Abstract
The present study was carried out to investigate the role of hypertension in the genesis and localization of intimal lesions and medial remodelling found in the prestenotic segment in relation to a severe stenosis of the abdominal aorta just below the diaphragm. Male young rats were divided randomly into operated group, animals submitted to surgical abdominal aorta stenosis, and sham-operated group, a control group of animals submitted to sham operation to simulate abdominal aorta stenosis. Aortas in the hypertensive prestenotic segment with increased circumferential wall tension associated with normal tensile stress, laminar flow/normal wall shear stress were characterized by enlarged heterogeneous endothelial cells elongated in the direction of the blood flow, diffusely distributed conspicuous neointimal plaques and medial thickening. The immunohistochemical analysis revealed an increased expression of eNOS, iNOS, nitrotyrosine and transforming growth factor-β (TGF-β) in endothelial cells and/or smooth muscle cells in this segment. Our findings suggest that increased circumferential wall tension due to hypertension plays a pivotal role in the remodelling of the prestenotic segment through biomechanical effects on oxidative stress and increased TGF-β expression. Further studies are needed to clarify the intrinsic pathogenetic mechanism of focal distribution of the neointimal plaques in the hypertensive segment.
Keywords: atherosclerosis, circumferential wall tension, hypertension, intimal and medial remodelling, neointimal plaque
Atherosclerosis is a focal disease that affects medium and large sized arteries. The typical lesions are characterized by asymmetric focal thickening of the intima composed of a lipid core and connective tissue (Ross 1999). These lesions have a non-uniform distribution in the vasculature and are prone to develop at bends, branches, and bifurcation of the aorta and its conduits (Cooke 2003). These high-susceptibility sites are thought to be conditioned by heamodynamic parameters, particularly associated with regions of low wall shear stress (WSS), oscillatory flow, or turbulent flow (VanderLaan et al. 2004). Hypertension, one of the most prevalent diseases in the industrialized world, represents a major risk factor for the development of atherosclerosis (Dai et al. 2004). High-blood pressure affects elastic and muscular arteries causing intimal and medial thickening and increase in connective tissue content (Touyz 2000) and seems to accelerate atherogenesis in man and experimental animals. The exact underlying mechanism of the association between hypertension and atherosclerosis is, however, not fully understood (Li & Chen 2005).
There is a pressing need for an increased understanding of the mechanisms by which the structural and functional changes occur within the vascular wall in response to sustained increased blood pressure. A central question is how the haemodynamic forces and mechanical factors are sensed by the cells of the blood vessel wall in response to an increase in blood pressure and then translated into pathophysiologically relevant changes. The present study was carried out to investigate the role of hypertension in the genesis and localization of intimal lesions and medial remodelling found in the prestenotic segment in relation to a severe stenosis of the abdominal aorta just below the diaphragm. This model was previously employed in our laboratory to induce pressure overload and cardiac hypertrophy (Rossi & Peres 1992).
Materials and methods
Experimental protocol
Male Wistar albino rats, weighing an average of 150 g, were obtained from the breeding colony of the Faculty of Medicine. The rats were fed solid laboratory rat food in stainless steel feeding dishes and liquid in Richter graduated drinking tubes, both freely available to all animals. Their liquid intake and solid food consumption were recorded twice weekly. The animals were divided randomly into two sets: operated group, animals submitted to surgical abdominal aorta stenosis, and sham-operated group, a control group of animals submitted to sham operations to simulate abdominal aorta stenosis. The animals were weighed weekly. All protocols were approved by the Committee on Animal Research of the University of São Paulo.
Animal surgery
With the animals under ether anaesthesia, the abdominal aorta was narrowed just below the diaphragm as previously described (Rossi & Peres 1992). Briefly, the aorta was exposed through a left flank incision and a 0.94 mm in diameter blunted probe was placed next to the vessel. The aorta was constricted with a ligature of cotton thread around the needle, which was immediately removed, thus reducing the vessel lumen to the diameter of the probe. The sham-operated animals underwent an identical surgical procedure, but aortic constriction was omitted.
Harvesting and preparation of hearts
On the day 28 after surgery the animals were weighed, anaesthetized with ether, and the thoracic cavity was opened to expose the still beating heart. The hearts were rapidly removed, rinsed in ice-cold 0.9% saline solution, blotted, weighed, and fixed as a whole in phosphate-buffered 10% formalin, for histological study. Both ventricles from each heart were isolated and cut into two fragments by a midi ventricular coronal section. Each block was serially cut in the same direction, and sections were stained with haematoxylin and eosin. The absolute thicknesses of the septum and left and right ventricular wall and the areas of each ventricular chamber were measured. For this morphometric study, the public-domain software NIH ImageJ (developed at the U.S. National Institute of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/) was used.
Harvesting and preparation of aortas for high resolution light microscopy
The aortas were rapidly excised from the trunk down to the iliac bifurcation, washed at a pressure-perfusion of 100 mmHg with phosphate-buffered saline (PBS) through the ascending aorta and followed by perfusion-fixation with phosphate buffered 10% formalin and then immersed in the same fixative for 24 h at room temperature. After fixation the adventitial tissue was removed and the aortic tube proximal or corresponding segments from sham-operated animals were transversally cut into 5–6 mm long fragments. The samples were then dehydrated, embedded in Historesin (Leica Instruments, GmbH, Heildelberg, Germany), serially cut at 2 μm, stained with toluidine blue, and examined by light microscopy. Fragments of the area of stenosis were processed for evaluation of the cross-sectional lumen reduction.
