braun ML, Brumfield RT. Enigmatic phylogeny of skuas: an alternative hypothesis. Proceedings of the Royal Society of London (Series B) 1998;265:995–999. [Google Scholar]
- Braun EL, Kimball RT. Examining basal avian divergences with mitochondrial sequences: model complexity, taxon sampling, and sequence length. Systematic Biology. 2002;51:614–625. doi: 10.1080/10635150290102294. [DOI] [PubMed] [Google Scholar]
- Bremer K. Branch support and tree stability. Cladistics. 1994;10:295–304. [Google Scholar]
- Bremer K. Estimating branch support. Cladistics. 1997;13:165–166. [Google Scholar]
- Bridge ES, Jones AW, Baker AJ. A phylogenetic framework for the terns (Sternini) inferred from mtDNA sequences: implications for taxonomy and plumage evolution. Molecular Phylogenetics and Evolution. 2005;35:459–469. doi: 10.1016/j.ympev.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Briggs JC. Fishes and birds: Gondwana life rafts reconsidered. Systematic Biology. 2003;52:548–553. doi: 10.1080/10635150390218385. [DOI] [PubMed] [Google Scholar]
- Brochu CA. Progress and future directions in archosaur phylogenetics. Journal of Paleontology. 2001;75:1185–1201. [Google Scholar]
- Brochu CA, Norell MA. Temporal congruence and the origin of birds. Journal of Vertebrate Paleontology. 2000;20:197–200. [Google Scholar]
- Brochu CA, Norell MA. Time and trees: a quantitative assessment of temporal congruence in the birds origins debate. In: Gauthier J, Gall LF, editors. New perspectives on the origin and early evolution of birds. New Haven, CT: Yale University Press; 2001. pp. 511–535. [Google Scholar]
- Brochu CA, Sumrall CD, Theodor JM. When clocks (and communities) collide: estimating divergence time from molecules and the fossil record. Journal of Palaeontology. 2004;78:1–6. [Google Scholar]
- Brodkorb P. Catalogue of fossil birds: Part 1 (Archaeopterygiformes through Ardeiformes) Bulletin of the Florida State Museum. 1963;7:179–293. [Google Scholar]
- Brodkorb P. Catalogue of fossil birds: Part 2 (Anseriformes through Galliformes) Bulletin of the Florida State Museum. 1964;7:295–335. [Google Scholar]
- Brodkorb P. Catalogue of fossil birds: Part 3 (Ralliformes, Ichthyornithiformes, Charadriiformes) Bulletin of the Florida State Museum. 1967;11:99–220. [Google Scholar]
- Brodkorb P. Origin and evolution of birds. In: Farner DS, King JR, editors. Avian biology. Vol. 1. New York: Academic Press; 1971a. pp. 19–55. [Google Scholar]
- Brodkorb P. Catalogue of fossil birds: Part 4 (Columbiformes through Piciformes) Bulletin of the Florida State Museum. 1971b;15:163–266. [Google Scholar]
- Brodkorb P. Discovery of a Cretaceous bird, apparently ancestral to the orders Coraciiformes and Piciformes (Aves: Carinatae) Smithsonian Contributions to Paleobiology. 1976;27:67–73. [Google Scholar]
- Brodkorb P. Catalogue of fossil birds: Part 5 (Passeriformes) Bulletin of the Florida State Museum. 1978;23:139–228. [Google Scholar]
- Broham L. What can DNA tell us about the Cambrian explosion? Integrative and Comparative Biology. 2003;43:148–156. doi: 10.1093/icb/43.1.148. [DOI] [PubMed] [Google Scholar]
- Broham L, Woofit M, Lee MSY, Rambaut A. Testing the relationship between morphological and molecular rates of change along phylogenies. Evolution. 2002;56:1921–1930. doi: 10.1111/j.0014-3820.2002.tb00118.x. [DOI] [PubMed] [Google Scholar]
- Brom TG. Villi and the phyly of Wetmore’s Order Passeriformes (Aves) Zoological Journal of the Linnean Society. 1990;98:63–72. [Google Scholar]
- Bryant HN. Hypothetical ancestors and rooting in cladistic analysis. Cladistics. 1997;13:337–348. doi: 10.1111/j.1096-0031.1997.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Bryant HN. Character polarity and the rooting of cladograms. In: Wagner GP, editor. The character concept in evolutionary biology. San Diego, CA: Academic Press; 2001. pp. 319–342. [Google Scholar]
- Buckley TR, Simon C, Chambers GK. Exploring among-site rate variation models in a maximum-likelihood framework using empirical data: effects of model assumptions on estimates of topology, branch lengths, and bootstrap support. Systematic Biology. 2001;50:67–86. [PubMed] [Google Scholar]
- Bühler P. Schädelmorphologie und Kiefermechanik der Caprimulgidae (Aves) Zeitschrift für Morphologie der Tiere. 1970;66:337–399. [Google Scholar]
- Burbridge ML, Colbourne RM, Robertson HA, Baker AJ. Moleclar and other biological evidence supports the recognition of at least three species of brown kiwi. Conservation Genetics. 2003;4:167–177. [Google Scholar]
- Burke AC, Feduccia A. Developmental patterns and the identification of homologies in the avian hand. Science. 1997;278:666–668. [Google Scholar]
- Burness G, Chardine JW, Darveau C-A. Flight muscle enzyme activities do not differ between pelagic and near-shore foraging seabirds species. Comparative Biochemistry and Physiology (Part A) 2005;140:53–58. doi: 10.1016/j.cbpb.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Burt DW. Origin and evolution of avian microchromosomes. Cytogenetics and Genome Research. 2002;96:97–112. doi: 10.1159/000063018. [DOI] [PubMed] [Google Scholar]
- Burt DB. Plumage-based phylogenetic analyses of the Merops bee-eaters. Ibis. 2004;146:481–492. [Google Scholar]
- Burton PJK. Jaw and tongue features in Psittaciformes and other orders with special reference to the anatomy of the tooth-billed pigeon (Didunculus strigirostris) Journal of Zoology (London) 1974;174:255–276. [Google Scholar]
- Burton PJK. Anatomy and evolution of the feeding apparatus in the avian orders Coraciiformes and Piciformes. Bulletin of the British Museum (Natural History) (Zoology Series) 1984;47:331–443. [Google Scholar]
- Bybee PJ, Lee AH, Lamm E-T. Sizing the Jurassic theropod dinosaur Allosaurus: assessing growth stategy and evolution of ontogenetic scaling of limbs. Journal of Morphology. 2006;267:347–359. doi: 10.1002/jmor.10406. [DOI] [PubMed] [Google Scholar]
- Cao Y, Sorenson MD, Kumazawa Y, Mindell DP. Phylogenetic position of turtles among amniotes: evidence from mitochondrial and nuclear genes. Gene. 2000;259:139–148. doi: 10.1016/s0378-1119(00)00425-x. [DOI] [PubMed] [Google Scholar]
- Carrano MT, Biewener AA. Experimental alteration of limb posture in the chicken (Gallus gallus) and its bearing on the use of birds as analogs for dinosaur locomotion. Journal of Morphology. 1999;240:237–249. doi: 10.1002/(SICI)1097-4687(199906)240:3<237::AID-JMOR3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Carroll RL. Vertebrate paleontology and evolution. New York: W.H. Freeman; 1988. [Google Scholar]
- Carroll RL. Patterns and processes of vertebrate evolution. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- Caspers G-J, de Weerd DU, Wattel J, de Jong WW. α-crystallin sequences support a galliform/anseriform clade. Molecular Biology and Evolution. 1997;15:1637–1646. [Google Scholar]
- Caspers G-J, Wattel J, de Jong WW. α A-crystallin sequences group tinamou with ratites. Molecular Biology and Evolution. 1994;11:711–713. doi: 10.1093/oxfordjournals.molbev.a040150. [DOI] [PubMed] [Google Scholar]
- Chandler AC. A study of the structure of feathers, with reference to their taxonomic significance. Publications in Zoology of the University of California (Berkeley) 1916;13:243–446. [Google Scholar]
- Chatterjee S. The rise of birds. Washington, DC: Johns Hopkins University Press; 1997. [Google Scholar]
- Chen Z-Q, Ritzel RG, Lin CC, Hodgetts RB. Sequence conservation in avian CR1: an interspersed repetitive DNA family evolving under functional constraints. Proceedings of the National Academy of Science USA. 1991;88:5814–5818. doi: 10.1073/pnas.88.13.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Zhang Y, Jiang T-X, Barlow AJ, St Amand TR, Hu Y, Heaney S, Francis-West P, Chuon C-M, Maas R. Conservation of early odontogenic signaling pathways in Aves. Proceedings of the National Academy of Science USA. 2000;97:10044–10049. doi: 10.1073/pnas.160245097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheneval J, Escuillié F. New data concerning Palaelodus ambiguus (Aves: Phoenicopteriformes: Palaelodidae): ecological and evolutionary interpretations. Natural History Museum of Los Angeles County Science Series. 1992;36:208–224. [Google Scholar]
- Chiappe LM. Phylogenetic relationships among basal birds. In: Gauthier J, Gall LF, editors. New perspectives on the origin and early evolution of birds. New Haven, CT: Yale University Press; 2001. pp. 126–139. [Google Scholar]
- Chiappe LM. Basal bird phylogeny. In: Chiappe LM, Witmer LM, editors. Mesozoic birds: above the heads of dinosaurs. Berkeley, CA: University of California Press; 2002. pp. 448–472. [Google Scholar]
- Chiappe LM, Dyke GJ. The Mesozoic radiation of birds. Annual Review of Ecology and Systematics. 2002;33:91–124. [Google Scholar]
- Chiu C-H, Nonaka D, Xue L, Amemiya CT, Wagner GP. Evolution of Hoxa-11 in lineages phylogenetically positioned along the fin-limb transition. Molecular Phylogenetics and Evolution. 2000;17:305–316. doi: 10.1006/mpev.2000.0837. [DOI] [PubMed] [Google Scholar]
- Christian P, Christidis DL, Schodde R. Biochemical systematics of the Charadriiformes (shorebirds): relationships between Charadrii, Scolopaci, and Lari. Australian Journal of Zoology. 1992;40:291–302. [Google Scholar]
- Christidis L, Schodde LR, Shaw DD, Maynes SF. Relationships among the Australian–New Guinean parrots, lorikeets, and cockatoos (Aves: Psittaciformes: protein evidence. Condor. 1991;93:302–317. [Google Scholar]
- Chu PC. Historical examination of delayed plumage maturation in the shorebirds (Aves: Charadriiformes) Evolution. 1994;48:327–350. doi: 10.1111/j.1558-5646.1994.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Chu PC. Phylogenetic re-analysis of Strauch’s osteological data set for the Charadriiformes. Condor. 1995;97:174–196. [Google Scholar]
- Chubb AL. New nuclear evidence for the oldest divergence among neognath birds: the phylogenetic utility of ZENK (I) Molecular Phylogenetics and Evolution. 2004a;30:140–151. doi: 10.1016/s1055-7903(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Chubb AL. Nuclear corroboration of DNA–DNA hybridization in deep phylogenies of hummingbirds, swifts, and passerines: the phylogenetic utility of ZENK (II) Molecular Phylogenetics and Evolution. 2004b;30:128–139. doi: 10.1016/s1055-7903(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Cicero C, Johnson NK. The tempo of avian diversification: reply. Evolution. 2006;60:413–414. [PubMed] [Google Scholar]
- Clark HL. The classification of birds. Auk. 1901;18:370–381. [Google Scholar]
- Clark JM, Norell MA, Makovicky PJ. Cladistic approaches to the relationships of birds to other theropod dinosaurs. In: Chiappe LM, Witmer LM, editors. Mesozoic birds: above the heads of dinosaurs. Berkeley, CA: University of California Press; 2002. pp. 31–61. [Google Scholar]
- Clarke JA. Morphology, phylogenetic taxonomy, and systematics of Ichthyornis and Apatornis (Avialae: Ornithurae) Bulletin of the American Museum of Natural History. 2004;286:1–179. [Google Scholar]
- Clarke JA, Chiappe LM. A new carinate bird from the Late Cretaceous of Patagonia. American Museum Novitates. 2001;3323:1–23. [Google Scholar]
- Clarke JA, Norell MA. The morphology and phylogenetic position of Apsaravis ukhaana from the Late Cretaceous of Mongolia. American Museum Novitates. 2002;3387:1–46. [Google Scholar]
- Clarke JA, Norell MA. New avialan remains and a review of the known avifauna from the Late Cretaceous Nemegt Formation of Mongolia. American Museum Novitates. 2004;3447:1–12. [Google Scholar]
- Clarke JA, Norell MA, Dashzeveg D. New avian remains from the Eocene of Monogolia and the phylogenetic position of the Eogruidae (Aves, Gruoidea) American Museum Novitates. 2005a;3494:1–17. [Google Scholar]
- Clarke JA, Olivero EB, Puerta P. Description of the earliest fossil penguin from South American and first Paleogene vertebrate locality of Tierra del Fuego, Argentina. American Museum Novitates. 2003;3423:1–18. [Google Scholar]
- Clarke JA, Tambussi CP, Noriega JI, Ericson GM, Ketcham RA. Definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature. 2005b;433:305–308. doi: 10.1038/nature03150. [DOI] [PubMed] [Google Scholar]
- Coddington JA. The roles of homology and convergence in studies of adaptation. In: Eggleton P, Vane-Wright RI, editors. Phylogenetics and ecology. London, UK: Academic Press; 1994. pp. 53–78. [Google Scholar]
- Cohen BL, Baker AJ, Blechschmidt K, Dittmann DL, Furness RW, Gerwin JA, Helbig AJ, De Korte K, Marshall HD, Palma RL, Peter H-U, Ramli R, Siebold I, Willcox MS, Wilson RH, Zink RM. Enigmatic phylogeny of skuas (Aves: Stercorariidae) Proceedings of the Royal Society of London (Series B) 1997;264:181–190. doi: 10.1098/rspb.1997.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn JMW. The convergent flight mechanism of swifts (Apodi) and hummingbirds (Trochili) (Aves) Doctoral dissertation, Ann Arbor, MI: University of Michigan; 1968. [Google Scholar]
- Colbert EH. Evolution of the vertebrates: a history of the backboned animals through time. 3. New York: John Wiley and Sons; 1980. [Google Scholar]
- Collins TM, Wimberger PH, Naylor GJP. Compositional bias, character-state bias, and character-state reconstruction under parsimony. Systematic Biology. 1994;43:482–496. [Google Scholar]
- Cooke J. Developmental mechanism and evolutionary origin of vertebrate left/right asymmetries. Biological Reviews (Cambridge) 2004;79:377–407. doi: 10.1017/s1464793103006298. [DOI] [PubMed] [Google Scholar]
- Cooper A. Studies of avian ancient DNA: from Jurassic Park to modern island extinctions. In: Mindell DP, editor. Avian molecular evolution. San Diego, CA: Academic Press; 1997. pp. 345–373. [Google Scholar]
- Cooper A, Fortey R. Evolutionary explosions and the phylogenetic fuse. Trends in Ecology and Evolution. 1998;13:151–156. doi: 10.1016/s0169-5347(97)01277-9. [DOI] [PubMed] [Google Scholar]
- Cooper A, Lalueza-Fox C, Anderson S, Rambaut A, Austin J, Ward R. Complete mitochondrial genome sequences of two extinct moas clarify ratite evolution. Nature. 2001;409:704–707. doi: 10.1038/35055536. [DOI] [PubMed] [Google Scholar]
- Cooper A, Mourer-Chauviré C, Chambers GK, von Haeseler A, Wilson AC, Pääbo S. Independent origins of New Zealand moas and kiwis. Proceedings of the National Academy of Science USA. 1992;89:8741–8744. doi: 10.1073/pnas.89.18.8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A, Penny D. Mass survival of birds across the Cretaceous–Tertiary boundary: molecular evidence. Science. 1997;275:1109–1113. doi: 10.1126/science.275.5303.1109. [DOI] [PubMed] [Google Scholar]
- Cottam PA. The pelecaniform characters of the skeleton of the shoebill stork, Balaeniceps rex. British Museum (Natural History) Bulletin (Zoological Series) 1957;5:49–72. [Google Scholar]
- Cotton JA, Page RDM. Going nuclear: gene family evolution and vertebrate phylogeny reconciled. Proceedings of the Royal Society of London (Series B) 2002;269:1555–1561. doi: 10.1098/rspb.2002.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracraft J. On the systematic position of the boat-billed heron. Auk. 1967a;84:529–533. [Google Scholar]
- Cracraft J. Comments on homology and analogy. Systematic Zoology. 1967b;16:355–359. [Google Scholar]
- Cracraft J. The lacrimal–ectethmoid bone complex in birds: a single-character analysis. American Midland Naturalist. 1968;80:316–359. [Google Scholar]
- Cracraft J. Systematics and evolution of the Gruiformes (Class Aves): 1. The Eocene family Geranoididae and the early history of the Gruiformes. American Museum Novitates. 1969;2388:1–41. [Google Scholar]
- Cracraft J. Systematics and evolution of the Gruiformes (Class Aves): 2. Additional comments on the Bathornithidae, with descriptions of new species. American Museum Novitates. 1971a;2449:1–14. [Google Scholar]
- Cracraft J. The relationships and evolution of the rollers: families Coraciidae, Brachypteraciidae, and Leptosomatidae. Auk. 1971b;88:723–752. [Google Scholar]
- Cracraft J. The systematics and evolution of the Cathartidae in the Old World Tertiary. Condor. 1972a;74:272–283. [Google Scholar]
- Cracraft J. The relationships of the higher taxa of birds: problems in phylogenetic reasoning. Condor. 1972b;74:379–392. [Google Scholar]
- Cracraft J. Systematics and evolution of the Gruiformes (Class Aves): 3. Phylogeny of the suborder Grues. Bulletin of the American Museum of Natural History. 1973a;151:1–128. [Google Scholar]
- Cracraft J. Continental drift, paleoclimatology, and the evolution of birds. Journal of Zoology (London) 1973b;169:455–545. [Google Scholar]
- Cracraft J. Phylogeny and evolution of the ratite birds. Ibis. 1974a;116:494–521. [Google Scholar]
- Cracraft J. Phylogenetic models and classification. Systematic Zoology. 1974b;23:71–90. [Google Scholar]
- Cracraft J. Mesozoic dispersal of terrestrial faunas around the southern end of the world. Mémoires du Muséum National d’Histoire Naturelle (Nouvell Série A, Zoologie) 1975;88:29–54. [Google Scholar]
- Cracraft J. Covariation patterns in the postcranial skelton of moas (Aves, Dirnornithidae): a factor analytic study. Paleobiology. 1976a;2:166–173. [Google Scholar]
- Cracraft J. The hindlimb elements of the moas (Aves, Dinornithidae): a multivariate assessment of size and shape. Journal of Morphology. 1976b;150:495–526. [Google Scholar]
- Cracraft J. Avian evolution on southern continents: influences of palaeogeography and palaeoclimatology. In: Frith HJ, Calaby JH, editors. Proceedings of the 16th International Ornithological Congress. Canberra: Australian Academy of Science; 1976c. pp. 40–52. [Google Scholar]
- Cracraft J. Science, philosophy, and systematics. Systematic Zoology. 1978;27:213–216. [Google Scholar]
- Cracraft J. Phylogenetic analysis, evolutionary models, and paleontology. In: Cracraft J, Eldredge N, editors. Phylogenetic analysis and paleontology. New York: Columbia University Press; 1979. pp. 7–39. [Google Scholar]
- Cracraft J. Phylogenetic theory and methodology in avian paleontology: a critical appraisal. Contributions to Science of the Natural History Museum of Los Angeles County. 1980;330:9–16. [Google Scholar]
- Cracraft J. Toward a phylogenetic classification of the recent birds of the world (Class Aves) Auk. 1981;98:681–714. [Google Scholar]
- Cracraft J. Phylogenetic relationships and monophyly of loons, grebes, and hesperornithiform birds, with comments on the early history of birds. Systematic Zoology. 1982a;31:35–56. [Google Scholar]
- Cracraft J. Phylogenetic relationships and transantartic [sic] biogeography of some gruiform birds. Geobios Mémoire Spécial. 1982b;6:393–402. [Google Scholar]
- Cracraft J. Geographic differentiation, cladistics, and vicariance biogeography: reconstructing the tempo and mode of evolution. American Zoologist. 1982c;22:411–424. [Google Scholar]
- Cracraft J. A nonequilibrium theory for the rate-control of speciation and extinction and the origin of macroevolutionary patterns. Systematic Zoology. 1982d;31:348–365. [Google Scholar]
- Cracraft J. Conceptual and methodological aspects of the study of evolutionary rates, with some comments on bradytely in birds. In: Eldredge N, Stanley SM, editors. Living fossils. New York: Springer; 1984. pp. 95–104. [Google Scholar]
- Cracraft J. Monophyly and phylogenetic relationships of the Pelecaniformes: a numerical cladistic analysis. Auk. 1985;102:834–853. [Google Scholar]
- Cracraft J. The origin and early diversification of birds. Paleobiology. 1986;12:383–399. [Google Scholar]
- Cracraft J. DNA hybridization and avian phylogenetics. In: Hecht MK, Wallace B, Prance GT, editors. Evolutionary biology. Vol. 21. New York: Plenum Press; 1987. pp. 47–96. [Google Scholar]
- Cracraft J. The major clades of birds. In: Benton MJ, editor. The phylogeny and classification of the tetrapods, amphibians, reptiles, birds. Vol. 1. Oxford, UK: Systematics Association; 1988. pp. 339–361. [Google Scholar]
- Cracraft J. The species of the birds-of-paradise (Paradisaeidae): applying the phylogenetic species concept to a complex pattern of diversification. Cladistics. 1992a;8:1–43. doi: 10.1111/j.1096-0031.1992.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Cracraft J. Phylogeny and classification of birds: a study of molecular evolution [review] Molecular Biology and Evolution. 1992b;9:182–186. [Google Scholar]
- Cracraft J. Avian evolution, Gondwana biogeography and the Cretaceous–Tertiary mass extinction event. Proceedings of the Royal Society of London (Series B) 2001;268:459–469. doi: 10.1098/rspb.2000.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracraft J, Barker FK, Braun MJ, Harshman J, Dyke GJ, Feinstein J, Stanley S, Cibois A, Schikler P, Beresford P, García-Morena J, Sorenson MD, Yuri T, Mindell DP. Phylogenetic relationships among modern birds (Neornithes): toward an avian tree of life. In: Cracraft J, Donoghue MJ, editors. Assembling the tree of life. New York: Oxford University Press; 2004. pp. 468–489. [Google Scholar]
- Cracraft J, Barker FK, Cibois A. Avian higher-level phylogenetics and the Howard and Moore checklist of birds. In: Dickinson HC, editor. The Howard and Moore complete checklist of birds of the world. 3. Princeton, NY: Princeton University Press; 2003. pp. 16–21. [Google Scholar]
- Cracraft J, Clarke J. The basal clades of modern birds. In: Gauthier J, Gall LF, editors. new perspectives on the origin and early evolution of birds. New Haven, CT: Yale University Press; 2001. pp. 143–156. [Google Scholar]
- Cracraft J, Feinstein J. What is not a bird of paradise? Molecular and morphological evidence places Macgregoria in the Meliphagidae and the Cnemophilinae near the base of the corvoid tree. Proceedings of the Royal Society of London (Series B) 2000;267:235–241. doi: 10.1098/rspb.2000.0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracraft J, Mindell DP. The early history of modern birds: a comparison of molecular and morphological evidence. In: Fernholm B, Bremer K, Jornvall J, editors. The hierarchy of life. Amsterdam, The Netherlands: Exerptica Medica; 1989. pp. 389–403. [Google Scholar]
- Cracraft J, Rich PV. The systematics and evolution of the Cathartidae in the Old World Tertiary. Condor. 1972;74:272–283. [Google Scholar]
- Craw RC, Grehan JR, Heads MJ. Panbiogeography: tracking the history of life. New York: Oxford University Press; 1999. [Google Scholar]
- Crowe TM, Harley EH, Jakutowicz MB, Kowen J, Crowe AA. Phylogenetic, taxonomic, and biogeographical implications of genetic, morphological, and behavioral variation in francolins (Phasianidae: Francolinus) Auk. 1992;109:24–43. [Google Scholar]
- Cubo J. Evidence for speciational change in the evolution of ratites (Palaeognathae) Biological Journal of the Linnean Society. 2003;80:99–106. [Google Scholar]
- Cubo J, Arthur W. Patterns of correlated character evolution in flightless birds: a phylogenetic approach. Evolutionary Ecology. 2001;14:693–702. [Google Scholar]
- de Beer G. The evolution of ratites. British Museum (Natural History) Bulletin (Zoological Series) 1956;4:59–70. [Google Scholar]
- Dalla Vecchia FM, Chiappe LM. First avian skeleton from the Mesozoic of northern Gondwana. Journal of Vertebrae Paleontology. 2002;22:856–860. [Google Scholar]
- DeFilippis VR, Moore WS. Resolution of phylogenetic relationships among recently evolved species as a function of amount of DNA sequence: an empirical study based on woodpeckers (Aves: Picidae) Molecular Phylogenetics and Evolution. 2000;16:143–160. doi: 10.1006/mpev.2000.0780. [DOI] [PubMed] [Google Scholar]
- Del Hoyo J, Elliott A, Sardgatal J, editors. Handbook of the birds of the world. Vol. 1. Barcelona: Lynx Edicions; 1992. [Google Scholar]
- Del Hoyo J, Elliott A, Sardgatal J, editors. Handbook of the birds of the world. Vol. 6. Barcelona: Lynx Edicions; 2001. [Google Scholar]
- Delacour J, Mayr E. The family Anatidae. Wilson Bulletin. 1945;57:3–55. [Google Scholar]
- Delport W, Ferguson JWH, Bloomer P. Characterization and evolution of the mitochondrial DNA control region in hornbills (Bucerotiformes) Journal of Molecular Evolution. 2002;54:794–806. doi: 10.1007/s00239-001-0083-0. [DOI] [PubMed] [Google Scholar]
