Abstract
Chronic hypoxia results in pulmonary hypertension due to vasoconstriction and structural remodelling of peripheral lung blood vessels. We hypothesize that vascular remodelling is initiated in the walls of prealveolar pulmonary arteries by collagenolytic metalloproteinases (MMP) released from activated mast cells. Distribution of mast cells and their expression of interstitial collagenase, MMP-13, in lung conduit, small muscular, and prealveolar arteries was determined quantitatively in rats exposed for 4 and 20 days to hypoxia as well as after 7-day recovery from 20-day hypoxia (10% O2). Mast cells were identified using Toluidine Blue staining, and MMP-13 expression was detected using monoclonal antibody. After 4, but not after 20 days of hypoxia, a significant increase in the number of mast cells and their MMP-13 expression was found within walls of prealveolar arteries. In rats exposed for 20 days, MMP-13 positive mast cells accumulated within the walls of conduit arteries and subpleurally. In recovered rats, MMP-13 positive mast cells gathered at the prealveolar arterial level as well as in the walls of small muscular arteries; these mast cells stayed also in the conduit part of the pulmonary vasculature. These data support the hypothesis that perivascular pulmonary mast cells contribute to the vascular remodelling in hypoxic pulmonary hypertension in rats by releasing interstitial collagenase.
Keywords: mast cells, matrix-metalloproteinase 13, normobaric hypoxia, pulmonary hypertension, rat
Introduction
Exposure to chronic hypoxia induces hypoxic pulmonary hypertension (HPH), which results from structural remodelling of peripheral pulmonary blood vessels and lung vasoconstriction (Reeves & Herget 1984; Reid 1986). The vascular remodelling starts as early as in the first week of exposure (Rabinovitch et al. 1979) and is characterized by medial hypertrophy and proliferation of smooth muscle in peripheral lung arterioles and by fibrotization of vascular walls (Hislop & Reid 1976; Herget et al. 1978; Rabinovitch et al. 1979). The turnover of vascular collagen is increased (Bishop et al. 1990; Poiani et al. 1990). As a result of increased collagenolytic activity (Novotná & Herget 1998), low molecular weight fragments of collagen type I molecules accumulate in the walls of peripheral pulmonary arteries (Novotná & Herget 1998). Collagen breakdown is highest in the first days of exposure to hypoxia (Novotná & Herget 2001). Experimental inhibition of collagen metabolism reduced vascular remodelling in hypoxic rats and inhibited the development of HPH (Kerr et al. 1984, 1987; Poiani et al. 1991; Herget et al. 2003). Elastolytic activity is also elevated during the development of chronic hypoxic pulmonary hypertension, and blockade of this activity inhibits pulmonary hypertension (Rabinovitch 1999). We hypothesize that breakdown of matrix protein molecules induced by hypoxic tissue injury may be one of the triggering mechanisms of vascular remodelling in chronic hypoxia (Hampl & Herget 2000; Novotná & Herget 2002).
Important sources of collagenolytic enzymes are mast cells. Tozzi et al. (1998) have shown that an increase in collagenolytic activity in the lung mast cells plays an important role in the normalization of lung vascular structure during recovery from exposure to chronic hypoxia. The number of perivascular lung mast cells increases in hypoxic animals (Kay et al. 1974; Mungall 1976; Tucker et al. 1977; Williams et al. 1981; Migally et al. 1983). It is increased in human high altitude residents as well (Heath 1992). In chronically hypoxic rats, inhibition of mast cell degranulation reduced the development of hypoxic pulmonary hypertension (Mungall 1976; Kay et al. 1981).
We tested the hypothesis that in rats exposed to chronic hypoxia, collagenolytic metalloproteinases are released from perivascular mast cells that accumulate in the walls of peripheral pulmonary arteries.
Methods
Experimental animals
Fifty male Wistar rats (initial body weight 210 ± 8 g; Anlab, Prague, Czech Republic) started the experiment. Thirty animals were exposed to hypoxia (FiO2 = 0.1) in a normobaric hypoxic chamber (Hampl & Herget 1990). Experimental rats were examined after 4 (n = 10) and 20 (n = 8) days of hypoxic exposure (body weight 198 ± 5 g and 241 ± 8 g, respectively) as well as after 7-day recovery from 20-day hypoxia (n = 8; body weight 323 ± 23 g). Two groups of control animals (n = 10 and 9, body weight 245 ± 19 g and 374 ± 35 g, respectively) were housed at atmospheric air in the same room for 4 and 28 days, respectively. All groups of rats received standard rat diet and tap water ad libitum. During the night on the day 11 of hypoxic exposure, four animals died. All protocols and procedures employed in this study were reviewed and approved by the Animals Protection Expert Commission of the Faculty.
Pathologic anatomy
At the end of the experiment, all animals were anaesthetized with chloral hydrate (300 mg/kg b.w., i.p.; Tamda, Olomouc, Czech Republic) and killed by cutting the cervical vertebral column.
