Abstract
The atrophic effect of ligating the main duct of the right submandibular gland was examined in rat using a novel intraoral approach that did not include the chorda lingual (CL) nerve. Comparison was made with the effect of duct ligation including the attached CL nerve as carried out in previous studies. In all animals, the contralateral, unligated left submandibular gland was used as a control. At different times (1, 2, 7, 14 and 21 days) after ligation, glands were removed and weighed. Tissue was fixed for morphological analysis and homogenized for biochemical assay of secretory proteins. After 21 days, ligated glands showed a significant decrease in wet weight compared with unligated glands. Weight loss was the greatest (P < 0.05) in glands ligated with the CL nerve included. Light microscopy revealed that following ligation, an initial inflammatory reaction was followed by severe atrophy of acini and granular ducts. The atrophy was less severe when the CL nerve was not ligated. Secretory proteins were decreased from day 1 onwards following duct ligation in both groups. It can be concluded that most of the atrophy induced by duct ligation is independent of damage caused to the parasympathetic nerve supply, although the latter causes a greater atrophy presumably due to denervation.
Keywords: atrophy, chorda lingual nerve, denervation, ligation, salivary gland, secretory proteins
Secretion from human and most mammalian salivary glands studied occurs in response to neurotransmitter stimulation from both sympathetic and parasympathetic nerves. Secretory nerve impulses initiate the production of fluid by acinar cells, affect the absorption and secretion of certain ions in the ducts and stimulate exocytosis of storage granules containing proteins in both acinar and duct cells (Garrett & Proctor 1998).
The rat submandibular gland is a useful model for the study of salivary secretion. Previous studies have characterized the secretory responses of rat submandibular glands in vivo to different pharmacological agonists (Abe & Dawes 1979) or autonomic nerve stimulation (Garrett et al. 1991). Recent studies have utilized peroxidase and tissue kallikrein protein markers of acinar cell and duct cell protein secretions, respectively, in response to stimulation (Anderson et al. 1995; Proctor et al. 2003). Impulses from parasympathetic nerves also have a trophic effect on the gland, since parasympathetic denervation has been shown to cause an atrophy (Garrett 1999).
Experimental obstruction of the main excretory ducts of salivary glands causes acinar cell atrophy and other marked changes in tissue morphology, which have been well documented in previous studies in rat (Tamarin 1971a, b; Martinez et al. 1982), in mice (Takai et al. 1986), in rabbit (Smaje 1974) and in cat (Harrison & Garrett 1976).
Junqueira (1951) referred to salivary glands after duct ligation as resting glands, suggesting that secretion can occur if the glands are stimulated. However, Ohlin and Perec (1967) found that in the first week after duct ligation, the secretory responses of the rat submandibular gland to sialagogue drugs had deteriorated markedly; later, only very small amounts of secretion could be elicited by intense stimulation with pilocarpine, which evokes a lively flow of saliva from control glands. Thus, the ability of the acinar cells to secrete is almost completely lost after duct ligation, though the ducts, which are of great importance for the modification of saliva, remain histologically more or less unaffected (Standish & Shafer 1957). Martinez et al. (1982) found that ligation of the main excretory duct caused a progressive dysfunction in acinar and ductal cells in the rat submandibular gland and altered responsiveness to physiological regulators of secretion. In humans, sialolithiasis, the formation of stones within salivary glands, is associated with partial or complete obstruction to the flow of saliva and atrophic changes similar to those described following experimental duct ligation (Isacsson et al. 1981).
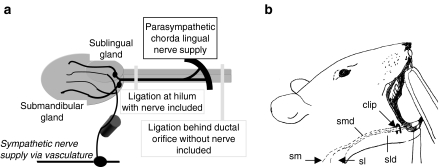
The chorda lingual (CL) nerve, which is a branch of the lingual nerve, crosses the submandibular duct and carries parasympathetic fibres to the hilum of the submandibular and sublingual glands. The nerve plays the major role in evoking the secretion of submandibular saliva. Most previous studies of duct ligation have ligated the duct at the hilum of the gland and consequently included the parasympathetic nerve supply in the ligature (Figure 1a). In the present study, we assessed the effect of excretory duct ligation on the secretory function of the rat submandibular gland with and without inclusion of the CL nerve. Some of the results were previously communicated and published in abstract form (Osailan et al. 2001).