- Dimcheff SV. Molecular phylogeny of grouse: individual and combined performance of W-linked, autosomal, and mitochondrial loci. Systematic Biology. 2002;51:930–945. doi: 10.1080/10635150290102500. [DOI] [PubMed] [Google Scholar]
- Dimcheff DE, Drovetski SV, Mindell DP. Molecular evolution and systematics of Tetraoninae and other Galliformes using mitochondrial 12S and ND2 genes. Molecular Phylogenetics and Evolution. 2002;24:203–215. doi: 10.1016/s1055-7903(02)00230-0. [DOI] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Ruiz MC, Michelangeli F. Evolutionary significance of foregut fermentation in the hoatzin (Opisthocomus hoazin; Aves: Opisthocomidae) Journal of Comparative Physiology (Part B) 1993;163:594–601. [Google Scholar]
- Donne-Goussé C, Laudet V, Hänni C. A molecular phylogeny of anseriforms based on mitochondrial DNA analysis. Molecular Phylogenetics and Evolution. 2002;23:339–356. doi: 10.1016/s1055-7903(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Doyle JL. Gene trees and species trees: molecular systematics as one-character taxonomy. Systematic Botany. 1992;17:144–163. [Google Scholar]
- Doyle JL. Homoplasy connections and disconnections: genes and species, molecules and morphology. In: Sanderson MJ, Hufford L, editors. Homoplasy: the recurrence of similarity in evolution. San Diego, CA: Academic Press; 1996. pp. 37–66. [Google Scholar]
- Drouin G, Moniz de Sá M. The concerted evolution of 5S ribosomal genes linked to the repeat units of other multigene families. Molecular Biology and Evolution. 1995;12:481–493. doi: 10.1093/oxfordjournals.molbev.a040223. [DOI] [PubMed] [Google Scholar]
- Dumbacher JP, Thane TK, Fleischer RC. Phylogeny of the owlet-nightjars (Aves: Aegothelidae) based on mitochondrial DNA sequence. Molecular Phylogenetics and Evolution. 2003;29:540–549. doi: 10.1016/s1055-7903(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Dyke GJ. The evolutionary radiation of modern birds: systematics and patterns of diversification. Geological Journal. 2001a;36:305–315. [Google Scholar]
- Dyke GJ. The fossil waterfowl (Aves: Anseriformes) from the Eocene of England. American Museum Novitates. 2001b;3354:1–15. [Google Scholar]
- Dyke GJ. A primitive swift from the London Clay and the relationships of fossil apodiform birds. Journal of Vertebrate Paleontology. 2001c;21:195–200. [Google Scholar]
- Dyke GJ. The fossil record and molecular clocks: basal radiations within Neornithes. In: Smith P, Donoghue MP, editors. Molecular clocks and the fossil record. London, UK: Taylor & Francis; 2003. pp. 263–278. [Google Scholar]
- Dyke GJ, Cooper JH. A new psittaciform bird from the London Clay (Lower Eocene) of England. Palaeontology. 2000;43:271–285. [Google Scholar]
- Dyke GJ, Dortangs RW, Jagt JWM, Mulder EWA, Schulp AS, Chiappe LM. Europe’s last Mesozoic bird. Naturwissenschaften. 2002;89:408–411. doi: 10.1007/s00114-002-0352-9. [DOI] [PubMed] [Google Scholar]
- Dyke GJ, Gulas BE, Crowe TM. Suprageneric relationships of galliform birds (Aves, Galliformes): a cladistic analysis of morphological characters. Zoological Journal of the Linnean Society. 2003;137:227–244. [Google Scholar]
- Dyke GJ, Mayr G. Did parrots exist in the Cretaceous period? Nature. 1999;399:317–318. [Google Scholar]
- Dyke GJ, Van Tuinen M. The evolutionary radiation of modern birds (Neornithes): reconciling molecules, morphology and the fossil record. Zoological Journal of the Linnean Society. 2004;141:153–177. [Google Scholar]
- Dyke GJ, Waterhouse DM. A mousebird (Aves: Coliiformes) from the Eocene of England. Journal für Ornithologie. 2000;141:1–9. [Google Scholar]
- Dzerzhinsky FY. Evidence for common ancestry of the Galliformes and Anseriformes. Courier Forschungsinstitut Senckenberg. 1995;181:325–336. [Google Scholar]
- Eberhard JR, Bermingham E. Phylogeny and comparative biogeography of Pionopsitta parrots and Pteroglossus toucans. Molecular Phylogenetics and Evolution. 2001;36:288–304. doi: 10.1016/j.ympev.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Eberhard JR, Bermingham E. Phylogeny and biogeography of the Amazona ochrocephala (Aves: Psittacidae) complex. Auk. 2004;121:318–332. [Google Scholar]
- Eberhard JR, Wright TF, Bermingham E. Duplication and concerted evolution of the mitochondrial control region in the parrot genus Amazona. Molecular Biology and Evolution. 2001;18:1330–1342. doi: 10.1093/oxfordjournals.molbev.a003917. [DOI] [PubMed] [Google Scholar]
- Edwards SV, Arctander P, Wilson AC. Mitochondrial resolution of a deep branch in the genealogical tree for perching birds. Proceedings of the Royal Society of London (Series B) 1991;243:99–107. doi: 10.1098/rspb.1991.0017. [DOI] [PubMed] [Google Scholar]
- Edwards SV, Boles WE. Out of Gondwana: the origin of passerine birds. Trends in Ecology and Evolution. 2002;17:347–349. [Google Scholar]
- Edwards SV, Fertil B, Giron A, Deschavanne PJ. A genomic schism in birds revealed by phylogenetic analysis of DNA strings. Systematic Biology. 2002;51:599–613. doi: 10.1080/10635150290102285. [DOI] [PubMed] [Google Scholar]
- Edwards SV, Jennings WB, Shedlock AM. Phylogenetics of modern birds in the era of genomics. Proceedings of the Royal Society of London. 2005;272:979–992. doi: 10.1098/rspb.2004.3035. Seris B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SV, Wilson AC. Phylogenetically informative length polymorphism and sequence variability in mitochondrial DNA of Australian songbirds (Pomatostomus) Genetics. 1990;126:695–711. doi: 10.1093/genetics/126.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman J, Ericson PGP. Out of Gondwanaland: the evolutionary history of cooperative breeding and social behaviour among crows, magpies, jays and allies. Proceedings of the Royal Society of London (Series B) 2006;273:1–9. doi: 10.1098/rspb.2005.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldredge N. Macroevolutionary dynamics: species, niches, and adaptive peaks. New York: McGraw-Hill; 1989. [Google Scholar]
- Eldredge N, Cracraft J. Phylogenetic patterns and the evolutionary process: method and theory in comparative biology. New York: Columbia University Press; 1980. [Google Scholar]
- Elliot DG. A study of the Pteroclidæ or family of the sand-grouse. Proceedings of the Zoological Society of London. 1878;1878:223–264. [Google Scholar]
- Elzanowski A. On the role of basipterygoid processes in some birds. Verhandlungen der Anatomischen Gesellschaft (Jena) 1977;71:1303–1307. [PubMed] [Google Scholar]
- Elzanowski A. Embryonic bird skeletons from the Late Cretaceous of Mongolia. Palaeontologia Polonica. 1981;42:147–176. [Google Scholar]
- Emslie SD. The fossil history and phylogenetic relationships of condors (Ciconiiformes: Vulturidae) in the New World. Journal of Vertebrate Paleontology. 1988;8:212–228. [Google Scholar]
- Ericson PGP. The skeletal evidence for a sister-group relationship of anseriform and galliform birds – a critical evaluation. Journal of Avian Biology. 1996;27:195–202. [Google Scholar]
- Ericson PGP. Systematic relationships of the Paleogene family Presbyornithidae (Aves: Anseriformes) Zoological Journal of the Linnean Society. 1997;121:429–483. [Google Scholar]
- Ericson PGP. New material of Juncitarsus (Phoenicopteriformes), with a guide for differentiating that genus from the Presbyornithidae (Anseriformes) Smithsonian Contributions to Paleobiology. 1999;89:245–251. [Google Scholar]
- Ericson PGP, Christidis L, Cooper A, Irestedt M, Jackson J, Johansson US, Norman JA. A Gondwanan origin of passerine birds supported by DNA sequences of the endemic New Zealand wrens. Proceedings of the Royal Society of London (Series B) 2002a;269:235–241. doi: 10.1098/rspb.2001.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson PGP, Christidis L, Irestedt M, Norman JA. Systematic affinities of the lyrebirds (Passeriformes: Menura), with a novel classification of the major groups of passerine birds. Molecular Phylogenetics and Evolution. 2002b;25:53–62. doi: 10.1016/s1055-7903(02)00215-4. [DOI] [PubMed] [Google Scholar]
- Ericson PGP, Envall L, Irestedt M, Norman JA. Interfamilial relationships of the shorebirds (Aves: Charadriiformes) based on nuclear DNA sequence data. BMC (Biomed Central) Evolutionary Biology. 2003a;3(16):1–14. doi: 10.1186/1471-2148-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson PGP, Irestedt M Johansson US. Evolution, biogeography, and patterns of diversification in passerine birds. Journal of Avian Biology. 2003b;34:3–15. [Google Scholar]
- Ericson PGP, Johansson US, Parsons TJ. Major divisions in oscines revealed by insertions in the nuclear gene c-myc: a novel gene in avian phylogenetics. Auk. 2000;117:1069–1078. [Google Scholar]
- Ericson PGP, Johansson US. Phylogeny of Passerida (Aves: Passeriformes) based on nuclear and mitochondrial sequence data. Molecular Phylogenetics and Evolution. 2003;29:126–138. doi: 10.1016/s1055-7903(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Ericson PGP, Parsons TJ, Johansson US. Morphological and molecular support for nonmonophyly of the Galloanserae. In: Gauthier JA, Gall LF, editors. New perspectives on the origin and early evolution of birds. New Haven, CT: Yale University Press; 1998. pp. 157–168. [Google Scholar]
- Ericson PGP, Zuccon D, Ohlson JI, Johansson US, Alvarenga H, Prum RO. Higher-level phylogeny and morphological evolution of tyrant flycatchers, cotingas, manakins, and their allies (Aves: Tyrannida) Molecular Phylogenetics and Evolution. 2006;40:471–483. doi: 10.1016/j.ympev.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A. Phylogenetic relationships among the trogons. Auk. 1998;115:937–954. [Google Scholar]
- Espinosa de los Monteros A. Higher-level phylogeny of Trogoniformes. Molecular Phylogenetics and Evolution. 2000;14:20–34. doi: 10.1006/mpev.1999.0683. [DOI] [PubMed] [Google Scholar]
- Evans SE. The early history and relationships of the Diapsida. In: Benton MJ, editor. The phylogeny and classification of the tetrapods, amphibians, reptiles, birds. Vol. 1. Oxford, UK: Systematics Association; 1988. pp. 221–260. [Google Scholar]
- Fain MG, Houde P. Parallel radiations in the primary clades of birds. Evolution. 2004;58:2558–2573. doi: 10.1111/j.0014-3820.2004.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Faith DP. Homoplasy as pattern: multivariate analysis of morphological convergence in Anseriformes. Cladistics. 1989;5:235–258. doi: 10.1111/j.1096-0031.1989.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Farris JS. A successive approximations approach to character weighting. Systematic Zoology. 1969;18:374–385. [Google Scholar]
- Farris JS. The logical basis of phylogenetic analysis. In: Platnick NI, Funk VA, editors. Advances in cladistics. Vol. 2. New York: Columbia University Press; 1982. pp. 7–36. [Google Scholar]
- Farris JS. The retention index and the rescaled consistency index. Cladistics. 1989;5:417–419. doi: 10.1111/j.1096-0031.1989.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Farris JS, Källersjö M, Crowe TM, Lipscomb D, Johansson U. Frigatebirds, tropicbirds, and Ciconiida: excesses of confidence probability. Cladistics. 1999;15:1–7. [Google Scholar]
- Farris JS, Källersjö M, De Laet JE. Branch lengths do not indicate support – even in maximum likelihood. Cladistics. 2001;17:298–299. doi: 10.1111/j.1096-0031.2001.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Fautin DG, Watling L. Review of textbooks of invertebrate zoology. American Zoologist. 1999;39:818–824. [Google Scholar]
- Feduccia A. Evolutionary trends in the Neotropical ovenbirds and woodhewers. Ornithological Monographs. 1973;13:1–69. [Google Scholar]
- Feduccia A. Osteological evidence for shorebird affinities of the flamingoes. Auk. 1976;93:587–601. [Google Scholar]
- Feduccia A. The whalebill is a stork. Nature. 1977a;266:719–720. [Google Scholar]
- Feduccia A. Hypothetical stages in the evolution of modern ducks and flamingos. Journal of Theoretical Biology. 1977b;67:715–721. doi: 10.1016/0022-5193(77)90256-9. [DOI] [PubMed] [Google Scholar]
- Feduccia A. A model for the evolution of perching birds. Systematic Zoology. 1977c;26:19–31. [Google Scholar]
- Feduccia A. The age of birds. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- Feduccia A. The morphological evidence for ratite monophyly. Fact or fiction? In: Ilyichev VD, Gavrilov VM, editors. Acta XVIII Congressus Internationalis Ornithologici. Vol. 1. Moscow, USSR: Academy of Science; 1985. pp. 184–190. [Google Scholar]
- Feduccia A. The scapulocoracoid of flightless birds: a primitive avian character similar to that of theropods. Ibis. 1986;128:128–132. [Google Scholar]
- Feduccia A. Explosive evolution in Tertiary birds and mammals. Science. 1995;267:637–638. doi: 10.1126/science.267.5198.637. [DOI] [PubMed] [Google Scholar]
- Feduccia A. The origin and evolution of birds. New Haven, CT: Yale University Press; 1996. [Google Scholar]
- Feduccia A. 1, 2, 3 = 2, 3, 4: accommodating the cladogram. Proceedings of the National Academy of Science USA. 1999;96:4740–4742. doi: 10.1073/pnas.96.9.4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduccia A. ‘Big bang’ for Tertiary birds? Trends in Ecology and Evolution. 2003;18:172–176. [Google Scholar]
- Feduccia A, Lingham-Soliar T, Hinchliffe JR. Do feathered dinosaurs exist? Testing the hypothesis on neontological and paleontological evidence. Journal of Morphology. 2005;266:125–166. doi: 10.1002/jmor.10382. [DOI] [PubMed] [Google Scholar]
- Feduccia A, Martin LD. The Eocene zygodactyl birds of North America (Aves: Piciformes) Smithsonian Contributions to Paleobiology. 1976;27:101–110. [Google Scholar]
- Felsenstein J. The number of evolutionary trees. Systematic Zoology. 1978;27:27–33. [Google Scholar]
- Felsenstein J. Alternative methods of phylogenetic inference and their interrelationship. Systematic Zoology. 1979;28:49–62. [Google Scholar]
- Felsenstein J. Parsimony in systematics: biological and statistical issues. Annual Review of Ecology and Systematics. 1983;14:313–333. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenetics: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Inferring phylogenies. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Felsenstein J, Kishino H. Is there something wrong with the bootstrap on phylogenies? A reply to Hillis and Bull. Systematic Biology. 1993;42:193–200. [Google Scholar]
- Fidler AE, Kuhn S, Gwinner E. Convergent evolution of strigiform and caprimulgiform dark-activity is supported by phylogenetic analysis using the arylakylamine N-acetyltransferase (Aanat) gene. Molecular Phylogenetics and Evolution. 2004;33:908–921. doi: 10.1016/j.ympev.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Figuerola J. A comparative study on the evolution of reversed size dimorphism in monogamous waders. Biological Journal of the Linnean Society. 1999;67:1–18. [Google Scholar]
- Fink WL. Phylogenetic analysis and the detection of ontogenetic patterns. In: McKinney ML, editor. Heterochrony in evolution: a multidisciplinary approach. New York: Plenum Press; 1988. pp. 71–91. [Google Scholar]
- Fisher DC. Stratigraphic parsimony. In: Maddison WP, Maddison DR, editors. MacClade: analysis of phylogeny and character evolution. Sunderland, MA: Sinauer Associates; 1992. pp. 124–129. [Google Scholar]
- Fitzpatrick JW. Why so many passerine birds? A response to Raikow. Systematic Zoology. 1988;37:71–76. [Google Scholar]
- Fjeldså J. The systematic affinities of sandgrouses, Pteroclidae. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening. 1976;139:179–243. [Google Scholar]
- Forbes WA. Note on the structure of the palate in the trogons (Trogonidæ) Proceedings of the Zoological Society of London. 1881;1881:836–837. [Google Scholar]
- Forbes WA. Description of the pterylosis of Mesites, with remarks on the position of that genus. Proceedings of the Zoological Society of London. 1882;1882:267–271. [Google Scholar]
- Forey PL, Kitching IJ. Experiments in coding multistate characters. In: Scotland R, Pennington RT, editors. Homology and systematics: coding characters for phylogenetic analysis. London, UK: Taylor & Francis; 2000. pp. 54–80. [Google Scholar]
- Freudenstein JV. Characters, states, and homology. Systematic Biology. 2005;54:965–973. doi: 10.1080/10635150500354654. [DOI] [PubMed] [Google Scholar]
- Friesen VL, Baker AJ, Piatt JF. Phylogenetic relationships within the Alcidae (Charadriiformes: Aves) inferred from total molecular evidence. Molecular Biology and Evolution. 1996;13:359–367. doi: 10.1093/oxfordjournals.molbev.a025595. [DOI] [PubMed] [Google Scholar]
- Fry CH. The evolutionary biology of kingfishers (Alcedinidae) Living Bird. 1980;18:113–160. [Google Scholar]
- Fry CH. The bee-eaters. Calton, UK: T & AD Poyser; 1984. [Google Scholar]
- Fujita M. Kinematic parameters of the walking of herons. ground-feeders, and waterfowl. Comparative Biochemistry and Physiology (Part A) 2004;139:117–124. doi: 10.1016/j.cbpb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Fürbringer M. Untersuchungen zur Morphologie und Systematik der Vögel, zugleich ein Beitrag zur Anatomie der Stütz- und Bewegungsorgane. Vol. 2. Amsterdam, Holland: T. J. Van Holkema; 1888. [Google Scholar]
- Fusco G. How many processes are responsible for phenotypic evolution? Evolution and Development. 2001;3:279–286. doi: 10.1046/j.1525-142x.2001.003004279.x. [DOI] [PubMed] [Google Scholar]
- Gadow H. Anatomie des Phoenicopterus roseus Pall. und seine Stellung in System. Journal für Ornithologie. 1877;25:382–397. [Google Scholar]
- Gadow H. On some points in the anatomy of Pterocles, with remarks on its systematic position. Proceedings of the Zoological Society of London. 1882;1882:312–332. [Google Scholar]
- Gadow H. Notes on the structure of Pedionomus torquatus, with regard to its systematic position. Records of the Australian Museum. 1891a;1:205–211. [Google Scholar]
- Gadow H. Crop and sternum of Opisthocomus cristatus: a contribution to the question of the correlation of organs and the inheritance of acquired characters. Proceedings of the Royal Irish Academy. 1891b;2:147–154. [Google Scholar]
- Gadow H. On the classification of birds. Proceedings of the Zoological Society of London. 1892;1892:229–256. [Google Scholar]
- Gadow H. Vögel, II. Systematischer Theil. In: Bronn HG, editor. Klassen und Ordnungen des Thierreichs. 4. Vol. 6. Leipzig: CF Winter; 1893. p. 303. [Google Scholar]
- Galis F. On the homology of structures and Hox genes: the vertebral column. In: Bock GR, Cardew G, editors. Homology. Chichester, UK: Academic Press; 1999. pp. 80–94. [DOI] [PubMed] [Google Scholar]
- Galis F, Kundrát M, Metz JAJ. Hox genes, digit identities and the theropod/bird transition. Journal of Experimental Zoology (Series B) 2005;304:198–205. doi: 10.1002/jez.b.21042. [DOI] [PubMed] [Google Scholar]
- Galis F, van Alphen JJM, Metz JAJ. Digit reduction: via repatterning or developmental arrest? Evolution and Development. 2002;4:249–251. doi: 10.1046/j.1525-142x.2002.02013.x. [DOI] [PubMed] [Google Scholar]
- García-Moreno J. Is there a universal mtDNA clock for birds? Journal of Avian Biology. 2004;35:1–4. [Google Scholar]
- García-Moreno J, Cortés N, Garciá-Deraqs GM, Hernández-Baños BE. Local origins and diversification among Lampornis hummingbirds: a Mesoamerican taxon. Molecular Phylogenetics and Evolution. 2006;38:488–498. doi: 10.1016/j.ympev.2005.08.015. [DOI] [PubMed] [Google Scholar]
- García-Moreno J, Sorenson MD, Mindell DP. Congruent avian phylogenies inferred from mitochondrial and nuclear DNA sequences. Journal of Molecular Biology. 2003;57:27–37. doi: 10.1007/s00239-002-2443-9. [DOI] [PubMed] [Google Scholar]
- Garrod AH. On certain muscles of the thigh of birds and on their value in classification. Proceedings of the Zoological Society of London. 1873a;1873:626–644. [Google Scholar]
- Garrod AH. On some points in the anatomy of Steatornis. Proceedings of the Zoological Society of London. 1873b;1873:526–535. [Google Scholar]
- Garrod AH. On certain muscles of the thigh of birds and on their value in classification. Part II. Proceedings of the Zoological Society of London. 1874;1874:111–124. [Google Scholar]
- Garrod AH. Notes on the anatomy of the colies (Colius) Proceedings of the Zoological Society of London. 1876;1876:416–419. [Google Scholar]
- Garrod AH. Notes on points in the anatomy of the hoatzin (Opisthocomus cristatus) Proceedings of the Zoological Society of London. 1879;1879:109–114. [Google Scholar]
- Gatesy SM. Hind limb scaling in birds and other theropods: implications for terrestrial locomotion. Journal of Morphology. 1991;209:83–96. doi: 10.1002/jmor.1052090107. [DOI] [PubMed] [Google Scholar]
- Gatesy SM, Biewiener AA. Bipedal locomotion: effects of speed, size and limb posture in birds and humans. Journal of Zoology (London) 1991;224:127–147. [Google Scholar]
- Geeta R. Structure trees and species trees: what they say about morphological development and evolution. Evolution and Development. 2003;5:609–621. doi: 10.1046/j.1525-142x.2003.03066.x. [DOI] [PubMed] [Google Scholar]
- Geffen E, Yom-Tov Y. Factors affecting the rate of intraspecific nest parasitism among Anseriformes and Galliformes. Animal Behaviour. 2001;62:1027–1038. [Google Scholar]
- Gelman A, Xiao-Li M. Applied Bayesian modeling and causal inference from incomplete-data perspectives. Chichester, UK: J. Wiley and Sons; 2004. [Google Scholar]
- Giebel CGA. Balaeniceps rex. Zeitschrift für die Gesamten Naturwissenschaften (Abteilung 1) 1873;7:350–354. [Google Scholar]
- Gittleman JL, Anderson CG, Kot M, Luh H-K. Comparative tests of evolutionary lability and rates using molecular phylogenies. In: Harvey PH, Brown AJ, Maynard Smith J, Nee S, editors. New uses for new phylogenies. Oxford, UK: Oxford University Press; 1996. pp. 289–307. [Google Scholar]
- Givnish TJ, Sytsma KJ. Homoplasy in molecular vs. morphological data: the likelihood of correct phylogenetic inference. In: Givnish TJ, Sytsma KJ, editors. Molecular evolution and adaptive radiation. Cambridge, UK: Cambridge University Press; 1997a. pp. 55–101. [Google Scholar]
- Givnish TJ, Sytsma KJ. Consistency, characters, and the likelihood of correct phylogenetic inference. Molecular Phylogenetics and Evolution. 1997b;7:320–333. doi: 10.1006/mpev.1997.0409. [DOI] [PubMed] [Google Scholar]
- Glenny FH. Antarctica as a center of origin of birds. Ohio Journal of Science. 1954;54:307–314. [Google Scholar]
- Goedert JL. A new Late Eocene species of Plotopteridae (Aves: Pelecaniformes) from northwestern Oregon. Proceedings of the California Academy of Science. 1988;45:97–102. [Google Scholar]
- Goedert JL. Giant late Eocene marine birds (Pelecaniformes: Pelagornithidae) from northwestern Oregon. Journal of Paleontology. 1989;63:939–944. [Google Scholar]
- Goedert JL, Cornish J. A preliminary report on the diversity and stratigraphic distribution of the Plotopteridae (Pelecaniformes) in Paleogene rocks of Washington state, USA. In: Zhou Z, Zhang F, editors. Proceedings of the 5th Symposium of the Society of Avian Paleontology and Evolution, 1–4 June 2000. Beijing, PRC: Science Press; 2002. pp. 63–76. [Google Scholar]
- Göhlich UB, Mourer-Chauviré C. Revision of the phasianids (Aves: Galliformes) from the lower Miocene of Saint-Gérand-le-Puy (Allier, France) Palaeontology. 2005;48:1331–1350. [Google Scholar]
- Goldring GB, Dean AM. The structural basis of molecular adaptation. Molecular Biology and Evolution. 1998;15:355–369. doi: 10.1093/oxfordjournals.molbev.a025932. [DOI] [PubMed] [Google Scholar]
- Goloboff PA. Analyzing large data sets in reasonable times: solutions for composite optima. Cladistics. 1999;15:415–428. doi: 10.1111/j.1096-0031.1999.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Díaz E, González-Solís J, Peinado MA, Page RDM. Phylogeography of the Calonectris shearwaters using molecular and morphometric data. Molecular Phylogenetics and Evolution. 2006;41:322–332. doi: 10.1016/j.ympev.2006.05.006. [DOI] [PubMed] [Google Scholar]
- González-Barba G, Schwennicke T, Goedert JL, Barnes LG. Earliest Pacific basin record of the Pelagornithidae (Aves: Pelecaniformes) Journal of Vertebrae Paleontology. 2002;22:722–725. [Google Scholar]
- Gould SJ. Ontogeny and phylogeny. Cambridge, MA: Belknap Press; 1977. [Google Scholar]
- Gould SJ, Eldredge N. Punctuated equilibria: the tempo and mode of evolution reconsidered. Paleobiology. 1977;3:115–151. [Google Scholar]
