Abstract
Adult hepatocytes and liver-cell progenitors play a role in restoring liver tissue after injury. For the study of progenitor cells in liver repair, experimental models included (a) surgical removal of liver tissue by partial hepatectomy; (b) acute injury by carbontetrachloride; (c) acute injury by d-galactosamine (GalN) and N-nitrosomorpholine (NNM); and (d) chemical hepatocarcinogenesis by feeding NNM in low and high doses. Serological and immunohistological detection of alpha-fetoprotein gene expression served to follow pathways of cellular differentiation. Stem cells were not required in models of surgical removal of parenchyma and in carbon tetrachloride intoxication of adult hepatocytes. In contrast, regeneration of liver occurred through biliary epithelial cells in injuries induced by GalN and NNM. These biliary epithelial cells, collectively called oval cells, are most probably derived from the canals of Hering. Proliferating bile duct cells reached a level of differentiation with reactivation of foetal genes and significant alpha-1-fetoprotein (AFP) synthesis signalling a certain degree of retrodifferentiation with potential stemness. Due to the same embryonic origin of bile ducts and hepatocytes, biliary epithelium and its proliferating progeny (oval cells) have a defined role in liver regeneration as a transit and amplification compartment. In their early proliferation stage, oval cells were heavily engaged in DNA synthesis ([3H]thymidine labelling). Pulse-chase experiments during experimental hepatocarcinogenesis exhibited their development into hepatocytes with high risk for transformation and leading to foci of altered hepatocytes. Hepatocellular carcinomas may arise either from proliferating/differentiating oval cells or from adult hepatocytes; both cell types have stem-like properties. AFP-positive and AFP-negative carcinomas occurred in the same liver. They may represent random clonal origin. The heterogeneity of phenotypic marker (AFP) correlated with a process of retrodifferentiation.
Keywords: cell differentiation, hepatocellular carcinoma, liver, oval cell, progenitor cell, stem cell
Introduction
The liver possesses the capacity to maintain its parenchyma in normal liver cell turnover or in restoring its cell mass during regeneration by switching of adult hepatocytes from a quiescent state to a proliferate state. There is now evidence that under certain conditions in which the proliferative capacity of normal adult hepatocytes is blocked, for example, in severe damage by hepatotoxic agents, stem-like populations of cells are also involved to replace lost liver mass (Oh et al. 2002; Yin et al. 2002). To this aim, the stem cell compartment and its progeny (bipotential progenitor cells) must be induced to proliferate and differentiate into hepatocytes and bile duct epithelia. At least proliferating oval cells are considered as facultative progenitor cells, which possess stem-like function. Such liver stem-like cells may be capable to generate new lineages of liver epithelia, i.e., the mature forms of the two hepatic epithelial cell lines, hepatocytes and cholangiocytes. The terminal bile ductular system is thought to be the main source of oval cells (Theise et al. 1999; Roskams et al. 2004), but oval cells were also described as to be derived from bone marrow (Petersen 2001; Crosby et al. 2002). The precise location of stem cells in the liver and their role in hepatocarcinogenesis is still under focus, especially with the latest issue that a stem cell progeny might be the differentiation product from an extra-hepatic compartment, for example the bone marrow.
The extraordinary research activity on adult stem cells is a challenge for the concepts of liver stem cells in which the role of hepatocytes, bile ductular epithelia and bone marrow cells needs to be clarified. Inclusive is the controversial question whether the major stem cells are epithelial cells which reside in the liver or are in part from the circulating pool of haematopoietic stem cells. Experimental approaches in the study of progenitor activation are mainly done with rodent models, and a large body of data have come from studies on the cellular origin of alpha-1-fetoprotein (AFP); these include also hepatocarcinogenesis (Kuhlmann 1978; Sell 2002, 2003). Until now, the ideal marker to trace the pathways of stem cell development does not exist, but under all circumstances, AFP is still a very promising candidate for the study of differentiation or retrodifferentiation by virtue of its strong correlation with foetal gene expression in ontogeny and in oncodevelopmental situations. We suggest AFP as a useful biological marker in the study of restitutive response of the liver following various injuries.
For exploiting the role of progenitor cells in liver repair, suitable animal models are needed because liver restoration after a variety of injuries will evoke a response of different cells in the hepatic lineage, and these cells will have the potential to differentiate into various cell types. It is hypothesized that, similar to other organ systems, lineage cells consist of stem cells (short-term and long-term stem cells), precursor cells, and mature cells (Sell 2001).
The purpose of different experimental models of injury on the basis of cellular loss by surgical means as well as by hepatotoxins including hepatocarcinogens was to evaluate the different cells in the hepatic lineage for restoration. AFP was employed as a typical marker of hepatoblasts and foetal hepatocytes with special reference to its postnatal repression and its resurgence in four different models of liver injury in which adult hepatocytes are either prolific during regeneration or are inhibited in their regenerative capacity by hepatotoxins. The models included (a) surgical removal of liver parenchyma by partial hepatectomy; (b) acute parenchymal injury by a hepatotoxic agent, i.e. carbon tetrachloride; (c) acute parenchymal injury by two further hepatoxic agents which can block the regenerative capacity of adult hepatocytes, i.e. d-galactosamine (GalN) and N-nitrosomorpholine (NNM); and (d) chemical hepatocarcinogenesis by feeding the genotoxic hepatocarcinogen NNM in either low or high doses.
Methods
Animals and experimental models
Twelve-week-old animals of male sex were used throughout. Rats of the inbred strain BD X and C3H/He mice were purchased from the Zentral-Institut für Versuchstierkunde (Hannover, Germany); BALB/cJ mice were obtained from The Jackson Laboratory (Bar Harbor, USA). All chemicals were of the highest grade available from commercial sources.
Rats were used because they are the preferred species for studies on liver regeneration and hepatocarcinogenesis. BD X inbred rats are a well characterized strain (Druckrey 1971). The very low incidence rate of spontaneous tumours make this strain useful for long-term studies on chemical carcinogenesis including hepatocarcinogenesis (Druckrey et al. 1967).
The two mouse strains were included in the studies on liver regeneration because both strains show striking differences in their ability to synthesize AFP which is due to strain-specific mechanisms of AFP gene control (Olsson et al. 1977; Lazarevich 2000).
The genotoxic hepatocarcinogen NNM (synthesized by the Division of Toxicology, German Cancer Research Center, Heidelberg, Germany) was applied in drinking water at two different doses. Rats were divided into two groups, 80–100 animals in each. The low dose consisted of 6 mg/kg/day and was given for 12 weeks. The high dose was 20 mg NNM/kg/day and was given for 6 weeks; details concerning LD50 and mean induction time are described earlier (Druckrey et al. 1967). Carcinogen intake was controlled by daily measurements of water drunk. From the beginning of NNM treatment, rats were bled weekly for AFP detection in sera. Moreover, rats were killed at 1-week intervals for histological and immunohistological analysis. Control animals were kept on a standard diet and tap water.
Studies on liver regeneration included experiments with partial hepatectomy (Higgins & Anderson 1931; Brues et al. 1936) and toxic injuries by use of carbon tetrachloride (Ruoslahti et al. 1974). In the case of carbon tetrachloride (CCl4) poisoning, rats and mice were given orally a single dose of 1 ml of 10% CCl4 in liquid paraffin/100 g body weight, directly into the esophagus, by a curved trochar and syringe. From each experimental model and each strain, three to five animals were bled and the livers dissected out 1–7 days after injury. Control animals were sham operated or sham treated by oral ingestion of liquid paraffin alone and kept on a standard diet and tap water.
Rat liver regeneration was also studied following d-galactosamine-HCl (GalN) injury: two intraperitoneal injections of GalN dissolved in 0.9% NaCl, 375 mg/kg/body weight per injection. The first dose was given at 8:30 a.m. and the second dose between 8:00 and 9:00 p.m. of the same day (Sell et al. 1976). Three to five rats per day were bled and the livers dissected out 1–10 days after the first GalN injection. Control rats were kept on a standard diet and tap water. All experiments are summarized in Table 1.
Table 1.
Experimental models for the study of hepatic progenitor cells
| Animal models | Treatment | Period of study |
|---|---|---|
| Normal controls | ||
| BALB/cJ mice | No treatment, animals kept on standard diet and tap water | 12 week old mice |
| C3H/He mice | 12 week old rats | |
| BD X rats | ||
| Partial hepatectomy | ||
| BALB/cJ mice | 70% resection | Daily for 7 days |
| C3H/He mice | Controls: sham operation | |
| BD X rats | ||
| CCl4 intoxication | ||
| BALB/cJ mice | 100 μl CCl4/100 g | Daily for 7 days |
| C3H/He mice | Controls: oral ingestion of liquid paraffin | |
| BD X rats | ||
| GalN injury | ||
| BD X rats | 375 mg/kg | Daily for 10 days |
| (a) First dose at 8:30 a.m. | ||
| (b) Second dose at 8.00 to 9.00 p.m. | ||
| Controls: no treatment | ||
| Hepatocarcinogenesis | ||
| BD X rats | NNM carcinogen schedules | Regular bleeding and histology at weekly intervals until hepatoma development (up to 200 days) |
| (a) Low dose feeding with 6 mg NNM/kg/day for 12 weeks | ||
| (b) High dose feeding with 20 mg NNM/kg/day for 6 weeks | ||
| After carcinogen diet, animals kept on standard diet and tap water | ||
CCl4, carbon tetrachloride; GalN, d-galactosamine; NNM, N-nitrosomorpholine.
