Introduction
The unusual name of this family of receptors, bombesin (Bn), comes from the original terminology used by V. Erspamer and his colleagues to name the first natural ligand described, bombesin, which was an amidated tetradecapeptide isolated from the skin of the European frog Bombina bombina (Erspamer et al., 1970; Erspamer et al., 1972) (Fig.1). They isolated many related peptides from other frog skins and most were named after the genus of frog from which it was isolated (Erspamer and Melchiorri, 1973; Erspamer, 1988). In terms of their structural similarities they were originally divided into three general groups (Fig.1): the bombesin group which all had a carboxyl terminus of Gly-His-Leu-Met-NH2 [bombesin, alytesin, [pGlu1] bombesin 6−14]; the ranatensin group which had a carboxyl terminus of Gly-His-Phe-Met-NH2 (ranatensin, ranatensin R and C, litorin, rodhei-litorin, [Glu (Ote) 2 or (Ome) 2] litorin) and the phyllolitorin group which had a carboxyl terminal Gly-Ser-Phe/Leu-Met-NH2 [phyllolitorin,[Leu8]phyllolitorin, [Thr5, Leu8]Phyllolitorin (Falconieri Erspamer et al., 1988; Erspamer, 1988)] (Fig.1). Recent molecular studies show the occurrence of these peptides in amphibian's skins is more complicated than originally thought with both Leu and Phe penultimate forms present in the same frog species in may cases (Nagalla et al., 1996; Spindel, 2006). For example in the skin of the frog, Bombina orientalis [Leu13] bombesin, [Phe13] bombesin and [Ser3,Arg10,Phe13] bombesin (SAP bombesin) are found and each of these three forms are derived from separate genes (Nagalla et al., 1996; Spindel, 2006).
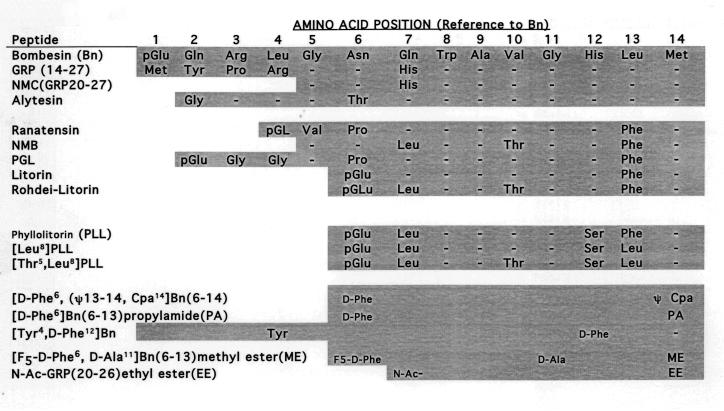
Figure 1.
Structures of GRP, NMB and Bn-related agonists and antagonists. The entire structures of the different peptides are shown except for GRP which has 27 amino acids and only the COOH terminal 14 amino acids are shown, which is the biologically active end. Both natural occurring agonists and some of the antagonists referred to in the text are shown. Abbreviations. ψ, –CONH peptide bond changed to -CH2NH- ; pGlu, pyroglutamic acid; Cpa, chlorophenylalanine; NMC, neuromedin C; F5, pentafluoro-
Subsequently, in mammals two Bn-like peptides were isolated, gastrin-releasing peptide (GRP) (McDonald et al., 1979) and neuromedin B (NMB) (Minamino et al., 1983). GRP, a 27 amino acid peptide was originally isolated from porcine stomach and shares the same seven carboxyl terminal amino acids with bombesin (McDonald et al., 1979) accounting for similar biological activity (Fig.1). The decapeptide of GRP was later isolated from porcine spinal cord and originally called neuromedin C (Minamino et al., 1984b), although it is recommended a more appropriate name is either GRP-10 or GRP18−27 (Anonymous, 1988). The mammalian equivalent of ranatensin, NMB, was isolated from porcine spinal cord and shown to be a decapeptide (McDonald et al., 1979), which also occurs in precursor forms of 30 and 32 amino acids (Minamino et al., 1985). The carboxyl terminal seven amino acids are identical in ranatensin, except for the replacement of threonine in NMB for valine in ranatensin at the penultimate position from the carboxyl terminus (Fig.1).
Studies of GRP and NMB immunoreactivity as well as mRNA studies have demonstrated these peptides and their mRNA are widely distributed in mammals in both the nervous system and peripheral tissues, especially the gastrointestinal tract (Penman et al., 1983; Wada et al., 1990; Battey and Wada, 1991; Spindel et al., 1993; Moody and Merali, 2004). In the alimentary tract GRP-like IR is found primarily in neurons as well as the submucosal and myenteric plexuses and not in endocrine cells (Penman et al., 1983). Using Northern blots the highest levels of mRNA occur in colon with lower amounts in the stomach and small intestine (Sunday et al., 1988). In the spinal cord GRP-IR was found in both the posterior and anterior horn and in the CNS, GRP-IR and mRNA is widely distributed in neurons with high levels in the hypothamic nuclei, forebrain and medullary nuclei that participate in autonomic functions, as well as in sensory nuclei (Panula et al., 1982; Panula et al., 1988; Wada et al., 1990; Battey and Wada, 1991; Spindel et al., 1993). NMB-IR and mRNA is found throughout the GI tract, but generally at lower levels than GRP except in the esophagus (Spindel et al., 1993). In general in the brain and spinal cord, NMB-IR is greater than GRP-IR (Minamino et al., 1984a), NMB mRNA is most abundant in the olfactory bulb, dentate gyrus and dorsal root ganglia, whereas GRP mRNA is highest in the forebrain and some hypothamic nuclei (Wada et al., 1990; Battey and Wada, 1991). In most brain regions the NMB mRNA distribution does not overlap with GRP (Wada et al., 1990; Battey and Wada, 1991; Moody and Merali, 2004; Ohki-Hamazaki et al., 2005).
The mammalian bombesin peptides, GRP and NMB demonstrate a broad spectrum of pharmacological and biological responses. GRP stimulates smooth muscle contraction in both the gastrointestinal tract and urogenital system and has profound effects on GI motility; stimulates release of numerous gastrointestinal hormones/neurotransmitters: stimulates secretion and/or hormone release from the pancreas, stomach, colon, and numerous endocrine organs; has potent effects on immune cells (macrophages, dendritic cells, lymphocytes, leukocytes) (Ruff et al., 1985; De la Fuente et al., 1991; van Tol et al., 1993; De la Fuente et al., 1993b; Del Rio and De la Fuente, 1994; Del Rio et al., 1994; Plaisancie et al., 1998; Makarenkova et al., 2003); has potent growth effects on both normal tissues and tumors; has potent CNS effects, including regulation of circadian rhythm, thermoregulation; regulation of anxiety and fear response, food intake, behavioral effects as well as involved in mediating numerous CNS effects on the GI tract (Tache et al., 1988; Bunnett, 1994; Martinez and Tache, 2000; Jensen et al., 2001; Jensen, 2003; Grider, 2004; Jensen and Moody, 2006). In many tissues the effects of NMB overlap with GRP, however NMB has specific effects in some tissues such as: contraction of smooth muscle; growth effects in various tissues (Moody et al., 2000; Matusiak et al., 2005); CNS effects including on feeding, thermoregulation; regulation of TSH release, stimulation of various CNS neurons, behavioral effects; and effects on spinal sensory transmission (von Schrenck et al., 1989; Rettori et al., 1992; Ladenheim et al., 1997b; Ohki-Hamazaki, 2000; Merali et al., 2006; Oliveira et al., 2006). GRP and to a lesser extent NMB affects the growth and/or differentiation of a number of important human tumors including colon, prostate, lung and some gynecologic cancers (Cuttitta et al., 1985; Schally et al., 2000; Jensen et al., 2001; Glover et al., 2003; Jensen and Moody, 2006).
Early studies on the biologic effects of the different bombesin peptides isolated from frog skins, primarily examining their effects on contraction of isolated smooth muscle preparations from various tissues, demonstrated markedly varying potencies, suggesting more than one subtype of bombesin receptor might exist (Falconieri Erspamer et al., 1988; Regoli et al., 1988; Severi et al., 1991). Binding studies and the development of highly selective antagonists established unequivocally the existence of two different classes of receptors in mammalian tissues mediating the actions of these peptides (Moody et al., 1978; Jensen et al., 1978; Jensen and Gardner, 1981; Coy et al., 1988; von Schrenck et al., 1989; Ladenheim et al., 1990; von Schrenck et al., 1990; Jensen and Coy, 1991; Metz et al., 1992). One class had a high affinity for GRP and a lower affinity for NMB (termed GRP-R, GRP receptor or GRP-preferring receptor) and the other class had a higher affinity for NMB than GRP (termed NMB-R, NMB receptor or NMB-preferring receptor) (Jensen and Gardner, 1981; Moody et al., 1988; von Schrenck et al., 1989; Ladenheim et al., 1990; von Schrenck et al., 1990; Moody et al., 1992; Wang et al., 1992; Ladenheim et al., 1992). Subsequently, two mammalian receptors with high affinity for GPR (Spindel et al., 1990; Battey et al., 1991) or NMB (Wada et al., 1991) have been cloned in addition to a closely related orphan receptor (Gorbulev et al., 1992; Fathi et al., 1993b) and one related receptor from amphibians (Nagalla et al., 1995) which will be discussed in more detail below (Table 1).
Table 1.
