Abstract
The reason why women sustain more soft tissue injury than men during physical activity is unknown. Connective tissue properties and extracellular matrix adaptability in human tendon were investigated in models that addressed biochemical, physiological and biomechanical aspects of tendon connective tissue in response to mechanical loading. Habitual training resulted in a larger patellar tendon in men but not in women. Following an acute bout of exercise, men had an elevated tendon collagen synthesis rate and this effect was less pronounced or absent in women. Moreover, levels of circulating oestrogen affected the acute exercise-related increase in collagen synthesis. Finally, the mechanical strength of isolated tendon collagen fascicles in men surpassed that of women. Thus, compared to men, women have (i) an attenuated tendon hypertrophy response to habitual training; (ii) a lower tendon collagen synthesis rate following acute exercise; (iii) a rate of tendon collagen synthesis which is further attenuated with elevated estradiol levels; and (iv) a lower mechanical strength of their tendons. These data indicate that tendons in women have a lower rate of new connective tissue formation, respond less to mechanical loading, and have a lower mechanical strength, which may leave the tissue more susceptible to injury.
Keywords: connective tissue, extracellular matrix, gender, mechanical properties, oestrogen
It is well established that women are more likely to sustain certain connective tissue injuries than men during physical activities (Jones et al. 1993; Bijur et al. 1997). For example, the incidence of anterior cruciate ligament injuries in women is reported to be up to six times that in men participating in similar physical activities (Bjordal et al. 1997; Hewett et al. 2001). The annual cost for these injuries is large (Griffin et al. 2000), and yet the underlying reasons for this gender-specific difference in connective tissue injury remains an enigma. Oestrogen receptor activity is modulated by estradiol (Ciana et al. 2003), and in vitro studies show that estradiol has an inhibiting effect upon collagen formation in ligaments(Liu et al. 1997; Yu et al. 2001), which gives us reason to believe that tendon tissue adaptation to mechanical loading may differ between men and women. We investigated whether a gender difference existed with regards to resting and exercise-induced collagen protein synthesis, tissue mechanical properties and morphology of human tendon, and to what extent this is influenced by circulating levels of estradiol.
Tendon size and physical training
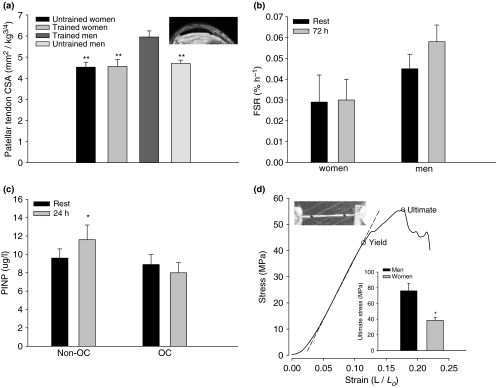
If long-term exercise produces tendon hypertrophy it would result in a lower stress on the tendon and thereby reduce the injury risk. We, therefore, used magnetic resonance imaging to determine the cross-sectional area (CSA) of the patellar tendon in well-trained men and women (running approximately 50 km/week over the last 5 years). Untrained age and gender matched individuals served as controls. Trained men had significantly larger (P < 0.01) patellar tendon CSA (Figure 1a) than untrained men, however, a similar difference could not be detected between trained and untrained women. This suggests that the human patellar tendon in men adapts to regular physical activity by hypertrophy while similar exercise had no detectable impact on the tendon size in women, consistent with gender-specific tendon adaptation.
Figure 1.
