Abstract
Aplastic anaemia (AA) in man is an often fatal disease characterized by pancytopenia of the peripheral blood and aplasia of the bone marrow. AA is a toxic effect of many drugs and chemicals (e.g. chloramphenicol, azathioprine, phenylbutazone, gold salts, penicillamine and benzene). However, there are no widely used or convenient animal models of drug-induced AA. Recently, we reported a new model of chronic bone marrow aplasia (CBMA = AA) in the busulphan (BU)-treated mouse: eight doses of BU (10.50 mg/kg) were administered to female BALB/c mice over a period of 23 days; CBMA was evident at day 91/112 post-dosing with significantly reduced erythrocytes, platelets, leucocytes and nucleated bone marrow cell counts. However, mortality was high (49.3%). We have now carried out a study to modify the BU-dosing regime to induce CBMA without high mortality, and investigated the patterns of cellular responses in the blood and marrow in the post-dosing period. Mice (n = 64/65) were dosed 10 times with BU at 0 (vehicle control), 8.25, 9.0 and 9.75 mg/kg over 21 days and autopsied at day 1, 23, 42, 71, 84, 106 and 127 post-dosing (n = 7–15); blood and marrow samples were examined. BU induced a predictable bone marrow depression at day 1 post-dosing; at day 23/42 post-dosing, parameters were returning towards normal during a period of recovery. At day 71, 84, 106 and 127 post-dosing, a stabilized, late-stage, nondose-related CBMA was evident in BU-treated mice, with decreased erythrocytes, platelets and marrow cell counts, and increased MCV. At day 127 post-dosing, five BU-treated mice showed evidence of lymphoma. In this study, mortality was low, ranging from 3.1% (8.25 mg/kg BU) to 12.3% (9.75 mg/kg BU). It is concluded that BU at 9.0 mg/kg (or 9.25 mg/kg) is an appropriate dose level to administer (10 times over 21 days) to induce CBMA at approximately day 50–120 post-dosing.
Keywords: aplastic anaemia, busulphan, chronic bone marrow aplasia, haematology, mouse, toxicity
Aplastic anaemia (AA) is a failure of haemopoiesis resulting from injury to haemopoietic stem cells; AA involves all the bone marrow-derived cell lineages, myeloid, erythroid and platelets (Young & Alter 1994; Jandl 1996). AA is characterized by a hypocellular marrow and peripheral blood pancytopenia; the condition carries a risk of life-threatening infection, severe anaemia and major haemorrhage (Jandl 1996; Young & Maciejewski 2000). In European countries, the incidence of AA is about 1.5–2.3 per million of the population per annum (Gordon-Smith & Issaragrissil 1992; Heimpel 2000; Young & Maciejewski 2000). In nonsevere AA, the rate of recovery is about 50%; in severe AA, the chances of spontaneous recovery are approximately 10%. However, in the recovered patient, the condition may be associated with later complications of relapse and clonal evolution to myelodysplasia, acute myeloid leukaemia and paroxysmal nocturnal haemoglobinuria (Jandl 1996; Tooze et al. 1999). Once the patient with AA has been stabilized with blood, platelet transfusions and antibiotics, the current mainstays of treatment are bone marrow transplantation and immunosuppression with antilymphocyte (antithymocyte) globulin with or without cyclosporin (Young & Alter 1994; Young 1995).
Although AA in man may be associated with several rare congenital or inherited conditions (Alter & Young 1998; Freedman 2000), the majority of cases are acquired (Heimpel & Heit 1980; Heimpel 2000). The principal causes of acquired AA are considered to be radiation, infectious agents, and drugs and chemicals (Alter et al. 1978; Young 1995; Young & Maciejewski 2000). Nevertheless, in as many as 70% of patients, no identifiable causal link can be determined (idiopathic AA) (Young & Maciejewski 1997). Apart from the predictable, dose-dependent bone marrow suppression that occurs following anticancer chemotherapy (Young & Alter 1994), and which is normally reversible (Reynolds 1989; Dollery 1999; Sweetman 2002), acquired AA is idiosyncratic in that it occurs unpredictably in a small proportion of the population exposed to a particular drug, chemical or other agent. This idiosyncratic nature of acquired AA may indicate an underlying genetic predisposition (Marsh et al. 1999) or evidence of a pre-existing bone marrow susceptibility resulting from an earlier marrow insult or defect.
AA is also seen in some animal species (e.g. cat, dog, bovine, sheep, pig, ferret, horse and chicken), where the aetiology is generally considered to be infectious agents, drugs, toxins and radiation, or the disease may have no known cause (idiopathic AA) (Weiss 2000). However, there are examples of experimentally induced AA in several animal species. Here, the agents used include radiation (Klassen et al. 1972; Speck & Kissling 1973; Knospe 1988), drugs (Morley & Blake 1974a; Krishna et al. 1981; Den Ottolander et al. 1982), chemicals (Moeschilin & Speck 1967; Lock et al. 1996), hormones (oestrogens) (Hart 1985; Sherrill & Gorman 1985; Hart 1990), antibodies (Nettlelship 1942; Knospe et al. 1983, 1994; Wolk et al. 1998) and infective agents (Haak 1980; Camitta et al. 1982; Binder et al. 1998). The animal species involved include the mouse, rat, rabbit, ferret, dog and cow. Although the fundamental pathophysiology of AA in man is not well understood, it is generally considered that none of the above experimental models is convenient to use (Alter et al. 1978; Haak 1980; Vincent 1984), and none has become widely employed in attempts to elucidate the pathogenesis of the human disease (Benestad 1979; Vincent 1986; Young & Maciejewski 1997). Similarly, no experimental models are currently used in preclinical toxicity studies in the pharmaceutical industry to assess the potential of new drugs to induce AA (see Young & Keisu 1996; Macharia et al. 1999a).
However, Morley and colleagues (Morley & Blake 1974a, 1974b; Morley et al. 1975) described a mouse model of busulphan (BU)-induced chronic hypoplastic marrow failure which showed late-stage marrow aplasia/hypoplasia, and the animals demonstrated evidence of ‘residual’ bone marrow injury. In this model, BU was administered to Swiss or BALB/c female mice on four occasions at 14-day intervals (i.e. over a 6-week period) at 20, 20, 20 and 10 mg/kg; the animals were then studied over the following 300–400 days. We have investigated these reports (Andrews et al. 1993, 1997, 1998; Andrews 2000) using modifications of the original protocols but have been unable to obtain results which compare closely with those of Morley and Blake (1974a). Accordingly, various regimens of BU dosing were re-examined in an attempt to produce an easily used mouse model of drug-induced AA, showing features of the human condition, and which developed within a relatively short period (about 120 days). These investigations, to induce chronic bone marrow aplasia (CBMA) in the mouse, have been reported (Diamanti et al. 1999; Macharia et al. 1999a, 1999b; Gibson et al. 2003). In essence, female BALB/c mice were treated with BU on eight occasions at 10.50 mg/kg over a 23-day period, and the animals were autopsied on five occasions over the following 112 days (days 1, 19, 41, 91 and 112 post-dosing). At 91/112 days post-dosing, mice showed evidence of CBMA with significantly reduced RBC, leucocytes, platelets and marrow-nucleated cell counts; MCV was increased. However, mortality in BU-treated mice was high (49.3%). Therefore, there was a need to modify the basic model further and define a more appropriate BU dosing regimen which would not cause high mortality. We also wished to examine the BU-induced changes in blood and marrow at a greater number of autopsy time points, as this would allow a clearer definition of the patterns of change in the various cellular responses during the post-dosing period. We now report a study to investigate these factors. A brief preliminary report has been published in abstract form (Sones et al. 2000).
Materials and methods
Animals
Female weanling BALB/c mice (A. Tuck and Son Ltd, Battlesbridge, Essex, UK) were caged in groups of 12–15, bedded on wood shavings, with diet (Rat and Mouse no. 1, SDS Ltd, Witham, Essex, UK) and mains drinking water provided ad libitum. A light : dark cycle of 12 : 12 h was maintained (lights on at 07.00 hours), with a temperature of 19–22 °C and a relative humidity of 45–65%. Animals were acclimatized for 10 days before the start of the experiment and were observed daily for evidence of ill health. Body weights were recorded twice weekly or at appropriate times. All procedures were conducted under local Ethical Committee guidelines and approval for Home Office Project and Personal licences, and followed the UK Home Office (1989) Code of Practice.
Administration of bulsulphan
Busulphan (Sigma Chemical, Poole, Dorset, UK) was dissolved in acetone at a concentration of 5–8 mg/ml following the technique of Gibson et al. (2003). Immediately prior to dosing, deionized water was added to the BU–acetone solution at a volume of approximately 5 ml of water to 1 ml of acetone solution. The solution was administered by intraperitoneal injection at a dose volume of 0.1–0.2 ml per mouse. Control animals were given acetone : water (vehicle) at the same dose volume and by the same route. The dose of drug administered during the 21-day dosing period was not corrected for changes in body weight.
