Abstract
Subendocardial remodelling of the left ventricular (LV) non-infarcted myocardium has been poorly investigated. Previously, we have demonstrated that low coronary driving pressure (CDP) early postinfarction was associated with the subsequent development of remote subendocardial fibrosis. The present study aimed at examining the role of CDP in LV remodelling and function following infarction. Haemodynamics were performed in Wistar rats immediately after myocardial infarction (MI group) or sham surgery (SH group) and at days 1, 3, 7 and 28. Heart tissue sections were stained with HE, Sirius red and immunostained for α-actin. Two distinct LV regions remote to infarction were examined: subendocardium (SE) and interstitium (INT). Myocyte necrosis, leucocyte infiltration, myofibroblasts and collagen volume fraction were determined. Compared with SH, MI showed lower CDP and LV systolic and diastolic dysfunction. Necrosis was evident in SE at day 1. Inflammation and fibroplasia predominated in SE as far as day 7. Fibrosis was restricted to SE from day 3 on. Inflammation occurred in INT at days 1 and 3, but at a lower grade than in SE. CDP correlated inversely with SE necrosis (r = −0.65, P = 0.003, at day 1), inflammation (r = −0.76, P < 0.001, at day 1), fibroplasia (r = −0.47, P = 0.04, at day 7) and fibrosis (r = −0.83, P < 0.001, at day 28). Low CDP produced progressive LV expansion. Necrosis at day 1, inflammation at days 3 and 7, and fibroplasia at day 7 correlated inversely with LV function. CDP is a key factor to SE integrity and affects LV remodelling and function following infarction.
Keywords: coronary driving pressure, myocardial infarction, myocardial remodeling, subendocardial fibrosis
Cardiac remodelling is characterized by molecular, cellular and interstitial changes of the myocardium resulting in changes in size, shape and function of the heart (Cohn et al. 2000). Remodelling that follows myocardial infarction manifests by wound healing at the site of wall necrosis and, depending on infarct size, by myocyte hypertrophy and extracellular matrix changes in the remote non-infarcted myocardium, where there is evidence of excessive accumulation of collagen fibers (Volders et al. 1993). Interstitial fibrosis is recognized as a key pathological change of remodelling and to predispose the heart to cardiac dysfunction (Wilke et al. 1996; Van Kerckhoven et al. 2000). The pathological changes that occur in the non-infarcted subendocardial region have been poorly investigated so far (Michel et al. 1988). In fact, subendocardial accumulation of fibrosis in the remote myocardium does occur and seems to be accounted for the impairment of the coronary perfusion pressure early in the course of infarction (de Carvalho Frimm et al. 2003). In infarcts of large magnitude, the haemodynamic changes that follow coronary artery occlusion, particularly the increase in left ventricular end-diastolic pressure concurrent with the decrease in systemic blood pressure, contribute to the impairment of coronary perfusion and may thus jeopardize the integrity of the non-infarcted myocardium. As the subendocardium is the myocardial layer most likely affected by perfusion deficits (Toyota et al. 2005), it is reasonable to suppose that in some yet unrecognized extent prolonged subendocardial ischaemia would further affect ventricular remodelling. In that case, subendocardial fibrosis may indeed represent a reparative process contrasting to reactive interstitial fibrosis that appears in the remainder layers of the non-infarcted myocardium (Weber et al. 1989). By interfering with myocardial integrity, subendocardial injury may therefore be implicated in postinfarction ventricular dysfunction.
The main objectives of the present study were twofold: (i) to investigate the role of coronary driving pressure in subendocardial remodeling, and (ii) to evaluate the role of subendocardial pathological changes in left ventricular function, using a rat model of myocardial infarction. Of particular additional interest was the evaluation of left ventricular remodelling, taking into account two distinct layers of the remote non-infarcted regions: the subendocardium and the interstitial space corresponding to the remainder myocardial layers.
Materials and methods
All procedures were carried out in accordance with the norms of the Brazilian College of Animal Research and The Universities Federation for Animal Welfare. Our Institutional Ethical Committee approved the protocol.
Male Wistar rats weighing 300–350 g were used. Four groups of rats with myocardial infarction (MI) and their respective sham controls (SH) were constituted according to the interval of follow-up: (i) early acute phase: MI 1 (n = 9) and SH 1 (n = 9), 1 day; (ii) late acute phase: MI 3 (n = 15) and SH 3 (n = 16), 3 days; (iii) subacute phase: MI 7 (n = 9) and SH 7 (n = 10), 7 days; and (iv) chronic phase: MI 28 (n = 11) and SH 28 (n = 11), 28 days.
Haemodynamic measurements
Initial and final haemodynamic measurements were determined after the experimental surgery and at the end of the follow-up respectively. Animals were anaesthetized with intraperitoneal Ketamine, 50 mg/kg, and Pentobarbital, 25 mg/kg. Systemic and left ventricular blood pressures were determined simultaneously via femoral and carotid catheters introduced into the abdominal aorta and into the left ventricular cavity respectively. The animals were put under mechanical ventilation (Model 683; Harvard Apparatus Inc., South Natick, MA, USA) and submitted to left coronary artery ligation or sham operation, according to a previously described technique (Selye et al. 1960). Haemodynamics were continuously recorded during 30 min after the closure of the chest wall. The average of each beat-to-beat measurement registered during the last 10 min was considered for analysis.
The following parameters were computed: mean arterial blood pressure (mmHg), left ventricular end-diastolic pressure (mmHg), left ventricular systolic pressure (mmHg), maximum rate of increase of left ventricular pressure (+dP/dt, mmHg/s), and maximum rate of decrease of left ventricular pressure (−dP/dt, mmHg/s). The last two were used to estimate left ventricular systolic and diastolic function respectively.
