Abstract
Non-alcoholic fatty liver disease (NAFLD) represents a histological spectrum of liver disease associated with obesity, diabetes and insulin resistance that extends from isolated steatosis to steatohepatitis and cirrhosis. As well as being a potential cause of progressive liver disease in its own right, steatosis has been shown to be an important cofactor in the pathogenesis of many other liver diseases. Animal models of NAFLD may be divided into two broad categories: those caused by genetic mutation and those with an acquired phenotype produced by dietary or pharmacological manipulation. The literature contains numerous different mouse models that exhibit histological evidence of hepatic steatosis or, more variably, steatohepatitis; however, few replicate the entire human phenotype. The genetic leptin-deficient (ob/ob) or leptin-resistant (db/db) mouse and the dietary methionine/choline-deficient model are used in the majority of published research. More recently, targeted gene disruption and the use of supra-nutritional diets to induce NAFLD have gained greater prominence as researchers have attempted to bridge the phenotype gap between the available models and the human disease. Using the physiological processes that underlie the pathogenesis and progression of NAFLD as a framework, we review the literature describing currently available mouse models of NAFLD, highlight the strengths and weaknesses of established models and describe the key findings that have furthered the understanding of disease pathogenesis.
Keywords: animal, diabetes, leptin, review, steatohepatitis
Introduction
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of liver disease encompassing steatosis (fatty change), non-alcoholic steatohepatitis (NASH) and cirrhosis in the absence of alcohol abuse. NASH has been reported worldwide and is increasingly recognized as the leading cause for liver dysfunction and cirrhosis in the non-alcoholic, viral hepatitis negative population in Europe and North America (Skelly et al. 2001; Angulo & Lindor 2002). Population studies show that NAFLD is strongly associated with obesity (Ludwig et al. 1980; Powell et al. 1990; Wanless & Lentz 1990; Ratziu et al. 2000), dyslipidaemia (Powell et al. 1990; Sanyal 2002), insulin resistance (IR) (Powell et al. 1990; Marchesini et al. 1999; Sanyal 2002) and type II (non-insulin dependent) diabetes mellitus (Powell et al. 1990; Sanyal 2002). Most authors now consider NAFLD to be a hepatic manifestation of the metabolic syndrome (Sanyal 2002; Marchesini et al. 2003).
Steatosis represents both an important cofactor that may determine of rate of progression of fibrosis in a range of liver diseases and a pathogenic process in its own right. The rate of progression of many liver diseases is accelerated when there are multiple coincidental insults [e.g. alcohol intake and chronic viral hepatitis (Poynard et al. 1997) or haemochromatosis (Fletcher et al. 2003)]. Similarly, the extent of hepatic steatosis has been shown to interact with several aetiological factors including chronic hepatitis C (Hourigan et al. 1999; Adinolfi et al. 2001; Hu et al. 2004) and alcohol (Teli et al. 1995; Reeves et al. 1996) as well as influencing the progression of lone steatohepatitis (Matteoni et al. 1999). Historically, lone hepatic NASH was considered a relatively benign disease in the majority of patients, however, currently available data suggest that NASH is progressive, leading to significant morbidity and may be a major cause of what was previously described as ‘cryptogenic cirrhosis’ (Caldwell et al. 1999; Poonawala et al. 2000; Reid 2001).
The development of NAFLD is determined by the interaction of genetic and environmental factors (Day 2002). Complex, polygenic disease traits, like NAFLD and IR, are now the focus of much research. Such diseases are difficult to study in humans due to genetic heterogeneity within populations, epistasis, gene–environment interaction and the low frequency of familial cases of diseases compared with the high overall frequency in the background population (Dragani 1998). Sharing many physiological, anatomical and metabolic similarities with the human, the laboratory mouse, Mus musculus, has been widely adopted as the primary model organism for research in this field. The many standardized and well-characterized inbred strains allow confounding factors such as genetic heterogeneity, gender, dietary or environmental variation and age to be eliminated as variables (Silver 1995). This allows researchers to study the effects of an intervention on a single homogeneous population.
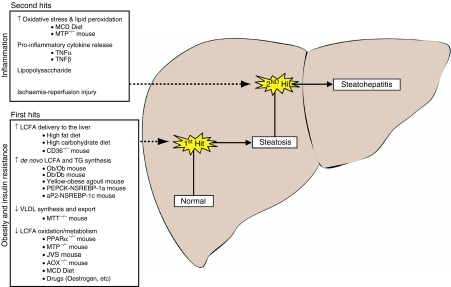
Despite recent advances in elucidating the genetic contribution and pathogenesis of related conditions, such as IR and type II diabetes mellitus (Kahn 1994; Almind et al. 2001), our understanding of the pathogenesis of steatosis and steatohepatitis remains incomplete (Koteish & Mae 2002). The use of animal models in this field has proved invaluable. Indeed, it was work-describing inducible steatohepatitis in an animal model that led Day and James to propose the ‘two-hit’ hypothesis for the pathogenesis of NASH which remains a foundation for research in this field (Day & James 1998). The first hit, steatosis, sensitizes the liver to the induction of inflammation by a second pathogenic insult that promotes oxidative stress and hence steatohepatitis (Figure 1). This model has subsequently been revised in recognition that a combination of ‘second hits’ (both environmental and genetic) may lead to the development of steatohepatitis (Day 2002).
Figure 1.
The ‘two-hit’ hypothesis for the pathogenesis on non-alcoholic fatty liver disease. The progression from normal healthy liver to steatohepatitis is in a stepwise fashion involving first the development of obesity and insulin resistance (which leads to fatty change) and later hepatic inflammation. Some of the available models and pathogenic processes are also summarized.
The pathogenesis of NAFLD
Steatosis and IR
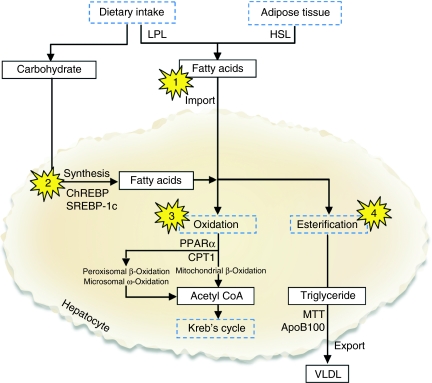
The liver plays a central role in whole body lipid and carbohydrate metabolism (shown in Figure 2). Disruption of the normal mechanisms for synthesis, transport and removal of long-chain fatty acids (LCFA) and triglycerides (TG) are the basis for the development of NAFLD and provide a scheme for reviewing the available animal models. The genesis of steatosis is closely related to the development of obesity and particularly IR, a near universal finding in patients with NAFLD (Day 2002). Indeed, IR is associated with NASH even in subjects with apparently normal glucose tolerance (Marchesini et al. 1999). Steatosis occurs when the rate of import or synthesis of fatty acids by hepatocytes exceeds the rate of export or catabolism (Koteish & Diehl 2001; Bradbury & Berk 2004). The accumulation of lipid within the liver may be produced by disrupting the normal physiological balance in one of four main ways:
Figure 2.
Hepatic lipid metabolism and the development of steatosis. In the absorptive state, dietary triglycerides (TG) are transported in the circulation as chylomicrons, LPL-mediated degradation releases LCFA. In the postabsorptive (fasting) state, insulin levels fall, and HSL releases LCFA from adipose tissue. In addition to absorbing circulating LCFA, hepatocytes synthesize LCFA from dietary carbohydrate under control of SREBP-1c and ChREBP. Hepatic clearance of fatty acids depends on oxidation in the mitochondria and peroxisomes (β-oxidation) or microsomes (ω-oxidation) to generate acetyl CoA for entry into Krebs cycle. Excess fatty acids are re-esterified to TG for export into the circulation as VLDL. Points at which disruption may produce non-alcoholic fatty liver disease are highlighted and discussed in the text. ApoB100, apolipoprotein B100; ChREBP, carbohydrate response element-binding protein; CPT1, carnitine palmonitoyl transferase 1; HSL, hormone sensitive lipase; LCFA, long-chain fatty acids; LPL, lipoprotein lipase; MTT, microsomal TG transfer protein; PPARα, peroxisome proliferator-activated receptor a; SREBP-1c, sterol regulatory element-binding protein 1c; VLDL, very low density lipoprotein.
