Abstract
Tendons are dense regular connective tissue structures that are defined based on their anatomical position of connecting muscle to bone. Despite these obvious commons features tendons from different locations within the body show remarkable variation in terms of their morphological, molecular and mechanical properties which relates to their specialized function. An appreciation of these differences is necessary to understand all aspects of tendon biology in health and disease. In our work, we have used a combination of mechanical assessment, histological measurements and molecular analysis of matrix in functionally distinct tendons to determine relationships between function and structure. We have found significant differences in material and molecular properties between spring-like tendons that are subjected to high strains during locomotion and positional tendons which are subjected to much lower strains. Furthermore, we have data to suggest that not only is the matrix composition different but also the ability of cells to synthesize and degrade the matrix (matrix turnover) varies between tendon types. We propose that these differences relate to the magnitude of strain that the tendon experiences during normal activities in life. Tendon cells may be preprogrammed during embryological development for the strain they will encounter in life or may simply respond to the particular strain environment they are subjected to. The elucidation of controlling mechanisms resulting in tendon cell specialization will have important consequences for cell based therapies and engineering strategies to repair damaged tendons.
Keywords: collagen, function, matrix, mechanics, metabolism, tendon
The main role of tendon is in transmitting muscle generated force to a bone resulting in movement around a joint. Tendons that function predominantly in this way are termed ‘positional’ tendons and their ability to do this requires them to be relatively inextensible under physiological loads. Other tendons have more specialized functions and one of these is the storage and release of elastic strain energy thereby increasing the efficiency of locomotion (Alexander 2002). The human Achilles tendon is an example of a tendon which works in this way (Lichtwark & Wilson 2005) while the equine superficial digital flexor tendon (SDFT) (Figure 1) has also been shown to have a spring-like function (Wilson et al. 2001). Unlike positional tendons, energy storing tendons are required to stretch and recoil under physiological loads to ensure efficient return of stored energy. Thus, energy storing tendons are subjected to relatively high strains during normal physiological activity while positional tendons experience much lower strains. For example, in vivo measurements in the human Achilles tendon have recorded strains of up to 10.3% during one-legged hopping (Lichtwark & Wilson 2005). Conversely, the anterior tibialis tendon, based on anatomical location, suggests a function as a positional tendon and in accordance with this in vivo measurements of strain have recorded maximum values of only 3.1% (Maganaris & Paul 2000).
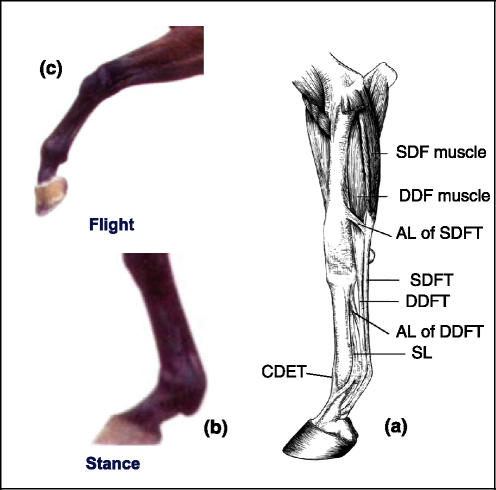
Figure 1.
Equine forelimb (a). The superficial digital flexor tendon (SDFT) experiences strains of up to 12% as the metacarpo-phalangeal joint undergoes hyperextension during the stance phase (b) while the common digital extensor tendon (CDET) experiences much lower strains positioning the limb during the flight phase (c). [Please refer to the electronic article for the colour version of this figure (http://www.blackwell-synergy.com/loi/iep)].