Immunological reagents and methods
Rat and mouse AFP were purified from amniotic fluid by preparative polyacrylamide-agarose gel electrophoresis (PAGE) and immunoadsorption techniques (Kuhlmann 1975). Rabbit IgG, rat IgG and mouse IgG were isolated from normal sera by DEAE ion exchange chromatography followed by preparative PAGE. Glucose oxidase enzyme (GOD) was purchased from Sigma-Aldrich (München, Germany). Antibodies against AFP, GOD, rat and mouse IgG were produced in rabbits by immunization with the respective antigens. Furthermore, sheep antibodies were obtained by immunization with AFP (mouse, rat) and rabbit IgG, respectively. Pure rabbit anti-AFP, sheep anti-AFP and sheep anti-rabbit IgG antibodies were isolated from hyperimmune sera by immunoadsorbent chromatography. IgG molecules were conjugated with horseradish peroxidase (HRP; Sigma-Aldrich, München, Germany) using glutaraldehyde (Avrameas & Ternynck 1971); another batch of sheep anti-rabbit IgG antibodies was conjugated with GOD in the same manner. Purification of antibody-enzyme conjugates was done on a Sephacryl S-200 column (Kuhlmann 1977). For immunohistology, HRP conjugates were also purified by Concanavalin A (Con A) affinity chromatography (Sepharose 4B). IgG fractions were prepared from rabbit anti-GOD, anti-mouse IgG and anti-rat IgG immune sera by DEAE ion exchange chromatography and used at concentrations of 0.05–0.1 mg/ml for immunohistological controls.
Alpha-1-fetoprotein content in sera was measured with two procedures: an enzyme enhanced electroimmunodiffusion method (Kuhlmann 1978) was used at the beginning of experimentation which was then replaced by a solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle (Engvall & Perlmann 1971; Voller et al. 1978). Rabbit anti-AFP coated to the solid phase (microtitration plate) served as the capture antibodies, and HRP conjugated sheep anti-AFP were used as detector antibodies. Assays were run in duplicate with serum dilutions, negative controls (sera from normal control animals) and standards with defined AFP concentrations. The latter were derived from pooled amniotic fluids which have been calibrated against purified AFP. The amount of AFP in test sera was then calculated by a curve fit programme of the photometer.
Immunohistochemistry and proliferation studies of liver cells
Immunoperoxidase labellings of AFP were performed on tissue sections of paraffin embedded specimens. Briefly, liver slices of about 0.5 cm thickness were fixed in 99% ethanol-1% acetic acid for 12–15 h at 0–4°C, dehydrated in absolute ethanol, cleared in benzene and embedded in paraffin. About 5–7 μm thick sections were mounted on acetone-cleaned glass slides, deparaffinated in xylene and passed from absolute ethanol into 0.1 M phosphate-buffered saline pH 7.2 (PBS). In some cases, small liver blocks were also fixed in cacodylate buffered 6% formaldehyde for 6–8 h at 0–4°C, followed by overnight washings with several changes of the buffer solution; tissue blocks were then dehydrated in ethanol series and embedded in epoxy resin. For immunohistology, 1–1.5 μm thick sections were mounted on glass slides and treated with sodium methoxide (Kuhlmann & Krischan 1981).
Prior to immunostaining, endogenous peroxidases were inhibited by treatment with 1% hydrogen peroxide (H2O2) in PBS for 1 h. Then, slides were washed in PBS supplemented with normal sheep serum (1:30) and reacted by an indirect staining method: (a) first incubation with unlabelled rabbit anti-AFP antibodies (purified by immunoaffinity chromatography, 0.005–0.01 mg IgG/ml) for 24 h at 4°C, followed by (b) second incubation with HRP conjugated sheep anti-rabbit IgG antibodies (purified by gel filtration and Concanavalin A affinity chromatography, 0.1 mg IgG/ml) for 20 min at room temperature. Unreacted antibodies were washed off by three successive washings, 5 min each in PBS supplemented with 1% bovine serum albumin and 0.5 M NaCl. Peroxidase activity was revealed by 3,3′diaminobenzidine and H2O2 (Graham & Karnovsky 1966). After washings in PBS, sections were optionally counterstained with Mayer's hemalum for 2 min. In addition, Gomori's silver impregnation and periodic acid-Schiff (PAS) reactions were also performed. Finally, sections were mounted under cover-glass.
Immunohistological specificity was controlled on tissue sections by incubation in (1) normal rabbit IgG globulins; (2) rabbit anti-AFP antibodies absorbed with an immunoadsorbent which was prepared by coupling of AFP to cyanogen bromide activated Sepharose 4B; (3) rabbit anti-mouse and anti-rat IgG antibodies; (4) rabbit anti-glucose oxidase antibodies. Each procedure was followed by HRP conjugates of sheep anti-rabbit IgG antibodies and enzyme substrate. These controls served to exclude false reactions, e.g. due to passive uptake of excreted AFP (and also uptake of serum IgG, a protein not being synthesized by hepatocytes) from extracellular spaces which could occur in liver areas of necroses by artefactual leakage of proteins into liver cells. Thus, tissue sites in which immunoreactivity for both AFP and serum IgG occurred were not taken into account. Finally, specificity of the reported AFP immunoreactivity was proven by these control reactions.
DNA synthesis was estimated by thymidine incorporation in vivo. The studies included intraperitoneal injections of [3H]thymidine according to one of these schedules: (a) 60 min pulse by application of 500 μCi/rat; (b) pulse over 24 h by three successive injections of 250 μCi each; (c) pulse for 60 min by injection of 500 μCi/rat followed by daily injections of unlabelled thymidine for 7 days. Livers were processed as described above. Autoradiography was performed according to the original method (Pelc 1947).
Results
Experimental data are summarized in Table 2. Under normal adult conditions, hepatocytes of BALB/cJ mice were weakly AFP immunoreactive, whereas no cellular AFP was seen in adult C3H/He mice or in BD X rats. In BALB/cJ mice, AFP was detected in the cytoplasm of mono- and binucleated hepatocytes in centrolobular areas and in intermediate zones.
Table 2.
Experimental results
| Animal models | AFP detection in seruma | Cellular AFP patternb |
|---|---|---|
| Normal controls | Normal serum values | Immunohistology |
| BALB/cJ mice | 0.3–0.9 | Hepatocytes |
| C3H/He mice | 0.05–0.1 | – |
| BD X rats | <0.1 | – |
| Partial hepatectomy | Peak values at days 3–4 | Immunohistology |
| BALB/cJ mice | 120–200 | Hepatocytes |
| C3H/He mice | 20–45 | Hepatocytes |
| BD X rats | <0.1–0.8 | – |
| CCl4 intoxication | Peak values at days 3–4 | Immunohistology |
| BALB/cJ mice | 300–700 | Hepatocytes |
| C3H/He mice | 40–110 | Hepatocytes |
| BD X rats | 0.1–3.6 | Hepatocytes (few and faint) |
| GalN injury | Peak values at days 3–5 | Immunohistology |
| BD X rats | 1.4–2.8 | Mainly oval cells and biliary epithelial cells; few hepatocytes |
| Hepatocarcinogenesis | Peak values during induction | Immunohistology |
| BD X rats | BD X rats | BD X rats |
| (a) With low dose NNM feeding at all days: <0.1 | (a) With low dose NNM feeding at all days: no AFP detected | |
| (b) With high dose NNM feeding at days 21–35: 1.1–2.3 | (b) With high dose NNM feeding at days 21–35: oval cells, biliary epithelial cells and biliary epithelial cells in transition towards hepatocytes | |
| Serum AFP at carcinoma stage with wide range: <0.1 to >4,000 | At the carcinoma stage: hepato-cellular carcinoma cells in distinct nodules; other nodules may not stain for AFP |
CCl4, carbon tetrachloride; GalN, d-galactosamine; NNM, N-nitrosomorpholine.
Range of AFP concentrations (μg/ml) in multiple samples.
Cellular detection of AFP by immunohistology; no cellular AFP detected.
Partial hepatectomy
In both strains of mices, AFP increase was slight at 24 h after partial hepatectomy, then it rose steadily and reached a maximum on day 4; afterwards, serum AFP decreased rapidly. Significant strain differences were observed in mice with serum AFP levels five to 10 times higher in the BALB/cJ than in the C3H/He strain. AFP levels were always higher in mice than in rats.
Histological aspects of liver regeneration were similar in all animal species. A few mitoses were seen at 24 h after hepatectomy, and mitotic peaks occurred on day 3 (about 24 h before serum AFP peak was measured) followed by rapid decline. Through the days 2–4, strong immunoexpression of AFP occurred in portal and periportal hepatocytes of mice livers (Figure 1); some hepatocytes in centrolobular and intermediate zones were weakly AFP immunoreactive. Generally, AFP immunoexpression was stronger in BALB/cJ than in C3H/He mice. In contrast to mice, partial hepatectomy in BD X rats led only to a slight increase in serum AFP, yet cellular AFP was not detected at any time.
