Abstract
Objective
Heat shock protein (HSP) 70, a conserved member of the stress protein family, is produced in almost all cell types in response to a wide range of stressful stimuli and their production has a survival value. Evidence suggests that extra-cellular HSP70 is involved in the activation of the innate and adaptive immune response. Furthermore, increased mRNA expression of HSP 70 was observed in human fetal membranes following endotoxin stimulation. This study was conducted to determine the changes in amniotic fluid HSP70 concentrations during pregnancy, term and preterm parturition, intra-amniotic infection (IAI), and histologic chorioamnionitis.
Study design
A cross-sectional study was conducted in 376 pregnant women in the following groups: 1) women with a normal pregnancy that were classified in the following categories: a) women in the mid-trimester (14–18 weeks) who underwent amniocentesis for genetic indications and delivered normal infants at term (n=72); b) women at term not in labor (n=23); and c) those at term in labor (n=48); 2) women with spontaneous preterm labor and intact membranes that were subdivided into the following categories: a) preterm labor who delivered at term without IAI (n=42), b) preterm labor who delivered preterm without IAI (n=57), and c) preterm labor and delivery with IAI (n=30); and 3) women with preterm prelabor rupture of membranes (PROM) with (n=50) and without (n=54) IAI. Among patients with preterm labor with intact membranes and preterm PROM who delivered within 72 hours of amniocentesis, placenta, umbilical cord and chorioamniotic membranes were collected and assessed for the presence or absence of acute inflammatory lesions in the extra-placental membranes (histologic chorioamnionitis) and/or umbilical cords (funisitis). HSP70 concentrations in amniotic fluid were determined using a sensitive and specific immunoassay. Non-parametric statistics were used for analysis. A p value <0.05 was considered statistically significant.
Results
Immunoreactive HSP70 was detected in 88% (332/376) of amniotic fluid samples. The median amniotic fluid HSP70 concentration was significantly higher in women at term without labor than in those in the mid-trimester (term no labor; median 34.9 ng/mL, range 0–78.1 ng/mL vs. mid-trimester; median 6.6 ng/mL, range 0–20.8 ng/mL; p<0.001). Among patients with spontaneous preterm labor and preterm PROM, those with IAI had a significantly higher median amniotic fluid HSP70 concentration than those without IAI (preterm labor with IAI: median 82.9 ng/ml, range 0–500 ng/ml vs. preterm labor without IAI: median 41.7 ng/ml, range 0–244 ng/ml; p=0.001; preterm PROM with IAI: median 86.5 ng/ml, range 0–428 ng/ml, vs. preterm PROM without IAI: median 55.9 ng/ml, range 14.9–299.9 ng/mL; p=0.007). There was no significant difference in the median amniotic fluid HSP70 concentration between patients with preterm labor who delivered preterm without IAI and those who delivered at term (p=0.6). However, among patients with preterm labor without IAI, there was an inverse relationship between amniotic fluid concentration of HSP70 and the amniocentesis-to-spontaneous delivery interval (Spearman’s Rho = −0.26; p=0.02). Patients with histologic chorioamnionitis/funisitis had a significantly higher median amniotic fluid HSP70 concentration than those without inflammation (inflammation: median 108.7 ng/ml, range 0–500 ng/ml vs. without inflammation: median 67.9 ng/ml, range 7.1–299.9 ng/ml; p=0.02). Women at term in labor had a median amniotic fluid concentration of HSP70 significantly higher than those not in labor (term in labor: median 60.7 ng/ml, range 0–359.9 ng/ml vs. term not in labor: median 34.9 ng/ml, range 0–78.1 ng/ml; p=0.02).
Conclusions
Intra-amniotic infection, histologic chorioamnionitis and term parturition are associated with elevated amniotic fluid HSP70 concentration. HSP70 plays a role in the host defense mechanism by activating the innate arm of the immune response in women with intrauterine infection. The mechanisms of preterm and term parturition in human may involve extra-cellular HSP 70.
Keywords: HSP70, intra-amniotic infection, preterm labor, preterm prelabor rupture of membranes, histologic chorioamnionitis, parturition
INTRODUCTION
Heat shock proteins (HSPs) are highly conserved molecules [1,2] that are present in almost all sub-cellular structures (eg: nucleus, mitochondria, endoplasmic reticulum and cytoplasm) of all cell types from prokayotes to eukaryotes [2]. HSPs regulate intracellular processes to maintain homeostasis during cell proliferation/differentiation and thus, function as molecular chaperones [3–6]. An increased expression of intracellular HSPs is observed following cell exposure to stressful stimuli such as hypoxia, ischemia and high temperature [7,8]. HSPs are categorized into several families according to their approximate molecular weight (eg: HSP40, HSP60, HSP70, HSP90 and HSP110). Among all HSPs, HSP70 is the best characterized [9].
HSPs also participate in innate and adaptive immune responses and originally, were considered to be exclusively intracellular proteins. Their presence in the extra-cellular compartment reflects tissue damage or “danger signals” [10]. HSPs released from necrotic cells have been proposed to activate monocytes through diverse cell-surface receptors (CD14, Toll-like receptor (TLR), CD40 etc.) [11–17]. which, in turn, stimulate production of pro-inflammatory cytokines [18–20]. HSPs participate in antigen processing and presentation by antigen presenting cells (APC), which elicit a robust T cell response in adaptive immunity [21]. However, recent evidence suggests that HSPs can be released from cells without necrosis [22,23]. Indeed, rat glia cells, [24], human islet cells [25], and human peripheral blood mononuclear cells [26], have been shown to release HSPs by exocytosis in the absence of detectable cell death [23]. HSP60 [27] and HSP70 [28] are normally present in serum of healthy individuals. However, psychological stressors have been shown to increase circulating HSPs in animal experiments [29,30] and changes in HSP concentration in blood have been reported in several pathological conditions. Elevation of serum HSP60 concentration was observed in patients with early atherosclerosis [31] and high serum concentrations of HSP70 were reported in patients with peripheral/renal vascular disease [32] and in those with preeclampsia [33].
Infection is an important mechanism of disease in preterm parturition [34–40]. Indeed, it is the only pathologic process for which a firm causal link with prematurity has been established and a defined molecular pathophysiology is known [41]. Moreover, intra-amniotic infection/inflammation has been implicated in the genesis of fetal and neonatal injury [42,43] leading to cerebral palsy [44] and chronic lung disease [45]. Several lines of evidence suggest a role for HSP70 in preterm labor. Among patients who were at risk for preterm delivery, the mean serum concentration of HSP70 was higher in patients who delivered preterm than in those who delivered at term [33]. Moreover, increased mRNA expression of HSP70 was observed in cultured human amnion following endotoxin stimulation [46]. Finally, HSP70 antigen antibody complexes were detected in the placenta of some patients who delivered preterm [47]. There is no previous information on HSP70 concentrations in the amniotic cavity of patients with preterm labor.
The aim of this study was to determine the changes of amniotic fluid HSP70 concentrations throughout gestation, during parturition in term and preterm pregnancies, in the presence of intra-amniotic infection (IAI) and histologic chorioamnionitis.
MATERIALS AND METHODS
Study design and population
A cross-sectional study was conducted by searching our clinical database and bank of biologic samples. A total of 376 women were classified into three groups: 1) women with a normal pregnancy that were divided into the following categories: a) women in the mid-trimester (14–18 weeks) who underwent amniocentesis for genetic indications and delivered normal infants at term (n=72), and b) women at term with (n=48) and without (n=23) labor; 2) women with spontaneous preterm labor and intact membranes that were subdivided into the following categories: a) preterm labor who delivered at term without IAI (n=42), b) preterm labor who delivered preterm without IAI (n=57), and c) preterm labor and delivery with IAI (n=30); and 3) women with preterm prelabor rupture of membranes (PROM) with (n=50) and without (n=54) IAI.
