Abstract
The functional role of neutral lipids in the lung is poorly understood. Lysosomal acid lipase (LAL) is a critical enzyme in hydrolysis of cholesteryl esters and triglycerides to generate free fatty acids and cholesterol in lysosomes. Human LAL was over-expressed in a doxycycline-controlled system in mouse respiratory epithelial cells to accelerate intracellular neutral lipid degradation and perturb the surfactant homeostasis in the lung. In this animal system, neutral lipid concentrations of pulmonary surfactant were reduced in bronchoalveolar lavage fluid (BALF) in association with decrease of surfactant protein C (SP-C) gene expression. The size and the number of lamellar bodies in alveolar type II epithelial cells (AT II cells) were significantly reduced accordingly. The number of macrophages required for surfactant recycling in BALF was also significantly reduced. As a result of these combinatory effects, emphysema of the alveolar structure was observed. Taken together, neutral lipid homeostasis is essential for maintenance of lamellar body genesis and the alveolar structure in the lung.
Keywords: alveolar structure, emphysema, lysosomal acid lipase, neutral lipids, pulmonary surfactant, transgenic mice
The lung contains the conducting airway and the distal alveolar structure for gas exchange between the air and blood to supply oxygen and remove carbon dioxide. In the mature lung, the surface of alveolar wall is covered by squamous alveolar type I (AT I) epithelial cells and round secretory Alveolar type II epithelial cells. AT II epithelial cells contain a highly distinctive vesicular structure called tubular myelin to form lamellar bodies (Yan & Du 2004). Lamellar bodies function as lipid storage and secretory organelles in many tissues (Schmitz & Muller 1991). Lamellar bodies contain apolipoproteins and lytic enzymes and have an acidic pH, which confers on them a lysosomal character. Under normal physiological conditions, the main function of lamellar bodies is to supply extracellular domains with specialized lipid components related to a specialized function. In lung AT II cells, the lamellar bodies synthesize and store the pulmonary surfactant. When pulmonary surfactant is released from AT II cells through the secretory pathway, they spread out to form a membrane that covers the surface of the interalveolar walls. Pulmonary surfactant reduces the surface tension force at the air–water interface, facilitates expansion of alveoli and prevents collapse of alveoli during respiratory cycles. A deficiency in alveolar surfactant due to immaturity of AT II epithelial cells and lamellar body genesis causes respiratory distress syndrome (RDS) (Whitsett & Weaver 2002).
Pulmonary surfactant is a complex mixture of structurally heterogeneous lipoproteins, including phospholipids, neutral lipids and proteins. The packaging of pulmonary surfactant utilizes the secretory pathway beginning with the endoplasmic reticulum and ending in the lamellar body. The composition of pulmonary surfactant is around 90–95% lipids and 5–10% surfactant proteins. Neutral lipids account for 10% of the composition of lipids in pulmonary surfactant. Cholesteryl esters and triglycerides are important components in neutral lipids. Lysosomal acid lipase (LAL) hydrolyses both compounds in the lysosome of cells to generate free cholesterol and free fatty acids. In humans, LAL deficiency produces two phenotypes, Wolman’s disease and CE storage disease (Sando & Rosenbaum 1985). Triglyceride and cholesteryl ester accumulation leads to death or disability during early life or adolescence (Burton et al. 1984). Recently, we demonstrated that blockage of this pathway in the LAL knock-out (lal−/−) mice results in neutral lipid accumulation in AT II cells and macrophages, massive pulmonary inflammation and severe alveolar remodelling in the lung (Lian et al. 2004). But, the functional role of neutral lipids has not been established in lamellar body genesis and alveolarization in the lung. Among surfactant proteins, surfactant protein B and C (SP-B and -C) are small, uniquely hydrophobic proteins that play important roles in the stability and spreading of surfactant lipids in the alveolus (Whitsett & Weaver 2002). Deletion or mutations in SP-B and C cause acute and chronic lung disease in neonates and adults (Clark et al. 1995; Glasser et al. 2003). SP-A and D play dual roles in pulmonary homeostasis, determining the structure of alveolar lipids and mediating the innate host defence system of the lung (Whitsett 2005; Wright 2005). Surfactant proteins are synthesized and packed with surfactant lipids in lamellar bodies.
To further evaluate the functional role of neutral lipids in lamellar body genesis and alveolar formation, hLAL was over-expressed in a doxycycline-controlled system in mouse respiratory epithelial cells to accelerate the neutral lipid degradation and perturb the surfactant homeostasis in the lung. In this animal system, ‘activator’ transgenic mice bear the reverse tetracycline-responsive transactivator (rtTA) fusion protein that is under the control of the Clara cell secretory protein (CCSP) promoter. The rtTA expression is restricted to AT II epithelial cells in transgenic mice. In a separate transgenic mouse line, the hLAL cDNA is under the control of the tet operator DNA binding sequence linked to a minimal promoter. After cross-breading, expression of the hLAL cDNA is precisely controlled by addition or removal of doxycycline in double transgenic mice (Tichelaar et al. 2000; Yang et al. 2003; Lian et al. 2005a). Here, we report that over-expressed hLAL reduced concentrations of cholesteryl esters and triglycerides in pulmonary surfactant. The size and the number of lamellar bodies in AT II cells were significantly reduced accordingly. SP-C gene expression was reduced. The numbers of macrophages required for surfactant recycling were also significantly reduced in bronchoalveolar lavage fluid (BALF). As a result of these combinatorial effects, severe alveolar destruction was observed. Taken together, neutral lipid homeostasis is essential for maintenance of the alveolar structure and normal lung function.
