Abstract
Background
Diabetic foot disease is characterized by progressive foot deformities that lead to amputation and disabling morbidity. The purpose is to investigate the classification of two distinct phenotypes of mid foot structural polymorphism in individuals using plantar kinetic and pressure distribution and tarsal bone density assessments.
Methods
Twenty-two individuals (26 ft) with diabetes mellitus, peripheral neuropathy and at least one mid foot deformity were compared to 29 age-, gender- and race-matched healthy controls (58 ft). Eleven subjects with diabetes mellitus and peripheral neuropathy (11 ft) had lateral deformity; 11 subjects (15 ft) had medial deformity. Each subject had calcaneal bone mineral density and plantar force and pressure assessments walking barefoot over an EMED-ST P-2 platform.
Findings
Control subjects had lower mid foot vertical forces and pressures despite significantly higher preferred walking speed. In subjects with diabetes and neuropathy, maximum vertical force was 6-fold greater, force–time integral 9.5-fold greater, peak pressure 6.7-fold higher, pressure–time integral was 9.7-fold greater, contact area 2-fold greater and contact time 1.9-fold higher than controls. Pressure values were larger in involved vs uninvolved (P ≤ 0.05). During stance in the mid foot, subjects with medial column phenotype showed greater pressure in the medial mask; subjects with lateral column phenotype had greater pressures in the lateral mask (P < 0.05). Calcaneal bone density was lower for the deformity foot vs the non-deformity foot; bone mineral density was lower in medial column phenotype vs lateral column phenotype (P = 0.02).
Interpretation
Diabetic foot disease can be classified as stereotypical, structurally-distinct phenotypes of deformities of the medial and lateral columns of the mid foot. Assessments of pedal bone density and plantar mid foot force and pressure during barefoot walking can characterize the structural polymorphic phenotypes and may assist the foot care specialist in clinical decision making.
Keywords: Mid foot deformity, Plantar pressure, Tarsal bone density
1. Introduction
Diabetic foot disease is characterized by insidious and progressive foot deformities that can lead to lower extremity amputation and disabling morbidity. Mid foot deformities in individuals with chronic diabetes mellitus (DM) and peripheral neuropathy (PN) can arise from acute fracture, dislocation or subluxation of the tarsal or metatarsal bones. Mid foot deformities commonly lead to plantar ulceration, foot infection and far too often lower extremity amputation (Pecoraro et al., 1990; Sinacore and Withrington, 1999).
The onset and progression of mid foot deformities remain incompletely understood (Armstrong et al., 1997; Armstrong and Lavery, 1998a,b). Repetitive stresses and trauma, even seemingly minor in nature such as incurred while walking on level surfaces, on an insensitive foot is believed to be a precipitating trigger leading to acute inflammation followed by a rapid and exaggerated disruption of the bones, ligaments and ultimately the entire structural architecture of the foot (Harris and Brand, 1966).
Harris and Brand (1966) described several patterns of rapid and severe tarsal disintegration in anesthetic feet in individuals with Hansen's disease. They further speculated that a similarly rapid and severe mid foot deformity occurs in individuals with insensitivity of the feet due largely to the synergistic combination of the anesthetic foot's posture and the abnormal forces and pressures incurred during walking. Unfortunately, Harris and Brand's seminal descriptions were well prior to the currently available technology of dynamic plantar force and pressure assessment which allows for a more accurate evaluation of the location as well as the magnitude of abnormal forces and pressures that contribute to the pattern of deformity in discreet regions of the foot including the mid foot. Likewise, only recently has the influence of lower extremity bone density been recognized and used to describe the pattern of diabetic neuropathic arthropathy (Herbst et al., 2004).
There have been no previous reports combining dynamic mid foot force and pressure distribution assessments with tarsal bone mineral density to characterize the phenotypic patterns of structural polymorphism in individuals with diabetic foot disease. Therefore, the purpose of this study is to investigate the classification of structural polymorphisms of the mid foot into two distinct phenotypes of deformities in individuals with diabetic foot disease. Each phenotype of mid foot deformity was classified by its stereotypical location of overt clinical pathology and is characterized by kinetic and pressure variables including patterns and magnitude of vertical force, force–time integral, pressure, pressure–time integral, contact area and contact time during barefoot walking as well as an estimate of tarsal bone mineral density.
2. Methods
2.1. Subjects
Twenty-two subjects [26 ft in 14 men, 8 women with an average age of 53 (9) years] with chronic diabetes and peripheral neuropathy and at least one fixed, non-reducible mid foot deformity were studied. Values obtained from the subjects with diabetes, neuropathy and fixed mid foot deformity were compared to twenty-nine age-, gender-and race-matched control subjects [58 ft in 14 men, 15 women with an average age of: 52 (14) years] who did not have DM, PN or mid foot deformity. Eleven subjects with DM & PN (11 ft) had deformity located in the lateral column bones (cuboid, 4th or 5th metatarsal) of the mid foot, while 11 subjects (15 ft) were classified as medial column deformity (involving the talus, navicular, cuneiforms, 1st, 2nd or 3rd metatarsal) based on overt clinical pathology consisting of one or both of the following criteria: (1) either a history or a recurrent plantar mid foot ulcer location or (2) the location of a neuropathic (Charcot) arthropathy with or without radiographic evidence of overt fracture.