Tissue sampling
Animals were killed by injection of pentobarbitone sodium (Sagatal, Rhône Mérieux, Harlow, Essex, UK). Blood was removed from the right ventricle following a thoracotomy incision. Blood (0.5 ml) was anticoagulated with 1.5 mg/ml dipotassium EDTA (Teklab Ltd, Sacriston, Durham, UK); the marrow contents of the right femur were aspirated into 1.0 ml of phosphate-buffered saline (PBS) to prepare a cell suspension for the measurement of the femoral marrow nucleated cell count (FNCC). A marrow smear was made from the contents of the right tibia (Smith et al. 1994). Here, the bone is exposed and the proximal epiphysis removed. Using a fine camel hair paint brush moistened in PBS, the brush is touched on the exposed marrow surface and the cells streaked as a series of parallel lines onto a glass slide. The preparation is left to air dry for 24 h, fixed in 100% methyl alcohol for 1 h and air dried prior to staining. For the measurement of levels of apoptosis in femoral marrow nucleated cells, the left femur was removed and the marrow contents flushed into 1 ml of Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Paisley, UK) containing 10% foetal calf serum (FCS; PAA Laboratories GmbH, Linz, Austria) and supplemented with 100 IU/ml of penicillin–streptomycin; samples were stored on ice. The spleen and sternum were removed and placed in 10.5% phosphate-buffered formalin fixative (14 days fixation); the thymus gland was removed from selected animals (five mice with gross evidence of lymphoma at autopsy and three control mice at 127 days post-dosing) and processed in a similar way. In the case of two of five BU-treated mice with gross changes at autopsy (day 127) which indicated the pressure of lymphoma, blood smears were prepared from the peripheral blood sample, and spleen impressions (touch preparations; imprints) were made from a transverse cut surface of the organ.
Analysis of blood, marrow suspensions and tissue samples
Blood was analysed using a Bayer H*I haematology analyser with mouse-specific software (Bayer Diagnostics UK, Newbury, Berks, UK). Reticulocyte analysis was performed with a Sysmex R-1000 (Sysmex UK, Milton Keynes, Bucks, UK). The FNCC of the marrow cell suspension in PBS was obtained from the basophil channel of the H*I analyser. Marrow and blood smears, and spleen impressions, were stained with May–Grünwald–Giesma. After fixation, the spleen was weighed and the weight expressed as absolute and relative weight (i.e. in relation to body weight). Sections of spleen, thymus and sternum from selected animals were prepared and stained with haematoxylin and eosin for histological assessment. The sternum was decalcified in buffered formic acid (Kristenson's solution) for 10 days at room temperature prior to staining (Bancroft & Stevens 1990).
Apoptosis in marrow mononuclear cells
Marrow samples in IMDM were analysed for apoptosis as described previously (Philpott et al. 1995a, 1995b). Diluted marrow was centrifuged on Ficoll-Hypaque (Amersham Pharmacia Biotech, St Albans, Herts, UK) at 400 g for 25 min at room temperature to obtain mononuclear cells (MNCs), which were washed twice in PBS supplemented with 1% foetal calf serum and 0.05% azide. Cell number and viability were assessed after trypan blue staining; bone marrow cell suspension in IMDM (10 µl) was mixed with 10 µl of trypan blue solution (0.4%; Sigma) and immediately loaded onto a haemocytometer slide for assessment of cell number and viability. MNCs (1 × 106) were incubated with 7-amino-actinomycin D (7-AAD; Philpott et al. 1995a, 1995b). 7-AAD (Calbiochem–Novabiochem, Nottingham, UK) was dissolved in acetone and diluted in PBS to a concentration of 200 µg/ml. This was kept at −20 °C and protected from light. 50 µl of 7-AAD solution was added to 106 cells, suspended in 1.0 mL PBS and mixed by vortexing. The cells were stained for 20 min at 4 °C, protected from light and pelleted by centrifugation. The supernatant was removed and cells resuspended in 500 µl of 2% paraformaldehyde solution (Sigma). Unstained cells were negative controls. Samples were analysed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA) within 30 min of fixation; control unstained cells were used to adjust the FACScan settings to measure specific 7-AAD fluorescence in positively stained samples. Data on 20,000 cells was acquired and processed using Lysys II software (Becton Dickinson). Regions were drawn around clear-cut populations having negative (R1), dim (R2), and bright (R3) 7-AAD fluorescence, corresponding to live, apoptotic and dead cells, respectively. The proportion of cells within each region was calculated.
Statistical analysis
Treated and control groups were routinely compared using Student's t-test for unpaired samples using Microsoft Excel (Microsoft Corporation, Microsoft UK, Reading, Berks, UK). For data on mortality and the incidence of lymphoma, results were analysed using a single-tailed Fisher's exact test. Data on blood parameters from individual animals were analysed (anova) using linear regression (least-squares) and tested using Pearson's correlation and t approximation (Microsoft Excel and Analyse-it Software Ltd, Leeds, Yorkshire, UK).
Experimental design
Two hundred and fifty-nine female BALB/c mice, mean body weight 15.0 g, were divided into four groups: group 1 (vehicle control), n = 65; group 2 (BU, 8.25 mg/kg), n = 64; group 3 (BU, 9.0 mg/kg), n = 65; group 4 (BU, 9.75 mg/kg), n = 65. Mice were dosed with vehicle or BU on 10 occasions over a period of 21 days (days 1, 3, 6, 8, 10, 13, 15, 17, 19 and 21). At 1, 23, 42, 71, 84, 106 and 127 days after the final BU dose, mice from each group (n = 7–15) were autopsied for blood and marrow examination.
Results
Clinical signs, mortality and body weight changes
In control (vehicle-dosed) and BU-treated (at all three dose levels) mice, there was no evidence of drug-induced toxicity during the 21-day dosing period. In the immediate post-dosing period (days 1–20 post-dosing), there was some evidence of a transient slight loss of condition in some BU animals (the fur becoming dull and dry), but such mice rapidly regained their normal appearance. However, over the whole post-dosing period (day 1–127), a small number of BU-treated animals showed an unexpected and rapid loss of condition. Such mice were killed when it was considered that they would not recover or, on occasion, some animals were found dead (Table 1). These mice, over a period of days, showed a rapid deterioration in the condition of the fur, a reduction in activity and responses, and an abnormal gait and hunched posture; often the ears, paws and tails of these individuals lost the normal pink colouration and became white. A total of 13 (of 194, i.e. 6.7%) mice were affected, two at 8.25 mg/kgBU, three at 9.0 mg/kg and eight at 9.75 mg/kg, giving some evidence of a dose-related effect. Mice appeared to become ill during two periods of the study, with a total of six animals affected from day 5–36 and seven animals from day 83–121 post-dosing.
Table 1.
Unexpected mortality† in female BALB/c mice treated with busulphan (BU) at 8.25, 9.0 and 9.75 mg/kg on 10 occasions over a period of 21 days‡ and studied for 127 days after the final BU dose
| BU group (mg/kg) | Number of mice in group§ | Number of mortalities (%)¶ |
|---|---|---|
| Control | 65 | 0 (0) |
| 8.25 | 64 | 2 (3.1) |
| 9.0 | 65 | 3 (4.6) |
| 9.75 | 65 | 8 (12.3)** |
P < 0.01 in comparison with controls; single-tailed Fisher's exact test.
Mice either became ill and were killed when it was considered that they would not recover or, on occasion, animals were found dead.
There were no mortalities during the dosing period.
Number of mice in each group at the beginning of the dosing period.
Days of mortalities: 8.25 mg/kg BU, days 12 and 113 post-dosing; 9.0 mg/kg, days 85, 93 and 121 post-dosing; 9.75 mg/kg, days 5, 13 and 19 (two mice), 36, 83, 93 and 98 post-dosing.
One mouse treated with BU at 9.0 mg/kg was found at autopsy (day 127 post-dosing) to have a cataract in the left eye.
All animals were weighed on 13 occasions throughout the study (on days 2, 7, 14 and 21 of the dosing period and on days 6, 16, 22, 41, 64, 70, 83, 105 and 125 of the post-dosing period). The mean weight of all animals at the beginning of the dosing period was 15.0 g. During the 21-day dosing period, and during the period from days 1–125 post-dosing, the overall mean body weight increases were 15.3 and 37.9% for the control group; 8.5 and 30.9% for the 8.25 mg/kg BU group; 5.8 and 33.6% for the 9.0 mg/kg group; and 7.6 and 33.5% for the 9.75 mg/kg group. Therefore, mean body weight increases were not related to the dose levels of BU administered. However, from days 64–105 post-dosing (involving four weighing points), the mean body weights of all BU-treated groups were significantly reduced in comparison with the mean weight of the control animals.
Haematology findings
Results are presented in Table 2. At day 1 post-dosing, BU at all dose levels caused a predictable bone marrow depression, but there was no clear evidence that the degree of change in the parameters affected was related to the BU dose levels. In general, there were BU-induced decreases in RBC and Hb and increases in MCV and MCH. The WBCs were reduced in BU-treated mice, as were individual leucocytes; platelet counts were reduced at all BU dose levels; the FNCC and relative spleen weights were decreased.
Table 2.