Methods
Animal care
All scientific protocols involving the use of animals in this study have been approved by the Institution Animal Care and Usage Committee (IACUC) of Cincinnati Children’s Hospital and Indiana University School of Medicine, and followed the guidelines established by the Panel on Euthanasia of the American Veterinary Medical Association. Protocols involving the use of recombinant DNA or biohazardous materials were approved by the Institutional Biosafety Committee and followed the guidelines established by the NIH. Animals were housed under IACUC-approved conditions in a secured animal facility at Cincinnati Children’s Hospital Research Foundation and Indiana University School of Medicine. Animals were regularly screened for common respiratory pathogens and murine viral hepatitis. Experiments involving animal sacrifice utilized CO2 narcosis to minimize animal discomfort.
Generation of doxycycline-controlled CCSP/hLAL transgenic mice
To generate the (TetO)7-CMV-hLAL transgenic mouse line, hLAL cDNA was amplified by PCR using a downstream primer (5′-AAGGAAAAAGCGGCCGCTTATCACTTGTCATCGTCGTCCTTGTAGTCCTGATATTTCCTCATTAG-3′) and an upstream primer (5′-CTAGACGCGTGCCACCA TGAAAATGCGGTTCTTGG-3′). The PCR product was digested with Mlu I/Not I and subcloned into pTRE2 vector that contains the cytomegalovirus (CMV) minimal promoter linked to seven Tet-operon responsive elements (tetO)7 (CLONTECH, Mountain View, CA, USA). The expression cassette, containing the (tetO)7, CMV minimal promoter, the hLAL cDNA and the β-globin polyadenylation signalling sequence, was dissected out and purified for microinjection into eggs of FVB/N mice by the Transgenic Core Facility at the University of Cincinnati, College of Medicine. Founder lines were identified by a pair of primers corresponding to a pTRE plasmid sequence (5′-ACGCCATCCACGCTGTTTTG-3′) and a hLAL cDNA coding region sequence (5′–AGACAACTGGTTTGGGA CCTTTG-3′). CCSP-rtTA/(tetO)7-CMV-hLAL bitransgenic mice were generated from cross-breeding of the CCSP-rtTA transgenic mice and (tetO)7-CMV-hLAL transgenic mice. The CCSP-rtTA transgenic mice were genotyped with an upstream primer corresponding to the CCSP promoter sequence (5′-ACTGCCCATTGCCCAAACAC-3′) and a downstream primer corresponding to the rtTA code region sequence (5′-AAAATCTTGCCAGCTTTCCCC-3′). For doxycycline treatment, animals were fed doxycycline water (5 mg/ml) replaced twice a week. Expression of hLAL mRNA in the lung of hLAL bitransgenic mice were measured either by RT-PCR (primer sequences are listed below), or real-time PCR with an upstream primer (5′-AATGAAGTTAATG GAAGCTGGTAGGT-3′) and a downstream primer (5′-GATGAATTCTGGGCTTTCAGTTATG-3′). To ensure that endogenous mLAL mRNA was not affected by doxycycline treatment, real-time PCR with an upstream primer (5′-GGC GGA AGA ACC ATT TTG G-3′) and a downstream primer (5′-GCA AGC CGT GCT GAA GAT ACA-3′) was also performed.
Lung tissue processing and histology
Mice were anesthetized and lungs were inflation fixed with 4% paraformaldehyde in PBS overnight at 4 °C. Lungs were washed with PBS and dehydrated through a series of ethanol, followed by paraffin embedding. Five-micrometre tissue sections were loaded onto slides for staining with haematoxylin and eosin (H&E).
Morphometric analysis
Measurements were performed on sections taken throughout various lobes of the lungs. Images were transferred by video camera to a computer screen using the metamorph imaging software (Universal Imaging Corp., Downingtown, PA, USA). To measure the number and size of alveolar, a threshold was applied to the spaces of the alveoli. Large vessels, and smaller arterioles and venules were excluded from the study. The number and area were measured using the integrated morphometry analysis tool. The areas were calibrated to micrometres using a 10x calibration. Three lungs per group were used for the statistical analysis. All data were exported to Microsoft excel using the log data tool, and the number of alveolar regions was analysed using excel.
Electron microscopy
Approximately 1-mm3 pieces of minced lung tissues were fixed in 3.0% buffered glutaraldehyde for 2 h at 4 °C. The fixed tissues were then rinsed three times in 0.15 M sodium cacodylate buffer. The tissues were postfixed in 1% osmic acid (OsO4) in 0.15 M sodium cacodylate for 1 h followed by dehydration in 70% ethanol for 5 min, 95% ethanol for 5 min two times, 100% ethanol for 15 min three times and propylene oxide for 5 min two times. Tissue samples were embedded and sectioned by the Core Facility in the Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, Indianapolis. Representative electron micrographs of six AT II cells/animal (n = 5) were taken from each sample group by a person without knowing the identity of samples. The numbers of lamellar bodies in each individual AT II cell were counted. Spot Advance Software was used to determine the diameter (d) of lamellar bodies by measuring 10 different straight lines across a lamellar body. The mean value of straight lines was used as diameter. The section area of a lamellar body was calculated by formula π(d/2)2.
