Abstract
Adenoid cystic carcinoma (ACC) is a frequent malignant salivary gland neoplasm presenting different growth patterns described as tubular, cribriform and solid, which represent distinct differentiation stages. Cell lines originated from ACCs grown inside three-dimensional environments have not been capable to reproduce all in vivo ACC growth patterns. As ACC cells in vivo present replicated basement membrane, to mimic this situation in vitro ACC cells (CAC2 cells) were grown on the top of a reconstituted basement membrane (Matrigel). Phenotype differences were assessed by light, fluorescence and transmission electron microscopy. The cultures grown on the top of Matrigel presented three-dimensional arrangement of cells intercepted by cellular cords. At these, cell nests pseudocyst formations were observed. This morphological structure entirely reproduced the cribriform growth pattern of ACC. We suggest that the cribriform differentiation of ACC in culture is dependent of proteins and growth factors associated in a bi-dimensional structure.
Keywords: adenoid cystic carcinoma, basement membrane, extracellular matrix, Matrigel, salivary gland neoplasms
Adenoid cystic carcinoma (ACC) is a frequent malignant salivary gland neoplasm. The neoplasm growth patterns are cribriform, tubular and solid and represent distinct differentiation stages. The cribriform pattern displays pseudo-cystic structures, formed by neoplastic cells of either myoepithelial or epithelial phenotype (Seifert & Sobin 1992). Electron microscopy of ACC shows both luminal and myoepithelial cells (Dardick 1996). These cells are often separated by extracellular material, such as pools of basal lamina, collagen fibres, elastin and glicosaminoglicans (Cheng et al. 1992; Seifert & Sobin 1992). A conspicuous finding in the cribriform variant of ACC is a thickened band of extensively reduplicated basement membrane (Cheng et al. 1992).
Morphogenetic studies of normal and neoplastic salivary glands have been carried out by culturing cells in matrix environment. Cell lines originated from ACCs grown inside three-dimensional environments of individual extracellular matrix proteins originated pseudocysts formation as well as ductlike structures (Munakata et al. 1996; Freitas & Jaeger 2002; Freitas et al. 2004). However, these in vitro assays have not been capable to reproduce all in vivo ACC growth patterns. As ACC cells in vivo present replicated basement membrane, to mimic this situation in vitro we decided to grow ACC cells (CAC2 cell line) on the top of a reconstituted basement membrane (Matrigel). Phenotype differences were assessed by light, fluorescence and transmission electron microscopy.
Material and methods
Histopathology
For light microscopy, following surgery, the specimen was immediately fixed in 10% neutral formalin for 24 h. Then, the specimen was embedded in paraffin, sectioned at 5 μm, and stained with haematoxylin and eosin.
Cell Culture
CAC2 cells (França et al. 1997) were cultured in Dulbecco’s modified Eagle medium (DMEM; Sigma Chemical Co., St Louis, MO, USA) supplemented by 10% of foetal bovine serum (Cultilab, Campinas, SP, Brazil) and 1% antibiotic-antimycotic solution (Sigma). The cells were maintained in 25 cm2 flasks in a humidified atmosphere of 5% CO2 in air at 37°C.
Cells were detached from the flasks using a 0.25% trypsin solution (Sigma) and plated either on glass coverslips (15 mm no. 1 round; Ted Pella Inc., Redding, CA, USA) or on polycarbonate filters of 4.7 cm2 diameter and 0.4 μm pore size (Transwell cell culture inserts; Costar Co., Cambridge, MA, USA).