- Grant T, Kluge AG. Transformation series as an ideographic character concept. Cladistics. 2004;20:23–31. doi: 10.1111/j.1096-0031.2004.00003.x. [DOI] [PubMed] [Google Scholar]
- Grau ET, Pereira SL, Silveira LF, Höfling E, Wajntal A. Molecular phylogenetics and biogeography of Neotropical piping guans (Aves: Galliformes): Pipile Bonaparte, 1856 is a synonym of Aburria Reichenbach, 1853. Molecular Phylogenetics and Evolution. 2005;35:637–645. doi: 10.1016/j.ympev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Graur D, Li W-H. Fundamentals of molecular evolution. 2. Sunderlund, MA: Sinauer Associates; 2000. [Google Scholar]
- Graybeal A. Evaluating the phylogenetic utility of genes: a search for genes informative about deep divergences among vertebrates. Systematic Biology. 1994;43:174–193. [Google Scholar]
- Graybeal A. Is it better to add taxa or characters to a difficult phylogenetic problem? Systematic Biology. 1998;47:9–17. doi: 10.1080/106351598260996. [DOI] [PubMed] [Google Scholar]
- Grellet-Tinner G, Norell M. An avian egg from the Campanian of Bayn Dzak, Mongolia. Journal of Vertebrate Paleontology. 2002;22:719–721. [Google Scholar]
- Griffiths CS. Syringeal morphology and the phylogeny of the Falconidae. Condor. 1994;96:127–140. [Google Scholar]
- Griffiths CS. Phylogeny of the Falconidae inferred from molecular and morphological data. Auk. 1999;116:116–130. [Google Scholar]
- Griffiths CS, Barrowclough GF, Groth JG, Mertz L. Phylogeny of the Falconidae (Aves): a comparison of the efficacy of morphological, mitochondrial, and nuclear data. Molecular Phylogenetics and Evolution. 2004;32:101–109. doi: 10.1016/j.ympev.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Groombridge JJ, Jones CG, Nichols RA, Carlton M, Bruford MW. Molecular phylogeny and morphological change in the Psittacula parakeets. Molecular Phylogenetics and Evolution. 2004;31:96–108. doi: 10.1016/j.ympev.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Groth JG, Barrowclough GF. Basal divergences in birds and the phylogenetic utility of the nuclear RAG-1 gene. Molecular Phylogenetics and Evolution. 1999;12:115–123. doi: 10.1006/mpev.1998.0603. [DOI] [PubMed] [Google Scholar]
- Gutiérrez RJ, Barrowclough GF, Groth JG. A classification of the grouse (Aves: Tetraoninae) based on mitochondrial DNA sequences. Wildlife Biology. 2000;6:205–211. [Google Scholar]
- Haccou P, Jagers P, Vatutin VA. Branching processes: variation, growth, and extinction of populations. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Hackett SJ, Griffiths CS, Bates JM, Klein N. Re: a commentary on the use of sequence data for phylogeny reconstruction. Molecular Phylogenetics and Evolution. 1995;4:350–353. doi: 10.1006/mpev.1995.1030. [DOI] [PubMed] [Google Scholar]
- Haddrath O, Baker AJ. Complete mitochondrial DNA genome sequences of extinct birds: ratite phylogenetics and the vicariance biogeography hypothesis. Proceedings of the Royal Society of London (Series B) 2001;268:939–945. doi: 10.1098/rspb.2001.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagey LR, Schteingart CD, Ton-Nu H-T, Rossi SS, Odell D, Hoffmann AF. β-phocacolic acid in bile; biochemical evidence that the flamingo is related to an ancient goose. Condor. 1990;92:593–597. [Google Scholar]
- Hall BK. Developmental processes underlying heterochrony as an evolutionary mechanism. Canadian Journal of Zoology. 1984;62:1–7. [Google Scholar]
- Hall BK. Introduction. In: Hall BK, editor. Homology: the hierarchical basis of comparative biology. San Diego, CA: Academic Press; 1994. pp. 1–19. [Google Scholar]
- Hall BK. Development of the clavicles in birds and mammals. Journal of Experimental Zoology. 2001;289:153–161. doi: 10.1002/1097-010x(20010215)289:3<153::aid-jez1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Hall BK. Descent with modification: the unity underlying homology and homoplasy as seen through an analysis of development and evolution. Biological Reviews (Cambridge) 2003;78:409–433. doi: 10.1017/s1464793102006097. [DOI] [PubMed] [Google Scholar]
- Hamrick MW. Developmental mechanisms of digit reduction. Evolution and Development. 2002;4:247–248. doi: 10.1046/j.1525-142x.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- Handt E, Höss M, Krings M, Pääbo S. Ancient DNA: methodological challenges. Experientia. 1994;50:524–529. doi: 10.1007/BF01921720. [DOI] [PubMed] [Google Scholar]
- Haring E, Kruckenhauser L, Gamauf A, Riesing MJ, Pinsker W. The complete sequence of the mitochondrial genome of Buteo buteo (Aves, Accipitridae) indicates an early split in the phylogeny of raptors. Molecular Biology and Evolution. 2001;18:1892–1904. doi: 10.1093/oxfordjournals.molbev.a003730. [DOI] [PubMed] [Google Scholar]
- Härlid A, Arnason U. Analyses of mitochondrial DNA nest ratite birds within the Neognathae: supporting a neotenous origin of ratite morphological characters. Proceedings of the Royal Society of London (Series B) 1998;266:305–309. [Google Scholar]
- Härlid A, Janke A, Arnason U. The mtDNA sequence of the ostrich and the divergence between palaeognathous and neognathous birds. Molecular Biology and Evolution. 1997;14:754–761. doi: 10.1093/oxfordjournals.molbev.a025815. [DOI] [PubMed] [Google Scholar]
- Härlid A, Janke A, Arnason U. The complete mitochondrial genome of Rhea americana and early avian divergences. Journal of Molecular Evolution. 1998;46:669–679. doi: 10.1007/pl00006347. [DOI] [PubMed] [Google Scholar]
- Harris TE. The theory of branching process. Mineola NY: Dover; 1963. [Google Scholar]
- Harrison CJO. The earliest parrot: a new species from the British Eocene. Ibis. 1982a;124:203–210. [Google Scholar]
- Harrison CJO. Cuculiform, piciform and passeriform birds in the lower Eocene of England. Tertiary Research. 1982b;4:71–81. [Google Scholar]
- Harrison GL, McLenachan PA, Phillips MJ, Slack KE, Cooper A, Penny D. Four new avian mitochondrial genomes help get to basic evolutionary questions in the late Cretaceous. Molecular Biology and Evolution. 2004;21:974–983. doi: 10.1093/molbev/msh065. [DOI] [PubMed] [Google Scholar]
- Harrison CJO, Walker CA. Birds of the British upper Eocene. Zoological Journal of the Linnean Society. 1976a;59:323–351. [Google Scholar]
- Harrison CJO, Walker CA. A reappraisal of Prophaethon shrubsolei Andrews (Aves) Bulletin of the British Museum (Natural History): Geology. 1976b;27:1–30. [Google Scholar]
- Harshman J. The effect of irrelevant characters on bootstrap values. Systematic Biology. 1994a;43:419–424. [Google Scholar]
- Harshman J. Reweaving the tapestry: What can we learn from Sibley and Ahlquist (1990)? Auk. 1994b;111:377–388. [Google Scholar]
- Harshman J, Braun EL, Braun MJ, Hackett SJ, Han K-J, Huddleston Kimball RT, Markes BD, Miglia KJ, Moore WA, Reddy S, Sheldon FH, Steadman D, Yri T, Witt C. Early Bird, an international collaboration in deep molecular phylogenetic of birds: Can four million bases resolve the tree [abstract]? Journal of Ornithology. 2006;147(Suppl. 1):42. [Google Scholar]
- Haszprunar G. Parsimony analysis as a specific kind of homology estimation and the implications for character weighting. Molecular Phylogenetics and Evolution. 1998;9:333–339. doi: 10.1006/mpev.1998.0496. [DOI] [PubMed] [Google Scholar]
- Hauser DL, Presch W. The effect of ordered characters on phylogenetic reconstruction. Cladistics. 1991;7:243–265. doi: 10.1111/j.1096-0031.1991.tb00037.x. [DOI] [PubMed] [Google Scholar]
- Hedges SB. Molecular evidence for the origin of birds. Proceedings of the National Academy of Science USA. 1994;91:2621–2624. doi: 10.1073/pnas.91.7.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges SB, Parker PH, Sibley CG, Kumar S. Continental breakup and the ordinal diversification of birds and mammals. Nature. 1996;381:226–229. doi: 10.1038/381226a0. [DOI] [PubMed] [Google Scholar]
- Hedges SB, Sibley CG. Molecules vs morphology in avian evolution: the case of the ‘pelecaniform’ birds. Proceedings of the National Academy of Science USA. 1994;91:9861–9865. doi: 10.1073/pnas.91.21.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges SB, Simmons MD, van Dijk AMA, Caspers G-J, deJong WW, Sibley CG. Phylogenetic relationships of the hoatzin, an enigmatic South American bird. Proceedings of the National Academy of Science USA. 1995;92:11662–11665. doi: 10.1073/pnas.92.25.11662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig AJ, Kocum A, Seibold I, Braun MJ. A multi-gene phylogeny of aquiline eagles (Aves: Accipitriformes) reveals extensive paraphyly at the genus level. Molecular Phylogenetics and Evolution. 2005;35:147–164. doi: 10.1016/j.ympev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Helbig A, Seibold I. Are storks and New World vultures paraphyletic? Molecular Phylogenetics and Evolution. 1995;4:315–319. doi: 10.1006/mpev.1996.0080. [DOI] [PubMed] [Google Scholar]
- Helfenbein KG, DeSalle R. Falsifications and corroborations: Karl Popper’s influence on systematics. Molecular Phylogenetics and Evolution. 2005;35:271–280. doi: 10.1016/j.ympev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Helm-Bychowski KM, Cracraft J. Recovering phylogenetic signal from DNA sequences: relationships within the corvine assemblage (Class Aves) as inferred from complete sequences of the mitochondrial DNA cytochrome-b gene. Molecular Biology and Evolution. 1993;10:1196–1214. doi: 10.1093/oxfordjournals.molbev.a040072. [DOI] [PubMed] [Google Scholar]
- Helm-Bychowski KM, Wilson AC. Rates of nuclear-DNA evolution in pheasant-like birds: evidence from restriction maps. Proceedings of the National Academy of Science USA. 1986;83:688–692. doi: 10.1073/pnas.83.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig W. Phylogenetic systematics. Urbana, IL: University of Illinois Press; 1966. [Google Scholar]
- Heyde CC. Quasi-likelihood and its application: a general approach to optimal parameter estimation. New York: Springer; 1997. [Google Scholar]
- Hill RV. Integration of morphological data sets for phylogenetic analysis of Amniota: the importance of integumentary characters and increased taxonomic sampling. Systematic Biology. 2005;54:530–547. doi: 10.1080/10635150590950326. [DOI] [PubMed] [Google Scholar]
- Hillis DM, Bull JJ. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology. 1993;42:182–192. [Google Scholar]
- Hillis DM, Wiens JJ. Molecules versus morphology in systematics. In: Wiens JJ, editor. Phylogenetic analysis of morphological data. Washington, DC: Smithsonian Institution Press; 2000. pp. 1–19. [Google Scholar]
- Hinchliffe JR. ‘One, two, three’ or ‘two, three, four’: an embryologist’s view of the homologies of the digits and carpus of modern birds. In: Hecht MK, Ostrom JH, Viohl G, Wellnhofer P, editors. The beginnings of birds. Willibaldsburg, Germany: Freunde des Jura-Museumd Eichstätt; 1985. pp. 141–147. [Google Scholar]
- Ho SYW, Phillips MJ, Cooper A, Drummond AJ. Time dependency of molecular rate estimates and systematic overestimation of recent divergence times. Molecular Biology and Evolution. 2005;22:1561–1568. doi: 10.1093/molbev/msi145. [DOI] [PubMed] [Google Scholar]
- Ho CY-K, Prager E, Wilson AC, Osuga DT, Feeney RE. Penguin evolution: comparisons demonstrate phylogenetic relationship to flying aquatic birds. Journal of Molecular Evolution. 1976;8:271–282. doi: 10.1007/BF01731000. [DOI] [PubMed] [Google Scholar]
- Hoff KM. A comparative study of the appendicular muscles of Strigiformes and Caprimulgiformes. Pullman, WA: Washington State University; 1966. [Google Scholar]
- Holdaway RN. An exploratory phylogenetic analysis of the genera of the Accipitridae, with notes on the biogeography of the family. In: Meyburn B-U, Chancellor RD, editors. Raptor conservation today. London, UK: World Working Group on Birds of Prey and Owls; 1994. pp. 601–649. [Google Scholar]
- Holman JA. Osteology of the living and fossil New World quails (Aves, Galliformes) Florida State Museum Bulletin (Biological Series) 1961;6:131–233. [Google Scholar]
- Holmquist R, Pearl D, Jukes TH. Non-uniform molecular divergence: the quantitative evolutionary analysis of genes and messenger RNAs under selective structural constraints. In: Goodman M, editor. Macromolecular sequences in systematic and evolutionary biology. New York: Plenum Press; 1983. pp. 281–315. [Google Scholar]
- Holtz TR., Jr A new phylogeny of the carnivorous dinosaurs. Gaia. 1998;15:5–61. [Google Scholar]
- Homberger DG. Functional morphology and evolution of the feeding apparatus in parrots, with secial reference to the Pesquet’s parrot, Psttrichas fuligidus (Lesson) In: Pasquier RF, editor. Conservation of New World parrots. Washington, DC: Smithsonian Institution Press; 1980. pp. 471–485. [Google Scholar]
- Hope S. The Mesozoic radiation of Neornithes. In: Chiappe LM, Witmer LM, editors. Mesozoic birds: above the heads of dinosaurs. Berkeley, CA: University of California Press; 2002. pp. 339–388. [Google Scholar]
- Houde PW. Critical evaluation of DNA hybridization studies in avian systematics. Auk. 1987;104:17–32. [Google Scholar]
- Houde PW. Palaeognathous birds form the early Tertiary of the Northern Hemisphere. Publications of the Nuttall Ornithological Club. 1988;22:1–148. [Google Scholar]
- Houde PW. Evolution of the Heliornithidae: reciprocal illumination by morphology, biogeography and DNA hybridization (Aves: Gruiformes) Cladistics. 1994;10:1–19. [Google Scholar]
- Houde PW, Cooper A, Leslie E, Strand AE, Montaño GA. Phylogeny and evolution of 12S rDNA in Gruiformes (Aves) In: Mindell DP, editor. Avian molecular evolution and systematics. San Diego, CA: Academic Press; 1997. pp. 121–158. [Google Scholar]
- Houde PW, Haubold H. Palaeotis weigelti restudied: a small Middle Eocene ostrich (Aves: Struthioniformes) Palaeovertebrata. 1987;17:27–42. [Google Scholar]
- Houde PW, Olson SL. Palaeognathous carinate birds from the Early Tertiary of North America. Science. 1981;214:1236–1237. doi: 10.1126/science.214.4526.1236. [DOI] [PubMed] [Google Scholar]
- Houde PW, Olson SL. A radiation of coly-like birds from the Eocene of North America (Aves: Sandcoleiformes new order) Natural History Museum of Los Angeles County, Science Series. 1992;36:137–160. [Google Scholar]
- Howard H. Gigantic ‘toothed’ marine birds from the Miocene of California. Bulletin of the Department of Geology of the Santa Barbara Museum of Natural History. 1957;1:1–23. [Google Scholar]
- Hudson GE, Lanzillotti PJ. Muscles of the pectoral limb in galliform birds. American Midland Naturalist. 1964;71:1–113. [Google Scholar]
- Hudson GE, Lanzillotti PJ, Edwards GD. Muscles of the pelvic limb in galliform birds. American Midland Naturalist. 1959;61:1–67. [Google Scholar]
- Hudson GE, Parker RA, Vanden Berge J, Lanzillotti PJ. A numerical analysis of the modifications of the appendicular muscles in various genera of gallinaceous birds. American Midland Naturalist. 1966;76:1–73. [Google Scholar]
- Huelsenbeck JP. Tree-length distribution skewness: an indicator of phylogenetic information. Systematic Zoology. 1991;40:257–270. [Google Scholar]
- Huelsenbeck JP, Bull JJ, Cunningham CW. Combining data in phylogenetic analysis. Trends in Ecology and Evolution. 1996;11:152–158. doi: 10.1016/0169-5347(96)10006-9. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Larget B, Miller RE, Ronquist F. Potential applications and pitfalls of Bayesian inference of phylogeny. Systematic Biology. 2002;51:673–688. doi: 10.1080/10635150290102366. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Nielsen R, Bollback JP. Stochastic mapping of morphological characters. Systematic Biology. 2003;52:131–158. doi: 10.1080/10635150390192780. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Rannala B. Using stratigraphic information in phylogenetics. In: Wiens JJ, editor. Phylogenetic analysis of morphological data. Washington, DC: Smithsonian Institution Press; 2000. pp. 165–191. [Google Scholar]
- Hughes JM. Phylogenetic analysis of the Cuculidae (Aves, Cuculiformes) using behavioral and ecological characters. Auk. 1996;113:10–22. [Google Scholar]
- Hughes JM. Monophyly and phylogeny of cuckoos (Aves, Cuculidae) inferred from osteological characters. Zoological Journal of the Linnean Society. 2000;130:263–307. [Google Scholar]
- Hughes JM, Baker AJ. Phylogenetic relationships of the enigmatic hoatzin (Opisthocomus hoazin) resolved using mitochondrial and nuclear gene sequences. Molecular Biology and Evolution. 1999;16:1300–1307. doi: 10.1093/oxfordjournals.molbev.a026220. [DOI] [PubMed] [Google Scholar]
- Humphries CJ, Parenti LR. Cladistic biogeographyInterpreting patterns of plant and animal distributions. 2. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- Hutchinson JR. The evolution of pelvic osteology and soft tissues on the line to extant birds (Neornithes) Zoological Journal of the Linnean Society. 2001a;131:123–168. [Google Scholar]
- Hutchinson JR. The evolution of femoral osteology and soft tissues on the line to extant birds (Neornithes) Zoological Journal of the Linnean Society. 2001b;131:169–197. [Google Scholar]
- Hutchinson JR. The evolution of hindlimb tendons and muscles on the line to crown-group birds. Comparative Biochemistry and Physiology (Part A) 2002;133:1051–1086. doi: 10.1016/s1095-6433(02)00158-7. [DOI] [PubMed] [Google Scholar]
- Huxley TH. On the classification of birds; and on the taxonomic value of the modifications of certain of the cranial bones observable in that class. Proceedings of the Zoological Society of London. 1867;1867:415–472. [Google Scholar]
- Insua DR, Ruggeri F, editors. Robust Bayesian analysis. New York: Springer; 2000. [Google Scholar]
- Irestedt M, Fjeldså J, Johansson US, Ericson PGP. Systematic relationships and biogeography of the tracheophone suboscines (Aves: Passeriformes) Molecular Phylogenetics and Evolution. 2002;23:499–512. doi: 10.1016/s1055-7903(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Irestedt M, Johansson US, Parsons TJ, Ericson PGP. Phylogeny of major lineages of suboscines (Passeriformes) analysed by nuclear DNA sequence data. Journal of Avian Biology. 2001;32:15–25. [Google Scholar]
- Iwabe N, Hara Y, Kumazawa Y, Shibamoto K, Saito Y, Miyata T, Kotah K. Sister group relationship of turtles to the bird-crocodilian clade revealed by nuclear DNA-coded proteins. Molecular Biology and Evolution. 2004;22:810–813. doi: 10.1093/molbev/msi075. [DOI] [PubMed] [Google Scholar]
- Jablonski D. Micro- and macroevolution: scale and hierarchy in evolutionary biology and paleobiology. Paleobiology. 2000;26(Suppl.):15–52. [Google Scholar]
- Jablonski D. Mass extinctions and macroevolution. Paleobiology. 2005;31(Suppl.):192–210. [Google Scholar]
- James HF. The osteology and phylogeny of the Hawaiian finch radiation (Fringillidae: Drepanidini), including extinct taxa. Zoological Journal of the Linnean Society. 2004;141:207–255. [Google Scholar]
- James HF. Palaeogene fossils and the radiation of fossil birds. Auk. 2005;122:1049–1054. [Google Scholar]
- Janke A, Arnason U. The complete mitochondrial genome of Alligator mississippiensis and the separation between Recent Archosauria (birds and crocodiles) Molecular Biology and Evolution. 1997;14:1266–1272. doi: 10.1093/oxfordjournals.molbev.a025736. [DOI] [PubMed] [Google Scholar]
- Jeffrey JE, Richarson MK, Coates MI, Bininda-Emonds ORP. Analyzing developmental sequences within a phylogenetic framework. Systematic Biology. 2002;51:478–491. doi: 10.1080/10635150290069904. [DOI] [PubMed] [Google Scholar]
- Jehl JR., Jr Relationships in the Charadrii (shorebirds): a taxonomic study based on color patterns of the downy young. Memoirs of the San Diego Society of Natural History. 1968;3:1–54. [Google Scholar]
- Jehl JR., Jr The color patterns of downy young ratites and tinamous. Transactions of the San Diego Society of Natural History. 1971;16:292–301. [Google Scholar]
- Jenkin PM. The filter-feeding and food of flamingoes (Phoenicopteri) Philosophical Transactions of the Royal Society of London (Series B) 1957;240:401–493. [Google Scholar]
- Jenner RA. Accepting partnership by submission? Morphological phylogenetics in a molecular millenium. Systematic Biology. 2004a;53:333–342. doi: 10.1080/10635150490423962. [DOI] [PubMed] [Google Scholar]
- Jenner RA. When molecules and morphology clash: reconciling conflicting phylogenies of the Metazoa by considering secondary character loss. Evolution and Development. 2004b;6:372–378. doi: 10.1111/j.1525-142X.2004.04045.x. [DOI] [PubMed] [Google Scholar]
- Johansson US, Ericson PGP. Molecular support for a sister group relationship between Pici and Galbulae (Piciformes sensu Wetmore 1960) Journal of Avian Biology. 2003;34:185–197. [Google Scholar]
- Johansson US, Ericson PGP. A re-evaluation of basal phylogenetic relationships within trogons (Aves: Trogonidae) Journal of Zoological Systematics and Evolutionary Research. 2004;43:166–173. [Google Scholar]
- Johansson US, Parsons TJ, Irestedt M, Ericson PGP. Clades within the ‘higher land birds’, evaluated by nuclear DNA sequences. Journal of Zoological Systematics and Evolutionary Research. 2001;39:37–51. [Google Scholar]
- Johnsgard PA. Hybridization in the Anatidae and its taxonomic implications. Condor. 1960;62:25–33. [Google Scholar]
- Johnsgard PA. The taxonomy of the Anatidae – a behavioural analysis. Ibis. 1961;103:71–85. [Google Scholar]
- Johnsgard PA. Behavioral isolating mechanisms in the family Anatidae. In: Sibley CG, editor. Proceedings of the 13th International Ornithological Congress. I. Washington, DC: American Ornithologists’ Union; 1963. pp. 531–543. [Google Scholar]
- Johnson KP. Taxon sampling and the phylogenetic position of Passeriformes: evidence from 916 avian cytochrome b sequences. Systematic Biology. 2001;50:128–136. [PubMed] [Google Scholar]
- Johnson NK, Cicero C. New mitochondrial DNA data affirm the importance of Pleistocene speciation in North American birds. Evolution. 2004;58:1122–1130. doi: 10.1111/j.0014-3820.2004.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Clayton DH. Nuclear and mitochondrial genes contain similar phylogenetic signal for pigeons and doves (Aves: Columbiformes) Molecular Phylogenetics and Evolution. 2000a;14:141–151. doi: 10.1006/mpev.1999.0682. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Clayton DH. A molecular phylogeny of the dove genus Zenaida: mitochondrial and nuclear DNA sequences. Condor. 2000b;102:864–870. [Google Scholar]
- Johnson KP, Goodman SM, Lanyon SM. A phylogenetic study of the Malagasy couas with insights into cuckoo relationships. Molecular Phylogenetics and Evolution. 2000;14:436–444. doi: 10.1006/mpev.1999.0715. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Sorenson MD. Comparing molecular evolution in two mitochondrial protein coding genes (cytochrome b and ND4) in the dabbling ducks (Tribe: Anatini) Molecular Phylogenetics and Evolution. 1998;10:82–94. doi: 10.1006/mpev.1997.0481. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Sorenson MD. Phylogeny and biogeography of dabbling ducks (genus Anas): a comparison of molecular and morphological characters. Auk. 1999;116:792–805. [Google Scholar]
- Johnson KP, Weckstein JD, Witt CC, Faucett RC, Moyle RG. The perils of using host relationships in parasite taxonomy: phylogeny of the Degeeriella complex. Molecular Phylogenetics and Evolution. 2002;32:150–157. doi: 10.1016/S1055-7903(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Jollie MT. Are the Falconiformes a monophyletic group? Ibis. 1953;95:369–371. [Google Scholar]
- Jollie MT. A contribution to the morphology and phylogeny of the Falconiformes. Evolutionary Theory. 1976;1:285–298. [Google Scholar]
- Jollie MT. A contribution to the morphology and phylogeny of the Falconiformes (part II) Evolutionary Theory. 1977a;2:115–208. [Google Scholar]
- Jollie MT. A contribution to the morphology and phylogeny of the Falconiformes (part III) Evolutionary Theory. 1977b;2:209–300. [Google Scholar]
- Jollie MT. A contribution to the morphology and phylogeny of the Falconiformes (part IV) Evolutionary Theory. 1977c;3:1–141. [Google Scholar]
- de Kloet RS, de Kloet SR. The evolution of the spindlin gene in birds: independent cessation of the recombination of sex chromosomes at the spindin gene in neognathous birds and tinamous, a palaeognathous avian family. Genetica. 2003;119:333–342. doi: 10.1023/b:gene.0000003842.72339.df. [DOI] [PubMed] [Google Scholar]
