Abstract
Irradiation has profound effects on the reproductive function. Our knowledge about radioprotective effects of melatonin against X-ray-induced testis damage is rudimentary. In this investigation, we hypothesized that melatonin can minimize germ-cell depletion and morphological features of cell damage in testis following X-ray irradiation (XRI). To examine these effects, and to test our hypothesis, an animal model comprised of 60 Albino rats was established. The animals were divided into five groups: Group 1, non-irradiated; Group 2, X-ray irradiated (XRI, 8 Grays); Group 3, XRI pretreated with solvent (ethanol and phosphate-buffered saline); Group 4, non-irradiated group treated with melatonin and Group 5, XRI pretreated with melatonin. The testes were evaluated for both histological (light microscopy) and ultrastructural changes (transmission electron microscopy). Histologically, there were marked depletions (66%) of the germinal epithelial cells, in XRI group (Groups 2 and 3), whereas these changes were almost absent in XRI testis of animals pretreated with melatonin (Group 5). The number of spermatogenic cells in XRI testis of animals pretreated with melatonin (Group 5) was comparable (95%) to that of non-irradiated group (Groups 1 and 4) but significantly (P < 0.05) higher than those in XRI testis (34%, Groups 2 and 3). Ultrastructurally, XRI testis (Groups 2 and 3) showed features of apoptosis (condensation of the nuclei, vacuolization of the cytoplasm, increased cytoplasmic density and apoptotic bodies). These features were absent in XRI testis of animals pretreated with melatonin (Group 5). Also, this Group showed features of an increased metabolic activity (large acrosomal vesicle, prominent Golgi, increased mitotic activity, increased complement of cytoplasmic organelles and appearance of nucleoli-like bodies). There was a minimal depletion of the Sertoli and Leydig cells following XRI. Also, morphological features of apoptosis were infrequent in these cells. Administration of melatonin (MEL) prior to irradiation can protect testis against its destructive effects. The protective effects include amelioration of germ-cell depletion and apoptotic changes. The clinical ramifications of these observations mandate further studies.
Keywords: testis, melatonin, X-ray irradiation
Irradiation has a profound effect on the reproductive function. The X-rays (electromagnetic ionizing radiation) are composed of massless particles of energy (photons) that disrupt electrons of atoms within cells and therefore affect cellular functions. X-ray irradiation (XRI) can affect normal cells, especially rapidly growing ones such as spermatogenic cells. The germ cells show very distinct sensitivity to X-irradiation during their development from spermatogonia to sperm. The most sensitive cell types are spermatogonia, which are rapidly depleted after irradiation. Other mature germ cells and a small subfraction of testicular stem cells survive. While mature germ cells are lost by maturation, the process of recolonization from stem cells is slow and may take up to several months (or even years in the human testis). However, survival rate of spermatogonia is the most crucial determinant of future fertility. Although X-ray is widely used for both imaging and therapeutic purposes, our knowledge about their possible early and acute injurious effects on the testis is still marginal. In lower doses, direct irradiation to testis can affect germinal epithelium. In larger doses, X-ray may cause either reversible or permanent aspermia (Shalet 1993). In animals, XRI of testis can produce changes in both serum luteinizing and follicle-stimulating hormones of rat testis (Bain & Keene 1975). Morphologically, XRI can produce arrest of spermatogenesis, desquamation, vacuolization of germinal cells and appearance of multinucleated giant cells. The type and extent of these changes depend on the dose, duration and frequency of XRI (Bansal et al. 1990). At the genetic level, testicular XRI can produce DNA damage in both primary spermatocytes and mature sperms (Cordelli et al. 2003). In human, XRI of the testis can produce a transient, but substantial, suppression of sperm counts (Clifton & Bremner 1983).
Melatonin, a secretory product of the pineal gland, can participate in the regulation of several physiological processes. It can scavenge many harmful free radicals such as hydroxyl, peroxyl radicals and peroxynitrite anions. Melatonin accumulates more in the nucleus than in the cytosol of the cells. Also, it is one of few antioxidants that can penetrate the mitochondrial membrane and enter the mitochondria, and thus, it has radioprotective roles. In support of this proposition, melatonin can improve the overall survival following total body irradiation and minimize the extent of DNA damage and the frequency of chromosomal abrasions (Hickman et al. 1999; Vijayalaxmi et al. 1999a; Hussein et al. 2005).
To date, whether melatonin has a radioprotective role against X-ray-induced acute and early testis damage is still unknown. In this investigation, we hypothesized that melatonin can minimize germ-cell depletion and morphological features of early and acute cell damage in the testis following XRI. This radioprotective effect would manifest: (i) on the histological level by preservation of the cellular counts and both stepwise development and association of the germinal epithelial cells and (ii) on the ultrastructural level by preservation of the nuclear and cytoplasmic features. To test our hypothesis and to fill this existing gap in literature, we carried out this investigation. To accomplish our goals, we established an animal model consisting of five different groups of Albino rats: (i) non-XRI, (ii) XRI, (iii) XRI pretreated with solvent, (iv) non-XRI pretreated with melatonin and (v) XRI pretreated with melatonin. We addressed two questions: (i) what are the histological and ultrastructural changes in XRI-testis and (ii) what are the effects of melatonin on these morphological changes?
Materials and methods
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the South Valley University, School of Medicine, Sohag, Egypt.
Rats and maintenance
Three-month-old Albino rats were obtained from Assuit University Animal Facility, Faculty of Medicine, Assuit University, Assuit, Egypt. They were housed in Animal Facility at Faculty of medicine, South Valley University, Sohag, Egypt, with room temperature maintained at 65–75 F, relative humidity of 50–70% and an airflow rate of 15 exchange/h. Also, a time-controlled system provided 0700–2100 h light and 2100–0700 h dark cycles. All rats were given ad libitum access to Taklad rodent chow diet and water from sanitized bottle fitted with stopper and sipper tubes. These conditions were adopted following other groups (Vijayalaxmi et al. 1999a; Vijayalaxmi et al. 1999b; Hussein et al. 2005).
X-ray irradiation
X-ray irradiation was carried out at The Department of Radiology and Oncology, Sohage University Hospitals, Egypt using a linear accelerator (Philips SL75.5). This device was adjusted to provide X-ray but not gamma irradiation, and therefore, no filters were used in these experiments. Each animal was placed separately in a special small box with adjustable width that can fairly accommodate the animal without allowing any movements. Each animal was exposed to a whole-body XRI dose of 8 Grays (Gy). The dose was delivered at a rate of 400 motor unit/minute. The X-ray irradiation dose for the testes was measured using special equation, and it was 8 Gy/testis.
Melatonin and X-ray irradiation
After a 7-day acclimatization period, a randomized block design based on the animal body weights was used to divide rats into five different groups. Five separate experiments were executed using a total of 60 rats. Each experiment had 12 rats in each of the following five subgroups: subgroup A, non-XRI; subgroup B, intraperitoneal injection of melatonin (100 mg/kg of body weight); subgroup C, XRI (8 Gy whole body); subgroup D, XRI pretreated with solvent (5% ethanol in phosphate-buffered saline 1 h before irradiation) and subgroup E, XRI pretreated with melatonin (100 mg/kg of body weight melatonin 1 h before irradiation). Non-irradiated subgroups (A and B) were initially evaluated as separate ones, and as no differences were found between them, they were summed together as one group (Group 1, non-XRI testis). Similarly, no differences were found between animals in subgroups C and D and thus considered as one group (Group 2, XRI testis). Animals in subgroup E, which received XRI and melatonin pretreatment, were considered as a separate group (Group 3, XRI + melatonin pretreatment testis). Therefore, animals in subgroups 1 and 4 served as controls for experimental animals in subgroups 2, 3 and 5. A summary of the experimental design is shown in Table 1.