Coronary driving pressure (mmHg) was calculated as the difference between mean arterial blood pressure and left ventricular end-diastolic pressure. It was used to estimate the pressure gradient for left ventricular blood flow (Cross et al. 1961).
At the end of each follow-up period (days 1, 3, 7 and 28), haemodynamics were repeated under the same conditions.
Heart morphometric study
After euthanasia with overdose anaesthesia and cadmium chloride administration, the heart was preserved by retrograde perfusion-fixation with 10% formalin in a phosphate-buffered saline solution. The perfusion pressure employed corresponded to the in vivo systemic diastolic blood pressure. The heart was then removed, the atria were trimmed away, and the ventricular weight index (mg/g) was calculated by normalizing ventricular weight to body weight. After fixation, a 1–2 mm coronal slice of the heart, including both ventricles at the equatorial plane, was embedded in paraffin and cut into 5 μm sections. Tissue sections were stained with haematoxylin–eosin (HE), Sirius red, alcian blue pH 2.5, and also underwent immunohistochemistry. A digital image analysis system (Leica Imaging Systems Ltd., Cambridge, UK) was used for morphometric measurements.
The ratio between endocardial infarct surface length and total left ventricular endocardial circumference and the ratio between epicardial infarct surface length and total left ventricular epicardial circumference were averaged to calculate infarct size (Pfeffer et al. 1985).
Left ventricular expansion index was calculated according to the following formula:
Left ventricular expansion index = (left ventricular cavity area/left ventricular total area) x (interventricular septum thickness/infarct wall thickness) (Whittaker et al. 1991).
The same formula was used to calculate left ventricular expansion index in sham animals, replacing the infarct wall thickness by the left ventricular free wall thickness.
Remote non-infarcted myocardium
Two regions were analysed separately – the subendocardium and the interstitium. The subendocardium was defined as corresponding to the inner third of the non-infarcted left ventricular region and the interstitium as the remainder outer two-thirds.
The amount of pathological changes in the subendocardium was estimated by the subendocardial lesion area (SE lesion, %) in HE-stained sections. It was calculated as the per cent ratio between the area of the subendocardium affected by inflammatory cell and/or collagen infiltration and the entire area of the remote non-infarcted left ventricular wall.
To estimate cardiomyocyte hypertrophy, HE-stained sections were examined under ×1000 magnification. The myocyte diameter (μm) around oval and central nuclei of longitudinally displayed myocytes was measured.
For each of the following measurements, the entire subendocardial region and a total of 20 interstitial microscopic fields were examined.
Myocyte necrosis (cells/mm2) was estimated in HE-stained sections under ×1000 magnification. Nuclear pyknosis and karyolysis as well as cytoplasmic changes including vacuolization, contraction bands and hypereosinophilia were taken into account altogether (Kumar et al. 2004).
Leucocyte cell infiltration (cells/mm2) was estimated in HE-stained sections under ×1000 magnification. Inflammatory cells were identified by nuclear and cytoplasmic morphological aspects. Cells with morphological characteristics of fibroblasts, cardiomyocytes, endothelial vascular cells and smooth muscle vascular cells were excluded.
Glycosaminoglycan fraction (%) was estimated in alcian blue-stained sections under ×1000 magnification. The main non-sulphated glycosaminoglycan identified by this method is hyaluronic acid (Baldwin et al. 1994). Glycosaminoglycan fraction was determined as the percentage of cyan blue-stained areas per total myocardial area, excluding perivascular regions.
Fibroplasia was estimated by measuring positive α-smooth muscle actin myofibroblasts (cells/mm2) detected by immunohistochemistry under ×1000 magnification. The tissue sections were incubated overnight with a 1:500 dilution of the mouse anti-human α- smooth muscle actin antibody (Sigma Chemical Co, St Louis, MO, USA) at 4 °C. Sections were then incubated with labelled streptavidin-biotin peroxidase kit (Dako LSAB+ kit; Dako Corporation, Carpinteria, CA, USA) and diaminobenzidine for colour development. Finally, the sections were faintly counterstained with haematoxylin. Vascular smooth muscle cells with intense staining were used as positive controls. Negative controls were obtained by omitting the primary antibody and using a non-immune bovine serum.
Collagen volume fraction (%) was estimated in Sirius red-stained sections under ×580 magnification. Collagen volume fraction was determined as the percentage of red-stained connective tissue areas per total myocardial area, excluding perivascular areas.
Statistical analysis
Data are expressed as mean ± SEM. One-way analysis of variance and one-way repeated measures analysis of variance complemented by the Wald test were used to compare the groups. The hypothesis of interaction between MI and SH groups and among the different intervals of follow-up was tested beforehand.
Linear regression was used to test the following potential relationships: (i) initial coronary driving pressure and left ventricular dilatation; (ii) initial coronary driving pressure and morphometric variables in remote non-infarcted myocardium; and (iii) morphometric variables and left ventricular function at the end of each follow-up. Normality and equal variance were tested in all analyses. Statistical significance was established at P < 0.05. All analyses were performed using SAS software (Statistical Analysis System, version 9.1; SAS Institute Inc., Cary, NC, USA).
Results
Haemodynamic variables
The initial haemodynamic parameters measured immediately after the experimental procedure and the final values obtained at the end of each follow-up period are depicted in Table 1.
Table 1.
