Abstract
Summary
Carbon tetrachloride (CCl4) was used to induce liver fibrosis in the rat. Using this model, we have identified changes in serum and urinary clinical chemistry parameters, and characterized histopathological lesions in the liver. Two experiments were conducted. In Experiment 1, rats were dosed at six levels of CCl4 (0.06–0.36 ml/kg) twice weekly for 6 weeks, followed by a 6-week non-dosing recovery period (week 12). Livers were removed for histology at 6 and 12 weeks and serum parameters analysed. In Experiment 2, rats were given seven dose levels of CCl4 (0.4–1.0 ml/kg) twice weekly for 6 weeks, followed by a 6-week recovery period (week 12); urine samples were analysed at 3, 6, 9 and 12 weeks using one-dimensional sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Liver fibrosis was evident at 6 weeks in Experiments 1 and 2, and the activity of serum enzymes (including alanine aminotransferase, aspartate aminotransferase and glutamate dehydrogenase) was increased. Sodium dodecyl sulphate–polyacrylamide gel electrophoresis analysis (Experiment 2) revealed a protein band at 18.4 kDa in urine from rats treated with CCl4, not present in control urine, which was identified as copper/zinc superoxide dismutase (Cu/Zn SOD). Western blotting revealed that SOD was increased in urine from rats treated with CCl4 at 3 and 6 weeks, but not at 9 and 12 weeks. We conclude that Cu/Zn SOD is a urinary marker of hepatic necrosis, but not hepatic fibrosis.
Keywords: carbon tetrachloride, hepatic fibrosis, rat, serum, superoxide dismutase, toxicity, urine
Introduction
In man, fibrosis is a common response of the liver to many types of chronic injury, for example alcohol consumption and viral hepatitis (Friedman 1993). The early stages of fibrosis in man consist of necrosis and inflammation, which may, in turn, initiate hepatocyte regeneration and repair. But, with continuing repeated injury, this response leads to an accumulation of matrix proteins, e.g. collagen I, III and IV, proteoglycans, and fibronectin and hyaluronic acid (Friedman 1993; Benyon et al. 1996; Kauser et al. 1998; Li & Friedman 1999), and the deposition of excess collagen fibres, resulting in the disruption of normal liver structure and function. With further continuing injury, cirrhosis may eventually develop.
Several rodent models of liver fibrosis/cirrhosis exist, including the repeat administration of carbon tetrachloride (CCl4), allyl alcohol, thioacetamide and dimethylnitrosamine (Fontana et al. 1996; Sanz et al. 1997; Ueki et al. 1999; Al-Bader et al. 2000; Jung et al. 2000; Sato et al. 2000; George et al. 2001). In the present studies, the rat CCl4 model was used and although CCl4 causes injury to a number of organs (e.g. the kidney and lungs) the major damage occurs in the liver. Carbon tetrachloride is metabolized in the liver endoplasmic reticulum (Slater 1966, 1978; Recknagel 1967; Castro et al. 1973; Recknagel & Glende 1973) particularly by the ethanol inducible cytochrome P-4502E1 (Edwards et al. 1993; de Zwart et al. 1998). Carbon tetrachloride induces liver injury progressing from steatosis to centrilobular necrosis, and following repeated administration, fibrosis and cirrhosis may develop (Luckey & Petersen 2001). Although liver fibrosis and cirrhosis were thought to be irreversible, evidence is accumulating to suggest that these conditions may be reversible following a period of non-exposure (Benyon & Iredale 2000; Arthur 2002,). The current studies therefore investigated the effect of a 6-week recovery period at the end of CCl4 dosing, during which time the rats received no treatment.
In a clinical setting, the current methods for assessing liver fibrosis are biopsy and histopathology, serum enzyme analysis and the assessment of other serum clinical chemistry parameters. Examples of serum markers include hyaluronic acid, procollagen III propeptide, collagen-IV, metalloproteinase, tissue inhibitors of metalloproteinase and plasma prolidase (Savolainen et al. 1988; Guéchot et al. 1994;Abraham et al. 2000; Olga & Nikolai 2003). Serum enzymes are also measured as markers of organ toxicity (Plaa & Charbonneau 2001), and for the determination of hepatic injury the enzymes commonly investigated include alanine aminotransferase (ALT), asparate aminotransferase (AST), glutamate dehydrogenase (GLDH) and sorbital dehydrogenase. In the present studies in the rat, a comprehensive range of serum parameters routinely used in toxicology was investigated. Unfortunately, many current markers are non-specific and fail to detect fibrosis until the later stages of the disease (Friedman 1999). There is also a need for non-invasive markers, which could be detected at an early stage of fibrosis development (Friedman 2003). Therefore, the present studies investigated the possibility of identifying a non- invasive marker for hepatic fibrosis by analysing the urine samples.
Urine analysis is a quick and effective means of identifying target organ toxicity, or the progression of a disease, and many biomarkers are measured in urine, for example urinary aldehydes, catecholamines, nitric oxide and isoprostane (Hermanns et al. 1998; Draper et al. 2000; Obata et al. 2000; Smith & Lassmann 2002; Banerjee et al. 2003). However, in general, these biomarkers are not organ-specific and there is a need for urinary markers that reflect a change in a specific organ or disease state. Studies have been carried out to identify urinary markers of bladder cancer (Kageyama et al. 2004), and Dare et al. (2002) identified parvalbumin as a urinary marker of skeletal muscle toxicity in rats.
In experiments reported here in the rat, two main studies were carried out. The first (Experiment 1) involved the repeat administration of relatively low-dose levels of CCl4 (0.06–0.36 ml/kg). A brief report of the results of this study is presented. In Experiment 2, higher dose levels of the agent were used (0.4–1.0 ml/kg). However, it was important that in both experiments there should be no evidence of an adverse effect on the kidney, and that the urinary system should be structurally normal. Therefore, as CCl4 in the rat is known to exert nephrotoxicity (Doi et al. 1991; Abraham et al. 1999; Ozturk et al. 2003), a preliminary experiment was conducted involving the administration of a single dose of CCl4, at dose levels from 0.4 to 2.8 ml/kg, with animals necropsied at 24 h post-dosing. The objective was to identify CCl4 dose levels that caused nephrotoxicity, but below which the kidney was structurally normal. In this way, any protein detected in the urine would be the result of hepatotoxicity, and not injury to the kidney. A brief account of the preliminary experiment is presented. Reports of the present studies have been presented in abstract form (Smyth et al. 2002, 2004).
Materials and methods
Animals, administration of CCl4 and collection of urine
Female Hanover Wistar rats (B and K Universal Ltd, Grimston, Aldbrough, Hull, UK) of 160–220 g were used. Animals were acclimatized for 7 days before each experiment, in communal cages, with diet (SDS Ltd, Wiltham, Essex, UK) and water ad libitum. The temperature was controlled at 19–21 °C with relative humidity of 45–65% and lighting on a 12-h light/dark cycle (lights on 7 am). Animals were weighed before dosing and at appropriate times, and observed daily for signs of ill health. Animal procedures were carried out under local Ethical Committee guidelines and approval and followed the Home Office (1989)‘Code of Practice for the Housing and Care of Animals Used in Scientific Procedures’. Carbon tetrachloride (Fluka Chemicals, New Road, Gillingham, Dorset, UK) was dissolved in vegetable oil and administered by gavage; the concentration of CCl4 was adjusted to give a maximum volume of 2 ml. Control rats received 2 ml of vegetable oil (vehicle). For urine collection, animals were placed individually in metabolism cages (Techniplast, Kettering, Northants, UK.), with water ad libitum, but not diet. Urine was collected for 24 h over ice and stored at −80 °C.
Necropsy procedure, serum and urine analysis
Animals were killed by an i.p. injection of pentobarbitone sodium (Sagatal; Rhone Merieux, Harlow, Essex, UK) and exsanguinated from the abdominal aorta. Blood was placed in Microtainers (Becton Dickinson and Co, Rutherford, NJ, USA) for separation of serum. After 2–3 h, microtainers were centrifuged at 3030 g for 5 min at room temperature. Serum was removed and stored at −80 °C. Liver and kidneys were removed and weighed. Sections of liver lobes and both kidneys were placed in 10.5% phosphate-buffered formalin, processed, sectioned and stained with haematoxylin and eosin (H&E) or Sirius red. Serum and urine samples were analysed spectrophotometrically at 37 °C on a Boehringer-Hitachi 917 automated clinical chemistry analyser according to manufacturer’s instructions (Roche Diagnostics Ltd, Lewes, Sussex, UK). The following serum parameters were measured; ALT, AST, alkaline phosphatase (ALP), GLDH, lactate dehydrogenase (LDH), creatine kinase (CK), total protein (TP), total-bilirubin (T-BIL), bile acids (BA), albumin (ALB), creatinine (CREA) and urea, triglycerides (TRIG) and cholesterol (CHOL), calcium (Ca) and glucose (GLU). Reagents were supplied by Roche Diagnostics Ltd. Superoxide dismutase (SOD) activity was determined in serum and urine using a Ransod assay kit (Randox Laboratories, Antrim, N. Ireland). Glutamate dehydrogenase activity was also assayed in serum and urine using the kinetic Deutsche Gesellschaft für Klinische Chemie method.
One-dimensional sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blotting
Sodium dodecyl sulphate gels were poured according to Laemmli (1970). A constant voltage of 200 V was applied for 1 h or until the dye front had reached the bottom of the resolving gel. Gels were further processed for Western blotting; gels were transferred to a polyvinylidene difluoride (PVDF) membrane (Perkin Elmer Life Sciences Inc, Boston, MA, USA) using the semi-dry transfer method. Following transfer, the PVDF membrane was stored in blocking solution: 1% Marvel in high salt tween buffer (HST; 20 mm Tris–HCl pH 7.4; 0.5 m NaCl; 0.5% tween 20) overnight at 4 °C. A polyclonal rabbit SOD-1 antibody (Santa Cruz Biotechnology Inc, supplied by Autogen Bioclear UK Ltd, Wiltshire, UK) was diluted 1:300 in 1% Marvel in HST. The secondary antibody was a goat anti-rabbit immunoglobulin G horseradish peroxidase conjugate (Bio-Rad Laboratories, Hemel Hempstead, Hertfordshire, UK), diluted 1:3000 in 1% Marvel in HST buffer. Protein bands were detected by using enhanced chemiluminescence detection reagents (Amersham BioSciences, Amersham, Buckinghamshire, UK). Images were recorded at 1-min intervals on a Synergy GeneGnome detector, the final image recorded was that taken after 5-min exposure time.