Bronchoalveolar lavage (BAL) cell purification
Bronchoalveolar lavage (BAL) fluids were collected by perfusing the lung with 1 ml of phosphate-buffered saline three times. BAL fluids were centrifuged for 5 min at 250 g in 4 °C to collect cell pellets. Cell pellets were resuspended in 0.5 ml of PBS. Cells in the same volume from different samples were spun down on glass slides by cytospin and cell numbers were determined by haemocytometry.
Western blot
Lungs were isolated and homogenized for protein extracts in RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate, and 1x PBS). Proteins were separated by electrophoresis on 10–20% gradient Tricine gels (Invitrogen, Novex, Carlsbad, CA, USA) in Tricine SDS running buffer and transferred to nitrocellular membrane. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline with Tween (TBST, 10 mM Tris, pH 8, 150 mM NaCl, 0.1% Tween 20) and incubated with purified rabbit IgG anti-hLAL antibody (1:1000), anti-SP-A antibody (Santa Cruz Bio. sc-7699, 1:1000), anti-SP-B antibody (Santa Cruz Bio. sc-7704, 1:1000), anti-SP-C antibody (Santa Cruz Bio. sc-13979, 1:1000), anti-CCSP antibody (Santa Cruz Bio. sc-25555, 1:1000), or anti-tubuline antibody (Santa Cruz Bio, sc-8035, 1:5000) followed by biotinylated secondary antibodies. Protein signals were visualized with a Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA).
Isolation of RNAs and reverse transcriptase-polymerase chain reaction
Whole lung tissues were homogenized in RLT lysis buffer for total RNA purification using the Qiagen total RNA purification kit as recommended by the manufacturer (QIAGEN Company, Maryland, USA). For semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) assays, 0.1–1 g of total RNAs was used to detect mRNA expression by the Super-Script One-Step RT-PCR Kit (Invitrogen). The RT reaction was performed at 45 °C for 30 min. PCR cycles (25–35) included 94 °C for 15 s, 55 °C for 30 s and 72 °C for 30 s. Primers for amplification of hLAL, SP-A, SP-B, SP-C, CCSP genes are listed below. The same reverse-transcribed cDNAs were used for PCR with a GAPDH primer pair as a control.
hLAL
Upstream primer: 5′-CAC ATT CTC CTG CTG GAA CTT CTG-3′
Downstream primer: 5′-CCT GAA AAC TTT GCC CCC TC-3′
SP-A
Upstream primer: 5′-AGTCCTCAGCTTGCAAGGATC-3′
Downstream primer: 5′-CGTTCTCCTCAGGATTCCTCG-3′
SP-B
Upstream primer: 5′-GGTCGCCGACAGGAGAATGGCTGC-3′
Downstream primer: 5′-AAGGTCGGGGCTGTGGATACACTG-3′
SP-C
Upstream primer: 5′-CATCGTTGTGTATGACTACCAGCG-3′
Downstream primer: 5′-GAATCGGACTCGGAACCAGTATC-3′
CCSP
Upstream primer: 5′-ATCACTGTGCTCATGCTGTCC-3′
Downstream primer: 5′-GCGTCGAATATCTCTGAAATC-3′
GAPDH
Upstream primer: 5′-CAGAAGACTGTGGATGGCCCC-3′
Downstream primer: 5′-GTCCACCACCCTGTTGCTGTAGCC-3′
Neutral lipid concentrations in BALF
Bronchoalveolar lavage fluids were collected by perfusing the lung with 1-ml aliquots of 0.9% sodium chloride and withdrawing back fluids for three times. BALFs were combined and centrifuged for 5 min at 250 g and 4 °C to remove cell pellets. After isolation of BALF from the lung, cholesteryl ester concentrations and triglyceride concentrations were measured as previously described (Rudel & Morris 1973; Du et al. 2001a,Du, 2001b). Briefly, total cholesterol concentrations (cholesteryl esters and free cholesterols) were determined by using Cholesterol/HP kit (Boehringer, GmbH, Indianapolis, IN, USA). Free cholesterol concentrations were determined by using COD-PAP kit (Wako Chemicals GmbH, Richmond, VA, USA). Cholesteryl ester concentrations were determined by subtraction of free cholesterol concentrations from total cholesterol concentrations. Triglyceride concentrations were determined by using Triglycerides/GB kit (Boehringer, GmbH).