Initial haemodynamic variables determined immediately after surgery and final haemodynamic variables determined at the end of 1, 3, 7, and 28 days of follow-up
| Groups (n) | SH 1 (9) | MI 1 (9) | SH 3 (16) | MI 3 (15) | SH 7 (10) | MI 7 (9) | SH 28 (11) | MI 28 (11) |
|---|---|---|---|---|---|---|---|---|
| MAP (mmHg) | ||||||||
| Initial | 114 ± 4 | 85 ± 6* | 107 ± 3 | 95 ± 6* | 100 ± 6 | 85 ± 6* | 108 ± 3 | 93 ± 5* |
| Final | 111 ± 5 | 86 ± 6* | 101 ± 4 | 86 ± 5* | 101 ± 4 | 79 ± 8* | 97 ± 9 | 94 ± 4* |
| LVSP (mmHg) | ||||||||
| Initial | 132 ± 5 | 104 ± 6* | 126 ± 4 | 117 ± 6* | 123 ± 5 | 103 ± 6* | 127 ± 5 | 112 ± 5* |
| Final | 133 ± 7 | 107 ± 6* | 125 ± 5 | 113 ± 5* | 128 ± 4 | 105 ± 8* | 127 ± 9 | 112 ± 4* |
| LVEDP (mmHg) | ||||||||
| Initial | 5.0 ± 0.8 | 12.6 ± 1.3* | 5.4 ± 0.5 | 11.2 ± 1.3* | 5.5 ± 0.7 | 10.1 ± 1.4* | 5.5 ± 0.4 | 10.2 ± 1.2* |
| Final | 5.3 ± 0.8 | 10.6 ± 1.0* | 4.9 ± 0.5 | 9.1 ± 1.5* | 6.0 ± 0.8 | 13.7 ± 3.4* | 7.3 ± 0.5 | 10.6 ± 2.1* |
| CDP (mmHg) | ||||||||
| Initial | 109 ± 4 | 72 ± 7* | 102 ± 4 | 83 ± 7* | 95 ± 6 | 75 ± 7* | 102 ± 3 | 83 ± 6* |
| Final | 106 ± 4 | 75 ± 5* | 96 ± 4 | 78 ± 4* | 95 ± 4 | 65 ± 9* | 90 ± 9 | 84 ± 5* |
| +dP/dt (mmHg/s) | ||||||||
| Initial | 8310 ± 570 | 5728 ± 336* | 7757 ± 411 | 6557 ± 518* | 7451 ± 718 | 5374 ± 376* | 8045 ± 448 | 6099 ± 406* |
| Final | 7730 ± 602 | 5907 ± 382* | 7432 ± 384 | 6673 ± 410* | 7574 ± 305 | 4973 ± 639* | 7002 ± 665 | 6065 ± 310* |
| −dP/dt (mmHg/s) | ||||||||
| Initial | 6648 ± 505 | 3248 ± 300* | 6269 ± 405 | 4112 ± 373* | 5446 ± 518 | 3316 ± 289* | 6113 ± 364 | 3822 ± 384* |
| Final | 6840 ± 376 | 4858 ± 303*† | 6524 ± 259 | 4775 ± 264* | 6750 ± 665 | 4045 ± 555*† | 6057 ± 818 | 5184 ± 512† |
SH, sham group; MI, myocardial infarction group; MAP, mean arterial pressure; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; CDP, coronary driving pressure; +dP/dt, maximum positive first derivative of left ventricular pressure; −dP/dt, maximum negative first derivative of left ventricular pressure.
P < 0.05 vs. SH.
P < 0.05 final vs. initial.
The mean arterial pressure was lower in all MI groups compared with SH, both initially and at the end of each follow-up. In each MI group, the final mean arterial pressure did not change from initial values and there was no significant difference of mean arterial pressure among the four succeeding follow-up periods. Correspondingly, left ventricular systolic pressure was also lower in all MI groups compared with SH. In each MI group, the initial and final left ventricular systolic pressure did not differ and there was no significant change among the succeeding follow-up periods.
Left ventricular end-diastolic pressure was 1.5- to 2.5-fold greater in MI groups compared with SH, both immediately after infarction and at the end of follow-up. In each MI group, final left ventricular end-diastolic pressure did not change from initial values and there was no significant difference of left ventricular end-diastolic pressure among the four succeeding follow-up intervals. Accordingly, coronary driving pressure followed the pattern observed for systemic blood pressure and left ventricular end-diastolic pressure. In average, it was 22% lower in MI groups compared with SH. Final and initial values did not differ and there was no significant difference of coronary driving pressure among the four succeeding follow-up intervals.
MI groups had +dP/dt 13% to 31% lower than that of SH. In each MI group, final +dP/dt did not differ from initial values and there was no significant difference of +dP/dt among the four succeeding follow-up intervals. As for −dP/dt, it was lower in all MI groups than SH except for final −dP/dt in MI 28. Final −dP/dt in this group (and also in MI 1 and MI 7) was higher than values taken immediately after infarction.
Morphometry
Left ventricular remodelling
Parameters of left ventricular remodelling are depicted in Table 2. Infarct size was comparable among all MI groups regardless of the period of follow-up. A twofold increase in left ventricular chamber size occurred as early as day 1 after infarction. Maximal left ventricular expansion occurred in MI rats at day 7, when left ventricular expansion index was fivefold greater than that of SH rats.
Table 2.