The amount a tendon stretches (strain) depends on the cross sectional area (CSA) of the tendon and the material properties of the tendon tissue, in addition to the force applied. During physiological activity, force is applied to the tendon via the associated muscle belly. In some cases, the muscle tissue generates force to counteract the effect of gravity and may undergo isometric or eccentric contraction. However, regardless of this, the tendon still cannot be subjected to more force than the muscle belly can withstand during normal physiological activity. Isolation of a single muscle in vivo to measure maximum force generation non-invasively is not possible, however, estimations of the maximum isometric force can be made using in vitro measurements of muscle volume and muscle fibre length (Wilson et al. 2001). Muscle architecture and the relationship between muscle CSA and tendon CSA differ between muscle–tendon units. The muscle bellies associated with tendons that are important as energy saving springs have relatively large physiological CSAs in relation to the CSA of the tendon and as a result large stresses act in these tendons (Ker et al. 2000). The physiological CSA of a muscle can be calculated by dividing muscle volume by muscle fibre length (Wilson et al. 2001) hence, a large physiological CSA value can be obtained by a multi-pennate arrangement of short muscle fibres. Increasing the pennation angle allows a larger number of short muscle fibres to be packed into the muscle resulting in greater force generation; although a high pennation angle can also reduce the force that is transmitted to the tendon. In contrast, muscles with longer fibres produce less force but over a greater range of muscle lengths hence, muscles with long fibres are able to work with greater joint excursions. Our preliminary studies (H.L. Birch & R.C. Payne, unpublished data) have indeed shown that the soleus muscle of the human Achilles tendon has very short multi-pennate muscle fibres of approximately 33 mm (Figure 2a) giving the muscle belly a relatively large physiological CSA in relation to the Achilles tendon. The Achilles tendon therefore will be subjected to large stresses during maximum contraction in keeping with its role as an energy storing structure. The anterior tibialis muscle belly had much longer muscle fibres of about 75 mm (Figure 2b) resulting in a smaller physiological CSA in relation to the tendon indicating lower stresses in the tendon during activity as expected for a positional tendon. Interestingly, the posterior tibialis muscle belly has short muscle fibres (31 mm) suggesting an energy storing role for this muscle–tendon unit. In the horse, muscle architectural measurements suggest that the SDF is able to generate a maximum isometric force of 9100 N while the common digital extensor (CDE) muscle only transmits a maximum of 1089 N to it associated ‘positional’ tendon (Brown et al. 2003). Although the CDET has a smaller CSA than the SDFT the ratio is not as great as that between the muscle bellies. Our work suggests a ratio of 3.5:1 for SDFT CSA relative to the CDET. Using this value, the maximum in vivo stress in the SDFT would be 2.4 times greater than the CDET indicating their respective roles as an energy storing tendon and positional tendon.
Figure 2.
Internal architecture of the human soleus muscle (a) and anterior tibialis muscle (b). The muscles have been sectioned through the muscle belly to reveal the arrangement of the fascicle bundles. (scale bar in cm).
Although differences in muscle architecture are well recognized, the associated tendons have only really been considered in terms of their size rather than possible differences in material properties (Ker 2002). However, our work has shown that in addition to muscle architectural adaptations for a specific function the associated tendons also show specialization.
In our studies, we collected the SDFT and CDET from the distal part of the forelimb from horses destroyed for reasons other than tendon injuries. Tendons were mounted in a hydraulic materials testing machine (Figure 3a) to determine the mechanical properties of the tendon as a structure. Prior to testing, the CSA was measured using dental embedding paste as described in Goodship and Birch (2005) allowing the properties of the tendon tissue (materials properties) to be determined. When force is applied to a tendon, a characteristic relationship between the load and elongation is obtained (Figure 3c). Initially, the tendon stretches relatively easily giving a toe region. This is followed by a linear region where the tendon obeys Hooke’s law and then a yield region where the gradient is reduced and finally the complete failure of the structure. These data allow the properties of the tendon at failure to be calculated and, more importantly when considering physiological functioning, the stiffness of the structure and stiffness of the tendon tissue (elastic modulus) in the linear region to be determined.
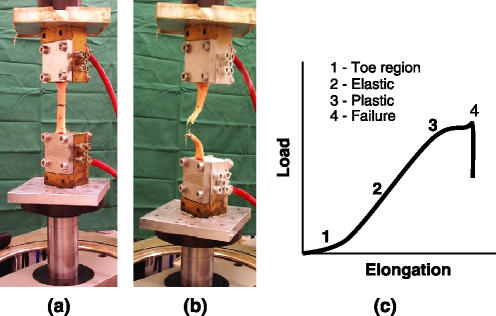
Figure 3.