Figure 1.
Regenerating mouse liver (BALB/cJ). Day 3 after partial hepatectomy; strong alpha-1-fetoprotein (AFP) immunoexpression in hepatocytes of portal areas (original magnification ×160).
Carbon tetrachloride intoxication
In all animal strains and on all days studied (days 1–7), AFP concentrations reached higher levels than after partial hepatectomy; peak values were found on days 3 and 4 as with partial hepatectomy. A significant strain difference between BALB/cJ and C3H/He mice was noted with AFP levels in the BALB/cJ strain with about 10-fold higher concentrations than in the C3H/He strain. At any time, AFP concentrations in rat sera were strikingly lower than in the corresponding mice sera.
Histotoxic patterns were similar in all animal strains. Cell and organelle oedema occurred within 24 h after poisoning followed by focal lesions with necrotic hepatocytes and cell infiltrates on the second day. The marginal zone between viable and necrotic areas contained most of the dividing mature hepatocytes; the highest mitotic activity was between day 2 and day 6 after a single oral dose of CCl4.
In mice, AFP staining occurred in hepatocytes of non-damaged livers within the portal and periportal zones. Strongest AFP immunoexpression was observed in hepatocytes of the intermediate zones adjacent to necrosis. In BALB/cJ mice, this typical AFP staining was already observed on day 1 after CCl4 poisoning and reached a maximum between days 3 and 4 (Figure 2). In C3H/He mice, AFP was not detected before day 2 but afterwards, AFP staining pattern corresponded to that of BALB/cJ mice, yet, with lower intensities than in the latter.
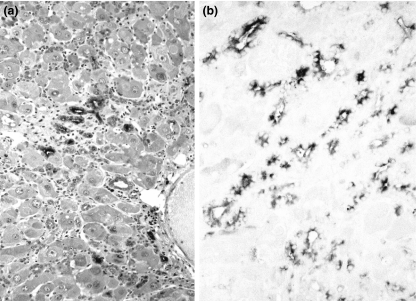
Figure 2.
Regenerating mouse liver (C3H/He), 3 days after carbon tetrachloride injury. Distribution of alpha-1-fetoprotein (AFP)-positive hepatocytes was comparable with that of injured BALB/cJ liver, but number of positive hepatocytes as well as their staining intensity was lower than in BALB/cJ mice. (a) Semithin Epon section (original magnification ×63). (b) Higher magnification view (original magnification ×250).
In the rat, cellular AFP was detected only on days 3 and 4; AFP positive hepatocytes were few in number and appeared either as single stained cells or in small groups in midlobular zones quite near to the necrotic zones and in portal areas.
Galactosamine injury in BD X rats
Serum AFP was slightly increased on day 2 after poisoning, then rose to the highest levels between day 3 and 5 which were followed by a rapid decline. Foci of hepatocellular necrosis appeared within 24 h after GalN injection; necroses were scattered but more extensive near the portal tracts than in the central vein area. Inflammatory infiltrates were present after 24 h, predominantly in the areas of necrosis. They consisted of histiocytes and neutrophilic and eosinophilic granulocytes; lymphocytes were present only in small numbers. The cellular composition of the inflammatory infiltrates changed very fast, but histiocytes were always the predominant cells throughout inflammation.
Beginning on day 2, mitotic activity was observed in both parenchymal and bile ductular cells, reaching a peak on day 3 and on day 4, respectively. In parallel to the increasing mitotic activity in biliary epithelial cells and the ductular structures, a striking increase of bile ductular cross-sections was seen along the borders of portal tracts, reaching a maximum on days 3 and 4.
Two days after GalN injections, AFP was localized for the first time in liver sections. AFP immunoreactivity was confined to the apical cytoplasm of epithelial cells of bile duct-like structures. On day 3, AFP positive cells became very numerous in the areas of increased bile ductular cross-sections (Figure 3). Here, ductular structures and rosettes with large numbers of small cells (oval cells) were seen. In addition, weakly AFP immunoreactive hepatocytes were seen on day 3 and day 4 at random distribution. Between day 6 and day 10, liver structure returned to normal, proliferation of bile ductular cells stopped and AFP staining disappeared.
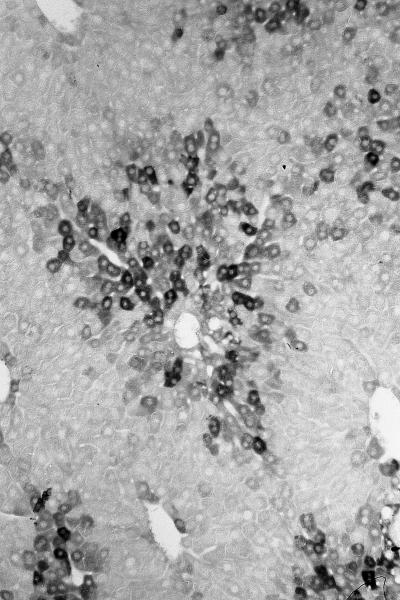
Figure 3.
Localization of alpha-1-fetoprotein (AFP) in bile ductular cells (oval cells) of rat liver on day 3 after GalN injury. (a) AFP stained section counterstained with Mayer's haematoxylin, note AFP immunoreactive cells in ductular reaction areas in portal and in periportal area (original magnification ×160). (b) AFP-positive bile ductular cells (original magnification ×160).
N-nitrosomorpholine hepatocarcinogenesis (induction phase) in BD X rats
In the course of low dose NNM feeding, no serum AFP elevation was measured in the carcinoma induction phase. In contrast, an increase of serum AFP was noted with the application of high NNM doses. At the time of the third bleeding (day 21), serum AFP levels ranged from 1.1 to 2.3 μg/ml and peak values were attained between days 21 and 35 from onset of NNM application. These concentrations were maintained until NNM feeding was stopped. Then, AFP levels dropped within 2 weeks and reached background concentrations as observed in control rats.
Histotoxic patterns were dose-dependent. In rats chronically fed low concentrations of NNM, necroses were rare and no proliferative activity occurred. Serum AFP remained at normal during the induction phase and no cellular AFP was detected.
In high dose NNM feeding experiments, extensive necrosis of hepatocytes developed within 14 days reaching portal areas. Then, inflammatory infiltrates were present and consisted mainly of histiocytes. From day 21, proliferation of small oval-shaped cells occurred within the zones of necroses exhibiting high [3H]thymidine incorporation (Figure 4). Hepatocytes were not involved in [3H]thymidine incorporation. When NNM feeding was stopped, necroses and oval cell proliferations decreased. Finally, livers showed heavy distortion of the original lobular architecture and a cirrhotic pattern.
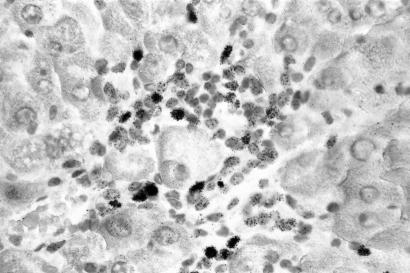
Figure 4.
Rat liver from day 28 during high dose NNM feeding, pulse labelling with [3H] thymidine. Thymidine incorporation in oval-shaped cells within the areas of ductular reaction (original magnification ×250, haematoxylin stain).
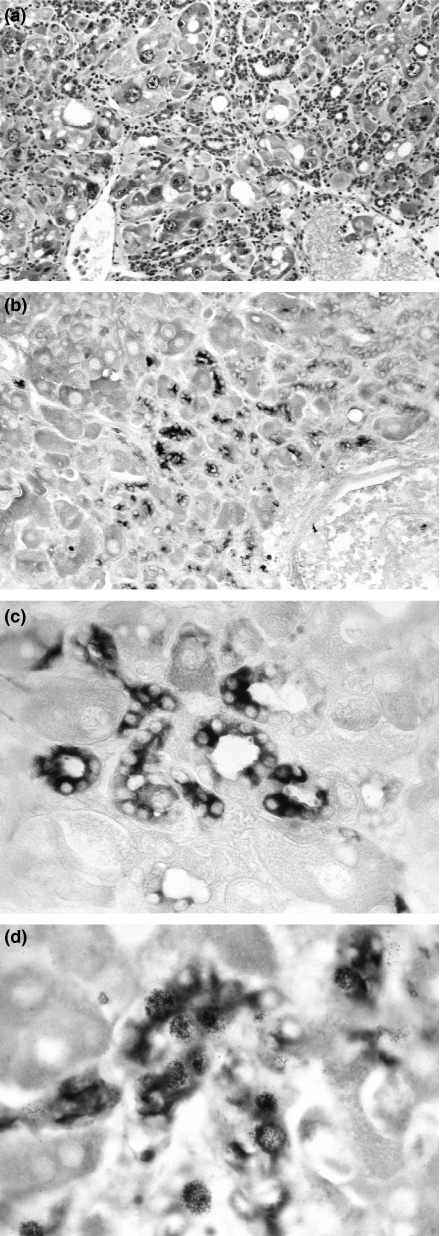
When AFP reappeared for the first time, the foetal protein was detected in the cytoplasm of the proliferating oval cells. Their morphology corresponded to the bile ductular cells described with the galactosamine experiments. AFP-positive oval cells were seen either as small single cells or as strings of cells which formed rosettes and ductular structures with bile duct aspect (Figure 5). During the following weeks, the phenotype of AFP-positive oval cells changed and indicated the development towards small-sized hepatocytes. While their bile ductular feature disappeared, foci of hyperplastic appearance occurred. At his developmental stage, AFP-staining vanished even if cells were proliferating: in pulse-labelling as well as in pulse-chase labelling experiments with [3H]thymidine, cells in the hyperplastic areas were still actively engaged in DNA synthesis. In the later stages, nodules with a clear-cut hepatocytic phenotype were found (Figure 6).
