Abstract
In chronic administration of sodium valproate to rats, significant disorders of structural integrity of the hippocampal gyrus and the neocortex of the temporal lobe, observed in the last two stages of the experiment (after 9 and 12 months), coexisted with increased number of microglial cells and, especially after 12 months, with intense phagocytic activity within these cells. At the ultrastructural level, phagocyte microglial cells were hypertrophied with several broadened processes. Their cytoplasm contained rich lysosomal apparatus, numerous lipofuscin-like structures, lipid droplets and multilaminated bodies. The nuclei of these cells were characteristic oval or round and sometimes triangle in shape with dense and highly clumped heterochromatin, distinctly accumulated under nuclear envelope, and sparse euchromatin. Microglia/macrophages were frequently present in a close vicinity of changed neuronal somata and also close to the altered elements of the neuropil pyramidal layer of the cortex. Microglial response may, together with abnormalities in neurones, astroglia and blood–brain barrier, play a significant role in the development of experimental valproate encephalopathy.
Keywords: hippocampal cortex, microglia/macrophages, neocortex, rats, ultrastructure, valproate
The term microglia refers to intrinsic cells of the central nervous system (CNS) of mesodermal origin, characteristic morphology, and antigen phenotype consistent with that of a specialized type of mononuclear phagocyte. These cells are distributed ubiquitously throughout the CNS and comprise up to 20% of the cell population in rodent brain. Microglia are the major components of macrophages found in the brain (Perry & Gordon 1988; Dickson et al. 1991; Dickson & Lee 1995).
The morphology of cells with microglia phenotype in vivo varies according to the location and to the microenvironmental factors. This ranges from compact amoeboid cells to widely ramified cells, with considerable variation in the length and complexity of branching of the processes (Giulian & Baker 1986; Perry & Gordon 1988; Dickson & Lee 1995).
Functionally, microglia are an important immune effector cell population in the brain (Perry & Gordon 1988; Streit et al. 1988; Graeber & Streit 1990; Gehrmann et al. 1992; McGeer et al. 1993) and have been implicated in the pathogenesis of several human neurological disorders, e.g. Alzheimer's disease, Parkinson's disease, multiple sclerosis, human immunodeficiency virus encephalopathy and amylotrophic lateral sclerosis (Dickson et al. 1991; McGeer et al. 1993; Dickson & Lee 1995; Le et al. 2001).
According to Banati (Banati 2002; Bonati et al. 1993a), the characteristic feature of microglia is their capacity for swift activation in response to neuronal stress and for site-directed phagocytosis. The most common way to study microglia in vitro is to use cultures derived from neonatal rat brain.
Under these experimental conditions, their differentiation into a macrophage-like morphology and immunophenotype has been demonstrated (Giulian & Baker 1986; Frei et al. 1987; Graeber et al. 1989; Banati et al. 1991). However, to study the differentiation of microglia into phagocytes in vivo, remote lesion models, e.g. facial nerve transection and facial nerve degeneration induced by the injection of toxic ricin (which leave the blood–brain barrier untouched), have been used (Streit et al. 1988). The phagocytic capacity of microglial cells has also been studied in other neuropathologies, such as in the deafferented dentate gyrus, which is a model of anterograde degeneration (Gehrmann et al. 1991).
In previous studies, we have investigated the effects of chronic valproate exposure on different cellular elements of selected CNS regions, of both neuroectodermal and mesenchymal origin, including the cerebellar cortex, the hippocampal gyrus and the neocortex of the temporal lobe. Long-term administration of the antiepileptic and antipsychotic drug sodium valproate (VPA) to rats caused significant ultrastructural degenerative changes in nerve cell perikarya and their processes, protoplasmic astrocytes and morphology of the blood–brain barrier in all the CNS sites mentioned above (Sendrowski et al. 2003; Sobaniec-Lotowska & Sobaniec 1996a; Sobaniec-Lotowska et al. 2000, 2001; Sobaniec-Lotowska 2001b, 2002, 2003).
