Abstract
Injuries to tendons are common in both human athletes as well as in animals, such as the horse, which are used for competitive purposes. Furthermore, such injuries are also increasing in prevalence in the ageing, sedentary population. Tendon diseases often respond poorly to treatment and require lengthy periods of rehabilitation. The tendon has a unique extracellular matrix, which has developed to withstand the mechanical demands of such tensile-load bearing structures. Following injury, any repair process is inadequate and results in tissue that is distinct from original tendon tissue. There is growing evidence for the key role of the tendon cell (tenocyte) in both the normal physiological homeostasis and regulation of the tendon matrix and the pathological derangements that occur in disease. In particular, the tenocyte is considered to have a major role in effecting the subclinical matrix degeneration that is thought to occur prior to clinical disease, as well as in the severe degradative events that occur in the tendon at the onset of clinical disease. Furthermore, the tenocyte is likely to have a central role in the production of the biologically inadequate fibrocartilaginous repair tissue that develops subsequent to tendinopathy. Understanding the biology of the tenocyte is central to the development of appropriate interventions and drug therapies that will either prevent the onset of disease, or lead to more rapid and appropriate repair of injured tendon. Central to this is a full understanding of the proteolytic response in the tendon in disease by such enzymes as metalloproteinases, as well as the control of the inappropriate fibrocartilaginous differentiation. Finally, it is important that we understand the role of both intrinsic and extrinsic cellular elements in the repair process in the tendon subsequent to injury.
Keywords: ADAMTS, degradation, MMP, tendon, TIMP
Tendons and their diseases in man
Tendons are a specialized form of fibrous connective tissue that are responsible not only for transmitting mechanical forces from skeletal muscle to bone but also as joint stabilizers and as ‘shock absorbers’ to limit muscle damage. By virtue of their matrix organization tendons are uniquely adapted to their function of resisting tensile forces. Tendons are composed predominantly of water (approximately 70%) with the remaining 30% dry matter consisting of collagen and a non-collagenous matrix. Type I collagen is the predominant collagen within tendon, although small amounts of other collagens (for example: collagen -II, -III, -IV and -V) are found within tendon. Collagen is arranged hierarchically within the tendon in longitudinal arrays, grouped as microfibrils, subfibrils and fibrils bound together into fascicles by the more loosely organized endotendon septa (Kastelic et al. 1978). The non-collagenous matrix of tendon consists of a wide range of glycoproteins including the proteoglycans, and a variety of other smaller molecules (Riley 2005; Corps et al. 2006). The cellular elements of tendon occupy only a small proportion of the tissue and they have been poorly defined to date (Fig. 1). They comprise morphologically different populations within different compartments of the tendon (intra- and inter-fascicular), different regions and in different ages (Kader et al. 2002). The ability of a developing tendon to produce a relevant matrix for its function has been unclear, although recently specialized cells have been identified in the developing tendon, which are likely to be responsible for the parallel longitudinal alignment of the collagen fibres (Canty et al. 2004).
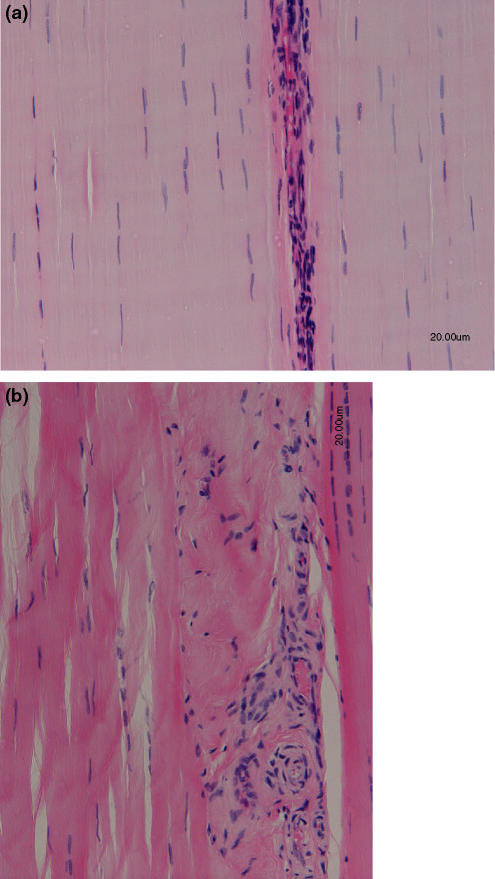
Figure 1.
(a) Longitudinal haemotoxylin and eosin stained section of a normal mid metacarpal (tensional) region of a superficial digital flexor tendon from an 8-year-old thoroughbred racehorse. Note the flattened nuclei of the tenocytes which lie between the parallel aligned collagen fibrils. The more cellular region is the endotenon which lies between tendon fascicles. (b) Longitudinal haemotoxylin and eosin stained section of a mid metacarpal (tensional) region of a superficial digital flexor tendon from an 8-year-old thoroughbred racehorse which sustained a tendon injury approximately 8 weeks previously. There appears to be a disorganized arrangement to the cells in the injured site and a loss of parallel alignment. The cells appear to be morphologically heterogeneous compared to the cellular element seen in normal tendon, with a distinct loss of cells with flattened nuclei at the site of pathology.
