Abstract
Various clinical trials have documented the therapeutic benefit of glucocorticoids (GCs) in enhancing muscle strength and slowing disease progression of Duchenne and Becker muscular dystrophies (DMD/BMD). We hypothesized that GCs may have relevance to the differential anti-inflammatory effect on mononuclear inflammatory cells (MICs) and Dendritic cells (DCs) infiltrating the dystrophic muscles. In this prospective study, two muscle biopsies were obtained (before and after 6-month prednisone therapy) from 30 patients with dystrophies (DMD = 18; BMD = 6; and limb girdle muscular dystrophies (LGMD) = 6). MICs and DCs infiltrating the muscles were examined using mouse monoclonal antibodies and immunoperoxidase staining methods. Muscle strength was evaluated monthly by manual testing, motor ability and timed tests. Prednisone therapy was associated with: (i) functional improvement of overall motor disability, in upper limbs of DMD (P < 0.001) and BMD (P < 0.01) and lower limbs of DMD (P < 0.001) and BMD (P < 0.05); (ii) histological improvement such as fibre size variation (DMD, P < 0.01; BMD, P < 0.05), internalization of nuclei (DMD, P < 0.05), degeneration and necrosis (DMD and BMD, P < 0.01), regeneration (DMD, P < 0.001; BMD, P < 0.01) and endomysial connective tissue proliferation (DMD, P < 0.01; BMD, P < 0.05) and (iii) reduction of total MICs (P < 0.01) and DCs (P < 0.01). There was a positive correlation between the degree of improvement in overall motor disability and reduction of DCs numbers (In upper limbs; r = 0.638, P < 0.01 for DMD and r = 0.725, P < 0.01 for BMD, in Lower limbs; r = 0.547, P < 0.05 for DMD and r = 0.576, P < 0.05 for BMD). Such improvements and changes of MICs/DCs were absent in LGMD. In DMD/BMD, prednisone therapeutic effect was associated with reduced MICs and DCs numbers. Whether this therapeutic effect reflects targeting of the deleterious immune response produced by these cells mandates further investigations.
Keywords: Dendritic cells, glucocorticoid, muscular dystrophy
In skeletal muscle, the dystrophin protein, a large subsarcolemmal protein (427 kDas), exists tightly associated with organized arrays of other structural proteins (Dystrophin- glycoprotein complex or DGC) that serves as an anchor and force-transmitting structure for the contractile proteins organized in the myofilaments, preventing muscle disruption due to shear forces during repeated cycles of muscle contraction and relaxation (Hoffman et al. 1987; Ervasti & Campbell 1991; Ohlendieck 1996). Disruption of DGC results in a disease state collectively known as muscular dystrophies. Duchenne muscular dystrophy (DMD) and its allelic type Becker muscular dystrophy (BMD) are the most common subtypes of muscular dystrophies that result from dystrophin gene mutation (Koenig et al. 1988; Hamed et al. in press; Hamed & Hoffman 2006) (http://www.dmd.nl/database.htm.). DMD is the most common devastating childhood X-linked disorder, resulting in early loss of ambulation between the ages of 7 and 13 years and death in the teens and twenties (http://www.dmd.nl/database.htm.) (Ben Hamida et al. 1983; Angelini et al. 1990; Matsumura et al. 1993).
The complete definition of the pathophysiologic cascade, which results in muscle cell death and its inability to regenerate, is extremely important for therapeutic approaches. Disruption of DGC is associated with alterations in the muscle cells microenvironment including complex and differential alteration of immune response with secondary deleterious progressive muscle degeneration (Ben Hamida et al. 1983; Lundberg et al. 1995; Hamed & Hoffman 2006; Hamed et al. in press).
It is well known that glucocorticoids (GCs), immunosuppressive drugs, have beneficial therapeutic roles in the treatment of muscular dystrophies (Merlini et al. 2003; Gaud et al. 2004; Mesa et al. 1991). This notion is supported by two observations. First, the targeted immunosuppression in dystrophic mice can delay the progression of the muscle damage (Takagi et al. 1998; Gosselin & McCormick 2004; Wehling-Henricks et al. 2004). Second, several GCs therapeutic clinical trials have documented the beneficial improvement in muscle strength, increased muscle mass and histological changes in DMD and BMD (Drachman et al. 1974; DeSilva et al. 1987; Mendell et al. 1989; Griggs et al. 1991). This beneficial effect of steroids may reflect immune response alteration of inflammatory cells infiltrating the damaged muscle. In support, (i) cellular immune responses by mononuclear inflammatory cell (MICs) contribute to muscle pathology in dystrophin-deficient muscle (Arahata & Engel 1984; Kissel et al. 1993; Spencer & Tidball 2001), (ii) Dendritic cells (DCs) and mast cells have been very recently suggested to act coordinately to mediate acute and chronic microenvironmental changes in dystrophic muscles (Sueki et al. 1993; Gorospe et al. 1994; Chen et al. 2000) and (iii) GCs can prevent activation of DCs by redirecting differentiation of a subset of cells, despite the presence of inflammatory cytokines (Matasic et al. 1999).
Therefore, it seems that there are some relationships among immunosuppressive effects of GCs, cellular immune responses (MICs and DCs) and muscle fibre loss in dystrophies. Nevertheless, our understanding of these relationships is still incomplete. In this investigation, we hypothesized that the beneficial therapeutic effects of GCs are associated with alterations of MICs and DCs infiltrating the dystrophic muscles. To test our hypothesis and to fill this existing gap in literature, we examined these cells in 30 patients with muscular dystrophies before and after GCs therapy.
Materials and methods
Patients
This prospective study included 30 patients with clinical and laboratory features suggestive of muscular dystrophies (DMD = 18, BMD = 6 and limb girdle muscular dystrophies (LGMD) = 6) recruited from the Department of Neurology, Assiut University Hospitals, Assiut, Egypt. Patients with primary neurologic diseases not diagnosed as primary muscular dystrophy were excluded. In this prospective study, clinical and laboratory data were evaluated twice before and at the end of the 6-month prednisone therapy (0.75 mg/kg/day). None of the patients were receiving immunosuppressive drugs till the time of the study. The experimental design was approved by the regional Ethics Committee. Informed consent was obtained from the patients and their parents before participation in this study.
Methods
Muscular dystrophy was diagnosed based on the clinical manifestations of muscle weakness and wasting, high creatine phosphokinase (CPK) values, myopathic electromyography (EMG) changes and dystrophic changes in muscle (Buchthal & Kamieniecka 1982; Bushby et al. 1993). The diagnosis and categorization of muscular dystrophies was confirmed by immunostaining with antibodies against dystrophin and other membrane proteins, as described before (Sealock et al. 1991; Hoshino et al. 2000; Sheriffs et al. 2001). These proteins can be detected by Western blotting or by immunohistochemistry; with the latter being thought to be the more sensitive technique. This is because of an apparent loss or breakdown of an epitope of dystrophin that occurs during processing for Western blotting (Nicholson et al. 1992). It has also been suggested that immunohistochemical analysis is more reliable than gene analysis using the multiplex polymer chain reaction, which only reveals approximately 70% of DMD mutations (Ozawa et al. 1998). Immunohistochemistry has become a powerful tool in distinguishing different muscular dystrophies, because of its speed, accuracy and the increasing availability of antibodies to dystrophin and its associated proteins.
