Abstract
When the body discovers a tumour cell (foreign antigen), several kinds of mechanisms and cells operate in what is called an immune response. The latter has evolved into two mechanisms: non-specific immunity and specific immunity, which are closely linked to and influence each other. The former represents the first line of defence against neoplastic cells. The adaptive (specific) immunity is orchestrated by antigen-specific T and B lymphocytes. The effector cells of innate immunity include granulocytes, macrophages and natural killer cells. Among these cells, macrophages represent the most important part of innate immunity against tumours. Tumour-associated macrophages (TAMs) are important antigen-presenting cells and as such an understanding of their interactions with tumour cells gives insights into novel therapeutic strategies. In tumours, the effect of TAMs is the outcome of their two concomitantly competing interactions: tumour growth reduction and tumour growth promotion. The macrophage (TAMs) content of melanoma ranges from 0 to 30% and their density increases with increasing tumour thickness. The melanoma cells and TAMs seem to interact with each other through the release of soluble factors that either prevent or enhance tumour growth. For instance, syngeneic macrophages from tumour-bearing mice can inhibit melanoma growth in the nude mice more than the control macrophages. Alternatively, metastatic B16 melanoma cells can produce some macrophage cytotoxic substances that help tumour cells not only escape the host immunosurveillance system but also prevent distant metastasis. Together, these observations suggest opposing effects for these soluble factors in melanoma. To date, little is available in the literature about the interactions between TAMs and melanoma cells. This viewpoint not only tries to examine these interactions but also provides relevant speculations.
Keywords: immune system, macrophages, melanoma
Introduction
Historical aspect
Melanocytes are cells derived from the neural crest that reside in the basal cell layer of the epidermis. Melanoma tumourigenesis is a multistep process that entails derangement of the immune mechanisms (Hussein 2004). Virchow first identified leucocytes in the tumour tissue in 1863. He proposed that their presence in melanoma may reflect the origin of cancer at sites of chronic inflammation. Subsequently, Handley reported that immune cell infiltration in melanoma may indicate a ‘regressive process’ (Mantovani et al. 1995; Mantovani et al. 2002). In 1973, Lejeune and colleagues reported the growth inhibitory effect of peritoneal macrophages on Harding Passey melanoma (Lejeune et al. 1973). Gauci and Alexander (1975) estimated that the macrophage content of the different tumours ranged from 0 to 30%. Shortly, Currie and Hedley (1977) reported a deficiency in macrophage precursors in melanoma patients. In 1978, Chalon and colleagues found higher histiocytes counts in smears made from the tracheobronchial washings of melanoma patients than those in a healthy control group. Leibovici and Hoenig (1985) reported that the density of macrophages could determine whether lysis or growth stimulation of melanoma cells would occur. In this respect, low macrophage numbers have lytic effects and high numbers can stimulate the growth of the melanoma. These conflicting observations may be reasoned to the pleiotropic nature and ill-defined functions of the macrophages.
Macrophage content in melanoma
Tumours contain varying numbers of macrophages. In some rat sarcomata, macrophage may represent up to 60% of the total cells of the tumour (Alexander 1976). A quantification of macrophage recruitment into neoplastic lesions in situ can be performed using a double-label histochemical method. This method is based on the fact that intratumoural macrophages can ingest colloidal iron particles from the interstitial fluid. As colloidal iron is retained in a stable form within these cells for a considerable time, new macrophages that emigrate into the tissue after injection of the colloidal iron are identified by their ability to ingest a second colloid (lanthanum). The latter can be reliably distinguished from the initial iron label. Pre-existing (colloidal iron label) and newly recruited macrophages (lanthanum label) are identified in serial sections by histochemical methods using hydrogen peroxide oxidation to detect iron (blue reaction product) and cleavage of phosphate esters to demonstrate lanthanum (Bugelski et al. 1983). In melanoma, the macrophage content ranges from 0 to 30%. Metastasizing melanomas, as well as metastatic lesions, all contain <10% of macrophages, whereas non-metastasizing tumours have widely varying numbers of macrophages (Gauci & Alexander 1975). Therefore, there seems to be an inverse correlation between the macrophage content of animal tumours and their capacity to metastasize. Also, by using this histochemical method, the macrophage content of experimental B16 melanoma metastases at different stages of their growth has been quantified. Analyses of 954 sections of 155 individual lung metastases showed that the macrophage content of individual B16 melanoma lung metastases (i) varies significantly among individual tumours and (ii) falls dramatically once metastases contain more than 700 tumour cells. Also, as metastases grow, the relative macrophage content falls, reaching uniformly low levels by the time the metastases are 0.5 mm in diameter (Bugelski et al. 1985; Bugelski et al. 1987).
Immune system
The immune system consists of two distinct arms serving distinct albeit interconnected functions; non-specific and acquired immunity. Macrophages and NK cells orchestrate the recognition and elimination of tumour cells. In this respect, activated macrophages can recognize and destroy several tumour cell types. Moreover, together with the dendritic cells, macrophage serves as a critical link between innate and acquired immunity (Hussein 2005).
Macrophages and immune response
Macrophages operate in specific immunity against tumours through several mechanisms including antigen presentation and interleukin-1 (IL-1) production. As antigen-presenting cells (APs), they have two properties: (i) expression of class II MHC molecules on their membranes and (ii) ability to deliver a co-stimulatory signal necessary for T-cell activation. Despite the evident immune defence mechanisms against melanoma cells and despite their strong antigenicity, these malignant cells grow progressively and spread through the body unrestricted by the immune system. Moreover, these cells grow eventually not only sending distant metastasis but also culminating in death of the host. This aggressive behaviour of melanoma may be reasoned to suppression of immune system exerted by the tumour (Janeway 1999). This suppression perhaps involves the alterations of macrophage activity at the steps of both tumour cytotoxicity and antigen presentation (Jager et al. 2002; Schmollinger et al. 2003). This eventually leads to specific immunological tolerance for the tumour cells and tumour progression. With this critical role of macrophages both in endogenous tumour defence and in tumour progression, an understanding of the molecular and cellular mechanisms involved in macrophage–melanoma interactions is important. Moreover, elucidation of the consequences of these interactions in induction of specific antimelanoma immune responses as well as the biological signal circuits controlling these interactions may represent a basis for an effective therapeutic intervention in melanoma.
Scope of the review
The function of the immune system is to defend the organism against what has been called ‘danger’ associated with ‘non-self’ antigens resulting from aberrant cellular structures arising in the body during the process of neoplastic transformation leading to cancer (Janeway 1999; Jager et al. 2001). Macrophages are widely distributed mononuclear phagocytes with protean homeostatic and immunological functions. As such, they provide an immediate defence against neoplastic elements. Melanoma is an aggressive malignancy with a tendency for recurrence and fatal dissemination. In this malignancy, despite these immune defence mechanisms against melanoma cells and despite their principal antigenicity, melanomas grow progressively and spread through the body unrestricted by the immune system, eventually resulting in death of the host. This is due to the suppression of immune functions exerted by the tumour, again primarily involving the control of macrophage activity at the step of both tumour cytotoxicity and antigen presentation, finally leading to specific immunological tolerance for the tumour cells and tumour progression. Unfolding studies suggest potential interactions between tumour-associated macrophages (TAMs) and melanoma cells (Alexander 1976; Jager et al. 2001). These studies suggest that both the number and phenotype of the macrophage affect the tumour growth and spread. This viewpoint tries to convey insights into these interactions and propose a model for them.