Tendons have high mechanical strength and elasticity necessary to perform their function. Tendons also exhibit viscoelasticity as they display properties of stress relaxation and creep (Sharma & Maffulli 2005). In man, loading of a tendon up to 2% strain leads to flattening of the crimp pattern in tendon. Between 2% and 4% strain, tendons deform in a linear fashion as a result of intramolecular sliding of collagen triple helices and fibres become more parallel. At strains less than 4% tendons behave in an elastic fashion and return to their original length when unloaded. Microscopic failure occurs when the strain exceeds 4%, and between 8% and 10% strain, macroscopic failure occurs. (Butler et al. 1978; Kastelic et al. 1978; Mosler et al. 1985; Sharma & Maffulli 2005).
A variety of tendons in man may be affected by overstrain injury, including elements of the rotator cuff and supraspinatus tendon in the shoulder, the forearm extensors, biceps brachii, the Achilles tendon in the lower limb, the tibialis posterior tendon in the foot, and the patella tendon in the knee (Kader et al. 2002; Rees et al. 2006). Tendon injuries present clinically either as acute or chronic disease and can be caused by both extrinsic (trauma) and intrinsic (overstrain) factors (Silva et al. 2002; Rees et al. 2006). Whilst spontaneous acute injuries do occur in tendon diseases, there is evidence that such injuries are often preceded by degeneration of the tendon matrix in the period prior to onset of clinical signs (Kannus & Jozsa 1991; Astrom & Rausing 1995). There is a growing body of evidence that proteolytic enzymes, such as matrix metalloproteinases, in part mediate such degeneration (Jones et al. 2006). In recent decades, the incidence of tendon injury has risen due to both an increase in an elderly population, and a rise in participation in recreational and competitive sporting activities (Rolf & Movin 1997; Kader et al. 2002).
Tendons and their diseases in horses
Tendon injuries are also a significant clinical issue in other species, particularly in horses, where tendinopathy of the superficial digital flexor tendon (SDFT) is a cause of significant economic loss to the racing industry. It has been estimated that the incidence of tendon injuries in populations of racehorses is in the region of 8–43% (Dowling et al. 2000) and 46% of all injuries that occur on UK racecourses are ligament or tendon injuries (Williams et al. 2001; Pinchbeck et al. 2004) (Figs 2 & 3). The SDFT in the horse is a weight-bearing tendon and has many similarities to the human Achilles tendon in both its structure and matrix composition (Dowling et al. 2000). This tendon acts as a spring, absorbing and releasing elastic energy during different phases of the stride, contributing to both the high efficiency of locomotion and, along with its associated muscle, also acting as a shock absorber for the limb (Wilson et al. 2001). The horse has maximized its ability to store energy for efficient locomotion by having maximal strains in the SDFT far higher than in humans, recorded at 16% at the gallop in horses (Minetti et al. 1999). In vitro measurements of tendon strain indicate that the failure limit of the equine SDFT is in the region of 10–20%, so that the normal SDFT is operating close to its functional limit in vivo (Goodship et al. 1994). Whilst the SDFT appears to be able to adapt during development, after skeletal maturity there appears to be little ability for the SDFT to remodel functionally (Smith et al. 1999). Furthermore, there is good evidence that with increasing age there is a trend towards tendon matrix degeneration. Such alterations in tendon matrix composition and arrangement due to matrix degeneration may result in a tendon which at maximal exercise is operating close to, or exceeding, its physiological limits, leading to clinical disease (Dowling et al. 2000).
Figure 2.
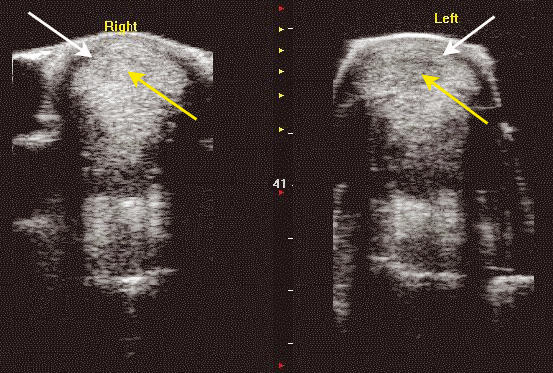
Transverse ultrasound scan of the mid-metacarpal region of the left and right superficial digital flexor tendon (SDFT) from an 9-year-old thoroughbred racehorse which sustained a tendon injury approximately 2 weeks previously. The SDFTs are identified by the white arrows. There is a loss of echogenicity in the central region of the tendons which is due to rupture of tendon fibres at that site (yellow arrow). The injury is more apparent on the left in comparison with the right limb. [Please refer to the electronic article for the colour version of this figure (http://www.blackwell-synergy.com/loi/iep)].