Increased delivery and uptake into hepatocytes of LCFA due to excess dietary intake or release of from adipose tissue stores
Adipose tissue is metabolically active and releases many biologically active products including mediators of carbohydrate metabolism (leptin, adiponectin and resistin), lipid metabolism (apolipoprotein E and lipoprotein lipase) and adipocytokines (TNFα, IL-6 and TGFβ) (Brunt 2004). As obesity develops, adipose TNFα expression has been shown to rise in patients and experimental models. This acts both in an autocrine and paracrine fashion activating IκB kinase β (Iκκβ) and inhibiting phosphorylation of insulin receptor substrates (IRS-1 and IRS-2). This in turn leads to failure of insulin-mediated suppression of hormone-sensitive lipase (HSL) and increased release of LCFA into the circulation (Day 2002; Shoelson et al. 2003). An explanation for the strong association between central obesity, characterized by predominant fat deposition in omental and mesenteric stores, and steatosis is that high concentrations of LCFAs are released directly into the portal circulation. This, coupled with TNFα-mediated upregulation of hepatic fatty acid translocase (Memon et al. 1998), leads to enhanced uptake by the liver. Studies in mouse models have shown that disruption of the type 1 TNFα receptor gene (Uysal et al. 1997), the Iκκβ gene, or administration of salicylates inhibits Iκκβ activity (Yuan et al. 2001) and ameliorates IR. However, there remains debate as to whether steatosis also abates (Koteish & Mae 2002).
Increased de novo hepatic LCFA and TG synthesis
Accumulation of excess oleic acid (a product of de novo fatty acid synthesis) has been demonstrated in both humans and murine models of steatosis (Shimomura et al. 1998a; Araya et al. 2004). Sterol regulatory element-binding protein-1c (SREBP-1c) is a key membrane-bound transcription factor through which insulin promotes hepatic lipid synthesis (Table 1). Similarly, rising glucose levels activate de novo hepatic lipid synthesis via carbohydrate response element-binding protein (ChREBP) (Dentin et al. 2005). High plasma glucose and insulin levels following a carbohydrate meal normally trigger rapid synthesis of LCFAs within hepatocytes. Elevated insulin levels also promote GLUT4-mediated uptake of plasma glucose by adipocytes, synthesis of lipid stores and inhibition of lipolysis in adipose tissue.
Table 1.
Selected transcription factors implicated in the pathogenesis of non-alcoholic fatty liver disease
| Transcription factor | Endogenous agonist | Exogenous agonist | Target genes | Effects |
|---|---|---|---|---|
| Peroxisome proliferator-activated receptor α (PPARα, NR1C1) | Fatty acids, oxidized phospholipids, leukotrienes (LTB4) | Fenofibrate, clofibrate, Wy-14643 (pirinixic acid) | Acyl-CoA synthase, fatty acid transporter 1, CPT1, ABCA1 | ↑ Fatty acid uptake, activation to acyl-CoA and mitochondrial β-oxidation. |
| Peroxisome proliferator-activated receptor γ (PPARγ, NR1C3) | Fatty acids, prostaglandin J2 | Thiazolidinediones (e.g. rosiglitazone, pioglitazone, troglitazone) | Lipoprotein lipase, fatty acid transporter 1, acyl-CoA synthase | Adipocyte differentiation. ↑ LPL mediated fatty acid release from chylomicrons. ↑ Adipose tissue fatty acid uptake and lipogenesis.↑ Adiponectin production |
| Sterol regulatory element-binding protein 1a (SREBP-1a) | Insulin and sterol depletion lead to ↑transcription and activation | HMG CoA synthase, HMG CoA reductase, fatty acid synthase | ↑ Cholesterol Synthesis and ↑ fatty acid/triglyceride synthesis | |
| Sterol regulatory element-binding protein 1c (SREBP-1c) | Insulin and LXRα activity lead to ↑transcription and activation | Glucokinase, acetyl CoA synthase and carboxylase, fatty acid synthase, ATP citrate lyase | ↑ Fatty acid/triglyceride synthesis; ↑ Lipogenesis | |
| Sterol regulatory element-bindingprotein 2 (SREBP-2) | Insulin and sterol depletion lead to ↑transcription and activation | HMG CoA synthase, HMG CoA reductase, LDL receptor | ↑ Cholesterol synthesis | |
| Carbohydrate response element-binding protein (ChREBP) | Glucose | Liver pyruvate kinase, fatty acid synthase, acetyl CoA carboxylase | ↑ Lipogenesis | |
| Liver X receptor α(LXRα, NR1H3) | Cholesterol derived oxysterols and LCFAs | T0901317, GW3965 | CYP7A1, ABCA1, ABCG1, SREBP-1c, liproprotein lipase, CETP, ApoE | ↑ Fatty acid synthesis, ↑ bile acid synthesis, ↑ lipoprotein assembly |
In obese individuals, there is less GLUT4 activity leading to greater postprandial hyperglycaemia, and further pancreatic insulin release (Carvalho et al. 2001). This, together with the increased release of TNFα from adipose tissue and consequent IR, drives further hepatic LCFA synthesis from acetyl CoA. The conversion of LCFAs to TGs by glycerol phosphate acyl transferase within hepatocytes is non-rate limiting, consequently increased availability of LCFAs will lead to increased hepatocyte TG synthesis and accumulation (Bradbury & Berk 2004).
Failure of very low-density lipoprotein synthesis and TG export
Hepatic clearance of LCFAs may only be accomplished by esterification to TG and export as very low-density lipoprotein (VLDL) or by oxidation. The transfer of lipid to apolipoprotein B100 by microsomal TG transfer protein (MTT) within the endoplasmic reticulum is the rate-limiting step in VLDL lipoprotein assembly. Studies in man have already demonstrated that mutations in the gene encoding this enzyme are responsible for the autosomal-recessive condition abetalipoproteinaemia that is characterized by severe hepatic steatosis which may lead to cirrhosis (Wetterau et al. 1992; Berriot-Varoqueaux et al. 2000). Several commonly used drugs (including amiodarone, doxycycline and tetracycline) are known to affect β-oxidation and have also been shown to inhibit MTT activity in animal models of drug-induced steatosis (Letteron et al. 2003; Bradbury & Berk 2004).
Failure of LCFA elimination due to impaired hepatic mitochondrial β-oxidation
Oxidation of fatty acids takes place in three cellular organelles: mitochondria, peroxisomes and endoplasmic reticulum (microsomes) (Rao & Reddy 2001). Mitochondrial β-oxidation is the main route for the metabolism of short, medium and long-chain fatty acids under normal physiological conditions (Reddy 2001). This process may be disrupted at several key enzymatic stages.
Carnitine palmonitoyl transferase 1 (CPT1)-mediated transesterification and import of fatty acids into the mitochondrial matrix is the key rate-limiting and regulatory step in this process. CPT1 is inhibited by malonyl CoA, the first intermediate in fatty acid synthesis, and so is sensitive to the effects of upregulated hepatic fatty acid synthesis (McGarry et al. 1977; McGarry 2001). If CPT1, and hence the oxidative capacity of the mitochondria, become overwhelmed, alternative oxidative pathways in other subcellular organelles process a greater proportion of the LCFA load.
