Abstract
Intragranulomatous necrosis is a primary feature in the natural history of human tuberculosis (TB). Unfortunately, this phenomenon is not usually seen in the experimental TB murine model. Artificial induction of this necrosis in pulmonary granulomas (INPG) may be achieved through aerosol inoculation of lipopolysaccharide (LPS) 3 weeks after Mycobacterium tuberculosis infection. At week 9 post-infection, the centre of primary granulomas became larger, showing eosinophilic necrosis. Interestingly, INPG induction was related to mice strains C57BL/6 and 129/Sv, but not to BALB/c and DBA/2. Furthermore, the same pattern was obtained with the induction of infection using a clinical M. tuberculosis strain (UTE 0335R) that naturally induces INPG. In all the mice strains tested, the study of pulmonary mRNA expression revealed a tendency to increase or to maintain the expression of RANTES, interferon-γ, tumour necrosis factor and iNOS, in both LPS- and UTE 0335R-induced INPG, thus suggesting that this response must be necessary but not sufficient for inducing INPG. Our work supports that INPG induction is a local phenomenon unrelated to the resistant (C57BL/6 and BALB/c) or susceptible (129/Sv and DBA/2) background of mice strains against M. tuberculosis infection.
Keywords: intragranulomatous necrosis, Koch's reaction, murine model, Mycobacterium tuberculosis, Shwartzman reaction
Intragranulomatous necrosis in pulmonary granulomas (INPG) is a characteristic feature in lesions induced by Mycobacterium tuberculosis infection in humans. These lesions are well structured, with a macrophage-based ring surrounding INPG and an outer lymphocyte-based layer well limited by a fibrotic container (Grange 1998). This is basic in the natural history of TB, because INPG is an important source of latent bacilli (Gomez & McKinney 2004). The difficulty in finding a successful short-course chemotherapy (Mitchison & Dickinson 1978) to control both TB and latent TB infection (as well as the 8000,000 annual incidental new cases and prevailing 2,000,000,000 infected people, respectively) lies in the presence of these bacilli (World Health Organization 2001). Unfortunately, the most commonly used experimental model of tuberculosis (TB) does not show INPG. At the most, similar lesions (named primary granulomas) (Cardona et al. 2000) with a macrophage-based centre surrounded by lymphocytes may be seen in the murine model of TB. After a few weeks, these lesions are also surrounded by a mantle of foamy macrophages profusely filling alveolar spaces immediately related to the granuloma.
A few years ago, our group described a technique to induce INPG in the murine model based on old data obtained with the experimental TB model in rabbits (Lurie 1932). As other authors did before (Rook & al Attiyah 1991), we supported the hypothesis that the origin of INPG was related to the Shwartzman reaction (Shwartzman 1937). This reaction was originally described as a local skin reaction triggered by an intradermal injection of endotoxin (preparatory injection), followed 24 h later by an intravenous injection (provoking injection). Approximately, 4 h later, a haemorrhagic skin necrosis developed at the intradermal injection site (Shwartzman 1937). In this regard, tumour necrosis factor (TNF) secreted by locally activated macrophages would cause haemorrhagic necrosis in mycobacteria-infected tissues reacting as a ‘prepared site’. Indeed, intranasal inoculation of lipopolysaccharide (LPS) 3 weeks after M. tuberculosis infection induced INPG in primary granulomas of mice, thus providing a kind of ‘human-like’ model of TB (Cardona et al. 2001). In this model, LPS inoculation caused the infiltration of lung parenchyma with neutrophils (PMNs) after 24 h. Necrosis could only be detected in primary granulomas at week 3 after LPS inoculation, when the presence of eosinophilic necrosis became apparent in the centre of the granulomas. At week 6 post-LPS inoculation, necrosis extended and presented a compact caseous consistency made by collagen (Cardona et al. 2001). However, this model was also associated with a high mortality rate in mice.
Further experiments showed the convenience of aerosol LPS inoculation to induce the same effects without such a high mortality rate. Searching for a model of ‘human TB disease’, and thus combining induction of INPG with spontaneous reactivation, resulted in new experiments with a susceptible mice strain, such as DBA/2 (Medina & North 1998). The outcome was clear after tests had been conducted using both intranasal and aerosol LPS inoculation: no INPG was induced in either case, and thus additional experiments were carried out with new mice strains. BALB/c and 129/Sv strains were chosen to search for strains that shared the same H-2 background but also had different susceptibility to M. tuberculosis infection, defined on the survival time after infection (Medina & North 1998).
