Abstract
Vitamin A and the retinoids play a unique role in mammalian embryonic and foetal development and are essential for both cellular differentiation and the establishment of normal morphogenesis. Vascular endothelial growth factor (VEGF) is a known potent mitogenic factor that plays a key role in lung development and function maintenance. In order to contribute to a better knowledge of the modulating effects of vitamin A in lung development, we investigated the effects of the antenatal administration of vitamin A on VEGF expression in lungs and plasma from foetuses and neonates. Pregnant mice were subjected to subcutaneous administration of vitamin A on the 12th gestational day. The lungs and plasma from foetuses and neonates were collected daily from the 15th gestational day till the day of birth. Our results show that vitamin A modulates VEGF concentrations both in lungs and plasma. Statistically significant differences were observed at gestational days 15 (P = 0.004 for lungs; P < 0.0001 for plasma), 16 (P < 0.0001 for lungs and plasma) and 18 (P < 0.0001 for lungs; P < 0.05 for plasma). Vitamin A tends to increase the expression of this factor in the lung, particularly during the critical period of perinatal adaptation to postnatal life. These effects seem to be spatial and temporally regulated, and point out to the important role of vitamin A during lung development.
Keywords: foetal development, lung, plasma, VEGF, vitamin A
Vitamin A and its related compounds, the retinoids, play a unique role in mammalian embryonic and foetal development and are essential for both cellular differentiation and the establishment of normal morphogenesis (Rodrigues et al. 2004).
Vessel development in the early lung determines lung structure maturation, and both undisturbed angiogenesis and vasculogenesis are necessary for the successful building of the organ (Healy et al. 2000; Voelkel et al. 2006). Several signalling pathways including the vascular endothelial growth factor (VEGF), glucocorticoid and retinoic acid (RA) have been implicated in regulating lung maturation and vascular development (Morrisey & Savani 2003).
It has been demonstrated that RA is required for the normal alveolar development in the mouse, and exogenous administration of RA restores alveolar architecture to the lungs of dexamethasone-treated mice (Hind & Maden 2004; Maden & Hind 2004; Maden 2006). Retinoic acid maintains alveolar development in mice treated with an angiogenesis inhibitor (Cho et al. 2005), and is required to control endothelial cell maturation and cell cycle progression during vasculogenesis and angiogenesis (Lai et al. 2003).
Vascular endothelial growth factor is a potent mediator of angiogenesis, which has multiple effects in lung development and physiology. It has been reported that lung presents the highest level of VEGF gene expression among normal tissues (Kaner et al. 2000; Voelkel et al. 2006). Vascular endothelial growth factor is also involved in the pathophysiology of some of the most common respiratory disorders (Papaioannou et al. 2006). One of these conditions is bronchopulmonary dysplasia (BPD). Infants dying from BPD have reduced pulmonary levels of VEGF (Bhatt et al. 2001), and intrauterine or postnatal intratracheal instillation of VEGF protects preterm mice against respiratory distress syndrome (RDS) (Compernolle et al. 2002). It was verified that VEGF plays a central role in the epithelial–endothelial interactions that are critical for normal lung development (Acarregui et al. 1999; Brown et al. 2001; Compernolle et al. 2002; Zhao et al. 2005). Vascular endothelial growth factor seems to interfere in a different manner, depending on its amount, the location and the underlying physiological or pathological process in lung tissue (Papaioannou et al. 2006). Recent studies have correlated RA and VEGF during lung development, hypothesizing that inhibition of alveolar development is associated with a block in angiogenesis due to the down-regulation of VEGF and VEGFR2, which is prevented by treatment with RA (Cho et al. 2005). It has been shown that RA regulates the expression of several signalling molecules including VEGF in tissues such as skin and brain development (Maden et al. 1996; Lachgar et al. 1999; Diaz et al. 2000). At this time, however, it is not known whether these RA functions are applicable during foetal lung development. On the other hand, clinical trials showed that vitamin A supplementation given largely to infants <1 kg resulted in a decrease in death and/or BPD without increasing mortality or neurodevelopment impairment at 18 to 22 months (Ambalavanan et al. 2005). Thus, interventions with vitamin A to treat infants with RDS and prevent its complications are promising, but further research must be conducted (Carlo et al. 2004). In order to contribute to a better knowledge of vitamin A effects and its influence in the lung maturation process we investigated if the antenatal administration of vitamin A could alter the lung and plasma concentrations of VEGF in foetuses and neonates.