Table 1.
The distribution of the animals in the study groups
| Groups | X-ray irradiation | Melatonin pretreatment | Solvent pretreatment |
|---|---|---|---|
| Non-XRI testis (control) | |||
| Subgroup #1 | – | – | – |
| Subgroup #2 | – | + | – |
| XRI testis | |||
| Subgroup #3 | + | – | – |
| Subgroup #4 | + | – | + |
| XRI + melatonin testis | |||
| Subgroup #5 | + | + | – |
+, received; –, not received.
Irradiation was carried out using a linear accelerator (Philips SL75.5). Animals in group 2, 3 and 5 were exposed to a whole-body XRI dose of 8 Gy. Animals in groups 4 and 5 were given an intraperitoneal injection of freshly prepared melatonin (sigma, St. Louis, MO, USA) in 1000 µl of 5% ethanol (made with phosphate-buffered saline). Following other groups, we selected this XRI-specific dose, as it can generate reactive oxygen radicles, induce apoptosis and alter cell-cycle protein expression in rapidly proliferating cells such as germ cells and basal cell keratinocytes (van Alphen et al. 1988; Kim et al. 2001; Sener et al. 2003; Hussein et al. 2005).
Morphological examination of testis
The animals were killed at 48 h after XRI. Immediately after killing, testis was removed, cut in two halves across the longitudinal axis. Two 1-g specimens of tissue were taken from the middle area of the parenchyma, fixed in Bouin's solution, conventionally dehydrated and embedded in paraffin. Sections of 4 µm were cut with a Leica sliding microtome (SM 2000R, Nussbach, Germany), and slides were stained with Haematoxylin and Eosin. The other half of the testis was processed for ultrastructural studies. The analyses included both quantitative and qualitative ones (Hussein et al. 2005).
Histological analysis of the testis
For histological analysis, spermatogenic series in the tubules were divided into the following groups: spermatogonia, primary spermatocytes, round spermatids and sperms. To characterize spermatogenic activity in the testis, spermatogenic cells is stage VII seminiferous tubules were counted according to Omura et al. (1999). The status of germinal epithelium was analysed for basal and adluminal portions of the tubules. Ten photomicrographs of stage VII seminiferous tubules were randomly taken under light microscope from one section of testis per rat with a digital camera (Fujix Digital Camera HC-300Z/CL, Olympus Co., Ltd, Tokyo, Japan). The digital pictures were printed out with a full-colour, high-resolution digital printer (PICTROGRAPHY 3000, Fuji Film Co., Ltd, Tokyo, Japan). The spermatogonia, spermatocytes, round spermatids and nuclei of Sertoli cells in the seminiferous tubule were counted. Counting of these cells was confirmed by direct observation under light microscope (at ×400 and ×1000 magnifications), as it was sometimes difficult to classify these cells on the printout. We examined 10 seminiferous tubules per rat, because the digital camera could take only 10 pictures, making it easy to avoid counting of the same seminiferous tubules twice. We adopted the number of 10 tubules following the recommendations of other groups (Creasy 1997; Zhao et al. 1998; Omura et al. 1999; Ponnapakkam et al. 2003). Elongated spermatids at stage VII were not counted because it was difficult to do so accurately. The numbers of the spermatogenic cells were expressed per tubule. A mean value of 10 tubules was treated as the representative value of each animal. For characterization of the Leydig cell area, a total of 10 interstitial areas that were surrounded by six neighbouring tubules were chosen at random, and Leydig cell nuclei were counted.
Transmission electron microscopy
Some tissue fragments were fixed in 2.5% glutradehyde in 0.1 m sodium cacodylate buffer at 4 °C and pH 7.2 for 24 h, washed in 0.1 m buffer and postfixed in osmium tetroxide in 0.2 m buffer for 1 h. The specimens were dehydrated in 70, 90 and 100% ethanol and then embedded in labelled capsules with freshly prepared resin and left to polymerize at 60 °C for 48 h. Several resin semithin sections were cut at approximately 1 μm using glass knives and ultramicrotome. The sections were stained with 1% toluidine blue in 1% borax solution for 1 min at 80 °C. The stain was rinsed off with distilled water, and sections were dried and examined. Selective areas from trimmed blocks were cut by using a diamond knife, with the ultramicrotome set to cut at around 50–70 nm using heat advances. The sections were picked up onto 300 mesh copper grids, stained with methanolic uranyl acetate and examined by transmission electron microscopy (TEM). Some of the examined fields were photographed (Hussein et al. 2003; Hussein et al. 2005; Zucker-Franklin et al).
Quantification of the ultrastructural features of cell damage
Quantification of features of cell damage was done by counting apoptotic bodies, cytoplasmic vacuoles, mitochondria as well as by estimation of cytoplasmic density in 20 random TEMs with final magnification of ×2500. The results were expressed as Mean ± SEM (Kerr et al. 1972; Hussein et al. 2003; Hussein et al. 2005).
Quantification of the morphometric parameters
The morphometric parameters were measured using image analyser program (Leica Q 500 MC), at The School of Veterinary Medicine, Assuit University, Assuit, Egypt. The measurements included: (i) volume proportion, diameter and epithelial height (μm) of the seminiferous tubules; (ii) volume proportions of interstitial tissue and (iii) nuclear surface area/total cell-surface area and nuclear diameter for Leydig cells.
Statistical analysis
anova with a statistical significance of P < 0.05 was used. Data were subjected to anova test of a completely randomized design according to other groups (Simpson et al. 1960; Petersen 1985). Examination of the statistical level of significance was performed with Student's t-test resulting from the anova tests. Level of significance (P) was considered as follows: (i) P > 0.05, none significant; (ii) P ≤ 0.05, significant and (iii) P ≤ 0.01, highly significant. Computations were performed with sas version 8.1 Software (SAS Institute Inc, Cary, NC, USA). All the analyses were performed in a blinded fashion by the authors.
Results
The morphological features included both histological and ultrastructural changes. As compared with non-irradiated testis, all values of morphometric parameters were statistically significantly reduced in the X-ray-irradiated testis (P < 0.05). In XRI testis with melatonin pretreatment, these values were relatively similar to non-irradiated group. A summary of these results is presented in Tables 2–5.
Table 2.
Morphometric changes following X-ray irradiation of the testis of Albino rats
| Aspects | Control (non-X-ray-irradiated testis) | X-ray-irradiated testis | X-ray-irradiated testis with melatonin pretreatment | P value |
|---|---|---|---|---|
| Weight of the testis (g) | 1.5 ± 0.04 | 0.9 ± 0.01 | 1.6 ± 0.08 | <0.05 |
| Volume proportions of interstitial tissue | 9.7% | 16.7% | 10.6% | <0.05 |
| Seminiferous tubules | ||||
| Volume proportion | 93.1% | 80.4% | 91.2% | <0.05 |
| Diameter (μm) | 272.1 ± 5.0 | 221.4 ± 8.0 | 251.3 ± 3.0 | <0.05 |
| Epithelial height (μm) | 95.8 ± 4.0 | 69.2 ± 3.0 | 87.6 ± 4.0 | <0.05 |
| Pachytene spermatocyte nuclear diameter (μm) | 11.2 ± 0.3 | 8.7 ± 0.2 | 9.9 ± 0.4 | NS |
| Leydig cells | ||||
| Nuclear surface area/total cell-surface area | 22.4% | 31.8% | 25.1% | <0.05 |
| Nuclear diameter | 7.24 ± 0.05 | 6.21 ± 0.04 | 7.12 ± 0.03 | NS |
| Mitotic figures (A-spermatogonia) | 18.8 ± 2.3 | 1.0 ± 0.3 | 30.2 ± 3.3 | <0.01 |
NS, none significant. P values (mentioned in the table) refer to the differences between the values for XRI testis and the values for XRI testis with melatonin pretreatment. Also, the differences between the values for non-irradiated testis and the values for XRI testis reached the level of statistical significance (P < 0.05).