The immune system
The immune system of higher vertebrates encompasses two distinct limbs serving distinct albeit closely linked functions – the non-specific or innate immune system which represents the first line of defence against tumour cells and the adaptive (specific) immunity which is orchestrated by antigen-specific T and B lymphocytes. The innate immunity comprises granulocytes, macrophages and natural killer (NK) cells as its effector cells. Among these cells, macrophages represent the most important part of innate immunity against tumours. As such, they have principal roles in recognition and elimination of newly arising cancer cells. In this sense, whereas the direct antitumour activity of NK cells is restricted to particular forms of neoplastic cells, activated macrophages can recognize and selectively destroy several types of tumour cells in a so-called macrophage-mediated tumour cytotoxicity (Janeway 1999). Moreover, together with the dendritic cells (powerful APs), macrophages (another APs) represent a critical link between innate and adaptive immunity. The second limb of the immune system is constituted by antigen-specific T and B lymphocytes (Janeway & Bottomly 1994). The macrophages and dendritic cells actually control the induction of specific, T lymphocyte-mediated immune responses and thus also govern specific immunity against tumour antigens, which is essential for final, long-term eradication of tumour cells in the body (Burdelya et al. 2005; Knutson & Disis 2005).
Non-specific immunity (innate immunity)
The innate immunity is characterized by being older phylogenetically, present at birth, does not require a previous encounter with the offending antigen and does not develop memory. Innate immunity includes both the natural barriers, such as the skin, and chemical protection, such as gastric acid. Also, there are two cellular components in the innate immunity: (i) the phagocytic system, whose function is to ingest and digest offending antigens and (ii) NK cells. The cells of the phagocytic system include neutrophils and monocytes (in the blood) and macrophages (in the tissues). Macrophages are the body's first line of defence and comprise a critical limb of the innate immune system. As such, macrophages are the first cells to recognize and engulf the antigens, break them down and present them (epitopes) to the T lymphocytes. The latter can recognize, respond to and remember antigens. They then activate the adaptive immune system by degrading the organisms and presenting the pathogen-derived MHC class II–bound peptides to T cells. Macrophages also produce substances called cytokines that help regulate the activity of lymphocytes. Also, the NK cells function to kill some tumour cells without previous sensitization. The soluble components of innate immunity consist of complement proteins, acute phase reactants and cytokines (Whiteside 1999; Hodi et al. 2002).
The human NK cells
The relative proportions of T and B cells vary among tissues. In peripheral blood, they account for about 75 and 10% of all lymphocytes, respectively. The remaining 15% of peripheral blood lymphocytes belong to separate lineage known as NK cells. In melanomas, the tumour infiltrating lymphocytes (TILs) include human NK cells. The latter have unique immunomorphologic features that distinguish them from T and B lymphocytes. Their activity is regulated by the products of both haematopoietic (lymphocytes and monocytes) and non-haematopoietic cells. Both are capable of regulating NK cell growth and activity (Hussein 2005). The NK cells can mediate spontaneous, non-major histocompatibility complex-restricted cytotoxicity and antibody-dependent cellular cytotoxicity (Schneeberger et al. 1999; Birk et al. 2001). The human NK cells can be expanded in culture in the presence of IL-2. The adherent lymphokine-activated killer (A-LAK) cells represent CD56+ IL-2-activated NK cells. The use of NK may have possible therapeutic ramifications in melanomas. This proposition is supported by several observations. First, A-LAK later can destroy melanoma cells both in vitro and in vivo. Second, expansion of peripheral blood NK cells correlates with clinical improvement in melanoma patients receiving recombinant subcutaneous IL-2 and interferon-α-2 (IFN-α-2) (Ha et al. 2004). Third, NK cells of the peripheral blood often have qualitative (function) rather than quantitative defects (Kamiryo et al. 2005). Finally, in the tumour tissues of melanoma, additional infiltration by NK cells (CD56+) during treatment was seen only in responding patients but not in those with progressive disease (Repp et al. 2000; Li et al. 2005).
Specific immunity
Specific (adaptive) immunity is characterized by learning, adaptability and memory. The cellular component is the lymphocyte, and immunoglobulins (Igs) are the soluble components. The specific immune response can confer life-long protective immunity to reinfection with the same pathogens. APs are of extreme importance for the lymphocytes to perform their functions. These APs make up a population of cells that are found in the lymphoid tissues (dendritic cells, DCs) and in the epidermis (Langerhans cells) (Viret & Janeway 1999).
Monocytes and macrophages
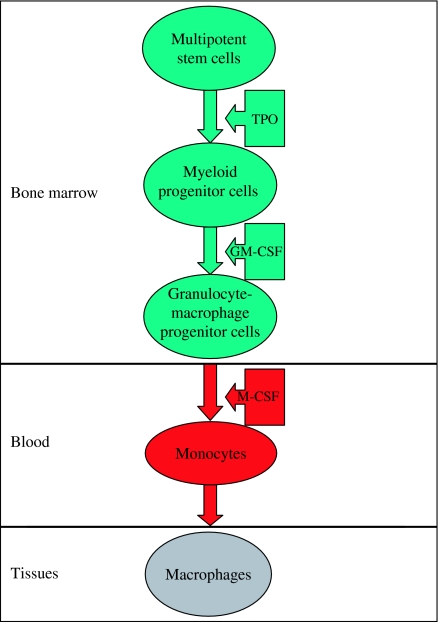
The monocytes and macrophages are cells of the reticulo-endothelial system. Their development in the bone marrow passes through the following steps: stem cell → committed stem cell → monoblast → promonocyte → monocyte (bone marrow) → monocyte (peripheral blood) → macrophage (tissues). The process of haematopoiesis is under the control of several growth factors such as IL−3 and granulocyte-macrophage colony-stimulating factor. The former helps generate differentiated progeny of all myeloid lineages. The latter helps the differentiated progeny to give rise to monocyte/macrophage-restricted progeny. The blood monocytes are young migratory cells that move into tissues and undergo further differentiation to become multifunctional tissue macrophages (Currie & Hedley 1977). The latter include both inflammatory and normal macrophages. When monocytes leave the bloodstream and enter the tissue, they differentiate into two types of macrophages. One type of macrophages that leave the bloodstream during inflammation are then trafficked to the site of infection and are therefore called wandering macrophages. The second type of macrophages are found fixed throughout the tissues and therefore called fixed macrophages. These fixed cells represent a part of the mononuclear phagocytic system. They are found mainly in lymph nodes, and the spleen where they not only encounter but also attack antigens. In a given tissue, the macrophage populations are maintained by three mechanisms: influx of monocytes from the circulating blood, local proliferation of progenitor cells and biological turnover (Whiteside 1999) (Figures 1 and 2).
Figure 1.
The formation of macrophages. The various blood cells are produced in the bone marrow. They arise from a single type of cell called multipotent stem cell. Thrombopoietin (TPO) stimulates the production of myeloid progenitors. Granulocyte-monocyte colony-stimulating factor (GM-CSF) sends cells down the road leading formation of granulocytes macrophage progenitors. Under the influence of macrophage colony-stimulating factor (M-CSF), the granulocyte/macrophage progenitor cells differentiate into monocytes. The latter migrated into the tissues (within 2 days) to form the tissue histiocytes (macrophages).
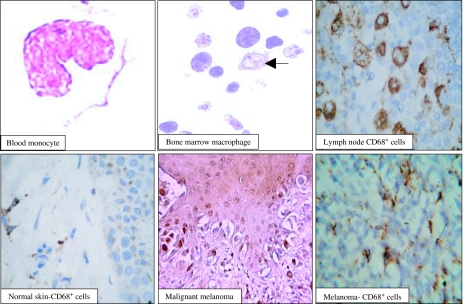
Figure 2.