Current bombesin receptor nomenclature and general characteristics
| Variable | Mammalian bombesin receptor | ||
|---|---|---|---|
| Receptor Code | BB1 | BB2 | BB3 |
| Previous Names | NMB-R, NMB preferring receptor | GRP-R, GRP preferring receptor | BRS-3, bombesin receptor subtype 3 |
| Cloned from mammals | Human, rat, mouse, monkey | Human, rat, mouse, monkey, chimpanzee, dog, sheep | Human, rat, mouse, monkey, sheep |
| Gene location | Chr 6p21 (human) | Chr Xp22 (human) | Chr Xq25 (human) |
| Structural information | 390 aa (human) | 384 aa (human) | 399 aa (human) |
| Natural ligands | NMB>GRP | GRP>NMB | Unknown (low affinity NMB, GRP, all Bn natural related peptides) |
| Selective agonist | NMB, NMB30 | GRP | [D-Tyr6, Apa-4Cl11, Phe13, Nle14] bombesin 6−14 ,Ac-Phe, Trp, Ala, His (tBzl), Nip, Gly, Arg-NH2 |
| Selective antagonists | PD 168368 | [D-Phe6, Cpa14, Ψ13−14]Bn6−14, JMV641, JMV594, BW2258U89, Ac-GRP20−26 methylester | None |
| Principal transduction | Gq/11 | Gq/11 | Gq/11 |
| Preferred radioligand | 125I−BH-[D-Tyr0]-NMB, 125I-[Tyr4]-Bn | 125I-[GRP], 125I-[Tyr4]-Bn, 125I-[D-Tyr6]-Bn 6−13 methyl ester | 125I- [D-Phe6, β-Ala11, Phe13, Nle14] Bn 6−14 |
| Tissue functions | CNS (regulate TSH release, satiety), GI tract (motility); regulate stress responses | CNS (thermoregulation, regulate circadian rhythm, satiety); GI tract (hormone release, motility, regulate secretions [pancreas, gastric acid, islets]): immunologic (chemoattractant, lymphocyte function); fetal development (lung), | Regulate energy homeostasis, glucose/insulin regulation; satiety; lung development and response to injury: present myenteric/submucosa ganglia, cells of Cajal proposed involved GI motility |
| Diseases | Altered hypo-, hyperthyroidism; autocrine tumor growth factor (lung/colon tumors, carcinoids, others) | Tumor growth effects- morphogen, autocrine growth factor (lung/colon//prostate/breast/. head-neck tumors, others); lung diseases (bronchopulmonary dysplasia, tobacco injury) | Tumor growth factor (lung, others) |
| Phenotype of knockout | Reduced hypothermic effect to NMB; abnormal behaviors; dysregulation of thyroid-pituitary axis; altered CNS 5-HT system with stress | Altered satiety, thermoregulation: abnormal behaviors, altered insulin release; | Mild obesity, hypertension, impaired glucose metabolism reduced metabolic rate, increased feeding behavior; altered lung response to injury |
References are in text under the indicated receptor
I. Molecular basis for nomenclature
Once the receptors were defined using binding studies, cross-linking studies and studies of biological activity (Kris et al., 1987; Tache et al., 1988; Sinnett-Smith et al., 1988; von Schrenck et al., 1989; Huang et al., 1990; Ladenheim et al., 1990; Lebacq-Verheyden et al., 1990), an active effort to clone the GRP-preferring receptor (GRP-R) was undertaken by Dr Eliot Spindel, Oregon Regional Primate Center and Dr James Battey, National Institutes of Health. In 1990 using electrophysiological and luminometric Xenopus oocyte expression assays, Spindel (Spindel et al., 1990) succeeded in cloning the GRP-R from murine Swiss 3T3 cells, which express high levels of this receptor (Rozengurt, 1988). The cDNA for the same receptor was isolated and described by Battey et al. in 1991 (Battey et al., 1991) by using an enriched library from Swiss 3T3 cells and specific oligonucleotide probes based on information from a partial sequence of the GRP-R in this cells obtained after solubilization and purification using wheat germ agglutinin-agarose and a ligand affinity chromatography (Feldman et al., 1990). Pharmacology studies demonstrated the cloned receptor preferred GRP to NMB and its activation was blocked by specific GRP-preferring receptor antagonists (Rozengurt, 1988; Battey et al., 1991). Subsequently, using low stringency conditions with a mouse GRP-R cDNA probe (Wada et al., 1991); the NMB preferring receptor (NMB-R) was cloned from a cDNA library made from the rat esophagus, a tissue that had been reported to have a high density of NMB-R (von Schrenck et al., 1989; von Schrenck et al., 1990). The structure of the cDNA of the human GRP-R and NMB-R were described from a small cell lung cancer cell line in 1991 (Corjay et al., 1991).
In 1992 a novel receptor was cloned from guinea-pig uterus (Gorbulev et al., 1992) which showed the highest amino acid identity to the GRP-R (52%) and the NMB-R (47%). This receptor bound GRP and NMB, but only with relatively low affinities (IC50-290 nM and 20,000 nM, respectively). The human analogue of this novel receptor was cloned in 1993 (Fathi et al., 1993b) and expression studies showed it was specifically activated by bombesin-related peptides, but only with low affinity and thus was classified as an orphan receptor. It was termed BRS-3 for bombesin receptor subtype 3 (Fathi et al., 1993b). Subsequent binding studies and signaling studies using synthetic ligands of bombesin with high affinity for hBRS-3 (Mantey et al., 1997), demonstrated that it not only had low affinity for GRP and NMB, but all known naturally occurring bombesin related peptides (Wu et al., 1996; Mantey et al., 1997; Pradhan et al., 1998; Ryan et al., 1998a; Ryan et al., 1998b) and therefore it remains an orphan receptor. Subsequently it was cloned from mouse (Ohki-Hamazaki et al., 1997a), rat (Liu et al., 2002) and sheep (Whitley et al., 1999).
Searching for receptors for bombesin-related peptides in amphibians (Nagalla et al., 1995), clones were isolated which had a similar sequence to the mammalian GRP-R and NMBR. A clone was isolated which encoded for a novel bombesin receptor, which had 61%, 56% and 70% amino acid identities to the human GRP-R, NMB-R and BRS-3 (Nagalla et al., 1995). This receptor had the highest affinity for [Phe13]bombesin, the form most prevalent in frog brain, and had lower affinity for GRP and NMB. This receptor was called BB4 for bombesin receptor subtype 4 (Nagalla et al., 1995). Subsequent detailed binding studies and studies of cell signaling confirmed these findings and showed this receptor had greater affinity for [Phe13] bombesin than any other naturally occurring bombesin-related peptide (Katsuno et al., 1999). At present no mammalian equivalent of this receptor has been described and therefore it is not included in the classification discussed below. Recently in chickens a receptor was cloned that had high amino acid identity to frog BB4 [fBB4](70%) as well as human BRS-3 (69%) and lower for human GRP-R (58%) and human NBR-R (52%) (Iwabuchi et al., 2003). When expressed this receptor had low affinity for GRP and NMB, but it retained high affinity for [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14(Iwabuchi et al., 2003), a synthetic analogue which has high affinity for hBRS-3, GRPR, NMBR and fBB4 (Mantey et al., 1997; Pradhan et al., 1998). It was proposed this receptor be termed chBRS-3.5 because of its resemble to both fBB4 and BRS-3. No mammalian equivalent of this receptor has been described and therefore it is also not included in the classification below.
Based on the above molecular studies, three classes of mammalian bombesin receptors are proposed for which the nomenclature and a few features are summarized in Table 1. Although the usual NC-IUPHAR nomenclature uses the endogenous mammalian ligand, the substantial historical use of the frog peptide bombesin in the field to describe this system was retained. The BB1-BB3 receptors will each be dealt with in more detail in the following sections, but a few important points will be briefly covered here. The BB1 receptor was previously referred to as the NMB receptor, NMB-R or NMB-preferring receptor. This terminology is the same used for this bombesin receptor subclass in the Sigma-RBI Handbook of Receptor Classification and Signal Transduction (Watling, 2007) and is the same as the BB1 in the BJP Guide to Receptors and Channels (Alexander et al., 2006). The BB2 receptor was previously referred to as the GRPR, GRP receptor or GRP-preferring receptor (Table 1). This terminology is the same used for this bombesin receptor subclass in the Sigma-RBI Handbook of Receptor Classification and Signal Transduction (Watling, 2007) and is the same as the BB2 subclass in the BJP Guide to Receptors and Channels (Alexander et al., 2006). The BB3 receptor was previously referred to as the BRS-3 receptor, BRS-3, and bombesin receptor subtype 3(Table 1). This terminology is the same used for this bombesin receptor classes in the Sigma-RBI Handbook of Receptor Classification and Signal Transduction (Watling, 2007) and is the same as the bb3 receptor in the BJP Guide to Receptors and Channels (Alexander et al., 2006). Finally, the amphibian BB4 receptor does not have a mammalian equivalent so is not included in this classification. This receptor was also not classified in the Sigma-RBI Handbook of Receptor Classification and Signal Transduction (Watling, 2007) or the BJP Guide to Receptors and Channels (Alexander et al., 2006).
II. BB1 Receptor
III.1. Early studies of the BB1 receptor
Prior to the identification of the BB1 in 1989 in rat esophageal muscle tissue sections by direct binding studies using 125I-Bolton-Hunter-labeled NMB and subsequent esophageal muscle strip contraction studies (von Schrenck et al., 1989), there were no early studies that unequivocally established the existence of BB1. Numerous previous studies had demonstrated the frog peptides ranatensin and litorin, which closely resembled NMB (Minamino et al., 1983), had potent effects on various tissues and especially on smooth muscle contraction, which in some classes had differences from bombesin (Falconieri Erspamer et al., 1988; Regoli et al., 1988). However, these differences were not significant enough to clearly establish the existence of a separate class of BB1 receptors (Minamino et al., 1983; Falconieri Erspamer et al., 1988; Regoli et al., 1988). Although there had been many binding studies to numerous tissues from the late 1970's, in almost all cases 125I-[Tyr4] bombesin or another radiolabeled bombesin analogue was used (Moody et al., 1978; Shapira et al., 1993; Ladenheim et al., 1993b). Unfortunately, bombesin has high affinity for both BB1 and BB2 making it more difficult to distinguish subtypes. Numerous classes of selective BB2 receptor antagonist were developed prior to the cloning of the BB1 and these also confirmed the presence of the BB1 on esophageal smooth muscle (von Schrenck et al., 1990). After the pharmacologic description of BB1 on esophageal muscle and prior to its cloning in 1991, using selective BB2 receptor antagonists or binding studies using radiolabeled NMB and selective agonists or BB2 receptor antagonists, BB1 receptors were demonstrated in the CNS (Ladenheim et al., 1990) and on gastric smooth muscle cells (Severi et al., 1991).
III.2. Cloned BB1 receptor and receptor structure
The human BB1 receptor is a 390 amino acid protein and it shows an 89% amino acid identity with the rat BB1 (Corjay et al., 1991). The human BB1 receptor has 55% amino acid identities with the human BB2 (Corjay et al., 1991) and 47% with the human BB3 receptor (Fathi et al., 1993b). The human BB1 receptor has two consensus sites for potential PKC phosphorylation and three potential N-linked glycosylation sites (Corjay et al., 1991). Hydropathy plots yielded results consistent with a seven transmembrane structure typical for a G-protein coupled receptor (Corjay et al., 1991). The BB1 receptor has been cloned from rat (Wada et al., 1991) (Fig. 2), mouse (Ohki-Hamazaki et al., 1997a) and the frog, bombina orientalis (Nagalla et al., 1995). Cross-linking studies demonstrate the mature human BB1 receptor had a molecular weight of 72 ± 1 kDa and when deglycosylated 43 ± 1 kDa (Benya et al., 1995b). Detailed cross-linking and serial deglycosylation studies using enzymatic digestion in the rat BB1 receptor demonstrated a molecular weight of 63 kDa in the membrane and showed there were no O-linked carbohydrates but that the mature BB1 receptor was a sialoprotein (Kusui et al., 1994). However, each of the potential N-linked glycosylation sites was, in fact, glycosylated, with tri-antennary and /or tetra-antennary complex oligosaccharide chains (Kusui et al., 1994).
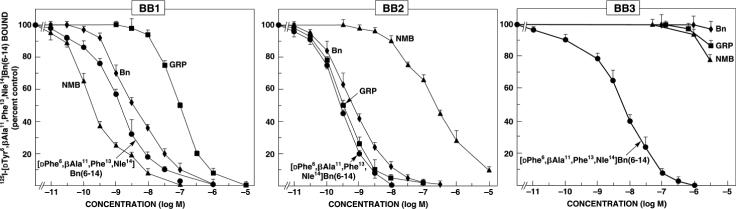
Figure 2.
Ability of the mammalian peptides, gastrin-releasing peptide (GRP) and neuromedin B NMB); the amphibian peptide, bombesin and the synthetic bombesin analogue, [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 to interact with the three human classes of bombesin receptors. Data are partially from (Benya et al., 1995b; Ryan et al., 1998b).