Morphometric parameters of left ventricular remodelling investigated at the end of each of the four periods of follow-up: 1, 3, 7, and 28 days
| Groups | SH 1 | MI 1 | SH 3 | MI 3 | SH 7 | MI 7 | SH 28 | MI 28 |
|---|---|---|---|---|---|---|---|---|
| IS (%) | 40 ± 4 | 37 ± 3 | 37 ± 5 | 39 ± 5 | ||||
| LVEI | 0.22 ± 0.03 | 0.47 ± 0.08* | 0.25 ± 0.03 | 0.64 ± 0.06* | 0.26 ± 0.04 | 1.32 ± 0.27*†† | 0.25 ± 0.04 | 1.44 ± 0.38* |
| SE lesion (%) | 0.35 ± 0.25 | 8.08 ± 3.36* | 0.24 ± 0.15 | 7.04 ± 2.71* | 0.32 ± 0.32 | 2.82 ± 1.37*†† | 0.52 ± 0.26 | 0.63 ± 0.21*††† |
| VW (mg/g) | 5.45 ± 0.23 | 4.89 ± 0.22 | 4.69 ± 0.26 | 3.65 ± 0.30 | 3.74 ± 0.12 | 4.00 ± 0.09 | 3.68 ± 0.13 | 3.84 ± 0.18 |
| Myocyte Ø (μm) | 10.69 ± 0.09 | 11.25 ± 0.26* | 9.70 ± 0.31 | 10.28 ± 0.38*† | 9.88 ± 0.19 | 11.67 ± 0.48*†† | 9.56 ± 0.21 | 11.02 ± 0.41* |
| Body weight (g) | 302 ± 3 | 301 ± 4 | 300 ± 3 | 302 ± 5 | 322 ± 5 | 310 ± 3 | 376 ± 6 | 380 ± 11 |
SH, sham group; MI, myocardial infarction group; IS, infarct size; LVEI, left ventricular expansion index; VW, ventricular weight; Ø, diameter; SE, subendocardial.
P < 0.05 vs. SH.
P < 0.05 vs. MI 1
P < 0.05 vs. MI 3
P < 0.05 vs. MI 7.
The subendocardial area of the remote non-infarcted left ventricular wall with pathological changes was largest at day 1 and then decreased progressively up to day 28. This result corresponds to a predominant cellular infiltration occupying a relatively larger endocardial region in the early phases postinfarction in contrast to a predominant fibrosis deposition occupying a relatively thinner endocardial region at the chronic phase.
Ventricular weight did not differ between MI and SH groups at any time point studied. Myocyte hypertrophy was already evident in the remote non-infarcted myocardium at day 1, was maximal at day 7 and then remained unchanged.
Remote non-infarcted myocardium
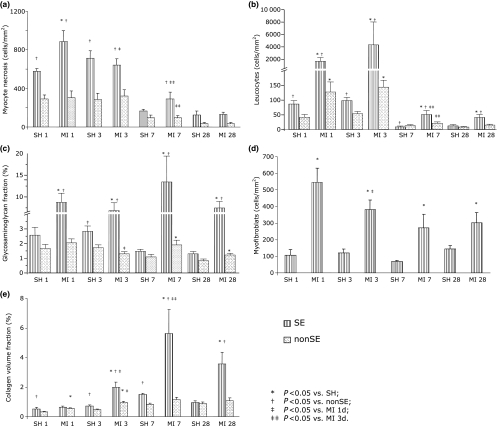
The main pathological changes that were investigated to distinguish the remodelling of the subendocardial region from that observed in the interstitium of the remainder layers of the non-infarcted left ventricle are represented in Figure 1.
Figure 1.
Pathological changes examined in two distinct non-infarcted myocardial regions, subendocardium and interstitium, at the end of 1, 3, 7 and 28 days of follow-up: (a), myocyte necrosis; (b), inflammation; (c), glycosaminoglycans; (d), fibroplasia and (e), fibrosis.
Myocyte necrosis was evident in the subendocardial region and was of greater magnitude in MI rats than in SH at day 1. Subendocardial myocyte necrosis decreased progressively up to day 7 in MI rats.
Leucocyte infiltration was greater in the subendocardial region of MI rats compared with SH at days 1, 3, 7 and 28. In the interstitium, leucocyte infiltration was much less evident but still more pronounced in MI rats than in SH at days 1 and 3. In MI rats, leucocyte infiltration within the subendocardium was maximal at days 1 and 3, decreased at day 7, and then remained unchanged. Similarly, in the interstitium, leucocyte infiltration was maximal at days 1 and 3, decreased at day 7, and then remained unchanged.
The increase in glycosaminoglycan fraction was again predominant in the subendocardial region, being greater in MI rats compared with SH regardless the follow-up intervals. The increase in subendocardial glycosaminoglycan fraction remained constant up to day 28. In the left ventricular interstitium, the glycosaminoglycan fraction of MI rats was greater than in SH at days 7 and 28.
Myofibroblasts were found almost exclusively in the subendocardium, where in MI rats they outnumbered SH by 3- to 5-fold regardless the follow-up interval. The highest numbers were observed at day 1, fell by 30% at day 3, and thereafter remained statistically unchanged.
The increase in collagen volume fraction observed in MI rats as compared with SH was again predominant in the subendocardial region and observed from day 3 on. In the interstitium, fibrosis deposition was much less evident. Moreover, interstitial collagen did not differ substantially between MI and SH groups.
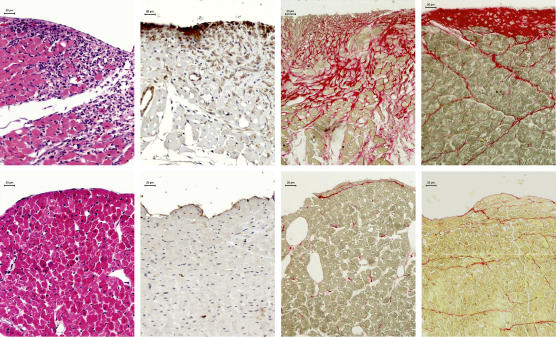
In Figure 2 are depicted the different phases of subendocardial remodelling.
Figure 2.
