Abstract
Chloramphenicol (CAP) is haemotoxic in man, inducing two types of toxicity. First, a dose-related, reversible anaemia with reticulocytopenia, sometimes seen in conjunction with leucopenia and thrombocytopenia; this form of toxicity develops during drug treatment. The second haemotoxicity is aplastic anaemia (AA) which is evident in the blood as severe pancytopenia. AA development is not dose-related and occurs weeks or months after treatment. We wish, in the longer term, to investigate CAP-induced AA in the busulphan-pretreated mouse. However, as a prelude to that study, we wanted to characterize in detail the reversible haemotoxicity of CAP succinate (CAPS), administered at high dose levels in the mouse, and follow the recovery of the bone marrow in the post-dosing period. Female B6C3F1 mice were gavaged with CAPS at 0, 2500 and 3500 mg/kg, daily, for 5 days and sampled (n = 5) at 1, 7, 14 and 21 days post-dosing. Blood, bone marrow and spleen samples were analysed and clonogenic assays carried out. At day 1 post-dosing, at both CAPS dose levels, decreases were seen in erythrocytes and erythrocyte precursors; marrow erythroid cells were reduced. Reductions were also evident in splenic nucleated cell counts, blood high fluorescence ratio (HFR) reticulocyte counts and total reticulocyte counts; burst-forming units-erythroid and colony-forming units-erythroid showed decreases. At day 7 post-dosing (2500 mg/kg CAPS), there was regeneration of erythrocyte production, with marked splenic erythropoietic activity, and raised blood HFR reticulocytes. At day 7, at 3500 mg/kg CAPS, erythrocyte and reticulocyte parameters remained depressed. At 14 days post-dosing (2500 mg/kg CAPS), many erythrocyte parameters had returned to normal; at 3500 mg/kg CAPS, there was erythroid regeneration. By 21 days post-dosing, at both CAPS dose levels, most erythrocytic parameters were equivalent to control values. For leucocyte parameters, there was some depression at day 1 post-dosing (at both CAPS dose levels) and signs of recovery at day 7. At days 14 and 21 post-dosing, most leucocyte parameters were close to control values. Marrow smears at day 1 post-dosing (at both CAPS dose levels) showed vacuolation of early normoblasts, of myeloid and of monocytic precursors. We conclude that the administration of CAPS at 2500 and 3500 mg/kg for 5 days induced significant myelotoxicity in female B6C3F1 mice, with cessation of erythropoiesis at day 1 post-dosing; recovery was seen over the following 7/14 days. The blood HFR reticulocyte count was a precise indicator of CAPS-induced depressive effects and subsequent recovery. It is concluded that the administration of five daily doses of CAPS at 2500 and 3500 mg/kg to the female B6C3F1 mouse induces an anaemia with reticulocytopenia, in conjunction with leucopenia, in the immediate post-dosing period; no evidence was seen at 21 days post-dosing of peripheral blood pancytopenia or a hypocellular/acellular bone marrow, which are both characteristic features of AA in man.
Keywords: chloramphenicol, haemotoxicity, mouse, myelotoxicity, reticulocyte, spleen
Aplastic anaemia (AA) in man is a disease characterized by pancytopenia of the peripheral blood and bone marrow hypocellularity (Young & Alter 1994; Jandl 1996). The condition is associated with a variety of aetiological agents including viruses, irradiation and a wide range of drugs and chemicals (Heimpel 2000; Young & Maciejewski 2000). However, the basic pathophysiology of AA is not clearly understood, and a factor that has contributed to this lack of understanding is that there are currently no animal models of the drug-induced disease that are convenient to use (Benestad 1979; Haak 1980; Vincent 1984; Young & Maciejewski 1997). Nevertheless, in a series of papers, Morley and his colleagues (Morley & Blake 1974a, b; Morley et al. 1975, 1976) developed and assessed a model of busulphan (BU)-induced chronic hypoplastic marrow failure (CHMF, i.e. AA) in the female Swiss and BALB/c mouse. In this model, BU was administered on four fortnightly occasions (i.e. over a 6-week period) at 20, 20, 20 and 10 mg/kg, respectively, and the animals were investigated over the following 300–400 days. An essential feature of this mouse model was that BU produced a form of ‘residual’ or ‘late stage’ marrow injury, which was characterized by mild or moderate marrow failure. Morley et al. (1976) proposed that such a residual marrow injury might be a factor in predisposing individuals to develop AA, following exposure to drugs such as the antibiotic chloramphenicol (CAP), a compound widely known to induce AA in man (Young & Alter 1994; Young & Maciejewski 2000). To investigate this proposal, Morley et al. (1976) evaluated the capacity of CAP succinate (CAPS) to induce CHMF in the female BALB/c mouse pretreated with BU. With CAPS administered in the drinking water at 5 mg/ml, the antibiotic was reported to cause a progressive fall in the number of pluripotent stem cells and granulocytic progenitor cells in BU-pretreated mice, but there was no effect of CAPS on the marrow cells of normal (i.e. not BU- pretreated) animals (Morley et al. 1976).
CAP is a highly active, broad-spectrum antibiotic. The drug became widely used in the treatment of serious infections including typhoid fever and other forms of salmonellosis, in severe infections due to Haemophilus influenzae (particularly meningitis), and in other life-threatening infections of the central nervous system and respiratory tract (Holt et al. 1993; Parfitt 1999). The therapeutic dose of CAP is generally 50 mg/kg daily, in divided doses; the time periods of treatment are often 10–15 days (Chaplin 1986). CAP is widely used today in the UK and USA in the topical treatment of ear and eye infections (Fraunfelder et al. 1993; Dollery 1999; Parfitt 1999) and is also used extensively in the developing world (Trevett et al. 1992; Kumar & Verma 1993; Kushwaha et al. 1994).
However, CAP is haemotoxic in man, inducing, in particular, two major effects on the blood (FAO/WHO 1988; IARC 1990; Young & Alter 1994). The first haemotoxicity is an often-occurring (i.e. predictable), mild anaemia with reticulocytopenia, sometimes seen in conjunction with leucopenia and thrombocytopenia (Yunis & Bloomberg 1964). This predictable myelosuppression, primary affecting erythropoiesis, is dose-related, develops during drug therapy (particularly with relatively high-dose levels given over a short time period), and is quickly reversible following drug withdrawal (Krakoff et al. 1955; Scott et al. 1965; Best 1967). The bone marrow generally shows a normal cellularity, but reduced numbers of late erythroid cells may be seen (Rubin et al. 1958; Chaplin 1986). The second haemotoxicity of CAP in man is AA (Rich et al. 1950; Welch et al. 1954; Hugley et al. 1961; Wallerstein et al. 1969). This toxicity is seen relatively infrequently (i.e. it is unpredictable) but is evident in the blood as a severe pancytopenia and a hypocellular or acellular marrow. CAP-induced AA does not generally appear to be related to the dose level of drug administered, develops weeks or months after treatment and is often irreversible and fatal (Yunis & Bloomberg 1964; Yunis 1978). The development of AA is unrelated to the reversible form of haemotoxicity (Yunis & Bloomberg 1964; Wallerstein et al. 1969).