The left lungs were fixed with Baker's fluid, longitudinally cut and embedded in paraffin. Parallel sections 4–6 μm thick were cut and a series of stainings was carried out on each specimen: haematoxylin & eosin (HE), cresyl fast blue (FB), aldehyde fuchsine (AF), and gomori silver stain. To identify the mast cells, Toluidine blue (TB) staining was used. The immunohistochemical detection of the rodent-type interstitial collagenase, matrix metalloproteinase-13 (MMP-13), on paraffin sections was performed after microwave-oven antigen retrieval (Thomas et al. 2000). Having blocked the endogenous alkaline phosphatase (AP) with levamizole and the non-specific binding with 10% bovine serum albumin (BSA) in tris-buffered saline (TBS), the sections were incubated with a monoclonal anti MMP-13 antibody (24 h at the room temperature in a humid chamber). The antibody was obtained as the ascitic fluid from mice at the Department of Medical Chemistry and Biochemistry (R. Vytášek and J. Novotná, personal communication) and diluted 1 : 50 with TBS. The secondary antibody, rabbit anti-mouse polyclonal AP-labelled (Sigma-Aldrich), was used in the second step, in the dilution 1 : 50 with TBS for 30 min. The binding reaction was visualized using Fast Red TR/Naphthol AS-MX Sigma FAST tablets (Sigma-Aldrich), slides were counterstained with haematoxylin and mounted in gelatin. For the control reaction, the primary antibody was omitted.
The right medial lung lobes were submerged in 4% cacodylate-buffered paraformaldehyde, immediately cut and tiny pieces of the central and peripheral portions of the lobes were fixed, dehydrated and embedded in medium-grade LR White Resin (London Resin Comp., Reading, UK) using a hot cure. Semi-thin sections were cut, mounted on slides using acetone and stained with TB to identify mast cells in perivascular locations. Then, selected serial semi-thin sections were incubated with 10% BSA in TBS, followed by incubation with the same monoclonal anti MMP-13 antibody as in the paraffin technique for 24 h at room temperature in a humid chamber. A goat anti-mouse polyclonal gold conjugate (Sigma-Aldrich) was used in the second step in the dilution 1 : 50 with TBS for 60 min, followed by intensification by reaction with silver using Silver Enhancer Kit (Sigma-Aldrich). Semi-thin sections were counterstained with TB to colocalize the metachromatic reaction of mast-cell granules and the metallic silver precipitate detecting MMP-13 sites.
Paraffin slides were quantitatively evaluated using Lucia G Image Analysis software (Laboratory Imaging, Prague, Czech Republic). The area of each section of the lung was measured (mm2) and mast cells in the subpleural, peribronchial and perivascular locations were counted. Mast cells found within walls of prealveolar arterioles, small muscular arterioles or arteries, and conduit arteries (Meyrick et al. 1978) were counted separately in both TB and anti-MMP-13 stainings. The same evaluation was performed in the large pulmonary veins, i.e. veins with myocardium within their walls (Paes de Almeida et al. 1975). Absolute numbers of mast cells in each six locations in both stainings were expressed as number per 50 mm2. The hearts were separated in parts and right ventricle to left ventricle plus septum weight index (RV/LV + S) was used as an indicator of presence of chronic pulmonary hypertension (Fulton et al. 1952).
Statistical analysis
Separately in TB- and anti-MMP-13-staining, data were compared between control and hypoxic groups using a one-way analysis of variance (anova) in conjunction with Tukey post-hoc test and Kruskal–Wallis one-way anova in conjunction with Dunn's method of the multiple comparison procedure, respectively, when appropriate (SigmaStat 2.0; SPSS Inc., Chicago, IL, USA). The results are presented as means ± SEM. Differences were considered significant at P < 0.05.
Results
Control animals
No pathological findings were observed in lungs of 4-day control animals. Mast cells were found scattered in subpleural, peribronchial and perivascular locations. Rare individual, MMP-13 positive neutrophilic granulocytes were found in alveolar septa or in subpleural, but never in perivascular locations. Similar findings were observed in 28-day control animals except one male in which we noticed increased cellularity of the lung interstitium and especially of the walls of bronchi and vessels, consisting of plasma cells, lymphocytes and neutrophilic granulocytes. Significant differences were encountered in numbers of his mast cells as well; that is why mast cell counts of this animal were discarded.