Cross-sections (n = 5–6), exactly perpendicular to the long axis of the aorta from each vascular segment were morphometrically evaluated. The absolute thickness of the intima and media, the cross-sectional area of the lumen, the perimeter, and the number of elastic layers in prestenotic segments and corresponding segments from sham-operated controls were measured (Rossi & Colombini-Netto 2001). The diameter was calculated according to the formula d = 2√a/π, where a is area expressed in mm2 and π is 3.14. The number of measurements to estimate the intima and media thickness was 50 and 20, respectively, around the vessel circumference for each portion of aorta from each rat. This number was chosen arbitrarily. Measurements were made by a skilled observer blinded to the treatment groups. The intima and media thicknesses and the number of elastic layers of the media were determined at a magnification of ×400, whereas the cross-sectional area and perimeter were determined at ×50. Findings were averaged for each group. Morphometric analysis was performed using videomicroscopy with the Leica Qwin software (Leica Imaging Systems Ltd, Cambridge, UK) in conjunction with a Leica microscope (Leica DMR; Leica Microsystems Wetzlar GmbH, Wetzlar, Germany), videocamera (Leica DC300F; Leica Microsystems AG, Heerbrugg, Switzerland), and an on-line computer.
Immunohistochemistry
For immunohistochemical staining, aortas (n = 5) were harvested as above and embedded in paraffin. Immunolabelling was performed with the following primary antibodies to demonstrate endothelial nitric oxide synthase (polyclonal rabbit anti-eNOS; LabVision, Fremont, CA, USA; diluted 1:150), inducible nitric oxide synthase (polyclonal rabbit anti-iNOS; LabVision; diluted 1:25), nitrotyrosine (NT) (polyclonal rabbit anti-NT; Upstate, Lake Placid, NY, USA; diluted 1:100), and transforming growth factors beta [polyclonal rabbit anti-transforming growth factor-β (TGF-β)1/2/3; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA; diluted 1:100]. Sections 5 μm thick were placed on silane-coated slides, deparaffinized, washed in PBS, and then submitted to heat-induced antigen, endogenous peroxidase inhibition, and nonspecific antibody binding block. After, the sections were incubated with the primary antibodies. Antigen was visualized with a labelled streptavidin biotin peroxidase technique (Vectastain ABC kit; Vector Laboratories Inc., Burlingame, CA, USA) with diaminobenzidine (DAB) substrate. Sections were then counterstained with haematoxylin, coverslipped, and examined by two skilled blinded observers, reflecting immunoreactivity intensity and extension. The evaluation of immunoreactivity was scored quali-quantitatively as absent (0), + (low), ++ (moderate), or +++ (strong) in the study groups. Each score reflects changes in the intensity and extension of staining.
Electron microscopy
Fragments of the proximal aorta and corresponding regions from sham-operated controls were processed for transmission electron microscopic study. After fixation with 2.5% glutaraldehyde in cacodylate buffer (pH 7.3) for 2 h and post-fixation with osmium tetroxide for 2 h, the fragments were dehydrated in ascending concentrations of acetone, and, subsequently, embedded in Araldite. Ultrathin sections were obtained from selected areas with a diamond knife in a Sorvall MT-5000 ultramicrotome (DuPont Co., Wilmington, DE, USA), double-stained with uranyl acetate and lead citrate, and examined in a Zeiss EM109 electron microscope (Carl Zeiss, Oberköchen, Germany) at 80 kV.
The aortas were also processed for scanning electron microscopy. Fragments 5–6 mm long, immediately before the stenosis and corresponding segments from sham controls, were fixed by immersion in Karnovsky fixative for 6 h at 4 °C, dehydrated in ascending concentrations of ethanol, and dried in liquid carbon dioxide by the critical point method. To improve our visualization of the endothelial cells, the specimens already fixed were put into 1% HCl for 30 s with gentle shaking. The dried specimens were then glued on aluminium stubs with silver paste, sputter-coated with gold, and examined in a Zeiss 940A scanning electron microscope (Carl Zeiss, Oberköchen, Germany) at 15 kV. The segments were fixed under atmospheric pressure rather than physiological pressure to prevent eventual artefacts due to overstretching. It is felt that qualitative assessment of these data are justified, as all segments were prepared under similar conditions.
Doppler
Aorta duplex ultrasonography (n = 5) under ether anaesthesia was performed 4 weeks after surgery using an Acuson Aspen apparatus (Acuson Corp., Mountain View, CA, USA) equipped with colour Doppler facility and multi-frequency linear electronic transducer at 11 MHz. The rats were lying in the supine position. The aorta was visualized by ultrasonography. Doppler was performed in the region of stenosis and upstream.