Morphological features of monocytes and macrophages in the peripheral blood, bone marrow, lymph node, normal skin and malignant melanomas. The immune system represents a complex network of cells collectively known as immune cells (such as macrophages) and organs (such as lymph nodes) that work together to defend the body against foreign substances (antigens) such as tumour cell. The immune cells are scattered throughout the body either as isolated cells or as non-encapsulated aggregations of cells in the skin, respiratory and other systems. The blood monocyte is the largest normal white cell found in the blood. It has an irregular lobulated nucleus and opaque grey-blue cytoplasm containing fine granules and irregular outlines. The bone marrow monocyte (arrow) has vesicular nuclei and abundant faint blue cytoplasm. In the lymph nodes, the macrophages occupy the sinuses and have positive reactivity for CD68. In normal skin, macrophage are found either singly or around the blood vessels (CD68+ cells). In melanomas, the content of macrophages (CD68+ cells) is about 3–7%.
Macrophages have numerous critical functions in the body defence against neoplasms. First, killing of neoplastic cells by phagocytosis. The latter is mediated through several receptors on the surfaces of these cells (Table 1). Macrophages that have engulfed tumour cells become activated by T-helper 1 cells, develop a ruffled cytoplasmic membrane and produce increased numbers of lysosomes. Second, antigen processing by macrophages can convert the antigen into several antigenic epitopes. The latter can be recognized by T lymphocytes. Macrophages engulf the tumour cells and degrade them with their lysosomes. Peptides from the tumour cells are then bound to a groove of MHC-II molecules produced by macrophages and dendritic cells. The peptide epitopes bound to the MHC II molecules are then put on the surface of the macrophage where they can be presented to T4 lymphocytes (Figure 3). Moreover, macrophages also secrete monokines that play a variety of roles in non-specific body defence. These roles include (i) promotion of inflammation, (ii) enhancement of phagocytosis, (iii) activation of resting T lymphocytes, (iv) attraction and activation of neutrophils and (v) stimulation of the replication of endothelial cells to form capillaries and fibroblasts to form connective scar tissue. Several cytokines are produced by macrophages including tumour necrosis factor (TNF)-α, IL-1, IL-6 and IL-8 (van Ravenswaay et al. 1992; Naama et al. 2001a; Naama et al. 2001b; Varney et al. 2002; Woodward et al. 2004).
Table 1.
Phagocytic receptors of the macrophages
| Receptor | Binds | Function |
|---|---|---|
| Fc receptor I | Monomers of IgG | ADCC; phagocytosis 1 |
| Fc receptor II | Aggregates of IgG | Phagocytosis |
| Fc receptor III | Aggregates of IgG | Phagocytosis |
| Mannose receptor | Oligosaccharides | Phagocytosis |
| Complement receptor 3 | C3b, fibrinogen | Adhesion; phagocytosis |
| Fibronectin receptor | Fibronectin oligomers | Adhesion, phagocytosis |
ADCC, antibody-dependent cellular cytotoxicity; Fc, fraction crystallizable; Ig, immunoglobulin.
Macrophages have numerous critical functions in the body defence against neoplasms. The killing of neoplastic cells is carried out through phagocytosis of these cells. The latter is mediated through several receptors on the surface of these cells including Fc receptor I, II and III, mannose receptor, complement receptor 3 and fibronectin receptor.
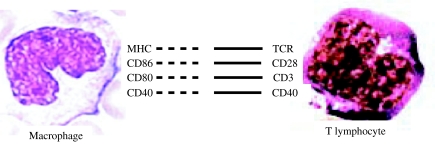
Figure 3.
The interactions between the macrophages and T lymphocytes. Antigen is recognized and processed intracellularly in the macrophages by means of proteolytic cleavage into short peptides. The latter are presented by major histocompatibility complex (MHC) molecules on the surface of macrophages to the T-cell receptors on the highly specific T cells. The latter become activated in the acquired immune response. Activation also causes macrophages to enhance their expression of co-stimulatory molecules. The latter are molecules that provide the signals necessary for lymphocyte activation in addition to those provided through the antigen receptor.
The interactions between the macrophages and T cells
Macrophages play a crucial role in the host response to neoplastic transformation. As such, they have immediate tumouricidal activities, as well as presenting and processing antigens for T-lymphocyte activation. The early response is probably non-specific killing by macrophages. More specific responses are initiated when macrophages act as APs for T lymphocytes. Such T lymphocytes can then produce cytokines that can activate macrophages. The macrophages/T cells signal exchange is two-way, with T cells inducing macrophage activation via cytokines, receptors and ligands. Alternatively, when a peptide MHC complex is recognized by T-cell receptors in the presence of co-stimulatory molecules, the transduced signals induce T-cell activation. Once become fully armed, T cells march out of the lymph node and shuttle to the bloodstream via the thoracic duct (Figures 3 and 4). Also, there is indirect interaction between specific antibody (produced by B cells) and macrophages (Dalton et al. 2003; Martin et al. 2004).
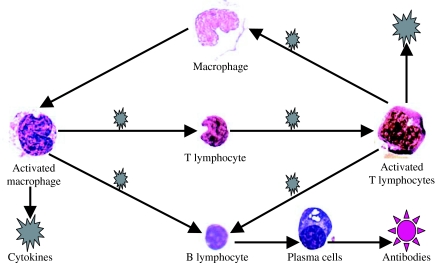
Figure 4.
The interactions between the macrophages and immune cells. The macrophages/T cells signal exchange is two-way, with T cells inducing macrophage activation via cytokines. Alternatively, when a peptide MHC complex is recognized by T-cell receptors in the presence of co-stimulatory molecules, the transduced signals induce T-cell activation. Once become fully armed, T cells march out of the lymph node and shuttle to the bloodstream via the thoracic duct. Also, there is indirect interaction between specific antibody (produced by B cells) and macrophages.
Immune effector cells chemoattractants in melanoma
Tumour infiltrating immune effector cells are critical for antitumour immune responses. Several molecules are involved in the regulation of immune cell infiltration of tumours such as Stat3, monocyte chemoattractant protein-1 (MCP-1), growth-related oncogene (GRO)-α, IL-8, Mig (monokine induced by IFN-γ), IP-10 (IFN-γ inducible protein 10) and RANTES (regulated on activation, normal T cells expressed and secreted) (Kilgore et al. 1997; Kunz et al. 1999; Nesbit et al. 2001; Burdelya et al. 2005). These molecules have attractant properties on the lymphocytes and monocytes and are therefore critical for the control of local melanoma tumour growth. Stat3 is constitutively activated in several human cancers. It promotes both tumour cell growth and survival. Tumour Stat3 activity affects recruitment of several immune effector cells. Also, it can be manipulated to activate the effector phase of innate immune responses. Abrogation of Stat3 signalling in tumours in vivo results in tumour growth inhibition. The latter involves killing of non-transfected tumour cells and infiltration of immune effector cells. This in turn suggests that Stat3 activity in tumour cells might affect immune cell recruitment. In isogenic murine melanomas, Burdelya and his colleagues showed that natural Stat3 activity is associated with tumour growth and reduction of T-cell infiltration. Blocking Stat3 signalling in the melanoma cells containing high Stat3 activity results in the expression of multiple chemoattractants, leading to increased migration of lymphocytes, NK cells, neutrophils and macrophages. In addition, blocking Stat3 induces tumour cells to produce soluble factors capable of activating macrophage production of nitric oxide. TNF-α and TNF-β are secreted by Stat3-inhibited tumour cells. These cytokines can activate macrophage nitric oxide production. Alternatively, neutralizing TNF-α in the tumour supernatant from Stat3-blocked tumour cells can abrogate nitrite production. Moreover, interrupting Stat3 signalling in tumour cells leads to macrophage-mediated, nitrite-dependent cytostatic activity against non-transduced tumour cells (Burdelya et al. 2005).
