Abstract
The aim of this study was to investigate scintigraphic, immunohistological and ultrastructural changes associated with radiation-induced dysfunction of the lachrymal gland in an established experimental animal model. Ten rabbits were randomized into two groups and used for the study; in the control as well as experimental group, the Schirmer-test, lachrymal gland scintigraphy, and immunohistological and ultrastructural investigations were carried out prior to irradiation and 72 h as well as 1 month after single-dose irradiation with 15 Gy. Seventy-two hours after irradiation, secretion reduction evaluated by the Schirmer-test was evident. At this phase, we could observe a decrease in the expression of α-SMA and a re-distribution of tenascin-C matrix. Ultrastructural changes of acinar and myoepithelial cells were noticed; simultaneously, disturbance in the primary 99mTcO4– uptake as well as significant reduction of the lachrymal ejection fraction was assessed scintigraphically. These changes were still evident 1 month following irradiation but became less intensive. Single-dose irradiation with 15 Gy implicates a functional impairment of the lachrymal gland, which is associated with early immunohistological and ultrastructural alterations. These changes may represent objective surrogate parameters for radiogenic dysfunction and prerequisites for further investigations on radioprotection of lachrymal glands during radiotherapy of the periorbital region.
Keywords: α-SMA, dysfunction, lachrymal gland, radiation, tenascin-C
The radiogenic induction of dry eye is an uncommon condition. Lack of lubrication caused by diminished lachrymal secretion may occur following radiotherapy of the orbital region for orbital or nasal sinus malignancies or in case of thyroid orbitopathy (Nakissa et al. 1983; Bartley & Gorman 2002). Lachrymal impairment may also be implicated by radioiodine for therapy of differentiated thyroid carcinoma, resulting in a significant reduction of tears (Zettinig et al. 2002). Associated histomorphological aspects of this functional alteration could only be found in a single study which investigated pure morphological light-microscopic changes of lachrymal gland tissue (Gazda et al. 1992). Recently, a clinical study demonstrated significant immunohistological changes in the conjunctival mucosa after brachiotherapy, postulating alteration of the extracellular matrix as an indicator of cellular damage (Heimann et al. 2001). However, immunohistological alterations and concomitant functional impairment have not yet been investigated contemporaneously. As such changes could provide evaluable surrogate parameters for radiation-induced functional impairment on the histological level, we investigated short and long-term immunohistological, ultrastructural and scintigraphic changes associated with tear reduction following a single-dose irradiation with 15 Gy in an established experimental animal model.
Materials and methods
Animals and study design
Ten healthy female New Zealand rabbits of 2.5–3.5 kg weight were used for the study. All animals were acclimatised for at least 1 week before starting the study and then randomised into two groups. Experimental procedures were approved by the local authority according to the current German law on the protection of animals. Ten of the rabbits underwent a first lachrymal gland scintigraphy as described below in addition to Schirmer-test. One week later the first group (n = 5) was then irradiated with a single dose of 15 Gy under general anaesthesia using a combination of 3 mg/kg (S)-Ketamin-hydrochloride (Ketanest-S®) and 0.1 mg/kg Xylazin-hydrochloride (Rompun®). Seventy-two hours after irradiation, a second scintigraphy and Schirmer-test were performed with a subsequent excision of a unilateral inferior lachrymal gland for histological examination. One month later, the same procedure was performed with removal of the contralateral lachrymal gland. The rabbits of the second group (n = 5) were sham-treated but kept unirradiated and provided control glandular tissue. They underwent the same scintigraphic evaluation performed in the first group.