The aim of the present study was the ultrastructural analysis of microglia which appear in the cortex of the hippocampal gyrus and in the neocortex of the temporal lobe of rat during the course of long-term VPA treatment. Until now, except for our preliminary studies (Sobaniec-Lotowska 2001a; Sobaniec-Lotowska & Sobaniec 1996b), such investigations have not been performed. This subject is of particular interest in the light of controversies, which surround morphogenesis and function of these cells in various experimental models of CNS damage and because of not yet fully elucidated morphological basis of valproate encephalopathy.
Materials and methods
Two groups of 3-month-old male Wistar rats (initial body mass 160–180 g) were preselected according to the standard pharmacological screening tests and used for the these studies. The animals were kept in a well sunlit room at 18–20 °C and fed standard granulated rat chow and tap water. All procedures were carried out in strict accordance with Helsinki Convention Guidelines for the care and use of laboratory animals.
Group I consisted of 30 rats receiving sodium valproate as a 20% solution of sodium salt of valproic acid daily in fasting state with an intragastric tube, at the effective dose of 200 mg/kg body weight for 1, 3, 6, 9 and 12 months (six animals in each time subgroup).
Group II consisted of 10 control animals matched in respect of age with experimental animals, receiving physiological saline in the same way as the Group I rats treated with VPA.
The rats of both the groups were subjected to behavioural examinations using Lat's test to evaluate the psychomotor and cognitive activity of the animals. The rats were weighed every 2 weeks to verify the correct dose of the antiepileptic drug.
Serum concentrations of sodium valproate in Group I were measured by gas chromatography and ranged between 60 and 135 µg/ml (mean 111.3333 µg/ml; SD 21.6131) (Sobaniec-Lotowska 2001b).
At the end of the experiment, 24 h after the termination of the final VPA administration, the animals were killed under Nembutal anaesthesia by intravital intracardiac perfusion with 2.5% glutaraldehyde in 0.1-m cacodylate buffer, pH 7.4, at constant pressure of 80 mmHg. Small specimens (six, 1 mm3) cut of the gyrus hippocampal cortex (using a magnifying glass) and neocortex of the temporal lobe were processed routinely for embedding in Epon 812. Semithin sections were stained with Toluidine Blue and examined in the light microscope. Ultrathin sections were double stained with uranyl acetate and lead citrate and examined using an Opton 900 PC transmission electron microscope (Zeiss, Oberkochen, Germany) (Sobaniec-Lotowska & Sobaniec 1996a; Sobaniec-Lotowska 2001b, 2002, 2003).
The material obtained from the cortex of CNS structures examined in the control group was processed using the same techniques as for the VPA-treated animals.
Results
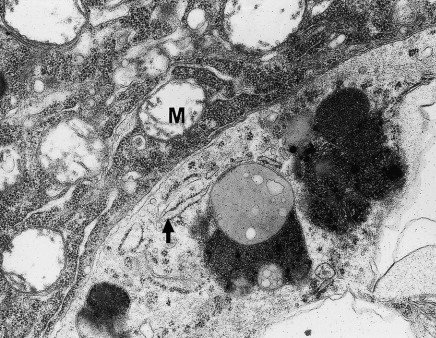
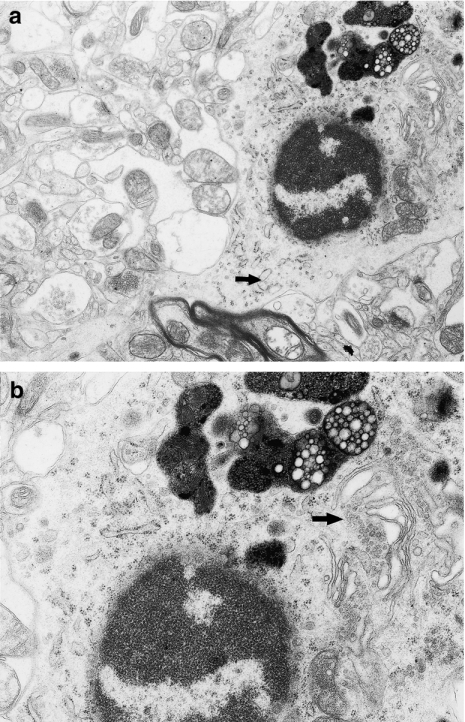
In previous studies of chronic administration of VPA to rats, the first discrete electron microscopic changes in structural elements of the cortex of the hippocampal gyrus and the neocortex of the temporal lobe were observed after 3 months, and these changes intensified with time, being most pronounced after 9 and 12 months. These changes include significant disorders of structural integrity of the CNS regions examined, and at 9 and 12 months, they coexisted with changes in microglial cells (Figures 1 and 2). These were increased in number especially after 12 months. These numerous, round or oval cells with features of microglial and macrophage cells were most frequently seen in the pyramidal layer of the cortex of the CNS regions examined, in close vicinity of damaged neuronal perikarya (identifiable as ischaemic neurones or sometimes as fragments of cells) (Figures 3a, 5 and 6) or injured neuropil elements (Figure 4a). As macrophages, these cells showed similar ultrastructural change, irrespective of location in CNS. These findings about the cells found in the cortex of the hippocampal gyrus and in the neocortex of the temporal lobe are presented together.