Haematological results from female BALB/c mice treated with busulphan (BU) at 8.25, 9.0 and 9.75 mg/kg on 10 occasions over a period of 21 days and sampled at days 1, 23, 42, 71, 84, 106 and 127 after the final dose
| Autopsy (days post-dosing) | BU group (mg/kg) | RBC | Hb | MCV | MCH | Retic | WBC | Neut | Lymph | Mono | Plt | FNCC | Spleen | n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Control | 10.77 | 16.0 | 46.6 | 14.8 | 257.7 | 2.7 | 0.54 | 1.99 | 0.07 | 811 | 12.96 | 5548 | 9 |
| 8.25 | 10.06** | 15.4 | 48.3 | 15.3 | 237.9 | 2.0* | 0.15*** | 1.76 | 0.02*** | 138*** | 6.20*** | 4766** | 7 | |
| 9.0 | 6.97** | 10.8* | 51.3* | 15.8* | 158.9** | 1.0*** | 0.09*** | 0.87** | 0.01*** | 107*** | 3.57*** | 4798* | 7 | |
| 9.75 | 8.22** | 13.0* | 50.1* | 16.0*** | 244.1 | 1.4*** | 0.11*** | 1.27** | 0.02*** | 135*** | 4.93*** | 4461* | 7 | |
| 23 | Control | 10.66 | 15.9 | 48.6 | 14.9 | 307.1 | 1.7 | 0.34 | 1.41 | 0.04 | 881 | 10.11 | 5044 | 7 |
| 8.25 | 10.17* | 16.6** | 53.5*** | 16.4*** | 282.5 | 1.5 | 0.32 | 1.05* | 0.04 | 474*** | 6.19*** | 4445 | 7 | |
| 9.0 | 8.98*** | 14.4* | 52.8 | 16.1 | 407.1 | 1.6 | 0.59 | 0.95* | 0.02 | 430** | 4.94** | 7293 | 7 | |
| 9.75 | 9.45** | 14.9** | 52.3* | 15.9* | 251.4 | 2.2 | 0.83* | 1.25 | 0.04 | 629* | 6.76* | 5871 | 7 | |
| 42 | Control | 10.25 | 15.6 | 49.8 | 15.3 | 224.4 | 1.9 | 0.40 | 1.24 | 0.06 | 797 | 10.79 | 5195 | 7 |
| 8.25 | 9.76* | 15.3 | 51.2* | 15.8** | 285.8* | 1.3* | 0.25** | 0.97* | 0.04 | 599*** | 8.83* | 4484* | 7 | |
| 9.0 | 9.44*** | 15.3 | 51.7** | 16.2** | 266.6 | 0.9*** | 0.22*** | 0.61*** | 0.02* | 610*** | 8.40* | 4225* | 7 | |
| 9.75 | 10.01 | 15.7 | 50.7* | 15.7** | 230.1 | 0.9*** | 0.21** | 0.63*** | 0.03 | 645*** | 9.11 | 4558* | 7 | |
| 71 | Control | 10.55 | 15.5 | 47.4 | 14.7 | 309.6 | 1.7 | 0.29 | 1.20 | 0.05 | 934 | 11.94 | 4521 | 7 |
| 8.25 | 10.40 | 15.8 | 49.1** | 15.2* | 257.0* | 1.5 | 0.31 | 1.08 | 0.04 | 568** | 10.19* | 4012* | 7 | |
| 9.0 | 9.77*** | 14.8** | 48.6* | 15.1* | 287.0 | 1.2 | 0.24 | 0.82 | 0.03 | 589** | 8.66** | 4217 | 7 | |
| 9.75 | 10.05** | 15.4 | 48.8* | 15.3** | 222.1** | 1.2* | 0.23 | 0.84* | 0.03 | 553*** | 9.30*** | 4328 | 7 | |
| 84 | Control | 10.31 | 15.3 | 47.7 | 14.8 | 270.7 | 1.9 | 0.40 | 1.27 | 0.08 | 932 | 15.24 | 4491 | 8 |
| 8.25 | 10.21 | 15.4 | 49.0*** | 15.1*** | 267.8 | 1.7 | 0.34 | 1.18 | 0.04** | 655*** | 11.49* | 3896*** | 8 | |
| 9.0 | 9.99* | 15.2 | 50.2*** | 15.2*** | 282.2 | 1.2* | 0.35 | 0.77* | 0.03** | 691*** | 10.49* | 3977** | 8 | |
| 9.75 | 9.73* | 14.9 | 50.5** | 15.4* | 271.6 | 1.4 | 0.32 | 0.92 | 0.05 | 566*** | 11.29 | 4368 | 8 | |
| 106 | Control | 10.39 | 15.7 | 47.9 | 15.1 | 250.7 | 1.4 | 0.29 | 0.93 | 0.05 | 935 | 13.13 | 4376 | 10 |
| 8.25 | 10.28 | 15.8 | 48.5* | 15.4** | 271.2 | 2.4** | 0.52** | 1.59** | 0.07 | 731*** | 9.26*** | 3614*** | 10 | |
| 9.0 | 9.31** | 14.7* | 50.7** | 15.7* | 286.5 | 1.0 | 0.26 | 0.62* | 0.03 | 598*** | 8.45*** | 4250 | 10 | |
| 9.75 | 9.94** | 15.5 | 48.9* | 15.7*** | 238.1 | 1.2 | 0.34 | 0.74 | 0.04 | 643*** | 9.70* | 3981* | 10 | |
| 127 | Control | 10.24 | 15.5 | 48.4 | 15.1 | 240.7 | 1.4 | 0.31 | 0.99 | 0.05 | 938 | 13.81 | 4086 | 15 |
| 8.25 | 9.91* | 15.2 | 49.0 | 15.4 | 272.4* | 1.5 | 0.39 | 0.96 | 0.05 | 658*** | 10.36*** | 3695* | 8 | |
| 9.0 | 9.77*** | 15.3 | 50.1*** | 15.6*** | 239.6 | 1.4 | 0.43* | 0.90 | 0.05 | 699*** | 9.39*** | 3520*** | 15 | |
| 9.75 | 9.85* | 15.3 | 49.3 | 15.3* | 239.7 | 1.3 | 0.38 | 0.76 | 0.04* | 599*** | 9.65*** | 3607** | 11 |
P < 0.05.
P < 0.01.
P < 0.001.
Values are means; P-values are significantly different to control animals.
Abbreviations and units: RBC, red blood cells (×106/µl); Hb, haemoglobin (g/dl); MCV, mean cell volume (fl); MCH, mean cell haemoglobin (pg); Retic, absolute reticulocyte count (×103/µl); WBC, white blood cells (×103/µl); Neut, neutrophils (×103/µl); Lymph, lymphocytes (×103/µl); Mono, monocytes (×103/µl); Plt, platelets (×103/µl); FNCC, femoral nucleated cell count (×106); Spleen, relative spleen weight (mg/kg body weight); n, number of mice per group. At day 127, lymphoma-bearing mice (n = 5) were not included in the analysis of the 8.25 and 9.0 mg/kg BU group results; blood samples from two mice sampled at day 127 (8.25 mg/kg BU) could not be analysed due to the presence of clots.
Three animals treated with BU and sampled at day 1 post-dosing out of a total of 21 BU-treated mice autopsied at this time point had erythrocyte counts below 6.0 × 106 µl (control mean 10.77 × 106 µl). Two of these three mice with significant bone marrow depression also showed very low platelet and WBC counts and high MCV values in the peripheral blood, and the bone marrow FNCC counts were also significantly reduced; the individual results for these two mice are shown (Table 3A).
Table 3.
(A) Individual haematology results from two mice (Mouse 1 and Mouse 2) treated with 10 doses of busulphan (BU) at 9.0 mg/kg and autopsied at day 1 after the final BU dose†, and (B) from two mice (Mouse 3 and Mouse 4) treated with BU at 9.75 and 9.0 mg/kg and autopsied on days 84 and 106 post-dosing, respectively‡
| A (Day 1) | B (Day 84/106) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | BU | Mouse 1 | Mouse 2 | Control | BU | Mouse 3 | Mouse 4 | |
| RBC | 10.77 | 6.97* | 2.15 | 2.83 | 10.39 | 9.31** | 7.92 | 6.33 |
| Hb | 16.0 | 10.8* | 3.8 | 4.6 | 15.7 | 14.7* | 13.2 | 10.9 |
| HCT | 0.511 | 0.345* | 0.133 | 0.150 | 0.497 | 0.469* | 0.437 | 0.367 |
| MCV | 46.6 | 51.3* | 62.0 | 52.9 | 47.9 | 50.7** | 55.2 | 58.0 |
| MCH | 14.8 | 15.8* | 17.7 | 16.3 | 15.1 | 15.7* | 16.7 | 17.3 |
| MCHC | 31.3 | 30.9 | 28.5 | 30.8 | 31.6 | 31.0* | 30.2 | 29.8 |
| Retic | 257.7 | 158.9** | 156.0 | 93.7 | 250.7 | 286.5 | 349.4 | 476.5 |
| Plt | 811 | 107*** | 112 | 50 | 935 | 598*** | 101 | 25 |
| WBC | 2.7 | 1.0*** | 0.2 | 0.2 | 1.4 | 1.0 | 0.5 | 0.3 |
| Neut | 0.54 | 0.09*** | 0.02 | 0.01 | 0.29 | 0.26 | 0.04 | 0.04 |
| Lymph | 1.99 | 0.87** | 0.22 | 0.14 | 0.93 | 0.62* | 0.44 | 0.26 |
| Mono | 0.07 | 0.01*** | 0.00 | 0.00 | 0.05 | 0.03 | 0.01 | 0.00 |
| Eo | 0.06 | 0.00*** | 0.00 | 0.00 | 0.11 | 0.09 | 0.01 | 0.00 |
| Baso | 0.01 | 0.00** | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| FNCC | 12.96 | 3.57*** | 0.7 | 0.9 | 13.13 | 8.45*** | 2.0 | 1.2 |
| Spleen | 5,548 | 4,798* | 5,917 | 5,071 | 4,376 | 4,250 | 5,714 | 5,095 |
P < 0.05.