Materials and methods
Animals
Mice, Mus musculus, strain ICR (CD-1®; Harlan, Spain) were housed at 22 °C, fed ad libitum, and exposed to day–night cycles alternatively every 12 h throughout the study period. Mating was performed according to a polygamist regimen (Broustail 1967). Timed-pregnant mice (visualization of vaginal plug at day 0) were obtained on days 15–18. Pregnant mice received a single subcutaneous injection of vitamin A (150 μl, 45,000 IU of retinyl palmitate; G. Streuli & Co., Uznach, Switzerland) on gestational day 12. At the same gestational day, pregnant mice of control groups received an equivalent volume of diluent alone (saturated median chain triglyceraldehydes, Dub MCT 55/45 EP ex Dubois, Georges Walther AG, Switzerland). Pregnant mice and pups delivered naturally at term gestation were anaesthetized in the same manner. For anaesthesia induction, each animal was placed alone in an induction chamber with gas input and exhaust output lines. Anaesthesia was induced with 5% isoflurane (Isoflo, Esteve Farma Lda., Carnaxide, Portugal) in 100% oxygen with a delivery rate of 5 l/min until loss of righting reflex. The mothers were sacrificed by neck dislocation and the foetuses were delivered by hysterectomy. Pups were sacrificed by cutting large thoracic vessels. The day of birth was designated day 0. Foetuses and neonates were randomized to study groups (treated vs. control) similar in number at each time point. All procedures and protocols were approved by the Animal Care and Use Committee of the ‘Direção Geral de Veterinária, Portugal’.
Sample collection
Blood was collected from foetuses and neonates by cutting large vessels in the thorax with a hand-modified 20G needle (Sterican 100, B. Braun, Germany) applied to a 1 ml/100 IU syringe (Ominifix 100 solo, B. Braun, Germany) (Pinto et al. 2007, personal communication). The needle and syringe were previously passed through a solution of ethylenediaminetetraacetic acid tripotassium salt dihydrate (Sigma, Steinheim, Germany) in NaCl 0.9% (B. Braun, Germany) at 25 mg/ml. Whole blood was centrifuged at 2500 g for 2 min (Minispin, Eppendorf, Hamburg, Germany). Plasma was collected and stored at −80 °C for further analysis. Lungs were collected, frozen and stored at −80 °C or processed for paraffin embedding.
ELISA for VEGF
Frozen lungs were placed in 5 ml ice-cold phosphate-buffered saline (PBS) and homogenized using a Potter-Elvjhem homogenizer. The homogenates were centrifuged at 5000 g for 5 min at +4 °C (Sigma 3K30, Steinheim, Germany) and the supernatant was used to assay VEGF concentration in lungs. ELISAs were performed with a commercial VEGF ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufactures’ instructions. Briefly, standards and samples were incubated in duplicate wells of a microplate coated with anti-VEGF polyclonal antibody. Unbound substances were washed from the plate, and horseradish peroxidase-linked anti-VEGF polyclonal antibody was added. After incubation and further washing, H2O2 and tetramethylbenzidine were added. The reaction was stopped with HCl, and optical densities were read at 450 nm (correction wavelength at 540 nm) on a multiskan plate reader (Labsystems® Multiskan MCC/340; Vantaa, Finland). Vascular endothelial growth factor concentration was calculated by comparison with a standard curve generated using a four-parameter logistic curve-fit and on-board software. Vascular endothelial growth factor concentrations were normalized to total protein concentrations determined by the bicinchoninic acid method. Each sample value is the result of three lungs, and 36 lungs (18 treated vs. 18 controls) were used at each time point. To assay the VEGF concentrations in plasma the same procedure was applied, but the samples were diluted (50 μl sample + 200 μl of calibrator diluent), and 16 individual values (8 treated vs. 8 controls) were obtained at each time point.
VEGF Immunostaining
Paraffin-embedded lung tissue sections (3 μm) were deparaffinized with xylene, and rehydrated through an ethanol–water series. Antigen retrieval was performed by microwaving sections in Tris–HCl (10 mM Tris–HCl, pH 10.0). Endogenous peroxidases activity was quenched using a 3% solution of hydrogen peroxide. Sections were blocked with ‘Large volume ultra V block’ (Labvision Corporation, Fremont, CA, USA). The slides were then incubated with primary antibody (rabbit polyclonal anti-VEGF Ab-1, 5 μg/ml, Labvision-Neomarkers, Fremont, CA, USA) overnight at +4 °C. Tissue sections were incubated with ‘Biotinylated goat anti-polyvalent secondary antibody’, washed, and incubated with ‘Large volume streptavidin peroxidase reagent’. After being rinsed, the sections were incubated with diaminobenzidine (Novocastra, UK) and counterstained with haematoxylin. A negative control, using non-immune goat serum instead of the primary antibody was used. Lungs were viewed under a Nikon FX Photomicroscope (Nikon, Japan) with a 40 × lens for immunohistochemical scoring. The degree of positive staining was evaluated by semi-quantitative scoring on a scale of 0 (negative cases) to 3 for intensity (I) and distribution (D) adapted from Ozer et al. (2000). Tissues with I × D ≤ 3 were considered weakly positive, and those with I × D > 3 were designated strongly positive.
