Abstract
Summary
Multiple organ failure is frequently associated with acute pancreatitis (AP). Our aim was to study pulmonary, hepatic and renal complications developed in the course of AP experimentally induced in rats by bile-pancreatic duct obstruction (BPDO), differentiating the complications caused by AP itself, from those directly caused by bile duct obstruction (BDO), after ligating the choledocus. N-acetylcysteine (NAC) was administered as a therapeutic approach. Myeloperoxidase activity revealed neutrophil infiltration in lungs from 12 h after BDO, even if AP was not triggered. Lactate dehydrogenase (LDH) activity indicated hepatocyte death from 48 h after BDO, and from 24 h following BPDO-induced AP onwards, an effect delayed until 48 h by NAC treatment. Rats with single cholestasis (BDO) and rats with BPDO-induced AP showed a significant increase in plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT) and bilirubin concentration from 12 h onwards, whose values were reduced by NAC treatment at early BPDO. No renal failure was found during 120 h of bile-pancreatic obstruction. Our results showed lung and liver impairment as a result of BDO, even if AP does not develop. Pancreatic damage and extrapancreatic complications during AP induced by BPDO were palliated by NAC treatment.
Keywords: acute pancreatitis, antioxidants, liver damage, lung impairment, renal failure
Acute pancreatitis (AP) is a disease with a relatively high morbidity and mortality (Bourke 1977; Winslet et al. 1992; Forsmark & Toskes 1995). Whatever the aetiology of AP, inflammation is developed in the pancreas as a result of the damage initiated within pancreatic acinar cells (Weber & Adler 2001; Frossard & Pastor 2002). It is mediated by pro-inflammatory factors released from acinar cells themselves and leucocytes infiltrated in the gland. An excessive production of these inflammatory mediators turns the local inflammation to a systemic response which could result in multiple organ dysfunction syndrome (MODS) (Davies & Hagen 1997). Respiratory, hepatic and renal failure contribute most significantly to the morbidity and mortality of patients with severe attacks of AP (Wilson & Imrie 1990; Lankisch et al. 1996). Lung injury, the first manifestation of MODS, is characterized by intrapulmonary sequestration of neutrophils and increased vascular permeability followed by oedema and vascular collapse (Guice et al. 1989; Murakami et al. 1995; Bhatia et al. 2000), leading to respiratory function impairment. Although most often in biliary pancreatitis (Van Gossum et al. 1984), liver injury has also been reported in pancreatitis induced by CDE diet and supramaximal doses of caerulein (Norman et al. 1997;He et al. 1999). Kidneys can also be altered during AP as a consequence of hypovolaemia due to both fluid extravasation and decrease in blood flow (Lucas & Ledgerwood 1982; Levy et al. 1986), both events contributing to hypotension associated with failure in glomerular function.
Although it is well established that the outcome of AP for individual patients is mostly dependent on systemic complications, there are few experimental studies aimed at an overall evaluation of the alterations which progressively appear in different organs during the course of AP. Thereby, our goal was to develop a detailed study to investigate the time-course and extent of alterations in lung, liver and kidney caused by acute pancreatitis induced in rats by bile-pancreatic duct obstruction (BPDO), an experimental model of relevant clinical interest as it simulates gallstone pancreatitis, the most frequent aetiology in humans (Cartmell & Kingsnorth 2000). As the pancreatic overproduction of reactive oxygen species (ROS) is a pathogenic factor in AP involved in local and systemic inflammation (Poch et al. 1999; Czako et al. 2000), we also investigated the effects of N-acetylcysteine (NAC), as antioxidant treatment, on the multi-organ impairment caused by BPDO-induced AP.
Materials and methods
Animals
Male Wistar rats (250–300 g) were used. They were kept in a controlled environment at 22 ± 1 °C using a 12-h light/dark cycle. The animals were housed individually in cages and fed with standard laboratory chow. All experiments were performed in accordance with European Community guidelines on ethical animal research (86/609/EEC). The study was approved by the Institutional Animal Care and Use Committee of the University of Salamanca (Spain).