Equine superficial digital flexor tendon mounted in a hydraulic materials testing machine (a) and tested to failure (b). When the force applied is plotted against the elongation of the tendon a characteristic curve is obtained (c). [Please refer to the electronic article for the colour version of this figure (http://www.blackwell-synergy.com/loi/iep)].
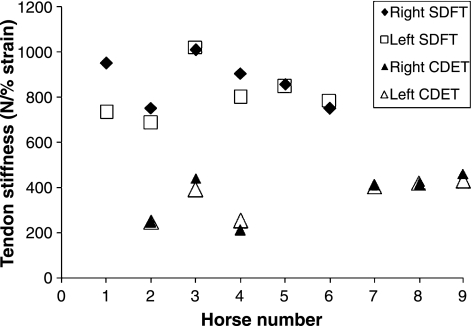
Our results showed that the ultimate strength of the SDFT (10465 ± 410 N) was significantly (P < 0.001, n = 12) greater than that of the CDET (3756 ± 241 N) as would be expected for a tendon with a larger CSA. The material strength (ultimate stress), however, was not significantly different between the two tendon types (SDFT – 115.74 ± 4.38 MPa; CDET – 136.94 ± 10.44 MPa; n = 12, P = 0.075). Although the ultimate stress did not differ significantly, the ultimate strain value was significantly (P = 0.018, n = 12) higher in the SDFT (25.98 ± 1.44%) than the CDET (20.45 ± 1.60%) indicating a more compliant structure over the whole loading curve. Analysis of the linear region of the load vs. elongation curve showed that the SDFT as a gross structure (839.8 ± 110.2 N/% strain) was stiffer than the CDET (358.0 ± 90.0 N/% strain), again as expected for a larger structure. However, the elastic modulus of the SDFT (970.8 ± 60.4 MPa) was significantly (P < 0.001) lower than the CDET (1236.3 ± 209.6 MPa) demonstrating a difference in the stiffness of the tendon tissue. Interestingly, we noted that, although there is considerable variation in tendon stiffness between individual horses, left and right tendons were very similar (Figure 4) for the SDFT and the CDET. The lower elastic modulus value obtained for the SDFT suggests that energy storing tendons are composed of a less stiff matrix than positional tendons. Giving support to this, in further experiments we measured the elastic modulus of the suspensory ligament (SL); another energy storing structure in the distal part of the equine limb and found that that the SL had a significantly lower elastic modulus (643 ± 130 MPa) than the SDFT (1165 ± 178 MPa) and that both were lower than the elastic modulus of the CDET (1510 ± 291 MPa) from the same horses (n = 6) (Smith 2006). Our in vitro mechanical testing data show a similar pattern for tendons from the distal part of the human leg. The ultimate tensile strength of the Achilles tendon (AT – 3.87 ± 1.61 kN) was significantly (P = 0.02, n = 4) higher than the anterior tibialis tendon (ATT – 1.54 ± 0.17 kN) although the ultimate stress values were not significantly different between the two tendon types (AT – 53.53 ± 19.77 MPa; ATT – 60.60 ± 9.34 MPa). The elastic modulus of the Achilles tendon (212 ± 109 MPa) was significantly (P = 0.04, n = 4) lower than that of the anterior tibialis tendon (426 ± 269 MPa) in the linear region of the loading curve demonstrating the more compliant nature of the matrix in the energy storing Achilles tendon.
Figure 4.
Stiffness of the right and left superficial digital flexor tendon (SDFT) and common digital extensor tendon (CDET) for individual horses.