Figure 3.
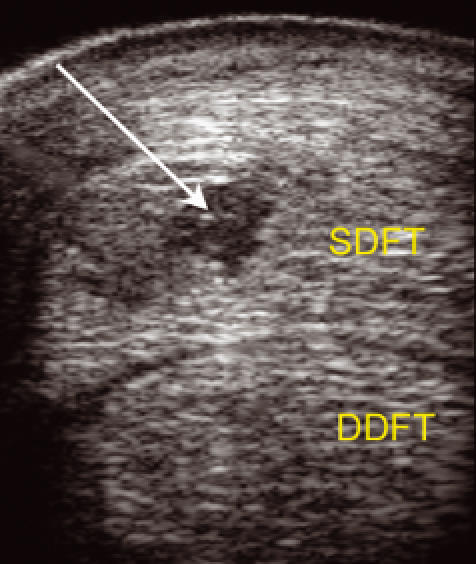
Transverse ulatrsonogram of the mid-metacarpal region of the left superficial digital flexor tendon (SDFT) and deep digital flexor tendon (DDFT) from an 6-year-old thoroughbred racehorse which sustained a tendon injury 10 days previously. There is an anechoic and hypoechoic region in the tendon as a result of a tendon injury at that site (white arrow). [Please refer to the electronic article for the colour version of this figure (http://www.blackwell-synergy.com/loi/iep)].
The tenocyte
The tenocyte was long considered to be a passive bystander in tendon diseases; however, there is now considerable evidence that the tenocyte has a central role in both the matrix degeneration that occurs prior to clinical tendinopathy, as well as the pathological response that occurs subsequent to clinical disease. The tenocytes lie between the longitudinally arranged collagen fibres and have elongated nuclei (Fig. 1). Little is currently known about the range of cell phenotype demonstrated by tenocytes, although a range of histological appearances have been reported (Jozsa et al. 1991; Kannus & Jozsa 1991; Goodship et al. 1994; Jarvinen et al. 1997; Jozsa & Kannus 1997; Kannus et al. 2000). Tenocytes have large cytoplasmic extensions, which connect adjacent cells via gap junctions both longitudinally and laterally throughout the tendon. The gap junctions are thought to be involved in effecting a coordinated cellular response to loading (McNeilly et al. 1996). It has long been recognized that tendon which receives compressive load, such as the region overlying the metacarpophalangeal joint, has a matrix which is much more fibrocartilaginous in nature. The cells in this site are more rounded and are reminiscent of chondrocytes with a more chondrogenic phenotype (Vogel & Koob 1989; Perez-Castro & Vogel 1999).
Proteinases and tendon disease
All connective tissues have mechanisms in place to maintain, adapt, remodel and repair their extracellular matrix. In particular, it is important in physiological remodelling and in repair processes that matrix molecules can be degraded to allow their removal from tissue. Whilst it is possible that some of this process may occur intracellularly following phagocytosis of matrix molecules (Creemers et al. 1998), there is strong evidence that extracellular degradation is the predominant mechanism of matrix degradation in tissues such as tendon. Whilst there may be involvement of serine proteinases in this extracellular matrix (ECM) degradation, most work has concentrated on the metalloproteinases, including the MMP (matrix metalloproteinases) the ADAM (A distegrin and metalloproteinases) and ADAMTS (A distegrin and metalloproteinases with thrombospondin motifs). It is thought that between them, these enzymes are capable of degrading key components of the tendon ECM. The MMP consist of a family of 23 genes in man, whilst the ADAM family consists of more than 30 members. The action of these proteinases is inhibited by tissue inhibitors of metalloproteinases (TIMP), which in man consists of four gene products (TIMP-1–4) (Cawston & Wilson 2006). It is considered that there is a balance in tissues between the enzyme and inhibitor, and alterations in this balance will result in pathological degradation. Whilst a key role of such proteinases is in ECM degradation, they also have major roles in aspects of cell physiology, for example in cleavage of cell surface molecules, cell proliferation and apoptosis (McCawley & Matrisian 2001). MMP are tightly controlled at a number of levels, including synthesis and secretion, activation of the zymogen, inhibition of the active enzyme and localization and clearance of the enzyme (Cawston & Wilson 2006).
Whilst proteinases are considered to be key effectors of the pathological process in tendon diseases, they also have important roles in normal physiological events in tendon homeostasis and repair. The therapeutic use of broad spectrum MMP inhibitors in clinical trials in man has been associated with clinical tendinopathy which rapidly resolves following cessation of drug therapy (Hutchinson et al. 1998; Jones et al. 1999; Tierney et al. 1999). This side effect is thought to occur due to MMP inhibition within the tendon (McCawley & Matrisian 2000). Furthermore, the importance of MMP in normal tendon repair is identified in a rat model of tendon transection. This demonstrated that transacted tendons from rats which had been treated with doxycycline, an MMP inhibitor, had significantly decreased force at failure and energy uptake when the tendons were mechanically tested (Pasternak et al. 2007).
