Abstract
Heat shock proteins are molecular chaperones that have an ability to protect proteins from damage induced by environmental factors such as free radicals, heat, ischaemia and toxins, allowing denatured proteins to adopt their native configuration. Heat shock protein-27 (Hsp27) is a member of the small Hsp (sHsp) family of proteins, and has a molecular weight of approximately 27 KDa. In addition to its role as a chaperone, it has also been reported to have many additional functions. These include effects on the apoptotic pathway, cell movement and embryogenesis. In this review, we have focused on its possible role in vascular disease.
Keywords: apoptosis, atherosclerosis, chaperonin, Hsp27, vascular cells
Introduction
When cells are exposed to environmental stresses of various kinds, several signalling pathways are activated. Some of these promote cell survival, whilst others initiate cell death. For example, exposure of cells to a high temperature causes an induction of a family of proteins termed heat shock proteins (HSPS) such as Hsp27, Hsp40, Hsp72 and Hsp90 that act to protect the cell. They have the ability to protect the structure and function of native macromolecules, particularly as they traffic across membranes. The upregulation in the expression of the HSPS is mediated by heat shock transcription factors (HSFs) reviewed by Christians et al. (2002), and it appears that the small HSPS confer thermo-tolerance when they are over-expressed (Mehlen et al. 1993; Iwaki et al. 1994; Lavoie et al. 1995) possibly via an effect on actin microfilament stability (Lavoie et al. 1995).
The HSPS have been divided into several categories on the basis of their molecular mass (Lindquist & Craig 1988). HSPS with low molecular masses of 15–30 kD (small HSPS) have been observed in most species. At least 10 mammalian small HSPS have been identified (Table 1), among these are Hsp27, αA- and αB-crystallin, p20 and MKBP (Hickey & Weber 1982; Ingolia & Craig 1982; Hickey et al. 1986a, b; Klemenz et al. 1991; Kato et al. 1994a; Suzuki et al. 1998; Kappe et al. 2003b).
Table 1.
Properties of members of the α-crystalline Hsp protein family
HspB11-B15 have been reported in non-mammalian species (Franck et al. 2004).
Heat shock protein-27 has a molecular weight of approximately 27 kDa, although it has been shown to form large aggregates of up to 800 kDa in the cytosol (Mehlen et al. 1997a). Hsp27 is induced during the stress response, and its expression has been shown to correlate with increased survival in cells exposed to cytotoxic stimuli (Chretien & Landry 1988; Mehlen et al. 1993; Lavoie et al. 1995). Hsp27 is found in several types of human cells, including tumour cells (Yoshida et al. 1999; Bruey et al. 2000b; Kabakov et al. 2003). It is a molecular chaperone with an ability to interact with a large number of different proteins. It has been shown to prevent cell death caused by a wide variety of toxic agents that promote apoptosis. Recent evidence has shown that Hsp27 interferes with apoptosis through its ability to interact with and inhibit key components of the apoptotic signalling pathway, including the caspase activation complex. Hsp27 plays a role in proteosome-mediated degradation of apoptosis-regulatory proteins. It may also participate in oncogenesis, as suggested by the observation that over-expression of heat shock proteins can increase the tumorigenic potential of tumour cells (Arrigo 2000; Bruey et al. 2000a; Charette et al. 2000). Hence, several mechanisms may account for the cytoprotective activity of Hsp27.
Gene structure and regulation
Analysis of the phylogeny of the small Hsp family of genes indicates that multiple α-crystallin-like HSPS were present in the last common ancestor of pro- and eukaryotes. It has been postulated that during eukaryote evolution, animal and non- animal α-HSPS originated from different ancestral gene copies, and that repeated gene duplications gave rise to the multiple alpha-HSPS present in most organisms (De Jong et al. 1998).
Ten small Hsp-like proteins have been identified from nucleotide sequence in the human genome (Kappe et al. 2003b). These comprise Hsp27/HspB1, HspB2, HspB3, alphaA-crystallin/HspB4, alphaB-crystallin/HspB5, Hsp20/HspB6, cvHsp/HspB7, H11/HspB8, HspB9 and HspB10, a sperm tail protein known as outer dense fibre protein 1 (ODF1). The HspB1-10 genes are dispersed over nine chromosomes, reflecting their ancient origin. Two of the genes (HspB3 and HspB9) are without introns, and the others have one or two introns at various positions. The transcripts of several sHsp genes, notably HspB7, display low levels of alternative splicing, which may result in the elaboration of small amounts of protein isoforms (Kappe et al. 2003b). The small HSPS are characterized by a conserved amino acid sequence of approximately 80–100 amino acids, known as the α-crystallin domain (MacRae 2000; Horwitz 2003; Augusteyn 2004). The beta sheet structure within the alpha crystallin domain is involved in dimerization, and overall there is a little alpha helical content.
The coding regions of the Hsp27 gene homologues are highly conserved across species. In mice, the coding region is interrupted by two introns of 128 bp and approximately 600 bp in length and which are found at identical positions as for the human Hsp27 gene. The 5′ flanking regions of the mouse and human genes are also very strongly conserved and contain several sequence motifs for the transcription factors, HSF and Spl (Frohli et al. 1993). In prokaryotes, the major Hsp genes are transcriptionally regulated by positively and negatively acting transcription factors. In eukaryotes, the genes encoding HSPS contain a regulatory DNA motif (inverted repeats of the pentameric sequence nGAAn) known as the heat shock element (reviewed by Ciocca et al. 1993). The first intron of the rat Hsp27 contains a consensus heat shock regulatory element (HSE), and this intronic HSE (i-HSE) is conserved among mammalian Hsp27 genes. i-HSE bind heat shock transcription factor-1 (HSF1) in a manner equivalent to that of HSE present in the Hsp27 promoter (p-HSE) (Cooper et al. 2000).
Post-translational modification
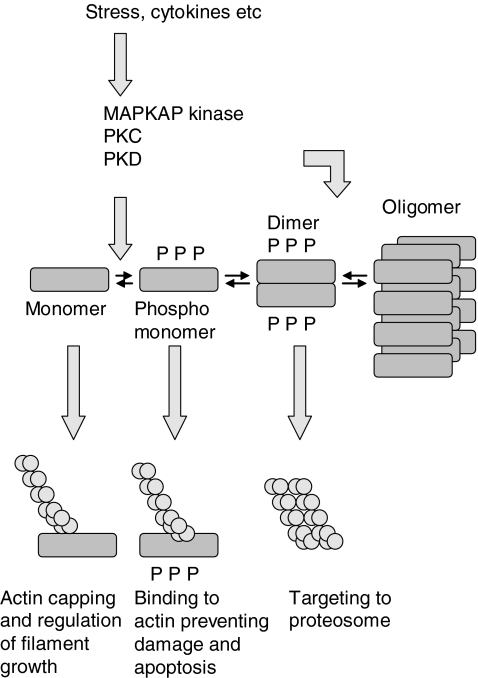
A summary of the post-translational processing of Hsp27 is shown in Figure 1 and Table 2 summarizes the factors that are known to modulate Hsp expression and function. Three forms of Hsp27 were originally identified by iso-electric focusing. The two most acidic forms were subsequently found to be phosphor–proteins representing post-translational modifications of the more basic species (Chretien & Landry 1988).
Figure 1.
Monomeric Hsp27 acts as a capping protein for actin, limiting filament growth. It is phosphorylated by several mechanisms, and the phosphorylated dimer is the active molecule in chaperone activity, being involved in the renaturation of damaged proteins, and in targeting denatured proteins to the proteasome. Dimers may also be generated by the phosphorylation of Hsp27 oligomers.
Table 2.
Modulators of Hsp27 expression and activity
| Agent | Effect | References |
|---|---|---|
| Angiotensin II | Upregulation | Chen et al. (2004) |
| Endothelin-1 | Upregulation | Kawamura et al. (1999) |
| Ethanol | Inhibits expression in response to heat shock | Iwaki et al. (1997); Fontaine et al. (2003) |
| Glutathione | Increased large aggregates | Mehlen et al. (1996a) |
| Heat shock | Upregulation | Hickey and Weber (1982); Inaguma et al. (1995) |
| Heavy metals (arsenite and cadmium) | Disassociation of aggregates | Hickey et al. (1986b); Kato et al. (2001) |
| Hydrogen peroxide | Increased phosphorylation | Gaitanaki et al. (2003) |
| IL-1α | Enhanced phosphorylation and disassociation | Kato et al. (1994b) |
| Ischaemia reperfusion | Upregulation | Li et al. (2003); Louapre et al. (2005) |
| LPS | Increased phosphorylation | Hirano et al. (2004a) |
| MG132 | Enhanced expression, phosphorylation and aggregation | Ito et al. (2002) |
| Midazolan | Inhibits vasopressin induced increase but enhances arsenic and heat-induced upregulation | Tanabe et al. (2002) |
| Okadaic acid | Enhanced phosphorylation and disassociation | Kato et al. (1994b) |
| PDGF-BB | Increased phosphorylation | Takenaka et al. (2004) |
| PMA | Enhanced phosphorylation and disassociation | Ito et al. (1997b) |
| Prostaglandins | Upregulationin rat glioma cells | Ito et al. (1997a) |
| Reactive oxygen species | Upregulation and increased phosphorylation | Mehlen et al. (1995) |
| Serum | Increased phosphorylation | Mehlen and Arrigo (1994) |
| Simvastatin | Upregulation in osteoblasts | Wang et al. (2003) |
| Sodium arsenite | Increased phosphorylation | Crete and Landry (1990); Ito et al. (1997a) |
| Sphingosine 1-phosphate | Upregulation | Kozawa et al. (1999) |
| Thrombin | Upregulation and increased phosphorylation | Hirade et al. (2002); Nakajima et al. (2005) |
| Thyroxine | Upregulation and increased phosphorylation | Pantos et al. (2003a); Pantos et al. (2003b) |
| TNFα | Phosphorylation and cellular redistribution | Mehlen et al. (1995) |
| Vasopressin | Upregulation and increased phosphorylation | Kaida et al. (1999); Akamatsu et al. (2004) |
| γ-Interferon | Downregulation | Yonekura et al. (2003) |
Human Hsp27 and mouse Hsp25 form large oligomers with molecular weights ranging from 100 to 800 kDa (Hickey et al. 1986a; Chaufour et al. 1996). Structural studies have been difficult because the oligomeric structure is highly dynamic, the high molecular weight oligomer being in equilibrium with dimers or tetramers and showing a high rate of subunit exchange. However, crystal structure analysis has revealed that the oligomers, composed of up to 24 subunits, form as a result of multiple interactions within the α-crystalline domain, stabilised in some instances by interactions with hydrophobic sequences in the amino terminal domain.
Phosphorylation of Hsp27 appears to be brought about by at least three mechanisms: via MAP kinase activated protein (MAPKAP) kinase 2/3, and protein kinases C (Kato et al. 2001) and D (Doppler et al. 2005). Phosphorylation alters the quaternary structure, and chaperone function of Hsp27. Mammalian sHSPS are rapidly phosphorylated at two or three serine residues in response to various extracellular stresses, and this has been reported to be important for their protective function. Rogalla et al. (1999) have shown that in vitro phosphorylation of recombinant sHsp causes a significant decrease in the size of oligomers. However, it has also been reported that both phosphorylated sHSPS and a mutant form of Hsp27 (Hsp27-S15D, S78D, S82D) which has a tertiary structure that mimics phosphorylated Hsp, have impaired chaperone function. Furthermore, the triple mutant was found to have an impaired ability to protect against oxidative stress when compared with the wild type molecule. Data suggest that large oligomers of sHSPS are necessary for chaperone action and resistance against oxidative stress, whereas phosphorylation downregulates these activities by dissociation of sHsp complexes to tetramers (Rogalla et al. 1999). Dissociation of the large oligomers of Hsp27 to small complexes is enhanced by incubation of cells with phorbol 12-myristate-13 acetate, interleukin-1α (IL-1α), tumour necrosis factor-α (TNFα) or okadaic acid, all of which are known to enhance or mimic the effects of phosphorylation of Hsp27 without stimulation of its synthesis. Exposure of cells to chemical stressors, namely, sodium arsenite and cadmium chloride also enhance the dissociation of the polymeric forms of Hsp27 (Hickey et al. 1986a; Kato et al. 1994b).
Heat shock protein-27 phosphorylation state, its intracellular distribution and structural organization are altered by cellular growth and differentiation. Hsp27 is dephosphorylated in starved cells and exists in the form of small structures (<200 kDa) in the soluble phase of the cytoplasm. Immediately after the addition of serum to starved cells, a rapid phosphorylation and complex changes in the intracellular distribution and structural organization of Hsp27 have been reported. Phosphorylation essentially occurs at the level of smaller Hsp27 structures (<200 kDa) and is concomitant with an increase in molecular mass (up to 700 kDa) of another intracellular pool. Serum treatment also induces the association of another fraction of dephosphorylated Hsp27, with other particulate cellular fractions. In contrast to Hsp27, another molecular chaperone, Hsp70 appears to relocalize to nucleoli during serum starvation (Hickey et al. 1986a; Mehlen & Arrigo 1994). In vascular smooth muscle cells (VSMCs) thrombin induces phosphorylation of Hsp27 at a point downstream from p38 MAP kinase via Akt, whereas the adenylyl cyclase-cAMP system is an upstream regulator of the Hsp27 phosphorylation in these cells (Nakajima et al. 2005).