Clinical assessment of the muscular dystrophies
In this study, clinical and lab assessment was done blindly. None of the patients was given placebo. The muscle strength was assessed before the start of prednisone therapy and every month during and at the end of the 6-month prednisone therapy (0.75 mg/kg/day). Grading of muscle power was done according to the Medical British Counsil or MBC (1–5) grading system. This evaluation was done using a battery of muscle force and function tests including manual testing, motor ability and timed tests (time in seconds to rise from the ground, time to climb up nine stairs and time to travel 10 meters as rapidly as possible) as described by others (Brooke et al. 1987; Mendell et al. 1989). The overall motor disability was evaluated in both upper and lower limbs as described before (Archibald & Vignos 1959).
Pathological evaluation
In this prospective study, pathological evaluation of muscle biopsies was done twice before and at the end of the 6-month prednisone therapy (0.75 mg/kg/day).
In the surgical theater and under complete aseptic conditions, muscle biopsy specimens were taken (open percutaneous biopsy specimen measuring at least 0.5 cm in diameter, and 1.0 cm in length along the longitudinal axis of the muscle fibres) from the most bulky part of the gastrocnemius muscle that was not subjected to EMG examination before, as needling was known to induce changes indistinguishable from inflammatory myopathy (Engel et al. 1996). Specimens then placed in 10% neutral buffered formalin solution and processed for paraffin embedding. Histological examination of sections was carried out utilizing haematoxylin and eosin (H&E) as well as Masson’s trichrome staining. Sections were examined by light microscopy, and the following pathological changes were described and assessed including; gapping of muscle bundles and fibres, splitting of muscle fibres, fibre size variation, scattered degenerating and regenerating fibres and connective tissue proliferation. These changes were examined in at least six different sections in each case, by two observers. In each section, at least five different fields were examined. Evaluation of the muscle changes (fibre size variation, internalization of nuclei, degeneration and necrosis, regeneration and endomysial connective tissue proliferation) was done using the following scoring system: no changes, 0; mild changes, 1; moderate changes, 2 and sever changes, 3. The results were reported as mean ± SD.
Immunohistochemical analysis
Immunohistochemical characterization of dystrophin and inflammatory cells
The definitive diagnosis of dystrophies can be made by means of immunostaining with antibodies against dystrophin and its associated protein immunostaining with dystrophin protein was done, as described by the manufacturer, utilizing a labelled streptavidin-biotin immunoenzymatic antigen detection system, Ultravision Detection System, Anti-Polyvalent, HRP/DAB, Catalogue number TP-015-HD, Laboratory Vision Corporation, Fermont, CA, USA. The primary antibody utilized was Dystrophin Ab-1, mouse monoclonal antibody (Clone 1808). This antibody is highly specific for dystrophin and shows no cross-reaction with C-protein (an isoform of µ-actinin), µ-actin or muscle spectrin. A biotinylated goat anti-polyvalent was utilized as secondary antibody.
Immunohistochemical characterization of inflammatory cells was carried out as previously described (Hussein & Ismael 2004). [Clones L26, UCHL-1, PG M1 and To5 for CD20 (B lymphocytes), CD3 (T lymphocytes), CD68 (histiocytes) and CD35 (DCs), DAKO corporation, CA, USA.] A catalysed signal amplification system (K1500, DAKO corp., USA) was used according to the manufacturer instructions.
Immunostaining was carried following the manufacturer instructions. Briefly, sections mounted on glass slides were deparaffinized and rehydrated through descending graded alcohols to water. Endogenous peroxidase activity was blocked with 0.6% H202. Sections were then immersed in the retrieval solution (10 mm sodium citrate buffer, pH 6.0) and subjected to heat-induced antigen retrieval for 20 min. The slides, in plastic Coplin jars containing retrieval solution, were microwaved in a microwave set at high (approximately 750 W) for four cycles of 5-min duration each. Non-specific protein binding was blocked with 10-min exposure to 10% normal goat serum. Sections were then incubated with mouse monoclonal antibodies for 30 min at 37 °C. A catalysed signal amplification system (for inflammatory cells) and Ultravision Detection System (for dystrophin) were used according to the manufacturer instructions. Sections were next treated with peroxidase-labelled streptavidin for 30 min at 37 °C and incubated with 14-diaminobenzidine for 5 min. They were counterstained with haematoxylin, dehydrated in alcohol, cleared in xylene and cover slipped. The slides were independently evaluated by two observers (Drs Mahmoud R. Hussein and Mohammed G. Mostafa). The experiments were done in triplicate. For each antibody, at least 10 different sections were examined in each case.
Positive controls
The positive control specimens consisted of normal muscle (dystrophin protein) and lymph nodes with reactive lymphoid hyperplasia (CD3, CD20, CD68 and CD35). DMD was diagnosed based on the absence of dystrophin in the muscle biopsies while BMD patients had reduced amounts of the dystrophin protein. Alternatively, the dystrophin-positive patients were defined as LGMD (Brooke et al. 1987; Hoffman et al. 1988; Hoshino et al. 2000; Sheriffs et al. 2001). In this study, we did not categorize different types of LGMD.
Negative controls
Additional sections running in parallel but with omission of the primary antibody served as the negative controls (Hussein & Ismael 2004).
Quantification of MICs in muscle biopsy
The cell infiltrate in the examined sections, whether routinely or immunohistochemically stained, was totally and differentially counted in five high-power fields (HPFs; × 400) in areas showing the most intense infiltrate, selected on low-power examination. Two pathologists recorded the average number of cells in the five selected HPFs by double-blind counting method. The cell counts in this work thus represented the mean (±SD or standard deviation) of the recordings reported by the two observers (Drs Mohmoud R. Hussein and Mohammed G. Mostafa).
Statistical analysis
Statistical comparison among different groups was evaluated using analysis of variance (anova). Calculations were done with the statistical package spss for windows, version 10.0. Statistical significance was defined as P < 0.05.
Results
Clinical characteristics of the dystrophic patients
Thirty patients (DMD = 18, BMD = 6 and LGMD = 6) (male/female; 27/3) carrying the clinical and laboratory features of muscular dystrophy were included in this study. Patients presented mainly by girdle-limb weakness, muscle wasting and hypertrophy. Muscle wasting was observed mainly in the biceps, supraspinatus, triceps and quadriceps muscles of all patients and deltoid gluteus maximus muscles in some patients. Hypertrophy was seen in calf, gluteus maximus and deltoid muscles. The mean age was statistically significantly higher in LGMD (11.1 ± 4.4 years, P < 0.05) than BMD (8.6 ± 1.3 years) or DMD (4.9 ± 1.3 years). Demographic data were summarized in Table 1.