- de Kloet RS, de Kloet SR. The evolution of the spindlin gene in birds: sequence analysis of an intron of the spindlin W and Z gene reveals four major divisions of the Psittaciformes. Molecular Phylogenetics and Evolution. 2005;36:706–721. doi: 10.1016/j.ympev.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Kadi F, Mouchiroud D, Sabeur G, Bernardi G. The compositional patterns of the avian genomes and their evolutionary implications. Journal of Molecular Evolution. 1993;37:544–551. [Google Scholar]
- Källersjö M, Albert VA, Farris JS. Homoplasy increases phylogenetic structure. Cladistics. 1999;15:91–93. [Google Scholar]
- Källersjö M, Farris JS, Kluge AG, Bolt C. Skewness and permutation. Cladistics. 1992;8:275–287. doi: 10.1111/j.1096-0031.1992.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Kardong KV, Zweers GA. Vertebrate evolution at geological boundaries: introduction. Zoology. 1997;100:121–127. [Google Scholar]
- Karhu A. Morphological divergence within the order Apodiformes as revealed by the structure of the humerus. Contributions to Science of the Natural History Museum of Los Angeles County. 1992;36:379–384. [Google Scholar]
- Karhu A. A new genus and species of the family Jungornithidae (Apodiformes) from the late Eocene of the northern Caucasus, with comments on the ancestry of hummingbirds. Smithsonian Contributions to Paleobiology. 1999;89:207–216. [Google Scholar]
- Karhu A. Convergence in the shoulder joint structure of hummingbirds, galliforms and tinamous. In: Kurochkin E, Rakchimov I, editors. Achievements and problems of ornithology of northern Eurasia at a boundary of centuries. Kazan, Tatarstan: Magarif Publishers; 2001. pp. 118–132. [Google Scholar]
- Kearney M. Fragmentary taxa, missing data, and ambiguity: mistaken assumptions and conclusions. Systematic Biology. 2002;51:369–381. doi: 10.1080/10635150252899824. [DOI] [PubMed] [Google Scholar]
- Kearney M, Clark JM. Problems due to missing data in phylogenetic analyses including fossils: a critical review. Journal of Vertebrate Paleontology. 2003;23:263–274. [Google Scholar]
- Kemp A. The Hornbills: Bucerotiformes. Oxford, UK: Oxford University Press; 1995. [Google Scholar]
- Kennedy M, Gray RD, Spencer HG. The phylogenetic relationships of the shags and cormorants: Can sequence data resolve a disagreement between behavior and morphology? Molecular Phylogenetics and Evolution. 2000;17:345–359. doi: 10.1006/mpev.2000.0840. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Holland BR, Gray RD, Spencer HG. Untangling long branches: identifying conflicting phylogenetic signals using spectral analysis, neighbor-net, and consensus networks. Systematic Biology. 2005;54:620–633. doi: 10.1080/106351591007462. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Page RDM. Seabird supertrees: combining partial estimates of procellariform phylogeny. Auk. 2002;119:88–108. [Google Scholar]
- Kennedy M, Spencer HG. Phylogenies of the frigatebirds (Fregatidae) and tropicbirds (Phaethontidae), two divergent groups of the traditional order Pelecaniformes, inferred from mitochondrial DNA sequences. Molecular Phylogenetics and Evolution. 2000;31:31–38. doi: 10.1016/j.ympev.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Spencer HG. Phylogenies of the frigatebirds (Fregatidae) and tropicbirds (Phaethontidae), two divergent groups of the traditional order Pelecaniformes, inferred from mitochondrial DNA sequences. Molecular Phylogenetics and Evolution. 2004;31:31–38. doi: 10.1016/j.ympev.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Spencer HG, Gray RD. Hop, step and gape: Do the social displays of the Pelecaniformes reflect phylogeny? Animal Behaviour. 1996;51:273–291. [Google Scholar]
- Kessler LG, Avise JC. A comparative description of mitochondrial DNA differentiation in selected avian and other vertebrate genera. Molecular Biology and Evolution. 1985;2:109–125. doi: 10.1093/oxfordjournals.molbev.a040339. [DOI] [PubMed] [Google Scholar]
- Kim J. General inconsistency conditions for maximum parsimony: effects of branch lengths and increasing numbers of taxa. Systematic Biology. 1996;45:363–374. [Google Scholar]
- Kim J. What do we know about the performance of estimators for large phylogenies? Trends in Ecology and Evolution. 1998;13:25–26. doi: 10.1016/s0169-5347(97)01270-6. [DOI] [PubMed] [Google Scholar]
- Kimball RT, Braun EL, Zwartjes PW, Crowe TM, Ligon JD. A molecular phylogeny of the pheasants and partridges suggests that these lineages are not monophyletic. Molecular Phylogenetics and Evolution. 1999;11:38–54. doi: 10.1006/mpev.1998.0562. [DOI] [PubMed] [Google Scholar]
- Kimmel M, Axelrod DE. Branching processes in biology. New York: Springer; 2002. [Google Scholar]
- Kirchman JJ, Hackett SJ, Goodman SM, Bates JM. Phylogeny and systematics of ground rollers (Brachypteraciidae) of Madagascar. Auk. 2001;118:849–863. [Google Scholar]
- Klassen GJ, Mooi RD, Kicke A. Consistency indices and random data. Systematic Zoology. 1991;40:446–457. [Google Scholar]
- Klicka J, Zink RM. The importance of recent ice ages in speciation: a failed paradigm. Science. 1997;277:1666–1669. [Google Scholar]
- Klingenberg CP. Heterochrony and allometry: the analysis of evolutionary change in ontogeny. Biological Reviews (Cambridge) 1998;73:79–123. doi: 10.1017/s000632319800512x. [DOI] [PubMed] [Google Scholar]
- Kluge AG. Testability and the refutation and corroboration of cladistic hypotheses. Cladistics. 1997a;13:81–96. doi: 10.1111/j.1096-0031.1997.tb00242.x. [DOI] [PubMed] [Google Scholar]
- Kluge AG. Sophisticated falsification and research cycles: consequences for differential character weighting in phylogenetic systematics. Zoologica Scripta. 1997b;26:349–360. [Google Scholar]
- Kluge AG. The science of phylogenetic systematics: explanation, prediction, and test. Cladistics. 1999;14:151–158. doi: 10.1111/j.1096-0031.1999.tb00279.x. [DOI] [PubMed] [Google Scholar]
- Kluge AG, Wolf AJ. Cladistics: What’s in a word? Cladistics. 1993;9:183–199. doi: 10.1111/j.1096-0031.1993.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Kochmer JP, Wagner RH. Why are there so many kinds of passerine birds? Because they are small. A reply to Raikow. Systematic Zoology. 1988;37:68–69. [Google Scholar]
- König C. Zur systematischen Stellung der Neuweltgeier (Cathartidae) Journal für Ornithologie. 1982;123:259–267. [Google Scholar]
- Kornegay JR, Kocher TD, Williams LA, Wilson AC. Pathways of lysozyme evolution inferred from the sequences of cytochrome b in birds. Journal of Molecular Evolution. 2003;37:367–379. doi: 10.1007/BF00178867. [DOI] [PubMed] [Google Scholar]
- Kornegay JR, Schilling JW, Wilson AC. Molecular adaptation of a leaf-eating bird: stomach lysozyme of the hoatzin. Molecular Phylogenetics and Evolution. 1994;11:921–928. doi: 10.1093/oxfordjournals.molbev.a040173. [DOI] [PubMed] [Google Scholar]
- Kozlova EB. Trends of evolution in waders of the family Charadriidae (on the base of a study of the skull structure) Trudy Zoological Institute of the Academy of Science USSR. 1961;24:183–212. [Google Scholar]
- Kraus F. An empirical evaluation of the use of the ontogeny polarization criterion in phylogenetic inference. Systematic Zoology. 1978;37:106–141. [Google Scholar]
- Krawjewski C, King DG. Molecular divergence and phylogeny: rates and patterns of cytochrome b evolution in cranes. Molecular Biology and Evolution. 1996;13:21–30. doi: 10.1093/oxfordjournals.molbev.a025558. [DOI] [PubMed] [Google Scholar]
- Kreitman M, Antezana M. The population and evolutionary genetics of codon bias. In: Singh RS, Kribas CB, editors. Evolutionary genetics: from molecules to morphology. Cambridge, UK: Cambridge University Press; 2000. pp. 82–101. [Google Scholar]
- Kristoffersen AV. An early Paleogene trogon (Aves: Trogoniformes) from the Fur Formation, Denmark. Journal of Vertebrate Paleontology. 2001;23:661–666. [Google Scholar]
- Ksepka DT, Bertelli S, Giannini NP. The phylogeny of the living and fossil Sphenisciformes (penguins) Cladistics. 2006;22:412–441. [Google Scholar]
- Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- Kumazawa Y, Nishida M. Variations in mitochondrial tRNA gene organization of reptiles as phylogenetic markers. Molecular Biology and Evolution. 1995;12:759–772. doi: 10.1093/oxfordjournals.molbev.a040254. [DOI] [PubMed] [Google Scholar]
- Kurochkin EN. Cretaceous birds of Mongolia and their significance for the study of the phylogeny of Class Aves. In: Kurochkin EN, editor. Trudy Sovmestnoi Sovetsko-Monglo’skoi Paleontologischeskoi Ekspeditsii. 1988. pp. 33–42. Fossil reptiles and birds of Mongolia. [Google Scholar]
- Kurochkin EN, Dyke GJ, Karhu AA. A new presbyornithid bird (Aves, Anseriformes) from the late Cretaceous of southern Mongolia. American Museum Novitates. 2002;3386:1–11. [Google Scholar]
- Kuroda N. On the classification and phylogeny of the order Tubinares, particularly the shearwaters (Puffinus), with special considerations on their osteology and habit differentiation (Aves) Tokyo: Herald Company; 1954. [Google Scholar]
- Lake JA. Phylogenetic inference: How much evolutionary history is knowable? Molecular Biology and Evolution. 1997;14:213–219. doi: 10.1093/oxfordjournals.molbev.a025757. [DOI] [PubMed] [Google Scholar]
- Lambrecht K. Handbuch der Palaeornithologie. Berlin: Gebrüder Borntraeger; 1933. [Google Scholar]
- Lang C. Das Cranium der Ratiten mit besonderer Berücksichtigung von Struthio camelus. Zeitschrift für Wissenschaftliche Zoologie. 1956;159:165–224. [Google Scholar]
- Lanyon SM. Phylogeny and classification of birds: a study in molecular evolution [review] Condor. 1992;94:304–307. [Google Scholar]
- Lanyon SM, Hall JG. Reexamination of barbet monophyly using mitochondrial-DNA sequence data. Auk. 1994;111:389–397. [Google Scholar]
- Lanyon SM, Zink RM. Genetic variation in piciform birds: monophyly and generic and familial relationships. Auk. 1987;104:724–732. [Google Scholar]
- Larhammar D, Milner RJ. Phylogenetic relationship of birds with crocodiles and mammals, as deduced from protein sequences. Molecular Biology and Evolution. 1989;6:693–696. doi: 10.1093/oxfordjournals.molbev.a040579. [DOI] [PubMed] [Google Scholar]
- Lauder GV. Homology, form, and function. In: Hall BK, editor. Homology: the hierarchical basis of comparative biology. San Diego, CA: Academic Press; 1994. pp. 151–196. [Google Scholar]
- Laurin M, Reisz R. New perspective on tetrapod phylogeny. In: Sumida SS, Martin KLM, editors. Amniote origins: completing the transition to land. San Diego, CA: Academic Press; 1997. pp. 1–59. [Google Scholar]
- Leach WE. Synopsis of the contents of the British Museum. 17. London: British Museum (Natural History); 1820. Eleventh room. [Google Scholar]
- Lecointre G, Philippe H, Ván Lé HL, Guyader HL. Species sampling has a major impact on phylogenetic inference. Molecular Phylogenetics and Evolution. 1993;2:205–224. doi: 10.1006/mpev.1993.1021. [DOI] [PubMed] [Google Scholar]
- Lecointre G, Philippe H, Ván Lé HL, Guyader HL. How many nucleotides are required to resolve a phylogenetic problem? The use of a new statistical method applicable to available sequences. Molecular Phylogenetics and Evolution. 1994;3:292–309. doi: 10.1006/mpev.1994.1037. [DOI] [PubMed] [Google Scholar]
- Lee MSY. Molecules, morphology, and phylogeny: a response to Hedges and Maxson. Molecular Phylogenetics and Evolution. 1997;7:394–395. doi: 10.1006/mpev.1996.0396. [DOI] [PubMed] [Google Scholar]
- Lee MSY. Molecular phylogenies become functional. Trends in Ecology and Evolution. 1999;14:177–178. doi: 10.1016/s0169-5347(99)01603-1. [DOI] [PubMed] [Google Scholar]
- Lee PLM, Clayton DH, Griffiths R, Page RDM. Does behavior reflect phylogeny in swiftlets (Aves: Apodidae)? A test using cytochrome b mitochondrial DNA sequences. Proceedings of the National Academy of Science USA. 1996;93:7091–7096. doi: 10.1073/pnas.93.14.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MSY, Doughty P. The relationship between evolutionary theory and phylogenetic analysis. Biological Reviews (Cambridge) 1997;72:471–495. doi: 10.1017/s0006323197005070. [DOI] [PubMed] [Google Scholar]
- Lee K, Feinstein J, Cracraft J. Phylogenetic relationships of the ratite birds: resolving conflicts between molecular and morphological data sets. In: Mindell DP, editor. Avian molecular evolution and systematics. New York: Academic Press; 1997. pp. 173–211. [Google Scholar]
- Lee MSY, Hugall AF. Model type, implicit weighting, and model averaging in phylogenetics. Molecular Phylogenetics and Evolution. 2005;38:847–857. doi: 10.1016/j.ympev.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Leeton PRJ, Christidis L, Westerman M, Boles WE. Molecular phylogenetic affinities of the night parrot (Geopsittacus occidentalis) and the ground parrot (Pezoporus wallicus) Auk. 1994;111:833–843. [Google Scholar]
- Leonard L, Dyke GJ, Van Tuinen M. A new specimen of the fossil palaeognath Lithornis from the Lower Eocene of Denmark. American Museum Novitates. 2005;3491:1–11. [Google Scholar]
- Lerner HRL, Mindell DP. Phylogeny of eagles, Old World vultures, and other Accipitridae based on nuclear and mitochondrial DNA. Molecular Phylogenetics and Evolution. 2005;37:327–346. doi: 10.1016/j.ympev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Levinton J. Genetics, paleontology, and macroevolution. Cambridge, UK: Cambridge University Press; 1988. [Google Scholar]
- Lewis PO. A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology. 2001;50:913–925. doi: 10.1080/106351501753462876. [DOI] [PubMed] [Google Scholar]
- Ligon JD. Relationships of the cathartid vultures. University of Michigan, Museum of Zoology Occasional Paper. 1967;651:1–26. [Google Scholar]
- Ligon JD. The evolution of avian breeding systems. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- Lipscomb DL. Parsimony, homology and the analysis of multistate characters. Cladistics. 1992;8:45–65. doi: 10.1111/j.1096-0031.1992.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Livezey BC. A phylogenetic analysis of Recent anseriform genera using morphological characters. Auk. 1986;105:681–698. [Google Scholar]
- Livezey BC. Morphometrics of flightlessness in the Alcidae. Auk. 1988;105:681–698. [Google Scholar]
- Livezey BC. Morphometric patterns in Recent and fossil penguins (Aves, Sphenisciformes) Journal of Zoology (London) 1989a;219:269–307. doi: 10.1111/j.1469-7998.1989.tb02582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livezey BC. Flightlessness in grebes (Aves, Podicipedidae): its independent evolution in three genera. Evolution. 1989b;43:29–54. doi: 10.1111/j.1558-5646.1989.tb04205.x. [DOI] [PubMed] [Google Scholar]
- Livezey BC. Phylogenetic relationships and incipient flightlessness of the extinct Auckland Islands merganser. Wilson Bulletin. 1989c;101:410–435. [Google Scholar]
- Livezey BC. Evolutionary morphology of flightlessness in the Auckland Islands Teal. Condor. 1990;92:639–673. [Google Scholar]
- Livezey BC. A phylogenetic analysis and classification of Recent dabbling ducks (Tribe Anatini) based on comparative morphology. Auk. 1991;108:471–508. [Google Scholar]
- Livezey BC. Morphological corollaries and ecological implications of flightlessness in the kakapo (Psittaciformes: Strigops habroptilus) Journal of Morphology. 1992a;213:105–145. doi: 10.1002/jmor.1052130108. [DOI] [PubMed] [Google Scholar]
- Livezey BC. Flightlessness in the Galápagos cormorant (Compsohalieus[Nannopterum]harrisi): heterochrony, giantism, and specialization. Zoological Journal of the Linnean Society. 1992b;105:155–224. [Google Scholar]
- Livezey BC. An ecomorphological review of the dodo (Raphus cucullatus) and solitaire (Pezophaps solitaria), flightless Columbiformes of the Mascarene Islands. Journal of Zoology (London) 1993;230:247–292. [Google Scholar]
- Livezey BC. The carpometacarpus of Aptornis[sic] Notornis. 1994;41:51–60. [Google Scholar]
- Livezey BC. Heterochrony and the evolution of avian flightlessness. In: McNamara KJ, editor. Evolutionary change and heterochrony. Chichester, UK: J. Wiley; 1995a. pp. 169–193. [Google Scholar]
- Livezey BC. A phylogenetic analysis of the whistling and white-backed ducks (Anatidae: Dendrocygninae) using morphological characters. Annals of Carnegie Museum. 1995b;64:65–97. [Google Scholar]
- Livezey BC. Phylogeny and evolutionary ecology of modern seaducks (Anatidae: Mergini) Condor. 1995c;97:233–255. [Google Scholar]
- Livezey BC. Phylogeny and comparative ecology of stiff-tailed Ducks (Anatidae: Oxyurini) Wilson Bulletin. 1995d;107:214–234. [Google Scholar]
- Livezey BC. A phylogenetic analysis of the geese and swans (Anseriformes: Anserinae), including selected fossil species. Systematic Biology. 1996a;45:415–450. [Google Scholar]
- Livezey BC. A phylogenetic reassessment of the tadornine-anatine divergence (Aves: Anseriformes, Anatidae) Annals of Carnegie Museum. 1996b;65:27–88. [Google Scholar]
- Livezey BC. A phylogenetic analysis of modern pochards (Anatidae: Aythyini) Auk. 1996c;113:74–93. [Google Scholar]
- Livezey BC. A phylogenetic analysis of basal Anseriformes, the fossil Presbyornis, and the interordinal relationships of waterfowl. Zoological Journal of the Linnean Society. 1997a;121:361–428. [Google Scholar]
- Livezey BC. An annotated phylogenetic classification of waterfowl (Aves: Anseriformes), including selected fossil species. Annals of Carnegie Museum. 1997b;67:457–496. [Google Scholar]
- Livezey BC. A phylogenetic analysis of modern shelducks and sheldgeese (Anatidae, Tadornini) Ibis. 1997c;139:51–66. [Google Scholar]
- Livezey BC. Erratum – A phylogenetic analysis of basal Anseriformes, the fossil Presbyornis, and the interordinal relationships of waterfowl. Zoological Journal of the Linnean Society. 1998a;124:397–398. [Google Scholar]
- Livezey BC. A phylogenetic analysis of the Gruiformes (Aves) based on morphological characters, with an emphasis on the rails (Rallidae) Philosophical Transactions of the Royal Society (Series B) 1998b;353:2077–2151. [Google Scholar]
- Livezey BC. Avian spirit collections: attitudes, importance and prospects. Bulletin of the British Ornithologists’ Club. 2003a;123(Suppl.):35–51. [Google Scholar]
- Livezey BC. Evolution of flightlessness in rails (Gruiformes: Rallidae): phylogenetic, ecomorphological, and ontogenetic perspectives. Ornithological Monographs. 2003b;53:1–654. [Google Scholar]
- Livezey BC. Millenial status report as debate wanes [review] Science. 2003c;299:1664–1665. [Google Scholar]
- Livezey BC, Humphrey PC. Flightlessness in steamer-ducks (Anatidae: Tachyeres): its morphological bases and probable evolution. Evolution. 1986;40:540–558. doi: 10.1111/j.1558-5646.1986.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Livezey BC, Martin LD. The systematic position of the Miocene anatid Anas[?]blanchardi Milne-Edwards. Journal of Vertebrate Paleontology. 1988;8:196–211. [Google Scholar]
- Livezey BC, Zusi RL. Higher-order phylogenetics of modern Aves based on comparative anatomy. Netherlands Journal of Zoology. 2001;51:179–206. [Google Scholar]
- Livezey BC, Zusi RL. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy: I. – Methods and characters. Bulletin of the Carnegie Museum of Natural History. 2006;37:1–556. [Google Scholar]
- Lockhart PJ, Steel MA, Hendy MD, Penny D. Recovering evolutionary trees under a more realistic model of sequence evolution. Molecular Biology and Evolution. 1994;11:605–612. doi: 10.1093/oxfordjournals.molbev.a040136. [DOI] [PubMed] [Google Scholar]
- Lowe PR. On the significance of certain characters in some charadriine genera, with a provisional classification of the order Charadriiformes. Ibis. 1922;64:475–495. [Google Scholar]
- Lowe PR. Notes on the systematic position of Ortyxelus, together with some remarks on the relationships of the turnicomorphs and the position of the seed-snipe (Thinocoridæ) and sand-grouse. Ibis. 1923;65:276–299. [Google Scholar]
- Lowe PR. On the anatomy and systematic position of the Madagascan bird Mesites (Mesœnas), with a preliminary note on the osteology of Monias. Proceedings of the Zoological Society of London. 1924;1924:1131–1152. [Google Scholar]
- Lowe PR. (1) On the systematic position of the Jacanidæ (Jaçanás), with some notes on a hitherto unconsidered anatomical character of apparent taxonomic value, (2) a preliminary note on the classification of the Charadriiformes (Limicolæ and Laro-Limicolæ) based on this character, viz., the morphology of the quadrato-tympanic articulation. Ibis. 1925;67:132–147. [Google Scholar]
- Lowe PR. Studies and observations bearing on the phylogeny of the ostrich and its allies. Proceedings of the Zoological Society of London. 1928;1928:185–247. [Google Scholar]
- Lowe PR. On the relationships of the Æpyornithes to the other Struthiones as revealed by a study of the pelvis of Mullerornis. Ibis. 1930;72:470–490. [Google Scholar]
- Lowe PR. An anatomical review of the ‘waders’ (Telmatomorphæ), with special reference to the families, subfamilies, and genera within the suborders Limicolæ, Grui-Limicolæ and Lari-Limicolæ. Ibis. 1931a;73:712–771. [Google Scholar]
- Lowe PR. On the relations of the Gruimorphæ to the Charadriimorphæ and Rallimorphæ, with special reference to the taxonomic position of Rostratulidæ, Jacanidæ, and Burhinidæ (Œdicnemidæ olim), with a suggested new order (Telmatomorphæ) Ibis. 1931b;73:491–534. [Google Scholar]
- Lowe PR. On the primitive characters of the penguins, and their bearing on the phylogeny of birds. Proceedings of the Zoological Society of London. 1933;102:483–541. [Google Scholar]
- Lowe PR. On the relationships of the Struthiones to the dinosaurs and the rest of the avian class, with special reference to the position of Archaeopteryx. Ibis. 1935;77:298–429. [Google Scholar]
- Lowe PR. On the systematic position of the swifts (Suborder Cypseli) and hummingbirds (Suborder Trochili), with special reference to their relation to the order Passeriformes. Transactions of the Zoological Society of London. 1939;24:307–348. [Google Scholar]
- Lowe PR. Some additional anatomical factors bearing on the phylogeny of the Struthiones. Proceedings of the Zoological Society of London. 1942;112:1–20. [Google Scholar]
- Lowe PR. Some notes on the anatomical differences obtaining between the Cuculidæ and the Musophagidæ, with special reference to the specialization of the œsophagus in Cuculus canorus Linnæus. Ibis. 1943;85:490–515. [Google Scholar]
- Lowe PR. Some additional comments on the phylogeny of the Struthiones. Ibis. 1944a;86:37–42. [Google Scholar]
- Lowe PR. An analysis of the characters of Archæopteryx and Archæornis. Were they reptiles or birds? Ibis. 1944b;86:517–543. [Google Scholar]
- Lowe PR. On the systematic position of the woodpeckers (Pici), honey-guides (Indicator), hoopoes and others. Ibis. 1946;88:103–127. [Google Scholar]
- Lowe PR. What are the Coraciiformes? Ibis. 1948;90:572–582. [Google Scholar]
- Lucchini V, Hoglund J, Klaus S, Swenson J, Randi E. Historical biogeography and mitochondrial DNA phylogeny of grouse and ptarmigan. Molecular Phylogenetics and Evolution. 2001;20:149–162. doi: 10.1006/mpev.2001.0943. [DOI] [PubMed] [Google Scholar]
- Lyons-Weiler J, Hoelzer GA. Escaping from the Felsenstein zone by detecting long branches in phylogenetic data. Molecular Phylogenetics and Evolution. 1997;8:375–384. doi: 10.1006/mpev.1997.0450. [DOI] [PubMed] [Google Scholar]
- Lyons-Weiler J, Hoelzer GA, Tausch RJ. Optimal outgroup analysis. Biological Journal of the Linnean Society. 1998;64:493–511. [Google Scholar]
- Mabee PM. The usefulness of ontogeny in interpreting morphological characters. In: Wiens JJ, editor. Phylogenetic analysis of morphological data. Washington, DC: Smithsonian Institution Press; 2000. pp. 84–114. [Google Scholar]
- Maddison DR. The discovery and importance of multiple islands of most-parsimonious trees. Systematic Zoology. 1991;40:315–328. [Google Scholar]
- Maddison WP. Missing data versus missing characters in phylogenetic analysis. Systematic Biology. 1993;42:576–581. [Google Scholar]
- Maddison WP. Gene trees in species trees. Systematic Biology. 1997;46:523–536. [Google Scholar]