Photomicrographs depicting non-infarcted subendocardial regions of the left ventricle in the rat model of infarction. Top panels represent rats with myocardial infarction and bottom panels sham animals. Left panels show inflammatory cell reaction in HE-stained tissue sections at day 3. Left middle panels show myofibroblasts positive to α-smooth muscle actin immunostaining (brown cytoplasm) in tissue sections co-stained with haematoxylin at day 7. The last two right panels show red stained fibrosis in Sirius red-stained tissue sections at days 7 and 28 respectively. Prominent differences in each of these phases of subendocardial remodelling of the non-infarcted left ventricular myocardium can be observed between infarcted and sham rats.
Relationship between initial coronary driving pressure and left ventricular remodelling
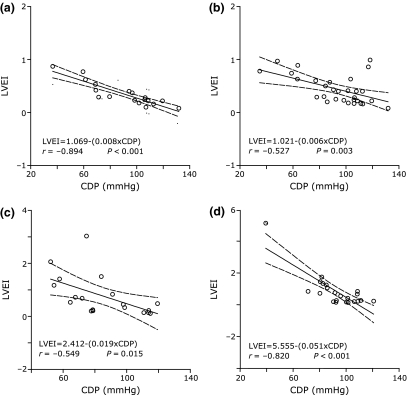
There were inverse correlations between the initial coronary driving pressure and the left ventricular expansion index at all follow-up intervals investigated: day 1, day 3, day 7, and day 28 (Figure 3).
Figure 3.
Relationships found between initial coronary driving pressure (CDP) and subsequent left ventricular expansion index (LVEI) measured at days 1 (panel A), 3 (panel B), 7 (panel C), and 28 (panel D). Lower initial CDP values were associated with larger left ventricular dimensions at each time point investigated.
Relationship between initial coronary driving pressure and remote non-infarcted myocardium
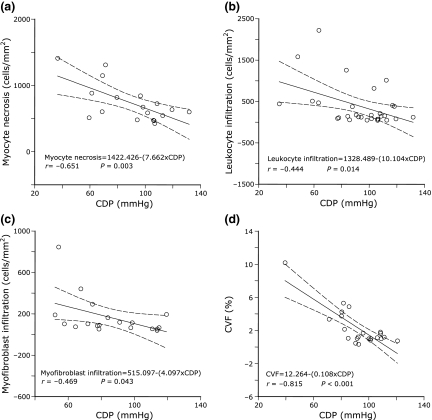
Initial coronary driving pressure was inversely related to different left ventricular subendocardial lesions according to the time point when each of these lesions was more pronounced. These results are represented in Table 3 and in Figure 4. Low coronary driving pressure was associated with more intense myocyte necrosis at day 1, with leucocyte cell infiltration during the first 7 days, and with fibrosis at days 7 and 28. While coronary driving pressure was inversely related to glycosaminoglycans at early and late phases of infarction, the association with fibroplasia was apparent during all time points investigated.
Table 3.
Relationships between initial coronary driving pressure and the different types of subendocardial lesions in the remote non-infarcted myocardium investigated at the end of each of the four periods of follow-up. Only correlation coefficients with statistical significance are represented
| day 1 | day 3 | day 7 | day 28 | |
|---|---|---|---|---|
| Initial coronary driving pressure vs. left ventricular subendocardial remodelling | ||||
| Myocyte necrosis | −0.65 | ns | ns | ns |
| Leucocyte infiltration | −0.76 | −0.44 | −0.53 | ns |
| Glycosaminoglycan fraction | −0.65 | ns | ns | −0.58 |
| Fibroplasia | −0.67 | −0.38 | −0.47 | −0.46 |
| Collagen volume fraction | ns | ns | −0.50 | −0.83 |
ns, non-significant; P > 0.05.
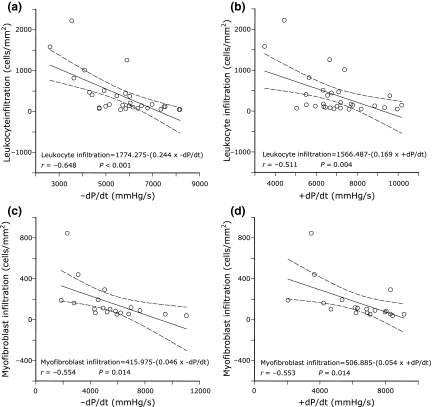
Figure 4.
Relationships found between initial coronary driving pressure (CDP) and subsequent subendocardial lesions representative of myocyte necrosis at day 1 (panel A), leukocyte infiltration at day 3 (panel B), myofibroblast infiltration at day 7 (panel C), and fibrosis estimated by collagen volume fraction (CVF, panel D) at day 28.
Initial coronary driving pressure was weakly correlated with interstitial leucocyte infiltration only at the late acute phase and with glycosaminoglycans and collagen only at the subacute and chronic phases.
Relationship between remote non-infarcted myocardium and left ventricular function
The following left ventricular subendocardial lesions were inversely associated with systolic function, assessed by +dP/dt: leucocyte cell infiltration at days 3 and 7, and glycosaminoglycan fraction, fibroplasia, and collagen volume fraction at day 7.
The following left ventricular subendocardial lesions were inversely associated with diastolic function, assessed by −dP/dt: myocyte necrosis at day 1, leucocyte cell infiltration at days 3 and 7, glycosaminoglycan fraction at days 1 and 7, fibroplasia at days 1, 3, and 7, and collagen volume fraction at days 3 and 7.
Interstitial collagen volume fraction was not related to either +dP/dt or −dP/dt at any of the follow-up intervals studied. In contrast, interstitial glycosaminoglycan fraction did relate to −dP/dt at day 7 (r = −0.63; P = 0.004) and to +dP/dt at days 7 (r = −0.47; P = 0.04) and 28 (r = −0.51; P = 0.03) (Table 4 and Figure 5).
Table 4.