P < 0.01.
P < 0.001.
Group mean results at day 1 post-dosing for control mice, and for animals treated with BU at 9.0 mg/kg, are included for comparison.
Group mean results at day 106 post-dosing for control mice, and for animals treated with BU at 9.0 mg/kg, are included for comparison.
Abbreviations and units: Baso, basophils (×103/µl); Eo, eosinophils (×103/µl); HCT, haematocrit (l/l); MCHC, mean cell haemoglobin concentration (g/dl). All other information as Table 2.
At 23 and 42 days post-dosing, the general pattern of change in BU mice was of a ‘period of recovery’ with a return of parameters towards normal (Table 2). However, at these time points, the values for RBC, platelets and FNCC in BU mice were still lower than in the controls, and MCV/MCH values were still raised. Nevertheless, the mean relative spleen weight was similar to the controls at day 23 post-dosing; however, in the case of two mice treated at 9.0 mg/kg BU, and 1 mouse at 9.75 mg/kg, the relative spleen weights were 194, 328 and 197% of the mean control value (control = 100%), respectively. At day 42 post-dosing, the mean relative spleen weight was reduced at all BU dose levels.
At the later stages of the study (days 71, 84, 106 and 127 post-dosing), the overall pattern of response in BU mice was of nondose-related significant reductions in RBC, platelets, FNCC and relative spleen weight, and elevated MCV/MCH values (Table 2).
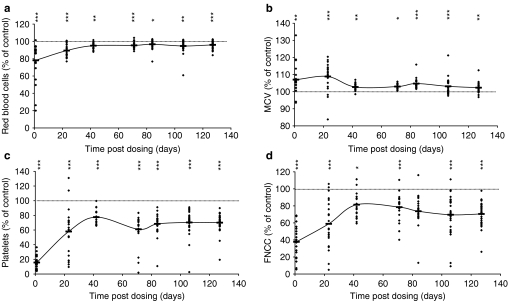
To investigate the changing patterns of cell responses, in time, where parameters were affected by BU administration (i.e. RBC, platelets, FNCC, MCV/MCH and spleen weight), results from individual animals were analysed at each BU dose level and the data expressed as percentage increase/decrease, in relation to the mean control value at each of the seven autopsy time points (data not shown). However, no consistent dose-related effects of BU on these affected parameters could be identified. Therefore, data for the three BU dose levels were pooled at each autopsy time point. Results are illustrated in Figure 1a–d for RBC, MCV, platelets and FNCC, respectively. It is seen that the general pattern of response consisted of an initial significant decrease (increase for MCV) at day 1 post-dosing, followed by a period of recovery with parameters returning towards normal, and a final late, stabilized, plateau phase (days 71, 84, 106 and 127) with mean values consistently decreased (increased for MCV). The average percentage reduction for RBC over the four late-stage autopsy points was to 95.7% of the control; for platelets the figure was 67.4%; for FNCC, 73.1%, and for relative spleen weight, 90.4%. The figure was 103.3% of control for MCV. From an examination of Figure 1a–d, it is considered that values for RBC, platelets, FNCC and MCV would have entered the late-stage, stabilized plateau phase at about day 50 post-dosing.
Figure 1.
Haematological parameters of individual mice, expressed as a percentage of the mean control value at each time point at 1, 23, 42, 71, 84, 106 and 127 days after busulphan (BU) dosing; data from animals treated with BU at 8.25, 9.0 and 9.75 mg/kg are included. (a) Red blood cells; (b) mean cell volume; (c) platelets and (d) femoral marrow nucleated cell count. Horizontal bars indicate group mean values of pooled data; *P < 0.05, **P < 0.01 and ***P < 0.001 are presented vertically above the data points.
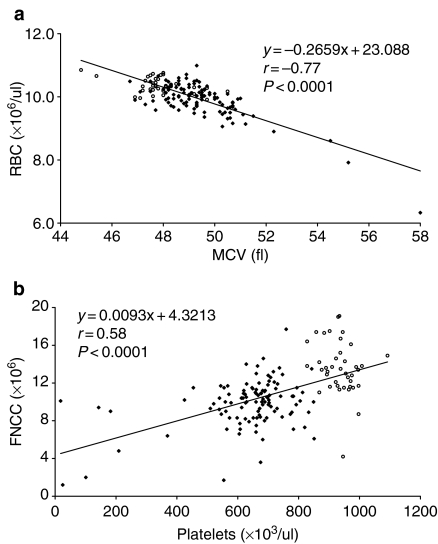
An analysis was carried out to examine the relationships between the various parameters affected by BU dosing in individual animals at days 71, 84, 106 and 127 post-dosing. In individual BU-treated mice with reduced RBC, there was evidence of a concomitant increase in MCV and a corresponding reduction in platelets and FNCC. Furthermore, the degree of the reduction in RBC, in individual animals, was related to the magnitude of the change in the other affected parameters. Two examples of these data are given (Figure 2a,b), for RBC : MCV and platelets : FNCC.
Figure 2.
Haematological parameters and line of best fit for individual mice sampled during the stabilized late plateau phase at 71, 84, 106 and 127 days after busulphan (BU) administration; results for control animals (open circles) and from animals treated with BU at 8.25, 9.0 and 9.75 mg/kg (solid diamonds) are included. (a) Relationship between red blood cell count and mean cell volume; (b) relationship between femoral marrow nucleated cell count and platelet count.
Two mice out of a total of 109 treated with BU and examined at days 71, 84, 106 and 127, had particularly low RBC, platelet, and FNCC values, and high MCV values; the reticulocyte counts and spleen weights of these two mice were slightly increased. The individual results for these two animals are presented in Table 3B.
At the autopsy at day 127 post-dosing, five BU-treated mice were found to have enlarged spleens (weight increases of up to sixfold over the control mean spleen weight) and enlarged thymus glands. The thymus glands from these animals, and from control mice, were taken for histological examination. The haematological investigation of these mice revealed the presence of lymphoma cells in the blood and marrow (Tables 4 and 5). Although the spleens of the five BU-treated mice were enlarged, the peripheral blood picture of each animal was relatively normal, except for some variability (compared to the control animals) in the reticulocyte, platelet, WBC, neutrophil and lymphocyte counts. However, the LUC counts in the peripheral blood showed an increase in each animal due to (it is assumed) the presence of circulating lymphoma cells. It is considered that these results suggest that the development of the lymphoma in each case was at a relatively early stage. No evidence of these various changes which may be associated with lymphoma had been seen in animals before the autopsy at day 127 post-dosing. A final diagnosis of lymphoma was made for the five BU-treated mice from the above evidence, in conjunction with information from the histology of spleen, thymus and sternal marrow and the marrow smears; in the case of two of the five animals, the appearance of the spleen impressions (touch preparations; imprints) and the blood smears was also helpful.
Table 4.
The number of mice with lymphoma in animals treated with busulphan (BU) at 8.25, 9.0 and 9.75 mg/kg on 10 occasions over 21 days and autopsied at 127 days after the final dose†
| BU group (mg/kg) | Number of mice sampled | Number of mice with lymphoma (%) |
|---|---|---|
| Control | 15 | 0 (0) |
| 8.25 | 14 | 4 (28.6)* |
| 9.0 | 16 | 1 (6.3) |
| 9.75 | 11 | 0 (0) |
P < 0.05 in comparison with controls; single-tailed Fisher's exact test.
No mice showed evidence of lymphoma before day 127.
Table 5.