Wall shear stress was calculated using the Poiseuille formula: τ = 4ηBFR/π(kr)3, where τ is WSS (dyne/cm2), η is the blood viscosity (0.03 poise), BFR is blood flow rate (ml/min), π is 3.14, k is 1.25 (the shrinkage index, which is the ratio of artery diameter before and after plastic embedding) (Zarins et al. 1986), and r is the arterial radius (cm) (Song et al. 2000).
Blood pressure
To assess the mean arterial blood pressure proximal to the aortic constriction in anaesthetized animals (ketamine 74 mg/kg i.p. and xylazine 8 mg/kg i.p.), carotid pressure was obtained from rats from each group 24 h and 15 and 28 days after surgery (number of animals per group = 8). Polyethylene catheters (PE-10) filled with heparinized saline were introduced and positioned in the carotid artery and exteriorized in the neck. Immediately after the surgical preparation, when haemodynamic was stable, mean carotid pressure was recorded by connecting the catheters to a PE-50 polyethylene catheter connected to the pressure transducer Powerlab (AD Instruments, Castle Hill, Australia). The mean carotid blood pressure of animals weighing 150 g, simulating the time 0 of the experiment immediately before surgery, was also measured.
Circumferential wall tension and tensile stress
Mean circumferential wall tension (CWT) was calculated by Laplace law according to the following formula: CWT = MBP × (ID/2), where CWT is expressed in dyne/cm, MBP is mean blood pressure (dynes/cm2) and ID is internal diameter (cm) (Carallo et al. 1999). The internal diameter of plastic embedded aorta was multiplied by 1.25, the shrinkage index after plastic embedding (Zarins et al. 1986).
Tensile stress (TS) was computed as TS = CWT/IMT, where TS is expressed in dyne/cm2, CWT is circumferential wall tension (dyne/cm) and IMT is intima-media thickness (cm) (Carallo et al. 1999).
Statistical analysis
Data were analysed using a GraphPad Prism statistic program (GraphPad Software Inc., San Diego, CA, USA). For analysis of differences between the two groups the Student's t-test was performed. Comparisons of immunoreactivity intensity grades of eNOS, iNOS, NT, and TGF-β were made using the Mann–Whitney test. A level of significance of 5% was chosen to denote differences between means.
Results
Animal growth
Experiments were conducted six to seven times with nine to 10 rats per group. Altogether, 19.5% of the animals from group operated and 5% from group sham-operated died in the first week of the experiment; this information was disregarded. Rats of both groups remained in good health, with no signs of nutritional deficiencies. Serum proteins and albumin levels were within the normal range for growing rats (data not shown).
Table 1 shows the mean initial and final body weights, the growth rates (g/day/rat) and the average consumption of solid food (g/day/rat) and water intake (ml/day rat) for both experimental groups.
Table 1.
Mean initial and final body weights, growth rates and the average consumption of solid food and water intake from sham-operated control and operated groups
| Body weight (g) | |||||
|---|---|---|---|---|---|
| Initial | Final | Growth rate (g/day/rat) | Water intake (ml/day/rat) | Solid food consumption (g/day/rat) | |
| Sham-operated | 147.6 ± 2.24 | 356.7 ± 10.64 | 6.97 ± 0.32 | 32.53 ± 1.93 | 26.67 ± 0.63 |
| Operated | 150.8 ± 1.00 | 319.1 ± 7.58** | 5.61 ± 0.27** | 31.29 ± 0.66 | 24.06 ± 0.86 |
Values are given as mean ± SE
P < 0.01.
Blood pressure
The increase in blood pressure was progressive after constriction of abdominal aorta. After 24 h, the increase was 7.8% (P > 0.05), after 15 days it was 13.2% (P < 0.01), and at the end of the experiment reached a maximum, 24.3% (P < 0.0001). The mean values of carotid blood pressures for both groups over the 4-week period are shown in Figure 1.
Figure 1.
Mean carotid blood pressure in operated and sham-operated rats during the 28 day period of study. The mean carotid blood pressure of constricted rats reached a maximum at the fourth week.
Heart morphology
The mean heart weight of rats from the operated group was 20.7% increased in comparison with that of sham-operated controls. As the body weight of animals is an important source of variability of organ weight, it seems obvious that the organ weight should be corrected for differences in body weights. Body and heart weight data on a large group of normal male rats in the body weight range of 50 and 450 g were collected, and expressed as a weight curve of heart relative to body weight (Rossi et al. 1981). By using this method, it was possible to compare the wet heart weight of rats from both groups to the wet heart weight of equal body weight controls. The wet heart weight of operated rats was 20% higher than the predicted heart weight of equal body weight controls, whereas the heart weight of sham-operated controls was not different from the wet heart weight of equal body weights controls. The heart ratio (heart weight/body weight expressed in g/kg) of operated rats was 36.5% higher than that observed in sham-operated controls (Table 2).
Table 2.
