Abstract
Endogenous PGE2 dynamically regulates membrane excitability, synaptic transmission and plasticity. Neonatal seizures are associated with a number of activity-dependent changes in brain development including altered synaptogenesis and synaptic plasticity as well as reduction in neurogenesis. Thus, it is reasonable to hypothesize that alteration of cyclooxygenase-2 (COX-2) expression induced by neonatal seizure may influence brain development. We evaluated the expression of COX-2 and microsomal prostaglandin E synthase (mPGES) by Western blot analysis and immnohistochemistry in flurothyl-induced neonatal seizure and also studied the effect of celecoxib on seizure induction. Seven to 10 days old Sprague–Dawley rats were used for control (n = 18) and experimental group (n = 30). Recurrent seizure group showed more increased level of COX-2 expression than control group. However, the level of mPGES-2 expression was similar in both groups, and mPGES-1 was not detected. Hippocampus of control rats showed endogenous COX-2 expression, which was localized mainly in CA3 region. This localization pattern was similar in recurrent seizure rats, but intensity of COX-2 expression was more increased than in control rats. Celecoxib treatment significantly delayed the seizure attack and also reduced COX-2 expression. In conclusion, this study suggests that COX-2 expression is related to epileptogenesis in flurothyl-induced neonatal seizure model and shows the possibility that its inhibition lessens functional impairments that occurred in neonatal seizure.
Keywords: celecoxib, COX-2, immature brain, seizure
Cyclooxygenase (COX) is a rate-limiting enzyme, which catalyses the metabolism of arachidonic acid to prostaglandins (PGs). Two isoforms of COX-1 and COX-2 have been characterized in mammalian and avian species. COX-1 is constitutively expressed in most tissues to maintain stable physiological conditions, whereas COX-2 is transiently induced by proinflammatory cytokines and growth factors and involved in inflammation (Hershman 1996). However, basal levels of COX-2 expression have been observed in neurones in the hippocampus as well as in other brain regions (Kaufmann et al. 1996). Prostaglandin E synthase (PGES), which is the terminal isomerase involved in the synthesis of PGE2, occurs in multiple forms in mammalian cells (Murakami et al. 2002).
Several studies have suggested that COX-2 and PGs may play a role in epilepsy. For example, COX-2 expression is induced in hippocampus after kindling (Tu & Bazan 2003), in the genetically susceptible El mice (Okada et al. 2001) and in kainate-induced seizures (Chen et al. 1995; Hirst et al. 1999). Transgenic mice overexpressing neuronal COX-2 are more susceptible to kainic acid excitotoxicity (Kelley et al. 1999). However, little is known about the relationship between COX-2 expression and seizure in immature or developing brain. Neonatal seizures are associated with a number of activity-dependent changes in brain development including altered synaptogenesis and synaptic plasticity as well as reduction in neurogenesis (Holmes 1994). Therefore, impairment of learning and memory as well as a lower-seizure threshold is frequently observed in adult period (Holmes et al. 1998). A recent study shows that endogenous PGE2 dynamically regulates membrane excitability, synaptic transmission and plasticity and that the PGE2-induced synaptic modulation is mediated via cAMP-PKA and PKC pathways in rat hippocampal pyramidal neurones (Chen & Bazan 2005). Thus, it is reasonable to hypothesize that alteration of COX-2 expression induced by neonatal seizure may influence brain development. In this work, we evaluated expression of COX-2 and microsomal PGES (mPGES) in flurothyl-induced neonatal seizure and also studied the effect of celecoxib on seizure induction.