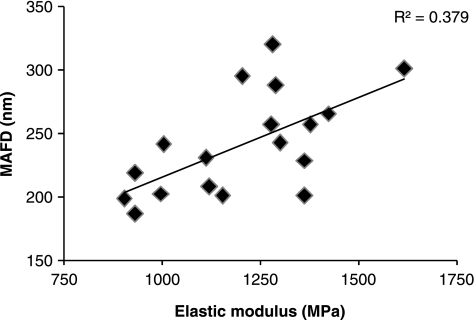
Differences in the material properties of distinct tendons must arise from variations in matrix composition or organization of the matrix components. Tendon is composed predominately of the fibrous protein collagen which accounts for approximately 75% of the dry weight of the tissue (Birch et al. 1999). Collagen molecules are precisely ordered within tendon to give a hierarchical structure from the collagen fibril to fibres, to fascicles and finally the whole tendon (Kastelic et al. 1978; Silver et al. 2003) bestowing high tensile on the tendon. Despite the key role that collagen plays in the mechanical properties of tendon tissue, we did not find a significant difference in the total collagen content between different tendon types. However, measurement of the collagen fibril diameters using transmission electron microscopy revealed a difference in the organization of the collagen content. The SDFT (169 ± 19 nm) had a significantly (P = 0.002, n = 6) lower mass average fibril diameter than the CDET (229 ± 36 nm) suggesting that larger fibril diameters might be responsible for a stiffer matrix in the CDET (Smith 2006). In keeping with this, the SL; an energy storing structure with a significantly lower elastic modulus than both the SDFT and CDET had the lowest mass average fibril diameter (122 ± 14 nm). As mentioned previously, the elastic modulus of the SDFT shows a marked variation between individual horses. We found that the elastic modulus of the SDFT from 18 different horses showed a significant positive correlation with the mass average fibril diameters for the SDFT from the same horse showing that the relationship between elastic modulus and collagen fibril diameters exists within a tendon type as well as between tendon types (Figure 5).
Figure 5.
Relationship between the mass average fibril diameter (MAFD) and elastic modulus in the equine superficial digital flexor tendon (n = 18).
Further analysis of the molecular composition of tissue from different equine tendon types has revealed considerable variation. The collagen molecules within a collagen fibril are linked to each other by chemical crosslinks which form in the telopeptide region of the molecule following conversion of lysine and hydroxylysine residues to aldehydes by the action of the enzyme lysyl oxidase. We have found that the profile of collagen crosslinks shows a marked difference between the different tendon types (H.L. Birch, unpublished data). The predominant mature crosslink detected in the SDFT, deep digital flexor tendon (DDFT) and SL samples was hydroxylysylpyridinoline (HP) however levels of HP were low in the CDET; the main crosslink found here being histidinohydroxymesodesmosine (HHMD). The SDFT and SL had similar levels of HP (SDFT – 0.47 ± 0.06 mol/mol collagen; SL – 0.46 ± 0.05 mol/mol collagen) which were significantly higher (P < 0.001, n = 11) than those found in the DDFT (0.35 ± 0.05 mol/mol collagen) and the CDET (0.04 ± 0.01 mol/mol collagen). HHMD was detected in high amounts in the CDET samples (1.24 ± 0.15 mol/mol collagen) and at trace levels in the DDFT samples but not in the SDFT or SL. Histidinohydroxylysinonorleucine, a major crosslink found in mature skin, was detected in all the CDET samples (0.069 ± 0.005 mol/mol collagen) and some of the DDFT samples at trace levels but not in the SDFT or SL. The divalent immature aldimine crosslink dehydro-hydroxylysinonorleucine (dehydro-HLNL) was detected in the CDET samples (0.04 ± 0.01 mol/mol collagen) but none of the other structures. The consequence of these differences in the crosslink profiles between tendon types is not known at present but is likely to impact on the mechanical properties of the matrix tissue.
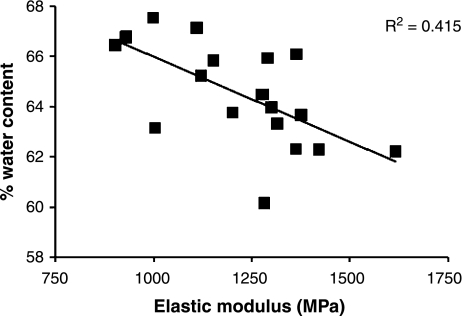
Water constitutes the main component of the wet weight of tendon tissue and we found small but consistent and significant (P < 0.001, n = 11) differences between tendon types (H.L. Birch, unpublished data). The energy storing SDFT and SL had the highest water contents (SDFT – 64.2 ± 0.8%; SL – 67.5 ± 1.2%) followed by the DDFT (61.6 ± 1.2%) and the positional CDET (57.1 ± 0.9%) with the lowest water content. The water content showed a significant negative correlation with the elastic modulus of the tendon (Figure 6) thus, higher water content is associated with a less stiff matrix (Smith 2006).
Figure 6.
