Abstract
The Ubiquitin-proteasome system has recently been shown to be involved in the regulation of cytokine expression. We tested the hypothesis of whether the in vivo administration of proteasome inhibitor MG-132 can modulate cytokine response and mortality in sepsis. Sepsis was induced in mice by caecal ligation and puncture (CLP). Animals were divided into four groups: control, CLP, CLP and 1 μg MG-132/g of b.w. intraperitoneally, and CLP and 10 μg MG-132/g of b.w. Plasma levels of interleukin (IL)-1, tumour necrosis factor-α (TNF-α, IL-6 and IL-10 were determined by ELISA 6 h after the induction of sepsis. CLP induced significant increase in plasma levels of all measured cytokines. MG-132 treatment resulted in lower increase in IL-1, TNF-α and IL-10 levels. IL-6 was not significantly affected. A mortality study revealed prolonged survival in MG-132 treated mice. We conclude that MG-132 treatment decreases inflammatory response and prolongs survival in the CLP model of sepsis.
Keywords: cytokine, inflammatory response, MG-132, proteasome inhibitor, sepsis
Introduction
Sepsis has become increasingly important over the past decades, septic shock has a very high and increasing mortality (Riedermann et al. 2003). Experimental data support the theory that an early intense proinflammatory response of the immune system after infection can cause harm or set the stage for subsequent organ damage. Therefore, interventions designed to attenuate inflammatory response might be clinically useful (Glauser 2000). Unspecific blocking of the physiological reaction [e.g. blocking of CD14, tumour necrosis factor (TNF)-α, TNF-αR or interleukin (IL)-1R] has been shown to decrease severity of symptoms in sepsis and mortality in lipopolysaccharide-induced septic shock. However, it does not influence or even increase mortality when subjects, both animals and humans, are exposed to alive microorganisms (Glauser 2000; Riedermann et al. 2003). This shows that our understanding of the inflammatory process and its regulation is still insufficient, and effective ways of how to beneficially modulate inflammatory response are still being intensively researched.
Proteasome inhibitors inhibit one of the major pathways of intracellular proteolysis – adenosine triphosphate (ATP) dependent ubiquitin-proteasome proteolytic pathway, which is highly activated in sepsis (Hobler et al. 1999). Proteasome inhibitors are perspective drugs, one of them is already used for treatment of haematological malignancies (Richardson et al. 2004), another is undergoing clinical trials for treatment of stroke and myocardial infarction (Di Napoli & Papa 2003) and their possible use in inflammatory diseases remains to be evaluated. From the use of these drugs in cancers and other disease states with high incidence of sepsis and from anti-inflammatory effects of proteasome inhibitors arises a need for knowledge of how the proteasome inhibitor treated organism reacts to pathogens. This study was designed to show whether in vivo administration of proteasome inhibitor MG-132 (Z-Leu-Leu-Leu-al) has any effect on cytokine response and mortality in sepsis induced by caecal ligation and puncture (CLP).
Methods
Female C57Bl/6 specified pathogen-free mice at the age of 6–8 weeks and body weight of 20–25 g were used in this study. The experiments were performed in adherence to the Guidelines on the Use of Laboratory Animals of the Kurume University.
As a proteasome inhibitor we used MG-132 (Sigma chemicals, St. Louis, MO, USA) that has been shown to inhibit proteasome in a dose-dependent manner in living mice (Lucker et al. 2003) and to decrease muscle proteolysis in muscles from normal and septic rats (Kadlčikováet al. 2004). MG-132 was dissolved in dimethyl sulphoxide (DMSO), diluted with physiologic saline to 1 ml and administered intraperitoneally. Sepsis was induced in ether anaesthesia by CLP. In brief, 80% of the cecum was ligated through a 1.5 cm abdominal midline incision (Singleton & Wischmeyer 2003). One through-and-through puncture was made using an 18 gauge needle, and a small amount of stool was expelled from the puncture to ensure leakage of the intestinal content. After reposition of the bowel, the abdomen was closed in two layers. Sham-operated animals underwent the same procedure without ligation and puncture of the cecum. Two separate studies were performed to assess the effect of proteasome inhibitor administration on cytokine response and survival in CLP model.
In the first study, we evaluated cytokine response in CLP after MG-132 administration. Animals were randomly divided into four groups – control (sham surgery and diluent administration) (n = 7), CLP and diluent administration (n = 11), CLP and 1 μg MG-132/g of body weight (n = 9), and CLP and 10 μg MG-132/g of b.w. (n = 10). MG-132 was administered 3 h before the surgery. Six hours after the induction of sepsis or sham surgery animals were anaesthetized with ether and blood samples were obtained by cardiac puncture. Plasma was obtained from these blood samples and levels of IL-1β, TNF-α, IL-6 and IL-10 were determined by ELISA kits purchased from Biosource International (Camarillo, CA, USA) according to manufacturers’ protocol. Absorbance was determined at 450 nm and values were calculated with Versamax Microplate Reader and SoftmaxPro (Molecular Device, Sunnyvale, CA, USA).