Rats and methods
Induction of seizures and preadministraion of celecoxib in neonatal rats
The ethics committee supervising the animal studies at the Dongguk University College of Medicine approved the experimental design. Seven to 10 days old Sprague–Dawley rats (Kist, Taejun, Korea) were used for control (n = 18) and experimental group (n = 30). Table 1 summarizes the detailed number of rats for experiment. Throughout the experiment, all rats were housed in a controlled environment with 12-h light/dark cycle and a temperature of 22 ± 2 °C. Seizures were induced in the neonatal rats with inhalant flurothyl (Sigma Chemical Co., St Louis, MO, USA). The experimental rats were put into a transparent plastic airtight box (40 × 20 × 20 cm), and then a dose of 0.1-ml flurothyl was administered through a hole in the box. The rats inhaled flurothyl continuously for 10 min. The rats showed signs of the seizure attacks, which were characterized by clonic movements of the four limbs and the loss of posture. To evaluate the effect of celecoxib on onset time of seizure, we divided seizure rats into three groups: five seizure control rats treated with only solvent treatment, five seizure rats treated with 10 mg/kg of celecoxib and five rats treated with 20 mg/kg of celecoxib. Celecoxib was dissolved in dimethyl sulfoxide and given to experimental rats by gavage feeding at 1 h before flurothyl administration. We measured the time of seizure onset in each experimental group.
Table 1.
The detailed number of rats for experiment
| Number of rats | ||
|---|---|---|
| Experiment | Control (18 rats) | Seizure (30 rats) |
| Seizure, 25 times | 6 | 6 |
| COX-2 expression related to age | 12 | |
| Seizure frequency | 9 | |
| Effect of celecoxib | 15 | |
COX-2, cyclooxygenase-2.
Immunohistochemistry and apoptotic staining
The animals were sampled by decapitation. The brains were removed immediately and were fixed in 10% neutral buffered formalin. Fixed–fixed tissues were embedded in paraffin. Tissue sections (4 µm) were prepared and spread on poly-L-lysine-coated slides. Paraffin sections were immersed in three changes of xylen and hydrated using a graded series of alcohol. Antigen retrieval was performed routinely by immersing the sections in 0.01 m citrate buffer (pH 6.0) in a pressure cooker by autoclaving for 15 min. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 15 min and then incubated with anti-COX-2 (1:500) (Cayman Chemical, Ann Arbor, MI, USA) for 2 h at room temperature. Staining was achieved using a DAKO LSAB + kit labelled with peroxidase (Dako Cytomation, Carpinteria, CA, USA) and developed with 3, 3′-diaminobenzidine tetrahydrochloride as a chromogen. Sections were counterstained for 3 min with Meyer's haematoxylin and then mounted. As a negative control, nonimmune rabbit IgG isotype was used instead of primary antibody. As a positive control, we used stomach cancer tissue expressing COX-2 (Jang 2004). We selected 50 neurones showing clearly brown colour at perinuclear area in five high-power fields and measured COX-2 intensity by assessing the optical density with Scion Image (beta 4.0.2). To assess apoptotic cells, we performed terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labelling (TUNEL). Briefly, the deparaffinized slides were treated with proteinase K for 15 min, and endogenous peroxidase activity was blocked by 5 min of incubation in 3% H2O2 in methanol. Cleaved DNA fragments were labelled with bromodeoxyuridine at the 3′ ends using terminal deoxynucleotidyl transferase and detected with biotinylated antibromodeoxyuridine antibody and streptavidin – horseradish peroxidase. 3, 3′-Diaminobenzidine tetrahydrochloride was used as a chromogen.