Heart weight, heart ratio, left and right ventricular chamber areas and septum and ventricles wall thicknesses from sham-operated control and operated groups
| Ventricular chamber area (mm2) | Wall thickness (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Heart weight (mg) | Heart ratio (g/Kg) | LV | RV | LV | RV | Septum | |
| Sham-operated | 1206 ± 48.66 | 3.37 ± 0.06 | 17.24 ± 1.53 | 7.46 ± 0.84 | 2.30 ± 0.10 | 0.84 ± 0.04 | 1.79 ± 0.09 |
| Operated | 1448 ± 63.47*** | 4.60 ± 0.28* | 18.66 ± 1.05 | 8.34 ± 0.55 | 2.46 ± 0.07** | 0.80 ± 0.03 | 2.03 ± 0.07*** |
| Predicted values† | 1212 ± 0.15 | 3.86 ± 0.12 | 18.54 ± 0.65 | 9.85 ± 0.74 | 2.07 ± 0.06 | 0.79 ± 0.05 | 1.50 ± 0.04 |
Values are given as mean ± SE.
LV, left ventricle; RV, right ventricle.
P < 0.05
P < 0.01
P < 0.0001; when compared with predicted values.
Predicted values for control animals with mean body weight equal to mean body weight of operated animals at the end of the experiment (319.1 ± 7.58 g).
The same way as we did with the heart weights, the experimental morphometric data were compared with those of equal body weight controls. Accordingly, the values of left and right ventricle chamber areas and septum and right and left wall thicknesses of equal body weight controls were obtained from a group of 12 animals with body weights close (314.5 ± 1.39 g) to the mean final body weight of operated animals. The morphometric analysis demonstrated that the areas of left and right ventricular chambers in operated rats were similar when compared with the respective values of equal body weight controls. On the other hand, the septum and left ventricle wall thicknesses were 35.3% and 18.8% increased, respectively, in comparison with the respective values of equal body weight control. The right ventricle wall thickness, however, was similar to that observed in equal body weight controls (Table 2).
Doppler
After 4 weeks of surgery, the blood flow rate in the prestenotic segment was similar to that observed in the aorta of sham-operated controls (Figure 2a and Table 3).
Figure 2.
(a) Blood flow rate (ml/min) in sham-operated and prestenotic aortas at day 28 of the experiment. The mean BFR values in both groups are similar. (b) Wall shear stress (WSS) mean values (dyne/cm2) in sham-operated and prestenotic aortas at day 28 of the experiment. The mean WSS values in both groups are similar. (c) Circumferential wall tension (CWT) (104 dyne/cm) in sham-operated and prestenotic aortas at day 28 of the experiment. The mean CWT level is increased in comparison to that of corresponding segments from control aortas. (d) Tensile stress (104 dyne/cm2) in sham-operated and prestenotic aortas at day 28 of the experiment. The mean tensile stress values in both groups are similar (□, Sham-operated; ▪, operated).
Table 3.
Blood flow rate, wall shear stress, circumferential wall tension and tensile stress of the aorta from sham-operated control and operated groups
| Blood flow rate (ml/min) | Wall shear stress (dyne/cm2) | Circumferential wall tension (104 dyne/cm) | Tensile stress (104 dyne/cm2) | |
|---|---|---|---|---|
| Sham-operated | 51.24 ± 3.60 | 43.72 ± 2.80 | 1.27 ± 0.02 | 127.5 ± 5.69 |
| Operated | 58.10 ± 3.72 | 51.31 ± 7.73 | 1.63 ± 0.05*** | 128.8 ± 5.28 |
Values are given as mean ± SE
P < 0.0001.
Colour Doppler showed a laminar flow in the control aorta. Similarly, colour Doppler in hypertensive segment of the operated rats could demonstrate a preserved laminar flow. The mean WSS value in the prestenotic segments was not different from that of control aortas (Figure 2b and Table 3).
Circumferential wall tension and tensile stress
The mean CWT level in the prestenotic segments was markedly increased in comparison with that of corresponding segments from control aortas (Figure 2c and Table 3). On the other hand, the mean TS from both operated and sham-operated animal were similar (Figure 2d and Table 3).
High resolution light microscopy and morphometry
The constriction of the abdominal aorta resulted in a mean reduction of 81% of the luminal infra-diaphragmatic aorta in comparison with the corresponding segment of control aortas.
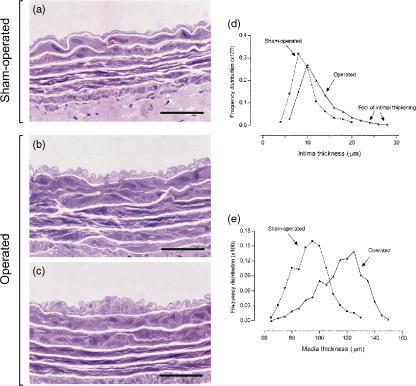
The use of plastic embedding allowed thin sections with adequate resolution of structural details. The light microscopic study of plastic-embedded aortas demonstrated striking changes in operated animals when compared with sham-operated controls. In the prestenotic segment, the changes were characterized by diffuse intimal thickening with enlarged endothelial cells (Figure 3b) and diffusely distributed conspicuous neointimal plaques composed of smooth muscle cells and occasional mononuclear cells with collagen and elastic fibres surrounding them and medial thickening (Figure 3c), contrasting with the delicate structure of the intima in the control group (Figure 3a). When the percentile frequency distribution of intima thickness in each group was plotted, shift to the right of the values in the prestenotic segment could be clearly seen in comparison with the values in corresponding segment in sham-operated animals. Moreover, the intimal thickening corresponding to numerous discrete neointimal plaques, absent in the control aortas, could be clearly demonstrated (Figure 3d). The same could be seen when the percentile frequency distribution of media thickness was plotted, demonstrating the medial thickening (Figure 3e).