Experimental design
Rats were randomly divided into the following groups:
Group 1: Sham-operated rats, surgically treated but without ligating the choledocus.
Group 2: Rats subjected to bile duct obstruction (BDO) by ligation of choledocus close to the liver.
Group 3: Rats with AP induced by BPDO by ligation of the common duct at the distal part closed to its exit to the duodenum.
Group 4: Rats with BPDO-induced AP receiving one intraperitoneal injection of NAC (50 mg/kg) 1 h before and 1 h after BPDO.
Four subgroups were designed within the different groups at the following time points: 12, 24, 48 and 120 h.
Surgical procedures were carried out under anaesthesia with 2–3% isofluorane (Abbot, Madrid, Spain). Afterwards, the abdominal cavity was closed in a double layer and rats were placed in their cages with free access to food and water. Postoperative analgesia was maintained by intramuscular injections of buprenorphine (0.2 mg/kg/8 h).
Collection of samples
After 12 h fasting but with free access to water, rats were re-anaesthetized with sodium pentobarbital (3 mg/100 g body weight, intraperitoneally) in order to collect the samples at the different time points. Blood samples were collected by cardiac puncture into heparinized tubes and plasma was separated by centrifuging at 1000 g for 10 min at 4 °C. Afterwards, laparotomy was performed and pancreas, lungs and liver were excised and weighed.
Plasma measurements
Amylase activity was determined by the method of Hickson (Hickson 1970). Concentrations of total bilirubina and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were determined, using a multiparametric commercial kit (Spotchem II Reagent strip; Menarini, Barcelona, Spain) in an automated analyzer (Spotchem™EZ SP-4430, Arkray Europe, Düsseldorf, Germany). Concentrations of creatinine were determined using a commercial kit from Roche Diagnostics (Mannheim, Germany) strictly following supplier’s recommendations. All samples were run in duplicate and averaged.
Tissue water content
The wet/dry weight ratio was calculated to evaluate interstitial oedema. For this purpose, freshly prepared pancreas, lung and liver tissue samples were weighed before and after drying freshly prepared tissue samples for 96 h at 100 °C.
Myeloperoxidase activity
Neutrophil infiltration was assessed in pancreas and lung and liver by measuring tissue myeloperoxidase (MPO) activity (Bhatia et al. 1998). Briefly, tissue samples were resuspended in 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (HDTAB) and homogenized. After four cycles of freezing and thawing, the homogenate was further disrupted by sonication. After centrifuging (10,000 g, 5 min, 4 °C) the supernatant was incubated with 3,3’,5,5’-tetramethylbenzidine (TMB) reagent for 110 s at 37 °C for the MPO assay. The reaction was stopped with 0.18 M H2SO4, and absorbance measured at 450 nm. MPO activity is expressed per unit of dry weight (fold increase over control rats).
Lactate dehydrogenase activity
Lactate dehydrogenase (LDH) activity was measured in samples of liver previously homogenized in ice-cold 81.3 mM Tris-HCl buffer (pH 7.2) containing 203.3 mM NaCl, according to the method of (Gutmann and Wahlefeld (1974)). Changes in absorbance due to NAD+ formation were recorded at 339 nm at 30 °C.
Statistical analysis
Results are expressed as means ± SEM. Statistical analysis was carried out using analysis of variance (anova) followed by Scheffé test. P-values lower than 0.05 were considered to be statistically significant.
Results
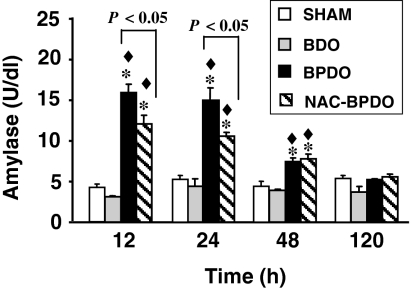
Figure 1 shows the kinetic of plasma amylase activity after surgical procedures. BDO rats displayed control values at all time periods. In contrast, a significant increase in plasma amylase activity was found during 48 h BPDO, with highest values at 12 and 24 h after inducing AP. Although NAC treatment failed to prevent hyperamylasemia, it was maintained at significantly lower levels at early stages of AP.