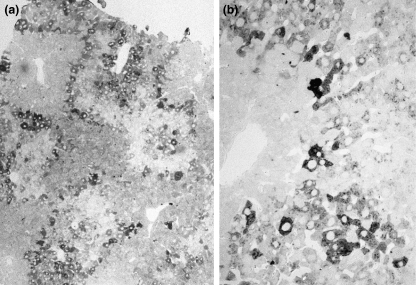
Figure 5.
Rat liver from day 28–35 at high dose NNM feeding. (a) Proliferation of biliary epithelial cells and occurrence of numerous oval cells within portal and periportal area (original magnification ×160 haematoxylin & eosin stained section). (b) Histological section immunostained for alpha-1-fetoprotein (AFP), note AFP-positive oval-shaped cells which form biliary epithelial structures (original magnification ×160). (c) Higher magnification view of proliferated biliary epithelial cells which are stained for AFP and which form tubular structures with typical bile duct appearance (original magnification ×540). (d) High magnification view of [3H]thymidine labelled AFP-positive oval cells (original magnification ×540).
Figure 6.
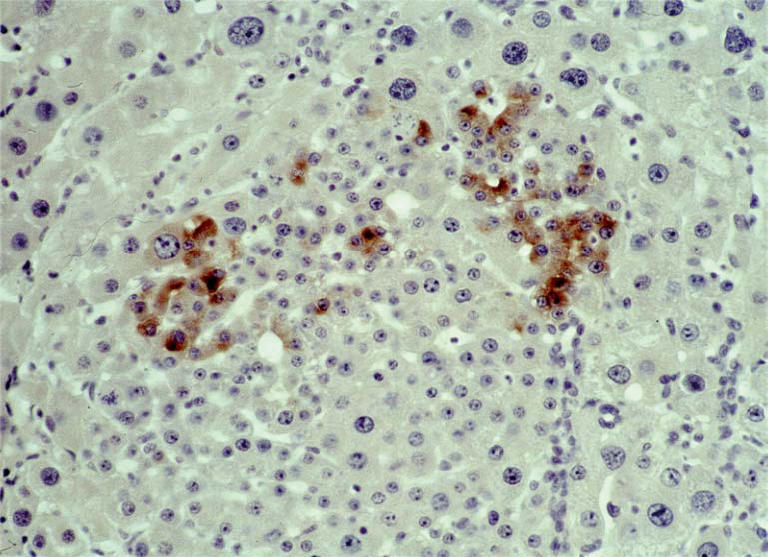
Rat liver from day 35 at high dose NNM feeding. Alpha-1-fetoprotein (AFP) immunoreactive ductular epithelial cells (oval cells) in transition towards hepatocytes giving rise to a nodule of hyperplastic appearance; note strong immunoexpression of AFP in both small oval cells and cells with the appearance of small intermediate-sized hepatocytes (original magnification ×160, haematoxylin counterstain).
NNM hepatocarcinogenesis (hepatoma stage)
The induction time of hepatocellular carcinomas in the BD X rats varied from 90 to 150 days. They usually developed later with low dose NNM than with high dose NNM feeding. In some rats with the experiments of low NNM doses, AFP-producing carcinomas were observed as late as 200 days after the carcinogen ingestion was started. At the carcinoma stage and when significant amounts of serum AFP were detected for the first time in weekly bleedings, its concentrations were in the range of 0.04–10 μg AFP/ml; the minimum detectable concentration of AFP was estimated to be 5 ng/ml. AFP levels rose steadily during the following 10–12 weeks and reached serum levels which were usually found between 50 and 1000 μg AFP/ml. In rare cases, concentrations even exceeded amounts of 4000 μg AFP/ml serum. The dynamics of serum AFP levels over the time were highly heterogenous as controlled by weekly measurements: slow but continuous increases within a period of several weeks, and sometimes very steep increases over the time. Increases up to 10-fold within 1 week were also observed. At a given concentration, AFP could level off to reach plateau values, mainly in the order of 102 and 103 μg AFP/ml. These were maintained for a short time (1–2 weeks) and then followed by further increases. Once AFP has appeared in the rats, AFP levels never dropped until the animals were sacrificed for histology. However, endpoint studies were not performed. For reasons of animal care, experiments were finished at least when rats showed signs of severe illness.
With the AFP appearance in serum, livers contained one or more distinct hepatocellular carcinomas. Some but not all of them would stain for AFP. AFP immunoexpression was always restricted to hepatocellular carcinomas with their neoplastic hepatocytes (Figure 7–8). Normal liver tissue, dysplastic foci and hyperplastic nodules did not stain for AFP. In serial tissue sections processed by conventional histological stains, AFP positive cells exhibited a basophilic character. They were free of glycogen (PAS negative). The high proliferation activity was revealed by incorporation of [3H]thymidine. Typically, up to 50% of the neoplastic hepatocytes became labelled in a 24 h pulse experiment while the percentage of labelled nuclei in normal hepatocytes stayed below 1%.
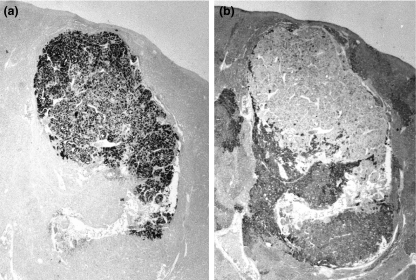
Figure 7.
Low magnification view of a hepatocellular carcinoma (original magnification ×16). (a) Section was immunostained for alpha-1-fetoprotein (AFP). (b) Serial section from same liver area with glycogen staining by periodic acid Schiff (PAS) reaction. Normal hepatocytes exhibit strong PAS reaction, while AFP-positive hepatocellular carcinoma remains PAS-negative.
Figure 8.
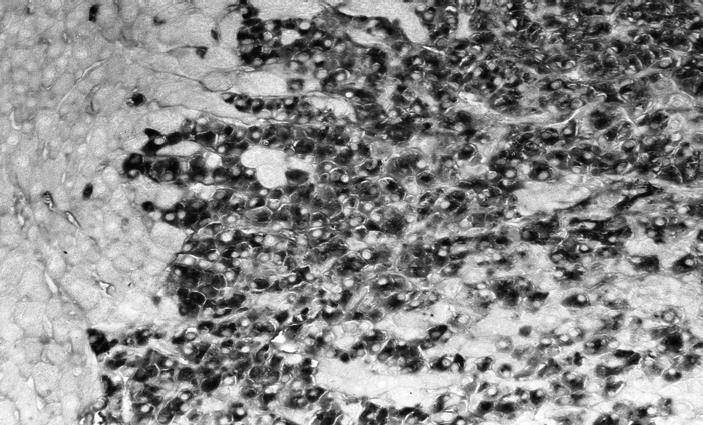
Hepatocellular carcinoma; note alpha-1-fetoprotein (AFP)-positive carcinoma cells, adjacent normal liver is AFP-negative (original magnification ×160).
Discussion
Foetal and normal adult liver
The search for stem cell-like liver cells has been the aim of many investigations using a variety of strategies and experimental designs. In this context, it turns out that AFP, mainly employed in combination with immunohistological staining techniques, remains a very promising marker not only for foetal development, but also for oncodevelopmental purposes. High levels of AFP transcription and translation usually occur in cells of the ventral foregut at the time when liver cords are formed. This step is taken as a signal for commitment toward the liver lineage (Shiojiri et al. 1991). During early liver morphogenesis, i.e. when hepatoblasts and foetal hepatocytes undergo gradual maturation, AFP is the characteristic hepatocyte marker and becomes also transiently expressed by early biliary cells. With regard to AFP expression or other lineage markers such as cytokeratins, cell surface markers, cytoplasmic enzymes and glycogen storage, hepatoblasts were shown to behave as bipotential precursors giving rise to parenchymal epithelial cells (hepatocytes) and biliary epithelial cells (intra- and extrahepatic bile ducts). At later stages of normal development, AFP becomes restricted to hepatocytes.
