Abstract
Spleen transplantation (SpTx) has established donor-specific tolerance in rodents, but not in large animals or humans. We report the histopathology of rejection in an established model of SpTx in major histocompatibility complex (MHC)-defined miniature swine. Of the 17 SpTx, rejection was observed in two grafts transplanted into untreated, MHC-matched, minor antigen-disparate recipients (group 1, n = 4), but not in the two that received a 12-day course of cyclosporin A (CyA). Rejection also occurred in five grafts transplanted into fully MHC-disparate recipients (group 2, n = 12), one of which was untreated and four of which received some form of immunosuppressive therapy. One recipient of an MHC class-I-mismatched spleen treated with 12 days of CyA did not show rejection. Following biopsy and/or necropsy, fixed allograft tissue sections were treated with multiple stains, immunohistochemical markers and TUNEL assay. Common features of rejection occurred in grafts from both groups, but with varying time courses. Necrosis developed as early as day 8 in group 2 and day 27 in group 1, ranging from focal fibrinoid necrosis of arteriolar walls and sinusoids to diffuse liquefactive necrosis, usually associated with haemorrhage. Other features of rejection included white pulp expansion by atypical cells and decreased staining of basement membranes and reticular fibres. A doubling of the baseline TUNEL index preceded histologically identifiable rejection. This study establishes histologic guidelines for diagnosing and, perhaps, in future studies, predicting acute rejection of splenic allografts transplanted across known histocompatibility barriers in a large-animal model.
Keywords: histopathology, pigs, rejection, spleen, transplantation
Since 1910, spleen allotransplantation (SpTx) has been performed for various reasons in rodents, large animals and humans (Dor et al. 2003). In some rodent models, there is clear evidence that a transplanted spleen can induce a state of donor-specific tolerance and allow long-term survival of both the transplanted spleen and another donor-specific organ without exogenous immunosuppressive therapy (Bitter-Suermann 1974; Suzuki et al. 1997; Dor et al. 2003). However, in large animals and in humans, its tolerogenic capacity has not been demonstrated (Marchioro et al. 1964; Booster et al. 1993; Dor et al. 2003). We are currently investigating whether SpTx can induce tolerance in major histocompatibility complex (MHC)-defined miniature swine, a clinically relevant large-animal model. No study of SpTx has been made previously in any large-animal model in which information on the MHC of both recipient and donor was known.
Bitter-Suermann described the morphologic features of SpTx in rats (Bitter-Suermann & Herbertson 1975), and there have been several studies on outbred dogs (Moore et al. 1960; Fisher et al. 1961; Dammin et al. 1962; Montague et al. 1962; Wheeler et al. 1962; Marchioro et al. 1964; Jordan et al. 1964). Most studies on human spleen allograft rejection have not included detailed histologic descriptions. In our current studies on miniature swine, histologic and immunohistochemical studies were performed in order to determine the course after SpTx with or without various immunosuppressive regimens. We, in this study, characterize previously unreported histologic features of spleen allograft rejection and suggest a systematic grading system based on histopathologic features, similar to those used for evaluating the rejection of transplanted hearts and kidneys. As the transplanted spleen can induce a state of tolerance to itself and to a second donor-specific organ (Dor et al. unpublished data), the histopathological interpretation of spleen biopsies may become clinically relevant.
Pigs and methods
Pigs
Pigs were selected from our herd of MHC-inbred miniature swine, of blood group O (Sachs 1992). Spleen donors were 5.1 ± 3.3 months old, weighed 31.9 ± 20 kg and were positive for the pig allelic antigen (PAA), an allelic non-histocompatibility marker with no known function (Fuchimoto et al. 1999). Weight-matched recipients were 5.2 ± 4.5 months old, weighed 31.2 ± 28.7 kg and were negative for PAA. SpTx has been performed over a minor antigen (group 1; n = 4), MHC class-I (n = 1) or full MHC (group 2; n = 12) histocompatibility barrier (Table 1).
Table 1.
Immunosuppressive regimens and outcome
| Immunosuppression | |||||
|---|---|---|---|---|---|
| Group | Pig # | WBI | ThI | Cyclosporin A | Outcome (day of rejection*) |
| MHC-matched | 14526 | − | − | − | Rejection† (28) |
| 14867 | − | − | − | Rejection (27) | |
| 14697 | − | − | days 0−11 | No rejection | |
| 14687 | − | + | days 0−11 | No rejection | |
| MHC class-I-mismatched | 15111 | − | + | days 0−11 | No rejection |
| Fully MHC- mismatched | 14534 | − | − | − | Rejection (8) |
| 14646 | − | − | CPP | Rejection (16) | |
| 14832 | − | + | days 0−11 | Rejection (47) | |
| 14840 | − | + | days 0−27 | Rejection (62) | |
| 15029 | − | + | days 0−27 | No rejection‡ | |
| 15051 | + | + | days 0−44 | No rejection | |
| 15242 | + | + | days 0−44 | No rejection | |
| 15311 | + | + | days 0−44 | Rejection (61) | |
| 15328 | + | + | days 0−44 | No rejection‡ | |
| 15399 | + | + | days 0−29 | No rejection | |
| 15410 | + | + | days 0−44 | No rejection | |
| 15446 | + | + | days 0−44 | No rejection | |
Cyclosporin A (CyA) at 10–30 mg/kg intravenously daily to maintain a whole blood trough level of 400–800 ng/ml (displayed is the number of days of CyA treatment; e.g. 12 days = days 0–11 after transplantation); cyclophosphamide (CPP) (3 × 20 mg/kg intravenously on days –5, –4 and –3); whole body irradiation (WBI) (100 cGy on day –2); thymic irradiation (ThI) (700 cGy on day –1).