Immunofluorescence
Cells grown on coverslips were fixed in paraformaldehyde 1% in phosphate-buffered saline (PBS) for 15 min, rinsed in PBS and permeabilized with Triton X-100 (Sigma) for 10 min. The cells were then subjected to an immunofluorescence protocol (Jaeger et al. 1995) to detect vimentin, AE-3, and smooth-muscle actin. Vimentin was detected by a mouse monoclonal antibody from Amersham (Amersham Co., Arlington Heights, IL, USA), diluted 1/100 in PBS. AE-3 was stained by a mouse monoclonal antibody from Biogenex, diluted 1/100 in PBS. Smooth-muscle actin was stained by a mouse monoclonal antibody from Biogenex, diluted 1/100 in PBS. A sheep anti-mouse fluorescein conjugate (Amersham) was used as secondary antibody. All incubations were done for 60 min at room temperature. The mounting medium was 0.1% paraphenylenediamine (Sigma) and 10% PBS in glycerol. Replacement of the primary antibody by PBS was used as negative control.
Immunofluorescence labelling of CAC2 cells was conducted at least five times, and a minimum of 100 cells were examined each time. The observations and photographic recording were carried out using a Zeiss Axiophot 2 fluorescence microscope with 63× Planapochromatic 1.4 NA, and 100× Planapochromatic, 1.4 NA objectives (Carl Zeiss, Oberköchem, Germany).
Transmission electron microscopy
For transmission electron microscopy, CAC2 cells grown on polycarbonate filters were fixed by immersion into 2% glutaraldehyde in 0.1 M sodium cacodylate buffer solution at pH 7.4 for 2 h, and postfixed in 1% osmium tetroxide in the same buffer, for 45 min. Then, filters were carefully removed from their supports, washed in distilled water, ‘en bloc’ stained with 0.5% uranyl acetate for 3 h, rinsed and dehydrated in graded ethanol. After immersion in propylene oxide, samples were embedded in epoxy resin (Epon 812; Ted Pella) and polymerized for 72 h at 60°C. Semithin sections (1 μm) were cut and stained with a mixture of 1% azure II, 2% methylene blue and 2% borax in distilled water. Ultrathin sections were stained with lead citrate and uranyl acetate and examined in a JEOL 1010 transmission electron microscope (Jeol In, Peabody, MA, USA).
Experiments
CAC2 cells after the nineth passage were used for the experiments. Cells were detached from the flasks using a 0.25% trypsin solution (Sigma), and plated on glass coverslips (Ted Pella), coated with Matrigel (Collaborative Research Inc., Bedford, MA, USA). Coating procedure was as follows:
Matrigel was thawed at 4°C overnight. Then, using cooled pipettes, Matrigel was homogenized and diluted in cold serum-free DMEM, to reach a final concentration of 3 mg/ml (stock solution 13 mg/ml). This diluted Matrigel was added (50 μl/cm2) to cold coverslips. Finally, these coated substrates were placed at 37°C for 30 min. CAC2 cells were then harvested from the culture flasks and plated on the top of this gel coating.
CAC2 cells plated on plain coverslips served as control. Both control and treated samples were studied by phase contrast and fluorescence microscopy.
Results
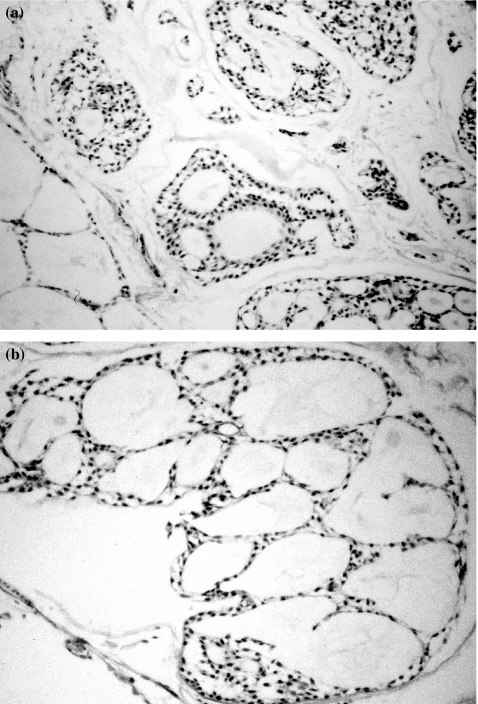
The original tumour exhibited the typical histopathological features of the ACC, characterized by a cribriform pattern, organized as variable sized nests and pseudo-cystic formations, separated by collagenized stroma (Figure 1a). Pseudo-cyst formations contained greyish blue to pinkish granulofibrillar material. Cells were uniform, relatively small and darkly stained (Figure 1b).