Relationships between the different types of left ventricular subendocardial lesions in the remote non-infarcted myocardium and left ventricular systolic and diastolic function at the end of each of the four periods of follow-up. Only correlation coefficients with statistical significance are represented
| +dP/dt | −dP/dt | |||||||
|---|---|---|---|---|---|---|---|---|
| day 1 | day 3 | day 7 | day 28 | day 1 | day 3 | day 7 | day 28 | |
| Myocyte necrosis | ns | ns | ns | ns | −0.53 | ns | ns | ns |
| Leucocyte infiltration | ns | −0.51 | −0.52 | ns | ns | −0.65 | −0.46 | ns |
| Glycosaminoglycan fraction | ns | ns | −0.63 | ns | −0.48 | ns | −0.54 | ns |
| Fibroplasia | ns | ns | −0.55 | ns | −0.61 | −0.72 | −0.55 | ns |
| Collagen volume fraction | ns | ns | −0.55 | ns | ns | −0.50 | −0.47 | ns |
+dP/dt, maximum positive first derivative of left ventricular pressure; −dP/dt, maximum negative first derivative of left ventricular pressure; ns, non-significant; P > 0.05.
Figure 5.
Relationships found between subendocardial lesions and left ventricular systolic function (+dP/dt) and diastolic function (−dP/dt). In panels A and B, the inverse correlations found between leucocyte infiltration and left ventricular function at day 3 are represented. In panels C and D, the inverse correlations between myofibroblast infiltration and left ventricular function at day 7 are represented.
Discussion
The present study demonstrated that coronary driving pressure lowers immediately after infarction and remains low for at least 28 days. The pathological changes in the remote non-infarcted regions were represented by myocyte necrosis, inflammatory cell infiltration, increased glycosaminoglycans, fibroplasia and fibrosis accumulation, all of them predominating in the subendocardial layer of the left ventricle. The decrease in coronary driving pressure was associated with the appearance of each of these different subendocardial lesions, according to the period when they were more pronounced. Moreover, low perfusion pressure was inversely related to the progressive dilatation of the left ventricle. Subendocardial changes implicated in left ventricular diastolic and systolic dysfunction at the acute and subacute phases post infarction. These findings corroborate the speculation that subendocardial damage occurs by an ischaemia-related mechanism and is followed by progressive left ventricular remodelling.
The prevailing haemodynamic changes observed at different intervals in this study characterize large magnitude infarctions often resulting in ventricular remodelling and dysfunction (Pfeffer et al. 1979). The average infarct size was 35% in all groups. In MI rats, ventricular weight was comparable with that of SH documenting, in view of myocyte enlargement, the development of hypertrophy of the remote non-infarcted myocardium. The appearance of left ventricular systolic dysfunction in large infarcts may seemingly play a pivotal role in further impairing coronary perfusion pressure as far as systemic blood pressure decreases and left ventricular filling pressure raises. Accordingly, a vicious haemodynamic cycle characterized by poor left ventricular function, low perfusion pressure and subendocardial injury is established immediately after infarction and gives rise to progressive cardiac chamber dilatation. In this circumstance, coronary driving pressure becomes critical, especially if we take into account that coronary flow reserve may be also impaired (Karam et al. 1990).
The observation of coagulation necrosis of myocytes, leucocyte cell infiltration, fibroplasia and fibrosis corroborates the assumption of ongoing ischaemia followed by a reparative process of wound healing taking place in the remote subendocardium. This region is reported as the myocardial layer most susceptible to underperfusion states (Toyota et al. 2005; Wang et al. 2005). Inflammation, although much more marked at the acute phases, was still present at the chronic phase when coronary driving pressure remained low. The relationship found between low coronary driving pressure and subendocardial damage at different intervals postinfarction suggests a key role for persistent subendocardial ischaemia in cardiac remodeling. Acute and subacute subendocardial changes, but not subendocardial fibrosis at day 28, explained left ventricular dysfunction. As the area of subendocardial lesion was larger at the early phases, when the granulation tissue predominated, than that found at the chronic phase, when fibrosis predominated, it is likely that the former better represents the amount of myocardial tissue damage in that region. In fact, as remodelling progresses subendocardial scar thinning develops. The abundant subendocardial changes implicated in acute and subacute left ventricular dysfunction were no more apparent at the chronic phase.
In addition to the large amounts of glycosaminoglycans identified by alcian blue staining in the subendocardial region, increased glycosaminoglycan fraction was also found in the interstitium. Interestingly, interstitial glycosaminoglycan fraction showed an inverse relationship with left ventricular function at subacute and chronic phases, probably pointing out to a role for extracellular matrix remodelling of the interstitium in sustaining cardiac dysfunction. The main non-sulphated glycosaminoglycan present in the extracellular matrix is hyaluronic acid. Hyaluronic acid plays a role in inflammatory processes by promoting interstitial oedema (Waldenstrom et al. 1991) and facilitating the migration and proliferation of macrophages (Savani et al. 2000). Recently, a hyaluronic acid-rich provisional matrix has been demonstrated to antedate fibrosis during infarct wound healing (Dobaczewski et al. 2006).
In contrast to many previous studies (Sun et al. 2000; Wei et al. 2000; Yu et al. 2001), there was no evidence of increased fibrillar collagen in the interstitium where myofibroblasts were likewise scarcely found. It is likely that a number of previous investigations by not examining separately subendocardial and interstitial regions have thus overestimated interstitial fibrosis. On the other hand, it is also likely that interstitial fibrosis may succeed subendocardial fibrosis and appear only during subsequent phases of remodelling. If that is the case, the excess of alcian blue staining observed in the interstitium at days 7 and 28 postinfarction may presumably antedate subsequent fibrosis deposition.