Figure 3.
High resolution light microscopy. Representative views of the prestenotic segments of the aorta from operated rats (b and c) and the corresponding segments from controls (a). In the prestenotic segment, there is diffuse intimal thickening with enlarged endothelial cells (b) and diffusely distributed minute foci of intimal thickening composed of smooth muscle cells and occasional mononuclear cells with collagen and elastic fibres surrounding then (c) and medial thickening (b and c), contrasting with the delicate structure of the intima in control group (a). The percentile frequency distribution of intimal thickness in the prestenotic segment demonstrates a shift to the right of the values in comparison with the values in corresponding segment in sham-operated animals. Moreover, the numerous discrete foci of intimal thickening, absent in the control aortas, can be clearly demonstrated (d). The same can be seen when the percentile frequency distribution of media thickness is plotted clearly, demonstrating the medial thickening in operated animals in comparison with controls (e). Scale bars, 20 μm (a–c).
Table 4 shows the data of the intima and media thickness, cross-sectional luminal area, perimeter, diameter and number of elastic layers.
Table 4.
Intima and media thickness, luminal area, perimeter, diameter, number of elastic layers of the media from sham-operated control and operated groups
| Intima thickness (μm) | Media thickness (μm) | Luminal area (mm2) | Diameter (mm) | Elastic layers of the media | |
|---|---|---|---|---|---|
| Sham-operated | 10.03 ± 0.60 | 93.69 ± 3.74 | 1.75 ± 0.07 | 1.49 ± 0.03 | 7.68 ± 0.26 |
| Operated | 12.68 ± 0.60* | 115.05 ± 4.49** | 1.71 ± 0.09 | 1.51 ± 0.04 | 7.58 ± 0.18 |
Values are given as mean ± SE
P < 0.05
P < 0.01.
Immunohistochemistry
The immunohistochemical analysis revealed an increased expression of eNOS in endothelial cells, iNOS and NT in endothelial and smooth muscle cells, and TGF-β in endothelial and, mainly, smooth muscle cells, in the prestenotic segment when compared with the corresponding segment of control aortas (Figure 4).
Figure 4.
Immunohistochemistry. Representative views of the prestenotic segments of the aorta from operated rats and the corresponding segments from controls. The arrow read (brown stained features) point out the expression of the endothelial nitric oxide synthase (eNOS), inducible nitric oxide synthase (iNOS), nitrotyrosine and transforming growth factor-β (TGF-β) in operated animals. The graphs represent the quali-quantitative evaluation of the immunohistochemical analysis revealing an increased expression of eNOS, iNOS, nitrotyrosine and TGFβ in the prestenotic segment in comparison with that of corresponding segments from control aortas. (□, Sham-operated; ▪, operated). Scale bars, 20 μm.
Transmission electron microscopy
The intima of control was composed of a continuous layer of endothelial cells sitting on a thin layer of fibrocollagenous support tissue. This is contiguous with the media, composed of smooth muscle cells reinforced by organized layers of elastic fibres forming elastic laminas and collagen fibres. Just below the intima, there is a broad continuous layer of organized elastic fibres interrupted by fenestras, the internal elastic lamina (Figure 5a). These findings did no differ from that reported in the literature (Rossi & Colombini-Netto 2001).
Figure 5.
Transmission electron microscopy. Representative aspects of the prestenotic segments of the aorta from operated rats and the corresponding segments from controls. The delicate structure of the intima from sham-operated rats contains flattened endothelial cells (end) and a very thin layer of support tissue (*). The intima is delimitated by the internal elastic lamina (iel) (a). In the prestenotic segment, the intima is thickened. The endothelial cells are heterogeneous, most of them with convoluted nuclei and cytoplasmic contours. Focal accumulation of mononuclear cells (mo) could be seen in the expanded intimal layer (b). Migration of smooth muscle cells from the media into the intima (arrow) through the fenestras of the internal elastic lamina (iel) can be also seen (c). The clusters of smooth muscle cells (smc) appear within the intima, randomly arranged and surrounded by collagen and elastic fibres (d). Scales bars = 10 μm.
In the hypertensive segment, the intima appeared diffusely expanded mainly due to enlarged endothelial cells showing irregular nuclear and cytoplasmic contours resting on a basement membrane-like material and delicate fibrocollagenous support tissue. Focally distributed discrete neointimal plaques could be also detected. They were composed of clusters of smooth muscle cells, randomly arranged, surrounded by basement membrane-like material, collagen and young elastic fibres (Figure 5c,d). Migration of smooth muscle cells from the media into the intima through the fenestras could be also seen (Figure 5c). Occasional mononuclear cells were detected in the expanded intimal layers (Figure 5b). The media remained intact, the medial smooth muscle cells appearing unaltered, retaining their orientation to the vessel.