The aim of this work was to obtain more extensive data on this ‘human-like’ model and the origin of intragranulomatous necrosis in TB; hence, we used different common inbred mouse strains with different resistance degrees against M. tuberculosis infection to test a more refined and equally effective model in which LPS mortality may be avoided using an aerosol instead of an intranasal LPS inoculation. INPG was not induced in all strains, appearing in H-2b mice strains (e.g. C57BL/6 and 129/Sv), but not in H-2d strains (e.g. BALB/c and DBA/2). Interestingly, this phenomenon was not related to the resistant (C57BL/6 and BALB/c) or susceptible (129/Sv and DBA/2) background of mice strains against M. tuberculosis infection (Medina & North 1998). The association between LPS inoculation and INPG was demonstrated by its induction in C3H/HeN mice, but not in LPS-resistant C3H/HeJ mice. Furthermore, the accidental finding of clinical M. tuberculosis strains that spontaneously induce INPG (Cardona et al. 2003) has led to similar histopathological findings. These data support the hypothesis of a Shwartzman reaction-like response as the origin of INPG in the experimental murine TB models of infection probably linked to the ‘provoking’ action of endotoxin-like molecules of the M. tuberculosis cell wall, like cord-factor. Finally, our data suggest that the induction of INPG is not related to the degree of resistance against M. tuberculosis infection.
Materials and methods
Mice
Parental inbred specific pathogen-free (spf) female mice, 6–8 weeks old, were obtained from Charles River Laboratories (St Germain sur l'Abresle, France). The parental strains were C57BL/6 (H-2b), 129/Sv (H-2b), BALB/c (H-2d), DBA/2 (H-2d), C3H/HeJ (H-2k) and C3H/HeN (H-2k). They were shipped in adequate travel conditions, with the corresponding certificate of health and origin. All the animals were kept under controlled conditions in a P3 High Security Facility with sterile food and water ad libitum.
Bacteria and infection
M. tuberculosis strains H37Rv NC007416 and UTE 0335R (Cardona et al. 2003a, b) were grown in Proskauer Beck medium containing 0.01% Tween 80 to mid-log phase and stored at −70 °C in 2 ml aliquots. Mice were placed in the exposure chamber of an airborne infection apparatus (Glas-Col Inc., Terre Haute, IN, USA). The nebulizer compartment was filled with 7 ml of a M. tuberculosis suspension at a previously calculated concentration (2.5 × 106 CFU/ml) to provide an approximate uptake of 20 viable bacilli within the lungs. Four mice were used for every time-point in every experimental group. The numbers of viable bacteria in the left lung and spleen homogenates were followed in weeks 9 and 18 by plating serial dilutions on nutrient Middlebrook 7H11 agar (Biomedics s.l., Madrid, Spain) and counting bacterial colony formation after incubation for 21 days at 37 °C. Special care was taken not to include hiliar lymph nodes when removing the left lung so as not to artificially increase colony-forming units (CFU) values. Lungs were immediately removed after euthanasia by means of a halothane overdose (Zeneca Farma, Pontevedra, Spain).
LPS aerosol inoculation
In Experiment B (Figure 1), mice were placed in the exposure chamber of an airborne infection apparatus (Glas-Col Inc.) at week 3 post-infection and submitted for half an hour to the aerosol obtained after filling the nebulizer compartment with 3 ml of distilled water with 100 mg of Escherichia coli 0111:B4 LPS (St Louis, MO, USA). Animals were then sacrificed at weeks 9 and 18 post-infection.
Figure 1.
Designs of the experiments conducted in this study. Experiment A is run with mice only infected with H37Rv; Experiment B with mice infected with H37Rv and inoculated with lipopolysaccharide (LPS), and Experiment C with mice infected with UTE 0335R.
Animal health
Mice were weighed once a week. They were supervised every day under a protocol paying attention to weight loss, apparent good health (bristled hair and wounded skin) and behaviour (signs of aggressiveness or isolation). Animals were euthanized with halothane (Fluothane, Zeneca Farma) overdose so as to avoid any suffering. Sentinel animals were used to check spf conditions in the facility. Tests for 25 known mouse pathogens were all negative. All experimental proceedings were approved and supervised by the Animal Care Committee of ‘Germans Trias i Pujol’ University Hospital in agreement with the European Union Laws for protection of experimental animals.