Statistical analysis
Data are expressed as mean ± SD. Statistical analysis was performed using spss 13.0 (Chicago, IL, USA). After checking both assumptions of normality and homogeneity of variances the one-way anova was used to analyse the different groups. Immunohistochemical scores were assessed by the χ2 test. Statistical significance was set at P < 0.05.
Results
Quantitative observations
VEGF pulmonary levels.
The ELISA values obtained for VEGF in the lungs of control and vitamin A treated foetuses and neonates are displayed in Figure 1. At gestational day 15, VEGF levels in the lung homogenates from control foetuses are higher (76.5 ± 15.1 ng VEGF/mg total lung protein; P = 0.004) than the ones observed in vitamin A treated foetuses (48.05 ± 10.55 ng/mg). From this day onwards, VEGF levels in the lungs from vitamin A treated foetuses increase gradually and reach a maximum of 192.7 ± 42.8 ng/mg at the 18th gestational day. On the opposite, VEGF levels in the lungs from control foetuses suffer a nearly three-fold decrease (26.8 ± 5.4 ng/mg) at the 16th gestational day. A statistically significant difference (P < 0.0001) between control and treated foetuses is observed at this day. Pulmonary VEGF levels increase to 70.2 ± 10.2 ng/mg in control foetuses of 17 gestational days, but decrease again to 48.4 ± 11.3 ng/mg by the 18th gestational day. A statistically significant difference (P < 0.0001) between control and treated foetuses is also observed at the 18th gestational day. However, at the day of birth, VEGF pulmonary levels in control and vitamin A treated neonates are similar (213.8 ± 37.8 for control and 188.7 ± 48.3 for treated). It is important to point out that pulmonary VEGF levels in vitamin A treated foetuses at the 18th gestational day are similar to those observed at the day of birth, a tendency that is not observed in control subjects (Table 1, Figure 1).
Figure 1.
Vascular endothelial growth factor (VEGF) pulmonary levels during foetal development expressed in ng of VEGF/mg of protein (mean ± SD). Sustained increase of VEGF in the lungs from vitamin A treated foetuses. In the last gestational day, VEGF levels are already similar to the ones observed in the neonate lung. On the opposite, control foetuses display reduced VEGF levels during the perinatal period. **P= 0.04 (vs. control); *P < 0.0001 (vs. control).
Table 1.
VEGF immunostaining scores (intensity × distribution)
| VEGF immunostaining scores | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | Vitamin A | |||||||
| Gestational days | None | Weak | Moderate | Strong | None | Weak | Moderate | Strong |
| 15 | 0 | 0 | 2 | 2 | 0 | 4* | 0 | 0 |
| 16 | 1 | 3 | 0 | 0 | 0 | 0 | 3* | 1* |
| 17 | 1 | 2 | 1 | 0 | 0 | 2 | 2 | 0 |
| 18 | 0 | 0 | 4 | 0 | 0 | 0 | 4 | 0 |
| 0† | 0 | 0 | 2 | 2 | 0 | 0 | 4 | 0 |
P< 0.05 (vs. control).
†Day of birth.
VEGF plasma levels.
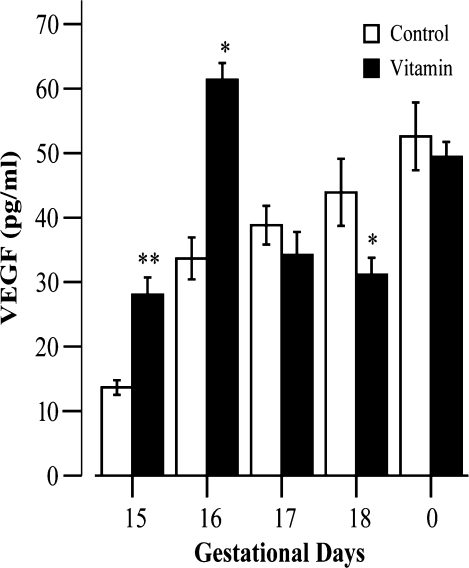
The results of VEGF quantification in plasma are presented in Figure 2. Vascular endothelial growth factor plasma levels in control foetuses and neonates show a gradual and sustained increase throughout development, ranging from 13.7 ± 3.2 pg/ml at the 15th gestational day to 52.6 ± 14.9 ng/ml at the day of birth. A significant increase takes place between the 15th and the 16th gestational days, in which VEGF plasma levels nearly triple (Figure 2). At the 15th gestational day, VEGF plasma levels in treated foetuses (28.1 ± 7.5 pg/ml) more than double the values observed in control foetuses, reaching a statistically significant difference (P < 0.0001). A similar difference at gestational day 16 is also observed (Table 2, Figure 2). A prominent decrease in VEGF plasma levels from treated foetuses occurs at the 17th gestational day. From this gestational day and until the day of birth, VEGF levels in treated foetuses increase gradually, despite being inferior to the ones observed in control (Table 2, Figure 2). The difference between control and treated groups is statistically significant (P < 0.05) at the 18th gestational day, the day before birth.