2.2. Procedures
In order to establish the presence of peripheral neuropathy, each subject with diabetes had their sensation assessed for three sensory modalities. Light touch and pressure at nine locations on the plantar surface of each foot was assessed using two thicknesses of Semmes Weinstein monofilaments, the 5.07 (10 g) and 6.10 (75 g) monofilaments (Diamond et al., 1989). If the subject was able to accurately sense the 5.07 filament at all nine locations, their sensation was graded as intact. If they were able to sense the 6.10 filament in at least one plantar location but unable to feel the 5.07 monofilament in at least one location, their sensation was graded as diminished, and if they were unable to sense the 6.10 filament at any single location, their sensation was graded as absent. Vibration sense was assessed using a 120-Hz tuning fork applied to both the dorsal surface of the middle cuneiform and to the medial distal phalanx of the great toe. Subjects were asked to report vibratory sensation for at least 5 s duration to each of these areas of their feet. Vibration sense was graded as either present or absent at each location. Joint position sense was determined both at the ankle joint and first metatarsal–phalangeal joint. The entire foot or distal phalanx of the great toe was passively moved into full plantar flexion then dorsal extension several times randomly having each subject determine the relative joint position with their eyes closed. Any incorrect response was graded as absent. Subjects were considered to have PN if one of the three sensory modalities was absent.
All subjects had a dynamic plantar force and pressure distribution assessment while barefoot walking at their preferred, self-selected walking speed using an EMED-ST P-2 pressure platform (Novel Inc., St Paul MN, USA). The two-step method of data collection was used for all trials as previously described (Meyers-Rice et al., 1994). Walking trials were performed over a 7-m raised walkway with the pressure platform embedded flush with the walkway surface. Control subjects performed three trials of walking for each foot while subjects with DM, PN and foot deformity typically performed two walking trials for each foot. Walking speed was determined using a stopwatch recorded to the nearest tenth of a second over the distance traversed.
Each subject also had their bone mineral density (BMD) assessed in each calcaneus using quantitative ultrasonometry (QUS-Sahara Clinical Bone Sonometer, Hologic Inc. Waltham, MA, USA). The same research assistant performed two trials for each calcaneus alternating right foot then left foot then right foot again followed by the left foot again. In our lab, the stability and precision of QUS measurements have been previously assessed over a 10 day interval in 20 healthy subjects (Sinacore et al., 2003). Intra-class correlation coefficient was 0.96 for BMD with a standard error of the measure equal to 0.4% indicating excellent reliability (Sinacore et al., 2003).
The study protocol was approved by the Washington University School of Medicine Human Subjects Committee. Written informed consent was obtained from each subject prior to voluntary participation in accordance with our Institutional Review Board. Subjects were not remunerated for their consent or participation.
2.3. Data processing and statistical analysis
Data was processed and analyzed in several stages. Each walking trial yielded a single plantar map (step). Each plantar map was divided (i.e., masked) into five masks using the Percent mask software from Novel Inc. First, each plantar map was masked into three horizontal masks reflecting anatomically distinct regions of the hind foot (at 33%), mid foot (at 63%) and forefoot (including the toes). Then, an additional vertical 50% mask of the mid foot through the forefoot was used to divide mid foot into medial and lateral masks. The 50% vertical mask most often bisected the second and third digits just lateral to the anatomical longitudinal axis of the foot in control subject's feet. After masking the plantar map, the magnitude of selected kinetic and pressure distribution variables of interest were averaged over the trial steps in each mask and summarized using the Groupmask evaluation software from Novel Inc. The average kinetic values for maximum vertical force, force–time integral, and pressure variables of peak pressure, pressure–time integral, contact area and contact time are reported in each mid foot mask.
The frequency of the pattern of dynamic center of pressure (CoP) line throughout the mid foot was judged by a blinded observer (KLB) and scored as occurring in the medial 50% mask, lateral 50% mask or split between the medial and lateral mask. Each pattern was tallied and a frequency count was calculated for each cohort and phenotype. The pattern of each foot was classified as occurring in the medial mask if the CoP line passed through and remained throughout the duration of mid foot loading medial to the 50% vertical mask line. If the CoP line passed through and remained throughout its loading duration lateral to the 50% vertical mid foot line, the pattern was classified as occurring in the lateral mask. If the CoP line crossed the vertical 50% mask line in the mid foot and occurred in both the medical and lateral masks, the pattern was classified as split.