MRNA quantification
The procedures are described elsewhere (Cardona et al. 2003b). In short, total RNA from the middle right lobe was removed with a commercial phenol-chloroform method, RNAzol (Cinna/Biotecx, Friendswood, TX, USA). After a Dnase treatment with DNA-free kit (Ambion, Woodward Austin, TX USA), a denaturing agarose gel was used to assess the stability of RNA. Five microgram of RNA was reverse transcribed using a Superscript RT kit (Gibco BRL, Grand Island, NY, USA) following the manufacturer's recommendations to obtain cDNA. The quantitative analysis for interferon-γ (IFN-γ), RANTES, iNOS and TNF was performed using a LightCycler™ System (Roche Biochemicals, Idaho Falls, ID, USA). A real-time PCR was carried out in glass capillaries to a final volume of 10 µl in the presence of 1 µl of ×10 reaction buffer (Taq Polymerase, dNTPs, MgCl2, SYBRGreen, Roche Biochemicals), 1 µl of cDNA (or water as negative control, which was always included), MgCl2 to a final concentration of 2–5 mm, and primers to a final concentration of 0.5 µm were also added. A single peak was obtained for each PCR product by melting the curve analysis, and only one band of the estimated size was observed on the agarose gel. Hypoxanthine guanine phosphoribosyl transferase (HPRT) mRNA expression was analysed for every target sample to normalize for efficiency in cDNA synthesis and RNA loading. A ratio based on the HPRT mRNA expression was obtained for each sample.
Histology and morphometry
Procedures have been described in previous works (Cardona et al. 2003b). Briefly, two right lung lobes from each mouse were fixed in buffered formalin and subsequently embedded in paraffin. Every sample was stained with haematoxylin and eosin (H&E). For histometry, 5-µm thick sections from each specimen were stained with H&E and photographed at ×6 using a Stereoscopic Zoom SMZ800 microscope (Nikon, Tokyo, Japan) and a Coolpix 990 digital camera (Nikon). Sections of eight lung lobes were studied in each case. A sequence of appropriate software programs was used, Scion Image (Scion Corporation, Frederick, MD, USA) and Photoshop 5.0 (Adobe Systems Incorporated, San José, CA, USA), to determine the area of each single lesion and the total tissue area on photomicrographs. Sections were blindly evaluated to get a more objective measurement.
Statistical analysis
Sigma Stat (Jandel Scientific Software, San Rafael, CA, USA) was used to compare values. Mann–Whitney Rank Sum Test or Kruskal–Wallis One Way Analysis of Variance on Ranks and All Pairwise Multiple Comparison Procedures (Student-Newman-Keuls Method) were used to determine the differences among experimental groups when comparisons were done between two groups or more, respectively. Differences were significant when marked with * for P < 0.05.
Results
Induction of INPG in pulmonary granulomas at week 9 after aerosol infection
Preliminary data revealed that LPS aerosol inoculation at week 3 post-infection with strain H37Rv caused a profuse PMN infiltration both in the granulomas and in the alveolar spaces after 24 h in all the mice strains tested but C3H/HeJ. Furthermore, this infiltration was not seen in mice infected only with either H37Rv or UTE 0335R strain (data not shown).
The presence of primary granulomas was detected in an average of one out of eight lobes in all the 5 µm sections examined (data not shown), as observed earlier (Cardona et al. 2001).
INPG was described as the presence of an eosinophilic necrosis with a scanty presence of macrophages in H&E staining in the enlarged centre of a primary granuloma. This lesion revealed a profuse presence of collagen as demonstrated by Masson's trichromic staining, thus reflecting scarring of an older necrotic process. The presence of these lesions may be detected at week 9 after infection, as described elsewhere when LPS was administered by intranasal inoculation at week 3 after infection (Cardona et al. 2001).
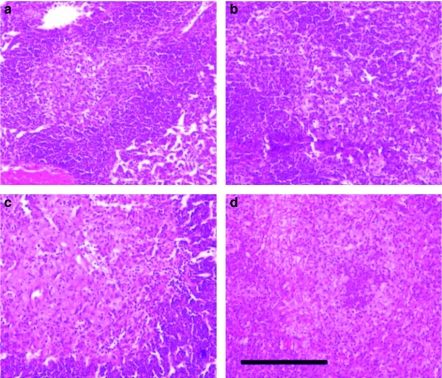
Figure 2 shows a primary granuloma at week 9 after infection with H37Rv strain both with and without LPS aerosol inoculation in C57BL/6 and 129/Sv mice, demonstrating the presence and the absence of INPG after H&E staining. These lesions may also be induced in C3H/HeN mice, but not in DBA/2, BALB/c and C3H/HeJ mice (pictures not shown).
Figure 2.
Induction of intragranulomatous necrosis in pulmonary granulomas (INPG) after haematoxylin and eosin stain at week 9 post-infection in mice infected with H37Rv (Experiment B) after LPS inoculation at week 3. INPG was described as the presence of eosinophilic necrosis with a scanty presence of macrophages in the enlarged centre of a primary granuloma. Panels (a) and (b) show C57BL/6 and 129/Sv control mice. Panels (c) and (d) show LPS-inoculated mice, C57BL/6 and 129/Sv, respectively. Bar represents 200 µm.