Once inside the mitochondrial matrix, acyl-CoA fatty acids are degraded by four sequential enzymatic reactions to release NADH, FADH2 and acetyl-CoA (which then enters the tricarboxylic cycle). Mitochondrial trifunctional protein is a hetero-octamer composed of four α and four β subunits associated with the inner mitochondrial membrane that catalyses the final three of these reactions (Ibdah et al. 2001). In man, autosomal recessive inherited mutations in mitochondrial trifunctional protein (MTP) are associated with a steatotic Reye–like syndrome, cardiomypoathy or sudden death. There is also evidence that foetal carriage of MTP mutations is associated with HELLP syndrome and acute fatty liver of pregnancy (Ibdah et al. 2001).
Steatosis to steatohepatitis
The two-hit hypothesis dictates that, once steatosis has developed, the liver is ‘sensitized’, and so an inflammatory response may be precipitated by a variety of stimuli. As is the case in the histologically similar condition alcoholic steatohepatitis, two mechanisms are thought to be pivotal to the genesis of NASH:
Oxidative stress, lipid peroxidation and cell death
Oxidative stress has been implicated as an aetiological factor in many progressive liver diseases including alcoholic steatohepatitis, Wilson's disease, hepatitis C and exposure to toxins such as carbon tetrachloride (McClain et al. 2004). The presence of biological markers of oxidative stress has been demonstrated in both patients and animal models of steatohepatitis (Sanyal et al. 2001; George et al. 2003).
Oxidation of fatty acids within hepatocytes is a major source of reactive oxygen species (ROS) including singlet oxygen molecules, hydrogen peroxide, hydroxyl radicles and superoxide anions. As described above, mitochondrial β- oxidation is the main route for the metabolism of short, medium and long-chain fatty acids under normal physiological conditions (Reddy 2001). Within the mitochondria, the electron transport chain safely dissipates the majority of electrons released; some react to form superoxide anions and other ROS. The enzymes that mediate mitochondrial β-oxidation are regulated by peroxisome-proliferator-activated receptor α (PPARα), a member of the nuclear receptor subfamily of ligand-activated transcription factors; however, PPARα preferentially induces extra-mitochondrial β-oxidation and ω-oxidation pathways (Aoyama et al. 1998; Rao & Reddy 2001). As mitochondrial oxidative capacity becomes overwhelmed, alternative pathways in the peroxisomes (β-oxidation) and endoplasmic reticulum (cytochrome P450 enzyme -4A and -2E1-catalysed ω-oxidation) assume a greater role in hepatic fatty acid oxidation. IR further enhances CYP2E1-mediated ω-oxidation. Peroxisomal β-oxidation is not linked to the electron transport chain and so generates hydrogen peroxide. Similarly, ω-oxidation produces dicarboxylic acid and other sources of ROS that further contribute to cellular oxidative stress (Reddy 2001). At times of hepatic fatty acid overload and mitochondrial dysfunction (e.g. diabetes mellitus and obesity), these physiologically minor pathways increase the hepatocyte ROS load.
When the production of ROS exceeds the antioxidant capacity of the cell, cellular macromolecules are damaged. This includes damage to nuclear and mitochondrial DNA, phospholipid membrane disruption by lipid peroxidation and the release of proinflammatory cytokines (Pessayre et al. 2001; Browning & Horton 2004). The production of oxidative stress is further amplified by mitochondrial damage that induces loss of cytochrome c which disables the electron transport chain (Pessayre et al. 2002; McClain et al. 2004). Several different mouse models of steatohepatitis (including methionine/choline-deficient model (MCD) and ob/ob mice) are characterized by increased ROS production, mitochondrial DNA damage (as assessed by mitochondrial 8-hydroxy-2′-deoxyguanosine levels) and reduced expression of the DNA mismatch repair enzyme MutY (Gao et al. 2004). Lipid peroxidation of polyunsaturated fatty acids generates toxic aldehyde by-products including malondyaldehyde and hydroxynonenal that are more persistent than ROS and so damage more distant intracellular organelles and may cause cell death. These products directly activate fibrogenic hepatic stellate cells and are chemotactic for neutrophils, recruiting immunologically active cells into the inflammatory process (George et al. 2003). ROS may also induce Fas ligand expression on hepatocytes and promote paracrine-induced apoptotic cell death (Pessayre et al. 2001).
Pro-inflammatory cytokine-mediated hepatocyte injury
Many of the proinflammatory cytokine abnormalities that were first described in alcoholic liver disease have now been reported in NASH (Tilg & Diehl 2000). The association between TNFα expression and IR has already been discussed. Studies also demonstrate greater expression of TNFα and TNFα type 1 receptor in patients with steatohepatitis than those with steatosis alone (Crespo et al. 2001; Kugelmas et al. 2003). In addition, products of lipid peroxidation (e.g. 4-hydroxynonenal) have been shown to increase TGF-β1 expression, promoting hepatic fibrogenesis.
Animal models of NAFLD
The study of existing animal models (summarized in Table 2) has provided vital insights into the pathogenesis of steatosis and steatohepatitis, but these remain incompletely understood (Koteish & Mae 2002). The literature contains numerous different rodent models that exhibit histological evidence of hepatic steatosis. The features of true steatohepatitis that should include ballooning hepatocyte degeneration in addition to simply fatty change and an inflammatory infiltrate (Kleiner et al. 2005), are less frequently demonstrated in these models. No existing model exhibits the entire NAFLD phenotype as encountered in clinical practice, and many differ from the human disease in all but gross histological appearance. These inconsistencies and the lack of a reliable model of progressive fibrosing steatohepatitis have hampered research in this field.
Table 2.
Current mouse models of non-alcoholic fatty liver disease
| Model | Description | DM/IR | Obese | Leptin | Fibrosis |
|---|---|---|---|---|---|
| Genetic: increased lipid import/synthesis | |||||
| ob/ob mouse | Leptin deficient. Steatosis may progress to NASH after ‘second hit’. | Y | Y | ↓ | N |
| db/db mouse | Leptin receptor deficient. Steatosis may progress to NASH after ‘second hit’. | Y | Y | ↑ | ?* |
| Yellow-obese agouti (Ay) mouse | Hyperphagic: no hypothalamic appetite suppression by melanocortin. | Y | Y | – | N |
| CD36–/– mouse | Reduced peripheral lipid uptake increases fatty acid delivery to liver. Steatosis. | N | N | – | N |
| PEPCK-NSREBP-1a mouse | Lipoatrophic model. Hepatic SREBP-1a overexpression. Develops steatosis. | Y | N | ↓ | N |
| aP2-NSREBP-1c mouse | Lipoatrophic model. SREBP-1c overexpression in adipose tissue. Steatosis. | Y | N | ↓ | N |
| aP2-Diptheria toxin mouse | Lipoatrophic model. Transgenic expression of diptheria toxin in WAT. | Y | N | ↓ | N |
| Genetic: reduced lipid export/catabolism | |||||
| PPARα–/– mouse | Impaired mitochondrial β-oxidation. Develops steatosis. | N | N | – | N |
| Acetyl CoA oxidase–/– mouse | Defective β-oxidation leads to transient steatosis. | N | N | – | N |
| Aromatase (Cyp 19)-deficient mouse | Female mice lack oestrogen. Develops steatosis. | N | Y | – | N |
| MTP–/– | Key enzyme for mitochondrial β-oxidation deleted. Develops steatohepatitis. | Y | ? | ? | ? |
| Juvenile visceral steatosis mouse | Carnitine deficiency ↓ fatty acid transport into mitochondria for β-oxidation. | N | N | – | N |
| Environmental: increased lipid import/synthesis | |||||
| High fat diet | Increased adiposity, fatty acid and insulin resistance. Steatosis and NASH. | Y | Y | ↑ | ? |
| High sucrose/fructose diet | Hepatic enzyme induction and increased fatty acid synthesis. Steatosis. | Y | Y | ↑ | ? |
| Argenine deficient | Increased lipid synthesis. Steatosis due to abnormal orotic acid metabolism. | N | N | – | N |
| Environmental: reduced lipid export/catabolism | |||||
| Methionine/choline-deficient diet | Impaired mitochondrial β-oxidation. Develops steatosis, NASH and fibrosis. | N | N | – | Y |
| Steroids, oestrogen, tamoxifen | Impaired mitochondrial β-oxidation and reduced hepatic triglyceride secretion. | Y | Y | – | – |
Fibrosis may be reduced in the db/db mouse model.