To examine the capacity of CAPS to induce AA in the BU-pretreated mouse, we carried out a series of experiments following the protocols of Morley and his co-workers (Morley & Blake 1974a; Morley et al. 1976) but using the female B6C3F1 mouse rather than the inbred BALB/c strain used by Morley & Blake (1974a). However, we were not able to demonstrate that CAPS caused AA (Andrews et al. 1993, 1997, 1998; Andrews 2000). In our studies, CAPS was administered in the drinking water at 4 mg/ml which was the maximum tolerated dose in the B6C3F1 strain of mouse (at dose levels of 5 and 6 mg/ml CAPS in the drinking water, there were significant reductions in body weight, a decrease in the reticulocyte count and an increase in RBC count, which were possible indications of a haemoconcentration effect). At 4 mg/kg, water consumption data demonstrated that over the course of a 13-month study, the animals consumed about 176–370 mg/kg/day of CAPS. It has not been possible to determine why our results contrasted with the earlier positive findings of Morley et al. (1976). However, it was subsequently shown that CAPS, administered at dose levels of 176–370 mg/kg/day in the drinking water, was not haemotoxic (Holt et al. 1998; Andrews 2000), and in, for example, the CD-1 mouse, dose levels of 1400–1700 mg/kg CAPS, administered by gavage, daily, were required to induce significant haemotoxicity (Turton et al. 1999). In a similar way, it was shown that in several inbred mouse strains, including the BALB/c, dose levels of 500–2500 mg/kg CAPS administered daily for 7 days by gavage are necessary to cause haemotoxicity (Festing et al. 2001).
Yunis & Bloomberg (1964), in a review of CAP-induced AA in man, observed that in 37 out of 46 cases of fatal marrow aplasia, the therapeutic administration of CAP to the patient had been on an intermittent basis, rather than as a continuous daily exposure. However, in 36 cases of the reversible form of anaemia and bone marrow depression, in only eight cases had CAP been taken intermittently (Yunis & Bloomberg 1964). It was therefore considered that our earlier failure to induce AA in the B6C3F1 mouse (Andrews et al. 1998; Andrews 2000) may have been related to the dosing of CAPS using a continuous low-level administration regimen in the drinking water. For this reason, it was thought possible that an intermittent dosing regimen with CAPS given in repeated cycles of dosing and recovery (i.e. dosing and non-dosing), at high (i.e. haemotoxic) dose levels, by gavage, might be successful in the induction of AA in the BU-pretreated B6C3F1 mouse.
As a prelude to carrying out such a study in the BU- pretreated mouse using the high-dose, intermittent, gavage administration of CAPS, we wished to investigate in detail the haemotoxicty of the drug given orally at 2500 and 3500 mg/kg daily for 5 days, followed by an observation of the haematological recovery in the following 21 day post-dosing period. It was considered that recovery would be complete by day 21 post-dosing, as Turton et al. (1999) had shown that in CD-1 mice given 1400 mg/kg CAPS daily for 10 days, there was a return to control values after 15 days. Furthermore, in man, erythropoietic recovery in the reversible form of CAPS haemotoxicity occurs at 1 week after drug administration (Saidi et al. 1961).
Materials and methods
Animals
Female B6C3F1 mice (Charles River UK, Margate, Kent), 12–14 weeks old, were housed in groups of five, bedded on wood shavings, and given diet (Rat and Mouse No.1, SDS, Witham, Essex) and drinking water ad libitum. A temperature of 22 °C ± 1 °C was maintained, with a relative humidity of 45–70% and a 12 : 12 h light : dark cycle (lights on at 06:00 hours). Animals were acclimatized for 5 days before the start of the experiment and were observed daily for signs of ill health.
Administration of CAPS
Solutions of CAPS (Sigma Chemical, Poole, Dorset) were prepared in water for irrigation (BP) and administered by gavage in dose volumes of approximately 10 ml/kg; control animals were given water at the same dose volume.
Tissue sampling
Mice were killed by CO2 overdose and 0.5 ml blood samples for cell counting were taken from the posterior vena cava and anticoagulated with 1.5 mg dipotassium EDTA/ml of blood (Teklab, Sacriston, Durham, UK). Bone marrow cellularity was assessed by flushing the contents of one femur into 10 ml of phosphate-buffered saline (PBS). A bone marrow smear was prepared from the contents of one tibia. The spleen was removed, weighed and sectioned to prepare spleen imprints (touch preparations). Here, the spleen is sectioned transversely with a razor blade and with each half held with forceps, the cut surface is lightly touched repeatedly on a glass microscope slide. Spleen cell suspensions were prepared by scissor-mincing after removal of the capsular material, and dispersion in PBS is achieved by cavitation in a 50 ml syringe. For bone marrow progenitor cell assays, the contents of one femur were flushed into 1.0 ml of Iscove's Modified Dulbecco's Medium (IMDM; Life Technologies, Paisley, UK) under sterile conditions.
Sample analysis
Peripheral blood: full blood count, differential leucocyte count and reticulocyte count
Full blood counts and differential leucocyte counts were obtained with a Bayer H*1 haematology analyser (Bayer Diagnostics, Newbury, Berkshire, UK). Leucocytes, excluding basophils, were differentiated by cluster analysis of peroxidase content and volume, measured by absorption of right-angle scatter, and forward scatter, of tungsten light, respectively, against an archetype for mouse leucocytes (Davies & Fisher 1991). Basophils were counted in a separate channel as phthalic acid-resistant intact cells; other cells in this channel were detected as stripped nuclei. The H*1, with mouse-specific software (Versions 1.0–3.0; Bayer, Swords, Dublin, Ireland), was validated in-house for use with mouse blood. Reticulocytes were counted using a Sysmex R-1000 reticulocyte analyser (Sysmex, Milton Keynes, Buckinghamshire, UK) (Tichelli et al. 1990), with photomultiplier voltage gain adjusted optimally for mouse blood (Fuchs & Eder 1991). Reticulocytes were differentiated from mature erythrocytes by fluorescence after staining with auramine O (Tichelli et al. 1990; Fuchs & Eder 1991). Reticulocyte maturity was assessed according to fluorescence intensity which is divided into three fractions: the high-, mid- and low-fluorescence ratio (HFR, MFR and LFR). In-house validation for the R-1000 has been performed for mouse blood. A small proportion of leucocytes fall into an area on the H*1 cytogram indicating a greater cell size than most lymphocytes, but having a lower peroxidase activity than monocytes. Such cells are classified as large unstained cells (LUC) (Andrews et al. 1997; Knoll 2000). In human blood, it has been shown that assigning half the LUC count to the lymphocytes and half to monocytes produces a differential leucocyte count very close to manual (i.e. visual) examination of the Romanowsky-stained smear (Kinsey & Watts 1988).
Bone marrow cellularity
The femoral bone marrow suspension in PBS was used to obtain a nucleated cell count from the basophil channel of the H*1. Where the presence of fat particles was evident in the analyser cytogram, correction of the count was performed according to Bentley et al. (1995).
Bone marrow smears, spleen touch preparations and spleen cell suspensions
Bone marrow smears were stained with May-Grünwald-Giemsa (MGG) stain (Dacie & Lewis 1975; Hall & Malia 1984; Smith et al. 1994; Freeman 2000) and examined microscopically for morphological assessment and the enumeration of myeloid, erythroid, lymphoid and ‘other’ cells in 200 cell-differential counts (other cells include monocytes and monocyte precursors, mast cells, megakaryocytes, plasma cells and unidentifiable cells). Spleen touch preparations (imprints) were stained with MGG stain and assessed histologically; erythropoietic activity was graded as: 0 = absent; 1 = very slight; 2 = slight; 3 = moderate; 4 = marked. The nucleated cell count of spleen cell suspensions in PBS was obtained from the basophil channel of the H*1.