Based on histological examination of the spleen graft.
Defined as the development of Grade 3 changes (coagulative necrosis, Table 2).
Died from infection.
All animal-care procedures were in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication no. 86-23, revised 1996), and were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Surgical procedures
The technique of SpTx has been described previously (Gollackner et al. 2003). Wedge biopsies of the donor spleen were taken immediately before explantation from the donor, after flushing, 2 h after reperfusion in the recipient, and subsequently through an abdominal flank incision on post-Tx days 14, 28 and then at increasing intervals. In one untreated recipient (#14534) in group 2, additional spleen biopsies were taken on days 4, 8 and 11. Biopsies were taken from various sites in the spleen on each occasion, working from distal to proximal. Bleeding could be readily controlled by means of cautery coagulation, and the application of pressure to the biopsy site for 10 min, sometimes with the use of Surgicel (Ethicon, Somerville, NJ, USA). Analysis of 106 biopsies forms the basis of this study. The grafts were evaluated macroscopically with regard to size, colour, consistency/texture, arterial pulsation and bleeding at the biopsy sites. All biopsies were examined histologically and by means of flow cytometric analysis for the presence of donor (PAA+) cells.
Euthanasia
All pigs were euthanized by means of an overdose of sodium pentobarbital, and necropsies were performed immediately thereafter, during which spleen grafts were harvested first. Reasons for euthanization of pigs included long-term survival of >150 days, frank rejection of the spleen graft or therapy-resistant disease in the pig. Some pigs were left to live after spleen rejection in order to obtain data for in vivo and in vitro studies at time points long after rejection. No pig demonstrated clinical symptoms of illness after spleen rejection.
Immunosuppressive therapy
The treatment of each recipient pig has been summarized in Table 1. Cyclosporin A (CyA) was administered intravenously daily for 12–45 days. A cobalt irradiator administered whole body and/or thymic irradiation (Fuchimoto et al. 2000; Huang et al. 2000).
Histological examination
Tissues were fixed in 10% formalin and were embedded in paraffin or were frozen in tissue medium (Tissue-Tek OCT 4583, Miles, Elkhart, IN, USA) and were stored at –80 °C. Histologic sections of 5 μ were cut from paraffin blocks; they were stained with hematoxylin and eosin (H&E) and were studied by means of light microscopy. In order to assess possible changes in elastic tissue, basement membrane integrity and collagen deposition, special stains included periodic acid-Schiff (PAS), trichrome, Verhoeff elastic and reticulin stains. Immunohistochemical studies included the use of monoclonal antibodies to α-smooth muscle actin (SMA; clone 1A4, Sigma, St Louis, MO, USA), vimentin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and proliferating cell nuclear antigen (PCNA; clone PC10, Dako, Carpinteria, CA, USA) on fixed tissue. Macrophage/L1 protein/calprotectin Ab-1 (clone Mac 387, NeoMarkers, Fremont, CA, USA) was used in order to label macrophages on 5-μ cryosections of tissue. Binding was visualized by using horseradish peroxidase-conjugated secondary antibodies (Vector, Burlingame, CA, USA). Bound antibodies were revealed by means of incubation of formalin-fixed and cryopreserved sections in aminoethylcarbazole (Dako). Sections were counterstained with hematoxylin solution (Richard-Allan Scientific, Kalamazoo, MI, USA). Negative controls omitted the primary antibodies.
Apoptosis was determined with the help of a terminal deoxyribonucleotidyl transferase-mediated, dUTP nick-end labelling (TUNEL) assay on paraffin sections. After deparaffinization, treatment with proteinase-K (Fisher Scientific, Fairlawn, NJ, USA) for 15 min at room temperature and quenching in 2% H2O2, tissue sections were incubated with biotinylated dUTP (Boehringer, Indianapolis, IN, USA) and terminal deoxyribonucleotidyl transferase (Promega, Madison, WI, USA) for 1 h at 37 °C. Slides were then blocked; they were washed with phosphate-buffered saline and were incubated with avidin–biotin complex horseradish peroxidase (Dako). Following development with diaminobenzidine (Zymed Laboratories, South San Francisco, CA, USA), slides were counterstained with methyl green. Positive controls included sections of human adenocarcinoma of the colon, in which apoptosis is common. For negative controls, distilled water was substituted for deoxyribonucleotidyl transferase. The TUNEL index (TI) was calculated as the mean number of TUNEL+ cells counted in 10 random microscopic fields at ×200 magnification.
Flow cytometry
Spleen biopsies were processed into single-cell suspensions, and red blood cells were lysed. The concentration was adjusted to approximately 1 × 107 cells/ml, and 100 µl (1 × 106 cells) was distributed into each staining tube. The following swine-specific antibodies were used: anti-CD1 (76-7-4, mouse IgG2aK), anti-CD3 (898H2-6-15, mouse IgG2aK), anti-CD4 (74-12-4, mouse IgG2bK), anti-CD8α (76-2-11, mouse IgG2aK), anti-CD16 (G7, mouse IgG1) and anti-PAA (1038H-10–9, mouse IgMK). Two-colour staining by using fluorescein isothiocyanate-conjugated CD1, CD3, CD4, CD8, CD16 and biotinylated PAA antibody was used in order to determine the proportion of donor vs. recipient lymphocytes, with appropriate negative isotype controls. A second-step antibody, phycoerythrin streptavidin (Pharmingen, San Diego, CA, USA), was also used. Data were acquired by using a Becton Dickinson FACScan (San Jose, CA, USA) and were analysed with WinList mode analysis software (Verity Software House, Topsham, ME, USA).