For statistical analysis, multivariate analysis of variance was performed to identify differences in all dependent variables between and within the two cohorts. Similarly multivariate analysis of variance was used to discern differences in mean values between control feet (combined left and right) to feet with deformity. Further, a comparison was performed on the involved feet with deformity classified as medial column phenotype to feet classified as lateral column phenotype using a t-test for independent samples. Lastly, a two-way chi-square test of association was used to compare the frequency of the pattern of dynamic center of pressure line in the mid foot masks among the two cohorts and two phenotypes of mid foot deformity. For all analyses, the alpha level was set at 0.05. Data are reported as mean, standard deviation or upper and lower 99% confidence interval of the mean for each cohort. The Systat version.11 software was used for all statistical analyses (Systat Software Inc., Richmond, CA, USA).
3. Results
Subjects with fixed, non-reducible mid foot deformity had a mean age of 53 (9) years, reported a history of chronic diabetes, and all had peripheral neuropathy that result in either a history of persistent or recurrent plantar ulceration, or a neuropathic (Charcot) arthropathy with or without radiographic evidence of overt fracture. Control subjects were well matched to subjects with DM, PN and mid foot deformity for age (mean age 52 (14) years), gender, race, shoe size and height (P ≥ 0.77, all not significant). Subjects with diabetes and neuropathy weighed more [107 (29) kg to 77 (14) kg, P < 0.0004] with a larger body mass index (BMI) [31.7 (5) to 26 (4) kg/m2, P < 0.0007] than controls. Twenty-three percent of subjects with diabetes have Type 1 DM [27 (8) years duration] while 77% have Type 2 DM [12 (9) years duration].
Sixty-four percent had absent sensation and 36% had diminished sensation. Only 9% had vibratory sensation, and 91% lacked joint position sense at the first metatarsal joint while only 5% lacked joint position sense at the ankle joint. Seven subjects (31%) had a previous plantar mid foot ulceration, nine subjects (42%) had a mid foot Charcot arthropathy without ulceration and six subjects (27%) had a plantar ulceration accompanying acute Charcot arthropathy.
Control subjects had significantly lower values for selected mid foot force and pressure variables during barefoot walking despite a significantly higher average preferred walking speed than subjects with DM & PN (Table 1). Furthermore, the mean values of mid foot force and pressure were generally larger in the involved foot with fixed mid foot deformity compared to the uninvolved foot without mid foot deformity, though the magnitude of the differences varied. The mean mid foot maximum vertical force in neuropathic subjects' foot with deformity was 2.3-fold larger than the uninvolved foot and 6-fold greater than control subjects' feet. The mean value for force–time integral was 3.4-fold higher in feet with mid foot deformity compared to the foot with no deformity and 9.5-fold higher than control feet. Peak pressure was similarly 1.7-fold and 6.7-fold larger in the mid foot with deformity compared to the uninvolved foot and control feet, respectively (Table 1). The mean value for pressure–time integral was 2.3-fold higher in feet with mid foot deformity compared to the foot with no deformity and 9.7-fold higher than control feet (Table 1). Contact time and contact area was 1.1-fold and 1.5-fold higher in the foot with deformity compared to the uninvolved foot without deformity and 1.9-fold and 2-fold higher than control feet (Table 1). For all mid foot force and pressure distribution variables, the 99% lower confidence interval limits of the foot with deformity exceed the upper 99% confidence interval limit for the control subjects.
Table 1.
Mid foot force and pressure variables, walking speed and calcaneal BMD for controls and subjects with DM & PN
| Controls N = 29 |
DM & PN N = 22 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Left and right n = 58 |
Uninvolved feet n = 17 |
Involved feet n = 26 |
||||||||
| Mean | 99% CI (LL) | 99% CI (UL) | Mean | 99% CI (LL) | 99% CI (UL) | Mean | 99% CI (LL) | 99% CI (UL) | P-value | |
| Maximum | ||||||||||
| Vertical force (N) | 53 | 30 | 76 | 134 | 18 | 249 | 318 | 209 | 428 | 0.00a,b,c |
| % Body weight | 7 | 13 | 30 | |||||||
| Force time integral (N s) | 16 | 8 | 24 | 55 | 1 | 110 | 153 | 89 | 218 | 0.00b,c |
| % Body weight | 1.6 | 5.6 | 11.2 | |||||||
| Peak pressure (N/cm2) | 9 | 6 | 12 | 16 | 3 | 29 | 61 | 38 | 83 | 0.00b,c |
| Pressure time integral [(N/cm2) s] | 3 | 2 | 4 | 7 | 1 | 12 | 29 | 16 | 42 | 0.00a,b,c |
| Contact area (cm2) | 12 | 8 | 16 | 18 | 9 | 27 | 24 | 18 | 31 | 0.01b,c |
| Contact time (ms) | 412 | 337 | 487 | 586 | 404 | 768 | 789 | 609 | 969 | 0.00a,b,c |
| Walking speed (m/min) | 69 | 60 | 78 | 56 | 47 | 65 | * | * | * | 0.02d |
| Calcaneal BMD (mg/cm2) | 501 | 435 | 568 | 484 | 398 | 570 | 406 | 335 | 477 | ≤0.02b |
Mean, 99% Confidence Interval lower limit [CI (LL)]; 99% Confidence Interval upper limit [CI (UL)]. N represents number of subjects; n represents the number of feet involved in analyses. P-value is Bonferroni pairwise probability after an overall significant F ratio.