Table 5.
Quantification of the ultrastructural features of cell damage in the seminiferous tubule of rats exposed to X-ray irradiation
| Aspect | Non-XRI testis | XRI testis | XRI testis with melatonin pretreatment |
|---|---|---|---|
| Apoptotic changes | Absent | 13.6 ± 0.8 | 2.3 ± 1.2 |
| Spermatogonia | |||
| Density of the cytoplasm | + | +++ | + |
| Number of cytoplasmic vacuolization | Absent | 2.5 ± 0.3 | 0.6 ± 0.3 |
| Number of mitochondria | 5.3 ± 0.8 | 4.0 ± 1.1 | 5.3 ± 1.2 |
| Spermatocytes | |||
| Density of the cytoplasm | + | ++ | + |
| Number of cytoplasmic vacuolization | Absent | 1.0 ± 0.5 | 0.6 ± 0.3 |
| Number of mitochondria | 6.7 ± 1.4 | 6.6 ± 0.8 | 6.0 ± 1.7 |
| Spermatids | |||
| Density of the cytoplasm | + | ++ | + |
| Number of cytoplasmic vacuolization | Absent | 2.0 ± 0.1 | Absent |
| Number of mitochondria | 13.6 ± 1.7 | 4.7 ± 0.8 | 10.6 ± 1.7 |
| Sertoli cells | |||
| Density of the cytoplasm | + | +++ | + |
| Number of cytoplasmic vacuolization | Absent | 20.1 ± 2.8 | 0.3 ± 0.3 |
| Number of mitochondria | 23.3 ± 3.5 | 13.0 ± 2.1 | 21.3 ± 2.7 |
| Leydig cells | |||
| Density of the cytoplasm | + | +++ | + |
| Number of cytoplasmic vacuolization | Absent | 14.3 ± 2.9 | 0.3 ± 0.3 |
| Number of mitochondria | 26.3 ± 2.8 | 14.0 ± 2.1 | 33.8 ± 0.8 |
In XRI testis, features of apoptosis (apoptotic bodies, cytoplasmic vacuoles and density) were statistically significantly more frequent as compared with either the non-irradiated group or to XRI testis in animals pretreated with melatonin (P < 0.05).
Table 3.
Ultrastructural changes following X-ray irradiation of the testis of Albino rats
| Aspect | X-ray-irradiated testis | X-ray-irradiated testis with melatonin pretreatment |
|---|---|---|
| Spermatogonia | The nuclei were condensed with irregular contour, increased heterochromatin, numerous apoptotic bodies. The basement membrane was thick. | The nuclei were almost similar to the non-irradiated cells. No apoptotic bodies were seen. The basement membrane was normal. |
| Primary spermatocytes | The nuclei were small with chromatinolysis, and electron lucent cytoplasm containing less numerous ribosomes. Some cells were large and swollen with multiple vacuoles | The cells had almost normal cytoplasm and nuclei with chromatin pattern similar to that of the non-irradiated group. |
| Spermatids | The cells were small in size with rounded nuclei. Their cytoplasm showed few mitochondria, small Golgi area and almost few tubules of smooth endoplasmic reticulum. Cells with acrosomes were rarely seen. | The cells showed large acrosomal vesicle, nucleoli-like bodies, prominent Golgi body, rough endoplasmic reticulum and mitochondria. |
| Sperms | Absent. | Numerous |
| Sertoli cells | The cells had indented nuclei and vacuolated prominent nucleoli, vacuolated cytoplasm. Tight junctions between the adjacent cell membranes were rarely demonstrated. | The cells were almost similar to those in the non-irradiated group. |
| Leydig cells | Few cells with irregular, dense nuclei and reduced numbers of smooth endoplasmic reticulum, presence of multiple vacuoles in their cytoplasm. | Aggregates of Leydig cells with most of them having a marked increase in the number of mitochondria and smooth endoplasmic reticulum. |
Table 4.
Ultrastructural changes indicative of apoptosis and increased metabolic activity in the X-ray-irradiated testis of Albino rats
| X-ray-irradiated testis | ||
|---|---|---|
| Features of cell damage | Features of apoptosis | XRI testis with melatonin pretreatment Features of increased metabolic activity |
| Depletion of the spermatogenic cells | Cytoplasmic vacuolization | Large acrosomal vesicle and nuclei |
| Thickened basement membrane | Condensation of the nuclear chromatin | Prominent Golgi |
| Loss or small sized acrosomes | Apoptotic bodies | Increased mitotic activity |
| Decreased complement of organellesin the cytoplasm | Prominence of the nucleoli | |
| Appearance of nucleoli-like bodies | ||
| Increased complement of cytoplasmicorganelles (mitochondria) | ||
Histological features
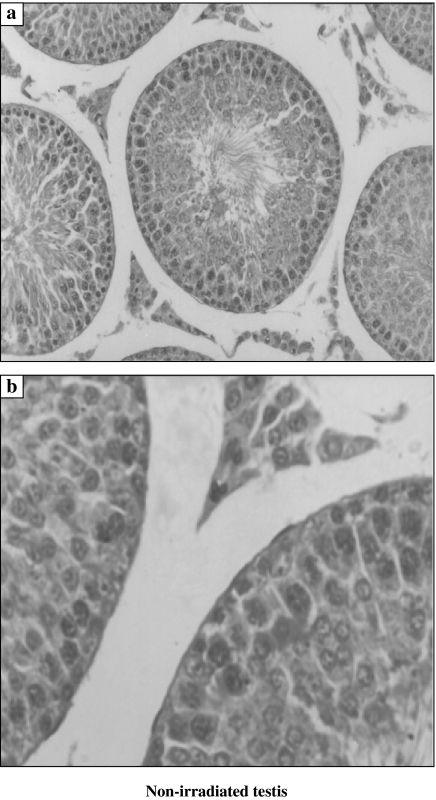
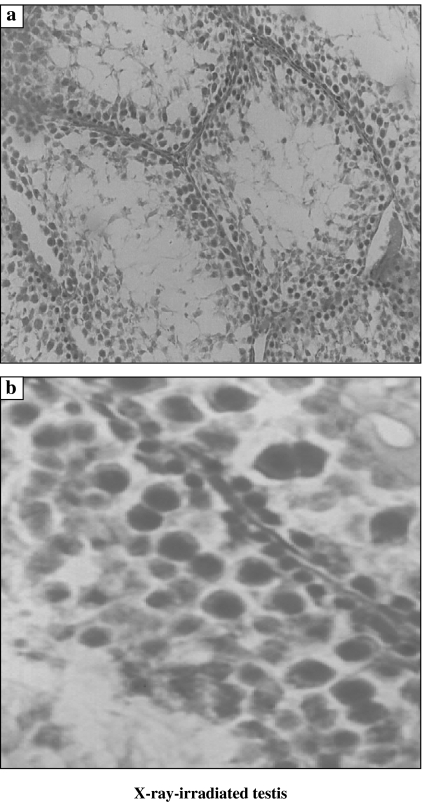
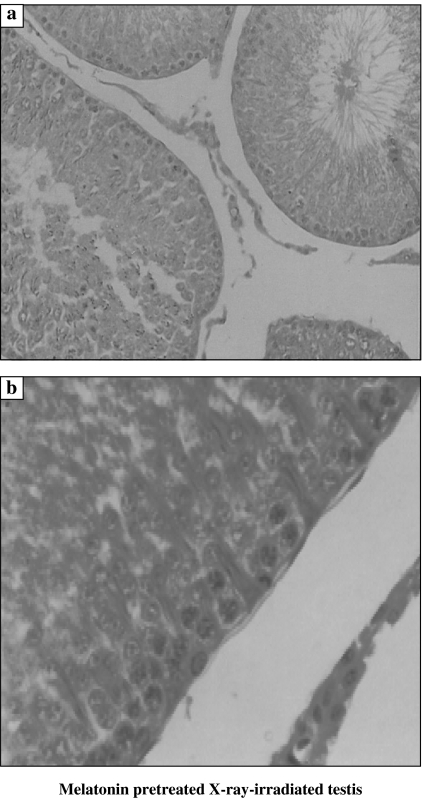