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) was performed on sex-mismatched grafts by using a paraffin pre-treatment reagent kit as recommended (Vysis, Downers Grove, IL, USA). Graft tissue sections of 5 μ were deparaffinized; they were digested with proteinase-K and were hybridized overnight in a Hybrite chamber with a porcine combined XF/YCy3 paint (CA-1685, Cambio, Cambridge, UK), with X labelled with fluorescein isothiocyanate (FITC) and Y labelled with cyanine-3 (Cy3). Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI). Sections were examined with a fluorescence microscope (Olympus, Model Bx60F-3, Tokyo, Japan) by using appropriate filters in order to detect green, red and blue fluorescence of the FITC, Cy3, and DAPI, respectively. Images were photographed with an enhanced digital camera (Spot, Model 130, Diagnostic Instruments, Sterling Heights, MI, USA); they were captured to a desktop computer (Compaq Deskpro DPENM P700 CCP, Hewlett Packard Co., Palo Alto, CA, USA) and were merged. Cells with intranuclear red signal were considered to be male-derived; cells with two intranuclear green signals were considered to be female-derived. Sections of lymph nodes from male and female pigs served as controls.
Identification of Howell–Jolly bodies in the blood
Blood was drawn into a K2-EDTA tube before SpTx and on a weekly basis thereafter. Blood smears were Wright-stained and were examined microscopically for Howell–Jolly bodies, which indicate a level of splenic dysfunction (Brigden & Pattullo 1999).
Results
Outcome of SpTx
A review of the Table 1 data suggests that when no or minimal immunosuppressive therapy was administered, rejection progressed faster after MHC-mismatched SpTx (group 2, when it occurred in 8 and 16 days) than in MHC-matched, minor-mismatched pairs (group 1, 27 or 28 days). Furthermore, greater pre- and post-Tx therapy was required in order to protect the graft from rejection and to induce tolerance in the MHC-mismatched pairs of group 2 than in the MHC-matched pairs of group 1. Results of experiments within group 2 suggest an increased graft survival and a greater incidence of tolerance induction when conditioning and therapy were increased. Whole body and thymic irradiation combined with 45 days of CyA prevented spleen rejection in six out of seven grafts. A regimen that did not contain all of these elements resulted in rejection in four out of five grafts; in a graft in which rejection did not occur, follow-up was limited to 23 days by the death of the recipient from infection. Our histological study is focused on the seven rejected grafts (n = 2 in group 1; n = 5 in group 2).
Macroscopic appearance of spleen grafts
Within 2 weeks of SpTx, spleens not undergoing rejection were enlarged to twice their normal size and demonstrated normal colour, a soft texture, good vascular pulsation and bleeding from the biopsy site. Long-term surviving grafts became dark purple in colour, but remained soft. Some capsular fibrosis and adhesions were identified. When rejection was occurring, early changes included those in colour (from aubergine to yellow-brown), texture (soft to firm), arterial pulsation (diminished or absent) and a lack of bleeding from biopsy sites. Advanced rejection was usually associated with shrinkage in spleen size to less than one-third of that at Tx.
Microscopic appearance of spleen grafts undergoing rejection
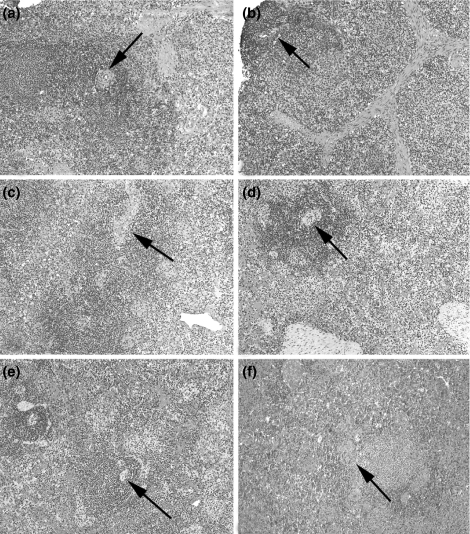
One to two hours after reperfusion, a slight increase in neutrophils in sinusoids of the red pulp was observed (Gollackner et al. 2003), but it was not indicative of adverse effects on graft survival. Figure 1 illustrates the normal histological appearance of a pig spleen before donor explantation and compares this histology with that of progressive biopsies of a graft that was not rejected.
Figure 1.
Histology of porcine spleen before transplantation of one graft and at progressive time points after transplantation in a graft that was not rejected. Normal features include those of white pulp (arrows mark central arteries in Malpighian corpuscles), red pulp, trabeculae and clusters of histiocytes. (a) Baseline biopsy, before transplantation (pig #14867, group 1) (H&E, ×100); biopsies of graft of pig #15111 (group 1) at (b) 1 h, (c) 15 days, (d) 27 days, (e) 139 days and (f) 362 days after SpTx (H&E, ×100). H&E, hematoxylin and eosin; SpTx, spleen transplantation.