Control feet vs DM, PN uninvolved feet.
Control feet vs DM, PN involved feet.
Uninvolved feet vs Involved feet.
Two-tailed t-test for independent samples, alpha level 6≤0.05.
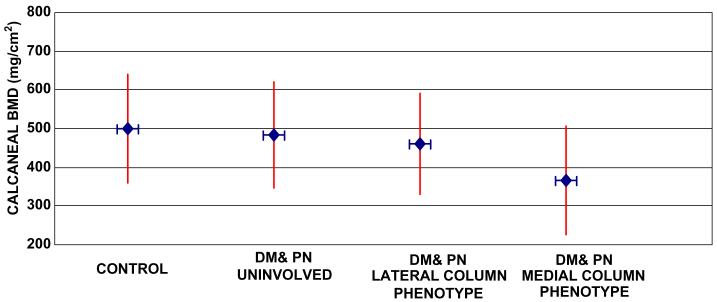
The control subjects' calcaneal BMD averaged nearly 13% higher than involved and uninvolved feet (combined) in subjects with diabetes and neuropathy (P = 0.02, Fig. 1). Calcaneal BMD was 16% lower for the involved foot with fixed mid foot deformity (combining phenotypes; 406 (14) mg/cm2) compared to the uninvolved foot without deformity (484 (13) mg/cm2, P = 0.04).
Fig. 1.
Whisker plot of calcaneal BMD in control feet (left and right combined), uninvolved foot without deformity, medial column and lateral column phenotypes with mid foot deformity. Diamonds with horizontal whiskers (lines) represent mean values. Vertical whiskers represent upper and lower 99% confidence limits of mean value. Statistical significance indicated in Tables 1 and 2.
Subjects with deformities classified as the medial column deformity phenotype showed a characteristic pattern of greater magnitude in all force and pressure variables in the medial 50% mid foot mask during the stance phase of barefoot walking compared to control subjects (Table 1) and subjects classified as lateral column phenotype (Table 2). Subjects with lateral column phenotype deformities had greater force and pressure values in the lateral mid foot mask during barefoot walking compared to control subjects, (P ≤ 0.006, Tables 1 and 2) though similar magnitudes of force and pressure variables as subjects with medial column phenotype deformities in medial and lateral masks (P ≥ 0.06, Table 2). Importantly, with the exception of peak pressure, there were no differences in plantar kinetic and pressure distribution in the lateral 50% mid foot mask between deformities classified as medial column or lateral column phenotypes (P ≥ 0.06). Calcaneal BMD was 21% lower in the subjects with medial column deformity phenotype (365 (14) mg/cm2) compared to subjects classified as lateral column phenotype (461 (13) mg/cm2, P = 0.02, (Table 2).
Table 2.
Mid foot force and pressure variables for DM+PN involved feet
| Calcaneal BMD (mg/cm2) | Medial column phenotype (n = 15) |
Lateral column phenotype (n = 11) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 99% CI (LL) | 99% CI (UL) | Mean | 99% CI (LL) | 99% CI (UL) | P-value | ||||||||
| 365 | 274 | 456 | 461 | 359 | 563 | 0.02 | ||||||||
| Medial mid foot mask |
Lateral mid foot mask |
Medial mid foot mask |
Lateral mid foot mask |
|||||||||||
| Mean | 99%CI (LL) | 99% CI (UL) | Mean | 99% CI (LL) | 99% CI (UL) | P-value | Mean | 99% CI (LL) | 99% CI (UL) | Mean | 99% CI (LL) | 99% CI (UL) | P-value | |
| Maximum | ||||||||||||||
| Vertical force (N) | 280 | 186 | 374 | 462 | 337 | 587 | 0.00a | 79 | 0 | 170 | 414 | 270 | 558 | 0.00a,b,c |
| Force time integral (N s) | 131 | 52 | 210 | 231 | 158 | 304 | 0.00a | 29 | 0 | 63 | 203 | 116 | 290 | 0.00a,b,c |
| Peak pressure (N/cm2) | 74 | 46 | 101 | 84 | 57 | 112 | NSa | 12 | 0 | 26 | 59 | 33 | 85 | 0.00a,b,c,d |
| Pressure time integral [(N/cm2) s] | 34 | 17 | 52 | 42 | 25 | 60 | NSa | 6 | 0 | 13 | 28 | 12 | 43 | 0.00a,b,c |
| Contact area (cm2) | 23 | 16 | 30 | 31 | 26 | 36 | NSa | 11 | 1 | 21 | 30 | 23 | 37 | 0.00a,b,c |
| Contact time (ms) | 875 | 651 | 1099 | 928 | 688 | 1168 | NSa | 484 | 246 | 721 | 787 | 624 | 951 | 0.00a,b,c |
Mean and 99% confidence interval lower limit [CI (LL)] – confidence interval upper limit [CI (UL)]. n Represents the number of feet involved. P-value is Bonferroni corrected pairwise probability after significant t test for independent samples.
