Abstract
In severe injury, liver-cell progenitors may play a role in recovery, proliferating, and subsequently differentiating into mature liver cells. Identifying these progenitors has major therapeutic potential for ex vivo pharmaceutical testing, bioartificial liver support, tissue engineering and gene therapy protocols. Potential liver-cell progenitors have been identified from bone marrow, peripheral blood, cord blood, foetal liver, adult liver and embryonic stem cells. Differences and similarities are found among cells isolated from rodents and humans. This review will discuss identifying markers and differentiation potential in in vitro and in vivo models of these putative progenitors in both humans and rodents.
Keywords: cell differentiation, liver, oval cell, progenitor cell, stem cell, stem cell plasticity
Background and significance
If adult liver receives mild or moderate injury, repair depends on the division of mature adult cells. In severe injury, however, liver-cell progenitors may play a role in recovery, proliferating and subsequently differentiating into mature liver cells. Bipotential liver-cell progenitors, for example, known as oval cells, capable of differentiating into either of the two epithelial cell populations of the liver, hepatocytes and biliary epithelial cells, occur in various liver injury models (Grisham & Hartroft 1961; Solt et al. 1977; Evarts et al. 1987). During foetal development, bipotential cells, known as hepatoblasts, are also present in the liver (Shiojiri et al. 1991). Maturation of these cells along the pathways into either hepatocytes or biliary epithelial cells has been demonstrated both in vivo and in vitro.
Identifying liver-cell progenitors, particularly hepatocyte precursors, has major therapeutic potential. They could be exploited ex vivo for pharmaceutical testing and liver support systems, and could be used in tissue engineering and gene therapy protocols. The proliferative potential that progenitor cells have is a major advantage for therapeutic exploitation – contrasting strikingly with mature hepatocytes, which have little proliferative potential in vitro.
Nomenclature
The nomenclature of stem-cell biology is complex. The liver is generally regarded as consisting largely of mature cells with a variety of phenotypes (hepatocytes, biliary epithelial cells, stellate cells, Kupffer cells and sinusoidal endothelial cells). They are mitotically quiescent, although they are all capable of undergoing division – for example – after partial resection of the liver. Indeed, in some transplantation experiments, notably the tyrosinaemic mouse model where implanted wild-type hepatocytes have a survival advantage over the endogenous mutant hepatocytes, these have huge proliferative potential (Overturf et al. 1996).
Progenitor cells are, generally, defined as cells, which can divide rapidly, and may be unipotential or multipotential; the oval cells both in adult liver and foetal hepatoblasts are clear examples of liver-cell progenitors. Strictly, these are not true stem cells, which are classically described as slowly cycling and self-renewing, and give rise to more than one cell lineage. Thus, when a true stem cell divides, it must give rise to one identical daughter cell, but can give rise to other daughters to become proliferating progenitor cells. However, the concept of progressive differentiation, in the development or repair, from stem cell to proliferating progenitor and eventually along one specific lineage to a terminally differentiated cell, has been revised. The concept of stem-cell plasticity – the differentiation of a stem cell associated with a particular tissue to yield a cell characteristic of a different tissue – has emerged (Herzog et al. 2003). Transdifferentiation – the adoption of a different phenotype by a cell apparently committed to a tissue-specific cell type – is now also well described, exemplified, for example, by studies in which pancreatic acinar cells changed, without cell division, in order to adopt a hepatocyte phenotype (Shen et al. 2000).
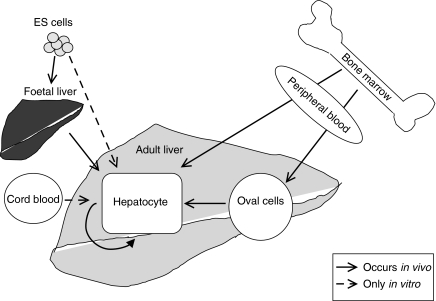
Recent research has indicated a number of sites from which hepatocyte precursors might be isolated (Figure 1). These include bone marrow, cord and peripheral blood, spleen, and foetal and adult liver. While some work has aimed at identifying a true undifferentiated multipotent stem cell, more has concentrated on identifying liver-cell progenitors, actively dividing and already partially determined along the pathway to liver epithelial cells, hepatocytic and/or biliary. There are many interpretative difficulties with current studies. In those which rely on transplantation of precursor cells into another host, the recognition that stem cells can fuse with other cell types has provided one such difficulty as discussed below (Terada et al. 2002). In studies, which depend on in vitro maturation of a putative precursor into a hepatocyte or biliary cell, while the issue of cell fusion may not arise, there are other difficulties. Criteria for recognizing liver-cell precursors before they express markers of the mature lineages are not defined. Manipulating an undifferentiated cell from an extrahepatic source to express liver-cell phenotype may reflect a process of transdifferentiation that regularly occurs in vivo, or be a rare event only occurring under the extreme conditions of experiment. Evidence of the adoption of a full hepatocytic phenotype is often lacking, with reported phenotypes often partial, for example, evidenced by mRNA expression rather than protein measurement, and without convincing adoption of liver-cell morphology. There are also differences in the cell markers expressed by rodent cells and human cells, yet many strategies to characterize progenitor cells in the human liver build on the characterization of analogous cells in rodents. Table 1 lists the various surface markers that have been used to characterize progenitor cells. Table 2 summarizes the marker combinations used in both human and rodent studies of potential liver-cell progenitors, discussed in this review.
Figure 1.
A schematic representation of various sources of hepatocytes both in vivo and in vitro.
Table 1.
A list of surface markers used in order to characterize progenitor cells
| Marker | Also known as | Function/expression |
|---|---|---|
| C1rRp | complement protein receptor | involved in classical complement pathway |
| CD7 | gp40 | expressed on pluripotent haematopoietic cells, T-cells and thymocytes |
| CD13 | gp150, aminopeptidase-N | expressed on pluripotent granulocytes and monocytes, bile ducts and bone marrow stroma |
| CD14 | lipopolysaccharide receptor (LPS-R) | expressed on monocytes and macrophages |
| CD29 | GP11a, VLA-β, β1 integrin subunit | expressed on hepatoblasts and maintained in maturation of both hepatocyte and biliary epithelial cells |
| CD34 | GP105-120, ligand for CD62 (L-selectin) | expressed on pluripotent haematopoietic cells, capillary endothelium and embryonic liver |
| CD38 | T-10, ADP-ribosylcyclase | augments B-cell proliferation and expressed on lymphoid progenitors |
| CD44 | H-CAM, Pgp-1, Hermes ag, ECMR II, HUTCH-1, gp85 | expressed on leucocytes and erythrocytes |
| CD45 | leucocyte common ag (LCA), B220, T200 | expressed on leucocytes |
| CD49α | VLA-1α, α1 integrin | expressed on hepatoblasts, maintained in hepatocyte and lost in biliary epithelial cell maturation |
| CD49β | VLA-2α, α2 integrin | expressed on mature biliary epithelial cells, associates with CD29 |
| CD49c | VLA-3α, α3 integrin | expressed on mature biliary epithelial cells |
| CD49e | VLA-4α, α4 integrin | expressed on hepatoblasts, maintained in hepatocyte and lost in biliary epithelial cell maturation |
| CD49f | VLA-6α, α6 integrin | expressed on hepatoblasts, maintained in biliary epithelial cells and lost in hepatocyte maturation |
| CD71 | T9, transferrin receptor | expressed on proliferating cells |
| CD106 | Vascular adhesion molecule-1 (VCAM-1), INCAM110 | ligand for VLA-4, expressed on endothelial cells |
| CD117 | c-kit, stem-cell factor receptor (SCFR) | expressed on haematopoietic progenitors, mast cells and liver stem cells |
| CD123 | interleukin-3 receptor (Il-3Rα) | expressed on bone marrow stem cells, granulocytes, monocytes and megakaryocytes |
| CD124 | interleukin-4 receptor (Il-4Rα) | expressed on haematopoietic precursors and mature B - and T-cells |
| CD133 | AC133, PROML1, haematopoietic stem cell ag | expressed on haematopoietic stem cells |
| c-met | hepatocyte growth factor receptor (HGFR) | receptor for HGF which is a mitogen, motogen and morphogen |
| CXCR-4 | stromal-derived factor 1 (SDF-1R) | role in attracting stem cells |
| Flk1 | vascular endothelial growth factor receptor 2 (VEGFR2), KDR | expressed on early haematopoietic progenitor cells |
| Flt1 | vascular endothelial growth factor receptor 1 (VEGF1) | expressed on early haematopoietic progenitor cells and monocytes and megakaryocyte precursors |
| lin | lineage marker | a combination of markers of haematological or lymphoid differentiation |
| MDR1, MRP1, MRP3 | ATP-binding cassette transporter proteins | ABC transporter proteins |
| OC.2, OV6, BD1, A6 ab | antibodies raised against rodent and human oval cells and biliary epithelial cells | |
| OX43 | expressed on macrophages, endothelial cells and red-cell precursors | |
| OX44 | CD53 | expressed on myeloid and peripheral lymphoid cells |
| Sca-1 | stem cell antigen-1, lymphocyte activation protein-6A (Ly-6A) | expressed on haematopoietic progenitor cells and oval cells |
| SSEA-1 | stage-specific embryonic antigen-1 | expressed on undifferentiated rodent ES cells and differentiated human ES cells |
| SSEA-3 | stage-specific embryonic antigen-3 | expressed on undifferentiated human ES cells and differentiated rodent ES cells |
| SSEA-4 | stage-specific embryonic antigen-4 | expressed on undifferentiated human ES cells and differentiated rodent ES cells |
| TER119 | erythroid precursor marker | |
| Thy-1 | CD90 | expressed on haematopoietic progenitors and liver progenitors (oval cells) |
| Tra-1-61 | keratan sulfate-associated antigen | expressed on undifferentiated ES cells |
| Tra-1-80 | keratan sulfate-associated antigen | expressed on undifferentiated ES cells |
Table 2.