Research models of NAFLD may be divided into two broad categories, those caused by either spontaneous or induced genetic mutation and those with an acquired NAFLD phenotype. The latter group may be produced by either dietary or pharmacological manipulation. The bulk of published research has employed the genetic leptin-deficient (ob/ob) or leptin-resistant (db/db) mouse and the dietary MCD model. However, these models differ significantly from the human phenotype in a number of pathogenically important ways and have led some to question whether observations made using many of these models are truly applicable to man. In this article, we will highlight the strengths and weaknesses of established models as well as summarizing those models that have been described since an earlier review (Koteish & Diehl 2001).
A model of genetically determined leptin deficiency (the ob/ob mouse)
The ob/ob mouse carries a spontaneous mutation first observed whilst intercrossing inbred mouse strains (Ingalls et al. 1950). This autosomal recessive trait renders animals hyperphagic, inactive, obese and severely diabetic with marked hyperglycaemia (Mayer et al. 1951a, b). Histological features include enlarged islets of Langerhans (consistent with compensatory hyperplasia due to IR) and hepatic fat deposition (Bleisch et al. 1952). Early parabiotic experiments demonstrated that ob/ob mice were unable to produce a satiety factor but could respond to such a factor from a donor animal (Coleman 1973; Coleman 1978). Positional cloning identified the leptin gene on chromosome 6 as the site of the ob mutation (Friedman et al. 1991; Zhang et al. 1994). This 16kDa adipokine is produced by white adipose tissue and acts on the hypothalamic ventral median nucleus to produce its prominent anorexic effects. In physiological states, leptin mediates an innate adaptive neuroendocrine response to starvation. As adipose tissue is depleted, leptin levels fall. This drives a desire to seek food and suppresses the thyroid, growth hormone, adrenal and reproductive endocrine axes (Ahima et al. 2000). Leptin influences numerous physiological processes and can directly affect the inflammatory response. Leptin-deficient ob/ob mice exist in a state of perceived starvation (Fantuzzi & Faggioni 2000). When give free access to standard chow, young ob/ob mice overeat, become obese and develop steatosis. This model has been extensively studied, although the ob mutation is not prevalent in the human obese, NASH population and leptin levels correlate poorly with the development of NASH (Chalasani et al. 2003).
Reflecting the plural activity of leptin, the ob/ob mouse is a complex model of obesity-related steatosis. Lipid and carbohydrate metabolism is deranged in several ways. The expanded white adipose tissue mass in ob/ob mice and increased expression of TNFα promote adipose tissue lipolysis releasing LCFAs. Increased circulating LCFAs are delivered to the liver (Figure 2, *1). In addition, SREBP-1c is activated and accumulates in ob/ob hepatocyte nuclei promoting fatty acid synthase activity and de novo synthesis (Figure 2, *2) (Shimomura et al. 1999). The increased synthesis and storage of lipid in the liver coincident with expanded adipose tissue stores contribute to hepatic steatosis and obesity.
There is conflicting evidence regarding the activity of hepatocyte fatty acid oxidation pathways in ob/ob mice (Figure 2, *3). Supporting the view that β-oxidation may not be appropriately upregulated in this setting, increased PPARα activity in ob/ob mice has not been demonstrated (Koteish & Diehl 2001). Although one proteomic study comparing ob/ob and lean mice demonstrates increased expression of peroxisomal 3-ketoacyl-CoA thiolase b and acyl-CoA oxidase and mitochondrial acyl-CoA dehydrogenase (Edvardsson et al. 2003), other studies indicate that there appears to be little increase in mitochondrial β-oxidation in ob/ob mice (Brix et al. 2002). However, investigators have demonstrated increased microsomal ω-oxidation activity (CYP2E1 and CYP4A) (Enriquez et al. 1999). Taken together, these findings suggest that there is little if any increase in hepatocyte β-oxidation in ob/ob mice, and that any increase is insufficient to dispose of the greater fatty acid load, necessitating the activation of the alternate microsomal ω-oxidation pathways.
Unlike the human NAFLD population, ob/ob mice do not spontaneously progress from steatosis to steatohepatitis. Ob mice require a ‘second hit’ to be administered to trigger progression to steatohepatitis. This may be provided by exposure to small doses of lipopolysaccharide endotoxin (LPS) that are insufficient to cause ill effects in control animals (Yang et al. 1997; Faggioni et al. 1999), ethanol exposure or hepatic ischaemia-reperfusion challenge which all provoke a severe steatohepatitis and frequently acute mortality (Chavin et al. 1999; Koteish & Diehl 2001). These findings underpin the assertion that steatosis sensitizes the liver to oxidative stress and free radical-mediated damage.
Leptin deficiency suppresses both the innate (monocyte/macrophage mediated) and acquired (T-lymphocyte mediated) immune responses. Hepatic depletion of natural killer T cells (NKT cells) favours a pro-inflammatory, anti-fibrotic type 1 T-helper cell (Th-1) lymphocyte polarization and so makes the steatotic liver vulnerable to LPS toxicity (Guebre-Xabier et al. 2000). It is widely accepted that TGFβ has an important regulatory role in the development of liver fibrosis (Friedman 2003). Defects in the production or post-translational modification of TGFβ prevent expression of TGFβ-dependent genes including pro-collagen type 1 and limit fibrogenesis. Studies demonstrate that leptin is necessary for the release of TNFα and the activation of TGFβ in response to liver injury. Hence, ob/ob mice are resistant to hepatic fibrosis, even when exposed to carbon tetrachloride (Honda et al. 2002; Leclercq et al. 2002; Leclercq et al. 2003). This protective effect may be reversed by concomitant leptin administration.
Using the ob/ob model, leptin treatment has also been shown to increase levels of norepinephrine which directly activates hepatic stellate cells. Similarly, treating ob/ob mice with norepinephrine has been shown to reset the Th-1/Th-2 balance in favour of a profibrotic Th-2 response with increased IL-4, IL-10 and TGFβ cytokine levels. Despite persistent leptin deficiency, norepinephrine treatment allows restitution of the depleted hepatic natural killer T-cell population and limits the Th-1-promoted LPS-induced toxicity while increasing collagen deposition and hepatic fibrosis (Oben et al. 2003; Li et al. 2004).
The effects of leptin deficiency on so many aspects of physiology increase the complexity of studies using this line. Similarly, the limited fibrotic capacity of a leptin-deficient model means that it is best suited to studies investigating the mechanisms behind the development of steatosis and the transition to steatohepatitis. Recent work demonstrates that the apparent flaws in this model can be turned to advantage, providing new insights into stellate cell function and the progression to fibrosis.