The phosphorylatable serine residues appear to be key elements affecting Hsp27 structural organization and its interaction with other cyto-skeletal elements. Treatment of endothelial cells with phosphatase inhibitors reduces Hsp27 de-phosphorylation and redistribution, and is associated with damage to the actin cytoskeleton, causing morphological changes to cells following ischaemia (Hickey et al. 1986a; Loktionova & Kabakov 2001). Only the large aggregates of Hsp27 are able to confer protection against reactive oxygen species (ROS). They also modulate cellular glutathione content and protect cells against TNFα-induced injury. Using drugs that modulate the intracellular level of glutathione, Mehlen et al. (1997a) have shown that an increase in glutathione by itself is sufficient to generate large Hsp27 structures, indicating a reciprocal regulatory process.
Small changes in pH appears to have a dramatic effect on ternary structure of Hsp27 and hence its function and localization within cells (Chernik et al. 2004). Oligomer dissociation, appears to only require Ser90 phosphorylation, and the replacement of Ser90 by Ala, which prevents the dissociation of the oligomer upon stress, also causes a severe defect in the protective activity of Hsp27. Dissociation, however, is not sufficient to activate Hsp27. Replacement of Ser15 by Ala, which causes a little effect on the oligomeric organization of the protein, also yields an inactive protein. Analyses of gene mutations of Hsp27 with short deletions in the amino terminus has identified the Hsp27-PF-rich domain as essential for cell protection, maintenance of the oligomeric structure, and in vitro chaperone activity of the protein. In the light of a three-dimensional model of Hsp27 based on the crystallographic structure of wheat Hsp16.9, Theriault et al. (2004) have proposed that the conserved WD/EPF motif of mammalian Hsp27 mediates important intra-molecular interactions with hydrophic surfaces of the alpha-crystallin domain of Hsp27. These interactions are destabilized by Ser90 phosphorylation, making the motif free to interact with heterologous molecular targets upon the additional phosphorylation of the nearby Ser15. Non-phosphorylated Hsp27 forms larger oligomeric complexes than the phosphorylated Hsp27. Interestingly, phosphorylation of Hsp27 seems not to play a role in its ability to protect adult rat cardiomyocytes against ischaemic damage (Martin et al. 1999).
Tissue and development related expression of Hsp27
In adults, Hsp27 is expressed at high levels in several normal tissues including breast, uterus, cervix, placenta, skin, lung, heart and platelets (Ciocca et al. 1993).
It is also expressed by various human tumour cells, including those derived from breast and prostate, and in some cases its expression has been reported to predict clinical outcome (Detoro & Luque 1997; Devaja et al. 1997; Garrido et al. 1999; Jaattela 1999; Soldes et al. 1999; Bruey et al. 2000a; Cornford et al. 2000).
The expression of Hsp27 and MKBP changes rapidly in the rat heart following birth, with a peak at 1 week after birth, and a subsequent rapid decline by 13 weeks postnatally. Human myocardium shows a similar age-dependency (Abu Shama et al. 1999).
In man, it has been reported that Hsp27 is expressed by cells of the developing jaw and kidney (Khan et al. 1996; Leonardi et al. 2004). In the neonatal piglets high levels of expression of Hsp27 were observed in heart, liver and lung. Hsp27 protein expression was observed in all the regions of brain studied (David et al. 2000). Work by Mehlen et al. (1997b) has indicated that Hsp27 may be involved in determining whether cells are directed to apoptosis, or to differentiation. They propose that this process may be modulated by the cellular glutathione content (Mehlen et al. 1997b). This may be important in embryogenesis as shown by Leonardi et al. (2004) who have suggested that the spatiotemporal-restricted expression of Hsp27 in craniofacial bones during development may be involved in the balance between differentiation and apoptosis, by modulating the viability of osteoblasts and chondrocytes.
Functions of Hsp27
Physiological stress may be induced by several processes (e.g. heat shock, ischaemia and haemodynamics) and induces many changes in a cell that affect metabolic processes and cellular structures. The ability of Hsp27 to facilitate recovery of protein synthesis and RNA synthesis following exposure to heat shock may provide the cell with a survival advantage (Carper et al. 1997). The elaboration of stress proteins is thought to be cyto-protective through their role as molecular chaperones. HSPS70, HSPS90, HSPS47, HSPS32 and HSPS27, in particular, appear to play an important role following cardiac ischaemia, ischaemic preconditioning, cardiac hypertrophy and vascular wall injury.
It has been proposed that the functions of Hsp27 include
chaperonin activity, facilitating the refolding of partially denatured proteins into active conformations (Ciocca et al. 1993; Jakob et al. 1993; Rogalla et al. 1999);
F-actin modulation, inhibiting F-actin polymerization (Lavoie et al. 1993; Guay et al. 1997);
protection against-apoptosis, acting by interfering with the cell death pathway (Garrido et al. 1999; Jaattela 1999; Bruey et al. 2000a);
involvement in the presentation of oxidized proteins to the proteosome degradation machinery (Arrigo 2001).
Hsp as a molecular chaperone
Chaperonins, including the small HSPS, are oligomeric proteins that assist in the folding of nascent or denatured proteins (Ciocca et al. 1993; Jakob et al. 1993; Carver et al. 2003). The chaperonins are expressed following heat shock (Hickey & Weber 1982; Chowdary et al. 2004), and their role in thermo-protection suggest that they may have a function in the formation or maintenance of the native conformation of cytosolic proteins (Mehlen et al. 1993; Lavoie et al. 1995). They prevent the aggregation, denaturation and precipitation of target proteins under stress by affecting the slow, off-folding protein pathway. It has been suggested that subunit exchange may be important in regulating chaperone activity; the dissociated form of the protein is probably the chaperone-active species rather than the aggregated species. Chaperone activity does not require the hydrolysis of ATP. Increased expression of sHSPS accompanies a range of diseases that arise from protein mis-folding and deposition of highly structured protein aggregates known as amyloid fibrils, e.g. Alzheimer's, Creutzfeldt–Jakob and Parkinson's diseases. There is an interaction between sHSPS and clusterin with fibril-forming species that prevent fibril formation (Carver et al. 2003).
From analysis of their crystal structures (Kim et al. 1998) and the effects of mutations of human Hsp genes (Irobi et al. 2004; Carra et al. 2005), it appears that the small HSPS normally interact with intermediate filaments, and these appear to be one of the key targets of their chaperone activity (Van Den Ijssel et al. 1999).
A growing number of intracellular signalling molecules, including various kinases, receptors and transcription factors, have also been constitutively associated with chaperone molecules (Rutherford & Zuker 1994). On the basis of these observations, the regulated folding or assembly of signalling molecules by chaperone molecules is beginning to be recognized as a general mechanism by which signal transduction pathways are modulated. Hsp27 was recently shown to bind and activate PKB/Akt/RAC-protein kinase in response to heat or oxidative stress (Konishi et al. 1997).
MG-132, an inhibitor of the proteasome pathway, caused an accumulation of Hsp27 and α-B-crystallin in both soluble and insoluble fractions in U373 MG. Enhanced expression of mRNAs for Hsp27 and α-B-crystallin was observed, suggesting transcriptional activation. Phosphorylation of Hsp27 and α-B-crystallin in cells treated with MG-132 was enhanced concomitantly with activation of p38 and p44/42 MAP kinase pathways. Immunofluorescence analysis has revealed that cells exposured to proteasome inhibitors develop aggresomes, to which Hsp27 and α-B-crystallin were recruited. However, phosphorylation was not required for this.
F-actin modulating activity and cell movement
The Hsp27 is homologous to alpha-crystallin and has strong sequence similarity with an in vitro inhibitor of actin polymerization. It appears to confer stress resistance, at least in part, by stabilizing the actin cytoskeleton (Iwaki et al. 1994; Lavoie et al. 1995). Hsp27 may regulate actin dynamics and cross-bridge function in response to activation of the p21-activated kinase and the p38 mitogen-activated protein kinase (MAPK) signalling pathway by signalling events linked to integrin proteins (Gerthoffer & Gunst 2001).
Heat shock protein-27, B-crystallin and p20 are highly expressed in muscle cells and together form large oligomeric complexes (Kato et al. 1994b). Hsp27 phosphorylation regulates actin microfilament dynamics, fluid phase pinocytosis and cell migration in human endothelial cells (Rousseau et al. 1997). The Hsp27 gene products directly affect lamellipodial microfilament polymerization, which in turn affects lamellipodia formation and morphology, and thus cell motility. The phosphorylation of Hsp27 additionally contributes to the regulation of microfilament dynamics following oxidative stress and may be involved in mediating an adaptive response to oxyradical-generating agents such as carcinogens, anticancer drugs and other xenobiotics (Huot et al. 1996).
The expression of a mutant form of Hsp27 has been found to result in aberrant lamellipodial microfilament structures, and an inhibition in the generation of microfilament-dependent cellular extensions. Immunofluorescence microscopy has shown that a pool of Hsp27 localizes to the leading edge of the lamellipodia of migrating cells, suggesting that wtHSP27 and muHSP27 have the potential to directly affect microfilament dynamics within the lamellipodia at these sites. Hsp27 homologues have been shown to be barbed-end capping proteins and have the potential to affect F-actin generation associated with lamella extension and cell motility. The localization of Hsp27 to the leading edge of the lamellipodia is consistent with the fact that lamellipodia microfilaments are orientated with the barbed ends at this edge. F-actin generation within the lamellipodia is crucial to cell migration; likely Hsp27 expression affects cell migration at this step. If Hsp27 activity is regulated by phosphorylation in vivo as it is in vitro, phosphorylation of Hsp27 within lamellipodia would be expected to release the inhibition of one factor controlling microfilament elongation. Studies have shown that Hsp27 phosphorylation does not result in translocation of membrane-associated Hsp27. Thus, enhanced expression of Hsp27 should result in an increase in the pool of phospho-Hsp27 and hence increase the potential for lamellipodia F-actin polymerization.
Vascular endothelial cell growth factor (VEGF) is a potent chemokine for endothelial cells that acts via the activation of p38 of the MAPK cascade. p38 activates MAPKAPK2 which is responsible for Hsp27 phosphorylation, and inhibition of p38 was found to abrogate the VEGF induction of stress fibres, the phosphorylation of Hsp27 and cell migration. In response to a contractile agonist, Hsp27 undergoes a rapid phosphorylation that may strengthen its interaction with tropomyosin in smooth muscle cells (Somara & Bitar 2004).
Agonist-induced phosphorylation of Hsp27 modulates actin–myosin interaction through thin-filament regulation of tropomyosin (Bitar 2002). Adenovirus-mediated expression of activated mutant MAPK kinase 6b(E), an upstream activator for p38 (MAPK), increased cell migration, whereas over-expression of a p38 alpha MAPK dominant negative mutant and an HSP27 phosphorylation mutant blocked cell migration completely. This indicates that activation of the p38 (MAPK) pathway by growth factors and pro-inflammatory cytokines regulates smooth muscle cell migration and may contribute to pathological states involving smooth muscle dysfunction (Hedges et al. 1999).
Proteomic analysis has shown that cofilin and Hsp27 may be involved in the inhibition of endothelial cell proliferation and migration, caused by endostatin and thrombospondin-1 (Keezer et al. 2003). Cells treated with these inhibitors had a more extensive network of actin stress fibres and more numerous focal adhesion plaques compared with untreated cells. This more adherent phenotype may explain their ability to block endothelial migratory signals (Keezer et al. 2003).
Non-phosphorylatable mutations of human Hsp27 have no effect on heat shock-induced change in solubility and cellular localization of the protein, indicating that phosphorylation is not involved in these processes (Lavoie et al. 1995). However, induction of Hsp27 phosphorylation by stressing agents or mitogens causes a reduction in the multimeric size of the wild-type protein, an effect which was not observed with the mutant protein. Hence, Lavoie et al. (1995) propose that early during stress, phosphorylation-induced conformational changes in the Hsp27 oligomers regulate the activity of the protein at the level of microfilament dynamics, resulting in both enhanced stability and accelerated recovery of the filaments. The level of protection provided by Hsp27 during heat shock may thus represent the contribution of better maintenance of actin filament integrity to overall cell survival.
The Hsp27 is associated with several intermediate filament networks. Heat shock treatment induces a collapse of intermediate filaments and associated with small heat shock proteins. The presence of Hsp27 does not prevent filament collapse. However, intermediate filaments form a gel in the absence of small heat shock proteins, whereas in the presence of Hsp27 gel formation is prevented, indicating that one of the major functions of the association of small heat shock proteins with intermediate filaments is to help manage the interactions that occur between filaments in their cellular networks. Intermediate filaments are protected against non-covalent interactions that may occur when they come into very close proximity, and which have the potential to induce filament aggregation that is seen in some disease processes (Perng et al. 1999).
