Abstract
Hepatic iron overload in hemochomatosis patients can be highly variable but in general it develops in older patients. The purpose of this study was to compare development of iron load in of β2m-/- and Hfe-/- mice paying special attention to liver pathology in older age groups. Liver iron content of β2m-/-, Hfe-/- and control B6 mice of different ages (varying from 3 weeks to 18 months) was examined. Additional parameters (haematology indices, histopathology, lipid content and ferritin expression) were also studied in 18-month-old mice. The β2m-/- strain presents higher hepatic iron content, hepatocyte nuclear iron inclusions, mitochondria abnormalities. In addition, hepatic steatosis was a common observation in this strain. In the liver of Hfe-/- mice, large mononuclear infiltrates positive for ferritin staining were commonly observed. The steatosis commonly observed the β2m-/- mice may be a reflection of its higher hepatic iron content. The large hepatic mononuclear cell infiltrates seen in Hfe-/- stained for ferritin, may point to the iron sequestration capacity of lymphocytes and contribute to the clarification of the differences found in the progression of hepatic iron overload and steatosis in older animals from the two strains.
Keywords: β2m, Hfe, iron overload, lymphocytes, steatosis
β2m-/- mice develop spontaneously hepatic iron overload with a distribution similar to that seen in the liver pathology of hereditary hemochromatosis (HH). They have become thus an excellent model for the study of iron overload disorders (De Sousa et al. 1994; Rothenberg & Voland 1996; Santos et al. 1996). With the discovery of the Hfe gene (a non-classical MHC class I gene) and the demonstration that the C282Y mutated form failed to bind to β2m (Feder et al. 1996), the explanation for the earlier findings in β2m KO mice was thought to lie in the impairment of Hfe function due to the lack of expression of the β2m molecule. However, subsequent studies of iron overload models in KO mice showed that this was insufficient to explain the pathology of β2m-/- mice. Mice double knockout (KO) for Hfe and β2m accumulate more tissue iron than KO mice for Hfe alone (Levy et al. 2000). Mhc class I Knockout animals have also higher liver iron levels, demonstrating the involvement of classical MHC class I molecules in the regulation of iron metabolism. The existence of additional β2m interacting molecules implicated in the regulation of iron absorption was further demonstrated by Miranda et al. (2004) showing that β2mRag1(-/-) and HfeRag1(-/-) have an exceptionally high-iron overload status. In addition, the analysis of the differential gene expression of genes involved in iron metabolism in β2m-/-and Hfe-/- mice using a specialized IronChip cDNA-based microarray revealed differences in iron gene expression profiles (Muckenthaler et al. 2003, 2004) between the two models. Although the increased expression of the duodenal iron transporters (DMT-1 and ferroportin 1), found by Muckenthaler et al. (2004), may explain the documented increase in iron absorption in β2m-/-mice (Santos et al. 1996), a similar situation was not observed in Hfe-/- mice with the same genetic background (Herrmann et al. 2004). Low hepcidin expression was also documented in that study in the β2m KO mice.
The majority of the studies carried out with animal models of iron overload have used young adult mice. Age, however, is a critical factor in the expression of HH and other iron overload disorders often associated with related liver disease in humans. In the present paper, we present the results of a comparative study of two models of spontaneous iron overload in mice older than 12 months. The models examined are β2m-/- and Hfe-/- mice kept on a normal diet. The study focuses on liver pathology and reveals differences in patterns of iron distribution within cells, in lipid accumulation and in mononuclear cell infiltration.
Animals, materials and methods
Mice
The β2m-/-, Hfe-/- mice (both backcrossed onto B6 background) and C57Bl/6 (B6) mice were bred and housed at the Institute for Molecular and Cell Biology (IBMC) animal facility. Mice used in this study were from several age groups (3 weeks, 3.5 months, 9 months and 18 months) and were fed with standard diet ad libitum. For the haematological parameters six to eight animals of the oldest age group (18 months) were killed. For the histological analysis, tissues from 11 to 18 animals were examined. All animal experiments were carried out in compliance with the animal ethics guidelines at the institute.