Lachrymal gland Scintigraphy
After intravenous administration of 50 MBq 99mTcO4– the rabbits of the first group underwent a sequential scintigraphy in a prone position and frontal projection of the head, using a single-head gamma camera (Picker CX 250 compact, LEHR collimator and field-of-view 25 cm). At the 20th minute, 0.01 mg/kg carbachol (Doryl®) was applied subcutaneously to stimulate lachrymal secretion, and the scintigraphy was continued for a further 25 min. Dynamic studies were acquired with 90 frames and 30 s per frame in 256 × 256 matrix with zoom 4. Percentage uptake of the administered activity was calculated for the 10th−19th and 36th−45th minute by summation of the appropriate frames and regions of interest (ROI) of the inferior lachrymal gland as previously described (Hakim et al. 2002). Time–activity curves were additionally registered and analysed. At the 50th minute, carbachol was antagonised by 0.1 mg/kg atropine administered subcutaneously. The second and third scintigraphy (3 and 30 days after irradiation, respectively) were carried out identically. A minimal interval of 1 week was respected between two subsequent scintigraphies in order to avoid any interaction or uptake disturbance by the rest of radiotracer injected previously. The lachrymal ejection fraction (LEF) has been defined as follows:
Schirmer-test
At the time of every scintigraphic investigation, the Schirmer-test was carried out twice for 5 min using only general anaesthesia mentioned above, without application of local anaesthetic fluids. The first Schirmer-test was performed directly prior to lachrymal gland scintigraphy and it measured unstimulated secretion; the second one was carried out after application of carbachol and it indicated, therefore, stimulated secretion. This procedure was carried out in control as well as in irradiated animals.
Irradiation
X-ray irradiation was performed under general anaesthesia using 10 MV MEVATRON 74 – Siemens teletherapy unit with a dose rate of 3 Gy/min. The rabbit was placed laterally and covered with a 1 cm thick tissue-equivalent bolus material; the treatment distance was 58 cm. An axial beam was directed from above towards the rabbit's head, including the periorbital region (field size 5 × 7.5 cm). A total dose of 15 Gy was applied.
Surgical harvesting of the inferior lachrymal gland
Surgical exposure and excision of the inferior lachrymal gland was carried out through a transconjunctival incision. Every gland was divided into three parts, one was fixed immediately with neutral phosphate-buffered 4% formalin; another was shock frozen in liquid nitrogen and preserved for immunohistochemical examination. The third portion was fixed for transmission electron microscopy.
Tissue preparation and immunohistochemistry
After a minimum of 48 h of fixation, the tissue was trimmed and processed by standard paraffin-embedding methods. Sections were cut at 4 µm, deparaffinized and then stained with haematoxylin and eosin to obtain conventional histologic sections. For immunohistochemical staining, monoclonal antibodies against the following proteins were used: alpha-smooth muscle actin (clone 1A4, diluted 1 : 40, Dako, Glostrup, Denmark) and monoclonal mouse anti-human tenascin (TN2, Dako). The APAAP technique was used for visualization of the bound primary antibodies. The secondary rabbit antimouse antibody and the APAAP complex were diluted 1 : 50 (both from Dako). Naphtol-AS-biphosphate (Sigma-Aldrich, Seelze, Germany) and new fuchsin (Merck, Darmstadt, Germany) were, respectively, used as substrate and developer. As negative control, the primary antibody was replaced by a non-immune serum. In order to evaluate the degree of α-SMA loss per acinus, a semiquantitative score was introduced as follows: no α-SMA loss, 0; 25% loss of α-SMA, +; significant α-SMA loss >50%, ++; more than 75% α-SMA loss, +++. For the evaluation of tenascin-C reaction, 10 view fields of each sample in every group were chosen at random and the area of positive reaction in relation to the whole view field was calculated using the professional Soft Imaging System software analysis®. Values were expressed as mean ± standard error of mean (SEM).
Transmission electron microscopy
Samples were immediately fixed by immersion in 0.1 m cacodylate buffer containing 2.5% glutaraldehyde and 2% paraformaldehyde at pH 7.4 for 24 h. The specimens were post-fixed in 1% OsO4 and stained en bloc with 2% uranylacetate. After dehydration in graded alcohol, the specimens were embedded in Araldite. Semi-thin sections were stained with methylene blue and azure II according to Richardson to visualize the ROI containing myoepithelial cells (MEC). Ultrathin sections were cut and stained with lead citrate and examined using a transmission electron microscope (Phillips, EM 109).