Bone marrow progenitor cell analysis
0.3 ml of femoral bone marrow cell suspension in IMDM was, within 1 h of sampling, resuspended in 3.0 ml of Methocult GF M3434 culture medium (Metachem Diagnostic, Piddington, Northampton, UK) containing 0.9% methylcellulose in IMDM, 15% fetal calf serum (PAA Laboratories GmbH, Linz, Austria), 10−4 M2-mercaptoethanol, 2 nm l-glutamine, 1% bovine serum albumin, 10 µg/ml bovine pancreatic insulin, 200 µg/ml human transferrin, 10 ng/ml recombinant murine IL-3, 10 ng/ml recombinant human IL-6, 50 ng/ml recombinant murine stem cell factor and 3 U/ml recombinant murine erythropoietin (all last ingredients: Sigma). The tubes were vortexed and allowed to stand (5 min; 37 °C), dispensed into cell culture multiwell plates and incubated at 37 °C. Burst-forming units-erythroid (BFU-E) were counted after 10 days incubation, and colony-forming units-erythroid (CFU-E) and colony-forming units-granulocyte-macrophage (CFU-GM) after 12 days, on the same plates. Each sample was assayed in triplicate.
Statistical analysis
Student's t-test was used to compare treated and control groups at the same time point using RS/1 software (BBN, Cambridge, MA, USA) and Microsoft Excel.
Experimental design
Sixty mice were divided into three equal groups and dosed orally with CAPS by gavage at 0, 2500 and 3500 mg/kg, daily for 5 days. On days 1, 7, 14 and 21 after the final dose, five mice per group were killed and samples of blood, femoral bone marrow and spleen were taken for a full blood count, nucleated cell count (marrow; spleen), femoral progenitor cell assays (BFU-E, CFU-E and CFU-GM) and microscopy of marrow smears and spleen touch imprints. Body weights of animals were determined twice weekly.
Results
Clinical observations
Control and CAPS-treated mice appeared well at all times and there was no evidence of drug-induced toxicity. One animal in the group receiving CAPS at 3500 mg/kg died on the first day of treatment following a dosing accident (oesophageal perforation).
Haematology
Erythroid series
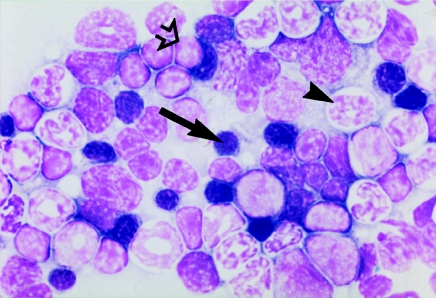
At 1 day post-dosing, marked reductions were seen in erythrocytes and bone marrow erythroid precursors at both 2500 and 3500 mg/kg CAPS (Table 1). HCT and Hb were also significantly decreased. MCV, MCH and MCHC were not affected. The femoral bone marrow erythroid cell count was reduced at 2500 mg/kg CAPS to 20.8% of the control mean value, and at 3500 mg/kg to 9.5% of the control mean. In the bone marrow smears, at both CAPS dose levels, the only representatives of the erythroid series were pronormoblasts and early normoblasts; the majority of early normoblasts contained a single large cytoplasmic vacuole (Figures 1 and 2).
Table 1.
Mean erythroid parameters in female B6C3F1 mice dosed with chloramphenicol succinate (CAPS) at 2500 and 3500 mg/kg on five consecutive days and sampled at 1, 7, 14 and 21 days after the final dose
| CAPS dose (mg/kg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 2500 | 3500 | ||||||||||
| Day post-dosing | 1 | 7 | 14 | 21 | 1 | 7 | 14 | 21 | 1 | 7 | 14 | 21 |
| RBC | 9.848 | 9.770 | 9.688 | 9.900 | 8.855** | 8.058** | 9.682 | 10.043 | 8.968** | 7.642** | 9.955 | 9.658 |
| HCT | 0.542 | 0.538 | 0.533 | 0.531 | 0.480** | 0.448** | 0.535 | 0.546 | 0.487** | 0.411** | 0.545 | 0.512 |
| Hb | 15.45 | 15.38 | 15.13 | 15.36 | 13.93** | 12.42** | 15.24 | 15.58 | 14.16** | 11.70** | 15.53 | 14.86 |
| MCV | 55.05 | 55.06 | 55.00 | 53.60 | 54.23 | 55.58 | 55.30 | 54.35 | 54.32 | 53.78** | 54.75 | 52.98 |
| MCH | 15.70 | 15.74 | 15.60 | 15.52 | 15.70 | 15.42 | 15.74 | 15.50 | 15.78 | 15.32* | 15.60 | 15.38 |
| MCHC | 28.53 | 28.60 | 28.35 | 28.94 | 28.98 | 27.74 | 28.42 | 28.58 | 29.02 | 28.50 | 28.48 | 29.04 |
| RDW | 11.60 | 11.84 | 11.85 | 11.66 | 11.10** | 12.70** | 12.86** | 12.71** | 10.92** | 11.32 | 13.80** | 13.26** |
| HDW | 1.500 | 1.514 | 1.558 | 1.492 | 1.430* | 1.550** | 1.622** | 1.660** | 1.392* | 1.410** | 1.665** | 1.708** |
| Retic | 318.4 | 274.1 | 280.9 | 249.4 | 6.0** | 414.1 | 275.4 | 249.2 | 6.1*** | 19.5*** | 397.8 | 212.6 |
| HFR | 77.5 | 54.1 | 49.6 | 39.4 | 0.1** | 170.8** | 50.8 | 39.2 | 0.2*** | 1.5*** | 77.1 | 28.3 |
| BM cells | 1.180 | 1.258 | 1.053 | 1.106 | 0.960 | 1.588 | 1.073 | 1.398 | 1.050 | 1.196 | 0.983 | 1.378 |
| BM eryth cells | 0.423 | 0.380 | 0.253 | 0.300 | 0.088*** | 0.558 | 0.345 | 0.400 | 0.040*** | 0.260 | 0.305 | 0.326 |
| BFU-E | 42.67 | nd | 45.33 | 30.00 | 33.67** | nd | 46.00 | 25.00* | 26.33*** | nd | 46.33 | 28.33 |
| CFU-E | 47.00 | nd | 48.67 | 60.00 | 35.00* | nd | 49.67 | 60.00 | 33.33* | nd | 39.00 | 58.67 |
| Splenic cells | 8.68 | 8.82 | 9.82 | 9.91 | 5.01*** | 17.44*** | 11.94 | 11.86 | 3.73*** | 8.31 | 11.65 | 10.59 |
| Splenic eryth | 3.2 | 1.8 | 1.8 | 2.0 | 0.0 | 4.0 | 1.6 | 2.0 | 0.0 | 2.6 | 3.0 | 2.2 |
| Plt | 847.3 | 970.4 | 1072.5 | 987.0 | 897.8 | 1261.8* | 1254.0 | 1084.0 | 770.4 | 1765.0* | 1066.0 | 973.0 |
Abbreviations and units: RBC, red blood cells (×1012/l); HCT, haematocrit (l/l); Hb, haemoglobin (g/dl); MCV, mean cell volume (fl); MCH, mean cell haemoglobin (pg); MCHC, mean cell haemoglobin concentration (g/dl); RDW, red cell (volume) distribution width femoral (%); HDW, haemoglobin (cellular concentration) distribution width (g/dl); Retic, absolute reticulocyte count (×109/l); HFR, high fluorescence ratio reticulocytes (×109/l); BM cells, bone marrow nucleated cell count (×107); BM eryth cells, femoral bone marrow erythroid cells (×107); BFU-E, burst-forming units-erythroid, absolute number of colonies per plate; CFU-E, colony-forming units-erythroid, absolute number of colonies per plate; Splenic cells, spleen suspension nucleated cell count (×107); Splenic eryth, splenic erythropoietic grading, histological grading: 0, absent; 1, very slight; 2, slight; 3, moderate; 4, marked; Plt, platelets, (×109/l); nd, not determined. n = 5 for control and CAPS groups at all time points, except n = 4 for 3500 mg/kg CAPS group at day 14 and for BFU-E and CFU-E where n = 3.