Over-expression of wild type Hsp27 enhances the migration of bovine endothelial cells compared with control cell transfectants, whereas expression of a mutant Hsp27 retarded migration. Homologues of the small heat shock protein inhibit F-actin polymerization in vitro and may affect F-actin distribution in vivo. It has been proposed that Hsp27 affects microfilament extension essential for cell motility. Expression of the wild type protein promotes the generation of long cellular extensions, whereas expression of the dominant negative mutant protein resulted in a marked reduction of lamellipodia and generated aberrant microfilament morphology. Phalloidin staining demonstrated the co-localization of the Hsp27 gene products with lamellipodial microfilament structures. These data suggest that Hsp27 regulates migration by affecting the generation of lamellipodia microfilaments (Piotrowicz et al. 1998).
The Hsp27 regulates fibroblast adhesion, elongation and migration (Hirano et al. 2004b). Hsp27 also confers resistance to TNFα-independent (probably free radical-mediated) lysis by monocytes. Moreover, Hsp27 may provide monocytes with a protective mechanism against their own toxicity (Jaattela & Wissing 1993).
Anti-apoptotic properties
The Hsp27 is an ATP-independent chaperone that can interfere with an apoptotic signal transduction at several steps (Figure 2). It is implicated in preserving mitochondrial integrity and reducing cytochrome c release; it can bind directly to cytochrome c, thus preventing activation of procaspase-9; and it can bind to procaspase-3 and inhibit its activation. Furthermore, Hsp27 can interact with Daxx, a component of Fas-induced apoptotic pathway, and this interaction was shown to be important for protection from apoptosis. However, which of these activities of Hsp27 are essential for protection against heat shock-induced cell death is yet to be clarified. Data suggest that the regulation of apoptosis by HSPS may not depend on its ability to cause the renaturation of protein. HSPS may be more directly involved by a role in the regulation of signalling pathways.
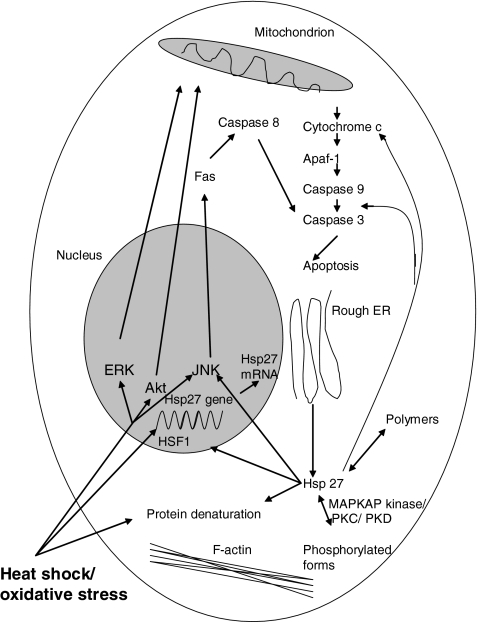
Figure 2.
Effects of heat shock on apoptosis related pathways. Heat shock stimulates apopotosis via several pathways. It also induces Hsp27 expression, and its phosphorylation. Hsp27 in turn acts to offset the potential damage induced by heat shock in part through inhibition of apoptosis. It does so at several steps in the apoptotic pathway.
In many cell types, although moderate heat shock does not cause apoptosis, cells die after several divisions and are unable to form colonies; this is known as clonogenic or reproductive cell death. Although reproductive cell death and apoptosis have distinct morphological characteristics and different mechanisms, the initial signalling events in these two types of cell death may be very similar.
Elevated temperatures and other stresses can trigger death-signalling pathways via activation of the stress kinase, c-Jun NH2-terminal kinase (JNK) and survival pathways via activation of Akt and extracellular signal-regulated kinases (ERK). The extent of activation of these pathways may determine the fate of cells exposed to heat shock. Inhibition of JNK has been shown to suppress heat-induced apoptosis of lymphoid cells and fibroblasts, whereas inhibition of Akt or ERK strongly increased apoptosis. A key process in the heat-activated apoptotic cascade is efflux from mitochondria of cytochrome c, which activates a cascade of caspases, including caspase-9 and caspase-3 leading to apoptosis (Figure 2).
c-Jun NH2-terminal kinase, Akt and ERK kinases can also regulate caspase-independent cell death pathways. When exposed to severe heat shock, human fibroblasts have been shown to undergo a death that is morphologically indistinguishable from apoptosis but is independent of caspases. However, these fibroblasts could survive such heat shock if JNK was inhibited. In contrast, if ERK or Akt kinases were inhibited, much milder heat shock that was not lethal to control cells, killed almost 100% of the treated cell population via a caspase-independent apoptosis. Furthermore, heat-induced clonogenic cell death was also reduced when JNK activity was inhibited and increased when the Akt pathway was blocked. Although the mechanisms of caspase-independent apoptosis and clonogenic cell death are still obscure, it seems that JNK, Akt and ERK cascades play major roles in regulation of these modes of death, as with caspase-dependent apoptosis. These data indicate that it was not heat shock-induced protein damage itself but rather activation of a death program (JNK) that evokes cell death, whereas activation of survival pathways (ERK and Akt) interfere with the death programme.
Small Hsp are expressed during the transition from cell division to differentiation and this process is likely to prevent the differentiating cells from undergoing apoptosis. The sHSPS are also known to interfere with programmed cell death induced by TNFα and Fas ligand (Arrigo 2000). Phosphorylated dimers of Hsp27 interact with Daxx, a mediator of Fas-induced apoptosis, preventing the interaction of Daxx with both Ask1 and Fas and blocking Daw-mediated apoptosis. This was not observed with an Hsp27 phosphorylation mutant that is only expressed as oligomers or when apoptosis was induced by transfection of a Daxx mutant lacking its Hsp27 binding domain. Hsp27 expression had no effect on Fas-induced FADD- and caspase-dependent apoptosis. However, Hsp27 blocked Fas-induced translocation of Daxx from the nucleus to the cytoplasm and Fas-induced Daxx- and Ask1-dependent apoptosis. These observations revealed a new level of regulation of the Fas pathway and suggest a mechanism for the phosphorylation-dependent protective function of Hsp27 during cellular stress and differentiation (Charette et al. 2000).
Tumour necrosis factor activates NF-κB, which does not drive any anti-apoptotic response, and p38, which plays an anti-apoptotic function probably through Hsp27 phosphorylation. Moreover, PKC and PI3K are involved in the control of survival pathways (Clermont et al. 2003).
Over-expression of Hsp27 prevents procaspase-9 activation (Garrido et al. 1999), and this is protective in the early stage of post-ischaemic reperfusion (Kabakov et al. 2003). Hsp27 inhibits cytochrome-c-mediated activation of caspases in the cytosol, However Hsp27 does not interfere with granzyme-B-induced activation of caspases, nor with apoptosis-inducing factor-mediated, caspase-independent, nuclear changes. Hsp27 binds to cytochrome c released from the mitochondria and prevents cytochrome-c-mediated interaction of Apaf-1 with procaspase-9. Thus, Hsp27 interferes specifically with the mitochondrial pathway of caspase-dependent cell death (Bruey et al. 2000a). Large oligomers of Hsp27 are the required for its antiapoptotic activity and cell–cell contacts induce the formation of large oligomers, whatever the status of phosphorylatable serines (Bruey et al. 2000b). Constitutive expression of human Hsp27 in murine L929 cells blocks Fas/APO-1-mediated cell death. Expression of human Hsp27 prevented anti-APO-1-induced DNA fragmentation and morphological changes. These results strongly suggest that Hsp27 acts as a cellular inhibitor of Fas/APO-1-induced apoptosis (Mehlen et al. 1996b).
Heat shock protein-27 over-expression in various cell types enhances the degradation of ubiquitinated proteins by the 26S proteasome in response to stressful stimuli, such as etoposide or TNFα. Hsp27 binds to polyubiquitin chains and to the 26S proteasome in vitro and in vivo. The ubiquitin-proteasome pathway is involved in the activation of transcription factor NF-κB by degrading its main inhibitor, IκB. Hsp27 over-expression increases NF-κB nuclear translocation, DNA binding and transcriptional activity induced by etoposide, TNFα and IL-1β. Hsp27 does not affect IκBα phosphorylation but enhances the degradation of phosphorylated IκBα by the proteasome. The interaction of Hsp27 with the 26S proteasome is required to activate the proteasome and the degradation of phosphorylated IκBα. A protein complex that includes Hsp27, phosphorylated IκBα, and the 26S proteasome is formed. On the basis of these observations, it has been propose that Hsp27, under stress conditions, favours the degradation of ubiquitinated proteins, such as phosphorylated IκBα. This function of Hsp27 may account for its anti-apoptotic properties through the enhancement of NF-κB activity (Parcellier et al. 2003).
Thermo-tolerance
Over-expression of Hsp27 generates heat-resistant variants of Chinese hamster. In these heat-resistant variants, the increased content of Hsp27 was correlated with a twofold increase in the constitutive level of the mRNA encoding Hsp27. Both the content and phosphorylation status of Hsp27 appear to determine the ability of cells to survive hyperthermic treatments (Chretien & Landry 1988). Arsenite, cycloheximide, A23187 and EGTA induce Hsp27 phosphorylation and thermoresistance in Chinese hamster cells (Crete & Landry 1990). A Hsp27 binding protein, Hic-5 (also known as ARA55) has recently been found. Hic-5 is also a focal adhesion protein and steroid receptor co-activator. The interaction between Hsp27 and Hic-5 was confirmed by co-immunoprecipitation, and critical protein–protein interaction regions were mapped to the Hic-5 LIM domains and the Hsp27 C-terminal domain. Initial analysis of the functional role of the Hsp27–Hic-5 interaction revealed that Hic-5 significantly inhibited the protection against heat-induced cell death conferred by Hsp27 over-expression (Jia et al. 2001).
Resistance to oxidative stress
The expression of sHSPS is associated with a decrease in the intracellular content of iron and hence reduced ability to generate hydroxyl radicals and oxidized proteins. In addition, it has been proposed that Hsp27 may have a role in the presentation of oxidized proteins to the proteasome degradation machinery. An analysis of several Hsp27 mutants suggests that the C-terminal part of Hsp27 is essential for its protective activity against oxidative stress (Arrigo et al. 2005). Stable transformants of an immortalized human fibroblast cell line were isolated by transfection of Hsp27 expression vectors. Surprisingly, clones expressing high levels of Hsp27 were reported to be more sensitive to growth inhibition by a low dose of hydrogen peroxide (0.1 mm) than those expressing low levels. Clones expressing high levels of Hsp27 did not acquire obvious resistance to high temperatures and cytotoxic agents, except for one clone, that was resistant to cytotoxic agents. Over-expression of a non-phosphorylatable mutant Hsp27 did not affect sensitivity to oxidative stress. These results suggested that a high constitutively expression of Hsp27 in this particular cell line make them more susceptible to oxidative stress resulting in growth arrest, and this may be due to an effect on Hsp27 phosphorylation (Arata et al. 1995). Inhibition of stress-induced Hsp27 dephosphorylation protects cells from ischaemia-induced damage (Loktionova & Kabakov 1998, 2001).
The Hsp27 stress protection requires neither its translocation into the nucleus nor the dissociation of its multimeric complex (Borrelli et al. 2002). Phosphorylation of Hsp27 is related to the regulation of microfilament dynamics following oxidative stress and may be involved in mediating an adaptive response to oxyradical-generating agents such as carcinogens, anticancer drugs and other xenobiotics (Huot et al. 1996).
Agents that lower cellular levels of glutathione increase the levels of mRNAs for Hsp27, αB crystallin and Hsp70 in response to stress (Ito et al. 1998).
The presence of the sHsp decrease the intracellular level of ROS, and also abolish the burst of intracellular ROS induced by TNFα (Mehlen et al. 1996a). Several downstream effects resulting from the TNFα-mediated ROS increment, such as NF-κ B activation, lipid peroxidation and protein oxidation, are also inhibited by sHsp expression. The expression of these sHsp is associated with a rise in the total cellular glutathione levels. This was essential for the sHsp-mediated decrease in ROS and resistance against TNFα.
Role in intracellular signalling
There is an interplay between the heat shock-induced HSPS and the heat shock-activated signalling pathways. The latter can modulate expression and chaperone function of HSPS, whereas HSPS can modulate signalling events. The main mechanism of induction of HSPS is through HSF1-mediated activation of transcription of HSPS genes. Denatured cell proteins, accumulate as a consequence of heat shock, interact with Hsp70 and Hsp90 releasing them from their complex with HSF1, thus activating the latter. The activity of HSF1 is also regulated by reversible phosphorylation at multiple sites. Many kinases are involved in this process, including heat-activated Akt, ERK and JNK kinases. For example, heat-induced activation of Akt was found to increase HSF1 activity, possibly through inhibition of glycogen synthase kinase-3β, a negative regulator of HSF1. On the other hand, heat shock-induced activation of ERK and JNK decreased HSF1 activity through phosphorylation at distinct sites. Therefore, it seems that regulation of Hsp transcription by stress-activated signalling pathways may be an important factor affecting the balance between cell death and survival pathways. Besides transcriptional regulation of HSF1, heat-activated kinases may be involved in post-transcriptional modulation of chaperone activity and, in phosphorylation of Hsp27. Hsp27 can be phosphorylated at serine residues by MAPKAP-2/3 kinase, a distal component of the p38 kinase pathway. Along with JNK and ERK, p38 kinase is a member of the mitogen-activated protein stress kinase (MAPK) family, which is strongly activated by heat shock and some other stresses. It seems that phosphorylation of Hsp27 is important for survival of cells after heat shock. Indeed, if over-expression of Hsp27 can confer thermo-resistance, over-expression of a mutant Hsp27 lacking phosphorylation sites did not protect cells from heat shock. Therefore, both transcription of HSPS and their activity appear to be regulated by stress-activated signalling pathways. HSPS, in their turn, can modulate cell signalling, providing a feedback loop. Hsp27 may confer protection against heat-induced killing when over-expressed, although this has not been found universally.