Non-irradiated testis showed normal cellular associations and was formed of multiple seminiferous tubules lined by both spermatogenic cells in stepwise stages of development (Type A spermatogonia, Type B spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids and sperms) and Sertoli cells (Figure 1). As compared to the non-irradiated testis, examination of XRI testis revealed presence of marked disorganization and depletion of the spermatogenic cells, especially spermatocytes and spermatids. The spermatozoa were completely depleted. The spermatogenic cells had dense irregular nuclei and acidophilic vacuolated cytoplasm. The spaces among the seminiferous tubules were reduced (Figure 2). Total number of Sertoli and Leydig cells was reduced to 71 and 66%, respectively, of the counts in non-irradiated group. As compared with XRI testis, XRI testis pretreated with melatonin showed relatively normal cellular associations and counts. The seminiferous tubules had relatively normal structure with resumption of complete spermatogenesis. The number of germ cells was 95% of non-irradiated group. Both Sertoli and spermatogenic cells had relatively normal architecture and cytological features. The seminiferous tubules were separated by scanty connective tissue containing interstitial cells of Leydig (Figure 3).
Figure 1.
Histological features of the non-irradiated testis. The seminiferous tubule is formed of the lining germinal epithelium (spermatogonia Type A and Type B, primary spermatocytes and spermatids) and the supporting Sertoli cells. Aggregates of interstitial cells are present in between tubules (a, ×200 and b, ×1000).
Figure 2.
Histological features of X-ray-irradiated testis featuring the presence of: (a) a marked reduction in the number of spermatogenic cells with absence of the sperms in the seminiferous tubules and (b) the cells have dense nuclei and acidophilic cytoplasm (×200).
Figure 3.
Histological features of X-ray irradiated testis from animals treated with melatonin (A) featuring the presence of relatively normal seminiferous tubules (a and b ×1000).
Ultrastructural features
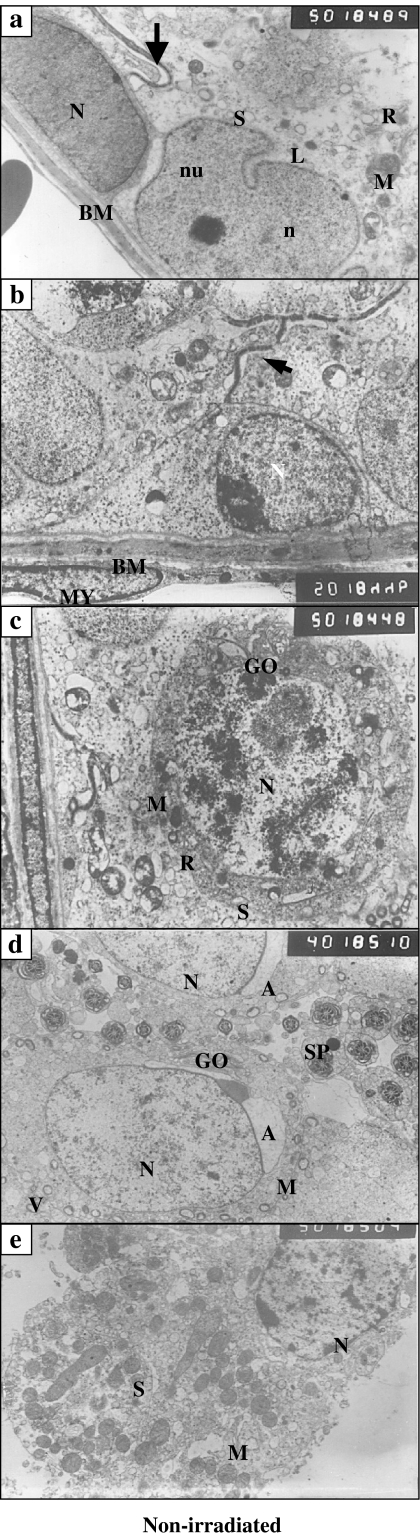
In non-XRI testis, Type A spermatogonia had oval nuclei and fine granular chromatin with scanty cytoplasm (Figure 1a). Type B spermatogonia had more rounded nuclei with coarsely clumped marginated heterochromatin (Figure 1b). The primary spermatocytes had large-sized nuclei with coarse chromatin threads that were evenly distributed within the granular chromatin. Their cytoplasm contained mitochondria, prominent Golgi bodies and numerous free ribosomes (Figure 4c). Spermatids appeared as rounded cells smaller than the primary spermatocytes. Their nuclei were rounded with evenly distributed chromatin. Their cytoplasm contained small spherical mitochondria with electron lucent centres. In late spermatides, the latter formed the acrosome. Transverse sections in sperms at different levels were frequently seen (Figure 4d). Leydig cells showed rounded nuclei and peripherally arranged heterochromatin. Prominent nucleoli were also observed. Their cytoplasm contained an abundance of vesicular and tubular profiles of agranular endoplasmic reticulum, varied numbers of lipid droplets, some mitochondria, a fairly large Golgi zone and vacuoles with some of them opening to the cell surface (Figure 4e).
Figure 4.
(a–e) Ultrastructural micrographs of the non-irradiated testis featuring the presence of: (a) Type A spermatogonia resting on the tubular basement membrane (BM) with its euchromatic oval nucleus (N) and scanty cytoplasm, Sertoli cell with irregular indented nucleus (n), prominent nucleolus (nu), cytoplasm full with mitochondria (M), smooth endoplasmic reticulum (S), strand of rough endoplasmic reticulum (R), small lipid droplets (L); a tight junction appears between the cell membranes of adjacent cells above the spermatogonia (arrow) (×5000); (b) Type B spermatogonia with its rounded nucleus (N)and course clumps of chromatin. Tight junction appears between the cell membranes of adjacent Sertoli cells above the spermatogonia (arrow). Myoid cell (MY) with oval, flattened nucleus is seen beneath the BM (×5000); (c) Primary spermatocyte with normal nucleus (N). The cytoplasm contains mitochondria, smooth endoplasmic reticulum, rough endoplasmic reticulum and Gogli body (GO). Note, the tight junction between the cell membranes of adjacent Sertoli cells below the spermatocyte (×5000); (d) transverse sections of the axoneme of the mid-piece of the sperm tail of some spermatozoa (SP), parts of normal spermatids with spherical nucleus (N) and fine granular chromatin. The cytoplasm contains parts of Golgi body residing close to the acrosomal vesicle (A), mitochondria and vesicles (v) (×4000); (e) interstitial cell of Leydig with rounded nucleus and peripherally arranged dense chromatin. The cytoplasm is predominantly filled with mitochondria and smooth endoplasmic reticulum (×5000).