Melanoma cell lines can secrete MCP-1, but not the normal melanocytes. Low-level MCP-1 secretion with modest monocyte infiltration can result in tumour formation. Alternatively, high MCP-1 secretion is associated with massive monocyte/macrophage infiltration into the tumour mass, leading to its destruction within a few days after injection into mice. Tumour growth stimulated by monocytes/macrophages is probably due to increased angiogenesis (Nesbit et al. 2001). Neutrophil-chemotactic peptides are a family of small basic peptides 70–80 amino acids in length. They contain four conserved cysteine residues, the first two spaced by one amino acid (C-X-C). These peptides include human IL-8, melanoma growth stimulatory activity (GRO-α), neutrophil-activating peptide-2, and epithelial-cell derived neutrophil-activating protein 78 (Watanabe et al. 1993; Rogivue et al. 1995).
Macrophage–tumour interactions
Despite our improved understanding of the roles of macrophages in tumour immunology and the identification of tumour antigens, the clinical application of immunotherapy in human neoplasia still has a limited rate of success. This may be reasoned in part to the fact that antitumour response of the host is complex with both the non-specific and specific immunity being operational. In this regard, monocytes and macrophages seem to play critical roles. The tumour-infiltrating macrophages (TIMs) may both inhibit and enhance tumour growth: this is the so-called macrophage balance hypothesis (Mantovani et al. 1992). This hypothesis proposes that macrophage may inhibit or enhance tumour growth. The macrophages can inhibit tumour growth by producing effector molecules. The latter include certain cytokines (TNF-α, IL-12 and IL-18) and reactive oxygen radicals. Also, macrophage can be involved in cytotoxic destruction of tumour cells (Zembala et al. 1994a; Mytar et al. 1999; Mytar et al. 2003). Alternatively, TIMs can inhibit the antitumour potential of macrophages by several mechanisms. The latter include both an IL-10-dependent mechanism and an IL-10-independent mechanism. The IL-10 is produced by the TIMs themselves or induced by them in macrophages. These actions are collectively called tumour-induced macrophage dysfunction or tumour-driven macrophage polarization (Elgert et al. 1998; Mantovani et al. 2002). Several other factors may be involved, such as TGF-β and the expression of surface molecules. The latter are important for monocyte–tumour cell interactions and include CD44, HLA-DR and CD29 molecules involved in signalling for TNF-α, reactive oxygen intermediates and reactive nitrogen intermediates generation (Zembala et al. 1994b; Mytar et al. 2001).
Histiocytic markers and melanoma
Melanoma immunoreactivity was seen for the histiocytic markers AAT, CD68, S100, tyrosinase HAM56, Mac387 and muramidase. Also, these tumours are commonly immunoreactive for histiocytic markers (α-1-antitrypsin or AAT, CD68/KP1, HAM56, Mac387 and muramidase) (Woodward et al. 2004) (Figure 2).
Melanoma–macrophage hybrids
A long time ago, it was hypothesized that metastases might be initiated through the generation of hybrids between primary tumour cells and tumour-infiltrating leucocytes such as macrophages. In this concept, the hybrids become metastatic through expression of the leucocyte motility phenotype. The macrophage × tumour cell hybridization could account for some of the most defining characteristics of metastatic cells such as aneuploidy, enhanced motility, aberrant glycosylation and phenotypic diversity (Pawelek 2000). Interestingly, melanoma–macrophage hybrids have increased metastatic potential. Therefore, as compared with the parental Cloudman S91 melanoma cells, melanoma–macrophage hybrids, produced by in vitro fusion of normal macrophages with Cloudman S91 melanoma cells, displayed marked metastatic potential in vivo, increased motility in vitro and altered N-glycosylation. The macrophage-specific glycosylation, especially increased β-1,6-N-acetylglucosaminyltransferase V (GnT-V) activity, β-1,6 branch formation in glycoproteins, is accompanied by enhanced metastatic potential in vivo and motility in vitro (Chakraborty et al. 2001a). Of note, GnT-V generated, β-1,6-branched polylactosamines are a common feature shared by normal granulocytes, monocytes and a variety of malignant cells. Furthermore, activation of GnT-V in oncogenic transformation induces invasiveness and metastatic potential in mice as well as in humans. These hybrids also express upregulated melanocortin-1 receptor (MC1-R) activity and exhibit increased motility after melanocyte-stimulating hormone (MSH) treatment. The MSH-mediated stimulation of motility is mediated through enhanced expression of c-Met proto-oncogene (Chakraborty et al. 2003). Similar observations have also been made in hybrids originating from mouse macrophage and mouse melanoma cells (Chakraborty et al. 2001b).
Melanoma–tumour-associated macrophage interactions
The interactions between the melanoma and TAMs occur through the release of certain soluble molecules both from the tumour cells and from macrophages. These substances enhance either tumour growth promotion or tumour growth reduction (Table 2).
Table 2.
Molecules involved in the interactions between melanoma and macrophages
| Molecules | Source | Action | References |
|---|---|---|---|
| Monocyte chemotactic protein | Melanoma cells | Increase macrophage recruitment | Kalish & Brody (1983) |
| Monocyte chemotactic protein (MCP-1) | Melanoma cells | Altered macrophage recruitment | Kalish & Brody (1983); Bottazzi et al. (1992) |
| Melanoma inhibitory activity | Melanoma cells | Enhance tumour progression and development of metastasis | Bosserhoff & Buettner (2002) |
| Macrophage migration inhibitory factor | Melanocytes/melanoma cells | Inhibit NK cell-mediated cytotoxicity andrandom migration of macrophages | Repp et al. (2000) |
| Macrophage inflammatory protein 1-α | Macrophages | Increase T-cell and dendritic cell recruitment | van Deventer et al. (2002) |
| Granulocyte-macrophage colony-stimulating factor | Bone marrow | Inhibit melanoma growth | Armstrong et al. (1996) |
| Cyclooxygenase-2 | Melanoma cells | Inhibit macrophage tumouricidal activity | Duff et al. (2003) |
Several molecules are involved in the interaction between the melanoma cells and the macrophages such as monocyte chemotactic protein, melanoma inhibitory activity, macrophage migration inhibitory factor, macrophage inflammatory protein 1-α, granulocyte–macrophage colony-stimulating factor, cyclooxygenase-2, direct cellular cytotoxicity and antibody-dependent cellular cytotoxicity.
Tumour growth promotion
Tumour growth promotion is achieved by several mechanisms including (i) decreased macrophage recruitment by the chemotactic factors such as monocyte chemotactic protein (MCP-1) (Kalish & Brody 1983; Bottazzi et al. 1992), (ii) inhibition of NK cell function by the macrophage migration inhibitory factor (MIF) (Repp et al. 2000), (iii) inhibition of melanoma cell attachment to the extracellular matrix by macrophage inhibitory factor (MIF) (Bosserhoff & Buettner 2002), (iv) increased expression of cyclooxygenase-2 and (v) release of substances which decrease macrophage effector molecule production and impair macrophage cytotoxicity (Duff et al. 2003). Several experimental observations support these mechanisms: (i) B16 melanoma cells can produce inhibitory and cytotoxic substances against macrophages, (ii) metastatic B16 melanoma cells produce substances that not only suppress nitric oxide production by macrophages but also kill macrophages and macrophage-like cell lines (Inoue et al. 2002) and (iii) subcutaneous injection of female C57BL/6 mice with 106 B16 melanoma cells can result in decreased macrophage production of nitric oxide, superoxide anion and TNF-α. Accordingly, macrophage-mediated cytotoxicity was significantly impaired (Naama et al. 2001a).