Data and statistical analysis
Each lachrymal gland was evaluated scintigraphically, immunohistochemically and ultrastructurally. When proved to follow a normal distribution, values of tracer uptake were expressed as mean ± SEM and the two-sided t-test for paired samples was used to compare scintigraphic data obtained prior to irradiation with those registered 3 days as well as 30 days afterwards. The Kruskal–Wallis test for unpaired samples was used to evaluate the difference of immunohistochemical reaction between the control glands and glands irradiated with 15 Gy. An alpha level of P < 0.05 was considered significant.
Results
Overall morphology
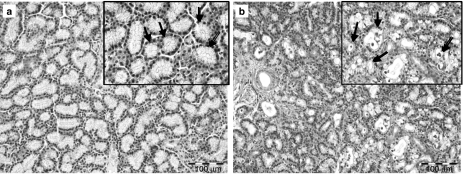
Histological findings derived from conventional histology of control lachrymal gland tissue showed the typical histological tubulo-acinar structure of the inferior lachrymal gland with the cubic regular shape of acinar cells and basally located nuclei (Figure 1a). Compared with control glands, secretory retention was observed in most acinar and tubular cells of the irradiated inferior lachrymal gland as well as intraluminally 3 days following irradiation. Furthermore, scattered vacuolopathy and an increase in the number of aberrant nuclei – anisomorphic pyknotic shapes – in apoptotic acinar cells was noticed along with an extracellular oedema and increased congestion of interlobular blood vessels (Figure 1b). Ductal cells were not affected by this process. Glandular tissue derived from sham-treated animals showed no sign of the cellular damage described above. One month later, most acute changes in the appearance of acinar cells were no longer evident. MEC were not evaluable at any time.
Figure 1.
Conventional haematoxylin-and-eosin-staining of the control lachrymal gland (a) shows the typical histological tubulo-acinar structure of the inferior lachrymal gland and the cubic regular shape of acinar cells with basally located nuclei (arrows). Three days after irradiation with 15 Gy, vacuolopathy and secretory retention were evident in the end-piece epithelium (b; arrows) while ductal cells stayed unaffected.
Scintigraphic data
The tracer uptake of the lachrymal glands prior to irradiation amounted 0.29 ± 0.06% of the applied activity before administration of carbachol. The mean LEF was 27.78 ± 0.83%. Three days after irradiation, the primary tracer uptake was reduced to 0.16 ± 0.02%, and the LEF was significantly lower. This reduction of LEF became statistically non-significant after 1 month; however, the primary uptake remained reduced. Original uptake data are summarized in Table 1.
Table 1.
The changes of the primary tracer uptake and the lachrymal ejection fraction (LEF) as well as alteration of native and stimulated tear secretion, investigated simultaneously with associated α-SMA loss and tenascin-C remodelling evaluated 3 and 30 days after single-dose irradiation with 15 Gy
| Investigation | Pre-radiation | After 3 days | P-value | After 30 days | P-value |
|---|---|---|---|---|---|
| Primary uptake (%) | 0.29 ± 0.06 | 0.16 ± 0.02 | <0.05 | 0.17 ± 0.03 | <0.05 |
| Remained activity (%) | 0.20 ± 0.04 | 0.14 ± 0.03 | <0.05 | 0.12 ± 0.02 | <0.05 |
| LEF (%) | 27.78 ± 0.83 | 11.66 ± 0.90 | <0.05 | 28.41 ± 0.77 | >0.05 |
| Native (mm) | 11.51 ± 7.84 | 6.42 ± 5.85 | <0.05 | 6.64 ± 2.46 | <0.05 |
| Stimulated (mm) | 20.88 ± 5.96 | 15.75 ± 8.42 | <0.05 | 17.07 ± 5.60 | <0.05 |
| Tenascin-C expression (%) | 10.97 ± 0.34 | 87.33 ± 1.06 | <0.05 | 56.31 ± 0.09 | <0.05 |
| α-SMA loss (score) | 0 | ++ | <0.05 | + (+) | <0.05 |
Schirmer-test
Compared with control glands, native unstimulated as well as stimulated lachrymal secretion 3 days after 15 Gy was significantly reduced. These changes were still evident after 30 days, although a slight non-significant recovery could be observed (Table 1).