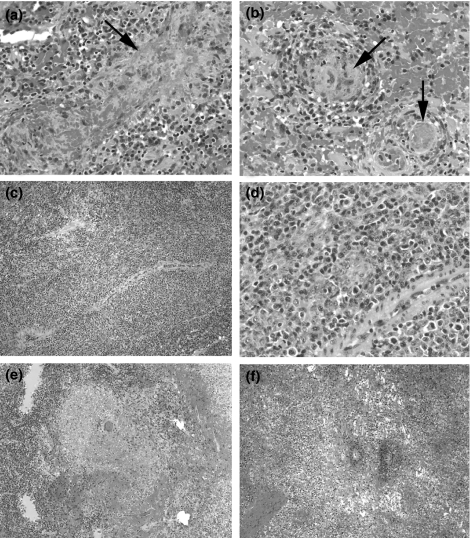
The speed of the development of rejection varied with the extent of MHC disparity between donor and recipient and the intensity of the conditioning/immunosuppressive therapy. Although the speed of the development of rejection was variable, the histopathological features of rejected grafts were similar. Figure 2 illustrates our histologic findings in grafts that were rejected. Multifocal fibrinoid necrosis was not necessarily followed by diffuse necrosis when found on biopsy. However, a marked expansion of periarteriolar lymphatic sheaths (PALS) of the white pulp by PCNA+ (not shown), pleomorphic, large, mitotically active lymphocytes was followed by coagulative necrosis on subsequent biopsies. This expansion was associated with an increased density of reticulin staining in the PALS (not shown), consistent with a reactive increase of collagen fibres in the white pulp. Similarly, increased SMA and vimentin staining in the peripheral aspects of the PALS (not shown) suggested a concomitant vascular proliferation or possible angiogenesis, or may reflect a proliferation of T-cell-zone fibroblasts, which are known to express SMA (Steiniger et al. 2003). The increase in proliferative activity was demonstrated by means of PCNA staining (not shown).
Figure 2.
Histology of splenic graft rejection at progressive stages. (a and b) Grade 1 (mild), focal, fibrinoid necrosis of sinusoidal walls (arrow), associated with fibrin thrombi in small vessels (pig #14526, group 1, 57 days after SpTx) (arrows). (c and d) Grade 2 (moderate), marked expansion of PALS around central artery (H&E, ×100), consisting of pleomorphic, atypical lymphoid cells (H&E, ×400) (pig #14867, group 1, 15 days after SpTx). Grade 3 borderline severe (e) and severe (f), focal to diffuse liquefactive necrosis, respectively (pig #14867, group 1, 28 days after SpTx) (H&E, ×100). H&E, hematoxylin and eosin; PALS, periarteriolar lymphatic sheaths; SpTx, spleen transplantation.
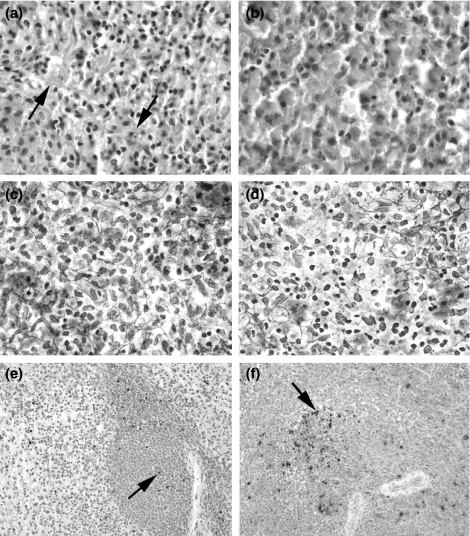
As necrosis developed, there was a loss of baseline staining with reticulin, SMA, vimentin and PCNA (data not shown). More subtle histological changes, which correlated with early, focal fibrinoid necrosis, included a diminished PAS and reticulin staining of reticular fibres in the red pulp (Figure 3). Before the appearance of irreversible histological features of rejection, there was at least a doubling of the TI, compared to that of baseline (Figure 3).
Figure 3.
Subtle histological changes, which preceded frank rejection. (a and b) The regular network of reticular fibres of the red pulp (arrows) is degraded (b), 4 days after SpTx (pig #14534, group 2) (PAS stain, ×600). The normal (c) and centrally disrupted (d) patterns of reticular fibres are again observed with further staining (pig #14526, group 1, 57 days after SpTx) (reticulin stain, ×600). A TUNEL index (TI) at baseline (e) on the day of SpTx (pig #14867, group 1) is increased more than two-fold (f) 15 days after SpTx, before a subsequent biopsy showed histologic evidence of rejection 28 days after SpTx (arrows mark TUNEL+ cells) (magnification: ×200). PAS, peridodic acid-Schiff; SpTx, spleen transplantation.
The onset of rejection, ranging from focal fibrinoid necrosis of arteriolar walls and sinusoids to liquefactive necrosis, was usually associated with variable degrees of haemorrhage (Figure 2). Acute vasculitis accompanied the process of necrosis in one graft (not shown), and, in another, extramedullary haematopoiesis was associated with patchy, liquefactive necrosis (not shown). Diffuse necrosis was the end result of rejection, and it developed as early as day 8 in untreated pigs in group 2 and by day 28 in group 1. Necrosis was accompanied by an increase in collagen and by either the first appearance or an increased density of elastic fibres in the capsule and trabeculae (not shown). Replacement fibrosis adjacent to geographical areas of necrosis was observed in one untreated group 1 graft on day 88 and in one treated group 2 graft on day 138. These areas of fibrous replacement consisted of a variably dense deposition of collagen and a diffuse population of SMA+ and vimentin+ spindle cells, most of which were PCNA-negative (not shown). This end-stage scarring corresponded to shrinkage in allograft size.