Figure 1.
(a) Anatomical relation between rat submandibular gland and parasympathetic chorda lingual nerve supply. The positions of the microclips for intraoral and extraoral ligation are shown. Extraoral ligation was performed using surgical approach from the ventral surface. (b) Position of the rat for intraoral duct ligation. A microclip was placed posterior to the ductal orifice (sm, submandibular gland; sl, sublingual gland; smd, submandibular duct; sld, sublingual duct).
Methods
Surgery
Adult, male rats of Wistar strain (Harlan Laboratories Ltd, Loughborough, England) were used. The animals weighed 230–350 g at the time of experiment and were given a standard pelleted diet and water ad libitum and kept in a 12-h light/dark cycle. Prior to duct ligation, the animals were anaesthetized with ketamine/xylazine intraperitoneally (0.75 ml/kg of each drug giving a dose of 75 + 15 mg/kg, intraperitoneally, respectively), given as a mixture of one part of each drug. A small intraoral incision was made in the floor of the mouth, behind the right submandibular (RSM) duct orifice. The RSM and right sublingual ducts were ligated less than 5 mm posterior to the ductal orifice with a metal microclip (SLS, Vitaltec International, Domalain, France), and the incision closed with 8/0 Ethilon suture (Johnson & Johnson International, Brussels, Belgium). In further groups of anaesthetized animals, the RSM duct was carefully dissected through a skin incision on the ventral surface of the neck and ligated less than 5 mm anterior to the hilum of the gland with a metal microclip, and the incision was sutured with 4/0 black silk suture. Animals were allowed to recover from anaesthesia in a cage maintained at a warm room temperature. Aseptic conditions were used throughout the surgical procedure of duct ligation to reduce the risk of infection. In all animals, the contralateral, unligated left submandibular (LSM) gland was used as a control. At different times (1, 2, 7, 14 and 21 days) after ligation, animals were anaesthetized (pentobarbitone; 60 mg/kg, intraperitoneally), the submandibular glands separated from the sublingual glands, removed and weighed and animals killed with an overdose of pentobarbitone. Experiments were conducted with approval from the local Animal Ethics and Welfare Committee and under a Home Office project licence.
Sample preparation
Following removal, each gland was divided into pieces and frozen on dry ice, then stored at −70 °C for later biochemical analyses or fixed in formal sucrose (4% formaldehyde, 7.5% sucrose, 0.08 m cacodylate buffer pH 7.2) for light microscopy.
Histochemical staining of tissue sections
Formal sucrose-fixed tissue was processed and embedded in paraffin wax. Five-micrometre sections were cut, mounted on slides and stained with Ehrlich haematoxylin and 1% eosin (H&E). For the demonstration of acinar cell protein secretory granules, Alcian Blue/periodic acid–Schiff′s reagent (AB/PAS) was used, and for the demonstration of ductal cell protein secretory granules, p-dimethylaminobenzaldehyde (DMAB) was used as described previously (Garrett et al. 1991).
Biochemical analysis
Pieces of LSM and RSM glands were homogenized in 50 mm phosphate buffer (pH 6.0) containing 0.02% Triton X-100, using a blade homogenizer (Ultra-Turrax, Janke & Kunkle, Staufen, Germany), at a speed of 20,000 rpm for two sets of 15 s during 2 min. An aliquot was taken out for DNA assay, the whole homogenate was then centrifuged at 10,000 × g for 3 min, and the supernatants (extracts) were collected and stored at −70 °C until analysis.
Total protein assay
The protein content of submandibular tissue extract was determined as described by Petersen (1977). Briefly, 1 µl of 4% (w/v) extract was diluted in distilled water to a volume of 100 µl and was mixed with 100 µl of reagent A (a mixture of equal volumes of distilled water, 10% SDS, copper-tartrate-carbonate solution and 0.8 m NaOH). After 10 min, 50 µl of reagent B (Folin-Ciocalteu phenol reagent, Sigma Chemicals, Poole, UK), diluted one volume in five volumes of distilled water, was added and after 20 min, absorbance was read using a Microplate Reader (Bio-Rad, Hemel Hempstead, UK) at 655 nm. Bovine serum albumin (100, 50, 25, 12.5 and 6.25 µg/ml) was used as a standard, and a calibration curve was constructed.