A summary of markers expressed by cells observed in or derived from various sources which have the potential to develop into hepatocytes
| From bone marrow (haematopoietic stem cells) |
| CD34+lin−(Theise) |
| Sca-1+Thy-1+CD34+lin−CD45+(Lagasse) |
| β2micro−Thy-1+Thy-1+Alb+AFP+CK8+CK18+CK19+C/EBPa+CYP3A2+HNF4+CD34−CD38−CD117−(Avital) |
| CD34+Thy-1+CD117+AFP+c-met+(Oh and Miyazaki) |
| Sca-1+Thy-1+(Okumota) |
| CXCR-4+AFP+Sca-1+c-met+CK19+(Ratajczak) |
| CD34+/–CD45+CD38−lin−C1rRp+ (Danet) |
| CD34+CD38−CD7− (Wang and Ge) |
| β2micro−Thy-1+C/EBP+Alb+ (Avital) |
| CD34+CD45+ (Fiegel) |
| CD34+CD133+AFP+CXCR-4+ (Ratajczak) |
| From bone marrow (mesenchymal stem cells) |
| CD45−glycophorin-A− (Schwartz) (Schwartz) (Jiang) |
| Str1+CD13+CD49a+ CD49b+CD29+CD44+CD71+Thy-1+CD106+CD124+ |
| CD34−CD44−CD45−CD117−MHC−β2microlowCD133lowFlklowFltlowCD13+CD49b+ |
| From peripheral blood |
| AFP+CK19+(Ratajczak) (Ratajczak) |
| CD14+ (Zhao) |
| From cord blood |
| CD34+/–CD45+CD38−lin−C1rRp+ (Danet) |
| CD34+CD38−CD7− (Wang and Ge) |
| CD34+/– (Kakinuma) |
| CD34+ or CD45+ (Ishikawa) |
| From foetal liver |
| AFP+Alb+GGT+AAT+glutathione-S-transferase+ |
| MHC class-I−ICAM-1+(Kubota and Reid) |
| OX43−OX44−(Fiegel) |
| CD45−TER119−CD49f+/lowCD29+CD117−c-met+(Suzuki) |
| CD117lowCD45−TER119−CD49f+/–alb+AFP+TTR+HGF+OSMR+ c-met+(Minguet) |
| DLK+Alb+AFP+CK19+(Tanimizu) |
| Alb+AFP+CK19+CD49f+Thy-1−CD45−(Yasuchika) |
| HepPar1+CK19+CK14+ (Haruna) |
| β1 and α1,5,6,9 integrin+ (Couvelard) |
| AFP+GGT+CK8+CK19+CD34+plasminogen activator inhibitor 1+ (Malhi) |
| From adult liver (Oval cells) |
| CK19+GGT+AFP+Thy-1+CD117+CD34+SCF+OV6+OC.2+BD1+CD45–/+Sca-1+A6+ |
| CXCR4+(Hatch) |
| β2micro−Thy-1+(Avital) |
| AFP+CD45−TER119−CD117−Thy-1−CD49f+CD29+(Fujikawa) |
| Alb+transferrin+CK8+CK18+(Mitaka) |
| OC.2+OC.5+CD34−Thy-1−AFP+CYP450−(Gordon) |
| SP+CD45+/– and minority CD34+CD117+Sca-1+Thy-1+(Wulf) |
| CK19+CD117+ (Theise) |
| CD117+ and/or CD34+ (Crosby) |
| OV6+ (Crosby) |
| CK14+CD117+CD34−Alb− (Seki) |
| From embryonic stem cells |
| SSEA-1+SSEA-3−SSEA-4− (Henderson) |
| SSEA-1−SSEA-3+SSEA-4+Tra-1-60+Tra-1-81+Thy-1+ (Henderson) |
| Oct-4+ (Nichols) (Reubinoff) |
| SSEA-1−SSEA-3+SSEA-4+Tra-1-60+Tra-1-81+Oct4+hTERT+CD133+ (Carpenter) |
Rodent studies are in italic and human studies are in normal text.
Bone marrow as a source of liver-cell progenitors
Bone marrow contains haematopoietic stem cells, endothelial cell progenitors and marrow stromal cells (which include mesenchymal stem cells). Many of the concepts applied to the liver derive from studies on haematopoietic cell differentiation. Haematopoietic stem cells in the bone marrow, functionally defined by their ability to reconstitute the bone marrow of a myeloablated host, are contained in a population expressing CD34 (CD34+). They also express CD117 (c-kit), the receptor for stem-cell factor (SCF) produced by marrow fibroblasts and endothelial cells (Smith et al. 2001). Suggestive evidence (summarized below) that haematopoietic stem cells may give rise to liver cells, particularly in the presence of severe liver damage, stimulated both transplantation experiments and in vitro studies in order to define the subpopulation of bone marrow cells capable of generating liver cells.
Rodent studies
The bipotential oval-cell population that develops in the periportal area of the liver in rats when liver damage is induced but hepatocyte proliferation is inhibited has already been mentioned. Petersen et al. (1999) used lethally irradiated female rats rescued with a bone marrow transplant from male rats, subsequently placed on a protocol to generate oval cells (2-acetylaminofluorene to prevent hepatocyte replication, followed by CCl4 to induce liver damage). They demonstrated that the host liver contained firstly oval cells and later hepatocytes carrying genetic markers (Y-chromosomes and the membrane protein DDPIV) derived from the implanted bone marrow cells. They proposed that bone marrow cells could, under certain circumstances, act as progenitors for several types of liver cells. Subsequently, many similar observations were made in mice. Theise et al. (2000b) demonstrated that as few as 200 CD34+ bone marrow cells which were lin– (i.e. lacking markers of haematological or lymphoid differentiation) transplanted into myeloablated mice could give rise to hepatocytes in the recipient liver (up to 2.2% of the total hepatocyte number). This was most strikingly shown in the fumarylacetoacetate hydrolase (FAH)–/– (tyrosinaemic) mouse strain. In this model, the FAH–/– hepatocytes can be protected from the toxic effects of endogenously generated succinylacetone by administering NTBC (2-(2-nitro-4-trifluoro-methyl-benzoyl)-1,3 cyclohexanedione), which blocks the metabolic pathway upstream of the enzyme defect and prevents the generation of the toxic metabolites – just as is used therapeutically in affected children. However, subsequent removal of the NTBC again exposes hepatocytes to the toxin, and transplanted wild-type liver cells with intact FAH activity have a striking survival advantage over the FAH–/– cells (Overturf et al. 1996). Remarkable repopulation of the host liver occurred following the transplantation of as few as 50 bone marrow cells with surface markers of haematopoietic stem cells (Sca-1+, Thy-1+, CD34+, lin– and CD45+) (Lagasse et al. 2000). They reported an initial engraftment, which occurred even if there was no selection pressure, followed by the proliferation of single engrafted cells when selective pressure was applied by NTBC withdrawal (Wang et al. 2002). However, as already discussed, recognition that stem cells may fuse with other cells has led to re-evaluation of such experiments. Subsequent publications from this group suggest that fusion between the bone marrow-derived transplanted cells and host liver cells was the major mechanism underlying apparent transdifferentiation of bone marrow-derived cells into liver phenotype in the FAH–/– mouse (Vassilopoulos et al. 2003; Wang et al. 2003d). It has now been established that myelomonocytic cells are the major source of hepatocyte fusion partners (Camargo et al. 2004; Willenbring et al. 2004). It is worth commenting, of course, that even if the mechanism is exclusively fusion, such bone marrow transplantation approaches retain exciting therapeutic potential if they prove to be exploitable in man. Characterizing the bone marrow cells and processes involved may, however, be more informative in respect of fusogenic potential and fusion mechanisms rather than true liver-cell progenitor status.
Purely in vitro studies, in which bone marrow cells express hepatocyte characteristics either on isolation or after culture, avoid the confounding issue of cell fusion, and demonstrate the potential of bone marrow cells to differentiate towards liver-cell phenotype. Most workers have studied bone marrow cells, which express haematopoietic stem-cell markers, particularly those which are also reported to be found on oval cells (e.g. Thy-1 and Sca-1).
Avital et al. (2001) identified β2micro–/Thy-1+ cells in rat bone marrow which immunestained for albumin, AFP, CK8, 18 and 19 and C/EBPα, and were RT-PCR+ for albumin, C/EBPα, CYP3A2 and HNF4. These cells lacked characteristic haematopoietic stem-cell markers, being CD34–, CD38– and CD117–, and the authors named them bone marrow-derived hepatocyte stem cells (BMDHSC). The proportion of bone marrow represented by such cells increased in rats with cholestasis. After co-culture with and subsequent separation from hepatocytes, BMDHSC from cholestatic rats could synthesize urea, and after transplantation into the portal system of rats of a different strain, under immunosuppression, they integrated into hepatic cords. By contrast, Oh et al. (2000) and Miyazaki et al. (2002) identified in normal rat bone marrow cells (approximately 3%) which co-expressed the haematopoietic stem cell markers CD34, Thy-1 and CD117, and expressed (by means of immunohistochemistry) AFP and the hepatocyte growth factor (HGF) receptor c-met. They also reported that unfractionated bone marrow cells cultured with HGF (40–100 ng/ml) and epidermal growth factor (EGF) (20 ng/ml) in 10% FCS gave rise within 2 weeks to colonies of polygonal cells staining for albumin, with RT-PCR evidence of expression of enzymes characteristic of terminal hepatocyte differentiation (tryptophan-2,3-deoxygenase and tyrosine aminotransferase). Okumoto et al. (2003) used negative selection to deplete rat bone marrow of non-stem cells in order to obtain a population (5% of original bone marrow cells) enriched for Sca-1, approximately 33% positive for Thy-1 (CD90). When these cells were cultured with HGF, they began to express (by means of RT-PCR) the liver-enriched transcription factor HNF1α and the hepatocyte-associated cytokeratin CK8. Similarly a subpopulation of murine mononuclear bone marrow cells isolated by chemotaxis to the α-chemokine stromal-derived factor SDF-1 expressed mRNA for AFP (Ratajczak et al. 2004), and a probably overlapping population enriched for Sca-1 expressed mRNA for AFP, c-met and the liver-biliary cytokeratin CK19.
In such studies, the proliferative potential, the repertoire and many quantitative aspects of hepatocyte function remain undefined. Taken together, they demonstrate that a subpopulation of bone marrow cells expresses some characteristics of liver cells, and that the expression of such characteristics can be enhanced in vitro by growth factors relevant to liver growth and development.