(a) The magnetic resonance imaging (MRI) determined patellar tendon cross-sectional area (CSA) for trained and untrained men and women normalized to body mass. Trained men had a greater CSA than untrained men (P < 0.01), however, note that trained women had a similar CSA compared to untrained women. An MRI of the patellar tendon (b). The fractional synthesis rate (FSR) of patellar tendon collagen, in vivo, at rest and 72 h after one-legged kicking exercise from tendon biopsy tissue after a flooding dose of unlabelled and labelled [15N] proline. Compared to the resting values for FSR of collagen in men (Miller et al. 2005), women had a substantially lower resting FSR. Further, in contrast to men who have an elevated FSR in response to an acute bout of exercise, women did not respond with an elevated FSR. (c) The interstitial concentration of aminoterminal propeptide of type I procollaen (PINP), in vivo, at rest and 24 h after 1-h one-legged kicking exercise in young women taking oral contraceptives (OC) and those who did not (non-OC). Non-OC responded to exercise with an elevated collagen synthesis (P < 0.05), while OC users did have a similar synthesis elevation. (d) The stress–strain data from the mechanical test of a single human patellar tendon fascicle indicating both the yield and the ultimate stress prior to complete tissue failure. The bar graph represents the average data form the mechanical testing of single isolated collagen fascicles obtained from the anterior portion of the patellar tendon of young men and women. Collagen fascicles from men reached greater peak stress than that from of women (P < 0.05), and the tangent modulus for fascicles from men (1231 ± 188 MPa) exceeded that of women (576 ± 86 MPa, P < 0.05). The picture shows a single isolated human fascicle in the mechanical testing device.
Tendon collagen synthesis and mechanical loading
To further explore a possible gender-specific collagen response to loading, we determined the rate of protein synthesis of patellar tendon collagen, in vivo, at rest and 72 h after one-legged kicking exercise by taking tendon biopsies 2 h after a flooding dose of unlabelled and labelled [15N] proline (Miller et al. 2005). The labelling in target proteins was determined by gas-chromatography mass-spectrometry (GC-MS)(blood) and Gas Chromatography–Combustion–Isotope Ratio Mass Spectrometry (GC-C-IRMS) (tendon). Resting fractional synthesis rate of collagen was markedly lower in women (Figure 1b) compared to the levels we have previously measured in men (Miller et al. 2005). Men have an elevated protein synthesis rate in response to exercise (Miller et al. 2005), but here we show that women do not respond to exercise with an elevated protein synthesis rate (Figure 1b), (P < 0.001). Interestingly, there was a tendency towards an elevated collagen synthesis in the women when tested in the early follicular phase (rest 0.0023 ± 0.003%/h, 72 h 0.0037 ± 0.017%/h) of the menstrual cycle when the level of estradiol is low (P = 0.09, n = 4). These data gave further support to the notion that gender, and therefore hormonal milieu, may influence connective tissue adaptability to physical activity.
Post-menopausal women have a greater Achilles tendon CSA than young women, although they load their tendons less, as indicated by their reduced muscle strength (Magnusson et al. 2003). This apparent contradiction may be related to the reduced inhibiting effect of estradiol on collagen synthesis. To more specifically study if circulating estradiol levels influenced the human collagen synthesis response to an acute boat of exercise, we measured a marker of collagen synthesis in young women taking oral contraceptives (OC) (n = 6) and those who did not (n = 6) (Figure 1c). A microdialysis catheter was introduced in the peritendinous tissue of the patellar tendon, in vivo, to sample the interstitial concentration of aminoterminal propeptide of type I procollaen (PINP), which reflects collagen type I synthesis, at rest and 24 h after 1-h one-legged kicking exercise (67% of workload maximum, Wmax) (Langberg et al. 1999). Non-users of OC tested in the early follicular phase of the menstrual cycle responded to the acute bout of exercise with an elevated collagen synthesis (P < 0.05), whereas the OC-users that have elevated circulating levels of estradiol did not demonstrate any change in PINP levels (Figure 1c). These data extend the notion that estradiol can influence collagen metabolism by reducing the acute exercise induced response of collagen synthesis.