Figure 1.
Haematoxylin and eosin stained section of the tumour that originated CAC2 cell line. Cells present a cribriform pattern, organized as nests and pseudo-cystic formations, separated by collagenized stroma (a). Pseudocysts contain granulofibrillar material. Cells are uniform, small and darkly stained (b). (original magnification a, 400×; b, 630×)
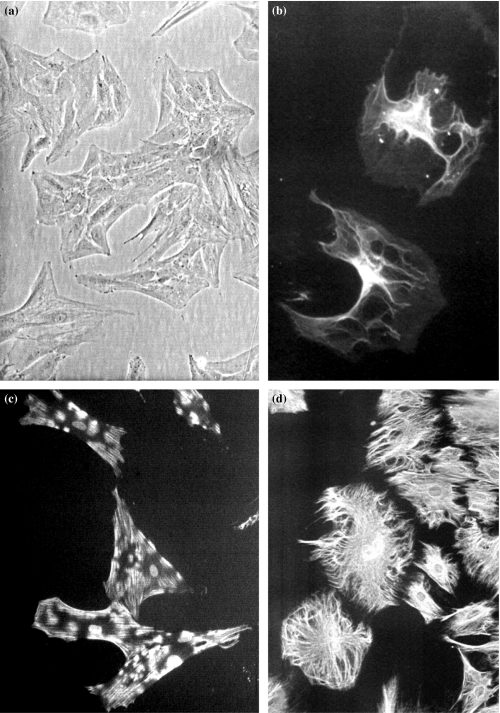
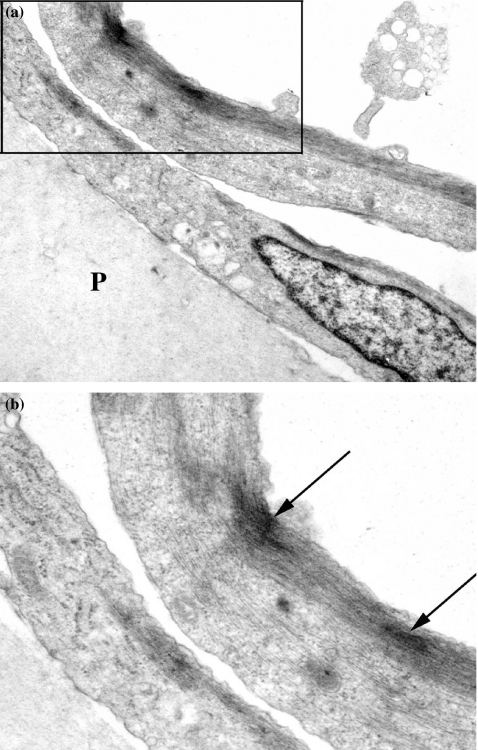
CAC2 cells, grown on substrates without Matrigel, were characterized by phase contrast light microscopy, immunofluorescence and transmission electron microscopy. The CAC2 cells were polygonal in shape and exhibited growth on plain substrates as monolayer (Figure 2a). Immunofluorescence showed expression of cytokeratin (AE3), smooth-muscle actin and vimentin (Figures 2b–d). Subcellular analysis of CAC2 cells showed spindle-shaped cells with endoplasmic reticulum, mitochondria, and Golgi apparatus (Figures 3a, b). Networks of linear elements were present, characterized by intermediate filaments and microfilaments (Figures 3a, b). An important feature was the detection of bundles microfilaments with dense bodies (Figure 3b), interpreted as myofilaments.