Our AFP immunostainings clearly showed the repression of AFP in portnatal liver in a graded fashion, i.e. a regional repression of AFP with zonal heterogeneity of AFP positive hepatocytes across the liver acinus which reflected quantitative differences in the level of AFP gene expression. Furthermore, immunostainings have shown that postnatal downregulation of AFP gene expression not only occurred gradually, but also with species and strain dependent strictness. Thus, the significant level of AFP in normal adult hepatocytes of BALB/cJ mice must be regarded as a constitutive gene expression. Heterogeneity of AFP expression in mouse liver was observed by us with immunoperoxidase labelling techniques (Kuhlmann 1979) and was later proved by in situ hybridization of transgenic mice (Emerson et al. 1992). Zonal expression of a variety of liver-characteristic proteins such as AFP, albumin and drug metabolizing enzymes is not unusual (Wolf et al. 1984; Buchmann et al. 1985; Poliard et al. 1986; Moorman et al. 1990; Buhler et al. 1992).
Alpha-1-fetoprotein gene expression is submitted to regulatory processes. A raf gene (regulation of alpha-fetoprotein) which affects the expression of the AFP gene after birth was originally described by screening inbred strains of mice (Olsson et al. 1977). Later on, AFP mRNA was shown to be under the control of at least two genetically unlinked trans-acting loci, termed raf (regulation of AFP) and Rif (regulation of induction of AFP). The raf gene determines the basal level AFP mRNA in adult liver, whereas the Rif locus determines the magnitude of the transient induction of AFP mRNA in response to liver damage (Belayew & Tilghman 1982; Pachnis et al. 1984; Vogt et al. 1987). Finally, within the mouse and rat AFP regulatory system, three distal enhancers, a promoter element, and a silencer have been defined (for review see Lazarevich 2000).
Regeneration of hepatocytes in response to injury
In the present studies on partial hepatectomy and carbon tetrachloride experiments, adult hepatocytes proved their inherent capacity for regeneration, and at no time was there progenitor cell activation which might have led to lineage regeneration. Thus, adult hepatocytes are believed to be functional stem cells as discussed earlier (Alison 1986; Fausto 2000). Furthermore, rising serum AFP levels and concomitant cellular AFP immunoexpression have shown that this function is coupled with induced AFP gene expression, with the extent of induced AFP gene expression being strongly species and strain dependent. The restriction of AFP immunostaining to hepatocytes underlined the reappearance of AFP as due to a cell-specific gene expression.
Because AFP was synthesized in small quantities before mitoses reached their maximum, a direct connection between AFP and DNA synthesis was not evident. On the other hand, some correlation between hepatocyte mitosis and amount of newly synthesized AFP will exist. In CCl4 intoxication, moderate increase of AFP mRNA by the remaining hepatocytes was described and suggested to be linked to their re-entry into the proliferative cycle (Tournier et al. 1988). Moreover, in Sprague–Dawley rat experiments with partial hepatectomy, moderate increase in AFP gene expression was observed in the course of regeneration which could be attributed to replicative cycles (Bernuau et al. 1988). It seems then that the cell cycle per se is linked with the regulating principle of AFP gene expression, however, the degree of this linkage remains to be defined. The great difference in the AFP levels during the recovery phase (partial hepatectomy and carbon tetrachloride) of both mouse strains used in this study and between mouse and the BD X rat strain must have further reasons. In any case, the AFP levels did not merely reflect the number of hepatocytes engaged in repair because liver injury and subsequent regeneration occurred to the same extent under both experimental conditions (and irrespective of strains and species). Some mechanism inherent to carbon tetrachloride as hepatotoxin must further contribute to the expression of AFP (Taketa et al. 1975).
Biliary epithelium as progenitor compartment
Putative stem cells are supposed to participate in liver regeneration of injury models other than partial hepatectomy and carbon tetrachloride intoxication. To this aim, conditions must be chosen in which cell damage is very extensive or chronic so that regeneration by mature hepatocytes is impeded. For example, GalN and NNM (at least in sufficiently high doses) are substances by which the biliary system proved to possess regenerative capacity with multilineage differentiation potential. While normal quiescent bile ducts failed to stain for AFP, proliferating biliary epithelial cells led to a marked AFP immunoexpression, and the bile duct system was regarded as a reservoir of tissue-specific stem and progenitor cells.
During proliferation of bile duct progenitors (oval cells), these cells will reach to a level of cytodifferentiation with reactivation of foetal genes. Significant AFP synthesis by oval cells is then signalling a certain degree of retrodifferentiation (reversal of ontogeny) and a potential stemness. This observation suggests to us the existence of a mechanism different from the above described AFP gene activation in adult hepatocytes during regeneration after partial hepatectomy and CCl4 induced liver injury: proliferation of cells from the biliary epithelium with concomitant AFP expression and the appearance of a new phenotype, i.e. the proliferation of oval cells. Under the microscope, the enhanced mitotic activity of biliary epithelial cells led to pictures of increased numbers of bile ductular cross-sections which may correspond to a higher amount of biliary ducts, extensive arborization of expanding ductules, or alternatively to prolongation of ductular structures. This phenomenon was related to the term oval cell proliferation that originates in the terminal branches of the bile ductular system and in the canals of Hering at the hepatocyte–biliary interface (Kuhlmann & Wurster 1980; Alison et al. 1997; Saxena et al. 1999; Sell 2003; Roskams et al. 2004). Support for the role of some intraportal stem cells in liver repair comes also from studies with allyl alcohol (Yavorkovsky et al. 1995). The authors concluded from their experiments that restitutive proliferation of periportal necrosis by allyl alcohol might be accomplished by proliferation of intraportal cells whose progeny differentiate and eventually repopulate the necrotic zone. Although proliferation of surviving hepatocytes adjacent to the injured zones could not be ruled out, proliferation of periportal and intraportal ‘stem cells’ was predominant to replace necrotic areas. The importance of hepatic oval cells to be facultative stem cells that arise as a result of certain forms of liver injury has also been found in mouse models (Petersen et al. 2003).
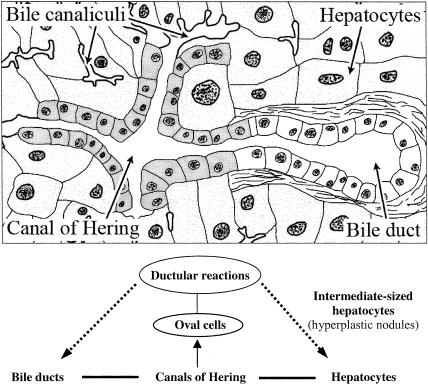
The significant role of bile ducts in restitutive response is strongly supported, and oval cells will function as facultative liver stem cells. This is in agreement with the concept that proliferating cells in the liver include the original tissue-determined stem cells which are represented in the adult organ by cells of the canal of Hering (Sell 2001). When oval cells can be regarded as functional progenitors for hepatocytes and cholangiocytes which can differentiate into hepatocytes or bile duct epithelia, i.e. the mature forms of the two hepatic epithelial cell lines, then, at least the terminal branches of the bile ductular system and the canals of Hering harbor the intrahepatic or ductular stem cells. With respect to the same embryonic origin of bile ducts and hepatocytes, the biliary epithelium and its proliferating oval cells have a defined role in liver regeneration as transit and amplification compartment (Figure 9).
Figure 9.
Schematic representation of normal liver structure and changes of its elements during disease and regeneration. (a) The lobular structure with the canal of Hering which drains bile from the bile canaliculi into the bile duct [modified from L.C. Junqueira & J. Carneiro (1980). In Basic Histology, Lange Medical Publications, p. 350]. (b) Oval cells can proliferate from the canals of Hering and lead to ductular epithelial structures. These proliferating cells are regarded as progenitor cells or intrahepatic stem cells which can differentiate via intermediate-sized hepatocytes into mature hepatocytes and bile duct epithelium; also, extra-hepatic stem cells of bone marrow origin are discussed to be a source of oval cells and provide progenitor cells for liver regeneration.
The fate of progenitor cells with respect to their differentiation into hepatocytes or bile ducts will be governed by the liver microenvironment. In this connection, the observed inflammatory stress with its associated cytokine secretions will certainly play a role. Cytokines were suggested in cross-regulation of epithelial and mesenchymal elements by the formation of a regenerative unit in which hepatopoiesis will take place (Craig et al. 2004). The importance of growth modulators and cytokine signalling to stimulate proliferation, migration and differentiation of liver cells is well established. For example, the onset of hepatocyte proliferation after partial hepatectomy was shown to be accompanied by increased NF-κB activity (FitzGerald et al. 1995). Moreover, NF-κB activity is required for HGF-induced proliferation in a hepatic stem-like cell line (Yao et al. 2004). Furthermore, at the time when hepatocyte proliferation is blocked by toxic agents and when oval cells begin to proliferate, an expression of stem cell factor (SCF) and its receptor (c-kit) can be observed in the oval cell compartment (Fujio et al. 1996). This suggests that the SCF/c-kit system in combination with other growth factor system, i.e. growth and transforming growth factors (e.g. HGF, EGF and TGF), cytokines [tumour necrosis factor (TNF), interleukin (IL), interferon-γ network] and signalling pathways, are involved in the activation of hepatic stem-like cells as well as in their expansion and differentiation (Hu et al. 1996; Lemaigre 2003; Lowes et al. 2003).