Chevalier et al. (2000) investigated the activation of the MAPK pathways in cultured porcine aortic VSMCs induced by stimulation with endothelin-1 (ET-1), phorbol 12-myristate 13-acetate (PMA), hydrogen peroxide or sodium arsenite. Multiple peaks of activity were revealed, phosphorylating ERK1, c-Jun and Hsp27.
Anti-inflammatory properties of Hsp27
Heat shock treatment was found to protect against Angiotensin II (Ang II)-induced hypertension and inflammation in rat aorta. This protection may be related to the interaction of HSPS with the NF-κB pathway (Chen et al. 2004). Heat shock treatment was found to block Ang II induced expression of IL-6 and intercellular adhesion molecule-1 (ICAM-1) in the heart and hence may play an important role in protecting the heart against Ang II-induced inflammation (Chen et al. 2004).
The Hsp27 is a potent activator of human monocyte IL-10 production, but only a modest inducer of TNFα. Although exogenous Hsp27 stimulation activated all three monocyte MAPK pathways [extracellular signal-regulated kinase (ERK) 1/2, c-Jun N-terminal kinase, and p38], only p38 activation was sustained and required for Hsp27 induction of monocyte IL-10, while both ERK 1/2 and p38 activation were required for induction of TNFα. Hsp27 induces IL-10 via activation of p38 signalling independently of TNFα activation and may be predominantly an anti-inflammatory monokine stimulus (De et al. 2000).
Experimental studies in vitro
Endothelial cells
Kabakov et al. (2003) have reported that transfection of human umbilical vein endothelial cells (HUVECs) with a vector promoting high expression of Hsp27 was cyto-protective when introduced before exposure to hypoxia but was also protective up to 2 h afterwards. The increase in Hsp27 levels in transfected cells correlated well with their resistance to apoptosis under reoxygenation. Hsp27 phosphorylation is also increased in cultured rat pulmonary arterial endothelial cells following treatment with TNFα, lipopolysaccharide (LPS), or hydrogen peroxide (Hirano et al. 2004a). Pretreatment with anti-TNFα antibody, which has been shown to reduce lung injury, blocked increases in Hsp27 phosphorylation at 3 h. This phosphorylation was also blocked by pretreatment with SB203580, an inhibitor of the upstream kinase, p38 MAP kinase.
In cultured aortic and saphenous vein human endothelial cells, heat shock and ATP-depletion led to the translocation of Hsp27 into the Triton X-100-insoluble cellular fraction. The staining pattern for Hsp27 was reported to be distinctive for each stress employed. Heating caused an association of Hsp27 with thick bundles of actin microfilaments (stress fibres), ATP depletion rapidly resulted in the appearance of Hsp27-containing compact granules in the nucleus. The changes in Hsp27 disposition were reversible in both cases. The stress-induced changes in Hsp27 isoform distribution indicate an increase in phosphorylation of Hsp27 in heat-shocked cells and its dephosphorylation in ATP-depleted cells. It has been suggested that these stresses affect the phosphorylation status of endothelial Hsp27, thus altering its localization, supra-molecular organization and functional activity toward actin (Loktionova et al. 1996). The cyto-skeletal and morphological changes resulting from lack of ATP were suppressed in heat-preconditioned (thermo-tolerant) cultures, this effect being sensitive to quercetin, a blocker of Hsp induction. The longer preservation of the cytosolic pool of phosphorylated Hsp27 during ATP depletion in the protein phosphatase inhibitor-treated or thermo-tolerant endothelial cells correlated with the acquired resistance of F-actin and morphology. These data suggest that protein phosphatase inhibitors as well as heat-inducible Hsp(s) can protect ischaemia-stressed cells by preventing the ATP loss-provoked protein dephosphorylation and breakdown of the actin cytoskeleton (Loktionova & Kabakov 1998).
Smooth muscle cells
The expression and phosphorylation of several α-crystallins, including Hsp27, is high in cultured, asynchronously growing arterial SMCs (Negre-Aminou et al. 2002). Oxidative stress induced by hydrogen peroxide caused membrane translocation of Rac(1), p38 phosphorylation and phosphorylation of Hsp27. In these cells, simvastatin, an HMG-CoA reductase inhibitor, blocked oxidative stress-induced membrane translocation of Rac(1) in a mevalonate-dependent way. Statin pretreatment before oxidative stress increased the levels of p38 phosphorylation, Hsp27 membrane translocation/phosphorylation, actin polymerization and apoptosis in these cells, in a mevalonate-dependent way. These results indicate that statin pretreatment has a stimulatory effect on the stress-activated p38/HSP27 pathway, despite its blocking effect on Rac(1) activation (Negre-Aminou et al. 2002).
Thrombin induces the phosphorylation of Hsp27 and an increase in the level of Hsp27 mRNA. Actinomycin D suppressed the thrombin-induced increase in mRNA level. Thrombin also stimulates the dissociation of the aggregated Hsp27 and an induction of Hsp27 via MAPK activation in aortic smooth muscle cells (Hirade et al. 2002).
Vasopressin (AVP) stimulates the induction of Hsp27 and the dissociation of the aggregated forms of Hsp27 through protein kinase C activation in aortic smooth muscle A10 cells (Kaida et al. 1999). In the A10 aortic smooth muscle cell line, AVP was found to markedly stimulate the phosphorylation of Hsp27 at Ser15 and Ser85. This was attenuated by SB203580 and PD169316, inhibitors of p38 MAPK, but not by PD98059, an MEK (mitogen-activated protein extracellular signal-related kinase) inhibitor. These results strongly suggest that AVP phosphorylates Hsp27 via MAPK in these cells (Akamatsu et al. 2004). Midazolam, an intravenous anaesthetic, has dual effects on the Hsp27 induction stimulated by various stresses in VSMCs. It was found to inhibit the accumulation of Hsp27 and level of its mRNA induced by vasopressin but enhanced the Hsp27-accumulation induced by heat or arsenite. Midazolam also enhances the heat-induced level of the mRNA for Hsp27 but no effect on the dissociation of the aggregated form of Hsp27 following stimulation by vasopressin, heat, or arsenite.
Cardiac myocytes
Several studies demonstrated that over-expression of Hsp27 protects cardiac myocyte against ischaemic injury (Bluhm et al. 1998; Yamboliev et al. 2000; Vander Heide 2002).
Studies in experimental animal models
When rats were immersed in a water bath at 42 °C for 20 min, the levels of Hsp27 in most tissues, including central nervous system, liver, lung, spleen, adrenal glands and hypophysis had increased dramatically by 8–16 h after the treatment. The increase in the levels of Hsp27 in response to heat stress was markedly inhibited when ethanol or an α1-adrenergic antagonist, prazosin, was administered before, but not after, the stress period. The expression of mRNA for Hsp27 was suppressed in the livers of rats that had received ethanol or prazosin. A β-adrenergic antagonist, propranolol and α2-adrenergic antagonist, yohimbine, did not inhibit induction of the synthesis of the two proteins (Inaguma et al. 1995).
Gaitanaki et al. (2003) investigated the activation of ERK, JNKs and p38-MAPK caused by oxidative stress in the isolated perfused amphibian heart. Stimulation of p38-MAPK and the consequent phosphorylation of Hsp27 may be important in cardioprotection under such conditions.
There is evidence that the cardio-protective effects of HSPS may be modified by ageing. Honma et al. (2003) showed that although a combination of heat shock and hypoxic preconditioning enhanced the translocation of PKC-delta in reperfused rat hearts from young animals, resulting in further improvement in functional recovery, this was not observed in older rats. Hypoxia was associated with a translocation of PKCδ from the membrane to the cytosolic fraction, but did not improve functional recovery. A combination of heat shock with hypoxia induced HSPS and the translocation of PKCδ from the cytosol to the nuclear fraction but did not protect function (Honma et al. 2003).
Pharmacological inducers of HSPS may have a therapeutic role to play in cardioprotection. Intimal hyperplasia is a major cause of re-stenosis postvascular intervention. Induction of HSPS by thermal preconditioning, reduces intimal hyperplasia. Connolly et al. (2003) showed herbimycin A, a pharmacological Hsp inducer, significantly attenuates intimal hyperplasia in the rat carotid balloon injury model. Hsp27 may be the Hsp involved in mediating this response. Treatment with thyroxine appears to increase the speed of response to ischaemia in the myocardium, and Hsp27 is thought to play an important role in this (Pantos et al. 2003a, b).
Sepsis may be associated with pulmonary oedema due to the formation of gaps between endothelial cells. This is followed by macrophage infiltration. Endothelial gap formation has been proposed to involve changes in the structure of the actin filament cytoskeleton. Hsp27 is believed to modulate actin filament dynamics or structure, in a manner dependent on its phosphorylation status. Hsp27 may play a role in endothelial gap formation, by affecting actin dependent on events in endothelial cells (Hirano et al. 2004a), and this may clearly be important in other vascular beds, and other disease processes, including atherogenesis. Following LPS injection, Hsp27was mainly localized to capillary endothelial cells of the alveolus, and in smooth muscle cells of pulmonary arteries. Hsp27 becomes significantly more phosphorylated at 3 h after LPS treatment, while the distribution of Hsp27 was reported to remain unchanged. The amount of actin associated with Hsp27 is reduced after treatment with LPS. Hirano et al. concluded that endothelial barrier dysfunction caused by LPS correlates with phosphorylation of Hsp27 in vivo.
In newborn piglets, hypoxia was associated with an increase in the expression of αB-crystallin. The hypoxia-associated factor HlF1α was also strongly and rapidly over-expressed. Other stress-associated proteins including Hsp27 were not induced under the same conditions (Louapre et al. 2005).
Role in human disease
Hsp27 and cancer
Heat shock proteins are over-expressed in a wide range of human cancers and are implicated in tumour cell proliferation, differentiation, invasion, metastasis, death and recognition by the immune system (Ciocca et al. 1993). Many lines of evidence indicate that the immortalization step is critical for the neoplastic transformation of normal human cells. Once normal human cells have been immortalized, they are easily transformed into neoplastic cells. Sakaguchi et al. found that the expression and phosphorylation levels of the Hsp27 were in general downregulated in the immortalized cells compared with those in their normal counterparts (Sakaguchi et al. 2001). Cellular stresses such as serum addition, treatment with a carcinogenic agent like 4-nitroquinoline-1-oxide, and a high osmotic pressure had no effects on the expression and phosphorylation of Hsp27 in the immortalized cells. These results suggest that an abnormal regulation of Hsp27 expression and phosphorylation may be one of the reasons for facilitated neoplastic transformation of immortalized cells.
Defects in the apoptosis signalling pathways are common in cancer cells. Such defects may play a role in tumour initiation because apoptosis is the normal means by which cells with damaged DNA or dysregulated cell cycle, i.e. cells with increased malignant potential, may be eliminated (Jaattela 1993; Jaattela & Wissing 1993). Impaired apoptosis may also enhance tumour progression and promote metastasis by enabling tumour cells to survive the process of migration in the bloodstream and to grow in ectopic sites. It may also make the cells resistant to the various modalities of therapy.
Heat shock protein concentrations may be useful biomarkers for assessing the degree of differentiation and the aggressiveness of some cancers. Hsp27 expression is associated with poor prognosis in gastric, liver and prostate carcinoma, and osteosarcomas. Increased Hsp expression may also predict the response to some anticancer treatments. High Hsp27 expression also predicts a poor response to chemotherapy in breast cancer and leukaemia, and may be related to oestrogen in breast and to oestrogen and progesterone in the endometrium. It has been shown that some but not all oestrogen positive breast cancers express Hsp27, and over-expression has been associated with the degree of tumour differentiation, and response to hormonal therapy (Tamoxifen). In endometrial carcinomas, the presence of Hsp27 is correlated with the degree of tumour differentiation as well as with the presence of oestrogen and progesterone receptors. However, the detection of Hsp27 in endometrial carcinoma, cannot be used to identify hormone-responsive tumours or indicator or presence of oestradiol receptors. In the cervix Hsp27 is a marker of cell differentiation, and is highly expressed during the process of squamous metaplasia. Expression in the ovary is still controversial and requires further confirmation of recent observations (Devaja et al. 1997).
Hsp27 and its phosphorylated forms are over-expressed in acute lymphoblastic leukaemia and may use as related markers for it. Identification of two related markers for common acute lymphoblastic leukaemia as heat shock proteins (Strahler et al. 1990, 1991).