Medial mid foot mask vs lateral mid foot mask within a phenotype.
medial mid foot mask vs medial mid foot mask between phenotypes.
medial mid foot mask vs lateral mid foot mask between phenotypes.
lateral mid foot mask vs lateral mid foot mask between phenotypes.
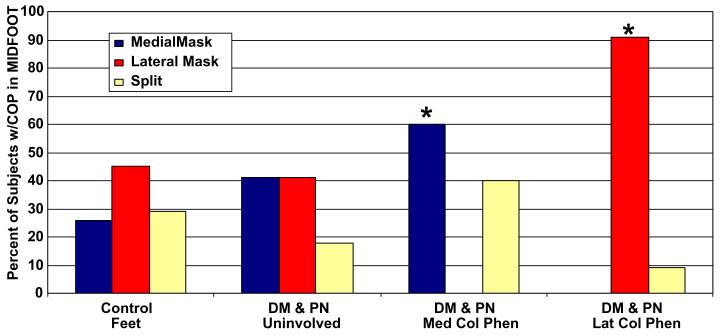
In control feet, the CoP line varied considerably though typically remained close to either side of the longitudinal axis of the foot (Fig. 2a). The CoP pattern through the mid foot was often split in the medial and lateral mid foot masks in control feet, though uniformly remained close to either side of the longitudinal axis of the foot (Fig. 3). The frequency of the CoP pattern through either the medial or lateral mid foot masks is not different between feet of control subjects and the foot without deformity in subjects with neuropathy and diabetes. However, there is a significant difference in the percent of feet with the pattern of CoP line in feet with fixed mid foot deformity compared to feet without deformity in subjects with DM & PN (χ2 = 24.1, df = 6, P = 0.001). Furthermore, the CoP pattern is markedly different among subjects with fixed mid foot deformity classified as either medial column phenotype compared to the lateral column phenotype. The majority of deformed feet (60%), the CoP line passed through the medial mask of the mid foot in subjects classified as the medial column phenotype, while the CoP pattern remained in the lateral mid foot mask in 91% of the subjects classified as the lateral column phenotype (Fig. 3).
Fig. 2.
Color-shaded pressure maps obtained during barefoot walking for each of three representative subjects' left foot. Mid foot is divided into 50% medial and lateral masks. (a) Depicts a left foot of a control subject with the CoP line indicated in the lateral 50% mask of the mid foot and close to the longitudinal axis of the foot; (b) depicts the left foot of a subject with DM & PN with a mid foot deformity classified as a medial column phenotype with CoP line indicated in the medial 50% mask; (c) depicts the left foot of a subject with DM & PN with mid foot deformity classified as lateral column phenotype with CoP line indicated in the lateral 50% mask of the mid foot though further away from the longitudinal axis of the foot than control subjects.
Fig. 3.
Percent of subjects with the pattern of center of pressure (CoP) line occurring in medial mask, lateral mask or split between the masks in the mid foot in control subjects' feet and feet of subjects with DM, PN in uninvolved feet without deformity and involved feet with deformity classified as medial column or lateral column phenotype. χ2 = 24.06, df = 6, P = 0.001.
4. Discussion
There have been no previous reports describing the pattern of dynamic CoP line and magnitude of plantar kinetic and pressure distributions to characterize neuropathic deformities of the mid foot. To our knowledge, the current study represents the first to characterize structural polymorphisms in diabetic foot disease using dynamic mid foot forces and pressures during barefoot walking combined with quantitative ultrasonography to assess the impact of tarsal bone density in each phenotype.
Armstrong and Lavery (1998a,b) have reported peak pressures in individuals with diabetes mellitus and several types of neuropathic foot disease. Peak pressure during barefoot walking in 21 patients with chronic diabetes and acute Charcot arthropathy of the mid foot averaged 100 (9) N/cm2, though these peak pressures were localized to the forefoot. Armstrong and Lavery did not report CoP patterns, did not mask the mid foot region or report force or pressure values specifically throughout the mid foot. They reasoned that despite the mid foot being the major site of involvement of the Charcot arthropathy, the high forefoot peak pressures are likely due to the forefoot functioning as a lever in the push off phase of walking forcing collapse of the more proximal mid foot. The high vertical force and peak pressures we report in the mid foot are most likely directly due to the fixed bony mid foot deformities during barefoot walking.