Scanning electron microscopy
The scanning electron microscopic evaluation revealed that the endothelial cells throughout the aorta of sham-operated control rats displayed typical cobblestone morphology, with a major axis parallel to the direction of the blood flow. Marginal folds marked the margins of the endothelial cells. The luminal surface had microvilli spread out, mainly clustered over the nuclear hillocks (Figure 6a). The endothelial cells of the prestenotic segments of operated animals were, comparatively, more elongated, in the direction of the flow, and more voluminous, with prominent nuclear hillocks. These cells were, however, ill-defined, with less evident margins due to paucity of marginal folds and microvilli on the cell surface (Figure 6b).
Figure 6.
Scanning electron microscopy. Representative views of the endothelial surface in sham-operated animals showing typical cobblestone morphology with major axis parallel to the direction of the blood flow (arrow). The prestenotic segment shows more voluminous and elongated endothelial cells with the major axis in the direction of the blood flow (arrow). Scale bars = 10 μm.
Discussion
Abdominal aortic constriction of about 80% created a defined proximal prestenotic hypertensive segment with an increased CWT associated with normal TS, laminar flow and normal WSS. Proximal hypertension reached a maximum at 28 days after surgery. The pressure elevation at the prestenotic segment (carotid artery blood pressure) seems to be related to mechanical obstruction. Activation of systemic renin–angiotensin system, with high levels of plasma renin concentration and activity, is an early response to aortic constriction, returning to normal levels after 7–10 days (Akers et al. 2000; Yayama et al. 2004).
Experimental abdominal aorta constriction has been postulated as a model of pressure overload cardiac hypertrophy (Swynghedauw & Delcayre 1982; Rossi & Peres 1992). The increased blood pressure proximal to the constriction is the stimulus for the development of cardiac hypertrophy. The absence of pleural or abdominal effusions supports the conclusion that the left ventricle overload was in a compensated state. The mean heart weights from rats of the operated group, expressed in absolute values or in g/kg of body weight, were significantly increased in comparison with those of controls. As well, the heart weights from operated group was significantly higher than the predicted heart weight of equal body weight controls, contrasting with the observation that heart weight from sham-operated controls was not different from the predicted heart weight of equal weight controls.
The morphological changes in the hypertensive segment consist of heterogeneous endothelial cells, most of them enlarged with convoluted nuclear and cytoplasmic contours. The scanning electron microscopic study clearly pointed out that the endothelial cells were elongated in the direction of the blood flow, voluminous, with prominent nuclear hillocks, though ill-defined with less evident margins due to paucity of marginal folds and microvilli over the cell surface. It is well known that arteries react to an increase of blood pressure with growth of vascular tissue, characterized by intima and media thickening and increase of several connective tissue components including collagen and elastin (Kowala et al. 1986; Chobanian 1992; Rachev et al. 1996). In the present investigation, we demonstrated diffuse intimal and medial thickening associated with diffusely distributed conspicuous neointimal plaques. These bulging plaques composed of randomly arranged smooth muscle cells and occasional mononuclear cells intermixed with membrane-like material and collagen fibres, are present in the tubular hypertensive aortic segment without curvatures, bifurcations or ramifications and associated with laminar flow and normal WSS. The focal nature of atherosclerotic plaques that develop in segments near arterial bifurcations, branch ostia and curvatures has been ascribed to blood flow disturbances such as non-uniform laminar flow, flow reversal and low WSS (Van der Vusse et al. 2000; VanderLaan et al. 2004; Cunningham & Gotlieb 2005). In contrast, it has been hypothesized that shear due to blood flow is probably not the major factor influencing atherogenesis, but that blood pressure-induced arterial wall stress and accompanying stretch are primary factors contributing to the localization of atherosclerotic lesions (Thubrikar & Robicsek 1995). Our results clearly indicate that the association of hypertension and stretch, characterized by increased CWT, could prime the focal localization of neointimal plaques and wall thickening. It could be suggested that the association of hypertension and increased CWT could act on certain endothelial genes modulating on a local level within the arterial wall to produce the neointimal plaques observed in this segment.
The medial thickening of 23% is likely a response to circumferential tension but not to circumferential deformation as diameter, perimeter, and luminal area of aortas in prestenotic segments were not different from those of corresponding segments of control vessels. This contrasts with previous study showing that medial thickening occurred in response to circumferential deformation but not to circumferential tension in a vein-graft model in which tensions and deformation were discriminated using a band to narrow the carotid artery proximal to the vein-graft (Dobrin 1995). Ours results are consistent with an investigation using healthy humans demonstrating CWT positively correlated with carotid intima-media thickness (Carallo et al. 1999). It has been reported that intraluminal pressure regulates artery thickness through its effects on wall tension and blood flow regulates arterial lumen diameter through changes in WSS (Dobrin 1995; Lehoux & Tedgui 1998; Carallo et al. 1999; VanderLaan et al. 2004). The increased wall thickness, mainly due to medial thickening, serve as a compensatory mechanism preventing increased arterial diameter and TS and change of the WSS. This medial thickening can be ascribed to the increased expression of TGF-β in both endothelial and smooth muscle cells in the hypertensive segments compared with discrete expression of TGF-β immunoreactivity in the corresponding segments in control aortas. TGF-β is a potent regulator of the cell cycle in many cells including vascular smooth muscle and endothelial cells (Massague 1998). This growth factor has been postulated to play an important, though largely undefined, role in vascular proliferative processes (Topper 2000). In addition, we noted that the thickness of the media layer was increased, whereas the number of elastic layers remained constant, thus indicating that hypertrophy and/or hyperplasia had occurred.