Human and other primate bone marrow studies
Alison et al. (2000) and Theise et al. (2000c) investigated livers from sex non-identical bone marrow transplant patients and also liver transplant patients. They demonstrated, as in rodents (Theise et al. 2000a), the appearance of Y-chromosomal markers of bone marrow origin in liver cells (both hepatocytes and biliary cells) in the liver of female patients. A number of other studies by using various markers have reported similar phenomena. While Theise's initial studies reported 4–43% of hepatocytes of bone marrow origin, others have indicated that this is numerically a much less significant phenomenon (e.g. 0–7%, Korbling et al. 2002; 1.6%, Ng et al. 2003). Although not the main subject of this review, evidence for the replacement of non-epithelial cells in donor livers by cells of recipient origin (notably sinusoidal and endothelial cells) emerges as common and indeed more frequent than replacement of hepatocytes or biliary epithelial cells. Thus, in the study by Ng et al. (2003), the low frequency of bone marrow-derived hepatocytes after orthotopic liver transplant contrasted with much higher proportions of tissue macrophage/ Kupffer cells. Hove et al. (2003) found endothelial cells of recipient origin in four of five sex-mismatched livers, biliary cells in three and hepatocytes in only one liver.
Transplantation into immunodeficient mice offers a more controlled approach to identifying the liver-cell potential of human bone marrow cells than observations made in the field of clinical transplantation. Differing techniques may utilize implantation into normal liver, damaged liver or liver subsequently damaged. Danet et al. (2002) indicated that CD34+ or CD34–, CD45+, CD38– and lin– cells from human bone marrow (or cord blood) expressing the complement protein receptor C1qrRp, injected into NOD/SCID mice, gave rise to clusters of human hepatocytes (identified by means of immunostaining for human albumin and c-met) in the mouse liver, although only at approximately 0.1% or less of liver cells. They used fluorescent in situ hybridization (FISH) analysis and argued that they had excluded cell fusion as a mechanism.
Wang et al. (2003c) used NOD/SCID and NOD/SCID/β-2microglobulin-deficient mice as recipients for CD34+ bone marrow (and cord blood) cells and for further-purified CD34+CD38–CD7– cells (a population which contains quiescent stem cells capable of maturing to both lymphoid and myeloerythroid progeny). A month after transplantation, liver damage was induced with carbon tetrachloride in some mice. Human albumin (but not AFP) mRNA was expressed in the mouse livers only after CCl4 damage and expression was enhanced if HGF was administered immediately after damage was induced, with human albumin present in the serum.
As in rodents, in vitro studies show that human bone marrow cells can express some hepatocytic characteristics and/or proliferate and differentiate into cells with liver-cell characteristics. Avital et al. (2001) reported similar Thyl+β2micro– cells in human bone marrow cells, as they found in rats, expressing albumin and C/EBPα by means of immunocytochemistry and RT-PCR. Fiegel et al. (2003a) cultured CD34+ cells from human bone marrow, most of which were CD45+, with serum, HGF, EGF and insulin, and found after 28 days a modest increase in cell number and expression of both albumin and CK19 mRNA. Ratajczak et al. (2004) purified human bone marrow cells by means of SDF-1 chemotaxis, as they did in mice. SDF-1 binds to the cell surface ligand CXCR-4, and is thought to play a role in attracting stem cells. They showed that the chemotactic cells were CD34+ and CD133+ and were highly enriched for AFP mRNA, but also that they appeared multipotential as they expressed some markers of muscle and neural cells. They considered this population of pluripotential cells distinct from the bone marrow mesenchymal stem-cell (MSC) population, as they differed from MSC by being CD34+ and non-adherent. Characterization and functional assessment were limited to RT-PCR data, without proliferation data. Kucia et al. (2004) subsequently developed the general concept of a circulating pool of tissue-specific progenitor cells, but which can also be stored in bone marrow and can be mobilized by agents, such as GCSF, and are recruited to tissues in need. Interestingly, SDF-1 expression by biliary epithelial cells is upregulated in inflammatory liver diseases (Kollet et al. 2003; Terada et al. 2003), and the homing of bone marrow-derived human CD34+ cells to murine liver was enhanced by local deposition of SDF-1 in the liver, abolished by neutralization of CXCR4, and the accumulating CD34+ cells could differentiate into albumin-producing cells (Kollet et al. 2003); pre-treatment of the haematopoietic cells with a combination of HGF and stem-cell factor (CD117 ligand) enhanced recruitment to the liver.
In contrast to these studies on starting populations with haematopoietic stem-cell characteristics, Schwartz et al. (2002) found in human (as well as in rodent) post-natal bone marrow a multipotent adult progenitor cell (MAPC) which co-purified with MSC, and could undergo a prolonged proliferative phase (35+ population doublings in humans and 80+ in mice) and subsequently differentiate into cells with a variety of tissue-specific characteristics, including hepatocytic. The relationship between MSC and MAPC is unclear. Both are adherent, and generated from mononuclear cells depleted of CD45+ and glycophorin-A+ cells – i.e. negative for common leucocyte antigen and erythroid-cell characteristics – and multipotential. MAPC can apparently proliferate indefinitely, perhaps relating to their expression of telomerase, whereas MSC have a finite life span. MSC can be differentiated into osteocytes, chondrocytes and adipocytes, and they express Str1, CD13, α-integrins CD49α and β, CD29, CD44, CD71, CD90 (Thy-1), CD106 (VCAM-1) and CD124 (Jiang et al. 2002). Because the culture of MAPC is fastidious, and has been difficult to replicate (but has been achieved in other laboratories), full comparisons between MSC and MAPC are not yet available. Schwartz et al. (2002) generated MAPC from a CD34–, CD44–, CD45–, CD117– and MHC– cell population, with low or very low levels of β2 microglobulin, CD133, Flk1 (VEGF receptor 2) and Flt1, and high levels of CD13 (membrane-bound amino-peptidase) and CD49b (α-2-integrin). When taken from the proliferative cultures and plated at high density in serum-free medium with HGF and FGF-4, on a complex extracellular matrix, cells stopped proliferating and progressively enlarged, and over 7–28 days adopted hepatocyte-like markers (expression of albumin, CK18 and HNF-3β,1α and HNF4 detected immunochemically and by means of RT-PCR), produced and secreted urea and albumin (the latter at a level similar to that seen in rat primary hepatocyte cultures) and demonstrated CYP450 enzyme activity and glycogen storage. These MAPC could also be induced with other growth factor combinations in order to express neuronal, adipocytic and other tissue characteristics.
In a non-human primate, the baboon, an ex vivo expanded mesenchymal stem-cell population transduced with green fluorescent protein (GFP) reinfused into adult baboons led to GFP expression in many organs including the liver, but as expression was demonstrated by means of RT-PCR rather than histology, the nature of expressing cells in the liver could not be judged (Devine et al. 2003).
Thus as in the rodent, there is, in man, more than one subpopulation of bone marrow cells that express some characteristics of liver cells, and such expression can be enhanced in vitro by growth factors relevant to liver growth and development.
Human peripheral and cord blood as a source of liver-cell progenitors
As accessible sources, peripheral blood and cord blood has obvious advantages. Some studies have used peripheral blood after mobilization of stem cells with growth factors, and indeed human recipients of such ‘stem-cell transplants’ for haematopoietic reconstitution show evidence of acquisition of liver cells with genetic evidence of bone marrow origin – similar to that seen after bone marrow transplantation protocols and subject to the same caveats concerning cell fusion in vivo already discussed.
Kakinuma et al. (2003) exposed primary cultures of cord blood to combinations of growth factors and identified a combination – FGF-1, FGF-2, LIF, SCF and HGF – as optimum for initiating albumin mRNA between 7 and 21 days in culture, associated with loss of spindle-shape and adoption of a rounded cell shape. Glutamine synthetase, CK-18 and AFP were also expressed to varying extents. A combination of CD34+ and CD34– cells was required. Transplantation of human cord blood cells into liver-injured SCID mice (2-AAF/partial hepatectomy protocol) led to the presence of human albumin mRNA and human X-chromosome-positive hepatocytes in mouse liver, and hepatocytes expressing a human hepatocyte antigen were detected immunochemically. The studies of Danet et al. (transplanting C1qRp+ cord cells or bone marrow cells into non-liver-injured NOD/SCID mice) and Wang and Ge (CD34+CD38–CD7– cord or bone marrow cells into NOD/SCID or NOD/SCID β-2-microglobulin– null mice subsequently treated with CCl4) have already been mentioned. Ishikawa et al. (2003) also transplanted CD34+ or CD45+ human cord blood cells into NOD/SCID/β2-micro– mice, leading to 45% of cells in the bone marrow being of human origin, and the emergence of human-derived hepatocytes (by means of RT-PCR for human albumin). Newsome et al. (2003) infused unsorted human cord blood mononuclear cells containing approximately 1% of CD34+ cells into sublethally irradiated NOD-SCID mice with normal livers, leading to bone marrow engraftment. They published histological evidence of hepatocytes identified by means of HepPar-1 antibody staining, and human–pattern heterochromatin staining characteristics, without evidence of fusion, as a rare event (<0.01% of cells) in the mouse liver from 4 weeks after infusion.
In peripheral blood of both mice and humans, and enhanced after treatment with G-CSF, Ratajczak et al. (2004) detected mRNA for AFP and CK19 (and also for muscle and neural markers). In mice, if mobilization with G-CSF was combined with inhibition of the CXCR4 receptor, there was a further increase in tissue-specific gene expression. More strikingly, Zhao et al. (2003) propagated a pluripotent stem cell from a subset of human peripheral blood monocytes by using only in vitro manipulation. CD14+ adherent cells, cultured with M-CSF, were then dispersed in single-cell cultures, and fibroblast-like cells formed colonies over 40–50 days in the presence of M-CSF, LIF and monocyte-conditioned medium. These colonies, CD14+, CD34+ and CD45+, expressed differentiation markers of lymphocytic, epithelial, neural and vascular endothelial lineages at low levels. Some 100 ng/ml of HGF caused cell rounding and ‘hepatocyte differentiation’, although evidence was limited to marked upregulation of the proportion of cells staining for albumin (8–75%), AFP (6–81%) and CK7 (biliary type).