Tumour growth reduction
The tumour growth reduction is achieved by several mechanisms including (i) increased macrophage chemotactic factors such as monocyte chemotactic protein (MCP-1), (ii) production of angiogenic factors (IL-8 and vascular endothelium growth factor) by the macrophages and melanoma cells (Torisu-Itakura et al. 2000), (iii) production of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Armstrong et al. 1996; Ciotti et al. 1999), (iv) direct cellular cytotoxicity (Duff et al. 2003), (v) antibody-dependent cellular cytotoxicity (te Velde & Figdor 1992), (vi) secretory products (cytotoxic/cytostatic) and (vii) macrophage-induced apoptosis.
Several experimental observations support these mechanisms: (i) growth inhibitory effect of peritoneal macrophages on Harding Passey melanoma (Lejeune et al. 1973), (ii) syngeneic macrophages from tumour-bearing mice can inhibit melanoma growth in the nude mice more than the control macrophages and (iii) local treatment of melanoma patients with GM-CSF enhanced the recruitment of dendritic cells (Vuylsteke et al. 2004).
The molecules involved in the interactions between the melanoma and macrophages
Several molecules are involved in the interactions between macrophages and melanoma including molecules that affect macrophage recruitment and tumour growth. Macrophage recruitment is under the control of chemotactic cytokines such as monocyte chemotactic protein (MCP-1). Melanoma cells produce these factors and therefore enhance macrophage recruitment (MCP-1). B16 cells produce MCP-1 that increases B16 tumour incidence in mice injected with a small number of B16 cells (Kalish & Brody 1983). Alternatively, as compared with paternal control clones, MCP-producing melanoma clones showed a marked increase in the percentage of TIMs (Bottazzi et al. 1992).
Macrophage migration inhibitory factor
Macrophage migration inhibitory factor (MIF) is a multifunctional cytokine. It was first discovered as a lymphokine involved in delayed hypersensitivity reactions and various macrophage functions, including phagocytosis, spreading and tumouricidal activity. MIF can inhibit the random migration of macrophages, modulates the immune response, acts as a growth and angiogenic factor and inhibits immediate NK cell activity. Therefore, it maintains a microenvironment of immune privilege for the tumour cells (Repp et al. 2000). Expression of MIF is much higher in melanoma cells than in normal melanocytes. As such, MIF can stimulate incessant growth and invasion of melanoma concomitant with neovascularization. In support, (i) transfection of the melanoma cell lines with an antisense MIF plasmid can suppress their growth and (ii) addition of anti-MIF antibody suppresses tumour-induced angiogenesis in melanoma (Shimizu et al. 1999). This tumourigenic of MIF in melanoma may be reasoned to the fact that it is a potent modulator of p53- and E2F-dependent pathways. The latter are activated in response to oncogenic signalling (Petrenko & Moll 2005).
Melanoma inhibitory activity
Melanoma inhibitory activity (MIA) is a 12-kDa small secreted protein that interacts with extracellular matrix protein substance produced by melanoma cells. It is produced by melanoma cells and can inhibit their attachment to extracellular matrix protein (fibronectin). The protein MIA was identified and isolated from the tissue culture supernatant of melanoma cells in vitro by its ability to inhibit thymidine incorporation by melanoma cell lines (Bosserhoff & Buettner 2002). Malignant transformation of melanocytes to melanoma cells closely parallels upregulation of MIA expression. Despite its ambiguous name, MIA production enhances tumour progression and development of metastatic potentialities in melanoma. In this respect, MIA can inhibit tumour cell attachment to the extracellular matrix (fibronectin) and therefore enhance their invasive potential. Macrophages secrete soluble factors that stimulate melanoma cells to enhance their production of MIA in vitro. The increased MIA production may, in turn, increase the invasive properties of the cells by modulating the attachment of melanoma cells to the extracellular matrix (Bosserhoff & Buettner 2002). In support, inhibiting MIA expression of the human melanoma cell line HMB2 via stable antisense MIA cDNA transfection can result in enhanced formation of cell–cell contacts, a re-induction of pigment synthesis and re-expression of tyrosinase-related protein 1 (Tatzel et al. 2005). Therefore, it is conceivable that inhibiting MIA functions in vivo may provide a novel therapeutic strategy for metastatic melanoma disease (Callejo et al. 2004).
Macrophage inflammatory protein 1-α
Macrophage inflammatory protein 1 (MIP-1)-α, a chemokine, is a chemoattractant for T cells and immature dendritic cells. It is an effective agent in preventing the initiation of metastasis. In this respect, its injection can reduce the number of pulmonary metastatic foci in the B16 F10 melanoma cells lines (van Deventer et al. 2002).
Granulocyte-macrophage colony-stimulating factor and melanoma
GM-CSF and its receptor protein are expressed in melanomas (Ciotti et al. 1999). Although GM-CSF receptor protein localizes to the cell surface and binds ligand, it lacks functional components or accessory factors needed to transduce signals inside the cells (Baldwin et al. 1991). The therapeutic potential for GM-CSF-1 in the treatment of malignancy is supported by several observations: (i) local treatment of melanoma patients with GM-CSF enhanced the recruitment of dendritic cells (Vuylsteke et al. 2004), (ii) addition of systemic infusion of M-CSF to the local therapy with TNF-α and IFN-γ delayed the progression of melanoma (Lasek et al. 1995), (iii) vaccination with irradiated tumour cells secreting GM-CSF stimulates potent, specific and long-lasting antitumour immunity and (iv) injection of recombinant human GM-CSF with autologous melanoma vaccine resulted in tumour rejection in melanoma patients (Leong et al. 1999). Interestingly, the HGH18 murine melanoma cell line transfected with the murine GM-CSF gene can produce varying amounts of GM-CSF. Mice inoculated with HFH18 melanoma cells develop large tumours lacking inflammatory cell infiltrate. By contrast, animals inoculated with melanoma clones producing GM-CSF either completely reject the tumour cells or develop small tumours densely infiltrated with neutrophils, macrophages, lymphocytes and dendritic cells expressing the B7-2 co-stimulatory molecule – the GM-CSF gene therapy in the treatment of advanced malignant melanoma, possibly by the recruitment of dendritic APs (Armstrong et al. 1996; Soiffer et al. 1998).
Tumour angiogenesis and macrophages in melanomas
Solid tumour cells are surrounded by desmoplastic stromal response formed of interstitial connective tissue, blood vessels and fibroblastic cells. Tumour angiogenesis plays a crucial role in the tumour development, growth, invasions and metastasis. Tumour angiogenesis is induced by increased production of angiogenic factors and decreased production of angiogenic inhibitors by macrophages, cancer cells, vascular endothelial cells and other stromal cell types. Also, interaction between the stroma and malignant cells appears to have a critical role in the development of tumour neovasculature. In melanomas, neovascularization in and around the tumour cells enhances the tumour growth and the haematogenous spread (metastasis). Thus, angiogenesis is a crucial prognostic factor in melanoma tumourigenesis. Once melanomas get access into the blood stream, there is no effective consistent cure for these lesions and prognosis is dismal one. Angiogenesis is enhanced by the expression of angiogenic factors such as IL-8, vascular endothelial growth factor and basic fibroblast growth factor. Alternatively, angiogenesis is suppressed by the expression of several inhibitors such as IFN, platelet factor-4 and angiostatin. Migration of the vascular endothelial cells to the stroma is a key step in the process of angiogenesis. Macrophages are the key effector cells in angiogenesis. They produce a number of stimulatory and inhibitory molecules of angiogenesis (Ono et al. 1999). Macrophages produce a number of potent angiogenic cytokines and growth factors such as vascular endothelial growth factor, TNF-α, IL-8 and basic fibroblast growth factor. Also, they control events in the extracellular matrix through the secretion of extracellular matrix-degrading enzymes and -modulating enzymes. Accordingly, macrophages seem to influence various stages of angiogenesis either positively or negatively (Ono et al. 1999; Skobe et al. 2001). The role for macrophage is supported by several experimental findings including (i) macrophage–tumour-associated conditioned media can induce angiogenesis in animal models, (ii) macrophage infiltration is associated with neovascularization, (iii) there is a close correlation between increased macrophage index, malignancy and high vascular grade in malignant melanoma, (iv) the numbers of macrophages and microvessels increased significantly with increasing depth of tumour and with tumour angiogenesis, (v) human melanoma cells can produce angiogenic factors in response to macrophage-derived cytokines such as TNF-α and IL-1-α, (vi) co-culture of human monocytes and human melanoma cells can significantly enhance production of IL-8 and VEGF, (vii) monocyte recruitment, activation and differentiation mediated by monocyte chemotactic protein-1 (MCP-1) and macrophage colony-stimulating factor (M-CSF) modulate the expression of the angiogenic factor, IL-8 and (viii) the pattern of macrophage populations changes with the stage of tumour progression (Ono et al. 1999; Torisu et al. 2000; Varney et al. 2002; Shan et al. 2004).