Figure 1.
Plasma amylase activity in sham-operated animals, rats with bile duct obstruction (BDO), rats with acute pancreatitis induced by bile-pancreatic duct obstruction (BPDO) and rats with BPDO treated with NAC (NAC-BPDO). Number of animals in each group and experimental period: 6. Values are mean ± SEM. anova followed by Scheffé test showed statistically significant differences vs. sham-operated (*), vs. BDO rats (♦) and between NAC-treated and non-treated BPDO rats.
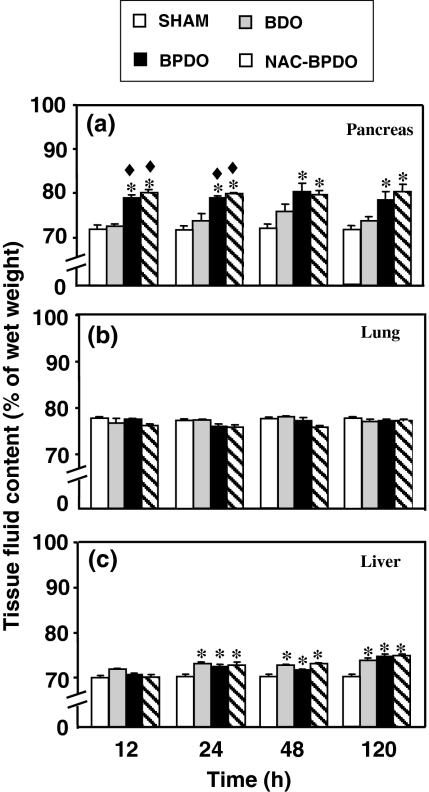
Pancreatic fluid content (Figure 2a) significantly increased during 120 h after inducing AP by BPDO in both NAC-treated and non-treated rats, but not in the BDO rats. No significant accumulation of fluid was found in the lung (Figure 2b) during 120 h either in BDO or BPDO rats. BDO significantly increased hepatic fluid content from 24 h onwards (Figure 2c). This effect did not vary in BPDO rats independently on whether or not they were treated with NAC.
Figure 2.
Tissue water content in pancreas (a), lung (b) and liver (c) from sham-operated animals, rats with bile duct obstruction (BDO), rats with acute pancreatitis induced by bile-pancreatic duct obstruction (BPDO) and rats with BPDO treated with NAC (NAC-BPDO). Number of animals in each group and experimental period: 6. Values are mean ± SEM. anova followed by Scheffé test showed statistically significant differences in pancreas vs. sham-operated (*) and vs. BDO rats (♦). No significant difference was found in the lung. Significant differences vs. sham-operated (*) were found in liver.
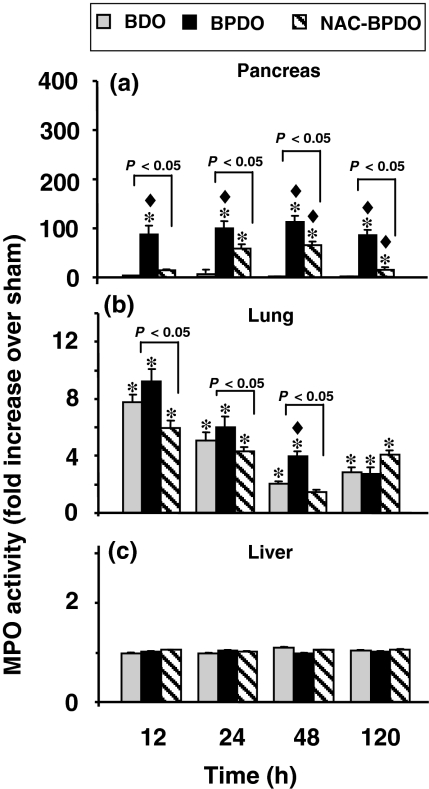
Neutrophil infiltration measured through MPO activity (Figure 3) significantly increased in the pancreas (Figure 3a) of BPDO rats from 12 h afterwards, an effect delayed and significantly reduced by NAC treatment. BDO led to neutrophil accumulation in the lung during 120 h after surgery (Figure 3b), with highest values at 12 h. Similar MPO values were found in rats with AP, except after 48 h BPDO, at which time neutrophil infiltration was significantly higher than in BDO rats. Lung neutrophil infiltration was significantly reduced by NAC treatment. No increase in MPO activity was found in the liver either in BDO or BPDO rats (Figure 3c).