Statistical analysis
Treated and control groups were compared by using Student’s t-test for unpaired samples using microsoft excel (Microsoft Ltd, Microsoft UK, Reading, UK).
Experimental design
Preliminary experiment
Forty rats (mean body weight 201.9 g) were divided into eight groups, n = 5 animals per group. Rats were gavaged with a single dose of vegetable oil (vehicle control) or CCl4 at 0.4, 0.8, 1.2, 1.6, 2.0, 2.4 and 2.8 ml/kg. Animals were autopsied at 24-h post-dosing; livers and kidneys removed for histological investigation and serum prepared for analysis.
Experiment 1
Eighty-four rats (mean weight 206.2 g) were divided into seven groups of 12 animals, and dosed by gavage with vegetable oil (vehicle control) or CCl4 in vegetable oil at 0.06, 0.12, 0.18, 0.24, 0.30 and 0.36 ml/kg. Rats were treated twice weekly (Monday and Thursday) for 6 weeks. At the end of dosing (week 6), six rats from each dose level group were killed and samples taken; the remaining six rats per group were left untreated for a further 6-week recovery period (to week 12).
Experiment 2
Ninety-six rats (mean weight 185.2 g) were divided into eight groups of 12 animals and gavaged with vegetable oil (vehicle control) or CCl4 at 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 and 1.0 ml/kg. Animals were treated twice weekly (Monday and Thursday) for 6 weeks. At the end of dosing (week 6), six rats from each dose level group were killed, the remaining six rats per group were left untreated for a further 6-week recovery period (to week 12). After 3 weeks of CCl4 dosing, six rats from the control group, six from the 0.7 ml/kg group and six from the 1.0 ml/kg group were placed in metabolism cages and urine collected for 24 h; they were then returned to communal cages. At the end of the 6-week dosing period, the same rats were placed in metabolism cages and urine again collected for 24 h. Urine was collected from the same six rats at 3 weeks into the non-dosing recovery period (i.e. week 9) and at the end of the 6-week recovery period (i.e. week 12).
Results
Preliminary experiment
CCl4-induced nephrotoxicity
Rats were given a single administration of CCl4 at dose levels from 0.4 to 2.8 ml/kg. Briefly, organ weights, serum clinical chemistry analysis and histopathology demonstrated that liver injury was induced at all dose levels of CCl4. For the kidney, histopathology showed that CCl4 at 0.4 and 0.8 ml/kg did not cause nephrotoxicity, but at 1.2 ml/kg and above, dose-related changes, including degeneration and necrosis of the proximal-convoluted tubules, were evident.
Experiment 1
Clinical observations, body weight changes, and liver and kidney weights
Throughout the 6-week period of CCl4 administration, at dose levels from 0.06 to 0.36 ml/kg, there were no clinical signs of CCl4 toxicity. However, animals in the CCl4-treated groups did not gain as much weight as the control (vehicle-treated) rats. At 12 weeks (i.e. after the 6-week non-dosing recovery period), the mean body weights of all groups of CCl4-treated rats were similar to the controls.
At the 6-week necropsy, the mean relative liver weights showed significant dose-related increases with the highest CCl4 dose group (0.36 ml/kg CCl4) having the heaviest liver weight. At the 12-week necropsy, livers from CCl4-treated animals were heavier (relative weights) than the controls and the increases were significant at the 0.18 (P < 0.05) and 0.24 (P < 0.05) ml/kg dose levels. The mean relative kidney weights of CCl4-treated rats at the necropsies at 6 and 12 weeks were directly comparable to the mean concurrent control kidney weights.
Serum clinical chemistry: sampling at 6 weeks
At the 6-week necropsy, at the end of CCl4 dosing, mean serum ALT, aspartate aminotransferase (AST) and GLDH levels were increased in all groups of CCl4-treated rats; the increases were greater in the higher-dose level groups. At the lowest CCl4 dose administered (0.06 ml/kg), there were significant increases for ALT (P < 0.001) and GLDH (P < 0.05); AST levels were significantly raised at 0.18 ml/kg CCl4 (P < 0.01) and at all higher CCl4 levels. Other serum parameters that were increased at 6 weeks were ALP, BA and T-BIL. Mean ALP activity was significantly raised at the lowest dose level (0.06 ml/kg) of CCl4 (P < 0.05) and enzyme activity increased with increasing dose levels of CCl4. Mean BA levels were significantly increased at 0.06 ml/kg CCl4, and in all the other CCl4-treated dose groups. Total-bilirubin was raised in all CCl4-treated groups and the increases were statistically significant at 0.12 ml/kg CCl4 and above. Serum ALB was decreased in rats treated with CCl4 at all dose levels, when compared with the mean control level, and this reduction was significant at 0.30 (P < 0.05) and 0.36 ml/kg (P < 0.05).
Sampling at 12 weeks
At the 12-week necropsy, at the end of the 6-week recovery period, serum enzyme analysis revealed that, in general, the mean levels of ALT, AST and GLDH in CCl4-treated rats were similar to control levels. However, mean AST levels were significantly lower than the controls in the 0.24, 0.30 and 0.36 ml/kg CCl4 groups. ALP activity and levels of BA, T-BIL and ALB in the serum at 12 weeks were similar in CCl4-treated rats and in the controls.
Histology
After 6 weeks of dosing with CCl4, the livers of treated rats showed hepatocellular degeneration and necrosis at all dose levels from 0.06 ml/kg and above (Figure 1). Hepatocytes in centrilobular regions showed cytoplasmic swelling and vacuolation, with cytoplasmic and nuclear degeneration and necrosis. Inflammatory cells were sometimes present. Around the centrilobular veins and in the periportal regions, immature and mature fibrosis was present, which extended into the surrounding parenchyma along the sinusoidal tracks. The amount of fibrosis showed considerable variation between CCl4 dose level groups, and also between individual rats in each dose level group. However, there was a trend for the degree of fibrosis to increase with increasing CCl4 dose level. Liver sections were chosen at random for Sirius red staining. This revealed the presence of collagen fibres in some liver sections. The fibrosis was often perivascular in distribution and sometimes included delicate ‘bridging’ between the centrilobular and periportal areas. In some sections, the capsular region was also involved. However, in general, the induced fibrosis was relatively mild. At week 12, after the 6-week recovery period, the livers appeared to have returned to normal; there was no clear or consistent evidence of fibrosis on Sirius red staining. Histological examination of kidney sections demonstrated no CCl4-treatment-related findings at 6 weeks, and no evidence of CCl4-induced changes at the end of the 6-week period of recovery (week 12).
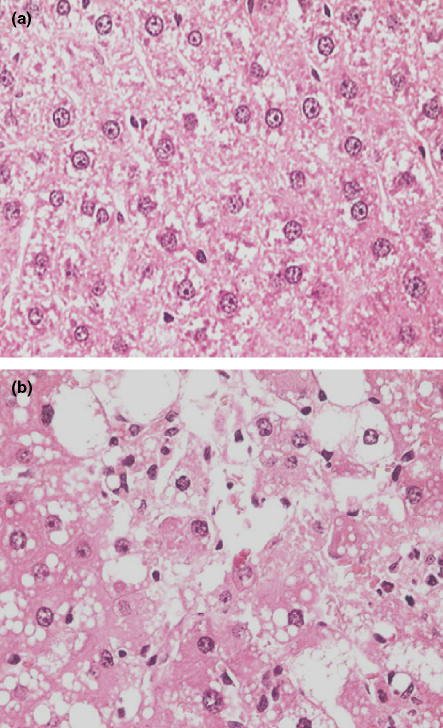
Figure 1.
Histology of the liver from a rat treated with carbon tetrachloride (CCl4) twice a week for 6 weeks and sampled at 6 weeks. (a) Liver from a control rat to show the normal arrangement and appearance of hepatocytes. (b) Centrilobular region in the liver of a rat treated with CCl4 at 0.36 ml/kg twice weekly for 6 weeks showing diffuse, marked, hepatocellular vacuolation and degeneration, with necrotic cells. Original magnification of images; ×400 (haematoxylin and eosin).
Experiment 2
Clinical observations, body weight changes, and liver and kidney weights
Throughout the 6-week period of CCl4 dosing at levels from 0.4 to 1.0 ml/kg, all animals appeared healthy and there were no clinical signs of CCl4 toxicity. However, during this period, the animals in CCl4-treated groups did not gain as much weight as control rats (Table 1). Nevertheless at 12 weeks, after the 6-week non-dosing recovery period, body weights of CCl4-treated rats were, in general, approximately similar to control animals. The percentage increases in body weight from the beginning of the experiment to week 12 were, for example, 30.87%, 31.77%, 30.60%, 30.02% and 25.82% for the groups treated with CCl4 at 0 (vehicle control), 0.4, 0.7, 0.9 and 1.0 ml/kg respectively.
Table 1.