Relationship between the % water content and elastic modulus in the equine superficial digital flexor tendon (n = 19).
The organization of collagen within tendon depends, in part, on interactions with other non-collagenous proteins within the matrix. Proteoglycans such as decorin, fibromodulin and biglycan are able to interact with collagen fibres and may regulate collagen fibril diameter (Danielson et al. 1997; Svensson et al. 1999; Chakravarti 2002). Proteoglycans are composed of a protein core and glycosaminoglycan (GAG) sidechain. In our studies, we found that the levels of sulphated GAG were significantly lower in the CDET (2.5 ± 0.4 μg/mg dry weight tissue) than the SDFT (9.1 ± 1.5 μg/mg), DDFT (10.6 ± 3.0 μg/mg) and SL (13.2 ± 1.6 μg/mg) while the levels of sulphated GAG in the SDFT were significantly lower than those in the SL but not significantly different to the DDFT (H.L. Birch, unpublished data). These differences in sulphated GAG levels indicate variations in the type and amount of proteoglycan in the tendon matrix and are likely to play a role in regulating collagen fibril diameters in functionally distinct tendons. Cellularity also appears to differ between tendons, based on DNA content. In our analysis of equine tendon tissue, the SL had the highest levels of DNA (2.27 ± 0.29 μg DNA/mg dry weight tissue) followed by the SDFT (1.51 ±0.16 μg/mg) then the DDFT (0.80 ± 0.15 μg/mg) while the CDET (0.47 ±0.18 μg/mg) had the lowest levels.
Our matrix composition analysis of human tendon tissue shows a reflection of the patterns observed in equine tendons (Birch et al. 2001). The energy storing Achilles tendon when compared with the positional anterior tibialis tendon had a higher water content (AT – 69.0 ± 2.9%; ATT – 67.5 ± 1.2%), higher sulphated GAG content (AT – 8.17 ± 1.49 μg/mg; ATT – 5.30 ± 1.72 μg/mg) and higher DNA content (AT – 0.61 ± 0.05 μg/mg; ATT – 0.18 ± 0.05 μg/mg) while total collagen content (AT – 77.2 ± 1.3%; ATT – 76.8 ± 3.7%) was not significantly different.
The relationship between tendon function and mechanical, morphological and molecular properties is of interest in human medicine for several reasons. In the late stages of tendon dysfunction, or in the case of complete tendon rupture, surgical intervention is often required (Kohls-Gatzoulis et al. 2004; Vora et al. 2005). Surgical procedures involve reconstruction and require transfer of tendon tissue from alternative sites. For example, the Achilles tendon is often reconstructed using the flexor hallicus longus tendon (Wapner et al. 1993) while the posterior tibialis tendon is repaired using the anatomically near but functionally distinct flexor digitorum longus tendon or a split anterior tibialis tendon (Kohls-Gatzoulis et al. 2004). However, functional competence of the tendon is likely to be impaired, as our studies have shown that the tissue properties are very different between functionally distinct tendons. An alternative approach, of great interest currently, is to use bioengineered scaffolds and/or cell based therapies to treat tendinopathies. A better understanding of structure function relationships will inform bioengineering strategies and allow refinement of bioengineered constructs and cell based therapies for treatment of tendon disease thus improving functional outcome.
Furthermore, of clinical concern, is that some tendons are particularly prone to tendinopathies and rupture. Tendons which appear to be most susceptible are the energy storing structures such as the human Achilles tendon and equine SDFT, while their positional counterparts, the anterior tibialis tendon and CDET, are rarely injured. In a recent study in horses, 93% of tendon and ligament injuries reported were to the SDFT and 7% to the SL while there were no reported injuries to CDET (Ely et al. 2004). Epidemiological studies have shown that the incidence of Achilles tendon rupture is increasing and that older age individuals are more at risk (Moller et al. 1996). Several epidemiological studies have shown that, as in human tendons, increasing horse age is a factor associated with increasing risk of tendon injury (Ely et al. 2004; Kasashima et al. 2004; Perkins et al. 2005). In tendons prone to injury, it is now generally accepted that degenerative changes to the matrix precede clinical injury in human (Kannus & Jozsa 1991; Riley et al. 1994) and equine subjects. Our previous work on equine tendons has shown that this central core degeneration is associated with a reddish discolouration and increased levels of type III collagen, sulphated GAG, cellularity and rate of matrix turnover (Birch et al. 1998).