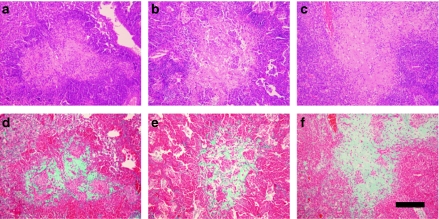
Figure 3 shows primary granulomas in C57BL/6, C3H/HeN and 129/Sv mice at week 9 after infection with the M. tuberculosis clinical strain UTE 0335R (Cardona et al. 2003a), which may naturally induce INPG. In this case, images obtained after Masson's trichromic staining are also shown to demonstrate the presence of collagen in the INPG at this point.
Figure 3.
Intragranulomatous necrosis in pulmonary granulomas in mice C57B/6 (a, d), C3H/HeN (b, e) and 129/Sv (c, f) induced by the Mycobacterium tuberculosis strain UTE 0335R at week 9 post-infection (Experiment C). Panels (a–c) represent cuts stained with haematoxylin and eosin (H&E). Panels (d–f) represent cuts stained with Trichromic of Masson which reveal a profuse presence of collagen (in green) in the enlarged centre of a primary granuloma, where H&E stain shows eosinophilic necrosis. Bar represents 200 µm.
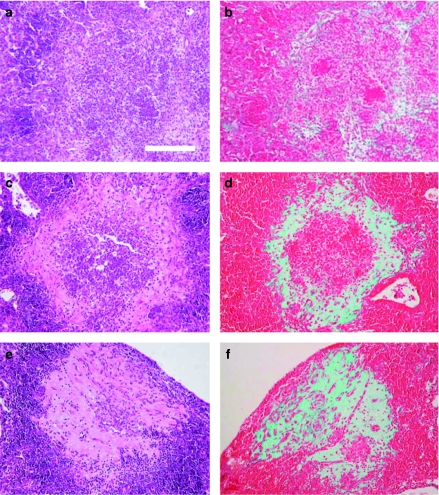
Figure 4 shows the evolution through time of the collagen-based fibrotic tissue from the periphery to the necrotic centre in C57BL/6 mice infected with M. tuberculosis clinical strain UTE 0335R, which is also representative to what happens in 129/Sv and C3H/HeN mice strains, and also to the same mice strains after being infected with H37Rv strain and inoculated with LPS aerosol at week 3 post-infection. Trichromic of Masson reveals the progressive occupation of the centre of primary granulomas by a fibrotic tissue. This centre is initially filled by a mixture of monocytes and neutrophils (week 3) and becomes mostly necrotic and occupied by debris, karyorrhexis, fibrin and some neutrophils by week 6. At week 9, this centre finally forms a compact eosinophilic scar.
Figure 4.
Evolution of intragranulomatous necrosis in pulmonary granulomas (INPG) through time in mice C57B/6 infected by the Mycobacterium tuberculosis strain UTE 0335R at week 3 (a, b), 6 (c, d) and 9 (c, f) post-infection (Experiment C). Panels (a), (c), (e) represent cuts stained with haematoxylin and eosin (H&E). Panels (b), (d), (f) represent cuts stained with Trichromic of Masson which reveals the progression from the periphery to the centre of the collagen-based fibrotic tissue (in green) to occupy the necrotic centre of a primary granuloma. Haematoxylin and eosin stain shows a mixture of monocytes and neutrophils at week 3; a centre occupied by debris, karyorrhexis, fibrin and some neutrophils at week 6 and a compact eosinophilic scar at week 9. Bar represents 100 µm.
PGIP is not related to the induction of INPG
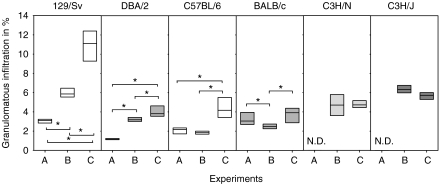
Figure 5 shows pulmonary granulomatous infiltration percentage (PGIP), which is the ratio obtained by dividing the area of granulomatous infiltration by the total area of the lung section, multiplied by 100. LPS inoculation in H37Rv-infected mice to strains 129/Sv and DBA/2 caused a significant increase of PGIP compared with those mice non-inoculated with LPS. Furthermore, infection with UTE 0335R generated a significantly higher percentage of infiltration in all strains but C3H/HeN and C3H/HeJ, compared with mice infected with H37Rv and inoculated with LPS.
Figure 5.