Haematology results from five female BALB/c mice treated with 10 doses of busulphan (BU) over a period of 21 days and showing evidence of lymphoma at autopsy (day 127 post-dosing)*
| Control mice (SD)† | Mice with lymphoma‡ | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| RBC | 10.24 (0.25) | 12.03 | 10.39 | 10.50 | 9.84 | 11.28 |
| Hb | 15.5 (0.4) | 16.8 | 15.6 | 16.1 | 15.0 | 16.0 |
| HCT | 0.496 (0.013) | 0.562 | 0.494 | 0.503 | 0.469 | 0.560 |
| MCV | 48.4 (1.3) | 46.7 | 47.5 | 47.9 | 47.7 | 49.7 |
| MCH | 15.1 (0.4) | 14.0 | 15.0 | 15.3 | 15.3 | 15.0 |
| MCHC | 31.3 (0.7) | 29.9 | 31.7 | 32.0 | 32.0 | 30.2 |
| Retic | 240.7 (42.0) | 436.4 | 82.1 | 158.0 | –§ | 221.1 |
| Plt | 938 (64) | 549 | 70 | 418 | 509 | 384 |
| WBC | 1.4 (0.4) | 11.1 | 3.7 | 3.9 | 3.5 | 22.8 |
| Neut | 0.31 (0.09) | 1.27 | 2.07 | 2.02 | 1.05 | 13.79 |
| Lymph | 0.99 (0.32) | 6.15 | 1.24 | 1.70 | 1.87 | 6.62 |
| Mono | 0.05 (0.02) | 0.25 | 0.18 | 0.07 | 0.21 | 0.78 |
| Eo | 0.07 (0.04) | 0.06 | 0.00 | 0.01 | 0.17 | 0.08 |
| Baso | 0.00 (0.01) | –¶ | 0.04 | 0.02 | 0.04 | –¶ |
| LUC | 0.01 (0.01) | 3.38** | 0.21** | 0.11** | 0.20** | 0.95** |
| FNCC | 13.81 (1.98) | 3.2 | 8.9 | 8.1 | 9.4 | 9.0 |
| Spleen | 4,086 (426) | 19,222 | 26,176 | 12,800 | 26,783 | 17,684 |
The initial diagnosis of lymphoma was on the basis of the presence of an enlarged spleen and thymus, and significant numbers of lymphoma cells in the blood, marrow or spleen. No mice with lymphoma were identified before day 127 post-dosing.
Mean (SD) results from 15 control mice autopsied at day 127 are included for comparison. All other information as Tables 2 and 3, except: LUC, large unstained cells (×103/µl).
A total of 15 control mice were sampled at day 127, 14 mice at 8.25 mg/kg BU, 16 mice at 9.0 mg/kg BU, and 11 mice at 9.75 mg/kg BU. Mice 1, 2, 3 and 4 were given BU at 8.25 mg/kg and mouse 5 at 9.0 mg/kg. All other information as Table 4.
Inadequate sample volume for analysis.
Basophil counts are not presented due to interference from lymphoma cells.
LUC cells in lymphoma-bearing mice include lymphoma cells and atypical lymphocytes.
Histological examination of tissues and marrow smears
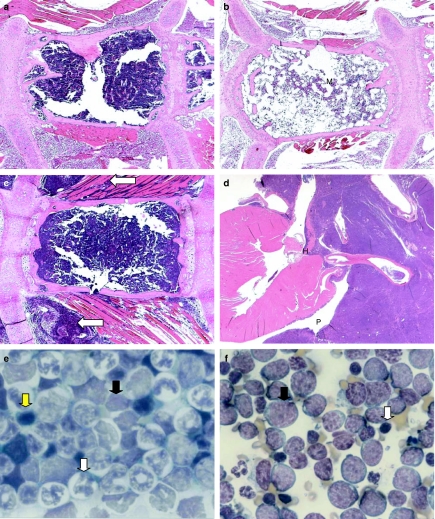
Sections of sternum, spleen and thymus, and marrow smears from selected animals (control and BU-treated) were examined histologically. At day 1 post-dosing, in BU-treated mice, the sternal bone marrow showed a depletion of all cell types (Figure 3a,b). At day 23, in most cases, the cellularity of the sternal marrow was seen to be returning towards normal, but in a few animals, significant marrow depletion was still evident. At 42 days post-dosing, normal levels of marrow cellularity were seen in all BU-treated animals examined. Lymphomatous infiltrates were apparent in the sternal marrow of several BU-treated animals at day 127 post-dosing.
Figure 3.
Haematoxylin and eosin-stained sections of mouse tissues (a–d), and May–Grünwald–Giemsa-stained tibial bone marrow smears (e, f), from control (vehicle-treated) and BU-treated animals. (a) Control mouse sternum at day 1 post-dosing showing (M) normal marrow cellularity [original magnification (OM) ×40]. (b) BU-treated (9.0 mg/kg) mouse at day 1 post-dosing illustrating (M) marked hypocellularity of the sternal bone marrow (OM ×40). (c) BU-treated (8.25 mg/kg) mouse at day 127 post-dosing; the thoracic muscle fibres are infiltrated with lymphoma cells (white arrows) (OM ×40). (d) BU-treated (8.25 mg/kg) mouse at 127 days post-dosing; lymphoma cells originating in the thymus are invading the base of the heart (H) and pericardial sac (P) (OM ×10). (e) Bone marrow smear from a control mouse at 127 days post-dosing illustrating the normal distribution of myeloid (white arrow), erythroid (yellow arrow) and lymphoid (black arrow) elements (OM ×1000). (f) Marrow smear from a BU-treated mouse (9.0 mg/kg) at day 127 post-dosing to show numerous lymphoma cells (black arrow); some normal lymphoid elements (white arrow) are also present (OM ×1000).
In the spleen, in all BU-treated animals at day 1 post-dosing, normal (i.e. minimal) levels of haemopoiesis were present. However, levels of haemopoiesis were significantly increased (i.e. to marked levels) at day 23; at day 42 post-dosing, the levels had decreased considerably (i.e. to moderate levels). At day 127, levels of splenic haemopoiesis in some BU-treated mice were normal (i.e. at a minimal level, as specified above), but in other cases haemopoiesis had not returned to the base line control levels.
Lymphoma was observed in the thymus gland and spleen of several BU-treated mice at day 127 post-dosing, and in some cases the tumour had extended into the sternal marrow and adjacent soft tissues (trachea, thoracic muscle and heart; Figure 3c,d). The tumours were of a lymphocytic type (i.e. lymphocytic lymphoma) in that they comprised lymphocytes of a well-differentiated appearance.
Examination of the tibial marrow smears from mice at day 127 post-dosing demonstrated that morphologically the lymphoma cell was a large oval cell with intense cytoplasmic basophilia, a high nuclear : cytoplasmic ratio, a relatively immature nucleus with nucleoli present and occasional cytoplastic vacuolation (Figure 3e,f).
A unilateral cataract was present in the eye of a mouse autopsied at day 127 after BU treatment.
Marrow mononuclear cell apoptosis
Studies on the levels of apoptosis in the nucleated cells of the femoral marrow were carried out in control mice and animals treated with BU at 8.25, 9.0 and 9.75 mg/kg on days 106 and 127 post-dosing. However, no significant or consistent patterns of change in the levels of apoptosis could be identified in BU-dosed animals.
Discussion
In a recent report, we described a new model of CBMA in the BU-treated BALB/c mouse (Gibson et al. 2003). In this model, BU was administered on eight occasions over a 23-day period at 10.50 mg/kg (and 5.25 mg/kg), and the animals were autopsied at 1, 19, 41, 91 and 112 days after dosing. BU induced a predictable bone marrow depression at day 1 post-dosing, with a return of values towards normal at days 19 and 41; however, at 91 and 112 days post-dosing there was evidence of a late-stage bone marrow depression with reduced peripheral blood RBC, platelets, individual leucocytes and decreased marrow nucleated cell counts. Nevertheless, BU administration at 10.50 mg/kg caused high mortality (49.3%). The main objectives of the present investigation were therefore, first, to identify a dose level of BU that could be used to induce CBMA but which did not cause significant mortality, and second, to define clearly the patterns of response (in time) of the various parameters affected by BU administration. The results presented here demonstrate that the administration of BU on 10 occasions over a 21-day period at a dose level of 9.0 mg/kg induced only a low mortality (4.6%). Also, the patterns of response for parameters affected by BU administration (RBC, platelets and FNCC) consisted of an initial predictable depression (day 1 post-dosing), followed by a period of recovery lasting until approximately day 50 post-dosing; a late-stage, stabilized, plateau phase was then evident, with depressed RBC, platelets and FNCC counts; this ‘plateau phase’ period was apparent from about day 50 post-dosing until the end of the study. Such changes in RBC, platelets and marrow cell counts, in conjunction with an increased MCV, are important and characteristic features of AA in man.
In the present study, levels of mortality appeared to be related to the three dose levels of BU administered (Table 1). A comparable dose-related effect was found in the earlier investigation (Gibson et al. 2003), where mortality at 5.25 mg/kg BU was 0%, and at 10.50 mg/kg BU, 49.3%. Morley and Blake (1974a) also reported significant mortality in their repeat-dose BU-treated mouse study; 494 animals began BU treatment, 472 (95.5%) survived BU dosing, and at day 60 post-dosing, 325 (65.8%) were alive.
Table 2 demonstrated that BU administered at 8.25, 9.0 and 9.75 mg/kg did not cause consistent dose-related haematological changes at day 1 post-dosing, or during the late-stage plateau phase (days 71, 84, 106 and 127 post-dosing). This contrasts with the earlier investigation (Gibson et al. 2003) where clear dose-related effects were seen in many haematological parameters with BU administered at 5.25 and 10.50 mg/kg.