Figure 2.
Vascular endothelial growth factor (VEGF) plasma levels during foetal development expressed in pg of VEGF/ml (mean ± SD). Gradual increase of VEGF plasma levels throughout development in control subjects. A prominent increase at the 15th gestational day is observed, both in control and in vitamin A treated foetuses. In the last gestational day, VEGF plasma levels are higher in control foetuses, reaching a significant statistical difference. At the day of birth, however, control and treated animals display similar VEGF plasma levels. **P < 0.05 (vs. control); *P < 0.0001 (vs. control).
Qualitative and semi-quantitative observations
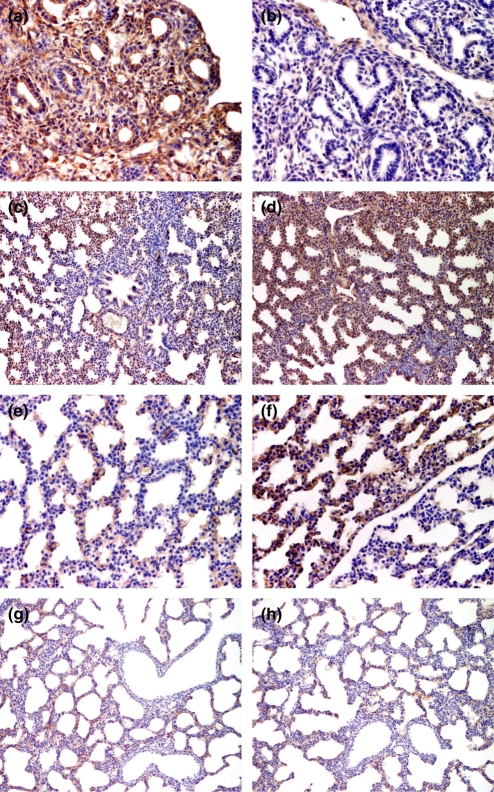
Immunostaining for VEGF protein revealed that in the foetal and neonate lung VEGF is observed both in epithelial and mesenchymal cells. Lungs from control foetuses with 15 gestational days display a strong immunoexpression for VEGF regarding both intensity and distribution patterns (Table 1, P < 0.05 vs. treated foetuses). Positive cells include columnar and cuboidal cells in the tubular epithelium and the surrounding mesenchymal cells (Figure 3a). At the same age, VEGF immunoexpression in the lungs of treated foetuses is weak, but it is more pronounced in the mesenchyme and in the areas where the interlobar septa will develop (Figure 3b). At the late canalicular stage (16 gestational days) VEGF pulmonary immunoexpression is weak in control subjects, but lungs from treated foetuses display a strong immunoexpression for VEGF, in particular regarding the distribution pattern (Table 1, P < 0.05 vs. control foetuses). Immunoexpression in the vascular endothelium was also observed. At the 17th gestational day, both control and treated groups exhibit strong pulmonary VEFG immunoexpression (Figure 3c,d), however, the distribution of VEGF positive cells throughout lung parenchyma is not homogeneous. The central areas have fewer VEGF expressing cells when compared with the peripheral lung areas, a finding specially pronounced in the control group (Figure 3d). In the lungs of treated foetuses at the late saccular period (18 gestational days) VEGF immunoexpression intensity in the saccular parenchyma reaches a peak (Figure 3f), but again the distribution pattern is not homogeneous, and there are areas where the expression is scarce or even absent (Figure 3e,f). At this day, VEGF immunoexpression intensity is high in the muscle cells of the vascular walls, but it is weak or even absent in the cuboidal epithelium of the distal airways, both in control and treated foetuses. At the day of birth, VEGF pulmonary immunoexpression is very similar to that observed on the last prenatal day, but there is a trend towards a higher intensity in control animals, and a more homogeneous distribution pattern in treated ones. At this day, VEGF immunoexpression is observed in the epithelial and endothelial cells of lung parenchyma (Figure 3g,h), as well as in muscle cells of the walls of vascular and large airways.
Figure 3.