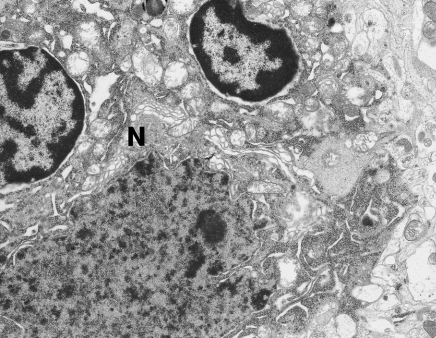
Figure 1.
Two microglial cells adhering to dark neurone (N). The nuclei of these cells contain dense heterochromatin distinctly accumulated under the nuclear envelope. Hippocampal cortex. Nine months of valproate administration. Original magnification ×4400.
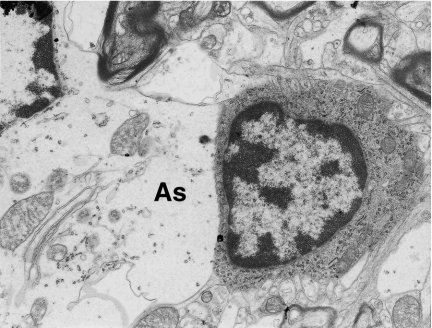
Figure 2.
A well-preserved microglial cell with a triangle-shaped nucleus in a close vicinity of swollen protoplasmic astrocyte (As). Neocortex. Nine months of valproate administration. Original magnification ×7000.
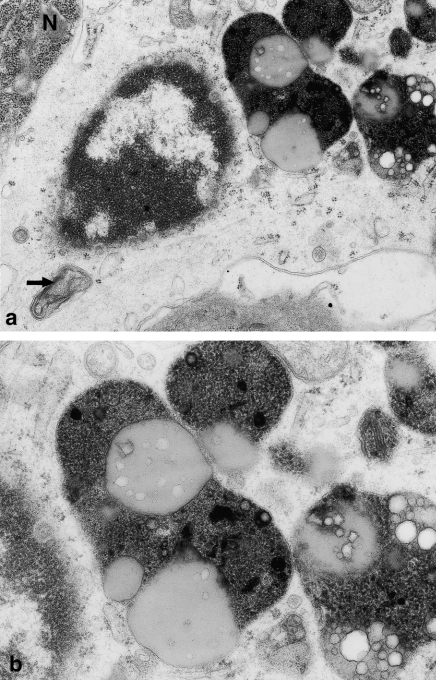
Figure 3.
(a) Fragment of microglial cell perikaryon showing features of marked phagocytic activity; the nucleus contains dense, highly clumped heterochromatin; and under nucleus visible multilaminated body (→). N – small fragment of dark neurone. Hippocampal cortex. Nine months of valproate administration. Original magnification ×12,000. (b) Higher magnification (×20,000) of microglial cell perikaryon, showing lipofuscin-like structures and frothy lipophagosomes of phagocytic microglial cell.
Figure 5.
Phagocytic microglial cell adhering to the pericaryon of damaged pyramidal neurone – channels of granular endoplasmic reticulum within neuronal cytoplasm markedly broadened. Hippocampal cortex. Twelve months of valproate administration. Original magnification ×7000.
Figure 6.
Fragment of phagocytic microglial cell in a close vicinity of injured pyramidal neurone. The cytoplasm of phagocyte contains channels of granular endoplasmic reticulum (→). M – altered neuronal mitochondria. Neocortex. Twelve months of valproate administration. Original magnification ×12,000.