Four-day hypoxia
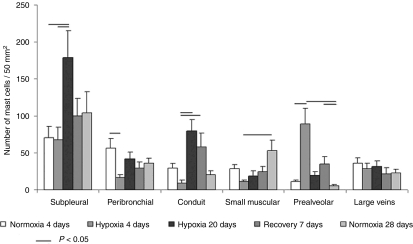
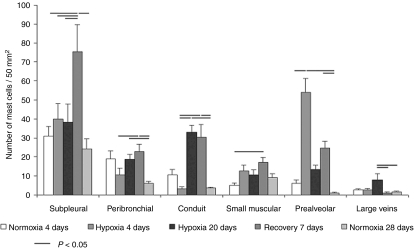
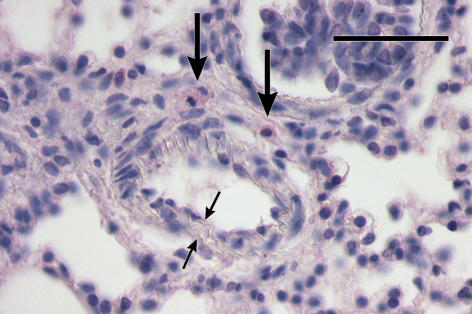
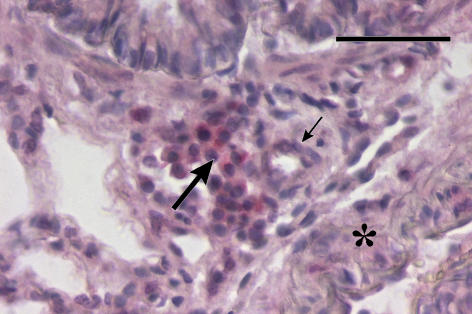
In rats exposed to 4-day hypoxia, mast cells significantly accumulated in the prealveolar portion of pulmonary vasculature (Figure 1). Similarly, in contrast to the two control groups, a significantly higher number of mast cells in prealveolar vessels expressed MMP-13 (Figure 2). Newly muscularized peripheral pulmonary arteries were observed at the prealveolar level, usually accompanied with MMP-13 positive mast cells (Figure 3). Mast cells relatively often surrounded the initial portion of a small muscular artery branched from a conduit one (supernumerary arteries, Elliott & Reid 1965) (Figure 4). MMP-13 positive neutrophilic granulocytes were found not numerous near mast cells in adventitia of some prealveolar and small muscular arteries and bronchioles. Similar granulocytes were found in several bronchioles. The number of mast cells detected peribronchially was low (Figure 1), and they rarely expressed MMP-13 (Figure 2). Occasionally, we observed MMP-13 positive alveolar macrophages. Four days of hypoxia were too short to produce haemodynamically significant pulmonary hypertension, as RV/LV + S weight ratio did not differ between 4 days hypoxic rats and normoxic controls.
Figure 1.
Distribution of pulmonary Toluidine Blue-detected mast cells in control and hypoxic rats. Means (± SEM) of absolute number of mast cells per 50 mm2 of the left lung section.
Figure 2.
Distribution of pulmonary MMP-13-expressing mast cells in control and hypoxic rats. Means (± SEM) of absolute number of mast cells expressing MMP-13 per 50 mm2 of the left lung section.
Figure 3.
Newly muscularized double-laminated (small arrows) prealveolar arteriole with MMP-13-positive mast cells (long arrows) in its adventitia. 4-day hypoxic rat. Anti-MMP-13/Fast Red and Haematoxylin. Bar = 50 μm.
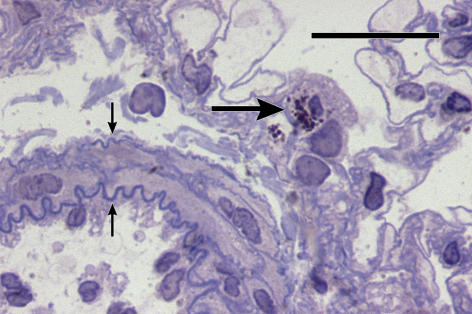
Figure 4.
A group of MMP-13-positive mast cells (long arrow) in adventitia of a newly muscularized supernumerary arteriole (small arrow) outbranched from a conduit artery (asterisk). 4-day hypoxic rat. Anti-MMP-13/Fast Red and Haematoxylin. Bar = 50 μm.
Twenty-day hypoxia
The rats exposed to hypoxia for 20 days developed hypoxic pulmonary hypertension, their RV/LV + S weight index was significantly higher than in normoxic controls. It was 0.28 ± 0.02 in controls and 0.39 ± 0.23 in experimental animals (P = 0.0006). The major difference from 4-day hypoxia group, however, was that the mast cells accumulated in the conduit part of the pulmonary vasculature and subpleurally (Figure 1). Many mast cells surrounding conduit pulmonary arteries expressed MMP-13 (Figures 2 and 5). The numbers of mast cells at the prealveolar level and around the small pulmonary arteries in rats exposed to hypoxia for 20 days, however, did not differ from normoxic controls in spite of their slight increase (Figure 2). MMP-13 was expressed also in smooth muscle cells in tunica media of some conduit arteries. The number of the MMP-13 positive mast cells in the walls of the large veins significantly increased in comparison with other groups (Figure 2).
Figure 5.
Small muscular artery with well developed elastic laminae and elastic network within its tunica media (small arrows) and a MMP-13-positive mast cell with released granules (long arrow). 20-day hypoxic rat. Anti-MMP-13/gold conjugate/silver enhanced and Toluidine Blue, semi-thin section. Bar = 20 μm.
Multiple atelectatic foci were occasionally encountered within lung parenchyma in all rats of the 20-day hypoxia group. In these foci, some alveoli were filled with clotted plasma and MMP-13 positive macrophages with abundant foamy cytoplasm, sometimes accompanied with red blood cells. A few conduit and small muscular arteries presented inflammatory infiltration composed of neutrophilic granulocytes or lymphocytes in their adventitia. Focally, pleural thickening occurred together with subpleural accumulation of mast cells; these mast cells were mostly MMP-13-negative.