- Maddison WP, Donoghue MJ, Maddison DR. Outgroup analysis and parsimony. Systematic Zoology. 1984;33:83–103. [Google Scholar]
- Maddison WP, Maddison DR. MacClade. Sunderland, MA: Sinauer Associates; 1992. Version 3. [Google Scholar]
- Manegold A. Zur Phylogenie und Evolution dere ‘Racken’-, Specht-, und Sperlingsvögel (‘Coraciiformes’, Piciformes und Passeriformes: Aves) Doctoral dissertation, Berlin: Freie Universität; 2005. [Google Scholar]
- Manegold A, Mayr G, Mourer-Chauviré C. Miocene songbirds and the composition of the European passeriform avifauna. Auk. 2004;121:1155–1160. [Google Scholar]
- Marceliano MLV. Estudo osteológico e miológico do crânio de Opisthocomus hoazin (Müller, 1776) (Aves: Opisthocomidae), comparado com algumas espécies de Cracidae, Musophagidae e Cuculidae. Boletim do Museum Paraense Emílio Goeldi (Série Zoologia) 1996;12:95–246. [Google Scholar]
- Mariaux J, Braun MJ. A molecular phylogenetic survey of the nightjars and allies (Caprimulgiformes) with special emphasis on the potoos (Nyctibiidae) Molecular Phylogenetics and Evolution. 1996;6:228–244. doi: 10.1006/mpev.1996.0073. [DOI] [PubMed] [Google Scholar]
- Marks BD, Willard DE. Phylogenetic relationships of the Madagascan pygmy kingfisher (Ispidina madagascariensis) Auk. 2005;122:1271–1280. [Google Scholar]
- Marques AC, Gnaspini P. The problem of characters susceptible to parallel evolution in phylogenetic reconstructions: suggestion of a practical method and its application to cave animals. Cladistics. 2001;17:371–381. [Google Scholar]
- Marshall JT., Jr Relationships of certain owls around the Pacific. Natural History Bulletin of the Siam Society. 1966;21:235–242. [Google Scholar]
- Marshall CR. The fossil record and estimating divergence times between lineages: maximum divergence times and the importance of reliable phylogenies. Journal of Molecular Evolution. 1990;30:400–408. doi: 10.1007/BF02101112. [DOI] [PubMed] [Google Scholar]
- Martin LD. Origin and early evolution of birds. In: Brush AH, Clarke GA Jr, editors. Perspectives in ornithology: essays presented for the centennial of the American Ornithologists’ Union. Cambridge, UK: Cambridge University Press; 1983. pp. 291–353. [Google Scholar]
- Maryanska T, Osmólska H, Wolsan M. Avialian status for Oviraptorosauria. Acta Palaeontologica Polonica. 2002;42:361–371. [Google Scholar]
- Maurer BA. The evolution of body size in birds. I. Evidence for non-random diversification. Evolutionary Ecology. 1998;12:925–934. [Google Scholar]
- Maurer DR, Raikow RJ. Appendicular myology, phylogeny, and classification of the avian order Coraciiformes (including Trogoniformes) Annals of Carnegie Museum. 1981;50:417–434. [Google Scholar]
- Mayr E. The sequence of songbird families. Condor. 1958;60:194–195. [Google Scholar]
- Mayr G. Ein Archaeotrogon (Aves: Archaeotronidae) aus dem Mittel-Eozän der Grube Messel (Hessen, Deutschland)? Journal of Ornithology. 1998a;139:121–129. [Google Scholar]
- Mayr G. ‘Coraciiforme’ und ‘piciforme’ Kleinvögel aus dem Mittel-Eozän der Grube Messel (Hessen, Deutschland) Courier Forschungsinstitut Senckenberg. 1998b;205:1–101. [Google Scholar]
- Mayr G. A new family of Eocene zygodactyl birds. Senckenbergiana Lethaea. 1998c;78:199–209. [Google Scholar]
- Mayr G. A new trogon from the middle Oligocene of Céreste, France. Auk. 1999a;116:427–434. [Google Scholar]
- Mayr G. Caprimulgiform birds from the Middle Eocene of Messel (Hessen, Germany) Journal of Vertebrate Paleontology. 1999b;19:521–532. [Google Scholar]
- Mayr G. A new raptor-like bird from the lower Eocene of North America and Europe. Senckenbergiana Lethaea. 2000a;80:59–65. [Google Scholar]
- Mayr G. A new mousebird (Coliiformes: Coliidae) from the Oligocene of Germany. Journal of Ornithology. 2000b;141:85–92. [Google Scholar]
- Mayr G. New or previously unrecorded avian taxa from the middle Eocene of Messel (Hessen, Germany). Mitteilungen aus dem. Museum Naturkunde in Berlin (Geowissenschaftliche Reihe) 2000c;3:207–219. [Google Scholar]
- Mayr G. Tiny hoopoe-like birds from the middle Eocene of Messel (Germany) Auk. 2000d;117:964–970. [Google Scholar]
- Mayr G. New specimens of the middle Eocene fossil mousebird Selmes absurdipes Peters 1999. Ibis. 2001a;143:427–434. [Google Scholar]
- Mayr G. A second skeleton of the early Oligocene trogon Primotrogon wintersteini Mayr 1999 (Aves: Trogoniformes: Trogonidae) in an unusual state of preservation. Senckenbergiana Lethaea. 2001b;81:335–338. [Google Scholar]
- Mayr G. A cormorant from the late Oligocene of Enspel, Germany (Aves, Pelecaniformes, Phalacrocoracidae) Senckenbergiana Lethaea. 2001c;81:329–333. [Google Scholar]
- Mayr G. The earliest fossil record of a modern-type piciform from the late Oligocene of Germany. Journal of Ornithology. 2001d;142:2–6. [Google Scholar]
- Mayr G. A new specimen of the tiny middle Eocene bird Gracilitarsus mirabilis (New Family: Gracilitarsidae) Condor. 2001e;103:78–84. [Google Scholar]
- Mayr G. The relationships of fossil apodiform birds – a comment on Dyke (2001) Senckenbergiana Lethaea. 2001f;81:1–2. [Google Scholar]
- Mayr G. Comments on the systematic position of the putative Lower Eocene parrot Pulchrapollia gracilis. Senckenbergiana Lethaea. 2001g;81:339–341. [Google Scholar]
- Mayr G. Osteological evidence for paraphyly of the avian order Caprimulgiformes (nightjars and allies) Journal of Ornithology. 2002a;143:82–97. [Google Scholar]
- Mayr G. Comments on the osteology of Masillapodargus longipes Mayr 1999 and Paraprefica major Mayr 1999, caprimulgiform birds form the middle Eocene of Messel (Hessen, Germany) Neues Jahrbuch für Geologie und Paläontologie, Monatshefte (Stuttgart) 2002b;2001:65–76. [Google Scholar]
- Mayr G. On the osteology and phylogenetic affinities of the Pseudasturidae – lower Eocene stem-group representatives of parrots (Aves, Psittaciformes) Zoological Journal of the Linnean Society. 2002c;136:715–729. [Google Scholar]
- Mayr G. A skull of a new pelecaniform bird from the middle Eocene of Messel, Germany. Acta Palaeontologica Polonica. 2002d;47:507–512. [Google Scholar]
- Mayr G. The phylogenetic affinities of the shoebill (Balaeniceps rex) Journal für Ornithologie. 2003a;144:157–175. [Google Scholar]
- Mayr G. On the phylogenetic relationships of trogons (Aves, Trogoniformes) Journal of Avian Biology. 2003b;34:81–88. [Google Scholar]
- Mayr G. Phylogeny of early Tertiary swifts and hummingbirds (Aves: Apodiformes) Auk. 2003c;120:145–151. [Google Scholar]
- Mayr G. A partial skeleton of a new fossil loon (Aves, Gaviiformes) from the early Oligocene of Germany with preserved stomach content. Journal of Ornithology. 2004a;145:281–286. [Google Scholar]
- Mayr G. Tertiary plotopterids (Aves, Plotopteridae) and a novel hypothesis on the phylogenetic relationships of penguins (Spheniscidae) Journal of Zoological Systematics and Evolutionary Research. 2004b;10:1–11. [Google Scholar]
- Mayr G. Morphological evidence for sister group relationship between flamingos (Aves: Phoenicopteridae) and grebes (Podicipedidae) Zoological Journal of the Linnean Society. 2004c;140:157–169. [Google Scholar]
- Mayr G. Old World fossil record of modern-type hummingbirds. Science. 2004d;304:861–864. doi: 10.1126/science.1096856. [DOI] [PubMed] [Google Scholar]
- Mayr G. The phylogenetic relationships of the early Tertiary Primoscenidae and Sylphornithidae and the sister taxon of crown group piciform birds. Journal of Ornithology. 2004e;15:188–198. [Google Scholar]
- Mayr G. Phylogenetic relationships of the early Tertiary Messel rails (Aves Messelornithidae) Senckenbergiana Lethaea. 2004f;88:317–322. [Google Scholar]
- Mayr G. The Paleogene fossil record of birds in Europe. Biological Reviews (Cambridge) 2005a;80:515–542. doi: 10.1017/S1464793105006779. [DOI] [PubMed] [Google Scholar]
- Mayr G. The postcranial osteology and phylogenetic position of the middle Eocene Messelastur gratulator Peters, 1994 – a morphological link between owls (Strigiformes) and ‘falconiform’ birds? Journal of Vertebrate Paleontology. 2005b;25:635–645. [Google Scholar]
- Mayr G. A chicken-sized crane precursor from the early Oligocene of France. Naturwissenschaften. 2005c;92:389–393. doi: 10.1007/s00114-005-0007-8. [DOI] [PubMed] [Google Scholar]
- Mayr G. A new Eocene Chascacocolius-like mousebird (Aves: Coliiformes) with a remarkable gaping adaptation. Organisms, Diversity and Evolution. 2005d;5:167–171. [Google Scholar]
- Mayr G. New trogons from the early Tertiary of Germany. Ibis. 2005e;147:512–518. [Google Scholar]
- Mayr G. The Paleogene Old World potoo Paraprefica Mayr, 1999 (Aves, Nyctibiidae): its osteology and affinities to the New World Preficinae Olson, 1987. Journal of Systematic Palaeontology. 2005f;3:359–370. [Google Scholar]
- Mayr G. A, new cypselomorph bird from the middle Eocene of Germany and the early diversification of avian aerial insectivores. Condor. 2005g;107:342–352. [Google Scholar]
- Mayr G. A, tiny barbet-Iike bird from the lower Oligocene of Germany: the smallest species and earliest substantial fossil record of the Pici (woodpeckers and allies) Auk. 2005h;122:1055–1063. [Google Scholar]
- Mayr G. Phylogenetic affinities and composition of the early Eocene Gracilitarsidae (Aves, ?Piciformes) Neues Jahrbuch für Geologie und Paläontologie Monatshefte. 2005i;2005:1–16. [Google Scholar]
- Mayr G. New specimens of the early Eocene stem group galliform Paraortygoides (Gallinuloididae), with comments on the evolution of a crop in the stem lineage of Galliformes. Journal of Ornithology. 2006a;147:31–37. [Google Scholar]
- Mayr G. The renaissance of avian paleontology and its bearing on the higher-level phylogeny of birds: Are there missing links between modern higher-level taxa [abstract]? Journal of Ornithology. 2006b;147(Suppl.):42. [Google Scholar]
- Mayr E, Amadon D. A classification of Recent birds. American Museum Novitates. 1951;1496:1–42. [Google Scholar]
- Mayr G, Clarke J. The deep divergences of neornithine birds: a phylogenetic analysis of morphological characters. Cladistics. 2003;19:527–553. doi: 10.1111/j.1096-0031.2003.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Mayr G, Daniels M. Eocene parrots from Messel (Hessen, Germany) and the London Clay of Walton-on-the-Naze (Essex, England) Senckenbergiana Lethaea. 1998;78:157–177. [Google Scholar]
- Mayr G, Daniels M. A new short-legged landbird from the early Eocene of Wyoming and contemporaneous European sites. Acta Palaeontologica Polonica. 2001;46:393–402. [Google Scholar]
- Mayr G, Ericson PGP. Evidence for a sister group relationship between the Madagascan mesites (Mesitornithidae) and the cuckoos (Cuculidae) Senckenbergiana Biologica. 2004;84:1–17. [Google Scholar]
- Mayr G, Göhlich UB. A new parrot from the Miocene of Germany, with comments on the variation of hypotarsus morphology in some Psittaciformes. Belgian Journal of Zoology. 2004;134:47–54. [Google Scholar]
- Mayr G, Manegold A. The oldest European fossil songbird from the early Oligocene of Germany. Naturwissenshaften. 2004;91:173–177. doi: 10.1007/s00114-004-0509-9. [DOI] [PubMed] [Google Scholar]
- Mayr G, Manegold A, Johansson US. Monophyletic groups within ‘higher land birds’– comparison of morphological and molecular data. Journal of Zoological Systematics and Evolutionary Research. 2003;41:233–248. [Google Scholar]
- Mayr G, Manegold A, Johansson US. Erratum – monophyletic groups within ‘higher land birds’– comparison of morphological and molecular data. Journal of Zoological Systematics and Evolutionary Research. 2004a;42:173–174. [Google Scholar]
- Mayr G, Mourer-Chauviré C. Rollers (Aves: Coraciiformes s. s.) from the middle Eocene of Messel (Germany) and upper Eocene of the Quercy (France) Journal of Vertebrate Paleontology. 2000;20:533–546. [Google Scholar]
- Mayr G, Mourer-Chauviré C, et al. Auk. 2003;120:202–203. Phylogeny and fossil record of the Brachypteraciidae: a comment on Kirchman. [Google Scholar]
- Mayr G, Mourer-Chauviré C. Unusual tarsometatarsus of a mousebird from the Paleogene of France and the relationships of Selmes Peters, 1999. Journal of Vertebrate Paleontology. 2004;24:366–372. [Google Scholar]
- Mayr G, Mourer-Chauviré C, Weidig I. Osteology and systematic position of the Eocene Primobucconidae (Aves, Coraciiformes sensu stricto), with first records from Europe. Journal of Systematic Paleontology. 2004b;2:1–12. [Google Scholar]
- Mayr G, Peters DS. The mousebirds (Aves: Coliiformes) from the middle Eocene of Grube Messel (Hessen, Germany) Senckenbergiana Lethaea. 1998;78:179–197. [Google Scholar]
- Mayr G, Pohl B, Peters DS. A well-preserved Archaeopteryx specimen with theropod features. Science. 2005;310:1483–1486. doi: 10.1126/science.1120331. [DOI] [PubMed] [Google Scholar]
- Mayr G, Smith R. A new record of the Prophaethontidae (Aves: Pelecaniformes) from the middle Eocene of Belgium. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique. 2002;72:135–138. [Google Scholar]
- Mayr G, Weidig I. The early Eocene bird Gallinuloides wyomingensis– a stem group representativve of Galliformes. Acta Palaeontologica Polonica. 2004;49:211–217. [Google Scholar]
- McCall RA, Nee AS, Harvey PH. The role of wing length in the evolution of avian flightlessness. Evolutionary Ecology. 1998;12:569–580. [Google Scholar]
- McCracken KG, Harshman J, McClellan DA, Afton AD. Data set incongruence and correlated character evolution: an example of functional convergence in the hind-limbs of stifftail diving ducks. Systematic Biology. 1999;48:683–714. doi: 10.1080/106351599259979. [DOI] [PubMed] [Google Scholar]
- McCracken KG, Sheldon FH. Avian vocalizations and phylogenetic signal. Proceedings of the National Academy of Science USA. 1997;94:3833–3836. doi: 10.1073/pnas.94.8.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KG, Sorenson MD. Is homoplasy or lineage sorting the source of incongruent mtDNA and nuclear gene trees in the stiff-tailed ducks (Nomonyx-Oxyura) Systematic Biology. 2005;54:35–54. doi: 10.1080/10635150590910249. [DOI] [PubMed] [Google Scholar]
- McDowell S. The bony palate of birds. Part I, the Palaeognathæ. Auk. 1948;65:520–549. [Google Scholar]
- McKinney ML, McNamara KJ, Zachos LG. Heterochronic hierarchies: application and theory in evolution. Historical Biology. 1990;3:269–287. [Google Scholar]
- McKitrick MC. Forelimb myology of loons (Gaviiformes), with comments on the relationship of loons and tubenoses (Procellariiformes) Zoological Journal of the Linnean Society. 1991a;102:115–152. [Google Scholar]
- McKitrick MC. Phylogenetic analysis of avian hindlimb musculature. University of Michigan, Museum of Zoology Miscellaneous Publications. 1991b;179:1–85. [Google Scholar]
- McKitrick MC. Trends in the evolution of hindlimb musculature in aerial-foraging birds. Auk. 1993;110:189–206. [Google Scholar]
- Meekangvan P, Barhorst AA, Burton TD, Chatterjee S, Schovanec L. Nonlinear dynamical model and response of avian cranial kinesis. Journal of Theoretical Biology. 2006;240:32–47. doi: 10.1016/j.jtbi.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Meier R. A test and review of the empirical performance of the ontogenetic criterion. Systematic Biology. 1997;46:699–721. [Google Scholar]
- Meier R, Richter S. Suggestions for a more precise usage of proper names of taxa – ambiguities related to the stem lineage concept. Zeitschrift für Zoologische Systematik und Evolutionsforschung. 1992;30:81–88. [Google Scholar]
- Meyer A, Zardoya R. Recent advances in the (molecular) phylogeny of vertebrates. Annual Review of Ecology and Systematics. 2003;34:311–338. [Google Scholar]
- Middleton KM, Gatesy SM. Theropod forelimb design and evolution. Zoological Journal of the Linnean Society. 2000;128:149–187. [Google Scholar]
- Miller AH. The syringeal structure of the Asiatic owl Phodilus. Condor. 1965;67:536–538. [Google Scholar]
- Milne-Edwards A. Observations sur les affinites zoologiques du genre Phodilus et description d’un nouveau genre de rapace. Nouveau Archives du Musée d’Histoire Naturelle (Série 2) 1878a;1:185–199. [Google Scholar]
- Milne-Edwards A. Remarques sur le genre Mesites et sure la place qu’il doit occuper dans la série ornithologique. Annales des Sciences Naturelles (Série 6, Zoologie) 1878b;7:1–13. [Google Scholar]
- Mindell DP. Phylogeny and classification of birds: a study in molecular evolution [review] Systematic Biology. 1992;41:126–134. [Google Scholar]
- Mindell DP, Knight A, Baer C, Huddleston CJ. Slow rates of molecular evolution in birds and the metabolic rate and body temperature hypotheses. Molecular Biology and Evolution. 1996;13:422–426. [Google Scholar]
- Mindell DP, Sorenson MD, Dimcheff DE. Multiple independent origins of mitochondrial gene order in birds. Proceedings of the National Academy of Science USA. 1998;95:10693–10697. doi: 10.1073/pnas.95.18.10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell DP, Sorenson MD, Dimcheff DE, Hasegawa M, Ast JC, Yuri T. Interordinal relationships of birds and other reptiles based on whole mitochondrial genomes. Systematic Biology. 1999;48:138–152. doi: 10.1080/106351599260490. [DOI] [PubMed] [Google Scholar]
- Mindell DP, Sorenson MD, Huddleston CJ, Miranda HC, Jr, Knight A, Swachuk SJ, Yuri T. Phylogenetic relationships among and within select avian orders based on mitochondrial DNA. In: Mindell DP, editor. Avian molecular evolution and systematics. San Diego, CA: Academic Press; 1997. pp. 213–247. [Google Scholar]
- Minelli A. Molecules, developmental modules, and phenotypes: a combinatorial approach to homology. Molecular Phylogeny and Evolution. 1998;9:340–347. doi: 10.1006/mpev.1997.0490. [DOI] [PubMed] [Google Scholar]
- Mishler BD. Cladistic analysis of molecular and morphological data. American Journal of Physical Anthropology. 1994;94:143–156. doi: 10.1002/ajpa.1330940111. [DOI] [PubMed] [Google Scholar]
- Mishler BD. The logic of the data matrix in phylogenetic analysis. In: Albert VA, editor. Parsimony, phylogeny and genomics. Oxford, UK: Oxford University Press; 2005. pp. 57–70. [Google Scholar]
- Mitchell PC. A contribution to the anatomy of the hoatzin (Opisthocomus cristatus) Proceedings of the Zoological Society of London. 1896;1896:618–628. [Google Scholar]
- Mitchell PC. Observations on the anatomy of the shoebill (Balæniceps rex) and allied birds. Proceedings of the Zoological Society of London. 1913;1913:644–703. [Google Scholar]
- Mitchell PC. Anatomical notes on the gruiform birds Aramus giganteus Bonap. & Rhinochetos kagu. Proceedings of the Zoological Society of London. 1915;1915:413–423. [Google Scholar]
- Mitsiadis TA, Caton J, Cobourne M. Waking-up the Sleeping Beauty: recovery of the ancestral bird ontogeneic program. Journal of Experimental Zoology (Series B) 2006;306:1–7. doi: 10.1002/jez.b.21094. [DOI] [PubMed] [Google Scholar]
- Miyaki CY, Matioli SR, Burke T, Wajntal A. Parrot evolution and paleographical events: mitochondrial DNA evidence. Molecular Biology and Evolution. 1998;15:544–551. [Google Scholar]
- Monroe BL. Response to E. Mayr [review] Auk. 1989;106:515–516. [Google Scholar]
- Mooers AØ, Cotgreave P. Sibley and Ahlquist’s tapestry dusted off. Trends in Ecology and Evolution. 1994;9:458–459. [Google Scholar]
- Mooers AØ, Harvey PH. Metabolic rate, generation time, and the rate of molecular evolution in birds. Molecular Phylogenetics and Evolution. 1994;3:344–350. doi: 10.1006/mpev.1994.1040. [DOI] [PubMed] [Google Scholar]
- Moore WS. Inferring phylogenies from mtDNA variation: mitochondrial gene trees versus nuclear gene trees. Evolution. 1995;49:718–726. doi: 10.1111/j.1558-5646.1995.tb02308.x. [DOI] [PubMed] [Google Scholar]
- Moore WS, deFilippis VR. The window of taxonomic resolution for phylogenies based on mitochondrial cytochrome b. In: Mindell DP, editor. Avian molecular evolution and systematics. San Diego, CA: Academic Press; 1997. pp. 83–119. [Google Scholar]
- Mort M, Soltis PS, Soltis DE, Mabry ML. Comparison of three methods for estimating internal support on phylogenetic trees. Systematic Biology. 2000;49:160–171. doi: 10.1080/10635150050207456. [DOI] [PubMed] [Google Scholar]
- Moum T, Arnason U, Arnason E. Mitochondrial DNA sequence evolution and phylogeny of the Atlantic Alcidae, including the extinct great auk (Pinguinus impennis) Molecular Biology and Evolution. 2002;19:1434–1439. doi: 10.1093/oxfordjournals.molbev.a004206. [DOI] [PubMed] [Google Scholar]
- Moum T, Johansen S, Erikstad KE, Piatt JF. Phylogeny and evolution of the auks (subfamily Alcinae) based on mitochondial DNA sequences. Proceedings of the National Academy of Sciences USA. 1994;91:7912–7916. doi: 10.1073/pnas.91.17.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourer-Chauviré C. The Archaeotrogonidae of the Eocene and Oligocene Phosphorites du Quercy (France) Contributions to Science of the Natural History Museum of Los Angeles County. 1980;330:17–31. [Google Scholar]
- Mourer-Chauviré C. Les Todidae (Aves, Coraciiformes) des Phosphorites du Quercy (France) Verhandlungen der Koninklijke Nederlandse Akademie Van Wetenschappen (Série B) 1985;88:407–414. [Google Scholar]
- Mourer-Chauviré C. Une nouvelle famille de perroquets (Aves: Psittaciformes dans l’Éocène Supérieur des phosphorites du Quercy, France. Geobios Mémoire Spéciale. 1992;14:169–177. [Google Scholar]
- Mourer-Chauviré C. A new species of Ameripodius (Aves: Galliformes: Quercymegapodiidae) from the lower Miocene of France. Palaeontology. 2000;43:481–493. [Google Scholar]
- Mourer-Chauviré C, Balouet JC. Alcover JA, Bover P, editors. Description of the skull of the genus Sylviornis Polin, 1980 (Galliformes, Sylviornithidae new family), a giant extinct bird from the Holocene of New Caledonia. Insular vertebrate evolution: the paleontological approach. Monografies de la Societat d’Història Natural de les Balears. 2005;12:205–118. [Google Scholar]
- Mourer-Chauviré C, Bertjet D, Hugueney M. The late Oligocene birds of the Créchy quarry (Allier, France), with a description of two new genera (Aves: Pelecaniformes: Phalacrocoracidae, and Anseriformes: Anseranatidae) Senckenbergiana Lethaea. 2004;84:303–315. [Google Scholar]
- Moyle RG. Phylogenetics of barbets (Aves: Piciformes) based on nuclear and mitochondrial DNA sequence data. Molecular Phylogenetics and Evolution. 2004;30:187–200. doi: 10.1016/s1055-7903(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Moyle RG. Phylogeny and biogeographical history of Trogoniformes, a pantropical bird order. Biological Journal of the Linnean Society. 2005;84:725–738. [Google Scholar]
- Moyle RG. A molecular phylogeny of kingfishers (Alcedinidae) with insights into early biogeographic history. Auk. 2006;123:487–499. [Google Scholar]
- Müller HJ. Die Morphologie und Entwicklung des Craniums von Rhea americana Linné. I. Das knorpelige Neurocranium. Zeitschrift für Wissenschaftliche Zoologie. 1961a;165:221–319. [Google Scholar]
- Müller HJ. Über strukturelle Ähnlichkeiten der Ohr- und Occipitalregion bei Vogel und Säuger. Zoologischer Anzeiger. 1961b;166:391–402. [Google Scholar]
- Müller HJ. Die Morphologie und Entwicklung des Craniums von Rhea americana Linné. II. Viszeralskelett, Mittelohr und Osteocranium. Zeitschrift für Wissenschaftliche Zoologie. 1963;168:35–118. [Google Scholar]
- Müller K. PRAP – computation of Bremer support for large data sets. Molecular Phylogenetics and Evolution. 2004;31:780–782. doi: 10.1016/j.ympev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Müller K. The efficiency of different search strategies for estimating parsimony jackknife, bootstrap, and Bremer support. BMC (Biomed Central) Evolutionary Biology. 2005;5(57):1–10. doi: 10.1186/1471-2148-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller GB, Newman SA. Generation, integration, autonomy. Three steps in the evolution of homology. In: Bock GR, Cardew G, editors. Homology. Chichester, UK: J. Wiley and Sons; 1999. pp. 65–79. [DOI] [PubMed] [Google Scholar]
- Murie J. On the dermal and visceral structures of the kagu, sun-bittern, and boatbill. Transactions of the Zoological Society of London. 1871;7:465–492. [Google Scholar]
- Murie J. On the genus Colius, its structure and systematic position. Ibis. 1872a;14:262–280. [Google Scholar]
- Murie J. On the motmots and their affinities. Ibis. 1872b;14:383–412. [Google Scholar]