A model of genetically determined leptin resistance (the db/db mouse)
Mutations in the diabetes (db) gene, mapped to mouse chromosome 4, result in an autosomal recessive diabetic, obese phenotype similar to the ob/ob mouse (Hummel et al. 1966). The db/db mice (and the fa/fa rat) have normal or elevated levels of leptin but are resistant to its effects. Studies have shown that the db gene encodes the leptin receptor (OB-R) which is structurally similar to a class I cytokine receptor (Tartaglia et al. 1995). On binding leptin, the receptors form tetrameric complexes (two receptors with two molecules of leptin) and the intracellular domain undergo ligand-induced conformational change. Several alternative splice variants with a single transmembrane domain and a cytoplasmic region of variable length have been described (Fantuzzi & Faggioni 2000). The short OB-Ra isoform has not been shown to have any signalling activity. In contrast, the OB-Rb isoform has a long intracytoplasmic region that contains signal transduction motifs that activate the JAK/STAT protein kinase signal transduction cascade (Ghilardi et al. 1996). C57BL/Ks db/db mice carry a sequence insertion at the 3′ end of the mRNA transcript exactly where the OB-Ra and OB-Rb transcripts diverge. This insertion contains a stop codon that leads to the premature termination of the OB-Rb long intracellular signalling domain, loss of function and consequently leptin resistance (Chen et al. 1996).
Models of genetically determined increased circulating LCFAs
The models described so far demonstrate the genesis of steatosis associated with obesity, but fatty acid delivery to the liver in excess of oxidative requirements can induce steatosis, irrespective of adipose tissue mass. This has been demonstrated by modifying how fatty acids are partitioned between body tissues in non-obese animals. The transmembrane protein CD36 (fatty acid translocase) is an important fatty acid transporter expressed in peripheral tissues including muscle and adipose tissue. CD36 null mouse exhibits elevated circulating LCFA and TG levels and hepatic IR due to impaired fatty acid storage; affected mice develop steatosis and fail to suppress hepatic gluconeogenesis (Coburn et al. 2000; Goudriaan et al. 2003). In contrast, HSL-deficient animals and mice with targeted over expression of muscle lipoprotein lipase have reduced levels of circulating LCFAs and are not steatotic (Voshol et al. 2001; 2003).
Models of acquired increased hepatic LCFA uptake and de novo lipogenesis
The strong association between obesity and NAFLD has prompted study of the effects of diet on the development of steatosis and steatohepatitis in mouse models. Increased dietary supply of fat or carbohydrate to the liver may promote steatosis by increasing hepatic lipid uptake or de novo synthesis (Figure 2, *1 and *2). Complex traits such as obesity and fatty liver disease are influenced as much by subtle genetic variations between the strains studied (Silver 1995) as by the formula of the diet they are fed. The effects of sustained consumption of an elevated fat content diet (HFD) on inbred mouse strains are reported elsewhere, however, much of the existing literature is primarily concerned with effects on insulin sensitivity, glucose tolerance and obesity rather than hepatic deposition (West et al. 1992). A comprehensive database describing the biochemical and hepatic effects of 8-week consumption of an ‘atherogenic’ 15% fat diet in 42 different inbred strains of mouse and 8 mutant lines may be found in the Mouse Phenome Database (http//:www.jax.org/phenome) (Grubb et al. 2004).
HFD fed C57BL/6 mice are considered a valuable tool for investigating the metabolic syndrome (Collins et al. 2004; Winzell & Ahren 2004). With ageing, C57BL/6 mice are genetically prone to obesity, hyperinsulinaemia and glucose intolerance irrespective of diet. This phenotype may be exacerbated and florid mixed micro- and macrovesicular steatosis provoked by feeding a 55% HFD for 6 months. These observations may in part be explained by a spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene carried by C57BL/6 mice which induces glucose intolerance (Toye et al. 2005). In contrast, 129S6/SvEvTac mice develop a microsteatotic phenotype only after receiving HFD (Biddinger et al. 2005). Expression of SREBP-1c, SREBP-2 and Stearoyl-CoA desaturase 1 (an enzyme marker of hepatic lipid synthesis) was increased by HFD in both groups, but C57BL/6 mice was more sensitive to its effects (Biddinger et al. 2005). Studies using gastrostomy-based HFD overfeeding of C57BL/6 mice also demonstrate inducible steatohepatitis associated with IR, obesity, glucose intolerance and raised plasma ALT levels are associated with induction of SREBP-1c, peroxisome proliferator receptor γ (PPARγ) and Liver X receptor α (LXRα) expression and reduced expression of PPARa (Deng et al. 2005).
Diets with elevated carbohydrate content have also been used to provoke steatosis in mouse models. C57BL/6 mice fed a 65% sucrose diet for 8 weeks have been shown to exhibit obesity, IR and macrovesicular steatosis (Feldstein et al. 2003). HFD and/or high sucrose diet induced steatosis in C57BL/6 mice leads to hepatic α cell depletion, a similar Th-1 polarization to that seen in ob/ob mice and exaggerated LPS sensitivity (Li et al. 2005). This observation is significant, because it confirms that pathogenic processes underlying increased LPS sensitivity in ob/ob mice may be replicated in an aetiologically appropriate model to man, however, the mechanisms for the NKT cell depletion remain undefined (Jones 2005). HFD-induced steatohepatitis is also associated with increased hepatic expression of the endotoxin-induced macrophage receptor with a collagenous structure (MARCO) and portal endotoxin levels in some mouse strains suggesting that HFD may not only sensitize the liver to LPS but also increase portal delivery of LPS (Yoshimatsu et al. 2004).
Models of genetically determined hepatic lipogenesis
Targeted over expression of the insulin-controlled transcription factor SREBP-1 promotes hepatic lipogenesis. Two non-obese transgenic mouse models (PEPCK-nSREBP-1a mice and aP2-nSREBP-1c mice) with severe hepatic steatosis have been described (Shimano et al. 1996; Shimomura et al. 1998b). PEPCK-nSREBP-1a mice over express a truncated version of SREBP-1a in the liver under the control of a phosphoenolpyruvate carboxykinase promoter (Shimano et al. 1996). These animals exhibit histological steatosis but not steatohepatitis or dyslipidaemia, although ALT levels are elevated. aP2-nSREBP-1c mice over express SREBP-1c in adipose tissue. These animals have a lipoatrophic phenotype associated with steatosis (Shimomura et al. 1998b). Studies have shown that SREBP-1-mediated hepatic lipogenesis proceeds, even in the presence of profound IR (Shimomura et al. 1999).
Carbohydrate-mediated lipogenesis is transcriptionally regulated by ChREBP (Yamashita et al. 2001). Targeted disruption of the ChREBP gene in a mouse model has been shown to produce a 50% reduction in the expression of key enzymes that mediate lipogenesis (Iizuka et al. 2004). These data suggest that both hyperglycaemia and hyperinsulinaemia (even on a background of IR) promote hepatic steatosis. The study of rodent models has also identified that PPARγ contributes to the development of steatosis and that liver-specific deletion of PPARγ reduces steatosis.
Models of genetically determined reduced β-oxidation
Several induced and spontaneous genetic mutations that are associated with steatosis due to defective β-oxidation have been described. The mutations all affect key regulatory transcription factors, the import of LCFAs into mitochondria and enzymatic activity within the oxidative cascade. Although these models establish causality in that these mutations may provoke steatosis, few exhibit steatohepatitis or provide a stable tool for NAFLD research.
This problem with these models is exemplified by the naturally occurring juvenile visceral steatosis (JVS) mouse. In this model, systemic carnitine deficiency is induced by a mutation in the carnitine transporter gene Octn2 and leads to failure of fatty acid transport into mitochondria for β-oxidation (Kuwajima et al. 1991). This model develops extreme steatosis within days of birth. Similarly, acyl-CoA oxidase (AOX–/–) mutant mice carry a deletion in a key enzyme in peroxisomal β-oxidation. Initially, animals are phenotypically normal but over an 8-week period, mice develop severe steatosis, but this then spontaneously resolves as steatotic hepatocytes are replaced. Mice carrying a homozygote PPARα knockout do not accumulate fat under normal fed conditions but fail to upregulate fatty acid oxidation and so develop severe steatosis when fatty acid delivery to the liver is increased by fasting (Kersten et al. 1999; Koteish & Diehl 2001).