Twenty-day hypoxia and 7-day recovery
After 20-day period of hypoxia, a group of rats were given 7-day recovery period in normoxic conditions. In this group, the moderate increase in number of the mast cells at the prealveolar arterial level was encountered, again, even MMP-13 positive. Nevertheless, the mast cells, even MMP-13 positive, again accumulated in the conduit part of the pulmonary vasculature. Increased number of MMP-13 positive mast cells occurred also in the walls of small muscular arteries (Figures 1 and 2).
Minor isolated atelectatic foci were occasionally encountered within lung parenchyma in three rats of the recovery group. In these foci, some alveoli were filled with clotted plasma, alveolar macrophages were rare and mostly MMP-13 negative; tiny intra-alveolar haemorrhage was sometimes found.
Common features
The total number of mast cells in the left lung was not significantly different among all groups, although it was slightly higher in the rats exposed to hypoxia for 20 days. This difference was due to the increase in number of the subpleurally located mast cells. No differences were encountered among all groups in numbers of the total mast cells found in walls of the large veins, either. Silver-enhanced visualization of MMP-13 on semi-thin sections stained with toluidine blue verified the presence of MMP-13 in mast cell granules (Figure 5).
Discussion
The main finding of the present study is that in 4 days of exposure to hypoxia, the interstitial collagenase (MMP-13) producing mast cells conspicuously accumulated in the small prealveolar pulmonary arteries. Later, after 20 days of exposure, when hypoxic pulmonary hypertension is fully developed, the majority of MMP-13 producing mast cells reside in the walls of conduit portion of pulmonary vasculature. Seven-day recovery after 20-day hypoxia causes again moderate increase in number of MMP-13 producing mast cells at the levels of prealveolar and small muscular arteries; accumulation of these mast cells is still encountered in the walls of conduit arteries as well.
We exposed rats to the hypoxic environment (10% O2) in a normobaric hypoxic chamber. According to our repeated experience, this procedure in <3 weeks consistently induces stable pulmonary hypertension, characterized by an increase in pulmonary arterial blood pressure, increase in the weight of the right heart ventricle, and hypertrophy of media and fibrotization in the walls of peripheral pulmonary arteries (Herget et al. 1978; Hampl & Herget 1990; Herget et al. 2003). Thus, the increase in the right to left ventricles weight ratio in the current experiment confirms the presence of pulmonary hypertension in the group exposed to hypoxia for 20 days. Previous findings of our and other groups demonstrated that significant morphological, biochemical and haemodynamic changes can be detected even during the first week of hypoxic exposure (Rabinovitch et al. 1979; Herget et al. 2000; Lachmanováet al. 2005). Increase in pulmonary arterial blood pressure and right heart weight, however, is usually moderate or not present at this time.
The accumulation of mast cells in the pulmonary vasculature in chronic hypoxia was described as early as three decades ago (Kay et al. 1974; Tucker et al. 1977) in studies associated with a search for a mediator of pulmonary vasoconstriction induced by hypoxia. In the present study, we did not find significant increase of the total number of mast cell (toluidine blue detected); on the other hand in the three experimental groups, the numbers of MMP-13 expressing mast cells increased. Tucker et al. (1977) in their original study found that the increase in the total number of perivascular mast cells was present only in species with the highest chronic hypoxia-induced pulmonary hypertension (calves and pigs). In rats and other species with moderate hypoxic pulmonary hypertension, the lung accumulation of mast cells was small. They concluded that the increase in mast cell density is related to the increase in pulmonary blood pressure rather than to the lung hypoxia. We hypothesize that the accumulation of mast cells is the result of hypoxic lung injury, which is a main cause of structural remodelling of peripheral pulmonary arteries and hypoxic pulmonary hypertension (Herget et al. 2000; Novotná & Herget 2002). One would assume that greater hypoxic lung injury in early phases of exposure in hypoxia would induce more prominent remodelling of vascular walls and more severe hypoxic pulmonary hypertension. Therefore, the positive correlation between the mast cells density and the severity of pulmonary hypertension is not surprising even if the mechanism is not directly linked to the increase in intravascular pressure. In fact, both mechanisms – hypoxic lung injury and increase in intravascular blood pressure – may participate. They may have, however, different importance in different stages of hypoxic pulmonary hypertension. In the first days of exposure, injury to the walls of prealveolar blood vessels attracts mast cells into this region, and they participate in the mechanism of remodelling of peripheral portion of pulmonary vascular bed. In the developed hypoxic pulmonary hypertension (after 20 days of exposure in our study), mast cells accumulate in the walls of conduit arteries, which are exposed to the increased intravascular pressure due to developed pulmonary hypertension. An increase in wall tension of conduit pulmonary arteries is known to stimulate matrix protein turnover (Riley & Gullo 1988). A similar mechanism may explain the increase in the number of mast cells localized subpleurally. Exposure to chronic hypoxia results in sustained increase in functional lung capacity (Barer et al. 1978), which may cause mechanical stress of the lung pleura. Why the vascular and subpleural mast cells differ in the expression of interstitial collagenase is not clear. Finally, the return to normoxia causes again gradual vascular wall remodelling in both prealveolar and conduit arteries. However, the splitting behaviour of the interstitial collagenase is now different as evidenced by Novotnáet al. (2001).