- Murie J. On the skeleton of Todus, with remarks as to its allies. Proceedings of the Zoological Society of London. 1872c;1872:664–680. [Google Scholar]
- Murie J. On the Upupidæ and their relationships. Ibis. 1873;15:181–211. [Google Scholar]
- Murray PR, Megirian D. The skull of dromornithid birds: anatomical evidence for their relationship to Anseriformes. Records of the South Australian Museum. 1998;31:51–97. [Google Scholar]
- Murray PR, Vickers-Rich P. Magnificent Mihirungs: the colossal flightless birds of the Australian dreamtime. Bloomington, IN: Indiana University Press; 2004. [Google Scholar]
- Neff NA. A rational basis for a priori character weighting. Systematic Zoology. 1986;35:110–123. [Google Scholar]
- Nelson G. Homology and systematics. In: Hall BK, editor. Homology: the hierarchical basis of comparative biology. San Diego, CA: Academic Press; 1994. pp. 101–149. [Google Scholar]
- Nielsen C. Animal evolution: interrelationships of the living phyla. Oxford, UK: Oxford University Press; 1995. [Google Scholar]
- Nielsen KK, Arctander P. Recombination among multiple mitochondrial pseudogenes from a passerine genus. Molecular Phylogenetics and Evolution. 2001;18:362–369. doi: 10.1006/mpev.2000.0885. [DOI] [PubMed] [Google Scholar]
- Nixon KC. The parsimony ratchet, a new method for rapid parsimony analysis. Cladistics. 1999;15:407–414. doi: 10.1111/j.1096-0031.1999.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Norell MA. Taxic origin and temporal diversity: the effect of phylogeny. In: Novacek MJ, Wheeler QD, editors. Extinction and phylogeny. New York: Columbia University Press; 1992. pp. 89–118. [Google Scholar]
- Norell MA, Clark JN, Dasheveg D, Barsbold R, Chiappe LM, Davidson AR, McKenna MC, Perle A, Novacek MJ. A theropod dinosaur embryo and the affinities of the Flaming Cliffs dinosaur eggs. Science. 1994;266:779–782. doi: 10.1126/science.266.5186.779. [DOI] [PubMed] [Google Scholar]
- Norell MA, Clarke JA. Fossil that fills a critical gap in avian evolution. Nature. 2001;409:181–184. doi: 10.1038/35051563. [DOI] [PubMed] [Google Scholar]
- Norell MA, Wheeler W. Missing entry replacement data analysis: a replacement approach to dealing with missing data in paleontological and total evidence data sets. Journal of Vertebrate Paleontology. 2003;23:275–283. [Google Scholar]
- Noriega JI, Tambussi CO. A late Cretaceous Presbyornithidae (Aves: Anseriformes) from Vega Island, Antarctic peninsula: paleobiogeographic implications. Ameghiniana. 1995;32:57–61. [Google Scholar]
- Nudds RL, Rayner JMV. Scaling of body frontal area and body width in birds. Journal of Morphology. 2006;267:341–346. doi: 10.1002/jmor.10409. [DOI] [PubMed] [Google Scholar]
- Nunn GB, Cooper J, Jouventin P, Robertson CJR, Robertson GG. Evolutionary relationships among extant albatrosses (Procellariiformes: Diomedeidae) established from complete cytochrome-b gene sequences. Auk. 1996;113:784–801. [Google Scholar]
- Nunn GB, Cracraft J. Phylogenetic relationships among major lineages of the birds-of-paradise (Paradisaeidae) using mitochondrial DNA gene sequences. Molecular Phylogenetics and Evolution. 1996;5:445–459. doi: 10.1006/mpev.1996.0041. [DOI] [PubMed] [Google Scholar]
- Nunn GB, Stanley SE. Body size effects and rates of cytochrome b evolution in tube-nosed seabirds. Molecular Biology and Evolution. 1998;15:1360–1371. doi: 10.1093/oxfordjournals.molbev.a025864. [DOI] [PubMed] [Google Scholar]
- Ödeen A, Håstad O. Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Molecular Biology and Evolution. 2003;20:855–861. doi: 10.1093/molbev/msg108. [DOI] [PubMed] [Google Scholar]
- Ogilvie-Grant WR. On the genus Turnix. Ibis. 1889;31:446–475. [Google Scholar]
- Oliveira CA, Silva RM, Santos MM, Mahecha GAB. Location of the uretal openings in the cloacas of tinamous, some ratite birds, and crocodilians: a primitive character. Journal of Morphology. 2004;260:234–246. doi: 10.1002/jmor.10217. [DOI] [PubMed] [Google Scholar]
- Olson SL. Taxonomic comments on the Eurylaimidae. Ibis. 1971;113:507–516. [Google Scholar]
- Olson SL. Oligocene fossils bearing on the origins of the Todidae and the Momotidae (Aves: Coraciiformes) Smithsonian Contributions to Paleobiology. 1976;27:111–119. [Google Scholar]
- Olson SL. A lower Eocene frigatebird from the Green River Formation of Wyoming (Pelecaniformes, Fregatidae) Smithsonian Contributions to Paleobiology. 1977;35:1–33. [Google Scholar]
- Olson SL. Multiple origins of the Ciconiiformes. Proceedings of Conference of the Colonial Waterbird Group. 1978;1978:165–170. [Google Scholar]
- Olson SL. A new genus of penguin-like pelecaniform bird from the Oligocene of Washington (Pelecaniformes: Plotopteridae) Natural History Museum of Los Angeles County Contributions in Science. 1980;330:51–57. [Google Scholar]
- Olson SL. A critique of Cracraft’s classification of birds. Auk. 1982;99:737–759. [Google Scholar]
- Olson SL. Evidence for a polyphyletic origin of the Piciformes. Auk. 1983a;100:126–133. [Google Scholar]
- Olson SL. A new genus of tropicbird (Pelecaniformes: Phaethontidae) from the middle Miocene Calvert Formation of Maryland. Proceedings of the Biological Society of Washington. 1983b;98:851–855. [Google Scholar]
- Olson SL. The fossil record of birds. In: Farner DS, King JR, Parkes KC, editors. Avian biology. Vol. 8. New York: Academic Press; 1985. pp. 79–238. [Google Scholar]
- Olson SL. An early Eocene oilbird from the Green River Formation of Wyoming (Caprimulgiformes: Steatornithidae) Documents des Laboratoires de Géologie de Lyon. 1987;99:57–99. [Google Scholar]
- Olson SL. Neogaeornis wetzeli Lambrecht, a Cretaceous loon from Chile (Aves: Gaviidae) Journal of Vertebrate Paleontology. 1992a;12:122–124. [Google Scholar]
- Olson SL. A new family of primitive landbirds from the lower Eocene Green River Formation of Wyoming. Natural History Museum of Los Angeles County, Science Series. 1992b;36:127–136. [Google Scholar]
- Olson SL. A giant Presbyornis (Aves: Anseriformes) from the Palaeocene Aquia Formation of Maryland. Proceedings of the Biological Society of Washington. 1994;107:429–435. [Google Scholar]
- Olson SL. A new species of pelican (Aves: Pelecanidae) from the lower Pliocene of North Carolina and Florida. Proceedings of the Biological Society of Washington. 1999a;112:503–509. [Google Scholar]
- Olson SL. The anseriform relationships of Anatalavis Olson and Parris (Anseranatidae), with a new species of the London Clay. Smithsonian Contributions to Paleobiology. 1999b;89:231–243. [Google Scholar]
- Olson SL. Why so many kinds of passerine birds? Bioscience. 2001;51:268–269. [Google Scholar]
- Olson SL, Feduccia A. Presbyornis and the origin of the Anseriformes (Aves: Charadriomorphae) Smithsonian Contributions to Zoology. 1980a;323:1–24. [Google Scholar]
- Olson SL, Feduccia A. Relationships and evolution of flamingos (Aves: Phoenicopteridae) Smithsonian Contributions to Zoology. 1980b;316:1–73. [Google Scholar]
- Olson SL, Hasegawa Y. Fossil counterparts of giant penguins from the North Pacific. Science. 1979;206:688–689. doi: 10.1126/science.206.4419.688. [DOI] [PubMed] [Google Scholar]
- Olson SL, Hasegawa Y. A new genus and two new species of gigantic Plotopteridae from Japan (Aves: Pelecaniformes) Journal of Vertebrate Paleontology. 1996;16:742–751. [Google Scholar]
- Olson SL, Houde P. Small arboreal nonpasserine birds from the Early Tertiary of western North America. In: Ouellet H, editor. Acta XIX Congressua Internationalia Ornithologici. Ottawa, Ontario: University of Ottawa Press; 1989. pp. 2030–2036. [Google Scholar]
- Olson SL, Matsuoka H. New specimens of the early Eocene frigatebirds Limnofregata (Pelecaniformes: Fregatidae), with the description of a new species. Zootaxa. 2005;1046:1–15. [Google Scholar]
- Olson SL, Parris DC. The Cretaceous birds of New Jersey. Smithsonian Contributions to Paleobiology. 1987;63:1–22. [Google Scholar]
- Olson SL, Rasmussen PC. Miocene and Pliocene birds from the Lee Creek Mine, North Carolina. Smithsonian Contributions to Paleobiology. 2001;90:233–365. [Google Scholar]
- Olson SL, Steadman DW. The relationships of the Pedionomidae (Aves: Charadriiformes) Smithsonian Contributions to Zoology. 1981;337:1–25. [Google Scholar]
- Omland KE. Correlated rates of molecular and morphological evolution. Evolution. 1997a;51:1381–1393. doi: 10.1111/j.1558-5646.1997.tb01461.x. [DOI] [PubMed] [Google Scholar]
- Omland KE. Examining two standard assumptions of ancestral reconstructions: repeated loss of dichromatism in dabbling ducks (Anatini) Evolution. 1997b;51:1636–1646. doi: 10.1111/j.1558-5646.1997.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Ovenden JR, Mackinlay AG, Crozier RH. Systematics and mitochondrial genome evolution of Australian rosellas (Aves: Platycercidae) Molecular Biology and Evolution. 1987;4:526–543. [Google Scholar]
- Overton LC, Rhoads DD. Molecular phylogenetic relationships based on mitochondrial and nuclear gene sequences for the todies (Todus, Todidae) of the Caribbean. Molecular Phylogenetics and Evolution. 2004;32:524–538. doi: 10.1016/j.ympev.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Owen R. Description of the skull of a dentigerous bird (Odontopteryx toliapicus, Ow.) from the London Clay of Sheppey. Quarterly Journal of the Geological Society of London. 1873;29:511–522. [Google Scholar]
- Padian K, Chiappe LM. The origin and early evolution of birds. Biological Reviews (Cambridge) 1998;73:1–42. [Google Scholar]
- Page RDM. On islands of trees and efficacy of different methods of branch swapping in finding most-parsimonious trees. Systematic Biology. 1993;42:200–209. [Google Scholar]
- Page RDM, Charleston M. From gene to organismal phylogeny: reconciled trees and the gene tree/species tree problem. Molecular Phylogenetics and Evolution. 1997;7:231–240. doi: 10.1006/mpev.1996.0390. [DOI] [PubMed] [Google Scholar]
- Page RDM, Lee PLM, Becher SA, Griffiths R, Clayton DH. A different tempo of mitochondrial DNA evolution in birds and their parasitic lice. Molecular Phylogenetics and Evolution. 1998;9:276–293. doi: 10.1006/mpev.1997.0458. [DOI] [PubMed] [Google Scholar]
- Pamilo P, Nei M. Relationships between gene trees and species trees. Molecular Biology and Evolution. 1988;5:568–583. doi: 10.1093/oxfordjournals.molbev.a040517. [DOI] [PubMed] [Google Scholar]
- Parker WK. Abstract of notes on the osteology of Balæniceps rex. Proceedings of the Zoological Society of London. 1860;1860:324–330. [Google Scholar]
- Parker WK. On the osteology of Balæniceps rex (Gould) Transactions of the Zoological Society of London. 1861;4:269–351. [Google Scholar]
- Parker WK. Abstract of a memoir on the osteology of the genera Pterocles, Syrrhaptes, Hemopodius, and Tinamus. Proceedings of the Zoological Society of London. 1862;1862:253–260. [Google Scholar]
- Parker WK. On the osteology of the kagu (Rhinochetus jubatus) Transactions of the Zoological Society of London. 1869;8:501–521. [Google Scholar]
- Parker WK. On the ‘manus’ of Phœnicopterus. Ibis. 1889a;31:183–185. [Google Scholar]
- Parker WK. On the osteology of Steatornis caripensis. Proceedings of the Zoological Society of London. 1889b;1889:161–190. [Google Scholar]
- Parker WK. On the morphology of a reptilian bird, Opisthocomus cristatus. Transactions of the Zoological Society of London. 1891;13:43–85. [Google Scholar]
- Parsons CW. Note on the embryonic development of the hoatzin (Opisthocomus hoazin) Proceedings of the Royal Society of Edinburgh (Series B) 1954;65:205–212. [Google Scholar]
- Patak AE, Baldwin J. Pelvic limb musculature in the emu Dromaius novaehollandiae (Aves: Struthioniformes: Dromaiidae): adaptations to high-speed running. Journal of Morphology. 1998;238:23–37. doi: 10.1002/(SICI)1097-4687(199810)238:1<23::AID-JMOR2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Paterson AM, Gray RD. Analytical tests of host/parasite cospeciation. In: Clayton DH, Moore J, editors. Coevolutionary biology of birds and parasites. Oxford, UK: Oxford University Press; 1996. pp. 236–259. [Google Scholar]
- Paterson AM, Gray RD, Wallis GP. Parasites, petrels and penguins: Does louse presence reflect seabird phylogeny? International Journal of Parasitology. 1993;23:515–526. [Google Scholar]
- Paterson AM, Wallis GP, Gray RD. Penguins, petrels, and parsimony: Does cladistic analysis of behavior reflect seabird phylogeny? Evolution. 1995;49:974–989. doi: 10.1111/j.1558-5646.1995.tb02332.x. [DOI] [PubMed] [Google Scholar]
- Paton TA, Baker AJ. Sequences from 14 mitochondrial genes provide a well-supported phylogeny of the charadriiform birds congruent with the nuclear RAG-1 tree. Molecular Phylogenetics and Evolution. 2006;39:657–667. doi: 10.1016/j.ympev.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Paton TA, Baker AJ, Groth JG, Barrowclough GF. RAG−1 sequences resolve phylogenetic relationships within charadriiform birds. Molecular Phylogenetics and Evolution. 2003;29:268–278. doi: 10.1016/s1055-7903(03)00098-8. [DOI] [PubMed] [Google Scholar]
- Paton TA, Haddrath O, Baker AJ. Complete mitochondrial DNA genome sequences show that modern birds are not descended from transitional shorebirds. Proceedings of the Royal Society of London (Series B) 2002;269:839–846. doi: 10.1098/rspb.2002.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C, Williams DM, Humphries CJ. Congruence between molecular and morphological phylogenies. Annual Review of Ecology and Systematics. 1993;24:153–188. [Google Scholar]
- Paxinos EE, James HF, Olson SL, Sorenson MD, Jackson J, Fleischer RC. mtDNA from fossils reveals of radiation of Hawaiian geese recently derived from the Canada goose (Branta canadensis) Proceedings of the National Academy of Science USA. 2002;99:1399–1404. doi: 10.1073/pnas.032166399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira SL, Baker AJ. Vicariant speciation of curassows (Aves, Cracidae): a hypothesis based on mitochondrial DNA phylogeny. Auk. 2004;121:682–694. [Google Scholar]
- Pereira SL, Baker AJ. Multiple gene evidence for parallel evolution and retention of ancestral morphological states in the shanks (Charadriiformes: Scolopacidae) Condor. 2005;107:514–526. [Google Scholar]
- Pereira SL, Baker AJ. A molecular timescale for galliform birds accounting for uncertainty in time estimate and heterogeneity of rates of DNA substitutions across lineages and sites. Molecular Phylogenetics and Evolution. 2006a;38:499–509. doi: 10.1016/j.ympev.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Pereira SL, Baker AJ. A mitogenomic timescale for birds detects variable phylogenetic rates of molecular evolution and refutes the standard molecular clock. Molecular Biology and Evolution. 2006b;23:1731–1740. doi: 10.1093/molbev/msl038. [DOI] [PubMed] [Google Scholar]
- Pereira SL, Baker AJ, Wajntal A. Combined nuclear and mitochondrial DNA sequences resolve generic relationships within the Cracidae (Galliformes, Aves) Systematic Biology. 2002;51:94–958. doi: 10.1080/10635150290102519. [DOI] [PubMed] [Google Scholar]
- Perrin JB. On the myology of Opisthocomus cristatus. Transactions of the Zoological Society of London. 1875;9:353–370. [Google Scholar]
- Peters JL. Check-list of birds of the world. II. Cambridge, MA: Harvard University Press; 1934. [Google Scholar]
- Philippe H, Laurent J. How good are deep phylogenetic trees? Current Opinions on Genetic Development. 1998;8:616–623. doi: 10.1016/s0959-437x(98)80028-2. [DOI] [PubMed] [Google Scholar]
- Philippe H, Lecointre G, Vân Lê HL, Guyader JL. A critical study of homoplasy in molecular data with the use of a morphologically based cladogram, and its consequences for character weighting. Molecular Biology and Evolution. 1996;13:1174–1186. [Google Scholar]
- Philippe H, Yan Z, Brinkmann H, Rodrigue N, Delsuc F. Heterotachy and long-branch attraction in phylogenetics. BMC (Biomed Central) Evolutionary Biology. 2005;5(50):1–8. doi: 10.1186/1471-2148-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A, Janies D, Wheeler W. Multiple sequence alignment in phylogenetic analysis. Molecular Phylogenetics and Evolution. 2000;16:317–330. doi: 10.1006/mpev.2000.0785. [DOI] [PubMed] [Google Scholar]
- Pickett KM, Randle CP. Strange Bayes indeed: uniform topological priors imply non-uniform clade priors. Molecular Phylogenetics and Evolution. 2005;34:203–211. doi: 10.1016/j.ympev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Pickett KM, Tolman GL, Wheeler WC, Wenzel JW. Parsimony overcomes statistical inconsistency with the addition of more data from the same gene. Cladistics. 2005;21:438–445. doi: 10.1111/j.1096-0031.2005.00076.x. [DOI] [PubMed] [Google Scholar]
- Pisani D, Yates AM, Langer MC, Benton MJ. A genus-level supertree of the Dinosauria. Proceedings of the Royal Society of London (Series B) 2002;269:915–921. doi: 10.1098/rspb.2001.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitra C, Lieckfeldt D, Frahnert S, Fickel J. Phylogenetic relationships and ancestral areas of the bustards (Gruiformes: Otididae), inferred from mitochondrial DNA and nuclear intron sequences. Molecular Phylogenetics and Evolution. 2002;23:63–74. doi: 10.1006/mpev.2001.1078. [DOI] [PubMed] [Google Scholar]
- Ploeger PL. Geographical differentiation in arctic Anatidae as a result of isolation during the last glacial. Ardea. 1968;56:1–159. [Google Scholar]
- Poe S, Chubb AL. Birds in a bush: five genes indicate explosive evolution of avian orders. Evolution. 2004;58:404–415. [PubMed] [Google Scholar]
- Pol D, Norell MA, Siddall ME. Measures of stratigraphic fit to phylogeny and their sensitivity to tree size, tree shape, and scale. Cladistics. 2004;20:64–75. doi: 10.1111/j.1096-0031.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- Pollock DD, Zwickl DJ, McGuire JA, Hillis DM. Increased taxon sampling is advantageous for phylogenetic inference. Systematic Biology. 2002;51:664–671. doi: 10.1080/10635150290102357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons J-M, Hassanin A, Crochet P-A. Phylogenetic relationships within the Laridae (Charadriiformes: Aves) inferred from mitochondrial markers. Molecular Phylogenetics and Evolution. 2005;37:686–699. doi: 10.1016/j.ympev.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Poplin F, Mourer-Chauviré C. Sylviornis neocaledoniae (Aves, Galliformes, Megapodidae), oiseau géant éteint de l’Ile des Pins (Nouvelle-Calédonie) Geobios. 1985;18:73–97. [Google Scholar]
- Posso SR, Donatelli RJ. Cranial osteology and systematic implications in Crotophaginae (Aves, Cuculidae) Journal of Zoological Systematics and Evolutionary Research. 2001;39:247–256. [Google Scholar]
- Prager EM, Wilson AC. Slow evolutionary loss of the potential for interspecific hybridization in birds: a manifestation of slow regulatory evolution. Proceedings of the National Academy of Science USA. 1975;72:200–204. doi: 10.1073/pnas.72.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Wilson AC. Congruency of phylogenies derived from different proteins: a molecular analysis of the phylogenetic position of cracid birds. Journal of Molecular Evolution. 1976;9:45–57. doi: 10.1007/BF01796122. [DOI] [PubMed] [Google Scholar]
- Prager EM, Wilson AC, Osuga DT, Feeney RE. Evolution of flightless land birds on southern continents: transferrin comparison shows monophyletic origin of ratites. Journal of Molecular Evolution. 1976;8:283–294. doi: 10.1007/BF01731001. [DOI] [PubMed] [Google Scholar]
- Prendini L. Species or supraspecific taxa as terminals in cladistic analysis? Groundplans versus exemplars revisted. Systematic Biology. 2003;50:290–300. doi: 10.1080/10635150118650. [DOI] [PubMed] [Google Scholar]
- Primmer CR, Ellegren H. Patterns of molecular evolution in avian microsatellites. Molecular Biology and Evolution. 1998;15:997–1008. doi: 10.1093/oxfordjournals.molbev.a026015. [DOI] [PubMed] [Google Scholar]
- Prum RO. Phylogenetic interrelationships of the barbets (Capitonidae) and toucans (Ramphastidae) based on morphology with comparisons to DNA-DNA hybridization. Zoological Journal of the Linnean Society. 1988;92:313–343. [Google Scholar]
- Prum RO. Phylogeny, biogeography, and evolution of the broadbills (Eurylaimidae) and asites (Philepittidae) based on morphology. Auk. 1993;110:304–324. [Google Scholar]
- Prychitko TM, Moore WS. The utility of DNA sequences of an intron from the β-fibrinogen gene in phylogenetic analysis of woodpeckers (Aves: Picidae) Molecular Phylogenetics and Evolution. 1997;8:193–204. doi: 10.1006/mpev.1997.0420. [DOI] [PubMed] [Google Scholar]
- Prychitko TM, Moore WS. Alignment and phylogenetic analysis of β-fibrinogen intron 7 sequences among avian orders reveal conserved regions within the intron. Molecular Biology and Evolution. 2003;20:762–771. doi: 10.1093/molbev/msg080. [DOI] [PubMed] [Google Scholar]
- Quicke DL, Taylor J, Provis A. Changing the landscape: a new strategy for estimating large phylogenies. Systematic Biology. 2001;50:60–66. [PubMed] [Google Scholar]
- Quinn TW. The genetic legacy of Mother Goose – phylogeographic patterns of lesser snow goose Chen caerulescens caerulescens maternal lineages. Molecular Ecology. 1992;1:1–5–711. doi: 10.1111/j.1365-294x.1992.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Raff RA, Parr BA, Parks AL, Wray GA. Heterochrony and other mechanisms of radical evolutionary change in early development. In: Nitecki MH, editor. Evolutionary innovations. Chicago, IL: University of Chicago Press; 1990. pp. 71–98. [Google Scholar]
- Raff EC, Raff RA. Dissociability, modularity, evolvability. Evolution and Development. 2000;2:235–237. doi: 10.1046/j.1525-142x.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- Rafinesque CS. Analyse de la nature, ou tableau de l’univers et des corps organisés. Palaermo: Published privately; 1815. [Google Scholar]
- Raikow RJ. Old birds and new ideas: progress and controversy in paleornithology [review] Wilson Bulletin. 1981;93:407–412. [Google Scholar]
- Raikow RJ. Monophyly of the Passeriformes: test of a phylogenetic hypothesis. Auk. 1982;99:431–445. [Google Scholar]
- Raikow RJ. Problems in avian classification. In: Johnston RF, editor. Current ornithology. Vol. 2. New York: Plenum Press; 1985a. pp. 187–212. [Google Scholar]
- Raikow RJ. Locomotor system. In: King AS, McLelland J, editors. Form and function in birds. Vol. 3. London, UK: Academic Press; 1985b. pp. 57–147. [Google Scholar]
- Raikow RJ. Why are there so many kinds of passerine birds? Systematic Zoology. 1986;35:255–259. [Google Scholar]
- Raikow RJ. The analysis of evolutionary success. Systematic Zoology. 1988;37:76–79. [Google Scholar]
- Raikow RJ. A phylogeny of the woodcreepers (Dendrocolaptinae) Auk. 1994a;111:104–114. [Google Scholar]
- Raikow RJ. Climbing adaptations in the hindlimb musculature of the woodcreepers (Dendrocolaptinae) Condor. 1994b;96:1103–1106. [Google Scholar]
- Raikow RJ, Bledsoe AH. Phylogeny and evolution of passerine birds. Bioscience. 2000;50:487–499. [Google Scholar]
- Raikow RJ, Borecky SR, Berman SL. The evolutionary reestablishment of a lost ancestral muscle in the bowerbird assemblage. Condor. 1980;81:203–206. [Google Scholar]
- Raikow RJ, Cracraft J. Monophyly of the Piciformes: a reply to Olson. Auk. 1983;100:134–138. [Google Scholar]
- Randi E, Fusco G, Lorenzini R, Spina F. Allozyme divergence and phylogenetic-relationships within the Strigiformes. Condor. 1991;93:295–301. [Google Scholar]
- Rawal UM, Bhatt PL. Comparative morphology of the feeding apparatus in a few representative birds from order Coraciiformes. Vidya (Series B) 1974;17:1–21. [Google Scholar]
- Rea AM. Cathartid affinities: a brief overview. In: Wilbur SR, Jackson JA, editors. Vulture biology and management. Berkeley, CA: University of California Press; 1983. pp. 26–54. [Google Scholar]
- Redelings BD, Suchard MA. Joint Bayesian estimation of alignment and phylogeny. Systematic Biology. 2005;54:401–408. doi: 10.1080/10635150590947041. [DOI] [PubMed] [Google Scholar]
- Ree RH, Donoghue MJ. Step matrices and the interpretation of homoplasy. Systematic Biology. 1998;47:582–588. doi: 10.1080/106351598260590. [DOI] [PubMed] [Google Scholar]
- Rees J, Lindgren J. Aquatic birds from the upper Cretaceous (lower Campanian) of Sweden and the biology and distribution of hesperornithiforms. Palaeontology. 2005;48:1321–1329. [Google Scholar]