Microscopic appearance of spleen grafts surviving long-term
Eight grafts (group 1, n = 3; group 2, n = 4; and the one MHC class-I-mismatched) remained viable 40–367 days after SpTx. Five of them showed atrophy of the white pulp from days 28–160, i.e. a paucity of lymphoid cells, giving the histiocyte-rich sheaths around central arteries a pale look on H&E stain. Accompanying the depletion of white pulp was replacement of normal red pulp by vacuolated histiocytes and SMA+ cells, presumably smooth muscle cells migrating in from adjacent trabeculae (data not shown).
Correlation of histopathology with spleen cell chimerism on flow cytometry
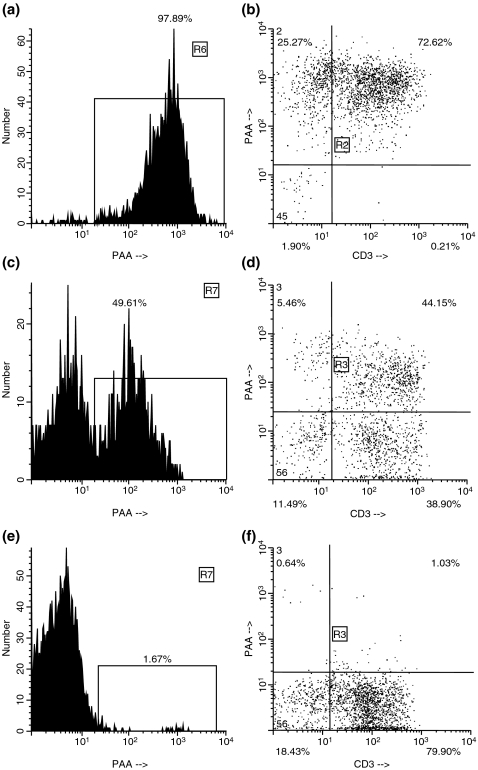
In both groups, a significant loss of PAA+ cells in the spleen either preceded or occurred concomitantly with the development of histological features of rejection. Before explantation from the donor and immediately after reperfusion in the recipient, the donor spleen demonstrated 96.6 ± 2.0% of PAA positivity (Figure 4), which was comparable to the PAA positivity of leucocytes in the blood of the donor pigs (96.2 ± 2.2%). In untreated pigs of group 1, the percentage of donor (PAA+) cells remaining in the spleen had fallen to 0–4% by day 15, being replaced by host (PAA-) cells (CD3+, CD8+ and CD16+). In immunosuppressed pigs in group 1, the percentage of PAA+ cells remained at >15% on day 29, and at 4% on day 215.
Figure 4.
Flow cytometry of a donor (PAA+) spleen (donor to pig #15410) before explantation, demonstrating the percentage of PAA+ cells (97.89%) (a) and the profile of CD3+PAA+ cells (b). Flow cytometry of an accepted spleen graft after cessation of CyA (pig #15051, group 2). Replacement with recipient cells is indicated by the presence of PAA– cells. The spleen graft still consists of 49.61% of donor cells (c), but some CD3+PAA– recipient cells can be seen (d). Almost complete repopulation of the spleen graft by recipient cells occurred (pig #15410, group 2, 183 days after SpTx). Only 1.67% of the cells are still of donor origin (e). Virtually, all CD3+ cells (f) are of recipient type (PAA–). CyA, cyclosporin A; PAA, pig allelic antigen; SpTx, spleen transplantation.
In untreated recipients in group 2, PAA+ donor cells were completely replaced by recipient cells within 8 days. In treated group 2 recipients that eventually rejected their grafts, 30% of cells in the graft were PAA+ during CyA therapy but, when rejection occurred 2–3 weeks later, all PAA+ cells were rapidly lost from the graft. The FISH technique demonstrated recipient-derived cells populating a sex-mismatched graft (Figure 5), supporting the flow cytometric data.
Figure 5.
Section of the spleen, which was transplanted from a female donor into a male recipient (pig #14840, group 2, 43 days after SpTx). A notable number of recipient-derived cells with Y chromosomes (red signal) have populated the graft at the time of expanded white pulp (day 43) (FISH technique, ×400). FISH, fluorescence in situ hybridization; SpTx, spleen transplantation.
In group 2 recipients that did not reject their grafts, PAA+ donor cells decreased by about 50% within the first post-Tx month, after which the spleen graft was slowly replaced almost entirely with recipient cells, which usually occurred within 100 days post-Tx. The chimerism in the spleen graft reflected the extent of lymphoid chimerism in blood (Dor et al. 2003b).
Presence of Howell–Jolly bodies
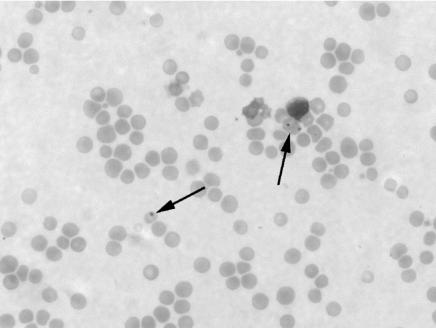
After combined native splenectomy and survival of a viable graft, Howell–Jolly bodies were never seen in the blood, indicating good splenic graft function. When rejection developed, however, Howell–Jolly bodies appeared in the blood within a week (Figure 6).