Western blot analysis
Hippocampus was washed with cold PBS and suspended in a lysis buffer [10 mm Tris-HCl (pH 7.4), 1 mm EDTA and 0.25 m sucrose, 1% Triton X-100] supplemented with complete mini protease inhibitor mixture tablets (Boehringer Mannheim, Mannheim, Germany) on ice for 1 h. After removing cell debris by centrifugation, cell lysate protein concentrations were determined using BCA protein assay reagent (Pierce, Rockford, IL, USA) and bovine serum albumin as a standard. Forty micrograms of protein were separated in 12% SDS polyacrylamide gel and transferred to a nitrocellulose membranes. Membranes were blocked with 5% skimmed milk in Tris-buffered saline (TBS) for 1 h at room temperature and probed with primary antibodies [mPGES-1 (1:1000, Cayman Chemical) and antimPGES-2 (1:1000, Cayman Chemical), COX-2 (1:750, Cayman Chemical), caspase-3 (1:1000, Upstate, Charlottesville, VA, USA) and β-actin (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA)] overnight at 4 °C. Anti-caspase-3 antibody detects pro- and cleaved product, and we confirmed them in gastric cancer cells with apoptosis (Jang et al. 2004). After washing with TBS 0.05% Tween 20, the blots were treated with horseradish peroxidase-conjugated anti-rabbit IgG antibody (1:3000; Zymed Laboratories, San Francisco, CA, USA) for 1 h at room temperature. Detection was performed by enhanced chemiluminescence (Pierce) and autoradiography. We examined optical density compared with β-actin by using Scion Image.
Statistical analysis
Kruskal–Wallis and Mann–Whitney U tests were used. Statistical significance was assumed if a P value was less than 0.05. Data were expressed as mean ± standard deviation.
Results
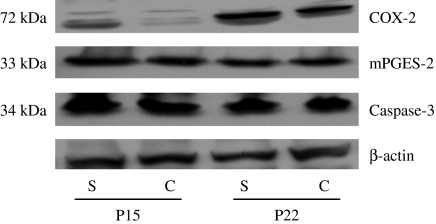
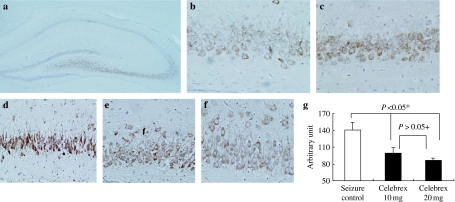
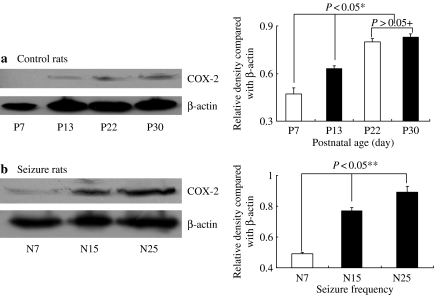
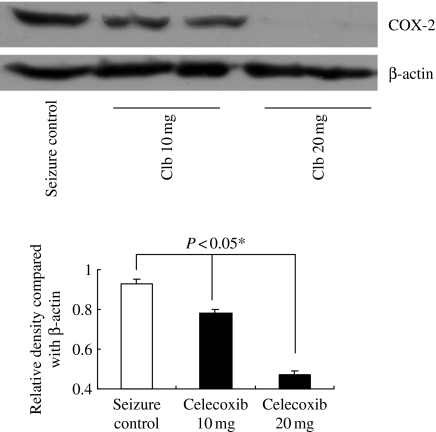
We induced seizure 25 times (5 times a day for consecutive days) in 10 days old rats (n = 6) and sacrificed them at postnatal day 15 (P15) and P22. We examined the expression of COX-2 and mPGES in hippocampus (Figure 1). Recurrent seizure group showed more increased level of COX-2 expression than control group at P15 and P22. However, the level of mPGES-2 expression was similar in both groups, and mPGES-1 was not detected (data not shown). We evaluated histology and the localization of COX-2 in hippocampus (Figure 2). No significant pathologic findings were found in recurrent seizure rats, and apoptotic cells were also rarely observed in both groups (data not shown). Active subunits of caspase-3 were not found in both groups (Figure 1). Hippocampus of control rats showed endogenous COX-2 expression, which was localized mainly in CA3 region. This localization pattern was similar in recurrent seizure rats, but intensity of COX-2 expression was more increased than in control rats. As shown in Figure 1, COX-2 expression was greater in the older animals than in younger ones; hence, we examined the relation between COX-2 expression and age in 12 control rats. COX-2 expression level significantly increased with age (P < 0.05), was highly expressed at P30 and negligible at P7 (Figure 3a). We evaluated the relationship between seizure frequency and COX-2 expression in nine rats. COX-2 expression was also found to be seizure frequency dependent (Figure 3B) (P < 0.05). Thereafter, we evaluated the effect of celecoxib on onset time of seizure and COX-2 expression. The onset time was 126 ± 37 s in five rats treated with only solvent, 144 ± 39 s in five rats treated with 10 mg/kg of celecoxib, 176 ± 42 s in five rats treated with 20 mg/kg of celecoxib (P < 0.05 vs. seizure control rats and rats with 10 mg/kg of celecoxib, respectively). Therefore, celecoxib treatment significantly delayed the seizure attack. COX-2 expression was markedly decreased in rats treated with 20 mg/kg of celecoxib compared with other groups (Figure 4) (P < 0.05). As shown in Figure 2, intensity of COX-2 expression was highest in CA3 region of rats treated with dimethyl sulfoxide and decreased in proportion to dose of celecoxib.