III.3. BB1 receptor genomic organization
The human BB1 receptor gene is localized at human chromosome 6p21-qter and in the mouse on chromosome 10 (Table 1). Both the human, rat and mouse genes contained three exons with two introns (Corjay et al., 1991; Wada et al., 1991; Ohki-Hamazaki et al., 1997a; Ohki-Hamazaki, 2000). In the mouse the gene for BB1 receptor spanned more than 10 kb with exon 1 of the BB1 gene separated from exon 2 by 6 kb and this in turn separated from exon 3 by 3 kb (Ohki-Hamazaki et al., 1997a). In human and mouse the first intron of the BB1 gene was located between transmembrane domains 3 and 4 and the second between transmembrane domains 5 and 6 (Corjay et al., 1991; Ohki-Hamazaki et al., 1997a). The first intron interrupted a codon for arginine located immediately COOH terminal to the transmembrane domain 3, and the second intron was located between glutamine and methionine codons in both the mouse and human BB1 gene (Corjay et al., 1991; Ohki-Hamazaki et al., 1997a). The positions of the first and second introns were identical in the mouse and human BB1 receptor gene (Corjay et al., 1991; Ohki-Hamazaki et al., 1997a).
III.4. BB1 receptor expression
Expression levels of BB1 receptor mRNA has been reported in human, mouse, rat, and monkey (Corjay et al., 1991; Wada et al., 1991; Ohki-Hamazaki et al., 1997a; Sano et al., 2004). In the monkey where it was studied in detail, highest levels of BB1 mRNA are found in the CNS and in the testis (Sano et al., 2004). In the CNS the BB1 receptor was expressed widely in different brain regions including the amydala, caudate nucleus, hippocampus, hypothalamus, thalamus, brain stem, spinal cord and in peripheral tissues in addition to the testis and the stomach, which is a similar distribution to that found in rats and mice (Wada et al., 1991; Ohki-Hamazaki et al., 1997a; Ohki-Hamazaki, 2000; Sano et al., 2004). In the rat and mouse, BB1 mRNA is present in high amounts in the olfactory region and esophagus (Wada et al., 1991; Ohki-Hamazaki et al., 1997a). Binding studies and studies of biological activity provide evidence for BB1 on both gastrointestinal and urogenital smooth muscle cells (von Schrenck et al., 1989; Severi et al., 1991; Bitar and Coy, 1992; Kim et al., 1993). Binding studies have confirmed the widespread distribution of BB1 in the brain showing especially high levels in the olfactory tract of the rat (Ladenheim et al., 1990; Ladenheim et al., 1992; Ladenheim et al., 1993a).
Using binding studies and/or assessment of BB1 mRNA, BB1 receptors have been shown to exist on a large number of different tumors (Reubi et al., 2002; Jensen and Moody, 2006) including CNS tumors (glioblastomas) (Wada et al., 1991; Wang et al., 1992), small cell and nonsmall cell lung cancers (Corjay et al., 1991; Moody et al., 1992; Toi-Scott et al., 1996; Siegfried et al., 1997; Moody et al., 2000; Jensen and Moody, 2006), carcinoids (intestinal, thymic, bronchial) (Reubi et al., 2002), human ovarian epithelial cancers (Sun et al., 2000b) and pancreatic cancer cell lines (Jensen and Moody, 2006).
III.5. BB1 receptor pharmacology
III.5.a. BB1 receptor agonists
The human BB1 receptor (Moody et al., 1992; Benya et al., 1995b; Reubi et al., 2002) as well as the rat BB1 receptor (von Schrenck et al., 1989; von Schrenck et al., 1990; Wang et al., 1992; Ladenheim et al., 1992; Ladenheim et al., 1993a) has a greater than a 100-fold higher affinity for NMB than GRP (Fig. 1,2). Bombesin and the frog peptides, ranatensin and litorin also had relatively high affinity for the BB1 receptor (affinities 1−10 fold less than NMB) (Wang et al., 1992; Mantey et al., 1997; Katsuno et al., 1999) (Table 1 and 2). The synthetic bombesin analogue [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14(Mantey et al., 1997), which has high affinity for the human BB3 receptor also has a high affinity for the human BB1 receptor as well as the human BB2 receptor and fBB4 (Mantey et al., 1997; Pradhan et al., 1998) (Table 2).
Table 2.
Affinity of bombesin receptor subtypes for various agonist/antagonists.
| Affinity (nM)a |
|||
|---|---|---|---|
| Variable | BB1 | BB2 | BB3 |
| I. Natural occurring Agonist | |||
| GRP | 440 | 18 | >10,000 |
| NMB | 4 | 248 | >10,000 |
| Bombesin (Bn) | 34 | 4 | >10,000 |
| Litorin | 7 | 6 | >10,000 |
| Ranatensin | 13 | 2 | >10,000 |
| Alytesin | 460 | 62 | >10,000 |
| Phyllolitorin | 47 | 240 | >10,000 |
| Neuromedin C (GRP 18−27) | 140 | 20 | >10,000 |
| [Phe13 ]Bombesin | 350 | 0.77 | >10,000 |
| II. Synthetic Agonists | |||
| [D-Phe6, β-Ala11, Phe13, Nle14] Bn 6−14 | 0.36 | 0.99 | 4.2 |
| [D-Tyr6, (R)-Apa11, Phe13, Nle14] Bn 6−14b | 7200 | >1900 | 8.2 |
| [D-Tyr6, Apa-4Cl11, Phe13, Nle14] Bn 6−14b | 2400 | 151 | 2.8 |
| Ac-Phe, Trp, Ala, His (tBzl), Nip, Gly, Arg-NH2b | 3800 | 5000 | 259 |
| [D-Phe6] Bn 6−14 | 14 | 2 | >10,000 |
| [D-Phe6, D-Ala11, Leu14] Bn 6−14 | 7600 | 13 | >10,000 |
| II. Antagonists | |||
| [D-Phe6] Bn 6−13 methyl ester | 7500 | 1.1 | >10,000 |
| N-propionyl- [D-Ala11] GRP 20−26 methyl ester | 13,660 | 3.4 | >10,000 |
| PD 168368 | 39 | 1300 | 1010 |
| D-Nal, Cys, Tyr, D-Trp, Lys, Val, Cys, Nal-NH2 | 59 | 2780 | >10,000 |
| [[Tyr4, D-Phe12] Bn 6−14 | 1900 | >10,000 | >10,000 |
| [Leu13, ψ13−14 ,Leu14]Bn6−14 | >10,000 | 430 | >10,000 |
| [D-Phe6, Leu13, Cpa14, ψ13−14]Bn6−14 | 2700 | 42 | 6800 |
| BW2258U89 | >10,000 | 0.74 | >10,000 |
| [D-Arg1, D-Trp7,9, Leu11]substance P | 4,100 | 11,300 | >10,000 |
| JMV594 | >10,000 | 2.2 | >10,000 |
| JMV641 | 1500 | 0.46 | >10,000 |
All data are for rat BB1, mouse BB2 and human BB3 except data indicated in footnote b. Data are from (Coy et al., 1992b; Mantey et al., 1997; Pradhan et al., 1998; Ryan et al., 1998b; Katsuno et al., 1999; Ryan et al., 1999; Tokita et al., 2001b)
Data are fro human BB1, BB2 and BB3 and are from (Mantey et al., 2001; Mantey et al., 2004; Mantey et al., 2006) Definition of compound structures are in text for each specific for receptor.
III.5.b. BB1 receptor antagonists
Whereas the search for high affinity receptor antagonists for the BB2 receptor has been very successful (see section below) (Jensen and Coy, 1991; Jensen et al., 1993; de Castiglione and Gozzini, 1996), the results with BB1 receptor have been much less successful and only a few high affinity receptor antagonists are available. None of the strategies used for making high affinity BB2 antagonists were successful with the BB1 receptor including the synthesis of bombesin or NMB COOH terminal pseudopeptide analogues, COOH terminal truncated analogues or [des-Met10] NMB amides, alkylamides or esters (Lin et al., 1995). Subsequently, it was discovered that certain substituted somatostatin (SS) analogues selectively antagonized the BB1 receptor compared to the BB2 receptor (Orbuch et al., 1993). The most potent analogue was cyclo-SS-octa [D-Nal-Cys-Tyr- D-Trp-Lys-Val-Cys-Nal-NH2], which had a 100-fold higher affinity for the BB1 receptor than the BB2 receptor (Ki −230 vs. 3000 nM) (Orbuch et al., 1993; Ryan et al., 1999) (Table 2). Unfortunately this analogue also interacted with high affinity with somatostatin receptors (IC50-0.80 nM) and mu opioid receptors (IC50-430 nM) (Orbuch et al., 1993). Substitution of an ornithine for Lys greatly reduced the affinity for somatostatin receptors, and a related analogue, (BIM-23127), [D-Nal-Cys-Tyr- D-Trp-Orn-Val-Cys-Nal-NH2] inhibited NMB cell signaling in rat BB1 receptor transfected Rat-1 cells (Lach et al., 1995) and selectively reversed NMB feeding suppression, but had no effect on the action of GRP (Ladenheim et al., 1997b). However, a recent study reports BIM-23127 also functions as a receptor antagonist of both human and rat urotensin-II receptors (Herold et al., 2003) limiting its utility. Peptoid antagonists of BB1 have been described including PD 165929 (Eden et al., 1996) and PD 168368 (Ryan et al., 1999), which have high affinity and selectivity for BB1. In a detailed comparison of bombesin receptors from different species, PD 168368 was found to have a similar high affinity (Ki-15−45 nM) for BB1 receptors from each species, a 30- to 60-fold lower affinity for the BB2 receptor from different species and a >300 fold lower affinity for the BB3 receptor or fBB4 (Ryan et al., 1999) (Table 2). It also inhibited NMB-stimulated cellular signaling in a competitive manner (Ryan et al., 1999) as well as inhibiting NMB-induced proliferation of rat C6 glioblastoma cells (Moody et al., 2000) and NMB stimulation of NCI-H1299 lung cancer cell proliferation (Moody et al., 2000).
III.6. BB1 receptor structural basis of receptor binding/activation
III.6.a. BB1 receptor agonist binding/activation
Structure-function studies of NMB demonstrate the COOH terminal octapeptide is the minimal peptide length required for BB1 receptor activation and the full decapeptide was required for full affinity for the BB1 receptor (Lin et al., 1996). NMB differs from GRP in the COOH octapeptide, which is the biologically active end (Broccardo et al., 1976; Lin et al., 1996), at 3 residues; substitution of a leucine in NMB for a histidine in GRP at position 3, a threonine for valine at position 6 and a phenylalanine for leucine at position 9 of NMB from the amino terminus (Minamino et al., 1983; Lin et al., 1996) (Fig.1). Structure-function studies of all naturally occurring bombesin-related peptides for BB1 and BB2 receptors suggested the presence of the phenylalanine instead of leucine, as the penultimate amino acid from the COOH terminus in NMB was not important for selectivity for the BB1 receptor, Single amino acid substitutions in NMB demonstrated the Leu for His substitution in position 3 was the most important for determining high affinity and selectivity for the BB1 receptor (Lin et al., 1996) (Fig.1.