The first report that identified MMP production in tendon described MMP-1 and TIMP-1 synthesis by human rotator cuff tendon in culture, although the study showed no difference in enzyme or inhibitor production between normal and degenerate tissue (Dalton et al. 1995). An immunohistochemical study demonstrated MMP-1 to be localized at the edge of tears within the supraspinatus tendon (Gotoh et al. 1997). Later, Riley showed increased levels of MMPs in the supraspinatus tendon in comparison to the distal biceps brachii tendon, and this corresponded with higher levels of collagen turnover in the supraspinatus tendon. It was concluded that these changes were due to a repair or maintenance function in the more highly loaded supraspinatus tendon (Riley et al. 2002).
More recently, studies have investigated alterations in proteinase production by tenocytes by identification of proteinase and TIMP transcription. Such studies are often powerful as they produce very specific and accurate data, however as the enzyme activity is controlled at a number of levels, not only at the level of transcription, such studies do not necessarily reflect the active proteinase profile within tissues. Whilst cDNA arrays have the potential to provide large amounts of data on gene expression variations between normal and diseased tendons, the two published array studies in tendons have only yielded limited information (Ireland et al. 2001; Alfredson et al. 2003). This is in part due to the fact that the array data could not always be confirmed by quantitative real-time PCR assay. A semi-quantitative study of MMP and TIMP gene expression in torn rotator cuff tendons identified elevation of MMP-13 gene expression and a decrease in MMP-3, and TIMP-1, -2, -3 & -4 gene expression in diseased compared with normal samples. Furthermore, the study identified increased amounts of MMP-13 protein in pathological specimens by western blotting (Lo et al. 2004). Most recently, Riley’s group performed a large-scale study of quantitative gene expression changes in normal compared with painful and ruptured Achilles tendons. Painful tendons demonstrated significantly lower levels of MMP-3 & -10 & TIMP-3 and higher levels of ADAM-12 and MMP-23 compared with normal tendons. Ruptured tendons had lower levels of MMP-3 & -7 and TIMPs-2, -3 & –4, and higher levels of ADAMs-8, -12, MMP-1, -9, -19 & -25 and TIMP-1 compared with normal tendons. The data indicated that extracellular proteolytic activity varied with different pathological states (Jones et al. 2006). A variety of both in-vitro culture systems, and tissue localization within tendon, have been used to identify proteinase catabolism of a variety of proteoglycans in tendon and such studies have identified both MMP and ADAMTS mediated catabolism of such matrix molecules (Rees et al. 2000, 2005, 2007; Samiric et al. 2004a,b).
Recently we have investigated the expression of several MMP, ADAMTS and TIMP genes in bilateral SDFTs of normal horses (n = 9), those suffering from acute tendon disease (within 6 weeks of injury) (n = 9) and chronic tendon disease (n = 9) (Clegg PD, unpublished observations). For each tendon, two biopsies were sampled, one from the peripheral margins of the mid-metacarpal SDFT, and one from the central region of the mid-metacarpal SDFT. It is the tensional region of the mid-metacarpal SDFT that is the most common site of SDFT injury in the horse, and such injuries tend to be localized to the central region of the tendon. In all tendons, we measured relative gene expression levels for a variety of MMP, ADAMTS and TIMP using quantifiable real-time PCR. In normal tendon, we showed no significant differences in gene expression between the central and peripheral sites of the mid-metacarpal SDFT with the exception of MMP-3, which was expressed at significantly (approximately 10-fold) lower levels in the peripheral site, which is at a lower risk of tendon injury compared with the central region at that site. In abnormal tendon, there were obvious perturbations in gene expression. MMP-13 gene expression was elevated substantially in acute tendon injury tendon (over 1000-fold) and MMP-1 to a lesser extent (10-fold). In chronic tendon injury the marked elevation of MMP-13 gene expression was maintained, although MMP-1 expression returned to levels that were comparable to normal tendon. As in studies in man (Lo et al. 2004; Jones et al. 2006) there was a decrease in expression of MMP-3 (approximately 50-fold decrease) in both acute and chronic tendon disease. Expression levels of ADAMTS-4 were unchanged in disease, whilst ADAMTS-5 levels were substantially decreased in acute tendon disease (over 100-fold), and were unchanged in chronic tendon disease in comparison with normal tissue. There were no changes in expression patterns of TIMP-1 & -2 between normal tendon and any disease states, although TIMP-3 gene expression was significantly decreased in acute tendon disease in comparison with normal or chronically injured tendon. If this pattern of gene expression accurately reflects the output of protein, these observations suggest that there is a net ‘over-activity’ of MMPs after injury. The perturbations in gene expression were most marked in the central region of the tendon, although significant variations were observed in peripheral tissue. Interestingly, in horses with tendonitis the contralateral uninjured tendon showed a similar, though less marked, pattern of altered gene expression, although there were no overt signs of pathology (Clegg PD, unpublished observation). These observations suggest that there is a temporal change in proteinase activities at different stages of tendon disease, with obvious perturbations in cell phenotype persisting into the chronic phases of the disease. Furthermore, there appears to be distinct regulation of different proteinase gene products in pathology, rather than a universal up- or down-regulation of a particular class of genes in disease. Further work is now required to extend the investigation to further proteinase genes, as well as investigations to identify alterations in cell phenotype in specific sub-populations in tenocytes, as the biopsy approach used here is likely to be quantifying an ‘average’ phenotype from what is likely to be a cell population with some degree of heterogeneity.