Significantly different from control animals
P < 0.05
P < 0.01
P < 0.001. Data for splenic erythropoietic grading was not analysed statistically.
Figure 1.
Romanowsky-stained bone marrow smear from a control (vehicle-treated) mouse at day 1 post-dosing to illustrate the normal distribution of erythroid (arrow), myeloid (arrowhead) and lymphoid (open arrow) cells; original magnification ×1000.
Figure 2.
Vacuolation in a Romanowsky-stained bone marrow smear from a mouse treated with chloramphenicol succinate at 3500 mg/kg for 5 days and sampled at day 1 post-dosing; early normoblasts are present and often contain a single cytoplasmic vacuole (arrow). There is a relative depletion of late normoblasts. Later myeloid forms appear normal (arrowhead). The lymphoid series is also represented (open arrow). No megakaryocytes are present. Original magnification ×1000.
On day 1 post-dosing, the splenic nucleated cell count was reduced to 57.7% and 43.0% of the mean control value at 2500 and 3500 mg/kg CAPS, respectively (Table 1); examination of the spleen touch preparations (erythropoietic grading) showed an absence of erythroid activity at both CAPS dose levels (Table 1). Similarly, the total reticulocyte count and the HFR reticulocyte count (i.e. the most immature reticulocytes) were both markedly decreased at each dose level. RDW and HDW were also significantly reduced at this time in both CAPS treatment groups, reflecting the depletion of immature erythrocytes in the peripheral circulation. Femoral bone marrow BFU-E and CFU-E were reduced at day 1 post-dosing at both CAPS dose levels. Platelet counts were not affected at either CAPS dose level at this time point.
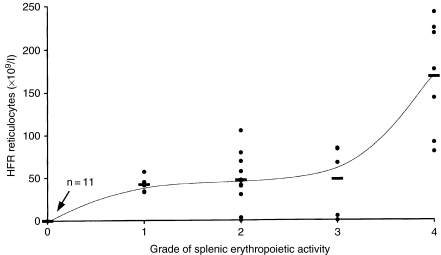
At day 7 after CAPS administration at 2500 mg/kg, the regeneration of erythrocyte production was evident. Assessment of the spleen touch preparations for erythropoietic grading at this dose level showed marked erythropoietic activity (Table 1), and there were significant increases in the splenic nucleated cell count; HFR reticulocytes and RDW were raised in value (Table 1). The relationship between the grade of splenic erythropoietic activity and the peripheral blood HFR reticulocyte count is illustrated in Figure 3. The total reticulocyte count and the femoral bone marrow erythroid count were both also elevated at this time in animals treated at 2500 mg/kg, but the increases were not statistically significant.
Figure 3.
Comparison of the grade of splenic erythropoietic activity in spleen touch preparations and the peripheral blood high fluorescence ratio (HFR) reticulocyte count, in individual mice treated with chloramphenicol succinate at 2500 and 3500 mg/kg for 5 days, and sampled at day 1, 7, 14 and 21 after the final dose. Splenic erythropoietic activity was graded in spleen touch preparations as: 0, absent; 1, very slight; 2, slight; 3, moderate; 4, marked. Horizontal bars indicate the mean HFR reticulocyte value at each grade of splenic erythropoietic activity.
At 3500 mg/kg CAPS, at day 7 post-dosing, significant depressions of the total reticulocyte count and the HFR reticulocyte count were still evident. Similarly, many other erythrocyte parameters including RBC, HCT and Hb were also still significantly depressed at this time in the 3500 mg/kg CAPS group. However, the nucleated cell count of the spleen compared with the control figure at day 7 post-dosing, but the splenic erythropoietic grading (in touch preparations) showed only slight or moderate activity (Table 1). However, platelet counts were significantly elevated at both 2500 and 3500 mg/kg CAPS on day 7 post-dosing, and at 3500 mg/kg the increase was to 181.9% of the control value.
On day 14 after CAPS administration, at 2500 mg/kg, many erythrocyte parameters had returned to normal (Table 1). At 3500 mg/kg CAPS, there was a similar trend with evidence of regeneration; the splenic nucleated cell count, the total reticulocyte count and the HFR reticulocyte count all showed elevations (to 118.6, 141.6 and 155.4% of the control values, respectively), but these increases were not statistically significant. However, at both 2500 and 3500 mg/kg CAPS, at day 14 post-dosing, RDW and HDW were significantly increased.
In overall terms, therefore, by day 21 post-dosing, at both CAPS levels, parameters had returned to normal; however, RDW and HDW still remained significantly elevated at this time.
Leucocytes
At the 2500 mg/kg dose level of CAPS, on day 1 post-dosing, the total WBC count was statistically significantly depressed, as was the count for CFU-GM (Table 2). However, the femoral bone marrow myeloid count at day 1 post-dosing was normal. The numbers of ‘other’ femoral marrow cells (this included especially monocytes and monocyte precursors), and peripheral blood LUC counts, were both elevated at day 1 post-dosing in mice treated at 2500 mg/kg, but these increases did not reach statistical significance. Peripheral blood neutrophils, monocytes, eosinophils and basophils all showed reductions at day 1 post-dosing at 2500 mg/kg, but these decreases did not reach statistical significance. At day 7 post-dosing in the 2500 mg/kg CAPS group, there were significant elevations in LUC and basophil counts; monocytes and ‘other’ femoral bone marrow cells were also increased, but not statistically significantly. At 14 and 21 days post-dosing, in the 2500 mg/kg CAPS mice, the majority of leucocyte parameters were close to control values.
Table 2.
Mean leucocyte parameters in female B6C3F1 mice dosed with chloramphenicol succinate (CAPS) at 2500 and 3500 mg/kg on five consecutive days and sampled at 1, 7, 14 and 21 days after the final dose
| CAPS dose (mg/kg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 2500 | 3500 | ||||||||||
| Day post-dosing | 1 | 7 | 14 | 21 | 1 | 7 | 14 | 21 | 1 | 7 | 14 | 21 |
| WBC | 4.43 | 4.54 | 4.78 | 5.80 | 360** | 6.44 | 5.60 | 5.80 | 2.60** | 5.66 | 4.35 | 6.06 |
| Neut | 0.510 | 0.530 | 0.563 | 0.652 | 0.265 | 0.643 | 0.754 | 0.660 | 0.116** | 0.708 | 0.640 | 0.690 |
| Lymph | 3.635 | 3.704 | 3.862 | 4.762 | 3.158 | 4.884 | 4.388 | 4.843 | 2.374* | 4.000 | 3.416 | 5.012 |
| Mono | 0.108 | 0.125 | 0.075 | 0.120 | 0.048 | 0.295 | 0.107* | 0.097 | 0.078 | 0.244 | 0.088 | 0.107 |
| Eo | 0.100 | 0.080 | 0.100 | 0.140 | 0.065 | 0.084 | 0.070 | 0.100 | 0.040* | 0.109 | 0.120 | 0.140 |
| Baso | 0.010 | 0.020 | 0.010 | 0.020 | 0.000 | 0.090* | 0.020 | 0.020 | 0.000 | 0.020 | 0.010 | 0.020 |
| LUC | 0.028 | 0.024 | 0.078 | 0.064 | 0.065 | 0.070* | 0.070 | 0.060 | 0.010 | 0.036* | 0.053 | 0.048 |
| BM myel cells | 0.440 | 0.478 | 0.433 | 0.404 | 0.426 | 0.506 | 0.380 | 0.460 | 0.598 | 0.608 | 0.363 | 0.604 |
| BM lymph cells | 0.403 | 0.378 | 0.323 | 0.360 | 0.280 | 0.472 | 0.323 | 0.498 | 0.315 | 0.274 | 0.288 | 0.422 |
| BM other cells | 0.035 | 0.022 | 0.045 | 0.044 | 0.084 | 0.052 | 0.028 | 0.046 | 0.100* | 0.058 | 0.028 | 0.026 |
| CFU-GM | 28.7 | nd | 30.3 | 30.7 | 19.0* | nd | 27.3 | 29.0 | 14.3* | nd | 26.7 | 35.3 |
Abbreviations and units: WBC, white blood cells (×109/l); Neut, neutrophils (×109/l); Lymph, lymphocytes (×109/l); Mono, monocytes (×109/l); Eo, eosinophils (×109/l); Baso, basophils (×109/l); LUC, large unstained cells (×109/l); BM myel cells, femoral bone marrow myeloid cells (×107); BM lymph cells, femoral bone marrow lymphoid cells (×107); BM other cells, femoral bone marrow other cells (×107); CFU-GM, colony-forming units-granulocyte-macrophage, absolute number of colonies per plate; nd, not determined. All other information as Table 1, except n = 3 for CFU–GM. Cells categorized as ‘other’ include monocytes and monocyte precursors, mast cells, megakaryocytes, plasma cells and unidentifiable cells.