Table 1.
Demographic, clinical and laboratory data of muscular dystrophy group of patients
| Demographic data | DMD (n = 18) | BMD (n = 6) | LGMD (n = 6) |
|---|---|---|---|
| Age of onset | |||
| Range (Mean ± SD), years | 3–7 (4.9 ± 1.3) | 7–14 (8.6 ± 1.3) | 8–20 (11.1 ± 4.4) |
| Age at sampling | |||
| Range (Mean ± SD), years | 4–14 (5.7 ± 1.0) | 9–18 (9.9 ± 1.6) | 11–22 (14.1 ± 2.4) |
| Age at presentation | |||
| Range (Mean ± SD), years | 5–10 (5.6 ± 2.4) | 8–18 (13.0 ± 2.3) | 18–35 (2.6 ± 4.1) |
| Male/Female | 18/0 | 6/0 | 3/3 |
| Duration of illness | |||
| Range (Mean ± SD), years | 0.6–5 (2.4 ± 0.8) | 1–8 (4.60 ± 1.71) | 1–13 (5.60 ± 1.9) |
| Similar condition in the family | 8 (44.4%) | 3 (50%) | 3 (50%) |
| Brother/cousin | 6/2 | 3/3 | 2/1 |
| CPK | |||
| Pre-treatment: Mean ± SD; IU/l | 7388.9 ± 4191.6 | 6258.0 ± 2821.1 | 1270.9 ± 673.5 |
| Post-treatment: Mean ± SD; IU/l | 7690.7 ± 4297.4 | 6223.3 ± 2931.5 | 1138.5 ± 821.1 |
| LDH | |||
| Pre-treatment: Mean ± SD; IU/l | 969.2 ± 552.7 | 868.0 ± 354.9 | 465.9 ± 235.3 |
| Post-treatment: Mean ± SD; IU/l | 998.2 ± 542.7 | 792.0 ± 302.0 | 407.6 ± 241.6 |
BMD, Becker muscular dystrophies; CPK, creatine phosphokinase; DMD, Duchenne muscular dystrophies; LDH, lactate dehydrogenase; LGMD, limb girdle muscular dystrophies.
Functional changes of the muscle after 6-month prednisone therapy in DMD and BMD
Most DMD (89%) and BMD (60%) patients exhibited improvement of motor function during the 6-months prednisone therapy (peaked in the first 3 months of therapy). This was obvious in (i) improvement of the muscle power (Table 2) (ii) timed function of motor assessment including, time in seconds to rise up from the ground to standing position, time in seconds for going up nine stairs and time in seconds to travel 10 meters as rapid as possible (Table 3) and (iii) overall motor disability in both upper and lower limbs (Table 3). Improvement in overall motor disability was inversely related to the duration of illness (in upper limbs; r = –0.618, P < 0.01 for DMD and r = –0.823, P < 0.01 for BMD, in lower limbs; r = –0.518, P < 0.05 for DMD and r = –0.580, P < 0.05 for BMD).
Table 2.
Assessment of the muscle power of the studied group of patients pre- and at the end of 6-month prednisone therapy
| DMD Range (Mean ± SD) | BMD Range (Mean ± SD) | LGMD Range (Mean ± SD) | ||||
|---|---|---|---|---|---|---|
| Muscle power assessment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment |
| Muscle power assessment | ||||||
| Deltoid | 3–5 (3.8 ± 0.7) | 3–5 (4.3 ± 0.6)*** | 3–4a (3.9 ± 0.5) | 4b–5 (4.8 ± 0.3)** | 3–4b (3.5 ± 0.7) | 3–4a (3.4 ± 0.3) |
| Supraspinatus | 3–5 (3.8 ± 0.7) | 3–5 (4.3 ± 0.6)*** | 3–4a (3.8 ± 0.5) | 4b–5 (4.5 ± 0.2)** | 3–4b (3.4 ± 0.5) | 3–4b (3.5 ± 0.4) |
| Trapezius | 3–5 (3.8 ± 0.6) | 3–5 (4.3 ± 0.5)*** | 3–4a (3.8 ± 0.5) | 4a–5 (4.6 ± 0.3)** | 3–4b (3.5 ± 0.7) | 3–4a (3.6 ± 0.5) |
| Flexors of the shoulder | 2–5 (4.1 ± 0.8) | 2–5 (4.4 ± 0.7)*** | 4a–4b (4.7 ± 0.2) | 4b–5 (4.7 ± 0.3) | 3–4b (3.8 ± 0.6) | 3–4a (3.7 ± 0.7) |
| Extensors of the shoulder | 2–5 (4.0 ± 0.7) | 2–5 (4.3 ± 0.8)*** | 4a–4b (4.2 ± 0.3) | 4b–5 (4.6 ± 0.2)** | 3–4b (3.5 ± 0.7) | 3–4b (3.2 ± 1.0) |
| Adductors of the shoulder | 3–5 (3.9 ± 0.7) | 2–5 (4.4 ± 0.5)** | 4a–4b (4.1 ± 0.2) | 4b–5 (4.7 ± 0.3)*** | 3–4b (4.1 ± 0.5) | 2–4b (3.7 ± 0.7) |
| Biceps | 3–5 (4.3 ± 0.5) | 3–5 (4.5 ± 0.6)*** | 3–4b (4.0 ± 0.6) | 4b–5 (4.8 ± 0.3)** | 3–5 (4.1 ± 0.6) | 3–5 (4.1 ± 0.6) |
| Triceps | 3–5 (4.4 ± 0.5) | 3–5 (4.6 ± 0.5)** | 4a–4b (4.3 ± 0.3) | 4b–5 (4.8 ± 0.3)*** | 3–5 (4.3 ± 0.7) | 4a–5 (4.4 ± 0.5) |
| Iliopsoas | 1–4b (3.5 ± 0.8) | 1–4b (4.2 ± 0.8)*** | 1–4b (4.2 ± 0.8) | 4b–5 (4.8 ± 0.3) | 2–4b (3.5 ± 0.9) | 3–4b (3.5 ± 0.9) |
| Gluteus maximus | 1–4b (3.3 ± 0.8) | 1–4b (4.1 ± 0.9)*** | 3–4a (3.7 ± 0.5) | 4b (4.5 ± 0.0)* | 2–4a (2.6 ± 1.1) | 2–4b (2.9 ± 0.7) |
| Gluteus medius | 1–5 (3.7 ± 0.9) | 1–5 (4.1 ± 0.9)** | 3–4b (4.0 ± 0.6) | 4b–5 (4.8 ± 0.3) | 2–4b (3.3 ± 1.1) | 2–4b (3.1 ± 0.9) |
| Adductors of the thigh | 1–5 (3.6 ± 0.9) | 1–5 (4.1 ± 0.9)** | 3–4b (4.0 ± 0.6) | 4b–5 (4.8 ± 0.3) | 2–4b (3.2 ± 0.9) | 2–4b (3.1 ± 0.7) |
| Quadriceps | 1–5 (3.9 ± 0.8) | 1–5 (4.4 ± 0.7)** | 4a–4b (4.3 ± 0.3) | 4b–5 (4.8 ± 0.3)** | 3–5 (4.0 ± 0.6) | 4a–5 (3.8 ± 0.8) |
| Hamstring | 2–5 (3.9 ± 0.7) | 2–5 (4.4 ± 0.7)** | 4a–4b (4.3 ± 0.3) | 4b–5 (4.7 ± 0.3)** | 2–5 (3.5 ± 1.0) | 2–5 (3.6 ± 0.9) |
| Calves | 1–5 (4.2 ± 0.9) | 1–5 (4.5 ± 0.9)** | 4a–5 (4.7 ± 0.4) | 4b–5 (4.8 ± 0.3) | 4a–5 (4.7 ± 0.3) | 4a–5 (4.6 ± 0.5) |
| Tibialis anterior | 2–5 (4.3 ± 0.8) | 2–5 (4.6 ± 0.7)** | 4a–5 (4.7 ± 0.4) | 4b–5 (4.8 ± 0.3) | 4b–5 (4.7 ± 0.4) | 4b–5 (4.8 ± 0.3) |
BMD, Becker muscular dystrophies; DMD, Duchenne muscular dystrophies; LGMD, limb girdle muscular dystrophies.