Mean (±SD) body weight changes, and relative liver and kidney weights of female Hanover Wistar rats treated with carbon tetrachloride (CCl4) at dose levels from 0.4 to 1.0 ml/kg twice weekly for 6 weeks, and at week 12 following a 6-week recovery period without dosing†
| Dose of CCl4 (ml/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week | 0 (control) | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | |
| Body weight§ (g) | 0‡ | 185.17 ± 17.68 | 164.67 ± 10.67 | 174.67 ± 21.69 | 166.50 ± 6.98 | 172.67 ± 5.39 | 177.00 ± 14.87 | 177.67 ± 9.31 | 178.83 ± 7.88 |
| 6 | 234.00 ± 23.19 | 194.67 ± 14.90 | 203.67 ± 26.79 | 193.83 ± 11.11 | 200.33 ± 5.82 | 202.83 ± 16.25 | 200.83 ± 12.53 | 201.50 ± 9.14 | |
| 12 | 242.33 ± 21.87 | 216.33 ± 16.81 | 228.83 ± 34.20 | 218.67 ± 9.99 | 225.50 ± 5.79 | 232.50 ± 17.63 | 231.00 ± 14.67 | 225.00 ± 7.51 | |
| Relative liver weight(g/kg body weight) | 6 | 35.89 ± 1.93 | 44.68*** ± 2.17 | 44.57*** ± 3.70 | 46.91*** ± 3.10 | 51.33*** ± 4.63 | 50.12*** ± 5.09 | 46.95*** ± 2.93 | 54.64*** ± 5.78 |
| 12 | 24.30 ± 2.82 | 26.03 ± 0.79 | 26.93 ± 1.47 | 27.06 ± 1.30 | 27.38* ± 1.31 | 28.02* ± 1.80 | 27.34 ± 2.52 | 26.59 ± 1.24 | |
| Relative kidney weight¶(g/kg body weight) | 6 | 2.88 ± 0.15 | 3.15** ± 0.14 | 3.08* ± 0.15 | 3.26* ± 0.29 | 3.36** ± 0.31 | 3.2* ± 0.22 | 3.15* ± 0.25 | 3.50*** ± 0.24 |
| 12 | 2.67 ± 0.16 | 2.67 ± 0.16 | 2.71 ± 0.15 | 2.77 ± 0.14 | 2.82 ± 0.17 | 2.75 ± 0.17 | 2.73 ± 0.08 | 2.71 ± 0.18 | |
Rats were treated with CCl4 by gavage. *Significantly different from control at the same time point P < 0.05, **P < 0.01, ***P < 0.001. n = 6 rats per dose level group at each time point.
Body weight at the time of administration of the first dose of CCl4.
Body weight data were not analysed statistically.
Mean weight of left and right kidney.
Mean relative liver weights for rats treated with CCl4 twice a week for 6 weeks were significantly heavier than those from control animals at all CCl4 dose levels (P < 0.001 for all groups) (Table 1). At the lowest dose (0.4 ml/kg), the relative liver weights from CCl4-treated rats were 24.49% heavier than the controls, and there was a 52.24% increase in relative liver weight in rats treated with 1.0 ml/kg CCl4 compared to the controls. After the 6-week recovery period, at 12-week post-dosing, the mean relative liver weights for CCl4-treated rats were still heavier than the controls (Table 1), but the only statistically significant increases were in the 0.7 and 0.8 ml/kg groups (P < 0.05 for both groups).
The mean relative kidney weights from CCl4-treated rats at the 6-week necropsy were slightly but significantly heavier than the controls at all dose levels (Table 1); the increase in rats treated at 1.0 ml/kg CCl4, for example, was 9.38% greater than the control animals. However, at the 12-week necropsy, there were no statistically significant differences between the mean relative kidney weights of control and CCl4-treated rats (Table 1), although the weights at all dose levels were equal to, or greater than, the controls.
Serum clinical chemistry: Sampling at 6 weeks
After 6 weeks of dosing, serum ALT, AST and GLDH activities were significantly increased in many CCl4-treated groups compared to the controls (Table 2). The mean ALT activity in control rats was 39.50 U/l and this increased 4.71-fold in the 0.4 ml/kg CCl4 group to 225.67 U/l (not significant). Alanine aminotransferase activity then increased as the dose level of CCl4 rose until at 1.0 ml/kg activity had risen 38.10-fold to 1544.33 U/l. Asparate aminotransferase activity was significantly increased in all CCl4-treated groups except at 0.7 ml/kg (Table 2). Mean AST activity in control rats was 123.17 U/l and this increased 1.64-fold in the 0.4 ml/kg group to 325.50 U/l. Asparate aminotransferase activity then rose as the dose of CCl4 increased until at 1.0 ml/kg activity had risen 14.53-fold to 1912.33 U/l. The mean control GLDH activity was 4.63 U/l, this increased 22.90-fold in the 0.4 ml/kg group to 110.65 U/l. Activity then increased as the CCl4 dose level rose until at 1.0 ml/kg, activity had risen 155.00-fold to 722.25 U/l.
Table 2.
Mean (±SD) of serum clinical chemistry parameters of female Hanover Wistar rats treated with carbon tetrachloride (CCl4) at dose levels from 0.4 to 1.0 ml/kg twice weekly for 6 weeks†
| Serum chemistry parameter | Dose of CCl4 (ml/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 (control) | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | |
| ALT | 39.50 ± 9.35 | 225.67 ± 222.97 | 576.17* ± 416.95 | 502.40*** ± 158.41 | 1088.67 ± 1280.35 | 995.83* ± 770.51 | 789.83* ± 705.70 | 1544.33** ± 948.40 |
| AST | 123.17 ± 15.20 | 325.50* ± 192.29 | 704.17* ± 590.27 | 557.20** ± 228.61 | 1095.17 ± 1316.29 | 1019.33* ± 724.55 | 867.50* ± 805.10 | 1912.33* ± 1509.21 |
| GLDH | 4.63 ± 0.98 | 110.65 ± 145.51 | 296.53 ± 409.14 | 279.26* ± 213.69 | 714.38 ± 1140.30 | 502.20* ± 444.16 | 433.07* ± 387.32 | 722.25* ± 574.37 |
| LDH | 3035.67 ± 841.80 | 2380.33 ± 687.20 | 3927.50 ± 2133.18 | 2945.00 ± 2006.42 | 3448.50 ± 2286.28 | 1944.67* ± 622.36 | 1825.17 ± 111.63 | 2644.17 ± 969.37 |
| CK | 1150.33 ± 304.01 | 830.50 ± 237.27 | 1026.67 ± 531.30 | 658.40* ± 293.53 | 660.00* ± 356.85 | 573.83** ± 204.11 | 575.67* ± 342.04 | 468.83*** ± 134.72 |
| ALP | 204.67 ± 28.19 | 316.33* ± 103.57 | 393.83*** ± 60.17 | 421.20*** ± 77.55 | 676.17* ± 411.27 | 592.00*** ± 167.64 | 526.00** ± 205.41 | 555.83* ± 281.13 |
| T-BIL | 3.28 ± 0.17 | 4.73** ± 0.92 | 4.40* ± 0.98 | 4.94*** ± 0.72 | 7.05 ± 4.44 | 5.08 ± 2.35 | 5.33*** ± 0.73 | 6.23* ± 2.40 |
| BA | 35.28 ± 15.06 | 84.68*** ± 18.36 | 128.60** ± 56.31 | 148.24** ± 56.91 | 127.28** ± 55.82 | 133.67** ± 60.17 | 110.28** ± 44.81 | 135.60** ± 57.47 |
| ALB | 38.02 ± 0.97 | 36.77 ± 1.72 | 35.62* ± 1.75 | 36.80 ± 1.04 | 35.67* ± 1.74 | 35.18*** ± 0.93 | 36.43 ± 1.65 | 34.80* ± 2.57 |
| UREA | 7.43 ± 0.37 | 9.63** ± 1.17 | 9.79*** ± 0.91 | 9.84*** ± 0.80 | 9.42** ± 1.08 | 9.24* ± 1.40 | 9.19** ± 1.17 | 9.15*** ± 0.78 |
| CREA | 57.83 ± 2.71 | 56.67 ± 1.97 | 56.50 ± 3.94 | 54.50* ± 0.89 | 51.17* ± 4.45 | 52.17* ± 3.54 | 56.17 ± 3.43 | 51.67* ± 5.13 |
| SOD | 386.67 ± 55.72 | 593.50* ± 179.42 | 772.50*** ± 175.77 | 739.20*** ± 34.10 | 612.33* ± 237.89 | 701.67** ± 170.36 | 760.50*** ± 147.87 | 726.50*** ± 98.64 |
| TP | 64.42 ± 2.20 | 60.38** ± 1.01 | 59.18** ± 1.96 | 61.22* ± 1.63 | 58.37** ± 2.76 | 57.83*** ± 2.44 | 55.63 ± 14.21 | 57.33** ± 4.45 |
| Ca | 2.52 ± 0.05 | 2.51 ± 0.08 | 2.53 ± 0.08 | 2.54 ± 0.10 | 2.52 ± 0.10 | 2.47 ± 0.12 | 2.52 ± 0.09 | 2.54 ± 0.15 |
| CHOL | 1.56 ± 0.25 | 1.24* ± 0.23 | 1.16* ± 0.20 | 1.14** ± 0.14 | 1.35 ± 0.40 | 1.30 ± 0.21 | 1.28* ± 0.09 | 1.35 ± 0.46 |
| TRIG | 0.79 ± 0.27 | 0.52 ± 0.29 | 0.38** ± 0.11 | 0.29** ± 0.06 | 0.65 ± 0.58 | 0.35** ± 0.04 | 0.31** ± 0.04 | 0.34** ± 0.08 |
| GLU | 8.42 ± 0.81 | 6.78** ± 0.66 | 5.69*** ± 0.52 | 6.25*** ± 0.45 | 6.02** ± 1.50 | 5.87*** ± 0.73 | 6.31*** ± 0.74 | 5.40*** ± 0.89 |
ALT, alanine aminotransferase (U/l); AST, asparate aminotransferase (U/l); GLDH, glutamate dehydrogenase (U/l); LDH, lactate dehydrogenase (U/l); CK, creatine kinase (U/l); ALP, alkaline phosphatase (U/l); T-BIL, total bilirubin (μmol/l); BA, bile acids (mmol/l); ALB, serum albumin (g/l); Urea (mmol/l); CREA, creatinine (μmol/l); SOD, superoxide dismutase (U/l); TP, total protein (g/l); Ca, calcium (mmol/l); CHOL, cholesterol (mmol/l); TRIG, triglyerides (mmol/l); GLU, glucose (mmol/l).