Figure 1.
Western blot analysis of cyclooxygenase-2 (COX-2), microsomal prostaglandin E synthase (mPGES)-2 and caspase-3 in both control and seizure rats at postnatal day 15 and 22. Forty microgram of protein was separated by 10% SDS polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The bottom represents β-actin, which was used as a loading control. C, control rats; P, postnatal day; S, seizure rats.
Figure 2.
Immunohistochemical staining of cyclooxygenase-2 (COX-2) in the hippocampus of control (a, b) and seizure rats (c–f) (interaural 5.70 mm). (a) Hippocampus of control rats showed endogenous COX-2 expression, which was localized mainly in CA3 region. (b) Higher magnification of CA3 area in control rats revealed immunoreactivity of COX-2 in perinuclear area of neurone. (c) CA3 area of seizure rats displayed higher COX-2 expression than that of control rats (b). Intensity of COX-2 expression was the highest in CA3 region of rat treated with dimethyl sulfoxide (d) and decreased in proportion to dose of celecoxib (e, 10 mg/kg; f, 20 mg/kg). (g) Quantification of COX-2 expression intensity in d–f. *Significantly different from seizure control vs. celecoxib 10 mg and celecoxib 20 mg (P < 0.05). +Not significantly different from celecoxib 10 mg and celecoxib 20 mg (P > 0.05).
Figure 3.
Effect of age and seizure frequency on cyclooxygenase-2 (COX-2) expression in both control and seizure rats. (a) Western blot analysis of COX-2 in control rats according to age. *Significantly different from P7, P15 and both P22 and P30 (P < 0.05).+No significantly different from P22 and P30 (P > 0.05). (b) Western blot analysis of COX-2 in seizure rats according to seizure frequency. ** Significantly different from N7, N15 and N22 (P < 0.05). Forty microgram of protein was separated by 10% SDS polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The bottom represents β-actin, which was used as a loading control. N, number of seizure; P, postnatal day.
Figure 4.
Western blot analysis of cyclooxygenase-2 (COX-2) in seizure rats according to celecoxib treatment. *Significantly different from seizure control, celecoxib 10 mg and celecoxib 20 mg (P < 0.05). Forty microgram of protein was separated by 10% SDS polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The bottom represents β-actin, which was used as a loading control. Clb, celecoxib.
Discussion
In the normal brain, COX-2 expression is observed in neurones and may be associated with the N-methyl-D-aspartate (NMDA) receptor-related excitability and increases in several animal seizure models (Adams et al. 1996). Several studies demonstrated that COX-2 inhibitor reduced the severity of seizure and protected brain from irreversible damage induced by kainate in rats (Baran et al. 1994; Paoletti et al. 1998; Kunz & Oliw 2001). These studies support our results, which show that COX-2 expression increases in flurothyl-induced neonatal seizure model and celecoxib treatment delays seizure onset by reducing COX-2 expression. These findings suggest that increased COX-2 expression may be related to neonatal epileptogenesis. However, several studies have reported that COX-2 inhibitor promotes seizure activity (Forstermann et al. 1982; Baik et al. 1999). It is necessary to further explore the exact roles of each PG and COX isozymes on seizure activity.