In mice treated at 3500 mg/kg CAPS, on day 1 post-dosing, significant reductions were evident in total leucocyte, neutrophil, lymphocyte, eosinophil and CFU-GM counts (Table 2). Basophils and LUC were also decreased, but not significantly. Femoral marrow myeloid cells, however, were slightly increased (NS), but other femoral marrow cells were increased to nearly 300% of the control value (P < 0.05). At 7 days post-dosing with 3500 mg/kg CAPS, all peripheral blood WBC counts except the basophil count were elevated; however, only the elevation of the LUC count was statistically significant (P < 0.05). The femoral marrow count of other cells was elevated to about 260% of the control value at 7 days post-dosing (NS).
At 14 days and 21 days post-dosing in the 3500 mg/kg CAPS group, most leucocyte parameters were close to the corresponding control values.
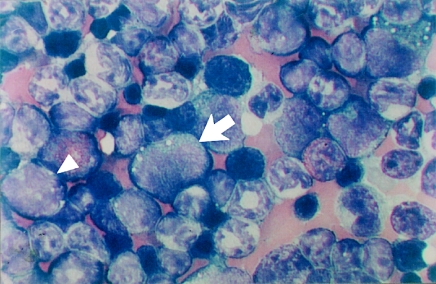
An examination of the marrow smears from mice at day 1 post-dosing (2500 and 3500 mg/kg CAPS) showed that many myeloblasts, promyelocytes, myelocytes and monocyte precursors contained a small number of cytoplasmic vacuoles. Furthermore, bone marrow taken from the mouse killed accidentally after a single dose of CAPS at 3500 mg/kg illustrated marked vacuolation of the myeloid precursors (Figure 4).
Figure 4.
Bone marrow smear (Romanowsky-stained) from a mouse given a single dose of chloramphenicol succinate at 3500 mg/kg and which died on the day of treatment (following a dosing accident). Cytoplasmic vacuolation is evident in the myeloid (arrow) and monocytic (arrowhead) precursors. Original magnification, ×1000.
Discussion
In the present study with female B6C3F1 mice, significant myelotoxicity was induced with CAPS using a dosing regimen of drug administration by gavage at 2500 and 3500 mg/kg for five consecutive days. At both CAPS dose levels, cessation of erythropoiesis was evident at day 1 post-dosing. A recovery was seen at day 7 post-dosing at the 2500 mg/kg CAPS dose level and at between 7 and 14 days at the 3500 mg/kg dose level (Table 1). Myelotoxicity was most pronounced in the erythroid series at each dose level. However, toxic effects were not restricted to the erythroid lineage, as some reductions in CFU-GM counts and peripheral blood leucocyte counts were also evident at both CAPS dose levels (Table 2). In addition, although cytoplasmic vacuolation was demonstrated in cells of the erythroid series (Figure 2), precursors of the myeloid lineage were also affected (Figure 4). Although femoral marrow BFU-E and CFU-E were depressed by CAPS administration at day 1 post-dosing, the myelosupressive effect of the drug was more clearly identified in the morphologically recognizable committed erythroid precursor cells in the bone marrow and in the cellularity of the spleen.
However, of the many parameters investigated in the present study, the CAPS-induced depressive change was most marked in the response of the HFR reticulocyte fraction, that is, in the most immature reticulocytes. The HFR reticulocyte count was also the most sensitive indicator of erythroid recovery and showed a maximal amplitude of response as erythropoiesis returned to normal in the post-dosing period.
The pattern of CAPS-induced erythroid suppression and recovery in the present experiment compares closely with the reversible toxicity to cells of the erythroid series reported in man (Volini et al. 1950; Saidi et al. 1961; Jiji et al. 1963; Scott et al. 1965). The maturation arrest (and vacuolation) at the level of the pronormoblast and early normoblast in the present study is also consistent with findings in man (Rosenbach et al. 1960; Scott et al. 1965; Yunis et al. 1970; Skinnider & Ghadially 1976), and also in the rat (Schrober et al. 1972), and in the mouse (Miura et al. 1980).
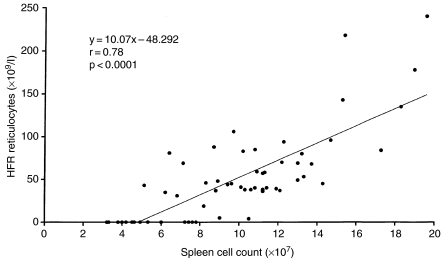
In the present experiment with CAPS, and in previous investigations with other myelotoxic drugs such as methotrexate (Andrews 1995), a clear relationship has been shown to exist between the peripheral blood HFR reticulocyte count and the histological assessment of splenic erythropoiesis in touch preparations (Figure 3). These results also find a parallel in the relationship between the HFR reticulocyte count and the splenic nucleated cell count; this is illustrated in Figure 5. However, it was demonstrated previously (Andrews 2000) that changes in the response of the peripheral blood HFR reticulocyte levels tend to lag behind the response of the spleen and marrow by about 1 day. A delay in response of the blood HFR reticulocyte fraction was also seen in the present study, for mice which had received 3500 mg/kg CAPS, at day 7 post-dosing, showed slight/moderate erythropoietic activity in spleen touch preparations, whereas the HFR reticulocyte counts in the peripheral blood at this time remained exceptionally low (Table 1).
Figure 5.
Relationship between splenic nucleated cell count and the peripheral blood high fluorescence ratio reticulocyte count (with line of best fit) for individual control mice and animals treated at 2500 and 3500 mg/kg chloramphenicol succinate for 5 days and sampled at 1, 7, 14 and 21 days after the final dose.
In the post-dosing recovery period, erythroid parameters exhibited a rebound effect, with some values increasing to levels above those in the control animals. For example, in mice treated with 2500 mg/kg CAPS at 7 days post-dosing, the HFR and total reticulocyte counts, and the spleen cellularity, all showed some evidence of a rebound effect to values above control levels (Table 1); this pattern of response was also seen in the bone marrow erythroid cells. Similarly, at the 3500 mg/kg CAPS dose level, at 14 days post-dosing, some evidence of a rebound effect was seen in the HFR and total reticulocyte counts. Rebound reticulocytosis in the BALB/c and CD-1 mouse strains in the recovery period after CAPS administration was also reported by Havard et al. (1999a) and Turton et al. (1999, 2000); a similar response was also evident after CAPS dosing in the guinea pig (Havard et al. 1999b; Turton et al. 2003) and after CAP administration in the duck (Rigdon et al. 1954). In the report of Rigdon et al. (1954), an analysis of reticulocyte morphology suggested that the response involved the most immature reticulocyte forms, and this result therefore confirms the changes seen in the HFR reticulocyte fraction in the present study.