Figure 3.
Myeloperoxidase (MPO) activity in pancreas (a) lung (b) and liver (c) from sham-operated animals, rats with bile duct obstruction (BDO), rats with acute pancreatitis induced by bile-pancreatic duct obstruction (BPDO) and rats with BPDO treated with NAC (NAC-BPDO). Number of animals in each group and experimental period: 6. Values are mean ± SEM. anova followed by Scheffé test showed statistically significant differences in pancreas and lung vs. sham-operated (*), vs. BDO rats (♦) and between NAC-treated and non-treated BPDO rats. No differences were found in liver.
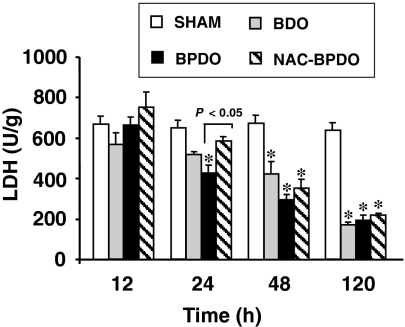
Liver necrosis determined by LDH activity in tissue homogenates (Figure 4) was detected in BPDO rats from 24 h onwards, an effect delayed until 48 h after AP induction in rats treated with NAC. LDH activity was significantly reduced in BDO rats from 48 h after ligation of the choledocus.
Figure 4.
Lactate dehydrogenase (LDH) activity in liver from sham-operated animals, rats with bile duct obstruction (BDO), rats with acute pancreatitis induced by bile-pancreatic duct obstruction (BPDO) and rats with BPDO treated with NAC (NAC-BPDO). Number of animals in each group and experimental period: 6. Values are mean ± SEM. anova followed by Scheffé test showed statistically significant differences vs. sham-operated (*) and between NAC-treated and non-treated BPDO rats.
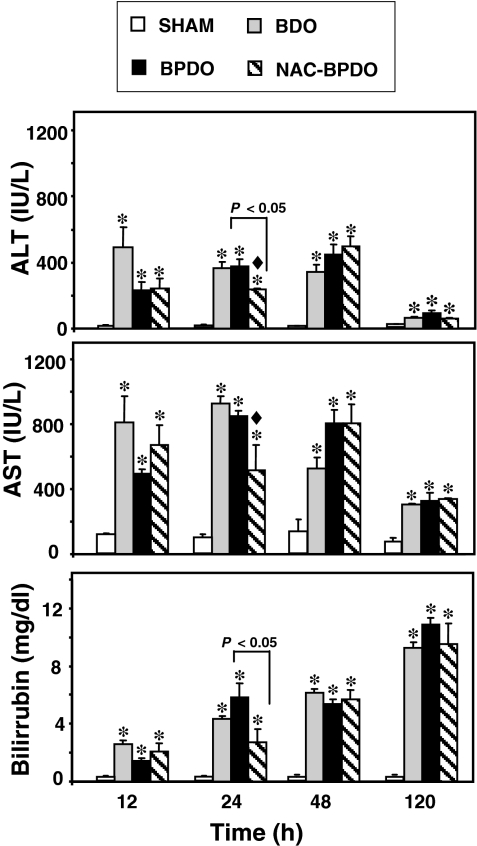
To assess the liver function hepatic enzyme activity and bilirubin concentration were measured in plasma (Figure 5). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) significantly increased in BDO and BPDO rats from 12 h afterwards, but a tendency to return to control values was found at 120 h. Lower enzyme activity was detected 24 h after BPDO in rats treated with NAC. A significant increase in plasma bilirubin concentration was found in BDO and BPDO rats, which resulted progressively higher from 12 to 120 h after obstruction. NAC treatment significantly reduced the hyperbilirubinemia found 24 h after BPDO.