Peroxidase assay
Peroxidase was assayed enzymically as previously described by Proctor and Chan (1994) using the substrate-activated 2,7-dichlorofluorescin diacetate (LDADCF; Molecular Probes, Eugene, OR, USA). Samples of submandibular gland extract were diluted to 1 : 200 with phosphate buffer, and 20 µl of the diluted samples was mixed with 1 ml of the phosphate buffer containing 1 mm potassium thiocyanate at 37 °C. Fifty microlitres of hydrogen peroxide (1 : 2000 dilution of 30% stock) was added, and the reaction was started immediately by adding 25 µl of the substrate (LDCF). Following incubation at 37 °C for 5 min, the reaction was stopped by adding 0.5 ml of 1 m sodium hydroxide. Fluorescence of the product dichlorofluorescein (DCF) was read in a fluorimeter (Perkin Elmer, Boston, MA, USA) set at 480 nm excitation and 530 nm emission. DCF (Sigma Chemicals) was used as a standard.
Tissue kallikrein assay
Tissue kallikrein activity was assayed by means of the peptide substrate DVLR-AFC (d-valine-leucine-arginine-7-amino-4-trifluoromethylcoumarin) as previously described by Shori et al. (1992). Twenty microlitres of 4% (w/v) tissue extracts diluted 1 : 1000 in 50 mm Tris–HCl buffer (pH 8.0) was added to 160 µl of Tris buffer in tubes pre-incubated at 30 °C in a water bath. The assay was started by adding 20 µl of DVLR-AFC substrate, incubated for 10 min, then stopped by adding 1.8 ml of 1% acetic acid. Fluoresence of the product AFC was read at 405 nm excitation and 505 nm emission. Free AFC standard (0.4 mm) was used to create a calibration curve.
DNA assay
The DNA content of the submandibular tissue homogenates was quantified in a fluorimeter with a fluorescent dye (Hoechst 33258). Hoechst 33258, a bisbenzimide DNA intercalator, binds to double-stranded DNA that excites in the UV region (360 nm) and emits in the blue region (450 nm) as described by Cesarone et al. (1979). Briefly, 25 µl of 4% (w/v) submandibular tissue homogenate was diluted to a volume of 1 ml with 15 mm trisodium citrate (SSc) buffer pH 7, then 0.5 ml of 0.15 mm Hoechst 33258 (Sigma Chemicals) was added to the sample and the mixture allowed to stand for 15 min in the dark before fluorescence was read at 360 nm excitation and 450 nm emission. Calf thymus DNA (Sigma Chemicals) standard in the range 1–100 µg/ml was used to construct a calibration curve.
Statistical analysis
Results are expressed as means ± SEM and were statistically compared by paired or unpaired Student's t-test for two groups following one-way ANOVA for multiple comparisons. P < 0.05 was considered statistically significant.
Results
Body weight
The increase in body weight following submandibular duct ligation with or without the inclusion of the CL nerve was similar to that of normal controls and indicates normal feeding and growth (data not shown).
Gland weights
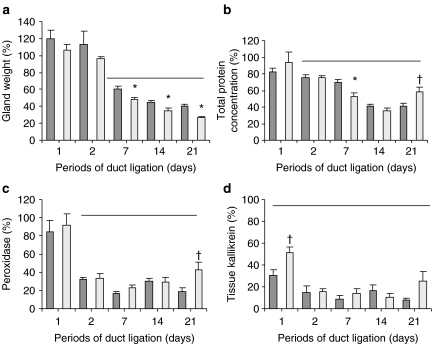
Following duct ligation, RSM gland weight initially increased slightly in the first 2 days. Mean weight of ligated glands (0.257 ± 0.04 g) increased (P < 0.05) compared with mean unligated, contralateral gland weight (0.215 ± 0.01 g) at 1 day following ligation without inclusion of the CL. Figure 2a shows weights of glands ligated with and without inclusion of CL compared with contralateral, unligated control glands. When the CL nerve was included in the ligation, mean gland weight was significantly less (P < 0.05) than that in the group where the nerve was excluded from the ligature (Figure 2a).