Clinical definitions
Preterm labor was diagnosed by the presence of at least two regular uterine contractions every 10 minutes associated with cervical changes that required admission to the hospital before 37 weeks gestation. IAI was defined as an amniotic fluid culture that was positive for microorganisms. The results of the amniotic fluid analyses were used for clinical management. Women at term not in labor underwent amniocentesis for the assessment of fetal lung maturity prior to cesarean section. Women at term in labor consisted of women who were suspected to have preterm labor because of uncertain dates and had an amniocentesis for the assessment of microbial invasion and fetal lung maturity. However, if they delivered a neonate greater than 2,500 grams without complications of prematurity, they were considered likely to represent patients in spontaneous labor at term. PROM was defined as amniorrhexis before the onset of spontaneous labor. Membrane rupture was diagnosed by vaginal pooling, ferning, or a positive nitrazine test.
Sample Collection
Amniotic fluid collection was performed by trans-abdominal amniocentesis under ultrasonographic guidance. Amniotic fluid was transported to the laboratory and cultured for aerobic/anaerobic bacteria as well as genital Mycoplasmas. White blood cell (WBC) count, glucose concentration, and Gram stain for microorganisms were performed in amniotic fluid. Among patients with preterm labor with intact membranes and preterm PROM, placenta, umbilical cords and chorioamniotic membranes were collected. The presence or absence of acute inflammatory lesions in the extra-placental membranes (histologic chorioamnionitis) and/or umbilical cords (funisitis) in those who delivered within 72 hours of amniocentesis was assessed as previously described [48]. This period of time was selected to preserve a meaningful temporal relationship between amniotic fluid HSP70 concentrations and membrane pathologic findings.
All women provided a written informed consent prior to the collection of samples. The Institutional Review Boards of Wayne State University, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS) approved the collection and utilization of samples for research purposes. Many of these samples have been employed to study the biology of cytokines [49–52], chemokines [53], antimicrobial peptides [54], and growth factors [55] in normal pregnant women and in those with pregnancy complications.
HSP70 immunoassays
The immunoassay kits (Stressgen Biotechnology Corporation, Victoria, BC, Canada) are specific for both native and recombinant HSP70. The HSP70 immunoassay was validated for human amniotic fluid in our laboratory. Briefly, amniotic fluid samples were incubated in duplicate wells, pre-coated with monoclonal antibodies to an inducible form of HSP70. The HSP70 protein were detected by a biotinylated monoclonal antibody. The final step involved signal amplification based on a biotin-avidin coupling in which avidin was linked to horseradish peroxidase. The amount of HSP70 was measured upon addition of tetramethylbenzidine utilizing a programmable spectrophotometer (Ceres 900 Micro plate Workstation, Bio-Tek Instruments, Winooski, VT) set to read absorbance at 450 nm. Amniotic fluid HSP70 concentrations were derived from interpolating the absorbance readings from a standard curve generated from known concentrations of HSP70. The inter- and intra-assay coefficients of variations were 6.7% and 4.4% respectively. The sensitivity was 0.7 ng/ml.
Statistical analysis
Kruskal Wallis and Mann-Whitney U tests were used to determine the differences in the median amniotic fluid HSP70 concentration among and between groups, respectively. Spearman rank correlation was utilized to assess correlations between amniotic fluid concentrations of HSP70, glucose, and WBC count. A p value of <0.05 was considered statistically significant. Analysis was performed with SPSS software version 12.0 (SPSS Inc, Chicago, Illinois).
RESULTS
Demographic and clinical characteristics
The median gestational age at amniocentesis in patients with preterm labor and intact membranes without IAI, who delivered preterm, was significantly lower than in those who delivered at term (p<0.001, Table I). There was no significant difference in the median gestational age at amniocentesis between patients who delivered preterm with and without IAI (p=0.2, Table I). Similarly, there was no significant difference in the median gestational age at amniocentesis between patients with preterm PROM with and without IAI (p=0.3, Table I). Demographic and clinical characteristics of women in the mid-trimester, women at term not in labor, and women at term in labor are displayed in Table II.
Table I.
Demographic and clinical characteristics of patients with preterm labor and intact membranes (PTL) and patients with preterm prelabor rupture of membranes (PROM)
| Characteristics | PTL who delivered at term without IAI (n=42) | p | PTL who delivered preterm without IAI (n=57) | pa | PTL with IAI (n=30) | pb | Preterm PROM without IAI (n=54) | pc | Preterm PROM with IAI (n=50) |
|---|---|---|---|---|---|---|---|---|---|
| Maternal age (years) | 21 (16–39) | 0.2 | 23 (15–38) | 0.5 | 25 (16–33) | 0.3 | 24 (15–41) | 0.1 | 28 (17–39) |
| 0.2 | 0.4 | 0.04* | 0.9 | ||||||
| Nulliparous | 10 (24%) | 21 (37%) | 14 (47%) | 18 (33%) | 16 (32%) | ||||
| Race | |||||||||
| African-American | 37 (88%) | 0.8 | 49 (86%) | 0.7 | 27 (90%) | 0.4 | 43 (80%) | 0.2 | 46 (92%) |
| Caucasian | 3 (7%) | 5 (9%) | 2 (7%) | 10 (18%) | 4 (8%) | ||||
| Hispanic | 2 (5%) | 2 (3%) | 0 | 1 (2%) | 0 | ||||
| Other | 0 | 1 (2%) | 1 (3%) | 0 | 0 | ||||
| Gestational age at amniocentesis (weeks) | 30.6 (23.3–34.0) | <0.001* | 27.3 (20.6–34.0) | 0.2 | 25.9 (20.0–33.3) | <0.001* | 30.5 (20.0–33.7) | 0.3 | 29.9 (20.0–33.9) |
| 0.003* | |||||||||
| Gestational age at delivery (weeks) | 39.0 (37.0–41.8) | 30.7 (20.9–36.7) | 26.1 (20.3–34.4) | 31.8 (20.0–35.0) | 29.9 (20.1–34.1) | ||||
| <0.001* | 0.002* | <0.001* | |||||||
| 1.0 | 0.3 | 0.09 | |||||||
| Amniotic fluid HSP 70 detectable | 42 (100%) | 56 (98%) | 28 (93%) | 54 (100%) | 46 (92%) | ||||
| 0.05 | |||||||||
IAI: intra-amniotic infection; Values are expressed as median (range) or numbers (percent);
p<0.05;
p comparison between PTL who delivered preterm without IAI and PTL with IAI;
p comparison between PTL who delivered at term without IAI and PTL with IAI;
p comparison between preterm PROM with and without IAI
Table II.