Histology and electron microscopy
Samples of liver, spleen and pancreas of 18-month-old mice [B6, Hfe-/- and β2m-/-] were fixed in buffered formaldehyde. After routine histology, the paraffin sections were stained by haematoxylin–eosin. Ferric iron was detected by Perls’ blue staining.
For the electron microscopy, small pieces of liver from the same animal groups were fixed in 2.5% glutaraldehyde (v/v) in cacodylate buffer (0.1 M, pH 7.2) for at least 24 h at 4°C. The tissue was washed twice with cacodylate buffer and postfixed in 1% (w/v) OsO4 in the same buffer. The samples were embedded in Epon resin (TAAB Lab Equipment Ltd, Berkshire, Berkshire, UK) after dehydration in a graded series of ethanol. Semithin sections (1 μm) were performed and were stained with methylene blue. Ultrathin sections (60 nm) were cut and contrasted with 2.5% uranyl acetate followed by lead citrate. Ultrastructure analysis was done under a Zeiss EM10 electron microscope (Zeiss, Oberkochen, Germany).
Iron measurements
Briefly, liver samples from the age groups studied of the B6, Hfe-/- and β2m-/- strains were collected and stored at −70°C for further use. Liver non-haeme iron was measured by the bathophenanthroline method (Torrance & Bothwell 1980).
Transferrin saturation and haematological measurements
The blood samples were obtained by cardiac puncture from 18-month-old mice [B6, Hfe-/- and β2m-/-] under anaesthesia. For the erythroid parameters, blood was harvested in EDTA tubes and haemoglobin, erythrocyte counts, haematocrit (HCT) and mean corpuscular volume (MCV) assessed on a Coulter-S counter (Coulter Electronics, Fullerton, CA). For Iron levels, 200 μl of serum from each animal was used to determine the serum iron and total iron binding capacity (TIBC) by the ferrozine method using a Cobas Fara chemical analyser. Transferrin saturation was calculated as (serum iron/TIBC) × 100.
Liver ferritin immunohistochemistry
Liver samples were fixed in buffered formaldehyde, paraffin sections were immmunostained with polyclonal antibodies for H and L mouse ferritin subunitits (P. Arosio). All sections were stained using the Avidin Biotin Complex method. Before immunohistochemical staining, slides were deparaffinized with xylene, and passed through graded alcohols and treated for epitope retrieval by incubation in a microwave oven for 20 min in EDTA 1 mM, pH 8 (759 W). The samples were placed on tris-buffered saline (TBS) and to reduce non-specific binding, normal pig serum diluted 1:5 was applied for 20 min. Subsequently, the sections were incubated over night with polyclonal rabbit anti-Fer-H or Fer-L at a 1:100 dilution. Then, after removing the excess of serum and washing in TBS, pig anti-rabbit immunoglobulin conjugated with biotin (DAKO, Glostrup, Denmark) was applied for 30 min diluted 1:200. After additional washes, colour was developed with the dy-amine benzidine (DAB) subsbstrate (DAKO) for 2 min. After washing, the sections were counterstained with Mayer's hematoxylin for 30 min and mounted in Aquatex® (Merck, Darmstadt, Germany).
Quantitative lipid vacuoles microscopy analyses
A Leica DMLB microscope (Leica Cambridge Lda., Cambridge, UK) equipped with a colour charge-coupled device (CCD) camera was used to examine the liver sections under a ×100 magnification. In LeicaQWin image analyser (Leica Cambridge, Lda.) a square image frame (with an area of 26276.5 μm2) was used. For each mice, five similar (i.e. in the same zone of the section) microscopic fields, in randomly chosen hepatic lobuli, were evaluated for vacuoles. The results were expressed as the mean ± SEM (area μm2). Computerized image analysis of lipids content was done using a routine developed in the LeicaQWin program. Threshold levels of brightness were set and a temporary binary colour image was super-imposed on the digitalized image. The areas of interest (white) were outlined in a pseudocolour. This allows the operator to check the accuracy of the measured area. Thus, the mean of positive areas was obtained and expressed as mean ± SEM.