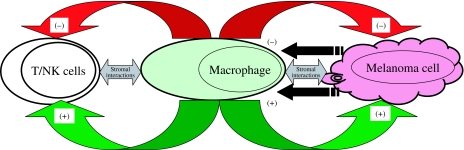
Proposed mechanisms of interaction between the TAMs and melanoma cells
As TAMs normally have defensive and beneficial roles, they are thought to have anticancer effects. However, spontaneous regression is a rare event in melanoma; therefore, it is tempting to speculate that the anticancer effects of TAMs are jeopardized in melanoma cells. In this respect, initially, the melanoma cells attract macrophages by producing chemotactic agents. Melanoma cells provide two types of signals that can induce effector functions in TAMs to produce either tumour growth promotion or tumour growth reduction. Depending upon the balance between these activation signals, TAMs can either enhance (tumour growth promotion) or inhibit (tumour growth reduction) melanoma growth (Figure 5). The tumour growth reduction effectors include direct cellular cytotoxicity, antibody-dependent cellular cytotoxicity, increased macrophage chemotactic factors and GM-CSF. Tumour growth promotion effectors include decreased macrophage recruitment, inhibition of NK cell function and melanoma cell attachment to the extracellular matrix and increased the expression of cyclooxygenase-2. Also, macrophage influence on tumour stroma (angiogenesis, fibrosis, etc.) is vital for effective tumour growth.
Figure 5.
The proposed mechanism of interactions between the melanoma cells and macrophages. Melanoma cells provide two types of signals that can induce effector functions in tumour-associated macrophages (TAMs) to produce either tumour growth promotion or tumour growth reduction. Depending upon the balance between these activation signals, TAMs can either enhance (+, tumour growth promotion) or inhibit (–, tumour growth reduction) melanoma growth. Also, macrophages may be key effectors in the modulation of the stromal response and angiogenesis (↔).
Conclusions
Macrophages (also called mononuclear phagocytes) arise from bone marrow stem cells, which give rise to promonocytes, which develop into monocytes. The latter are released into the blood stream. Monocytes make up 3–7% of the circulating white blood cells. Within 2 days or so, the blood stream monocytes (wondering macrophages) emigrate into the tissues where they reside, enlarge and become fixed macrophages (tissue histiocytes). The latter have a strong phagocytic potential. Macrophages are more active in phagocytosis as they develop many more granules containing hydrolytic enzymes. New macrophages can develop either by cell division or by maturation of the blood monocytes. The TAMs are found in the malignant melanomas and have apparent paradoxical effects. These cells have various functions according to the microenvironment. TAMs influence several aspects of melanoma growth including angiogenesis and vascularization, stroma formation and dissolution, and modulation of tumour cell growth (promotion/reduction). Activated TAMs can eradicate the neoplastic cells through cytotoxicity, apoptosis and alterations of the tumour microvasculature. It is tempting to speculate that in melanoma, TAMs can induce tumour growth reduction by non-specific antitumour cytotoxic mechanisms or induction of specific cell lytic effects. Alternatively, TAMs may be reprogrammed to trigger immune suppression of host defence mechanisms, through release of certain cytokines, prostanoids and some humoural mediators. In turn, this deranged immune response hinders effective cell-mediated immune mechanism against the melanoma cells. Moreover, TAMs produce tumour growth promoting factors. Collectively, these mediators induce tumour growth promotion. The possible underlying mechanisms of these interactions are poorly understood and their possible therapeutic ramifications await further investigations.
References
- Alexander P. Macrophages and tumours. Schweiz. Med. Wochenschr. 1976;106(3):1345–1350. [PubMed] [Google Scholar]
- Armstrong CA, Botella R, Galloway TH, et al. Antitumor effects of granulocyte-macrophage colony-stimulating factor production by melanoma cells. Cancer Res. 1996;56(9):2191–2198. [PubMed] [Google Scholar]
- Baldwin GC, Golde DW, Widhopf GF, Economou J, Gasson JC. Identification and characterization of a low-affinity granulocyte-macrophage colony-stimulating factor receptor on primary and cultured human melanoma cells. Blood. 1991;78(3):609–615. [PubMed] [Google Scholar]
- Birk RW, Gratchev A, Hakiy N, et al. Alternative activation of antigen-presenting cells: concepts and clinical relevance. Hautarzt. 2001;52(3):193–200. doi: 10.1007/s001050051289. [DOI] [PubMed] [Google Scholar]
- Bosserhoff AK, Buettner R. Expression, function and clinical relevance of MIA (melanoma inhibitory activity) Histol. Histopathol. 2002;17(1):289–300. doi: 10.14670/HH-17.289. [DOI] [PubMed] [Google Scholar]
- Bottazzi B, Walter S, Govoni D, Colotta F, Mantovani A. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J. Immunol. 1992;148(4):1280–1285. [PubMed] [Google Scholar]
- Bugelski PJ, Corwin SP, North SM, Kirsh RL, Nicolson GL, Poste G. Macrophage content of spontaneous metastases at different stages of growth. Cancer Res. 1987;47(15):4141–4145. [PubMed] [Google Scholar]
- Bugelski PJ, Kirsh RL, Poste G. New histochemical method for measuring intratumoral macrophages and macrophage recruitment into experimental metastases. Cancer Res. 1983;43(11):5493–5501. [PubMed] [Google Scholar]
- Bugelski PJ, Kirsh RL, Sowinski JM, Poste G. Changes in the macrophage content of lung metastases at different stages in tumor growth. Am. J. Pathol. 1985;118(3):419–424. [PMC free article] [PubMed] [Google Scholar]
- Burdelya LM, Kujawski G, Niu B, et al. Stat3 activity in melanoma cells affects migration of immune effector cells and nitric oxide-mediated antitumor effects. J. Immunol. 2005;174(7):3925–3931. doi: 10.4049/jimmunol.174.7.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejo SA, Marshall JC, Cools-Lartigue J, Saraiva VS, Burnier MN., Jr Macrophage-derived soluble factor enhances melanoma inhibitory activity expression by uveal melanoma cells in vitro. Melanoma Res. 2004;14(2):91–95. doi: 10.1097/00008390-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, de Freitas Sousa J, Espreafico EM, Pawelek JM. Human monocyte x mouse melanoma fusion hybrids express human gene. Gene. 2001b;275(1):103–106. doi: 10.1016/s0378-1119(01)00647-3. [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, Kolesnikova N, Sousa Jde F, Espreafico EM, Peronni KC, Pawelek J. Expression of c-Met proto-oncogene in metastatic macrophage x melanoma fusion hybrids: implication of its possible role in MSH-induced motility. Oncol. Res. 2003;14(3):163–174. doi: 10.3727/000000003771013062. [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, Pawelek J, Ikeda Y, et al. Fusion hybrids with macrophage and melanoma cells up-regulate N-acetylglucosaminyltransferase V, beta1–6 branching, and metastasis. Cell Growth Differ. 2001a;12(12):623–630. [PubMed] [Google Scholar]