This difference in susceptibility to degeneration and rupture between tendon types may be attributed to mechanical factors. As discussed above, energy storing tendons are subjected to high stresses during physiological activities resulting in high strains. Strains of up to 16.6% have been recorded for the equine SDFT in vivo at gallop (Stephens et al. 1989). In contrast, the equine CDET experiences maximum strains in vivo of approximately 3%, based on muscle architectural measurements (Brown et al. 2003) and our data for tendon stiffness (see above). In human Achilles tendon a strain of 8% was recorded during voluntary isometric contraction of the gastrocnemius (Magnusson et al. 2003) and strains of up to 10.3% during one-legged hopping (Lichtwark & Wilson 2005) while in vivo measurements of strain in the anterior tibialis tendon have recorded maximum values of only 3.1% (Maganaris & Paul 2000). These data show a lower safety margin in energy storing tendons and suggest that they may occasionally be loaded beyond their linear elastic region into the ‘yield’ phase of the stress/strain curve hence suffering micro-damage during strenuous activity. As a result, energy storing tendons may have a higher demand for matrix renewal to repair micro-damage than positional tendons, a concept supported by the higher cellulartiy in the SDFT and Achilles tendon compared to the CDET and anterior tibialis tendon. This has important clinical implications as mechanically induced micro-damage has been proposed as a mechanism (Rees et al. 2006) for the high incidence of degeneration and subsequent rupture suffered by the SDFT and AT. However, mechanical factors alone do not explain the increased incidence of tendinopathies in older age which suggests additional changes to cell activity and as a consequence, matrix composition.
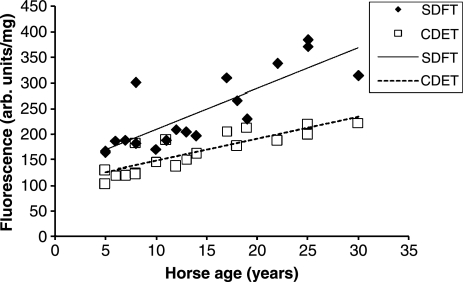
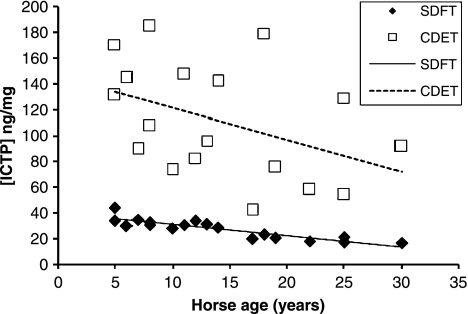
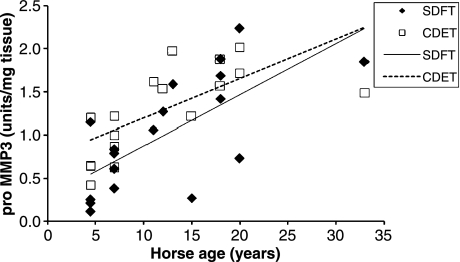
We have measured a number of features in equine tendons to give an indication of the rate of matrix turnover (H.L. Birch, unpublished data). Tissue fluorescence is a consequence of the addition of sugar residues to relatively long-lived proteins such as collagen. We have shown previously that collagen linked fluorescence increases with horse age in a linear fashion (Birch et al. 1999) in the SDFT. Our recent data from SDFT and CDET tissue from horses with a wide range of ages confirmed that collagen linked fluorescence increases with increasing age as expected but at all ages the CDET had significantly lower levels of fluorescence than the SDFT (Figure 7) suggesting that there may be a more rapid turnover of the matrix in the CDET. Determination of the levels of the crosslinked carboxyterminal telopeptide of type I collagen (ICTP) in the tissue showed a linear increase with age in both tendons (Figure 8) and significantly (P < 0.001, n = 18) higher levels of ICTP in the CDET supporting a higher rate of collagen turnover in the matrix of the CDET. Levels of matrix metalloproteinases (MMPs), a family of enzymes responsible for degradation of the matrix, give an indication of the potential for matrix breakdown in tendon. In our studies, gelatin zymography revealed no significant differences in the levels of MMP2 and MMP9 activities between tendon types while casein zymography showed significantly (P = 0.007) higher levels of MMP3 activity in the CDET compared to the SDFT and an increase in enzyme levels in both tendons with increasing age (Figure 9). Gene expression studies by Clegg and colleagues (P.O. Clegg, personal communication) showed significantly higher levels of mRNA for the α1 polypeptide chain of type I collagen, the α1 polypeptide chain of type III collagen, fibromodulin and superficial zone protein in CDET tissue compared to SDFT tissue. Taken together these data suggest, contrary to our predictions, a higher rate of matrix turnover in the CDET than the SDFT and a decrease in turnover with increasing age in both tendon types. Increased