Demographic and clinical characteristics of women at midtrimester, women at term not in labor and women at term in labor
| Characteristics | Women at midtrimester (n=72) | Women at term, not in labor (n=23) | Women at term, in labor (n=48) |
|---|---|---|---|
| Maternal age (years) | 37 (24–42) | 27 (17–40) | 23 (16–37) |
| Gestational age at amniocentesis (weeks) | 16 (14 –18) | 39 (38–42) | 39 (37–41) |
| Gestational age at delivery (weeks) | 40 (37–42) | 39 (38–42) | 39 (37–41) |
| Amniotic fluid HSP 70 detectable | 41 (57%) | 21 (91%) | 44 (92%) |
Values are expressed as median (range) or numbers (percent)
Changes in amniotic fluid HSP70 concentration during normal pregnancy
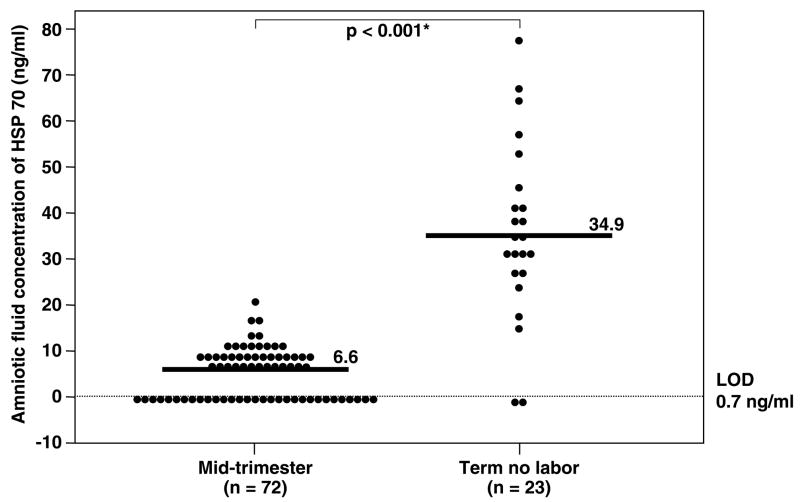
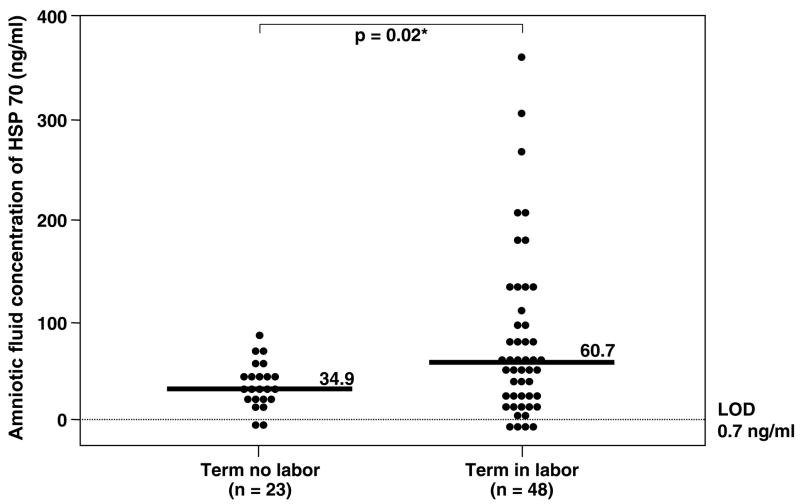
Immunoreactive HSP70 was detected in 88% (332/376) of all amniotic fluid samples. However, HSP70 was detected in only 57% (41/72) of women in the mid-trimester. The median amniotic fluid HSP70 concentration was significantly higher in women at term not in labor than in those in the mid-trimester (term no labor: median 34.9 ng/ml, range 0–78.1 ng/ml vs. mid-trimester: median 6.6 ng/ml, range 0–20.8 ng/ml; p<0.001; Figure 1). Women at term in spontaneous labor had a significantly higher median amniotic fluid HSP70 concentration than those not in labor (term in labor: median 60.7 ng/ml, range 0–359.9 ng/ml vs. term not in labor: median 34.9 ng/ml, range 0–78.1 ng/ml; p=0.02; Figure 2).
Figure 1.
Amniotic fluid heat shock protein (HSP) 70 concentration in women at mid-trimester and at term gestation not in labor. The median amniotic fluid concentration of HSP70 in women at term not in labor was significantly higher than in women at mid-trimester (term not in labor: median 34.9 ng/ml, range 0–78.1 ng/ml vs. mid-trimester: median 6.6 ng/ml, range 0–20.8 ng/ml; p<0.001). LOD: limit of detection. *p<0.05.
Figure 2.
Amniotic fluid heat shock protein (HSP) 70 concentration in women at term gestation. Women in spontaneous labor had a median amniotic fluid HSP70 concentration significantly higher than those not in labor (term in labor: median 60.7 ng/ml, range 0–359.9 ng/ml vs. term not in labor: median 34.9 ng/ml, range 0–78.1 ng/ml; p=0.02). LOD: limit of detection. *p<0.05.
Changes in amniotic fluid HSP70 concentration during preterm labor and preterm PROM
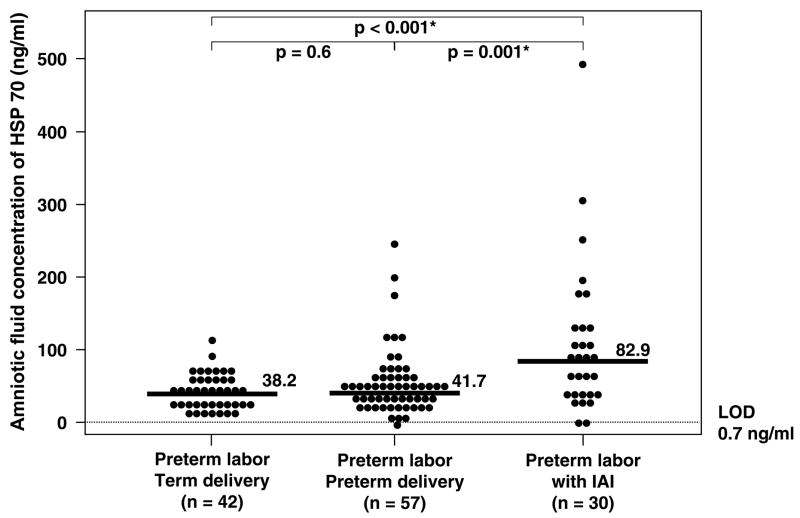
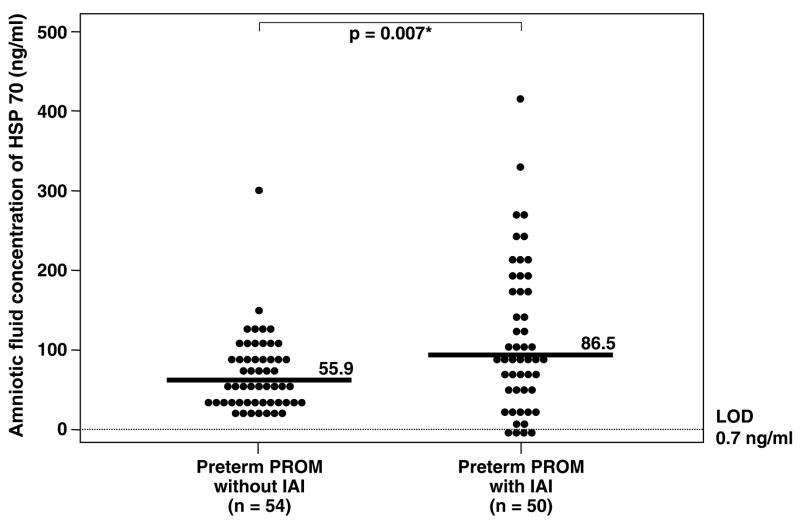
Patients with IAI and either intact or ruptured membranes had a significantly higher median amniotic fluid HSP70 concentration than those without IAI (preterm labor with IAI: median 82.9 ng/ml, range 0–500 ng/ml vs. preterm labor who delivered preterm without IAI: median 41.7 ng/ml, range 0–244 ng/ml; p=0.001) and (preterm PROM with IAI: median 86.5 ng/ml, range 0–428 ng/ml vs. preterm PROM without IAI: median 55.9 ng/ml, range 14.9–299.9 ng/ml; p=0.007; Figures 3 and 4). No significant difference in the median amniotic fluid HSP70 concentration was found between patients with preterm labor and sterile amniotic fluid who delivered preterm and those who delivered at term (preterm labor who delivered preterm without IAI: median 41.7 ng/ml, range 0–244 ng/ml vs. preterm labor who delivered at term without IAI: median 38.2 ng/ml, range 7.8–110.7 ng/ml; p=0.6; Figure 3). Similar results were obtained after adjusting for duration of sample storage and gestational age at amniocentesis using analysis of covariance (p=0.4). However, among patients with preterm labor who delivered at term and preterm without IAI, there was an inverse relationship between amniotic fluid concentration of HSP70 and the length of the amniocentesis-to-spontaneous delivery interval (Spearman’s Rho = −0.26; p=0.02).
Figure 3.