P < 0.05
P < 0.01
P < 0.001.
Table 3.
Assessment of the timed function/second of the studied group of patients pre- and at the end of 6-month prednisone therapy
| DMD Range (Mean ± SD) | BMD Range (Mean ± SD) | LGMD Range (Mean ± SD) | ||||
|---|---|---|---|---|---|---|
| Timed function/ second and Overall disability | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment |
| Timed function/second | 8.9 ± 2.5 | 6.1 ± 2.3*** | 7.3 ± 0.8 | 4.8 ± 1.2*** | 7.6 ± 2.5 | 8.4 ± 2.9 |
| Time in seconds (Mean ± SD) to rise upfrom the ground to standing position 2 to <14 s | ||||||
| Time in seconds (Mean ± SD) for going up 9 stairs; 4 to <31 s | 14.9 ± 5.8 | 10.1 ± 4.7*** | 10.2 ± 1.9 | 6.9 ± 1.6*** | 15.4 ± 8.7 | 16.1 ± 8.0 |
| Time in seconds to travel 10 meters as rapidas possible; 4 to <22 s | 10.7 ± 3.4 | 7.7 ± 2.5*** | 9.2 ± 2.7 | 5.5 ± 2.1** | 12.1 ± 7.7 | 13.3 ± 8.8 |
| Overall disability | ||||||
| In upper limbs | 5.7 ± 0.6 | 6.4 ± 0.7*** | 5.8 ± 0.4 | 6.7 ± 0.5** | 6.0 ± 1.0 | 5.9 ± 1.5 |
| In lower limbs | 8.2 ± 1.5 | 9.4 ± 1.3*** | 9.0 ± 0.0 | 9.7 ± 0.5* | 8.9 ± 0.7 | 8.7 ± 1.0 |
BMD, Becker muscular dystrophies; DMD, Duchenne muscular dystrophies; LGMD, limb girdle muscular dystrophies.
P < 0.05
P < 0.01
P < 0.001.
No difference was observed in the level of CPK and lactate dehydrogenase (LDH) before and after treatment with prednisone in all groups of the patients studied (Table 1).
Histological changes in the muscle following prednisone therapy in DMD/BMD
Before prednisone treatment, the histological changes were consistent with muscle dystrophy including fibre size variation, internalization of nuclei, degeneration, myofibre necrosis, regeneration and endomysial connective tissue proliferation. Muscle biopsies from prednisone-treated DMD and BMD, respectively, revealed amelioration of histological features of muscle damage including: fibre size variation (2.4 ± 0.5 vs. 1.7 ± 0.7, P < 0.01; 2.3 ± 0.5 vs. 1.3 ± 0.8, P < 0.05), internalization of nuclei (2.1 ± 0.8 vs. 1.1 ± 1.1, P < 0.05; 1.5 ± 0.8 vs. 0.8 ± 0.7, P < 0.05), degeneration and necrosis (2.5 ± 0.8 vs. 1.4 ± 1.3, P < 0.001; 2.2 ± 0.7 vs. 0.6 ± 0.4, P < 0.01), regeneration (0.6 ± 0.9 vs. 1.9 ± 0.6, P < 0.001; 0.5 ± 0.8 vs. 2.2 ± 0.4, P < 0.01) and endomysial connective tissue proliferation (2.1 ± 0.8 vs. 1.1 ± 1.1, P < 0.01; 2.2 ± 0.7 vs. 0.8 ± 0.8, P < 0.05) (Table 4 and Fig. 1).
Table 4.
Histopathological changes in dystrophic muscles pre- and at the end of 6-month prednisone therapy. The micrometer (µm) represents measurement unit of muscle fibre size
| DMD Range (Mean ± SD) | BMD Range (Mean ± SD) | LGMD Range (Mean ± SD) | ||||
|---|---|---|---|---|---|---|
| Histopathological changes | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment |
| Fibre size variation | 2.4 ± 0.5 | 1.7 ± 0.7** | 2.3 ± 0.5 | 1.3 ± 0.8* | 2.0 ± 0.6 | 2.3 ± 0.5 |
| Internalization of nucle | 2.1 ± 0.8 | 1.1 ± 1.1* | 1.5 ± 0.8 | 0.8 ± 0.7 | 0.8 ± 1.0 | 1.0 ± 0.8 |
| Degeneration and necrosis | 2.5 ± 0.8 | 1.4 ± 1.3** | 2.2 ± 0.7 | 0.6 ± 0.4** | 2.2 ± 0.4 | 2.0 ± 0.6 |
| Regeneration | 0.6 ± 0.9 | 1.9 ± 0.6*** | 0.5 ± 0.8 | 2.2 ± 0.4** | 1.0 ± 1.0 | 0.8 ± 1.0 |
| Endomysial connective tissue proliferation | 2.1 ± 0.8 | 1.1 ± 1.1** | 2.2 ± 0.7 | 0.8 ± 0.8* | 1.7 ± 1.2 | 2.2 ± 1.5 |
BMD, Becker muscular dystrophies; DMD, Duchenne muscular dystrophies; LGMD, limb girdle muscular dystrophies.
P < 0.05
P < 0.01
P < 0.001.
Figure 1.