An increased expression of constitutive endothelial (eNOS) and inducible (iNOS) isoforms of nitric oxide synthase could be detected. It is likely that endothelial cells respond to hypertensive stress with augmentation of eNOS and iNOS expression as a compensatory mechanism aiming to increase production of nitric oxide (NO). In a similar model, marked upregulation of eNOS in the aortic segment proximal to coarctation compared with the corresponding segments in sham-operated control was found (Barton et al. 2001). It has been suggested that NO produced in large amounts by iNOS is a toxic-damaging agent (Hernandez-Pando et al. 2001), whereas eNOS is a protective enzyme (Heeringa et al. 2002). The increased presence of NT in endothelial cells and, mainly, smooth muscle cells, when compared with the absence or discrete expression of NT immunoreactivity in the endothelial cells of controls indicates an increased production of NO and superoxide (O2−) that interact to produce peroxynitrite, a powerful oxidant causing damage to multiple cell components including proteins (Nucci et al. 2003). The significant accumulation of NT, which is the footprint of NO oxidation/inactivation by reactive oxygen species (Reiter et al. 2000), supports the supposition of endothelial dysfunction in the face of marked upregulation of eNOS and iNOS in this model, consistent with avid NO inactivation by oxidative stress in the affected vascular bed. This agrees with previous study showing marked increase in NT in the aortic segment proximal to constriction above the renal arteries compared with corresponding segment from sham-operated controls (Barton et al. 2001).
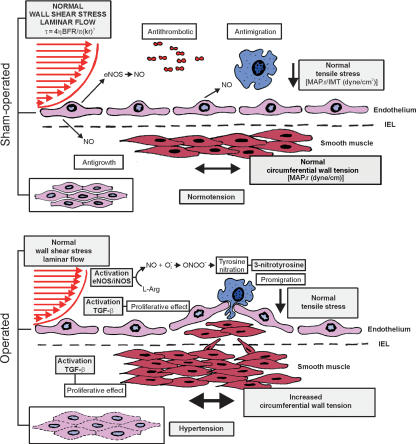
In summary, the aorta remodelling observed in the hypertensive prestenotic segment with increased CWT associated with normal TS, laminar flow/normal WSS was characterized by enlarged heterogeneous endothelial cells, elongated in the direction of the blood flow, diffusely distributed conspicuous neointimal plaques, and medial thickening. Our findings suggest that increased CWT due to hypertension plays a pivotal role in the remodelling of the prestenotic segment through biomechanical effects on oxidative stress and increased TGF-β expression. Further studies are needed to clarify the intrinsic pathogenetic mechanism of focal distribution of the neointimal plaques in the hypertensive segment (Figure 7).
Figure 7.
Sham-operated aortas demonstrate normal wall shear stress and laminar flow, normal tensile stress and circumferential wall stress. The endothelial cells display typical cobblestone morphology, with well-defined margin and the media remained intact. The aorta remodelling observed in the hypertensive prestenotic segment with increased circumferential wall tension associated with normal tensile stress, laminar flow/normal wall shear stress was characterized by enlarged heterogeneous endothelial cells, elongated in the direction of the blood flow, diffusely distributed conspicuous neointimal plaques. The endothelial cells are ill-defined and the media is thickened. Our findings suggest that increased circumferential wall tension due to hypertension plays a pivotal role in the remodelling of the prestenotic segment through biomechanical effects on oxidative stress and increased TGF-β expression.
Acknowledgments
The authors thank Lígia B. Santoro, Maria E. Riul, Mônica A. Abreu, for excellent technical assistance. This study was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP no. 01/09879-8, no. 06/52882-3) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Prof. Rossi is Senior Investigator of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- Akers WS, Cross A, Speth R, Dwoskin LP, Cassis LA. Renin-angiotensin system and sympathetic nervous system in cardiac pressure-overload hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2797–H2806. doi: 10.1152/ajpheart.2000.279.6.H2797. [DOI] [PubMed] [Google Scholar]
- Barton CH, Ni Z, Vaziri ND. Effect of severe aortic banding above the renal arteries on nitric oxide synthase isotype expression. Kidney Int. 2001;59:654–661. doi: 10.1046/j.1523-1755.2001.059002654.x. [DOI] [PubMed] [Google Scholar]
- Carallo C, Irace C, Pujia A, et al. Evaluation of common carotid hemodynamic forces. Relations with wall thickening. Hypertension. 1999;34:217–221. doi: 10.1161/01.hyp.34.2.217. [DOI] [PubMed] [Google Scholar]
- Chobanian AV. Vascular effects of systemic hypertension. Am. J. Cardiol. 1992;69:3E–7E. doi: 10.1016/0002-9149(92)90010-v. [DOI] [PubMed] [Google Scholar]