A recently described model of NAFLD exhibits a strong phenotypic resemblance to the human disease. MTP is a key enzyme mediating mitochondrial β-oxidation. Homozygous carriage of an MTPα mutation causes perinatal death. Recently published data demonstrate that carriage of the heterozygous mutation is associated with a progressive, age-related elevation of serum ALT levels. By 9–10 months, mutant mice exhibit hepatic steatosis, basal hyperinsulinaemia and evidence of IR and impaired glucose tolerance during glucose tolerance testing (Ibdah et al. 2001; Ibdah et al. 2005). Mutant animals have also been shown to have higher antioxidant activity of total superoxide dismutase and lower glutathione levels as well as increased CYP2E1 expression, consistent with increased hepatic oxidative stress.
Models of acquired reduced β-oxidation (methionine/choline deficiency)
Impaired β-oxidation may be induced by drugs including the oestrogen antagonist Tamoxifen or the CPT1 inhibitor etomoxir (Koteish & Diehl 2001), however, the main research model in which an acquired defect in mitochondrial β- oxidation is used to induce steatosis is based on feeding a diet deficient in methionine and choline.
Choline is an FDA-classified essential nutrient with roles in cell membrane integrity, transmembrane signalling, phosphatidylcholine synthesis, neurotransmission and methyl metabolism. The role of dietary choline deficiency in promoting hepatic steatosis and reduced plasma VLDL levels is well established in the literature [reviewed in (Zeisel & Blusztajn 1994)]. This was thought to be due to impaired synthesis of phosphatidylcholine resulting in diminished VLDL assembly and secretion and consequently reduced TG clearance (Yao & Vance 1990) (Figure 2, *4). Recent observations now cast doubt on this explanation and suggest further mechanistic study is required to define the role of choline deficiency in steatosis (Kulinski et al. 2004). Mice fed a diet that is deficient in both choline and the essential amino acid methionine (MCD) develop inflammation and hepatic fibrosis in addition to simple steatosis (Weltman et al. 1996). The magnitude of the effect of this diet varies according to the species, strain and gender of animals studied (Kirsch et al. 2003). Evidence suggests that MCD impairs mitochondrial β-oxidation and leads to induction of alcohol-inducible CYP2E1 expression (Weltman et al. 1996): a finding later confirmed in a cohort of NASH patients (Weltman et al. 1998) (Figure 2, *3). These findings support the hypothesis that alcoholic and non- alcoholic steatohepatitis may share pathogenic mechanisms. ROS produced by CYP2E1 ω-oxidation, coupled with depletion of hepatic anti-oxidants (e.g. reduced glutathione and s-adenosylmethionine), promotes oxidative stress and induces an histological steatohepatitis. The histological features are associated with elevated plasma TNFα levels (Chawla et al. 1998) and may be ameliorated by treatment with pentoxifylline (Koppe et al. 2004). The MCD diet induces significantly greater ROS production, mitochondrial DNA damage and apoptotic cell death than many of the other mouse models of NAFLD (Gao et al. 2004).
The MCD model is arguably the best-established model with which to study the inflammatory and fibrotic elements of the NAFLD spectrum. Despite this, there is little evidence to support the assertion that this model replicates either the phenotype or the pathogenic mechanisms of metabolic syndrome-related NAFLD. In contrast to human fatty liver disease, animals fed the MCD diet are cachectic (50% weight loss compared with control mice by 10 weeks), have low plasma TG levels and reduced liver weight/body weight ratio. The histological distribution of hepatic steatosis differs from the pattern seen in humans where a periportal rather than perivenous deposition is seen (Koteish & Diehl 2001). MCD fed mice also exhibit a markedly more elevated plasma ALT level than that seen in patients with NASH. This model is also not overtly insulin resistant (Rinella & Green 2004), although there has been some recently published data indicating that CYP2E1 expression and consequent oxidative stress may impair insulin signalling by reducing tyrosine phosphorylation and increasing serine phosphorylation of IRS-1 (Schattenberg et al. 2005).
Combined models of genetically and environmentally determined NAFLD
Several groups have attempted to bridge the phenotype gap that exists between available models and the human disease. This work has involved combining genetic and dietary manipulation to produce more severe, accelerated steatohepatitis or fibrosing steatohepatitis. The literature reports a number of such combination models including Abcb11 mutant mice fed an MCD diet (Sundaram et al. 2005), apolipoprotein E mutant mice fed an high fat diet (Tous et al. 2005) and PPARα null mice fed MCD (Kashireddy & Rao 2004). These models are all characterized by the development of histological steatohepatitis.
Feeding steatotic db/db mice, the MCD diet is shown to produce an accelerated fibrosing steatohepatitis (Sahai et al. 2003). In this model, hepatic pro-collagen type 1 mRNA levels were found to be 10-fold increased in db/db mice while non-steatotic db/m mice had a 4-fold rise, and ob/ob mice showed no increase in fibrogenesis with MCD feeding. The authors conclude that their data demonstrate the development of an obese/diabetic experimental model of progressive NASH and suggest an important role for the short-form leptin receptor in steatohepatitis.
Our own group has approached this problem in a different way. To identify relevant pathogenic genes and novel models of steatohepatitis, we have collaborated in a random N-ethyl-N-nitrosourea chemical mutagenesis screen and assayed offspring for insulin resistant, diabetic and NASH phenotypes. Initial phenotypic data from one line derived from this screen demonstrate a stable model of impaired glucose tolerance/IR with increased body weight and histological evidence of NASH strongly resembling the human NASH phenotype (Anstee et al. 2003).
Conclusion
The increasing prevalence of obesity, diabetes and IR and NAFLD within Western society makes research in this field imperative. It is only through better understanding of pathogenic mechanisms that novel therapies targeting both the steatotic/steatohepatitic process and fibrogenesis may be discovered. Animal models of NAFLD have provided phenotypically consistent tools with which disease pathogenesis may be dissected allowing us to appreciate NAFLD as one facet of a systemic disorder rather than an organ-specific phenomenon.