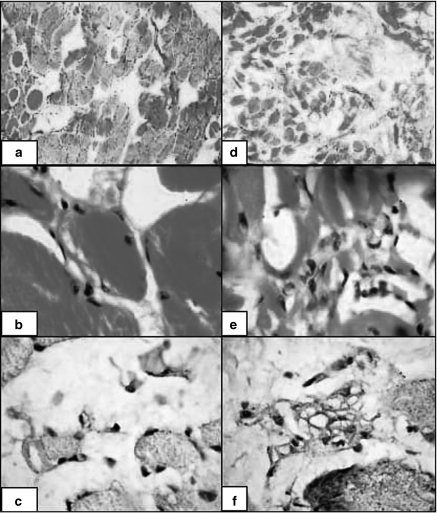
Pathologic evaluation of the dystrophic muscles before (d, e and f) and after (a, b and c) prednisone therapy. The upper left section of the figure (a) shows histopathological features of improvement (regeneration, appearance of striations, decrease in fibre size variation, and endomysial connective tissue proliferation). The middle left section of the figure (b) demonstrates reduction in the inflammatory cells before and after 6-months treatment with prednisone. The lower left section of the figure (c) reveals marked reduction of Dendritic cells (CD35+ cells) in response to prednisone.
Numbers of MICs and DCs following prednisone therapy in DMD/BMD
As compared with values before therapy, total and differential MICs were generally reduced in DMD and BMD. In DMD, there were a reduction of total MICs (23.2 ± 10.1 vs. 18.0 ± 6.0, P < 0.01) and DCs (3.4 ± 0.5 vs. 1.8 ± 0.8, P < 0.01) for before and after treatment, respectively. In BMD, only the reduction in DCs, after therapy, reached the level of statistical significance (2.2 ± 0.7 vs. 1.8 ± 0.6, P < 0.05). However, no significant difference was observed in lymphocyte (3.6 ± 1.7 vs. 2.7 ± 1.1) and histiocytes (12.4 ± 0.9 vs. 9.0 ± 3.4) for before and after treatment, respectively (Table 5 and Fig. 1).
Table 5.
Inflammatory cells count in dystrophic muscles pre- and at the end of 6-month prednisone therapy
| DMD Range (Mean ± SD) | BMD Range (Mean ± SD) | LGMD Range (Mean ± SD) | ||||
|---|---|---|---|---|---|---|
| Inflammatory cells | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment |
| Total MICs (H&E) | 23.2 ± 10.1 | 18.0 ± 6.0** | 18.3 ± 5.5 | 18.3 ± 7.6 | 20.0 ± 7.5 | 20.7 ± 7.5 |
| Lymphocytes (CD3 and CD20) | 3.6 ± 1.7 | 2.7 ± 1.1 | 3.3 ± 2.1 | 3.7 ± 1.2 | 2.5 ± 2.1 | 4.0 ± 1.4 |
| Hitiocytes (CD68) | 12.4 ± 0.9 | 9.0 ± 3.4 | 12.3 ± 1.59.7 ± 4.2 | 13.0 ± 6.2 | 11.7 ± 4.5 | |
| Dendritic cells (CD35) | 3.4 ± 0.5 | 1.8 ± 0.8** | 2.2 ± 0.7 | 1.8 ± 0.6* | 2.5 ± 0.7 | 1.5 ± 0.7 |
BMD, Becker muscular dystrophies; DMD, Duchenne muscular dystrophies; H&E, haematoxylin and eosin; LGMD, limb girdle muscular dystrophies, MIC, mononuclear inflammatory cell.
P < 0.05
P < 0.01
P < 0.001.
Functional, histological and immunohistochemical changes in LGMD
None of the LGMD patients exhibited improvement in motor function after 6-months prednisone therapy (Tables 2.3). Muscle biopsies from prednisone-treated LGMDs revealed no differences in the histological features of muscle damage before and after treatment (Table 4). Moreover, there were no reductions of MICs or DCs counts following therapy (Table 5).
Correlation between the functional and histological improvements and changes in MICs/DCs
There was positive correlation between the degree of clinical improvement (overall motor disability) and reduction of DCs numbers (in upper limbs; r = 0.638, P < 0.01 for DMD and r = 0.725, P < 0.01 for BMD, in lower limbs; r = 0.547, P < 0.05 for DMD and r = 0.576, P < 0.05 for BMD).
Discussion
Transient inflammatory response is a normal homeostatic reaction to muscle damage as a result of muscle-lengthening exercises (Gosselin & McCormick 2004). In contrast to normal muscles, the dystrophic muscles are more vulnerable to contraction-induced damage with persistent inflammatory response that leads to an altered extracellular environment, including an increased presence of inflammatory cells (e.g. B, CD4+ helper/inducer T lymphocytes, natural killer cells and macrophages) and various inflammatory cytokines (e.g. TNF-α, TGF-β). The majorities of these cells expressed classes I and II major histocomptability complex (MHC) antigens and are, therefore, considered to be activated. The recruitment of infiltrating lymphocytes occurs in collaboration with other mononuclear cells, namely the DCs, the expression of the class I and II MHC molecules at the periphery of muscle fibres, the presence of chemoattractants as well as costimulatory molecules. These variables are orchestrated by delicate interactions mediating muscle damage in muscular dystrophies (Arahata & Engel 1984; Morrison et al. 2000).
Unfolding studies suggest the possible relationships among immunosuppressive effects of GCs, muscle damage and MICs. However, to date, knowledge about the status of MICs and DCs in dystrophies is still lacking. In this investigation, we decided to examine these issues as well as to test the hypothesis that the therapeutic effects of GCs are associated with alterations of MICs and DCs infiltrating the dystrophic muscles. To achieve our goals, 30 patients with dystrophies were included in this study. Our study clearly demonstrated that 6-month prednisone therapy is associated with several changes in DMD/BMD, including (i) functional and histological improvements of the muscle (ii) reduced numbers of MICs/DCs and (iii) positive correlation between the muscle improvements and the reduction of DCs. None of the above changes were observed in LGMD.
Functional and histological improvement of the dystrophic muscles following prednisone therapy in DMD/BMD
The functional and histological improvement of the dystrophic muscles in our series (DMD and BMD) not only concurs with previous studies but also confirms the beneficial therapeutic roles of GCs (Drachman et al. 1974; Mendell et al. 1989; Burrow et al. 1991; Griggs et al. 1991; Griggs et al. 1993; Johnsen 2001; Merlini et al. 2003). Also, in agreement with previous reports, none of our patients had to be withdrawn from medication due to side-effects from prednisone therapy (Fenichel et al. 1991). It has been suggested that steroid treatment slows the progress of DMD for at least 3 years (Fenichel et al. 1991; Kang 1996). GCs therapy was associated with decreased muscle breakdown, enhanced muscle regeneration, with concomitant increase in muscle mass (Fenichel et al. 1991). The exact underlying mechanisms of these beneficial effects are unknown. However, it is possible that GCs can affect the immune responses through (i) reducing the number of T cells especially the cytotoxic/suppressor cells and therefore reducing the number of muscle fibre infiltrated by lymphocytes (McDouall et al. 1990; Kissel et al. 1991), (ii) down-regulating monocytes/macrophages, the expression of many cytokine genes at the transcriptional and post-transcriptional levels (Almawi et al. 1996), including IL-1 (Snyder & Unanue 1982; Lee et al. 1988), IL-6 (Amano et al. 1993), TNF (Beutler et al. 1986), IL-10 (Fushimi et al. 1997) and macrophage inflammatory protein-1 (Hawrylowicz et al. 1994), (iii) ameliorating the immunologic relevant activities of the inflammatory cells (Hawrylowicz et al. 1994; Colotta et al. 1996) and (iv) modulating cell adhesion molecules expression (Wehling-Henricks et al. 2004).