In the present study, during the late-stage plateau phase, no reductions in WBC or individual leucocytes (neutrophils, lymphocytes and monocytes) were evident. However, in the earlier investigation (Gibson et al. 2003), WBC, neutrophils, lymphocytes and monocytes were significantly reduced at day 91/112 post-dosing in animals treated at 10.50 mg/kg BU, but not at 5.25 mg/kg. It is considered possible therefore that the lack of an effect on leucocytes in the present study may be related to the lower dose levels of BU used (i.e. being below 10.50 mg/kg). Similarly, the lack of an effect of BU on levels of apoptosis in marrow nucleated cells at day 106/127 in the present study contrasts with the findings in the earlier investigation (Gibson et al. 2003), where apoptosis was significantly increased at day 91/112 post-dosing. Again, the absence of a change in levels of marrow cell apoptosis may related to the lower levels of BU used in the present study.
In the report of Morley and Blake (1974a) where a mouse model of BU-induced chronic hypoplastic marrow failure is described, animals at day 60 post-dosing were reported as entering a latent phase during which they exhibited only minor haematological changes in comparison with the control animals. However, Morley and Blake (1974a) stated that between day 60 and day 313 post-dosing, a small number of individual latent mice developed a significant bone marrow aplasia with body weight loss, pallor and loss of condition, and a marked reduction in all peripheral blood cells and marrow counts; such animals were referred to as ‘aplastic’ mice. Comparing the observations of Morley and Blake (1974a, 1974b) and Morley et al. (1975) with the results of the present study, mice sampled at days 71, 84, 106 and 127 post-dosing may equate to latent phase mice. However, two mice out of a total of 109 autopsied at these last four time points, showed more significant bone marrow depression (Table 3B). As the neutrophil and platelet counts of these individual mice were particularly low, it is possible that a more severe aplasia was developing, and these animals may therefore compare with (Morley & Blake 1974a) aplastic animals.
At the autopsy at day 127, of a total of 41 BU-treated animals sampled, five mice (12.2%) were identified with evidence of lymphoma (Table 4 and 5). These animals showed features of an enlarged thymus and splenomegaly, infiltration of the sternal marrow with lymphoma cells and the presence of lymphoma cells in the thymus gland, spleen, tibial marrow smear and peripheral blood. There was no evidence that the incidence of animals with lymphoma was related to the dose level of BU administered (Table 4).
Morley & Blake (1974a) described the presence of lymphoma in 15 of 494 (3.0%) BU-treated Swiss mice given four doses of the drug at 14-day intervals; in the investigation of Gibson et al. (2003), where female BALB/c mice were given eight doses of BU, one of the seven mice autopsied at day 112 post-dosing showed evidence of lymphoma. The development of lymphoma in BU-treated female B6C3F1 mice was also described in the earlier studies of Andrews (2000); in this investigation, where mice were given four doses of BU (10–40 mg/kg), lymphoma cells were identified in 21 animals between 174 and 437 days post-dosing. Using cytochemical techniques, Andrews (2000) described a localized pattern of cytoplasmic staining for alpha-naphthyl acetate esterase and acid phosphatase in the lymphoma cells and considered this indicated that the cells were of a T-cell lineage (after descriptions of Krueger 1990). The morphological features of the lymphoma cell in the present investigation compare closely with the report of Andrews (2000) and suggest therefore that the cell identified here may also possibly be of T-cell origin. However, it is not possible to state with certainty which organ may have been the site of origin for neoplastic development.
The induction of lymphomas in BU-treated mice in the present investigation at day 127 post-dosing is consistent with the findings of other workers (Upton et al. 1961; Conklin et al. 1965; Robin et al. 1981; Bhoopalam et al. 1986). However, the identification of tumours at 127 days is considerably earlier than reported previously (Andrews 2000). Also, the presence of lymphoma in the thymus in all tumour cases in the present study is understandable, in the light of the work of Bhoopalam et al. (1986) who demonstrated that lymphomas induced by BU were of a T-cell origin. Nevertheless, spontaneous lymphoblastic and lymphocytic lymphoma does occur commonly in mice (Frith & Wiley 1981; Wogan 1984; Scales & Andrews 1991); in the BALB/c strain, spontaneous incidences of up to 6.7% in ageing animals are reported (Frith et al. 1983; Krueger 1990). In the B6C3F1 mouse, the incidence of lymphoma in ageing animals is given as 8.3 (males) and 16.8% (females) (Ward et al. 1979) or 8.3 (males) and 20.9% (females) (Haseman et al. 1998). In Eppley Swiss mice, the incidence in older animals is reported to be about 12% in males and about 27% in females (Clayson 1984).
One BU-treated mouse autopsied at day 127 was found to have a unilateral cataract, but on histological examination, no other lesions were found to be present in the eye. BU is known to be a potent cataractogenic agent (Solomon et al. 1955; Grimes et al. 1964; Grimes & Von Sallmann 1966; Gehring 1971), and there is evidence for BU-induced cataract in man (Podos & Canellos 1969; Ravindranathan et al. 1972; Potts 1986). The mechanism of these changes is considered to be a drug-induced reduction in the mitotic activity and proliferation of the lens epithelium with the lens eventually becoming entirely opaque (Von Sallmann 1957; Gehring 1971; Potts 1986). It is considered that the finding of a cataract in one mouse in the present study was probably related to BU treatment, as lenticular opacities have been recorded following BU administration (Conklin et al. 1965). However, this cannot be definitely stated as similar lesions are known to occasionally develop spontaneously in the BALB/c strain (Frith et al. 1983).
The present study has demonstrated that the administration of BU to female BALB/c mice on 10 occasions over 21 days induces a stabilized and long-lasting mild CBMA from about day 50 post-dosing. Animals showed significantly reduced RBC, platelets and marrow nucleated cells, and an increased MCV; these changes parallel several of the important features of AA in man. From the results of mortality at 8.25, 9.0 and 9.75 mg/kg BU (Table 1), it is suggested that administration of the drug at 9.0 (or 9.25) mg/kg would be expected to induce only a very low mortality. The mild, but statistically significant, nature of the haematological changes induced during the late-stage plateau phase make this model of CBMA particularly suitable for assessing the effect of the administration of a second agent from approximately day 50 post-dosing (Macharia et al. 1999a). For example, the administration of a drug with a reported potential to induce AA in man (e.g. phenylbutazone, gold salts, azathioprine, chloramphenicol, penicillamine, etc.) or an agent with therapeutic potential in the treatment of the human disease (e.g. cyclosporin and antilymphocyte globulin). However, the generally moderate nature of the late-stage changes induced in the marrow and blood is in contrast to the often severe bone marrow depression seen in the human condition. Therefore, the lack of a pronounced neutropenia, and the absence of a profound anaemia with reticulocytopenia, may be considered as relative disadvantages in investigations to elucidate the mechanisms of the pathogenesis of AA in man. Furthermore, the generally moderate nature of the late-stage changes does raise a question of terminology, and it is appreciated that there are reasons to describe the late-stage condition in the BU-treated mouse as ‘chronic bone marrow hypoplasia’ rather than ‘chronic bone marrow aplasia’. It is of interest to note here that Morley and colleagues described the condition induced in their animals as chronic hypoplastic marrow failure (Morley et al. 1975; Morley et al. 1978; Pugsley et al. 1978).
Experiments are now in progress to address the generally moderate nature of the late-stage changes, and several approaches are being examined to develop a further-improved mouse model of human AA. For example, first, the use of drugs other than BU could be employed to induce marrow injury. There is, for example, evidence to suggest that chlorambucil, melphalan, mitomycin and carmustine (BCNU) may be appropriate agents to consider (Trainor & Morley 1976; Trainor et al. 1979; Morley 1980). Second, a re-examination of the BU-dosing regimens could be considered, with repeat doses of the drug being administered on a daily, or weekly, or fortnightly basis, and finally, other more wide-ranging possibilities could be investigated such as the use of a more susceptible inbred mouse strain or another rodent species (e.g. rat, hamster or guinea pig), or the use of BU in conjunction with other antineoplastic agents, or BU in conjunction with radioactivity, or in association with the modulation of the immune response.
Acknowledgments
We acknowledge with thanks the assistance of the technical staff at the School of Pharmacy for their care of the animals. We appreciate the work of Janet Andrew in the preparation of the manuscript. CMA thanks the staff of the Clinical Pathology Unit, GlaxoSmithKline for technical assistance in the analysis of the blood and marrow samples. JAT acknowledges the continued support of GlaxoSmithKline Research and Development, Ware. The Aplastic Anaemia Trust (GM, SR), The Leukaemia Research Fund (FMG) and the School of Pharmacy (GM) supported this work.