Immunostaining
Alpha smooth muscle actin (α-SMA)
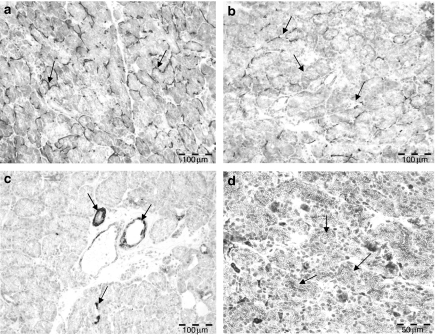
In controls, lachrymal glands MEC were stained around the acini forming a hem or basket-like figure, whereas the MEC of the intercalated and secretory ducts as well as blood vessels displayed a circular distribution (Figure 2a).
Figure 2.
(a) Immunohistochemical expression pattern of α-SMA in the control lachrymal glands showing a basket-like pattern in the acinar region (arrows). (b) The inferior lachrymal gland 72 h after irradiation with 15 Gy shows a marked loss of α-SMA reaction in the acinar region, the labelling displayed an irregular pattern and is limited to scattered acini. (c) Tenascin-C expression is observed in the duct system and blood vessels of control lachrymal glands (arrows); the acinar cells themselves are not labelled. (d) Seventy-two hours following irradiation, the acinar cells display an excessive intracellular expression of tenascin-C (arrows). Bar = 100 µm.
Inferior lachrymal glands investigated 72 h after irradiation showed a special pattern of staining: the MEC of the end-pieces were irregularly stained and a lot of acinar MEC were not stained at all (Figure 2b); there was a significant loss of α-SMA staining in all irradiated glands. On the contrary, the MEC of the intercalated and secretory ducts remained unaffected. Acinar cells themselves were not labelled in control or irradiated lachrymal glands. One month after irradiation, the overall expression was still reduced, but the pattern of α-SMA labelling was similar to that investigated prior to irradiation. Original semiquantitative data of α-SMA evaluation are shown in Table 1.
Tenascin-C
In the control lachrymal glands, a tenascin-C matrix was observed, and labelling was limited to the basal membrane of the intercalated and secretory ducts and some scattered acinar cells (10.97 ± 0.34% of field view; Figure 2c). Irradiated glands investigated 72 h later showed a remarkable re-distribution of the tenascin-C staining. A significant excessive expression of tenascin-C (87.33 ± 1.06%) was evident intracellularly in acinar and tubular cells (Figure 2d). This expression underwent partial normali-sation after 1 month but was still significantly changed (Table 1).
Electron microscopic findings
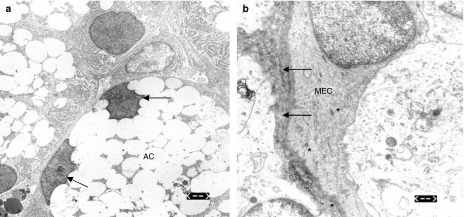
Three days after irradiation lachrymal glands showed an intracellular retention of secretory granula with subsequent displacement of the acinar nuclei (Figure 3a). Furthermore, a thickening of the basal membrane and condensation of the myofilaments in the MEC as well as apoptotic acinar nuclei were observed (Figure 3b). One month after irradiation, partial remission was noticed including reduction of secretory retention and reorganization of the myofilaments in MEC, which appeared intact without nuclear aberration. Only the cell membrane thickening and scattered pyknotic nuclei in apoptotic acinar cells were still evident at this time.
Figure 3.
(a) Electron microscopic appearance of the lachrymal acinar cells (AC) 72 h after irradiation showing granula retention and peripheral displacement of the cell nuclei (arrows). Bar = 2.5 µm. (b) Thickening of the basal membrane (arrows) as well as condensation of the myofilaments in the myoepithelial cells (MECs) noticed 72 h after irradiation (asterisks). Bar = 0.6 µm.