The onset and progression of foot deformities in individuals with diabetes and neuropathy remain incompletely understood. The Charcot foot is easily characterized by tarsal collapse of the medial or lateral longitudinal and transverse arches (Armstrong et al., 1997; Harris and Brand, 1966; Sinacore, 2001). Diabetic neuropathic mid foot deformities may be considered an accelerated occurrence of adult acquired flatfoot deformities. However, unlike other forms of adult acquired foot deformities, diabetic neuropathic mid foot deformities may progress exceedingly rapid due to a synergistic interaction of neuropathy-induced bone loss and rapid alterations in foot kinetics during walking or prolonged weight bearing (Greisberg et al., 2003). Therefore it is likely that neuropathic mid foot deformities represent a similar continuum of disease progression that begins with joint inflammation (Jeffcoate et al., 2005), progressing rapidly to arthropathy with partial or complete joint subluxation or dislocation with loss of arch height, manifesting as severe valgus inclination of the hind foot and excessive pronation of the mid foot and finally to fixed bony deformity with abduction of the forefoot on the mid foot or hind foot.
The rate of structural collapse in the neuropathic mid foot has been vastly understudied. Though seemingly rapid in some cases, other phenotypes of deformity appear more insidious. The role of reduced lower extremity bone mass on accelerating neuropathic foot and ankle deformities has only recently been implicated (Herbst et al., 2004). Herbst et al. have reported that lower extremity bone density (assessed at the contralateral femoral neck using dual-energy X-ray absorptiometry-bone density assessment) appears to have a significant role in the odds of developing a future Charcot joint with a fixed deformity as well as predicting the overall pattern of development. They report subjects with a fracture pattern onset of Charcot arthropathy of the ankle or forefoot were 9.5 times more likely (mean age-adjusted odds ratio = 9.5) to develop Charcot joints than either mid foot or hind foot patterns of Charcot arthropathy. While their study is seemingly different than the current study, Herbst and colleagues did not assess pedal bone density nor did they assess the impact of foot function on the pattern of deformity. The current data do support the conclusions by Herbst et al., namely that peripheral osteopenia is likely a strong predictor of who may likely develop foot deformity from Charcot arthropathy.
It remains uncertain whether elevated plantar forces or pressures cause or result in pedal bone loss. These results may be interpreted as suggesting that abnormally high plantar forces and pressures may precede tarsal bone loss since calcaneal BMD is only slightly lower (3.4%, P > 0.05) in the uninvolved foot without deformity compared to age- gender- and race-matched control feet or to feet with deformities classified as lateral column phenotype (Fig. 1). By contrast, indices of plantar mid foot forces and pressures are already significantly elevated in uninvolved feet without fixed deformity in subjects with neuropathy and diabetes compared to control feet (Table 1). Subjects with medial column phenotype had significantly lower calcaneal BMD and higher or equally high plantar force and pressures as subjects classified as lateral column phenotype (Fig. 1 and Table 2). Therefore, tarsal bone density affects structural phenotype and may have an important role in the onset, progression and pattern of some neuropathic mid foot deformities. Excessively elevated plantar stresses during walking in individuals with neuropathic feet can cause a persistent hyperemic, inflammatory osteolysis leading to excessive bony resorption with resultant bone loss that contributes to mid foot deformity (Armstrong and Lavery, 1998b; Jeffcoate et al., 2005). The threshold for pedal bone density associated with neuropathic fracture or mid foot deformity has yet to be identified though whether such a threshold truly exists deserves further study.
In addition to the magnitude of mid foot kinetic and pressure distributions during barefoot walking and tarsal BMD, the pattern of CoP through the mid foot may reflect the structural polymorphism in mid foot deformity. In control feet, the CoP line through the mid foot varied considerably though typically remained close to either side of the longitudinal axis of the foot (Fig. 2a). In fact, the dynamic CoP line pattern in control feet varied from 26% occurring in the medial mask to 45% occurring in the lateral mask and 29% split into both medial and lateral mask (Fig. 3). The close proximity of the CoP line to the anatomical longitudinal axis in control feet may suggest the mid foot's bony and structural architecture was maintained. The considerable variation in the pattern of the CoP line in control subject's feet has been reported previously (Fuller, 1999; Cornwall and McPoil, 2003; McPoil and Cornwall, 1998). Most likely the variability in CoP pattern is due to the variability in foot structure encountered in asymptomatic individuals with differences in mid foot arch heights (Morag and Cavanagh, 1997). Importantly, all control subjects' feet had mid foot kinetic indices that were very low or were recorded as zero in the vertical loading. These force and pressure distribution characteristics combined with dynamic CoP patterns suggest the control subjects' mid foot bony architecture and foot structure was maintained allowing smooth transfer of force and pressure progression through the tarsal bones from hind foot to forefoot throughout the stance phase of barefoot walking. In uninvolved, non-deformed feet of subjects with diabetes and neuropathy, the CoP pattern was similar to the control subjects' feet (Fig. 3) though it appeared to deviate noticeably further from the longitudinal axis of the foot either in the lateral mask or in the medial mask. However, unlike control subjects' feet, the magnitude of the mid foot force and pressure indices were significantly elevated (i.e., doubled) despite having no obvious deformity or clinical pathology. By contrast, the involved foot with mid foot deformity in subjects with diabetes and neuropathy showed the CoP line to be located more frequently (60% of feet) in the medial half of the mid foot in subjects classified as the medial column phenotype, while the CoP line remained in the lateral mid foot mask in 91% of subjects classified as the lateral column phenotype (Fig. 3). The average mid foot force and pressure distribution in subjects with deformities classified as medial column phenotype were significantly greater (nearly 10-fold) than control subjects' feet and nearly 2-fold greater than contralateral uninvolved feet without deformity (Tables 1 and 2). Although variable in control subjects' feet, we observe the CoP pattern becomes more stereotypical of the structural phenotype in feet with fixed mid foot deformities.