Interstitial fibrosis is attributable among other factors to local renin-angiotensin system activation (Sun et al. 1994; Weber et al. 1995; de Carvalho Frimm et al. 1997) leading to enhanced collagen production by cardiac fibroblasts (Sun & Weber 1996). There is activation of tissue metalloproteinases (Deten et al. 2001), ventricular dilatation and myocardial fibrosis accumulation. As the extracellular matrix changes in the subendocardial layer seem to antedate those appearing in the interstitium, we speculate that interstitial fibrosis may result from prior subendocardial injury. By affecting the integrity of the subendocardial layer of the remote myocardium, the decrease in coronary driving pressure may indeed impair diastolic function, further augment left ventricular end-diastolic pressure and diastolic stress and thus result in left ventricular dilatation and dysfunction.
Study limitations
The pathological changes occurring in the subendocardial non-infarcted region most likely account for a reparative process to myocyte necrosis. In fact, nuclear pyknosis and karyolysis, cytoplasmic vacuolization and hypereosinophilia were usually found in that region at the acute phases. It has to be acknowledged, however, that nuclear pyknosis and cytoplasmic hypereosinophilia appear in both cell necrosis and programmed cell death processes (Saxena et al. 2002). The means utilized in the present study to identify myocyte cell death were exclusively morphological and thus not reliable to differentiate cell necrosis from apoptosis. Accordingly, myocyte necrosis may have been overestimated. In addition, the participation of apoptosis in subendocardial injury remains unclear.
Non-sulphated glycosaminoglycans alone were contemplated in the present study. The recognized role of proteoglycans and sulphated glycosaminoglycans in remodelling remain to be addressed taking into account subendocardial and interstitial regions of the remote non-infarcted myocardium (Endo et al. 1997; Doi et al. 2000). The latest follow-up interval examined was 28 days when glycosaminoglycans, but not collagen, appeared in greater amounts in the non-infarcted interstitial myocardium. The hypothesis that this may represent an intermediary phase in the remodelling process taking place in the interstitium needs to be addressed using longer periods of follow-up.
In the present study, the pathological changes observed in the subendocardium represent an indirect evidence of an ongoing ischaemic process affecting that region. It remains to be established whether or not low perfusion pressure actually translates into impaired myocardial blood flow to the subendocardium, in particular. As the coronary flow reserve may also be impaired after infarction (Karam et al. 1990; Kalkman et al. 1996), it remains unsettled as to what extent the decrease in the coronary driving pressure might be counterbalanced by the vasodilating capacity of coronary resistance vessels.
Clinical implications
The present findings suggest that the use of vasodilator drugs following infarction may be potentially harmful provided myocardial perfusion is jeopardized by the reduction in systemic blood pressure. In cases of heart failure and elevated left ventricular filling pressure, the assessment of coronary driving pressure may be useful to tailor the degree to which left ventricular end-diastolic pressure may be reduced without impairing perfusion pressure.
Conclusions
Distinctive subendocardial and interstitial remodelling occurs in the remote non-infarcted myocardium. The pathological changes observed indicate that subendocardial fibrosis represents a reparative process in response to early and ongoing low perfusion pressure. The impairment in coronary driving pressure seems to be related to left ventricular dysfunction by contributing to progressive ventricular remodelling.
References
- Baldwin HS, Lloyd TR, Solursh M. Hyaluronate degradation affects ventricular function of the early postlooped embryonic rat heart in situ. Circ. Res. 1994;74:244–252. doi: 10.1161/01.res.74.2.244. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- Cross C, Riechen P, Salisbury P. Coronary driving pressure and vasomotor tonus as determinants of coronary blood flow. Circ. Res. 1961;9:589–600. doi: 10.1161/01.res.9.3.589. [DOI] [PubMed] [Google Scholar]
- de Carvalho Frimm C, Sun Y, Weber KT. Angiotensin II receptor blockade and myocardial fibrosis of the infarcted rat heart. J. Lab. Clin. Med. 1997;129:439–446. doi: 10.1016/s0022-2143(97)90077-9. [DOI] [PubMed] [Google Scholar]
- de Carvalho Frimm C, Koike MK, Cúri M. Subendocardial fibrosis in remote myocardium results from reduction of coronary driving pressure during acute infarction in rats. Arq. Bras. Cardiol. 2003;80:515–520. [PubMed] [Google Scholar]
- Deten A, Holz A, Leicht M, Barth W, Zimmer HG. Changes in extracelular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J. Mol. Cell. Cardiol. 2001;33:1191–1207. doi: 10.1006/jmcc.2001.1383. [DOI] [PubMed] [Google Scholar]
- Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006;324:475–488. doi: 10.1007/s00441-005-0144-6. [DOI] [PubMed] [Google Scholar]
- Doi M, Kusachi S, Murakami T, et al. Time-dependent changes of decorin in the infarct zone after experimentally induced myocardial infarction in rats: comparison with biglycan. Pathol. Res. Pract. 2000;196:23–33. doi: 10.1016/S0344-0338(00)80018-7. [DOI] [PubMed] [Google Scholar]