Immunohistochemistry for vascular endothelial growth factor (VEGF) in the lungs of control (a, c, e, g) and vitamin A treated (b, d, f, h) foetuses and neonates. Higher intensity and distribution of VEGF immunoexpression patterns in control foetuses at the 15th gestational (a, b). Strong VEGF immunoexpression, both for control and for treated foetuses at the 17th gestational day (c, d). The central area depicted in (c) displays fewer immunoexpressing cells. Higher immunoexpression in treated foetuses at the 18th gestational day (f). The intensity and distribution expression patterns are different in contiguous areas of the saccular parenchyma. Similar immunoexpression for VEGF in control and treated neonates (g, h). Notice the weak or absent immunoexpression in the cuboidal epithelium of the distal airways. Original magnification ×400 (a, b and e, f). Original magnification ×200 (c, d and g, h).
Discussion
Lung-branching morphogenesis and epithelial development have been the subject of intense investigation over the past 50 years (Warburton et al. 2000; Cardoso 2001; Demayo et al. 2002). Emerging data indicate that the formation of pulmonary vascular units directs epithelial morphogenesis but the precise signalling pathways involved in this process are still to be determined (Warburton et al. 2000; Demayo et al. 2002; Cardoso & Lu 2006). A biologically active derivative of vitamin A, RA, plays an important role in both the biochemical (surfactant) and structural maturation (alveolarization) of the developing lung (Torday & Rehan 2004). A large number of physiological effects of VEGF pertain particularly to the lung, which is one of the organs with the highest expression of VEGF in animal systems (Voelkel et al. 2006).
This study was conducted to investigate if the antenatal administration of vitamin A, in the period where its teratogenic effects are no longer present, could alter VEGF expression in lungs and plasma from foetuses and neonates.
Our results show that vitamin A modulates VEGF expression in plasma and lungs of foetuses and neonates. Vitamin A treated foetuses present higher VEGF pulmonary levels at all but the 15th gestational day. A particularly critical and delicate period, the antenatal day (18th gestational day), presents the most striking difference in VEGF pulmonary concentrations between control and treated foetuses. In fact, VEGF pulmonary levels in treated foetuses are nearly four times higher than the levels observed in control subjects. In control foetuses, there is a peak in pulmonary VEGF concentrations at gestational day 17. This finding differs from the reports made by Ng et al. (2001) and Chang et al. (2004) who suggest that there is an increase in VEGF expression throughout lung development, peaking at the 16th gestational day. This discrepancy may be explained by the fact that the commercial ELISA kit used in our study quantifies 120 and 164 mouse VEGF protein isoforms, leaving out the VEGF188 isoform, and that we have quantified proteins and not VEGF mRNAs isoforms, as in the previous studies.
The precise regulation of VEGF pulmonary levels is of critical importance since lung VEGF overexpression causes lung dysmorphogenesis (Akeson et al. 2003). Decrease in lung VEGF as a consequence of VEGF neutralization or gene deletion results in poor septal formation and an emphysematous pattern (Gerber et al. 1997). Disruption of the VEGF/VEGF receptor signalling not only reduces pulmonary arterial density, but also leads to impaired alveolarization, emphysema and pulmonary hypertension (Jakkula et al. 2000; Kasahara et al. 2000; Lassus et al. 2001). Furthermore, exogenous RA increases alveolar septal formation in adult tight skin mice, a genetic model associated with a failure in alveolar septation resulting in emphysema (Massaro & Massaro 2000).
In our study, the morphologic observations revealed that the lungs of treated animals did not display any abnormal features. Previous studies made by our group (data not published) have already indicated that the elastic fibres of the lung’s interstitial compartment significantly increased in foetuses and neonate mice from vitamin A treated mothers, which is in accordance with the recent studies published by Pierce et al. (2007).
The results of our study suggest that the beneficial effects of vitamin A administration seem to work in a synergetic manner with the modulating action of VEGF in lung tissue. This is not surprising taking into account that RA indirectly regulates visceral endoderm survival and production of soluble signals, including VEGF (Bohnsack et al. 2004). Moreover, the presence of all-trans RA in rat lung cultured endothelial cells increases their nitric oxide production, which is one of the mediators of the VEGF effects (Uruno et al. 2005). Additionally, it was also shown recently that all-trans RA induces VEGF secretion in umbilical vein endothelial cells culture (Saito et al. 2007).
Recent studies also demonstrate that RA attenuates the mild hyperoxic lung injury of newborn mice, with effects on VEGF mRNA expression (Zimováet al. 2007). Vascular endothelial growth factor acts as a morphogen, inducing a significant increase in both epithelial- and endothelial-branching morphogenesis (Del Moral et al. 2006), and accelerating lung growth in rat foetal lung explants (Shinkai et al. 2006).