Statistical analyses
All statistical analysis was done using the software MINTAB. The effect of treatment was analysed using the Mann–Whitney U-test with subsequent Bonferroni corrections for multiple comparisons for continuous variables and chi-square test for proportions. Results are presented as means ± SEM. Differences were considered significant at P < 0.05.
Results
Liver iron content
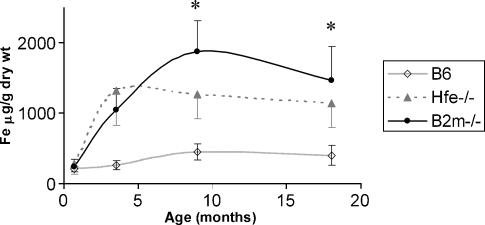
At 3 weeks of age no significant differences were seen between control and knock-out strains (Figure 1). At 3.5 months, both the Hfe-/- and β2m-/- mice had developed iron overload when compared with the B6. Between 3.5 and 9 months the β2m-/- mice further increased the hepatic iron concentration, whereas the Hfe-/- maintained the value observed at 3.5 months. These differences remained in 18-month-old mice (Table 2). The kinetics of the change in hepatic iron content with age is shown in Figure 1. Both strains show a similar starting point at 3 weeks, with a rapid increase in both Hfe-/- and β2m-/- strains, followed by a plateau in older Hfe-/- mice with further increase in iron concentration in the older β2m-/- animals. Older β2m-/- mice present statistically significantly higher iron content than the Hfe-/- counterpart (Figure 1).
Figure 1.
Hepatic iron concentration vs. age in B6, Hfe-/- and β2m-/- strains. Liver samples (n = 8−12) were collected at 0.7, 3.5, 9 and 18 months from all strains. For the iron quantification, the bathophenanthroline method was used. *Statistically significant difference (P < 0.05) between the B6 strain and the Hfe-/- and β2m strains.
Table 2.
Liver histological analysis, iron content of 18-month-old mice
| Histological analysis | Iron | |||||
|---|---|---|---|---|---|---|
| Strain | n | Mononuclear infiltrates, % | Steatosis foci, % | Generalized steatosis, % | Vacuoles area, μm2 per field | Fe, μg/g dry wt |
| B6 | 18 | 7 (39) a | 6 (33) a | 2 (11) a | 130 ± 172 a | 399 ± 140 a |
| Hfe-/- | 11 | 8 (72) b | 3 (27) a | 1 (9) a | 81 ± 75 a | 1142 ± 344 b |
| β2m-/- | 15 | 5 (33) a | 9 (60) b | 7 (47) b | 1782 ± 2752 b | 1468 ± 479 c |
Liver iron content and vacuoles area are presented as mean ± SE.
Within each column different letters indicate significant differences between groups at P < 0.05.
Haematological indices
The evaluation of the erythroid parameters, demonstrated that the 18-month-old β2m-/- mice had significantly higher values of red blood cells (RBC) and haemoglobin when compared with mice of the B6 or Hfe-/- strains (Table 1). The HCT level showed an increase from B6 to Hfe-/- and from Hfe-/- to β2m-/-. However, the MCV values were significantly higher in the Hfe-/- followed by the β2m-/- that in turn were higher than the B6 (Table 1).
Table 1.
Haematological indices of 18-month-old mice
| Erythroid parameter | Serum iron levels | |||||||
|---|---|---|---|---|---|---|---|---|
| Strain | n | RBC, ×1012l | Hb, mmol/l | HCT, % | MCV, fl | SI, μg/dl | TIBC, μg/dl | Tf Sat, % |
| B6 | 8 | 8.12 ± 0.37 a | 12.1 ± 0.6 a | 36.0 ± 2.0 a | 44.3 ± 0.8 a | 167 ± 32 a | 440 ± 43 a | 37.8 ± 4.8 a |
| Hfe-/- | 6 | 8.20 ± 0.13 a | 13.1 ± 0.3 a | 39.4 ± 1.2 b | 48.0 ± 1.0 b | 329 ± 31 b | 383 ± 28 b | 87.4 ± 1.9 b |
| β2m-/- | 8 | 9.82 ± 0.72 b | 15.3 ± 0.9 b | 45.2 ± 2.9 c | 46.0 ± 0.9 c | 403 ± 50 b | 539 ± 46 c | 73.4 ± 12.0 b |
Values are presented as mean ± SE.
