Abstract
A toxic dose of the nitric oxide (NO) donor S-nitrosoglutathione (GSNO; 1 mM) promoted apoptotic cell death of RAW 264.7 macrophages, which was attenuated by cellular preactivation with a nontoxic dose of GSNO (200 μM) or with lipopolysaccharide, interferon-γ, and NG-monomethyl-l-arginine (LPS/IFN-γ/NMMA) for 15 h. Protection from apoptosis was achieved by expression of cyclooxygenase-2 (Cox-2). Here we investigated the underlying mechanisms leading to Cox-2 expression. LPS/IFN-γ/NMMA prestimulation activated nuclear factor (NF)-κB and promoted Cox-2 expression. Cox-2 induction by low-dose GSNO demanded activation of both NF-κB and activator protein-1 (AP-1). NF-κB supershift analysis implied an active p50/p65 heterodimer, and a luciferase reporter construct, containing four copies of the NF-κB site derived from the murine Cox-2 promoter, confirmed NF-κB activation after NO addition. An NF-κB decoy approach abrogated not only Cox-2 expression after low-dose NO or after LPS/IFN-γ/NMMA but also inducible protection. The importance of AP-1 for Cox-2 expression and cell protection by low-level NO was substantiated by using the extracellular signal-regulated kinase inhibitor PD98059, blocking NO-elicited Cox-2 expression, but leaving the cytokine signal unaltered. Transient transfection of a dominant-negative c-Jun mutant further attenuated Cox-2 expression by low-level NO. Whereas cytokine-mediated Cox-2 induction relies on NF-κB activation, a low-level NO–elicited Cox-2 response required activation of both NF-κB and AP-1.
INTRODUCTION
Nitric oxide (NO) exerts a number of activities that encompass endothelium-dependent relaxation, neurotransmission, and cell-mediated immune responses. Its scope of action touches on the functions of many organ systems and cellular responses (Ignarro, 1990; Nathan, 1992). Signal transmission and target interactions are achieved via redox and additive chemistry (Stamler, 1994) that is the basis for communication in which NO-sensitive targets serve both sensory and regulatory elements in mediating functional responses (Nüssler and Billiar, 1993).
The oxidation of l-arginine proceeds to the formation of citrulline in addition to NO. Constitutive and inducible NO synthase (NOS) isoforms are characterized as low- versus high-NO output systems. Besides NOS activation and/or induction, NO donors such as S-nitrosoglutathione (GSNO) are valuable tools to study NO-transducing pathways because they liberate NO upon decomposition (Sandau and Brüne, 1996).
During macrophage activation with lipopolysaccharide and interferon-γ (LPS/IFN-γ), up-regulation of NOS-II and formation of large amounts of NO are considered naturally occurring, nonspecific cellular immune responses directed against invading pathogens or tumor cells (Lander, 1997). Activation of macrophages is a key component of the complex pathophysiology of inflammation in which cells serve as an effector and generator system of chemokines, cytokines, and free radicals. Apparently, macrophages not only produce radicals such as NO but are affected as well in a self-destructing feedback loop, which leads to NO-mediated apoptotic cell death (Albina et al., 1993; Sarih et al., 1993). Macrophage apoptosis is accompanied by chromatin condensation, DNA fragmentation, p53 accumulation, and caspase activation (Messmer and Brüne, 1996; Messmer et al., 1996) and is blocked by NOS inhibitors such as NG-monomethyl-l-arginine (NMMA) that underscores a death-promoting role of NO.
Numerous reports focused on the interaction between NO and the prostaglandin pathway (Salvemini et al., 1993; Vane et al., 1994; Landino et al., 1996). It has been reported that NO stimulated prostaglandin biosynthesis in vivo, in perfused organs, and in macrophages (Salvemini et al., 1996). Mechanistically, NO may cause direct activation of cyclooxygenase-2 (Cox-2), although conflicting reports regarding the ability of NO to stimulate the purified protein exist (Tetsuka et al., 1996a). In previous experiments it became apparent that low, nonapoptotic concentrations of NO or the addition of LPS/IFN-γ/NMMA promoted Cox-2 up-regulation in RAW 264.7 macrophages in close association with blocking high-dose GSNO–mediated apoptotic alterations (von Knethen and Brüne, 1997). The requirement of Cox-2 was demonstrated in cells that overexpress an active Cox-2 enzyme, was substantiated in Cox-2 antisense-expressing clones, and was ensured further by pharmacological intervention with the Cox-2 inhibitor NS398. Mechanistically, the antiapoptotic action of Cox-2 is transmitted via the formation of cAMP (von Knethen et al., 1998). However, the mechanism responsible for NO-mediated Cox-2 expression remained elusive. There are indications that activation of the nuclear factor (NF)-κB is involved in Cox-2 expression because there is an NF-κB consensus site in the upstream promoter region (−600/+1) of the murine Cox-2 gene (Yamamoto et al., 1995). In addition activator protein-1 (AP-1) has been linked to Cox-2 expression (Xie and Herschman, 1995), although a complete AP-1 site in the Cox-2 promoter is missing. This discrepancy is rationalized by the observation that AP-1 in this case activates a rather homologous cAMP response element site (Xie et al., 1994). AP-1 is induced, among other stimuli, by NO (Pilz et al., 1995; Robinson and Cobb, 1997; Sciorati et al., 1997). Mechanistically, activation of AP-1 is achieved via the mitogen-activated protein kinase (MAPK) pathway that is diverted into the extracellular-regulated (ERK), the c-jun amino-terminal kinase/stress–activated protein kinase, and the p38 pathway (Robinson and Cobb, 1997). Detailed mechanisms of AP-1 activation by these phosphorylation cascades remain elusive, but obviously all three MAPK cascades are involved (Karin et al., 1997).
The available information encouraged us to explore the molecular mechanism of Cox-2 induction by NO. We established NO-mediated activation of the transcription factor NF-κB and AP-1 by NO-releasing compounds such as GSNO in close association with Cox-2 induction. Our results suggest activation of NF-κB and AP-1 by NO as a macrophage rescue system, which in turn abrogates apoptotic signaling mechanisms.
MATERIALS AND METHODS
Materials
Diphenylamine, pyrrolidine dithiocarbamate (PDTC), and LPS (Escherichia coli serotype 0127:B8) were purchased from Sigma (Deisenhofen, Germany). The Cox-2 antibody was bought from Transduction Laboratories (Lexington, KY); the p50- and p65-supershift antibodies as well as the IκB-α antibody were obtained from Santa Cruz Biotechnology (Heidelberg, Germany). NMMA was from Alexis (Grünberg, Germany). Recombinant murine IFN-γ and the β-tubulin antibody were provided by Boehringer Mannheim (Mannheim, Germany). RPMI 1640, cell culture supplements, and fetal calf serum were ordered from Biochrom (Berlin, Germany). The luciferase assay kit was obtained from Promega (Mannheim, Germany), and the β-galactosidase detection kit was from Tropix (Mannheim, Germany). Oligonucleotides (± fluorescein labeling) were provided by Eurogentec (Seraing, Belgium). PD98059 and SB203580 were from Biomol (Hamburg, Germany). All other chemicals were of the highest grade of purity commercially available.
Cell Culture
The mouse monocyte/macrophage cell line RAW 264.7 was maintained in RPMI 1640 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal calf serum (complete RPMI). All experiments were performed using complete RPMI. GSNO, LPS, NMMA, and PDTC were dissolved in water and added as indicated. PD98059 and SB203580 were dissolved in DMSO.
Cell Survival
The number of alive RAW 264.7 macrophages, after treatment with different agents, was determined by the trypan blue dye exclusion assay.