Rats were treated with CCl4 by gavage.
Significantly different from control, P < 0.05,
P < 0.01,
P < 0.001. n = 6 rats per dose level group.
At the 6-week autopsy, mean serum ALP activity was increased in all CCl4-treated groups (Table 2). At the lowest dose administered (0.4 ml/kg), ALP activity was 0.55-fold greater than the control group (P < 0.05). Alkaline phosphatase activity tended to rise in the serum as the CCl4 dose increased up to the 0.7 ml/kg dose level. At 1.0 ml/kg CCl4, mean ALP activity was 1.72-fold greater than in the controls. Total-bilirubin and BA also tended to increase in CCl4-treated rats compared to control levels. Bile acid measurements in the serum revealed that at the lowest dose of CCl4 (0.4 ml/kg), the value was significantly higher (P < 0.001) in the CCl4-treated rats than in the controls. At the highest CCl4 dose level (1.0 ml/kg), the mean BA value was 2.84-fold greater in the CCl4-treated rats than in the controls. The mean TP levels in serum from rats treated with CCl4 were decreased at all dose levels; however, the decrease was not significant in the 0.9 ml/kg group. The serum ALB level in the serum of rats treated with CCl4 was also decreased in all treatment groups compared to the control value; this decrease was significant at 0.5, 0.7, 0.8 and 1.0 ml/kg CCl4. Serum superoxide dismutase activity was significantly increased in all CCl4-treated groups. Creatine kinase levels were significantly decreased in the higher dose CCl4 groups (i.e. 0.6 to 1.0 ml/kg) and there was some evidence of a general dose–response relationship; at the highest CCl4 dose (1.0 ml/kg), the mean CK level was reduced to 40.76% of the control value. The LDH activity in CCl4-treated rats was decreased in all groups (except at 0.5 and 0.7 ml/kg), but was only significantly reduced in the 0.8 ml/kg group. There was no difference in serum Ca levels between the control and CCl4-treated rats at any dose level. Cholesterol measurements were decreased in all CCl4-treated groups, but this was not statistically significant in the 0.7, 0.8 and 1.0 ml/kg groups. Triglyceride levels were significantly decreased in all CCl4-treated groups except at 0.4 and 0.7 ml/kg. Glucose values were decreased to statistically significant levels in all CCl4-treated groups. Serum urea values were significantly increased in all CCl4-treated groups, but the levels did not clearly increase with increasing dose levels of CCl4 (Table 2). Creatinine levels were significantly decreased in rats treated with CCl4 at 0.6, 0.7, 0.8 and 1.0 ml/kg.
Sampling at 12 weeks
Serum analysis at 12 weeks (i.e. after the 6-week recovery period) revealed that mean ALT and GLDH activities were similar to the controls at all CCl4 dose levels (Table 3). However, rats that had received doses of 0.7 ml/kg CCl4 or higher showed a significantly lower serum AST activity after the 6-week period of recovery; serum ALP and T-BIL levels, in general, showed a return to control values. Serum BA levels after the 6-week recovery period were highly variable in the different CCl4 dose groups and showed significant increases in CCl4-treated animals at 0.5, 0.6, 0.7 and 1.0 ml/kg. Following the 6-week recovery period, mean TP and ALB levels in general showed a return to control values, as did TRIG and GLU values. Superoxide dismutase activity was significantly decreased in the 0.7, 0.8 and 0.9 ml/kg CCl4 groups (P < 0.05). Lactate dehydrogenase and CK activities were significantly lower than the controls in all CCl4-treated groups except at 0.4 and 0.5 ml/kg. Mean CHOL levels were significantly increased above control values in all CCl4-treated groups except at 1.0 ml/kg. Urea levels in rats treated with CCl4 were greater than the control level at 0.4 and 0.6 ml/kg. However, in the higher dose CCl4 groups, urea levels were lower than the controls, and the mean value was significantly decreased at 1.0 ml/kg CCl4. Control and CCl4-treated serum CREA levels at 12 weeks were similar.
Table 3.
Mean (±SD) of serum clinical chemistry parameters of female Hanover Wistar rats treated with carbon tetrachloride (CCl4) at dose levels from 0.4 to 1.0 ml/kg twice weekly for 6 weeks followed by a 6-week recovery period without dosing (week 12)†
| Serum chemistry parameter | Dose of CCl4 (ml/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 (control) | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | |
| ALT | 45.50 ± 7.53 | 43.33 ± 9.69 | 43.67 ± 8.59 | 56.17 ± 26.42 | 41.67 ± 8.14 | 35.67 ± 11.33 | 40.00 ± 7.38 | 42.00 ± 7.01 |
| AST | 144.50 ± 14.01 | 133.67 ± 18.03 | 134.00 ± 18.89 | 126.67 ± 26.61 | 117.17** ± 15.33 | 99.67* ± 37.53 | 102.33*** ± 10.33 | 123.50* ± 14.63 |
| GLDH | 5.97 ± 6.60 | 4.60 ± 4.95 | 2.88 ± 0.69 | 4.13 ± 2.92 | 2.13 ± 1.03 | 2.02 ± 1.24 | 3.03 ± 2.63 | 6.17 ± 7.80 |
| LDH | 3298.83 ± 242.74 | 2896.33 ± 492.15 | 2939.83 ± 725.70 | 2578.67** ± 343.31 | 2506.33** ± 347.69 | 1849.17** ± 781.07 | 1759.83*** ± 633.69 | 2217.83* ± 905.5 |
| CK | 1416.83 ± 130.17 | 1225.83 ± 202.97 | 1233.17 ± 321.34 | 1046.33** ± 153.52 | 959.33*** ± 125.70 | 775.00*** ± 316.59 | 720.17*** ± 233.58 | 795.50*** ± 240.55 |
| ALP | 65.33 ± 5.92 | 66.67 ± 4.37 | 65.67 ± 6.35 | 69.17 ± 25.10 | 68.83 ± 20.85 | 75.83 ± 27.55 | 77.33 ± 28.39 | 76.83 ± 8.26 |
| T-BIL | 3.68 ± 0.36 | 3.37 ± 0.27 | 3.77 ± 0.36 | 3.97 ± 0.39 | 4.33 ± 0.65 | 4.25 ± 1.37 | 4.28 ± 0.57 | 3.87 ± 0.69 |
| BA | 29.65 ± 6.04 | 41.67 ± 16.57 | 51.18* ± 19.84 | 54.12* ± 23.95 | 42.45* ± 8.96 | 34.35 ± 10.92 | 49.27 ± 21.61 | 50.07* ± 19.49 |
| ALB | 38.13 ± 1.77 | 37.50 ± 1.44 | 36.88 ± 1.64 | 38.47 ± 1.54 | 38.03 ± 1.47 | 37.22 ± 1.24 | 36.55 ± 1.26 | 35.63* ± 1.13 |
| UREA | 7.48 ± 0.36 | 8.17** ± 0.24 | 8.65 ± 1.38 | 9.09** ± 1.13 | 7.20 ± 1.36 | 6.80 ± 1.37 | 6.93 ± 0.87 | 6.40* ± 1.02 |
| CREA | 57.50 ± 13.17 | 63.33 ± 5.79 | 63.83 ± 4.54 | 64.50 ± 3.27 | 60.67 ± 5.43 | 55.67 ± 11.47 | 56.00 ± 3.74 | 58.17 ± 3.76 |
| SOD | 622.67 ± 139.03 | 513.83 ± 113.99 | 444.33 ± 143.27 | 494.50 ± 201.06 | 471.67* ± 48.50 | 473.17* ± 76.49 | 455.17* ± 85.92 | 616.67 ± 113.05 |
| TP | 66.05 ± 2.69 | 64.05 ± 2.35 | 63.80 ± 3.33 | 65.73 ± 2.72 | 66.75 ± 2.70 | 63.63 ± 2.54 | 64.07 ± 1.93 | 62.92 ± 3.41 |
| Ca | 2.45 ± 0.08 | 2.39 ± 0.07 | 2.42 ± 0.10 | 2.44 ± 0.03 | 2.46 ± 0.09 | 2.45 ± 0.06 | 2.47 ± 0.08 | 2.44 ± 0.06 |
| CHOL | 1.03 ± 0.22 | 1.69** ± 0.34 | 1.56* ± 0.40 | 1.52** ± 0.29 | 1.62** ± 0.26 | 1.65*** ± 0.13 | 1.78*** ± 0.19 | 1.19 ± 0.20 |
| TRIG | 0.56 ± 0.19 | 0.52 ± 0.23 | 0.78 ± 0.40 | 0.66 ± 0.39 | 0.44 ± 0.07 | 0.47 ± 0.23 | 0.60 ± 0.49 | 0.43 ± 0.10 |
| GLU | 4.68 ± 2.00 | 3.85 ± 1.21 | 4.31 ± 1.26 | 4.52 ± 2.09 | 5.36 ± 1.08 | 4.43 ± 1.44 | 4.48 ± 1.01 | 4.54 ± 1.23 |
ALT, alanine aminotransferase (U/l); AST, asparate aminotransferase (U/l); GLDH, glutamate dehydrogenase (U/l); LDH, lactate dehydrogenase (U/l); CK, creatine kinase (U/l); ALP, alkaline phosphatase (U/l); T-BIL, total bilirubin (μmol/l); BA, bile acids (mmol/l); ALB, serum albumin (g/l); Urea (mmol/l); CREA, creatinine (μmol/l); SOD, superoxide dismutase (U/l); TP, total protein (g/l); Ca, calcium (mmol/l); CHOL, cholesterol (mmol/l); TRIG, triglyerides (mmol/l); GLU, glucose (mmol/l).
Rats were treated with CCl4 by gavage.
Significantly different from control, P < 0.05,
P < 0.01,
P < 0.001. n = 6 rats per dose level group.
Histology
At the 6- and 12-week necropsies, livers and kidneys were removed for histopathological assessment. Tissues were stained with H&E and Sirius red. Haematoxylin and eosin staining of liver tissues at 6 weeks revealed in the CCl4-treated animals hepatocellular vacuolation in the centrilobular region and fibrosis with capsular pitting (Figure 2). Haematoxylin and eosin staining of kidney sections at 6 weeks did not reveal treatment-related changes.