A chimeric receptor approach (Fathi et al., 1993a) and homology screening after computer alignment of bombesin receptor family members (Sainz et al., 1998), followed by site-directed mutagenesis studies have been used to explore the molecular basis of NMB high affinity and selectivity for the BB1 receptor over the BB2 receptor (Fig. 3). A study of BB1/BB2 chimeric receptors (Fathi et al., 1993a) demonstrated that differences in the amino terminus of the two receptors were of minimal importance for high affinity NMB interaction. High affinity and selectivity for the BB1 receptor was primarily determined by differences in transmembrane domain 5 (TM5)(Fathi et al., 1993a)(Fig. 3). Site-directed mutagenesis of the amino acid differences between the BB1 receptor and the BB2 receptor in this region demonstrated the substitution of an Ile216 instead of Ser in the comparable position of the TM5 of the BB2 receptor was the critical difference accounting for high affinity NMB interaction with the BB1 and not the BB2 receptor (Fathi et al., 1993a). A second study (Sainz et al., 1998) used a different approach to select potentially important amino acids for NMB selectivity for the BB1 receptor and further study. Using amino acid sequence alignment of bombesin receptor family members and identifying conserved amino acids in members with similar peptide affinities (Akeson et al., 1997), four amino acids were identified that could be important for high affinity bombesin binding to either the BB1 or BB2 receptor (Akeson et al., 1997)(i.e. in the BB1 receptor: Gln123, Pro200, Arg290, Ala310 and in the BB2 receptor: Gln121,Pro199,Arg288,Ala 308). Possible gain of affinity mutants were made in the BB3 receptor, which has a low affinity for NMB (Mantey et al., 1997; Ryan et al., 1998a; Ryan et al., 1998b), by substituting alone or in combination each of these four BB1 receptor amino acids for the comparable amino acid(s) of the BB3 receptor (Arg127, Ser205, His294, Ser315) (Fig. 3). It was found that each of these four amino acids are important for determining NMB affinity because the affinity for NMB of the BB3 mutants with these BB1 receptor amino acids substituted one at a time were increased (Sainz et al., 1998). The substitution of all 4 amino acids for the comparable amino acid in the BB3 receptor, which has a low very affinity for NMB (i.e. Ki-3450 nM) increased the affinity and the potency for NMB, almost up to that seen with the native BB1 receptor (Sainz et al., 1998). This study helps to define the binding pocket for NMB by identifying four amino acids needed for high affinity NMB interaction in markedly different BB1 regions [transmembrane domain 2 (Gln123), extracellular domain 2 (Pro200), extracellular domain 3 (Arg290) and transmembrane region 7 (Ala310) (Fig. 3) (Sainz et al., 1998).
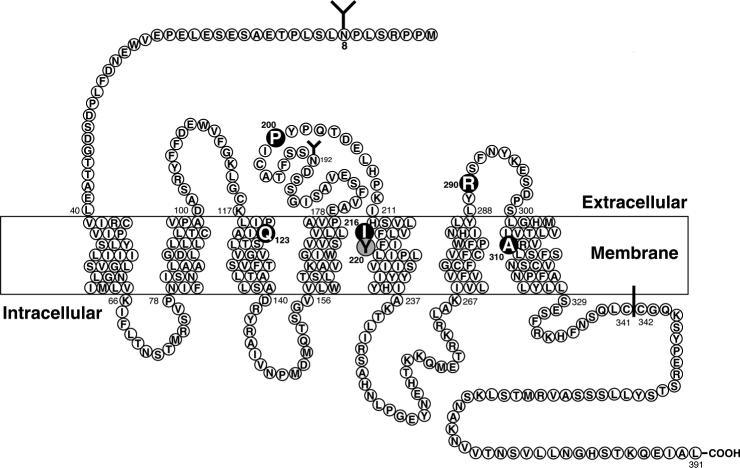
Figure 3.
Schematic representation of the rat BB1 receptor showing the postulated transmembrane topology, sites for NH2-linked glycosylation, possible palmitoylated cysteines in the cytoplasmic tail, and the key amino acids for high affinity NMB interaction (dark) or interaction with the BB1 receptor specific peptoid antagonist PD 168368 (shaded). Amino acid data is from (Wada et al., 1991), NMB high affinity sites form (Fathi et al., 1993a; Sainz et al., 1998) and the PD 168368 from (Tokita et al., 2001a).
III.6.b. BB1 receptor antagonist binding
Using a chimeric receptor approach combined with site-directed mutagenesis and receptor modeling the molecular basis of selectivity of the BB1 receptor antagonist, PD 168,368 was studied (Tokita et al., 2001a)(Fig. 3). PD 168,368 is a new class of antagonists described as a peptoid, because this group of antagonists is nonpeptide ligands, which were designed using the chemical structure of the mammalian neuropeptide of interest as a stating point (Horwell et al., 1994; Horwell, 1995). This approach has yielded antagonists for cholecystokinin, somatostatin, tachykinins and bombesin receptors (Boden et al., 1993; Horwell et al., 1994; Boyle et al., 1994; Horwell, 1995; Eden et al., 1996; Tran et al., 1998; Tokita et al., 2001a). However, little is known about the molecular basis of their affinity and whether they resemble peptide or other nonpeptide ligands in the basis of their selectivity and affinity (Tokita et al., 2001a). The receptor extracellular domains were shown not to be important for the selectivity of PD 168,369 by studying both loss-of-affinity BB1 receptor chimeras in which the extracellular domains of the BB1 were replaced by those from BB2, one at a time or the reverse study performed by making PD 168,368 gain-of-affinity chimeras in the BB2 receptor (Tokita et al., 2001a). Additional PD 168,368 loss and gain-of-affinity chimeric studies made by exchanging the upper transmembrane regions of BB1 and BB2 receptors showed that differences in the upper transmembrane 5 (TM5) were the key determinants of selectivity of PD 168,368 (Tokita et al., 2001a). Site-directed mutagenesis studies of the different amino acids between the BB1 receptor and the BB2 receptor in the upper TM5 region demonstrated the substitution of Tyr at position 220 of BB1 for Phe in the comparable position in BB2 was the critical difference (Tokita et al., 2001a)(Fig. 3). Three-dimensional modeling studies showed the critical Tyr220 was facing the interior of a large binding pocket formed primarily by transmembrane domains 3−7 and minimum energy conformation of the ligand showed that it was dominated by a large hydrogen bond accepting region around the nitrophenyl group (Tokita et al., 2001a). It was concluded that the Tyr220 hydroxyl of the BB1 receptor was critical for interacting with the nitrophenyl group of PD 168,368, likely primarily by hydrogen bonding. This result showed the binding of this peptoid antagonist was similar to that reported with other non-peptide antagonists of GRPR's, in that it was primarily dependent on interaction with transmembrane regions (Tokita et al., 2001a).
III.7.BB1 receptor signaling, activation, and modulatory processes (internalization, down-regulation, desensitization)
The human BB1 receptor (Moody et al., 1986; Corjay et al., 1991; Moody et al., 1992; Moody et al., 1995a; Benya et al., 1995b), as well as the rat BB1 receptor (Wada et al., 1991; Wang et al., 1992; Jones et al., 1992; Dobrzanski et al., 1993; Lach et al., 1995; Akeson et al., 1997; Vigne et al., 1997; Tsuda et al., 1997b; Hou et al., 1998) is coupled to phospholipase C resulting in breakdown of phosphoinositides, mobilization of cellular calcium and activation of protein kinase C. BB1 receptor activation also result in the stimulation phospholipase A2 (Moody et al., 1995a) and phospholipase D by a PKC-dependent and independent mechanism (Tsuda et al., 1997b), but does not activate adenylate cyclase (Benya et al., 1992). BB1 receptor stimulation also results in activation of tyrosine kinases (Lach et al., 1995; Tsuda et al., 1997b) stimulating tyrosine phosphorylation of p125FAK by a phospholipase C independent mechanism which requires p21rho and the integrity of the actin cytoskeleton (Tsuda et al., 1997b). BB1 receptor activation also stimulated tyrosine phosphorylation of paxillin and MAP kinase activation (Lach et al., 1995). The native as well as transfected rat BB1 receptor in BALB 3T3 cells has been shown to behave in a similar manner in their binding and signaling cascades (Benya et al., 1992) demonstrating the usefulness of this cell line for studying BB1 receptor interaction and signaling.
The BB1 receptor is coupled to heterotrimeric guanine-nucleotide binding proteins in both native and BALB 3T3 transfected cells (Benya et al., 1992; Wang et al., 1993). In a Xenopus oocyte assay with the injection of antisense oligonucleotides, Gαq was identified as a mediator of the BB1 receptor response (Shapira et al., 1994). With an in situ reconstitution assay with purified G protein α subunits, it was found that cells expressing the BB1 receptor activated Gαq, but not Gαt or Gαi/o (Jian et al., 1999). This activation was enhanced by βγ dimers with a relative potency of: βγ> β1γ2>> β1γ1. In this study (Jian et al., 1999) these results were contrasted with the BB2 receptor and differences were found in their kinetics of activation, preference for Gαq proteins from different sources and for βγ dimers demonstrating distinct coupling mechanisms for these two closely related receptors (Jian et al., 1999).
In contrast to the BB2 receptor there have been few studies of BB1 receptor modulatory processes (internalization, down-regulation, or desensitization). Both the human (Benya et al., 1995b) and rat BB1 receptors (Benya et al., 1992; Wang et al., 1993; Benya et al., 1994c) are rapidly internalized with receptor activation of the rat BB1 receptor. The rat BB1 receptor internalized 60−80%% of the bound ligand and human BB1 receptors 70% of the bound ligand. In addition to being rapidly internalized by BB1 receptor bearing cells, the ligand is rapidly degraded by these cells (Benya et al., 1992; Wang et al., 1993). Protease inhibitors markedly decreased ligand degradation by either rat native or rat BB1 receptor transfected BALB 3T3 cells (Benya et al., 1992; Wang et al., 1993) with the acid proteinase inhibitor, leupeptin being the most potent followed by bacitracin>chymostatin> phosphoramidon>> bestatin, amastatin. The BB1 receptor also undergoes desensitization, which is mediated by receptor down-regulation and internalization (Benya et al., 1994c). Pre-incubation for 3 hours with 3 nM NMB markedly attenuated the ability of a maximally effective concentration of NMB (1 uM) to subsequently stimulate either native or BB1 transfected BALB 3T3 cells, but did not alter the response to other stimulants (Benya et al., 1994c). This desensitization was associated with a rapid decrease in BB1 receptors due to internalization of the receptors. Restoration of receptor number and response recovered over a 6-hour period and it was not dependent on new protein synthesis, but was due to receptor recycling, because it was inhibited by the recycling inhibitor, monesin, a monocarboxylic acid cation ionophore (Benya et al., 1994c).
III.8.BB1 receptor function in various tissues and in vivo
One of the main difficulties in assessing the effects of BB1 receptor activation in the CNS as well as peripheral tissues, especially in older studies, is that bombesin was frequently used as the agonist, and it interacts with both BB1 and BB2 receptor with relatively high affinity. Furthermore, many tissues possess both BB1 and BB2 receptors and therefore it was difficult to assess whether a particular response was due to activation of the BB1 or BB2 receptors present.
Numerous effects of NMB in both in vivo and in vitro studies have been reported, but it is not clear in many cases which are physiological and which are pharmacological. Studies comparing the potencies of NMB to GRP as well as binding studies or antagonist studies provide evidence that BB1 receptor can stimulate contraction of urogenital and gastrointestinal smooth muscle (esophageal, gastric, colonic, gallbladder) (Regoli et al., 1988; von Schrenck et al., 1989; von Schrenck et al., 1990; Severi et al., 1991; Kilgore et al., 1993; Parkman et al., 1994; Milusheva et al., 1998); potently inhibit thyrotropin release from the pituitary acting as an autocrine and paracrine regulator (Rettori et al., 1992; Pazos-Moura et al., 1996; Ortiga-Carvalho et al., 2003); and have potent CNS effects including inhibiting food intake independent of BB2 stimulation (Ladenheim et al., 1994; Ladenheim et al., 1996b; Ladenheim et al., 1997b; Merali et al., 1999; Ladenheim and Knipp, 2007), mediating aspects of the stress and fear responses as well as various behaviors such as spontaneous activity (Merali et al., 2002; Merali et al., 2006).