Marked reduction in the number of MICs and DCs following prednisone therapy in DMD/BMD
In this study, we demonstrated a statistically significant reduction in the number of MICs and DCs following prednisone therapy. It has been very recently suggested that Dendritic and mast cells may act coordinately to mediate acute and chronic microenvironmental changes in dystrophic muscles. These findings may have relevance to immunosuppressive effect of chronic administration of steroids in treatment of DMD and BMD (Chen et al. 2000). Genome profiling showed that inflammation in the mdx mice is robust including a substantial and coordinated inflammatory/repair response. The nature and breadth of immune function markers (approximately 30% of differentially expressed genes) indicated a chronic persistent inflammatory reaction. The major participating cellular elements were identified by significant elevations in mRNA pathognomonic of mast cells, macrophages, T cells and B cells (Porter et al. 2002). Mast cell degranulation increases local blood flow/vascular permeability and causes direct proteolysis of dystrophin-deficient myofibres (Gorospe et al. 1994). Injury of dystrophin-deficient muscle causes cytokine release by mast cells, fibroblast and damaged muscle fibres, eliciting coordinated vascular response and mononuclear cell accumulation (Gorospe et al. 1994; Lefaucheur et al. 1996; Porter et al. 2002).
DCs are the most potent antigen-presenting cells, which act as a commander of the immune system army that includes in addition T and B lymphocytes and natural killer cells. DCs are equipped to capture antigens and to produce large numbers of immunogenic MHC-peptide complexes. In the presence of maturation-inducing stimuli, such as inflammatory cytokines (TNF, IL-1), DCs upregulate adhesion and costimulatory molecules to become a more potent stimulator of T-cell immunity (Sallusto et al. 1995). Little is known about how GC influences the initiation of the specific immune response at the level of DCs. The significant reduction of DCs in our series may be due to the suppressive effects of GCs and thereby inhibit the induction of primary T-cell responses. GCs can modulate DCs differentiation, terminal maturation and function (Piemonti et al. 1999; Vanderheyde et al. 1999). In support (i) GCs can prevent activation of DCs as well as reducing their numbers by redirecting differentiation of a subset of them (Matasic et al. 1999), (ii) in addition, GC may further inhibit T-cell-mediated inflammation indirectly via the suppression of IL-12 production by DC and also block IL-4-driven differentiation of monocytes into DC (Pedro et al. 1998) and (iii) the inhibitory effect of steroids on the expression of costimulatory molecules and the antigen-presenting capacity of DC is also considered (Pan et al. 2001).
Positive correlation between the functional and histological improvements and the reduction of DCs number
In our study, the presence of a positive correlation between the functional and histological improvements and the reduction of DCs count suggest a critical role for DCs in the development of dystrophies. Moreover, it suggests (i) DCs counts as a possible prognostic marker in the evaluation and follow up of patients with dystrophies and (ii) reduction in DCs and muscle improvements are mediated by common mechanisms.
Absence of functional and histological improvement in the dystrophic muscle in LGMD
None of the LGMD patients exhibited significant improvement in motor function after 6-months prednisone therapy. Several clinical therapeutic trials have been performed on different types of muscular dystrophies. Of all, only DMD and BMD have generated positive clinical improvement in muscle strength or muscle mass and histopathological changes in response to GCs (Drachman et al. 1974; DeSilva et al. 1987; Mendell et al. 1989; Griggs et al. 1991).
Despite the apparent similarity of the muscle microenvironmental alterations in dystrophinopathies and LGMD (Ohlendieck 1996; Hamed et al. in press) (http://www.dmd.nl/database.htm.), the inability of GCs to induce both functional and histological improvements in the dystrophic muscles of LGMD patients may be explained by several possibilities. First, lack of a central role for the immune cells (targets of GCs suppressive effects) in the development of this disorder. Second, the evolution of LGMD is mediated by, yet unknown, cells that is unresponsive to GCs. To our knowledge, a functional and quantitative significant improvement in muscle strength was reported in a trial treatment with prednisone carried out for a female carrying the diagnosis of LGMD due to primary a-sarcoglycan deficiency. She maintained stable strength over 3 years of treatment. The timing and the degree of benefit in strength were similar to those seen in boys with DMD who are treated with prednisone (Connolly et al. 1998; Fisher et al. 2005). Future controlled studies on larger number of patients are required.
To conclude, GCs therapy can induce functional and histological improvement in the dystrophic muscle (DMD/BMD) possibly through immunologic mechanisms involving MICs and DCs. Our data indicated a significant positive correlation between reduction of DCs number and these improvements. Thus they suggest a critical role for DCs in the evolution of muscle dystrophies. One hypothesis to explain these findings, which has to be tested, is that the reduction of DCs numbers is associated with a reduced activation of naive T lymphocytes. This in turn protects the muscle against their detrimental effects. An alternative hypothesis, which has to be tested, is that prednisone therapy may suppress the ability of DCs to stimulate the naive T lymphocytes, i.e. prednisone affects the antigen-presenting capabilities of DCs. The DCs/T-cells signal exchange is two-way, with T cells inducing DCs maturation and activation via CD40 ligand binding to CD40, and via cytokines such as granulocyte macrophage colony-stimulating factor. Our data also suggest a potential-targeted immunotherapeutic benefit for DCs in DMD/BMD. Furthermore, it may help improve molecular strategies for successful dystrophin myoblast transplantation. The underlying mechanisms of altered dystrophin expression in muscles of prednisone-treated DMD patients are still open for further investigations.