- Endo C, Kusachi S, Ninomiya Y, et al. Time dependent increases in syndecan-1 and fibroglycan messenger RNA expression in the infarct zone after experimentally induced myocardial infarction in rats. Coron. Artery Dis. 1997;8:155–161. doi: 10.1097/00019501-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Kalkman EA, Bilgin YM, van Haren P, van Suylen RJ, Saxena PR, Schoemaker RG. Determinants of coronary reserve in rats subjected to coronary artery ligation or aortic banding. Cardiovasc. Res. 1996;32:1088–1095. doi: 10.1016/s0008-6363(96)00166-6. [DOI] [PubMed] [Google Scholar]
- Karam R, Healy BP, Wicker P. Coronary reserve is depressed in postmyocardial infarction reactive cardiac hypertrophy. Circulation. 1990;81:238–246. doi: 10.1161/01.cir.81.1.238. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Fausto N. Robbins and Cotran’s Pathologic Basis of Disease. Philapdelphia, PA: Elsevier Health Sciences; 2004. pp. 19–26. [Google Scholar]
- Michel JB, Lattion AL, Salzmann JL, et al. Hormonal and cardiac effects of converting enzyme inhibition in rat myocardial infarction. Circ. Res. 1988;62:641–650. doi: 10.1161/01.res.62.4.641. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Pfeffer JM, Fishbein MC. Myocardial infarct size and ventricular function in rats. Circ. Res. 1979;44:503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the heart. Circ. Res. 1985;57:84–95. doi: 10.1161/01.res.57.1.84. [DOI] [PubMed] [Google Scholar]
- Savani RC, Hou G, Liu P, et al. A role of hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2000;23:475–484. doi: 10.1165/ajrcmb.23.4.3944. [DOI] [PubMed] [Google Scholar]
- Saxena A, McMeekin JD, Thomson DJ. Expression of Bcl-x, Bcl-2, Bax, and Bak in endarterectomy and atherectomy specimens. J. Pathol. 2002;196:335–342. doi: 10.1002/path.1040. [DOI] [PubMed] [Google Scholar]
- Selye H, Bajuusj E, Grasso S, Mendell P. Simple techniques for the surgical occlusion of coronary vessels in the rat. Angiology. 1960;11:398–407. doi: 10.1177/000331976001100505. [DOI] [PubMed] [Google Scholar]
- Sun Y, Weber KT. Angiotensin converting enzyme and myofibroblasts during tissue repair in the rat heart. J. Mol. Cell. Cardiol. 1996;28:851–858. doi: 10.1006/jmcc.1996.0080. [DOI] [PubMed] [Google Scholar]
- Sun Y, Cleutjens JP, Diaz-Arias AA, Weber KT. Cardiac angiotensin converting enzyme and myocardial fibrosis in the rat. Cardiovasc. Res. 1994;28:1423–1432. doi: 10.1093/cvr/28.9.1423. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang JQ, Zhang J, Lamparter S. Cardiac remodeling by fibrous tissue after infarction in rats. J. Lab. Clin. Med. 2000;135:316–323. doi: 10.1067/mlc.2000.105971. [DOI] [PubMed] [Google Scholar]
- Toyota E, Ogasawara Y, Hiramatsu O, et al. Dynamics of flow velocities in endocardial and epicardial coronary arterioles. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1598–1603. doi: 10.1152/ajpheart.01103.2003. [DOI] [PubMed] [Google Scholar]
- Van Kerckhoven R, Kalkman EAJ, Saxena PR, Schoemaker RG. Altered cardiac collagen and associated changes in diastolic function of infarcted rats hearts. Cardiovasc. Res. 2000;46:316–323. doi: 10.1016/s0008-6363(99)00427-7. [DOI] [PubMed] [Google Scholar]
- Volders PG, Willems IE, Cleutjens JP, Arends JW, Havenith MG, Daemen MJ. Interstitial collagen is increased in the non-infarcted human myocardium after myocardial infarction. J. Mol. Cell. Cardiol. 1993;25:1317–1323. doi: 10.1006/jmcc.1993.1144. [DOI] [PubMed] [Google Scholar]
- Waldenstrom A, Martinussen HJ, Gerdin B, Hallgren R. Accumulation of hyaluronan and tissue edema in experimental myocardial infarction. J. Clin. Invest. 1991;88:1622–1628. doi: 10.1172/JCI115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Abraham TP, Korinek J, Urheim S, McMahon EM, Belohlavek M. Delayed onset of subendocardial diastolic thinning at rest identifies hypoperfused myocardium. Circulation. 2005;111:2943–2950. doi: 10.1161/CIRCULATIONAHA.104.482984. [DOI] [PubMed] [Google Scholar]
- Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP. Patterns of myocardial fibrosis. J. Mol. Cell. Cardiol. 1989;21(Suppl 5):121–131. doi: 10.1016/0022-2828(89)90778-5. [DOI] [PubMed] [Google Scholar]
- Weber KT, Sun Y, Katwa LC, Cleutjens JP, Zhou G. Connective tissue and repair in the heart. Potential regulatory mechanisms. Ann. N. Y. Acad. Sci. 1995;752:286–299. doi: 10.1111/j.1749-6632.1995.tb17438.x. [DOI] [PubMed] [Google Scholar]
- Wei S, Chow LTC, Sanderson JE. Effect of carvedilol in comparison with metoprolol on myocardial collagen postinfarction. J. Am. Coll. Cardiol. 2000;36:276–281. doi: 10.1016/s0735-1097(00)00671-9. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Boughner DR, Kloner RA. Role of collagen in acute myocardial infarct expansion. Circulation. 1991;84:2123–2134. doi: 10.1161/01.cir.84.5.2123. [DOI] [PubMed] [Google Scholar]
- Wilke A, Funck R, Rupp H, Brilla CG. Effect of the renin-angiotensin-aldosterone system on the cardiac interstitium in heart failure. Basic Res. Cardiol. 1996;91(Suppl 2):79–84. doi: 10.1007/BF00795367. [DOI] [PubMed] [Google Scholar]
- Yu CM, Tipoe GL, Wing-Hon LK, Lau CP. Effects of combination of angiotensin-converting enzyme inhibitor and angiotensin receptor antagonist on inflammatory cellular infiltration and myocardial interstitial fibrosis after acute myocardial infarction. J. Am. Coll. Cardiol. 2001;38:1207–1215. doi: 10.1016/s0735-1097(01)01518-2. [DOI] [PubMed] [Google Scholar]