The findings in the present investigation on CAPS myelotoxicity support the observation that in states of drug- and chemical-induced myelosuppression in the mouse, compensatory erythropoiesis occurs in the spleen. In mice which developed marrow aplasia following the administration of 89Sr (Klassen et al. 1972), normal levels of reticulocyte production and 59Fe incorporation were maintained by splenic erythropoiesis, with splenectomized animals dying with pancytopenia. Likewise, Anagnostou et al. (1976) reported that in mice treated with estradiol benzoate, reduction in the uptake of 59Fe into the bone marrow occurred, but significantly increased uptake took place in the spleen, which maintained a normal haematocrit and red cell mass in the peripheral blood. Similarly, in mice treated with the antibiotic thiamphenicol (TAP) (Goris et al. 1990) or human granulocyte colony-stimulating factor (de Hann et al. 1992), suppression of femoral marrow BFU-E and CFU-E was accompanied by the stimulation of splenic erythroid progenitors; this response was interpreted as a net migration of erythroid precursor cells from the marrow to the spleen.
At day 7 post-dosing in mice treated with CAPS at both 2500 and 3500 mg/kg, there was a significant increase in platelet count (Table 1). A similar effect on platelet numbers has previously been reported in the CAPS- and TAP-treated mouse (Festing et al. 2001; Turton et al. 2002). This thrombocytosis, in the period after a drug-mediated inhibition of erythrocyte maturation, is consistent with the suggestion that this change is an erythropoietin (EPO)-stimulated effect and appears to relate to the frequently observed clinical finding of an inverse correlation between HCT and platelet count (Jackson et al. 1974). An explanation for the response may be that in an anaemia, EPO production by the kidney increases, and EPO as well as stimulating RBC output from the marrow also has an enhancing effect on megakaryopoiesis (Sakaguchi et al. 1987; Hunt 1995).
Peripheral blood total leucocyte counts were significantly depressed at day 1 post-dosing with CAPS given at 2500 and 3500 mg/kg in the present study, returning to normal values at day 7. However, this was in contrast to erythroid counts which only returned to control values at day 14 post-dosing. This suggests a lower specificity of CAPS toxicity for cells of the myeloid series, possibly in association with the shorter maturation time of mouse leucocytes (Filmanowicz & Gurney 1960; Lee et al. 1979). However, although counts of CFU-GM were also significantly reduced at day 1 post-dosing at both CAPS dose levels (Table 2), the numbers of morphologically recognizable myeloid precursors in the bone marrow were not decreased. Indeed, monocytes and monocyte precursors (recorded as ‘other’ bone marrow cells, Table 2), were present at increased levels in marrow smears at both 2500 (NS) and 3500 mg/kg CAPS (P < 0.05) at day 1 post-dosing, and this was followed at day 7 by increased peripheral blood monocyte (NS) and LUC (P < 0.05) counts. Adams (1983) described a neutrophilia following CAP administration in a patient with chronic neutropenia, and a stimulation of human CFU-GM levels was demonstrated in vitro with sub-therapeutic concentrations of CAP by Bostrom et al. (1986). Turton et al. (2000) also described elevated neutrophil counts in the immediate post-dosing period in BALB/c mice treated with CAPS at 2000 mg/kg for 17 days.
In the present study, the formation of cytoplasmic vacuoles in early normoblasts, and in myeloblasts, promyelocytes and myelocytes, and in monocyte precursors, is of interest. CAP-induced cytoplasmic and nuclear vacuolation has been described in patients treated with the antibiotic (Krakoff et al. 1955; Rosenbach et al. 1960; Saidi et al. 1961; Jiji et al. 1963; Scott et al. 1965; Ferrari & Pajola 1981), with the change generally involving the erythroblast, but granulocytes, megakaryocytes and plasma cells may also be affected. Schrober et al. (1972), reported the induction of vacuoles in the pronormoblasts of rats dosed with 150 mg/kg CAP twice daily for 5 weeks, and Turton et al. (2000, 2002) described the formation of TAP-induced vacuoles in the bone marrow precursors of the mouse and in the CAPS-treated guinea pig (Turton et al. 2003).
The present experiment was carried out to characterize in detail the myelotoxicity of CAPS administered at high-dose levels, with a view to using repeated cycles of a 5-day dosing period followed by a 16- or 23-day non-dosing recovery period (i.e. an intermittent dosing regimen), in a later study to investigate the possible development of CAPS-induced AA in the BU-pretreated B6C3F1 mouse. In general terms, all the blood and marrow parameters in the present study returned to normal by 14 days post-dosing at both the 2500 and 3500 mg/kg CAPS dose level. However, the particularly sensitive indices of erythrocyte volume (RDW) and intracellular haemoglobin distribution (HDW) still illustrated some residual effects at day 21 post-dosing, reflecting the earlier perturbation of erythropoiesis. It was therefore considered that in a future experiment with BU-pretreated mice, an intermediate dose level of 3000 mg/kg CAPS would be well tolerated, with repeated 21 day cycles consisting of a 5-day dosing period followed by a 16-day recovery period (Andrews 2000).
Acknowledgments
CMA thanks colleagues in the BioResources Section and the Clinical Pathology Unit, GlaxoSmithKline for their assistance with the in vivo studies and the analysis of samples. JAT thanks GlaxoSmithKline, Ware for continued support, and Ms Vicky Welsh and Ms Janet Andrew for assistance in the the preparation of the manuscript.