RBC, red blood cells; Hb, haemoglobin; HCT, haematocrit; MCV, mean corpuscular volume; SI, serum iron; TIBC, total iron binding capacity; Tf Sat, transferrin saturation.
Within each column different letters indicate significant differences between groups at P < 0.05.
As expected, the serum iron and transferrin saturation levels were significantly elevated in the Hfe-/- and β2m-/- mice compared with the B6 counterpart. Interestingly, the levels of TIBC were high in β2m-/- (539 ± 46) followed by the B6 (440 ± 43) and low in the Hfe-/- mice (383 ± 28). The respective differences were statistically significant (Table 1).
Histology and EM
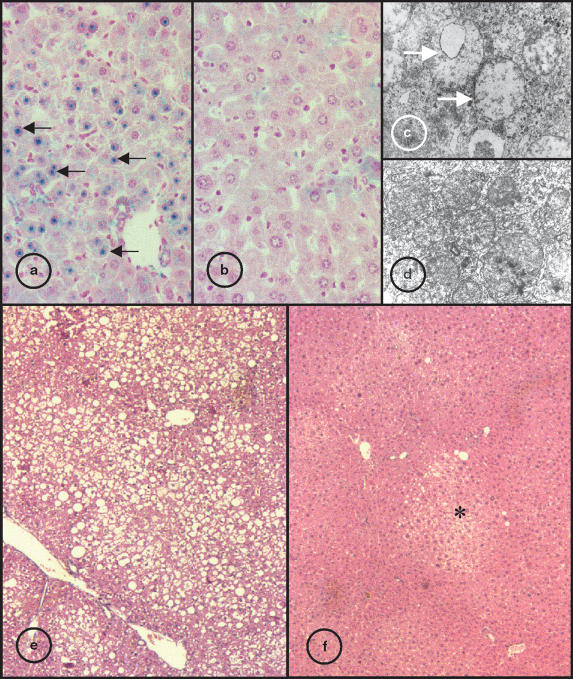
From the analysis of liver sections stained with Perls from 18-month-old mice it was clear that both β2m-/- and Hfe-/- had stainable iron in hepatocytes but not in Kupffer cells, indicative of an iron overload pattern similar to that of human HH, a situation not observed in the B6 mice (data not shown). However, the intensity of staining was often higher in the β2m-/- animals when compared with the Hfe-/- in agreement with the iron content values (Table 2). Moreover, in the β2m-/- mice visible blue staining in the nucleus of the hepatocytes was always seen, a situation not found in the Hfe-/- or B6 strains (Figure 2a,b). In addition, the ultrastructural analysis of the liver often revealed abnormal mitochondria (enlarged and without visible inner cristae) in the β2m-/- hepatocytes (Figure 2c,d).
Figure 2.
Liver sections of 18-month-old β2m and Hfe-/mice. Sections stained for iron by Perls method, the blue stain indicates heavy iron deposition: (a) β2m-/- strain, black arrow shows blue stained hepatocyte nuclear inclusions; (b) Hfe-/- mice (original magnification ×400). Electron photomicrograph of hepatic parenchyma cells; (c) β2m-/- mice, white arrow indicate abnormal mitochondria without visible cristae; (d) Hfe-/- strain (original magnification ×8000). Liver sections stained with HE; (e) β2m-/- mice, large vacuoles visible trough the parenchyma indicative of generalized steatosis; (f) Hfe-/- strain, asterisk indicate steatosis foci (original magnification ×40).
An analysis of liver sections showed that hepatic steatosis was more frequent in the β2m-/- mice than in the Hfe-/- or B6 strains. Although steatosis foci could be observed in the three groups, the number of animals where generalized steatosis was observed, was significantly higher in β2m-/- mice (Figure 2e,f and Table 2). Moreover, morphometric analysis of the area occupied by lipids identified by oil red stain indicative of lipid content was significantly larger in the β2m-/- (Table 2).