Amniotic fluid heat shock protein (HSP) 70 concentration in women with preterm labor and intact membranes. The median amniotic fluid HSP70 concentration in woman with preterm labor with intra-amniotic infection (IAI) was significantly higher than in those without IAI who delivered preterm (preterm labor with IAI: median 82.9 ng/ml, range 0–500 ng/ml vs. preterm labor who delivered preterm without IAI: median 41.7 ng/ml, range 0–244 ng/ml; p=0.001). There was no significant difference in the median amniotic fluid HSP70 concentration between women with preterm labor who delivered preterm without IAI and those who delivered at term (preterm labor who delivered preterm: median 41.7 ng/ml, range 0–244 ng/ml vs. preterm labor who delivered at term: median 38.2 ng/ml, range 7.8–110.7 ng/ml; p=0.6). LOD: limit of detection. *p<0.05.
Figure 4.
Amniotic fluid heat shock protein (HSP) 70 concentration in women with preterm prelabor rupture of membranes (preterm PROM). The median amniotic fluid concentration of HSP70 was significantly higher in women with preterm PROM with intra-amniotic infection (IAI) than in those without IAI (IAI: median 86.5 ng/ml, range 0–428 ng/ml vs. without IAI: median 55.9 ng/ml, range 14.9–299.9 ng/mL; p=0.007). LOD: limit of detection. *p<0.05.
Among patients with preterm labor and preterm PROM, there was a positive correlation between the amniotic fluid concentration of HSP70 and WBC count (Spearman’s rho=0.4; p<0.001), and a negative correlation between the amniotic fluid concentration of HSP70 and glucose (Spearman’s rho= −0.3; p<0.001).
Amniotic fluid HSP70 concentration and histologic chorioamnionitis
Placental pathology was available in 92% (36/39) of patients with spontaneous preterm labor and in 98% (53/54) of those with preterm PROM who delivered within 72 hours of amniocenteses. Patients with evidence of inflammation in the extra-placental membranes (histologic chorioamnionitis) and/or umbilical cords (funisitis) (n=66) had a significantly higher median amniotic fluid HSP70 concentration than those without inflammation (n=23) (inflammation: median 108.7 ng/mL, range 0–500 ng/mL vs. without inflammation: median 67.9 ng/mL, range 7.1–299.9 ng/mL; p=0.02).
DISCUSSION
Principal findings of this study
1) Immunoreactive HSP70 was present in the amniotic fluid and its concentration increased at term gestation compared to that in the mid-trimester; 2) patients with IAI (with either intact or ruptured membranes) had a higher median amniotic fluid HSP70 concentration than those without IAI; 3) amniotic fluid HSP70 concentrations correlated with indirect amniotic fluid markers for intra-amniotic infection/inflammation (WBC count and glucose concentration); 4) similarly, histologic chorioamnionitis and/or funisitis were associated with higher median amniotic fluid HSP70 concentrations; and 5) women with spontaneous labor at term had a higher median amniotic fluid concentration of HSP70 than those at term not in labor.
Biological activities of HSPs
HSPs were discovered more than 30 years ago and received their names from the observations that the expression of this group of proteins could be induced by high temperatures [56–60]. HSPs are constitutively present in nearly all cell types and considered the most abundant group of molecules in living forms [61]. In humans, there are at least 17 genes encoding for the HSP70 protein family, which are located on various chromosomes [9]. The 73 kDa HSP (HSP73 or heat shock cognate protein 70) is present constitutively, where as the 72 kDa HSP (HSP72 or HSP70) is highly inducible and under the control of the transcriptional factor “heat shock factor” [62]. HSP expression is up-regulated by various factors including environmental (heat, ultraviolet radiation [63–69], amino acids [70], heavy metals [71,72]), physiological (growth factors, cell differentiation, hormonal stimulation) [73–75], pathological (viral, bacterial or parasitic infections [76–78], fever [79], inflammation [80–83], ischemia [84,85], or autoimmunity [77,86]) conditions [8,87,88].
HSPs function as intracellular molecular chaperones by regulating folding, transportation, translocation and translation of proteins, which promotes the recovery of cellular activities after stressful stimuli [4,6,19,89,90]. Moreover, HSPs have an anti-apoptotic activity by inhibiting caspases [91], a group of enzymes that induces programmed-cell death [92]. Evidence in support of this protective function of HSPs includes: 1) HSPs protect human gastric cells from oxidative injury [93], rabbit hearts from ischemic-reperfusion injury [94], and rat retinas from light injury [95]; 2) in an animal experiment, over-expression of HSP72 protects lungs from sepsis-induced injury [96] and is associated with a reduction in hepatocyte apoptosis induced by tumor necrosis factor (TNF)-alpha [97]; 3) an increased expression of intra-cellular HSP70 in monocytes and macrophages inhibit TNF-alpha production following endotoxin stimulation [98]; 4) a preceding heat shock environment, which leads to an increased expression of HSP70, reduces sepsis-induced organ dysfunction and mortality in animal models [99–101]; and 5) polymorphisms of HSP70 gene have been reported in patients with Parkinson’s disease, suggesting a protective role of HSP70 against neuronal damage from degenerative disease [102]. Indeed, HSP70 family genes were proposed as candidate genes associated with human longevity [9].
A role of HSP70 in innate and adaptive immunity
HSPs are released extra-cellularly by either passive or active mechanisms [103]. The passive release results from necrotic cells, while the active release is from viable non-necrotic cells. The release could be as free HSPs, or within exosomes, which are internal vesicles of multivesicular bodies fused with the cell surface [22]. Exosomes can be released as free exosomes or surface membrane bound HSPs [23]. Subsequently, HSPs bind to specific receptors on the surface of specialized cells including monocytes [104], macrophages [105], B cells [106], dendritic cells [107] and natural killer (NK) cells [103,108,109].
Extracellular HSPs can stimulate the innate component of the immune system independently from their chaperone properties. Hence the term “Chaparokine” is used to represent the dual roles of HSP [15,18,110,111]. Several studies provide evidence that HSP70 utilizes both TLR-2 (a receptor for Gram-positive bacteria) and TLR-4 (a receptor for Gram-negative bacteria) to induce nuclear factor-kappa B (NFκB) [112], and elicits pro-inflammatory responses in a CD14-dependent manner [12,19,20]. HSP70 also participates in antigen processing and presentation by antigen presenting cells [11], resulting in a robust T cell response in adaptive immunity [21]. Soluble as well as membrane bound HSP70, can directly activate the cytolytic and migratory capacity of NK cells [108,109,113].
The human defense mechanisms against many infectious diseases, especially from intra-cellular pathogens (i.e. Chlamydia trachomatis, Mycobacterium tuberculosis, Plasmodium falciparum), encompass HSP70 as the immunodominant antigen [21]. Immunization with HSPs purified from pathogens has been shown to protect against diseases such as tuberculosis [114,115], peptic ulcers induced by Helicobacter pylori [116], and infection with Yersinia enterocolitica [117]. Interestingly, the administration of HSP70 purified from tumor cells generates effective anti-tumor specific immunity in animals [118–120].
HSP70 was also proposed to participate in the mechanisms of several autoimmune diseases such as systemic lupus erythromatous [121,122], rheumatoid arthritis [123,124], Graves’ disease [125], and Hashimoto thyroiditis [126,127]. Due to the similarity between eukaryotic and the prokaryotic HSPs, immune recognition of cross-reactive epitopes of pathogens and self-HSPs might be a mechanism linking infections and autoimmune diseases [14]. However, the observations that there are differences in immune responses between pathogens and self-HSPs contradict this view. In an experiment conducted in T-cell lines from synovial fluid of patients with rheumatoid arthritis, T cells stimulated with self-HSP produced Th2 type cytokines (eg: interleukin-4 and 10), which were more protective than the Th1 type pro-inflammatory response (eg: interferon gamma) [128] that was released when T cells were stimulated with bacterial HSP [129].