Figure 5.
Plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST) and bilirubin concentration in sham-operated animals, rats with bile duct obstruction (BDO), rats with acute pancreatitis induced by bile-pancreatic duct obstruction (BPDO) and rats with BPDO treated with NAC (NAC-BPDO). Number of animals in each group and experimental period: 6. Values are mean ± SEM. anova followed by Scheffé test showed showed statistically significant differences vs. sham-operated (*), vs. BDO rats (♦) and between NAC-treated and non-treated BPDO rats.
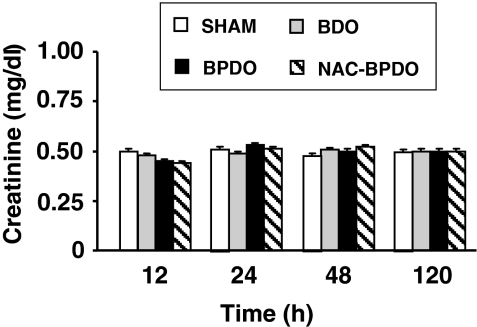
No renal failure was detected in BDO and BPDO rats during 120 h following surgery, as indicated unchanged concentrations of creatinine in plasma (Figure 6).
Figure 6.
Plasma creatinine in sham-operated animals, rats with bile duct obstruction (BDO), rats with acute pancreatitis induced by bile-pancreatic duct obstruction (BPDO) and rats with BPDO treated with NAC (NAC-BPDO). Number of animals in each group and experimental period: 6. Values are mean ± SEM. anova followed by Scheffé test did not show any significant difference among groups.
Discussion
As multisystemic alteration is the main cause of mortality in AP, it is of great interest for clinicians to ascertain the course of extrapancreatic complications in order to apply proper management in patients with AP. Experimental animal models of AP represent a useful tool for this proposal, specially BPDO-induced AP, as it is a corollary of gallstone-induced AP, the most common aetiology of AP in humans (Cartmell & Kingsnorth 2000). Moreover, this experimental model also allows us to investigate the effect of bile retention in the development of pancreatic and extrapancreatic alterations during the disease, whose results appear as clinically relevant in AP with biliary origin.
According to our results choledocus obstruction induced deleterious effects on the liver itself and in the lung. High concentrations of bilirubin found in plasma from 12 h after obstruction, suggest severe damage in hepatic secretory function. High AST and ALT plasma activity indicated, a relevant metabolic dysfunction from the same point time with levels tending to return to control values 5 days after ligating the bile duct. However, taking into account that LDH activity in liver revealed progressive cell death from 48 h after BDO onwards, the kinetic of plasma hepatic enzymes should not be interpreted as a tendency towards metabolic normalization but a consequence of a reduction in the number of viable hepatocytes capable of producing and releasing enzymes to plasma. Hepatic dysfunction was also found in rats with AP induced by BPDO, which suggests that liver failure is induced independently of where the obstruction occurs along the bile via. Nevertheless, it is noticeable that hepatocyte viability was significantly reduced from 24 h onwards in rats with BPDO-induced AP. Multiple mechanisms associated with AP would explain this premature cell death in the liver of the BPDO rats, such as impairment of the mitochondrial metabolism of hepatocytes by phospholipase A2 and lysolecithin released from the pancreas (Kitamura et al. 1973), reduced hepatic flow secondary to hypovolemic shock (Kobold & Thal 1963) and hepatotoxic factors present in pancreatitis-associated ascitic fluid able to induce necrosis and apoptosis in hepatocytes (Ueda et al. 1999; Yang et al. 2003).