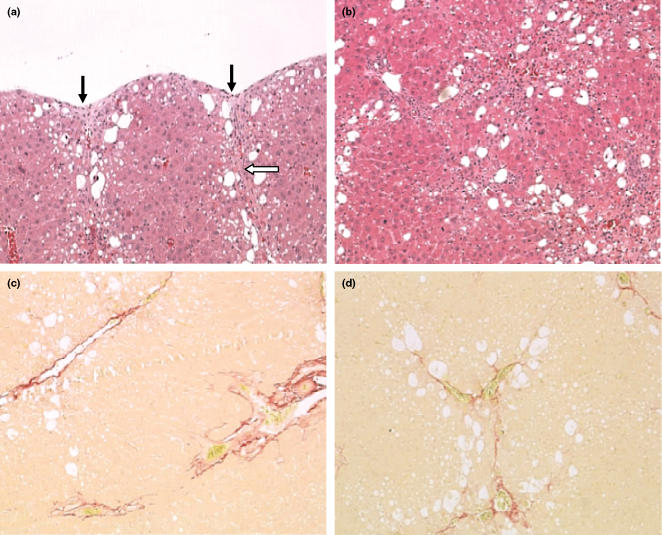
Figure 2.
Histopathology of the liver in rats treated with carbon tetrachloride (CCl4) twice a week for 6 weeks and sampled at that time. (a) Liver from a rat treated with CCl4 at 0.6 ml/kg twice weekly for 6 weeks. In the subcapsular region, there is hepatocellular vacuolation, degeneration and necrosis, with capsular pitting or contraction (black arrows). There is collagen formation in the capsular area which extends downwards into the liver parenchyma (open arrow). Original magnification of image; ×100 [haematoxylin and eosin (H&E)]. (b) Liver from a rat treated with CCl4 twice weekly for 6 weeks at 1.0 ml/kg. There is significant fibrosis which is tending to link centrilobular and periportal areas. Hepatocytes are vacuolated with evidence of degeneration. Original magnification of image; ×100 (H&E). (c) Liver from a rat treated with CCl4 twice weekly for 6 weeks at 0.7 ml/kg; there is perivascular fibrosis in the centrilobular and periportal areas, with some evidence of bridging along the sinusoidal tracts. Original magnification of image; ×100 (Sirius red). (d) Liver from a rat treated for 6 weeks, twice weekly at 1.0 ml/kg CCl4. Perivascular fibrosis is evident with bridging along sinusoidal tracts within the liver parenchyma. Original magnification of image; ×100 (Sirius red).
Liver tissues at 6 weeks were randomly selected for Sirius red staining; this showed evidence of fibrosis in all selected animals in all CCl4-treated groups (Figure 2). In all rats, there was perivascular fibrosis and delicate ‘bridging’ among centrilobular, periportal and capsular areas. There was no evidence of fibrosis in any kidney sections stained with Sirius red.
At the 12-week necropsy, after the 6-week recovery period, H&E staining of liver sections identified fibrosis in some animals. Also, in some animals, mature fibrosis was present in subcapsular regions associated with capsular pitting or contraction. Some liver sections also showed parenchymal fibrosis with fibrous tissue present around the centrilobular veins, the periportal regions and extending into the parenchyma along the sinusoidal tracks. In the kidney, at the 12-week necropsy, there were no treatment-related changes identified with H&E staining. Sirius red staining of liver sections from rats treated with CCl4 showed evidence of fibrosis in all randomly selected animals, although there was some intra-group variation in relation to the degree of fibrosis detected (Figure 3). The fibrotic response did not therefore return to normal during the 6-week recovery period.
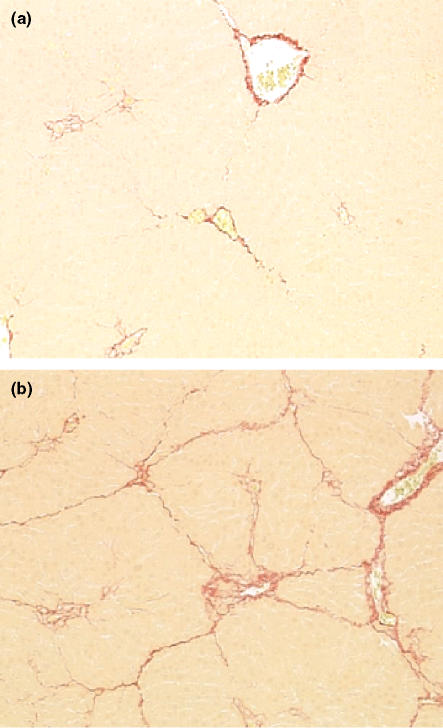
Figure 3.
Histopathology of the liver from rats treated with carbon tetrachloride (CCl4) twice a week for 6 weeks, then left untreated for a 6-week period of recovery. (a) Liver from a rat treated with CCl4 for 6 weeks at 0.4 ml/kg and left untreated for a 6-week recovery period. There is perivascular fibrosis with some evidence of delicate partial bridging within the liver parenchyma. (b) Liver from a rat treated with CCl4 for 6 weeks at 1.0 ml/kg and left untreated for a 6-week recovery period. There is perivascular fibrosis in centrilobular and portal regions, with delicate bridging of fibrosis between these areas. Original magnification of both images; ×100 (Sirius red).
Analysis of urine for novel protein markers of hepatic fibrosis
SDS–PAGE.Urine samples from control and 0.7 and 1.0 ml/kg CCl4 groups were collected during Experiment 2 at week 3 and 6 of the dosing period and at week 3 and 6 of the non-dosing recovery period (i.e. week 9 and 12 respectively). Samples were analysed by SDS–PAGE. The only difference identified between control and CCl4-treated rat urine was the presence of a band at 18.4 kDa in the CCl4-treated urine samples at week 3 and 6 of the dosing period. Previous studies (Smyth et al. 2004) had shown that following the administration of a single dose of CCl4 this same protein band had appeared in the urine of CCl4-treated rats and was identified by nano-scale reversed-phase liquid chromatography (nano-LC) combined with electrospray ionization tandem mass spectrometry (ESI-MS/MS) as copper/zinc superoxide dismutase (Cu/Zn SOD or SOD-1). In the present studies, the appearance of the SOD-1 band in the urine samples from rats repeatedly dosed with CCl4 at week 3 and 6 of dosing was confirmed by Western blotting with an SOD-1 antibody.
Western blotting with SOD-1 antibody
Urine samples collected after 3 weeks (i.e. 6 doses of CCl4) and 6 weeks (i.e. 12 doses of CCl4) at 0 (vehicle control) and the 0.7 ml/kg CCl4 dose level were Western blotted with SOD-1 antibody. This revealed that SOD-1 was greatly increased after both 3 and 6 weeks of dosing in the CCl4-treated rat urine samples compared with control animals (Figure 4a,b). However, when the urine samples collected from rats dosed with CCl4 at 0.7 ml/kg at 3 weeks into the non-dosing recovery period (i.e. week 9) and at the end of the 6-week period of recovery (i.e. week 12) were Western blotted with the SOD-1 antibody, SOD-1 could no longer be detected (Figure 4c).
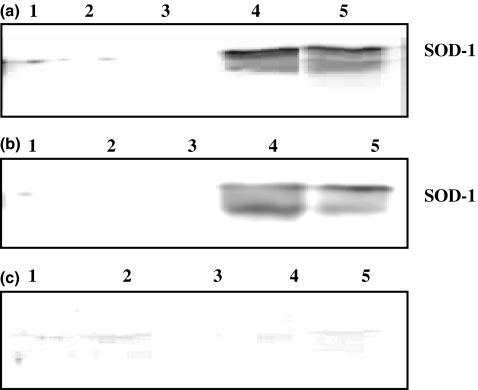
Figure 4.
Western blots with a superoxide dismutase (SOD-1) antibody of rat urine samples collected during the induction of liver fibrosis with carbon tetrachloride (CCl4) repeatedly dosed at 0.7 ml/kg. Samples for sodium dodecyl sulphate–polyacrylamide gel electrophoresis were prepared, with each sample containing 14 μl of urine. (a) Urine collected after 3 weeks of dosing twice a week with CCl4. (b) Urine collected after 6 weeks of dosing twice a week with CCl4. (c) Urine collected at the end of the 6-week non-dosing recovery period. In all blots: lane 1, 2, 3 = control urine from rats treated with vehicle, and lanes 4 and 5 = urine from rats treated with CCl4 at 0.7 ml/kg.
SOD and GLDH activity measurements
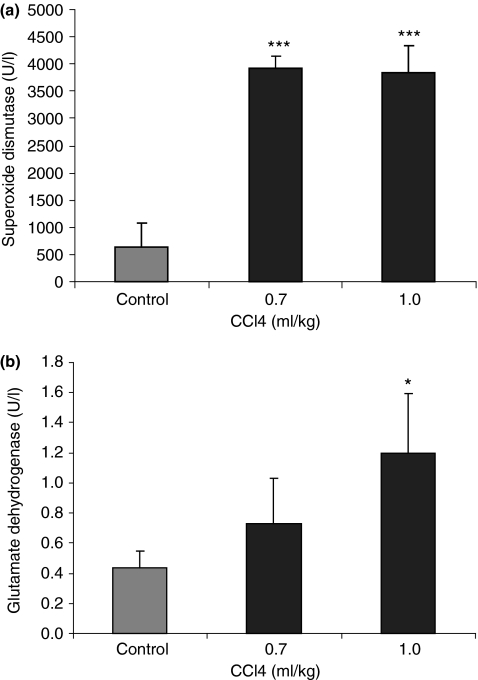
Urine samples collected after 6 weeks of dosing twice a week with CCl4 at 0, 0.7 and 1.0 ml/kg were assayed for SOD activity. Superoxide dismutase activity was increased in the CCl4-treated groups. The mean control SOD activity was 633.25 U/l; this was increased to 3911.83 and 3835.33 U/l in the 0.7 and 1.0 ml/kg groups (increases of 5.18-fold and 5.06-fold) respectively (P < 0.001 at both CCl4 dose levels) (Figure 5a). However, SOD activity did not appear to be related to the dose level of CCl4 administered. Glutamate dehydrogenase activity was also measured in the urine samples collected after 6 weeks of CCl4 dosing at 0.7 and 1.0 ml/kg. The GLDH activity in control samples was 0.43 U/l and this was increased in the 0.7 ml/kg group to 0.73 U/l (0.70-fold greater than control; NS). However, GLDH activity was increased to 1.20 U/l (1.79-fold greater than control; P < 0.05) in the urine samples from rats treated with CCl4 at 1.0 ml/kg (Figure 5b).