- Ciotti P, Pesce GP, Cafiero F, et al. Intercellular adhesion molecule-1 (ICAM-1) and granulocyte-macrophage colony stimulating factor (GM-CSF) co-expression in cutaneous malignant melanoma lesions. Melanoma Res. 1999;9(3):253–260. doi: 10.1097/00008390-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Currie GA, Hedley DW. Monocytes and macrophages in malignant melanoma. I. Peripheral blood macrophage precursors. Br. J. Cancer. 1977;36(1):1–6. doi: 10.1038/bjc.1977.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton JE, Pearson J, Scott P, Carding SR. The interaction of gamma delta T cells with activated macrophages is a property of the V gamma 1 subset. J. Immunol. 2003;171(12):6488–6494. doi: 10.4049/jimmunol.171.12.6488. [DOI] [PubMed] [Google Scholar]
- van Deventer HW, Serody JS, McKinnon KP, Clements C, Brickey WJ, Ting JP. Transfection of macrophage inflammatory protein 1 alpha into B16, F10 melanoma cells inhibits growth of pulmonary metastases but not subcutaneous tumors. J. Immunol. 2002;169(3):1634–1639. doi: 10.4049/jimmunol.169.3.1634. [DOI] [PubMed] [Google Scholar]
- Duff M, Stapleton PP, Mestre JR, et al. Cyclooxygenase-2 inhibition improves macrophage function in melanoma and increases the antineoplastic activity of interferon gamma. Ann. Surg. Oncol. 2003;10(3):305–313. doi: 10.1245/aso.2003.04.033. [DOI] [PubMed] [Google Scholar]
- Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J. Leukoc Biol. 1998;64(3):275–290. doi: 10.1002/jlb.64.3.275. [DOI] [PubMed] [Google Scholar]
- Gauci CL, Alexander P. The macrophage content of some human tumours. Cancer Lett. 1975;1(1):29–32. doi: 10.1016/s0304-3835(75)94826-0. [DOI] [PubMed] [Google Scholar]
- Ha ES, Hwang SH, Shin KS, et al. Anti-metastatic activity of glycoprotein fractionated from Acanthopanax senticosus, involvement of NK-cell and macrophage activation. Arch. Pharm. Res. 2004;27(2):217–224. doi: 10.1007/BF02980109. [DOI] [PubMed] [Google Scholar]
- Hodi FS, Schmollinger JC, Soiffer RJ, et al. ATP6S1 elicits potent humoral responses associated with immune-mediated tumor destruction. Proc. Natl Acad. Sci. U S A. 2002;99(10):6919–6924. doi: 10.1073/pnas.102025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein MR. Genetic pathways to melanoma tumorigenesis. J. Clin. Pathol. 2004;57(8):797–801. doi: 10.1136/jcp.2003.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein MR. Dendritic cells and melanoma tumorigenesis: an insight. Cancer Biol. Ther. 2005;4(5):501–505. doi: 10.4161/cbt.4.5.1780. [DOI] [PubMed] [Google Scholar]
- Inoue M, Ohno T, Ogihara Y. Suppression of macrophage function by substances with a molecular weight lower than 3000 Da in B16 melanoma-conditioned medium. Biol. Pharm. Bull. 2002;25(7):907–912. doi: 10.1248/bpb.25.907. [DOI] [PubMed] [Google Scholar]
- Jager E, Hohn H, Necker A, et al. Peptide-specific CD8+ T-cell evolution in vivo: response to peptide vaccination with Melan-A/MART-1. Int. J. Cancer. 2002;98(3):376–388. doi: 10.1002/ijc.10165. [DOI] [PubMed] [Google Scholar]
- Jager D, Jager E, Knuth A. Vaccination for malignant melanoma: recent developments. Oncology. 2001;60(1):1–7. doi: 10.1159/000055289. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr The role of self-recognition in receptor repertoire development. Members of the Janeway Laboratory. Immunol. Res. 1999;19(2–3):107–118. doi: 10.1007/BF02786480. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76(2):275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Kalish R, Brody NI. The effects of tumor facilitating factor of B16 melanoma on the macrophage. J. Invest. Dermatol. 1983;80(3):162–167. doi: 10.1111/1523-1747.ep12533308. [DOI] [PubMed] [Google Scholar]
- Kamiryo Y, Yajima T, Saito K, et al. Soluble branched (1,4) -beta-D-glucans from Acetobacter species enhance antitumor activities against MHC class I-negative and – positive malignant melanoma through augmented NK activity and cytotoxic T-cell response. Int. J. Cancer. 2005;115(5):769–776. doi: 10.1002/ijc.20934. [DOI] [PubMed] [Google Scholar]
- Kilgore KS, Imlay MM, Szaflarski JP, et al. Neutrophils and reactive oxygen intermediates mediate glucan-induced pulmonary granuloma formation through the local induction of monocyte chemoattractant protein-1. Lab Invest. 1997;76(2):191–201. [PubMed] [Google Scholar]
- Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 2005 doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz M, Toksoy A, Goebeler M, Engelhardt E, Brocker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. J. Pathol. 1999;189(4):552–558. doi: 10.1002/(SICI)1096-9896(199912)189:4<552::AID-PATH469>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Lasek W, Wankowicz A, Kuc K, et al. Potentiation of antitumor effects of tumor necrosis factor alpha and interferon gamma by macrophage-colony-stimulating factor in a MmB16 melanoma model in mice. Cancer Immunol. Immunother. 1995;40(5):315–321. doi: 10.1007/BF01519632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovici J, Hoenig S. Lysis and growth stimulation of a murine melanoma determined by density of macrophage populations. Anticancer Res. 1985;5(5):545–551. [PubMed] [Google Scholar]
- Lejeune FJ, Beaumont E, Garcia Y. Growth inhibitory effect of peritoneal macrophages on Harding Passey melanoma, its impairment by macrophage lysosome overloading. Br. J. Cancer. 1973;28(1):80. doi: 10.1038/bjc.1973.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SP, Enders-Zohr P, Zhou YM, et al. Recombinant human granulocyte macrophage-colony stimulating factor (rhGM-CSF) and autologous melanoma vaccine mediate tumor regression in patients with metastatic melanoma. J. Immunother. 1999;22(2):166–174. doi: 10.1097/00002371-199903000-00008. [DOI] [PubMed] [Google Scholar]
- Li H, Cao MY, Lee Y, et al. Virulizin, a novel immunotherapy agent, activates NK cells through induction of IL-12 expression in macrophages. Cancer Immunol. Immunother. 2005 doi: 10.1007/s00262-005-0698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol. Today. 1992;13(7):265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bottazzi B, Sozzani S, et al. Cytokine regulation of monocyte recruitment. Arch. Immunol. Ther. Exp.(Warsz) 1995;43(2):149–152. [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Martin JG, Suzuki M, Ramos-Barbon D, Isogai S. T cell cytokines: animal models. Paediatr. Respir. Rev. 2004;5(Suppl. A):S47–S51. doi: 10.1016/s1526-0542(04)90010-3. [DOI] [PubMed] [Google Scholar]
- Mytar B, Siedlar M, Woloszyn M, Colizzi V, Zembala M. Cross-talk between human monocytes and cancer cells during reactive oxygen intermediates generation: the essential role of hyaluronan. Int. J. Cancer. 2001;94(5):727–732. doi: 10.1002/ijc.1530. [DOI] [PubMed] [Google Scholar]