Discussion
The influence of ionising radiation of the head and neck region on parenchymal organ functions is a well-known phenomenon, especially in salivary glands (Vissink et al. 2003; Munter et al. 2004). Since the orbital region still represents an area which has to be frequently unshielded during radiotherapy, there is only poor data on the side effects of this therapeutic procedure on the lachrymal function and subsequent alteration of the ocular surface. Radiotherapy may also be indicated in cases of orbital tumours such as lymphoma, uveal melanoma or adjacent malignant neoplasms (Heimann et al. 2001). Thyroidal orbitopathy and age-related macular degeneration are further conditions in which radiotherapy may be indicated, albeit in a lower dose (Eter & Schuller 2001). The therapeutic procedure frequently reported to induce xerophthalmia is radioiodine therapy, due to the radioiodide used which is also trapped by lachrymal parenchyma (Solans et al. 2001; Zettinig et al. 2002). On the contrary, there is no data concerning functional impairment of lachrymal glands and concomitant structural and immunohistochemical changes following external beam radiotherapy of head and neck malignancies. Simultaneous evaluation of both immunohistochemical and functional aspects gains a considerable value in this field since it elucidates the pathogenesis of such impairment. We chose the single-dose irradiation with 15 Gy in order to investigate the influence of radiotherapy of adjacent head regions on the lachrymal secretion. The use of single-dose irradiation has been proven to have a comparable effect with a fractionated pattern on serous acinar cells (Stephens et al. 1986).
Considering functional scintigraphic data, two phenomena could be revealed. First, the short-term reduction of primary tracer uptake 72 h following radiation. This could be attributed to the alteration of the basal membrane of acinar cells, as the latter represents the site of tracer uptake in acinar cells by the sharing mechanism of the Na+/K+/Cl– cotransporter (Helman et al. 1987). The increased tenascin-C expression observed at this time supports this suggestion, since alteration of the baso-lateral membrane has been proven to induce tenascin-C expression (Jones et al. 1995). Thickening of the basal membrane, evident electronmicroscopically (Figure 3b), confirms this hypothesis.
The significant reduction of the LEF represents the second phenomenon which could be attributed to a decrease or a non-response of the MEC to the neurosecretory stimulus carbachol. It has been proven that reduced capability of excretion indicates impaired contractile function of the MEC (Garrett 1987; Satoh et al. 1997); hence, altered reactivity of α-SMA as well as ultrastructural changes of MEC shown 72 h after radiation may represent the histological correlate of this phenomenon.
The contradiction between scintigraphic and Schirmer-test results noticed 1 month after irradiation is explained by the relative nature of the LEF value. Since both primary uptake and excretion fraction were reduced at 1 month, the LEF may stay unaffected although a significant lachrymal reduction is still evident using the Schirmer-test. These results are supported by the immunohistochemical changes in samples investigated after 1 month, reflecting a partial recovery of myoepithelial cells' staining with α-SMA. These results are thus in accordance with Eter and Schuller who could observe a transient impairment followed by a partial recovery of lachrymal secretion after irradiation (Eter & Schuller 2001). Our ultrastructural investigations provide a further explanation for functional deficiencies at this stage, albeit these were not as distinct as those observed 72 h after irradiation.
Although corneal epithelium itself is considered a vulnerable target site for radiation which induces keratopathy (Kwok et al. 1998), the results presented above provide unequivocal evidence that significant tear reduction may additionally occur at a lower dose, implicating lack of ocular lubrication and consequently dry eye symptoms.
As far as we know, this is the first study to investigate concomitant immunohistological changes of functionally radiogenic-impaired lachrymal glands. The immunohistological alteration which is proven to have morphological and functional correlation may provide surrogate parameters of secretory dysfunction and prerequisites for further therapeutic approaches in the field of radioprotection of lachrymal glands in experimental and clinical studies.