Values of pulmonary granulomatous infiltration percentage (PGIP) in all experiments (A to C). PGIP values were obtained after dividing the area of granulomatous infiltration by the total area of the lobes multiplied by 100. Data are graphed as a box representing statistical values. The boundary of the box closest to zero indicates the 25th percentile, a line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Differences between mice groups were significant when marked with * (P < 0.05).
One way analysis on the variance on ranks and all pairwise multiple comparison procedures (Student-Newman-Keuls Method) revealed significant differences among all mice strains but except for the comparison of 129/Sv and BALB/c mice in Experiment A. No differences were seen only among 129/Sv, C3H/HeN and C3H/HeJ in Experiment B. In Experiment C, differences were only significant when comparing 129/Sv and C3H/HeJ strains to the other strains.
These data established that infection with H37Rv induces different PGIP values that are not related to the H-2 background, the Th1 or Th2 type or the resistance of the strains against M. tuberculosis infection. This trend is also seen in LPS-challenged mice. However, PGIP induced by UTE 0335R seems not to be related to the infected mice strain and shows similar values, except for the significant highest infiltration found in strain 129/Sv. This analysis indicates that the increase in granulomatous infiltration is not related to the induction of INPG.
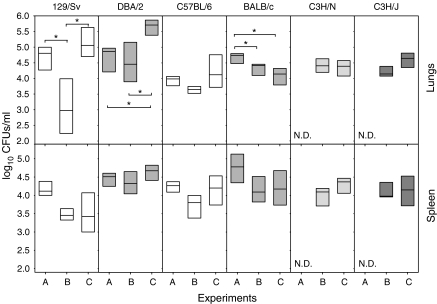
Inoculation of LPS transiently reduces bacillary concentration in the lungs of H-2b haplotype mice
Bacillary concentration in the lungs and spleen tended to decrease after LPS administration, although these differences were only significant in the lungs of 129/Sv and BALB/c (Figure 6). These differences were not observed when bacillary concentration was assessed at week 18 after infection (data not shown). Interestingly, UTE 0335R-infected mice tended to show the opposite pattern when comparing with H37Rv-infected mice, but in the case of BALB/c, that showed lower values.
Figure 6.
Bacillary concentration in the lungs at week 9 post-infection in all experiments (A to C). Data are graphed as a box representing statistical values. The boundary of the box closest to zero indicates the 25th percentile, a line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Differences between mice groups were significant when marked with * (P < 0.05).
Furthermore, there were almost no significant differences among the values obtained in the same experiment. The only differences were observed in the lungs from Experiment C between DBA/2 and the other mice, save for 129/Sv (P < 0.05). In the spleen, differences were only significant among strains 129/Sv and DBA/2 in Experiment B (P < 0.05).
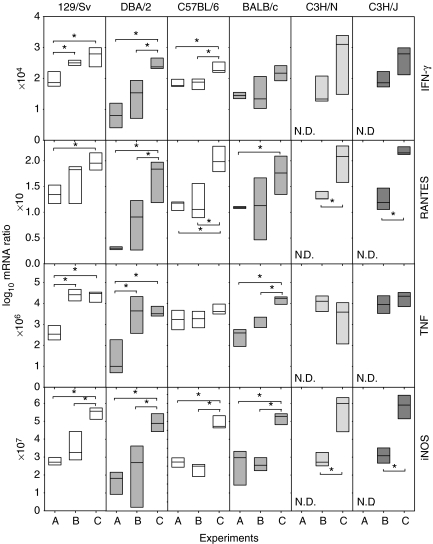
Expression of mRNA in the lungs
Expression of IFN-γ, RANTES, TNF and iNOS tended to increase in LPS-challenged mice and to be even higher in UTE 0335R-infected mice, when comparing with mice only infected with H37Rv (Figure 7). Usually, mice infected with UTE 0335R present higher expression values than mice infected with H37Rv (LPS-challenged or not). This difference is somewhat correlated with PIPG, although the relation is the opposite in C3H/HeJ, because PIPG is lower. Again, the intensity level of the expression of these parameters has no correlation with the induction of INPG.
Figure 7.
Evolution of mRNA expression of Th1 response related cytokines, TNF and iNOS in the lungs at week 9 post-infection. These are expressed as the log10 of the ratio obtained after dividing every value by the expression of HPRT in each sample and multiplying it by a factor (ranging from 10 to 107). Data are graphed as a box representing statistical values. The boundary of the box closest to zero indicates the 25th percentile, a line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Differences between mice groups were significant when marked with * (P < 0.05).
Significant differences between the strains in the same experiment group were not found in any on the parameters investigated, save for TNF expression in Experiment B between 129/Sv and C57BL/6 strains (P < 0.05).