References
- Adams P. Chloramphenicol-responsive chronic neutropenia. N. Engl. J. Med. 1983;309:1039–1041. doi: 10.1056/NEJM198310273091707. [DOI] [PubMed] [Google Scholar]
- Anagnostou A, Zander A, Barone J, Fried W. Mechanisms of the increased splenic erythropoiesis in mice treated with estradiol benzoate. J. Lab. Clin. Med. 1976;88:700–706. [PubMed] [Google Scholar]
- Andrews CM. Sensitivity of reticulocyte high fluorescence ratio to splenic erythropoiesis. Comp. Haematol. Int. 1995;5:210. [Google Scholar]
- Andrews CM. University of London; Studies on the haemotoxicity of busulphan and chloramphenicol in the B6C3F1 mouse. PhD Thesis. [Google Scholar]
- Andrews CM, Dash LM, Williams TC, Criag Gray J, Turton JA. Long-term effects of busulphan on lymphocyte subpopulations in female B6C3F1 mice. Comp. Haematol. Int. 1997;7:230–237. [Google Scholar]
- Andrews CM, Spurling NW, Turton JA. Characterisation of busulphan-induced myelotoxicity in B6C3F1 mice using flow cytometry. Comp. Haematol. Int. 1993;3:102–115. [Google Scholar]
- Andrews CM, Williams TC, Turton JA. Long-term haematological alterations in female B6C3F1 mice treated with busulphan. Comp. Haematol. Int. 1998;8:125–138. [Google Scholar]
- Benestad H. Drug mechanisms in marrow aplasia. In: Geary CG, editor. Aplastic Anaemia. London: Balliere-Tindall; 1979. pp. 26–42. [Google Scholar]
- Bentley SA, Taylor MA, Killian DE, et al. Correction of bone marrow nucleated cell counts for the presence of fat particles. Am. J. Clin. Pathol. 1995;104:60–64. doi: 10.1093/ajcp/104.1.60. [DOI] [PubMed] [Google Scholar]
- Best WR. Chloramphenicol-associated blood dyscrasias. J. Am. Med. Assoc. 1967;201:99–106. [Google Scholar]
- Bostrom B, Smith K, Ramsey NKC. Stimulation of human committed bone marrow stem cells (CFU-GM) by chloramphenicol. Exp. Haematol. 1986;14:156–161. [PubMed] [Google Scholar]
- Chaplin S. Bone marrow depression due to mianserin, phenylbutazone, oxyphenbutazone, and chloramphenicol-part II. Adverse Drug React. Acute Poisoning Rev. 1986;3:181–196. [PubMed] [Google Scholar]
- Dacie JV, Lewis SM. Practical Haematology. 5. London: Churchill Livinstone; 1975. [Google Scholar]
- Davies DT, Fisher GV. The evaluation and application of the Techicon H*1 for the complete automated evaluation of laboratory animal haematology. Comp. Haematol. Int. 1991;1:803–808. [Google Scholar]
- Dollery C. Therapeutic Drugs. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- FAO/WHO. Toxicological Evaluation of Certain Veterinary Drug Residues in Food (WHO Food Additives Series 23) World Health Organization: Geneva; 1988. Expert on food additives. Chloramphenicol; pp. 1–71. [Google Scholar]
- Ferrari V, Pajola E. Types of haemopoietic inhibition by chloramphenicol and thiamphenicol. In: Najean Y, Tognoni G, Yunis AA, editors. Safety Problems Related to Chloramphenicol and Thiamphenicol. New York: Raven Press; 1981. pp. 43–59. [Google Scholar]
- Festing MFW, Diamanti P, Turton J. Strain differences in haematological response to chloramphenicol succinate in mice: implications for toxicological research. Food Chem. Toxicol. 2001;39:375–383. doi: 10.1016/s0278-6915(00)00149-6. [DOI] [PubMed] [Google Scholar]
- Filmanowicz E, Gurney CW. Studies on erythropoiesis XV1. Response to a single dose of erythropoietin in polycythemic mice. J. Lab. Clin. Med. 1960;57:65–72. [PubMed] [Google Scholar]
- Fraunfelder FT, Morgan RL, Yunis AA. Blood dyscrasias and topical ophthalmic chloramphenicol. Am. J. Ophthalmol. 1993;115:812–813. doi: 10.1016/s0002-9394(14)73653-0. [DOI] [PubMed] [Google Scholar]
- Freeman KP. Bone marrow evaluation. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm's Veterinary Haematology. 5. Philadelphia: Lippincott Williams; 2000. pp. 29–32. [Google Scholar]
- Fuchs A, Eder H. Automated reticulocyte analysis of blood in different species. Sysmex J. Int. 1991;1:34–38. [Google Scholar]
- Goris H, Bungart B, Loeffler M, Schmitz S, Nijhof W. Migration of stem cells and progenitors between marrow and spleen following thiamphenicol treatment of mice. Exp. Haematol. 1990;18:400–407. [PubMed] [Google Scholar]
- Haak HL. Experimental drug-induced aplastic anaemia. Clinics Haematol. 1980;9:621–639. [PubMed] [Google Scholar]
- Hall R, Malia RG. Medical Laboratory Haematology. London: Butterworths; 1984. [Google Scholar]
- de Hann G, Loeffler M, Nijhof W. Long-term recombinant human granulocyte colony-stimulating factor (rhG-CSF) treatment severely depresses murine marrow erythropoiesis without causing an anaemia. Exp. Haematol. 1992;20:600–604. [PubMed] [Google Scholar]
- Havard A, Andrews M, Holt D, Williams T, Turton JA. Chloramphenicol-induced reticulocytopenia and anaemia in the mouse: depressed parameters return to normal with continued dosing. Hum. Exp. Toxicol. 1999a;18:532. [Google Scholar]
- Havard A, Andrews CM, Willliams TC, Turton JA. Chloramphenicol induces reversible dose-related reticulocytopenia, not aplastic anaemia, in the guinea pig. Hum. Exp. Toxicol. 1999b;18:764. [Google Scholar]
- Heimpel H. Epidemiology and etiology of aplastic anaemia. In: Schrezenmeier H, Bacigalupo A, editors. Aplastic Anaemia; Pathophysiology and Treatment. Cambridge: Cambridge University Press; 2000. pp. 97–116. [Google Scholar]
- Holt DE, Andrews CM, Payne JP, Williams TC, Turton JA. The myelotoxicity of chloramphenicol: in vitro and in vivo studies: II in vivo myelotoxicity in the B6C3F1 mouse. Hum. Exp. Toxicol. 1998;17:8–17. doi: 10.1177/096032719801700102. [DOI] [PubMed] [Google Scholar]
- Holt D, Harvey D, Hurley R. Chloramphenicol toxicity. Adverse Drug React. Toxicol. Rev. 1993;12:83–95. [PubMed] [Google Scholar]
- Hugley CM, Erslev AJ, Bergsagel DE. Drug related blood dyscrasias. J. Am. Med. Assoc. 1961;177:23–26. doi: 10.1001/jama.1961.73040270010004a. [DOI] [PubMed] [Google Scholar]
- Hunt P. A bipotential megakaryocyte/erythrocyte progenitor cell: the link between erythropoiesis and megakaryopoiesis becomes stronger. J. Lab. Clin. Med. 1995;125:303–304. [PubMed] [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risk of Chemicals to HumansChloramphenicol. Vol. 50. Lyon: IARC; 1990. pp. 169–193. [PMC free article] [PubMed] [Google Scholar]
- Jackson CW, Simone JV, Edwards CC. The relationship of anaemia and thrombocytosis. J. Lab. Clin. Med. 1974;84:357–368. [PubMed] [Google Scholar]
- Jandl H. Blood; Textbook of Haematology. Boston: Little Brown; 1996. [Google Scholar]
- Jiji RM, Gangarosa EJ, De la Macorra F. Chloramphenicol and its sulfamoyl analogue. Arch. Intern. Med. 1963;111:116–127. doi: 10.1001/archinte.1963.03620250074011. [DOI] [PubMed] [Google Scholar]
- Kinsey SE, Watts MJ. Accurate automated leucocyte differential counts despite profound leucopemia. J. Clin. Pathol. 1988;41:1236–1239. doi: 10.1136/jcp.41.11.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen LW, Birks J, Allen E, Gurney CW. Experimental medullary aplasia. J. Lab. Clin. Med. 1972;80:8–17. [PubMed] [Google Scholar]