Figure 5.
Mean superoxide dismutase (SOD) (a) and glutamate dehydrogenase (GLDH) (b) levels in the urine of rats treated with carbon tetrachloride at 0.7 and 1.0 ml/kg twice a week for 6 weeks. Superoxide dismutase activity was determined by using a Ransod assay kit; GLDH activity was measured by using the kinetic Deutsche Gesellschaft für Klinische Chemie method. Values are means (error bars represent SD) of six animals. Values that differ from control by Student’s t-test are shown, *P < 0.05, ***P < 0.001.
Discussion
In the present studies to induce hepatic fibrosis, CCl4 was administered using a repeat dose regimen at a series of dose levels and a comprehensive range of serum enzymes and other clinical chemistry parameters measured at each dose level. Urine samples were also analysed in an attempt to identify a non-invasive marker of hepatic fibrosis. A preliminary experiment was carried out to identify the lowest dose level of CCl4 which, when administered as a single dose (0.4–2.8 ml/kg), caused liver but not kidney injury. Carbon tetrachloride induced histopathological and clinical chemistry evidence of hepatotoxicity at 0.4 ml/kg and above, whereas kidney injury was only evident at 1.2 ml/kg and above. It was therefore decided that in Experiments 1 and 2, rats would be dosed with CCl4 at levels below 1.2 ml/kg, and thus any proteins detected in the urine were likely to result from liver injury, not kidney injury. However, as CCl4 in Experiments 1 and 2 was to be given repeatedly, twice a week for 6 weeks, and as the effect of summating CCl4 doses on the liver and kidney were unclear, it was decided to err towards using lower doses of CCl4; for this reason, dose levels in Experiment 1 were set at levels of 0.06–0.36 ml/kg. Nevertheless, in Experiment 1, only mild fibrosis was induced, and therefore in Experiment 2, higher dose levels of CCl4 were used, between 0.4 and 1.0 ml/kg. However, Experiment 1 produced results of interest, demonstrating that the very low-dose level of 0.06 ml/kg CCl4 induced significant hepatotoxicity, and was above the ‘no toxic effect’ or ‘threshold’ level for CCl4 administration using the present dosing regimen.
In Experiment 1, liver weights were increased at all CCl4 dose levels from 0.06 to 0.36 ml/kg and the increases in liver weight were closely related to CCl4 dose. Serum chemistry results showed significant increases in ALT, AST and GLDH and as the dose level of CCl4 increased, the serum levels of the three enzymes increased. Park et al. (2000) found significant increases in serum ALT and AST after 4 weeks of dosing rats with 1 ml/kg CCl4 twice a week. However, studies by Jiang et al. (1992) in which rats were dosed i.p. with 1 ml/kg CCl4 only demonstrated a two-fold increase in serum ALT activity. In Experiment 1, serum ALP and T-BIL levels were also increased in rats treated with CCl4. These results suggest an impairment of bile flow, possibly because of the deposition of collagen fibres disrupting normal liver structure and function (Zimmerman 1978; Gaudio et al. 1997).
The amount of serum ALB in rats treated with CCl4 twice a week for 6 weeks (Experiment 1) was reduced in relation to the control animals at all dose levels, and this decrease was significant in the 0.30 and 0.36 ml/kg CCl4 groups. The reduction in ALB levels finds a parallel in reports of other authors. Serum ALB levels tend to be reduced in chronic liver injury as a result of the impaired ability of liver cells to synthesize protein (Horne et al. 1973). Giménez et al. (1994) reported a decrease in serum ALB following 16 weeks of treatment with CCl4 by inhalation. Similarly, studies by Hernandez-Munoz et al. (2001) in which liver cirrhosis was induced in rats demonstrated that after 10 weeks of repeated dosing with CCl4, serum ALB levels were significantly reduced.
Histopathological examination of liver tissues in Experiment 1 after 6 weeks of CCl4 dosing showed the presence of fibrosis, but this was not a consistent response throughout the CCl4-treated rats. Furthermore, even at the highest dose (0.36 ml/kg), the fibrosis induced was mild. Sirius red staining of randomly selected liver samples showed that collagen fibres were present, but again there was variation in the degree of fibrosis induced. Examination of the literature demonstrated that CCl4-induced hepatic fibrosis has been induced in several studies by the administration of larger doses of CCl4, or by dosing for longer periods. For example, Luckey and Petersen (2001) induced fibrotic changes after 4 weeks of dosing rats with 1.0 ml/kg CCl4 twice a week; however, the injury was more evident after 6 weeks of dosing, with greater deposition of extracellular matrix and the appearance of bridging fibrosis. Similarly, Park et al. (2000) administered CCl4 orally at 1.0 ml/kg twice a week for 4 weeks; this regimen induced the extensive accumulation of connective tissue resulting in the destruction of the lobular architecture and the formation of septa. Paradis et al. (1999) induced fibrosis by dosing 2.0 ml/kg CCl4 twice a week for 3 weeks, whereas Iredale et al. (1998) administered 2.0 ml/kg CCl4 to male rats twice a week for 4 weeks and observed nodular fibrosis with the deposition of fibrotic septa. In the present Experiment 1, following the 6-week non-dosing recovery period (i.e. at 12 weeks), liver weights remained heavier than in the control animals and the increases were statistically significant at 0.18 and 0.24 ml/kg CCl4. At the 12-week necropsy, serum clinical chemistry results showed ALT and GLDH activities similar to the controls in all dose level groups. However, AST activities were significantly reduced compared to the controls in the 0.24, 0.30 and 0.36 ml/kg groups, but comparable to control values at all the lower dose levels of CCl4. In general, there were no significant differences between the control and CCl4-treated groups for the other serum parameters measured (e.g. ALP, ALB and T-BIL). These results suggest that in the 6-week recovery period the liver underwent a period of repair. Histology results confirmed that following the 6-week recovery period the livers had returned to a normal structure with no evidence of fibrosis.
The findings of Experiment 2 at 6 weeks, in general, compared with Experiment 1; increases in liver weights (Table 1) and serum clinical chemistry results (Table 2) tended to follow similar patterns of change. Elevations of relative liver weights, as a result of CCl4 administration, are generally considered to be related to hepatocyte cytoplasmic swelling as a result of cellular injury and degeneration, and the infiltration of inflammatory cells; also, with repeated CCl4-induced cellular damage, the deposition of collagen in the development of the fibrotic response would also play a part. Thus, the general overall change is an increase in hepatocyte size, an increase in infiltrating inflammatory cell numbers, and an increase in matrix tissue deposition. Alanine aminotransferase, AST and GLDH were also increased in Experiment 2 at all dose levels of CCl4 (Table 2) and dose–response relationships were apparent. At all CCl4 dose levels, AST activity was greater than ALT indicating the induction of chronic injury. Alkaline phosphatase activity was significantly elevated in all CCl4 groups, as were BA measurements. Total-bilirubin values were also greater than the controls at all levels of CCl4. Vollmar et al. (1999) reported that after administering 0.15 ml CCl4/100 g rat body weight (i.e. 1.5 ml/kg) twice a week for 8 weeks, marked increases were observed in serum ALT and AST. Wang et al. (1996) found ALT activity in female rats treated with CCl4 for 6 weeks was four times greater than in controls. In these two studies, serum T-BIL was also measured and was significantly greater in CCl4-treated rats than in control animals. Serum CREA measurements at 6 weeks were reduced in all CCl4-treated groups, with significant decreases at 0.6, 0.7, 0.8 and 1.0 ml/kg CCl4 (Table 2). Reductions of serum CREA generally result from decreases in muscle mass, or from a diet that is inadequate in protein (York, pers. comm.). Lopez-Lirola et al. (2003) reported muscle atrophy in CCl4-induced cirrhosis; however, this response was due to protein deficiency and not the result of induced cirrhosis. At 12 weeks, after the 6-week non-dosing recovery period, most serum parameters had returned to control levels (Table 3), indicating that no new hepatocellular injury was occurring and therefore enzymes and other biomarkers were at normal blood levels.
Histopathological study of the CCl4-treated livers from Experiment 2 revealed that fibrosis had been induced, with capsular pitting (Figure 2). Sirius red staining revealed perivascular fibrosis and delicate bridging between the centrilobular veins, periportal and capsular areas. However, even at the higher dose levels of CCl4 used in this experiment, there was still variation between, and within, treatment groups. Histopathological examination of livers at 12 weeks (Figure 3) revealed the presence of fibrosis in the subcapsular regions, which extended into the parenchyma along the sinusoids. Therefore, Experiment 2 demonstrated that the fibrosis had not reversed during the 6-week recovery period. Other workers have reported a significant fibrotic response after dosing rats with CCl4 twice a week for 8 and 12 weeks at 0.2 ml/kg by i.p. injection (Ohishi et al. 2001). Here, after 8 weeks of dosing, assessment of the livers demonstrated periportal fibrosis. By 12 weeks, the fibrosis had extended along the sinusoids with bridging between portal tracts. Ferréet al. (1999) induced cirrhosis in rats by the i.p. injection of 0.5 ml/kg CCl4 twice a week for 9 weeks. In another study (Rivera et al. 2001), rats were dosed intragastrically with CCl4 once a week for 9 weeks. The outcome was extensive collagen deposition and bridging between portal regions. Cabréet al. (2000) administered 0.5 ml CCl4 by i.p. injection to rats twice a week for 9 weeks. By 2 weeks after the commencement of dosing, fibrosis was observed in all animals. In a similar study by Varela-Moreiras et al. (1995), steatosis was evident after 3 weeks of CCl4 dosing and cirrhosis after 9 weeks. It is therefore considered that in the present studies more significant fibrosis may have been achieved by dosing for a longer period or with higher CCl4 doses.