Fibrochondrogenic differentiation and tendon disease
As documented above, tendon at sites of compressive loading is more fibrocartilaginous in nature (Vogel & Koob 1989; Perez-Castro & Vogel 1999). It has been long recognized that in human tendon disease there is an increase in glycosaminoglycan (GAG) content, of which chondroitin sulphate is the major GAG present (Riley et al. 1994). More recently it has been identified that gene expression for the proteoglycans aggrecan and biglycan are increased in painful human Achilles tendons, whilst levels of versican and decorin were not altered in comparison with normal tendon (Corps et al. 2006). Analysis of versican gene expression in normal and pathological tendon has identified that there are alterations in the balance of spliced variants of this gene in pathological compared with normal tendon, and alterations in its gene expression relative to collagen (Corps et al. 2004). Tendon is known to contain a wide variety of proteoglycans, including the large aggregating proteoglycans as a well as a variety of small leucine-rich proteoglycans (SLRPs) (Berenson et al. 1996; Waggett et al. 1998; Rees et al. 2000). Recently we have investigated the expression of several key proteoglycan genes, as well as other genes assocated with fibrochondrogenic differentiation (Coll-I, -II, -IX, -X and -XI and SOX9) in the tensional region of the mid-metacarpal bilateral SDFTs of normal horses (n = 9), those suffering from acute tendon disease (within 6 weeks of injury) (n = 9) and chronic tendon disease (n = 9) (Clegg PD, unpublished observations). We confirmed that fibrochondrogenic differentiation is the specific phenotype in chronic tendon disease, with elevations of Collagen-II, -IX, -X, -XI, SOX9, aggrecan, biglycan gene expression all being a consistent finding in chronically injured compared with normal tendons. In acute tendon injury, there was a lack of a consistent switch to a more fibrochondrogenic phenotype. Whilst some genes associated with such a cartilaginous phenotype, such as aggrecan, were elevated in early disease, many were unchanged (Collagen-II, -IX and -XI, SOX9) or even decreased (lumican) (Clegg PD, unpublished observations). Interestingly in a subset of samples, we assessed cell phenotypic changes relating to chondrogenesis in the compressional region of normal and both acutely and chronically injured SDFTs. It is this site that is normally fibrocartilaginous in nature, although in the horse pathological changes are rare in this region of the tendon (Dowling et al. 2000). In acute tendon disease compared with normal tendon there was a decrease in expression levels of a variety of genes associated with the cartilaginous phenotype in this region, in particular Collagen-II, SOX9, aggrecan and biglycan. However by the time the disease was chronic, such phenotypic markers had returned to levels similar to those seen in normal tendon at that site (Clegg PD, unpublished observations). We propose that this alteration in the phenotype observed at this site in acute disease could be due to decreased weight bearing following acute injury, resulting in altered compressive forces on the tenocytes leading to a lack of chondrogenic stimuli necessary to fully maintain the cell phenotype at this site. In chronic disease, once weight bearing has returned more towards normal, relevant physiological loading is enough to maintain this phenotype. It is uncertain what stimuli, either mechanical or biochemical, are leading to chondrogenic differentiation in tension-bearing tendons following injury. However it is clear that such differentiation is the preferred cellular response in chronic injury, and some distinct elements of chondrogenic differentiation can be observed early in clinical tendon disease.