Mechanical properties of human tendon
To explore if the observed gender-specific differences in collagen synthesis and hypertrophy also meant that were differences in tissue strength, we performed mechanical testing of single isolated collagen fascicles obtained during elective surgery (anterior cruciate ligament reconstruction) from the anterior portion of the patellar tendon of young men (n = 6) and women (n = 6). The collagen fascicles (ø approximately 330 μm) were tested in a mechanical rig, placed under a stereoscopic microscope equipped with a digital camera that recorded elongation (Haraldsson et al. 2005) (Figure 1d). Collagen fascicles from men reached greater ultimate stress than those from women (P < 0.05). Strain did not differ, but the tangent modulus for fascicles from men (1231 ± 188 MPa) exceeded that of women (576 ± 86 MPa, P < 0.05)(Figure 1d). These results suggest that in addition to gender-specific differences in tendon collagen metabolism there are also differences in the mechanical properties of the tendon fascicles. The extracellular component responsible for the distinction in mechanical properties remains unknown, but differences in collagen fibril diameter within a human tendon can leave it more susceptible to injury (Magnusson et al. 2002) and is therefore a likely explanation.
Conclusion
In summary, connective tissue properties and extracellular matrix adaptability were investigated in men and women. Habitual training resulted in a larger patellar tendon in men, but not women. Following an acute bout of exercise, men had an elevated tendon collagen synthesis rate compared to women. Moreover, circulating oestrogen inhibited the acute exercise-related increase in tendon collagen synthesis. Finally, the mechanical strength of isolated collagen fascicles from men surpassed that of women. Collectively, these data show that women have a lower rate of new connective tissue formation in tendon tissue, respond less to mechanical loading, and have a lower mechanical strength, which may leave the tissue more susceptible to injury.
References
- Bijur PE, Horodyski M, Egerton W, Kurzon M, Lifrak S, Friedman S. Comparison of injury during cadet basic training by gender. Arch. Pediatr. Adolesc. Med. 1997;151:456–461. doi: 10.1001/archpedi.1997.02170420026004. [DOI] [PubMed] [Google Scholar]
- Bjordal JM, Arnøy F, Hannestad B, Strand T. Epidemiology of anterior cruciate ligament injuries in soccer. Am. J. Sports Med. 1997;25:341–345. doi: 10.1177/036354659702500312. [DOI] [PubMed] [Google Scholar]
- Ciana P, Raviscioni M, Mussi P, et al. In vivo imaging of transcriptionally active estrogen receptors. Nat. Med. 2003;9:82–86. doi: 10.1038/nm809. [DOI] [PubMed] [Google Scholar]
- Griffin LY, Agel J. Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J. Am. Acad. Orthop. Surg. 2000;8:141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- Haraldsson BT, Aagaard P, Krogsgaard M, Alkjaer T, Kjaer M, Magnusson SP. Region specific mechanical properties of the human patella tendon. J. Appl. Physiol. 2005;98:1006–1012. doi: 10.1152/japplphysiol.00482.2004. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer T E, Ford KR. Prevention of anterior cruciate ligament injuries. Curr. Womens Health Rep. 2001;1:218–224. [PubMed] [Google Scholar]
- Jones BH, Bovee MW, Harris JM, III, Cowan DN. Intrinsic risk factors for exercise-related injuries among male and female army trainees. Am. J. Sports Med. 1993;21:705–710. doi: 10.1177/036354659302100512. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bülow J, Kjær M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J. Physiol. 1999;521(Pt 1):299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SH, Al-shaikh RA, Panossian V, Finerman GAM, Lane JM. Estrogen affects the cellular metabolism of the anterior cruciate ligament: a potential explanation for female athletic injury. Am. J. Sports Med. 1997;25:704–709. doi: 10.1177/036354659702500521. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Qvortrup K, Larsen JO, et al. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol. 2002;21:369–377. doi: 10.1016/s0945-053x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjær M. Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J. Gerontol. A Biol. Sci. Med. Sci. 2003;58:123–127. doi: 10.1093/gerona/58.2.b123. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J. Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin. Orthop. 2001;383:268–281. doi: 10.1097/00003086-200102000-00031. [DOI] [PubMed] [Google Scholar]