A recent study reported that mPGES-1 expression level was negligible in adult rat hippocampus but induced in hippocampus in kainite-induced seizure (Ciceri et al. 2002). mPGES-1 was also induced in brain endothelial cells of adult rats during endotoxin-induced fever (Yamagata et al. 2001). In our work, however, mPGES-1 was not detected. We cannot exclude the possibility that immature hippocampus does not express endogenous mPGES-1, which is not also induced by neonatal seizure model using flurothyl. mPGES-2 is functionally coupled with both COX-1 and COX-2 and was expressed in cells and tissues relatively constitutively (Murakami et al. 2003). PGE2 mediates its effects, in part, through G protein-coupled PGE receptors, designated EP1, EP2, EP3 and EP4 (Ashby 1998). In mouse brain, EP3 mRNA is widely distributed in neurones of the cortex, hippocampus, thalamus, hypothalamus, midbrain and lower brain stem (Ushikubi et al. 1998) In our work, mPGES-2 expressed similarly in both seizure and control rats. We suggest that increased PGE2 via COX-2 and mPGES-2 may be involved in neonatal epileptogenesis.
The developing brain differs from the adult brain in its susceptibility to seizures, seizure characteristics and response to antiepileptic drug (Holmes 1994). In contrast to developed brain, developing brain is resistant to acute seizure-induced cell death, but there are functional abnormalities following seizures, resulting in impairment of visual spatial memory and reduced seizure threshold (Holmes et al. 1998). It is known that these abnormalities are related to synaptic modification. Recently, Chen et al. (2002) have demonstrated that COX-2, which is expressed in postsynaptic dendritic spines, regulates PGE2 signalling in activity-dependent long-term synaptic plasticity at hippocampus. PGE2-induced synaptic modulation is mediated via cAMP-PKA and PKC pathways in rat hippocampal pyramidal neurones (Chen & Bazan 2005). We found that celecoxib delayed seizure attack and reduced COX-2 expression. This result suggests that COX-2 inhibitor may mitigate functional abnormalities of neurones by modulating synaptic plasticity.
In conclusion, this study suggests that COX-2 expression is related to epileptogenesis in flurothyl-induced neonatal seizure model and shows the possibility that its inhibition lessens functional impairments that occurred in neonatal seizure. Further study is necessary to demonstrate the possibility.
Acknowledgments
This work was supported by the research fund of Dongguk University.
References
- Adams J, Collaco-Moraes Y, de Belleroche J. Cyclooxygenase-2 induction in cerebral cortex: an intracellular response to synaptic excitation. J. Neurochem. 1996;66:6–13. doi: 10.1046/j.1471-4159.1996.66010006.x. [DOI] [PubMed] [Google Scholar]
- Ashby B. Co-expression of prostaglandin receptors with opposite effects: a model for homeostatic control of autocrine and paracrine signaling. Biochem. Pharmacol. 1998;55:239–246. doi: 10.1016/s0006-2952(97)00241-4. [DOI] [PubMed] [Google Scholar]
- Baik EJ, Kim EJ, Lee SH, Moon C. Cyclooxygenase-2 selective inhibitors aggravate kainic acid induced seizure and neuronal cell death in the hippocampus. Brain Res. 1999;843:118–129. doi: 10.1016/s0006-8993(99)01797-7. [DOI] [PubMed] [Google Scholar]
- Baran H, Vass K, Lassmann H, Hornykiewicz O. The cyclooxygenase and lipoxygenase inhibitor BW755C protects rats against kainic acid-induced seizures and neurotoxicity. Brain Res. 1994;646:201–206. doi: 10.1016/0006-8993(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Chen C, Bazan NG. Endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 2005;93:929–941. doi: 10.1152/jn.00696.2004. [DOI] [PubMed] [Google Scholar]
- Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J. Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- Chen J, Marsh T, Zhang JS, Graham SH. Expression of cyclooxygenase 2 in rat brain following kainate treatment. Neuroreport. 1995;6:245–248. [PubMed] [Google Scholar]
- Ciceri P, Zhang Y, Shaffer AF, et al. Pharmacology of celecoxib in rat brain after kainate administration. J. Pharmacol. Exp. Ther. 2002;302:846–852. doi: 10.1124/jpet.302.3.846. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Heldt R, Knappen F, Hertting G. Potential anticonvulsive properties of endogenous prostaglandins formed in mouse brain. Brain Res. 1982;240:303–310. doi: 10.1016/0006-8993(82)90225-6. [DOI] [PubMed] [Google Scholar]
- Hershman HR. Prostaglandin synthase 2. Biochim. Biophys. Acta. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Young KA, Newton R, Allport VC, Marriott DR, Wilkin GP. Expression of COX-2 by normal and reactive astrocytes in the adult rat central nervous system. Mol. Cell. Neurosci. 1999;13:57–68. doi: 10.1006/mcne.1998.0731. [DOI] [PubMed] [Google Scholar]
- Holmes GL. Neonatal seizures. Semin. Pediatr. Neurol. 1994;1:72–82. [PubMed] [Google Scholar]
- Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann. Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Jang TJ. Expression of proteins related to prostaglandin E2 biosynthesis is increased in human gastric cancer and during gastric carcinogenesis. Virchows Arch. 2004;445:564–571. doi: 10.1007/s00428-004-1104-3. [DOI] [PubMed] [Google Scholar]
- Jang TJ, Kang HJ, Kim JR, Yang CH. Non-steroidal anti-inflammatory drug activated gene (NAG-1) expression is closely related to death receptor-4 and -5 induction, which may explain sulindac sulfide induced gastric cancer cell apoptosis. Carcinogenesis. 2004;25:1853–1858. doi: 10.1093/carcin/bgh199. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc. Natl. Acad. Sci. USA. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KA, Ho L, Winger D, et al. Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am. J. Pathol. 1999;155:995–1004. doi: 10.1016/S0002-9440(10)65199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainite-induced cell death in the rat hippocampus. Eur. J. Neurosci. 2001;13:569–575. doi: 10.1046/j.1460-9568.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nakashima K, Kamei D, et al. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenase-1 and-2. J. Biol. Chem. 2003;278:37937–37947. doi: 10.1074/jbc.M305108200. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nakatani Y, Tanioka T, Kudo I. Prostaglandin E synthase. Prostaglandins Other Lipid Mediat. 2002;68–69:383–399. doi: 10.1016/s0090-6980(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Okada K, Yuhi T, Tsuji S, Yamashita U. Cyclooxygenase-2 expression in the hippocampus of genetically epilepsy susceptible El mice was increased after seizure. Brain Res. 2001;894:332–335. doi: 10.1016/s0006-8993(01)02019-4. [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Piccirilli S, Costa N, Rotiroti D, Bagetta G, Nistico G. Systemic administration of N omega-nitro-L-arginine methyl ester and indomethacin reduces the elevation of brain PGE2 content and prevents seizures and hippocampal damage evoked by LiCl and tacrine in rat. Exp. Neurol. 1998;149:349–355. doi: 10.1006/exnr.1997.6741. [DOI] [PubMed] [Google Scholar]
- Tu B, Bazan NG. Hippocampal kindling epileptogenesis upregulates neuronal cyclooxygenase-2 expression in neocortex. Exp. Neurol. 2003;179:167–175. doi: 10.1016/s0014-4886(02)00019-5. [DOI] [PubMed] [Google Scholar]
- Ushikubi F, Segi E, Sugimoto Y, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Matsumura K, Inoue W, et al. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J. Neurosci. 2001;21:2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]