Results
Generation of CCSP-rtTA/(tetO)7-CMV-hLAL bitransgenic mice
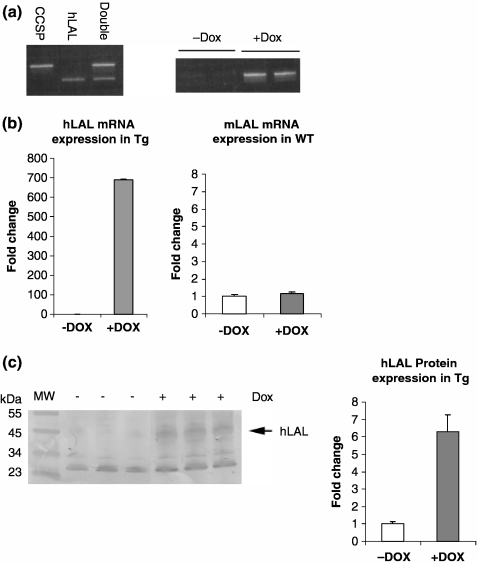
The expression cassette of the (tetO)7-CMV-hLAL construct was microinjected into eggs of FVB/N mice. Six positive founders were obtained by PCR using a pair of primers covering the hLAL cDNA and pTRE sequences. These founders are designated as (tetO)7-CMV-hLAL. Three founder lines were crossbred with CCSP-rtTA transgenic line (Tichelaar et al. 2000) to generate bitransgenic mice (Figure 1a). After treatment of CCSP-rtTA/(tetO)7-CMV-hLAL bitransgenic mice with doxycycline food for 1 month, the increased hLAL mRNA expression level of transgene was detected in the lung by RT-PCR (Figure 1a) or real-time PCR (Figure 1b). In bitransgenic mice without doxycycline treatment, no hLAL mRNA expression was detected. To assure the induction is not an effect of dietary change, expression of hLAL mRNA in the lung of wild-type mice was also studied. No hLAL mRNA expression was detected in doxycycline treated wild-type mouse lung (data not shown). In addition, the expression level of endogenous mLAL mRNA was not altered in hLAL bitransgenic lungs (Figure 1b). Therefore, the biological effects of this bitransgenic line were due to the induction of the hLAL transgene, not due to the endogenous mLAL gene. To assess if the hLAL protein is produced and secreted in the lung, hLAL bitransgenic mice were treated with or without doxycycline for 6 months. Proteins from lung parenchyma tissues were extracted and analysed by Western blot with anti-hLAL antibody. As demonstrated in Figure 1c, the hLAL protein level was induced significantly by densitometric measurements in the lungs of doxycycline-treated hLAL bitransgenic mice in comparison with doxycycline-untreated control mice.
Figure 1.
Generation of CCSP-rtTA/(tetO)7-CMV-hLAL transgenic mice. (a) PCR genotyping of CCSP-rtTA, (tetO)7-CMV-hLAL and CCSP-rtTA/(tetO)7-CMV-hLAL transgenic mice (left panel). Expression of hLAL mRNA in the lung of hLAL bitransgenic mice with (+Dox) or without (−Dox) doxycycline treatment (right panel). (b) Quantitative real-time PCR analysis of endogenous mLAL mRNA expression in the lung of wild-type (WT) and hLAL mRNA expression in the lung of hLAL bitransgenic (Tg) mice with (+Dox) or without (−Dox) doxycycline treatment. (c) Total proteins were isolated from doxycycline treated (+) or untreated (−) hLAL bitransgenic lungs and analysed by Western Blot for hLAL protein expression (left panel). The signal intensity of hLAL protein was analysed by densitometric measurements (right panel). The result from three independent mice was analysed by anova. The data are represented as the means ± SD (P < 0.05).
Reduction of total neutral lipid concentration in BALF of hLAL bitransgenic mice
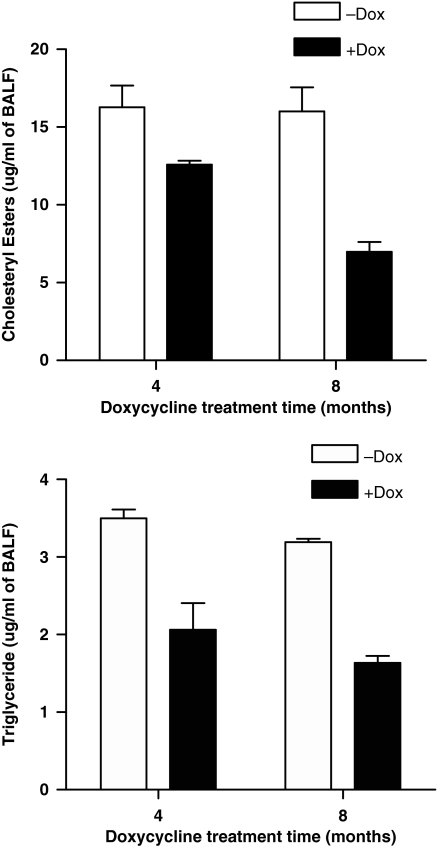
Induction of the hLAL protein in hLAL bitransgenic mice can accelerate degradation of neutral lipids in pulmonary surfactant homeostasis. BALF was collected from hLAL bitransgenic mice that were treated with or without doxycycline for 6 months. Concentrations of cholesteryl esters and triglycerides were measured in pulmonary surfactant collected from BALF samples. After over-expression of hLAL in doxycycline-treated hLAL bitransgenic mice, concentrations of cholesteryl esters and triglycerides were significantly lower in BALF (Figure 2), indicating a direct correlation between hLAL increase and neutral lipid decrease in the alveoli of hLAL bitransgenic lung.
Figure 2.
Reduction of neutral lipid concentration in hLAL bitransgenic mice. Concentrations of triglycerides and cholesteryl esters were measured in BALF of hLAL bitransgenic mice without (−Dox) and with (+Dox) treatment of doxycycline food for 4 and 8 months. Reduction of neutral lipid concentrations was observed in doxycycline-treated lungs. Differences between +Dox and −Dox samples were analysed by anova (P < 0.05). Values are mean ± SD (n = 5 per group).