Foetal liver as a source of liver progenitors
Rodent studies
In mice, the liver buds from the foregut endoderm at 7.5–8.5 days post-coitus, and is engrafted by haematopoietic progenitors at 9–10 days. The foetal liver is, thus, rich in haematopoietic cells as well as the site of proliferating and differentiating liver-cell precursors. Expression of AFP (at that stage regarded as an endodermal marker) occurs by 9 days and of albumin by 9.5 days. FGF1 and FGF2 are important in driving albumin expression. During the period of 9–12 days, hepatoblasts progressively express AFP, albumin, GGT, α-1-anti-trypsin and glutathione-S-transferase, and diverge along hepatocytic or biliary pathways thereafter. Oncostatin-M and glucocorticoids favour differentiation along the hepatic pathway (Kinoshita et al. 1999), with hepatoblasts committed to hepatocyte or biliary differentiation by 16 days, and continuing to divide. Cells with morphological similarity to hepatocytes at 13 days are capable of differentiating into biliary duct cells after transplantation and re-implantation (Shiojiri 1984). Strick-Marchand and Weiss (2002) derived bipotential liver cell lines from 14-day mouse embryos; initially, the colonies expressed hepatocyte-associated transcription factors but not albumin or other liver-specific products. The cells could be differentiated towards biliary phenotype by means of Matrigel, and towards hepatocyte phenotype by means of aggregation, while maintaining proliferation and removal of the environmental manipulation (re-plating or disaggregation) reversed the differentiation. Gene expression studies on hepatoblasts from foetal rat liver have documented the differences between foetal and adult liver and serial changes during gestation (Petkov et al. 2004).
Recent studies have demonstrated the proliferative capacity and differentiation of liver-cell progenitors from foetal rat liver after transplantation into adult livers. Cantz et al. (2003) tracked the maturation of 13.5-day hepatoblasts after transplantation into adult (mouse) liver, with the development of mature adult phenotype over 2–6 weeks. Proliferation of transplanted progenitors was encouraged, when normal hepatocyte replication was inhibited (Dabeva et al. 2000; Sandhu et al. 2001; Oertel et al. 2003) or when transplanted cells had a survival advantage (Cantz et al. 2003). Foetal hepatoblasts proliferated strongly even after transplantation into normal liver, contrasting with the lack of proliferation of transplanted adult hepatocytes under the same circumstances (Sandhu et al. 2001).
Hepatoblasts proliferate in vitro. Kubota and Reid (2000) digested rat foetal liver and established clonogenic bipotential hepatoblasts by culture on a feeder cell layer in a hormonally defined medium, the progenitors being MHC class-1– ICAM-1+. Identification of cell markers of hepatoblasts, in order to discriminate them from haematopoietic cell progenitors in foetal liver, is clearly a major interest. Such identification can be aided by strategies, such as depletion, by means of magnetic cell sorting to exclude OX43+ (macrophages, endothelial cells and red-cell precursors) and OX44+ (anti-CD53, myeloid and peripheral lymphoid cells) in rats (Fiegel et al. 2003b). Suzuki et al. (2000) studied CD45–TER119– (i.e. positive for leucocyte common antigen CD45 and erythroid precursors) cells from mouse embryos, initially sorting cells for α6 (CD49f) and β1 (CD29) integrin subunits, and CD117(c-kit). They identified c-kit–, CD49f+CD29+ cells as markedly enriched for the development of hepatic colony-forming units, particularly when cultured on extracellular matrix-coated plates in the presence of HGF (Zheng et al. 2000; Suzuki et al. 2003). These colonies arose within 5 days, reaching several hundred cells by 20 days. Both hepatocytic and cholangiocytic markers were expressed in the colonies. Similarly, Minguet et al. (2003) identified approximately 10% of liver cells in 11-day mouse embryos as c-kitlowCD45–TER119–; they were bimodal with respect to the expression of α6 integrin (CD49f), and expressed albumin, AFP, TTR, HGF, OSMR and c-met. They could be maintained in vitro by long-term proliferation, particularly in the presence of HGF or FGF-1, enhanced by oncostatin-M and demonstrated bipotentiality by maturation into functional hepatocytes (albumin+ and CK19–) and biliary cells (albumin– and CK19+). These cells could also repopulate cell-depleted hepatic organoids.
Subsequently, Suzuki et al. (2002) refined their selection strategy and used single-cell assays in order to demonstrate that CD45–TER119–c-kit–c-met+CD49f+/low cells from 13-day mouse livers (0.3% of liver cells) were 560-fold enriched for hepatic colony-forming units, compared to starting liver. Single cells cultured on laminin-coated plates in the presence of HGF/EGF and foetal liver-cell-conditioned medium gave rise to colonies of up to 100 cells at 5 days; on longer culture, cells expressing only both or only one of hepatocytic (albumin) and cholangiolar (CK19) characteristics emerged, while multipotential cells also persisted. Multipotentiality was demonstrated by the ability to differentiate in vitro into hepatocyte, biliary, gastric and intestinal epithelium and to reconstitute cells in liver, pancreas and intestine after transplantation into those organs. At this stage, in some colonies, c-kit, Thy-1 and CD34, markers associated with progenitor cells developing in damaged adult rodent liver, became detectable. Early differentiation into albumin expression was aided by HGF and associated with CEBP activation, and inhibition of CEBP stopped differentiation and restored proliferation (Suzuki et al. 2003). The later differentiation into mature hepatocytes expressing tryptophan-2,3-dioxygenase was enhanced by oncostatin. The significance of this study lies not only in the characterization of a multipotential (including hepatocyte) progenitor by using single-cell assays, but in the demonstration that some cell markers regarded as ‘early’ are phasically expressed.
An alternative cell marker for hepatoblasts was defined by Tanimizu et al. (2003) who identified a strong expression of DLK, a membrane protein with 6 EGF-like repeats in its extracellular domain, on mouse foetal liver cells between 10.5 and 16.5 days. Cells sorted with this marker expressed albumin and AFP (immunochemically and by means of RT-PCR), and CK19, and proliferated in response to HGF. Such cells from a GFP-transgenic mouse could differentiate into hepatocytes after transplantation into mouse spleen. Yasuchika et al. (2002) investigated cells from 13.5-day mouse livers that formed aggregates in suspension culture and demonstrated that these cells included hepatic progenitors, shown by albumin, AFP and CK19 expression by means of RT-PCR and immunochemistry. The progenitors were CD49f+Thy1–CD45–, but maturation into more terminal hepatocyte differentiation (tyrosine aminotransferase and tryptophan oxidase expression) was helped by means of co-culture with Thy1+ cells that also clustered within the aggregates (Hoppo et al. 2004).
Human and other primate studies
As in rodents, human foetal liver contains both haematopoietic and liver precursors. Cells co-expressing markers conventionally associated with haematopoiesis (CD34+, CD117+ and CD123+) are present, in fact in an increasing number, between 6 and 24 weeks (Gilles et al. 1997). Haruna et al. (1996) identified cells with a combination of hepatocytic and biliary lineage markers (HepPar1 and CK19) as early as 4-week gestation. These, subsequently, expressed CK14. Some cells then lost CK14 and 19 by 14–16 weeks, with persisting HepPar1 expression; others maintained CK19, lost HepPar1 and CK14, and expressed vimentin. The expression of cellular integrins also evolves as bipotential hepatoblasts mature along one or other lineage (Couvelard et al. 1998). Hepatoblasts express β1 and α1,5,6,9 integrins; maturation along hepatocyte lineage is associated with the maintenance of β1, α1, 5 and 9 and eventual loss of α6 (by 30 weeks). Biliary cells lose α1, upregulate α6 and maintain β1, but acquire β4, and α2 and α3. These authors comment on the relationship between these integrin expression patterns and local expression of extracellular matrix, notably laminin, in the ductal plate area with biliary cells strongly expressing the laminin ligand, α6β4.
Epithelial progenitor cells expressing markers of hepatocytes, biliary cells and oval cells, capable of maturing into hepatocytes in vitro, have been isolated from foetal human livers of 17–24 weeks of gestation (Malhi et al. 2002). The starting material contained approximately 50% of cells expressing both hepatocytic and biliary markers, and the precise origin of the cells giving rise to proliferating cultures is unclear. Co-expression of biliary and hepatocytic markers AFP, GGT, CK 8 and 19, and CD34 and plasminogen activator inhibitor 1 (together reminiscent of oval cells) persisted for several passages. Limiting dilution cloning studies were successful, when cells were grown on an irradiated feeder layer of autologous cells with a collagen overlay, and again cells were bipotential. Transplantation into either the peritoneum or via the spleen into the liver of immunodeficient mice demonstrated the adoption of hepatocyte morphology. Subsequently, such foetal cells have been immortalized by using telomerase reconstitution, maintaining their differentiative potential without senescence over >300 cell doublings (Wege et al. 2003). The hepatocytic functional profile included the expression of C/EBPα and HNF-4, glycogen storage and glucose-6-phosphatase activity by means of histochemistry, CYP expression by means of RT-PCR and quantification of urea production at >100 pg/cell/3 days (one would calculate 540 pg for cells from adult liver). In analogous experiments, Allain et al. (2002) used SV40 large T transfection in order to immortalize primate cynomolgus monkey foetal liver cells, and identified one clone (from 144 initially derived) that survived long term, proliferated, had appropriate morphology and expressed albumin+, AFP+CK8/18 and CK7/19.
Lazaro et al. (2003) disaggregated human foetal liver at various ages from 8 to 18 weeks of gestation and grew hepatocytes by attachment on collagen and culturing with EGF. Cells expressing AFP, albumin, transferrin and anti-trypsin were cultured with both CK18 and CK19 expression, interpreted as being hepatocytes at a late stage of differentiation still maintaining some biliary markers. Oncostatin increased proliferation and enhanced hepatocytic function (glycogen deposition, G6Pase activity, biliary canalicular formation, increased albumin and decreased AFP expression). Cultures proliferated over several passages over months; after time, ‘blast-like’ cells, which were CD34–, CD90 (Thy-1)–, OV-6+ and β-micro– were identified.
Adult liver as a source of progenitor cells
Rodent studies
Hepatic progenitor cells in adult liver were first recognized to proliferate when liver mass is diminished; the normal reparative response of the liver involving mature hepatocytes was inhibited (Solt et al. 1977). The proliferation of oval cells, adjacent to the portal tracts, has now been stimulated by a variety of protocols, such as the administration of 2-aceto-aminofluorine (2-AAF) followed by partial hepatectomy, 2-AAF plus carbon tetrachloride, 0.1%-diethoxycarbonyl-1,4-dihydrocollidine (DDC) (which affects hepatocyte apoptosis but not replication), choline deficiency, galactosamine and allyl alcohol. Oval-cell proliferation can be enhanced by HGF in vivo (Shiota et al. 2000), or (in a half choline-deficient diet model) by α-1-adrenoreceptor antagonists (Oben et al. 2003).