On the other hand, neutrophil infiltration was found in pancreas and lung of rats with AP from 12 h after BPDO onwards. This effect has been widely described in different models of AP (Guice et al. 1989; Murakami et al. 1995; Bhatia et al. 1998; Folch et al. 2000; Fujita et al. 2001) as a result of an initial oxidative stress locally developed within the pancreas which leads to the production of inflammatory mediators, involved in turn in the activation of circulating leucocytes and their subsequent interaction with endothelial vascular cells (Frossard & Pastor 2002). Interestingly, a similar pulmonary leukocyte recruitment was found in rats subjected to BDO. Previous studies (Folch et al. 2000; Liu et al. 2006) have pointed to Kupffer cells, the resident macrophages in liver, as responsible for lung injury in necrotizing acute pancreatitis through the production of cytokines in response to substances released by the pancreas during AP. Our results revealed lung inflammation in the absence of AP, which suggests that Kuppfer cells are activated to produce inflammatory mediators as a result of the hepatic insult developed in the liver by cholestasis, therefore playing a key role in the development of lung injury in rats with BDO. These results suggest that exclusion of bile into duodenum would delay the lung damage in the course of AP.
In line with results previously reported in rats with AP induced by sodium taurocholate (Folch et al. 1998), liver neutrophil infiltration does not occurs despite of the damage detected in this organ. This finding could be explained by the fact of that the liver contains Kupffer cells, the major component of mononuclear phagocytic system. These resident macrophages could be able, by themselves, to develop an initial inflammatory response, in such a way that neutrophil recruitment is not required.
No renal failure was found during BPDO-induced AP. No kidney injury was reported in rats with AP-induced by caerulein, although significant renal damage was found in necrotizing AP-induced by sodium taurocholate (Fujita et al. 2001). Taken together, our results lead us to the conclusion that high severity of AP would be required to extend organ dysfunction to the kidney.
Oxidative stress is a key pathogenic factor in AP. ROS produced within the pancreas from early stages by acinar cells (Uruñuela et al. 2002) and infiltrating leucocytes (Poch et al. 1999) trigger the activation of signalling pathways regulating gene expression of inflammatory mediators (Sen & Packer 1996). It results in an increased production of cytokines and chemokines (Chen et al. 2002), which leads to the progression of local pancreatic inflammation to a systemic inflammatory reaction in which different organs may be affected. On this basis antioxidant treatments could be helpful to prevent extrapancreatic complications during AP. In line with this, NAC has been shown to reduce pancreatic oxidative stress and has proved to have beneficial effects by reducing pancreatic injury in BPDO-induced AP (Sevillano et al. 2003). In addition, in this AP model, NAC was able to inhibit signalling pathways involved in the generation of inflammatory mediators, thereby reducing circulating cytokine levels (Ramudo et al. 2005). These findings would explain why extrapancreatic manifestations (lung and liver impairment) were reduced in rats with BPDO-induced AP after treatment with NAC. As ROS are over- produced from the beginning of AP (Uruñuela et al. 2002), our data would support the effectiveness of antioxidant treatments to lessen local and systemic alterations if they were applied at early stages in the course of AP, a fact not always possible in clinical setting.
In summary, our study shows lung neutrophil infiltration and hepatic failure as a result of bile duct obstruction, even if AP is not triggered. Because of its antioxidant effects, NAC treatment reduced pulmonary damage and liver dysfunction and delayed the hepatocyte death in rats with AP induced by BPDO. The results give an overview of clinically relevant organ complications developed not only as a result of AP of bile origin but also by obstructing the high bile duct, and suggests the use of antioxidants as a therapeutic approach to palliate the organ failure.
Acknowledgments
Thanks are due to Mark Anderson for this linguistic assistance. This study was supported by grants from FEDER-FIS (Fondo de Investigacion Sanitaria), Spain (PI05/0025) and Junta Castilla-León, Spain (SA 033A06).