levels of MMP3 activity in older tendons does not correspond with a decline in turnover with increasing age, but suggests that non-collagenous components may be differentially regulated to the collagenous components of tendon. A slower rate of matrix turnover in the SDFT may result in accumulation of micro-damage and explain why this tendon is particularly prone to degeneration and subsequent rupture. It remains unclear however as to why a tendon subjected to relatively high stresses and strains should have a lower matrix metabolism rate than a low strain tendon although it has been suggested that structures which require high mechanical strength are protected from remodelling which would weaken the structure (Laurent 1987).
Figure 7.
Collagen linked fluorescence levels in superficial digital flexor tendon (SDFT) and common digital extensor tendon (CDET) tissue plotted against horse age (n = 18).
Figure 8.
Crosslinked carboxyterminal telopeptide of type I collagen (ICTP) in superficial digital flexor tendon (SDFT) and common digital extensor tendon common digital extensor tendon (CDET) tissue plotted against horse age (n = 18).
Figure 9.
Pro MMP3 levels in superficial digital flexor tendon (SDFT) and common digital extensor tendon (CDET) tissue plotted against horse age (n = 18).
In summary, our work has shown marked differences in matrix composition and turnover in functionally distinct tendons. We believe that these differences relate to the magnitude of strain that the tendon experiences during normal physiological function. Tendon cells may be pre-set during embryological development to function at a specific strain suitable for their anatomical position. Alternatively, cells may simply respond to the imposed strain environment by expressing a phenotype suitable for that environment. We propose that the former case is true; that tendon cells are programmed for their strain environment and respond to changes by synthesizing specific matrix components to counteract any change. Recent research in the field of dermatology has demonstrated that skin fibroblasts have ‘positional memory’ and express specific gene patterns that relate to their topographic identity (Rinn et al. 2006). Tendon specialization is a fascinating area with much yet to be discovered. Determining which of these possible theories is correct has great consequence for cell based therapies and tissue engineering strategies in musculoskeletal health care and is particularly relevant at the present time due to the huge interest in and expectations for tissue regeneration into the next decade.
References
- Alexander RM. Tendon elasticity and muscle function. Comp. Biochem. Physiol., A Mol. Integr. Physiol. 2002;133:1001–1011. doi: 10.1016/s1095-6433(02)00143-5. [DOI] [PubMed] [Google Scholar]
- Birch HL, Bailey AJ, Goodship AE. Macroscopic ‘degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular matrix composition. Equine Vet. J. 1998;30:534–539. doi: 10.1111/j.2042-3306.1998.tb04530.x. [DOI] [PubMed] [Google Scholar]
- Birch HL, Bailey JV, Bailey AJ, Goodship AE. Age-related changes to the molecular and cellular components of equine flexor tendons. Equine Vet. J. 1999;31:391–396. doi: 10.1111/j.2042-3306.1999.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Birch HL, Smith TJ, Tasker T, Goodship AE. Transactions of the 47th Annual Meeting of the Orthopaedic Research Society. San Francisco, CA, USA: 2001. Age related changes to mechanical and matrix properties in human Achilles tendon. [Google Scholar]
- Brown NA, Kawcak CE, McIlwraith CW, Pandy MG. Architectural properties of distal forelimb muscles in horses. Equus caballus. J. Morphol. 2003;258:106–114. doi: 10.1002/jmor.10113. [DOI] [PubMed] [Google Scholar]
- Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj. J. 2002;19:287–293. doi: 10.1023/A:1025348417078. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely ER, Verheyen KL, Wood JL. Fractures and tendon injuries in National Hunt horses in training in the UK: a pilot study. Equine Vet. J. 2004;36:365–367. doi: 10.2746/0425164044890607. [DOI] [PubMed] [Google Scholar]