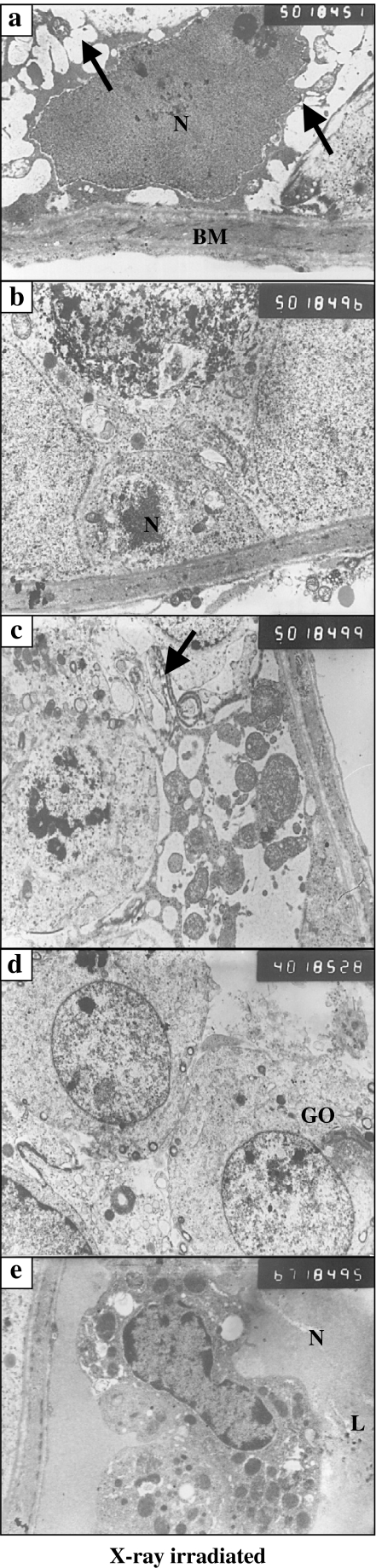
As compared with the non-irradiated testis, the spermatogenic cells of XRI testis had several features indicative of apoptosis. Type A spermatogonia had dense irregular nuclei and condensed chromatin. Their electron-dense cytoplasm was fragmented into numerous apoptotic bodies seen in vicinity of these cells (Figure 5a). Type B spermatogonia had dense heterochromatic nuclei with ill-defined nuclear membranes (Figure 3b). The primary spermatocytes had small nuclei with electron lucent cytoplasm containing less numerous ribosomes as compared with those in non-irradiated group (Figure 5c). The spermatids were small in size with rounded nuclei. Their cytoplasm showed few mitochondria, small Golgi area and almost few tubules of SER (Figure 5d). The Leydig cells showed signs of impaired function in the form of decreased numbers of SER, increased numbers of lysosomes and multiple vacuoles in their cytoplasm. Their nuclei appeared irregular and dense in comparison with the control group (Figure 5e).
Figure 5.
(a–e) Ultrastructural characteristics of X-ray-irradiated testis featuring the presence of: (a) Type A spermatogonia with irregular, large dense pyknotic nucleus (N), numerous apoptotic bodies (arrows) and the thickened basement membrane (BM) (×5000); (b) Type B spermatogonia with dense heterochromatic nucleus and ill-defined nuclear membrane (×5000); (c) primary spermatocyte with small nucleus and electron lucent cytoplasm containing few ribosomes, numerous apoptotic bodies and having an abnormal junction with widened intercellular space (arrow) (×5000); (d) two small spermatids with rounded nuclei and the cytoplasm containing few mitochondria, small Golgi area (GO). Few number of tubules of smooth endoplasmic reticulum (×4000); (e) interstitial cell of Leydig with irregular nucleus (N), mitochondria (M), lipid droplets (L) and a dilated nuclear membrane (×6700).
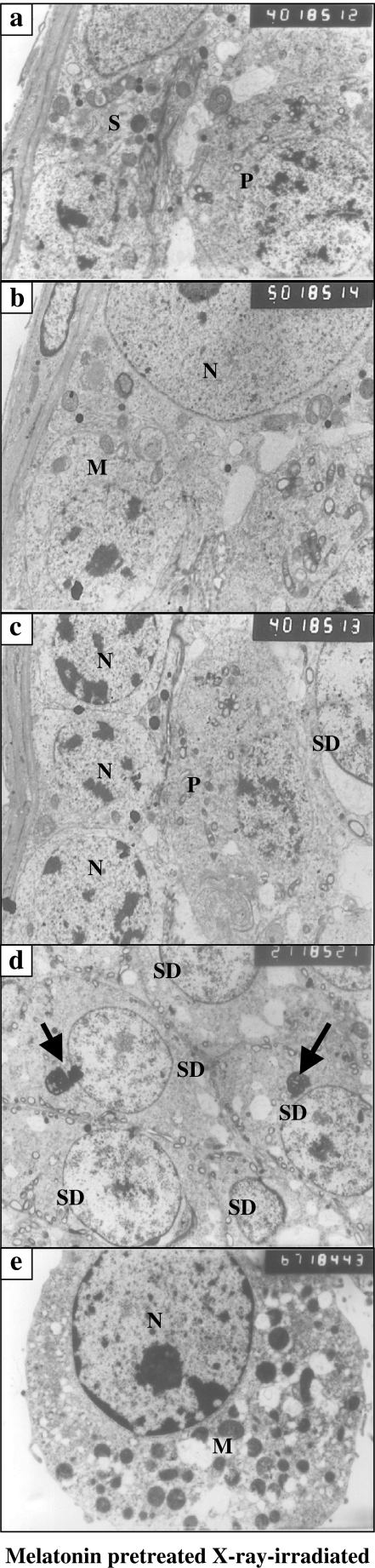
As compared with XRI testis, testis from animals pretreated with melatonin prior to XRI had features of an increased metabolic activity with absence of changes indicative of apoptosis (cell damage). Most of spermatogenic cells had relatively normal morphology. Spermatogonia type A and B had normal morphology with normal underlying basement membrane (Figure 6a,c). The primary spermatocytes had nuclei with chromatin pattern similar to that of cells in non-irradiated group (Figure 6c). The spermatids showed large acrosomal vesicle, granule, prominent Golgi body, SER and mitochondria. The appearance of nucleoli-like bodies near the nucleus was a frequent feature in the cytoplasm of the spermatids (Figure 6d), that is, feature of an increased metabolic activity in these cells. Numerous sperms (transverse sections) were frequently seen in the lumens of seminiferous tubules. Aggregates of Leydig cells were seen with a marked increase in the number of mitochondria and SER as compared with the non-irradiated group (Figure 6e).
Figure 6.
(a–e) Ultrastructural features of X-ray-irradiated testis from animals treated with melatonin featuring the presence of: (a) Type A and Type B spermatogonia within the cytoplasm of a Sertoli cell (S). The primary spermatocyte (P) has normal nucleus and cytoplasm (×4000); (b) Type B spermatogonia with rounded nucleus and cytoplasm containing multiple mitochondria (M). A part of a Sertoli cell with large nucleus (N), prominent nucleolus and a cytoplasm containing mitochondria, smooth endoplasmic reticulum, lysosomes and strands of rough endoplasmic reticulum (×5000); (c) primary spermatocyte (P) and a part from a spermatid (SD) with characteristic acrosomal vesicle. Note the presence of type B spermatogonia with rounded nuclei (N) and clumps of hetrochromatin (×4000); (d) group of spermatids (SD) with normal rounded nuclei and cytoplasm containing nucleolus-like bodies (arrows) (×2700) and (e) Leydig cell with rounded nucleus (N), prominent nucleolus and peripherally arranged dense chromatin. The cytoplasm contains numerous mitochondria and smooth endoplasmic reticulum (×6700).