Nuclear Protein Extraction
Preparation of crude nuclear extract was basically as described (Schoonbroodt et al., 1997). Briefly, after cell activation for the times indicated, 4 × 106 RAW 264.7 macrophages were washed in 1 ml of ice-cold PBS, centrifuged at 1000 × g for 5 min, resuspended in 400 μl of ice-cold hypotonic buffer (10 mM HEPES/KOH, 2 mM MgCl2, 0.1 mM EDTA, 10 mM KCl, 1 mM DTT, 0.5 mM PMSF, pH 7.9), left on ice for 10 min, vortexed, and centrifuged at 15,000 × g for 30 s. Pelleted nuclei were gently resuspended in 50 μl of ice-cold saline buffer (50 mM HEPES/KOH, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 10% glycerol, 1 mM DTT, 0.5 mM PMSF, pH 7.9), left on ice for 20 min, vortexed, and centrifuged at 15,000 × g for 5 min at 4°C. Aliquots of the supernatant that contained nuclear proteins were frozen in liquid nitrogen and stored at −70°C. Protein was determined using a Bio-Rad II Kit (Richmond, CA).
Electrophoretic Mobility Shift Assays (EMSAs)
An established EMSA method, with slight modifications, was used (Camandola et al., 1996). Nuclear protein (5 μg) was incubated for 20 min at room temperature with 20 μg of bovine serum albumin, 2 μg of poly(dI-dC) from Pharmacia (Uppsala, Sweden), 2 μl of buffer D (20 mM HEPES/KOH, 20% glycerol, 100 mM KCl, 0.5 mM EDTA, 0.25% Nonidet P-40, 2 mM DTT, 0.5 mM PMSF, pH 7.9), 4 μl of buffer F (20% Ficoll-400, 100 mM HEPES/KOH, 300 mM KCl, 10 mM DTT, 0.5 mM PMSF, pH 7.9), and 20,000 cpm of a [32P]-labeled oligonucleotide in a final volume of 20 μl. Supershift antibodies (2 μg) were added as indicated. DNA–protein complexes were resolved at 180 V for 4 h in a taurine-buffered, native 6% polyacrylamide gel (4% for supershifts), dried, and visualized (with autoradiography using a Fuji x-ray film; Siemens, Erlangen, Germany). Oligonucleotide probes were labeled by a filling reaction using the Klenow fragment (Boehringer Mannheim). One picomole of oligonucleotide was labeled with 50 μCi of [α-32P]-dCTP (3000 Ci/mmol, Amersham, Braunschweig, Germany) in cold nucleotides (dATP, dTTP, and dGTP from GIBCO, Eggenstein, Germany), purified on a CHROMA SPIN-10 column (Clontech, Heidelberg, Germany), and stored at −20°C until use. The following oligonucleotide sequences were used: the NF-κB site (−401/−393, bold letters) from the murine Cox-2 promoter (Yamamoto et al., 1995),
5′-GAG GTG AGG GGA TTC CCT TAG-3′; 3′-AC TCC CCT AAG GGA ATC AATC-5′; a mutated NF-κB site,
5′-GAG GTG AGG GCC TTC CCT TAG-3′; 3′-AC TCC CGG AAG GGA ATC AATC-5′; the AP-1 site from the human collagenase gene (Angel et al., 1987),
5′-AGC TAA AGC ATG AGT CAG ACA GCC T-3′; 3′-TT TCG TAC TCA GTC TGT CGG ATC GA-5′ (this oligonucleotide was kindly provided by Dr. Peter Angel, Deutsches Krebsforschungs-zentrum, Heidelberg, Germany).
Immunoblot Analysis
Cell lysis was achieved with lysis buffer (50 mM Tris, 5 mM EDTA, 150 mM NaCl, 0.5% Nonidet-40, 1 mM PMSF, pH 8.0) and sonication (Branson sonifier, Plainview, NY; 20 s, duty cycle 100%, output control 60%). After centrifugation (14,000 × g, 5 min), protein was determined. Proteins (100 μg) were resolved on 10% polyacrylamide gels and blotted onto nitrocellulose. Equal loading was confirmed by Ponceau S staining. Filters were incubated overnight at 4°C with the Cox-2 antibody (1:250, Dianova, Hamburg, Germany), the p53 antiserum (hybridoma supernatant, clone PAb122, 1:5, kindly provided by Dr. H. Stahl, Homburg/Saar, Germany), or the IκB-α antibody (1:500). Proteins were detected by a horseradish peroxidase–conjugated polyclonal antibody (1:10,000) with the ECL method (Amersham, Braunschweig, Germany).
Quantitation of DNA Fragmentation
DNA fragmentation was measured with the diphenylamine assay as reported elsewhere (McConkey et al., 1989). Briefly, after incubations, cells were scraped off the culture plates, resuspended in 250 μl of 10 mM Tris, 1 mM EDTA, pH 8.0 (TE buffer), and incubated with an additional 250 μl of lysis buffer (5 mM Tris, 20 mM EDTA, pH 8.0, 0.5% Triton X-100) for 30 min at 4°C. After lysis, intact chromatin (pellet) was separated from DNA fragments (supernatant) by centrifugation for 15 min at 13,000 × g. Pellets were resuspended in 500 μl of TE buffer, and samples were precipitated overnight by adding 500 μl of 10% trichloroacetic acid at 4°C. DNA was pelleted by centrifugation (4000 × g, 10 min), and the supernatant was removed. After addition of 300 μl of 5% trichloroacetic acid, samples were boiled for 15 min. DNA contents were quantitated using the diphenylamine reagent (Burton, 1956). The percentage of fragmented DNA was calculated as the ratio of the DNA content in the supernatant to the amount in the pellet.
Transient Transfection of a Dominant-negative c-jun Mutant into RAW 264.7 Macrophages
Targeting transcription factor activation by transient transfection of upstream signaling components requires high transfection efficiency and/or selection of cells expressing the mutant protein. One day before transfection, cells were seeded at a density of 1 × 106 cells/ml into 10-cm noncell culture plates. RAW 264.7 macrophages were transiently transfected with 15 μg of the expression vector (TAM-67), which contains the sequence of a dominant-negative c-jun mutant (kindly provided by Dr. E. Gulbins, Tübingen, Germany) (Brown et al., 1993, 1994). For positive selection, 5 μg of the vector pMACS4, designed to express a truncated human CD4 molecule, were cotransfected. Transfection was achieved using a Pro Gentor II electroporator (Hoeffer, San Francisco, CA). Cells (3 × 106) were resuspended in 400 μl of complete medium, transferred to a cuvette, and pulsed (260 V, 1080 μF, 26 ms). Transfected cells were pooled and seeded in 10 ml of complete medium into a 10-cm noncell culture Petri dish. Cells cultured overnight for 15 h were harvested, and CD4-positive clones were enriched using a Mini-MACS system (Miltenyi Biotech GmbH, Bergisch-Gladbach, Germany) according to the manufacturer’s instructions. Briefly, transfected cells were harvested in PBS supplemented with 5 mM EDTA. Cells (107) were resuspended in 320 μl of PBS, 0.5% BSA, and 5 mM EDTA (PBE) and 80 μl of MACSelect 4 Microbeads to achieve magnetic labeling of transfected cells. After 15 min on ice, the volume was adjusted to 2 ml with PBE. Cells were applied to a positive selection column (MS+) that was placed in the magnetic field of a Mini-MACS separator. Unbound cells were washed out (2 ml of PBE), the column was removed from the separator, and positive cells were collected, pooled, and seeded. In control examinations, 15 h has been determined as the most effective period for allowing the CD4 surface marker expression in RAW 264.7 macrophages.