Various regimes lead to the proliferation of cell populations with some differences in properties. In general, oval cells are small (10 µm) with oval nuclei and a high nuclear–cytoplasmic ratio. They proliferate and radiate from the periportal region as ductular-like structures with poorly defined lumens. Rat oval cells express CK19, GGT, and AFP, Thy-1, c-kit, CD34, and also SCF, and can be recognized by a series of monoclonals (e.g. OV6, OC.2 and BD1). They are CD45– (Petersen et al. 1998). Some of these markers are shared by biliary epithelial cells (e.g. GGT, CK19 and CD34). Mouse oval cells induced by the DDC protocol express Sca-1 and CD34 and in this protocol are CD45+. They express AFP and the marker A6 (shared by biliary cells and oval cells) (Petersen et al. 2003).
Pulse-chase experiments have demonstrated the differentiation of oval cells into hepatocytes, as well as biliary cells, in vivo (Evarts et al. 1996). After transplantation, oval cells have been therapeutic in rescuing the FAH–/– mouse (Wang et al. 2003b), and have demonstrated their multipotentiality by apparent transdifferentiation into macro- and microglia after transplantation into neonatal mouse brain (although fusion was not rigorously excluded as a mechanism) (Deng et al. 2003). There is a dispute whether oval cells arise purely from a dormant precursor in adult liver. Study by Wang et al. on the FAH–/– mouse could find no evidence of a bone marrow origin of those oval cells which were successful in rescue. However, other work already mentioned – before the impact of cell fusion was appreciated – indicated a bone marrow origin of oval cells under some conditions (Petersen et al. 1999). Hatch et al. (2002) showed that mouse oval cells express CXCR4, and in severe (but not mild) injury hepatocytes express SDF-1-α, the only known ligand for CXCR4, offering a mechanism for the homing of bone marrow-derived oval cells to the liver (the potential role of SDF-1 in attracting bone marrow CD34+ cells has already been observed (Kollet et al. 2003).
Maturation of presumed oval cells into hepatocytes in vitro has been the subject of a report with an important sting in the tail. From the livers of rats treated 3 days before with a single dose of allyl alcohol, but without other manipulation, and no use of carcinogens, etc., colonies of proliferating epithelial cells grew within 10 days on feeder cell layers, and were subsequently passaged long term and clonal lines were obtained (Yin et al. 2002). Some clones co-expressed CK14, c-kit, albumin and AFP, with other lines/clones expressing some but not all of these. Most cells expressed all of CD34+, CD45+ and Thy-1+; one cell line also expressed three oval-cell markers OC.10, OV1 and OV6. On removing from feeder cells, loss of CD34 and Thy-1 and adoption of hepatocyte-specific phenotype (e.g. CYPIAII expression) were observed, enhanced by the addition of oncostatin-M. However, subsequent work identified that these markers were associated with cells likely derived from the mouse embryonic feeder layer rather than the rat (Zhang et al. 2003; Leffert & Sell 2004).
Identifying (and particularly culturing) liver-cell progenitors in and from normal liver is more problematic than identifying oval cells generated experimentally. Normal rat liver (including immediately postnatal rat liver) was demonstrated by Avital to contain a few β2micro-Thy-1+ cells analogous to those they reported in bone marrow, with strikingly increased numbers in cholestasis (Avital et al. 2001). Liver cells from a strain of GFP+ transgenic mice, in which GFP expression was marked in hepatocytes but not in non-parenchymal cells, could be sorted to yield GFP+AFP+ cells with high growth potential which were CD45–TER119– side scatter low, c-kit–, Thy-1–, integrin α6+β1+, and capable of differentiating into both hepatocytes and biliary epithelial cells (Fujikawa et al. 2003). Wang et al. (2003a) used a straightforward perfusion and centrifugation technique in order to yield progenitors which proliferated and matured into hepatocytes in vitro, expressing oval-cell antigens peaking at 2 weeks in culture, with a progressive increase in albumin expression. Recently, Azuma et al. (2003) designed a complex culture technique, involving shaking non-parenchymal cells from adult mouse liver under hypoxic conditions. Cell aggregates, constituted 95% by vascular endothelial cells and 5% by rapidly dividing small epithelial cells, were obtained. The proliferating epithelial cells demonstrated hepatocyte-like morphology (including biliary canaliculi) and expressed mature hepatocyte functions (by means of RTPCR) over several weeks after culture with dexamethasone and dimethylsulfoxide.
Mitaka and colleagues have particularly emphasized the proliferative potential of small hepatocytes, 1–2% of the adult hepatocyte population in rats (Mitaka et al. 1998; Mizuguchi et al. 2001) identifying small hepatocytes as potential originators of hepatocyte clones. Over 10 days in culture, these initially albumin+ transferrin+ CK8+ and CK18+ cells adopted the characteristics of terminal hepatocyte differentiation (tryptophan 2,3 dioxygenase and connexin-32 expression). These cells are reminiscent of those described by Gordon et al. (2000b) which were induced to proliferate in rats when most hepatocytes were inhibited from proliferation by the alkaloid retrorsine, and partial hepatectomy performed or hepatic necrosis initiated. Under these circumstances, rather than oval cells proliferating, there was a rapid expansion of small hepatocyte-like progenitor cells, arising in any area of the hepatic lobule. They expressed some oval-cell/bile-duct-associated antigens (OC.2 and OC.5), lacked CD34 and Thy-1 and expressed mRNA for AFP and some liver-associated transcription factors. They did not, however, proliferate in culture, although after transplantation they could insert into the hepatic cords (Gordon et al. 2002). They lacked CYP450 expression, and the authors hypothesized that this enabled them to avoid the toxic effect of the alkaloid (Gordon et al. 2000a).
Stem cells have been delineated by their ability to exclude the Hoechst dye 33342. The property reflects the possession of one of the ABC-binding cassette transporter pumps (Zhou et al. 2001). On fluorescent-activated cell sorting, these cells then appear as a separate ‘side-sorted’ population. Wulf et al. (2003) exploited side scattering in order to isolate putative stem cells from normal mouse liver, and directly injected the side population into mouse liver in which hepatocyte growth had been altered by the oval-cell DDC regime. The side population comprised approximately 1% of liver mononuclear cells, 75% were CD45+ (although CD45– cells had the greatest dye-effluxing potential) and a minority expressed various other antigens (CD34, c-kit, SCA-1 and Thy-1). They could give rise to mixed haematopoietic and hepatocytic colonies on culture with haematopoietic growth factors (IL3, IL6, erythropoietin and SCF) but not by EGF, HGF or TGF-α. This group then demonstrated (albeit by Y-chromosome marking) that these cells could contribute to hepatocyte and biliary populations after direct injection into the liver, or indirectly via reconstituting bone marrow in myeloablated animals. Side scattering, associated with the ATP-binding cassette transporter ABCG2/BCRP1, has also been identified as a property of rat oval cells induced by the 2AAF partial hepatectomy protocol (Shimano et al. 2003; Ros et al. 2003b).
Human studies
A number of classical histological studies on diseased liver – both chronic (De Vos & Desmet 1992) and acute (Tan et al. 2002; Craig et al. 2004) – indicate the presence of likely progenitors in man. These include small epithelial cells at the margin of regenerating nodules and in fibrous septa in chronic viral hepatitis (Xiao et al. 2003), cells in liver malignancies in chronic hepatitis with small hyperchromatic oval nuclei (Theise et al. 2003) and similar cells in areas of focal nodular hyperplasia (Roskams et al. 1996) and adenomas (Libbrecht et al. 2001). Theise's careful histological studies indicated the presence of a CK19+ cell population and c-kit+ cells in the Canal of Hering in normal liver in adults (Theise et al. 1999). In acute liver necrosis, such as after acetaminophen poisoning, the periportal area shows CK19+ cells taken as the correlate of proliferating oval cells. Scarce c-kit+CD34+ cells have been identified in both normal and diseased human liver, predominantly around bile ducts but occasionally integrated within bile duct epithelium (Crosby et al. 2001). In chronic liver disease (primary biliary cirrhosis and primary sclerosing cholangitis), OV6+ cells have been identified. Other studies have shown CK14 and c-kit+ ductule-like cells negative for CD34 and albumin in acute liver failure (Seki et al. 2003). Regenerating ductules in submassive hepatic necrosis stain strongly for a number of ATP-binding cassette transporter proteins (MDR1, MRP1 and MRP3), relevant, therefore, to a putative side-scattering population in man (Ros et al. 2003a).
Crosby et al. (2001) cultured CD34+ and/or c-kit+ cells from human liver (normal or diseased) for up to 8 days and identified biliary differentiation. Selden et al. (2003) identified (as a rare event and after some weeks quiescence) colonies proliferating from the non-parenchymal cell population isolated from a liver with subacute hepatic necrosis, which expressed a combination of hepatocytic and biliary markers as well as the embryonic stem-cell marker Oct-4, and secreted albumin and α-anti-trypsin into the medium over several months.