Figure 2.
Atrophy of the rat submandibular gland following ligation of the main submandibular duct. Each histogram expresses values in the right (ligated) and left (unligated) submandibular glands as a percentage of values in control rats examined at day 0 without duct ligation (100%). Ligation without inclusion of the parasympathetic chorda lingual nerve supply (dark columns) or with inclusion of the nerve (light columns) are compared. (a) Glands weight, (b) total protein concentration of gland extracts, (c) peroxidase activities of gland extracts and (d) tissue kallikrein activities of gland extracts. Values are means ± SEM (n = 4). Bars indicate values statistically significantly (P < 0.05) less than normal control rats (day 0). *Statistically significantly (P < 0.05) less than gland ligated without inclusion of nerve at same time point. †Statistically significantly (P < 0.05) more than gland ligated without inclusion of nerve at same time point.
Total protein
Mean total protein concentration in extracts of glands was significantly decreased at day 2 following duct ligation with or without inclusion of the CL compared with contralateral control glands. Decreases in total protein concentration showed a similar pattern whether the CL was included or excluded from the duct ligation. However, when the nerve was included, total protein concentration was significantly less at 7 days and significantly more at 21 days compared with that when the CL nerve was excluded (Figure 2b).
Peroxidase and tissue kallikrein
Peroxidase activity in glandular extracts rapidly decreased following duct ligation, and by day 2, peroxidase activity was approximately only 30% of activity in control glands and remained at this level at the later time points (Figure 2c). Ductal cell tissue kallikrein levels rapidly and significantly decreased from the first day of ligation in both groups and then showed a further decrease at day 2 and remained reduced up to 21 days (Figure 2d).
Light microscopy
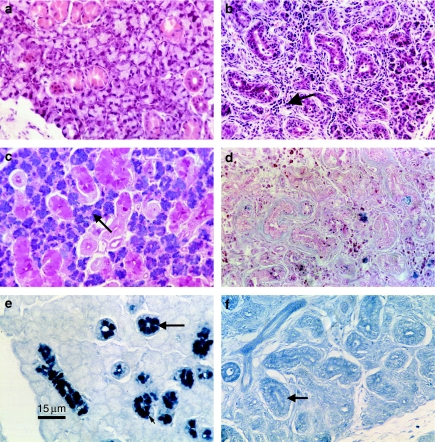
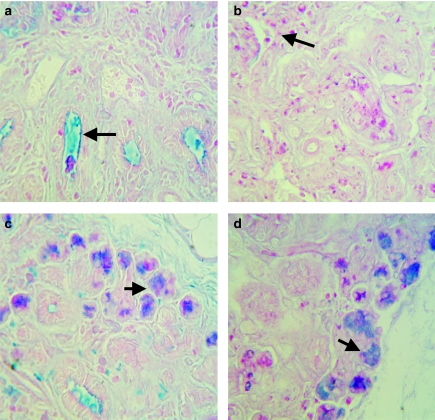
At days 1 and 2, ligated glands (RSM) showed inflammatory cells, mostly neutrophils and mononuclear inflammatory cells, between and within gland lobules and inside ductal lumena. Some eosinophils were shown by H&E staining. Acinar cells were shrunken and contained fewer granules as shown by AB/PAS staining. By day 7, ligated glands (RSM) showed a great atrophy in the acinar and ductal cells (H&E), and intralobular macrophages and inflammatory cells were still present (compare Figure 3a,b). AB/PAS staining showed that little secretory material remained following 7 days of ligation compared with the unligated gland (LSM) which showed granules clearly inside the acinar cells (Figure 3c,d). DMAB staining showed that at 7 days following ligation, very little secretory material remained in glandular duct cells (Figure 3e,f). However, PAS/AB staining showed that secretory material was present in ducts by 7, 14 and 21 days following duct ligation without the CL nerve included (Figure 4a) and very little material, when the nerve was included (Figure 4b). Even after an extended period of duct ligation (21 days), acini containing secretory granules were observed on the periphery of lobules in both groups (Figure 4c,d).
Figure 3.