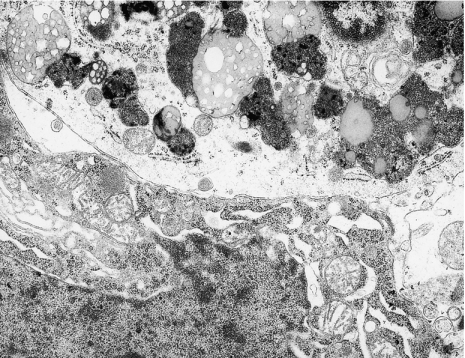
Figure 4.
(a) Phagocytic microglial cell in a close vicinity of swollen neuropil elements. The nucleus of this cell is round and contains dense heterochromatin, highly clumped under nuclear envelope and sparse euchromatin; in the swollen cytoplasm visible profiles of granular endoplasmic reticulum (→). Neocortex. Nine months of valproate administration. Original magnification ×4000. (b) Higher magnification (×12,000) of phagocytic microglial cell. Well-developed frothy lipophagosomes and lipofuscin-like bodies and dilated Golgi apparatus (→).
The phagocytic microglial cells were enlarged (with a high nuclear to cytoplasmic ratio) with expanded cytoplasm and broad processes. They showed prominent features of phagocytic activity, such as abundant lysosomes with characteristic, well-developed, highly polymorphic, frothy lipophagosomes, numerous deposits of lipofuscin-like material, lipid droplets and multilaminated bodies (Figures 3–6).
The nuclei were characteristic oval or round and sometimes triangular in shape with dense and highly clumped heterochromatin, accumulated distinctly under the nuclear envelope. The euchromatin was sparse (Figures 3 and 4a,b).
The perinuclear region showed few conspicuous organelles, usually including a few strands of granular endoplasmic reticulum and dilated Golgi apparatus (Figures 4a,b and 6). Some mitochondria were normal, but others were swollen with degenerative changes.
At 12 months, the phagocytic cells showed similar general features, but some cells contained more phagolysosomes than were seen at 9 months, and also showed features suggesting degradation of ingested material.
Discussion
At the end of chronic VPA administration, i.e. after 9 and 12 months, significant abnormalities in the structural elements of the cortex of the hippocampal gyrus and the neocortex of the temporal lobe were accompanied by increased number of microglial cells and by evidence suggesting intense phagocytic activity within these cells.
Microglia/macrophages were present frequently in a close vicinity of damaged neuronal cells and in the neighbourhood of altered elements of the neuropil pyramidal layer of the cortex of all areas studied.
The morphological features of microglial reaction described in the present study were not specific for VPA. Similar ultrastructure of microglial and macrophagic cells in vivo in various regions of CNS has been observed under other conditions, e.g. in the arcuate nucleus after neurotoxic injury induced by a single subcutaneous injection of monosodium glutamate in neonatal rats (Pelaez et al. 1999); in the lizard medial cortex, after intraperitoneal injection of the neurotoxin 3-acetylopyridine (Nacher et al. 1999); in the transgenic mouse model for CNS primary demyelination and motor disease, where the cytokine interleukin-3 is expressed in astrocytes (Chiang et al. 1996; Powell et al. 1999); in the developing cerebellum of rats exposed to X-ray irradiation (Das 1976); and in the cerebellar cortex of rats following an episode of total ischaemia (Walski & Borowicz 1995).
The differentiation of microglia into brain macrophages in response to neuronal stress is accompanied by the release of several secretory products. They include lysosomal proteinases, such as cathepsin B/L (Banati et al. 1993b); cytokines (e.g. interleukin-1, interleukin-6, neurokines, tumour necrosis factor-alpha; Griffin et al. 1989; Dickson et al. 1991; Banati 2002); colony-stimulating factors (CSFs), particularly macrophage CSF and granulocyte macrophage CSF (Giulian & Ingeman 1988; Suzumura et al. 1990); and excitatory amino acids like glutamate (Giulian et al. 1990; Piani et al. 1991).