Antibodies to the Hsp27 are present in some women with ovarian and endometrial cancers but not in women with non-malignant conditions or healthy women (Korneeva et al. 2000a, b). The appearance of these antibodies suggests that Hsp27 may be present in an extracellular form in gynecologic cancer patients. Synthesis of Hsp27 is upregulated in gynecologic cancers and inhibits the induction of apoptosis. Cell-free Hsp27 and Hsp27-cytochrome c complexes can be detected in the lower genital tract of women with ovarian and endometrial cancers. Identification of these biomarkers may be beneficial in the early diagnosis of these malignancies, and circulating autoantibodies to the Hsp27 were associated with malignancies of the female genital tract (Korneeva et al. 2000b).
Hsp27 and atherosclerosis
Atherosclerosis is a chronic inflammatory process due to the endothelial reaction to stress risk factors, only some of which are known. Positive associations have been reported between exposure to several specific pathogens and future risk of coronary heart disease. An immune response mounted against antigens on a pathogenic organism may cross-react with homologous host proteins in a form of molecular mimicry. Cross-reaction between the Hsp of infectious origin and the endogenous protein produced by the endothelium as a consequence of stress due to risk factor (reviewed by Lamb et al. 2003). Alternatively, activation of innate immune surveillance may occur due to the synthesis and exposure of HSPS on the surface of infected endothelial cells. HSPS are involved in the assembly of molecules which play important roles in the immune system. It is not surprising that due to their wide distribution and their homology among different species, HSPS represent target antigens of the immune response. Chronic exposure to Hsp antigens may convert the immune response to one directed against host antigens and promote autoimmune disease. Bacterial chaperonins are strongly immunogenic and can cause tissue pathology and have been implicated in infection, autoimmune disease, and idiopathic or multifactorial diseases, such as arthritis and atherosclerosis. We have recently assessed the relationship between the immune responses to HSPS and subsequent atherosclerosis in a rabbit model (Lamb & Ferns 2002). Rabbits were immunized with BCG vaccine or saline. Plasma levels of IgG specific for mycobacterial antigen A60 and human Hsp60, but not for human Hsp70, rose following BCG immunization, reaching a peak after 8 weeks. The percentage aortic area covered by atherosclerotic plaque was greater in animals immunized with BCG compared with saline treated animals.
Hsp27 in human heart disease
Martin-Ventura et al. (2004) observed that Hsp27 expression was decreased in complicated atherosclerotic plaques. They also reported that plasma Hsp27 levels were decreased in patients with atherosclerosis compared with healthy subjects. They suggested plasma sHsp27 levels could be a potential index of atherosclerosis, although further validation in large patient cohorts is required.
In humans with cardiac allograft rejection, it has been suggested that increased expression of Hsp27, could be important for cardiac self-protection (Schimke et al. 2000). Tanonaka et al. (2001) have reported that during the development of heart failure, changes in the production of myocardial HSPS occurs. Despite an increase in the myocardial expression of Hsp27, which may bind to cytoskeletal elements of the cardiac myocytes, only the increase in myocardial Hsp60 production was associated with the development of heart failure.
Hsp27 and platelets
Recent studies suggest an important role for HSPS in platelet function. Phosphorylation of Hsp27 in human platelets, following treatment with thrombin for example, is mediated by activation of a protein kinase cascade involving the p38 MAPK, the MAPKAP-K2 kinase, as well as PRAK, or p38-regulated protein kinase. Intriguingly, platelet Hsp27 can associate with platelet factor XIII, suggesting a role for Hsp27 in the regulation of transglutaminase activity in stabilizing fibrin–platelet clots. Hsp27 is also phosphorylated by cGMP-dependent protein kinase (cGK), a signalling system important for the inhibition of platelet aggregation (Shu et al. 2005). Phosphorylation of Hsp27 and Hsp27-dependent regulation of actin microfilaments contribute to the inhibitory effects of cGK on platelet function (Butt et al. 2001). Hsp70 and Hsp90 are also present in platelets, being found in a large phosphorylated complex that contains the catalytic and myosin-targeting subunits of protein phosphatase 1. Platelet adhesion to collagen via the α2β1-integrin causes the rapid dissociation of this complex and dephosphorylation of its components. Hence, it has been proposed that Hsp70 and Hsp90 act as signalling scaffolds, regulating platelet adhesion and spreading via modulation of protein phosphatase activity, whereas Hsp27 is involved in controlling actin polymerization during the platelet shape change and subsequent aggregation (Polanowska-Grabowska & Gear 2000).
Hsp27 and macrophages
Hsp27 expression was examined during phorbol ester (PMA)-induced macrophage differentiation of the human HL-60 promyelocytic leukaemic cell line. Although Hsp27 was expressed at low levels constituitively, PMA rapidly induced its phosphorylation which preceded an increase in Hsp27 protein levels at 24–48 h. In contrast to other agents that induce macrophage differentiation, PMA induced Hsp27 steady state mRNA and protein levels that were regulated concordantly in response to macrophage differentiation. These changes were transient, and there was a concomitant downregulation of cellular proliferation and the onset of G1 phase cell cycle arrest (Spector et al. 1993).
Hsp27 antibodies and heart disease
Although there is a considerable the literature relating antibody titres to Hsp65 to peripheral and coronary atherosclerosis in man (Xu et al. 1993; Hoppichler et al. 1996; Metzler et al. 1997; Xu et al. 1999) and in animal models of atherosclerosis (George et al. 1999, 2001; Afek et al. 2000; Lamb & Ferns 2002), there are a few data for other HSPS, including the small HSPS. Now, it does not appear that Hsp27 antibody titres are related to any coronary risk factors (Vaidya et al. 2005). A summary of the possible roles of Hsp27 in vascular disease is outlined in Figure 3 and Table 3.
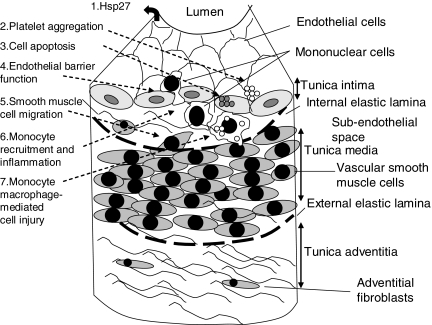
Figure 3.
Possible involvement of Hsp27 in atherogenesis. 1, Hsp27 is expressed by endothelial cells and may stimulate an autoimmune response; 2, Hsp27 is phosphorylated in platelets following treatment with aggregating agents, and may be involved in fibrin clot stabilization; 3, Hsp27 inhibits cell apoptosis; 4, Hsp27 helps maintain endothelial cell barrier function, this would tend to reduce the influx of plasma proteins such as LDL that may be involved in atherogenesis; 5, Hsp27 stabilises the actin cytoskeleton, and regulates actin microfilament dynamics and polymerization, and hence cell migration; 6, Hsp27 affects the expression of IL-10 by monocyte macrophages, and is thought to be anti-inflammatory; 7, Hsp inhibits monocyte/macrophage-mediated cell injury.
Table 3.
Effects of Hsp27 in processes potentially involved in atherosclerosis
| Process | References |
|---|---|
| Maintaining intracellular redox status | Mehlen et al. (1997a); Arrigo et al. (2005) |
| Cell apoptosis | Bruey et al. (2000a); Charette et al. (2000); Paul et al. (2002); Kabakov et al. (2003) |
| Smooth muscle cell migration | Hedges et al. (1999) |
| NF-kB inhibition | Chen et al. (2004) |
| Endothelial cell migration | McMullen et al. (2005); Piotrowicz et al. (1998) |
| Platelet actin polymerization and aggregation | Polanowska-Grabowska and Gear (2000) |
| Endothelial cell barrier function | Hirano et al. (2004a) |
| Development of intimal hyperplasia | Connolly et al. (2003) |
| Cardiac transplant rejection | Schimke et al. (2000) |
| Monocyte-mediated cell injury | Jaattela & Wissing (1993) |
| Established plaque | Martin-Ventura et al. (2004) |
| Oxidative stress in smooth muscle cells | Negre-Aminou et al. (2002) |
| Sheer stress in endothelial cells | Yoo et al. (2005) |
| Anti-inflammatory via IL-10 induction | De et al. (2000) |
Conclusions
The Hsp27 is a ubiquitous molecular chaperone that has several other potentially important roles in cell biology. These include VSMC migration, apoptosis, resistance to oxidant stress, endothelial barrier function and modulation of inflammation. All of these functions may have an impact on the process of atherogenesis, and indeed Hsp27 is expressed within arterial lesions. Antibodies to Hsp27 have been detected in patients with cancer and coronary disease, although levels do not associate with known coronary risk factors in the latter.
Acknowledgments
The authors acknowledge the support of the British Heart Foundation for a grant supporting Dr Shafi and Tehran University of Medical Sciences for providing Dr Shams with a Sabbatical grant.
References
- Abu Shama KM, Suzuki A, Harada K, et al. Transient up-regulation of myotonic dystrophy protein kinase- binding protein, MKBP, and HSP27 in the neonatal myocardium. Cell. Struct. Funct. 1999;24:1–4. doi: 10.1247/csf.24.1. [DOI] [PubMed] [Google Scholar]
- Afek A, George J, Gilburd B, et al. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J. Autoimmun. 2000;14:115–121. doi: 10.1006/jaut.1999.0351. [DOI] [PubMed] [Google Scholar]
- Akamatsu S, Nakajima K, Ishisaki A, et al. Vasopressin phosphorylates HSP27 in aortic smooth muscle cells. J. Cell. Biochem. 2004;92:1203–1211. doi: 10.1002/jcb.20148. [DOI] [PubMed] [Google Scholar]
- Arata S, Hamaguchi S, Nose K. Effects of the overexpression of the small heat-shock protein, Hsp27, on the sensitivity of human fibroblast cells exposed to oxidative stress. J. Cell. Physiol. 1995;163:458–465. doi: 10.1002/jcp.1041630305. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. sHsp as novel regulators of programmed cell death and tumorigenicity. Pathol. Biol. (Pari.) 2000;48:280–288. [PubMed] [Google Scholar]
- Arrigo AP. Hsp27: novel regulator of intracellular redox state. Iubmb Life. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, AZ-Latoud C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxidants Redox Signal. 2005;7:414–424. doi: 10.1089/ars.2005.7.414. [DOI] [PubMed] [Google Scholar]
- Assimakopoulou M, Sotiropouloubonikou G, Maraziotis T, Varakis I. Prognostic significance of HSP-27 in astrocytic brain tumors: an immunohistochemical study. Anticancer. Res. 1997;17:2677–2682. [PubMed] [Google Scholar]
- Augusteyn RC. Dissociation is not required for alpha-crystallin's chaperone function. Exp. Eye. Res. 2004;79:781–784. doi: 10.1016/j.exer.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Bex A, Geis C, Metz K, et al. Expression of heat shock proteins 27 and 70 in renal cell cancer and normal renal tissue: an immunohistochemical study. Onkologie. 1999;22:236–239. [Google Scholar]
- Bitar KN. HSP27 phosphorylation and interaction with actin-myosin in smooth muscle contraction. Am. J. Physiol. Gastrointest. Liver. Physiol. 2002;282:G894–G903. doi: 10.1152/ajpgi.00141.2001. [DOI] [PubMed] [Google Scholar]
- Bluhm WF, Martin JL, Mestril R, Dillmann WH. Specific heat shock proteins protect microtubules during simulated ischemia in cardiac myocytes. Am. J. Physiol.-Heart Circulat. Physiol. 1998;44:H2243–H2249. doi: 10.1152/ajpheart.1998.275.6.H2243. [DOI] [PubMed] [Google Scholar]
- Borrelli MJ, Bernock LJ, Landry J, et al. Stress protection by a fluorescent Hsp27 chimera that is independent of nuclear translocation or multimeric dissociation. Cell Stress Chaperones. 2002;7:281–296. doi: 10.1379/1466-1268(2002)007<0281:spbafh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2000a;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Paul C, Fromentin A, et al. Differential regulation of HSP27 oligomerization in tumor cells grown in vitro and in vivo. Oncogene. 2000b;19:4855–4863. doi: 10.1038/sj.onc.1203850. [DOI] [PubMed] [Google Scholar]
- Butt E, Immler D, Meyer HE, Kotlyarov A, Laass K, Gaestel M. Heat shock protein 27 is a substrate of cGMP-dependent protein kinase in intact human platelets – phosphorylation-induced actin polymerization caused by Hsp27 mutants. J. Biol. Chem. 2001;276:7108–7113. doi: 10.1074/jbc.m009234200. [DOI] [PubMed] [Google Scholar]