Histological changes 7 days after duct ligation of rat submandibular salivary glands. Ligated right submandibular glands (RSM; b, d and f) are compared to control left submandibular glands (LSM; a, c and e). Haematoxylin and eosin stained sections (a and b) show a normal appearance of ductal and acinar cells in the LSM, while the RSM shows a great atrophy in acini as well as ductal cells and inflammatory cells present within lobes (arrow). Alcian Blue/periodic acid–Schiff's staining (c and d) clearly shows secretory glycoprotein containing storage granules inside acinar cells of LSM (arrow), while granules are absent from RSM. Dimethylaminobenzaldehyde (e and f) staining shows secretory tissue kallikrein containing storage granules in granular duct cells of the LSM (arrow) and an absence of such granules from the RSM (arrow). All micrographs are ×312.5 magnification, and the bar in (e) is equivalent to 15 µm.
Figure 4.
Secretory glycoproteins in rat submandibular glands following 21 days of duct ligation. (a) Right submandibular gland (RSM) ligated without the chorda lingual (CL) nerve included shows accumulation of secretory material in duct lumena (arrow). (b) RSM ligated with the CL nerve shows absence of secretory material in duct lumena. Acini in the periphery of lobes of RSM ligated without (c) and with (d) the CL nerve included (arrows). All sections have been stained with Alcian Blue/periodic acid–Schiff's reagent, and micrographs are ×500 magnification.
Discussion
In the present study, duct ligation with inclusion of the parasympathetic CL nerve caused a progressive decrease in gland wet weight as found previously in rat (Tamarin 1971a, b; Shiba et al. 1972) and in cat (Emmelin et al. 1974; Harrison & Garrett 1976). When duct ligation was performed without including the CL nerve, achieved by using an intraoral approach and a surgical metal clip, atrophy was still observed. In previous studies of the feline submandibular gland, inclusion of the CL nerve in the ligature has been shown to cause neurological damage (Harrison et al. 2001). The parasympathetic nerve has a trophic effect on salivary glands, and nerve damage is likely to cause atrophy as seen in experimental studies when the parasympathetic nerve is cut (Garrett 1999; Proctor 1999). The results of the present study suggest that there was a more extensive atrophy when the parasympathetic supply was included in the duct ligature.
Salivary gland atrophy was preceded by an initial increase in gland wet weight which has also been observed previously (Ekström 1975; Martinez et al. 1982). Light microscopy showed the presence of a cellular inflammatory infiltrate in the first couple of days following ligation. The initial increase in weight is most likely due to accumulation of secretory product and inflammatory infiltrate. When the nerve was excluded from the ligature, the increase in gland wet weight was statistically significant compared with the contralateral gland. The weight increase was not statistically significant when the nerve was included in duct ligation. The difference may be due to a greater residual secretory activity in ligated glands with an intact parasympathetic nerve supply because ligation of the nerve is likely to interfere with nerve impulse traffic and therefore reflex secretory activity of the gland. Light microscopy showed remaining alcianophilic secretory material within duct lumena at day 7 when the nerve was excluded from ligation but not with nerve inclusion. This observation again suggests that the gland may retain a greater residual secretory activity when the CL nerve remains intact.
Following 7 days of duct ligation, there were significant decreases in gland wet weight, and this coincided with an atrophy of secretory cells as shown morphologically using specific histological stains for the contents of secretory granules. Changes in secretory cells, as indicated by loss of secretory granules, were obvious by day 2 of duct ligation and preceded the decrease in gland weight. Biochemical assay of gland extracts revealed that the level of secretory proteins showed similar patterns of decrease to the disappearance of secretory granules shown by morphology. However, a statistically significant decrease in level of tissue kallikrein, a ductal cell secretory protein, was seen earlier than was apparent by morphology. Such an early decrease in secretory enzyme activity is most likely due to loss of secretory protein from cells, even though observation by light microscopy did not suggest an equivalent loss of secretory granules. It is also possible that there was some inhibition of enzyme activity during this period. The widespread loss of ductal cell secretory protein within 24 h is unlikely to be due to cell death at such an early time point and suggests that loss of secretory protein is being triggered by an unsubstantiated stimulus. Because early loss of secretory protein occurred whether the nerve was included in the ligation or not, it is unlikely to be due to the degeneration of parasympathetic nerve fibres and release of neurotransmitters as observed in earlier denervation studies (Ekström 1999). Activity of peroxidase, an acinar cell secretory protein, was also significantly decreased during the first 2 days of ligation, when alcianophilic secretory material was still abundant morphologically. The extent of the reduction in peroxidase at day 1 following ligation was not as great as that seen with tissue kallikrein, but it may be that peroxidase activity assayed during the early period of inflammation was influenced by increased inflammatory cell-derived myeloperoxidase (Schierwagan et al. 1990).