In the second study, we assessed the impact of proteasome inhibitor on survival in CLP model. MG-132 in a dose 10 μg/g of b.w. was administered 3 h before or 3 h after the induction of CLP or sham operation; there were 12 animals in each group. During the experiment, animals received standard chow and water ad libitum and were observed for survival.
One-way anova followed by Tukey–Kramer test was used for statistical analysis of inflammatory response. Kaplan–Meier survival curves and Logrank test was used to analyse mortality study. Significance was accepted at *P < 0.05. Data are expressed as mean ± SEM.
Results
Plasma levels of all measured cytokines (IL-1β, TNF-α, IL-6 and IL-10) were not detectable in control animals. CLP induced increase in plasma levels of IL-1β to 128 ± 16 pg/ml, TNF-α to 109 ± 25 pg/ml, IL-6 to 19696 ± 3310 pg/ml and IL-10 to 3258 ± 673 pg/ml, respectively. As shown on Table 1, MG-132 treatment caused, in a dose dependent manner, significantly lower increase in plasma levels of IL-1β, TNF-α and IL-10. However, the effect on plasma IL-6 levels was insignificant.
Table 1.
Plasma cytokine levels 6 h after caecal ligation and puncture (CLP)
| Experimental group | |||
| Cytokine | CLP only (n = 11) | CLP and low dose MG-132 (n = 9) | CLP and high dose MG-132 (n = 10) |
|---|---|---|---|
| IL-1 (pg/ml) | 128 ± 16 | 62 ± 10** | 56 ± 6*** |
| TNF-α (pg/ml) | 109 ± 25 | 44 ± 8* | 24 ± 6* |
| IL-6 (pg/ml) | 19696 ± 3310 | 11786 ± 2659 | 13877 ± 3702 |
| IL-10 (pg/ml) | 3258 ± 673 | 341 ± 61*** | 198 ± 38*** |
Plasma levels of cytokines in control animals were not detectable.
Data are given as mean ± SEM. anova followed by Tukey–Kramer test.
Low dose MG-132, 1 μg/g of body weight; high dose MG-132, 10 μg/g of body weight.
P < 0.05;
P < 0.01;
P < 0.001.
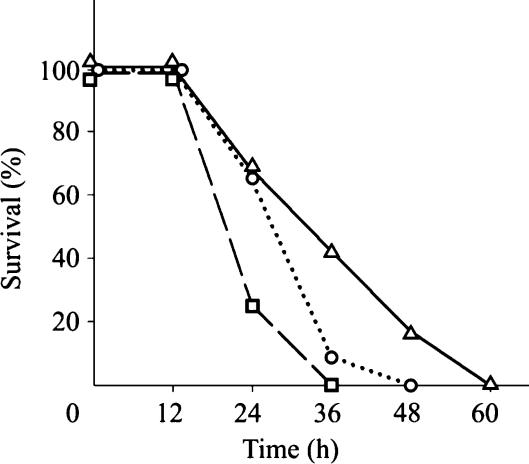
Figure 1 shows the effect of MG-132 administration on survival after CLP. Survival was significantly longer in both groups that received proteasome inhibitor and longest in a group that received MG-132 3 h after induction of CLP. Median times of survival for the three groups (CLP only, MG-132 3 h before CLP, MG-132 3 h after CLP) were 24, 36 and 36 h, respectively.
Figure 1.
Survival curves. Groups: caecal ligation and puncture (CLP) only, MG-132 administration 3 h before CLP, and MG-132 administration 3 h after CLP. In MG-132 treated groups, survival was significantly longer compared with CLP group. Logrank test, P = 0.011, 12 animals in each group.
Discussion
We have shown that in vivo administration of proteasome inhibitor MG-132 significantly decreased production of TNF-α, IL-1β and IL-10; production of IL-6 decreased insignificantly. This finding suggests that proteasome is involved in signal trasnsduction in CLP model of sepsis.
The reason why we observed a lower decrease in IL-6 production after proteasome inhibition might by explained by different pathways of interleukine activation. IL-6 production is, at least partially, proteasome and NF-κB independent (Haddad & Fahlman 2002), and its expression is not very sensitive to proteasome inhibition. This is consistent with recent finding that proteasome inhibition attenuates lipopolysaccharide-induced IκB degradation resulting in impaired NF-κB activation with subsequent decrease in TNF-α levels and no change in IL-6 levels in cultured macrophage (Cuschieri et al. 2004).