Luciferase Plasmid Expression Containing the NF-κB Site of the Mouse Cox-2 Promoter
NF-κB reporter constructs were cloned into the pGL3-basic plasmid (Promega) and contained four copies of the NF-κB element taken from the murine Cox-2 promoter (NF-κB–sense) or its mutated form (NF-κB–mut) (see EMSA), inserted upstream of a TK minimal promoter, driving a luciferase gene. Corresponding sequences were verified by DNA sequencing. A further NF-κB reporter construct containing four copies of the NF-κB site of the porcine E-selectin promoter (NF-κB–selectin) was kindly provided by Dr. F. Bach (Sandoz Center for Immunobiology, Boston, MA) (Bach et al., 1997). RAW 264.7 macrophages were transiently transfected using the DEAE-dextran method as described previously (Arakawa et al., 1996). Cell selection or electroporation was unnecessary because the synthesis of two, generally not in macrophages, expressed proteins was analyzed. Briefly, 1 d before transfection, cells were seeded in suspension at a density of 1 × 106 cells/ml. Cells (1 × 107) were harvested, washed twice with PBS, and incubated for 3 h at 37°C in 1 ml of RPMI 1640 supplemented with 50 mM Tris-HCl, pH 7.3, 400 μg of DEAE-dextran, 20 μg of luciferase reporter construct (NF-κB–sense, NF-κB–mut, NF-κB–selectin), and 5 μg of cytomegalovirus-β-galactosidase plasmid as an internal control. Cells were washed twice with PBS to discard the DNA/DEAE-dextran mixture and were seeded at a density of 1 × 106 cells/ml and cultured for 24 h. Afterward cells were stimulated for 12 h with 200 μM GSNO or with LPS/IFN-γ/NMMA. Cell extracts were assayed for luciferase and β-galactosidase activity. For calculations, luciferase activity was normalized for β-galactosidase using the following formula: luciferase activity/β-galactosidase activity.
Decoy Approach
RAW 264.7 cells were exposed to an NF-κB or a mutated NF-κB oligonucleotide. One day before exposition, cells were seeded at a density of 1 × 106 cells/well into six-well plates. Oligonucleotides (3 μM) were added 24 h before cell stimulation. After the medium was changed, cell stimulation was performed as indicated. Oligonucleotide sequences were identical to those used for EMSA.
Statistical Analysis
Each experiment was performed at least three times, and statistical analysis was performed using the two-tailed Student’s t test. Otherwise representative data are shown.
RESULTS
Cox-2 Expression and p53 Accumulation Are Inversely Related in RAW 264.7 Macrophages
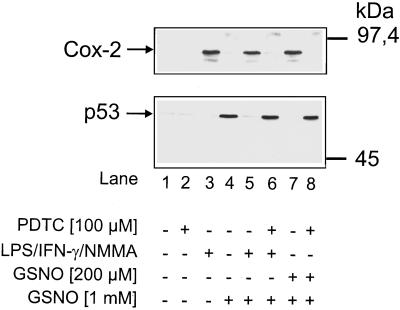
Within a 4-h incubation period, NO-releasing compounds such as GSNO (1 mM) evoked a massive tumor suppressor p53 accumulation in RAW 264.7 macrophages (Figure 1, lane 4) but no induction of Cox-2. As revealed by Western blot analysis, p53 expression and Cox-2 were absent in controls (Figure 1, lane 1). In corroboration with earlier experiments, macrophages exposed to a combination of LPS/IFN-γ/NMMA (Figure 1, lane 3) responded with Cox-2 expression. For these experiments, the NOS inhibitor NMMA was necessary to prevent endogenous NO generation that is known to initiate apoptotic cell death in macrophages (Sarih et al., 1993). Inhibition of macrophage inducible NOS by NMMA and inhibition of the NO formation have been shown before (Messmer et al., 1995). When RAW 264.7 cells were prestimulated with LPS/IFN-γ/NMMA for 15 h, the ability to up-regulate p53 during a subsequent 4-h incubation period with 1 mM GSNO was suppressed (Figure 1, lane 5 compared with lane 4). Further experiments established that the addition of 100 μM PDTC during the preincubation period inhibited Cox-2 expression and restored a functional, GSNO-mediated p53 response (Figure 1, lane 6 compared with lane 5). PDTC by itself neither affected 1 mM GSNO-evoked p53 accumulation (our unpublished results) nor promoted a Cox-2 or p53 response (Figure 1, lane 2). Moreover, cell viability as judged by trypan blue exclusion was unaltered (our unpublished results).
Figure 1.
Inverse expression of Cox-2 and p53 in RAW 264.7 macrophages. Western blot analysis of Cox-2 and p53 in RAW 264.7 macrophages is shown. Cells were stimulated with a combination of LPS/IFN-γ/NMMA (LPS, 10 μg/ml; IFN-γ, 100 U/ml; NMMA, 1 mM) or with 200 μM GSNO in the presence or absence of 100 μM PDTC for 15 h. After the addition of 1 mM GSNO or vehicle, incubations continued for an additional 4 h (total incubation period of 19 h). GSNO-mediated p53 accumulation (lane 4) was measured after 4 h. Details are described in MATERIALS AND METHODS. The blot is representative of three similar experiments.
Prestimulation of macrophages with a low and thus nontoxic dose of GSNO (200 μM) for 19 h promoted Cox-2 expression. At the same time, low-dose NO prestimulation abrogated the p53 response elicited by 1 mM GSNO (Figure 1, lane 7). In turn, the addition of PDTC reversed cellular responses. PDTC effectively restored p53 accumulation after the addition of 1 mM GSNO and inhibited protein expression of Cox-2 (Figure 1, lane 8).
These results point to efficient Cox-2 expression in response to LPS/IFN-γ/NMMA or low-dose GSNO in RAW 264.7 macrophages, whereas PDTC diminished these alterations. Moreover, Cox-2 expression and p53 accumulation are inversely related in RAW 264.7 macrophages.
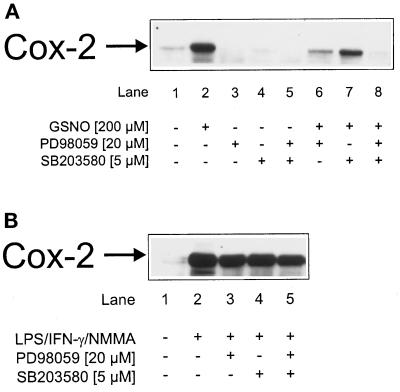
The involvement of AP-1 in NO-mediated Cox-2 induction was analyzed by the use of specific MAPK inhibitors. Cells were incubated with low-dose GSNO (200 μM), the ERK kinase inhibitor PD98059, the p38-specific inhibitor SB203580, and a combination thereof or remained as controls. After low-dose NO prestimulation, only PD98059 inhibited Cox-2 expression (Figure 2A, lane 6). Attenuating the p38 pathway by SB203580 affected NO-induced Cox-2 expression marginally (Figure 2A, lane 7). A combination of PD98059 and SB203580 abrogated Cox-2 expression completely (Figure 2A, lane 8). In control experiments the omission of GSNO (Figure 2A, lanes 1 and 3–5) revealed no Cox-2 induction.
Figure 2.
Attenuated Cox-2 induction by MAPK inhibitors. Western blot analysis of Cox-2 in RAW 264.7 macrophages is shown. Cells were stimulated with (A) 200 μM GSNO or (B) a combination of LPS/IFN-γ/NMMA (LPS, 10 μg/ml; IFN-γ, 100 U/ml; NMMA, 1 mM) in the presence or absence of 20 μM PD98059 or 5 μM SB203580 for 15 h. Details are described in MATERIALS AND METHODS. The blot is representative of three similar experiments.
Preactivation of cells with LPS/IFN-γ/NMMA promoted Cox-2 expression (Figure 2B, lane 2) in agreement with a previous report (von Knethen and Brüne, 1997). However, the addition of PD98059 or SB203580 left Cox-2 expression unaltered (Figure 2B, lanes 3–5). Evidently AP-1 activation is dispensable for Cox-2 induction after LPS/IFN-γ/NMMA stimulation but is needed after low-dose NO (200 μM GSNO) prestimulation.