Embryonic stem cells
Embryonic stem (ES) cells, continuously growing stem cells first isolated from the inner cell mass of blastocysts, are capable of indefinite continuous culture and are multipotential for every cell type. Mouse ES cells can be maintained in their proliferative undifferentiated state on mouse embryonic fibroblasts or by culture with leukaemia inhibitory factor (LIF); removal from the feeder layer or of LIF leads to aggregation into embryonic bodies and differentiation into three interacting germ layers. Hamazaki et al. (2001) demonstrated that mouse ES cells cultured as hanging drops to induce EB formation and then plated on collagen began to express transthyretin, followed by AFP, α-1-anti-trypsin and albumin; further differentiation required the addition of growth factors, notably HGF, or oncostatin, dexamethasone and insulin/transferrin/selenium. Jones et al. (2002) confirmed these observations, although they documented a faster onset of differentiation, utilizing ES cells carrying a gene trap vector insertion into an ankyrin-repeat-containing gene, resulting in the expression of β-galactosidase when hepatocyte differentiation commences. Kuai et al. (2003) reported that β-nerve growth factor also promoted hepatocytic differentiation evidenced by means of immunohistochemistry and RT-PCR for hepatocyte-specific proteins. Yamada et al. (2002) identified indocyanin-green (ICG) uptake by some cells differentiated from mouse embryoid bodies (by using an ES cell line expressing GFP), and reported the expression of a panel of liver-specific mRNAs by the ICG-accumulating cells. These cells (subsequently tracked by GFP) were incorporated into hepatocyte-cords after transplantation into mice via the portal vein. Other transplantation experiments demonstrated that cells isolated from embryoid bodies 9 or more days after LIF removal expressed a panel of hepatocyte mRNAs, and could synthesize urea, and when these cells were implanted into a damaged liver they survived and expressed albumin (tracked by Y-chromosome markers) (Chinzei et al. 2002). Yamamoto et al. (2003) described an improved liver function when ES cells were transplanted into mice with CCl4-induced injury. Tabei et al. (2003) report the derivation of a hepatocyte cell line isolated from rat embryonic stem cells, which, grown with LIF by using a collagen sponge gel system, produced albumin and bilirubin.
ES-derived cells have a propensity to develop teratomas on implantation into animals. While this introduces a major caveat in respect of any naïve utilization of them clinically, this has been used in order to demonstrate their hepatocytic potential, with the teratoma tissue developing in the spleens of nude mice after injection of mouse ES cells showing some areas of hepatocyte cord formation (Choi et al. 2002).
ES cells express a wide variety of antigens, the function of many of which remains unclear. These include stage-specific embryonic antigens (SSEA) 1, 3 and 4 and keratan sulfate-associated antigens Tra-1-60 and Tra-1-81. SSEA-1 is a lactoseries oligosaccharide; SSEA-3 and SSEA-4 are globoseries glycolipids. Undifferentiated mouse ES cells express SSEA-1 and increase the expression of SSEA-3 and SSEA-4 when differentiating. However, the opposite is seen with human ES cells. Undifferentiated human ES cells express SSEA-3, SSEA-4, Tra-1-60, Tra-1-81 and Thy-1 and differentiation is characterized in the increased expression of SSEA-1 and downregulation of SSEA-3 and SSEA-4 (Thomson et al. 1998; Reubinoff et al. 2000; Draper et al. 2002; Henderson et al. 2002). ES cells also express the transcription factor Oct-4, a POU domain transcription factor, essential for the development of pluripotential cells in the mouse embryo, and which can either repress or activate target gene transcription. In ES cells, the expression of Oct-4 is downregulated with differentiation. Oct-4-deficient embryos develop to the blastocyst stage, but the inner cell mass cells are not pluripotent (Nichols et al. 1998; Reubinoff et al. 2000). We have already mentioned the expression of Oct-4 in the presumed progenitor colonies we cultured from adult human explant liver (Selden et al. 2003). It is a reasonable hypothesis that progenitor cells in adult tissues may re-express ES-associated proteins.
Carpenter et al. (2003) reviewed 26 human ES cell lines and showed that all retain normal karyotype, had similar expression patterns of SSEA-3, SSEA-4, Tra-1-60 and Tra-1-81 as well as markers associated with pluripotency, e.g. Oct-4. The cells express high levels of telomerase activity and hTERT expression and appear to have indefinite growth potential. Furthermore, Carpenter et al. observed CD133 expression in all lines tested, although the marker was only expressed by 50–60% of cells, compared to 70–100% expression of SSEA-4, Tra-1-60 and Tra-1-81.
Human ES cell lines are gradually being developed, which though still requiring feeder layers or feeder cell-conditioned medium for culture, are ‘laboratory-friendly’ as they are trypsin–stable and antibiotic-resistant (Cowan et al. 2004). Human feeder layers are being developed (Richards et al. 2002), as is the use of extracellular matrix (Xu et al. 2001). As in the mouse, ES-derived cell lines that have formed embryoid bodies can also differentiate into derivatives of all three germ layers and demonstrate receptors for a variety of growth factors. Schuldiner et al. (2000) showed that HGF and nerve growth factor encouraged differentiation into the three embryonic germ layers. However, of eight growth factors inducing differentiation (including also EGF, TGF-β1 and βFGF), none induced the differentiation into one specific cell type. Rambhatla et al. (2003) used sodium butyrate in order to induce hepatocyte differentiation in human ES cells, shown by morphology, albumin and α-1-anti-trypsin and CK8 and CK18 expression and inducible CYP450 expression and glycogen accumulation. Levenberg et al. (2003) used biodegradable scaffolds in order to induce tissue-like structures after seeding with embryonic stem cells or cells from embryoid bodies, and found hepatocytic differentiation under the influence of activin-A and IGF. Ishizaka et al. (2002) were able to produce hepatocyte-like cells from human ES cells after transfection with HNF-3β gene. The cells were cultured with FGF-2 in a three-dimensional culture system; they produced albumin and triacylglycerol and could synthesize urea.
Cells from other organs
Cells within the pancreas, notably the exocrine acinar cells, have the capacity after manoeuvres, such as copper depletion and repletion in vivo, to undergo metaplasia to hepatocyte phenotype. In organ cultures of pancreatic buds, these exocrine cells were shown to undergo true transdifferentiation rather than activation of a progenitor stem-cell population, as this could occur without cell division (Shen et al. 2000). For transdifferentiation into hepatocyte phenotype, the expression of CEBPβ was shown to be a critical master-switch. A rat pancreatic cell line, the parent cell line of which had been shown to express neuron-like properties after treatment with activin-A (Ohnishi et al. 1995), can be differentiated in vitro into hepatocyte phenotype, expressing albumin and functional cytochrome P450s, after treatment with dexamethasone (Shen et al. 2000; Marek et al. 2003). Finally, in the FAH–/– model, wild-type pancreatic cells lead to the rescue of tyrosinaemic recipients with the appearance of hepatocytes carrying donor genetic material (Wang et al. 2001). As this effect was not enhanced with ductal cell-enriched transplants, and not seen with purified ducts, the authors argued that a progenitor cell, rather than mature ductal cells, be responsible for the rescue. The paper derives from before the time that the significance of fusion was appreciated.
Conclusions
As in all exciting biological science, this survey of the origins and potential of hepatocyte precursors in animals and in man reflects ‘unfinished business’. Over the last decade, the simple concept that regeneration and repair in adult liver depends exclusively on the division of mature hepatocytes has been significantly revised. It is important to observe that the most striking deviations from this dogma have been demonstrated in extremely adverse circumstances or in highly contrived experimental conditions. The hepatocytic potential of bone marrow cells – whether true stem-cell plasticity or manifestation of cell fusion with pre-existing hepatocytes – is most strikingly demonstrated in the unique FAH–/– model; in man, highly contrived conditions, such as bone marrow or liver grafting, are required. It remains an open question whether a far more subtle, low level of transdifferentiation or fusion takes place during a normal life span when the liver responds to far less dramatic stresses, or whether such events contribute to normal cell turnover in the liver. Furthermore, the generation of oval cells in man is best documented in clinical conditions when it has been an unsuccessful survival strategy – i.e. in explant livers removed when liver transplantation has been performed for acute liver failure. Nonetheless, the potential of manipulating hepatocyte precursors, to aid in strategies for tissue repair and gene therapy, to provide cellular tools both for biological liver support or tools for the assessment of drug metabolism and toxicity, remains a hugely attractive challenge.
Acknowledgments
Joanna Laurson is the holder of a PhD Studentship supported by The Liver Group Charity.