- Mytar B, Siedlar M, Woloszyn M, Ruggiero I, Pryjma J, Zembala M. Induction of reactive oxygen intermediates in human monocytes by tumour cells and their role in spontaneous monocyte cytotoxicity. Br. J. Cancer. 1999;79(5)(6):737–743. doi: 10.1038/sj.bjc.6690118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mytar B, Woloszyn M, Szatanek R, et al. Tumor cell-induced deactivation of human monocytes. J. Leukoc Biol. 2003;74(6):1094–1101. doi: 10.1189/jlb.0403140. [DOI] [PubMed] [Google Scholar]
- Naama HA, Mack VE, Smyth GP, Stapleton PP, Daly JM. Macrophage effector mechanisms in melanoma in an experimental study. Arch. Surg. 2001a;136(7):804–809. doi: 10.1001/archsurg.136.7.804. [DOI] [PubMed] [Google Scholar]
- Naama HA, McCarter MD, Mack VE, et al. Suppression of macrophage nitric oxide production by melanoma: mediation by a melanoma-derived product. Melanoma Res. 2001b;11(3):229–238. doi: 10.1097/00008390-200106000-00004. [DOI] [PubMed] [Google Scholar]
- Nesbit M, Schaider H, Miller TH, Herlyn M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J. Immunol. 2001;166(11):6483–6490. doi: 10.4049/jimmunol.166.11.6483. [DOI] [PubMed] [Google Scholar]
- Ono M, Torisu H, Fukushi J, Nishie A, Kuwano M. Biological implications of macrophage infiltration in human tumor angiogenesis. Cancer Chemother. Pharmacol. 1999;43(Suppl.):S69–S71. doi: 10.1007/s002800051101. [DOI] [PubMed] [Google Scholar]
- Pawelek JM. Tumour cell hybridization and metastasis revisited. Melanoma Res. 2000;10(6):507–514. doi: 10.1097/00008390-200012000-00001. [DOI] [PubMed] [Google Scholar]
- Petrenko O, Moll UM. Macrophage migration inhibitory factor MIF interferes with the Rb-E2F pathway. Mol Cell. 2005;17(2):225–236. doi: 10.1016/j.molcel.2004.11.052. [DOI] [PubMed] [Google Scholar]
- van Ravenswaay Claasen HH, Kluin PM, Fleuren GJ. Tumor infiltrating cells in human cancer. On the possible role of CD16+ macrophages in antitumor cytotoxicity. Lab. Invest. 1992;67(2):166–174. [PubMed] [Google Scholar]
- Repp AC, Mayhew ES, Apte S, Niederkorn JY. Human uveal melanoma cells produce macrophage migration-inhibitory factor to prevent lysis by NK cells. J. Immunol. 2000;165(2):710–715. doi: 10.4049/jimmunol.165.2.710. [DOI] [PubMed] [Google Scholar]
- Rogivue C, Car BD, Allmann-Iselin I, Zwahlen RD, WalZ A. Bovine melanoma growth stimulatory activity: a new monocyte-macrophage-derived cytokine of the IL-8 family. Partial structure, function, and expression in acute pulmonary inflammation. Lab. Invest. 1995;72(6):689–695. [PubMed] [Google Scholar]
- Schmollinger JC, Vonderheide RH, Hoar KM, et al. Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc. Natl. Acad. Sci. U.S.A. 2003;100(6):3398–3403. doi: 10.1073/pnas.0530311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger A, Koszik F, Schmidt W, Kutil R, Stingl G. The tumorigenicity of IL-2 gene-transfected murine M-3D melanoma cells is determined by the magnitude and quality of the host defense reaction: NK cells play a major role. J. Immunol. 1999;162(11):6650–6657. [PubMed] [Google Scholar]
- Shan S, Robson ND, Cao Y, et al. Responses of vascular endothelial cells to angiogenic signaling are important for tumor cell survival. FASEB J. 2004;18(2):326–328. doi: 10.1096/fj.03-0765fje. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, Nishihira J. High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem. Biophys. Res. Commun. 1999;264(3):751–758. doi: 10.1006/bbrc.1999.1584. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hamberg LM, Hawighorst T, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am. J. Pathol. 2001;159(3):893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc. Natl. Acad. Sci. U.S.A. 1998;95(22):13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatzel J, Poser I, Schroeder J, Bosserhoff AK. Inhibition of melanoma inhibitory activity (MIA) expression in melanoma cells leads to molecular and phenotypic changes. Pigment Cell Res. 2005;18(2):92–101. doi: 10.1111/j.1600-0749.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- Torisu H, Ono M, Kiryu H, et al. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int. J. Cancer. 2000;85(2):182–188. [PubMed] [Google Scholar]
- Torisu-Itakura H, Furue M, Kuwano M, Ono M. Co-expression of thymidine phosphorylase and heme oxygenase-1 in macrophages in human malignant vertical growth melanomas. Jpn J. Cancer Res. 2000;91(9):906–910. doi: 10.1111/j.1349-7006.2000.tb01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varney ML, Olsen KJ, Mosley RL, Bucana CD, Talmadge JE, Singh RK. Monocyte/macrophage recruitment, activation and differentiation modulate interleukin-8 production: a paracrine role of tumor-associated macrophages in tumor angiogenesis. In Vivo. 2002;16(6):471–477. [PubMed] [Google Scholar]
- te Velde AA, Figdor CG. Monocyte mediated cytotoxic activity against melanoma. Melanoma Res. 1992;1(5–6):303–309. doi: 10.1097/00008390-199201000-00001. [DOI] [PubMed] [Google Scholar]
- Viret C, Janeway MHC and T cell development. Rev. Immunogenet. 1999;1(1):91–104. [PubMed] [Google Scholar]
- Vuylsteke RJ, Molenkamp BG, Gietema HA, et al. Local administration of granulocyte/macrophage colony-stimulating factor increases the number and activation state of dendritic cells in the sentinel lymph node of early-stage melanoma. Cancer Res. 2004;64(22):8456–8460. doi: 10.1158/0008-5472.CAN-03-3251. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Iida M, Takaishi K, et al. Chemoattractants for neutrophils in lipopolysaccharide-induced inflammatory exudate from rats are not interleukin-8 counterparts but gro-gene-product/melanoma-growth-stimulating-activity-related factors. Eur J. Biochem. 1993;214(1):267–270. doi: 10.1111/j.1432-1033.1993.tb17920.x. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. Signaling defects in T lymphocytes of patients with malignancy. Cancer Immunol. Immunother. 1999;48(7):346–352. doi: 10.1007/s002620050585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J, Sisley K, Reeves G, et al. Evidence of macrophage and lymphocyte, but not dendritic cell, infiltration in posterior uveal melanomas, whilst cultured uveal melanomas demonstrate pluripotency by expressing CD68 and CD163. Int. J. Exp. Pathol. 2004;85(1):35–43. doi: 10.1111/j.0959-9673.2004.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembala M, Siedlar M, MarcinkiewicZ J, Pryjma J. Human monocytes are stimulated for nitric oxide release in vitro by some tumor cells but not by cytokines and lipopolysaccharide. Eur. J. Immunol. 1994a;24(2):435–439. doi: 10.1002/eji.1830240225. [DOI] [PubMed] [Google Scholar]
- Zembala M, Siedlar M, Ruggiero I, et al. The MHC class-II and CD44 molecules are involved in the induction of tumour necrosis factor (TNF) gene expression by human monocytes stimulated with tumour cells. Int. J. Cancer. 1994b;56(2):269–274. doi: 10.1002/ijc.2910560221. [DOI] [PubMed] [Google Scholar]