References
- Alter BP, Potter NU, Li FP. Classification and aetiology of the aplastic anaemias. Clin. Haematol. 1978;7:431–465. [PubMed] [Google Scholar]
- Alter BP, Young NS. The bone marrow failure syndromes. In: Nathan DG, Orkin SH, editors. Haematology of Infancy and Childhood. Philadelphia: W.B. Saunders; 1998. pp. 111–222. [Google Scholar]
- Andrews CM. PhD Thesis. University of London; 2000. Studies on the haemotoxicity of busulphan and chloramphenicol in the B6C3F1 mouse. [Google Scholar]
- Andrews CM, Spurling NW, Turton JA. Characterisation of busulphan-induced myelotoxicity in B6C3F1 mice using flow cytometry. Comp. Haemtol. Int. 1993;3:102–115. [Google Scholar]
- Andrews CM, Dash LM, Williams TC, Craig Gray J, Turton JA. Long-term effects of busulphan on lymphocyte subpopulations in female B6C3F1 mice. Comp. Haematol. Int. 1997;7:230–237. [Google Scholar]
- Andrews CM, Williams TC, Turton JA. Long-term haematological alterations in female B6C3F1 mice treated with busulphan. Comp. Haematol. Int. 1998;8:125–138. [Google Scholar]
- Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. 3. Edinburgh: Churchill Livingstone; 1990. [Google Scholar]
- Benestad H. Drug mechanisms in marrow aplasia. In: Geary CG, editor. Aplastic Anaemia. London: Ballière-Tindall; 1979. pp. 26–42. [Google Scholar]
- Bhoopalam N, Price K, Norgello H, Barone-Varelas J, Fried W. Busulphan and chloramphenicol induced T cell lymphoma: cell surface characteristics and functional properties. Clin. Exp. Immunol. 1986;64:646–655. [PMC free article] [PubMed] [Google Scholar]
- Binder D, van den Broek MF, Kagi D, et al. Aplastic anaemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus. J. Exp. Med. 1998;187:1903–1920. doi: 10.1084/jem.187.11.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camitta BM, Storb R, Thomas ED. Aplastic anaemia. Pathogenesis, diagnosis, treatment and prognosis. N. Eng. J. Med. 1982;306:645–652. doi: 10.1056/NEJM198203183061105. [DOI] [PubMed] [Google Scholar]
- Clayson DB. Modulation of the incidence of murine leukaemia and lymphoma. CRC Crit. Rev. Toxicol. 1984;13:183–195. doi: 10.3109/10408448409034081. [DOI] [PubMed] [Google Scholar]
- Conklin JW, Upton AC, Christenberry KW. Further observations on late somatic effects of radiomimetic chemicals and X-rays in mice. Cancer. Res. 1965;25:20–28. [PubMed] [Google Scholar]
- Den Ottolander GT, te Velde J, Veenhof W, Kleiverda K, Haak HL, Spaander PJ. Busulphan aplasia in rabbits: a model for human aplastic anaemia. Br. J. Haematol. 1982;51:265–276. doi: 10.1111/j.1365-2141.1982.tb02780.x. [DOI] [PubMed] [Google Scholar]
- Diamanti V, Turton JA, Sharpe S, et al. Induction of chronic bone marrow aplasia with busulphan: studies on marrow cell counts and colony forming assays in a new experimental model in the BALB/c mouse. Hum. Exp. Toxicol. 1999;18:763. [Google Scholar]
- Dollery C. Therapeutic Drugs. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- Freedman MH. Inherited forms of bone marrow failure. In: Hoffman R, et al., editors. Haematology: Basic Principles and Practice. New York: Churchill Livingstone; 2000. pp. 260–297. [Google Scholar]
- Frith CH, Wiley LD. Morphologic classification and correlation of incidence of hyperplastic and neoplastic hematopoietic lesions in mice with age. J. Gerontol. 1981;36:534–545. doi: 10.1093/geronj/36.5.534. [DOI] [PubMed] [Google Scholar]
- Frith CH, Highman B, Burger G, Sheldon WD. Spontaneous lesions in virgin and retired breeder BALB/c and C57BL6 mice. Lab. Anim. Sci. 1983;33:273–286. [PubMed] [Google Scholar]
- Gehring PJ. The cataractogenic activity of chemical agents. CRC Crit. Rev. Toxicol. 1971;1:93–118. doi: 10.3109/10408447109104302. [DOI] [PubMed] [Google Scholar]
- Gibson FM, Andrews CM, Diamanti P, et al. A new model of busulphan-induced chronic bone marrow aplasia in the female BALB/c mouse. Int. J. Exp. Pathol. 2003;84:31–47. doi: 10.1046/j.1365-2613.2003.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Smith EC, Issaragrissil S. Epidemiology of aplastic anaemia. Baillieres Clin. Haematol. 1992;5:475–491. doi: 10.1016/s0950-3536(11)80028-4. [DOI] [PubMed] [Google Scholar]
- Grimes P, Von Sallmann L. Interference with cell proliferation and induction of polyploidy in rat lens epithelium during prolonged myleran treatment. Exp. Cell Res. 1966;42:265–273. doi: 10.1016/0014-4827(66)90289-8. [DOI] [PubMed] [Google Scholar]
- Grimes P, Von Sallmann L, Frichette A. Influence of myleran on cell proliferation in the lens epithelium. Invest. Ophthalmol. 1964;3:566–576. [PubMed] [Google Scholar]
- Haak HL. Experimental drug-induced aplastic anaemia. Clin. Haematol. 1980;9:621–639. [PubMed] [Google Scholar]
- Hart JE. Endocrine factors in haematological changes seen in dogs and ferrets given oestrogens. Med. Hypotheses. 1985;16:159–163. doi: 10.1016/0306-9877(85)90070-2. [DOI] [PubMed] [Google Scholar]
- Hart JE. Endocrine pathology of estrogens: species differences. Pharmacol. Ther. 1990;47:203–218. doi: 10.1016/0163-7258(90)90087-i. [DOI] [PubMed] [Google Scholar]
- Haseman JK, Hailey JR, Morris RW. Spontaneous neoplasm incidences in Fischer 344 rats and B6C3F1 mice in two-year carcinogenicity studies: a National Toxicology Programme update. Toxicol. Pathol. 1998;26:428–441. doi: 10.1177/019262339802600318. [DOI] [PubMed] [Google Scholar]
- Heimpel H. Epidemiology and etiology of aplastic anaemia. In: Schrezenmeier H, Bacigalupo A, editors. Aplastic Anaemia: Pathophysiology and Treatment. Cambridge: Cambridge University Press; 2000. pp. 97–116. [Google Scholar]
- Heimpel H, Heit W. Drug-induced aplastic anaemia: clinical aspects. In: Gordon-Smith EC, editor. Clinics in Haematology; Haematological Effects of Drug Therapy. 3. Vol. 9. London: Saunders; 1980. pp. 641–662. [PubMed] [Google Scholar]
- Home Office. Code of Practice for the Housing and Care of Animals used in Scientific Procedures. London: Her Majesty's Stationery Office; 1989. [Google Scholar]
- Jandl H. Blood: Textbook of Haematology. Boston: Little Brown; 1996. [Google Scholar]
- Klassen LW, Birks J, Allen E, Gurney CW. Experimental medullary aplasia. J. Lab. Clin. Med. 1972;80:8–17. [PubMed] [Google Scholar]
- Knospe WH. Long-term bone marrow damage after irradiation. In: Testa NG, Gale RR, editors. Hematopoiesis: Long-Term Effects of Chemotherapy and Radiation. New York: Marcel Dekker; 1988. pp. 93–130. [Google Scholar]
- Knospe WH, Steinberg D, Speck B. Experimental immunologically mediated aplastic anaemia in H-2k identical, Mls locus deficient mice. Exp. Hematol. 1983;11:542–552. [PubMed] [Google Scholar]
- Knospe WH, Husseini SG, Chiu KM, Fried W. Immunologically mediated aplastic anaemia in mice: evidence of haematopoietic stromal injury and injury to haematopoietic stem cells. Exp. Hematol. 1994;22:573–581. [PubMed] [Google Scholar]
- Krishna G, Aykac I, Siegel D. Recent studies on the mechanisms of chloramphenicol activation responsible for aplastic anamia. In: Najean Y, Togoni G, Yunis AA, editors. Safety Problems Related to Chloramphenicol and Thiamphenicol. New York: Raven Press; 1981. pp. 5–16. [Google Scholar]
- Krueger GRF. Lymphoblastic lymphomas, mouse. In: Jones TC, Ward JM, Mohr U, Hunt RD, editors. Monographs on Pathology of Laboratory Animals; Hemopoietic System. Berlin: Springer-Verlag; 1990. pp. 264–275. [Google Scholar]
- Lock EA, Sani Y, Moore RB, Finkelstein MB, Anders MW, Seawright AA. Bone marrow and renal injury associated with haloalkene cysteine conjugates in calves. Arch. Toxicol. 1996;70:607–619. doi: 10.1007/s002040050319. [DOI] [PubMed] [Google Scholar]