Mast cells are detected by various methods. The reliable and most common technique is metachromatic staining with toluidine blue, metachromatic blue or thionin (Williams et al. 1977; Churukian & Schenk 1981; Henwood 2002; Masuda et al. 2003; Menétrey et al. 2003). Other methods, e.g. polychromatic (Unna's methylene blue or Giemsa) or mucopolysaccharide (alcian blue, aldehyde fuchsin and acridine orange) methods (Henwood 2002; Chong et al. 2003) are used in special needs as well as immunohistochemical evidence of mast cell tryptase (Cai et al. 2003), especially in immunofluorescent co-localization. Chloroacetate esterase activity is detected in both mast cells and neutrophilic granulocytes (Gomori 1953). Cytoplasmic granules of mast cells contain several biologically active substances, which may be involved in hypoxia-induced remodelling of peripheral pulmonary vasculature (e.g. biogenic amines, proteolytic enzymes, neutral proteases, metalloproteinases and cytokines). As MMPs are potentially dangerous for extracellular matrix, they are strictly regulated. They are usually not stored but transcribed under the influence of cytokines immediately before their secretion. Moreover, they are secreted as precursors activated by cleaving. Mast cells express interstitial collagenase (Di Girolamo & Wakefield 2000), which can be activated by mast cell neutral proteases, tryptase and chymase (Gruber et al. 1988, 1989; Saarinen et al. 1994). In active MMPs, the ratio between MMP and the tissue inhibitors of metalloproteinases (TIMPs) decides of the proper activity. Finally, sites of MMPs’ activity are usually compartmentalized (Elkington & Friedland 2006). The interstitial collagenase, in the rat species identified as MMP-13 (rodent-like interstitial collagenase), is the principal enzyme responsible for initiation of collagen breakdown. In previous studies, we observed that extracts from the walls of peripheral pulmonary arteries of rats exposed to hypoxia had increased activity of MMP-13 (Novotná & Herget 1998; Herget et al. 2003) and a consequent presence of collagen type I cleavages (Novotná & Herget 1998). Pharmacological inhibition of collagenolytic activity partly inhibited the development of hypoxic pulmonary hypertension (Herget et al. 2003). Atkinson et al. (2001) observed collagen type I degradation also by the membrane type 1 matrix metalloproteinase (MT-1–MMP) in a transfected human fibrosarcoma cell line in co-operation with MMP-2. As MT-1–MMP is membrane-bound, this breakdown can be highly localized. In addition, MT-1–MMP forms a complex with TIMP-2 on the cell surface, which activates MMP-2. Than, MMP-2 can serve as an activator of other MMPs, namely MMP-13 (Li et al. 2000). MT-1–MMP expression in mast cells is thus another candidate to be traced because it could be a part of the collagenolytic cascade in the walls of pulmonary vessels during their remodelling.
According to our hypothesis (Hampl & Herget 2000; Herget et al. 2000), collagen degradation products may represent one of the mechanisms, which stimulate growth of the vascular smooth muscle (Bačákováet al. 1997) and fibroproduction (Gardi et al. 1994) in peripheral pulmonary blood vessels in hypoxia. In addition, it was shown that release of tryptase from activated mast cells can also directly stimulate production of collagen type I (Cairns & Walls 1997).
Mast cells play a key role in the early inflammatory response of systemic microvasculature to hypoxia (Dix et al. 2003; Steiner et al. 2003). The mechanism of activation of mast cells in systemic hypoxia is explained by alteration of balance between oxygen radicals (ROS) and nitric oxide (NO) production (Steiner et al. 2002). Similarly, in the pulmonary blood vessels, an increase in ROS and NO production seems to be one of the triggering mechanisms of pulmonary hypertension induced by chronic hypoxia (Hampl & Herget 2000).
Administration of an antioxidant (N-acetylcysteine) inhibits the development of hypoxic pulmonary hypertension most effectively if it is applied in a short period at the beginning of exposure to hypoxia (Lachmanováet al. 2005). Antioxidant therapy is therefore most effective at the time when mast cells accumulate in the prealveolar pulmonary vessels. Frantz et al. (1988) concluded that mast cell products are not important mediators of the short-time hypoxia-induced pulmonary hypertension because 20 min-intravenous infusion of cromolyn sodium did not inhibit 10 min hypoxia-induced pulmonary hypertension in newborn and young lambs.
For the cellular response to hypoxia, hypoxia-inducible factor-1 (HIF-1) plays a master role. Under normoxic conditions, the turnover of its subunit HIF-1α is controlled by ubiquitination and degradation in proteasomes. In hypoxia, HIF-1α is stabilized by ROS (Griffiths et al. 2005) and binds the HIF-1β subunit in the nucleus. Thus, HIF-1 mediates transcription of genes encoding proteins engaged in reactions to hypoxia. At least two of these proteins, inducible NO-synthase and vascular endothelial growth factor certainly participate in mechanism of the chronic hypoxia – induced pulmonary vascular remodelling.