Figure 2.
Morphology and immunofluorescence of CAC2 cells grown on plain substrate (glass). Phase contrast shows a monolayer of polyedrical cells. Nuclei are round, with two to three nucleoli, and the cytoplasm are abundant (a). Immunofluorescence exhibits cytokeratin, smooth-muscle actin and vimentin (b–d). (original magnification a, 200×; b–d, 400×).
Figure 3.
Subcellular analysis of CAC2 cells grown on plain polycarbonate filter (P) showed spindle-shaped cells with endoplasmic reticulum, mitochondria and Golgi apparatus (a, b). Networks of linear elements were present, characterized by intermediate filaments and microfilaments (a, b). An important feature was the detection of bundles microfilaments with dense bodies (B-arrows) (original magnification a, 4000×; b, 8000×).
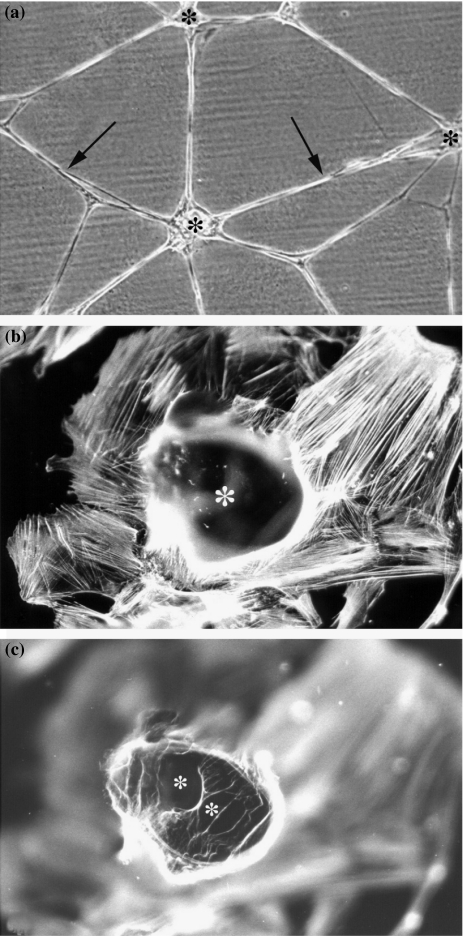
Bi-dimensional preparation of Matrigel created remarkable alterations in the phenotype of CAC2 cells. Phase contrast microscopy showed a monolayer of dendritic cells, with several thin, long and intercommunicating processes branching out from the central body (Figure 4a). This phenotype resembled pseudo-cystic formations, a characteristic feature of ACC. Fluorescence microscopy provided more information on the pseudo-cystic formations (Figures 4b, c). Cells grown on Matrigel were labelled with the specific fluorescent probe to actin, rhodamine phalloidin, as described elsewhere (9). The rationale to use this probe was to detect the actin cytoskeleton that lies immediately beneath the plasma membrane, which indirectly outlines the profile of cells. Our previous experience (9) shows that rhodamine phalloidin yields a strong and specific fluorescence signal, emanating from the cell edges at different focal planes. This feature facilitates three-dimensional studies, even in the absence of a confocal microscope. The fluorescence image obtained in Matrigel-treated samples allowed a three-dimensional analysis of the pseudocysts (Figures 4b, c). These structures were formed by cells in close relationship to each other. At the top of the sample, CAC2 cells delineated a large and round space (Figure 4b). Signal coming from inside the pseudocyst revealed at least two other cavities (Figure 4c).
Figure 4.
(a)Response of CAC2 cells to bi-dimensional preparation of Matrigel. Phase contrast microscopy displays a monolayer of dendritic cells, with thin, long and intercommunicating processes (arrows) branching out from the central body. (b, c) Distribution of actin, labelled by Rhodamine Phalloidin, in Matrigel-treated samples presents groups of cells surrounding a pseudocystic space (*). More spaces are observed inside the pseudocyst (C, *) (original magnification a, 100×; b, c, 400×).