Stem cells of extra-hepatic origin in liver regeneration
Oval cells are considered as progeny of intrahepatic stem cells. Now, there is also some evidence that haematopoietic stem cells can contribute to the development of hepatocytes. Indeed, a link between haematopoietic and hepatic cells is likely, at least during foetal development where the liver is the principal haematopoietic organ (Timens & Kamps 1997). In adult life, haematopoiesis can re-emerge in liver during extreme stress and, this phenomenon may suggest the existence of a common stem cell (Masson et al. 2004). In any case, the observation of transdifferentiation or so-called plasticity of adult stem cells is of considerable interest. Most experiments which could show plasticity usually used cells derived from bone marrow with some evidence of reprogrammed adult stem cells to differentiate into other cell types, e.g. into hepatocytes and other epithelial cells including hepatic oval cells (Bjornson et al. 1999; Petersen et al. 1999; Alison et al. 2000; Clarke et al. 2000; Lagasse et al. 2000; Anderson et al. 2001; Krause et al. 2001; Holden & Vogel 2002; Korbling et al. 2002; Oh et al. 2002; Schwartz et al. 2002; Wagers & Weissman 2004). Apart from these findings, recent data have shown that the sources of oval cells are endogenous liver progenitors and that they do not arise through transdifferentiation from bone marrow cells (Menthena et al. 2004).
Some support of relationship between liver lineage cells and bone marrow comes from facts inasmuch as hepatic oval cells and haematopoietic stem cells share common markers such as CD34, Thy-1 and C-kit mRNA and protein (Fujio et al. 1994; Omori et al. 1997; Petersen et al. 1998; Crosby et al. 2002). Furthermore, the relationship between haematopoietic stem cells and liver could be deduced from combined transplantion and liver injury studies; for example, after transplantation of male bone marrow into lethally irradiated syngeneic females, the male Y-chromosome could be observed in the hepatocytes of the female recipient animal after liver injury; or in transplantation experiments with different donor/recipient expression of marker molecules where the marker molecules were detected in the recipient hepatocytes. Hence, an extrahepatic source for liver repopulation seems possible (Petersen et al. 1999; Alison et al. 2000; Theise et al. 2000a,b).
An alternative explanation for the development of plasticity may be the formation of hybrids by spontaneous cell fusion which gives rise to heterokaryons (Terada et al. 2002). Transplantation experiments and cytogenetic analyses support the possibility that hepatocytes being derived from bone marrow will arise from cell fusion instead of differentiation of haematopoietic stem cells. Such cells were able to divide and, also, the expression of previously silent genes became induced (Ying et al. 2002; Wang et al. 2003).
Liver regeneration and development of hepatomas in chemical carcinogenesis
Hepatocarcinogenesis reflects a special situation in the study of BD X rat liver injury because the inducing agents are carcinogenic and eventually toxic at the same time. Apart from dose-dependent acute toxicity which is followed by regeneration, a multistep process of hepatoma induction is started. In the toxic stages with NNM application, hepatocyte necrosis was accompanied by massive proliferation of ductular epithelial cells together with AFP reappearance. In contrast to this observation, low doses of NNM did not result in oval cell proliferation or cellular AFP staining, and no significant increase in serum AFP levels was observed. Since both NNM schedules lead to hepatocellular carcinomas, oval cell proliferation and transitory AFP synthesis cannot be regarded as a prerequisite for conversion to cancer. Merely, oval cells resulted from restitution of damaged liver due to acute toxic injury by high dose NNM feeding. However, these present findings do not exclude that oval cells can be also a target for hepatoma development.
Histological and immunohistological features of oval cell proliferation together with the ductular reactions could be compared with the observations made in the GalN model. AFP-positivity occurred throughout the areas of ductular reactions. In their early proliferation stage, oval cells and ductular-like cells were heavily engaged in DNA synthesis ([3H]thymidine labelling experiments). Moreover, pulse-chase experiments gave evidence for their development into hepatocytes. While still forming ductular-like structures, the AFP-positive cells reached the appearance of small-sized hepatocytes. Finally, areas of hyperplasia and nodular structure were found in cirrhotic livers. At this stage, DNA synthesis has come to a standstill and glycogen accumulation occurred.
From the above, we have seen that oval cells will function as facultative progenitor cells for hepatocytes and biliary tract cells. In experimental hepatocarcinogenesis, they can also give rise to regenerated hepatocytes with a high risk for transformation, and, also, to foci of altered hepatocytes (Dunsford et al. 1989). The latter are usually considered to be preneoplastic (Pitot 1990) with growth advantage over normal cells (Rabes et al. 1982). These properties are indicators of distinct stages of carcinogenesis. The role of oval cells in the histogenesis of liver carcinomas, however, is still debated. Some reports have evidence of an important role of oval cells in this direction (Tian et al. 1997; Libbrecht & Roskams 2002), while other experiments (without signs of liver injury and oval cell proliferation) concluded that precursor lesions will not originate from oval cells. Then, early foci and nodules must be derived from resistant hepatocytes (Anilkumar et al. 1995). The same conclusion was drawn from experiments in which parenchymal necrosis and massive oval cell proliferation were produced, but the development of foci of altered hepatocytes and hepatocellular adenomas led to phenotypes without the expression of cytokeratin 19 (a marker for bile duct epithelia). Consequently, this observation was reported as not to support a precursor–product relationship between oval and parenchymal cells; only the hepatocyte cell lineage being involved in the development of hepatocellular tumours (Steinberg et al. 1991).
At the carcinoma stage when AFP appeared in our rat sera, AFP was stained in the cytoplasm of cells which by routine histology were typical basophilic and PAS-negative neoplastic hepatocytes. AFP was unequivocally caused by the carcinoma cells, and these cells were easily distinguished by their size and shape from normal adult hepatocytes. Most often, both AFP-positive and AFP-negative hepatocellular carcinomas were observed as circumscribed areas within the liver of a given animal. Hence, not every carcinoma was an AFP producer. Moreover, AFP-positive and AFP-negative nodules could be observed side by side. In [3H]thymidine pulse labelling experiments, both AFP-positive and AFP-negative carcinoma cells showed active proliferation. Based on the observations in the present study, we suggest that the AFP-positive population represents distinct neoplastic hepatocytes with a certain degree of differentiation and of clonal origin. Serum AFP levels are the result from synthesis, secretion and turnover of this marker, respectively, and the rising rates of serum AFP in the developmental course of hepatocellular carcinomas are indeed useful indicators for production and secretion of this oncofoetal protein by the carcinoma cells. The heterogeneity of phenotypic cell markers and differences in growth rates of foci and precancerous nodules is known from most of the experimental models, and this heterogeneity will point to different cellular origins as well as to variations of malignant potency of preneoplastic lesions. In any case, the possibility of a random clonal origin of hepatocellular carcinomas from mature hepatocytes was definitively shown by a recently published method which used genetic labelling of hepatocytes (Bralet et al. 2002).
Neoplasia may be preceded or accompanied by molecular and morphologic patterns which are characteristic for cells with variable degree of maturity. Thus, foetal patterns of gene expression are observed which led to the hypothesis that the emergence of tumours correlates with a process of retrodifferentiation (Uriel 1976). Retrodifferentiation is inverse to differentiation, i.e. reversing the maturation process and programming of mature cells backwards along the normal developmental pathway. This formulation was inferred from the dynamics of fetospecific antigens and isozymic patterns during ontogenic and neoplastic growth. Although foetal antigens such as AFP are re-expressed in hepatoma cells, they are not considered as characteristic of malignancy because such molecules can emerge in tissues undergoing non-malignant growth. Hence, the term ‘transitory cell antigens’ appears to be more significant for these biomolecules which are usually restricted to a transient period of cell differentiation. Clonality and retrodifferentiation succeed in selecting cell populations with highest autonomy and unresponsiveness to regulatory principles operating in normal organisms.
Conclusions
From all the injury and hepatocarcinogenesis studies, it was deduced that cells at different levels of the hepatic lineage will become involved in both regenerative repair and generation of carcinomas. The role of adult hepatocytes and canalicular epithelial cells including the biliary epithelium as transit compartment in regenerative processes is evident. The cell types which respond are (a) the normal adult and differentiated hepatocytes; (b) the bipotential stem-like cells in the canals of Hering giving rise to populations of oval cells as progenitor cells. The possible role of multipotent progenitor cells of presumed extra-hepatic origin (haematopoietic stem cells, bone marrow) is discussed.
Postnatal AFP gene repression occurs with species and strain dependent strictness. When hepatocytes regenerate, AFP levels will rise. In partial hepatectomy and carbon tetrachloride injury, adult hepatocytes are prolific and regenerative. Stem cells with multilineage differentiation ability are not required in this type of liver regeneration. The concomitant rise of serum AFP is due to its synthesis by adult hepatocytes. The degree of AFP gene expression is also species and strain dependent.
In severe injury when regenerative capacity of hepatocytes is blocked (GalN and NNM at high doses), reconstitution of livers occur through biliary epithelial cells which are collectively named oval cells. They are derived most probably from the canals of Hering and the small interlobular bile ducts (as source of intraorgan stem cells). Oval cells exhibit multilineage differentiation potential. It is not yet clear, however, if the regenerated structures differentiate either from stem cells as defined by their capability of self-renewal and multiple differentiation or from lineage-committed cells. At least, oval cells are a population with differentiation potential to functionally mature hepatocytes and bile duct epithelia. Proliferating cells of the bile duct system reach to a level of differentiation with reactivation of foetal genes and significant AFP synthesis (as sign of reversal of ontogeny and of potential stemness).