The classification of the mid foot deformities are based on the location of the overt clinical pathology rather than a strict anatomical classification based on the bone and joints primarily affected as determined by standard radiograph (Brodsky, 1999; Cofield et al., 1983). This method of classification of foot deformity based on the location of overt clinical pathology has been previously reported (Armstrong et al., 1997; Mueller et al., 1990). Mueller et al. have shown a strong relationship between the location of plantar ulceration to several types of foot deformities including mid foot deformities in subjects with neuropathy and diabetes (Mueller et al., 1990). They reported 6/7 (86%) of individuals classified as having Charcot foot deformities had mid foot plantar ulcerations, though they did not report whether the medial column or lateral column was more affected. Armstrong (Armstrong et al., 1997) classified 55 subjects with diabetes and an acute Charcot arthropathy of the foot or ankle by a combination of radiography, dermal thermometry and location of clinical signs. They report 45 of 55 subjects (82%) had patterns of tarsal involvement localized to either the tarso-metatarsal joints (i.e., Lisfranc joints) or the transverse tarsal joints (i.e., Chopart's joint), though neither the medial or lateral column involvement was specified. Regardless of the classification scheme used, these studies confirm the high prevalence of tarsal involvement in individuals with diabetes and neuropathy and emphasize the need to focus more study in understanding the structural phenotypes and their clinical consequences.
4.1. Clinical implications
Mid foot deformities have been recognized as contributing to significant impairments in foot function as well as physical disability (Sinacore and Withrington, 1999). Mid foot deformities are particularly troublesome as they most often are multi-plane rotational deformities (i.e., occurring simultaneously in three-planes) therefore difficult to correct with surgery or manage successfully with conservative methods such as therapeutic footwear and orthotic therapy. Mid foot force and pressures in diabetic, neuropathic subjects that exceed the upper 99% confidence limits for age-, gender- and race-matched control subjects should be used to signal the early need for off-loading particularly if feet are at risk for lower extremity amputation. A comparison of the magnitude and location of kinetic and pressure variables while walking barefoot and in therapeutic footwear may allow for a more precise evaluation of the ability of therapeutic shoes, bracing or a combination of both to protect the insensitive foot from further joint destruction, skin ulceration and lower extremity amputation (Bauman et al., 1963; Myerson et al., 1994). Elevated plantar forces and pressures during walking in subjects with neuropathy combined with bone loss may signal the onset of rapid and progressive deterioration in mid foot structure and alert the foot care specialist to prescribe improved methods of management to maintain walking while limiting disability.
5. Conclusion
Diabetic foot disease manifests as stereotypical, structurally-distinct phenotypes of deformities of the medial and/or lateral columns of the mid foot. An assessment of pedal bone densitometry and mid foot kinetic and pressure patterns during barefoot walking can distinctly classify each structural polymorphism in diabetic foot disease and may be useful for recommending and prescribing therapeutic footwear for effective off-loading to prevent further trauma, recurrent skin breakdown and subsequent lower extremity amputation in high-risk diabetic, neuropathic feet with mid foot deformity.
Acknowledgements
Supported by a National Institutes of Health/National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK RO1 DK 59224-05 (Sinacore) & Washington University School of Medicine Diabetes Research & Training Center 5 P60 DK 20579. The authors thank Dr. Michael J. Mueller for his assistance with data analysis and support in preparation of this manuscript.