References
- Alison M, Poulsom R, Jeffery R, et al. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- Allain JE, Dagher I, Mahieu-Caputo D, et al. Immortalization of a primate bipotent epithelial liver stem cell. Proc. Natl. Acad. Sci. USA. 2002;99:3639–3644. doi: 10.1073/pnas.062038599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital I, Inderbitzin D, Aoki T, et al. Isolation, characterization, and transplantation of bone marrow-derived hepatocyte stem cells. Biochem. Biophys. Res. Commun. 2001;288:156–164. doi: 10.1006/bbrc.2001.5712. [DOI] [PubMed] [Google Scholar]
- Azuma H, Hirose T, Fujii H, et al. Enrichment of hepatic progenitor cells from adult mouse liver. Hepatology. 2003;37:1385–1394. doi: 10.1053/jhep.2003.50210. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Finegold M, Goodell MA. Hematopoietic myelomonocytic cells are the major source of hepatocyte fusion partners. J. Clin. Invest. 2004;113(9):1266–1270. doi: 10.1172/JCI21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantz T, Zuckerman DM, Burda MR, et al. Quantitative gene expression analysis reveals transition of fetal liver progenitor cells to mature hepatocytes after transplantation in uPA/RAG-2 mice. Am. J. Pathol. 2003;162:37–45. doi: 10.1016/S0002-9440(10)63796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MK, Rosler E, Rao MS. Characterization and differentiation of human embryonic stem cells. Cloning Stem Cells. 2003;5:79–88. doi: 10.1089/153623003321512193. [DOI] [PubMed] [Google Scholar]
- Chinzei R, Tanaka Y, Shimizu-Saito K, et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36:22–29. doi: 10.1053/jhep.2002.34136. [DOI] [PubMed] [Google Scholar]
- Choi D, Oh HJ, Chang UJ, et al. In vivo differentiation of mouse embryonic stem cells into hepatocytes. Cell Transplant. 2002;11:359–368. [PubMed] [Google Scholar]
- Couvelard A, Bringuier AF, Dauge MC, et al. Expression of integrins during liver organogenesis in humans. Hepatology. 1998;27:839–847. doi: 10.1002/hep.510270328. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Klimanskaya I, Mcmahon J, et al. Derivation of embryonic stem-cell lines from human blastocysts. N. Engl. J. Med. 2004;350:1353–1356. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- Craig CE, Quaglia A, Selden C, Lowdell M, Hodgson H, Dhillon AP. The histopathology of regeneration in massive hepatic necrosis. Semin. Liver Dis. 2004;24:49–64. doi: 10.1055/s-2004-823101. [DOI] [PubMed] [Google Scholar]
- Crosby HA, Kelly DA, Strain AJ. Human hepatic stem-like cells isolated using c-kit or CD34 can differentiate into biliary epithelium. Gastroenterology. 2001;120:534–544. doi: 10.1053/gast.2001.21175. [DOI] [PubMed] [Google Scholar]
- Dabeva MD, Petkov PM, Sandhu J, et al. Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am. J. Pathol. 2000;156:2017–2031. doi: 10.1016/S0002-9440(10)65074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danet GH, Luongo JL, Butler G, et al. C1qRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc. Natl. Acad. Sci. USA. 2002;99:10441–10445. doi: 10.1073/pnas.162104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos R, Desmet V. Ultrastructural characteristics of novel epithelial cell types identified in human pathologic liver specimens with chronic ductular reaction. Am. J. Pathol. 1992;140:1441–1450. [PMC free article] [PubMed] [Google Scholar]
- Deng J, Steindler DA, Laywell ED, Petersen BE. Neural trans-differentiation potential of hepatic oval cells in the neonatal mouse brain. Exp. Neurol. 2003;182:373–382. doi: 10.1016/s0014-4886(03)00058-x. [DOI] [PubMed] [Google Scholar]
- Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- Draper JS, Pigott C, Thomson JA, Andrews PW. Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J. Anat. 2002;200:249–258. doi: 10.1046/j.1469-7580.2002.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts RP, Hu Z, Omori N, Omori M, Marsden ER, Thorgeirsson SS. Precursor-product relationship between oval cells and hepatocytes: comparison between tritiated thymidine and bromodeoxyuridine as tracers. Carcinogenesis. 1996;17:2143–2151. doi: 10.1093/carcin/17.10.2143. [DOI] [PubMed] [Google Scholar]
- Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Fiegel HC, Lioznov MV, Cortes-Dericks L, et al. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003a;21:98–104. doi: 10.1634/stemcells.21-1-98. [DOI] [PubMed] [Google Scholar]
- Fiegel HC, Park JJ, Lioznov MV, et al. Characterization of cell types during rat liver development. Hepatology. 2003b;37:148–154. doi: 10.1053/jhep.2003.50007. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Hirose T, Fujii H, et al. Purification of adult hepatic progenitor cells using green fluorescent protein (GFP)-transgenic mice and fluorescence-activated cell sorting. J. Hepatol. 2003;39:162–170. doi: 10.1016/s0168-8278(03)00237-x. [DOI] [PubMed] [Google Scholar]
- Gilles JM, Divon MY, Bentolila E, et al. Immunophenotypic characterization of human fetal liver hematopoietic stem cells during the mid-trimester of gestation. Am. J. Obstet. Gynecol. 1997;177:619–625. doi: 10.1016/s0002-9378(97)70155-8. [DOI] [PubMed] [Google Scholar]
- Gordon GJ, Butz GM, Grisham JW, Coleman WB. Isolation, short-term culture, and transplantation of small hepatocyte-like progenitor cells from retrorsine-exposed rats. Transplantation. 2002;73:1236–1243. doi: 10.1097/00007890-200204270-00008. [DOI] [PubMed] [Google Scholar]
- Gordon GJ, Coleman WB, Grisham JW. Temporal analysis of hepatocyte differentiation by small hepatocyte-like progenitor cells during liver regeneration in retrorsine-exposed rats. Am. J. Pathol. 2000a;157:771–786. doi: 10.1016/S0002-9440(10)64591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GJ, Coleman WB, Hixson DC, Grisham JW. Liver regeneration in rats with retrorsine-induced hepatocellular injury proceeds through a novel cellular response. Am. J. Pathol. 2000b;156:607–619. doi: 10.1016/S0002-9440(10)64765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham JW, Hartroft WS. Morphologic identification by electron microscopy of “oval” cells in experimental hepatic degeneration. Lab. Invest. 1961;10:317–332. [PubMed] [Google Scholar]
- Hamazaki T, Iiboshi Y, Oka M, et al. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett. 2001;497:15–19. doi: 10.1016/s0014-5793(01)02423-1. [DOI] [PubMed] [Google Scholar]
- Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. Identification of bipotential progenitor cells in human liver development. Hepatology. 1996;23:476–481. doi: 10.1002/hep.510230312. [DOI] [PubMed] [Google Scholar]
- Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- Henderson JK, Draper JS, Baillie HS, et al. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells. 2002;20:329–337. doi: 10.1634/stemcells.20-4-329. [DOI] [PubMed] [Google Scholar]
- Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- Hoppo T, Fujii H, Hirose T, et al. Thy1-positive mesenchymal cells promote the maturation of CD49f-positive hepatic progenitor cells in the mouse fetal liver. Hepatology. 2004;39:1362–1370. doi: 10.1002/hep.20180. [DOI] [PubMed] [Google Scholar]
- Hove WR, van Hoek B, Bajema IM, Ringers J, van Krieken JH, Lagaaij EL. Extensive chimerism in liver transplants: vascular endothelium, bile duct epithelium, and hepatocytes. Liver Transpl. 2003;9:552–556. doi: 10.1053/jlts.2003.50116. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Drake CJ, Yang S, et al. Transplanted human cord blood cells give rise to hepatocytes in engrafted mice. Ann. N. Y. Acad. Sci. 2003;996:174–185. doi: 10.1111/j.1749-6632.2003.tb03245.x. [DOI] [PubMed] [Google Scholar]
- Ishizaka S, Shiroi A, Kanda S, et al. Development of hepatocytes from ES cells after transfection with the HNF-3beta gene. FASEB J. 2002;16:1444–1446. doi: 10.1096/fj.01-0806fje. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Jones EA, Tosh D, Wilson DI, Lindsay S, Forrester LM. Hepatic differentiation of murine embryonic stem cells. Exp. Cell Res. 2002;272:15–22. doi: 10.1006/excr.2001.5396. [DOI] [PubMed] [Google Scholar]
- Kakinuma S, Tanaka Y, Chinzei R, et al. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21:217–227. doi: 10.1634/stemcells.21-2-217. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Sekiguchi T, Xu MJ, et al. Hepatic differentiation induced by oncostatin M attenuates fetal liver hematopoiesis. Proc. Natl. Acad. Sci. USA. 1999;96:7265–7270. doi: 10.1073/pnas.96.13.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollet O, Shivtiel S, Chen YQ, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J. Clin. Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbling M, Katz RL, Khanna A, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N. Engl. J. Med. 2002;346(10):738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- Kuai XL, Cong XQ, Li XL, Xiao SD. Generation of hepatocytes from cultured mouse embryonic stem cells. Liver Transpl. 2003;9:1094–1099. doi: 10.1053/jlts.2003.50207. [DOI] [PubMed] [Google Scholar]
- Kubota H, Reid LM. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc. Natl. Acad. Sci. USA. 2000;97:12132–12137. doi: 10.1073/pnas.97.22.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol. Dis. 2004;32:52–57. doi: 10.1016/j.bcmd.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Lazaro CA, Croager EJ, Mitchell C, et al. Establishment, characterization, and long-term maintenance of cultures of human fetal hepatocytes. Hepatology. 2003;38:1095–1106. doi: 10.1053/jhep.2003.50448. [DOI] [PubMed] [Google Scholar]
- Leffert HL, Sell S. Unexpected artifacts raise new caution in stem cell culture research. Hepatology. 2004;39:258. doi: 10.1002/hep.20013. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc. Natl. Acad. Sci. USA. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbrecht L, De Vos R, Cassiman D, Desmet V, Aerts R, Roskams T. Hepatic progenitor cells in hepatocellular adenomas. Am. J. Surg. Pathol. 2001;25:1388–1396. doi: 10.1097/00000478-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Malhi H, Irani AN, Gagandeep S, Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J. Cell Sci. 2002;115:2679–2688. doi: 10.1242/jcs.115.13.2679. [DOI] [PubMed] [Google Scholar]
- Marek CJ, Cameron GA, Elrick LJ, Hawksworth GM, Wright MC. Generation of hepatocytes expressing functional cytochromes P450 from a pancreatic progenitor cell line in vitro. Biochem. J. 2003;370:763–769. doi: 10.1042/BJ20021545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguet S, Cortegano I, Gonzalo P, et al. A population of c-Kit(low)(CD45/TER119)- hepatic cell progenitors of 11-day postcoitus mouse embryo liver reconstitutes cell-depleted liver organoids. J. Clin. Invest. 2003;112:1152–1163. doi: 10.1172/JCI17409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitaka T, Mizuguchi T, Sato F, Mochizuki C, Mochizuki Y. Growth and maturation of small hepatocytes. J. Gastroenterol. Hepatol. 1998;13:S70–S77. doi: 10.1111/jgh.1998.13.s1.70. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Akiyama I, Sakaguchi M, et al. Improved conditions to induce hepatocytes from rat bone marrow cells in culture. Biochem. Biophys. Res. Commun. 2002;298:24–30. doi: 10.1016/s0006-291x(02)02340-9. [DOI] [PubMed] [Google Scholar]
- Mizuguchi T, Hui T, Palm K, et al. Enhanced proliferation and differentiation of rat hepatocytes cultured with bone marrow stromal cells. J. Cell Physiol. 2001;189:106–119. doi: 10.1002/jcp.1136. [DOI] [PubMed] [Google Scholar]
- Newsome PN, Johannessen I, Boyle S, et al. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891–1900. doi: 10.1016/s0016-5085(03)00401-3. [DOI] [PubMed] [Google Scholar]