Our immunohistochemical results, regarding the intensity scores from our study, are in accordance with the results from the studies made by Zhao et al. (2005). However, there is a slight discrepancy regarding the type of cells displaying VEGF expression that may be explained by the different techniques used. In the study by Zhao et al. (2005) it is mentioned that at the 18.5th gestational day VEGF expression is restricted to a subpopulation of cells that line the saccular walls, and there is no mention of VEGF expression by the muscle cells of the vascular walls, as seen in our study. Even so, they mention that they cannot rule out the expression by other cells. The other cells would include endothelial cells, as we already observed at the 16th gestational day, despite the close proximity of epithelial and endothelial cells. Immunostaining of the vascular endothelium in the lung of foetuses and preterm infants was also observed by Lassus et al. (2001). It is interesting to notice that in term infants this was not apparent, and that VEGF increased rapidly in the tracheal aspirates from preterm infants during the first days of life (Lassus et al. 1999). Due to these findings the authors suggested that VEGF may be indicative of pulmonary maturity. Our observations revealed VEGF immunostaining of the vascular endothelium also in term mice (day 0 of life), which can be a normal feature of the mice lung.
To the best of our knowledge, this is the first report that presents results on plasma VEGF levels in foetuses and neonates. Vascular endothelial growth factor plasma levels were previously determined by Lassus et al. (1999) in infants with clinically diagnosed RDS. These authors demonstrated that they remained constant during the first postnatal week. Our results show that VEGF plasma levels suffer variations during foetal development, and are modified by the maternal administration of vitamin A. It is interesting to notice that during our study, the days were significantly statistical differences were observed regarding both VEGF plasma and pulmonary levels, where precisely the same (15, 16 and 18 gestational days). Regarding this particular aspect, it is interesting to consider the hypothesis raised by Kaner and Crystal (2001), that high levels of VEGF protein on the respiratory epithelial surface may function as a physiological reservoir. If this is correct, we could then assume that the variations of plasma VEGF levels during foetal development would reflect VEGF variations in the developing lung, the physiological reservoir. The same authors suggested that in healthy humans VEGF protein levels in alveoli are 500 times higher than in plasma. In the present study we cannot directly compare VEGF plasma levels with the ones obtained in the lung homogenates, since these last were normalized to total protein concentrations. Nevertheless, there is a tendency for the plasma and pulmonary VEGF levels to change inversely, which could point out to the role of reservoir displayed by the lung, but further studies need to be made to confirm this.
Blood VEGF data is affected by several confounding circumstances, including circadian rhythm and hypoxia. Hypoxia is one of the major regulators of VEGF production (Papaioannou et al. 2006; Voelkel et al. 2006). However, Oltmanns et al. (2006) found that in contrast with in vitro studies (Ferrara et al. 1992; Shweiki et al. 1992; Namiki et al. 1995; Fredriksson et al. 2000; Mick et al. 2002), hypoxia decreases plasma concentrations in adult human healthy subjects. Therefore, systemic VEGF concentration may be differently regulated than the expression on a cellular basis. Whether this is also true for foetal development, and if the physiologic ‘hypoxic environment’ of the developing foetus decreases VEGF in plasma is still to be addressed. Circadian rhythm can also affect VEGF blood levels, but in order to avoid variations related to this factor the sacrifice and sample collection were consistently made at the same hour of the day.
Despite very recent studies having demonstrated that the repression of VEGF expression by premature birth and mechanical ventilation was not altered by vitamin A supplementation (Pierce et al. 2007), we demonstrated that maternal vitamin A administration influences foetal and neonate VEGF pulmonary and plasma levels, and that VEGF/RA is one of the signalling pathways involved in this process. Furthermore, our data support the hypothesis that the maternal antenatal vitamin A administration can enhance foetal lung development. We may therefore speculate that, despite the differences between lung alveolar development in mice and humans, vitamin A antenatal administration could be beneficial for the human foetus. However, further studies and clinical trials must be performed to address this question. Bearing in mind this perspective, the present results could make an important contribution towards future appropriately designed strategies to prevent the risk of RDS and BPD in premature neonates.
Acknowledgments
We thank Lígia Lourenço for technical assistance.