Abnormal genesis of lamellar bodies in AT II cells of hLAL bitransgenic mice
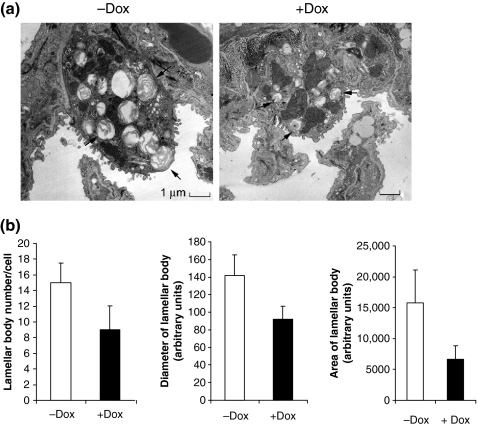
As surfactant is produced and stored primarily in lamellar bodies, we speculate that decrease of neutral lipids affects lamellar body genesis in AT II cells. After treatment of hLAL bitransgenic mice with or without doxycycline for 6 months, lung parenchyma were prepared and sectioned for an electron microscopy analysis. Over-expression of hLAL severely diminished lamellar body genesis with less surfactant content in AT II cells of doxycycline-treated hLAL bitransgenic mice (Figure 3a). By quantitative measurement, the number of lamellar bodies was significantly reduced in AT II cells of doxycycline-treated hLAL bitransgenic mice (Figure 3b). In addition, the size of lamellar bodies was significantly smaller in the same AT II cells. Therefore, the normal concentration of neutral lipids seems essential for lamellar body genesis. As illustrated in the same magnification in Figure 3a, the size of AT cells was also significantly smaller in doxycycline-treated hLAL bitransgenic mice.
Figure 3.
Abnormal formation of lamellar bodies in the lung of hLAL bitransgenic mice. (a) AT II cells were analysed by electron microscope. Lamellar bodies (pointed by arrows) were significantly reduced in the doxycycline-treated (+Dox) hLAL bitransgenic AT II cells vs. the not treated (−Dox) AT II cells. The scale bar is 1 μm. The original magnification was ×8900. (b) Quantitative analysis of lamellar body size and number in AT II cells. EM pictures of six AT II cells from doxycycline-treated (+Dox) or not treated (−Dox) hLAL bitransgenic mice (n = 5 per group) were measured for numbers, diameters and areas of lamellar bodies. Ten lamellar bodies were measured for each AT II cell. Significant reduction of lamellar body number and size were observed in doxycycline-treated lungs. Differences between +Dox and −Dox samples were analysed by anova (P < 0.05). Values are mean ± SD.
Disruption of the alveolar structure in hLAL bitransgenic mice
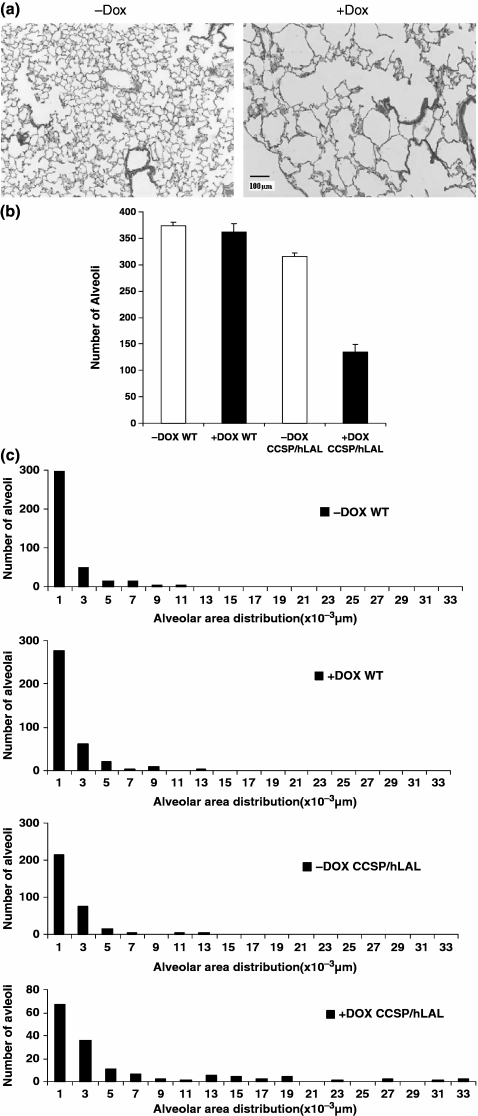
Surfactant synthesis and secretion is essential for maintenance of the alveolar structure in the lung. Decrease of lamellar body formation with less content of pulmonary surfactant in AT II cells can cause malformation of alveoli. To assess the impact of neutral lipid decrease on alveolar formation, lung sections were prepared from hLAL bitransgenic mice with or without doxycycline treatment. After 6 months’ treatment of hLAL bitransgenic mice with doxycycline, over-expression of hLAL resulted in emphysematous lungs (Figure 4a). Based on morphometric analysis, the total alveolar numbers were decreased (Figure 4b) and the size of alveoli were increased (Figure 4c) in hLAL bitransgenic lungs after doxycycline treatment. This observation is similar to RDS in clinical cases that are caused by deficiency in pulmonary surfactant and immaturity of AT II cells, and strongly indicates that the normal turnover of neutral lipids in pulmonary surfactant is required for normal alveolar formation and function. These changes obviously were not due to dietary change because doxycycline treatment had no effect on wild-type control animals (Figure 4b and c).