Redistribution of mast cells to the peripheral parts of pulmonary vasculature and expression and release of interstitial collagenase is in concordance with our hypothesis that increased collagenolysis in peripheral pulmonary arteries is probably one of the important mechanisms that triggers pulmonary vascular remodelling in chronic hypoxia.
Acknowledgments
The work was supported by the grants from the Grant Agency of the Czech Republic (Nos 304/02/1348 and 305/05/0672) and Grant Agency of the Charles University, Prague (No 53/2002). We are also obliged to Erik B. Johnson, B.C. Pharm. (Department of Physiology, Charles University 2nd Medical Faculty) for his kind language review and correction.
References
- Atkinson SJ, Patterson ML, Butler MJ, Murphy G. Membrane type 1 matrix metalloproteinase and gelatinase A synergistically degrade type I collagen in a cell model. FEBS Lett. 2001;491:222–226. doi: 10.1016/s0014-5793(01)02204-9. [DOI] [PubMed] [Google Scholar]
- Bačáková L, Wilhelm J, Herget J, Novotná J, Eckhardt A. Oxidized collagen stimulates proliferation of vascular smooth muscle cells. Exp. Molec. Pathol. 1997;64:185–194. doi: 10.1006/exmp.1997.2219. [DOI] [PubMed] [Google Scholar]
- Barer GR, Herget J, Sloan PJM, Suggett AJ. The effect of acute and chronic hypoxia in the thoracic gas volume in anaesthetized rats. J. Physiol. 1978;277:177–192. doi: 10.1113/jphysiol.1978.sp012268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JE, Guerreiro D, Laurent J. Changes in the composition and metabolism of arterial collagens during the development of pulmonary hypertension in rabbits. Am. Rev. Resp. Dis. 1990;141:450–455. doi: 10.1164/ajrccm/141.2.450. [DOI] [PubMed] [Google Scholar]
- Cai Y, Bjermer L, Halstensen TS. Bronchial mast cells are the dominating LTC4S-expressing cells in aspirin-tolerant asthma. Am. J. Respir. Cell Mol. Biol. 2003;29:683–693. doi: 10.1165/rcmb.2002-0174OC. [DOI] [PubMed] [Google Scholar]
- Cairns JA, Walls AF. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J. Clin. Invest. 1997;99:1313–1321. doi: 10.1172/JCI119290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong LK, Suvarna K, Chess-Williams R, Peachell PT. Desensitization of β2-adrenoceptor-mediated responses by short-acting β2-adrenoceptor agonists in human lung mast cells. Brit. J. Pharmacol. 2003;138:512–520. doi: 10.1038/sj.bjp.0705050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churukian CJ, Schenk EA. A toluidine blue method for demonstrating mast cells. J. Histotechnol. 1981;4:85–86. [Google Scholar]
- Dix R, Orth T, Allen J, Wood JG, Gonzalez NC. Activation of mast cells by systemic hypoxia, but not by local hypoxia, mediates increased leukocyte-endothelial adherence in cremaster venules. J. Appl. Physiol. 2003;95:2495–2502. doi: 10.1152/japplphysiol.00735.2003. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, Wakefield D. In vitro and in vivo expression of interstitial collagenase/MMP-1 by human mast cells. Dev. Immunol. 2000;7:131–142. doi: 10.1155/2000/82708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington PTG, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259–266. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott FM, Reid L. Some new facts about the pulmonary artery and its branching pattern. Clin. Radiol. 1965;16:193–198. doi: 10.1016/s0009-9260(65)80042-3. [DOI] [PubMed] [Google Scholar]
- Frantz EG, Schreiber MD, Soifer SJ. Cromolyn sodium does not prevent hypoxia-induced pulmonary hypertension in newborn and young lambs. J. Dev. Physiol. 1988;10:555–565. [PubMed] [Google Scholar]
- Fulton RM, Hutchinson EC, Jones AM. Ventricular weight in cardiac hypertrophy. Brit. Heart J. 1952;14:413–420. doi: 10.1136/hrt.14.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardi D, Calzoni P, Marcolongo P, Vavarra E, Vanni L, Lungarella G. Collagen breakdown and lung collagen metabolism: an in vitro study on fibroblast cultures. Thorax. 1994;49:312–318. doi: 10.1136/thx.49.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomori G. Chloroacyl esters as histochemical substrates. J. Histochem. Cytochem. 1953;1:469–470. doi: 10.1177/1.6.469. [DOI] [PubMed] [Google Scholar]
- Griffiths EA, Pritchard SA, Welch IM, Price PM, West CM. Is the hypoxia-inducible factor pathway important in gastric cancer. Eur. J. Cancer. 2005;41:2792–2805. doi: 10.1016/j.ejca.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Gruber BL, Schwartz LB, Ramamurthy NS, Irani AM, Marchese MJ. Activation of latent rheumatoid synovial collagenase by human mast cell tryptase. J. Immunol. 1988;140:3936–3942. [PubMed] [Google Scholar]