References
- Acarregui MJ, Penisten ST, Goss KL, Ramirez K, Snyder JM. Vascular endothelial growth factor gene expression in human fetal lung in vitro. Am. J. Respir. Cell Mol. Biol. 1999;20:14–23. doi: 10.1165/ajrcmb.20.1.3251. [DOI] [PubMed] [Google Scholar]
- Akeson AL, Greenberg JM, Cameron JE, et al. Temporal and spatial regulation of VEGF-A controls vascular patterning in the embryonic lung. Dev. Biol. 2003;264:443–455. doi: 10.1016/j.ydbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Ambalavanan N, Tyson JE, Kennedy KA, et al. Vitamin A supplementation for extremely low birth weight infants: outcome at 18 to 22 months. Pediatrics. 2005;115:249–254. doi: 10.1542/peds.2004-1812. [DOI] [PubMed] [Google Scholar]
- Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- Bohnsack BL, Lai L, Dolle P, Hirschi KK. Signalling hierarchy downstream of retinoic acid that independently regulates vascular remodeling and endothelial cell proliferation. Genes Dev. 2004;8:1345–1358. doi: 10.1101/gad.1184904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broustail M. La souris de laboratoire et son élevage, Réproduction, son aspect biologique. Paris: Vigot Fréres Éditeurs; 1967. pp. 13–27. Troisiéme Édition, Réproduction, son aspect génétique. [Google Scholar]
- Brown KRS, England KM, Goss KL, Snyder JM, Acarregui MJ. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L1001–L1010. doi: 10.1152/ajplung.2001.281.4.L1001. [DOI] [PubMed] [Google Scholar]
- Cardoso WV. Molecular regulation of lung development. Annu. Rev. Physiol. 2001;63:471–494. doi: 10.1146/annurev.physiol.63.1.471. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Carlo WA, Prince LS, St John EB, Ambalavanan N. Care of very low birth weight infants with respiratory distress syndrome: an evidence-based review. Minerva Pediatr. 2004;56:373–380. [PubMed] [Google Scholar]
- Chang R, Andreoli S, Ng Y, et al. VEGF expression is downregulated in nitrogen-induced congenital diaphragmatic hernia. J. Pediatr. Surg. 2004;39:825–828. doi: 10.1016/j.jpedsurg.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Cho SJ, George CLS, Snyder JM, Acarregui MJ. Retinoic acid and erythropoietin maintain alveolar development in mice treated with an angiogenesis inhibitor. Am. J. Respir. Cell Mol. Biol. 2005;33:622–628. doi: 10.1165/rcmb.2005-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compernolle V, Brusselmans K, Acker T, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat. Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- Del Moral P-M, Sala FG, Tefft D, et al. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev. Biol. 2006;290:177–188. doi: 10.1016/j.ydbio.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Demayo F, Minoo P, Plopper CG, Schuger L, Shannon J, Torday JS. Mesenchymal-epithelial interactions in lung development and repair: are modeling and remodeling the same process? Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L510–L517. doi: 10.1152/ajplung.00144.2002. [DOI] [PubMed] [Google Scholar]
- Diaz BV, Lenoir M, Ladoux A, Frelin C, Démarchez M, Michel S. Regulation of vascular endothelial growth factor expression in human keratinocytes. J. Biol. Chem. 2000;275:642–650. doi: 10.1074/jbc.275.1.642. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr. Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Fredriksson JM, Lindquist JM, Bronnikov GE, Nedergaard JT. Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a beta adrenoreceptor/cAMP/Protein Kinase A pathway involving Src but independently of Erk1/2. J. Biol. Chem. 2000;275:13802–13811. doi: 10.1074/jbc.275.18.13802. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J. Biol. Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- Healy AM, Margenthau L, Zhu X, Farber HW, Cardoso WV. VEGF is deposited in the subepithelial matrix at the leading edge of branching airways and stimulates neovascularization in the murine embryonic lung. Dev. Dyn. 2000;219:341–352. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1061>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hind M, Maden M. Retinoic acid induces alveolar regeneration in the adult mouse lung. Eur. Respir. J. 2004;23:20–27. doi: 10.1183/09031936.03.00119103. [DOI] [PubMed] [Google Scholar]
- Jakkula M, Le Cras TD, Gebb S, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- Kaner RJ, Crystal RG. Compartmentalization of vascular endothelial growth factor to the epithelial surface of the human lung. Mol. Med. 2001;7:240–246. [PMC free article] [PubMed] [Google Scholar]
- Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am. J. Respir. Cell Mol. Biol. 2000;22:657–664. doi: 10.1165/ajrcmb.22.6.3779. [DOI] [PubMed] [Google Scholar]
- Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J. Clin. Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachgar S, Charveron M, Gall Y, Bonafe JL. Inhibitory effects of retinoids on vascular endothelial growth factor production by cultured human skin keratinocytes. Dermatology. 1999;199:25–27. doi: 10.1159/000051374. [DOI] [PubMed] [Google Scholar]