Figure 6.
Red blood cells (arrows) contain Howell–Jolly bodies in a peripheral blood smear of a recipient of a rejected (Grade 3) spleen allograft (pig #15311, group 2, 75 days after SpTx, 2 weeks after spleen graft rejection (charcterized by coagulative necrosis) (Wright's stain, ×640). SpTx, spleen transplantation.
Discussion
To our knowledge, this is the first study describing the histopathology of spleen allograft rejection in a large-animal model with known MHC barriers. In miniature swine, our observation was that common features of rejection occurred in minor-mismatched and fully MHC-disparate grafts, but with varying time courses. This correlates with the course of rejection in other organ transplants, where the pattern of rejection is similar, yet the rapidity of its development varies depending on the extent of MHC incompatibility between donor and recipient (Gianello & Sachs 1996).
Histologic findings that did not persist and, thus, seemed unimportant as predictors of rejection include scattered foci of an increased number of neutrophils at 1 h post-reperfusion of the graft, congested red pulp and fibrinoid necrosis which was limited to a subcapsular focus. An early indicator of rejection was the expansion of white pulp, as described in other studies (Wheeler et al. 1962; Bitter-Suermann & Herbertson 1975; Starzl et al. 1984). Some (Bitter-Suermann & Herbertson 1975; Starzl et al. 1984) have attributed the expansion of PALS to an increased number of donor cells. However, in our model, correlation with flow cytometry indicated that expansion of the white pulp was because of infiltration by recipient cells. Atrophy of the white pulp, on the other hand, perhaps reflects a ‘burned out’ immune response to the allograft, allowing relatively long-term graft survival.
Based on our histologic findings and on data reported by others previously, a histologic grading system for acute cellular rejection in spleen allografts is proposed (Table 2). Focal or multifocal fibrinoid necrosis of sinusoidal and/or arteriolar walls (Grade 1) did not preclude long-term graft survival. Expansion of white pulp by large, atypical and mitotically active lymphoid cells (Grade 2) was invariably followed by borderline-severe or severe rejection (Grades 3A and B), manifested by focal or diffuse coagulative necrosis, respectively. The TI may be a helpful indicator of spleen graft survival; in one untreated group 2 spleen, although the graft looked normal histologically on day 4, the TI had increased almost four-fold (12.6–40.0) from baseline; by day 8, the graft demonstrated diffuse necrosis. In future studies, these histological changes may have a predictive significance and aid in the treatment or prevention of rejection.
Table 2.
Grading scheme for splenic allograft rejection
| Grade | Rejection | Histological features |
|---|---|---|
| 0 | None | Normal splenic architecture |
| 1 | Mild | Focal or multifocal fibrinoid necrosis of sinusoidal and/or arteriolar walls, with or without haemorrhage |
| 2 | Moderate | Expansion of white pulp by large, atypical, and mitotically active lymphoid cells |
| 3A | Borderline severe | Focal* coagulative necrosis |
| 3B | Severe | Diffuse coagulative necrosis |
Focal is involvement of a small fraction of tissue in a high-power field.
Regarding the sequence of events during rejection, multifocal fibrinoid necrosis was not necessarily followed by diffuse necrosis when found on biopsy. In pig 15242, patchy fibrinoid necrosis was found on a biopsy at pod 46 and again at autopsy of the graft on pod 60. In pig 14526, foci of fibrinoid necrosis were present at pod 57 but not seen in the viable graft at harvest on pod 160. We have interpreted the finding of patchy fibrinoid necrosis as a feature of mild and potentially reversible rejection. On the other hand, when expansion of white pulp with atypical cells was seen on biopsy in two grafts, subsequent biopsies in both grafts showed diffuse necrosis, i.e. frank rejection (pig 14867, pods 15 and 28; pig 14840, pods 43 and 62). Normal biopsies were followed by biopsies showing diffuse necrosis in grafts (pig 14534, pods 4 and 8; pig 14646, pods 0 and 16; pig 14832, pods 11 and 22). The apparent absence of expansion of white pulp by atypical cells before diffuse necrosis, in these grafts may reflect a more rapid onset of frank rejection and/or an issue of timing of biopsies. Further studies should clarify the influence of MHC disparity and therapeutic regimens on the sequence and rapidity of rejection episodes. The scoring system outlined in Table 2, therefore, reflects our findings to date and is proposed as a novel guide for characterization of splenic allograft rejection.
It is unclear why extramedullary haematopoiesis occurred in one of our pig allografts (Montague et al. 1962; Bitter-Suermann & Herbertson 1975). The spleen is known, however, to be an organ capable of haematopoiesis in certain disease states (Kraus et al. 1998; Mesa et al. 2001), and, in other studies, we have observed that the naive spleen has haematopoietic stem cell activity (Dor et al. 2004).
In rodents, spleen grafts from untreated PVG rats to untreated AGUS rat recipients were rejected within 2–3 weeks (Bitter-Suermann & Herbertson 1975). The grafts enlarged to six times of normal size, and became firm and white. Histologically, a slight expansion of the PALS occurred as early as 2 days after SpTx, approximately 50% of the lymphocytes becoming enlarged with pyroninophilic cytoplasm (Bitter-Suermann & Herbertson 1975); the authors do not specify whether these lymphocytes were of donor or recipient origin. There was also an increased cellularity of the red pulp. After 12 days, foci of necrosis enlarged and coalesced until the whole spleen became necrotic.