- Gruber BL, Marchese MJ, Suzuki K, Schwartz LB, Okada Y, Nagase H, Ramamurthy NS. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J. Clin. Invest. 1989;84:1657–1662. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampl V, Herget J. Perinatal hypoxia increases hypoxic pulmonary vasoconstriction in adult rats recovering from chronic exposure to hypoxia. Am. Rev. Respir. Dis. 1990;142:619–624. doi: 10.1164/ajrccm/142.3.619. [DOI] [PubMed] [Google Scholar]
- Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol. Rev. 2000;80:1337–1384. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- Heath D. Mast cells in the human lung at high altitude. Int. J. Biometeorol. 1992;36:210–213. doi: 10.1007/BF02726399. [DOI] [PubMed] [Google Scholar]
- Henwood A. Improved demonstration of mast cells using alcian blue tetrakis (methylpyridium) chloride. Biotech. Histochem. 2002;77:93–94. [PubMed] [Google Scholar]
- Herget J, Suggett AJ, Leach E, Barer GR. Resolution of pulmonary hypertension and other features induced by chronic hypoxia in rats during complete and intermittent normoxia. Thorax. 1978;33:468–473. doi: 10.1136/thx.33.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget J, Wilhelm J, Novotná J, Eckhardt A, Vytášek R, Mrázková L, Ošt’ádal M. A possible role of oxidant tissue injury in the development of pulmonary hypertension. Phys. Res. 2000;49:493–501. [PubMed] [Google Scholar]
- Herget J, Novotná J, Bíbová J, Povýšilová V, Vaňková M, Hampl V. Metalloproteinase inhibition by Batimastat attenuates pulmonary hypertension in chronically hypoxic rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L199–L208. doi: 10.1152/ajplung.00167.2002. [DOI] [PubMed] [Google Scholar]
- Hislop A, Reid L. New findings in pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. Br. J. Exp. Pathol. 1976;57:542–554. [PMC free article] [PubMed] [Google Scholar]
- Kay JM, Waymire JC, Grover RF. Lung mast cell hyperplasia and pulmonary histamine-forming capacity in hypoxic rats. Am. J. Physiol. 1974;226:178–184. doi: 10.1152/ajplegacy.1974.226.1.178. [DOI] [PubMed] [Google Scholar]
- Kay JM, Suyama KL, Keane PM. Mast cell stabilizing compound FPL 55618 reduces right ventricular hypertrophy and lung mast cell hyperplasia in chronically hypoxic rats. Experientia. 1981;37:75–76. doi: 10.1007/BF01965579. [DOI] [PubMed] [Google Scholar]
- Kerr JS, Riley DJ, Frank MM, Trelstad RL, Frankel HM. Reduction of chronic hypoxic pulmonary hypertension in the rat by beta-aminopropionitrile. J. Appl. Physiol. 1984;57:1760–1766. doi: 10.1152/jappl.1984.57.6.1760. [DOI] [PubMed] [Google Scholar]
- Kerr JS, Ruppert CL, Tozzi CA, Neubauer JA, Frankel HM, Yu SY, Riley DJ. Reduction of chronic hypoxic pulmonary hypertension in the rat by an inhibitor of collagen production. Am. Rev. Resp. Dis. 1987;135:300–306. doi: 10.1164/arrd.1987.135.2.300. [DOI] [PubMed] [Google Scholar]
- Lachmanová V, Hniličková O, Povýšilová V, Hampl V, Herget J. N-acetylcysteine inhibits hypoxic pulmonary hypertension most effectively in the initial phase of chronic hypoxia. Life Sci. 2005;77:175–182. doi: 10.1016/j.lfs.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc. Res. 2000;46:214–224. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- Masuda T, Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, Nagai H. Mast cells play a partial role in allergen-induced subepithelial fibrosis in a murine model of allergic asthma. Clin. Exp. Allergy. 2003;33:705–713. doi: 10.1046/j.1365-2222.2003.01588.x. [DOI] [PubMed] [Google Scholar]
- Menétrey D, Dubayle D, Serviere J. Intrathalamic Mast Cell Mobilisation is Under Sumatriptan Influences. Prague: Abstract Book of the 6th IBRO Congress; 2003. p. 421. [Google Scholar]
- Meyrick B, Hislop A, Reid L. Pulmonary arteries of the normal rat: the thick walled oblique muscle segment. J. Anat. 1978;2:209–221. [PMC free article] [PubMed] [Google Scholar]
- Migally NB, Tucker A, Greenlees K, Wright M, Zambernard J. Density and ultrastructure of mast cells in lung vessels of aging rats exposed to and recovering from chronic hypoxia. Cell Tissue Res. 1983;232:601–608. doi: 10.1007/BF00216432. [DOI] [PubMed] [Google Scholar]