In conclusion, ductal ligation with or without inclusion of the CL nerve causes atrophy of the rat submandibular gland. When the CL nerve was excluded from ligation, the extent of glandular atrophy was less, and there was evidence of minor residual secretory activity within the gland. Biochemical assay revealed that substantial losses of secretory protein occur as early as 24 h following ligation. In future studies, we aim to examine the influence of an intact parasympathetic nerve supply on the recovery of gland function following removal of ductal obstruction.
Acknowledgments
This study was funded by the government of Saudi Arabia. The authors thank Professor John Garrett and Dr Guy Carpenter for helpful discussions regarding the study.
References
- Abe K, Dawes C. The effect of electrical and pharmacological stimulation on the types of proteins secreted by the rat parotid and submandibular glands. Arch. Oral Biol. 1979;23:367–372. doi: 10.1016/0003-9969(78)90094-8. [DOI] [PubMed] [Google Scholar]
- Anderson LC, Garrett JR, Zhang XS. Differential secretion of proteins by rat submandibular acini and granular ducts on graded autonomic nerve stimulations. J. Physiol. 1995;485:503–511. doi: 10.1113/jphysiol.1995.sp020746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarone CF, Bolognesi C, Santi LP. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal. Biochem. 1979;100:188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Ekström J. Compensatory increase in choline acetyltransferase activity in salivary glands and diaphragm muscle of the rat. Acta Physiol. Scand. 1975;56:1–5. doi: 10.1111/j.1748-1716.1975.tb05844.x. [DOI] [PubMed] [Google Scholar]
- Ekström J. Degeneration secretion and supersensitivity in salivary glands following denervations, and effects on choline acetyltransferase activity. In: Garrett JR, Ekström J, Anderson LC, editors. Neural Mechanisms of Salivary Gland Secretion, Frontiers of Oral Biology. Vol. 11. Basel: Karger; 1999. pp. 166–184. [Google Scholar]
- Emmelin N, Garrett JR, Ohlin P. Secretory activity and the myoepithelial cells of salivary glands after duct ligation in cats. Arch. Oral Biol. 1974;19:275–283. doi: 10.1016/0003-9969(74)90188-5. [DOI] [PubMed] [Google Scholar]
- Garrett JR. Effects of autonomic denervations on parenchymal structure and nerves in salivary glands. In: Garrett JR, Ekström J, Anderson LC, editors. Neural Mechanisms of Salivary Glands Secretion, Frontiers of Oral Biology. Vol. 9. Basel: Karger; 1999. pp. 131–149. [Google Scholar]
- Garrett JR, Proctor GB. Control of salivation. In: Garrett JR, Ekström J, Anderson LC, editors. The Scientific Basis of Eating, Frontiers of Oral Biology. Vol. 9. Basel: Karger; 1998. pp. 135–155. [Google Scholar]
- Garrett JR, Suleiman AM, Anderson LC, Proctor GB. Secretory responses in granular ducts and acini of submandibular glands in vivo to parasympathetic or sympathetic nerve stimulation in rats. Cell Tissue Res. 1991;264:117–126. doi: 10.1007/BF00305729. [DOI] [PubMed] [Google Scholar]
- Harrison JD, Garrett JR. The effects of ductal ligation on the parenchyma of salivary glands of cat studied by enzyme histochemical methods. Histochem. J. 1976;8:35–44. doi: 10.1007/BF01004003. [DOI] [PubMed] [Google Scholar]
- Harrison JD, Fouad HMA, Garrett JR. Variation in the response to ductal obstruction of feline submandibular and sublingual salivary glands and the importance of the innervation. J. Oral Pathol. Med. 2001;30:29–34. doi: 10.1034/j.1600-0714.2001.300105.x. [DOI] [PubMed] [Google Scholar]