- Knoll JS. Clinical automated haematology systems. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm's Veterinary Haematology. 5. Philadelphia: Lippincott William; 2000. pp. 3–11. [Google Scholar]
- Krakoff IH, Karnofsky DA, Burchenal JH. Effects of large doses of chloramphenicol on human subjects. N. Engl. J. Med. 1955;253:7–10. doi: 10.1056/NEJM195507072530102. [DOI] [PubMed] [Google Scholar]
- Kumar P, Verma IC. Antibiotic therapy for bacterial meningitis in children in developing countries. Bull. Wld. Hlth. Org. 1993;71:183–188. [PMC free article] [PubMed] [Google Scholar]
- Kushwaha KP, Verma RB, Singh YD, Rathi AK. Surveillance of drug induced diseases in children. Indian J. Pediatr. 1994;61:357–365. doi: 10.1007/BF02751889. [DOI] [PubMed] [Google Scholar]
- Lee M, Durch S, Dale D, Finch C. Kinetic of tumour-induced murine neutrophilia. Blood. 1979;53:619–632. [PubMed] [Google Scholar]
- Miura AB, Yoshida K, Yamaguchi A, Fukuda M. The effect of chloramphenicol and thiamphenicol on the ultrastructive of erythroblasts in mouse splenic colonies. Tohuku J. Exp. Med. 1980;130:335–340. doi: 10.1620/tjem.130.335. [DOI] [PubMed] [Google Scholar]
- Morley A, Blake J. An animal model of chronic hypoplastic marrow failure. Late marrow failure after busulphan. Blood. 1974a;44:49–56. [PubMed] [Google Scholar]
- Morley A, Blake J. Hemopoietic precursor cells in experimental hypoplastic marrow failure. Aust. J. Exp. Biol. Med. Sci. 1974b;52:909–914. doi: 10.1038/icb.1974.90. [DOI] [PubMed] [Google Scholar]
- Morley A, Trainor K, Blake J. A primary stem cell lesion in experimental chronic hypoplastic marrow failure. Blood. 1975;45:681–688. [PubMed] [Google Scholar]
- Morley A, Trainor K, Remes J. Residual marrow damage: possible explanation for idiosyncrasy to chloramphenicol. Br. J. Haematol. 1976;32:525–531. doi: 10.1111/j.1365-2141.1976.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Parfitt K. Martindale: the Complete Drug Reference. 32. London: Pharmaceutical Press; 1999. pp. 182–184. [Google Scholar]
- Rich ML, Ritterhoff RJ, Hoffmann RJ. A fatal case of aplastic anaemia following chloramphenicol (chloromycetin) therapy. Ann. Intern. Med. 1950;33:1459–1467. doi: 10.7326/0003-4819-33-6-1459. [DOI] [PubMed] [Google Scholar]
- Rigdon RH, Crass G, Martin N. Anaemia produced by chloramphenicol (chloromycetin) in the duck. A. M. A. Arch. Pathol. 1954;58:85–93. [PubMed] [Google Scholar]
- Rosenbach LM, Caviles AP, Mitus WJ. Chloramphenicol toxicity: reversible vacuolization of erythroid cells. N. Engl. J. Med. 1960;263:274–278. doi: 10.1056/NEJM196010132631503. [DOI] [PubMed] [Google Scholar]
- Rubin D, Weisberger AS, Botti RE, Storaosli JP. Changes in iron metabolism in early chloramphenicol toxicity. J. Clin. Invest. 1958;37:1286–1292. doi: 10.1172/JCI103716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi MD, Wallerstein RO, Aggeler PM. Effect of chloramphenicol on erythropoiesis. J. Lab. Clin. Med. 1961;57:247–256. [PubMed] [Google Scholar]
- Sakaguchi M, Kawakita M, Matsushita J, Shibaya K, Koishihara Y, Takatsuki K. Human erythropoietin stimulates murine megakarypoiesis in serum-free culture. Exp. Haematol. 1987;15:1028–1034. [PubMed] [Google Scholar]
- Schrober R, Kosek JC, Wolf PL. Chloramphenicol-induced vacuoles. Their ultrastructure in bone marrow pronormoblasts and immature myeloid cells. Arch. Pathol. 1972;94:298–392. [PubMed] [Google Scholar]
- Scott JL, Finegold SM, Belkin GA, Lawrence JS. A controlled double blind study of the haematologic toxicity of chloramphenicol. N. Engl. J. Med. 1965;272:1137–1142. doi: 10.1056/NEJM196506032722201. [DOI] [PubMed] [Google Scholar]
- Skinnider LF, Ghadially FN. Chloramphenicol-induced mitochondrial and ultrastructural changes in haemopoietic cells. Arch. Pathol. Lab. Med. 1976;100:601–605. [PubMed] [Google Scholar]
- Smith CA, Andrews CM, Collard JK, Hall DE, Walker AK. A Color Atlas of Comparative Diagnostic and Experimental Haematology. London: Wolfe; 1994. [Google Scholar]
- Tichelli A, Gratwohl A, Driessen A, et al. Evaluation of the SysmexR-1000: an automated reticulocyte analyser. Am. J. Clin. Pathol. 1990;93:70–78. doi: 10.1093/ajcp/93.1.70. [DOI] [PubMed] [Google Scholar]
- Trevett AJ, Naraqi S, Wambri J. Typhoid fever complicated by chloramphenicol toxicity, ataxia and psychosis. Papua New Guinea Med. J. 1992;35:205–209. [PubMed] [Google Scholar]
- Turton JA, Andrews CM, Havard AC, et al. Haemotoxicity of thiamphenicol in the BALB/c mouse and Wistar Hanover rat. Food Chem. Toxicol. 2002;40:1849–1861. doi: 10.1016/s0278-6915(02)00178-3. [DOI] [PubMed] [Google Scholar]
- Turton JA, Andrews CM, Havard AC, Williams TC. Studies on the haemotoxicity of chloramphenicol in the Dunkin Hartley guinea pig. Int. J. Exp. Pathol. 2003;83:1–14. doi: 10.1046/j.1365-2613.2003.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton JA, Havard AC, Robinson S, et al. An assessment of chloramphenicol and thiamphenicol in the induction of aplastic anaemia in the BALB/c mouse. Food Chem. Toxicol. 2000;38:925–938. doi: 10.1016/s0278-6915(00)00087-9. [DOI] [PubMed] [Google Scholar]
- Turton JA, Yallop D, Andrews M, Fagg R, York M, Willaims TC. Haemotoxicity of chloramphenicol succinate in the CD-1 mouse and Wistar Hanover rat. Hum. Exp. Toxicol. 1999;18:566–576. doi: 10.1191/096032799678845098. [DOI] [PubMed] [Google Scholar]
- Vincent PC. In vitro evidence of drug action in aplastic anaemia. Blut. 1984;49:3–12. doi: 10.1007/BF00320378. [DOI] [PubMed] [Google Scholar]
- Volini IF, Greenspan L, Ehrlich L, Gunner JA, Felsenfeld O, Schwartz SO. Hemopoietic changes during administration of chloramphenicol (chloromycetin) J. Am. Med. Assoc. 1950;142:1333–1355. doi: 10.1001/jama.1950.02910350003002. [DOI] [PubMed] [Google Scholar]
- Wallerstein RO, Condit PK, Kasper CK, Brown JW, Morrison FR. Statewide study of chloramphenicol therapy and fatal aplastic anaemia. J. Am. Med. Assoc. 1969;208:2045–2050. [PubMed] [Google Scholar]
- Welch H, Lewis CN, Kerlan I. Blood dyscrasias. A nationwide survey. Antibiot. Chemother. 1954;4:607–623. [PubMed] [Google Scholar]
- Young NS, Alter BP. Aplastic Anaemia: Acquired and Inherited. Philadelphia: W.B. Saunders; 1994. [Google Scholar]
- Young NS, Maciejewski JP. The pathophysiology of acquired aplastic anaemia. N. Engl. J. Med. 1997;336:1365–1372. doi: 10.1056/NEJM199705083361906. [DOI] [PubMed] [Google Scholar]
- Young NS, Maciejewski JP. Aplastic anaemia. In: Hoffman R, et al., editors. Haematology: Basic Principles and Practice. New York: Churchill Livingstone; 2000. pp. 297–331. [Google Scholar]
- Yunis AA. Mechanisms underlying marrow toxicity from chloramphenicol and thiamphenicol. In: Silver R, Le Bue J, Gordon AS, editors. The Year in Haematology. New York: Plenum Press; 1978. pp. 143–170. [Google Scholar]
- Yunis AA, Bloomberg GR. Chloramphenicol toxicity, clinical features and pathogenesis. Prog. Haematol. 1964;4:138–159. [PubMed] [Google Scholar]
- Yunis AA, Smith US, Restreppo A. Reversible bone marrow suppression from chloramphenicol: a consequence of mitochondrial injury. Arch. Intern. Med. 1970;126:272–275. [PubMed] [Google Scholar]