References
- Almawi WY, Beyhum HN, Rahme AA, et al. Regulation of cytokine and cytokine receptor expression by glucocorticoids. J Leukoc Biol. 1996;60:563–572. doi: 10.1002/jlb.60.5.563. [DOI] [PubMed] [Google Scholar]
- Amano Y, Lee SW, Allison AC. Inhibition by glucocorticoids of the formation of interleukin-1 alpha, interleukin-1 beta, and interleukin-6: mediation by decreased mRNA stability. Mol Pharmacol. 1993;43:176–182. [PubMed] [Google Scholar]
- Angelini C, Beggs AH, Hoffman EP, et al. Enormous dystrophin in a patient with Becker muscular dystrophy. Neurology. 1990;40:808–812. doi: 10.1212/wnl.40.5.808. [DOI] [PubMed] [Google Scholar]
- Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear cells in myopathies. I: quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol. 1984;16:193–208. doi: 10.1002/ana.410160206. [DOI] [PubMed] [Google Scholar]
- Archibald K, Vignos P. A study of contractures in muscular dystrophy. Arch Phys Med Rehabil. 1959;40:150–157. [PubMed] [Google Scholar]
- Ben Hamida M, Fardeau M, Attia N. Severe childhood muscular dystrophy affecting both sexes and frequent in Tunisia. Muscle Nerve. 1983;6:469–480. doi: 10.1002/mus.880060702. [DOI] [PubMed] [Google Scholar]
- Beutler B, Krochin N, Milsark IW, et al. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986;232:977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Fenichel GM, Griggs RC, et al. Clinical investigation of Duchenne muscular dystrophy. Interesting results in a trial of prednisone. Arch Neurol. 1987;44:812–817. doi: 10.1001/archneur.1987.00520200016010. [DOI] [PubMed] [Google Scholar]
- Buchthal F, Kamieniecka Z. The diagnostic yield of quantified electromyography and quantified muscle biopsy in neuromuscular disorders. Muscle Nerve. 1982;5:265–280. doi: 10.1002/mus.880050403. [DOI] [PubMed] [Google Scholar]
- Burrow KL, Coovert DD, Klein CJ, et al. Dystrophin expression and somatic reversion in prednisone-treated and untreated Duchenne dystrophy. CIDD Study Group. Neurology. 1991;41:661–666. doi: 10.1212/wnl.41.5.661. [DOI] [PubMed] [Google Scholar]
- Bushby KM, Gardner-Medwin D, Nicholson LV, et al. The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy. II. Correlation of phenotype with genetic and protein abnormalities. J Neurol. 1993;240:105–112. doi: 10.1007/BF00858726. [DOI] [PubMed] [Google Scholar]
- Chen YW, Zhao P, Borup R, et al. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol. 2000;151:1321–1336. doi: 10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Saccani S, Giri JG, et al. Regulated expression and release of the IL-1 decoy receptor in human mononuclear phagocytes. J Immunol. 1996;156:2534–2541. [PubMed] [Google Scholar]
- Connolly AM, Pestronk A, Mehta S, et al. Primary alpha-sarcoglycan deficiency responsive to immunosuppression over three years. Muscle Nerve. 1998;21:1549–1553. doi: 10.1002/(sici)1097-4598(199811)21:11<1549::aid-mus30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- DeSilva S, Drachman DB, Mellits D, et al. Prednisone treatment in Duchenne muscular dystrophy. Long-term benefit. Arch Neurol. 1987;44:818–822. doi: 10.1001/archneur.1987.00520200022012. [DOI] [PubMed] [Google Scholar]
- Drachman DB, Toyka KV, Myer E. Prednisone in Duchenne muscular dystrophy. Lancet. 1974;2:1409–1412. doi: 10.1016/s0140-6736(74)90071-3. [DOI] [PubMed] [Google Scholar]
- Engel AG, Yamamoto M, Fischbeck KH. Dystrophinopathies. In: Engel AG, Franzini-Armstrong C, editors. Myology Badsic and clinical. 2. New York: McGraw-Hill; 1996. pp. 1133–1186. [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Fenichel GM, Mendell JR, Moxley RT, III, et al. A comparison of daily and alternate-day prednisone therapy in the treatment of Duchenne muscular dystrophy. Arch Neurol. 1991;48:575–579. doi: 10.1001/archneur.1991.00530180027012. [DOI] [PubMed] [Google Scholar]
- Fisher I, Abraham D, Bouri K, Hoffman EP, Muntoni F, Morgan J. Prednisolone-induced changes in dystrophic skeletal muscle. FASEB J. 2005;19:834–836. doi: 10.1096/fj.04-2511fje. [DOI] [PubMed] [Google Scholar]
- Fushimi T, Okayama H, Seki T, et al. Dexamethasone suppressed gene expression and production of interleukin-10 by human peripheral blood mononuclear cells and monocytes. Int Arch Allergy Immunol. 1997;112:13–18. doi: 10.1159/000237425. [DOI] [PubMed] [Google Scholar]
- Gaud A, Simon JM, Witzel T, et al. Prednisone reduces muscle degeneration in dystrophin-deficient Caenorhabditis elegans. Neuromuscul Disord. 2004;14:365–370. doi: 10.1016/j.nmd.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Gorospe JR, Tharp MD, Hinckley J, et al. A role for mast cells in the progression of Duchenne muscular dystrophy? Correlations in dystrophin-deficient humans, dogs, and mice. J Neurol Sci. 1994;122:44–56. doi: 10.1016/0022-510x(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, McCormick KM. Targeting the immune system to improve ventilatory function in muscular dystrophy. Med Sci Sports Exerc. 2004;36:44–51. doi: 10.1249/01.MSS.0000106185.22349.2C. [DOI] [PubMed] [Google Scholar]
- Griggs RC, Moxley RT, III, Mendell JR, et al. Prednisone in Duchenne dystrophy. A randomized, controlled trial defining the time course and dose–response. Clinical Investigation of Duchenne Dystrophy Group. Arch Neurol. 1991;48:383–388. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
- Griggs RC, Moxley RT, Mendell JR, et al. Duchenne dystrophy: randomized, controlled trial of prednisone (18 months) and azathioprine (12 months) Neurology. 1993;43:520–527. doi: 10.1212/wnl.43.3_part_1.520. [DOI] [PubMed] [Google Scholar]
- Hamed SA, Hoffman EP. Automated sequence screening of the entire dystrophin cDNA in Duchenne dystrophy: point mutation detection. Am J Med Genet B Neuropsychiatr Genet. 2006;5:44–50. doi: 10.1002/ajmg.b.30234. [DOI] [PubMed] [Google Scholar]
- Hamed SA, Sutherland-Smith AJ, Gorospe JR, Kendrick-Jones J, Hoffman EP. DNA sequence analysis for structure/function and mutation studies in Becker muscular dystrophy. Clin Genet. in press. [DOI] [PubMed]
- Hawrylowicz CM, Guida L, Paleolog E. Dexamethasone up-regulates granulocyte-macrophage colony-stimulating factor receptor expression on human monocytes. Immunology. 1994;83:274–280. [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Fischbeck KH, Brown RH, et al. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne’s or Becker’s muscular dystrophy. N Engl J Med. 1988;318:1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Knudson CM, Campbell KP, et al. Subcellular fractionation of dystrophin to the triads of skeletal muscle. Nature. 1987;330:754–758. doi: 10.1038/330754a0. [DOI] [PubMed] [Google Scholar]