Urine samples collected during Experiment 2 were analysed by 1D SDS–PAGE. This revealed that the only protein band apparent in the urine of CCl4-treated rats, not detected in control urine, was an 18.4-kDa band (Figure 4). Previous studies (Smyth et al., 2004) had demonstrated the same 18.4-kDa band in rat urine in response to a single dose of CCl4. This protein had been identified using nano-electrospray tandem mass spectrometry (nano-ESI-MS/MS) as Cu/Zn SOD (SOD-1) (Smyth et al. 2004). In the present studies, the identification of Cu/Zn SOD in urine was confirmed, but in this case in repeat-dosed CCl4 rats by Western blotting with SOD-1 antibody.
Superoxide dismutase belongs to a group of metalloenzymes that are responsible for catalysing the dismutation of superoxide anions (O2•−) into oxygen and hydrogen peroxide (McCord & Fridovich 1969). Therefore, SOD forms part of the antioxidant defence system (McCord et al. 1971). Carbon tetrachloride is metabolized by the cytochrome P450 oxidase system to produce the trichloromethyl free radical. This free radical initiates lipid peroxidation, whereby a number of reactive oxygen species and free radicals are generated (de Zwart et al. 1998).
Superoxide dismutase-1 was identified in urine from rats treated with CCl4 after both 3 and 6 weeks of dosing (Figure 4). However, SDS–PAGE analysis did not detect SOD-1 in urine samples collected at 3 weeks into the recovery period (i.e. week 9) nor at 6 weeks of the recovery period (week 12). Western blotting with SOD-1 antibody showed SOD-1 was greatly increased in the urine from rats treated with CCl4, but was barely visible in control urine samples (Figure 4). However, after 6 weeks of no CCl4 treatment, Western blotting demonstrated that SOD-1 levels were similar to the control animals in all urine samples from CCl4-treated rats.
The activity of SOD was also measured in urine from rats treated with CCl4 twice a week for 6 weeks (Figure 5a). Superoxide dismutase activity was approximately 5-fold greater in the urine from rats treated with CCl4 at 0.7 ml/kg than in control urine. However, SOD activity was not further increased in rat urine at the higher CCl4 dose level of 1.0 ml/kg (Figure 5a). The activity of GLDH was also measured in urine samples from rats after dosing with CCl4 twice a week for 6 weeks (Figure 5b). A clear dose–response relationship is seen, with GLDH activity in urine increasing with an increasing dose of CCl4. Glutamate dehydrogenase activity was approximately 1.8 times greater in urine from rats treated with CCl4 at 1.0 ml/kg than in the controls. However, although GLDH is present at high concentrations in the liver, it can also be identified in the kidney and skeletal muscle. Therefore, GLDH is not a liver-specific biomarker. The activity of GLDH is higher in the centrilobular area of the hepatic lobule than in other regions and the enzyme therefore appears to be a good marker of centrilobular necrosis. Urinary SOD activity values showed greater fold increases in rat urine following CCl4 treatment, compared with the fold increases for GLDH levels; however, GLDH activity may be a more sensitive urinary marker than SOD activity as GLDH demonstrated a dose–response relationship to the dose levels of CCl4 administered.
Superoxide dismutase-1, as well as showing raised levels in urine, was also increased in the serum from rats treated with CCl4 for 6 weeks (Table 2). However, although the mean levels of the enzyme in serum were significantly greater than in the controls at all CCl4 dose levels, SOD-1 appears to be a less sensitive serum biomarker in comparison with other conventional biomarkers of liver injury such as ALT, AST and GLDH.
The present studies show that at week 12, on removal of the stimulus for fibrosis (i.e. CCl4 administration), hepatocytes were not being injured, and therefore SOD-1 levels and levels of other biomarker enzymes were, in general, at base-line (control) levels in the blood (Table 3), and SOD-1 was also absent from the urine (Figure 4c). Nevertheless, at this time (week 12), fibrosis remained clearly evident (Figure 3). Therefore, it is concluded that SOD-1 is a urinary marker of hepatocyte injury (degeneration and necrosis), but not a marker of the established fibrotic state. However, as SOD-1 is found in tissues other than the liver, e.g. the kidney and heart (Frederiks & Bosch 1997), the enzyme cannot be considered as a liver-specific urinary biomarker.
Acknowledgments
We acknowledge the assistance of the technical staff at the School of Pharmacy for their care of the animals. We thank Dr Catherine S. Lane (School of Pharmacy) for the initial identification of SOD by mass spectrometry. JAT acknowledges the continued support of GlaxoSmithKline. GlaxoSmithKline and the School of Pharmacy funded this work.
References
- Abraham P, Wilfred G, Cathrine SP. Oxidative damage to the lipids and proteins of the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clim. Chim. Acta. 1999;289:177–179. doi: 10.1016/s0009-8981(99)00140-0. [DOI] [PubMed] [Google Scholar]
- Abraham P, Wilfred G, Ramakrishna B. Plasma prolidase may be an index of liver fibrosis in the rat. Clin. Chim. Acta. 2000;295:199–202. doi: 10.1016/s0009-8981(00)00183-2. [DOI] [PubMed] [Google Scholar]
- Al-Bader A, Mathew TC, Abul H, Al-Sayer H, Singal PK, Dashti HM. Cholangiocarcinoma and liver cirrhosis in relation to changes due to thioacetamide. Mol. Cell Biochem. 2000;208:1–10. doi: 10.1023/a:1007082515548. [DOI] [PubMed] [Google Scholar]
- Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525–1528. doi: 10.1053/gast.2002.33367. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Madhusoodana UK, Nayak S, Jacob J. Urinary hydrogen peroxide: a probable marker of oxidative stress in malignancy. Clin. Chim. Acta. 2003;334:205–209. doi: 10.1016/s0009-8981(03)00236-5. [DOI] [PubMed] [Google Scholar]
- Benyon RC, Iredale JP. Is liver fibrosis reversible? Gut. 2000;46:443–446. doi: 10.1136/gut.46.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821–831. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- Cabré M, Camps J, Paternain JL, Ferré N, Joven J. Time-course of changes in hepatic lipid peroxidation and glutathione metabolism in rats with carbon tetrachloride-induced cirrhosis. Clin. Exp. Pharmacol. Physiol. 2000;27:694–699. doi: 10.1046/j.1440-1681.2000.03322.x. [DOI] [PubMed] [Google Scholar]
- Castro JA, Diaz Gomez, De Ferreyra EC, De Castro CR, D’Acosta N, De Fenos CM. Differences in the carbon tetrachloride-induced damage to components of the smooth and rough endoplasmic reticulum from rat liver. Biochem. Biophys. Res. Commun. 1973;50:337–343. doi: 10.1016/0006-291x(73)90845-0. [DOI] [PubMed] [Google Scholar]
- Dare TO, Davies HA, Turton JA, Lomas L, Williams TC, York MJ. Application of surface-enhanced laser desorption/ionization technology to the detection and identification of urinary parvalbumin-alpha: a biomarker of compound-induced skeletal muscle toxicity in the rat. Electrophoresis. 2002;23:3241–3251. doi: 10.1002/1522-2683(200209)23:18<3241::AID-ELPS3241>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Doi K, Kurabe S, Shimazu N, Inagaki M. Systemic histopathology of rats with CCl4-induced hepatic cirrhosis. Lab Anim. 1991;25:21–25. doi: 10.1258/002367791780808121. [DOI] [PubMed] [Google Scholar]
- Draper HH, Csallany AS, Hadley M. Urinary aldehydes as indicators of lipid peroxidation in vivo. Free Radic. Biol. Med. 2000;29:1071–1077. doi: 10.1016/s0891-5849(00)00367-1. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Keller BJ, Kauffman FC, Thurman RG. The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol. Appl. Pharmacol. 1993;119:275–279. doi: 10.1006/taap.1993.1069. [DOI] [PubMed] [Google Scholar]
- Ferré N, Girona J, Cabre M, et al. Hepatic production of apolar aldehydes in rats with carbon tetrachloride-induced cirrhosis. Mol. Cell Biochem. 1999;198:57–60. doi: 10.1023/a:1006998028528. [DOI] [PubMed] [Google Scholar]
- Fontana L, Moreira E, Torres MI, et al. Serum amino acid changes in rats with thioacetamide-induced liver cirrhosis. Toxicology. 1996;106:197–206. doi: 10.1016/0300-483x(95)03177-h. [DOI] [PubMed] [Google Scholar]
- Frederiks WM, Bosch KS. Localization of superoxide dismutase activity in rat tissues. Free Radic. Biol. Med. 1997;22:241–248. doi: 10.1016/s0891-5849(96)00328-0. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N. Engl. J. Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Evaluation of fibrosis and hepatitis C. Am. J. Med. 1999;107:27S–30S. doi: 10.1016/s0002-9343(99)00377-0. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Liver fibrosis – from bench to bedside. J. Hepatol. 2003;38:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Gaudio E, Onori P, Franchitto A, Sferra R, Riggio O. Liver metabolic zonation and hepatic microcirculation in carbon tetrachloride-induced experimental cirrhosis. Dig. Dis. Sci. 1997;42:167–177. doi: 10.1023/a:1018813911469. [DOI] [PubMed] [Google Scholar]