Quantification of the histological and ultrastructural changes
As compared with non-irradiated testis, spermatogenic cell counts were markedly reduced in XRI testis. The spermatogenic cell counts of XRI testis in animals pretreated with melatonin was relatively similar to those in the non-irradiated group. Alternatively, both Sertoli and Leydig cell counts were mildly reduced in XRI testis. The differences between the values for XRI testis and the values for XRI testis with melatonin pretreatment were statistically significant (P < 0.05). Similarly, differences between the values for non-irradiated testis and the values for XRI testis were significant (P < 0.05). In XRI testis, features of apoptosis (apoptotic bodies, cytoplasmic vacuoles and density) were statistically significantly more frequent as compared with either non-irradiated group or XRI testis in animals pretreated with melatonin (P < 0.05). Further quantification of features of increased metabolic activity revealed that they were merely present in XRI testis from animals pretreated with melatonin (Tables 2–6).
Discussion
Although XRI-induced testicular damage was examined by previous investigations, our knowledge about radioprotective effects of melatonin against XRI-induced testicular damage (early and acute ones) is still lacking. In this investigation, we hypothesized that melatonin can minimize cell injury (early and acute ones) associated with XRI possibly through its antioxidant and DNA-reparative effects. These effects would manifest as amelioration of both germ-cell depletion and morphological features of cell damage. To test our hypothesis and to fill this existing gap in literature, we carried out this investigation. To accomplish our goals, we established an animal model consisting of non-irradiated XRI and XRI pretreated with melatonin. Our study clearly demonstrated, for the first time, that (i) XRI of testis was associated with a marked depletion of spermatogenic cells, minimal depletion of both Sertoli and Leydig cells as well as with an increased frequency of apoptotic changes and (ii) administration of melatonin was able not only to minimize these changes but also to enhance metabolic activity of the spermatogenic cells.
X-ray irradiation of testis was associated with marked depletion of germinal epithelial cells
In XRI testis, the presence of morphological features of cellular damage (apoptosis and cell depletion) agrees with previous studies (Hatier et al. 1982; Clifton & Bremner 1983; Pinon-Lataillade & Maas 1985; Pinon-Lataillade et al. 1985; Allan et al. 1988; Pineau et al. 1989; Bansal et al. 1990; Shalet 1993; Evdokimov et al. 1997; Sawada & Esaki 2003; Songthaveesin et al. 2004; Zichner & Engel 1971). These changes may be due to induction of both oxidative mechanisms and apoptotic pathways (West & Lahdetie 2001; West et al. 2002; Hussein et al. 2003; Hussein et al. 2005). We speculate that XRI can induce these apoptotic pathways by inducing expression of both proapoptotic Bcl-2 homology domains-3 only proteins and p53 protein which in turn force cells to commit apoptosis (Beumer et al. 1997; Hussein et al. 2003; Hussein 2005; Hussein et al. 2005). The marked depletion of spermatogenic cells may be due to their rapid proliferation and therefore enhanced intake of more radiants (Vachhrajani & Dutta 1992). Alternatively, the absence of sperms in seminiferous tubules of XRI testis may be reasoned to the failure of the secondary spermatocytes to complete transitions destined to sperms. The minimal damage to Sertoli cells agrees with previous reports (Giwercman et al. 1991; Guitton et al. 1999; Guitton et al. 2000) and suggests their resistance to XRI probably due to increased transferrin, IL-6 production (Guitton et al. 1999).
Administration of melatonin was able to minimize X-ray-induced testis damage
In our series, administration of melatonin was associated with both absence of X-ray-induced germ-cell depletion and amelioration of associated apoptotic changes. These observations are not only in accord with our recent findings in the skin (Hussein et al. 2005) but also support reports indicating a radioprotective role for melatonin in rapidly proliferating cells (Mornjakovic et al. 1991; Mornjakovic et al. 1998; Undeger et al. 2004). In this respect, Mornjakovic and colleagues determined the volume density of the seminiferous epithelium, lumen of tubules and testis interstitium in sham pinealectomized adult Wistar rats after melatonin treatment and whole-body irradiation with 8 Gy of gamma rays. They found that melatonin cannot only modify the quantitative characteristics of seminiferous tubules but also reduce effects originally produced by irradiation (Mornjakovic et al. 1991). Also, melatonin administration significantly can reduce the notorious effects of irradiation on Leydig cells (Mornjakovic et al. 1998). Taken collectively, these observations raise the notion that melatonin has a radioprotective effects against X-ray irradiation. Several observations support this notion. First, melatonin administration prior to irradiation prevented radiation damage on peripheral blood cells (Koc et al. 2002). Second, 6 and 8 Gy XRI of rats were associated with increased MDA, MPO, nitric oxide and decreased GSH levels (Sener et al. 2003). All these indices were reduced with melatonin pretreatment (Taysi et al. 2003). Therefore, melatonin by its free radical scavenging and antioxidant properties ameliorates irradiation-induced cell damage (Sener et al. 2003).
We propose that the ability of melatonin to ameliorate cell damage may be reasoned to its ability to easily enter not only cells but also their subcellular compartments, a feature not shared by most antioxidants (Reiter et al. 2003). Melatonin can specifically enter the nucleus where it protects DNA from oxidative damage (Ressmeyer et al. 2003). Melatonin can also improve cellular communication between normal and proliferating cells and alter the intracellular redox state (Reiter et al. 2003). Moreover, melatonin can ameliorate alterations in membrane fluidity and lipid peroxidation in microsomal membranes (Karbownik et al. 2000). Also, in view of close interactions and dependence of germinal epithelial cells and Sertoli cells, it is possible that the lack of functional impairment of Sertoli cells may result in the resumption of spermatogenesis following melatonin administration (Vachhrajani & Dutta 1992).
Melatonin administration was associated with ultrastructural features of increased metabolic activity
As compared with XRI testis, XRI testis from animals pretreated with melatonin showed features of an increased metabolic activity. These features included large acrosomal vesicle, prominent Golgi, increased mitotic activity, increased complement of cytoplasmic organelles and appearance of nucleoli-like bodies. The presence of both prominent Golgi and an increased complement of cytoplasmic organelles is suggestive of an enhanced metabolic activity following irradiation (Bessis 1985 and Ghadially et al. 1985). The presence of an increased mitotic activity in irradiated spermatogenic cells is indicative of increased DNA replication, rapid growth and increased metabolism (Love & Soriano 1971; Ghadially et al. 1985; Montironi et al. 1991; Teodori et al. 2000). Moreover, the presence of nucleoli-like bodies is indicative of increased protein synthesis and nucleocytoplasmic exchange (Beltran & Stuckey 1972). This nucleolar segregation probably reflects DNA binding and inhibition of DNA-dependent RNA synthesis (Reddy & Svoboda 1968; Zatsepina et al. 1989).
To summarize, to the best of our knowledge, this study is the first to report the histological and ultrastructural changes (both quantitative and qualitative analyses) following administration of melatonin in XRI testis. Our data suggest a radioprotective role for melatonin against XRI-induced testis damage. The presence of morphological changes indicative of apoptosis following XRI supports the detrimental effects of these rays. The underlying mechanisms of our observations as well as their possible clinical and therapeutic ramifications mandate further investigations.