Discussion
The cell line derived from human ACC (CAC2 cells) grown on the top of Matrigel presented three-dimensional arrangement of cells intercepted by cellular cords. At these cell nests pseudocyst formations were observed. This morphological structure entirely reproduced the cribriform growth pattern of ACC.
We have a previous experience on the in vitro influence of extracellular matrix on salivary gland neoplasms derived from the intercalated duct, such as pleomorphic adenoma and myoepithelioma (Jaeger et al. 1995, 1997; França et al. 1997, 2000, 2001; Oliveira et al. 2001). Using the three and bi-dimensional environment to grow these tumour cells we suggested that pleomorphic adenoma would be derived from a ‘reserve’ cell, still capable of differentiation into either directions, epithelial or myoepithelial (Jaeger et al. 1997), whereas myoepithelioma would be derived from a cell already committed to myoepithelial differentiation (Oliveira et al. 2001).
The arrangement of CAC2 cells on Matrigel mimicked the architectural growth pattern of the original tumour. Phase contrast microscopy and fluorescence microscopy showed pseudo-cystic structures, similar to those found in the cribriform subtype of ACC. We believe that culturing CAC2 cells on Matrigel would provide important information on ACC biology. This reconstituted basement membrane has been used in many laboratories as means of preserving, enhancing or inducing phenotypes of a variety of epithelial cells (Kleinman et al. 1986; Boudreau & Bissell 1998). Matrigel has in its composition important morphoregulatory molecules, such as laminin and type IV collagen, playing important roles either in cell proliferation or in cell differentiation (Kleinman et al. 1986).
Cell lines originated from ACCs grown inside three-dimensional environments of individual extracellular matrix proteins originated pseudocysts formation as well as ductlike structures (Munakata et al. 1996; Freitas & Jaeger 2002; Freitas et al. 2004). Differently from the results observed in these studies that only obtained ductlike structures and pseudocyst formations in a sparse arrangement of CAC2 cells inside a three dimensional laminin-1 or laminin enriched with SIKVAV (Freitas & Jaeger 2002; Freitas et al. 2004) in our study the cribriform pattern of the ACC was entirely obtained. The ultimate differentiation of CAC2 cells originating the cribriform pattern of the tumour development was only obtained in cultures grown in bi-dimensional arrangement on the top of Matrigel. This complex reconstituted basement membrane has proteins and growth factors that associated to each other were capable to induce this fully tumour differentiation in vitro. This reconstituted basement membrane has been used in many laboratories as means of preserving, enhancing or inducing phenotypes of a variety of epithelial cells (Kleinman et al. 1986; Boudreau & Bissell 1998).
Cheng et al. (1992) studied the biosynthesis of basement membrane molecules by ACC cells in culture. They found that these cells secrete basement membrane proteins, such as laminin and type IV collagen. These authors also suggested that the architecture of ACC, represented by pseudocystic formations, would result from their own secretion of basement membrane molecules. Our results provided direct evidence that basement membrane molecules influence ACC cell phenotype. This tumour shows in vivo a thickened reduplicated basement membrane at the outer margin of myoepithelial cells which make up the pseudocysts (Dardick 1996). Our assay, by placing Matrigel in contact with only one surface of CAC2 cells, simulated in vitro the neoplastic environment in vivo. We assume that the basement membrane observed in ACC is probably secreted by neoplastic cells themselves. Thus, we may infer that basement membrane molecules, mostly laminin and type IV collagen, may act as autocrine factors, determining the morphogenetic changes of CAC2 cells.