Figure 4.
Destruction of the alveolar structure in hLAL bitransgenic mice (a) H&E staining of the lungs from doxycycline-treated (+Dox) or not treated (−Dox) hLAL bitransgenic mice. Severe destruction was observed in the doxycycline-treated lungs. The original magnification was ×100. (b) Metamorphic analysis of alveolar numbers in doxycycline-treated (+Dox) or not treated (−Dox) wild-type (WT) or hLAL bitransgenic (CCSP/hLAL) mice (n = 3). The differences were analysed by anova and represented as the means ± SD (P < 0.05). (c) Metamorphic analysis of alveolar size distribution in doxycycline-treated (+Dox) or not treated (−Dox) wild-type (WT) or hLAL bitransgenic (CCSP/hLAL) mice.
Surfactant protein gene expression in hLAL bitransgenic mice
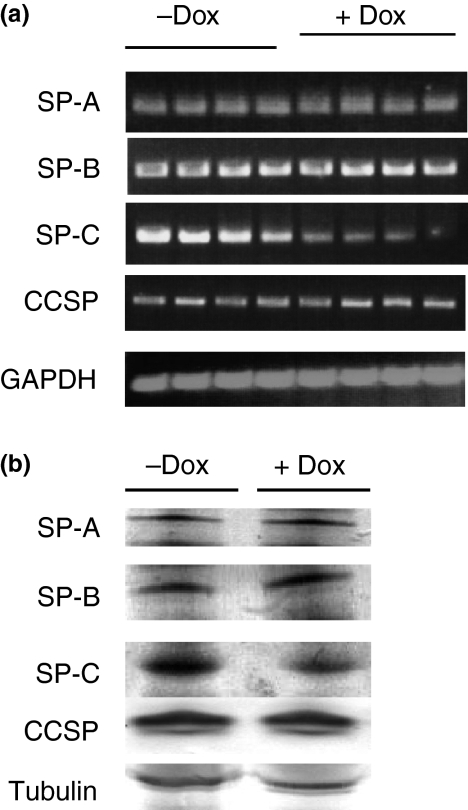
In addition to lipid components, surfactant proteins are essential for lamellar body genesis and surfactant homeostasis in the lung. As lamellar body was significantly reduced, it is important to determine which concentrations of surfactant proteins are reduced. To see how over-expression of hLAL affects surfactant protein expression, hLAL bitransgenic mice were treated with (+Dox) or without (−Dox) doxycycline for 6 months. Total RNAs were isolated from the lungs. Using specific primers, SP-A, SP-B, SP-C and CCSP mRNA expression were studied by RT-PCR analysis. While SP-A, SP-B and CCSP remained unchanged, over-expression of hLAL significantly reduced SP-C mRNA expression in doxycycline-treated hLAL bitransgenic mice (Figure 5a). As a control, GAPDH mRNA was unchanged in these samples. In a Western blot assay, only SP-C protein concentration was reduced in doxycycline-treated hLAL bitransgenic lung (Figure 5b). This is in agreement with the mRNA study. The tubuline control sample was unchanged.
Figure 5.
Surfactant protein expression in hLAL bitransgenic mice. (a) The mRNA samples (four per group) were isolated from doxycycline-treated (+Dox) or not treated (−Dox) hLAL transgenic lungs and analysed by RT-PCR for SP-A, SP-B, SP-C, CCSP and GAPDH gene expression. The SP-C mRNA expression was reduced in doxycycline-treated samples. (b) The protein samples were isolated from doxycycline-treated (+Dox) or not treated (−Dox) hLAL bitransgenic lungs and analysed by Western blot for SP-A, SP-B, SP-C, CCSP and tubuline gene expression. The SP-C protein expression was reduced in doxycycline-treated samples.
Macrophage reduction in hLAL bitransgenic mice
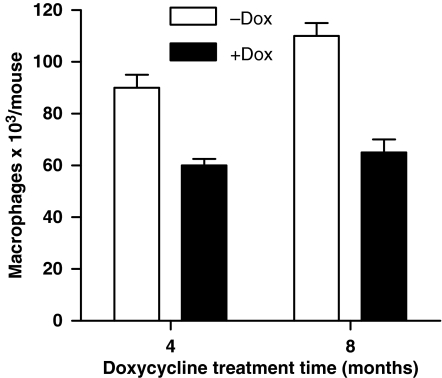
It has been well established that macrophages play an important role in surfactant recycling during alveolar homeostasis. Due to reduced lamellar body formation, which affects surfactant production, it is possible that fewer macrophages are required for less demanding of surfactant recycling. To test this assumption, macrophages were collected from BALF of hLAL bitransgenic mice that were treated with or without doxycycline for 4 and 8 months. Over-expression of hLAL in epithelial cells significantly reduced macrophage numbers by 33% in 4-month doxycycline-treated and by 41% in 8-month doxycycline-treated hLAL bitransgenic mice (Figure 6). Unlike LAL deficiency mice (Lian et al. 2004), no major inflammatory cell (e.g. neutrophils) infiltration was observed in the lung of doxycycline-treated hLAL bitransgenic mice by immunohistochemical staining (data not shown).