Acknowledgments
Q. M. Anstee is a Medical Research Council funded Clinical Research Fellow.
References
- Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- Almind K, Doria A, Kahn CR. Putting the genes for type II diabetes on the map. Nat. Med. 2001;7:277–279. doi: 10.1038/85405. [DOI] [PubMed] [Google Scholar]
- Angulo P, Lindor KD. Treatment of non-alcoholic steatohepatitis. Best Pract. Res. Clin. Gastroenterol. 2002;16x:797–810. doi: 10.1053/bega.2002.0327. [DOI] [PubMed] [Google Scholar]
- Anstee QM, Wright M, Goldin R, et al. A Novel murine model of non-alcoholic steatohepatitis generated using a sensitised ENU mutagenesis screen. Hepatology. 2003;1(Supplement):469A. [Google Scholar]
- Aoyama T, Peters JM, Iritani N, et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J. Biol. Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n – 6/n – 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin. Sci. (Lond.) 2004;106:635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 2000;20:663–697. doi: 10.1146/annurev.nutr.20.1.663. [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes. 2005;54:1314–1323. doi: 10.2337/diabetes.54.5.1314. [DOI] [PubMed] [Google Scholar]
- Bleisch VR, Mayer J, Dickie MM. Familial diabetes mellitus in mice, associated with insulin resistance, obesity, and hyperplasia of the islands of langerhans. Am. J. Pathol. 1952;28:369–385. [PMC free article] [PubMed] [Google Scholar]
- Bradbury MW, Berk PD. Lipid metabolism in hepatic steatosis. Clin. Liver Dis. 2004;8:639–671. doi: 10.1016/j.cld.2004.04.005. xi. [DOI] [PubMed] [Google Scholar]
- Brix AE, Elgavish A, Nagy TR, Gower BA, Rhead WJ, Wood PA. Evaluation of liver fatty acid oxidation in the leptin-deficient obese mouse. Mol. Genet. Metab. 2002;75:219–226. doi: 10.1006/mgme.2002.3298. [DOI] [PubMed] [Google Scholar]
- Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt EM. Nonalcoholic steatohepatitis. Semin. Liver Dis. 2004;24:3–20. doi: 10.1055/s-2004-823098. [DOI] [PubMed] [Google Scholar]
- Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- Carvalho E, Jansson PA, Nagaev I, Wenthzel AM, Smith U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin- stimulated glucose transport. FASEB J. 2001;15:1101–1103. [PubMed] [Google Scholar]
- Chalasani N, Crabb DW, Cummings OW, et al. Does leptin play a role in the pathogenesis of human nonalcoholic steatohepatitis? Am. J. Gastroenterol. 2003;98:2771–2776. doi: 10.1111/j.1572-0241.2003.08767.x. [DOI] [PubMed] [Google Scholar]
- Chavin KD, Yang S, Lin HZ, et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J. Biol. Chem. 1999;274:5692–5700. doi: 10.1074/jbc.274.9.5692. [DOI] [PubMed] [Google Scholar]
- Chawla RK, Watson WH, Eastin CE, Lee EY, Schmidt J, McClain CJ. S-adenosylmethionine deficiency and TNF-alpha in lipopolysaccharide-induced hepatic injury. Am. J. Physiol. 1998;275:G125–G129. doi: 10.1152/ajpgi.1998.275.1.G125. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9:294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- Coleman DL. Obese and diabetes: two mutant genes causing diabetes–obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol. Behav. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Crespo J, Cayon A, Fernandez-Gil P, et al. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- Day CP. Pathogensis of steatohepatitis. Best. Pract. Res. Clin. Gastroenterol. 2002;16:663–678. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- Day CP, James OF. Steatohepatitis: a tale of two ‘hits’? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- Deng QG, She H, Cheng JH, et al. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42:905–914. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87:81–86. doi: 10.1016/j.biochi.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Dragani TA, editor. Human Polygenic Diseases: Animal Models. Australia: Harwood; 1998. [Google Scholar]
- Edvardsson U, von Lowenhielm HB, Panfilov O, Nystrom AC, Nilsson F, Dahllof B. Hepatic protein expression of lean mice and obese diabetic mice treated with peroxisome proliferator-activated receptor activators. Proteomics. 2003;3:468–478. doi: 10.1002/pmic.200390061. [DOI] [PubMed] [Google Scholar]
- Enriquez A, Leclercq I, Farrell GC, Robertson G. Altered expression of hepatic CYP2E1 and CYP4A in obese, diabetic ob/ob mice, and fa/fa Zucker rats. Biochem. Biophys. Res. Commun. 1999;255:300–306. doi: 10.1006/bbrc.1999.0202. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Fantuzzi G, Gabay C, et al. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am. J. Physiol. 1999;276:R136–R142. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J. Leukoc. Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J. Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- Fletcher LM, Bridle KR, Crawford DH. Effect of alcohol on iron storage diseases of the liver. Best Pract. Res. Clin. Gastroenterol. 2003;17:663–677. doi: 10.1016/s1521-6918(03)00020-9. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Liver fibrosis – from bench to bedside. J. Hepatol. 2003;38(Suppl. 1):S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Leibel RL, Siegel DS, Walsh J, Bahary N. Molecular mapping of the mouse ob mutation. Genomics. 1991;11:1054–1062. doi: 10.1016/0888-7543(91)90032-a. [DOI] [PubMed] [Google Scholar]
- Gao D, Wei C, Chen L, Huang J, Yang S, Diehl AM. Oxidative DNA damage and DNA repair enzyme expression are inversely related in murine models of fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G1070–G1077. doi: 10.1152/ajpgi.00228.2004. [DOI] [PubMed] [Google Scholar]
- George J, Pera N, Phung N, Leclercq I, Yun Hou J, Farrell G. Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis. J. Hepatol. 2003;39:756–764. doi: 10.1016/s0168-8278(03)00376-3. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan JR, Dahlmans VE, Teusink B, et al. CD36 deficiency increases insulin sensitivity in muscle, but induces insulin resistance in the liver in mice. J. Lipid Res. 2003;44:2270–2277. doi: 10.1194/jlr.M300143-JLR200. [DOI] [PubMed] [Google Scholar]
- Grubb SC, Churchill GA, Bogue MA. A collaborative database of inbred mouse strain characteristics. Bioinformatics. 2004;20:2857–2859. doi: 10.1093/bioinformatics/bth299. [DOI] [PubMed] [Google Scholar]
- Guebre-Xabier M, Yang S, Lin HZ, Schwenk R, Krzych U, Diehl AM. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000;31:633–640. doi: 10.1002/hep.510310313. [DOI] [PubMed] [Google Scholar]
- Honda H, Ikejima K, Hirose M, et al. Leptin is required for fibrogenic responses induced by thioacetamide in the murine liver. Hepatology. 2002;36:12–21. doi: 10.1053/jhep.2002.33684. [DOI] [PubMed] [Google Scholar]
- Hourigan LF, Macdonald GA, Purdie D, et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. 1999;29:1215–1219. doi: 10.1002/hep.510290401. [DOI] [PubMed] [Google Scholar]
- Hu KQ, Kyulo NL, Esrailian E, et al. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J. Hepatol. 2004;40:147–154. doi: 10.1016/s0168-8278(03)00479-3. [DOI] [PubMed] [Google Scholar]
- Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- Ibdah JA, Paul H, Zhao Y, et al. Lack of mitochondrial trifunctional protein in mice causes neonatal hypoglycemia and sudden death. J. Clin. Invest. 2001;107:1403–1409. doi: 10.1172/JCI12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibdah JA, Perlegas P, Zhao Y, et al. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology. 2005;128:1381–1390. doi: 10.1053/j.gastro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J. Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- Jones DE. Fat is an immuno-regulatory issue. Hepatology. 2005;42:755–758. doi: 10.1002/hep.20908. [DOI] [PubMed] [Google Scholar]
- Kahn CR. Banting Lecture: Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- Kashireddy PV, Rao MS. Lack of peroxisome proliferator-activated receptor alpha in mice enhances methionine and choline deficient diet-induced steatohepatitis. Hepatol. Res. 2004;30:104–110. doi: 10.1016/j.hepres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch R, Clarkson V, Shephard EG, et al. Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J. Gastroenterol. Hepatol. 2003;18:1272–1282. doi: 10.1046/j.1440-1746.2003.03198.x. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Koppe SW, Sahai A, Malladi P, Whitington PF, Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J. Hepatol. 2004;41:592–598. doi: 10.1016/j.jhep.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Koteish A, Diehl AM. Animal models of steatosis. Semin. Liver Dis. 2001;21:89–104. doi: 10.1055/s-2001-12932. [DOI] [PubMed] [Google Scholar]