BB1 receptor knockout mice are now available and have undergone a limited number of investigations for actions of NMB (Ohki-Hamazaki et al., 1999; Oeffner et al., 2000; Yamano et al., 2002; Yamada et al., 2002b; Yamada et al., 2003) (Table 1). In these mice the hypothermic effect of NMB was reduced by 50% without a change in the GRP response supporting a possible BB1 receptor mediated role in thermoregulation: NMB-mediated gastric smooth muscle contraction was not effected suggesting this is mediated not through BB1 receptors; and no effect on feeding could be confirmed, although NMB did not have an effect in the control animals (Ohki-Hamazaki et al., 1999). BB1 receptor's satiety effects are mediated through different peripheral neural pathways than BB2 receptor's satiety effects, because only BB1 receptors satiety effect is inhibited by capsaicin treatment suggesting the involvement of primary sensory afferent neurons (Ladenheim and Knipp, 2007). Recently, NMB has found to be expressed in human and rodent adipose tissue and to be regulated by changes in energy balance. It was proposed that because of the known anorectic effects of NMB centrally, it may form part of a new adipose tissue-hypothalamic regulating system for food intake (Hoggard et al., 2007). In the BB1 receptor knockout mice dysregulation of the thyroid occurs suggesting BB1 receptor pathways are importantly involved in both TSH gene regulation and function (Oliveira et al., 2006); dysfunction in response to stress was seen (Yamano et al., 2002; Yamada et al., 2002b), impairment in the modulation of the CNS 5-HT system in response to stress occurred (Yamano et al., 2002) and an impairment of learning and memory was seen (Yamada et al., 2003). The alterations in the CNS 5-HT and stress in these animals is particularly interesting, because the dorsal raphe nucleus is one of the brain regions that has a preponderance of BB1 receptors (Wada et al., 1990; Ladenheim et al., 1992; Pinnock et al., 1994; Merali et al., 2006), which are located on 5-HT neurons, and stimulation of this nucleus by NMB stimulates release of 5-HT resulting in anxiogenesis (Merali et al., 2006). In a study in rats using BB1 and BB2 receptor agonists and antagonists (Bedard et al., 2007) data was provided that both GRP and NMB affect the stress response. NMB affected both anxiety and fear responses, whereas GRP affected only fear responses (Bedard et al., 2007).
Whereas the growth effects of the BB2 receptor on normal and especially in neoplastic tissues have received the most attention, stimulation of the BB1 receptor and/or administration of NMB has been shown to have growth promoting effects in a number of neoplastic tissues. NMB is an autocrine growth factor for non-small cell lung cancer (NSCLC) with 14/14 such cell lines possessing BB1 receptors in one study (Siegfried et al., 1997) and in 4 NSCLC cell lines examined in detail NMB was synthesized and released into the media by the tumor cell in 7−15 times greater amounts than GRP (Siegfried et al., 1997). Blockade of the BB2 receptor only partially blocked the proliferative effect of NMB on there cells demonstrating the importance of BB1 receptor activation for the proliferative effects in these tumor cells (Siegfried et al., 1997). Furthermore, in human colon cancers NMB and the BB1 receptor are co-expressed and they act in an autocrine growth fashion (Matusiak et al., 2005). Activation of BB1 receptors causes proliferation of rat C6 glioblastoma cells (Moody et al., 1995a), BB1 receptor transfected RAT-1 cells (Lach et al., 1995), small cell lung cancers (Moody et al., 1992) and adrenal zona fasciculata cells (Malendowicz et al., 1996).
III.9.BB1 receptor in diseases
At present no disease has been shown to be caused specifically by alterations in the BB1 receptor. Activation of the BB1 receptor in various human cancers due to an autocrine growth pathway may have an important effect on their growth particularly human small cell lung cancers, nonsmall cell lung cancers, colonic cancer, and various carcinoid tumors (Moody et al., 1992; Moody and Jensen, 1996; Siegfried et al., 1999; Matusiak et al., 2005; Jensen and Moody, 2006). In various studies BB1 receptors are over-expressed by 55% of small cell lung cancers, 67% of non-small cell lung cancers, 46% of intestinal carcinoids, and a proportion of colon cancers, prostate cancers and CNS tumors such as glioblastomas (Moody et al., 1995a; Reubi et al., 2002; Matusiak et al., 2005; Jensen and Moody, 2006).
Numerous studies (Rettori et al., 1992; Pazos-Moura et al., 1996; Ortiga-Carvalho et al., 2003) including BB1 receptor knockout studies (Oliveira et al., 2006) support the conclusion that NMB plays an important physiological role in the regulation of thyrotropin release primarily having an inhibitory effect. NMB is produced in the pituitary (Jones et al., 1992) and it is proposed that NMB functions as a tonic inhibitor of TSH secretion, acting as an autocrine/paracrine regulator (Rettori et al., 1992; Oliveira et al., 2006) (Table 1). Conditions with increased TSH release such as hypothyroidism are associated with decreased pituitary NMB levels (Jones et al., 1992; Ortiga-Carvalho et al., 2003), while in hyperthyroidism where the TSH levels are suppressed; there is an increased pituitary NMB level (Jones et al., 1992; Ortiga-Carvalho et al., 1997). These results suggest NMB could play an important role in human thyroid disorders causing hyper- of hypo-function.
The role of NMB in human feeding disorders is unclear at present. Two genetic studies have suggested that the NMB gene is a possible candidate for eating disorders and predisposition to obesity (Oeffner et al., 2000; Bouchard et al., 2004).
III. BB2 Receptor
IV.1. Early studies of the BB2
Many of the early studies provide limited information on the BB2 receptor, as discussed in III.1 above for the BB1 receptor. This occurred because many of the tissues studied are now known to possess both BB2 and BB1 receptor and in most studies bombesin analogues were used which have high affinity for both subclasses of receptors. This continued to occur after the isolation of GRP in 1978 (McDonald et al., 1979), even though it had greater selectivity than bombesin analogues for the BB2 over the BB1 (von Schrenck et al., 1989; Lin et al., 1995; Benya et al., 1995b; Reubi et al., 2002) because of its limited availability. In vivo studies were even more difficult to interprete because numerous studies demonstrated that GRP-related peptides can both have a direct action on tissues as well as indirect action because they are potent at stimulating the release of many hormones (gastrin, insulin, somatostatin, CCK, pancreatic polypeptide, enteroglucagon, pancreatic glucagon, gastric inhibitory peptide) (McDonald et al., 1979; Modlin et al., 1981; Ghatei et al., 1982; McDonald et al., 1983; Greeley, Jr. et al., 1986; Pettersson and Ahren, 1987; Knuhtsen et al., 1987; Kawai et al., 1988; Hermansen and Ahren, 1990). With the development of selective BB2 receptor antagonists (von Schrenck et al., 1990; Jensen and Coy, 1991; Benya et al., 1995b) and the increased use of BB2 selective ligands such as GRP, it became clear that a separate GRP-preferring receptor existed, even before the cloning of the mouse and human BB2 receptor in the early 1990's(Spindel et al., 1990; Corjay et al., 1991; Battey et al., 1991) (Table 2). It subsequently became clear that a number of the tissues that had been extensively used to characterize bombesin receptors/responses such as pancreatic acinar cells (Jensen et al., 1978; Jensen, 1994) and Swiss 3T3 cells (Rozengurt, 1988) possessed only BB2 receptors, whereas others such as the CNS (Battey and Wada, 1991; Ladenheim et al., 1992) and smooth muscle preparations possessed both BB1 and BB2 receptors (Severi et al., 1991).
IV.2. Cloned BB2 and receptor structure
The human BB2 receptor has 384 amino acids and shows high homology (90% amino acid identities) with the mouse BB2 receptor (Corjay et al., 1991) (Fig. 4). The human BB2 receptor has 55% amino acid identities with the human BB1 receptor (Corjay et al., 1991) and 51% with human BB3 receptor (Fathi et al., 1993b). Hydropathy analysis of the predicted human BB2 structure revealed seven regions of hydrophobic amino acids consistent with a seven transmembrane structure typical for G-protein coupled receptors (Corjay et al., 1991). There were two consensus sites of potential PKC phosphorylation and two potential sites for N-linked glycosylation in the human BB2 receptor (Corjay et al., 1991). The BB2 receptor has been completely or partially cloned from 21 species (Baldwin et al., 2007) and the most highly conserved regions are in the transmembrane domains and the third intracellular domain (Baldwin et al., 2007). The presence of a likely disulphide bond between cysteines at the end of the extracellular domain1 and middle of extracellular domain 2 (Cys113 and Cys196 in human BB2) is preserved in all non-insect species (Baldwin et al., 2007)(Fig. 4). Solubilization studies as well as cross-linking studies demonstrate the mature human BB2 receptor has a molecular weight greater than that predicted from the structure (Kris et al., 1987; Rozengurt, 1988; Feldman et al., 1990; Huang et al., 1990; Staley et al., 1993; Williams and Schonbrunn, 1994; Kusui et al., 1994; Benya et al., 1994b; Benya et al., 1995b). Cross-linking studies demonstrate the mature human BB2 receptor has a molecular weight of 60 ±1 kDa, the mouse BB2 receptor 82 ± 2 kDa and when each is deglycosylated 43 ±1 kDa (Kris et al., 1987; Rozengurt, 1988; Huang et al., 1990; Williams and Schonbrunn, 1994; Kusui et al., 1994; Benya et al., 1994b; Benya et al., 1995b). These results demonstrate that 35% of the mature human BB2 receptor's molecular weight is due to glycosylation, whereas in the mouse BB2 receptor it is 47%. This difference is likely due to the existence of 2 potential sites of N-linked glycosylation in the human BB2 receptor compared to four potential sites in the mouse BB2 receptor (Spindel et al., 1990; Corjay et al., 1991; Battey et al., 1991; Benya et al., 1995b)(Fig. 4). Using cross-linking studies with serial deglycosylation by enzymatic digestion (Kusui et al., 1994; Kusui et al., 1995), as well as a molecular approach involving mutating the four potential N-linked glycosylation sites either alone or in combination in the murine BB2 receptor followed by receptor expression and cross-linking analysis (Benya et al., 1994d), the murine BB2 receptor was shown to be glycosylated at all 4 potential N-linked sites (Asn5, Asn20, Asn24 and Asn191) (Kusui et al., 1994; Benya et al., 1994d; Kusui et al., 1995). The extent of glycosylation varied however, with carbohydrate residues of 12 kDa on Asn5, 10 kDa on Asn20, 5 kDa on Asn24 and 9 kDa on Asn191 (Benya et al., 1994d). The presence of the glycosylation on Asn24 and Asn191 were especially important for sorting and expression of the murine BB2 receptor on the plasma membrane (Benya et al., 1994d). Digestion of the cross-linked receptor with different enzymes demonstrated the murine BB2 receptor was not a sialoprotein, contained no O-linked glycosylation, and had four tri-antennary and or tetra-antennary complex oligosaccharide chains (Kusui et al., 1994). Studies using baculovirus expression of the BB2 receptor (Kusui et al., 1995) demonstrated that neither full glycosylation was needed for receptor expression on the cell surface nor did the glycosylation have to be tri- or tetra-antennary for expression, because in the baculovirus only 11 kDa of glycosylation was seen on different sites and the glycosylation was entirely biantennary complex oligosaccharide chains (Kusui et al., 1995).