- Reinhardt J. On the affinities of Balæniceps. Proceedings of the Zoological Society of London. 1860;1860:377–380. [Google Scholar]
- Reinhardt J. Some remarks on the genus Balæniceps. Ibis. 1862;4:158–175. [Google Scholar]
- Ribas CC, Gaban-Lima R, Miyaki CY, Cracraft J. Historical biogeography and diversification within the Neotropical parrot genus Pionopsitta (Aves: Psittacidae) Journal of Biogeography. 2005;32:1409–1427. [Google Scholar]
- Rice KA, Donoghue MJ, Olmstead RG. Analyzing large data sets: rbcL 500 revisited. Systematic Biology. 1997;46:554–563. doi: 10.1093/sysbio/46.3.554. [DOI] [PubMed] [Google Scholar]
- Rich PV. The Dromornithidae, an extinct family of large ground birds endemic to Australia. Bulletin of Bureau of Natural Resources, Geology and Geophysics. 1979;184:1–196. [Google Scholar]
- Rich PV. The Australian Dromornithidae: a group of large extinct ratites. Los Angeles County Natural History Museum Contributions to Science. 1980;330:93–103. [Google Scholar]
- Richardson MK, Jeffrey JE, Tabin CJ. Proximodistal patterning of the limb: insights from evolutionary morphology. Evolution and Development. 2004;6:1–5. doi: 10.1111/j.1525-142x.2004.04008.x. [DOI] [PubMed] [Google Scholar]
- Richardson MK, Minelli A, Coates KI. Some problems with typological thinking in evolution and development. Evolution and Development. 1999;1:5–7. doi: 10.1046/j.1525-142x.1999.00112.x. [DOI] [PubMed] [Google Scholar]
- Rieppel O. Homology and logical fallacy. Journal of Evolutionary Biology. 1992;5:701–715. [Google Scholar]
- Rieppel O. Homology, topology, and typology: the history of modern debates. In: Hall BK, editor. Homology: the hierarchical basis of comparative biology. San Diego, CA: Academic Press; 1994. pp. 63–100. [Google Scholar]
- Rodriguez-Trelles F, Tarrio R, Ayala FJ. A methodological bias toward overestimation of molecular evolutionary time scales. Proceedings of the National Academy of Science USA. 2002;99:8112–8115. doi: 10.1073/pnas.122231299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer AS. Notes and comments on vertebrate paleontology. Chicago, IL: University of Chicago Press; 1968. [Google Scholar]
- Rotthowe K, Starck JM. Evidence for a phylogenetic position of button quails (Turnicidae: Aves) among the Gruiformes. Journal of Zoological Systematics and Evolutionary Research. 1998;36:39–51. [Google Scholar]
- Roulin A. The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biological Reviews (Cambridge) 2004;79:815–848. doi: 10.1017/s1464793104006487. [DOI] [PubMed] [Google Scholar]
- Russello MA, Amato G. A molecular phylogeny of Amazona: implications for Neotropical parrot biogeography, taxonomy, and conservation. Molecular Phylogenetics and Evolution. 2004;30:421–477. doi: 10.1016/s1055-7903(03)00192-1. [DOI] [PubMed] [Google Scholar]
- Ruta M, Coates MI, Quicke DLJ. Early tetrapod relationships revisited. Biological Reviews (Cambridge) 2003;78:251–345. doi: 10.1017/s1464793102006103. [DOI] [PubMed] [Google Scholar]
- Salter LA. Complexity of the likelihood surface for a large DNA dataset. Systematic Biology. 2001;50:970–978. doi: 10.1080/106351501753462902. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ. In search of homoplastic tendencies: statistical inference of topological patterns in homoplasy. Evolution. 1991;45:351–358. doi: 10.1111/j.1558-5646.1991.tb04409.x. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ. Objections to bootstrapping phylogenies: a critique. Systematic Biology. 1995;44:299–320. [Google Scholar]
- Sanderson MJ. A nonparametric approach to estimating divergence times in the absence of rate constancy. Molecular Biology and Evolution. 1997;14:1218–1231. [Google Scholar]
- Sanderson MJ, Donoghue MJ. Patterns of variation in levels of homoplasy. Evolution. 1989;43:1781–1795. doi: 10.1111/j.1558-5646.1989.tb02626.x. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ, Donoghue MJ. The relationship between homoplasy and confidence in a phylogenetic tree. In: Sanderson MJ, Hufford L, editors. Homoplasy: the recurrence of similarity in evolution. San Diego, CA: Academic Press; 1996. pp. 67–89. [Google Scholar]
- Sangster G. A name for the flamingo–grebe clade. Ibis. 2005;147:612–615. [Google Scholar]
- Santini F, Stellwag EJ. Phylogeny, fossils, and model systems in the study of evolutionary developmental biology. Molecular Phylogenetics and Evolution. 2002;24:379–383. doi: 10.1016/s1055-7903(02)00209-9. [DOI] [PubMed] [Google Scholar]
- Sarich VM, Schmid CW, Marks J. DNA hybridization as a guide to phylogenies: a critical analysis. Cladistics. 1989;5:3–32. doi: 10.1111/j.1096-0031.1989.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Saunders DA, Smith GT, Campbell NA. The relationship between body weight, egg weight, incubation period, nestling period and nest site in the Psittaciformes, Falconiformes, Strigiformes, and Columbiformes. Australian Journal of Zoology. 1984;32:57–67. [Google Scholar]
- Scherer S, Hilsberg T. Hybridisierung und Verwandtschaftsgrade innerhalb der Anatidae – eine systematische und evolutionstheoretische Betrachtung. Journal für Ornithologie. 1982;123:357–380. [Google Scholar]
- Schluter D. The ecology of adaptive radiation. Oxford, UK: Oxford University; 2000. [Google Scholar]
- Schulmeister S, Wheeler WC. Comparative and phylogenetic analysis of developmental sequences. Evolution and Development. 2004;6:50–57. doi: 10.1111/j.1525-142x.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- Schweitzer MH, Jackson FD, Chiappe LM, Schmitt JG. Late Cretaceous avian eggs with embryos from Argentina. Journal of Vertebrae Paleontology. 2002;22:191–195. [Google Scholar]
- Scotland RW, Olmstead RG, Bennett JR. Phylogeny reconstruction: the role of morphology. Systematic Biology. 2003;52:539–548. doi: 10.1080/10635150390223613. [DOI] [PubMed] [Google Scholar]
- Seebohm H. An attempt to diagnose the suborders of the great Gallino-Gralline group of birds, by the aid of osteological characters alone. Ibis. 1888;30:415–435. [Google Scholar]
- Seebohm H. An attempt to diagnose the suborders of the ancient Ardeino-Anserine assemblage of birds by the aid of osteological characters alone. Ibis. 1889;31:92–104. [Google Scholar]
- Seebohm H. An attempt to diagnose the subclass Coraciiformes and the orders, suborders, and families comprised therein. Ibis. 1890a;32:200–205. [Google Scholar]
- Seebohm H. The classification of birds: an attempt to classify the subclasses, orders, suborders, and some of the families of existing birds. London, UK: R. H. Porter; 1890b. [Google Scholar]
- Seebohm H. An attempt to diagnose the Pico-Passerine group of birds and the suborders of which it consists. Ibis. 1890c;32:29–37. [Google Scholar]
- Seebohm H. Classification of birds: an attempt to classify the subclasses, orders, suborders, and some of the families of existing birds (Supplement) London, UK: R. H. Porter; 1895. [Google Scholar]
- Seibel DE. A phylogenetic analysis of the Cuculiformes and Opisthocomus, based on postcranial skeletal characters. Doctoral dissertation, Lawrence, KS: University of Kansas; 1988. [Google Scholar]
- Seibold I, Helbig AJ. Evolutionary history of New and Old world vultures inferred from nucleotide sequences of the mitochondrial cytochrome b gene. Philosophical Transactions of the Royal Society of London (Series B) 1995;150:163–178. doi: 10.1098/rstb.1995.0150. [DOI] [PubMed] [Google Scholar]
- Seibold I, Helbig AJ. Phylogenetic relationships of the sea eagles (genus Haliaetus): reconstruction based on morphology, allozymes, and mitochondrial DNA sequences. Journal of Zoological Systematics and Evolutionary Research. 1996;34:103–112. [Google Scholar]
- Semple C, Steel M. Phylogenetics. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- Sepkowski JJ., Jr . Rates of speciation in the fossil record. In: Magurran AE, May RM, editors. Evolution of biological diversity. Oxford, UK: Oxford University Press; 1999. pp. 260–282. [Google Scholar]
- Sereno PC. The pectoral girdle and forelimb of the basal theropod Herrerasaurus ischigualastensis. Journal of Vertebrate Paleontology. 1994;13(1993):425–450. [Google Scholar]
- Sereno PC. Alvarezsaurids: birds or ornithomimosaurs? In: Gauthier J, Gall LF, editors. New perspectives on the origin and early evolution of birds. New Haven, CT: Yale University Press; 2001. pp. 69–98. [Google Scholar]
- Sereno PC, Novas FE. The skull and neck of the basal theropod Herrerasaurus ischigualastensis. Journal of Vertebrate Paleontology. 1994;13(1993):451–476. [Google Scholar]
- Shafer HB, Clark JM, Kraus F. When molecules and morphology clash: a phylogenetic analysis of the North American ambystomatid salamanders (Caudata: Ambystomatidae) Systematic Zoology. 1991;40:284–305. [Google Scholar]
- Shapiro B, Sibthorpe D, Rambaut A, Austin J, Wragg GM, Bininda-Emonds ORP, Lee PLM, Cooper A. Flight of the dodo. Science. 2002;295:1683. doi: 10.1126/science.295.5560.1683. [DOI] [PubMed] [Google Scholar]
- Sharkey MJ. A hypothesis-independent method of character weighting for cladistic analysis. Cladistics. 1989;5:63–86. doi: 10.1111/j.1096-0031.1989.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Sheehan PM, Fastovsky DE. Major extinctions of land-dwelling vertebrates at the Cretaceous–Tertiary boundary, eastern Montana. Geology. 1992;20:556–570. [Google Scholar]
- Sheldon FH, Jones CE, McCracken KG. Relative patterns and rates of evolution in heron nuclear and mitochondrial DNA. Molecular Biology and Evolution. 2000;17:437–450. doi: 10.1093/oxfordjournals.molbev.a026323. [DOI] [PubMed] [Google Scholar]
- Shetty S, Griffin DK, Graves JAM. Comparative painting reveals strong chromosome homology over 80 million years of bird evolution. Chromosome Research. 1999;7:289–295. doi: 10.1023/a:1009278914829. [DOI] [PubMed] [Google Scholar]
- Shields GF, Wilson AC. Calibration of mitochondrial DNA evolution in geese. Journal of Molecular Evolution. 1987;24:212–217. doi: 10.1007/BF02111234. [DOI] [PubMed] [Google Scholar]
- Shpak M, Churchill GA. The information content of a character under a Markov model of evolution. Molecular Phylogenetics and Evolution. 2000;17:231–243. doi: 10.1006/mpev.2000.0846. [DOI] [PubMed] [Google Scholar]
- Shubin NH. The phylogeny of development and the origin of homology. In: Grande L, Rieppel O, editors. Interpreting the hierarchy of nature: from systematic patterns to evolutionary process theories. New York: Academic Press; pp. 210–225. [Google Scholar]
- Shufeldt RW. Contribution to the comparative osteology of the Trochilidæ, Caprimulgidæ, and Cypselidæ. Proceedings of the Zoological Society of London. 1885;1885:886–915. [Google Scholar]
- Shufeldt RW. Note on the anserine affinities of the flamingoes. Science. 1889a;14:224–225. doi: 10.1126/science.ns-14.347.224-b. [DOI] [PubMed] [Google Scholar]
- Shufeldt RW. Studies of the Macrochires, morphological and otherwise, with the view of indicating their relationships and defining their several positions in the system. Journal of the Linnean Society of London. 1889b;20:299–394. [Google Scholar]
- Shufeldt RW. On the osteology of the Striges (Strigidæ and Bubonidæ) Proceedings of the American Philosophical Society. 1900;39:665–722. [Google Scholar]
- Shufeldt RW. Notes on the osteology of Scopus umbretta and Balaeniceps rex. Journal of Anatomy (Series 2) 1901a;15:405–412. [PMC free article] [PubMed] [Google Scholar]
- Shufeldt RW. Osteology of the flamingoes (Odontoglossæ). Family: Phoenicopteridæ, sp. P. ruber. Annals of Carnegie Museum. 1901b;1:295–324. [Google Scholar]
- Shufeldt RW. On the systematic position of the sand-grouse (Pterocles; Syrrhaptes) American Naturalist. 1901c;35:11–16. [Google Scholar]
- Shufeldt RW. Notes on the osteology of the young of the hoatzin (Opisthocomus cristatus) and other points on its morphology. Journal of Morphology. 1918;31:599–606. [Google Scholar]
- Sibley CG. The evolutionary and taxonomic significance of sexual dimorphism and hybridization in birds. Condor. 1957;59:166–191. [Google Scholar]
- Sibley CG. The relationships of the lyrebirds. Emu. 1974;74:65–79. [Google Scholar]
- Sibley CG, Ahlquist JE. A comparative study of the egg white proteins of passerine birds. Peabody Museum of Natural History Bulletin. 1970;32:1–131. [Google Scholar]
- Sibley CG, Ahlquist JE. A comparative study of the egg white proteins of non-passerine birds. Peabody Museum of Natural History Bulletin. 1972;39:1–276. [Google Scholar]
- Sibley CG, Ahlquist JE. The relationships of the hoatzin. Auk. 1973;90:1–13. [Google Scholar]
- Sibley CG, Ahlquist JE. The phylogeny and relationships of the ratite birds as indicated by DNA-DNA hybridization. In: Scudder GGE, Reveal JL, editors. Evolution today. Pittsburgh, PA: Carnegie-Mellon University; 1981. pp. 301–335. [Google Scholar]
- Sibley CG, Ahlquist JE. Avian phylogeny reconstructed from comparisons of the genetic material, DNA. In: Paterson C, editor. Molecules and morphology in evolution: conflict or compromise? Cambridge, UK: Cambridge University Press; 1987. pp. 95–121. [Google Scholar]
- Sibley CG, Ahlquist JE. Phylogeny and classification of birds: a study in molecular evolution. New Haven, CT: Yale University Press; 1990. [Google Scholar]
- Sibley CG, Ahlquist JE, Monroe BL., Jr A classification of the living birds of the world based on DNA–DNA hybridization studies. Auk. 1988;105:409–423. [Google Scholar]
- Sibley CG, Ahlquist JE, Monroe BL., Jr . Distribution and taxonomy of birds of the world. New Haven, CT: Yale University Press; 1990. [Google Scholar]
- Sibley CG, Ahlquist JE, DeBenedictus P. The phylogenetic relationships of the rails, based on DNA comnparisons. Journal of the Yamashina Institute of Ornithology. 1993;25:1–11. [Google Scholar]
- Sibley CG, Frelin C. The egg white protein evidence for ratite affinities. Ibis. 1972;114:377–387. [Google Scholar]
- Siegel-Causey D. Phylogeny of the Phalacrocoracidae. Condor. 1988;90:885–905. [Google Scholar]
- Siegel-Causey D. Phylogeny of the Pelecaniformes: molecular systematics of a privative group. In: Mindell DP, editor. Avian molecular evolution and systematics. San Diego, CA: Academic Press; 1997. pp. 159–171. [Google Scholar]
- Simmons NB. Misleading results from the use of ambiguity coding to score polymorphisms in higher-level taxa. Systematic Biology. 2001;50:613–620. doi: 10.1080/10635150120578. [DOI] [PubMed] [Google Scholar]
- Simmons MP, Pickett KM, Miya M. How meaningful are Bayesian support values? Molecular Biology and Evolution. 2004;21:188–199. doi: 10.1093/molbev/msh014. [DOI] [PubMed] [Google Scholar]
- Simmons MP, Reeves A, Davis JI. Character-states space versus rates of evolution in phylogenetic inferences. Cladistics. 2004;20:191–204. doi: 10.1111/j.1096-0031.2004.00014.x. [DOI] [PubMed] [Google Scholar]
- Simon J, Laurent S, Grolleau G, Thoraval P, Soubieux D, Rasschaert D. Evolution of preproinsulin gene in birds. Molecular Phylogenetics and Evolution. 2004;30:755–766. doi: 10.1016/S1055-7903(03)00254-9. [DOI] [PubMed] [Google Scholar]
- Simon C, Nigro L, Sullivan J, Holsinger K, Martin A, Grapputo A, Franke A, McIntosh C. Large differences in substitutional pattern and evolutionary rate of 12S ribosomal RNA genes. Molecular Biology and Evolution. 1996;13:923–932. doi: 10.1093/oxfordjournals.molbev.a025660. [DOI] [PubMed] [Google Scholar]
- Simonetta AM. Cinesi e morfologiea del cranio negli uccelli non passeriformi. Studio su varie tendenze evolutive. Parte Ia. Archivio Zoologico Italiano (Torino) 1963;48:53–135. [Google Scholar]
- Simpson GG. Tempo and mode in evolution. New York: Columbia University Press; 1944. [Google Scholar]
- Simpson SF, Cracraft J. The phylogenetic relationships of the Piciformes (Class Aves) Auk. 1981;98:481–494. [Google Scholar]
- Slack KE, Delsuc F, McLenachan PA, Arnason U, Penny D. Resolving the root of avian mitogenomic tree by breaking up long branches. Molecular Phylogenetics and Evolution. 2006a;42:1–13. doi: 10.1016/j.ympev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Slack KE, Janke A, Penny D, Amason U. Two new avian mitochondrial genomes (penguin and goose) and a summary of bird and reptile mitogenomic features. Gene. 2003;302:43–52. doi: 10.1016/s0378111902010533. [DOI] [PubMed] [Google Scholar]
- Slack KE, Jones CM, Ando T, Harrison GL, Fordyce RE, Arnason U, Penny D. Early penguin fossils, plus mitochondrial genomes, calibrate avian evolution. Molecular Biology and Evolution. 2006b;23:1144–1155. doi: 10.1093/molbev/msj124. [DOI] [PubMed] [Google Scholar]
- Slikas B. Phylogeny of the avian family Ciconiidae (storks) based on cytochrome b sequences and DNA-DNA hybridization distances. Molecular Phylogenetics and Evolution. 1997;8:275–300. doi: 10.1006/mpev.1997.0431. [DOI] [PubMed] [Google Scholar]
- Slikas B. Recognizing and testing homology of courtship displays in storks (Aves: Ciconiiformes: Ciconiidae) Evolution. 1998;52:884–893. doi: 10.1111/j.1558-5646.1998.tb03713.x. [DOI] [PubMed] [Google Scholar]
- Smith GA. Systematics of parrots. Ibis. 1975;117:18–68. [Google Scholar]
- Smith AB. Systematics and the fossil record: documenting evolutionary patterns. Oxford, UK: Blackwell Scientific Publications; 1994. [Google Scholar]
- Smith AB. What does palaeontology contribute to systematics in a molecular world? Molecular Phylogenetics and Evolution. 1998;9:437–447. doi: 10.1006/mpev.1998.0488. [DOI] [PubMed] [Google Scholar]
- Smith EJ, Li S, Zhijian T. Gallus gallus aggrecan gene-based phylogenetic analysis of selected avian taxonomic groups. Genetica. 2005;124:23–32. doi: 10.1007/s10709-004-5184-4. [DOI] [PubMed] [Google Scholar]
- Smith VS, Page RDM, Johnson KP. Data incongruence and the problem of avian louse phylogeny. Zoologica Scripta. 2004;33:239–259. [Google Scholar]
- Smith AB, Peterson KJ. Dating the time of origin of major clades: molecular clocks and the fossil record. Annual Review of Earth and Planetary Science. 2002;30:65–88. [Google Scholar]
- Snively E, Russell AP, Powell GL. Evolutionary morphology of the coelurosaurian arctometatarsus: descriptive, morphometric and phylogenetic approaches. Zoological Journal of the Linnean Society. 2004;142:525–553. [Google Scholar]
- Sober E. Parsimony methods in systematics. In: Platnick NI, Funk VA, editors. Advances in cladistics. Vol. 2. New York: Columbia University Press; 1982. pp. 37–48. [Google Scholar]
- Sober E. Parsimony and its presuppositions. In: Albert VA, editor. Parsimony, phylogeny, and genomics. Oxford, UK: Oxford University Press; 2005. pp. 43–56. [Google Scholar]
- Sommer RJ. Convergence and the interplay of evolution and development. Evolution and Development. 1999;1:8–10. doi: 10.1046/j.1525-142x.1999.99022.x. [DOI] [PubMed] [Google Scholar]
- Sorenson MD. TreeRot. Boston, MA: Boston University; 1999. Version 2. [Google Scholar]
- Sorenson MD, Cooper A, Paxinos EE, Quinn TW, James HF, Olson SL, Fleischer RC. Relationships of the extinct moa-nalos, flightless Hawaiian waterfowl, based on ancient DNA. Proceedings of the Royal Society of London (Series B) 1999;266:2187–2194. doi: 10.1098/rspb.1999.0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson MD, O’Neal E, García-Moreno J, Mindell DP. More taxa, more characters: the hoatzin problem is still unresolved. Molecular Biology and Evolution. 2003;20:1484–1499. doi: 10.1093/molbev/msg157. [DOI] [PubMed] [Google Scholar]
- Sorenson MD, Payne RB. Molecular systematics: cuckoo phylogeny inferred from mitochondrial DNA sequences. In: Payne RB, editor. Bird families of the world: cuckoos. Oxford, UK: Oxford University Press; 2003. pp. 68–108. [Google Scholar]
- Spicer GS, Dunipace L. Molecular phylogeny of songbirds (Passeriformes) inferred from mitochondrial 16S ribosomal RNA gene sequences. Molecular Phylogenetics and Evolution. 2004;30:325–335. doi: 10.1016/s1055-7903(03)00193-3. [DOI] [PubMed] [Google Scholar]
- Springer MS. Molecular clocks and the incompleteness of the fossil record. Journal of Molecular Evolution. 1995;41:531–538. [Google Scholar]
- Sraml M, Christidis L, Easteal S, Horn P, Collet C. Molecular relationships between Australian waterfowl (Anseriformes) Australian Journal of Zoology. 1996;44:47–58. [Google Scholar]
- Stanley SE, Cracraft J. Higher-level systematic analysis of birds: current problems and possible solutions. In: DeSalle R, Giribet G, Wheeler W, editors. Molecular systematics and evolution: theory and practice. Basel, Switzerland: Birkhäuser-Verlag; 2002. pp. 31–43. [DOI] [PubMed] [Google Scholar]
- Stanley SE, Harrison RG. Cytochrome b evolution in birds and mammals: an evaluation of the avian constraint hypothesis. Molecular Biology and Evolution. 1999;16:1575–1585. doi: 10.1093/oxfordjournals.molbev.a026070. [DOI] [PubMed] [Google Scholar]
- Stapel SO, Leunissen JA, Versteeg M, Wattel J, de Jong W. Ratites as oldest offshoot of avian stem – evidence from α-crystallin A sequences. Nature. 1984;311:257–259. doi: 10.1038/311257a0. [DOI] [PubMed] [Google Scholar]
- Starck D. Die endocraniale Morphologie der Ratiten, besonders der Apterygidae und Dinornithidae. Gegenbaurs Morphologisches Jahrbuch. 1955;96:14–72. [Google Scholar]
- Starck D. Parallel development and specialisation during the evolution of the bird skull. Annale Universiteit Van Stellenbosch (Série A) 1969;44:217–228. [Google Scholar]
- Starck JM, Sutter E. Patterns of growth and heterochrony in moundbuilders (Megapodiidae) and fowl (Phasianidae) Journal of Avian Biology. 2000;31:527–547. [Google Scholar]
- Steel M, Penny D. Maximum parsimony and the phylogenetic information in multistate characters. In: Albert VA, editor. Parsimony, phylogeny, and genomics. Oxford, UK: Oxford University Press; 2005. pp. 163–180. [Google Scholar]
- Steel M, Pickett KM. On the impossibility of uniform priors on clades. Molecular Phylogenetics and Evolution. 2006;38:585–586. doi: 10.1016/j.ympev.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Stegmann BK. [Über die Eigenheiten des Fluges der Pterocliden] Zoologichesky Zhurnal. 1957; 36:1521–1529. [in Russian, German summary] [Google Scholar]
- Stegmann BK. [Some structual peculiarities of the skull and vertebral column in pigeons and sand-grouses] Zoologichesky Zhurnal. 1959;38:1049–1059. [in Russian, English summary] [Google Scholar]
- Steinbacher J. Bemerkungen zur Brutbiologie, Morphologie und Anatomie von Kagu-Jungen (Rhynochetos jubatus Verreaux & Des Murs) Bonner Zoologische Beiträge. 1968;19:198–205. [Google Scholar]
- Stevens PF. On characters and character states: Do overlapping and non-overlapping variation, morphology and molecules all yield data of the same value? In: Scotland R, Pennington RT, editors. Homology and systematics: coding characters for phylogenetic analysis. London: Taylor & Francis; 2000. pp. 81–105. [Google Scholar]
- Stidham TA. A lower jaw from a Cretaceous parrot. Nature. 1998;396:29–30. [Google Scholar]
- Stolpe M. Colymbus, Hesperornis, Podiceps: ein Vergleich ihrer hinteren Extremität. Journal für Ornithologie. 1935;83:115–128. [Google Scholar]
- Stonor CR. Some features of the variation of the birds of paradise. Proceedings of the Zoological Society of London. 1938;108:417–481. [Google Scholar]
- Storer RW. The fossil loon, Colymboides minutus. Condor. 1956;58:413–426. [Google Scholar]
- Storer RW. The classification of birds. In: Marshall AJ, editor. Biology and comparative physiology of birds. Vol. 1. New York: Academic Press; 1960a. pp. 57–93. [Google Scholar]
- Storer RW. Evolution in the diving birds. In: Bergman G, Donner KO, von Haartman L, editors. Proceedings of the XII International Ornithological Congress. Vol. 2. Helsinki, Finland: Tilgmannin Kirjapaino; 1960b. pp. 694–707. [Google Scholar]
- Storer RW. Adaptive radiation of birds. In: Farner DS, King JR, editors. Avian biology. Vol. 1. New York: Academic Press; 1971a. pp. 149–188. [Google Scholar]
- Storer RW. Classification of birds. In: Farner DS, King JR, editors. Avian biology. Vol. 1. New York: Academic Press; 1971b. pp. 1–18. [Google Scholar]
- Storer RW. The metazoan parasite fauna of grebes (Aves: Podicipediformes) and its relationship to the birds’ biology. Miscellaneous Publications of the University of Michigan Museum of Zoology. 2000;188:1–74. [Google Scholar]