- Carper SW, Rocheleau TA, Cimino D, Storm FK. Heat shock protein 27 stimulates recovery of RNA and protein synthesis following a heat shock. J. Cell. Biochem. 1997;66:153–164. [PubMed] [Google Scholar]
- Carra S, Sivilotti M, Zobel ATC, Lambert H, Landry J. HspB8, a small heat shock protein mutated in human neuromuscular disorders, has in vivo chaperone activity in cultured cells. Hum. Mol. Genet. 2005;14:1659–1669. doi: 10.1093/hmg/ddi174. [DOI] [PubMed] [Google Scholar]
- Carver JA, Rekas A, Thorn DC, Wilson MR. Small heat-shock proteins and clusterin: intra- and extracellular molecular chaperones with a common mechanism of action and function. Iubmb Life. 2003;55:661–668. doi: 10.1080/15216540310001640498. [DOI] [PubMed] [Google Scholar]
- Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol. Cell. Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaufour S, Mehlen P, Arrigo AP. Transient accumulation, phosphorylation and changes In the oligomerization of Hsp27 during retinoic acid-induced differentiation of HL-60 cells: possible role in the control of cellular growth and differentiation. Cell Stress Chaperones. 1996;1:225–235. doi: 10.1379/1466-1268(1996)001<0225:tapaci>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ross BM, Currie RW. Heat shock treatment protects against angiotensin II-induced hypertension and inflammation in aorta. Cell Stress Chaperones. 2004;9:99–107. doi: 10.1379/CSC-1R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernik IS, Panasenko OO, Li Y, Marston SB, Gusev NB. pH induced changes of the structure of small heat shock proteins with molecular mass 24/27 kDa (HspB1) Biochem. Biophys. Res. Common. 2004;324:1199–203. doi: 10.1016/j.bbrc.2004.09.176. [DOI] [PubMed] [Google Scholar]
- Chevalier D, Thorin E, Allen BG. Simultaneous measurement of ERK, p38, and JNK MAP kinase cascades in vascular smooth muscle cells. J. Pharmacol. Toxicol. Methods. 2000;44:429–439. doi: 10.1016/s1056-8719(00)00118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D, Boyle GM, Williams RM, et al. Alpha B-crystallin, a new independent marker for poor prognosis in head and neck cancer. Laryngoscope. 2005;115:1239–1242. doi: 10.1097/01.MLG.0000164715.86240.55. [DOI] [PubMed] [Google Scholar]
- Chowdary TK, Raman B, Ramakrishna T, Rao CM. Mammalian Hsp22 is a heat-inducible small heat-shock protein with chaperone-like activity. Biochem. J. 2004;381:379–387. doi: 10.1042/BJ20031958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien P, Landry J. Enhanced constitutive expression of the 27-Kda heat-shock proteins in heat-resistant variants from chinese-hamster cells. J. Cell. Physiol. 1988;137:157–166. doi: 10.1002/jcp.1041370119. [DOI] [PubMed] [Google Scholar]
- Christians ES, Yan LJ, Benjamin IJ. Heat shock factor 1 and heat shock proteins: critical partners in protection against acute cell injury. Crit. Care. Med. 2002;30:S43–S50. [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Oesterreich S, Chamness GC, Mcguire WL, Fuqua SAW. Biological and clinical implications of heat-shock protein 27000 (Hsp27) – a review. J. Natl. Cancer. Inst. 1993;85:1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- Clermont F, Adam E, Dumont JE, Robaye B. Survival pathways regulating the apoptosis induced by tumour necrosis factor-alpha in primary cultured bovine endothelial cells. Cell. Signal. 2003;15:539–546. doi: 10.1016/s0898-6568(02)00145-6. [DOI] [PubMed] [Google Scholar]
- Connolly EM, Kelly CJ, Chen G, et al. Pharmacological induction of HSP27 attenuates intimal hyperplasia in vivo. Eur. J. Vasc. Endovasc. Surg. 2003;25:40–47. doi: 10.1053/ejvs.2002.1793. [DOI] [PubMed] [Google Scholar]
- Cooper LF, Uoshima K, Guo ZY. Transcriptional regulation involving the intronic heat shock element of the rat Hsp27 gene. Biochim. Biophys. Acta-Gene Struct. Expr. 2000;1490:348–354. doi: 10.1016/s0167-4781(00)00005-1. [DOI] [PubMed] [Google Scholar]
- Cornford PA, Dodson AR, Parsons KF, et al. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer. Res. 2000;60:7099–7105. [PubMed] [Google Scholar]
- Crete P, Landry J. Induction of Hsp27 phosphorylation and thermoresistance in Chinese hamster-cells by arsenite, cycloheximide, A23187, and egta. Radiat. Res. 1990;121:320–327. [PubMed] [Google Scholar]
- David JC, Landry A, Grongnet LF. Perinatal expression of heat-shock protein 27 in brain regions and nonneural tissues of the piglet. J. Mol. Neurosci. 2000;15:109–120. doi: 10.1385/jmn:15:2:109. [DOI] [PubMed] [Google Scholar]
- De AK, Kodys KM, Yeh BS, Miller-Graziano C. Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J. Immunol. 2000;165:3951–3958. doi: 10.4049/jimmunol.165.7.3951. [DOI] [PubMed] [Google Scholar]
- De Jong WW, Caspers GJ, Leunissen JAM. Genealogy of the alpha-crystallin – small heat-shock protein superfamily. Int. J. Biol. Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- De Wit NJW, Verschuure P, Kappe G, et al. Testis-specific human small heat shock protein HSPB9 is a cancer/testis antigen, and potentially interacts with the dynein subunit TCTEL1. Eur. J. Cell. Biol. 2004;83:337–345. doi: 10.1078/0171-9335-00396. [DOI] [PubMed] [Google Scholar]
- Detoro MMM, Luque EH. Lack of relationship between the expression of Hsp27 heat shock estrogen receptor-associated protein and estrogen receptor or progesterone receptor status in male breast carcinoma. J. Steroid. Biochem. Mol. Biol. 1997;60:277–284. doi: 10.1016/s0960-0760(96)00221-x. [DOI] [PubMed] [Google Scholar]
- Devaja O, King RJB, Papadopoulos A, Raju KS. Heat-shock protein 27 (HSP27) and its role in female reproductive organs. Eur. J. Gynaecol. Oncol. 1997;18:16–22. [PubMed] [Google Scholar]
- Doppler H, Storz P, Li J, Comb MJ, Toker A. A phosphorylation state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J. Biol. Chem. 2005;280:15013–15019. doi: 10.1074/jbc.C400575200. [DOI] [PubMed] [Google Scholar]
- Fontaine JM, Rest JS, Welsh MJ, Benndorf R. The sperm outer dense fiber protein is the 10th member of the superfamily of mammalian small stress proteins. Cell Stress Chaperones. 2003;8:62–69. doi: 10.1379/1466-1268(2003)8<62:tsodfp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck E, Madsen O, Van Rheede T, Ricard G, Huynen MA, De Jong WW. Evolutionary diversity of vertebrate small heat shock proteins. J. Mol. Evol. 2004;59:792–805. doi: 10.1007/s00239-004-0013-z. [DOI] [PubMed] [Google Scholar]
- Frohli E, Aoyama A, Klemenz R. Cloning of the mouse Hsp25 gene and an extremely conserved Hsp25 pseudogene. Gene. 1993;128:273–277. doi: 10.1016/0378-1119(93)90574-m. [DOI] [PubMed] [Google Scholar]
- Gaitanaki C, Konstantina S, Chrysa S, Beis I. Oxidative stress stimulates multiple Mapk signalling pathways and phosphorylation of the small HSP27 in the perfused amphibian heart. J. Exp. Biol. 2003;206:2759–2769. doi: 10.1242/jeb.00483. [DOI] [PubMed] [Google Scholar]
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB. J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- George J, Shoenfeld Y, Afek A, et al. Enhanced fatty streak formation in C57BL/6J mice by immunization with heat shock protein-65. Arterioscler. Thromb. Vasc. Biol. 1999;19:505–510. doi: 10.1161/01.atv.19.3.505. [DOI] [PubMed] [Google Scholar]
- George J, Greenberg S, Barshack I, et al. Accelerated intimal thickening in carotid arteries of balloon-injured rats after immunization against heat shock protein 70. J. Am. Coll. Cardiol. 2001;38:1564–1569. doi: 10.1016/s0735-1097(01)01579-0. [DOI] [PubMed] [Google Scholar]
- Gerthoffer WT, Gunst SJ. Signal transduction in smooth muscle – invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J. Appl. Physiol. 2001;91:963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Perng MD, Quinlan RA, Drenckhahn D. Comparison of the small heat shock proteins alpha B-crystallin, MKBP, HSP25, HSP20, and cvHSP in heart and skeletal muscle. Histochem. Cell. Biol. 2004;122:415–425. doi: 10.1007/s00418-004-0711-z. [DOI] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingrasbreton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase- mediated phosphorylation of heat shock protein 27. J. Cell. Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Gusev NB, Bukach OV, Marston SB. Structure, properties, and probable physiological role of small heat shock protein with molecular mass 20 kD (Hsp20, HspB6) Biochem-Moscow. 2005;70:629–637. doi: 10.1007/s10541-005-0162-8. [DOI] [PubMed] [Google Scholar]
- Hedges JC, Dechert MA, Yamboliev IA, et al. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J. Biol. Chem. 1999;274:24211–24219. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- Hickey ED, Weber LA. Modulation of heat-shock polypeptide-synthesis in hela-cells during hyperthermia and recovery. Biochemistry. 1982;21:1513–1521. doi: 10.1021/bi00536a008. [DOI] [PubMed] [Google Scholar]
- Hickey E, Brandon SE, Potter R, Stein G, Stein J, Weber LA. Sequence and organization of genes encoding the human 27 kDa heat-shock protein. Nucleic Acids Res. 1986a;14:4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E, Brandon SE, Sadis S, Smale G, Weber LA. Molecular-cloning of sequences encoding the human heat-shock proteins and their expression during hyperthermia. Gene. 1986b;43:147–154. doi: 10.1016/0378-1119(86)90018-1. [DOI] [PubMed] [Google Scholar]
- Hirade K, Kozawa O, Tanabe K, et al. Thrombin stimulates dissociation and induction of HSP27 via p38 MAPK in vascular smooth muscle cells. Am. J. Physiol.-Heart Circulat. Physiol. 2002;283:H941–H948. doi: 10.1152/ajpheart.00060.2001. [DOI] [PubMed] [Google Scholar]
- Hirano S, Rees RS, Yancy SL, et al. Endothelial barrier dysfunction caused by LPs correlates with phosphorylation of HSP27 in vivo. Cell. Biol. Toxicol. 2004a;20:1–14. doi: 10.1023/b:cbto.0000021019.50889.aa. [DOI] [PubMed] [Google Scholar]
- Hirano S, Shelden EA, Gilmont RR. HSP27 regulates fibroblast adhesion, motility, and matrix contraction. Cell Stress Chaperones. 2004b;9:29–37. doi: 10.1379/471.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma Y, Tani M, Yamamura K, Takayama M, Hasegawa H. Preconditioning with heat shock further improved functional recovery in young adult but not in middle-aged rat hearts. Exp. Gerontol. 2003;38:299–306. doi: 10.1016/s0531-5565(02)00199-7. [DOI] [PubMed] [Google Scholar]
- Hoppichler F, Lechleitner M, Traweger C, et al. Changes of serum antibodies to heat-shock protein 65 in coronary heart disease and acute myocardial infarction. Atherosclerosis. 1996;126:333–338. doi: 10.1016/0021-9150(96)05931-x. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Exp. Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer. Res. 1996;56:273–279. [PubMed] [Google Scholar]
- Inaguma Y, Hasegawa K, Goto S, Ito H, Kato K. Induction of the synthesis of Hsp27 and alpha-B-crystallin in tissues of heat-stressed rats and its suppression by ethanol or an alpha(1)-adrenergic antagonist. J. Biochem. (Tokyo) 1995;117:1238–1243. doi: 10.1093/oxfordjournals.jbchem.a124850. [DOI] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. 4 Small Drosophila heat-shock proteins are related to each other and to mammalian alpha-crystallin. Proc. Natl. Acad. Sci. U. S. A.-Biol. Sci. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irobi J, Van Impe K, Seeman P, et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat. Genet. 2004;36:597–601. doi: 10.1038/ng1328. [DOI] [PubMed] [Google Scholar]
- Ito H, Okamoto K, Kato K. Prostaglandins stimulate the stress-induced synthesis of Hsp27 and alpha B crystallin. J. Cell. Physiol. 1997a;170:255–262. doi: 10.1002/(SICI)1097-4652(199703)170:3<255::AID-JCP6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. Phosphorylation of alpha B-crystallin in response to various types of stress. J. Biol. Chem. 1997b;272:29934–29941. doi: 10.1074/jbc.272.47.29934. [DOI] [PubMed] [Google Scholar]
- Ito H, Okamoto K, Kato K. Enhancement of expression of stress proteins by agents that lower the levels of glutathione in cells. Biochim. Biophys. Acta-Gene Struct. Expr. 1998;1397:223–230. doi: 10.1016/s0167-4781(98)00010-4. [DOI] [PubMed] [Google Scholar]
- Ito H, Kamei K, Iwamoto I, et al. Inhibition of proteasomes induces accumulation, phosphorylation, and recruitment of HSP27 and alpha B- crystallin to aggresomes. J. Biochem. (Tokyo) 2002;131:593–603. doi: 10.1093/oxfordjournals.jbchem.a003139. [DOI] [PubMed] [Google Scholar]