References
- Bhatia M, Saluja AK, Hofbauer B, et al. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Brady M, Shokuhi S, et al. Inflammatory mediators in acute pancreatitis. J. Pathol. 2000;190:117–125. doi: 10.1002/(SICI)1096-9896(200002)190:2<117::AID-PATH494>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bourke JB. Incidence and mortality of acute pancreatitis. Br. Med. J. 1977;2:1668–1669. doi: 10.1136/bmj.2.6103.1668-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell MT, Kingsnorth AN. Acute pancreattis. Hosp. Med. 2000;61:382–385. doi: 10.12968/hosp.2000.61.6.1350. [DOI] [PubMed] [Google Scholar]
- Chen X, Ji B, Han B, et al. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448–457. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- Czako L, Takacs T, Varga IS, et al. Oxidative stress in distant organs and the effects of allopurinol during experimental acute pancreatitis. Int. J. Pancreatol. 2000;27:209–216. doi: 10.1385/IJGC:27:3:209. [DOI] [PubMed] [Google Scholar]
- Davies MG, Hagen PO. Systemic inflammatory response syndrome. Br. J. Surg. 1997;84:920–935. doi: 10.1002/bjs.1800840707. [DOI] [PubMed] [Google Scholar]
- Folch E, Gelpí E, Roselló-Catafau J, et al. Free radicals generated by xanthine oxidase mediate pancreatitis-associated organ failure. Dig. Dis. Sci. 1998;43:2405–2410. doi: 10.1023/a:1026617812283. [DOI] [PubMed] [Google Scholar]
- Folch E, Prats N, Hotter G, et al. P-selectin expression and Kupffer cell activation in rat acute pancreatitis. Dig. Dis. Sci. 2000;45:1535–1544. doi: 10.1023/a:1005552725243. [DOI] [PubMed] [Google Scholar]
- Forsmark CE, Toskes PP. Acute pancreatitis. Medical management. Crit. Care. Clin. 1995;11:295–309. [PubMed] [Google Scholar]
- Frossard JL, Pastor CM. Experimental acute pancreatitis: new insights into the pathophysiology. Front. Biosci. 2002;7:275–287. doi: 10.2741/A727. [DOI] [PubMed] [Google Scholar]
- Fujita M, Masamune A, Satoh A, et al. Ascites of rat experimental model of severe acute pancreatitis induces lung injury. Pancreas. 2001;22:409–418. doi: 10.1097/00006676-200105000-00012. [DOI] [PubMed] [Google Scholar]
- Guice KS, Oldham KT, Caty MG, et al. Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann. Surg. 1989;210:740–747. doi: 10.1097/00000658-198912000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann I, Wahlefeld AW. (L-(T)-lactato determination with lactatodehydrogenase and NAD. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York, NY: Academic Press Inc; 1974. pp. 1464–1468. [Google Scholar]
- He ZJ, Matikainen MP, Alho H, et al. Extrapancreatic organ impairment in caerulein induced pancreatitis. Ann. Chir. Gynaecol. 1999;88:112–117. [PubMed] [Google Scholar]
- Hickson JC. The secretion of pancreatic juice in response to stimulation of the vagus nerves in the pig. J. Physiol. 1970;206:275–297. doi: 10.1113/jphysiol.1970.sp009013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Ozawa K, Honjo I. Alterations of liver metabolism associated with experimental acute pancreatitis. Am. J. Surg. 1973;126:379–382. doi: 10.1016/s0002-9610(73)80128-x. [DOI] [PubMed] [Google Scholar]
- Kobold EE, Thal AP. Quantitation and identification of vasoactive substances liberated during various types of experimental and clinical intestinal ischemia. Surg. Gynecol. Obstet. 1963;117:315–322. [PubMed] [Google Scholar]
- Lankisch PG, Burchard-Reckert S, Petersen M, et al. Morbidity and mortality in 602 patients with acute pancreatitis seen between the years 1980-1994. Z. Gastroenterol. 1996;34:371–377. [PubMed] [Google Scholar]