HSPs in normal pregnancy
The findings that more than half (57%) of normal pregnant women in the mid-trimester and almost all women (91%) at term not in labor had detectable HSP70 in amniotic fluid, support the view that HSP70 can be released extra-cellularly under physiologic conditions. Our finding is consistent with two previous studies which reported the presence of HSP70 in amniotic fluid during the mid-trimester [130,131]. Our study also found significantly higher amniotic fluid concentrations of HSP70 in patients at term than in those in the mid-trimester. This phenomenon could be beneficial to pregnant women since HSP70 might function as a “chaperokine” inside the amniotic cavity during growth and development of the fetus. In contrast, there are conflicting reports concerning the changes in HSP70 in maternal serum during pregnancy [33,132]. While Molvarec et al [132], in a recent large study, reported a significant increase in serum HSP70 concentration with advancing gestational age, Fukushima et al [33] did not find significant changes in the mean serum HSP 70 concentrations among the three trimesters. The median serum concentration of HSP70 is lower in pregnant than in non pregnant women [132].
A role of HSP70 in spontaneous labor at term
Our finding that the median amniotic fluid concentration of HSP70 is increased in women with spontaneous labor at term is novel and consistent with a previous observation that HSP70 mRNA expression in sheep myometrium was increased during spontaneous labor [133]. It is noteworthy that the increase in the median amniotic fluid concentration of HSP70 in women at term in labor is modest when compared to the increase observed in women with preterm labor with IAI (60.7 and 82.9 ng/ml respectively). The proposed mechanism that links an elevation of HSP70 mRNA expression in myometrium and spontaneous labor [133] is that the intra-cellular HSP70, by binding to the progesterone receptor, functions as a co-repressor of this receptor and suppresses progesterone binding to the nuclear response element [134–136]. However, the precise mechanism leading to an increased HSP70 concentration in amniotic fluid (which is an extra-cellular compartment) in spontaneous labor at term remains unknown. It is possible that the release of HSP70 from the intracellular compartment might be related to a mild inflammatory response and tissue remodeling process that is frequently observed in the reproductive tract during parturition at term [137–141]. Alternatively, extra-cellular HSP70 could directly stimulate prostaglandin (PG) production leading to delivery, since HSP70 has been shown to induce cyclooxygenase enzyme (COX)-2 protein expression and PGE2 production in human umbilical vein endothelial cells [142]. However, there was no information regarding whether HSP70 could stimulate PG production in human amnion.
A role of HSP70 in IAI in preterm labor and preterm PROM
The major finding of this study is that IAI is associated with a higher median amniotic fluid concentration of HSP70. This could be interpreted as reflecting a “danger signal” [143,144] or that HSP70 was released into the amniotic cavity following microbial invasion. This is consistent with the report of Jean-Pierre et al [131]], in which the recovery of Mycoplasma hominis from mid-trimester amniotic fluid was associated with an elevated median intra-amniotic HSP70 concentration. Bacterial endotoxins and HSP70 could engage with TLR-2 and TLR-4 to activate NFκB, and induce the production of pro-inflammatory cytokines including interleukin (IL)-1, IL-6 and TNF-alpha by mononuclear cells and macrophages leading to PG production and preterm delivery [15,18,110,111]. The relationship between the WBC count and the concentration of HSP70 in amniotic fluid supports this view.
What is the origin of HSP70 in amniotic fluid?
HSP70 protein and mRNA expression has been identified in the epithelium cells in large airways of fetal sheep [145], villous trophoblast, decidua, as well as human chorion and amnion [146]. Menon et al [46] demonstrated an increased HSP70 mRNA expression in cultured human chorioamniotic membranes after adding endotoxin. These observations suggest that HSP70 could be stimulated and released from chorioamniotic membranes following IAI. Our finding that patients with evidence of inflammation in the extra-placental membranes (histologic chorioamnionitis) and/or umbilical cords (funisitis) had a higher median amniotic fluid HSP70 concentration than those without inflammation supports this hypothesis. Similarly, Fukushima et al [33] reported that the mean serum concentration of HSP70 was higher in patients who delivered preterm than in those who delivered at term. However, there was no information regarding how many of these patients had intra-amniotic infection. In contrast, Divers et al [147] could not find any changes in protein expression of HSP70, HSP60 and HSP90 in trophoblasts of the basal plate and decidua of women with preterm delivery compared to those with term delivery. Similarly, a study [47] conducted in placentae from 12 women with preterm and 10 with term birth found no difference in protein expression of HSP70, HSP60 and HSP90 in all specimens. Thus, it is likely that the amnion, not the placenta, is the main source of an increased HSP70 concentration in the amniotic fluid of patients with IAI who delivered preterm.
Interestingly, Ziegert et al proposed that HSP70 antibody might involve in the mechanism of preterm labor [47]. In their study, HSP70 antigen-antibody complexes were localized in 4 of the 12 preterm placentae, but in none of the term placentae [47]. Moreover, maternal serum anti-HSP70 immunoglobulin G (IgG) was present in 4 cases of preterm birth and in no women at term in labor [47]. Indeed, there was a relationship between the concentration of HSP70 IgG and TNF–α, interferon gamma and secretory leukocyte protease inhibitor in mid-trimester amniotic fluid suggesting that antibodies to HSP70 might modulate inflammatory responses inside the amniotic fluid cavity [131]. Moreover, a case-control study reported a higher median serum concentration of HSP70 antibody at 16 weeks of gestation in mothers whose neonates were subsequently born with birth defects (cleft lip, cleft palate and neural tube defects) than that in mothers who gave birth to healthy neonates [148]. Collectively, the roles of HSP70 antibody or antigen-antibody complex in preterm labor requires further investigation.
Conclusion
In summary, we report herein that intra-amniotic infection, histologic chorioamnionitis and term parturition are associated with increased amniotic fluid HSP70 concentrations. HSP70 plays a role in the host defense mechanism by activating the innate arm of the immune response in women with intrauterine infection and may participate in the mechanisms of preterm and term parturition.
Acknowledgments
This research was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Bardwell JC, Craig EA. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci USA. 1984;81:848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert J. Evolution of heat shock protein and immunity. Dev Comp Immunol. 2003;27:449–464. doi: 10.1016/s0145-305x(02)00160-x. [DOI] [PubMed] [Google Scholar]
- 3.Ellis J. Proteins as molecular chaperones. Nature. 1987;328:378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- 4.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 5.Haslbeck M. sHsps and their role in the chaperone network. Cell Mol Life Sci. 2002;59:1649–1657. doi: 10.1007/PL00012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelham HR. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986;46:959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- 7.Pelham HR. Hsp70 accelerates the recovery of nucleolar morphology after heat shock. EMBO J. 1984;20(3):3095–3100. doi: 10.1002/j.1460-2075.1984.tb02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- 9.Singh R, Kolvraa S, Rattan SI. Genetics of human longevity with emphasis on the relevance of HSP70 as candidate genes. Front Biosci. 2007;12:4504–4513. doi: 10.2741/2405. [DOI] [PubMed] [Google Scholar]