- George J, Rao KR, Stern R, Chandrakasan G. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156:129–138. doi: 10.1016/s0300-483x(00)00352-8. [DOI] [PubMed] [Google Scholar]
- Giménez A, Pares A, Alie S, et al. Fibrogenic and collagenolytic activity in carbon-tetrachloride-injured rats: beneficial effects of zinc administration. J. Hepatol. 1994;21:292–298. doi: 10.1016/s0168-8278(05)80304-6. [DOI] [PubMed] [Google Scholar]
- Guéchot J, Poupon RE, Giral P, Balkau B, Giboudeau J, Poupon R. Relationship between procollagen III aminoterminal propeptide and hyaluronan serum levels and histological fibrosis in primary biliary cirrhosis and chronic viral hepatitis C. J. Hepatol. 1994;20:388–393. doi: 10.1016/s0168-8278(94)80013-8. [DOI] [PubMed] [Google Scholar]
- Hermanns RC, de Zwart LL, Salemink PJ, Commandeur JN, Vermeulen NP, Meerman JH. Urinary excretion of biomarkers of oxidative kidney damage induced by ferric nitrilotriacetate. Toxicol. Sci. 1998;43:241–249. doi: 10.1006/toxs.1998.2429. [DOI] [PubMed] [Google Scholar]
- Hernandez-Munoz R, Diaz-Munoz M, Suarez-Cuenca JA, et al. Adenosine reverses a preestablished CCl4-induced micronodular cirrhosis through enhancing collagenolytic activity and stimulating hepatocyte cell proliferation in rats. Hepatology. 2001;34:677–687. doi: 10.1053/jhep.2001.27949. [DOI] [PubMed] [Google Scholar]
- London: Her Majesty's Stationery Office; Home Office Code of Practice for the Housing and Care of Animals used in Scientific Procedures. [Google Scholar]
- Horne CH, Scott CA, Busuttil A, Macsween RN. Effect of the production of experimental cirrhosis in rats on serum protein levels. Biomedicine. 1973;19:3–6. [PubMed] [Google Scholar]
- Iredale JP, Benyon RC, Pickering J, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, You DY, Chen XC, Wu J. Monitoring of serum markers for fibrosis during CCl4-induced liver damage. Effects of anti-fibrotic agents. J. Hepatol. 1992;16:282–289. doi: 10.1016/s0168-8278(05)80658-0. [DOI] [PubMed] [Google Scholar]
- Jung SA, Chung YH, Park NH, et al. Experimental model of hepatic fibrosis following repeated periportal necrosis induced by allylalcohol. Scand. J. Gastroenterol. 2000;35:969–975. doi: 10.1080/003655200750023057. [DOI] [PubMed] [Google Scholar]
- Kageyama S, Isono T, Iwaki H, et al. Identification by proteomic analysis of calreticulin as a marker for bladder cancer and evaluation of the diagnostic accuracy of its detection in urine. Clin. Chem. 2004;50:857–866. doi: 10.1373/clinchem.2003.027425. [DOI] [PubMed] [Google Scholar]
- Kauser CA, Iredale JP, Winwood PJ, Arthur MJP. Rat hepatic stellate cell expression of α2-macroglobulin is a feature of cellular activation: implications for matrix remodelling in hepatic fibrosis. Clin. Sci. 1998;95:179–186. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J. Gastroenterol. Hepatol. 1999;14:618–633. doi: 10.1046/j.1440-1746.1999.01928.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Lirola A, Gonzalez-Reimers E, Martin Olivera R, et al. Protein deficiency and muscle damage in carbon tetrachloride induced liver cirrhosis. Food Chem. Toxicol. 2003;41:1789–1797. doi: 10.1016/s0278-6915(03)00218-7. [DOI] [PubMed] [Google Scholar]
- Luckey SW, Petersen DR. Activation of Kupffer cells during the course of carbon tetrachloride-induced liver injury and fibrosis in rats. Exp. Mol. Pathol. 2001;71:226–240. doi: 10.1006/exmp.2001.2399. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- McCord JM, Keele BB, Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc. Natl. Acad. Sci. U.S.A. 1971;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Tomaru K, Nagakura T, Izumi Y, Kawamoto T. Smoking and oxidant stress: assay of isoprostane in human urine by gas chromatography-mass spectrometry. J. Chromatogr. B. Biomed. Sci. Appl. 2000;746:11–15. doi: 10.1016/s0378-4347(00)00182-1. [DOI] [PubMed] [Google Scholar]
- Ohishi T, Saito H, Tsusaka K, et al. Anti-fibrogenic effect of an angiotensin converting enzyme inhibitor on chronic carbon tetrachloride-induced hepatic fibrosis in rats. Hepatol. Res. 2001;21:147–158. doi: 10.1016/s1386-6346(01)00102-4. [DOI] [PubMed] [Google Scholar]
- Olga OZ, Nikolai DY. Invasive and non-invasive monitoring of hepatitis C virus-induced liver fibrosis: alternatives or complements? Curr. Pharm. Biotechnol. 2003;4:195–209. doi: 10.2174/1389201033489810. [DOI] [PubMed] [Google Scholar]
- Ozturk F, Ucar M, Ozturk IC, Vardi N, Batcioglu K. Carbon tetrachloride-induced nephrotoxicity and protective effect of betaine in Sprague–Dawley rats. Urology. 2003;62:353–356. doi: 10.1016/s0090-4295(03)00255-3. [DOI] [PubMed] [Google Scholar]
- Paradis V, Dargere D, Vidaud M, et al. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology. 1999;30:968–976. doi: 10.1002/hep.510300425. [DOI] [PubMed] [Google Scholar]
- Park EJ, Nan JX, Kim JY, et al. The ethanol-soluble part of a hot-water extract from Artemisia iwayomogi inhibits liver fibrosis induced by carbon tetrachloride in rats. J. Pharm. Pharmacol. 2000;52:875–881. doi: 10.1211/0022357001774561. [DOI] [PubMed] [Google Scholar]
- Plaa GL, Charbonneau M. Detection and evaluation of chemically induced liver injury. In: Hayes W, editor. Principles and Methods of Toxicology. 4. Philadelphia: Taylor and Francis; 2001. pp. 1145–1187. Chapter 24. [Google Scholar]
- Recknagel RO. Carbon tetrachloride hepatotoxicity. Pharmacol. Rev. 1967;19:145–208. [PubMed] [Google Scholar]
- Recknagel RO, Glende EA., Jr Carbon tetrachloride hepatotoxicity: an example of lethal cleavage. CRC Crit. Rev. Toxicol. 1973;2:263–297. doi: 10.3109/10408447309082019. [DOI] [PubMed] [Google Scholar]
- Rivera CA, Bradford BU, Hunt KJ, et al. Attenuation of CCl4 induced hepatic fibrosis by GdCl3 treatment or dietary glycine. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G200–G207. doi: 10.1152/ajpgi.2001.281.1.G200. [DOI] [PubMed] [Google Scholar]
- Sanz N, Diez-Fernandez C, Valverde AM, Lorenzo M, Benito M, Cascales M. Malic enzyme and glucose 6-phosphate dehydrogenase gene expression increases in rat liver cirrhogenesis. Br. J. Cancer. 1997;75:487–492. doi: 10.1038/bjc.1997.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Kakubari M, Kawamura M, Sugimoto J, Matsumoto K, Ishii T. The decrease in total collagen fibers in the liver by hepatocyte growth factor after formation of cirrhosis induced by thioacetamide. Biochem. Pharmacol. 2000;59:681–690. doi: 10.1016/s0006-2952(99)00359-7. [DOI] [PubMed] [Google Scholar]
- Savolainen ER, Brocks D, Ala-Kokko L, Kivirikko KI. Serum concentrations of the N-terminal propeptide of type III procollagen and two type IV collagen fragments and gene expression of the respective collagen types in liver in rats with dimethylnitrosamine-induced hepatic fibrosis. Biochem. J. 1988;249:753–757. doi: 10.1042/bj2490753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater TF. Necrogenic action of carbon tetrachloride in the rat: a speculative mechanism based on activation. Nature. 1966;209:36–40. doi: 10.1038/209036a0. [DOI] [PubMed] [Google Scholar]
- Slater TF. Mechanisms of protection against the damage produced in biological systems by oxygen-derived radicals. Ciba Found. Symp. 1978;65:143–176. doi: 10.1002/9780470715413.ch9. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1:232–241. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Smyth R. Investigation of novel urinary markers of hepatotoxicity. University of London; 2004. Ph.D. Thesis. [Google Scholar]
- Smyth R, Turton JA, Clarke CJ, et al. Proteomic identification of superoxide dismutase in the urine of the rat following carbon tetrachloride-induced hepatoxicity. Comp. Clin. Path. 2002;11:197. [Google Scholar]
- Smyth R, Turton JA, Clarke CJ, et al. The identification of superoxide dismutase in rat urine following carbon tetrachloride-induced hepatotoxicity. Toxicology. 2004;194:271–272. [Google Scholar]
- Ueki T, Kaneda Y, Tsutsui H, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat. Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- Varela-Moreiras G, Alonso-Aperte E, Rubio M, et al. Carbon tetrachloride-induced hepatic injury is associated with global DNA hypomethylation and homocysteinemia: effect of S-adenosylmethionine treatment. Hepatology. 1995;22:1310–1315. [PubMed] [Google Scholar]
- Vollmar B, Siegmund S, Richter S, Menger MD. Microvascular consequences of Kupffer cell modulation in rat liver fibrogenesis. J. Pathol. 1999;189:85–91. doi: 10.1002/(SICI)1096-9896(199909)189:1<85::AID-PATH399>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Sun ZQ, Quan QZ, Juan-Ji YU. Fat- storing cells and liver fibrosis. China Natl. J. New Gastroenterol. 1996;2:53–57. [Google Scholar]
- Zimmerman HJ. Drug-induced liver disease. Drugs. 1978;16:25–45. doi: 10.2165/00003495-197816010-00002. [DOI] [PubMed] [Google Scholar]
- de Zwart LL, Hermanns RC, Meerman JH, Commandeur JN, Salemink PJ, Vermeulen NP. Evaluation of urinary biomarkers for radical-induced liver damage in rats treated with carbon tetrachloride. Toxicol. Appl. Pharmacol. 1998;148:71–82. doi: 10.1006/taap.1997.8310. [DOI] [PubMed] [Google Scholar]