- Storer RW. The Metazoan parasite fauna of loons (Aves: Gaviiformes), its relationship to the birds’ evolutionary history and biology, and a comparison with the parasite fauna of grebes. University of Michigan Museum of Zoology Miscellanious Publications. 2002;191:1–36. [Google Scholar]
- Storer RW. The grebe-flamingo connection: a rebuttal. Auk. 2006;123:1183–1184. [Google Scholar]
- Strauch JG., Jr The phylogeny of the Charadriiformes (Aves): a new estimate using the method of character compatibility analysis. Transactions of the Zoological Society of London. 1978;34:263–345. [Google Scholar]
- Strauch JG., Jr The phylogeny of the Alcidae. Auk. 1985;102:520–539. [Google Scholar]
- Stresemann E. Aves. In: Kükenthal W, Krumbach T, editors. Handbuch der Zoologie. Vol. 7. Berlin, Germany: Walter de Gruyter; 1934. part 2. [Google Scholar]
- Stresemann E. The status of avian systematics and its unsolved problems. Auk. 1959;76:269–280. [Google Scholar]
- Stucchi M, Urbina M. Ramphastosula (Aves, Sulidae): a new genus from the early Pliocene of the Pisco Formation, Peru. Journal of Vertebrate Paleontology. 2004;24:974–978. [Google Scholar]
- Sumrall CD, Brochu CA, Merck JW., Jr Global lability, regional resolution, and majority-rule consensus bias. Paleobiology. 2001;27:254–261. [Google Scholar]
- Suzuki Y, Glazko GV, Nei M. Overcredibility of molecular phylogenies obtained by Bayesian phylogenetics. Proceedings of the National Academy of Science USA. 2002;99:16138–16143. doi: 10.1073/pnas.212646199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Laskowski M, Jr, Lee YC. Phylogenetic expression of Galα1–4Gal on avian glycoproteins: glycan differentiation inscribed in the early history of modern birds. Proceedings of the National Academy of Science USA. 2004;101:9023–9028. doi: 10.1073/pnas.0402822101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierczewski EV, Raikow RJ. Hindlimb morphology, phylogeny, and classification of the Piciformes. Auk. 1981;98:466–480. [Google Scholar]
- Swofford DL. When are phylogeny estimates from molecular and morphological data incongruent? In: Miyamoto MM, Cracraft J, editors. Phylogenetic analysis of DNA sequences. Oxford, UK: Oxford University Press; 1991. pp. 295–333. [Google Scholar]
- Swofford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods) Sunderlund, MA: Sinauer Associates; 2002. Version 4. [Google Scholar]
- Tavares ES, Baker AJ, Pereira SL, Miyaki CY. Phylogenetic relationships and historical biogeography of Neotropical parrots (Psittaciformes: Psittacidae: Arini) inferred from mitochondrial and nuclear DNA sequences. Systematic Biology. 2006;55:454–470. doi: 10.1080/10635150600697390. [DOI] [PubMed] [Google Scholar]
- Taylor PG. Reproducibility of ancient DNA sequences from extinct Pleistocene fauna. Molecular Biology and Evolution. 1996;13:283–285. doi: 10.1093/oxfordjournals.molbev.a025566. [DOI] [PubMed] [Google Scholar]
- Telford MJ. Turning Hox‘signatures’ into synapomorphies. Evolution and Development. 2000;2:360–364. doi: 10.1046/j.1525-142x.2000.00075.x. [DOI] [PubMed] [Google Scholar]
- Templeton AR. Phylogenetic inference from restriction endonuclease cleavage site maps with particular reference to the evolution of humans and apes. Evolution. 1983;37:221–244. doi: 10.1111/j.1558-5646.1983.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Temrin H, Sillén-Tullberg B. The evolution of avian mating systems: a phylogenetic analysis of male and female polygamy and length of pair bond. Biological Journal of the Linnean Society. 1994;52:121–149. [Google Scholar]
- Temrin H, Sillén-Tullberg B. A phylogenetic analysis of the evolution of avian mating systems in relation to altricial and precocial young. Behavioral Ecology. 1995;6:296–307. [Google Scholar]
- Thomas GH, Wills MA, Székely T. Phylogeny of shorebirds, gulls, and alcids (Aves: Charadrii) from the cytochrome-b gene: parsimony, Bayesian inference, minimum evolution, and quartet puzzling. Molecular Phylogenetics and Evolution. 2004a;30:516–526. doi: 10.1016/S1055-7903(03)00222-7. [DOI] [PubMed] [Google Scholar]
- Thomas GH, Wills MA, Székely T. A supertree approach to shorebird phylogeny. BMC (Biomed Central) Evolutionary Biology. 2004b;4(28):1–18. doi: 10.1186/1471-2148-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen HA, den Tex R-J, de Bakker MAG, Povel GDE. Phylogenetic relationships amongst swifts and swiftlets: a multi-locus approach. Molecular Phylogenetics and Evolution. 2005;37:264–277. doi: 10.1016/j.ympev.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Thomassen HA, Wiersema AT, de Bakker MAG, de Knijff P, Hetebrij E, Povel GDE. A new phylogeny of swiftlets (Aves: Apodidae) based on cytochrome-b DNA. Molecular Phylogenetics and Evolution. 2003;29:86–93. doi: 10.1016/s1055-7903(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Thorne JL, Kishno H, Painter LS. Estimating the rate of evolution of the rate of evolution. Molecular Phylogenetics and Evolution. 1998;15:1647–1657. doi: 10.1093/oxfordjournals.molbev.a025892. [DOI] [PubMed] [Google Scholar]
- Thornton J. Gene tree phylogenetics: tracing protein evolution on trees. In: DeSalle R, Giribet G, Wheeler W, editors. Molecular systematics and evolution: theory and practice. Basel, Switzerland: Birkhäuser-Verlag; 2002. [Google Scholar]
- Tsaousis AD, Martin DP, Ladoukakis ED, Posada D, Zouros E. Widespread recombination in published animal mtDNA sequences. Molecular Biology and Evolution. 2005;22:925–933. doi: 10.1093/molbev/msi084. [DOI] [PubMed] [Google Scholar]
- Tullberg BS, Ah-King M, Temrin H. Phylogenetic reconstruction of parental–care systems in the ancestors of birds. Philosophical Transactions of the Royal Society of London (Series B) 2002;357:251–257. doi: 10.1098/rstb.2001.0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler C. A study of the egg shells of the Gaviiformes, Procellariiformes, Podicipitiformes [sic] and Pelecaniformes. Journal of Zoology (London) 1969;159:395–412. [Google Scholar]
- Unwin DM. Aves. In: Benton MJ, editor. The fossil record 2. London, UK: Chapman & Hall; 1993. pp. 717–737. Aves. [Google Scholar]
- Valentine JW. The macroevolution of clade shape. In: Ross RM, Allmon WD, editors. Causes of evolution: a paleontological perspective. Chicago, IL: Chicago University Press; 1990. pp. 128–150. [Google Scholar]
- Van Tets GF. Comparative study of some social communiation patterns in the Pelecaniformes. Ornithological Monographs. 1965;2:1–88. [Google Scholar]
- Van Tuinen M. Electronic encyclopedia of life sciences. London: Nature Publishing Group; 2002. Relationships of birds – molecules versus morphology. [Google Scholar]
- Van Tuinen M, Butvill DB, Kirsch JAW, Hedges SB. Convergence and divergence in the evolution of aquatic birds. Proceedings of the Royal Society of London (Series B) 2001;268:1–6. doi: 10.1098/rspb.2001.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tuinen M, Dyke GJ. Calibration of galliform molecular clocks using multiple fossils and genetic partitions. Molecular Phylogenetics and Evolution. 2004;30:74–88. doi: 10.1016/s1055-7903(03)00164-7. [DOI] [PubMed] [Google Scholar]
- Van Tuinen M, Hadly EA. Error in estimation of rate and time inferred from the early amniote fossil record and avian molecular clocks. Journal of Molecular Evolution. 2004;59:267–276. doi: 10.1007/s00239-004-2624-9. [DOI] [PubMed] [Google Scholar]
- Van Tuinen M, Hedges SB. Calibration of avian molecular clocks. Molecular Biology and Evolution. 2001;18:206–213. doi: 10.1093/oxfordjournals.molbev.a003794. [DOI] [PubMed] [Google Scholar]
- Van Tuinen M, Hedges SB. The effect of external and internal fossil calibrations on the avian evolutionary timescale. Journal of Paleontology. 2004;78:45–50. [Google Scholar]
- Van Tuinen M, Sibley CG, Hedges SB. Phylogeny and biogeography of ratite birds inferred from DNA sequences of the mitochondrial ribosomal genes. Molecular Biology and Evolution. 1998;15:370–376. doi: 10.1093/oxfordjournals.molbev.a025933. [DOI] [PubMed] [Google Scholar]
- Van Tuinen M, Sibley CG, Hedges SB. The early history of modern birds inferred from DNA sequences of nuclear and mitochondrial ribosomal genes. Molecular Biology and Evolution. 2000;17:451–457. doi: 10.1093/oxfordjournals.molbev.a026324. [DOI] [PubMed] [Google Scholar]
- Van Tuinen M, Stidham TA, Hadly EA. Tempo and mode of modern bird evolution observed with large-scale taxonomic sampling. Historical Biology. 2006;18:205–221. [Google Scholar]
- Van Tuinen M, Waterhouse D, Dyke GJ. Avian molecular systematics on the rebound: a fresh look at modern shorebird phylogenetic relationships. Journal of Avian Biology. 2004;35:191–194. [Google Scholar]
- Vargas AO, Fallon JF. Birds have dinosaur wings: the molecular evidence. Journal of Experimental Zoology (Series B) 2005a;304:86–90. doi: 10.1002/jez.b.21023. [DOI] [PubMed] [Google Scholar]
- Vargas AO, Fallon JF. The digits of the wing of birds are 1, 2, and 3: a review. Journal of Experimental Zoology (Series B) 2005b;304:206–219. doi: 10.1002/jez.b.21051. [DOI] [PubMed] [Google Scholar]
- Vazquez RJ. Functional osteology of the avian wrist and the evolution of flapping flight. Journal of Morphology. 1992;211:259–268. doi: 10.1002/jmor.1052110303. [DOI] [PubMed] [Google Scholar]
- Verheyen R. Note systématique sur Opisthocomus hoazin (St. Müller) Bulletin de l’Institut Royal des Sciences Naturelles de Belgique. 1956a;32(32):1–8. [Google Scholar]
- Verheyen R. Note sur l’anatomie et al classification des Coliiformes. Bulletin de l’Institut Royal des Sciences, Naturelles de Belgique. 1956b;32(47):1–7. [Google Scholar]
- Verheyen R. Les plongeons (Gaviae) et les grèbes (Podicitides) dans les systémes de classification. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique. 1959a;35(44):1–12. [Google Scholar]
- Verheyen R. Les Striges, les Trogones et les Caprimulgi dans la systématique moderne. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique. 1959b;32(3):1–31. [Google Scholar]
- Verheyen R. Contribution à ostéologie et à la systématique des Ratitae. Bulletin de l’Societe Royal des Zoologique d’Anvers. 1960a;36(56):1–19. [Google Scholar]
- Verheyen R. Les Pelecaniformes et le paille-en-quene (Phaethon) Bulletin de l’Institut Royal des Sciences Naturelles de Belgique. 1960b;36(25):1–18. [Google Scholar]
- Verheyen R. A new classification for the non-passerine birds of the world. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique. 1961;37(27):1–36. [Google Scholar]
- Vermeij GJ. The evolutionary success of passerines: a question of semantics? Systematic Zoology. 1988;37:69–71. [Google Scholar]
- Veron G. Phylogénie des touracos (Aves, Musophagidae). Analyse des caractères morphologiques. Journal of Zoological Systematics and Evolutionary Research. 1999;37:39–48. [Google Scholar]
- Vickers-Rich P, Trusler P, Rowley MJ, Cooper A, Chambers GK, Bock WJ, Millener PR, Worthy TH, Yaldwyn JC. Morphology, myology, collagen and DNA of a mummified upland moa, Megalapteryx didinus (Aves: Dinornithiformes), from New Zealand. Tuhinga: Records of the Museum of New Zealand Te Papa Tongarewa. 1995;4:1–26. [Google Scholar]
- Von Nathusius W. Über die Structur der Eischale von Opisthocomus cristatus und deren Beziehungen zu diesen Verhältnissen bei den Hühnern. Journal für Ornithologie. 1881;29:334–336. [Google Scholar]
- Waddell PJ, Cau Y, Hasegawa M, Mindell DP. Assessing the Cretaceous superordinal divergence times within birds and placental mammals by using whole mitochondrial protein sequences and an extended statistical framework. Systematic Biology. 1999;48:119–137. doi: 10.1080/106351599260481. [DOI] [PubMed] [Google Scholar]
- Wägele JW. On the information content of characters in comparative morphology and molecular systematics. Journal of Zoological Systematics and Evolutionary Research. 1995;33:42–47. [Google Scholar]
- Wägele JW. First principles of phylogenetic systematics, a basis for numerical methods used for morphological and molecular characters. Vie Milieu. 1996;46:125–138. [Google Scholar]
- Wagner GP. The origin of morphological characters and the biological basis of homology. Evolution. 1989;43:1157–1171. doi: 10.1111/j.1558-5646.1989.tb02566.x. [DOI] [PubMed] [Google Scholar]
- Wagner GP. Homology and the mechanisms of development. In: Hall BK, editor. Homology: the hierarchical basis of comparative biology. San Diego, CA: Academic Press; 1994. pp. 273–299. [Google Scholar]
- Wagner PJ. The quality of the fossil record and the accuracy of phylogenetic inferences about sampling and diversity. Systematic Biology. 2000a;49:65–86. doi: 10.1080/10635150050207393. [DOI] [PubMed] [Google Scholar]
- Wagner PJ. Exhaustion of morphologic character states among fossil taxa. Evolution. 2000b;54:365–386. doi: 10.1111/j.0014-3820.2000.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Gauthier JA. 1, 2, 3 = 2, 3, 4: a solution to the problem of the homology of the digits in the avian hand. Proceedings of the National Academy of Science USA. 1999;96:5111–5116. doi: 10.1073/pnas.96.9.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake DB. Homoplasy, homology, and the problem of ‘sameness’ in biology. In: Bock GR, Cardew G, editors. Homology. Chichester, UK: J. Wiley and Sons; 1999. pp. 24–46. [DOI] [PubMed] [Google Scholar]
- Wakeley J. Substitution-rate variation among sites and the estimation of transition bias. Molecular Biology and Evolution. 1994;11:436–442. doi: 10.1093/oxfordjournals.molbev.a040124. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Nikaido M, Tsuda TT, Inoko H, Mindell DP, Murata K, Okada N. The rise and fall of the CR1 subfamily in the lineage leading to penguins. Gene. 2006;365:57–66. doi: 10.1016/j.gene.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Wattel J, Stapel S, de Jong WW. Amino acid sequences of the eye-lens protein alpha-crystallin A in avian taxonomy. In: Ouellet H, editor. Acta XIX Congressua Internationalia Ornithologici. Vol. 2. Ottawa, Canada: University of Ottawa Press; 1988. pp. 1905–1911. [Google Scholar]
- Webb DM, Moore WS. A phylogenetic analysis of woodpeckers and their allies using 12S, cyt b, and COI nucleotide sequences (Class Aves: Order Piciformes) Molecular Phylogenetics and Evolution. 2005;36:233–248. doi: 10.1016/j.ympev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Weber E. Zur Kraniogenese bei der Lachmöwe (Larus ridibundus L.), zugleich ein Beitrag zur Rekonstuktion des Grundplans der Vögel. Gegenbaurs Morphologisches Jahrbuch (Leipzig) 1990;136:335–387. [PubMed] [Google Scholar]
- Weber E. Zur Evolution basicranialer Gelenke bei Vögeln, insbesondere bei Hühner- und Entenvögeln (Galloanseres) Zeitschrift für Zoologische Systematik und Evolutionsforschung. 1993;31:300–317. [Google Scholar]
- Wechstein JD. Molecular phylogenetics of the Ramphastos toucans: implications for the evolution of morphology, vocalizations, and coloration. Auk. 2005;122:1191–1209. [Google Scholar]
- Weibel AC, Moore WS. Molecular phylogeny of a cosmopolitan group of woodpeckers (genus Picoides) based on COI and cyt b mitochondrial gene sequences. Molecular Phylogenetics and Evolution. 2002a;22:65–75. doi: 10.1006/mpev.2001.1010. [DOI] [PubMed] [Google Scholar]
- Weibel AC, Moore WS. A test of a mitochondrial gene-based phylogeny of woodpeckers (genus Picoides) using an independent nuclear gene, β-fibrinogen intron 7. Molecular Phylogenetics and Evolution. 2002b;22:247–257. doi: 10.1006/mpev.2001.1062. [DOI] [PubMed] [Google Scholar]
- Weldon WFR. On some points in the anatomy of Phoenicopterus and its allies. Proceedings of the Zoological Society of London. 1883;1883:638–652. [Google Scholar]
- Welten MCM, Verbeek FJ, Meijer AH, Richardson MK. Gene expression and digit homology in the chicken embryo wing. Evolution and Development. 2005;7:18–28. doi: 10.1111/j.1525-142X.2005.05003.x. [DOI] [PubMed] [Google Scholar]
- Wesolowski T. On the origin of parental care and the early evolution of male and female parental roles in birds. American Naturalist. 1994;143:39–58. [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- Wetmore A. Fossil birds from the Green River deposits of eastern Utah. Annals of Carnegie Museum. 1926;16:391–402. [Google Scholar]
- Wetmore A. A systematic classification for the birds of the world. Proceedings of the United States National Museum. 1930;76:1–8. [Google Scholar]
- Wetmore A. A classification for the birds of the world. Smithsonian Miscellaneous Collection. 1960;139:1–37. [Google Scholar]
- Wheeler QD. Character weighting and cladistic analysis. Systematic Zoology. 1986;38:102–109. [Google Scholar]
- Wheeler WC, Gatesy J, DeSalle R. Elision: a method for accommodating multiple molecular sequence alignment-ambiguous sites. Molecular Phylogenetics and Evolution. 1995;4:1–9. doi: 10.1006/mpev.1995.1001. [DOI] [PubMed] [Google Scholar]
- Whittingham LA, Sheldon FH, Emlen ST. Molecular phylogeny of jaçanas and its implications for morphologic and biogeographic evolution. Auk. 2000;117:22–32. [Google Scholar]
- Wiens JJ. Incomplete taxa, incomplete characters, and phylogenetic accuracy: Is there a missing data problem? Journal of Vertebrate Paleontology. 2003;23:297–310. [Google Scholar]
- Wiens JJ. The role of morphological data in phylogeny reconstruction. Systematic Biology. 2004;53:653–661. doi: 10.1080/10635150490472959. [DOI] [PubMed] [Google Scholar]
- Wiens JJ. Can incomplete taxa rescue phylogenetic analyses from long-branch attraction? Systematic Biology. 2005;54:731–742. doi: 10.1080/10635150500234583. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Chippindale PT, Hillis DM. When are phylogenetic analyses misled by convergence? A case study in Texas cave salamanders. Systematic Biology. 2003;52:501–514. doi: 10.1080/10635150390218222. [DOI] [PubMed] [Google Scholar]
- Wiley EO. Phylogenetics: the theory and practice of phylogenetic systematics. New York: J. Wiley; 1981. [Google Scholar]
- Wilkinson M. Coping with abundant missing entries in phylogenetic inference using parsimony. Systematic Biology. 1995;44:501–514. [Google Scholar]
- Wilkinson M. Missing entries and multiple trees: instability, relationships, and support in parsimony analysis. Journal of Vertebrate Paleontology. 2003;23:311–323. [Google Scholar]
- Wilkinson M, LaPointe F-J, Gower DJ. Branch lengths and support. Systematic Biology. 2003;52:127–130. doi: 10.1080/10635150390132939. [DOI] [PubMed] [Google Scholar]
- Wilson SJ. A higher-order parsimony method to reduce long-branch attraction. Molecular Biology and Evolution. 1999;16:694–705. [Google Scholar]
- Wimberger PH, de Queiroz A. Comparing behavioral and morphological characters. In: Martins EP, editor. Phylogenies and the comparative method in animal behavior. Oxford, UK: Oxford University Press; 1996. pp. 206–223. [Google Scholar]
- Wink M. Phylogeny of Old and New World vultures (Aves: Accipitridae and Cathartidae) inferred from nucleotide sequences of the mitochondrial cytochrome b gene. Zeitschrift der Naturforschenden (Abteilung C) 1995;50:868–882. doi: 10.1515/znc-1995-11-1220. [DOI] [PubMed] [Google Scholar]
- Wink M, Heidrich P. Molecular evolution and systematics of the owls (Strigiformes) In: König C, Wieck F, Becking JH, editors. Owls: a guide to the owls of the world. Sussex, UK: Pica Press; 1999. pp. 39–57. [Google Scholar]
- Winkler DW, Sheldon FH. Evolution of nest construction in swallows (Hirundinidae): a molecular phylogenetic perspective. Proceedings of the National Academy of Science USA. 1993;90:5705–5707. doi: 10.1073/pnas.90.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnepennincks B, Backeljau T. 18S rRNA alignments derived from different secondary structure models can produce alternative phylogenies. Journal of Zoological Systematics and Evolutionary Research. 1996;34:135–143. [Google Scholar]
- Witmer LM. Perspectives on avian origins. In: Schultze H-P, Trueb L, editors. Origins of the higher groups of tetrapods. Ithaca, NY: Comstock Publishers; 1991. pp. 427–466. [Google Scholar]
- Worthy TH, Holdaway RN. The lost world of the moa: prehistoric life of New Zealand. Bloomington, IN: Indiana University Press; 2002. [Google Scholar]
- Wyles JS, Kunkel JG, Wilson AC. Birds, behavior, and anatomical evolution. Proceedings of the National Academy of Science USA. 1983;80:4394–4397. doi: 10.1073/pnas.80.14.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Xie Z, Kjer KM. 18S ribosomal RNA and tetrapod phylogeny. Systematic Biology. 2003;52:283–295. doi: 10.1080/10635150390196948. [DOI] [PubMed] [Google Scholar]
- Yang Z, Bielawski JP. Statistical methods for detecting molecular adaptation. Trends in Ecology and Evolution. 2000;15:496–503. doi: 10.1016/S0169-5347(00)01994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Rannala B. Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds. Molecular Biology and Evolution. 2006;23:212–226. doi: 10.1093/molbev/msj024. [DOI] [PubMed] [Google Scholar]
- Yeates DK. Groundplans and exemplars: paths to the tree of life. Cladistics. 1995;11:343–357. doi: 10.1111/j.1096-0031.1995.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Yuri T, Mindell DP. Molecular phylogenetic analysis of Fringillidae, ‘New World nine-primaried oscines’ (Aves: Passeriformes) Molecular Phylogenetics and Evolution. 2002;23:229–243. doi: 10.1016/S1055-7903(02)00012-X. [DOI] [PubMed] [Google Scholar]
- Zardoya R, Cao Y, Hasegawa M, Meyer A. Searching for the closest living relative(s) of tetrapods through evolutionary analyses of mitochondrial and nuclear data. Molecular Biology and Evolution. 1998;15:506–517. doi: 10.1093/oxfordjournals.molbev.a025950. [DOI] [PubMed] [Google Scholar]
- Zardoya R, Meyer A. Phylogenetic performance of mitochondrial protein-coding genes in resolving relationships among vertebrates. Molecular Biology and Evolution. 1996;13:933–942. doi: 10.1093/oxfordjournals.molbev.a025661. [DOI] [PubMed] [Google Scholar]
- Zeffer A, Johansson C, Marmebro Å. Functional correlation between habitat use and leg morphology in birds (Aves) Biological Journal of the Linnean Society. 2003;79:461–484. [Google Scholar]
- Zeffer A, Norberg L. Leg morphology and locomotion in birds: requirements for force and speed during ankle flexion. Journal of Experimental Zoology (Series B) 2003;206:1085–1097. doi: 10.1242/jeb.00208. [DOI] [PubMed] [Google Scholar]
- Zelditch ML, Fink WL. Heterochrony and heterotopy: stability and innovation in the evolution of form. Paleobiology. 1996;22:241–254. [Google Scholar]
- Zhou Z, Zhang F. Discovery of an ornithurine bird and its implication for early Cretaceous avian radiation. Proceedings of the National Academy of Science USA. 2005;102:18998–19002. doi: 10.1073/pnas.0507106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink RM, Klicka J. The tempo of avian diversification: a comment on Johnson and Cicero. Evolution. 2006;60:411–412. [PubMed] [Google Scholar]
- Zusi RL. Patterns of diversity in the avian skull. In: Hanken J, Hall BK, editors. The skull, patterns of structural and systematic diversity. Vol. 2. Chicago, IL: University of Chicago Press; 1993. pp. 391–437. [Google Scholar]
- Zusi RL, Livezey BC. Homologies and phylogenetic implications of some enigmatic cranial features in galliform and anseriform birds. Annals of Carnegie Museum. 2000;69:157–193. [Google Scholar]
- Zusi RL, Livezey BC. Variation in the os palatinum and its structural relation to the palatum osseum of birds (Aves) Annals of Carnegie Museum. 2006;75 in press. [Google Scholar]
- Zusi RL, Storer RW. Osteology and myology of the head and neck of the pied-billed grebes (Podilymbus) University of Michigan Miscellaneous Publications of the Museum of Zoology. 1969;139:1–49. [Google Scholar]
- Zusi RL, Warheit KI. On the evolution of intraramal mandibular joints in pseudodontorns (Aves: Odontopterygia) In: Campbell KE, editor. Papers in avian paleontology honoring Pierce Brodkorb. Los Angeles, CA: Natural History Museum of Los Angeles County; 1992. pp. 351–360. [Google Scholar]
- Zweers GA, Vanden Berge JC. Evolutionary transitions in the trophic system of the wader-waterfowl complex. Netherlands Journal of Zoology. 1997a;47:255–287. [Google Scholar]
- Zweers GA, Vanden Berge JC. Birds at geological boundaries. Zoology. 1997b;100:183–202. [Google Scholar]
- Zweers GA, Vanden Berge JC, Berkhoudt H. Evolutionary patterns of avian trophic diversification. Zoology. 1997;100:25–57. [Google Scholar]