- 10.Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology. 2003;110:1–9. doi: 10.1046/j.1365-2567.2003.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 12.Wallin RP, Lundqvist A, More SH, von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–135. doi: 10.1016/s1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 14.Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- 15.Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- 16.Lazarevic V, Myers AJ, Scanga CA, Flynn JL. CD40, but not CD40L, is required for the optimal priming of T cells and control of aerosol M. tuberculosis infection. Immunity. 2003;19:823–835. doi: 10.1016/s1074-7613(03)00324-8. [DOI] [PubMed] [Google Scholar]
- 17.Guzhova I, Margulis B. Hsp70 chaperone as a survival factor in cell pathology. Int Rev Cytol. 2006;254:101–49. 101–149. doi: 10.1016/S0074-7696(06)54003-3. [DOI] [PubMed] [Google Scholar]
- 18.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 19.Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol. 2002;270:169–184. doi: 10.1007/978-3-642-59430-4_11. [DOI] [PubMed] [Google Scholar]
- 20.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 21.Zugel U, Kaufmann SH. Immune response against heat shock proteins in infectious diseases. Immunobiology. 1999;201:22–35. doi: 10.1016/s0171-2985(99)80044-8. [DOI] [PubMed] [Google Scholar]
- 22.Fleshner M, Johnson JD. Endogenous extra-cellular heat shock protein 72: releasing signal(s) and function. Int J Hyperthermia. 2005;21:457–471. doi: 10.1080/02656730500088211. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- 24.Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- 25.Child DF, Williams CP, Jones RP, Hudson PR, Jones M, Smith CJ. Heat shock protein studies in type 1 and type 2 diabetes and human islet cell culture. Diabet Med. 1995;12:595–599. doi: 10.1111/j.1464-5491.1995.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 26.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 27.Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4:29–35. doi: 10.1054/csac.1998.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- 29.Campisi J, Fleshner M. Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J Appl Physiol. 2003;94:43–52. doi: 10.1152/japplphysiol.00681.2002. [DOI] [PubMed] [Google Scholar]
- 30.Fleshner M, Campisi J, Amiri L, Diamond DM. Cat exposure induces both intra- and extracellular Hsp72: the role of adrenal hormones. Psychoneuroendocrinology. 2004;29:1142–1152. doi: 10.1016/j.psyneuen.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Pockley AG, Wu R, Lemne C, Kiessling R, De FU, Frostegard J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. 2000;36:303–307. doi: 10.1161/01.hyp.36.2.303. [DOI] [PubMed] [Google Scholar]
- 32.Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessels. 2000;15:18–22. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]
- 33.Fukushima A, Kawahara H, Isurugi C, Syoji T, Oyama R, Sugiyama T, Horiuchi S. Changes in serum levels of heat shock protein 70 in preterm delivery and pre-eclampsia. J Obstet Gynaecol Res. 2005;31:72–77. doi: 10.1111/j.1447-0756.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 34.Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 35.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 37.Ledger WJ. Infection and premature labor. Am J Perinatol. 1989;6:234–236. doi: 10.1055/s-2007-999583. [DOI] [PubMed] [Google Scholar]
- 38.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 39.Brocklehurst P. Infection and preterm delivery. BMJ. 1999;318:548–549. doi: 10.1136/bmj.318.7183.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 41.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann NY Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 42.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 43.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 44.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 45.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, Jun JK. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–779. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 46.Menon R, Gerber S, Fortunato SJ, Witkin SS. Lipopolysaccharide stimulation of 70 kilo Dalton heat shock protein messenger ribonucleic acid production in cultured human fetal membranes. J Perinat Med. 2001;29:133–136. doi: 10.1515/JPM.2001.017. [DOI] [PubMed] [Google Scholar]
- 47.Ziegert M, Witkin SS, Sziller I, Alexander H, Brylla E, Hartig W. Heat shock proteins and heat shock protein-antibody complexes in placental tissues. Infect Dis Obstet Gynecol. 1999;7:180–185. doi: 10.1002/(SICI)1098-0997(1999)7:4<180::AID-IDOG3>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 49.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, Gomez R, Kuivaniemi H. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405–416. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1138–1143. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 51.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, Romero R. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999;181:1142–1148. doi: 10.1016/s0002-9378(99)70097-9. [DOI] [PubMed] [Google Scholar]
- 52.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon BH, Edwin SS. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2000;182:135–141. doi: 10.1016/s0002-9378(00)70502-3. [DOI] [PubMed] [Google Scholar]
- 53.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Mazor M, Adashi EY. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–373. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 54.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 55.Seubert DE, Maymon E, Pacora P, Gervasi MT, Berry SM, Torry DS, Romero R. A study of the relationship between placenta growth factor and gestational age, parturition, rupture of membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;182:1633–1637. doi: 10.1067/mob.2000.107437. [DOI] [PubMed] [Google Scholar]
- 56.Tissieres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- 57.Lewis M, Helmsing PJ, Ashburner M. Parallel changes in puffing activity and patterns of protein synthesis in salivary glands of Drosophila. Proc Natl Acad Sci USA. 1975;72:3604–3608. doi: 10.1073/pnas.72.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKenzie SL, Henikoff S, Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci USA. 1975;72:1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McKenzie SL, Meselson M. Translation in vitro of Drosophila heat-shock messages. J Mol Biol. 1977;117:279–283. doi: 10.1016/0022-2836(77)90035-3. [DOI] [PubMed] [Google Scholar]
- 60.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 61.Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 62.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 63.Williams KJ, Landgraf BE, Whiting NL, Zurlo J. Correlation between the induction of heat shock protein 70 and enhanced viral reactivation in mammalian cells treated with ultraviolet light and heat shock. Cancer Res. 1989;49:2735–2742. [PubMed] [Google Scholar]
- 64.Brunet S, Giacomoni PU. Heat shock mRNA in mouse epidermis after UV irradiation. Mutat Res. 1989;219:217–224. doi: 10.1016/0921-8734(89)90003-9. [DOI] [PubMed] [Google Scholar]
- 65.Muramatsu T, Tada H, Kobayashi N, Yamji M, Shirai T, Ohnishi T. Induction of the 72-kD heat shock protein in organ-cultured normal human skin. J Invest Dermatol. 1992;98:786–790. doi: 10.1111/1523-1747.ep12499953. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki K, Watanabe M. Augmented expression of HSP72 protein in normal human fibroblasts irradiated with ultraviolet light. Biochem Biophys Res Commun. 1992;186:1257–1264. doi: 10.1016/s0006-291x(05)81541-4. [DOI] [PubMed] [Google Scholar]
- 67.Maytin EV. Differential effects of heat shock and UVB light upon stress protein expression in epidermal keratinocytes. J Biol Chem. 1992;267:23189–23196. [PubMed] [Google Scholar]
- 68.Trautinger F, Kindas-Mugge I, Barlan B, Neuner P, Knobler RM. 72-kD heat shock protein is a mediator of resistance to ultraviolet B light. J Invest Dermatol. 1995;105:160–162. doi: 10.1111/1523-1747.ep12317003. [DOI] [PubMed] [Google Scholar]
- 69.Ohnishi K, Matsumoto H, Takahashi A, Wang X, Ohnishi T. Heat shock transcription factor, HSF, is activated by ultraviolet irradiation. Photochem Photobiol. 1996;64:949–952. [PubMed] [Google Scholar]