Figure 6.
Reduction of macrophages in bitransgenic mice. Macrophages were isolated from BALF of 4 months or 8 months doxycycline-treated (+Dox) or not treated (−Dox) hLAL transgenic lungs (n = 4) and counted after cytospin. The number of macrophages in BALF was significantly reduced in doxycycline-treated samples. Differences between +Dox and −Dox samples were analysed by anova (P < 0.05). Values are mean ± SD.
Discussion
Neutral lipids account for 10% components of pulmonary surfactant. Their roles in the lung are not very clear. To assess their function in alveolar homeostasis, hLAL was overly expressed in CCSP-rtTA/(tetO)7-CMV-hLAL conditional transgenic mice to accelerate the degradation process of neutral lipid metabolism. Over-expression of hLAL in this animal model reduced the concentrations of cholesteryl esters and triglycerides in BALF. As a consequence, pathological assessment revealed alveolar destruction in the lung. This can be caused by multiple factors. First, as neutral lipids account for 10% components of pulmonary surfactant, lowering concentrations of neutral lipids altered the composition of pulmonary surfactant in hLAL bitransgenic mice. This change may weaken the ability of the surfactant membrane to support the alveolar structure. During the respiratory cycles, the weakened surfactant membrane was unable to prevent the collapse of alveoli. Second, surfactant proteins are required for maintaining surfactant homeostasis to sustain the normal function of alveoli. They facilitate lipid membrane spreading along the alveolar wall during expansion of alveoli. Reduced SP-C gene expression by hLAL over-expression in hLAL bitransgenic mice may weaken the ability of surfactant spreading and contribute to the collapse of alveoli during respiratory cycles. SP-C is the most hydrophobic protein in the proteome and consists of a 35-amino-acid polypeptide rich in valine, leucine and isoleucine. SP-C precursor protein is routed to multivesicular bodies, where both are processed and packaged into lamellar bodies for secretion into the air space along with lipids (Whitsett & Weaver 2002). SP-C accounts for approximately 4% of surfactant by weight and is selectively expressed in AT II cells in the alveoli of the lung. SP-C also enhances the reuptake of surfactant phospholipids and plays a role in surfactant catabolism. In SP-C-deficient mice, severe emphysema was observed (Glasser et al. 2003). Decreased SP-C expression in the hLAL bitransgenic lung is a secondary effect due to accelerated neutral lipid degradation. As neutral lipid derivatives control gene regulation by serving as hormonal ligands for nuclear receptors, it is possible that SP-C gene regulation is under the control of unknown nuclear receptors. This needs to be determined in future. Third, lamellar bodies are the storage and secretory organelle for surfactant synthesis in AT II cells. It seems that hLAL over-expression significantly reduced lamellar body biogenesis. In the hLAL over-expression lung, the lamellar body number was fewer and the lamellar body size was smaller. This implicates the reduced synthesis and storage of surfactant in AT II cells. Insufficient amount of surfactant production and secretion can lead to the collapse of alveolar structure during respiratory cycles. As the lamellar bodies are known for recycling of degraded surfactant debris in the lung, the reduced number and size of lamellar body may also reflect the reduced ability of surfactant clearance.
There was no major pulmonary inflammation observed in hLAL bitransgenic mice. The numbers of macrophages were significantly reduced in BALF. As surfactant synthesis and secretion were decreased due to reduced lamellar body genesis, the demand for surfactant clearance was also reduced. Macrophages are major cells to clear degraded surfactant debris. It is not surprising that the macrophage number was decreased for less recycling demand in hLAL over-expressing bitransgenic mice. In an opposite observation, the macrophage number was significantly increased in LAL deficient mice (Lian et al. 2004). Therefore, neutral lipid concentrations contribute to macrophage recruiting in the lung to maintain alveolar homeostasis and function.
Taken together, normal concentrations of neutral lipids are essential for surfactant homeostasis, surfactant protein gene expression, lamellar body genesis and macrophage recruitment to maintain normal pulmonary function. Disturbing of neutral lipid metabolism by over-expression of LAL enzymatic activity unbalances the alveolar homeostasis and leads to disruption of the alveolar structure. As we reported previously, neutral lipids play multiple roles in the lung. In contrast to the hLAL over-expression in CCSP-rtTA/(tetO)7-CMV-hLAL bitransgenic mice, pulmonary inflammation and tissue remodelling were observed during LAL deficiency in the lal−/− animal model (Lian et al. 2004, 2005b; Yan et al. 2006), indicating that neutral lipids also play an essential role in balancing pro- and anti-inflammatory responses, cell proliferation and tissue remodelling in the lung. In the lal−/− animal system, Oil red-O staining demonstrated massive neutral lipid accumulation in AT II cells and macrophages. The pathophysiologic phenotypes resembled the so-called ‘smoking model’ in the lung. The phenotypes in lal−/− mice include massive neutrophil influx, foamy macrophage accumulation, unwanted tissue remodelling and Clara cell hypertrophy and hyperplasia. Affymetrix GeneChip microarray analysis identified unique molecular pathways that are responsible for these phenotypes (Lian et al. 2005b). The changed genes can be categorized into inflammatory cytokines/chemokines, endopeptidases (e.g. matrix metalloproteinases or MMPs), apoptosis inhibitors and oncogenes. These gene profile changes correlated well with pulmonary pathogenic progression in the lal−/− lung in an age-dependent fashion. In a combinatorial manner, up- and down-regulation of these molecules regulates pathogenic progression in the lal−/− lung.