- Isacsson G, Ahlner B, Lundquist PG. Chronic sialadenitis of submandibular gland. Arch. Otorhinolaryngol. 1981;232:91–100. doi: 10.1007/BF00661007. [DOI] [PubMed] [Google Scholar]
- Junqueira LCU. Cytological, cytochemical and biochemical observations on secreting and resting salivary glands. Exp. Cell Res. 1951;2:327–338. [Google Scholar]
- Martinez JR, Bylund DB, Cassity N. Progressive secretory dysfunction in the rat submandibular gland after excretory duct ligation. Arch. Oral Biol. 1982;27:443–450. doi: 10.1016/0003-9969(82)90082-6. [DOI] [PubMed] [Google Scholar]
- Ohlin P, Perec C. Secretory responses and choline acetylase of the rat's submaxillary gland after duct ligation. Experientia. 1967;23:1–4. doi: 10.1007/BF02135662. [DOI] [PubMed] [Google Scholar]
- Osailan SM, Proctor GB, McGurk M. Intraoral duct ligation of the rat submandibular salivary gland. J. Dent. Res. 2001;80:1168. [Google Scholar]
- Petersen GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Proctor GB. Effects of autonomic denervation on protein secretion and synthesis by salivary glands. In: Garrett JR, Ekström J, Anderson LC, editors. Neural Mechanisms of Salivary Gland Secretion, Frontiers of Oral Biology. Vol. 11. Basel: Karger; 1999. pp. 150–165. [Google Scholar]
- Proctor GB, Chan KM. A fluorometric assay of peroxidase activity utilizing 2′,7′-dichlorofluorescein with thiocyanate: application to the study of salivary secretion. J. Biochem. Biophys. Methods. 1994;28:329–336. doi: 10.1016/0165-022x(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Proctor GB, Garrett GH, Carpenter GH, Ebersole LE. Salivary secretion of immunoglobulin A by submandibular glands in response to autonomimetic infusions in anaesthetized rats. J. Neuroimmunol. 2003;136:17–24. doi: 10.1016/s0165-5728(02)00466-6. [DOI] [PubMed] [Google Scholar]
- Schierwagan C, Bylund-fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J. Pharmacol. Methods. 1990;23:179–186. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- Shiba R, Hamada T, Kawakatsu K. Histochemical and electron microscopical studies on the effect of duct ligation of rat salivary glands. Arch. Oral Biol. 1972;17:299–309. doi: 10.1016/0003-9969(72)90213-0. [DOI] [PubMed] [Google Scholar]
- Shori DK, Proctor GB, Chao J, Chan K-M, Garrett JR. New specific assays for tonin and tissue kallikrein activities in rat submandibular glands. Biochem. Pharmacol. 1992;43:1209–1217. doi: 10.1016/0006-2952(92)90494-4. [DOI] [PubMed] [Google Scholar]
- Smaje LH. Spontaneous secretion in the rabbit submaxillary gland. In: Thorn NA, Petersen OH, editors. Secretory Mechanisms of Exocrine Glands. Copenhagen: Munksgaard; 1974. pp. 608–622. [Google Scholar]
- Standish S, Shafer WG. Serial histological effects of rat submaxillary and sublingual salivary gland duct and blood vessel ligation. J. Dent. Res. 1957;36:866–879. doi: 10.1177/00220345570360060801. [DOI] [PubMed] [Google Scholar]
- Takai Y, Tatemoto Y, Noda Y, Sumitomo S, Tanaka T, Mori M. Immunohistochemical observations of epidermal growth factor, keratin proteins and lectin binding on effects of testosterone administration in duct-ligated submandibular glands of mice. Acta Histochem. Cytochem. 1986;19:481–495. [Google Scholar]
- Tamarin A. Submaxillary gland recovery from obstruction. I. Overall changes and electron microscopic alterations of granular duct cells. J. Ultrastruct. Res. 1971a;34:276–287. doi: 10.1016/s0022-5320(71)80072-2. [DOI] [PubMed] [Google Scholar]
- Tamarin A. Submaxillary gland recovery from obstruction. II. Electron microscopic alterations of acinar cells. J. Ultrastruct. Res. 1971b;34:288–302. doi: 10.1016/s0022-5320(71)80073-4. [DOI] [PubMed] [Google Scholar]