- Iwaki T, Iwaki A, Tateishi J, Sakaki Y, Goldman JE. Alpha-B-crystallin and 27-kD heat-shock protein are regulated by stress conditions in the central-nervous-system and accumulate in rosenthal fibers. Am. J. Pathol. 1993;143:487–495. [PMC free article] [PubMed] [Google Scholar]
- Iwaki T, Iwaki A, Tateishi J, Goldman JE. Sense and antisense modification of glial alpha-B-crystallin production results in alterations of stress fiber formation and thermoresistance. J. Cell. Biol. 1994;125:1385–1393. doi: 10.1083/jcb.125.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki A, Nagano T, Nakagawa M, Iwaki T, Fukumaki Y. Identification and characterization of the gene encoding a new member of the alpha-crystallin small Hsp family, closely linked to the alpha B-crystallin gene in a head-to-head manner. Genomics. 1997;45:386–394. doi: 10.1006/geno.1997.4956. [DOI] [PubMed] [Google Scholar]
- Jaattela M. Overexpression of major heat-shock protein Hsp70 inhibits tumor necrosis factor-induced activation of phospholipase-A(2) J. Immunol. 1993;151:4286–4294. [PubMed] [Google Scholar]
- Jaattela M. Escaping cell death: survival proteins in cancer. Exp. Cell. Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- Jaattela M, Wissing D. Heat-shock proteins protect cells from monocyte cytotoxicity – possible mechanism of self-protection. J. Exp. Med. 1993;177:231–236. doi: 10.1084/jem.177.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat-shock proteins are molecular chaperones. J Biol. Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Jia YF, Ransom RF, Shibanuma M, Liu CH, Welsh MJ, Smoyer WE. Identification and characterization of hic-5/ARA55 as an Hsp27 binding protein. J. Biol. Chem. 2001;276:39911–39918. doi: 10.1074/jbc.M103510200. [DOI] [PubMed] [Google Scholar]
- Kabakov AE, Budagova KR, Bryantsev AL, Latchman DS. Heat shock protein 70 or heat shock protein 27 overexpressed in human endothelial cells during posthypoxic reoxygenation can protect from delayed apoptosis. Cell Stress Chaperones. 2003;8:335–347. doi: 10.1379/1466-1268(2003)008<0335:hspohs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida T, Kozawa O, Ito T, et al. Vasopressin stimulates the induction of heat shock protein 27 and alpha B-crystallin via protein kinase C activation in vascular smooth muscle cells. Exp. Cell. Res. 1999;246:327–337. doi: 10.1006/excr.1998.4277. [DOI] [PubMed] [Google Scholar]
- Kappe G, Verschuure P, Philipsen RLA, et al. Characterization of two novel human small heat shock proteins: protein kinase-related HspB8 and testis-specific HspB9. Biochim. Biophys. Acta-Gene Struct. Expr. 2001;1520:1–6. doi: 10.1016/s0167-4781(01)00237-8. [DOI] [PubMed] [Google Scholar]
- Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JAM, De Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1–10. Cell Stress Chaperones. 2003a;8:53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JAM, De Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1–10. Cell Stress Chaperones. 2003b;8:53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Shinohara H, Kurobe N, Goto S, Inaguma Y, Ohshima K. Immunoreactive alpha A crystallin in rat nonlenticular tissues detected with a sensitive immunoassay method. Biochim. Biophys. Acta. 1991;1080:173–180. doi: 10.1016/0167-4838(91)90146-q. [DOI] [PubMed] [Google Scholar]
- Kato K, Goto S, Hasegawa K, Shinohara H, Inaguma Y. Responses to heat-shock of alpha-B-crystallin and Hsp28 in U373-Mg human glioma-cells. Biochim. Biophys. Acta. 1993;1175:257–262. doi: 10.1016/0167-4889(93)90214-a. [DOI] [PubMed] [Google Scholar]
- Kato K, Goto S, Inaguma Y, Hasegawa K, Morishita R, Asano T. Purification and characterization of a 20-Kda protein that is highly homologous to alpha-B-crystallin. J. Biol. Chem. 1994a;269:15302–15309. [PubMed] [Google Scholar]
- Kato K, Hasegawa K, Goto S, Inaguma Y. Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, Hsp27. J. Biol. Chem. 1994b;269:11274–11278. [PubMed] [Google Scholar]
- Kato K, Ito H, Iwamoto I, Iida K, Inaguma Y. Protein kinase inhibitors can suppress stress-induced dissociation of Hsp27. Cell Stress Chaperones. 2001;6:16–20. doi: 10.1379/1466-1268(2001)006<0016:pkicss>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H, Otsuka T, Matsuno H, et al. Endothelin-1 stimulates heat shock protein 27 induction in osteoblasts: involvement of p38 MAP kinase. Am. J. Physiol-Endocrinol. Metab. 1999;277:E1046–E1054. doi: 10.1152/ajpendo.1999.277.6.E1046. [DOI] [PubMed] [Google Scholar]
- Kawanishi K, Shiozaki H, Doki Y, et al. Prognostic significance of heat shock proteins 27 and 70 in patients with squamous cell carcinoma of the esophagus. Cancer. 1999;85:1649–1657. doi: 10.1002/(sici)1097-0142(19990415)85:8<1649::aid-cncr2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Keezer SM, Ivie SE, Krutzsch HC, Tandle A, Libutti SK, Roberts DD. Angiogenesis inhibitors target the endothelial cell cytoskeleton through altered regulation of heat shock protein 27 and cofilin. Cancer. Res. 2003;63:6405–6412. [PubMed] [Google Scholar]
- Khan W, Mcguirt JP, Sens MA, Sens DA, Todd JH. Expression of heat shock protein 27 in developing and adult human kidney. Toxicol. Lett. 1996;84:69–79. doi: 10.1016/0378-4274(95)03613-x. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- King KL, Li AFY, Chau GY, et al. Prognostic significance of heat shock protein-27 expression in hepatocellular carcinoma and its relation to histologic grading and survival. Cancer. 2000;88:2464–2470. doi: 10.1002/1097-0142(20000601)88:11<2464::aid-cncr6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Klemenz R, Frohli E, Steiger RH, Schafer R, Aoyama A. Alpha-B-crystallin is a small heat-shock protein. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Matsuzaki H, Tanaka M, et al. Activation of protein kinase B (Akt/Rac-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS. Lett. 1997;410:493–498. doi: 10.1016/s0014-5793(97)00541-3. [DOI] [PubMed] [Google Scholar]
- Korneeva I, Bongiovanni AM, Girotra M, Caputo TA, Witkin SS. IgA antibodies to the 27-kDa heat-shock protein in the genital tracts of women with gynecologic cancers. Int. J. Cancer. 2000a;87:824–828. doi: 10.1002/1097-0215(20000915)87:6<824::aid-ijc11>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Korneeva I, Bongiovanni AM, Girotra M, Caputo TA, Witkin SS. Serum antibodies to the 27-kd heat shock protein in women with gynecologic cancers. Am. J. Obstet. Gynecol. 2000b;183:18–21. doi: 10.1067/mob.2000.105431. [DOI] [PubMed] [Google Scholar]
- Kozawa O, Tanabe K, Ito H, et al. Sphingosine 1-phosphate regulates heat shock protein 27 induction by a p38 MAP kinase-dependent mechanism in aortic smooth muscle cells. Exp. Cell. Res. 1999;250:376–380. doi: 10.1006/excr.1999.4536. [DOI] [PubMed] [Google Scholar]
- Kozawa O, Matsuno H, Niwa M, Hatakeyama D, Kato K, Uematsu T. Alpha B-crystallin, a low-molecular-weight heat shock protein, acts as a regulator of platelet function. Cell Stress Chaperones. 2001;6:21–28. doi: 10.1379/1466-1268(2001)006<0021:bcalmw>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozawa O, Matsuno H, Niwa M, et al. HSP20, low-molecular-weight heat shock-related protein, acts extracellularly as a regulator of platelet functions: a novel defense mechanism. Life. Sci. 2002;72:113–124. doi: 10.1016/s0024-3205(02)02144-6. [DOI] [PubMed] [Google Scholar]
- Lam WY, Tsui SKW, Law PTW, et al. Isolation and characterization of a human heart cDNA encoding a new member of the small heat shock protein family – HSPL27. Biochim. Biophys. Acta-Mol Cell. Res. 1996;1314:120–124. doi: 10.1016/s0167-4889(96)00121-8. [DOI] [PubMed] [Google Scholar]
- Lamb DJ, Ferns GAA. The magnitude of the immune response to heat shock protein-65 following BCG immunisation is associated with the extent of experimental atherosclerosis. Atherosclerosis. 2002;165:231–240. doi: 10.1016/s0021-9150(02)00244-7. [DOI] [PubMed] [Google Scholar]
- Lamb DJ, EL-Sankary W, Ferns GAA. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis. 2003;167:177–185. doi: 10.1016/s0021-9150(02)00301-5. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid-phase pinocytosis by phosphorylation of heat-shock protein-27. J. Biol. Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat-shock protein-27. Mol. Cell. Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Ndisang D, Patel C, et al. Expression of the Brn-3b transcription factor correlates with expression of HSP-27 in breast cancer biopsies and is required for maximal activation of the HSP-27 promoter. Cancer. Res. 2005;65:3072–3080. doi: 10.1158/0008-5472.CAN-04-2865. [DOI] [PubMed] [Google Scholar]
- Lenne C, Block MA, Garin J, Douce R. Sequence and expression of the messenger-RNA encoding Hsp22, the mitochondrial small heat-shock protein in pea leaves. Biochem. J. 1995;311:805–813. doi: 10.1042/bj3110805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi R, Barbato E, Paganelli C, Lo Muzio L. Immunolocalization of heat shock protein 27 in developing jaw bones and tooth germs of human fetuses. Calcif. Tissue. Int. 2004;75:509–516. doi: 10.1007/s00223-004-0077-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Roth S, Laser M, Ma JX, Crosson CE. Retinal preconditioning and the induction of heat-shock protein 27. Invest. Ophthalmol. Vis. Sci. 2003;44:1299–1304. doi: 10.1167/iovs.02-0235. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu. Rev. Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lo Muzio L, Leonardi R, Mariggio MA, et al. HSP27 as possible prognostic factor in patients with oral squamous cell carcinoma. Histol. Histopathol. 2004;19:119–128. doi: 10.14670/HH-19.119. [DOI] [PubMed] [Google Scholar]
- Loktionova SA, Kabakov AE. Protein phosphatase inhibitors and heat preconditioning prevent Hsp27 dephosphorylation, F-actin disruption and deterioration of morphology in ATP-depleted endothelial cells. FEBS. Lett. 1998;433:294–300. doi: 10.1016/s0014-5793(98)00920-x. [DOI] [PubMed] [Google Scholar]
- Loktionova SA, Kabakov AE. Phosphatase inhibitors prevent HSP27 dephosphorylation, destruction of stress fibrils, and morphological changes in endothelial cells during ATP depletion. Bull. Exp. Biol. Med. 2001;132:914–917. doi: 10.1023/a:1013199508296. [DOI] [PubMed] [Google Scholar]
- Loktionova SA, Ilyinskaya OP, Gabai VL, Kabakov AE. Distinct effects of heat shock and ATP depletion on distribution and isoform patterns of human Hsp27 in endothelial cells. FEBS. Lett. 1996;392:100–104. doi: 10.1016/0014-5793(96)00792-2. [DOI] [PubMed] [Google Scholar]
- Louapre P, Grongnet JF, Tanguay RM, David JC. Effects of hypoxia on stress proteins in the piglet heart at birth. Cell Stress Chaperones. 2005;10:17–23. doi: 10.1379/CSC-74R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/alpha-crystalline proteins: established concepts and emerging ideas. Cell. Mole. Life. Sci. 2000;57:899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Hickey E, Weber LA, Dillmann WH, Mestril R. Influence of phosphorylation and oligomerization on the protective role of the small heat shock protein 27 in rat adult cardiomyocytes. Gene. Expr. 1999;7:349–355. [PMC free article] [PubMed] [Google Scholar]
- Martin-Ventura JL, Duran MC, Blanco-Colio LM, et al. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation. 2004;110:2216–2219. doi: 10.1161/01.CIR.0000136814.87170.B1. [DOI] [PubMed] [Google Scholar]
- McLemore EC, Tessier DJ, Thresher J, Komalavilas P, Brophy CM. Role of the small heat shock proteins in regulating vascular smooth muscle tone. J. Am. Coll. Surg. 2005;201:30–36. doi: 10.1016/j.jamcollsurg.2005.03.017. [DOI] [PubMed] [Google Scholar]