Putative progenitor cell niches in tendon
In recent years, there has been considerable interest in tissue engineering techniques to improve methods of tissue repair. These techniques could be used to manipulate either exogenously implanted stem cells, or endogenous resident cells to enhance repair. Such techniques are highly relevant to tissues such as tendon, which produces an abnormal fibrocartilaginous scar tissue after injury, which is distinct in biochemical composition and mechanical properties to native tendon tissue. Because of these differences, the healed tendon is at a much higher risk of re-injury (Dowling et al. 2000). Most mesenchymal tissues are considered to contain specific populations of precursor or progenitor cells, with such populations being defined by their multipotent differentiation capability (Caplan & Bruder 2001). These multipotent cells have been demonstrated in a variety of mesenchymal tissues including muscle (Bosch et al. 2000), cartilage (Barbero et al. 2003) and bone (Noth et al. 2002). It is important that we are able to identify, and in the future potentially manipulate, such populations to enhance tissue repair subsequent to injury. There is evidence that tendon does contain cells with some degree of multipotent differentiation capability. Specific cell lines that were established from single cell clones derived from murine tendon cells have been demonstrated to undergo multipotent differentiation capability to a variety of mesenchymal phenotypes with appropriate stimuli (Salingcarnboriboon et al. 2003). Recently, a study using tendon tissues derived from adolescent humans concluded that explanted human tendon cells have an intrinsic differentiation capability and could to some extent be caused to differentiate into a variety of mesenchymal cell phenotypes (de Mos et al. 2007). However, recent work in our laboratories suggests that adult tendon-derived cells in the horse, whilst having some degree of multipotent differentiation capability, are less capable than bone marrow-derived mesenchymal cells for differentiating into different cell lines, other than possibly their own (Strassburg et al. 2006). The exact niche and phenotype of these precursor/progenitor cells is not known in the tendon, but we hypothesize that such cells may be associated with the intrinsic vasculture of the tendon, as in other tissues (Farrington-Rock et al. 2004). Further work is now required to fully identify the intrinsic cellular elements that are involved in tissue repair and regeneration in tendon. Currently, the cellular repair response in injured tendon is suboptimal and the aim of therapeutic interventions should be to promote relevant cellular differentiation in damaged tendon.
Conclusions
There is good evidence that the tenocyte has an important role in both the response of the tendon to any pathological insult, and the failure of the repair process following injury to fully recapitulate the native tendon structure. The normal physiological balance of matrix synthesis and turnover is beneficial for tendon health, but once the tendon is injured there is a substantial change in the phenotype of the cells present within the tendon leading to substantive matrix degradation and inappropriate matrix synthesis. It remains far from clear how this process is controlled, and what signals result in the fibrochondrogenic differentiation, which is characteristic of the chronically injured tendon. Furthermore, there is a lack of knowledge concerning the cellular aspects of tissue healing following injury and the role of intrinsic tendon-derived progenitor cells in this process. It is likely that in both physiology and in pathology the tenocyte population will show considerable phenotypic heterogeneity, and work is necessary to identify the level of cellular diversity within tendon and how this alters with ageing, subclinical injury and disease. Compared with other diseases where inappropriate matrix degradation is a key feature, such as cartilage degradation by the chondrocyte in osteoarthritis, there is still much to learn about the role of the tenocytes in tendon injury and repair but, as a corollary to this, great potential to influence repair once this knowledge is gained.
Acknowledgments
The authors would like to acknowledge the Wellcome Trust, The Horserace Betting Levy Board and the Pet Plan Charitable Trust who have provided financial support for our studies.
References
- Alfredson H, Lorentzon M, Backman S, Backman A, Lerner UH. cDNA-arrays and real-time quantitative PCR techniques in the investigation of chronic achilles tendinosis. J. Orthop. Res. 2003;21:970–975. doi: 10.1016/S0736-0266(03)00107-4. [DOI] [PubMed] [Google Scholar]
- Astrom M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin. Orthop. Relat. Res. 1995;316:151–164. [PubMed] [Google Scholar]
- Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315–1325. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- Berenson MC, Blevins FT, Plaas AHK, Vogel KG. Proteoglycans of human rotator cuff tendons. J. Orthop. Res. 1996;14:518–525. doi: 10.1002/jor.1100140404. [DOI] [PubMed] [Google Scholar]
- Bosch P, Musgrave DS, Lee JY, et al. Osteoprogenitor cells within skeletal muscle. J. Orthop. Res. 2000;18:933–944. doi: 10.1002/jor.1100180613. [DOI] [PubMed] [Google Scholar]