Development of hepatocellular carcinomas is a multistep process. Tumors arise from a pool of differentiating oval cells or by dedifferentiation of mature hepatocytes because both cell types have stem-like properties. The lineage and phenotype of chemically induced liver carcinoma may arise from a single cell through genetic and epigenetic alterations; a clonal origin and expansion is probable. AFP resurgence is associated with the appearance of hepatocellular carcinoma and reflects a process of retrodifferentiation. Clonality and retrodifferentiation succeed in selecting cell populations with highest autonomy.
The lack of a unique stem cell marker in the research of stem cells in general and also in liver regeneration remains a problem. The possibility of lineage plasticity and transdifferentiation of potential hepatic stem cells is still a matter of controversy, but the value of stem cells seems to be considerable in either conventional studies of differentiation or in studies on self-renewing potential and in therapy.
Acknowledgments
The authors wish to thank Prof. K. Wurster, Institut für Pathologie, Krankenhaus München Schwabing, for histopathological inspection of hepatotoxin injured livers, and Prof. E.W. Hahn, UT Southwestern Medical Center at Dallas, TX, USA for helpful discussions.
References
- Alison MR. Regulation of hepatic growth. Physiol. Rev. 1986;66:499–541. doi: 10.1152/physrev.1986.66.3.499. [DOI] [PubMed] [Google Scholar]
- Alison M, Golding M, Lalani EN, Nagy P, Thorgeirsson S, Sarraf C. Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J. Hepatol. 1997;26:343–352. doi: 10.1016/s0168-8278(97)80051-7. [DOI] [PubMed] [Google Scholar]
- Alison MR, Poulsom R, Jeffery R, et al. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat. Med. 2001;7:393–395. doi: 10.1038/86439. [DOI] [PubMed] [Google Scholar]
- Anilkumar TV, Golding M, Edwards RJ, Lalani EN, Sarraf CE, Alison MR. The resistant hepatocyte model of carcinogenesis in the rat: the apparent independent development of oval cell proliferation and early nodules. Carcinogenesis. 1995;16:845–853. doi: 10.1093/carcin/16.4.845. [DOI] [PubMed] [Google Scholar]
- Avrameas S, Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971;8:1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Belayew A, Tilghman SM. Genetic analysis of alpha-fetoprotein synthesis in mice. Mol. Cell Biol. 1982;2:1427–1435. doi: 10.1128/mcb.2.11.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernuau D, Poliard A, Feldmann G. In situ cellular analysis of alpha-fetoprotein gene expression in regenerating rat liver after partial hepatectomy. Hepatology. 1988;8:997–1005. doi: 10.1002/hep.1840080504. [DOI] [PubMed] [Google Scholar]
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Bralet MP, Pichard V, Ferry N. Demonstration of direct lineage between hepatocytes and hepatocellular carcinoma in diethylnitrosamine-treated rats. Hepatology. 2002;36:623–630. doi: 10.1053/jhep.2002.35540. [DOI] [PubMed] [Google Scholar]
- Brues AM, Drury DR, Brues MC. A quantitative study of cell growth in regenerating liver. Arch. Pathol. 1936;22:658–673. [Google Scholar]
- Buchmann A, Kuhlmann W, Schwarz M, et al. Regulation and expression of four cytochrome P-450 isoenzymes, NADPH-cytochrome P-450 reductase, the glutathione transferases B and C and microsomal epoxide hydrolase in preneoplastic and neoplastic lesions in rat liver. Carcinogenesis. 1985;6:513–521. doi: 10.1093/carcin/6.4.513. [DOI] [PubMed] [Google Scholar]
- Buhler R, Lindros KO, Nordling A, Johansson I, Ingelman-Sundberg M. Zonation of cytochrome P450 isozyme expression and induction in rat liver. Eur. J. Biochem. 1992;204:407–412. doi: 10.1111/j.1432-1033.1992.tb16650.x. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Johansson CB, Wilbertz J, et al. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- Craig CE, Quaglia A, Selden C, Lowdell M, Hodgson H, Dhillon AP. The histopathology of regeneration in massive hepatic necrosis. Semin. Liver Dis. 2004;24:49–64. doi: 10.1055/s-2004-823101. [DOI] [PubMed] [Google Scholar]
- Crosby HA, Nijjar SS, de Goyet Jde V, Kelly DA, Strain AJ. Progenitor cells of the biliary epithelial cell lineage. Semin. Cell Dev. Biol. 2002;13:397–403. doi: 10.1016/s108495210200126x. [DOI] [PubMed] [Google Scholar]
- Druckrey H. Genotypes and phenotypes of ten inbred strains of BD-rats. Arzneimittel-Forschung. 1971;21:1274–1278. [PubMed] [Google Scholar]
- Druckrey H, Preussmann R, Ivankovic S, Schmahl D. Organotropic carcinogenic effects of 65 various N-nitroso- compounds on BD rats. Z. Krebsforsch. 1967;69:103–201. [PubMed] [Google Scholar]
- Dunsford HA, Karnasuta C, Hunt JM, Sell S. Different lineages of chemically induced hepatocellular carcinoma in rats defined by monoclonal antibodies. Cancer Res. 1989;49:4894–4900. [PubMed] [Google Scholar]
- Emerson JA, Vacher J, Cirillo LA, Tilghman SM, Tyner AL. The zonal expression of alpha-fetoprotein transgenes in the livers of adult mice. Dev. Dyn. 1992;195:55–66. doi: 10.1002/aja.1001950106. [DOI] [PubMed] [Google Scholar]
- Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration. J. Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- FitzGerald MJ, Webber EM, Donovan JR, Fausto N. Rapid DNA binding by nuclear factor kappa B in hepatocytes at the start of liver regeneration. Cell Growth Differ. 1995;6:417–427. [PubMed] [Google Scholar]
- Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab. Invest. 1994;70:511–516. [PubMed] [Google Scholar]
- Fujio K, Hu Z, Evarts RP, Marsden ER, Niu CH, Thorgeirsson SS. Coexpression of stem cell factor and c-kit in embryonic and adult liver. Exp. Cell Res. 1996;224:243–250. doi: 10.1006/excr.1996.0134. [DOI] [PubMed] [Google Scholar]
- Graham RC, Jr, Karnovsky MJ. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J. Histochem. Cytochem. 1966;14:291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Higgins GM, Anderson RM. Experimental pathology of the liver: restoration of the liver of the white rat following surgical removal. Arch. Pathol. 1931;12:186–202. [Google Scholar]
- Holden C, Vogel G. Stem cells. Plasticity: time for a reappraisal. Science. 2002;296:2126–2129. doi: 10.1126/science.296.5576.2126. [DOI] [PubMed] [Google Scholar]
- Hu Z, Evarts RP, Fujio K, et al. Expression of transforming growth factor alpha/epidermal growth factor receptor, hepatocyte growth factor/c-met and acidic fibroblast growth factor/fibroblast growth factor receptors during hepatocarcinogenesis. Carcinogenesis. 1996;17:931–938. doi: 10.1093/carcin/17.5.931. [DOI] [PubMed] [Google Scholar]
- Korbling M, Katz RL, Khanna A, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N. Engl. J. Med. 2002;346:738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Kuhlmann WD. Purification of mouse alpha-1-fetoprotein and preparation of specific peroxidase conjugates for its cellular localization. Histochemistry. 1975;44:155–167. doi: 10.1007/BF00494077. [DOI] [PubMed] [Google Scholar]
- Kuhlmann WD. Ultrastructural immunoperoxidase cytochemistry. Prog. Histochem. Cytochem. 1977;10:1–57. doi: 10.1016/s0079-6336(77)80002-8. [DOI] [PubMed] [Google Scholar]
- Kuhlmann WD. Localization of alpha-1-fetoprotein and DNA-synthesis in liver cell populations during experimental hepatocarcinogenesis in rats. Int. J. Cancer. 1978;21:368–380. doi: 10.1002/ijc.2910210319. [DOI] [PubMed] [Google Scholar]
- Kuhlmann WD. Immunoperoxidase labelling of alpha-1-fetoprotein (AFP) in normal and regenerating livers of a low and a high AFP producing mouse strain. Histochemistry. 1979;64:67–75. doi: 10.1007/BF00493355. [DOI] [PubMed] [Google Scholar]
- Kuhlmann WD, Krischan R. Resin embedment of organs and postembedment localization of antigens by immunoperoxidase methods. Histochemistry. 1981;72:377–389. doi: 10.1007/BF00501780. [DOI] [PubMed] [Google Scholar]
- Kuhlmann WD, Wurster K. Correlation of histology and alpha-1-fetoprotein resurgence in rat liver regeneration after experimental injury by galactosamine. Virchows Arch. A Pathol. Anat. Histol. 1980;387:47–57. doi: 10.1007/BF00428428. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Lazarevich NL. Molecular mechanisms of alpha-fetoprotein gene expression. Biochemistry (Mosc) 2000;65:117–133. [PubMed] [Google Scholar]
- Lemaigre FP. Development of the biliary tract. Mech. Dev. 2003;120:81–87. doi: 10.1016/s0925-4773(02)00334-9. [DOI] [PubMed] [Google Scholar]
- Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin. Cell Dev. Biol. 2002;13:389–396. doi: 10.1016/s1084952102001258. [DOI] [PubMed] [Google Scholar]
- Lowes KN, Croager EJ, Olynyk JK, Abraham LJ, Yeoh GC. Oval cell-mediated liver regeneration: role of cytokines and growth factors. J. Gastroenterol. Hepatol. 2003;18:4–12. doi: 10.1046/j.1440-1746.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- Masson S, Harrison DJ, Plevris JN, Newsome PN. Potential of hematopoietic stem cell therapy in hepatology: a critical review. Stem Cells. 2004;22:897–907. doi: 10.1634/stemcells.22-6-897. [DOI] [PubMed] [Google Scholar]
- Menthena A, Deb N, Oertel M, et al. Bone marrow progenitors are not the source of expanding oval cells in injured liver. Stem Cells. 2004;22:1049–1061. doi: 10.1634/stemcells.22-6-1049. [DOI] [PubMed] [Google Scholar]
- Moorman AF, De Boer PA, Evans D, Charles R, Lamers WH. Expression patterns of mRNAs for alpha-fetoprotein and albumin in the developing rat: the ontogenesis of hepatocyte heterogeneity. Histochem. J. 1990;22:653–660. doi: 10.1007/BF01047449. [DOI] [PubMed] [Google Scholar]
- Oh SH, Hatch HM, Petersen BE. Hepatic oval ‘stem’ cell in liver regeneration. Semin. Cell Dev. Biol. 2002;13:405–409. doi: 10.1016/s1084952102001271. [DOI] [PubMed] [Google Scholar]
- Olsson M, Lindahl G, Ruoslahti E. Genetic control of alpha-fetoprotein synthesis in the mouse. J. Exp. Med. 1977;145:819–827. doi: 10.1084/jem.145.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori N, Omori M, Evarts RP, et al. Partial cloning of rat CD34 cDNA and expression during stem cell-dependent liver regeneration in the adult rat. Hepatology. 1997;26:720–727. doi: 10.1002/hep.510260325. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Belayew A, Tilghman SM. Locus unlinked to alpha-fetoprotein under the control of the murine raf and Rif genes. Proc. Natl. Acad. Sci. U. S. A. 1984;81:5523–5527. doi: 10.1073/pnas.81.17.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelc SR. Autoradiograph technique. Nature. 1947;160:749–750. doi: 10.1038/160749b0. [DOI] [PubMed] [Google Scholar]
- Petersen BE. Hepatic ‘‘stem’’ cells: coming full circle. Blood Cells Mol. Dis. 2001;27:590–600. doi: 10.1006/bcmd.2001.0422. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433–445. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Grossbard B, Hatch H, Pi L, Deng J, Scott EW. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology. 2003;37:632–640. doi: 10.1053/jhep.2003.50104. [DOI] [PubMed] [Google Scholar]
- Pitot HC. Altered hepatic foci: their role in murine hepatocarcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1990;30:465–500. doi: 10.1146/annurev.pa.30.040190.002341. [DOI] [PubMed] [Google Scholar]
- Poliard AM, Bernuau D, Tournier I, et al. Cellular analysis by in situ hybridization and immunoperoxidase of alpha-fetoprotein and albumin gene expression in rat liver during the perinatal period. J. Cell Biol. 1986;103:777–786. doi: 10.1083/jcb.103.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabes HM, Bucher T, Hartmann A, Linke I, Dunnwald M. Clonal growth of carcinogen-induced enzyme-deficient preneoplastic cell populations in mouse liver. Cancer Res. 1982;42:3220–3227. [PubMed] [Google Scholar]
- Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pihko H, Vaheri A, Seppala M, Virolainen M, Konttinen A. Alpha fetoprotein: structure and expression in man and inbred mouse strains under normal conditions and liver injury. Johns Hopkins Med. J. 1974;3(Suppl.):249–255. [PubMed] [Google Scholar]
- Saxena R, Theise ND, Crawford JM. Microanatomy of the human liver-exploring the hidden interfaces. Hepatology. 1999;30:1339–1346. doi: 10.1002/hep.510300607. [DOI] [PubMed] [Google Scholar]
- Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J. Clin. Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S. The role of progenitor cells in repair of liver injury and in liver transplantation. Wound Repair Regen. 2001;9:467–482. doi: 10.1046/j.1524-475x.2001.00467.x. [DOI] [PubMed] [Google Scholar]
- Sell S. Cellular origin of hepatocellular carcinomas. Semin. Cell Dev. Biol. 2002;13:419–424. doi: 10.1016/s1084952102001295. [DOI] [PubMed] [Google Scholar]
- Sell S. The hepatocyte: heterogeneity and plasticity of liver cells. Int. J. Biochem. Cell Biol. 2003;35:267–271. doi: 10.1016/s1357-2725(02)00182-6. [DOI] [PubMed] [Google Scholar]
- Sell S, Becker FF, Leffert HL, Watabe L. Expression of an oncodevelopmental gene product (alpha-fetoprotein) during fetal development and adult oncogenesis. Cancer Res. 1976;36:4239–4249. [PubMed] [Google Scholar]
- Shiojiri N, Lemire JM, Fausto N. Cell lineages and oval cell progenitors in rat liver development. Cancer Res. 1991;51:2611–2620. [PubMed] [Google Scholar]
- Steinberg P, Hacker HJ, Dienes HP, Oesch F, Bannasch P. Enzyme histochemical and immunohistochemical characterization of oval and parenchymal cells proliferating in livers of rats fed a choline-deficient/DL-ethionine-supplemented diet. Carcinogenesis. 1991;12:225–231. doi: 10.1093/carcin/12.2.225. [DOI] [PubMed] [Google Scholar]
- Taketa K, Watanabe A, Kosaka K. Different mechanisms of increased alpha-fetoprotein production in rats following CCl4 intoxication and partial hepatectomy. Ann. N. Y. Acad. Sci. 1975;259:80–84. doi: 10.1111/j.1749-6632.1975.tb25404.x. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Theise ND, Saxena R, Portmann BC, et al. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- Theise ND, Badve S, Saxena R, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000a;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- Theise ND, Nimmakayalu M, Gardner R, et al. Liver from bone marrow in humans. Hepatology. 2000b;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- Tian YW, Smith PG, Yeoh GC. The oval-shaped cell as a candidate for a liver stem cell in embryonic, neonatal and precancerous liver: identification based on morphology and immunohistochemical staining for albumin and pyruvate kinase isoenzyme expression. Histochem. Cell Biol. 1997;107:243–250. doi: 10.1007/s004180050109. [DOI] [PubMed] [Google Scholar]
- Timens W, Kamps WA. Hemopoiesis in human fetal and embryonic liver. Microsc. Res. Tech. 1997;39:387–397. doi: 10.1002/(SICI)1097-0029(19971201)39:5<387::AID-JEMT1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Tournier I, Legres L, Schoevaert D, Feldmann G, Bernuau D. Cellular analysis of alpha-fetoprotein gene activation during carbon tetrachloride and D-galactosamine-induced acute liver injury in rats. Lab. Invest. 1988;59:657–665. [PubMed] [Google Scholar]
- Uriel J. Cancer, retrodifferentiation, and the myth of Faust. Cancer Res. 1976;36:4269–4275. [PubMed] [Google Scholar]
- Vogt TF, Solter D, Tilghman SM. Raf, a trans-acting locus, regulates the alpha-fetoprotein gene in a cell-autonomous manner. Science. 1987;236:301–303. doi: 10.1126/science.2436297. [DOI] [PubMed] [Google Scholar]
- Voller A, Bartlett A, Bidwell DE. Enzyme immunoassays with special reference to ELISA techniques. J. Clin. Pathol. 1978;31:507–520. doi: 10.1136/jcp.31.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Wolf CR, Moll E, Friedberg T, et al. Characterization, localization and regulation of a novel phenobarbital-inducible form of cytochrome P450, compared with three further P450-isoenzymes, NADPH P450-reductase, glutathione transferases and microsomal epoxide hydrolase. Carcinogenesis. 1984;5:993–1001. doi: 10.1093/carcin/5.8.993. [DOI] [PubMed] [Google Scholar]
- Yao P, Zhan Y, Xu W, et al. Hepatocyte growth factor-induced proliferation of hepatic stem-like cells depends on activation of NF-kappaB. J. Hepatol. 2004;40:391–398. doi: 10.1016/j.jhep.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Yavorkovsky L, Lai E, Ilic Z, Sell S. Participation of small intraportal stem cells in the restitutive response of the liver to periportal necrosis induced by allyl alcohol. Hepatology. 1995;21:1702–1712. [PubMed] [Google Scholar]
- Yin L, Lynch D, Ilic Z, Sell S. Proliferation and differentiation of ductular progenitor cells and littoral cells during the regeneration of the rat liver to CCl4/2-AAF injury. Histol. Histopathol. 2002;17:65–81. doi: 10.14670/HH-17.65. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]