No expression of IL-4 and IL-10 was detected in any mice strains, either in those infected with H37Rv and challenged or not with LPS or in those infected with UTE 0335R (data not shown).
Aerosol LPS inoculation and infection with UTE 0335R did not affect the animal welfare
Aerosol LPS inoculation and infection with UTE 0335R did not induce any suffering to the animals. End point protocol was not required in any case. No weight loss or spontaneous mortality was detected in any experimental protocol.
Discussion
This study aimed to improve the ‘human-like’ model of experimental TB in C57BL/6 mice described elsewhere (Cardona et al. 2001). In this model, INPG may be induced by intranasal LPS inoculation, thus resembling the Shwartzman reaction because TB granuloma would be considered to be a ‘prepared zone’ due to high levels of TNF (Rook & al Attiyah 1991). In this context, local inoculation of LPS suddenly increased neutrophil count in mice BAL, whereas INPG was first detected at week 6 after infection (3 weeks after intranasal LPS inoculation) and was well structured by week 9 (Cardona et al. 2001). Unfortunately, this procedure resulted in a high mortality rate in mice (up to 20%) and thus required further refining.
We have demonstrated that the induction of INPG is not related to the resistance against M. tuberculosis infection. Moreover, this study raises some questions on the mechanism of INPG in human TB, because it has been related to the activity of TNF in a Th2 environment (Rook & al Attiyah 1991; Rook et al. 2004). Our data show that INPG has been associated to C57BL/6 and 129/Sv strains but not to the BALB/c and DBA/2 ones. This is very interesting because several authors suggest that these strains tend to polarize towards Th1 and Th2-type responses, respectively (Mattner et al. 1996; Mills et al. 2000). In this regard, future studies need to be carried out to further determine whether H-2 background or Th1-Th2 response is responsible for INPG.
The experimental model in which the participation of the Th2 response in the necrotic process was demonstrated is completely different from the model presented in this study. Therefore, it is difficult to compare both models. In fact, in this study, we could not produce necrotic granulomas in BALB/c; however, the model in which the influence of IL-4 combined with TNF induced necrosis was actually achieved using this mouse strain (Hernandez-Pando et al. 1996). Besides, that model used a very high infecting dose by intratracheal inoculation, and necrosis was exclusively observed during the progressive phase of infection. Nevertheless, in our model, the infecting dose was very low, and animals developed a very slow progressive disease, with very few CFU that is usually related with a strong Th1 response and where survival is prolonged for 2 years, instead of half a year. In fact, it is quite difficult to induce an evident Th2 response when the CFU is very low, and the lungs exclusively show granulomas, but not pneumonia. In this regard, it must be stated that the model used by Hernandez-Pando et al. (1996) tries to induce a model of developed TB, whereas our model tries to resemble the natural M. tuberculosis infection by aerosol, which also induces necrosis in humans (Grange 1998).
There are other experimental models that use a low dose aerosol, which are able to induce extensive INPG. In these models, immunodeficient mice strains are used that lack clue Th1 cytokines (i.e. IFN-γ or IL-12), aβ T cells or TNF (Cooper et al. 1993; North & Jung 2004). Unfortunately, these are not good models for looking at latent bacilli, as they induce extensive INPG, but growing bacilli are easily seen in the necrotic tissue (Cooper et al. 1993). In this case, INPG is much related to the presence of a high bacillary concentration.
Previous studies carried out to establish the virulence of different clinical strains of M. tuberculosis revealed a natural induction of INPG (Cardona et al. 2003b), and thus new experiments were conducted to determine whether an association existed between artificial induction caused by LPS inoculation and INPG induced by one of the strains. The time point chosen for these experiments was week 9 after infection again. Interestingly, the UTE 0335R strain showed the same histological INPG pattern in the same mice strains, as seen with the LPS inoculation.
Clear differences can be established between M. tuberculosis susceptible (129/Sv and DBA/2) and resistant (C57BL/6 and BALB/c) mice strains. In susceptible strains, LPS inoculation induces an increase in PGIP and a decrease in pulmonary CFUs (although not significant in DBA/2). Regarding mRNA expression, there is an increase in TNF, and in IFN-γ (only significant in 129/Sv strain). After infection with UTE 0335R, a different scenario can be seen, as PGIP raises, as well as the expression of IFN-γ, RANTES, TNF and iNOS, but the important fact is that pulmonary CFUs also tend to increase.
In resistant mice strains, LPS challenge does not modify PGIP (in fact, it decreases in BALB/c). The expression of IFN-γ, RANTES, TNF and iNOS does not vary either, although there is a tendency to decrease bacillary concentration in the lungs. Regarding UTE 0335R infection, there is a tendency to increase PGIP (at least in C57BL/6) as well as the expression of IFN-γ, RANTES, TNF and iNOS. Nonetheless, the most important point is that bacillary concentration in the lungs is controlled.