References
- Allan DJ, Gobe GC, Harmon BV. Sertoli cell death by apoptosis in the immature rat testis following x-irradiation. Scanning Microsc. 1988;2:503–512. [PubMed] [Google Scholar]
- van Alphen MM, van de Kant HJ, de Rooij DG. Depletion of the spermatogonia from the seminiferous epithelium of the rhesus monkey after X irradiation. Radiat Res. 1988;113:473–486. [PubMed] [Google Scholar]
- Bain J, Keene J. Further evidence for inhibin: change in serum luteinizing hormone and follicle-stimulating hormone levels after x-irradiation of rat testis. J. Endocrinol. 1975;66:279–280. doi: 10.1677/joe.0.0660279. [DOI] [PubMed] [Google Scholar]
- Bansal MR, Kaul A, Tewari M, Nehru B. Spermatogenesis and epididymal sperm after scrotal gamma irradiation in adult rats. Reprod Toxicol. 1990;4:321–324. doi: 10.1016/0890-6238(90)90044-v. [DOI] [PubMed] [Google Scholar]
- Beltran G, Stuckey WJ. Nuclear lobulation and cytoplasmic fibrils in leukemic plasma cells. Am. J. Clin. Pathol. 1972;58:159–164. doi: 10.1093/ajcp/58.2.159. [DOI] [PubMed] [Google Scholar]
- Bessis M. Role of the cytoplasm fibrils in lobulation of the cell nucleus (formation of Rieder's cells) C R. Acad. Sci. Hebd Seances Acad. Sci. D. 1985;261:1392–1393. [PubMed] [Google Scholar]
- Beumer TL, Roepers-Gajadien HL, Gademan LS, Rutgers DH, de Rooij DG. P21 (Cip1/WAF1) expression in the mouse testis before and after X irradiation. Mol Reprod Dev. 1997;47:240–247. doi: 10.1002/(SICI)1098-2795(199707)47:3<240::AID-MRD2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Clifton DK, Bremner WJ. The effect of testicular x-irradiation on spermatogenesis in man. A comparison with the mouse. J. Androl. 1983;4:387–392. doi: 10.1002/j.1939-4640.1983.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Cordelli E, Fresegna AM, Leter G, Eleuteri P, Spano M, Villani P. Evaluation of DNA damage in different stages of mouse spermatogenesis after testicular X irradiation. Radiat Res. 2003;160:443–451. doi: 10.1667/rr3053. [DOI] [PubMed] [Google Scholar]
- Creasy DM. Evaluation of testicular toxicity in safety evaluation studies: the appropriate use of spermatogenic staging. Toxicol. Pathol. 1997;25:119–131. doi: 10.1177/019262339702500201. [DOI] [PubMed] [Google Scholar]
- Evdokimov VV, Kodentsova VM, Vrzhezinskaia OA, Iakushina LM, Erasova VI, Kirpatovskii VI, Sakharov I. [Effect of irradiation on the vitamin status and spermatogenesis in rats] Biull Eksp Biol. Med. 1997;123:524–527. [PubMed] [Google Scholar]
- Ghadially FN, Senoo A, Fuse Y. A serial section study of nuclear pockets containing nuclear material. J. Submicrosc Cytol. 1985;17:687–694. [PubMed] [Google Scholar]
- Giwercman A, von der Maase H, Berthelsen JG, Rorth M, Bertelsen A, Skakkebaek NE. Localized irradiation of testes with carcinoma in situ: effects on Leydig cell function and eradication of malignant germ cells in 20 patients. J. Clin. Endocrinol. Metab. 1991;73:596–603. doi: 10.1210/jcem-73-3-596. [DOI] [PubMed] [Google Scholar]
- Guitton N, Brouazin-Jousseaume V, Dupaix A, Jegou B, Chenal C. Radiation effect on rat Sertoli cell function in vitro and in vivo. Int. J. Radiat Biol. 1999;75:327–333. doi: 10.1080/095530099140500. [DOI] [PubMed] [Google Scholar]
- Guitton N, Touzalin AM, Sharpe RM, Cheng CY, Pinon-Lataillade G, Meritte H, Chenal C, Jegou B. Regulatory influence of germ cells on sertoli cell function in the pre-pubertal rat after acute irradiation of the testis. Int. J. Androl. 2000;23:332–339. doi: 10.1046/j.1365-2605.2000.00248.x. [DOI] [PubMed] [Google Scholar]
- Hatier R, Grignon G, Touati F. Ultrastructural study of seminiferous tubules in the rat after prenatal irradiation. Anat Embryol (Berl) 1982;165:425–435. doi: 10.1007/BF00305578. [DOI] [PubMed] [Google Scholar]
- Hickman AB, Klein DC, Dyda F. Melatonin biosynthesis: the structure of serotonin N-acetyltransferase at 2.5 A resolution suggests a catalytic mechanism. Mol Cell. 1999;3:23–32. doi: 10.1016/s1097-2765(00)80171-9. [DOI] [PubMed] [Google Scholar]
- Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update. 2005;11:162–177. doi: 10.1093/humupd/dmi001. [DOI] [PubMed] [Google Scholar]
- Hussein MR, Abu-Dief EE, Abd El-Reheem MH, Abd-Elrahman A. Ultrastructural evaluation of the radioprotective effects of melatonin against X-ray-induced skin damage in Albino rats. Int. J. Exp Pathol. 2005;86:45–55. doi: 10.1111/j.0959-9673.2005.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein MR, Hassan M, Wood GS. Morphological changes and apoptosis in radial growth phase melanoma cell lines following ultraviolet-B irradiation. Am. J. Dermatopathol. 2003;25:466–472. doi: 10.1097/00000372-200312000-00003. [DOI] [PubMed] [Google Scholar]
- Karbownik M, Reiter RJ, Qi W, Garcia JJ, Tan DX, Manchester LC Vijayalaxmi. Protective effects of melatonin against oxidation of guanine bases in DNA and decreased microsomal membrane fluidity in rat liver induced by whole body ionizing radiation. Mol Cell Biochem. 2000;211:137–144. doi: 10.1023/a:1007148530845. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BC, Shon BS, Ryoo YW, Kim SP, Lee KS. Melatonin reduces X-ray irradiation-induced oxidative damages in cultured human skin fibroblasts. J. Dermatol. Sci. 2001;26:194–200. doi: 10.1016/s0923-1811(01)00088-3. [DOI] [PubMed] [Google Scholar]
- Koc M, Buyukokuroglu ME, Taysi S. The effect of melatonin on peripheral blood cells during total body irradiation in rats. Biol. Pharm. Bull. 2002;25:656–657. doi: 10.1248/bpb.25.656. [DOI] [PubMed] [Google Scholar]
- Love R, Soriano RZ. Correlation of nucleolini with fine structural nucleolar constituents of cultured normal and neoplastic cells. Cancer Res. 1971;31:1030–1037. [PubMed] [Google Scholar]
- Montironi R, Braccischi A, Matera G, Scarpelli M, Pisani E. Quantitation of the prostatic intra-epithelial neoplasia. Analysis of the nucleolar size, number and location. Pathol Res. Pract. 1991;187:307–314. doi: 10.1016/S0344-0338(11)80789-2. [DOI] [PubMed] [Google Scholar]
- Mornjakovic Z, Alicelebic S, Bilalovic N, Susko I. [Morphometric characteristics of Leydig cells after total irradiation of rats treated with melatonin] Med. Arh. 1998;52:183–184. [PubMed] [Google Scholar]
- Mornjakovic Z, Scepovic M, Kundurovic Z. [Morphometric aspects of seminiferous tubules in rats treated with melatonin and whole body irradiation (stereologic analysis) ] Med. Arh. 1991;45:9–10. [PubMed] [Google Scholar]