Thus, the microglia/macrophages found in the CNS in the present study following chronic administration of VPA and/or its metabolites participate in the removal/phagocytosis of injured nervous tissue. Together with abnormalities in neurones, astroglia and blood–brain barrier, cells must play a role in the development of experimental valproate encephalopathy, and with the toxic effects of VPA in humans treated with these drugs. However, the ultrastructural findings favour a reactive phagocytic role, rather than a secretory role, for these cells during the development of the neuropathology.
References
- Banati RB. Brain plasticity and microglia: is transsynaptic glial activation in the thalamus after limb denervation linked to cortical plasticity and central sensitisation. J Physiol Paris. 2002;96:289–299. doi: 10.1016/s0928-4257(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Banati RB, Gehrmann JG, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993a;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Banati RB, Rothe G, Kreutzberg GW. Detection of lysosomal proteinases in microglia: flow cytometric measurement and histochemical localization of cathepsin B and L. Glia. 1993b;7:183–191. doi: 10.1002/glia.440070208. [DOI] [PubMed] [Google Scholar]
- Banati FB, Rothe G, Valet G, Kreutzberg GW. Respiratory burst in brain macrophages: a flow cytometric study on cultured rat microglia. Neuropathol Appl Neurobiol. 1991;17:223–230. doi: 10.1111/j.1365-2990.1991.tb00718.x. [DOI] [PubMed] [Google Scholar]
- Chiang CS, Powell HC, Gold LH, Samimi A, Campbell IL. Macrophage/microglial-mediated primary demyelination and motor disease induced by the central nervous system production of interleukin-3 in transgenic mice. J Clin Invest. 1996;97:1512–1524. doi: 10.1172/JCI118574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das GD. Resting and reactive macrophages in the developing cerebellum: an experimental ultrastructural study. Virchows Arch B Cell Pathol. 1976;20:287–298. doi: 10.1007/BF02890347. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Lee SC. Microglia. In: Vinken PJ, Bruyn GW, Dewolff FA, editors. Handbook of Clinical Neurology. Vol. 65. Amsterdam: Elsevier; 1995. pp. 165–178. [Google Scholar]
- Dickson DW, Mattiace LA, Kure K, Hutchins K, Lyman DW, Brosnan CF. Microglia in human disease, with an emphasis on acquired immune deficiency syndrome. Lab Invest. 1991;64:135–156. [PubMed] [Google Scholar]
- Frei K, Siepl C, Groscurth P, Bodmer S, Schwerdel C, Fontana A. Antigen presentation and tumor cytotoxicity by intrerferon-gamma-treated microglial cells. Eur J Immunol. 1987;17:1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Schoen SW, Kreutzberg GW. Lesion of the rat entorhinal cortex leads to a rapid microglial reaction in the dentate gyrus. A light and electron microscopical study. Acta Neuropathol (Berl) 1991;82:442–455. doi: 10.1007/BF00293378. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Bonnekoh P, Takahito M, Hossmann KA, Kreutzberg GW. Immunocytochemical study of an early microglia activation in ischemia. J Cereb Blood Flow Metab. 1992;12:257–269. doi: 10.1038/jcbfm.1992.36. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Ingeman JE. Colony-stimulating factors as promotors of ameboid microglia. J Neurosci. 1988;8:4707–4717. doi: 10.1523/JNEUROSCI.08-12-04707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: immune network in the brain. Brain Pathol. 1990;1:2–5. doi: 10.1111/j.1750-3639.1990.tb00630.x. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Banati RB, Streit WJ, Kreutzberg GW. Immunophenotypic characterization of rat brain macrophages in culture. Neurosci Lett. 1989;103:241–246. doi: 10.1016/0304-3940(89)90106-7. [DOI] [PubMed] [Google Scholar]
- Griffin WST, Stanley LC, Ling C, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le W, Rowe D, Xie W, Ortiz I, He Y, Appel SH. Microglial activation of dopaminergic cell injury: an in vitro model relevant to Parkinson's disease. J Neurosci. 2001;21:8447–8455. doi: 10.1523/JNEUROSCI.21-21-08447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Kawamata T, Walker DG, Akiyama H, Tooyama I, McGeer E. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- Nacher J, Ramirez C, Palop JJ, Molowny A, Luis De La Iglesia JA, Lopez-Garcia C. Radial glia and cell debris removal during lesion – regeneration of the lizard medial cortex. Histol Histopathol. 1999;14:89–101. doi: 10.14670/HH-14.89. [DOI] [PubMed] [Google Scholar]