- Ng IO, Chan KL, Shek WH, et al. High frequency of chimerism in transplanted livers. Hepatology. 2003;38:989–998. doi: 10.1053/jhep.2003.50395. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Oben JA, Roskams T, Yang S, et al. Sympathetic nervous system inhibition increases hepatic progenitors and reduces liver injury. Hepatology. 2003;38:664–673. doi: 10.1053/jhep.2003.50371. [DOI] [PubMed] [Google Scholar]
- Oertel M, Rosencrantz R, Chen YQ, et al. Repopulation of rat liver by fetal hepatoblasts and adult hepatocytes transduced ex vivo with lentiviral vectors. Hepatology. 2003;37:994–1005. doi: 10.1053/jhep.2003.50183. [DOI] [PubMed] [Google Scholar]
- Oh SH, Miyazaki M, Kouchi H, et al. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem. Biophys. Res. Commun. 2000;279:500–504. doi: 10.1006/bbrc.2000.3985. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Ohgushi N, Tanaka S, et al. Conversion of amylase-secreting rat pancreatic AR42J cells to neuronlike cells by activin A. J. Clin. Invest. 1995;95:2304–2314. doi: 10.1172/JCI117922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumoto K, Saito T, Hattori E, et al. Differentiation of bone marrow cells into cells that express liver-specific genes in vitro: implication of the Notch signals in differentiation. Biochem. Biophys. Res. Commun. 2003;304:691–695. doi: 10.1016/s0006-291x(03)00637-5. [DOI] [PubMed] [Google Scholar]
- Overturf K, Al Dhalimy M, Tanguay R, et al. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I [published erratum appears in Nat Genet 1996 April; 12 (4): 458] Nat. Genet. 1996;12:266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433–445. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Grossbard B, Hatch H, Pi L, Deng J, Scott EW. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology. 2003;37:632–640. doi: 10.1053/jhep.2003.50104. [DOI] [PubMed] [Google Scholar]
- Petkov PM, Zavadil J, Goetz D, et al. Gene expression pattern in hepatic stem/progenitor cells during rat fetal development using complementary DNA microarrays. Hepatology. 2004;39:617–627. doi: 10.1002/hep.20088. [DOI] [PubMed] [Google Scholar]
- Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1–11. doi: 10.3727/000000003783985179. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Kucia M, Reca R, Majka M, Janowska-Wieczorek A, Ratajczak J. Stem cell plasticity revisited: CXCR4-positive cells expressing mRNA for early muscle, liver and neural cells ‘hide out’ in the bone marrow. Leukemia. 2004;18:29–40. doi: 10.1038/sj.leu.2403184. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- Ros JE, Libbrecht L, Geuken M, Jansen PL, Roskams TA. High expression of MDR1, MRP1, and MRP3 in the hepatic progenitor cell compartment and hepatocytes in severe human liver disease. J. Pathol. 2003a;200:553–560. doi: 10.1002/path.1379. [DOI] [PubMed] [Google Scholar]
- Ros JE, Roskams TA, Geuken M, et al. ATP binding cassette transporter gene expression in rat liver progenitor cells. Gut. 2003b;52:1060–1067. doi: 10.1136/gut.52.7.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskams T, De Vos R, Desmet V. ‘Undifferentiated progenitor cells’ in focal nodular hyperplasia of the liver. Histopathology. 1996;28:291–299. doi: 10.1046/j.1365-2559.1996.d01-438.x. [DOI] [PubMed] [Google Scholar]
- Sandhu JS, Petkov PM, Dabeva MD, Shafritz DA. Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am. J. Pathol. 2001;159:1323–1334. doi: 10.1016/S0002-9440(10)62519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J. Clin. Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S, Kitada T, Sakaguchi H, et al. Expression of progenitor cell markers in livers with fulminant massive necrosis. Hepatol. Res. 2003;25:149–157. doi: 10.1016/s1386-6346(02)00205-x. [DOI] [PubMed] [Google Scholar]
- Selden C, Chalmers SA, Jones C, et al. Epithelial colonies cultured from human explanted liver in subacute hepatic failure exhibit hepatocyte, biliary epithelial, and stem cell phenotypic markers. Stem Cells. 2003;21:624–631. doi: 10.1634/stemcells.21-6-624. [DOI] [PubMed] [Google Scholar]
- Shen CN, Slack JM, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat. Cell Biol. 2000;2:879–887. doi: 10.1038/35046522. [DOI] [PubMed] [Google Scholar]
- Shimano K, Satake M, Okaya A, et al. Hepatic oval cells have the side population phenotype defined by expression of ATP-binding cassette transporter ABCG2/BCRP1. Am. J. Pathol. 2003;163:3–9. doi: 10.1016/S0002-9440(10)63624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojiri N. The origin of intrahepatic bile duct cells in the mouse. J. Embryol. Exp. Morphol. 1984;79:25–39. [PubMed] [Google Scholar]
- Shiojiri N, Lemire JM, Fausto N. Cell lineages and oval cell progenitors in rat liver development. Cancer Res. 1991;51:2611–2620. [PubMed] [Google Scholar]
- Shiota G, Kunisada T, Oyama K, et al. In vivo transfer of hepatocyte growth factor gene accelerates proliferation of hepatic oval cells in a 2-acetylaminofluorene/partial hepatectomy model in rats. FEBS Lett. 2000;470:325–330. doi: 10.1016/s0014-5793(00)01337-5. [DOI] [PubMed] [Google Scholar]
- Smith MA, Court EL, Smith JG. Stem cell factor: laboratory and clinical aspects. Blood Rev. 2001;15:191–197. doi: 10.1054/blre.2001.0167. [DOI] [PubMed] [Google Scholar]
- Solt DB, Medline A, Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am. J. Pathol. 1977;88:595–618. [PMC free article] [PubMed] [Google Scholar]
- Strick-Marchand H, Weiss MC. Inducible differentiation and morphogenesis of bipotential liver cell lines from wild-type mouse embryos. Hepatology. 2002;36:794–804. doi: 10.1053/jhep.2002.36123. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Iwama A, Miyashita H, Nakauchi H, Taniguchi H. Role for growth factors and extracellular matrix in controlling differentiation of prospectively isolated hepatic stem cells. Development. 2003;130:2513–2524. doi: 10.1242/dev.00459. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Zheng Y, Kondo R, et al. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology. 2000;32:1230–1239. doi: 10.1053/jhep.2000.20349. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Zheng Yw YW, Kaneko S, et al. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J. Cell Biol. 2002;156:173–184. doi: 10.1083/jcb.200108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabei I, Hashimoto H, Ishiwata I, et al. New approach for the establishment of an hepatocyte cell line derived from rat early embryonic stem cells. Hum. Cell. 2003;16:39–46. doi: 10.1111/j.1749-0774.2003.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Tan J, Hytiroglou P, Wieczorek R, et al. Immunohistochemical evidence for hepatic progenitor cells in liver diseases. Liver. 2002;22:365–373. doi: 10.1034/j.1600-0676.2002.01622.x. [DOI] [PubMed] [Google Scholar]
- Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J. Cell Sci. 2003;116:1775–1786. doi: 10.1242/jcs.00388. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Terada R, Yamamoto K, Hakoda T, et al. Stromal cell-derived factor-1 from biliary epithelial cells recruits CXCR4-positive cells: implications for inflammatory liver diseases. Lab. Invest. 2003;83:665–672. doi: 10.1097/01.lab.0000067498.89585.06. [DOI] [PubMed] [Google Scholar]
- Theise ND, Badve S, Saxena R, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000a;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- Theise ND, Badve S, Saxena R, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000b;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- Theise ND, Nimmakayalu M, Gardner R, et al. Liver from bone marrow in humans. Hepatology. 2000c;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- Theise ND, Saxena R, Portmann BC, et al. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- Theise ND, Yao JL, Harada K, et al. Hepatic ‘stem cell’ malignancies in adults: four cases. Histopathology. 2003;43:263–271. doi: 10.1046/j.1365-2559.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- Wang X, Al Dhalimy M, Lagasse E, Finegold M, Grompe M. Liver repopulation and correction of metabolic liver disease by transplanted adult mouse pancreatic cells. Am. J. Pathol. 2001;158:571–579. doi: 10.1016/S0002-9440(10)63999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Clark JB, Rhee GS, Fair JH, Reid LM, Gerber DA. Proliferation and hepatic differentiation of adult-derived progenitor cells. Cells Tissues Organs. 2003a;173:193–203. doi: 10.1159/000070375. [DOI] [PubMed] [Google Scholar]
- Wang X, Foster M, Al Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc. Natl. Acad. Sci. USA. 2003b;100:11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ge S, Mcnamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003c;101:4201–4208. doi: 10.1182/blood-2002-05-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Montini E, Al Dhalimy M, Lagasse E, Finegold M, Grompe M. Kinetics of liver repopulation after bone marrow transplantation. Am. J. Pathol. 2002;161:565–574. doi: 10.1016/S0002-9440(10)64212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003d;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Wege H, Le HT, Chui MS, et al. Telomerase reconstitution immortalizes human fetal hepatocytes without disrupting their differentiation potential. Gastroenterology. 2003;124:432–444. doi: 10.1053/gast.2003.50064. [DOI] [PubMed] [Google Scholar]
- Willenbring H, Bailey AS, Foster M, et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat. Med. 2004;10(7):744–748. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- Wulf GG, Luo KL, Jackson KA, Brenner MK, Goodell MA. Cells of the hepatic side population contribute to liver regeneration and can be replenished with bone marrow stem cells. Haematologica. 2003;88:368–378. [PubMed] [Google Scholar]
- Xiao JC, Ruck P, Adam A, Wang TX, Kaiserling E. Small epithelial cells in human liver cirrhosis exhibit features of hepatic stem-like cells: immunohistochemical, electron microscopic and immunoelectron microscopic findings. Histopathology. 2003;42:141–149. doi: 10.1046/j.1365-2559.2003.01544.x. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Yamada T, Yoshikawa M, Kanda S, et al. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20:146–154. doi: 10.1634/stemcells.20-2-146. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Quinn G, Asari A, et al. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology. 2003;37:983–993. doi: 10.1053/jhep.2003.50202. [DOI] [PubMed] [Google Scholar]
- Yasuchika K, Hirose T, Fujii H, et al. Establishment of a highly efficient gene transfer system for mouse fetal hepatic progenitor cells. Hepatology. 2002;36:1488–1497. doi: 10.1053/jhep.2002.36951. [DOI] [PubMed] [Google Scholar]
- Yin L, Sun M, Ilic Z, Leffert HL, Sell S. Derivation, characterization, and phenotypic variation of hepatic progenitor cell lines isolated from adult rats. Hepatology. 2002;35:315–324. doi: 10.1053/jhep.2002.31355. [DOI] [PubMed] [Google Scholar]
- Zhang M, Sell S, Leffert HL. Hepatic progenitor cel lines from allyl alcohol-treated adult rats are derived from gamma-irradiated mouse STO cells. Stem Cells. 2003;21:449–458. doi: 10.1634/stemcells.21-4-449. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2003;100:2426–2431. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YW, Taniguchi H, Suzuki A, et al. Effects of four extracellular matrices associated with growth factors on clonal culture and proliferation of murine fetal hepatocytes. Transplant Proc. 2000;32:2498–2499. doi: 10.1016/s0041-1345(00)01765-6. [DOI] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]