- Butler DL, Grood ES, Noyes FR, Zernicke RF. Biomechanics of ligaments and tendons. Exerc. Sport Sci. Rev. 1978;6:125–181. [PubMed] [Google Scholar]
- Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J. Cell Biol. 2004;165:553–563. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- Cawston TE, Wilson AJ. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract. Res. Clin. Rheumatol. 2006;20:983–1002. doi: 10.1016/j.berh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Corps AN, Robinson AHN, Movin T, et al. Versican splice variant messenger RNA expression in normal human Achilles tendon and tendinopathies. Rheumatology. 2004;43:969–972. doi: 10.1093/rheumatology/keh222. [DOI] [PubMed] [Google Scholar]
- Corps AN, Robinson AHN, Movin T, Costa ML, Hazleman BL, Riley GP. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology. 2006;45:291–294. doi: 10.1093/rheumatology/kei152. [DOI] [PubMed] [Google Scholar]
- Creemers LB, Jansen IDC, Docherty AJP, Reynolds JJ, Beertsen W, Everts V. Gelatinase A (MMP-2) and cysteine proteinases are essential for the degradation of collagen in soft connective tissue. Matrix Biol. 1998;17:35–46. doi: 10.1016/s0945-053x(98)90123-8. [DOI] [PubMed] [Google Scholar]
- Dalton S, Cawston TE, Riley GP, Bayley IJL, Hazleman BL. Human shoulder tendon biopsy samples in organ-culture produce procollagenase and tissue inhibitor of metalloproteinases. Ann. Rheum. Dis. 1995;54:571–577. doi: 10.1136/ard.54.7.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling BA, Dart AJ, Hodgson DR, Smith RK. Superficial digital flexor tendonitis in the horse. Equine Vet. J. 2000;32:369–378. doi: 10.2746/042516400777591138. [DOI] [PubMed] [Google Scholar]
- Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- Goodship AE, Birch HL, Wilson AM. The pathobiology and repair of tendon and ligament injury. Vet. Clin. North Am. Equine Pract. 1994;10:323–349. doi: 10.1016/s0749-0739(17)30359-0. [DOI] [PubMed] [Google Scholar]
- Gotoh M, Hamada K, Yamakawa H, Tomonaga A, Inoue A, Fukuda HO. Significance of granulation tissue in torn supraspinatus insertions: an immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. J. Orthop. Res. 1997;15:33–39. doi: 10.1002/jor.1100150106. [DOI] [PubMed] [Google Scholar]
- Hutchinson JW, Tierney GM, Parsons SL, Davis TRC. Dupuytren’s disease and frozen shoulder induced by treatment with a matrix metalloproteinase inhibitor. J. Bone Joint Surg Br. 1998;80B:907–908. doi: 10.1302/0301-620x.80b5.8464. [DOI] [PubMed] [Google Scholar]
- Ireland D, Harrall R, Curry V, et al. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20:159–169. doi: 10.1016/s0945-053x(01)00128-7. [DOI] [PubMed] [Google Scholar]
- Jarvinen M, Jozsa L, Kannus P, Jarvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scand. J. Med. Sci. Sports. 1997;7:86–95. doi: 10.1111/j.1600-0838.1997.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Jones L, Ghaneh P, Humphreys M, Neoptolemos JP. The Matrix metalloproteinases and their inhibitors in the treatment of pancreatic cancer. Ann. N. Y. Acad. Sci. 1999;880:288–307. doi: 10.1111/j.1749-6632.1999.tb09533.x. [DOI] [PubMed] [Google Scholar]
- Jones G, Corps A, Pennington C, et al. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum. 2006;54:832–842. doi: 10.1002/art.21672. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P. Histopathological findings in spontaneous tendon ruptures. Scand. J. Med. Sci. Sports. 1997;7:113–118. doi: 10.1111/j.1600-0838.1997.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P, Balint JB, Reffy A. Three-dimensional ultrastructure of human tendons. Acta Anat. (Basel) 1991;142:306–312. doi: 10.1159/000147207. [DOI] [PubMed] [Google Scholar]
- Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: some aspects of basic science and clinical management. Br. J. Sports Med. 2002;36:239–249. doi: 10.1136/bjsm.36.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J. Bone Joint Surg. Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- Kannus P, Jozsa L, Jarvinnen M. Basic science of tendon. In: Gordon SL, Blair SJ, Fine LJ, editors. Repetitive motion disorders of the upper extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2000. pp. 21–37. [Google Scholar]
- Kastelic J, Galeski A, Baer A. The multicomposite structure of tendon. Connect. Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Lo IKY, Marchuk LL, Hollinshead R, Hart DA, Frank CB. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am. J. Sports Med. 2004;32:1223–1229. doi: 10.1177/0363546503262200. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol. Med. Today. 2000;6:149–156. doi: 10.1016/s1357-4310(00)01686-5. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr. Opin. Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J. Anat. 1996;189:593–600. [PMC free article] [PubMed] [Google Scholar]
- Minetti AE, ArdigO LP, Reinach E, Saibene F. The relationship between mechanical work and energy expenditure of locomotion in horses. J. Exp. Biol. 1999;202:2329–2338. doi: 10.1242/jeb.202.17.2329. [DOI] [PubMed] [Google Scholar]