- Lai L, Bohnsack BL, Niederreither K, Hirschi KK. Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development. 2003;130:6465–6474. doi: 10.1242/dev.00887. [DOI] [PubMed] [Google Scholar]
- Lassus P, Ristimäki A, Ylikorkala O, Viinikka L, Andersson S. Vascular endothelial growth factor in human preterm lung. Am. J. Respir. Crit. Care Med. 1999;159:1429–1433. doi: 10.1164/ajrccm.159.5.9806073. [DOI] [PubMed] [Google Scholar]
- Lassus P, Turanlahti M, Heikkila P, et al. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am. J. Respir. Crit. Care Med. 2001;164:1981–1987. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoids have different efficacies on alveolar regeneration in a dexamethasone-treated mouse. Am. J. Respir. Cell Mol. Biol. 2006;35:260–267. doi: 10.1165/rcmb.2006-0029OC. [DOI] [PubMed] [Google Scholar]
- Maden M, Hind M. Retinoic acid in alveolar development, maintenance and regeneration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:799–808. doi: 10.1098/rstb.2004.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M, Gale E, Kostetskii I, Zile M. Vitamin A-deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 1996;6:417–426. doi: 10.1016/s0960-9822(02)00509-2. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D. Retinoic acid treatment partially rescues failed septation in rats and in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L955–L960. doi: 10.1152/ajplung.2000.278.5.L955. [DOI] [PubMed] [Google Scholar]
- Mick GJ, Wang X, McCormicK K. White adipocyte vascular endothelial growth factor: regulation by insulin. Endocrinology. 2002;143:948–953. doi: 10.1210/endo.143.3.8673. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Savani RC. Midkine. A potential bridge between glucocorticoid and retinoid effects on lung vascular development. Am. J. Respir. Cell Mol. Biol. 2003;28:5–8. doi: 10.1165/rcmb.F255. [DOI] [PubMed] [Google Scholar]
- Namiki A, Brogi E, Kearney M, et al. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J. Biol. Chem. 1995;270:31189–31195. doi: 10.1074/jbc.270.52.31189. [DOI] [PubMed] [Google Scholar]
- Ng Y-S, Rohan R, Sunday ME, DeMello DE, d’Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev. Dyn. 2001;220:112–121. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Oltmanns KM, Gehring H, Rudolf S, et al. Acute hypoxia decreases plasma VEGF concentration in healthy humans. Am. J. Physiol. Endocrinol. Metab. 2006;290:E434–E439. doi: 10.1152/ajpendo.00508.2004. [DOI] [PubMed] [Google Scholar]
- Ozer E, Sis B, Ozen E, Sakizli M, Canda T, Sarioglu S. BRCA1 c-erB-2 and H-ras gene expressions in young woman with breast cancer: an immunohistochemical study. Appl. Immunohistochem. Mol. Morphol. 2000;8:12–18. doi: 10.1097/00129039-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Papaioannou A, Kostikas K, Kollia P, Gourgoulianis K. Clinical implications for vascular endothelial growth factor in the lung: friend or foe? Respir. Res. 2006;7:128. doi: 10.1186/1465-9921-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RA, Joyce B, Officer S, et al. Retinoids increase lung elastin expression but fail to alter morphology or angiogenesis genes in premature ventilated baboons. Pediatr. Res. 2007;61:703–709. doi: 10.1203/pdr.0b013e318053661d. [DOI] [PubMed] [Google Scholar]
- Rodrigues P, Gonçalves C, Honório A, Bairos VA. Metabolic pathways and modulating effects of vitamin A. Curr. Med. Chem. Immunol. Endocrinol. Metab. Agents E. 2004;4:119–142. [Google Scholar]
- Saito A, Sugawara A, Uruno A, et al. All-trans retinoic acid induces in vitro angiogenesis via retinoic acid receptor: possible involvement of paracrine effects of endogenous vascular endothelial growth factor signaling. Endocrinology. 2007;148:1412–1423. doi: 10.1210/en.2006-0900. [DOI] [PubMed] [Google Scholar]
- Shinkai M, Shinkai T, Montedonico S, Puri P. Effect of VEGF on the branching morphogenesis of normal and nitrogen-induced hypoplastic fetal rat lung explants. J. Pediatr. Surg. 2006;41:781–786. doi: 10.1016/j.jpedsurg.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Torday JS, Rehan VK. Does “A” stand for alveolization? Eur. Respir. J. 2004;23:3–4. doi: 10.1183/09031936.03.00109803. [DOI] [PubMed] [Google Scholar]
- Uruno A, Sugawara A, Kanatsuka H, et al. Upregulation of nitric oxide production in vascular endothelial cells by all-trans retinoic acid through the phosphoinositide 3-kinase/Akt pathway. Circulation. 2005;112:727–736. doi: 10.1161/CIRCULATIONAHA.104.500959. [DOI] [PubMed] [Google Scholar]
- Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L209–L221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech. Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wanga K, Ferrara N, Vu TH. Vascular endothelial growth factor co-ordinates proper development of lung epithelium and vasculature. Mech. Dev. 2005;122:877–886. doi: 10.1016/j.mod.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Zimová M, Myslivecek J, Potmesil P. Retinoic acid attenuates the mild hyperoxic lung injury in newborn mice. Physiol. Res. 2007 doi: 10.33549/physiolres.930794. (Epub ahead of print. [DOI] [PubMed] [Google Scholar]