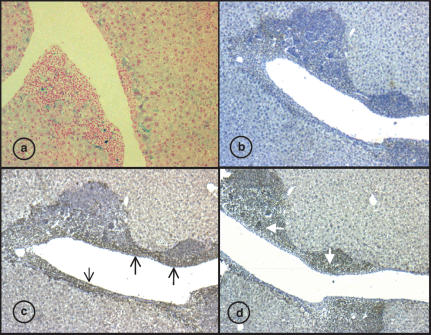
The liver histology of 18-month-old mice revealed also the presence of mononuclear infiltrates often associated with large vessels. Interestingly, the frequency of mononuclear liver infiltrates on Hfe-/- mice was significantly higher (eight of 11 animals) when compared with β2m-/- (5/15) or B6 (7/18) (see Table 2). In addition, it was possible to detect sporadic cells positive for Perls blue indicative of iron content in the mononuclear infiltrate (Figure 3a).
Figure 3.
Hepatic mononuclear infiltrates of 18-months-old Hfe-/mice. (a) Liver sections stained for iron by Perls method, the dark blue stain indicates heavy iron deposition within the infiltrates (original magnification ×100). (b–d) Anti-Ferritin immunohistochemistry (original magnification ×100). (b) conjugate antibody only. (c) Anti-mouse Fer-L staining showing heavy Ferritin L staining in the mononuclear infiltrate endothelial region (black arrow) and in the hepatocytes. (d) Anti-mouse Fer-H staining showing a clear staining in the mononuclear infiltrates (white arrow) but not in the endothelial and parenchyma area.
The study of heart and pancreas sections stained with Perls method showed that some of the 18-month-old Hfe-/- mice had cardiac iron deposits, a situation not observed in the β2m-/- and B6 mice at similar ages.
Immunohistochemistry
To answer the question whether the presence of the mononuclear cells could be associated with the differences observed in the iron content changes seen between β2m-/- and the Hfe-/- mice, paraffin sections of livers available from mice with infiltrates were stained with polyclonal antibodies against L and H Ferritin. The immunohistochemistry staining revealed a clear separation of two zones within the infiltrate itself (Figure 3).
The liver sections labelled with anti-Fer-L revealed a visible staining of the parenchyma and a very strong labelling of the infiltrates. The Fer-L positive mononuclear infiltrate area was confined mainly to the endothelial and periendothelial region (Figure 3c). By contrast, the anti-Fer-H sections showed a heavy staining of the mononuclear cells themselves, located in the outer region of the aggregate. No strong labelling for H-Ferritin was observed in the hepatocytes (Figure 3d). Collectively, these results indicate that the mononuclear infiltrates contain both H and L ferritin.
Discussion
The results of the present comparative study between older β2m-/- and Hfe-/- mice reveal the existence of differences in iron distribution, degree of iron overload, liver mononuclear cell infiltration and hepatic steatosis.
The fact that the plateau of hepatic iron concentration was reached at different ages for the Hfe-/- and the β2m-/- strains (at 3.5 and 9 months, respectively) may indicate differences at the level of iron absorption. In addition, after the stabilization of hepatic iron, the β2m-/- mice showed higher liver iron content than the Hfe-/- counterpart a situation that might explain results reported in other studies (Levy et al. 2000). Abnormally, high iron absorption has been reported both in β2m-/- (Santos et al. 1998) and in the Hfe-/- mice (Bahram et al. 1999). Since none of those studies compared the two strains at the same time, in the same housing conditions, it is unclear whether they have a similar degree of iron absorption disruption. The expression profile studies carried out by Muckenthaler et al. (2003, 2004) in β2m-/- and Hfe-/- duodenal tissue showed that both duodenal iron transporters (DMT1 and Ferroportin) are upregulated in the β2m-/-, a situation not observed in Hfe-/- mice. The importance of the differences seen cannot, however, be overlooked. Alternatively, the enhanced iron accumulation in β2m-/- mice could be explained by the lack of β2m binding molecules, other than the Hfe itself, as concluded also by the study of Muckenthaler et al. (2004). Recently, it was demonstrated that the absence of classical MHC itself in mice leads to moderate hepatic iron overload (Cardoso et al. 2002) pointing to the importance of the MHC proteins and other unsuspected β2m binding molecules in iron metabolism.