- Omura M, Romero Y, Zhao M, Inoue N. Histopathological evidence that spermatogonia are the target cells of 2-bromopropane. Toxicol. Lett. 1999;104:19–26. doi: 10.1016/s0378-4274(98)00350-6. [DOI] [PubMed] [Google Scholar]
- Petersen RG. Design and Analysis of Experiments. New york: Marcil Dekker,Inc; 1985. [Google Scholar]
- Pineau C, VeleZ de la Calle JF, Pinon-Lataillade G, Jegou B. Assessment of testicular function after acute and chronic irradiation: further evidence for an influence of late spermatids on Sertoli cell function in the adult rat. Endocrinology. 1989;124:2720–2728. doi: 10.1210/endo-124-6-2720. [DOI] [PubMed] [Google Scholar]
- Pinon-Lataillade G, Maas J. Continuous gamma-irradiation of rats: dose-rate effect on loss and recovery of spermatogenesis. Strahlentherapie. 1985;161:421–426. [PubMed] [Google Scholar]
- Pinon-Lataillade G, Viguier-MartineZ MC, Maas J. Endocrinological and histological changes induced by continuous low dose gamma-irradiation of the rat testis. Acta Endocrinol. (Copenh) 1985;109:558–562. doi: 10.1530/acta.0.1090558. [DOI] [PubMed] [Google Scholar]
- Ponnapakkam TP, Sam GH, Iszard MB. Histopathological changes in the testis of the Sprague Dawley rat following orally administered manganese. Bull. Environ. Contam Toxicol. 2003;71:1151–1157. doi: 10.1007/s00128-003-8694-3. [DOI] [PubMed] [Google Scholar]
- Reddy J, Svoboda D. The relationship of nucleolar segregation to ribonucleic acid synthesis following the administration of selected hepatocarcinogens. Laboratory Invest. 1968;19:32–45. [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Mayo JC, SainZ RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003;50:1129–1146. [PubMed] [Google Scholar]
- Ressmeyer AR, Mayo JC, Zelosko V, SainZ RM, Tan DX, Poeggeler B, Antolin I, Zsizsik BK, Reiter RJ, Hardeland R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003;8:205–213. doi: 10.1179/135100003225002709. [DOI] [PubMed] [Google Scholar]
- Sawada H, Esaki M. Electron microscopic observation of 137Cs-irradiated rat testis: production of basal laminae for germ cells, despite their absence. J. Electron Microsc (Tokyo) 2003;52:391–397. doi: 10.1093/jmicro/52.4.391. [DOI] [PubMed] [Google Scholar]
- Sener G, Jahovic N, Tosun O, Atasoy BM, Yegen BC. Melatonin ameliorates ionizing radiation-induced oxidative organ damage in rats. Life Sci. 2003;74:563–572. doi: 10.1016/j.lfs.2003.05.011. [DOI] [PubMed] [Google Scholar]
- Shalet SM. Effect of irradiation treatment on gonadal function in men treated for germ cell cancer. Eur Urol. 1993;23:148–151. doi: 10.1159/000474584. discussion 152. [DOI] [PubMed] [Google Scholar]
- Simpson GG, Roe A, Lewontin RC. Quantitative Zoology. New York,Harcourt: Brace & World. Inc.; 1960. [Google Scholar]
- Songthaveesin C, Saikhun J, Kitiyanant Y, Pavasuthipaisit K. Radio-protective effect of vitamin E on spermatogenesis in mice exposed to gamma-irradiation: a flow cytometric study. Asian J. Androl. 2004;6:331–336. [PubMed] [Google Scholar]
- Taysi S, Koc M, Buyukokuroglu ME, Altinkaynak K, Sahin YN. Melatonin reduces lipid peroxidation and nitric oxide during irradiation-induced oxidative injury in the rat liver. J. Pineal Res. 2003;34:173–177. doi: 10.1034/j.1600-079x.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- Teodori L, Tagliaferri F, Stipa F, Valente MG, Coletti D, Manganelli A, Guglielmi M, D'Angelo LS, Schafer H, Gohde W. Selection, establishment and characterization of cell lines derived from a chemically-induced rat mammary heterogeneous tumor, by flow cytometry, transmission electron microscopy, and immunohistochemistry. In Vitro Cell Dev Biol. Anim. 2000;36:153–162. doi: 10.1290/1071-2690(2000)036<0153:SEACOC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Undeger U, Giray B, Zorlu AF, Oge K, Bacaran N. Protective effects of melatonin on the ionizing radiation induced DNA damage in the rat brain. Exp Toxicol. Pathol. 2004;55:379–384. doi: 10.1078/0940-2993-00332. [DOI] [PubMed] [Google Scholar]
- Vachhrajani KD, Dutta KK. Stage specific effect during one seminiferous epithelial cycle following ethylene glycol monomethyl ether exposure in rats. Indian J. Exp Biol. 1992;30:892–896. [PubMed] [Google Scholar]
- Vijayalaxmi Meltz ML, Reiter RJ, Herman TS. Melatonin and protection from genetic damage in blood and bone marrow: whole-body irradiation studies in mice. J. Pineal Res. 1999a;27:221–225. doi: 10.1111/j.1600-079x.1999.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi Seaman RL, Belt ML, Doyle JM, Mathur SP, Prihoda TJ. Frequency of micronuclei in the blood and bone marrow cells of mice exposed to ultra-wideband electromagnetic radiation. Int. J. Radiat Biol. 1999b;75:115–120. doi: 10.1080/095530099140870. [DOI] [PubMed] [Google Scholar]
- West A, Lahdetie J. X-irradiation – induced changes in the progression of type B spermatogonia and preleptotene spermatocytes. Mol Reprod Dev. 2001;58:78–87. doi: 10.1002/1098-2795(200101)58:1<78::AID-MRD11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- West A, Priante G, Lahdetie J. Stage-specific expression of Gadd45 induced by X-irradiation in rat spermatogenesis. Int. J. Radiat Biol. 2002;78:29–39. doi: 10.1080/09553000110089982. [DOI] [PubMed] [Google Scholar]
- Zatsepina OV, Voronkova LN, Sakharov VN, Chentsov YS. Ultrastructural changes in nucleoli and fibrillar centers under the effect of local ultraviolet microbeam irradiation of interphase culture cells. Exp Cell Res. 1989;181:94–104. doi: 10.1016/0014-4827(89)90185-7. [DOI] [PubMed] [Google Scholar]
- Zhao M, Omura M, Tokunaga S, Romero Y, Inoue N. Histopathological changes within the testis caused by allyl chloride exposure in mice. Bull. Environ. Contam Toxicol. 1998;60:494–501. doi: 10.1007/s001289900652. [DOI] [PubMed] [Google Scholar]
- Zichner L, Engel D. Electron microscopial examination of the ultra-sonic effect on the rabbit's synovial membrane. ZGesamte Exp Med. 1971;154:1–13. doi: 10.1007/BF02048761. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D, Melton JW, 3rd Quagliata F. Ultrastructural, immunologic, and functional studies on Sezary cells: a neoplastic variant of thymus-derived (T) lymphocytes. Proc. Natl Acad. Sci. U S A. 1974;71:1877–1881. doi: 10.1073/pnas.71.5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]