The aim to use C3H mice strains, which are also susceptible against M. tuberculosis infection (Medina & North 1998), was to obtain a negative control of LPS inoculation, as C3H/HeJ is a LPS-resistant strain. Interestingly, the only difference between both strains was that C3H/HeN developed INPG. In this case, the parameters were almost the same as those used to compare LPS or natural induction of INPG, although the latter also induced a greater inflammatory and immunological response, because it triggered a higher mRNA expression of RANTES and iNOS. Once again, these data support the hypothesis that the increase in the inflammatory and immunological response, PGIP and bacillary concentration in the lungs are not paramount for the induction of INPG.
Regarding on the increase in the mRNA expression of RANTES, TNF, IFN-γ and iNOS, we must take into account that the expression of the former tends to favour Th1 response (Cardona et al. 2003b); TNF is a key factor that induces the formation of granulomas and synergizes with IFN-γ in the activation of infected macrophages to trigger a bactericidal response against bacilli. The expression of iNOS is a marker of macrophage activation (Rhoades & Orme 1997) involved in the production of reactive nitrogen intermediates (RNIs). Because all these parameters tend to increase, a bacillary control after LPS inoculation is not surprising, although this response is observed 6 weeks after challenge. These data may be interpreted as an external induction of an inflammatory response, where LPS favours the host in its fight against infection as previously described (Cardona et al. 2001), although the difference between resistant and susceptible strains is that the former do not increase PGIP. This is a crucial factor, because the survival of mice infected with M. tuberculosis is directly correlated with the control of PGIP, and these animals finally die due to complete infiltration of the lungs (Cardona et al. 2000, 2003b; Medina & North 1998). Hence, only resistant strains would take advantage from this external agent in terms of survival.
Besides, infection with UTE 0335R also increased these parameters, but in this case, the bacillary load in the lungs was not reduced, thus indicating that the increase in these values does not always imply a control of bacilli. In fact, in the progression of M. tuberculosis infection, values always rise after an increase in bacillary load (Cardona et al. 2000, 2001, 2003a, b). In other words, bacilli always take the initiative. However, as an external factor, LPS inoculation stimulates this expression differently from the natural infection: it enhances an extra production of Th1 response not proportional to the magnitude of bacillary concentration, thus tending to favour the host (although, as mentioned above, PIPG may increase too much and be deleterious for the host).
The time when the immunological response is triggered is also crucial: it must be triggered at the precise moment. For example, in the evolution of iNOS expression, this parameter is related to the activation of macrophages, but also to the induction of local immunosuppression, thus being a factor that favours chronicity in infection. For instance, in a susceptible mice strain like DBA/2, this parameter tends to increase spectacularly at week 18–22 post-infection as a consequence of its accumulation in the foamy macrophages surrounding the granulomas. These cells are not sufficiently stimulated and may not induce levels of iNOS high enough to produce enough RNIs (Rhoades & Orme 1997). These micobacteriostatic levels allow bacilli to survive inside them and may also induce local immunosuppression, thus favouring the survival of bacilli, which may potentially reactivate with time (Cardona et al. 2003b).
At all events, these data are obtained when INPG takes place and, despite providing information on the later stages of INPG, they do not allow us to fully understand the direct mechanisms that induce it. In this case, the parameters related to Th1 response (RANTES and IFN-γ) and macrophage activation (TNF and iNOS) are maintained or increased, whereas Th2 expression is not found, thus not supporting previous hypothesis (Rook & al Attiyah 1991).
Moreover, the presence of PMNs is not crucial for the induction of INPG, as this reaction is induced both after favouring its local increase (with the LPS inoculation) and without such increase (after UTE 0335R infection).
Finally, the most interesting fact is that, in both cases, no histopathological differences were found between LPS and natural induction of INPG by the M. tuberculosis strain UTE 0335R, thus supporting the hypothesis that the generation of INPG may be a localized phenomena temporally limited at the beginning of infection. These data also support the hypothesis that the Koch reaction is related to the Shwartzman reaction (Shwartzman 1937) and thus to a nonspecific response triggered against a ‘provoking’ factor, like endotoxin-like molecules of M. tuberculosis, such as cord-factor (Roach et al. 1993), and not to a delayed-type hypersensitivity (Dannenberg 1991). Differences in the composition and quantity of these molecules in M. tuberculosis strains may explain why some of these molecules spontaneously induce INPG while others do not. This opens a new field to study new aspects related to the virulence of M. tuberculosis. Nevertheless, this aspect needs to be further studied by searching for more strains able to induce INPG and studying their endotoxin-like molecules.