- 70.Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- 71.Ovelgonne JH, Souren JE, Wiegant FA, Van WR. Relationship between cadmium-induced expression of heatshock genes, inhibition of protein synthesis and cell death. Toxicology. 1995;99:19–30. doi: 10.1016/0300-483x(94)02990-c. [DOI] [PubMed] [Google Scholar]
- 72.Piano A, Valbonesi P, Fabbri E. Expression of cytoprotective proteins, heat shock protein 70 and metallothioneins, in tissues of Ostrea edulis exposed to heat and heavy metals. Cell Stress Chaperones. 2004;9:134–142. doi: 10.1379/483.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ting LP, Tu CL, Chou CK. Insulin-induced expression of human heat-shock protein gene hsp70. J Biol Chem. 1989;264:3404–3408. [PubMed] [Google Scholar]
- 74.Zhang W, Drach J, Andreeff M, Deisseroth A. Proliferation of hematopoietic cells is accompanied by suppressed expression of heat shock protein 70. Biochem Biophys Res Commun. 1992;183:733–738. doi: 10.1016/0006-291x(92)90544-u. [DOI] [PubMed] [Google Scholar]
- 75.Yaar M, Gilani A, DiBenedetto PJ, Harkness DD, Gilchrest BA. Gene modulation accompanying differentiation of normal versus malignant keratinocytes. Exp Cell Res. 1993;206:235–243. doi: 10.1006/excr.1993.1143. [DOI] [PubMed] [Google Scholar]
- 76.Kaufmann SH, Schoel B, van Embden JD, Koga T, Wand-Wurttenberger A, Munk ME, Steinhoff U. Heat-shock protein 60: implications for pathogenesis of and protection against bacterial infections. Immunol Rev. 1991;121:67–90. 67–90. doi: 10.1111/j.1600-065x.1991.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 77.Feige U, van EW. Infection, autoimmunity and autoimmune disease. EXS. 1996;77:359–73. 359–373. doi: 10.1007/978-3-0348-9088-5_24. [DOI] [PubMed] [Google Scholar]
- 78.Stewart GR, Young DB. Heat-shock proteins and the host-pathogen interaction during bacterial infection. Curr Opin Immunol. 2004;16:506–510. doi: 10.1016/j.coi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Cosgrove JW, Brown IR. Heat shock protein in mammalian brain and other organs after a physiologically relevant increase in body temperature induced by D-lysergic acid diethylamide. Proc Natl Acad Sci USA. 1983;80:569–573. doi: 10.1073/pnas.80.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polla BS. A role for heat shock proteins in inflammation? Immunol. Today. 1988;9:134–137. doi: 10.1016/0167-5699(88)91199-1. [DOI] [PubMed] [Google Scholar]
- 81.Jacquier-Sarlin MR, Fuller K, nh-Xuan AT, Richard MJ, Polla BS. Protective effects of hsp70 in inflammation. Experientia. 1994;50:1031–1038. doi: 10.1007/BF01923458. [DOI] [PubMed] [Google Scholar]
- 82.Vignola AM, Chanez P, Polla BS, Vic P, Godard P, Bousquet J. Increased expression of heat shock protein 70 on airway cells in asthma and chronic bronchitis. Am J Respir Cell Mol Biol. 1995;13:683–691. doi: 10.1165/ajrcmb.13.6.7576706. [DOI] [PubMed] [Google Scholar]
- 83.Polla BS, Cossarizza A. Stress proteins in inflammation. EXS. 1996;77:375–91. 375–391. doi: 10.1007/978-3-0348-9088-5_25. [DOI] [PubMed] [Google Scholar]
- 84.Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91:299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee WC, Wen HC, Chang CP, Chen MY, Lin MT. Heat shock protein 72 overexpression protects against hyperthermia, circulatory shock, and cerebral ischemia during heatstroke. J Appl Physiol. 2006;100:2073–2082. doi: 10.1152/japplphysiol.01433.2005. [DOI] [PubMed] [Google Scholar]
- 86.Cohen IR. Autoimmunity to chaperonins in the pathogenesis of arthritis and diabetes. Annu Rev Immunol. 1991;9:567–89. 567–589. doi: 10.1146/annurev.iy.09.040191.003031. [DOI] [PubMed] [Google Scholar]
- 87.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 88.Morimoto RI, Sarge KD, Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem. 1992;267:21987–21990. [PubMed] [Google Scholar]
- 89.Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- 90.Zugel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998;17:6124–6134. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thornberry NA. Caspases: key mediators of apoptosis. Chem Biol. 1998;5:R97–103. doi: 10.1016/s1074-5521(98)90615-9. [DOI] [PubMed] [Google Scholar]
- 93.Barton SG, Rampton DS, Winrow VR, Domizio P, Feakins RM. Expression of heat shock protein 32 (hemoxygenase-1) in the normal and inflamed human stomach and colon: an immunohistochemical study. Cell Stress Chaperones. 2003;8:329–334. doi: 10.1379/1466-1268(2003)008<0329:eohsph>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Currie RW, Tanguay RM, Kingma JG., Jr Heat-shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993;87:963–971. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- 95.Barbe MF, Tytell M, Gower DJ, Welch WJ. Hyperthermia protects against light damage in the rat retina. Science. 1988;241:1817–1820. doi: 10.1126/science.3175623. [DOI] [PubMed] [Google Scholar]
- 96.Ribeiro SP, Villar J, Downey GP, Edelson JD, Slutsky AS. Sodium arsenite induces heat shock protein-72 kilodalton expression in the lungs and protects rats against sepsis. Crit Care Med. 1994;22:922–929. doi: 10.1097/00003246-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 97.Takano M, Arai T, Mokuno Y, Nishimura H, Nimura Y, Yoshikai Y. Dibutyryl cyclic adenosine monophosphate protects mice against tumor necrosis factor-alpha-induced hepatocyte apoptosis accompanied by increased heat shock protein 70 expression. Cell Stress Chaperones. 1998;3:109–117. doi: 10.1379/1466-1268(1998)003<0109:dcampm>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ding XZ, Fernandez-Prada CM, Bhattacharjee AK, Hoover DL. Over-expression of hsp-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine. 2001;16:210–219. doi: 10.1006/cyto.2001.0959. [DOI] [PubMed] [Google Scholar]
- 99.Ryan AJ, Flanagan SW, Moseley PL, Gisolfi CV. Acute heat stress protects rats against endotoxin shock. J Appl Physiol. 1992;73:1517–1522. doi: 10.1152/jappl.1992.73.4.1517. [DOI] [PubMed] [Google Scholar]
- 100.Hotchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol. 1993;265:R1447–R1457. doi: 10.1152/ajpregu.1993.265.6.R1447. [DOI] [PubMed] [Google Scholar]
- 101.Villar J, Ribeiro SP, Mullen JB, Kuliszewski M, Post M, Slutsky AS. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med. 1994;22:914–921. [PubMed] [Google Scholar]
- 102.Wu YR, Wang CK, Chen CM, Hsu Y, Lin SJ, Lin YY, Fung HC, Chang KH, Lee-Chen GJ. Analysis of heat-shock protein 70 gene polymorphisms and the risk of Parkinson's disease. Hum Genet. 2004;114:236–241. doi: 10.1007/s00439-003-1050-1. [DOI] [PubMed] [Google Scholar]
- 103.Asea A. Initiation of the Immune Response by Extracellular Hsp72: Chaperokine Activity of Hsp72. Curr Immunol Rev. 2006;2:209–215. doi: 10.2174/157339506778018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol Cell Physiol. 2004;286:C739–C744. doi: 10.1152/ajpcell.00364.2003. [DOI] [PubMed] [Google Scholar]
- 105.Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002;158:1277–1285. doi: 10.1083/jcb.200208083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Foy SP, Matsuuchi L. Association of B lymphocyte antigen receptor polypeptides with multiple chaperone proteins. Immunol Lett. 2001;78:149–160. doi: 10.1016/s0165-2478(01)00256-5. [DOI] [PubMed] [Google Scholar]
- 107.Ueda G, Tamura Y, Hirai I, Kamiguchi K, Ichimiya S, Torigoe T, Hiratsuka H, Sunakawa H, Sato N. Tumor-derived heat shock protein 70-pulsed dendritic cells elicit tumor-specific cytotoxic T lymphocytes (CTLs) and tumor immunity. Cancer Sci. 2004;95:248–253. doi: 10.1111/j.1349-7006.2004.tb02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elsner L, Muppala V, Gehrmann M, Lozano J, Malzahn D, Bickeboller H, Brunner E, Zientkowska M, Herrmann T, Walter L, et al. The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol. 2007;179:5523–5533. doi: 10.4049/jimmunol.179.8.5523. [DOI] [PubMed] [Google Scholar]
- 110.Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptidembearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones. 2000;5:425–431. doi: 10.1379/1466-1268(2000)005<0425:hpbapn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 112.Malhotra V, Eaves-Pyles T, Odoms K, Quaid G, Shanley TP, Wong HR. Heat shock inhibits activation of NF-kappaB in the absence of heat shock factor-1. Biochem Biophys Res Commun. 2002;291:453–457. doi: 10.1006/bbrc.2002.6470. [DOI] [PubMed] [Google Scholar]