Figure 4.
Schematic representation of the murine BB2 receptor showing the postulated transmembrane topology, sites for NH2-linked glycosylation, possible palmitoylated cysteines in the cytoplasmic tail, and the key amino acids for high affinity GRP interaction or signaling (dark) or interaction with the BB2 selective antagonist statin analogue JMV594 or the pseudopeptide analogue JMV641. Amino acid data is from (Spindel et al., 1990; Battey et al., 1991), GRP high affinity sites from (Akeson et al., 1997; Donohue et al., 1999; Lin et al., 2000; Carroll et al., 2000b; Tokita et al., 2002; Glover et al., 2003; Nakagawa et al., 2005) and the JMV594 and JMV641 from (Tokita et al., 2001b).
IV.3. BB2 genomic organization
The human BB2 receptor gene was localized to Xp22 (Maslen and Boyd, 1993; Xiao et al., 2001) and the murine BB2 receptor gene to X chromosome between the Pdha-1 and Amg loci (Maslen and Boyd, 1993). Both the human (Xiao et al., 2001) and murine (Weber et al., 2000) BB2 receptor gene organization have been studied in detail. The human BB2 receptor gene has three exons (Corjay et al., 1991; Xiao et al., 2001) spanning more than 27 kb with intron 1 and intron 2 being 23 and 1.6 kb (Xiao et al., 2001). Exon one encodes the first three membrane-spanning domains of the BB2 receptor and the splice site is located in the proximal second intracellular loop (residue 137). Exon 2 encodes for the transmembrane regions 4 and 5 and most of the 3rd intracellular loop with the splice site located at residue 254. Exon 3 encodes for the transmembrane domains 5 as well as the cytoplasmic carboxyl terminus of the BB2 receptor (Xiao et al., 2001). Two major transcription start sites for the human BB2 receptor gene were found in gastrointestinal and breast cancer cells located 43 and 36 bp downstream of a TTTAAA motif which is identified 407 to 402 bp upstream of the ATG start codon (Xiao et al., 2001). Truncation studies of the transfected promoter region suggested a cyclic AMP response element (CRE) motif located 112 bp upstream of the major transcription start site is required to confer basal BB2 receptor promoter activity in duodenal cancer cells (Xiao et al., 2001).
IV.4. BB2 receptor expression
Expression levels of BB2 receptor mRNA has been reported in human, mouse and monkey (Spindel et al., 1990; Corjay et al., 1991; Battey et al., 1991; Ohki-Hamazaki et al., 1997a; Sano et al., 2004). BB2 receptor mRNA distribution was studied in detail in the monkey where it is found in the greatest amount in the pancreas and lesser amounts in the stomach, prostate, skeletal muscle and in the CNS (Sano et al., 2004). This result generally agrees with studies of location of the human BB2 receptor gene where a very strong signal was found in the normal pancreas with 4 specific transcripts of 9, 4.6, 3.1 and 2.1kb sizes, a weaker signal in the stomach with 2 transcripts of 9 and 3.1 kb and very weak 9 kb transcript signal in whole brain and adrenal (Xiao et al., 2001). In the monkey CNS the BB2 receptor mRNA was widely expressed with the highest amounts in hippocampus, hypothalamus, amydala and pons (Sano et al., 2004). In the mouse BB2 receptor mRNA was present in high amounts in the digestive tract in the colon, but not in the stomach or small intestine (Battey et al., 1991). Detailing mapping in the rat brain was reported which showed BB2 receptor expression in all brain regions, with the highest amounts of BB2 receptor mRNA in the hypothalamus, particularly the suprachiasmatic and supraoptic nuclei as well in the magnocellular preoptic nucleus in the basal ganglia and the nucleus of the lateral olfactory tract (Battey and Wada, 1991).
Detailed CNS location of the murine BB2 receptor has beenreported using a specific BB2 receptor antibody (Kamichi et al., 2005). The BB2 receptor was widely distributed in the mouse brain in the isocortex, hippocampal formation, pyriform cortex, amydala, hypothalamus and brain stem (Kamichi et al., 2005). Strong BB2 immunoreactivity was observed in many nuclei of the amygdala and in the nucleus tractus solitarius (Kamichi et al., 2005). Double labeling studies in the amydala demonstrated subpopulations of BB2 receptors present in the GABAergic neurons providing support for a possible role of BB2 receptors mediating memory by modulating neurotransmitter release in the local GABAergic network (Kamichi et al., 2005).
Binding studies have confirmed the widespread distribution of BB2 receptors in the brain showing high levels in the cortex as well as the suprachiasmatic and supraoptic nuclei of the rat (Ladenheim et al., 1990; Ladenheim et al., 1992; Ladenheim et al., 1993a; Moody and Merali, 2004). Binding studies and studies of biological activity provide evidence for BB2 on both gastrointestinal and urogenital smooth muscle cells (Severi et al., 1991; Kilgore et al., 1993; Ladenheim et al., 1997a; Milusheva et al., 1998; ter Beek et al., 2004; Fleischmann et al., 2005). BB2 receptors in the gastrointestinal tract are also found in gastric antral G cells (Giraud et al., 1987), other gastric mucosa cells (D cell, mucus cell, parietal cell) (Nakamura et al., 1988) and pancreatic acinar cells (Jensen et al., 1978; Jensen et al., 1988a; Jensen, 1994). In the epithelial cells lining the normal human gastrointestinal tract, BB2 receptor mRNA was only found in the antrum with the esophagus, jejunum, ileum and not in the descending colon (Ferris et al., 1997).
BB2 receptors are present on a large number of different tumors using binding studies, immunohistochemical localization using specific receptor antibodies and/or assessment of BB2 receptor mRNA. BB2 receptors have been widely studied in prostate cancer (Reubi et al., 2002; Jensen and Moody, 2006; Patel et al., 2006), small cell lung cancer (Corjay et al., 1991; Toi-Scott et al., 1996; Jensen and Moody, 2006; Patel et al., 2006), non small cell lung cancer (Corjay et al., 1991; Toi-Scott et al., 1996; Siegfried et al., 1997; Jensen and Moody, 2006), breast cancer (Gugger and Reubi, 1999; Reubi et al., 2002; Jensen and Moody, 2006; Patel et al., 2006), head and neck squamous cell cancer (Lango et al., 2002; Jensen and Moody, 2006), colon cancer (Carroll et al., 1999b; Carroll et al., 2000a; Jensen et al., 2001; Glover et al., 2003; Patel et al., 2006), uterine cancer (Fleischmann et al., 2005), various CNS/neural tumors [glioblastomas, neuroblastomas] (Jensen and Moody, 2006), ovarian cancer (Sun et al., 2000b), gastrointestinal carcinoid tumors (Reubi et al., 2002; Scott et al., 2004) and renal cell cancers (Reubi et al., 2002; Heuser et al., 2005).
IV.5. BB2 receptors pharmacology
IV.5.a. BB2 receptors agonists
The human BB2 receptor (Frucht et al., 1992; Benya et al., 1995b; Reubi et al., 2002) as well as in the rat (von Schrenck et al., 1990; Ladenheim et al., 1992; Ladenheim et al., 1993a; Lin et al., 1996; Katsuno et al., 1999; Ryan et al., 1999), mouse (Huang et al., 1990; Ryan et al., 1999) and guinea pig BB2 receptors (Jensen and Gardner, 1981; Mantey et al., 1993) have greater than 50-fold higher affinity for GRP than NMB (Fig. 2). Bombesin and various frog peptides, including ranatensin litorin, PG-L and [Phe13] bombesin also have high affinities for the BB2 receptor, where as other frog peptides such as phyllolitorin, [Leu8] phyllolitorin, SAP bombesin and Xenopus NMB have low affinity for this receptor (Jensen and Gardner, 1981; Frucht et al., 1992; Mantey et al., 1997; Katsuno et al., 1999) (Fig.1) (Table 2). The synthetic bombesin analogue, [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14(Iwabuchi et al., 2003), which has high affinity for human BB3 receptor, also has high affinity for the BB2 receptor as well as the BB1 receptor and fBB4 (Mantey et al., 1997; Pradhan et al., 1998; Ryan et al., 1998b).
IV.5.b. BB2 receptor antagonists, partial agonists and biased agonists
IV.5.b.1. BB2 receptor antagonists
There have been a large number of different compounds reported to function as BB2 receptor antagonists (Jensen and Coy, 1991; Jensen et al., 1993; de Castiglione and Gozzini, 1996). They can be divided into six general classes of BB2 receptor antagonists (Jensen and Coy, 1991; Jensen et al., 1993; de Castiglione and Gozzini, 1996)(Table 2). All classes are peptides or peptoid antagonists, except for Class 6, which are flavone derivatives, isolated from extracts of the mulberry tree Morus bombycis (Mihara et al., 1995). These six classes include substituted substance P analogues (Class 1); [D-Phe12] bombesin analogues (Class 2); modified position 13−14 bombesin or position 26−27 GRP analogues (Class 3); desMet14 or GRP27 analogues (Class 4); peptoids (Class 5) and finally the nonpeptide analogues, kuwanon G and H (Class 6) (Fig.1).
Jensen and co-workers described in 1984 that the D-amino acid substituted substance P (SP) analogue, [D-Arg1, D-Pro2, DTrp7,9, Leu13] SP, not only functioned as a substance P receptor antagonist, but also inhibited both radiolabeled bombesin binding and bombesin-stimulated amylase release from guinea pig pancreatic acini, which are now known to possess only BB2 receptors. Later, they showed various D-amino acid substituted substance P analogues had broad inhibitory activity against a number of GPCR (Zhang et al., 1988; Jensen et al., 1988b). The inhibition of the action of bombesin by [D-Arg1, D-Pro2, D-Trp7,9, Leu13] SP was competitive in nature with a Schild plot having a slope of 0.996 and the inhibition was specific for the substance P and BB2 receptor, because it did not inhibit VIP, secretin or carbamylcholine-stimulated secretion (Jensen et al., 1984). Subsequent studies demonstrated numerous D-amino acid substance P and SP4−11 analogues including [D-Arg1 , D-Phe5, D-Trp7, 9, Leu13] SP functioned as BB2 receptor antagonists (Woll and Rozengurt, 1988b; Jensen et al., 1988b; de Castiglione and Gozzini, 1996). These analogues were reported to inhibit bombesin-stimulated growth of lung cancer cells and Swiss 3T3 cells (Woll and Rozengurt, 1988a; Woll and Rozengurt, 1988b) as well as a number of other bombesin-stimulated changes in the CNS and peripheral tissues (Jensen and Coy, 1991). This class of BB2 receptor antagonists is now rarely used, not only because of their relatively low affinities for the BB2 receptor (1−40 uM), but also because of their lack of selectivity for the BB2 over the BB1 receptor. In addition, some show agonist activity in various tissues(von Schrenck et al., 1990; Jensen and Coy, 1991; Patel and Schrey, 1991; Lin et al., 1995; Mantey et al., 1997; Katsuno et al., 1999) (Table 2). These various D-amino acid substituted SP analogues were reported not only to inhibit the action of bombesin, but also to function as antagonists of substance P, cholecystokinin, vasopressin and endothelin (Zhang et al., 1988; Langdon et al., 1992; Jarpe et al., 1998). Subsequent detailed studies of the mechanism of action of these substance P analogues provided evidence that they were functioning as biased agonists rather than antagonists. This will be discussed below in the next section dealing with biased agonists.