NF-κB Activation by LPS/IFN-γ/NMMA and by GSNO
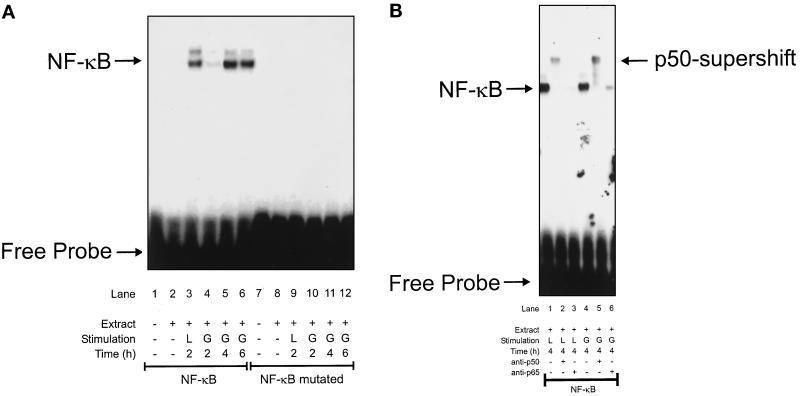
To study NF-κB activation in RAW 264.7 macrophages, we exposed cells to cytokines and NO-generating compounds followed by EMSA. In a first set of experiments, cells were exposed for 2–6 h to LPS/IFN-γ/NMMA or to low-dose GSNO (Figure 3A). LPS/IFN-γ/NMMA activated NF-κB in a time-dependent manner. Active NF-κB became detectable 2 h after agonist challenge (Figure 3A, lane 3), revealed stronger activation at 4 and 6 h (our unpublished results), but was absent in unstimulated controls (Figure 3A, lane 2). We then investigated NF-κB activation by a nontoxic dose of GSNO (200 μM) (Figure 3A). GSNO led to a weak NF-κB activation 2 h after cell stimulation, promoted stronger activation at 4 h, and demonstrated a slightly decreased NF-κB activity after 6 h (Figure 3A, lanes 4–6).
Figure 3.
Activation of NF-κB in RAW 264.7 macrophages. Activation of NF-κB was analyzed by EMSA using a specific (NF-κB) or mutated (NF-κB mutated) oligonucleotide derived from the mouse Cox-2 promoter as described in MATERIALS AND METHODS. (A) Macrophages were stimulated with a combination designated L (LPS, 10 μg/ml; IFN-γ, 100 U/ml; NMMA, 1 mM) or G (GSNO, 200 μM) for the times indicated. EMSA was performed with sense or mutated oligonucleotides. For controls the addition of nuclear extracts (extract) or cell stimulation was omitted. (B) Supershift analysis of the active NF-κB complex was performed as described in MATERIALS AND METHODS. Macrophages were stimulated with L or G for 4 h. For supershift analysis a p50 antibody (lanes 2 and 5) or a p65 antibody (lanes 3 and 6) was included. NF-κB activation without antibody addition (lanes 1 and 4) is shown. Data are representative of three similar experiments.
To demonstrate specific NF-κB binding to the corresponding oligonucleotide derived from the mouse Cox-2 promoter, we performed EMSA experiments using a mutated oligonucleotide in parallel. With the mutated oligonucleotide, no NF-κB complex binding in response to LPS/IFN-γ/NMMA or to GSNO became apparent (Figure 3A, lanes 7–12). This ensures specificity of the oligonucleotide from the mouse Cox-2 promoter and NF-κB binding.
To show activation of NF-κB further, we used the NF-κB–blocking agent PDTC (our unpublished results). Addition of 100 μM PDTC in combination with LPS/IFN-γ/NMMA or with GSNO completely suppressed NF-κB–oligonucleotide complex formation.
The activation of NF-κB by exogenously added NO was concentration dependent. GSNO concentrations higher than 200 μM led to minor or no NF-κB activation (our unpublished results). This is rationalized by the ability of NO to interfere with DNA binding at higher concentrations (Park et al., 1997) or to promote enhanced IκB synthesis (Umansky et al., 1998).
The identity of the activated NF-κB–oligonucleotide complex in RAW 264.7 macrophages was determined further by supershift analysis (Figure 3B). Binding of activated NF-κB to antisera against the p50 and p65 subunits of NF-κB was performed. Addition of the p50 antiserum during NF-κB–oligonucleotide binding shifted the complex to a higher molecular weight (Figure 3B, lanes 2 and 5). In accordance with previous studies (Newton et al., 1997), the p65 antiserum led to decreased complex formation (Figure 3B, lanes 3 and 6) that implicated inhibition of the DNA-binding ability of the p65 NF-κB subunit after antibody addition. Conclusively, NF-κB activation in response to LPS/IFN-γ/NMMA or to GSNO resembles the p50/p65 heterodimer NF-κB complex.
NF-κB Activation Is Accompanied by IκB-α Degradation
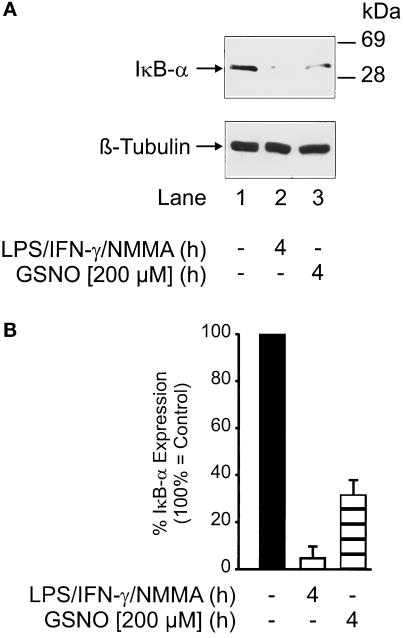
Activation of NF-κB required dissociation and degradation of the inhibitory protein IκB-α. Western blot analysis revealed significantly decreased IκB-α expression at 4 h after LPS/IFN-γ/NMMA (Figure 4A, Atop, lane 2) or GSNO (Figure 4A, top, lane 3) stimulation. Equal loading was confirmed by β-tubulin staining (Figure 4A, bottom). As a result of multiple examinations, it seems that LPS/IFN-γ/NMMA reduced IκB-α expression to values <10% compared with controls, whereas GSNO attenuated expression to 30% compared with controls (Figure 4B). IκB-α degradation further supported NF-κB activation.
Figure 4.
IκB-α degradation after RAW 264.7 macrophage activation. (A) Top, IκB-α Western blot analysis in RAW 264.7 macrophages after stimulation with a combination of LPS/IFN-γ/NMMA (LPS, 10 μg/ml; IFN-γ, 100 U/ml; NMMA, 1 mM) or with 200 μM GSNO for 4 h. Bottom, β-tubulin Western blot analysis performed to ensure equal sample loading. Blots are representative of three similar experiments. (B) Densitometric analysis of multiple examinations as described in A. Details are outlined in MATERIALS AND METHODS. Data are means ± SD of the individual experiments.
The Murine Cox-2 NF-κB Site Is Activated by Low-Dose NO as well as by LPS/IFN-γ/NMMA
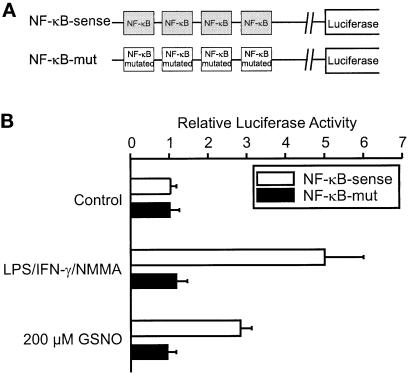
To determine whether the Cox-2 NF-κB site is involved functionally in Cox-2 promoter activation after low-dose NO addition, we transiently transfected luciferase reporter plasmids into RAW 264.7 macrophages. Luciferase activity of two reporter plasmids, containing four copies of either the NF-κB site derived from the murine Cox-2 promoter or its mutated counterpart (Figure 5A), was low in the absence of agonists (Figure 5B). LPS/IFN-γ/NMMA as well as low-dose NO led to increased luciferase activity in cells containing the NF-κB–sense construct. Luciferase activity was absent in cells transfected with the mutated plasmid (NF-κB–mut) (Figure 5B).