- Mungall IPF. Hypoxia and lung mast cells: influence of disodium cromoglycate. Thorax. 1976;31:94–100. doi: 10.1136/thx.31.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotná J, Herget J. Exposure to chronic hypoxia induces qualitative changes of collagen in the walls of peripheral pulmonary arteries. Life Sci. 1998;62:1–12. doi: 10.1016/s0024-3205(97)01032-1. [DOI] [PubMed] [Google Scholar]
- Novotná J, Herget J. Small collagen cleavage fragments present in peripheral pulmonary arteries (ppa) of rats exposed to 4 days hypoxia disappear in chronic hypoxic exposure. Phys. Res. 2001;50:P21. [Google Scholar]
- Novotná J, Herget J. Possible role of matrix metalloproteinases in reconstruction of peripheral pulmonary arteries induced by hypoxia. Phys. Res. 2002;51:323–334. [PubMed] [Google Scholar]
- Novotná J, Bíbová J, Hampl V, Deyl Z, Herget J. Hyperoxia and recovery from hypoxia alter collagen in peripheral pulmonary arteries similarly. Phys. Res. 2001;50:153–163. [PubMed] [Google Scholar]
- Paes de Almeida O, Bohm CM, de Paula Carvalho M, Paes de Carvalho A. The cardiac muscle in the pulmonary vein of the rat: a morphological and electrophysiological study. J. Morphol. 1975;145:409–433. doi: 10.1002/jmor.1051450403. [DOI] [PubMed] [Google Scholar]
- Poiani GJ, Tozzi CA, Yohn SA, Pierce RA, Belsky SA, Berg RA, Yu SY, Deak SB, Riley DJ. Collagen and elastin metabolism in hypertensive pulmonary arteries of rats. Circ. Res. 1990;66:968–978. doi: 10.1161/01.res.66.4.968. [DOI] [PubMed] [Google Scholar]
- Poiani GJ, Wilson FJ, Fox DJ, Sumka JM, Peng BW, Liao W-C, Tozzi CA, Riley DJ. Liposome-entrapped antifibrotic agent prevents collagen accumulation in hypertensive pulmonary arteries of rats. Circ. Res. 1991;70:912–922. doi: 10.1161/01.res.70.5.912. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M. EVE and beyond, retro and prospective insights. Am. J. Physiol. 1999;277:L5–L12. doi: 10.1152/ajplung.1999.277.1.L5. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Gamble W, Nadas AS, Miettinene OS, Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am. J. Physiol. 1979;236:818–827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Herget J. Experimental models of pulmonary hypertension. In: Weir EK, Reeves JT, editors. Pulmonary Hypertension. New York: Futura Publishing Co; 1984. pp. 361–391. [Google Scholar]
- Reid LM. Structure and function in pulmonary hypertension. New perceptions. Chest. 1986;89:279–288. doi: 10.1378/chest.89.2.279. [DOI] [PubMed] [Google Scholar]
- Riley D, Gullo J. Pressure applied to cultured pulmonary artery endothelial cells causes release of fibroblast mitogen and induces a proto-oncogene. FASEB J. 1988;2:A300. [Google Scholar]
- Saarinen J, Kalkkinen N, Welgus HG, Kovanen PT. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J. Biol. Chem. 1994;269:18134–18140. [PubMed] [Google Scholar]
- Steiner DR, Gonzalez NC, Wood JG. Interaction between reactive oxygen species and nitric oxide in the microvascular response to systemic hypoxia. J. Appl. Physiol. 2002;93:1411–1418. doi: 10.1152/japplphysiol.00251.2002. [DOI] [PubMed] [Google Scholar]
- Steiner DR, Gonzalez NC, Wood JG. Mast cells mediate the microvascular inflammatory response to systemic hypoxia. J. Appl. Physiol. 2003;94:325–334. doi: 10.1152/japplphysiol.00637.2002. [DOI] [PubMed] [Google Scholar]
- Thomas P, Khokha R, Shepherd FA, Feld R, Tsao M-S. Differential expression of matrix metalloproteinases and their inhibitors in non-small cell lung cancer. J. Pathol. 2000;190:150–156. doi: 10.1002/(SICI)1096-9896(200002)190:2<150::AID-PATH510>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Tozzi CA, Thakker-Varia S, Yu SY, Bannett RF, Peng BW, Poiani GJ, Wilson FJ, Riley DJ. Mast cell collagenase correlates with regression of pulmonary vascular remodeling in the rat. Am. J. Respir. Cell. Mol. Biol. 1998;18:497–510. doi: 10.1165/ajrcmb.18.4.2536. [DOI] [PubMed] [Google Scholar]
- Tucker A, McMurtry I, Alexander AF, Reeves JT, Grover RF. Lung mast cell density and distribution in chronically hypoxic animals. J. Appl. Physiol. 1977;42:174–178. doi: 10.1152/jappl.1977.42.2.174. [DOI] [PubMed] [Google Scholar]
- Williams A, Heath D, Kay JM, Smith P. Lung mast cells in rats exposed to acute hypoxia, and chronic hypoxia with recovery. Thorax. 1977;32:287–295. doi: 10.1136/thx.32.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Heath D, Harris P, Williams D, Smith P. Pulmonary mast cells in cattle and llamas at high altitude. J. Pathol. 1981;134:1–6. doi: 10.1002/path.1711340102. [DOI] [PubMed] [Google Scholar]