Early bombesin structure-function studies demonstrated that Trp8 and His12 in the COOH terminus of bombesin were essential for biologic activity (Broccardo et al., 1976; Rivier and Brown, 1978; Marki et al., 1981). The substitution of a number of D-amino acids (DPhe, D-chloro-phenylalanine, D-Tyr) for His12 in bombesin analogues produced antagonists (Class 2) (Heinz-Erian et al., 1987; Saeed et al., 1989) (Fig.1). These antagonists inhibited bombesin-stimulated amylase release from pancreatic acini (Heinz-Erian et al., 1987; Saeed et al., 1989) and the satiety effect of bombesin in rats (Flynn, 1997) which were both due to BB2 receptor activation. The use of these antagonists is limited by their relatively low affinities for the BB2 receptor (0.4−10 uM), their low aqueous solubility and their low selectivity for BB2 over BB1 receptors (Lin et al., 1995; Mantey et al., 1997; Katsuno et al., 1999).
Numerous studies have demonstrated that the biologically active portion of GRP or bombesin is the COOH terminus (Broccardo et al., 1976; Rivier and Brown, 1978; Heimbrook et al., 1988; Lin et al., 1996). In 1988 Coy and coworkers reported a new class of BB2 receptor antagonists by substituting into the COOH terminus of bombesin, pseudopeptide bonds (ψ bonds) (i.e. each CONH group one at a time replaced by CH2NH), a strategy that had been used successfully to make antagonists for gastrin, secretin and substance P (Martinez et al., 1985; Rodriquez et al., 1986; Coy et al., 1988; Qian et al., 1989; Haffar et al., 1991) (Table 2) (Fig.1). Two of the pseuodopeptides were antagonists with the ψ 13−14 analogues having a higher affinity than the ψ 9−10 bond analogue. This ψ13−14 bombesin analogue was the first bombesin receptor antagonist described with an affinity <0.1 uM (Coy et al., 1988). Subsequent studies demonstrated this analogue had 50−100-fold selectivity for the BB2 receptor in human or rat than the BB1 receptor (Benya et al., 1995b; Ryan et al., 1999). This antagonist was shown to inhibit a number of BB2 receptor-stimulated processes including bombesin stimulated enzyme secretion from isolated acini and growth of Swiss 3T3 cells as well as of various small cell lung cancer cell lines (Trepel et al., 1988; Coy et al., 1988; Coy et al., 1989; Liu et al., 2002). A subsequent study described short chain pseudopeptide bombesin receptor antagonists ([such as [D-Phe6, Cpa14, ψ13−14]Bn6−14 ) that had fewer proteolytic sites, and could be more easily synthesized (Coy et al., 1989; Coy et al., 1990; Jensen and Coy, 1991; Coy et al., 1992a)(Table 2). Furthermore, some of the ψ13−14 analogues had partial agonist activity in some species (particularly the rat) which was not seen in a number of the newer, shortened substituted pseudopeptide analogues such as [D-Phe6, Cpa14, ψ13−14]Bn6−14 (Dickinson et al., 1988; Coy et al., 1990; Houben and Denef, 1991; Coy et al., 1992a) (Fig.1). A number of the shortened D-Phe substituted [ψ13−14] Bn6−14 analogues are > 100 fold selective for the BB2 over the BB1 receptor (von Schrenck et al., 1990; Mantey et al., 1997; Katsuno et al., 1999). Subsequently, a particularly potent group of pseudopeptide antagonists were described having a D-Pro-ψ (CH2NH)-Phe-NH2 moiety at the COOH terminus of GRP (Leban et al., 1993). One of the most potent and widely used analogue in this series is: (3-PhPr)-His, Trp, Ala, Val, D-Ala, His, D-Pro-ψ (CH2NH)-Phe-NH2 [Ki-0.001 nM murine BB2 (Leban et al., 1993); 0.7 nM rat BB2 (Mantey et al., 1997), 10 nM human BB2 (Moody et al., 1996a)] (BW2258U89). BW2258U89 has >10,000 fold selectivity for the rat BB2 over the rat BB1 receptor (Mantey et al., 1997; Katsuno et al., 1999)(Table 2). BW2258U89 is reported to inhibit small cell lung cancer growth (Moody et al., 1995b), inhibit bombesin-stimulated gastrin release in vivo in dogs and rats (Singh et al., 1992) and blocked the satiety effect of bombesin in rats (Kirkham et al., 1994). An additional series of substituted pseudopeptide analogues with position 14 substitutions in addition to the ψ13−14 bond have been described and widely used by Schally's group for inhibition of various tumor cell growth (Radulovic et al., 1991a; Cai et al., 1992; Qin et al., 1994; Cai et al., 1994; Qin et al., 1995; Jungwirth et al., 1998; Bajo et al., 2004). Two analogues with high potency in this group include [D-Phe6, ψ13−14, Tac14] Bn6−14 (tac=thiazolidine-4-carboxylic acid)[RC-3950-II) (Cai et al., 1994) (Ki-0.078 nM, murine BB2 receptor and [D-Tpi6, ψ13−14] Bombesin6−14 (RC-3095) (Ki-0.92 nM, murine BB2 receptor (Reile et al., 1994; Qin et al., 1994; Qin et al., 1995)). A final group of potent antagonists in this class were synthesized by J. Martinez's group, with the most potent being JMV641 and JMV594 (Azay et al., 1996; Lamharzi et al., 1998). JMV641 [H-D-Phe, Gln, Trp, Ala, Val, Gly, His-NH-*CH [CH2-CH (CH3)2]-**CHOH-(CH2)3-CH3 [where *(S) and **92% of (S isomer)] contains a pseudopeptide bond that mimics the transition state analogue [Ki-murine BB2 0.85 nM](Azay et al., 1996) and has a >3000 fold selectivity for the BB2 over the BB1 receptor (Tokita et al., 2001b). JMV594 [DPhe6, statine13]Bn6−14](where statine=4-amino-3-hydroxy-6-methylhepatanonoic acid) also has a high affinity for the murine BB2 receptor (Ki-0.60 nM) (Azay et al., 1998; Llinares et al., 1999) and has >5000 fold selectivity for the BB2 over the BB1 receptor (Tokita et al., 2001b)(Table 2).
The fourth class of BB2 receptor antagonists are all [desMet14] Bn or [desMet27] GRP analogues (Jensen and Coy, 1991; Jensen et al., 1993; de Castiglione and Gozzini, 1996), but vary widely in chemical groups attached and they including desMet amides (Heimbrook et al., 1989; Wang et al., 1990a; Wang et al., 1990b), alkylamides (Camble et al., 1989; Heimbrook et al., 1989; Wang et al., 1990a; Wang et al., 1990b), esters (Heimbrook et al., 1989; Wang et al., 1990b; Coy et al., 1992b), hydrazides (Wang et al., 1990b) and with other COOH terminal groups attached (Heimbrook et al., 1989; Heimbrook et al., 1991)(Table 2) (Fig.1). A number of these analogues have high potency for the BB2 receptor in all species studied and have high selectivity for the BB2 over the BB1 receptor (Heimbrook et al., 1989; Jensen and Coy, 1991; Jensen et al., 1993; Benya et al., 1995b; de Castiglione and Gozzini, 1996; Mantey et al., 1997; Katsuno et al., 1999). Two widely used antagonists in this class are [D-Phe6]Bn6−13methylester or its analogues (Wang et al., 1990b; Coy et al., 1992b) and Ac-[N-GRP 20−26-ethylester (Heimbrook et al., 1989) with each having high affinity for the BB2 receptor [Ki-2−5 nM](Heimbrook et al., 1989; Wang et al., 1990b; Coy et al., 1992b; Benya et al., 1995b; Mantey et al., 1997; Katsuno et al., 1999) and having > 1000 fold selectivity for the BB2 over the BB1 receptor (von Schrenck et al., 1990; Katsuno et al., 1999). [D-Phe6]Bn6−13methylester and/or Ac- [N-GRP 20−26-ethylester are reported to inhibit GRP-stimulated mitogenesis in 3T3 cells (Heimbrook et al., 1989) (Fig.1). GRP-dependent acid secretion (Heimbrook et al., 1989), GRP-induced signaling in small cell lung cancer cells, GRP/Bn-induced smooth muscle contraction (Maggi et al., 1992), BB2 receptor mediated pancreatic enzyme secretion (Wang et al., 1990b) and in vivo to inhibit bombesin/GRP stimulated pancreatic enzyme secretion (Varga et al., 1991; Coy et al., 1992b), satiety (Stratford et al., 1995; Ladenheim et al., 1996a), hypothermia (Cai et al., 1994) and acid secretion (Weigert et al., 1997). In vivo a number of these antagonist were found to have a short duration of action (Alptekin et al., 1991; Coy et al., 1992b) and it was found that by adding a D-Ala11 in place of Gly11 in bombesin, as well as lipophilic moieties to the amino terminus, the in vivo stability was improved and analogues with long duration of action were obtained. [D-pentafluoro-Phe6, D-Ala11]Bn6−13methylester not only retained high affinity for the BB2 receptor (human BB2 0.9 nM; rat BB2 Ki-5 nM) it had >400 to 10,000 fold selectivity for the BB2 over the BB1 receptor in rat and human (Coy et al., 1992b; Benya et al., 1995b) it had a 15-fold longer duration of action in vivo (Coy et al., 1992b) (Fig.1). This analogue was subsequently used in a number of human studies (Guex and Peitsch, 1997; Hildebrand et al., 2001) which will be reviewed in Section IV.8. below.
In contrast to the BB1 receptor (Eden et al., 1996; Moody et al., 2000; Tokita et al., 2001a), there are no selective peptoid BB2 receptor antagonists (Class 5). However, PD 176252 is a peptoid antagonist that has nanomolar affinity for both the BB2 [Ki 1 nM] and BB1 receptor [Ki 0.1 nM](Ashwood et al., 1998; Moody et al., 2003b). Subsequent studies demonstrated PD 176252 inhibited the growth of lung cancer cells; potentiated the growth inhibitory effects of histone deacetylase inhibitors (Moody et al., 2006a); inhibited GRP/Bn-stimulated signaling in lung cancer cells [Ca2+ and tyrosine phosphorylation of p125FAK] as well as the stimulation of increases in c-fos mRNA (Moody et al., 2000) and growth (Moody et al., 2000); and in rats had an anxiolytic effect in vivo in rats(Merali et al., 2006).
The only nonpeptide, nonpeptoid antagonists of BB2 receptors reported are kuwanon G and kuwanon H which are two closely related flavone compounds that were isolated from the Mulberry tree, Morus bombcycis (Mihara et al., 1995). Only one study (Mihara et al., 1995) has examined their ability to interact with BB2 receptors on Swiss 3T3 cells. Kuwanon G and kuwanon H had affinities of 290 and 470 nM, respectively for the murine BB2 receptor and Kuwanon H had a 22 fold higher affinity for the murine BB2 receptor than the rat BB1 receptor (Mihara et al., 1995). Kuwanon H inhibited both bombesin-stimulated changes in cytosolic calcium and growth in Swiss 3T3 cells, which are both, mediated by BB2 receptors (Mihara et al., 1995).