References
- Bartley GB, Gorman CA. Perspective – Part I: the Mayo Orbital Radiotherapy for Graves Ophthalmopathy (ORGO) study: lessons learned. Ophthal. Plast. Reconstr. Surg. 2002;18:170–172. doi: 10.1097/00002341-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Eter N, Schuller H. External beam radiotherapy for age-related macular degeneration causes transient objective changes in tear-film function. Graefes Arch. Clin. Exp. Ophthalmol. 2001;239:923–926. doi: 10.1007/s00417-001-0391-5. [DOI] [PubMed] [Google Scholar]
- Garrett JR. The proper role of nerves in salivary secretion: a review. J. Dent. Res. 1987;66:387–397. doi: 10.1177/00220345870660020201. [DOI] [PubMed] [Google Scholar]
- Gazda MJ, Schultheiss TE, Stephens LC, Ang KK, Peters LJ. The relationship between apoptosis and atrophy in the irradiated lachrimal gland. Int. J. Radiat. Oncol. Biol. Phys. 1992;24:693–697. doi: 10.1016/0360-3016(92)90716-u. [DOI] [PubMed] [Google Scholar]
- Hakim SG, Geerling G, Lauer I, Sieg P. Scintigraphic evaluation of lachrimal glands using a rabbit experimental model. Ophthalmic Res. 2002;34:254–257. doi: 10.1159/000063878. [DOI] [PubMed] [Google Scholar]
- Heimann H, Coupland SE, Gochman R, Hellmich M, Foerster MH. Alterations in expression of mucin, tenascin-C and syndecan-1 in the conjunctiva following retinal surgery and plaque radiotherapy. Graefes Arch. Clin. Exp. Ophthalmol. 2001;239:488–495. doi: 10.1007/s004170100301. [DOI] [PubMed] [Google Scholar]
- Helman J, Turner RJ, Fox PC, Baum BJ. 99mTc-pertechnetate uptake in parotid acinar cells by the Na+/K+/Cl– co-transport system. J. Clin. Invest. 1987;79:1310–1313. doi: 10.1172/JCI112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Boudreau N, Myers CA, Erickson HP, Bissell MJ. Tenascin-C inhibits extracellular matrix-dependent gene expression in mammary epithelial cells. Localization of active regions using recombinant tenascin fragments. J. Cell Sci. 1995;108:519–527. doi: 10.1242/jcs.108.2.519. [DOI] [PubMed] [Google Scholar]
- Kwok SK, Ho PC, Leung SF, et al. An analysis of the incidence and risk factors of developing severe keratopathy in eyes after megavoltage external beam irradiation. Ophthalmology. 1998;105:2051–2055. doi: 10.1016/s0161-6420(98)91123-x. [DOI] [PubMed] [Google Scholar]
- Munter MW, Karger CP, Hoffner SG, et al. Evaluation of salivary gland function after treatment of head-and-neck tumors with intensity-modulated radiotherapy by quantitative pertechnetate scintigraphy. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:175–184. doi: 10.1016/s0360-3016(03)01437-8. [DOI] [PubMed] [Google Scholar]
- Nakissa N, Rubin P, Strohl R, Keys H. Ocular and orbital complications following radiation therapy of paranasal sinus malignancies and review of literature. Cancer. 1983;51:980–986. doi: 10.1002/1097-0142(19830315)51:6<980::aid-cncr2820510603>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Sano K, Habara Y, Kanno T. Effects of carbachol and catecholamines on ultrastructure and intracellular calcium-ion dynamics of acinar and MEC of lachrimal glands. Cell Tissue Res. 1997;289:473–485. doi: 10.1007/s004410050893. [DOI] [PubMed] [Google Scholar]
- Solans R, Bosch JA, Galofre P, et al. Salivary and lachrimal gland dysfunction (sicca syndrome) after radioiodine therapy. J. Nucl. Med. 2001;42:738–743. [PubMed] [Google Scholar]
- Stephens LC, Ang KK, Schultheiss TE, King GK, Brock WA, Peters LJ. Target cell and mode of radiation injury in rhesus salivary glands. Radiother. Oncol. 1986;7:165–174. doi: 10.1016/s0167-8140(86)80096-2. [DOI] [PubMed] [Google Scholar]
- Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit. Rev. Oral Biol. Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- Zettinig G, Hanselmayer G, Fueger BJ, et al. Long-term impairment of the lachrimal glands after radioiodine therapy: a cross-sectional study. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:1428–1432. doi: 10.1007/s00259-002-0969-0. [DOI] [PubMed] [Google Scholar]