Lysosomal acid lipase downstream free fatty acid derivative compounds serve as ligands for nuclear receptors such as peroxisome proliferator-activated receptor gamma (PPARγ). To prove that depletion of free fatty acid metabolites initiates pulmonary inflammation and pathogenesis in the lal−/− lung, LAL downstream hormonal ligands for PPARγ significantly improved pathogenesis of the lal−/− lung (Lian et al. 2005b). Abnormal neutrophil infiltration and macrophage accumulation were significantly reduced. Aberrant over-expression of characteristic marker genes was inhibited. PPARγ and ligands inhibited transcriptional expression of several characteristic marker genes in respiratory epithelial cells. Previous studies and the results outlined in this report support a concept that neutral lipid metabolism has a great impact on lung homeostasis.
Acknowledgments
C. Yan and H. Du were supported by National Institute of Health Grants HL-061803 and HL-067862. We thank Ms Marjorie E. Albrecht and Nathan Furr for technical assistance in electron microscopy analysis and in proofreading the manuscript.
Conflict of interest statement
There is no conflict of interest to declare.
References
- Burton BK, Remy WT, Rayman L. Cholesterol ester and triglyceride metabolism in intact fibroblasts from patients with Wolman’s disease and cholesterol ester storage disease. Pediatr. Res. 1984;18:1242–1245. doi: 10.1203/00006450-198412000-00003. [DOI] [PubMed] [Google Scholar]
- Clark JC, Wert SE, Bachurski CJ, et al. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc. Natl Acad. Sci. U.S.A. 1995;92:7794–7798. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Heur M, Duanmu M, et al. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J. Lipid Res. 2001a;42:489–500. [PubMed] [Google Scholar]
- Du H, Schiavi S, Levine M, Mishra J, Heur M, Grabowski G A. Enzyme therapy for lysosomal acid lipase deficiency in the mouse. Hum. Mol. Genet. 2001b;10:1639–1648. doi: 10.1093/hmg/10.16.1639. [DOI] [PubMed] [Google Scholar]
- Glasser SW, Detmer EA, Ikegami M, Na CL, Stahlman M T, Whitsett J A. Pneumonitis and emphysema in sp-C gene targeted mice. J. Biol. Chem. 2003;278:14291–14298. doi: 10.1074/jbc.M210909200. [DOI] [PubMed] [Google Scholar]
- Lian X, Yan C, Yang L, Xu Y, Du H. Lysosomal acid lipase deficiency causes respiratory inflammation and destruction in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:L801–807. doi: 10.1152/ajplung.00335.2003. [DOI] [PubMed] [Google Scholar]
- Lian X, Qin Y, Hossain SA, et al. Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J. Immunol. 2005a;174:7250–7256. doi: 10.4049/jimmunol.174.11.7250. [DOI] [PubMed] [Google Scholar]
- Lian X, Yan C, Qin Y, Knox L, Li T, Du H. Neutral lipids and peroxisome proliferator-activated receptor-{gamma} control pulmonary gene expression and inflammation-triggered pathogenesis in lysosomal acid lipase knockout mice. Am. J. Pathol. 2005b;167:813–821. doi: 10.1016/s0002-9440(10)62053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel L L, Morris M D. Determination of cholesterol using O-phthalaldehyde. J lipid Res. 1973;14:164–366. [PubMed] [Google Scholar]
- Sando G N, Rosenbaum L M. Human lysosomal acid lipase/cholesteryl ester hydrolase. Purification and properties of the form secreted by fibroblasts in microcarrier culture. J. Biol. Chem. 1985;260:15186–15193. [PubMed] [Google Scholar]
- Schmitz G, Muller G. Structure and function of lamellar bodies, lipid–protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 1991;32:1539–1570. [PubMed] [Google Scholar]
- Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- Whitsett JA. Surfactant proteins in innate host defense of the lung. Biol. Neonate. 2005;88:175–180. doi: 10.1159/000087580. [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N. Engl. J. Med. 2002;347:2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- Wright JR. Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- Yan C, Du H. Alveolus formation: what have we learned from genetic studies? J. Appl. Physiol. 2004;97:1543–1548. doi: 10.1152/japplphysiol.00286.2004. [DOI] [PubMed] [Google Scholar]
- Yan C, Lian X, Li Y, et al. Macrophage-specific expression of human lysosomal acid lipase corrects inflammation and pathogenic phenotypes in lal−/− mice. Am. J. Pathol. 2006;169:916–926. doi: 10.2353/ajpath.2006.051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Naltner A, Yan C. Overexpression of dominant negative retinoic cid receptor alpha causes alveolar abnormality in transgenic neonatal lungs. Endocrinology. 2003;144:3004–3011. doi: 10.1210/en.2002-0121. [DOI] [PubMed] [Google Scholar]