In addition to the elevated liver iron content found in the β2m-/- mice, it was apparent from the histological analysis that the hepatocytes had nuclear iron inclusions, a situation not observed in Hfe-/- mice, in this study, illustrated albeit not commented earlier (De Sousa et al. 1994).
Nuclear inclusions in hepatocytes of Hfe-/- mice have recently been extensively described by Magens et al. (2005). It must be stressed that in the present study animals were not overloaded by administration of iron. Nuclear inclusions in this and the previous report of De Sousa et al. (1994) iron overload developed spontaneously, thus mimicking the clinical natural history seen in human disease. Moreover, in the β2m-/- strain, electron microscopy studies showed frequently disrupted mitochondria with an abnormal internal organization. These findings further indicate the high severity of hepatic iron overload on β2m-/- liver when compared with the Hfe-/- counterpart.
The comparisons of the haematological indices from this study highlighted the differences between the mice strains at older age. With the B6 mice as baseline control, it becomes apparent that the Hfe-/- presents a profile resembling that seen in hemochromatosis patients, that is, normal levels of RBC counts and haemoglobin concentration and a elevated HCT, MCV and transferrin saturation. By contrast, the β2m-/- mice showed a significantly elevated number of RBC associated with higher haemoglobin concentration as has previously been reported (8). In addition, the β2m-/- mice presented an elevated HCT and MCV; however, the values were significantly different from those observed in the Hfe-/- mice. As expected both strains have elevated serum iron and transferrin saturation (Santos et al. 1996; Zhou et al. 1998). Interestingly, the TIBC observed in the β2m-/- was higher than in control mice. That observation contrasts with the significantly lower TIBC values registered in the Hfe-/- strain that is normally attributed to a response to the elevated iron stores. The fact that TIBC is elevated in the β2m-/- mice, unlike the Hfe-/- strain, may be due to the existence of a constitutive elevated iron absorption that surpasses the threshold of transferrin transport capacity leading to the possible mobilization of other iron binding proteins. It was recently demonstrated that haptoglobin, a plasma protein with high affinity for haemoglobin, modifies the hemochromatosis phenotype in mice, contributing significantly to iron loading observed in the Hfe-/- strain (Tolosano et al. 2005). It will be of interest to look at the circulating levels of such iron binding proteins in β2(-/-) as well as Hfe-/- mice.
The finding that liver steatosis was common in the β2m-/- old mice, a situation not observed in older Hfe-/- or B6 mice is also of considerable interest as a model of human liver disease. Iron-storage diseases in humans are believed to cause organ damage through generation of reactive oxygen species that can cause oxidative damage to lipids. Hepatic lipid peroxidation has been demonstrated in animals with overload (by iron-supplemented diet or intraperitoneal administration of iron dextran), through the increase of major products of lipid peroxidation, such as malondialdehyde (Valerio & Petersen 1998; Khan et al. 2002; Sochaski et al. 2002) or thiobarbituric acid reactive substances, observed in 18-month-old Hfe-/- mice (Lebeau et al. 2002). In addition, it has been shown that in iron overload mice the stearoyl coenzyme A desaturase 1 (SCD1) mRNA level and its corresponding activity were increased (Pigeon et al. 2001a). Since SCD1 is involved in the biosynthesis of unsaturated fatty acids, it was suggested that the increase of SCD1 expression and activity, could reflect a compensatory mechanism to a cellular need for renewing unsaturated fatty acids degraded by lipid peroxidation. Therefore, it is tempting to speculate that the high hepatic iron content seen in β2m-/- mice is linked to a pronounced lipid peroxidation in this strain. As response, lipid biosynthesis may be enhanced that in turn can lead to hepatic lipid accumulation in the form of exuberant hepatic steatosis. This was not observed in Hfe-/- mice.