Figure 5.
NF-κB–driven luciferase activity. (A) Schematic presentation of the constructs. (B) RAW 264.7 macrophages cotransfected with NF-κB luciferase plasmid constructs and with a plasmid encoding β-galactosidase. We analyzed for luciferase and β-galactosidase expression, after both activities were normalized as described in MATERIALS AND METHODS. Cells were stimulated with a combination of LPS/IFN-γ/NMMA (LPS, 10 μg/ml; IFN-γ, 100 U/ml; NMMA, 1 mM), GSNO (200 μM), or vehicle (control). Data are means ± SD of three individual experiments.
LPS/IFN-γ/NMMA–mediated induction was fivefold compared with a threefold induction with 200 μM GSNO. It was impossible to study luciferase activity in response to high NO concentrations (1 mM GSNO) because of the immediate resultant cytotoxicity. We observed induction of luciferase expression further using an NF-κB reporter taken from the porcine E-selectin promoter after LPS/IFN-γ/NMMA or 200 μM GSNO stimulation (our unpublished results). Our results suggest that binding of NF-κB to its cognition site transmitted LPS/IFN-γ/NMMA– and to a lower extent low-dose NO–mediated Cox-2 promoter activation.
AP-1 Activation by LPS/IFN-γ/NMMA and by GSNO
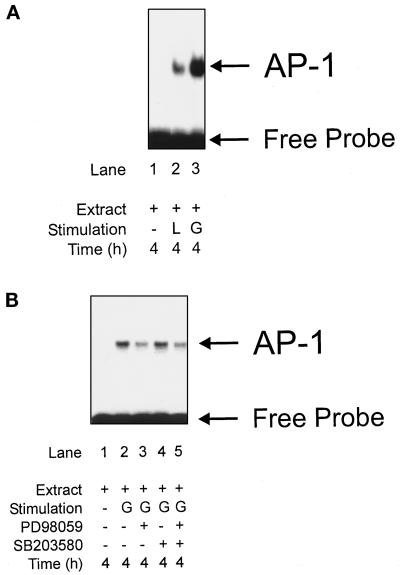
To study activation of AP-1, we stimulated RAW 264.7 macrophages with LPS/IFN-γ/NMMA or with GSNO. Nuclear extracts were analyzed in EMSA using oligonucleotides that contained the TRE site from the human collagenase promoter (Angel et al., 1987). In corroboration with previous studies, GSNO activated AP-1 in a dose-dependent manner (our unpublished results). Stimulation with LPS/IFN-γ/NMMA for 4 h achieved weak AP-1 activation (Figure 6A, lane 2) compared with the GSNO (200 μM)-elicited response (Figure 6A, lane 3).
Figure 6.
Activation of AP-1 by low-dose NO or by LPS/IFN-γ/NMMA. Activation of AP-1 was analyzed by EMSA using a specific AP-1 oligonucleotide, derived from the human collagenase promoter as described in MATERIALS AND METHODS. (A) Macrophages were stimulated with a combination designated L (LPS, 10 μg/ml; IFN-γ, 100 U/ml; NMMA, 1 mM), or G (GSNO, 200 μM) for 4 h. EMSA was performed using the AP-1 oligonucleotides. For a control, cell stimulation was omitted. (B) Macrophages were stimulated with G (GSNO, 200 μM) for 4 h in the presence or absence of PD98059 or SB203580. EMSA was performed using the AP-1 oligonucleotides. For a control, cell stimulation was omitted. Data are representative of three similar experiments.
To study activation of AP-1 further, we used the MAPK pathway inhibitors PD98059 and SB203580 (Figure 6B). Addition of 20 μM PD98059 in combination with 200 μM GSNO attenuated AP-1 activation (Figure 6B, lane 3), whereas 5 μM SB203580 left the NO response unaltered (Figure 6B, lane 4). A combination of PD98059 and SB203580 revealed no synergism (Figure 6B, lane 5), although both inhibitors efficiently blocked the specified kinase pathway (our unpublished results). We conclude that AP-1 induction by low-dose NO is mediated by the ERK pathway and therefore is blocked by PD98059.
NF-κB Decoy Oligonucleotides Attenuated Low-Dose GSNO– and LPS/IFN-γ/NMMA–mediated Cox-2 Induction, whereas Targeting of c-jun Abolished Induction of Cox-2 after Low-Dose NO Only
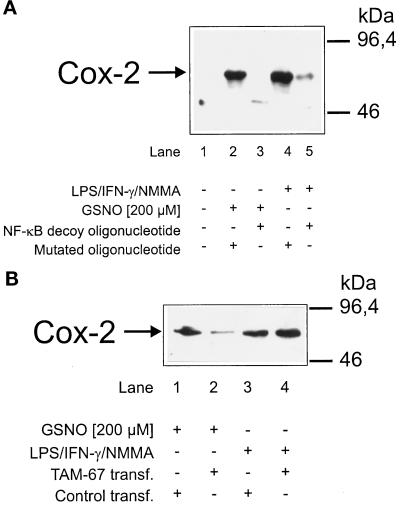
To demonstrate a transcriptionally active NF-κB in promoting Cox-2 expression, we used an NF-κB decoy approach. For these experiments RAW macrophages were incubated with oligonucleotides that have been specified for the EMSA. Oligonucleotide uptake was ∼50% (von Knethen et al., 1998), and fluorescence microscopy revealed oligonucleotide incorporation into the nucleus. LPS/IFN-γ/NMMA (Figure 7A, lane 4) or 200 μM GSNO (Figure 7A, lane 2) mediated Cox-2 induction in cells transfected with oligonucleotides containing a mutated murine Cox-2 NF-κB site.
Figure 7.
NF-κB decoy oligonucleotides as well as the targeting of c-jun attenuated Cox-2 induction after low-dose NO. (A) RAW 264.7 macrophages (1 × 106 cells) were exposed to oligonucleotides (3 μM) containing the murine Cox-2 promoter NF-κB site (decoy) or a mutated NF-κB site for 24 h. After the medium was changed, cells were stimulated with a combination of LPS/IFN-γ/NMMA (LPS, 10 μg/ml; IFN-γ, 100 U/ml; NMMA, 1 mM) or with GSNO (200 μM) for 15 h. Cox-2 expression was analyzed by Western Blot analysis. (B) Cells were transiently transfected and enriched as described in MATERIALS AND METHODS with an expression vector containing the sequence of a dominant-negative c-jun mutant to target c-jun (TAM-67 transf.). Cells were stimulated for 15 h with 200 μM GSNO or with a combination of LPS/IFN-γ/NMMA (LPS, 10 μg/ml; IFN-γ, 100 U/ml; NMMA, 1 mM). Cox-2 Western blot analysis was as described in MATERIALS AND METHODS. Blots are representative of three similar experiments.
In contrast, cells containing oligonucleotides with the murine Cox-2 NF-κB site attenuated Cox-2 expression after LPS/IFN-γ/NMMA (Figure 7A, lane 5) or 200 μM GSNO (Figure 7A, lane 3) stimulation. Conclusively, a transcriptionally active NF-κB is obligatory for Cox-2 induction by low-dose NO or LPS/cytokines.