It should be noted that MG-132 is not a specific proteasome inhibitor. To a lesser extent it also inhibits other proteases and its selectivity is shown by the fact that inhibition of calpains and cathepsins requires at least 10-fold higher concentration than inhibition of preteasomes (Tsubuki et al. 1996). However, we cannot exclude that part of the effects of MG-132 is mediated by other proteases.
Prolonged survival after administration of proteasome in our study shows that MG-132 inhibited the development of inflammatory response, but preserved the response to pathogens that is essential to combat the infection. The longest survival in a group that received MG-132 3 h after the induction of CLP, compared with MG-132 pretreatment, shows that it is not necessary to block preteasome before the onset of sepsis and that proteasome inhibitor might be used as a treatment. Prolonged survival was associated with a decrease in pro-inflammatory cytokines in our experiment; this is in agreement with Manley et al. (2005) and others who have shown that the expression of proinflammatory cytokines in the early phase of sepsis is related to outcome. It is interesting to note that Manley et al. (2005) also showed that higher levels of IL-10 in later phases of sepsis have some positive effects. In our experiment, prolonged survival was associated with lower expression of IL-10.
In conclusion, our data strongly suggests that proteasome regulates signal transduction after CLP and that regulation of proteasome activity by proteasome inhibitor could be an effective way to modulate the inflammatory response in the CLP model in mice.
Acknowledgments
We would like to thank Takeshita Kumi and Yamaguchi Emiko for their technical assistance and James Dykman for his English assistance. Roman Šafránek was partially supported by the grant of the Ministry of Health of the Czech Republic No. NB/7611-3 and by Research Project MSM 0021620820.
References
- Cuschieri J, Gourlay D, Garcia I, Jelacic S, Maier RV. Implications of proteasome inhibition: an enhanced macrophage phenotype. Cell. Immun. 2004;227:140–147. doi: 10.1016/j.cellimm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Papa F. MLN-519. Millennium/PAION. Curr. Opin. Investig. Drugs. 2003;4:333–341. [PubMed] [Google Scholar]
- Glauser MP. Pathophysiologic basis of sepsis: considerations for future strategies of intervention. Crit. Care Med. 2000;28:S4–8. doi: 10.1097/00003246-200009001-00002. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Fahlman CS. Nuclear factor-κB-independent regulation of lipopolysaccharide-mediated interleucin-6 biosynthesis. Biochem. Biophys. Res. Commun. 2002;291:1045–1051. doi: 10.1006/bbrc.2002.6556. [DOI] [PubMed] [Google Scholar]
- Hobler SC, Williams A, Fischer D, et al. Activity and expression of the 20S proteasome are increased in skeletal muscle during sepsis. Am. J. Physiol. 1999;277:R434–R440. doi: 10.1152/ajpregu.1999.277.2.R434. [DOI] [PubMed] [Google Scholar]
- Kadlčiková J, Holeček M, Šafránek R, Tilšer I, Kessler BM. Effects of proteasome inhibitors MG132, ZL3VS and AdaAhx3L3VS on protein metabolism in septic rats. Int. J. Exp. Path. 2004;85:365–371. doi: 10.1111/j.0959-9673.2004.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucker GD, Pica CM, Song J, Lucker KE, Piwnica-Worms D. Imaging 26S proteasome activity and inhibition in living mice. Nat. Med. 2003;9:969–973. doi: 10.1038/nm894. [DOI] [PubMed] [Google Scholar]
- Manley MO, O'Riordan MA, Levine AD, Latifi SQ. Interleukin 10 extends the effectiveness of standard therapy during late sepsis with serum interleukin 6 levels predicting outcome. Shock. 2005;23:521–526. [PubMed] [Google Scholar]
- Richardson PG, Hideshima T, Mitsiades C, Anderson K. Proteasome inhibition in hematologic malignancies. Ann. Med. 2004;36:304–314. doi: 10.1080/07853890410030877. [DOI] [PubMed] [Google Scholar]
- Riedermann NC, Guo RF, Ward PA. The enigma of sepsis. J. Clin. Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton KD, Wischmeyer PE. Distance of cecum ligated influences mortality, tumor necrosis factor-alpha and interleukin-6 expression following cecal ligation and puncture in the rat. Eur. Surg. Res. 2003;35:486–491. doi: 10.1159/000073387. [DOI] [PubMed] [Google Scholar]
- Tsubuki S, Saito Y, Tomioka M, Ito H, Kawashima S. Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine. J. Biochem. 1996;119:572–576. doi: 10.1093/oxfordjournals.jbchem.a021280. [DOI] [PubMed] [Google Scholar]