- Macharia G, Gibson F, Andrews M, et al. Chronic hypoplastic marrow failure in the mouse; a possible new model for the assessment of drugs with potential to induce aplastic anaemia (AA) J. Pharm. Pharmacol. 1999a;51(Suppl.):136. [Google Scholar]
- Macharia G, Andrews CM, Williams TC, et al. Peripheral blood changes in a new model of busulphan-induced chronic bone marrow aplasia (aplastic anaemia) in the BALB/c mouse. Hum. Exp. Toxicol. 1999b;18:764. [Google Scholar]
- Marsh JCW, Choudry J, Parry-Jones N, et al. Study of the association between cytochromes P450 2D6 and 2E1 genotypes and the risk of drug and chemical induced idiosyncratic aplastic anaemia. Br. J. Haematol. 1999;104:266–270. doi: 10.1046/j.1365-2141.1999.01190.x. [DOI] [PubMed] [Google Scholar]
- Moeschilin S, Speck B. Experimental studies on the mechanism of benzene on the bone marrow (radioautographic studies using 3H-thymidine) Acta Haematol. 1967;38:104–111. doi: 10.1159/000209006. [DOI] [PubMed] [Google Scholar]
- Morley A. Residual damage from cytotoxic drugs. Aust. N. Z. J. Med. 1980;10:569–571. doi: 10.1111/j.1445-5994.1980.tb04980.x. [DOI] [PubMed] [Google Scholar]
- Morley A, Blake J. An animal model of chronic hypoplastic marrow failure. Late marrow failure after busulphan. Blood. 1974a;44:49–56. [PubMed] [Google Scholar]
- Morley A, Blake J. Hemopoietic precursor cells in experimental hypoplastic marrow failure. Aust. J. Exp. Biol. Med. Sci. 1974b;52:909–914. doi: 10.1038/icb.1974.90. [DOI] [PubMed] [Google Scholar]
- Morley A, Trainor K, Blake J. A primary stem cell lesion in experimental chronic hypoplastic marrow failure. Blood. 1975;45:681–688. [PubMed] [Google Scholar]
- Morley AA, Trainor KJ, Seshadri RS. Chronic hypoplastic marrow failure and residual injury. Blood Cells. 1978;4:253–266. [PubMed] [Google Scholar]
- Nettlelship A. Bone-marrow changes produced by specific antibodies. Am. J. Pathol. 1942;18:689–697. [PMC free article] [PubMed] [Google Scholar]
- Philpott NJ, Turner AJC, Scopes J, et al. The use of 7-amino-actinomycin D in identifying apoptosis: simplicity of use and broad spectrum of application compared to other techniques. Blood. 1995a;47:2244–2251. [PubMed] [Google Scholar]
- Philpott NJ, Scopes J, Marsh JCW, Gordon-Smith EC, Gibson FM. Increased apoptosis in aplastic anaemia progenitor cells: possible pathophysiological significance. Exp. Haematol. 1995b;23:1642–1648. [PubMed] [Google Scholar]
- Podos SM, Canellos GP. Lens changes in chronic granulocytic leukaemia: possible relationships to chemotherapy. Am. J. Ophthalmol. 1969;68:500–504. doi: 10.1016/0002-9394(69)90722-3. [DOI] [PubMed] [Google Scholar]
- Potts AM. Toxic responses of the eye. In: Klaassen CD, Amdur MO, Doull J, editors. Casarett and Doull's Toxicology. New York: Macmillan; 1986. pp. 478–515. [Google Scholar]
- Pugsley CAJ, Forbes IJ, Morley AA. Immunologic abnormalities in an animal model of chronic hypoplastic marrow failure induced by busulphan. Blood. 1978;51:601–610. [PubMed] [Google Scholar]
- Ravindranathan MP, Paul VJ, Kuriakose ET. Cataract after busulphan treatment. Br. Med. J. 1972;1:218–219. doi: 10.1136/bmj.1.5794.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JEF. Martindale: The Extra Pharmacopoeia. 29. London: Pharmaceutical Press; 1989. [Google Scholar]
- Robin E, Berman M, Bhoopalam N, Cohen H, Fried W. Induction of lymphomas in mice by busulphan and chloramphenicol. Cancer Res. 1981;41:3478–3482. [PubMed] [Google Scholar]
- Scales MDC, Andrews ZE. The use of two compatible rodent strains to improve the sensitivity of carcinogenicity studies: a data review of spontaneous neoplasms. Adverse Drug React. Toxicol. Rev. 1991;10:99–111. [PubMed] [Google Scholar]
- Sherrill A, Gorman J. Bone marrow hypoplasia associated with estrus in ferrets. Lab. Anim. Sci. 1985;35:280–286. [PubMed] [Google Scholar]
- Smith CA, Andrews CM, Collard JK, Hall DE, Walker AK. A Colour Atlas of Comparative, Diagnostic and Experimental Haematology. London: Wolfe; 1994. [Google Scholar]
- Solomon C, Light AE, De Beer EJ. Cataracts produced in rats by 1,4-dimethanesulfonoxybutane (myleran) Arch. Ophthalmol. 1955;54:850–852. doi: 10.1001/archopht.1955.00930020856006. [DOI] [PubMed] [Google Scholar]
- Sones W, Andrews M, Williams T, Gibson F, Gordon-Smith E, Turton J. Induction of aplastic anaemia in the busulphan-treated mouse: further development of a new model of the human disease. Toxicol. Lett. 2000;116(Suppl. 1):32. [Google Scholar]
- Speck B, Kissling M. Studies on bone marrow transplantation in experimental 32P-induced aplastic anaemia after conditioning with antilymphocyte serum. Acta Haematol. 1973;50:193–199. doi: 10.1159/000208349. [DOI] [PubMed] [Google Scholar]
- Sweetman SC. Martindale: The Complete Drug Reference. 33. London: Pharmaceutical Press; 2002. [Google Scholar]
- Tooze J, Marsh JCW, Gordon-Smith EC. Clonal evolution of aplastic anaemia to myelodysplastic syndrome/acute myeloid leukaemia and paroxysmal nocturnal haemoglobinuria. Leuk. Lymphoma. 1999;33:231–241. doi: 10.3109/10428199909058423. [DOI] [PubMed] [Google Scholar]
- Trainor KJ, Morley AA. Screening of cytotoxic drugs for residual bone marrow damage. J. Natl. Cancer Inst. 1976;57:1237–1239. doi: 10.1093/jnci/57.6.1237. [DOI] [PubMed] [Google Scholar]
- Trainor KJ, Seshadri RS, Morley AA. Residual marrow injury following cytotoxic drugs. Leuk. Res. 1979;3:205–211. doi: 10.1016/0145-2126(79)90043-2. [DOI] [PubMed] [Google Scholar]
- Upton AC, Wolff FF, Sniffen EP. Leukemogenic effect of myleran on the mouse thymus. Proc. Soc. Exp. Biol. Med. 1961;108:464–467. doi: 10.3181/00379727-108-26966. [DOI] [PubMed] [Google Scholar]
- Vincent PC. In vitro evidence of drug action in aplastic anaemia. Blut. 1984;49:3–12. doi: 10.1007/BF00320378. [DOI] [PubMed] [Google Scholar]
- Vincent PC. Drug-induced aplastic anaemia and agranulocytosis. Incidence and mechanisms. Drugs. 1986;31:52–63. doi: 10.2165/00003495-198631010-00004. [DOI] [PubMed] [Google Scholar]
- Von Sallmann L. The lens epithelium in the pathogenesis of cataract. Am. J. Ophthalmol. 1957;44:159–170. doi: 10.1016/0002-9394(57)90001-6. [DOI] [PubMed] [Google Scholar]
- Ward JM, Goodman DG, Squire RA, Chu KC, Linhart MS. Neoplastic and non-neoplastic lesions in aging (C57BL/6N, x C3H/HEN) F1 (B6C3F1) mice. J. Natl. Cancer Inst. 1979;63:849–854. doi: 10.1093/jnci/63.3.849. [DOI] [PubMed] [Google Scholar]
- Weiss DJ. Aplastic anaemia. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm's Veterinary Haematology. 5. Philadelphia, Lippincott: Williams & Wilkins; 2000. pp. 212–215. [Google Scholar]
- Wogan GN. Tumours of the mouse haematopoietic system: their diagnosis and interpretation in safety evaluation tests. Report of a study group. CRC Crit. Rev. Toxicol. 1984;13:161–181. doi: 10.3109/10408448409034080. [DOI] [PubMed] [Google Scholar]
- Wolk A, Simon-Stoos K, Nami I, et al. A mouse model of immune-mediated aplastic anaemia [Abstract] Blood. 1998;92:639. [Google Scholar]
- Young N. Aplastic anaemia. Lancet. 1995;346:228–232. doi: 10.1016/s0140-6736(95)91273-8. [DOI] [PubMed] [Google Scholar]
- Young NS, Alter BP. Aplastic Anaemia: Acquired and Inherited. Philadelphia: W.B. Saunders; 1994. [Google Scholar]
- Young N, Keisu M. Preface: drug-related blood dyscrasias workshop. Eur. J. Haematol. 1996;57(Suppl.):5. [PubMed] [Google Scholar]
- Young NS, Maciejewski JP. The pathophysiology of acquired aplastic anaemia. N. Engl. J. Med. 1997;336:1365–1372. doi: 10.1056/NEJM199705083361906. [DOI] [PubMed] [Google Scholar]
- Young NS, Maciejewski JP. Aplastic anaemia. In: Hoffman R, et al., editors. Haematology: Basic Principles and Practice. New York: Churchill Livingstone; 2000. pp. 297–331. [Google Scholar]