In conclusion, the search for a new ‘human-like’ model has revealed a new feature of murine response against M. tuberculosis infection, i.e. that the induction of INPG is related to a Th1-type environment (as in mice C57BL/6 and 129/Sv), that it is a local phenomenon temporally limited at the beginning of infection and unrelated to an increased resistance or susceptibility against infection. This is associated with the presence of LPS or LPS-like molecules, thus linking the Koch phenomenon with the nonspecific Shwartzman reaction. Although further studies will clarify this aspect, this work opens a new field in which new experiences may help study this paramount pathological process in humans and will provide new data on the virulence of M. tuberculosis.
Acknowledgments
This work has been funded by Grants FIS 01/0644 and 01/3104. We thank Mrs Marga Ortiz, from the Center for Animal Experimentation, and the technicians of the Pathology Department, Hospital Universitari Germans Trias i Pujol, for their excellent technical support, and Mrs Deborah Cullell for her excellent work in English reviewing.
References
- Cardona PJ, Gordillo S, Amat I, et al. Catalase-peroxidase activity has no influence on virulence in a murine model of tuberculosis. Tuberculosis (Edinb) 2003a;83:351–359. doi: 10.1016/s1472-9792(03)00056-8. [DOI] [PubMed] [Google Scholar]
- Cardona PJ, Gordillo S, Diaz J, et al. Widespread bronchogenic dissemination makes DBA/2 mice more susceptible than C57BL/6 mice to experimental aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 2003b;71:5845–5854. doi: 10.1128/IAI.71.10.5845-5854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona PJ, Llatjos R, Gordillo S, et al. Evolution of granulomas in lungs of mice infected aerogenically with Mycobacterium tuberculosis. Scand. J. Immunol. 2000;52:156–163. doi: 10.1046/j.1365-3083.2000.00763.x. [DOI] [PubMed] [Google Scholar]
- Cardona PJ, Llatjos R, Gordillo S, et al. Towards a ‘human-like’ model of tuberculosis: intranasal inoculation of LPS induces intragranulomatous lung necrosis in mice infected aerogenically with Mycobacterium tuberculosis. Scand. J. Immunol. 2001;53:65–71. doi: 10.1046/j.1365-3083.2001.00842.x. [DOI] [PubMed] [Google Scholar]
- Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg AM., Jr Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol. Today. 1991;12:228–233. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 2004;84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Grange JM. Immunophysiology and immunopathology of tuberculosis. In: Davies PDO, editor. Clinical Tuberculosis. London: Chapman & Hall; 1998. pp. 129–152. [Google Scholar]
- Hernandez-Pando R, Pavon L, Arriaga K, Orozco H, Madrid-Marina V, Rook G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect. Immun. 1997;65:3317–3327. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie MB. The correlation between the histological changes and the fate of living tubercle bacilli in the organs of tuberculous rabbits. J. Exp Med. 1932;55:31–58. doi: 10.1084/jem.55.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner F, Magram J, Ferrante J, et al. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J. Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- Medina E, North RJ. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology. 1998;93:270–274. doi: 10.1046/j.1365-2567.1998.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- Mitchison DA, Dickinson JM. Bactericidal mechanisms in short-course chemotherapy. Bull. Int. Union Against Tuberc. 1978;53:254–259. [PubMed] [Google Scholar]
- North RJ, Jung YJ. Immunity to tuberculosis. Annu. Rev. Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- Rhoades ER, Orme IM. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect. Immun. 1997;65:1189–1195. doi: 10.1128/iai.65.4.1189-1195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach TIA, Barton CH, Chatterjee D, Blackwell JM. Macrophage activation: lipoarabinomannan from avirulent and virulent strains of Mycobacterium tuberculosis differentially induces the early genes C-fos, KC, JE and tumor necrosis factor-a. J. Immunol. 1993;150:1886–1896. [PubMed] [Google Scholar]
- Rook GA, al Attiyah R. Cytokines and the Koch phenomenon. Tubercle. 1991;72:13–20. doi: 10.1016/0041-3879(91)90019-o. [DOI] [PubMed] [Google Scholar]
- Rook GA, Hernandez-Pando R, Dheda K, Teng Seah G. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004;25:483–488. doi: 10.1016/j.it.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Shwartzman G. Phenomenon of Local Tissue Reactivity and its Immunological, Pathological and Clinical Significance. New York: Paul B. Hober; 1937. [Google Scholar]
- World Health Organization. Global Tuberculosis Control. Geneva, Switzerland: World Health Organization; 2001. WHO Report 2001. WHO/CDS/TB/2001.287. [Google Scholar]