It was also of interest that the H and L ferritin expression areas differ. The narrow perivascular, where iron accumulation has been reported in 12-month Hfe-/- mice (Pigeon et al. 2001b) was occupied by the L-fer positive mononuclear cells. It is tempting to speculate that the hepatic mononuclear cell infiltrates could be part of a response to iron load. This findings and previous results derived particularly from results with patients (Porto et al. 1994) and Rag(-/-) mice (Santos et al. 2000; Miranda et al. 2004) provide further evidence for an association in an experimental model of lymphocyte numbers in iron overload. However, in the experimental models, such as examined here, this conclusion is based on lymphocyte numbers from birth, examined in all compartments, whereas in patients, with a few exceptions (Cardoso et al. 2001) lymphocyte numbers are measured exclusively in the peripheral blood.
In conclusion, the comparison between the Hfe-/- and β2m-/- mice showed that the β2m-/- strain presents a severe hepatic iron overload picture, with higher hepatic iron content and other histocytological features such as nuclear iron inclusions and mitochondria abnormalities. The finding that liver steatosis was more common in the β2m-/- strain may be a reflection of the high hepatic iron content causing lipid peroxidation that in turn leads to lipid biosynthesis and its accumulation. The observation that large mononuclear infiltrates were commonly observed in Hfe-/- strain, and not in β2m-/-, may relate to the known constitutive low number of CD8 lymphocytes in the β2m-/- mice. Unfortunately, it was not possible to characterize the lymphocyte phenotype in the current study. The fact that these large sheets were positive for ferritin staining make it tempting to speculate that the iron sequestering capacity by lymphocytes is abrogated in β2m-/- mice, thus contributing for a more severe progression of hepatic iron overload and steatosis in older animals of this strain.
Acknowledgments
The authors would like to thank the help of Miguel Duarte (microscopy settings), Bianca Machado (animal dissection), Cidália Silva and Júlia Reis (haematological parameters). This study was supported by the EU QLG1-CT-1999-00665 Project; Calouste Gulbenkian Foundation/FCT Project on Hemochromatosis (Portugal) and the INNOVA Foundation/APBRF (USA).
References
- Bahram S, Gilfillan S, Kuhn LC, et al. Experimental hemochromatosis due to MHC class I HFE deficiency: immune status and iron metabolism. Proc. Natl. Acad. Sci. U. S. A. 1999;96:3312–13317. doi: 10.1073/pnas.96.23.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso EM, Hagen K, De Sousa M, Hultcrantz R. Hepatic damage in C282Y homozygotes relates to low numbers of CD8+ cells in the liver lobuli. Eur. J. Clin. Invest. 2001;31:45–53. doi: 10.1046/j.1365-2362.2001.00744.x. [DOI] [PubMed] [Google Scholar]
- Cardoso EM, Macedo MG, Rohrlich P, et al. Increased hepatic iron in mice lacking classical MHC class I molecules. Blood. 2002;100:4239–4241. doi: 10.1182/blood-2002-05-1565. [DOI] [PubMed] [Google Scholar]
- De Sousa M, Reimao R, Lacerda R, Hugo P, Kaufmann SH, Porto G. Iron overload in beta 2-microglobulin-deficient mice. Immunol. Lett. 1994;39:105–111. doi: 10.1016/0165-2478(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary hemochromatosis. Nat. Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- Herrmann T, Muckenthaler M, van der Hoeven F, et al. Iron overload in adult Hfe-deficient mice independent of changes in the steady-state expression of the duodenal iron transporters DMT1 and Ireg1/ferroportin. J. Mol. Med. 2004;82:39–48. doi: 10.1007/s00109-003-0508-x. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Tipnis UR, Ansari GA, Boor PJ. Protein adducts of malondialdehyde and 4-hydroxynonenal in livers of iron loaded rats: quantitation and localization. Toxicology. 2002;173:193–201. [PubMed] [Google Scholar]