Inhibition of c-jun by transient transfection of a dominant-negative c-jun mutant (TAM-67) suppressed Cox-2 after 200 μM GSNO (Figure 7B, lane 2), compared with results in cells that had been transfected with an unrelated control plasmid (Figure 7B, lane 1). In contrast, Cox-2 induction by LPS/IFN-γ/NMMA remained unaffected in TAM-67–transfected cells (Figure 7B, lane 4 compared with lane 3). These data substantiate an active role of AP-1 in Cox-2 induction after the addition of NO, which is attenuated by targeting c-jun, a basic component of the AP-1 complex.
PDTC, NF-κB Decoy Oligonucleotides, and a Dominant-negative c-jun Mutant Reversed Inducible Protection and Restored Apoptotic Cell Death
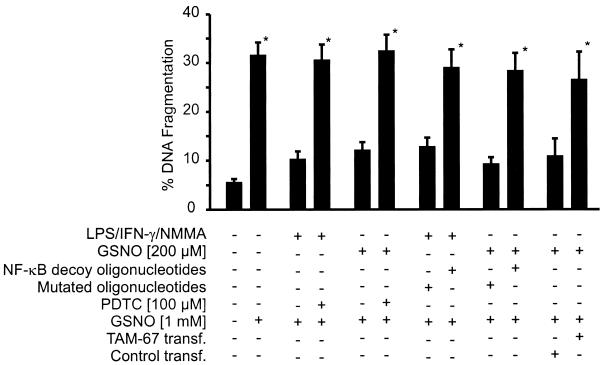
GSNO (1 mM) induced apoptotic DNA fragmentation in RAW 264.7 macrophages. Controls exhibited ∼5% fragmentation, whereas values rose to >30% after an 8-h incubation period with GSNO. As seen in these and previous experiments, prestimulation with LPS/IFN-γ/NMMA for 15 h suppressed GSNO-mediated DNA fragmentation when the NO donor was added for a subsequent 8-h incubation period (von Knethen and Brüne, 1997). When prestimulation was performed in the presence of NF-κB decoy oligonucleotides or PDTC, GSNO-initiated apoptotic DNA cleavage was restored (Figure 8). Control experiments performed with the mutated NF-κB oligonucleotide left protection unaltered. In close analogy, macrophage prestimulation with a nontoxic dose of GSNO (200 μM) for 15 h primarily blocked the subsequent apoptotic response of a high, toxic dose of the NO-releasing compound (1 mM GSNO). NF-κB decoy oligonucleotides or PDTC abrogated low-dose GSNO–mediated protection and allowed high-dose GSNO–evoked apoptosis. GSNO effects were unrelated to glutathione, and the apoptotic-initiating activity of GSNO was mimicked by other NO-releasing agents such as spermine NO. In addition low-level NO–initiated protection was achieved by other NO donors such as diethylenetriamine-nitric oxide (our unpublished results). NF-κB decoy oligonucleotides or PDTC was nontoxic to macrophages as judged by the trypan blue exclusion assay and did not promote DNA fragmentation (our unpublished results). Similarly, targeting of c-jun by transient transfection of a dominant-negative c-jun mutant abrogated protection, mediated by low-dose NO prestimulation (Figure 8).
Figure 8.
NF-κB decoy oligonucleotides, PDTC, and transfection of TAM-67 attenuated inducible protection in RAW 264.7 macrophages. DNA fragmentation was assessed as described in MATERIALS AND METHODS. Cells were prestimulated with a combination of LPS/IFN-γ/NMMA (LPS, 10 μg/ml; IFN-γ, 100 U/ml; NMMA, 1 mM), GSNO (200 μM), or vehicle in the absence or presence of NF-κB decoy and mutated oligonucleotides (3 μM) or of 100 μM PDTC or after transient transfection of TAM-67 for 15 h as indicated. After prestimulation, 1 mM GSNO was added for an additional 8-h incubation period to assay DNA fragmentation by the diphenylamine method. Data are means ± SD of three individual experiments (*p ≤ 0.005 vs inhibited controls).
In conclusion, protective mechanisms evoked by LPS/IFN-γ/NMMA or by GSNO in macrophages are reversed by NF-κB decoy oligonucleotides or the NF-κB inhibitor PDTC. Elimination of the major AP-1 component c-jun attenuated low-dose NO–mediated protection. Our results demonstrate that LPS/IFN-γ/NMMA–induced protection requires NF-κB, whereas low-dose NO–mediated protection demands both NF-κB and AP-1. These transcription factors are necessary components of the signaling cascade leading to Cox-2 expression.
DISCUSSION
A major function for naturally occurring oxygen- and nitrogen-derived radicals that are produced at least in part by specialized cells such as macrophages and neutrophils is immunological host defense. This is exemplified during their active antipathogenic or antitumorigenic roles (Wiseman and Halliwell, 1996; Lander, 1997). A delicate balance between formation and detoxification of radicals allows directing signaling pathways to physiological or pathological conditions. Overproduction of radicals is cytotoxic and refers to necrotic or apoptotic pathways (Jacobson, 1996; Wiseman and Halliwell, 1996). However, radicals also act as intracellular messengers. In agreement, redox-controlled transcription factors, oxidative-susceptible thiol groups or redox-sensitive phosphorylation events allow radicals to modulate and trigger communicating systems (Anderson et al., 1994; Lander, 1997; Quijano et al., 1997). The constitutive expression of defense systems permits cells to cope with oxidative conditions, whereas inducible protective systems make the adjustment to longer-lasting perturbations of the normal redox environment possible. For activated macrophages, a rescue mechanism exists that allows them to face high-level radical production without entering the naturally existing death program, known as apoptosis. Recently, we established up-regulation of Cox-2 in response to low-level NO or LPS/cytokine treatment that in turn conferred resistance to macrophage apoptotic cell death, normally elicited by a toxic dose of the NO donor GSNO. An active role of Cox-2 was established in Cox-2–overexpressing macrophages and further substantiated in cells that lost the ability to express Cox-2 by an antisense approach (von Knethen and Brüne, 1997). Protection was mediated by an increase of cAMP (von Knethen et al., 1998). Cox-2 overexpression and resistance to butyrate-induced apoptosis are causatively correlated for colon epithelial cells, and a general link between the ability of nonsteroidal anti-inflammatory drugs to prevent or reduce the occurrence of colorectal, gastrointestinal, and perhaps other cancers exists, thus supporting the notion that products of the prostanoid/Cox-2 pathways cause tumor promotion (Tsujii and DuBois, 1995; Levy, 1997; Sheng et al., 1997). This is important for the proposal that the occurrence of tumors reflects an impairment of apoptosis (Levy, 1997) and for further suggestions that NO may be tumorigenic (Ambs et al., 1997). However, details on NO-mediated Cox-2 expression remained elusive.
In this study we established formation of an active p50/p65 heterodimer NF-κB complex and Cox-2 expression in response to LPS/cytokine or low-level NO. A role of NF-κB during Cox-2 expression can be rationalized when we consider the putative NF-κB–binding site in the upstream Cox-2 promoter region (−600/+1) (Yamamoto et al., 1995). Further evidence stems from the ability of PDTC to block NF-κB activation (Tetsuka et al., 1996b) that has been proven in alveolar macrophages, in which PDTC suppressed Cox-2 expression in response to LPS (Hempel et al., 1994). To avoid pharmacological side effects of PDTC, we used an NF-κB decoy approach that unequivocally demonstrated an active NF-κB complex in promoting Cox-2 expression. Decoy oligonucleotide approaches have been used successfully to inhibit transcriptional activity (Roshak et al., 1996; Sharma et al., 1996; von Knethen et al., 1998) by scavenging active transcription factors. Our results are in agreement with the report of Schmedtje et al. (1997) in which NF-κB p65 decoy oligonucleotides down-regulated hypoxia-induced Cox-2 expression.