In large animals, most histological observations have been made in dogs (Moore et al. 1960; Fisher et al. 1961; Dammin et al. 1962; Montague et al. 1962; Wheeler et al. 1962; Marchioro et al. 1964; Jordan et al. 1964). Subcapsular splenic haemorrhage developed, with diffuse infiltration of polymorphonuclear leucocytes into red pulp. Subsequently, generalized haemorrhage was seen, with many pyroninophilic cells in the red pulp, followed by complete haemorrhagic infarction and necrosis.
In the few human SpTx studies, the histological picture of rejection remains uncertain (Marchioro et al. 1964; Hathaway et al. 1969; Groth et al. 1971; Raccuglia & Lansing 1973; Starzl et al. 1984; Peltenburgh et al. 1992; Booster et al. 1993; Dor et al. 2003). Within days, splenic grafts may contain an increased number of lymphocytes, histiocytes and reticulum cells. Red pulp congestion, immunoblast proliferation and intimal swelling may develop, with lymphoid expansion predominantly in the marginal zones. Grafts may develop extensive oedema, focal haemorrhage and cellular depletion in the PALS, with a generalized and massive infiltrate of large lymphoid cells. Extensive necrosis follows. Others have reported that, within 1–2 months, marked fibrosis, focal necrosis and intimal proliferation (causing arterial occlusion) were present and, after 7–9 months, complete atrophy had occurred (Booster et al. 1993).
The sequence of histopathological events leading to allograft rejection in our study is similar to those described in rodents and dogs. With the availability of special staining techniques and flow cytometry, however, we have been able to detect subtle morphologic changes that may help predict the course and ultimate outcome of the transplanted spleen. Our observations should prove valuable if SpTx, for the purpose of inducing tolerance, becomes a clinical reality.
Acknowledgments
We thank R.B. Colvin and D.H. Sachs for their valuable comments on this manuscript. We are grateful to Luisa Raleza for excellent secretarial support in the preparation of this manuscript. Cyclosporin A (Sandimmune) was a generous gift from Novartis Pharmaceutical Corporation, East Hanover, NJ, USA. F.J.M.F. Dor is a recipient of grants from the Ter Meulen Fund from the Royal Netherlands Academy of Arts and Sciences, the Professor Michaël-van Vloten Fund and the Netherlands–America Foundation. B. Gollackner is a recipient of grants from the Max Kade Foundation of the Austrian Academy of Sciences. D.K.C. Cooper is a consultant to Immerge BioTherapeutics, Inc. Work in our laboratory is supported, in part, by NIH Program Project 1PO1 A145897 and by a Sponsored Research Agreement between the Massachusetts General Hospital and Immerge BioTherapeutics, Inc.
References
- Bitter-Suermann H. Survival of unmodified spleen allografts in rats. Nature. 1974;247:465–466. doi: 10.1038/247465a0. [DOI] [PubMed] [Google Scholar]
- Bitter-Suermann H, Herbertson BM. Morphologic features of spleen allografts in rats. Graft rejection, acceptance, or lethal graft-versus-host-disease in the same donor-recipient pairing. Am. J. Pathol. 1975;81:283–294. [PMC free article] [PubMed] [Google Scholar]
- Booster MH, Wijnen RM, van Hooff JP, et al. The role of the spleen in pancreas transplantation. Transplantation. 1993;56:1098–1102. doi: 10.1097/00007890-199311000-00010. [DOI] [PubMed] [Google Scholar]
- Brigden ML, Pattullo AL. Prevention and management of overwhelming postsplenectomy infection – an update. Crit. Care. Med. 1999;27:836–842. doi: 10.1097/00003246-199904000-00050. [DOI] [PubMed] [Google Scholar]
- Dammin GJ, Wheeler HB, Montague ACW, Dealy JB, Greenberg JB, Moore FD. The splenic allograft: its course in the unmodified and modified canine recipient. Ann. N. Y. Acad. Sci. 1962;99:861–869. doi: 10.1111/j.1749-6632.1962.tb45366.x. [DOI] [PubMed] [Google Scholar]
- Dor FJMF, Gollackner B, Cooper DKC. Can spleen transplantation induce tolerance? A review of the literature. Transpl. Int. 2003a;16:451–460. doi: 10.1007/s00147-003-0640-0. 10.1007/s00147-003-0640-0. [DOI] [PubMed] [Google Scholar]
- Dor FJMF, Gollackner B, Kuwaki K, et al. Donor-specific unresponsiveness and mixed hematopoietic chimerism following allogeneic spleen transplantation in miniature swine. Am. J. Transplant. 2003b;3(Suppl. 5):263. [Google Scholar]
- Dor FJMF, Ramirez ML, Altman EL, et al. The spleen as a source of hematopoietic stem cell: potential for tolerance induction. Transplantation. 2004;78(Suppl. 1):590. [Google Scholar]
- Fisher B, Lee SH, Fisher ER. Observations concerning spleen homotransplantation in normal and irradiated animals. Surg. Gyn. Obst. 1961;112:455–462. [PubMed] [Google Scholar]