- Hoshino S, Ohkoshi N, Watanabe M, Shoji S. Immunohistochemical staining of dystrophin on formalin-fixed paraffin-embedded sections in Duchenne/Becker muscular dystrophy and manifesting carriers of Duchenne muscular dystrophy. Neuromuscul Disord. 2000;10(0):425–429. doi: 10.1016/s0960-8966(99)00116-9. [DOI] [PubMed] [Google Scholar]
- Hussein MR, Ismael HH. Alterations of p53, Bcl-2, and hMSH2 protein expression in the normal breast, benign proliferative breast disease in situ and infiltrating ductal breast carcinomas in the upper Egypt. Cancer Biol Ther. 2004;3:983–988. doi: 10.4161/cbt.3.10.1136. [DOI] [PubMed] [Google Scholar]
- Johnsen SD. Prednisone therapy in Becker’s muscular dystrophy. J Child Neurol. 2001;16:870–871. doi: 10.1177/08830738010160111406. [DOI] [PubMed] [Google Scholar]
- Kang J. [Glucocorticoid therapy in Duchenne muscular dystrophy] Rinsho Shinkeigaku. 1996;36:1338–1340. [PubMed] [Google Scholar]
- Kissel JT, Burrow KL, Rammohan KW, et al. Mononuclear cell analysis of muscle biopsies in prednisone-treated and untreated Duchenne muscular dystrophy. CIDD Study Group. Neurology. 1991;41:667–672. doi: 10.1212/wnl.41.5.667. [DOI] [PubMed] [Google Scholar]
- Kissel JT, Lynn DJ, Rammohan KW, et al. Mononuclear cell analysis of muscle biopsies in prednisone- and azathioprine-treated Duchenne muscular dystrophy. Neurology. 1993;43:532–536. doi: 10.1212/wnl.43.3_part_1.532. [DOI] [PubMed] [Google Scholar]
- Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–226. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Lee SW, Tsou AP, Chan H, et al. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci USA. 1988;85:1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Gjata B, Sebille A. Factors inducing mast cell accumulation in skeletal muscle. Neuropathol Appl Neurobiol. 1996;22:248–255. [PubMed] [Google Scholar]
- Lundberg I, Brengman JM, Engel AG. Analysis of cytokine expression in muscle in inflammatory myopathies, Duchenne dystrophy, and non-weak controls. J Neuroimmunol. 1995;63:9–16. doi: 10.1016/0165-5728(95)00122-0. [DOI] [PubMed] [Google Scholar]
- Matasic R, Dietz AB, Vuk-Pavlovic S. Dexamethasone inhibits dendritic cell maturation by redirecting differentiation of a subset of cells. J Leukoc Biol. 1999;66:909–914. doi: 10.1002/jlb.66.6.909. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tome FM, Ionasescu V, et al. Deficiency of dystrophin-associated proteins in Duchenne muscular dystrophy patients lacking COOH-terminal domains of dystrophin. J Clin Invest. 1993;92:866–871. doi: 10.1172/JCI116661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDouall RM, Dunn MJ, Dubowitz V. Nature of the mononuclear infiltrate and the mechanism of muscle damage in juvenile dermatomyositis and Duchenne muscular dystrophy. J Neurol Sci. 1990;99:199–217. doi: 10.1016/0022-510x(90)90156-h. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Moxley RT, Griggs RC, et al. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med. 1989;320:1592–1597. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- Merlini L, Cicognani A, Malaspina E, et al. Early prednisone treatment in Duchenne muscular dystrophy. Muscle Nerve. 2003;27:222–227. doi: 10.1002/mus.10319. [DOI] [PubMed] [Google Scholar]
- Mesa LE, Dubrovsky AL, Corderi J, et al. Steroids in Duchenne muscular dystrophy – deflazacort trial. Neuromuscul Disord. 1991;1:261–266. doi: 10.1016/0960-8966(91)90099-e. [DOI] [PubMed] [Google Scholar]
- Morrison J, Lu QL, Pastoret C, et al. T-cell-dependent fibrosis in the mdx dystrophic mouse. Lab Invest. 2000;80:881–891. doi: 10.1038/labinvest.3780092. [DOI] [PubMed] [Google Scholar]
- Nicholson L, Johnson M, Davison K, et al. Dystrophin or a ‘related protein’ in Duchenne muscular dystrophy. Acta Neurol Scand. 1992;86:8–14. doi: 10.1111/j.1600-0404.1992.tb08046.x. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K. Towards an understanding of the dystrophin-glycoprotein complex: linkage between the extracellular matrix and the membrane cytoskeleton in muscle fibers. Eur J Cell Biol. 1996;69:1–10. [PubMed] [Google Scholar]
- Ozawa E, Noguchi S, Mizuno Y, et al. From dystrophinopathy to sarcoglycanopathy: evolution of a concept of muscular dystrophy. Muscle Nerve. 1998;21:421–438. doi: 10.1002/(sici)1097-4598(199804)21:4<421::aid-mus1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Pan J, Ju D, Wang Q, et al. Dexamethasone inhibits the antigen presentation of dendritic cells in MHC class II pathway. Immunol Lett. 2001;76:153–161. doi: 10.1016/s0165-2478(01)00183-3. [DOI] [PubMed] [Google Scholar]
- Piemonti L, Monti P, Allavena P, et al. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162:6473–6481. [PubMed] [Google Scholar]
- Porter JD, Khanna S, Kaminski HJ, et al. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum Mol Genet. 2002;11:263–272. doi: 10.1093/hmg/11.3.263. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, et al. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealock R, Butler MH, Kramarcy NR, et al. Localization of dystrophin relative to acetylcholine receptor domains in electric tissue and adult and cultured skeletal muscle. J Cell Biol. 1991;113:1133–1144. doi: 10.1083/jcb.113.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriffs IN, Rampling D, Smith VV. Paraffin wax embedded muscle is suitable for the diagnosis of muscular dystrophy. J. Clin. Pathol. 2001;54:517–520. doi: 10.1136/jcp.54.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DS, Unanue ER. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982;129:1803–1805. [PubMed] [Google Scholar]
- Spencer MJ, Tidball JG. Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscul Disord. 2001;11:556–564. doi: 10.1016/s0960-8966(01)00198-5. [DOI] [PubMed] [Google Scholar]
- Sueki H, Whitaker D, Buchsbaum M, et al. Novel interactions between dermal dendrocytes and mast cells in human skin. Implications for hemostasis and matrix repair. Lab Invest. 1993;69:160–172. [PubMed] [Google Scholar]
- Takagi A, Watanabe T, Kojima S, et al. [Effect of long-term administration of prednisolone on serum creatine kinase and muscle pathology of mdx mouse] Rinsho Shinkeigaku. 1998;38:724–728. [PubMed] [Google Scholar]
- Vanderheyde N, Verhasselt V, Goldman M, et al. Inhibition of human dendritic cell functions by methylprednisolone. Transplantation. 1999;67:1342–1347. doi: 10.1097/00007890-199905270-00009. [DOI] [PubMed] [Google Scholar]
- Wehling-Henricks M, Lee JJ, Tidball JG. Prednisolone decreases cellular adhesion molecules required for inflammatory cell infiltration in dystrophin-deficient skeletal muscle. Neuromuscul Disord. 2004;14:483–490. doi: 10.1016/j.nmd.2004.04.008. [DOI] [PubMed] [Google Scholar]