- 113.Radons J, Multhoff G. Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc Immunol Rev. 2005;11:17–33. [PubMed] [Google Scholar]
- 114.Silva CL, Silva MF, Pietro RC, Lowrie DB. Protection against tuberculosis by passive transfer with T-cell clones recognizing mycobacterial heat-shock protein 65. Immunology. 1994;83:341–346. [PMC free article] [PubMed] [Google Scholar]
- 115.Silva CL, Lowrie DB. A single mycobacterial protein (hsp 65) expressed by a transgenic antigen-presenting cell vaccinates mice against tuberculosis. Immunology. 1994;82:244–248. [PMC free article] [PubMed] [Google Scholar]
- 116.Ferrero RL, Thiberge JM, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Noll A, Autenrieth IB. Immunity against Yersinia enterocolitica by vaccination with Yersinia HSP60 immunostimulating complexes or Yersinia HSP60 plus interleukin-12. Infect Immun. 1996;64:2955–2961. doi: 10.1128/iai.64.8.2955-2961.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Srivastava PK. Heat shock proteins in immune response to cancer: the Fourth Paradigm. Experientia. 1994;50:1054–1060. doi: 10.1007/BF01923461. [DOI] [PubMed] [Google Scholar]
- 119.Srivastava PK, Udono H. Heat shock protein-peptide complexes in cancer immunotherapy. Curr Opin Immunol. 1994;6:728–732. doi: 10.1016/0952-7915(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 120.Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995;182:885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Conroy SE, Tucker L, Latchman DS, Isenberg DA. Incidence of anti Hsp 90 and 70 antibodies in children with SLE, juvenile dermatomyositis and juvenile chronic arthritis. Clin Exp Rheumatol. 1996;14:99–104. [PubMed] [Google Scholar]
- 122.Tasneem S, Islam N, Ali R. Crossreactivity of SLE autoantibodies with 70 kDa heat shock proteins of Mycobacterium tuberculosis. Microbiol Immunol. 2001;45:841–846. doi: 10.1111/j.1348-0421.2001.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 123.Dhillon V, McCallum S, Wilks D, Twomey B, Latchman D, Isenberg D. The differential expression of heat shock proteins in rheumatic disease. Br J Rheumatol. 1993;32:883–892. doi: 10.1093/rheumatology/32.10.883. [DOI] [PubMed] [Google Scholar]
- 124.Detanico T, Rodrigues L, Sabritto AC, Keisermann M, Bauer ME, Zwickey H, Bonorino C. Mycobacterial heat shock protein 70 induces interleukin-10 production: immunomodulation of synovial cell cytokine profile and dendritic cell maturation. Clin Exp Immunol. 2004;135:336–342. doi: 10.1111/j.1365-2249.2004.02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heufelder AE, Wenzel BE, Bahn RS. Cell surface localization of a 72 kilodalton heat shock protein in retroocular fibroblasts from patients with Graves' ophthalmopathy. J Clin Endocrinol Metab. 1992;74:732–736. doi: 10.1210/jcem.74.4.1548335. [DOI] [PubMed] [Google Scholar]
- 126.Heufelder AE, Goellner JR, Wenzel BE, Bahn RS. Immunohistochemical detection and localization of a 72-kilodalton heat shock protein in autoimmune thyroid disease. J Clin Endocrinol Metab. 1992;74:724–731. doi: 10.1210/jcem.74.4.1548334. [DOI] [PubMed] [Google Scholar]
- 127.Appetecchia M, Castelli M, Delpino A. Anti-heat shock proteins autoantibodies in autoimmune thyroiditis. Preliminary study. J Exp Clin Cancer Res. 1997;16:395–400. [PubMed] [Google Scholar]
- 128.van Roon JA, van Roy JL, Duits A, Lafeber FP, Bijlsma JW. Proinflammatory cytokine production and cartilage damage due to rheumatoid synovial T helper-1 activation is inhibited by interleukin-4. Ann Rheum Dis. 1995;54:836–840. doi: 10.1136/ard.54.10.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Roon JA, van EW, van Roy JL, Lafeber FJ, Bijlsma JW. Stimulation of suppressive T cell responses by human but not bacterial 60-kD heat-shock protein in synovial fluid of patients with rheumatoid arthritis. J Clin Invest. 1997;100:459–463. doi: 10.1172/JCI119553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gelber SE, Bongiovanni AM, Jean-Pierre C, Linhares IM, Skupski DW, Witkin SS. Antibodies to the 70 kDa heat shock protein in midtrimester amniotic fluid and intraamniotic immunity. Am J Obstet Gynecol. 2007;197:278–4. doi: 10.1016/j.ajog.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 131.Jean-Pierre C, Perni SC, Bongiovanni AM, Kalish RB, Karasahan E, Ravich M, Ratushny V, Skupski DW, Witkin SS. Extracellular 70-kd heat shock protein in mid-trimester amniotic fluid and its effect on cytokine production by ex vivo-cultured amniotic fluid cells. Am J Obstet Gynecol. 2006;194:694–698. doi: 10.1016/j.ajog.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 132.Molvarec A, Rigo J, Jr, Nagy B, Walentin S, Szalay J, Fust G, Karadi I, Prohaszka Z. Serum heat shock protein 70 levels are decreased in normal human pregnancy. J Reprod Immunol. 2007;74:163–169. doi: 10.1016/j.jri.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 133.Wu WX, Derks JB, Zhang Q, Nathanielsz PW. Changes in heat shock protein-90 and -70 messenger ribonucleic acid in uterine tissues of the ewe in relation to parturition and regulation by estradiol and progesterone. Endocrinology. 1996;137:5685–5693. doi: 10.1210/endo.137.12.8940400. [DOI] [PubMed] [Google Scholar]
- 134.DeMarzo AM, Beck CA, Onate SA, Edwards DP. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proc Natl Acad Sci USA. 1991;88:72–76. doi: 10.1073/pnas.88.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bagchi MK, Tsai SY, Tsai MJ, O'Malley BW. Progesterone enhances target gene transcription by receptor free of heat shock proteins hsp90, hsp56, and hsp70. Mol Cell Biol. 1991;11:4998–5004. doi: 10.1128/mcb.11.10.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tuohimaa P, Pekki A, Blauer M, Joensuu T, Vilja P, Ylikomi T. Nuclear progesterone receptor is mainly heat shock protein 90-free in vivo. Proc Natl Acad Sci USA. 1993;90:5848–5852. doi: 10.1073/pnas.90.12.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, Athanassiadis AP, Mitchell MD. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35:235–238. [PubMed] [Google Scholar]
- 138.Halgunset J, Johnsen H, Kjollesdal AM, Qvigstad E, Espevik T, Austgulen R. Cytokine levels in amniotic fluid and inflammatory changes in the placenta from normal deliveries at term. Eur J Obstet Gynecol Reprod Biol. 1994;56:153–160. doi: 10.1016/0028-2243(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 139.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- 140.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 141.Keski-Nisula LT, Aalto ML, Kirkinen PP, Kosma VM, Heinonen ST. Myometrial inflammation in human delivery and its association with labor and infection. Am J Clin Pathol. 2003;120:217–224. doi: 10.1309/KC6K-DTX9-8LFY-B3J7. [DOI] [PubMed] [Google Scholar]
- 142.Zhang F, Hackett NR, Lam G, Cheng J, Pergolizzi R, Luo L, Shmelkov SV, Edelberg J, Crystal RG, Rafii S. Green fluorescent protein selectively induces HSP70-mediated up-regulation of COX-2 expression in endothelial cells. Blood. 2003;102:2115–2121. doi: 10.1182/blood-2003-01-0049. [DOI] [PubMed] [Google Scholar]
- 143.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. 991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 144.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 145.Kramer BW, Kramer S, Ikegami M, Jobe AH. Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2002;283:L452–L459. doi: 10.1152/ajplung.00407.2001. [DOI] [PubMed] [Google Scholar]
- 146.Shah M, Stanek J, Handwerger S. Differential localization of heat shock proteins 90, 70, 60 and 27 in human decidua and placenta during pregnancy. Histochem J. 1998;30:509–518. doi: 10.1023/a:1003259907014. [DOI] [PubMed] [Google Scholar]
- 147.Divers MJ, Bulmer JN, Miller D, Lilford RJ. Placental heat shock proteins: no immunohistochemical evidence for a differential stress response in preterm labour. Gynecol Obstet Invest. 1995;40:236–243. doi: 10.1159/000292344. [DOI] [PubMed] [Google Scholar]
- 148.Child DF, Hudson PR, Hunter-Lavin C, Mukhergee S, China S, Williams CP, Williams JH. Birth defects and anti-heat shock protein 70 antibodies in early pregnancy. Cell Stress Chaperones. 2006;11:101–105. doi: 10.1379/CSC-130R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]