- Koteish A, Mae DA. Animal models of steatohepatitis. Best. Pract. Res. Clin. Gastroenterol. 2002;16:679–690. doi: 10.1053/bega.2002.0332. [DOI] [PubMed] [Google Scholar]
- Kugelmas M, Hill DB, Vivian B, Marsano L, Mcclain CJ. Cytokines and Nash: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- Kulinski A, Vance DE, Vance JE. A choline-deficient diet in mice inhibits neither the CDP-choline pathway for phosphatidylcholine synthesis in hepatocytes nor apolipoprotein B secretion. J. Biol. Chem. 2004;279:23916–23924. doi: 10.1074/jbc.M312676200. [DOI] [PubMed] [Google Scholar]
- Kuwajima M, Kono N, Horiuchi M, et al. Animal model of systemic carnitine deficiency: analysis in C3H-H-2 degrees strain of mouse associated with juvenile visceral steatosis. Biochem. Biophys. Res. Commun. 1991;174:1090–1094. doi: 10.1016/0006-291x(91)91532-h. [DOI] [PubMed] [Google Scholar]
- Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J. Hepatol. 2002;37:206–213. doi: 10.1016/s0168-8278(02)00102-2. [DOI] [PubMed] [Google Scholar]
- Leclercq IA, Field J, Farrell GC. Leptin-specific mechanisms for impaired liver regeneration in ob/ob mice after toxic injury. Gastroenterology. 2003;124:1451–1464. doi: 10.1016/s0016-5085(03)00270-1. [DOI] [PubMed] [Google Scholar]
- Letteron P, Sutton A, Mansouri A, Fromenty B, Pessayre D. Inhibition of microsomal triglyceride transfer protein: another mechanism for drug-induced steatosis in mice. Hepatology. 2003;38:133–140. doi: 10.1053/jhep.2003.50309. [DOI] [PubMed] [Google Scholar]
- Li Z, Oben JA, Yang S, et al. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40:434–441. doi: 10.1002/hep.20320. [DOI] [PubMed] [Google Scholar]
- Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- Ludwig J, Viggiano TR, Mcgill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- Mayer J, Bates MW, Dickie MM. Hereditary diabetes in genetically obese mice. Science. 1951;113:746–747. doi: 10.1126/science.113.2948.746. [DOI] [PubMed] [Google Scholar]
- Mayer J, Dickie MM, Bates MW, Vitale JJ. Free selection of nutrients by hereditarily obese mice. Science. 1951;113:745–746. doi: 10.1126/science.113.2948.745-a. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Mokshagundam SP, Barve SS, et al. Mechanisms of non-alcoholic steatohepatitis. Alcohol. 2004;34:67–79. doi: 10.1016/j.alcohol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Mcgarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- Mcgarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J. Clin. Invest. 1977;60:265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon RA, Feingold KR, Moser AH, Fuller J, Grunfeld C. Regulation of fatty acid transport protein and fatty acid translocase mRNA levels by endotoxin and cytokines. Am. J. Physiol. 1998;274:E210–E217. doi: 10.1152/ajpendo.1998.274.2.E210. [DOI] [PubMed] [Google Scholar]
- Oben JA, Roskams T, Yang S, et al. Norepinephrine induces hepatic fibrogenesis in leptin deficient ob/ob mice. Biochem. Biophys. Res. Commun. 2003;308:284–292. doi: 10.1016/s0006-291x(03)01360-3. [DOI] [PubMed] [Google Scholar]
- Pessayre D, Berson A, Fromenty B, Mansouri A. Mitochondria in steatohepatitis. Semin. Liver Dis. 2001;21:57–69. doi: 10.1055/s-2001-12929. [DOI] [PubMed] [Google Scholar]
- Pessayre D, Mansouri A, Fromenty B. Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G193–G199. doi: 10.1152/ajpgi.00426.2001. [DOI] [PubMed] [Google Scholar]
- Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689–692. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- Rao MS, Reddy JK. Peroxisomal beta-oxidation and steatohepatitis. Semin. Liver Dis. 2001;21:43–55. doi: 10.1055/s-2001-12928. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- Reddy JK. Nonalcoholic steatosis and steatohepatitis. III. Peroxisomal beta-oxidation, PPAR alpha, and steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1333–G1339. doi: 10.1152/ajpgi.2001.281.6.G1333. [DOI] [PubMed] [Google Scholar]
- Reeves HL, Burt AD, Wood S, Day CP. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J. Hepatol. 1996;25:677–683. doi: 10.1016/s0168-8278(96)80238-8. [DOI] [PubMed] [Google Scholar]
- Reid AE. Nonalcoholic steatohepatitis. Gastroenterology. 2001;121:710–723. doi: 10.1053/gast.2001.27126. [DOI] [PubMed] [Google Scholar]
- Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J. Hepatol. 2004;40:47–51. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Sahai A, Malladi P, Green RM, Whitington PF. Steatohepatitis and liver fibrosis associated with upregulated osteopontin expression in diabetic/insulin-resistant db/db mice fed a methionine and choline deficient diet. Hepatology. 2003;38:497A. [Google Scholar]
- Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- Schattenberg JM, Wang Y, Singh R, Rigoli RM, Czaja MJ. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J. Biol. Chem. 2005;280:9887–9894. doi: 10.1074/jbc.M410310200. [DOI] [PubMed] [Google Scholar]
- Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J. Clin. Invest. 1996;98:1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Richardson JA, et al. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Shimano H, Korn BS, Bashmakov Y, Horton JD. Nuclear sterol regulatory element-binding proteins activate genes responsible for the entire program of unsaturated fatty acid biosynthesis in transgenic mouse liver. J. Biol. Chem. 1998;273:35299–35306. doi: 10.1074/jbc.273.52.35299. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J. Obes. Relat Metab Disord. 2003;27(Suppl. 3):S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- Silver LM. Mouse Genetics: Concepts and Applications. New York, Oxford: Oxford University Press; 1995. [Google Scholar]
- Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J. Hepatol. 2001;35:195–199. doi: 10.1016/s0168-8278(01)00094-0. [DOI] [PubMed] [Google Scholar]
- Sundaram SS, Whitington PF, Green RM. Steatohepatitis develops rapidly in transgenic mice overexpressing Abcb11 and fed a methionine-choline-deficient diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G1321–G1327. doi: 10.1152/ajpgi.00455.2004. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987–990. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N. Engl. J. Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- Tous M, Ferre N, Camps J, Riu F, Joven J. Feeding apolipoprotein E-knockout mice with cholesterol and fat enriched diets may be a model of non-alcoholic steatohepatitis. Mol. Cell Biochem. 2005;268:53–58. doi: 10.1007/s11010-005-2997-0. [DOI] [PubMed] [Google Scholar]
- Toye AA, Lippiat JD, Proks P, et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia. 2005;48:675–686. doi: 10.1007/s00125-005-1680-z. [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- Voshol PJ, Haemmerle G, Ouwens DM, et al. Increased hepatic insulin sensitivity together with decreased hepatic triglyceride stores in hormone-sensitive lipase-deficient mice. Endocrinology. 2003;144:3456–3462. doi: 10.1210/en.2002-0036. [DOI] [PubMed] [Google Scholar]
- Voshol PJ, Jong MC, Dahlmans VE, et al. In muscle-specific lipoprotein lipase-overexpressing mice, muscle triglyceride content is increased without inhibition of insulin-stimulated whole-body and muscle-specific glucose uptake. Diabetes. 2001;50:2585–2590. doi: 10.2337/diabetes.50.11.2585. [DOI] [PubMed] [Google Scholar]
- Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am. J. Physiol. 1992;262:R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- Wetterau JR, Aggerbeck LP, Bouma ME, et al. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992;258:999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl. 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Takenoshita M, Sakurai M, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ZM, Vance DE. Reduction in VLDL, but not HDL, in plasma of rats deficient in choline. Biochem. Cell Biol. 1990;68:552–558. doi: 10.1139/o90-079. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu M, Terasaki Y, Sakashita N, et al. Induction of macrophage scavenger receptor MARCO in nonalcoholic steatohepatitis indicates possible involvement of endotoxin in its pathogenic process. Int. J. Exp. Pathol. 2004;85:335–343. doi: 10.1111/j.0959-9673.2004.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu. Rev. Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]