- Levy M, Geller R, Hymovitch S. Renal failure in dogs with experimental acute pancreatitis: role of hypovolemia. Am. J. Physiol. 1986;251:F969–F977. doi: 10.1152/ajprenal.1986.251.6.F969. [DOI] [PubMed] [Google Scholar]
- Liu HB, Cui NQ, Li DH, Chen C. Role of Kupffer cells in acute hemorrhagic necrotizing pancreatitis-associated lung injury of rats. World J. Gastroenterol. 2006;12:403–407. doi: 10.3748/wjg.v12.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CE, Ledgerwood AM. Shock and renal failure. In: Bradley EZ III, editor. Complication of pancreatitis; medical and surgical management. Philadelphia, PA: WB Saunders; 1982. pp. 33–50. [Google Scholar]
- Murakami H, Nakao A, Kishimoto W, et al. Detection of O2-generation and neutrophil accumulation in rat lungs after acute necrotizing pancreatitis. Surgery. 1995;118:547–554. doi: 10.1016/s0039-6060(05)80372-1. [DOI] [PubMed] [Google Scholar]
- Norman JG, Fink GW, Denham W, et al. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig. Dis. Sci. 1997;42:1783–1788. doi: 10.1023/a:1018886120711. [DOI] [PubMed] [Google Scholar]
- Poch B, Gansauge F, Rau B, et al. The role of polymorphonuclear leukocytes and oxygen-derived free radicals in experimental acute pancreatitis: mediators of local destruction and activators of inflammation. FEBS Lett. 1999;461:268–272. doi: 10.1016/s0014-5793(99)01470-2. [DOI] [PubMed] [Google Scholar]
- Ramudo L, Manso MA, Sevillano S, De Dios I. Kinetic study of TNF-alpha production and its regulatory mechanisms in acinar cells during acute pancreatitis induced by bile-pancreatic duct obstruction. J. Pathol. 2005;206:9–16. doi: 10.1002/path.1747. [DOI] [PubMed] [Google Scholar]
- Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- Sevillano S, de la Mano AM, Manso MA, et al. N-acetylcysteine prevents intra-acinar oxygen free radical production in pancreatic duct obstruction-induced acute pancreatitis. Biochim. Biophys. Acta. 2003;1639:177–184. doi: 10.1016/j.bbadis.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ueda T, Ho HS, Anderson SE, Takeyama Y. Pancreatitis-induced ascitic fluid and hepatocellular dysfunction in severe acute pancreatitis. J. Surg. Res. 1999;82:305–311. doi: 10.1006/jsre.1998.5539. [DOI] [PubMed] [Google Scholar]
- Uruñuela A, Sevillano S, de la Mano AM, et al. Time-course of oxygen free radical production in acinar cells during acute pancreatitis induced by pancreatic duct obstruction. Biochim. Biophys. Acta. 2002;1588:159–164. doi: 10.1016/s0925-4439(02)00160-6. [DOI] [PubMed] [Google Scholar]
- Van Gossum A, Seferian V, Rodzynek JJ, et al. Early detection of biliary pancreatitis. Dig. Dis. Sci. 1984;29:97–101. doi: 10.1007/BF01317048. [DOI] [PubMed] [Google Scholar]
- Weber CK, Adler G. From acinar cell damage to systemic inflammatory response: current concepts in pancreatitis. Pancreatology. 2001;1:356–362. doi: 10.1159/000055834. [DOI] [PubMed] [Google Scholar]
- Wilson C, Imrie CW. Changing patterns of incidence and mortality from acute pancreatitis in Scotland, 1961–1985. Br. J. Surg. 1990;77:731–734. doi: 10.1002/bjs.1800770705. [DOI] [PubMed] [Google Scholar]
- Winslet M, Hall C, London NJ, Neoptolemos JP. Relation of diagnostic serum amylase levels to aetiology and severity of acute pancreatitis. Gut. 1992;33:982–986. doi: 10.1136/gut.33.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Fier A, Carter Y, et al. Liver injury during acute pancreatitis: the role of pancreatitis-associated ascitic fluid (PAAF), p38-MAPK, and caspase-3 in inducing hepatocyte apoptosis. J. Gastrointest. Surg. 2003;7:200–208. doi: 10.1016/s1091-255x(02)00134-8. [DOI] [PubMed] [Google Scholar]