- Cooke JP. Flow, NO, and atherogenesis. Proc. Natl Acad. Sci. U.S.A. 2003;100:768–770. doi: 10.1073/pnas.0430082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- Dai G, Kaazempur-Mofrad MR, Natarajan S, et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc. Natl Acad. Sci. U.S.A. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrin PB. Mechanical factors associated with the development of intimal and medial thickening in vein grafts subjected to arterial pressure. A model of arteries exposed to hypertension. Hypertension. 1995;26:38–43. doi: 10.1161/01.hyp.26.1.38. [DOI] [PubMed] [Google Scholar]
- Heeringa P, Steenbergen E, van Goor H. A protective role for endothelial nitric oxide synthase in glomerulonephritis. Kidney Int. 2002;61:822–825. doi: 10.1046/j.1523-1755.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pando R, Schon T, Orozco EH, Serafin J, Estrada-Garcia I. Expression of inducible nitric oxide synthase and nitrotyrosine during the evolution of experimental pulmonary tuberculosis. Exp. Toxicol. Pathol. 2001;53:257–265. doi: 10.1078/0940-2993-00182. [DOI] [PubMed] [Google Scholar]
- Kowala MC, Cuenoud HF, Joris I, Majno G. Cellular changes during hypertension: a quantitative study of the rat aorta. Exp. Mol. Pathol. 1986;45:323–335. doi: 10.1016/0014-4800(86)90021-3. [DOI] [PubMed] [Google Scholar]
- Lehoux S, Tedgui A. Signal transduction of mechanical stresses in the vascular wall. Hypertension. 1998;32:338–345. doi: 10.1161/01.hyp.32.2.338. [DOI] [PubMed] [Google Scholar]
- Li JJ, Chen JL. Inflammation may be a bridge connecting hypertension and atherosclerosis. Med. Hypotheses. 2005;64:925–929. doi: 10.1016/j.mehy.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-β signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Nucci C, Morrone L, Rombola L, Nistico R, Piccirilli S, Cerulli L. Multifaceted roles of nitric oxide in the lateral geniculate nucleus: from visual signal transduction to neuronal apoptosis. Toxicol. Lett. 2003;139:163–173. doi: 10.1016/s0378-4274(02)00430-7. [DOI] [PubMed] [Google Scholar]
- Rachev A, Stergiopulos N, Meister JJ. Theoretical study of dynamics of arterial wall remodeling in response to changes in blood pressure. J. Biomech. 1996;29:635–642. doi: 10.1016/0021-9290(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Teng RJ, Beckman JS. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J. Biol. Chem. 2000;275:32460–32466. doi: 10.1074/jbc.M910433199. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis - an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Colombini-Netto M. Chronic inhibition of NO synthesis per se promotes structural remodeling of the rat aorta. J. Hypertens. 2001;19:1567–1579. doi: 10.1097/00004872-200109000-00008. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Peres LC. Effect of captopril on the prevention and regression of myocardial cell hypertrophy and interstitial fibrosis in pressure overload cardiac hypertrophy. Am. Heart J. 1992;124:700–709. doi: 10.1016/0002-8703(92)90281-y. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Carillo SV, Oliveira JS. The effect of iron deficiency anemia in the rat on catecholamine levels and heart morphology. Cardiovasc. Res. 1981;15:313–319. doi: 10.1093/cvr/15.6.313. [DOI] [PubMed] [Google Scholar]
- Song RH, Kocharyan HK, Fortunato JE, Glagov S, Bassiouny HS. Increased flow and shear stress enhance in vivo transforming growth factor-beta 1 after experimental arterial injury. Arterioscler. Thromb. Vasc. Biol. 2000;20:923–930. doi: 10.1161/01.atv.20.4.923. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B, Delcayre C. Biology of cardiac overload. Pathobiol. Annu. 1982;12:137–183. [PubMed] [Google Scholar]
- Thubrikar MJ, Robicsek F. Pressure-induced arterial wall stress and atherosclerosis. Ann. Thorac. Surg. 1995;59:1594–1603. doi: 10.1016/0003-4975(94)01037-d. [DOI] [PubMed] [Google Scholar]
- Topper JN. TGF-β in the cardiovascular system: molecular mechanisms of a context-specific growth factor. Trends. Cardiovasc. Med. 2000;10:132–137. doi: 10.1016/s1050-1738(00)00061-x. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Molecular and cellular mechanisms regulating vascular function and structure-implications in the pathogenesis of hypertension. Can. J. Cardiol. 2000;16:1137–1146. [PubMed] [Google Scholar]
- Van der Vusse GJ, van Bilsen M, Glatz JF. Cardiac fatty acid uptake and transport in health and disease. Cardiovasc. Res. 2000;45:279–293. doi: 10.1016/s0008-6363(99)00263-1. [DOI] [PubMed] [Google Scholar]
- VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- Yayama K, Horii M, Hiyoshi H, et al. Up-regulation of angiotensin II type 2 receptor in rat thoracic aorta by pressure-overload. J. Pharmacol. Exp. Ther. 2004;308:736–743. doi: 10.1124/jpet.103.058420. [DOI] [PubMed] [Google Scholar]
- Zarins CK, Zatina MA, Glagov S. Correlation of postmortem angiography with pathologic anatomy: quantitation of atherosclerotic lesions. In: Bond MG, editor. Clinical Diagnosis of Atherosclerosis: Quantitative Methods of Evaluation. New York, NK: Springer-Verlag; 1986. pp. 283–306. [Google Scholar]