- McMullen ME, Bryant PW, Glembotski CC, Vincent PA, Pumiglia KM. Activation of p38 has opposing effects on the proliferation and migration of endothelial cells. J. Biol. Chem. 2005;280:20995–21003. doi: 10.1074/jbc.M407060200. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Arrigo AP. The serum-induced phosphorylation of mammalian Hsp27 correlates with changes in its intracellular-localization and levels of oligomerization. Eur. J. Biochem. 1994;221:327–334. doi: 10.1111/j.1432-1033.1994.tb18744.x. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Briolay J, Smith L, et al. Analysis of the resistance to heat and hydrogen-peroxide stresses in cos cells transiently expressing wild-type or deletion mutants of the Drosophila 27-kDa heat-shock protein. Eur. J. Biochem. 1993;215:277–284. doi: 10.1111/j.1432-1033.1993.tb18032.x. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Kretzremy C, Briolay J, Fostan P, Mirault ME, Arrigo AP. Intracellular reactive oxygen species as apparent modulators of heat-shock-protein-27 (Hsp27) structural organization and phosphorylation in basal and tumor-necrosis-factor-alpha-treated T47D human carcinoma-cells. Biochem. J. 1995;312:367–375. doi: 10.1042/bj3120367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Kretzremy C, Preville X, Arrigo AP. Human Hsp27, Drosophila Hsp27 and human alpha B-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNF alpha-induced cell death. EMBO. J. 1996a;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Schulzeosthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis – heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J. Biol. Chem. 1996b;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Hickey E, Weber LA, Arrigo AP. Large unphosphorylated aggregates as the active form of Hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNF alpha in NIH-3T3-ras cells. Biochem. Biophys. Res. Commun. 1997a;241:187–192. doi: 10.1006/bbrc.1997.7635. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Mehlen A, Godet J, Arrigo AP. Hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J. Biol. Chem. 1997b;272:31657–31665. doi: 10.1074/jbc.272.50.31657. [DOI] [PubMed] [Google Scholar]
- Metzler B, Schett G, Kleindienst R, et al. Epitope specificity of anti-heat shock protein 65/60 serum antibodies in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1997;17:536–541. doi: 10.1161/01.atv.17.3.536. [DOI] [PubMed] [Google Scholar]
- Michils A, Redivo M, De Beyl VZ, et al. Increased expression of high but not low molecular weight heat shock proteins in resectable lung carcinoma. Lung Cancer. 2001;33:59–67. doi: 10.1016/s0169-5002(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Hirade K, Ishisaki A, et al. Akt regulates thrombin-induced HSP27 phosphorylation in aortic smooth muscle cells: function at a point downstream from p38 MAP kinase. Life Sci. 2005;77:96–107. doi: 10.1016/j.lfs.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Negre-Aminou P, Van Leeuwen REW, Van Thiel GCF, et al. Differential effect of simvastatin on activation of Rac(1) vs. activation of the heat shock protein 27-mediated pathway upon oxidative stress, in human smooth muscle cells. Biochem. Pharmacol. 2002;64:1483–1491. doi: 10.1016/s0006-2952(02)01388-6. [DOI] [PubMed] [Google Scholar]
- O'neill PA, Shaaban AM, West CR, et al. Increased risk of malignant progression in benign proliferating breast lesions defined by expression of heat shock protein 27. Br. J. Cancer. 2004;90:182–188. doi: 10.1038/sj.bjc.6601449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padival AK, Crabb JW, Nagaraj RH. Methylglyoxal modifies heat shock protein 27 in glomerular mesangial cells. FEBS. Lett. 2003;551:113–118. doi: 10.1016/s0014-5793(03)00874-3. [DOI] [PubMed] [Google Scholar]
- Pantos C, Malliopoulou V, Mourouzis I, et al. Thyroxine pretreatment increases basal myocardial heat-shock protein 27 expression and accelerates translocation and phosphorylation of this protein upon ischaemia. Eur. J. Pharmacol. 2003a;478:53–60. doi: 10.1016/j.ejphar.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Pantos C, Malliopoulou V, Sfakianoudis K, et al. Long-term thyroxine administration results in heat shock protein 27 (HSP27) overexpression and protects the heart from ischaemia. Eur. Heart. J. 2003b;24:341. [Google Scholar]
- Parcellier A, Schmitt E, Gurbuxani S, et al. HSP27 is a ubiquitin-binding protein involved in I-kappa B alpha proteasomal degradation. Mol. Cell. Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome c release. Mol. Cell. Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng MD, Cairns L, Van Den Ijssel P, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and alpha B-crystallin. J. Cell. Sci. 1999;112:2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- Piotrowicz RS, Hickey E, Levin EG. Heat shock protein 27 kDa expression and phosphorylation regulates endothelial cell migration. FASEB. J. 1998;12:1481–1490. doi: 10.1096/fasebj.12.14.1481. [DOI] [PubMed] [Google Scholar]
- Polanowska-Grabowska R, Gear ARL. Heat-shock proteins and platelet function. Platelets. 2000;11:6–22. doi: 10.1080/09537100075742. [DOI] [PubMed] [Google Scholar]
- Posner M. A comparative view of alpha crystallins: the contribution of comparative studies to understanding function. Integr. Comp. Biol. 2003;43:481–491. doi: 10.1093/icb/43.4.481. [DOI] [PubMed] [Google Scholar]
- Pountney DL, Treweek TM, Chataway T, et al. alpha B-crystallin is a major component of glial cytoplasmic inclusions in multiple system atrophy. Neurotoxicity Res. 2005;7:77–85. doi: 10.1007/BF03033778. [DOI] [PubMed] [Google Scholar]
- Rao PV, Huang QL, Horwitz J, Zigler JS. Evidence that alpha-crystallin prevents non-specific protein aggregation in the intact eye lens. Biochim. Biophys. Acta-Gen. Subj. 1995;1245:439–447. doi: 10.1016/0304-4165(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, Preville X, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress tumor necrosis factor alpha by phosphorylation. J. Biol. Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Rousseau S, Houle F, Landry J, Huot J. p38 Map kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Zuker CS. Protein-folding and the regulation of signaling pathways. Cell. 1994;79:1129–1132. doi: 10.1016/0092-8674(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M, Miyazaki M, Kondo T, Tsuji T, Kouchi H, Namba M. Identification of a phosphoprotein that is downregulated in immortalized human fibroblasts. Electrophoresis. 2001;22:155–160. doi: 10.1002/1522-2683(200101)22:1<155::AID-ELPS155>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Schimke I, Lutsch G, Schernes U, et al. Increased level of HSP27 but not of HSP72 in human heart allografts in relation to acute rejection. Transplantation. 2000;70:1694–1697. doi: 10.1097/00007890-200012270-00005. [DOI] [PubMed] [Google Scholar]
- Shu E, Matsuno H, Akamastu S, et al. alpha B-crystallin is phosphorylated during myocardial infarction: Involvement of platelet-derived growth factor-BB. Arch. Biochem. Biophys. 2005;438:111–118. doi: 10.1016/j.abb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Soldes OS, Kuick RD, Thompson IA, et al. Differential expression of Hsp27 in normal oesophagus, Barrett's metaplasia and oesophageal adenocarcinomas. Br. J. Cancer. 1999;79:595–603. doi: 10.1038/sj.bjc.6690094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somara S, Bitar KN. Tropomyosin interacts with phosphorylated HSP27 in agonist-induced contraction of smooth muscle. Am. J. Physiol.-Cell Physiol. 2004;286:C1290–C1301. doi: 10.1152/ajpcell.00458.2003. [DOI] [PubMed] [Google Scholar]
- Spector NL, Ryan C, Samson W, Levine H, Nadler LM, Arrigo AP. Heat-shock protein is a unique marker of growth arrest during macrophage differentiation of Hl-60 cells. J. Cell. Physiol. 1993;156:619–625. doi: 10.1002/jcp.1041560322. [DOI] [PubMed] [Google Scholar]
- Strahler JR, Kuick R, Eckerskorn C, et al. Identification of 2 related markers for common acute lymphoblastic-leukemia as heat-shock proteins. J. Clin. Invest. 1990;85:200–207. doi: 10.1172/JCI114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahler JR, Kuick R, Hanash SM. Diminished phosphorylation of a heat-shock protein (Hsp-27) in infant acute lymphoblastic-leukemia. Biochem. Biophys. Res. Commun. 1991;175:134–142. doi: 10.1016/s0006-291x(05)81211-2. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Suzuki A, Kishikawa M, et al. Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J. Biol. Chem. 2000;275:1095–1104. doi: 10.1074/jbc.275.2.1095. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Sugiyama Y, Hayashi Y, et al. MKBP, a novel member of the small heat shock protein family, binds and activates the myotonic dystrophy protein kinase. J. Cell. Biol. 1998;140:1113–1124. doi: 10.1083/jcb.140.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka M, Matsuno H, Ishisaki A, et al. Platelet-derived growth factor-BB phosphorylates heat shock protein 27 in cardiac myocytes. J. Cell. Biochem. 2004;91:316–324. doi: 10.1002/jcb.10717. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Kozawa O, Niwa M, et al. Contrasting effects of midazolam on induction of heat shock protein 27 by vasopressin and heat in aortic smooth muscle cells. J. Cell. Biochem. 2002;84:39–46. doi: 10.1002/jcb.1264. [DOI] [PubMed] [Google Scholar]
- Tanigawa S, Rees R, Welsh M, Gilmont R. Differential tissue expression and phosphorylation of Hsp27 in LPS treated rats. Mol. Biol. Cell. 1997;8:2133. [Google Scholar]
- Tanonaka K, Yoshida H, Toga W, Furuhama K, Takeo S. Myocardial heat shock proteins during the development of heart failure. Biochem. Biophys. Res. Commun. 2001;283:520–525. doi: 10.1006/bbrc.2001.4801. [DOI] [PubMed] [Google Scholar]
- Taylor RP, Benjamin IJ. Small heat shock proteins: a new classification scheme in mammals. J. Mol. Cell. Cardiol. 2005;38:433–444. doi: 10.1016/j.yjmcc.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Theriault JR, Lambert H, Chavez-Zobel AT, Charest G, Lavigne P, Landry J. Essential role of the NH2-terminal WD/EPF motif in the phosphorylation-activated protective function of mammalian Hsp27. J. Biol. Chem. 2004;279:23463–23471. doi: 10.1074/jbc.M402325200. [DOI] [PubMed] [Google Scholar]
- Vaidya N, Ghayour M, Lamb DJ, Ferns G. Anti heat shock protein27 (Hsp27) titres and serum hs-CRP in patients with traditional coronary risk factors including metabolic syndrome (MS) Clin. Chim. Acta. 2005;355:S118. [Google Scholar]
- Van Den Ijssel P, Norman DG, Quinlan RA. Molecular chaperones: small heat shock proteins in the limelight. Curr. Biol. 1999;9:R103–R105. doi: 10.1016/s0960-9822(99)80061-x. [DOI] [PubMed] [Google Scholar]
- Vander Heide RS. Increased expression of HSP27 protects canine myocytes from simulated ischemia-reperfusion injury. Am. J. Physiol.-Heart. Circulat. Physiol. 2002;282:H935–H941. doi: 10.1152/ajpheart.00660.2001. [DOI] [PubMed] [Google Scholar]
- Verschuure P, Tatard C, Boelens WC, Grongnet JF, David JC. Expression of small heat shock proteins HspB2, HspB8, Hsp20 and cvHsp in different tissues of the perinatal developing pig. Eur. J. Cell. Biol. 2003;82:523–530. doi: 10.1078/0171-9335-00337. [DOI] [PubMed] [Google Scholar]
- Wang XD, Tokuda H, Hatakeyama D, et al. Mechanism of simvastatin on induction of heat shock protein in osteoblasts. Arch. Biochem. Biophys. 2003;415:6–13. doi: 10.1016/s0003-9861(03)00213-3. [DOI] [PubMed] [Google Scholar]
- Xu QB, Willeit J, Marosi M, et al. Association of serum antibodies to heat-shock protein-65 with carotid atherosclerosis. Lancet. 1993;341:255–259. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- Xu QB, Kiechl S, Mayr M, et al. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis – clinical significance determined in a follow-up study. Circulation. 1999;100:1169–1174. doi: 10.1161/01.cir.100.11.1169. [DOI] [PubMed] [Google Scholar]
- Yamboliev IA, Hedges JC, Mutnick JLM, Adam LP, Gerthoffer WT. Evidence for modulation of smooth muscle force by the p38 Map kinase/HSP27 pathway. Am. J. Physiol-Heart. Circul. Physiol. 2000;278:H1899–H1907. doi: 10.1152/ajpheart.2000.278.6.H1899. [DOI] [PubMed] [Google Scholar]
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-gamma downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313–322. doi: 10.1038/sj.cdd.4401169. [DOI] [PubMed] [Google Scholar]
- Yoo SY, Chung CN, Kim JK, Chang JK. Perinuclear translocation of Hsp27 in shear stress exposed human endothelial cells. Biotechnol. Lett. 2005;27:443–448. doi: 10.1007/s10529-005-1877-8. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Aki T, Harada K, et al. Translocation of HSP27 and MKBP in ischemic heart. Cell. Struct. Funct. 1999;24:181–185. doi: 10.1247/csf.24.181. [DOI] [PubMed] [Google Scholar]
- Zagorianakou N, Ioachim E, Mitselou A, et al. Immunohistochemical expression of Heat Shock Protein 27, in normal hyperplastic and neoplastic endometrium: correlation with estrogen and progesterone receptor status, p53, pRb and proliferation associated indices (PCNA, MIB1) Eur. J. Gynaecol. Oncol. 2003;24:299–304. [PubMed] [Google Scholar]