- Pelaez B, Blazquez JL, Pastor FE, Sanchez A, Amat P. Lectin histochemistry and ultrastructure of microglial response to monosodium glutamate-mediated neurotoxicity in the arcuate nucleus. Histol Histopathol. 1999;14:165–174. doi: 10.14670/HH-14.165. [DOI] [PubMed] [Google Scholar]
- Perry VH, Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988;11:273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Piani D, Frei K, Do KQ, Cuenod M, Fontana A. Murine brain macrophages included NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci Lett. 1991;133:159–162. doi: 10.1016/0304-3940(91)90559-c. [DOI] [PubMed] [Google Scholar]
- Powell HC, Garrett RS, Brett FM, et al. Response of glia, mast cells and the blood brain barrier, in transgenic mice expressing interleukin-3 in astrocytes, an experimental model for CNS demyelination. Brain Pathol. 1999;9:219–235. doi: 10.1111/j.1750-3639.1999.tb00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendrowski K, Sobaniec-Lotowska M, Sobaniec W. Ultrastructure of hippocampal capillaries in experimental valproate encephalopathy. Eur J Paediatr Neurol. 2003;7:A25–A26. (abstract). [Google Scholar]
- Sobaniec-Lotowska M. Ultrastructure of microglial response to prolonged application of valproate and after its terminating in temporal lobe neocortex. Virchows Arch. 2001a;439:474. (abstract). [Google Scholar]
- Sobaniec-Lotowska M. Ultrastructure of Purkinje perikarya and their dendritic processes in the rat cerebellar cortex in experimental encephalopathy induced by chronic application of valproate. Int J Exp Path. 2001b;8:337–348. doi: 10.1046/j.1365-2613.2001.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobaniec-Lotowska M. Ultrastructure of synaptic junctions in the cerebellar cortex in experimental valproate encephalopathy and after terminating chronic application of the antiepileptic. Folia Neuropathol. 2002;40:87–96. [PubMed] [Google Scholar]
- Sobaniec-Lotowska M. Ultrastructure of astrocytes in the cortex of the hippocampal gyrus and in the neocortex of the temporal lobe in experimental valproate encephalopathy and after valproate withdrawal. Int J Exp Path. 2003;84:115–126. doi: 10.1046/j.1365-2613.2003.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobaniec-Lotowska M, Sobaniec W. Morphological features of encephalopathy after chronic administration of the antiepileptic drug valproate to rats. A transmission electron microscopic study of capillaries in the cerebellar cortex. Exp Toxicol Pathol. 1996a;48:65–75. doi: 10.1016/S0940-2993(96)80094-7. [DOI] [PubMed] [Google Scholar]
- Sobaniec-Lotowska M, Sobaniec W. Ultrastructural picture of mononuclear phagocytes of the cerebellar cortex in rats in the course of chronic administration of basic antiepileptic valproate. Eur J Haematol Suppl. 1996b;57:48. (abstract). [Google Scholar]
- Sobaniec-Lotowska M, Sobaniec H, Sobaniec W, Sendrowski K. Electron microscopic studies of the capillaries in the temporal neocortex in experimental valproate encephalopathy. Aktuelle Neurol. 2000;27:159. (abstract). [Google Scholar]
- Sobaniec-Lotowska M, Sobaniec W, Augustynowicz A. Morphometric analysis of the cerebellar cortex capillaries in the course of experimental valproate encephalopathy and after chronic exposure to sodium valproate using transmission electron microscopy. Folia Neuropathol. 2001;39:277–280. [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Sawada M, Yamamoto H, Marunouchi T. Effects of colony stimulating factors on isolated microglia in vitro. J Neuroimmunol. 1990;30:111–120. doi: 10.1016/0165-5728(90)90094-4. [DOI] [PubMed] [Google Scholar]
- Walski M, Borowicz J. Brain phagocytes and microglia of the cerebellar cortex of rats following an incident of total ischaemia. J Brain Res. 1995;1:55–66. [PubMed] [Google Scholar]