- Lebeau A, Frank J, Biesalski HK, et al. Long-term sequelae of HFE deletion in C57BL/6 × 129/O1a mice, an animal model for hereditary haemochromatosis. Eur. J. Clin. Invest. 2002;32:603–612. doi: 10.1046/j.1365-2362.2002.01026.x. [DOI] [PubMed] [Google Scholar]
- Levy JE, Montross LK, Andrews NC. Genes that modify the hemochromatosis phenotype in mice. J. Clin. Invest. 2000;105:1209–1216. doi: 10.1172/JCI9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magens B, Dullmann J, Schumann K, Wulfhekel U, Nielsen P. Nuclear iron deposits in hepatocytes of iron-loaded HFE-knock-out mice: a morphometric and immunocytochemical analysis. Acta Histochem. 2005;107:57–65. doi: 10.1016/j.acthis.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Miranda CJ, Makui H, Andrews NC, Santos MM. Contributions of beta2-microglobulin-dependent molecules and lymphocytes to iron regulation: insights from HfeRag1(-/-) and beta2mRag1(-/-) double knock-out mice. Blood. 2004;103:2847–2849. doi: 10.1182/blood-2003-09-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckenthaler M, Roy CN, Custodio AO, et al. Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis. Nat. Genet. 2003;34:102–107. doi: 10.1038/ng1152. [DOI] [PubMed] [Google Scholar]
- Muckenthaler M, Rodrigues P, Macedo MG, et al. Molecular analysis of iron overload in B2-microglobulin-deficient mice. Blood Dis. Mol. 2004;33:125–131. doi: 10.1016/j.bcmd.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Pigeon C, Legrand P, Leroyer P, et al. Stearoyl coenzyme A desaturase 1 expression and activity are increased in the liver during iron overload. Biochim. Biophys. Acta. 2001a;1535:275–284. doi: 10.1016/s0925-4439(01)00024-2. [DOI] [PubMed] [Google Scholar]
- Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001b;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- Porto G, Reimão R, Gonçalves C, Vicente C, Justiça B, De Sousa M. Haemochromatosis as a window into the study of the immunological system in man: a novel correlation between CD8+ lymphocytes and iron overload. Eur. J. Haematol. 1994;52:283–290. doi: 10.1111/j.1600-0609.1994.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Rothenberg BE, Voland JR. Beta2 knockout mice develop parenchymal iron overload: a putative role for class I genes of the major histocompatibility complex in iron metabolism. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1529–1534. doi: 10.1073/pnas.93.4.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M, Schilham MW, Rademakers LH, Marx JJ, De Sousa M, Clevers H. Defective iron homeostasis in beta 2-microglobulin knockout mice recapitulates hereditary hemochromatosis in man. Exp. Med. 1996;184:1975–1985. doi: 10.1084/jem.184.5.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M, Clevers H, De Sousa M, Marx JJ. Adaptive response of iron absorption to anemia, increased erythropoiesis, iron deficiency, and iron loading in beta2-microglobulin knockout mice. Blood. 1998;91:3059–3065. [PubMed] [Google Scholar]
- Santos MM, De Sousa M, Rademakers LH, Clevers H, Marx JJ, Schilham MW. Iron overload and heart fibrosis in mice deficient for both beta2-microglobulin and Rag1. Am. J. Pathol. 2000;157:1883–1892. doi: 10.1016/s0002-9440(10)64827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochaski MA, Bartfay WJ, Thorpe SR, et al. Lipid peroxidation and protein modification in a mouse model of chronic iron overload. Metabolism. 2002;51:645–651. doi: 10.1053/meta.2002.30530. [DOI] [PubMed] [Google Scholar]
- Tolosano E, Fagoonee S, Garuti C, et al. Haptoglobin modifies the hemochromatosis phenotype in mice. Blood. 2005;105:3353–3355. doi: 10.1182/blood-2004-07-2814. [DOI] [PubMed] [Google Scholar]
- Torrance JD, Bothwell TH. Tissue iron stores. In: Cook JD, editor. Methods in Hematology. Vol. 1. New York, NY: Churchill Livingston Press; 1980. pp. 104–109. [Google Scholar]
- Valerio LG, Jr, Petersen DR. Formation of liver microsomal MDA-protein adducts in mice with chronic dietary iron overload. Toxicol. Lett. 1998;98:31–39. doi: 10.1016/s0378-4274(98)00100-3. [DOI] [PubMed] [Google Scholar]
- Zhou XY, Tomatsu S, Fleming RE, et al. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]