References
- Armstrong DG, Lavery LA. Acute Charcot's arthropathy of the foot and ankle. Phys. Ther. 1998a;78:74–80. doi: 10.1093/ptj/78.1.74. [DOI] [PubMed] [Google Scholar]
- Armstrong DG, Lavery LA. Elevated peak plantar pressure in patients with Charcot arthropathy. J. Bone Joint Surg. 1998b;80A:365–369. doi: 10.2106/00004623-199803000-00009. [DOI] [PubMed] [Google Scholar]
- Armstrong DG, et al. The natural history of acute Charcot's arthropathy in a diabetic foot specialty clinic. Diabetic Med. 1997;14:357–363. doi: 10.1002/(SICI)1096-9136(199705)14:5<357::AID-DIA341>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Bauman JH, et al. Plantar pressures and trophic ulceration: an evaluation of footwear. J. Bone Joint Surg. 1963;45B:652–673. [PubMed] [Google Scholar]
- Brodsky JW. The Diabetic Foot. In: Coughlin MJ, Mann RA, editors. Surgery of the Foot and Ankle. seventh ed. Mosby Yearbook; St Louis: 1999. pp. 895–969. [Google Scholar]
- Cofield RH, et al. Diabetic neuroarthropathy in the foot: patient characteristics and patterns of radiographic change. Foot Ankle. 1983;4:15–22. doi: 10.1177/107110078300400104. [DOI] [PubMed] [Google Scholar]
- Cornwall MW, McPoil TG. Reliability and validity of center of pressure quantification. J. Am. Podiat. Med. Assoc. 2003;93:142–149. doi: 10.7547/87507315-93-2-142. [DOI] [PubMed] [Google Scholar]
- Diamond JE, et al. Reliability of diabetic foot evaluation. Phys. Ther. 1989;69:797–802. doi: 10.1093/ptj/69.10.797. [DOI] [PubMed] [Google Scholar]
- Fuller EA. Center of pressure and its theoretical relationship to pathology. J. Am. Podiat. Med. Assoc. 1999;89:278–291. doi: 10.7547/87507315-89-6-278. [DOI] [PubMed] [Google Scholar]
- Greisberg J, et al. Deformity and degeneration in the hind foot and midfoot joints of the adult acquired flatfoot. Foot Ankle Int. 2003;24:530–534. doi: 10.1177/107110070302400704. [DOI] [PubMed] [Google Scholar]
- Harris JR, Brand PW. Patterns of disintegration of the tarsus in the anaesthetic foot. J. Bone Joint Surg. (Br) 1966;48:4–16. [PubMed] [Google Scholar]
- Herbst SA, et al. Pattern of diabetic neuropathic arthropathy associated with the peripheral bone mineral density. J. Bone Joint Surg. (Br) 2004;86:378–383. doi: 10.1302/0301-620x.86b3.14593. [DOI] [PubMed] [Google Scholar]
- Jeffcoate WJ, et al. The role of proinflammatory cytokines in the cause of neuropathic osteoarthropathy (acute Charcot foot) in diabetes. Lancet. 2005;366:2058–2061. doi: 10.1016/S0140-6736(05)67029-8. [DOI] [PubMed] [Google Scholar]
- McPoil TG, Cornwall MW. Variability of the center of pressure pattern integral during walking. J. Am. Podiat. Med. Assoc. 1998;88:259–267. doi: 10.7547/87507315-88-6-259. [DOI] [PubMed] [Google Scholar]
- Meyers-Rice B, et al. Comparison of three methods for obtaining plantar pressures in non-pathologic subjects. J. Am. Pod. Med. Assoc. 1994;84:499–504. doi: 10.7547/87507315-84-10-499. [DOI] [PubMed] [Google Scholar]
- Morag E, Cavanagh PR. Structural and functional predictors of regional peak pressures under the foot during walking. Biomechanics. 1997;32:359–370. doi: 10.1016/s0021-9290(98)00188-2. [DOI] [PubMed] [Google Scholar]
- Mueller MJ, et al. Relationship of foot deformity to ulcer location in patients with diabetes mellitus. Phys. Ther. 1990;70:356–362. doi: 10.1093/ptj/70.6.356. [DOI] [PubMed] [Google Scholar]
- Myerson MS, et al. Management of mid foot diabetic neuroarthropathy. Foot Ankle Int. 1994;15:233–241. doi: 10.1177/107110079401500502. [DOI] [PubMed] [Google Scholar]
- Pecoraro RE, et al. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care. 1990;13:513–521. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- Sinacore DR, Withrington NC. Recognition and management of acute neuropathic (Charcot) arthropathies of the foot and ankle. J. Orthop. Sports Phys. Ther. 1999;29:736–746. doi: 10.2519/jospt.1999.29.12.736. [DOI] [PubMed] [Google Scholar]
- Sinacore DR. Severe sensory neuropathy need not precede Charcot arthropathies of the foot or ankle: implications for the rehabilitation specialist. Physiother. Theor. Pract. 2001;17:39–50. [Google Scholar]
- Sinacore DR, et al. Precision of serial quantitative ultrasonometry measures of the calcaneus; (Abstract) Section on Research, Combine Section's Meeting; American Physical Therapy Association. Tampa, FL. Feb, 2003. [Google Scholar]