We further underscored an active role of NF-κB during NO-mediated Cox-2 activation by using a reporter plasmid with four copies of the NF-κB site derived from the murine Cox-2 or the porcine E-selectin promoter (Bach et al., 1997). Additionally, it is known that IκB-α acts as a natural NF-κB inhibitor (Lin et al., 1995). Consequently, IκB-α degradation results in NF-κB activation and concomitant expression of NF-κB–inducible genes (Traenckner et al., 1995; Baichwal and Baeuerle, 1997). This scenario is in agreement with our results achieved by low-dose NO and by LPS/IFN-γ/NMMA prestimulation.
The influence of NO or NO-releasing compounds on NF-κB activation is controversial. Although activation of NF-κB by NO donors has been described for lymphocytes (Lander et al., 1993) and has been established as a NO-responsive system during hemorrhagic shock (Hierholzer et al., 1998), other reports stated that NO inhibits activation of NF-κB, in part by hindering DNA binding (Park et al., 1997). The ability of NO or NO+ to interfere in the DNA-binding assay (EMSA) can be explained by S-nitrosation of critical thiol groups at the active NF-κB complex, which may not necessarily apply to the situation in intact cells but is in agreement with our observations that GSNO concentrations >200 μM attenuated NF-κB activation. These considerations are supported by the observation that NO is a potent coactivator of IκB-α kinase at low concentrations, whereas high doses of NO impaired the DNA-binding activity of NF-κB (Umansky et al., 1998). Mechanisms of NF-κB activation by NO are currently under investigation. We address the possible involvement of tyrosine phosphorylation that is known to be affected by redox active compounds, i.e. GSNO, and is considered as a mechanism for NF-κB activation (Menon et al., 1995; Traenckner et al., 1995).
NF-κB regulates a diverse group of genes that play important roles in immune and inflammatory responses as well as in apoptotic cell death (Baeuerle and Baltimore, 1996). Experimental evidence from tumor necrosis factor-α–initiated apoptosis in cells taken from NF-κB knockout mice or in cells that carry a mutant and thus suppressive form of IκB implied a general role for NF-κB in preventing apoptosis (Wang et al., 1996). For macrophages our results put NF-κB–mediated expression of Cox-2 as a protective gene product in place. Apoptotic parameters such as DNA fragmentation and p53 accumulation are suppressed when Cox-2 is transcribed (von Knethen and Brüne, 1997; von Knethen et al., 1998), whereas apoptosis proceeds when activation of NF-κB and the concomitant Cox-2 expression are compromised by PDTC or NF-κB decoy oligonucleotides. Although our experiments do not eliminate the involvement of other transcription factors or protective proteins, the inverse expression of Cox-2 and p53 in RAW 264.7 macrophages is striking.
In contrast to LPS/cytokine–induced Cox-2 expression, NF-κB appears to be only one component of low-dose NO signaling. Observing a dose-dependent AP-1 activation after NO donor addition is in agreement with previous reports that stated that NO mediated AP-1 activation (Pilz et al., 1995; Sciorati et al., 1997). As presented here, activation was attenuated by the ERK kinase inhibitor PD98059 but not by the p38 inhibitor SB203580. The inhibitory profile paralleled that of Cox-2 induction because PD98059 suppressed low-dose NO–mediated Cox-2 expression. The involvement of AP-1 is comprehensible because AP-1 activates the murine Cox-2 promoter via a cAMP response element (Xie et al., 1994; Xie and Herschman, 1995). Our experiments using a dominant-negative c-jun mutant (TAM-67) further support the notion that AP-1 causes Cox-2 induction in response to NO, thereby abrogating apoptotic cell death. Cytokine-elicited Cox-2 expression required NF-κB activation, whereas inhibition of MAPKs revealed no interference. The essential role of NF-κB is confirmed by studies from Hwang and coworkers that point to NF-κB activation and Cox-2 expression in LPS-stimulated macrophages. In LPS-activated macrophages, Cox-2 expression partially required the MAPK pathway (Hwang et al., 1997), whereas our results neglected any contribution of this system in LPS/IFN-γ–stimulated cells. The discrepancy may be the signal strength. Minor NF-κB activation evoked by LPS may require an additional pathway (i.e., the MAPK system for promoting Cox-2), whereas strong NF-κB activation that results from the combination of LPS and IFN-γ (von Knethen, unpublished results) relies on one signaling system only. These considerations may also apply for the NO system. NO causes minor NF-κB activation and therefore requires the MAPK system in parallel for Cox-2 induction.
Our study provides insights into how low-level NO– or LPS/cytokine–signaling mechanisms conferred cellular protection against NO-mediated apoptosis. LPS/cytokine–elicited protection demanded NF-κB activation and subsequent Cox-2 expression, whereas low-dose NO–induced protection required AP-1 in addition. Our results help us understand the dual role of NO in regulating apoptotic cell death and differentiate between an apoptotic initiating versus inhibitory action of NO.
ACKNOWLEDGMENTS
We thank Sabine Häckel for expert technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft and the Deutsche Krebshilfe.
REFERENCES
- Albina JE, Cui S, Mateo RB, Reichner JS. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- Ambs S, Hussain SP, Harris CC. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J. 1997;11:443–448. doi: 10.1096/fasebj.11.6.9194524. [DOI] [PubMed] [Google Scholar]
- Anderson MT, Staal FJ, Gitler C, Herzenberg LA. Separation of oxidant-initiated and redox-regulated steps in the NF-κ B signal transduction pathway. Proc Natl Acad Sci USA. 1994;91:11527–11531. doi: 10.1073/pnas.91.24.11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, Jonat C, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Laneuville O, Miller CO, Lakkides KM, Wingerd BA, DeWitt DL, Smith WL. Prostanoid receptors of murine NIH 3T3 and RAW 264.7 cells—structure and expression of the murine prostaglandin EP4 receptor gene. J Biol Chem. 1996;271:29569–29575. doi: 10.1074/jbc.271.47.29569. [DOI] [PubMed] [Google Scholar]
- Bach FK, Ferran C, Hechenleitner P, Mark W, Koyamada N, Miyatake T, Winkler H, Badrichani A, Candinas D, Hancock WW. Accommodation of vascularized xenografts: expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nat Med. 1997;3:196–204. doi: 10.1038/nm0297-196. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-κ B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Baichwal VR, Baeuerle PA. Activate NF-kappaB or die? Curr Biol. 1997;7:94–96. doi: 10.1016/s0960-9822(06)00046-7. [DOI] [PubMed] [Google Scholar]
- Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993;8:877–886. [PubMed] [Google Scholar]
- Brown PH, Chen TK, Birrer MJ. Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene. 1994;9:791–799. [PubMed] [Google Scholar]
- Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the estimation of DNA. Biochem J. 1956;62:315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camandola S, Leonarduzzi G, Musso T, Varesio L, Carini R, Scavazza A, Chiarpotto E, Baeuerle PA, Poli G. Nuclear factor κ B is activated by arachidonic acid but not by eicosapentaenoic acid. Biochem Biophys Res Commun. 1996;229:643–647. doi: 10.1006/bbrc.1996.1857. [DOI] [PubMed] [Google Scholar]
- Hempel SL, Monick MM, He B, Yano T, Hunninghake GW. Synthesis of prostaglandin H synthase-2 by human alveolar macrophages in response to lipopolysaccharide is inhibited by decreased cell oxidant tone. J Biol Chem. 1994;269:32979–32984. [PubMed] [Google Scholar]
- Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D, Jang BC, Yu G, Boudreau M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide: mediation through both mitogen-activated protein kinase and NF-kappaB signaling pathways in macrophages. Biochem Pharmacol. 1997;54:87–96. doi: 10.1016/s0006-2952(97)00154-8. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Jacobson MD. Reactive oxygen species and programmed cell death. Trends Biochem Sci. 1996;21:83–86. [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- Lander HM, Sehajpal P, Levine DM, Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993;150:1509–1516. [PubMed] [Google Scholar]
- Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci USA. 1996;93:15069–15074. doi: 10.1073/pnas.93.26.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy GN. Prostaglandin H synthases, nonsteroidal antiinflammatory drugs, and colon cancer. FASEB J. 1997;11:234–247. [PubMed] [Google Scholar]
- Lin Y-C, Brown K, Siebenlist U. Activation of NF-kappaB requires proteolysis of the inhibitor IkappaB-α: signal-induced phosphorylation of IkappaB-α alone does not release active NF-kappaB. Proc Natl Acad Sci USA. 1995;92:552–556. doi: 10.1073/pnas.92.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey DJ, Nicotera P, Hartzell P, Bellomo G, Wyllie AH, Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989;269:365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- Menon SD, Guy GR, Tan YH. Involvement of a putative protein-tyrosine phosphatase and I κ B-α serine phosphorylation in nuclear factor κ B activation by tumor necrosis factor. J Biol Chem. 1995;270:18881–18887. doi: 10.1074/jbc.270.32.18881. [DOI] [PubMed] [Google Scholar]
- Messmer UK, Brüne B. Nitric oxide (NO) in apoptotic versus necrotic RAW 264.7 macrophage cell death: the role of NO-donor exposure, NAD+ content, and p53 accumulation. Arch Biochem Biophys. 1996;327:1–10. doi: 10.1006/abbi.1996.0085. [DOI] [PubMed] [Google Scholar]
- Messmer UK, Lapetina EG, Brüne B. Nitric oxide-induced apoptosis in RAW 264.7 macrophages is antagonized by protein kinase C- and protein kinase A-activating compounds. Mol Pharmacol. 1995;47:757–765. [PubMed] [Google Scholar]
- Messmer UK, Reimer DM, Reed JC, Brüne B. Nitric oxide induced poly(ADP-ribose) polymerase cleavage in RAW 264.7 macrophage apoptosis is blocked by Bcl-2. FEBS Lett. 1996;384:162–166. doi: 10.1016/0014-5793(96)00311-0. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- Newton R, Kuitert LME, Bergmann M, Adcock IM, Barnes PJ. Evidence for involvement of NF-κB in the transcriptional control of Cox-2 gene expression by IL-1β. Biochem Biophys Res Commun. 1997;237:28–32. doi: 10.1006/bbrc.1997.7064. [DOI] [PubMed] [Google Scholar]
- Nüssler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukocyte Biol. 1993;54:171–178. [PubMed] [Google Scholar]
- Park SK, Lin HL, Murphy S. Nitric oxide regulates nitric synthase-2 gene expression by inhibiting NF-κ B binding to DNA. Biochem J. 1997;322:609–613. doi: 10.1042/bj3220609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz RB, Suhasini M, Idriss S, Meinkoth JL, Boss GR. Nitric oxide and cGMP analogs activate transcription from AP-1- responsive promoters in mammalian cells. FASEB J. 1995;9:552–558. doi: 10.1096/fasebj.9.7.7737465. [DOI] [PubMed] [Google Scholar]
- Quijano C, Alvarez B, Gatti RM, Augusto O, Radi R. Pathways of peroxynitrite oxidation of thiol groups. Biochem J. 1997;322:167–173. doi: 10.1042/bj3220167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Roshak AK, Jackson JR, McGough K, Chabot Fletcher M, Mochan E, Marshall LA. Manipulation of distinct NFkappaB proteins alters interleukin-1β-induced human rheumatoid synovial fibroblast prostaglandin E2 formation. J Biol Chem. 1996;271:31496–31501. doi: 10.1074/jbc.271.49.31496. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Currie MG, Mollace V. Nitric oxide-mediated cyclooxygenase activation. A key event in the antiplatelet effects of nitrovasodilators. J Clin Invest. 1996;97:2562–2568. doi: 10.1172/JCI118704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandau K, Brüne B. The dual role of S-nitrosoglutathione (GSNO) during thymocyte apoptosis. Cell Signal. 1996;8:173–177. doi: 10.1016/0898-6568(95)02051-9. [DOI] [PubMed] [Google Scholar]
- Sarih M, Souvannavong V, Adam A. Nitric oxide synthase induces macrophage death by apoptosis. Biochem Biophys Res Commun. 1993;191:503–508. doi: 10.1006/bbrc.1993.1246. [DOI] [PubMed] [Google Scholar]
- Schmedtje JF, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- Schoonbroodt S, Legrand Poels S, Best Belpomme M, Piette J. Activation of the NF-kappaB transcription factor in a T-lymphocytic cell line by hypochlorous acid. Biochem J. 1997;321:777–785. doi: 10.1042/bj3210777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorati C, Nistico G, Meldolesi J, Clementi E. Nitric oxide effects on cell growth: GMP-dependent stimulation of the AP-1 transcription complex and cyclic GMP-independent slowing of cell cycling. Br J Pharmacol. 1997;122:687–697. doi: 10.1038/sj.bjp.0701413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HW, Perez JR, Higgins Sochaski K, Hsiao R, Narayanan R. Transcription factor decoy approach to decipher the role of NF-κ B in oncogenesis. Anticancer Res. 1996;16:61–69. [PubMed] [Google Scholar]
- Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Tetsuka T, Daphna Iken D, Miller BW, Guan Z, Baier LD, Morrison AR. Nitric oxide amplifies interleukin 1-induced cyclooxygenase-2 expression in rat mesangial cells. J Clin Invest. 1996a;97:2051–2056. doi: 10.1172/JCI118641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsuka T, Srivastava SK, Morrison AR. Tyrosine kinase inhibitors, genistein and herbimycin A, do not block interleukin-1 β-induced activation of NF-κ B in rat mesangial cells. Biochem Biophys Res Commun. 1996b;218:808–812. doi: 10.1006/bbrc.1996.0144. [DOI] [PubMed] [Google Scholar]
- Traenckner EBM, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human IkappaB-α on serines 32 and 36 controls IkappaB-α proteolysis and NF-κ B activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Umansky V, Hehner SP, Dumont A, Hofmann TG, Schirrmacher V, Droge W, Schmitz ML. Costimulatory effect of nitric oxide on endothelial NF-kappaB implies a physiological self-amplifying mechanism. Eur J Immunol. 1998;28:2276–2282. doi: 10.1002/(SICI)1521-4141(199808)28:08<2276::AID-IMMU2276>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby DA. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci USA. 1994;91:2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Knethen A, Brüne B. Cyclooxygenase-2: an essential regulator of NO-mediated apoptosis. FASEB J. 1997;11:887–895. [PubMed] [Google Scholar]
- von Knethen A, Lotero A, Brüne B. Etoposide and cisplatin induced apoptosis in activated RAW264.7 macrophages is attenuated by cAMP-induced gene expression. Oncogene. 1998;17:387–394. doi: 10.1038/sj.onc.1201926. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Fletcher BS, Andersen RD, Herschman HR. v-src induction of the TIS10/PGS2 prostaglandin synthase gene is mediated by an ATF/CRE transcription response element. Mol Cell Biol. 1994;14:6531–6539. doi: 10.1128/mcb.14.10.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Herschman HR. v-src induces prostaglandin synthase 2 gene expression by activation of the c-Jun N-terminal kinase and the c-Jun transcription factor. J Biol Chem. 1995;270:27622–27628. doi: 10.1074/jbc.270.46.27622. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor κ B and nuclear factor-interleukin-6 in the tumor necrosis factor α-dependent induction of cyclooxygenase-2 in MC3T3–E1 cells. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]