In conclusion, we presented the effects of a bi-dimensional preparation of Matrigel on cells derived from a human salivary gland ACC. The cells grown on Matrigel presented remarkable morphological changes. It was possible to reproduce in vitro the cribriform tumour architecture demonstrating that basement membranes molecules are key modulators of ACC differentiation. New studies aiming to determine the particular molecules involved in this process must be done.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). We thank Ms Patricia Galdino for technical assistance in transmission electron microscopy.
References
- Boudreau N, Bissell MJ. Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr. Opin. Cell Biol. 1998;10:640–646. doi: 10.1016/s0955-0674(98)80040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Saku T, Okabe H, Furthmayr H. Basement membranes in adenoid cystic carcinoma – an immunohistochemical study. Cancer. 1992;69:2631–2640. doi: 10.1002/1097-0142(19920601)69:11<2631::aid-cncr2820691103>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Dardick I, editor. Salivary gland tumor pathology. New York: Lippincott Williams & Wilkins; 1996. pp. 149–161. [Google Scholar]
- França CM, Oliveira PT, Jaeger MMM, Jaeger RG. Salivary gland tumors: a cytodifferentiation in vitro study. J. Dent. Res. 1997;76:1001. [Google Scholar]
- França CM, Jaeger MMM, Jaeger RG, Araújo NS. The role of basement membrane proteins on the expression of neural cell adhesion molecule (NCAM) in an adenoid cystic carcinoma cell line. Oral. Oncol. 2000;36:248–252. doi: 10.1016/s1368-8375(99)00087-1. [DOI] [PubMed] [Google Scholar]
- França CM, Jaeger RG, Freitas VM, Araújo NS, Jaeger MM. Effect of N-CAN on in vitro invasion of human adenoid cystic carcinoma. Oral. Oncol. 2001;37:638–642. doi: 10.1016/s1368-8375(01)00007-0. [DOI] [PubMed] [Google Scholar]
- Freitas VM, Jaeger RG. The effect of laminin and its peptide SIKVAV on a human salivary gland adenoid cystic carcinoma cell line. Virchows Arch. 2002;441:569–576. doi: 10.1007/s00428-002-0678-x. [DOI] [PubMed] [Google Scholar]
- Freitas VM, Scheremeta B, Hoffman MP, Jaeger RG. Laminin-1 and SIKVAV a laminin-1 derived peptide, regulate the morphology an protease activity of a human salivary gland adenoid cystic carcinoma cell line. Oral. Oncol. 2004;40:483–489. doi: 10.1016/j.oraloncology.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Jaeger RG, Jaeger MMM, Araújo VC, Kachar B. Analysis of the interdependent localization of vimentin and micotubules in neoplastic myoepithelial cells. Cell Motil. Cytoskeleton. 1995;32:289–298. doi: 10.1002/cm.970320405. [DOI] [PubMed] [Google Scholar]
- Jaeger MMM, Araújo VC, Kachar B, Jaeger RG. The effect of spatial arrangement of the basement membrane on cultured pleomorphic adenoma cells. Study by immunocytochemistry, electron and confocal microscopy. Virchows Archiv. 1997;430:467–477. doi: 10.1007/s004280050057. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Hassell JR, et al. Basement membrane complexes with biological activities. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Munakata R, Irie T, Cheng J, Nakagima T, Saku T. Pseudocyst formation by adenoid cystic carcinoma cells in collagen gel culture. J. Oral. Pathol. Med. 1996;25:441–448. doi: 10.1111/j.1600-0714.1996.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Oliveira PT, Jaeger MMM, Miyagi SPH, Jaeger RG. The effect of a reconstituted basement membrane (Matrigel) on human salivary gland myoepithelioma cell line. Virchows Arch. 2001;439:571–578. doi: 10.1007/s004280000380. [DOI] [PubMed] [Google Scholar]
- Seifert G, Sobin LH. The World Health Organization’s histological classification of salivary gland tumors. A commentary on the second edition. Cancer. 1992;70:379–385. doi: 10.1002/1097-0142(19920715)70:2<379::aid-cncr2820700202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]