- de Mos M, Koevoet W, Jahr H, et al. Intrinsic differentiation potential of adolescent human tendon tissue: an in-vitro cell differentiation study. BMC Musculoskelet. Disord. 2007;8:16. doi: 10.1186/1471-2474-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosler E, Folkhard W, Knorzer E, Nemetschek-Gansler H, Nemetschek T, Koch MHJ. Stress-induced molecular rearrangement in tendon collagen. J. Mol. Biol. 1985;182:589. doi: 10.1016/0022-2836(85)90244-x. [DOI] [PubMed] [Google Scholar]
- Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J. Orthop. Res. 2002;20:1060–1069. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Pasternak B, Fellenius M, Aspenberg P. Doxycycline impairs tendon repair in rats. Acta Orthop. Belg. 2007;72:756–760. [PubMed] [Google Scholar]
- Perez-Castro AV, Vogel KG. In situ expression of collagen and proteoglycan genes during development of fibrocartilage in bovine deep flexor tendon. J. Orthop. Res. 1999;17:139–148. doi: 10.1002/jor.1100170120. [DOI] [PubMed] [Google Scholar]
- Pinchbeck GL, Clegg PD, Proudman CJ, Stirk A, Morgan KL, French NP. Horse injuries and racing practices in National Hunt racehorses in the UK: the results of a prospective cohort study. Vet. J. 2004;167:45–52. doi: 10.1016/s1090-0233(03)00141-2. [DOI] [PubMed] [Google Scholar]
- Rees SG, Flannery CR, Little CB, Hughes CE, Caterson B, Dent CM. Catabolism of aggrecan, decorin and biglycan in tendon. Biochem. J. 2000;350:181–188. [PMC free article] [PubMed] [Google Scholar]
- Rees SG, Curtis CL, Dent CM, Caterson B. Catabolism of aggrecan proteoglycan aggregate components in short-term explant cultures of tendon. Matrix Biol. 2005;24:219–231. doi: 10.1016/j.matbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology. 2006;45:508–521. doi: 10.1093/rheumatology/kel046. [DOI] [PubMed] [Google Scholar]
- Rees SG, Waggett AD, Dent CM, Caterson B. Inhibition of aggrecan turnover in short-term explant cultures of bovine tendon. Matrix Biol. 2007;26:280–290. doi: 10.1016/j.matbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Riley GP. Gene expression and matrix turnover in overused and damaged tendons. Scand. J. Med. Sci. Sports. 2005;15:241–251. doi: 10.1111/j.1600-0838.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Glycosaminoglycans of human rotator cuff tendons - changes with age and in chronic rotator cuff tendinitis. Ann. Rheum. Dis. 1994;53:367–376. doi: 10.1136/ard.53.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley GP, Curry V, DeGroot J, et al. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. doi: 10.1016/s0945-053x(01)00196-2. [DOI] [PubMed] [Google Scholar]
- Rolf CMT, Movin T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int. 1997;18:565–569. doi: 10.1177/107110079701800906. [DOI] [PubMed] [Google Scholar]
- Salingcarnboriboon R, Yoshitake H, Tsuji K, et al. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp. Cell Res. 2003;287:289–300. doi: 10.1016/s0014-4827(03)00107-1. [DOI] [PubMed] [Google Scholar]
- Samiric T, Ilic MZ, Handley CJ. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004a;23:127–140. doi: 10.1016/j.matbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Samiric T, Ilic MZ, Handley CJ. Large aggregating and small leucine-rich proteoglycans are degraded by different pathways and at different rates in tendon. Eur. J. Biochem. 2004b;271:3612–3620. doi: 10.1111/j.0014-2956.2004.04307.x. [DOI] [PubMed] [Google Scholar]
- Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J. Bone Joint surg. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Boyer MI, Gelberman RH. Recent progress in flexor tendon healing. J. Orthop. Sci. 2002;7:508. doi: 10.1007/s007760200090. [DOI] [PubMed] [Google Scholar]
- Smith RK, Birch H, Patterson-Kane J, et al. Should equine athletes commence training during skeletal development?: changes in tendon matrix associated with development, ageing, function and exercise. Equine Vet. J. Suppl. 1999;30:201–209. doi: 10.1111/j.2042-3306.1999.tb05218.x. [DOI] [PubMed] [Google Scholar]
- Strassburg S, Smith R, Goodship A, Hardingham T, Clegg P. Adult and late fetal equine tendons contain cell populations with weak progenitor properties in comparison to bone marrow derived mesenchymal stem cells. Trans. Orthop. Res. Soc. Annu. Meet. 2006;31:1113. [Google Scholar]
- Tierney GM, Griffin NR, Stuart RC, et al. A pilot study of the safety and effects of the matrix metalloproteinase inhibitor marimastat in gastric cancer. Eur. J. Cancer. 1999;35:563–568. doi: 10.1016/s0959-8049(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Vogel KG, Koob TJ. Structural specialization in tendons under compression. Int. Rev. Cytol. Surv. Cell Biol. 1989;115:267–293. doi: 10.1016/s0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- Waggett AD, Ralphs JR, Kwan APL, Woodnutt D, Benjamin M. Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biol. 1998;16:457–470. doi: 10.1016/s0945-053x(98)90017-8. [DOI] [PubMed] [Google Scholar]
- Williams RB, Harkins LS, Hammond CJ, Wood JL. Racehorse injuries, clinical problems and fatalities recorded on British racecourses from flat racing and National Hunt racing during 1996, 1997 and 1998. Equine Vet. J. 2001;33:478–486. doi: 10.2746/042516401776254808. [DOI] [PubMed] [Google Scholar]
- Wilson AM, McGuigan MP, Su A, van den Bogert AJ. Horses damp the spring in their step. Nature. 2001;414:895–899. doi: 10.1038/414895a. [DOI] [PubMed] [Google Scholar]