- Fuchimoto Y, Huang CA, Shimizu A, Seebach J, Arn JS, Sachs DH. An allelic non-histocompatibility antigen with wide tissue distribution as a marker for chimerism in pigs. Tissue Antigens. 1999;54:43–52. doi: 10.1034/j.1399-0039.1999.540105.x. 10.1034/j.1399-0039.1999.540105.x. [DOI] [PubMed] [Google Scholar]
- Fuchimoto Y, Huang CA, Yamada K, et al. Mixed chimerism and tolerance without whole body irradiation in a large animal model. J. Clin. Invest. 2000;105:1779–1789. doi: 10.1172/JCI8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianello PR, Sachs DH. Effect of major histocompatibility complex matching on the development of tolerance to primarily vascularized renal allografts: a study in miniature swine. Hum. Immunol. 1996;50:1–10. doi: 10.1016/0198-8859(96)00059-6. 10.1016/0198-8859(96)00059-6. [DOI] [PubMed] [Google Scholar]
- Gollackner B, Dor FJMF, Knosalla C, et al. Spleen transplantation in miniature swine. Surgical technique and initial results in MHC-matched pairs. Transplantation. 2003;75:1799–1806. doi: 10.1097/01.TP.0000063220.19441.5C. 10.1097/01.TP.0000063220.19441.5C. [DOI] [PubMed] [Google Scholar]
- Groth CG, Hagenfeldt L, Dreborg S, et al. Splenic transplantation in a case of Gaucher's disease. Lancet. 1971;1:1260–1264. doi: 10.1016/s0140-6736(71)91778-8. 10.1016/S0140-6736(71)91778-8. [DOI] [PubMed] [Google Scholar]
- Hathaway WE, Mull MM, Githens JH, Groth CG, Marchioro TL, Starzl TE. Attempted spleen transplant in classical hemophilia. Transplantation. 1969;7:73–75. doi: 10.1097/00007890-196901000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DM, Jr, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J. Clin. Invest. 2000;105:173–181. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan GL, Jr, Fiscus WG, Trentin JJ, Foster RP, Collins VP, Barton HL. Splenic transplantation in the dog. Ann. N. Y. Acad. Sci. 1964;120:612–625. doi: 10.1111/j.1749-6632.1964.tb34756.x. [DOI] [PubMed] [Google Scholar]
- Kraus MD, Bartlett NL, Fleming MD, Dorfman DM. Splenic pathology in myelodysplasia: a report of 13 cases with clinical correlation. Am. J. Surg. Pathol. 1998;22:1255–1266. doi: 10.1097/00000478-199810000-00011. 10.1097/00000478-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Marchioro TL, Rowlands DT, Rifkind D, Waddell WR, Starzl TE, Fudenberg H. Splenic homotransplantation. Ann. N. Y. Acad. Sci. 1964;120:626–651. doi: 10.1111/j.1749-6632.1964.tb34757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa RA, Li CY, Schroeder G, Tefferi A. Clinical correlates of splenic histopathology and splenic karyotype in myelofibrosis with myeloid metaplasia. Blood. 2001;97:3665–3667. doi: 10.1182/blood.v97.11.3665. [DOI] [PubMed] [Google Scholar]
- Montague ACW, Greenberg JJ, Dammin GJ, Moore FD. The effect of nitrogen mustard in altering the histocompatibility rejection sequence in splenic homotransplantation in the dog. J. Surg. Res. 1962;2:130–135. doi: 10.1016/s0022-4804(62)80008-0. [DOI] [PubMed] [Google Scholar]
- Moore FD, Wheeler HB, Demissianos HV, et al. Experimental whole-organ transplantation of the liver and the spleen. Ann. Surg. 1960;152:374–387. [PMC free article] [PubMed] [Google Scholar]
- Peltenburgh HG, Tiebosch A, Van den Berg-Loonen PM, et al. A positive T cell crossmatch and accelerated acute rejection of a pancreas-spleen allograft. Transplantation. 1992;53:226–228. [PubMed] [Google Scholar]
- Raccuglia G, Lansing A. Spleen transplantation in a leukaemic individual from his healthy identical twin. Clin. Exp. Immunol. 1973;14:1–18. [PMC free article] [PubMed] [Google Scholar]
- Sachs DH. MHC homozygous miniature swine. In: Swindle MM, Moody DC, Phillips LD, editors. Swine as Models in Biomedical Research. Ames, Iowa: State University Press; 1992. pp. 3–15. [Google Scholar]
- Starzl TE, Iwatsuki S, Shaw BW, Jr, et al. Pancreaticoduodenal transplantation in humans. Surg. Gynecol. Obstet. 1984;159:265–272. [PMC free article] [PubMed] [Google Scholar]
- Steiniger B, Rüttinger L, Barth PJ. The three-dimensional structure of human splenic white pulp compartments. J. Histochem. Cytochem. 2003;51:655–663. doi: 10.1177/002215540305100511. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Li XH, Miyamoto M, Sano T, Hattori Y, Yamashita A. Induction of transplantation tolerance in adult rats by vascularized spleen transplantation. Transplantation. 1997;64:650–653. doi: 10.1097/00007890-199708270-00018. 10.1097/00007890-199708270-00018. [DOI] [PubMed] [Google Scholar]
- Wheeler HB, Balankura O, Pendower JEH, Greenberg JB, Dammin GJ, Moore FD. The homograft response to whole-organ transplantation of the canine spleen. J. Surg. Res. 1962;2:114–123. doi: 10.1016/s0022-4804(62)80006-7. [DOI] [PubMed] [Google Scholar]