- Goodship AE, Birch HL. Cross sectional area measurement of tendon and ligament in vitro: a simple, rapid, non-destructive technique. J. Biomech. 2005;38:605–608. doi: 10.1016/j.jbiomech.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J. Bone Joint Surg. Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- Kasashima Y, Takahashi T, Smith RK, et al. Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese Thoroughbred flat racehorses in 1999. Equine Vet. J. 2004;36:346–350. doi: 10.2746/0425164044890580. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect. Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Ker RF. The implications of the adaptable fatigue quality of tendons for their construction, repair and function. Comp. Biochem. Physiol., A Mol. Integr. Physiol. 2002;133:987–1000. doi: 10.1016/s1095-6433(02)00171-x. [DOI] [PubMed] [Google Scholar]
- Ker RF, Wang XT, Pike AV. Fatigue quality of mammalian tendons. J. Exp. Biol. 2000;203:1317–1327. doi: 10.1242/jeb.203.8.1317. [DOI] [PubMed] [Google Scholar]
- Kohls-Gatzoulis J, Angel JC, Singh D, Haddad F, Livingstone J, Berry G. Tibialis posterior dysfunction: a common and treatable cause of adult acquired flatfoot. BMJ. 2004;329:1328–1333. doi: 10.1136/bmj.329.7478.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent GJ. Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am. J. Physiol. 1987;252:C1–C9. doi: 10.1152/ajpcell.1987.252.1.C1. [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Wilson AM. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J. Exp. Biol. 2005;208:4715–4725. doi: 10.1242/jeb.01950. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. In vivo human tendinous tissue stretch upon maximum muscle force generation. J. Biomech. 2000;33:1453–1459. doi: 10.1016/s0021-9290(00)00099-3. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Aagaard P, et al. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol. Scand. 2003;177:185–195. doi: 10.1046/j.1365-201X.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- Moller A, Astron M, Westlin N. Increasing incidence of Achilles tendon rupture. Acta Orthop. Scand. 1996;67:479–481. doi: 10.3109/17453679608996672. [DOI] [PubMed] [Google Scholar]
- Perkins NR, Reid SW, Morris RS. Risk factors for injury to the superficial digital flexor tendon and suspensory apparatus in Thoroughbred racehorses in New Zealand. N. Z. Vet. J. 2005;53:184–192. doi: 10.1080/00480169.2005.36503. [DOI] [PubMed] [Google Scholar]
- Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (Oxford) 2006;45:508–521. doi: 10.1093/rheumatology/kel046. [DOI] [PubMed] [Google Scholar]
- Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann. Rheum. Dis. 1994;53:359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 2003;36:1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Smith TJ. London, UK: University College London; 2006. The relationship between tendon morphology and function. PhD thesis. [Google Scholar]
- Stephens PR, Nunamaker DM, Butterweck DM. Application of a Hall-effect transducer for measurement of tendon strains in horses. Am. J. Vet. Res. 1989;50:1089–1095. [PubMed] [Google Scholar]
- Svensson L, Aszodi A, Reinholt FP, Fassler R, Heinegard D, Oldberg A. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J. Biol. Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- Vora AM, Myerson MS, Oliva F, Maffulli N. Tendinopathy of the main body of the Achilles tendon. Foot Ankle Clin. 2005;10:293–308. doi: 10.1016/j.fcl.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wapner KL, Pavlock GS, Hecht PJ, Naselli F, Walther R. Repair of chronic Achilles tendon rupture with flexor hallucis longus tendon transfer. Foot Ankle. 1993;14:443–449. doi: 10.1177/107110079301400803. [DOI] [PubMed] [Google Scholar]
- Wilson AM, McGuigan MP, Su A, van Den Bogert AJ. Horses damp the spring in their step. Nature. 2001;414:895–899. doi: 10.1038/414895a. [DOI] [PubMed] [Google Scholar]