IV.5.b.2. BB2 receptor partial agonists
None of the naturally occurring mammalian or frog bombesin related peptides is a partial agonist for the BB2 receptor (Jensen et al., 1978; Jensen et al., 1988a; von Schrenck et al., 1989; Lin et al., 1996). However, one of the main difficulties found with the various classes of peptide antagonists is that, in some species or some cellular systems they demonstrated partial agonist activity or even full agonist activity, whereas they are antagonists in other species or cell systems (Jensen and Coy, 1991; Coy et al., 1991b; Coy et al., 1992a). This was reported for both class 3 pseudopeptide analogues as well as for class 4 potent desMet14 bombesin analogues in a number of studies (Dickinson et al., 1988; Coy et al., 1990; Wang et al., 1990b; Houben and Denef, 1991; Coy et al., 1992a; Wu et al., 1995). Furthermore, some BB2 receptor antagonists functioned as partial agonists for BB1 receptors (Ryan et al., 1996). Detailed studies with both bombesin pseudopeptide and desMet14 analogues, which functioned as pure BB2 receptor antagonists in the guinea pig or mouse, demonstrated that many showed partial agonist activity in the rat BB2 receptor (Coy et al., 1990; Wang et al., 1990b; Jensen and Coy, 1991; Coy et al., 1991b). The conclusion from these studies was that there exist important differences in the ability of the same ligand to activate the BB2 receptor from different species with the rat having less stringent peptide structural requirements for BB2 receptor activation than the guinea pig or mouse. The expression level of the BB2 receptor can have a marked effect on the magnitude of various agonist responses such as phospholipase C activation with stimulation of phosphoinositide breakdown (Tsuda et al., 1997a) and calcium mobilization (Wu et al., 1995) or stimulation of mitogenesis (Wu et al., 1995). This may contribute to the presence or magnitude of the partial agonist activity of some of these compounds in different tissues.
IV.5.b.3. BB2 receptor biased agonists
As discussed above after the initial description (Jensen et al., 1984) in 1984 of the ability of D-amino acid substituted analogues of substance P to function as bombesin receptor antagonists, the same group reported that some of these analogues could function as broad-spectrum antagonists inhibiting the activation of a number of peptide hormone GPCR's (Zhang et al., 1988; Jensen et al., 1988b). It is now clear these compounds can inhibit activation of a wide range of different G-protein coupled receptors [i.e. substance P, cholecystokinin, vasopressin and endothelin](Zhang et al., 1988; Langdon et al., 1992; Jarpe et al., 1998). A number of subsequent studies have proposed different mechanisms for the ability for the substituted SP analogues to function as broad spectrum GPCR antagonists, with some studies, but not others, suggesting they function as biased agonists at the BB2 receptor (Jarpe et al., 1998; Sinnett-Smith et al., 2000; MacKinnon et al., 2001; Djanani et al., 2003). Initially it was shown (Jarpe et al., 1998) that the substance P analogue [D-Arg1, D-Phe5, D-Trp7,9, Leu11] SP at concentrations that inhibited bombesin-stimulated calcium mobilization at the BB2 receptor, stimulated c-jun kinase activation and cytoskeletal changes. To explain this unexpected result it was proposed (Jarpe et al., 1998) that the substance P analogue functions as a biased agonist in that it causes the BB2 receptor to preferentially activate Gα12 over Gαq and this results in activation of the Gα12 stimulated events (c-Jun kinase activation, changes in cytoskeletal events) and inhibition of the Gαq stimulated events (i.e. calcium mobilization). A latter study (Sinnett-Smith et al., 2000) challenged this hypothesis by providing evidence that D-amino acid substituted SP analogues prevented BB2, bradykinin and vasopressin receptor activation of both Gα12 and Gαq. A more recent study (MacKinnon et al., 2001) provides evidence that [D-Arg1, D-Phe5, D-Trp7,9, Leu11] SP differentially modulates the activation of the G proteins Gα12, Gαi and Gαq. This unique ability allows BB2 receptor activation to couple to Gαi and at the same time block Gαq supporting the proposal that [D-Arg1, D-Phe5, D-Trp7,9, Leu11] SP is functioning as a biased agonist at the BB2 receptor.
IV.6. BB2 receptors structural basis of receptor binding/activation
IV.6.a. BB2 receptors agonist binding/activation
Structure-function studies of GRP or bombesin demonstrate the COOH terminal heptapeptide is the minimal peptide length required for BB2 receptor activation and the COOH terminal nonapeptide is the minimal fragment required for full affinity for BB2 (Mazzanti et al., 1982; Heimbrook et al., 1988; Lin et al., 1996). GRP differs from NMB in 3 residues in the biologically active COOH decapeptide: a histidine in GRP 8 amino acids from the COOH terminus instead of Leu in NMB, at 5 amino acids from the COOH terminus a valine in GRP instead of a threonine and a leucine at the penultimate position of GRP instead of phenylalanine in NMB (Minamino et al., 1983; Lin et al., 1996). Structure-function studies of all natural occurring bombesin-related peptides for the BB2 and BB1 receptors suggested primarily the presence His for Leu and to a lesser extent the presence of Leu for Phe were the most important differences in GRP from NMB determining high affinity and selectivity for the BB1 receptor (Lin et al., 1996). Correlating biological activity with binding affinity, especially of antagonists, demonstrated the presence of a COOH terminal amino acid in position 14 of bombesin, is not essential for high affinity for the BB2 receptor, but it is essential for biologic activity (Coy et al., 1988; Wang et al., 1990a; Wang et al., 1992).
From studies correlating binding results with biological activity, especially for COOH terminal pseuodopeptides, a model was proposed for the biologically active conformation of GRP/Bn at the BB2 receptor (Coy et al., 1988; Wang et al., 1990a; Coy et al., 1991b). In a study (Coy et al., 1988) of the effects on bombesin's affinity and potency for the BB2 receptor of substitution of a pseudopeptide bond (ψ bond) (i.e. CH2NH2 instead of CONH) between each amino acid pair at the COOH terminus, it was found only a ψ13−14 and ψ8−9 substitution resulted in peptides that retained affinity for the BB2 receptor but did not activate it and thus functioned as antagonists. Because previous studies of somatostatin analogues had shown hydrogen bonding was the prime factor in stabilizing the peptide's conformation (Sasaki et al., 1987), the loss of efficacy with retention of affinity in these two bombesin pseuodopeptides suggested the elimination of these CO groups were likely having an effect on the conformation of the peptide due to both loss of a potential intramolecular hydrogen-bonding point and increased rotation about the C-N bond (Coy et al., 1988). The model proposed (Coy et al., 1988) was based on the known solution conformation of somatostatin in which the COOH terminus of bombesin had a β-bend beginning at Val10 and the rest of the amino acid chains arranged in an antiparallel β-pleated sheet. In this model the hydrogen bonding between Leu13-Leu14 CO groups and Ala9-Val10−CO groups is important and their destruction by a pseudopeptide bond would lead to a conformational shift and loss of efficacy. Support for this conformation has come from studies of both agonists and antagonists (Wang et al., 1990a; Coy et al., 1991a; Kull, Jr. et al., 1992). Only the agonist results will be discussed here with the antagonist result in the next section. The proposed folded conformation of the COOH terminus of GRP/bombesin was supported by findings from a study of various covalently cyclized analogues of the COOH terminus of bombesin (Coy et al., 1991a). Using such an approach both agonists and antagonists were identified supporting the proposal that both BB2 receptor agonists and antagonist likely adopted a folded conformation. A subsequent study (Lin et al., 1996) demonstrated one cyclized analogue, [D-Cys6, D-Ala11, Cys14]Bn6−14 had >400 fold greater potency at activation the BB2 receptor than the BB1 receptor, suggesting the constrained conformation induced by cyclization resembled more the active conformation for the BB2 than the BB1 receptor. It also suggested the active conformation for BB2 and BB1 receptor are significantly different (Lin et al., 1996). The substitution of D-Ala in position 11 of bombesin for glycine would be expected to stabilize the folding in the above proposed model and therefore not lead to a decrease it affinity/potency (Lin et al., 1996). The finding that [D-Ala11] bombesin was equipotent to native bombesin for the BB2 receptor, but resulted in a marked decrease in affinity for the BB1 receptor, supports both the folded conformation model proposed for the GRP/Bn COOH terminus (Coy et al., 1988) and also suggests the active conformation of bombesin for these two receptors is very different (Lin et al., 1996).
To elucidate the molecular basis of BB2 receptor agonist selectivity, high affinity and receptor activation both a chimeric receptor approach (Tseng et al., 1995a; Tseng et al., 1995b; Maughfling et al., 1997; Tokita et al., 2002) either alone or followed by site-directed mutagenesis (Tokita et al., 2002), a comparison of receptor selectivity for agonists combined with homology screening after computer alignment of bombesin receptor family members (Akeson et al., 1997; Nakagawa et al., 2005) and site-directed mutagenesis of specific residues (Benya et al., 1993; Slice et al., 1994; Benya et al., 1994d; Donohue et al., 1999; Lin et al., 2000; Schumann et al., 2003) has been used. A study (Maughfling et al., 1997) of chimeric BB2/BB1 receptors demonstrated receptor regions between the end of transmembrane (TM) domain 3 (TM3) and TM6 were responsible for the high affinity and selectivity of NMC (GRP18−27) for the BB2 receptor. A subsequent detailed study (Tokita et al., 2002) examined both GRP loss and gain of affinity chimeric BB2/BB1 receptors followed by site-directed mutagenesis, demonstrated differences in the extracellular (EC) domain 3 (EC3)[where the N terminus is EC1] was the specific critical region for determining GRP high affinity and selectivity (Fig. 4). Site-directed mutagenesis (Tokita et al., 2002) of each of the 20 amino acid differences between the BB2 and BB1 receptor in the EC3 demonstrated that two amino acid differences were the most important; the substitution of Phe185 in the BB2 receptor for Ile in the comparable position in the BB1 receptor and Ala198 in the BB2 for Ile in the comparable position of the BB1 receptor (Fig. 4). Additional point mutations in these positions (Tokita et al., 2002) demonstrated that an amino acid with an aromatic ring in position 185 of the BB2 receptor was the most important of these two changes, whereas the size of the backbone substitution in position 198 was the difference from the BB2 receptor at this position but it was less important that the position 185 difference for determining high affinity for GRP. The mechanism (Tokita et al., 2002) of the aromatic substitution's effect in position was not studied in detail, but it was proposed it might be due to cation-π or π−receptor interaction.
Important amino acids for GRP selectivity/high affinity were also identified using a different approach of comparison of receptor selectivity for agonists combined with homology screening after computer alignment of bombesin receptor family members (Akeson et al., 1997; Nakagawa et al., 2005). This approach made use of the fact that the BB2, BB1 and frog BB4 receptors all have relatively high affinity for bombesin, whereas the BB3 receptor has a very low affinity. In the first study (Akeson et al., 1997) nine amino acids were identified which were the same in BB1, BB2, and frog BB4 receptor, but differed in the BB3 receptor. Site-directed mutagenesis (Akeson et al., 1997) demonstrated the occurrence of Arg288 in the BB2 receptor or comparable position of the other receptors with high affinity for bombesin, instead of a histidine in the comparable position of the BB3 receptor (i.e. R288H change), a glutamine in position 121 instead of arginine (Q121R), a proline in position 199 instead of a serine (P199S change) and an alanine in position 308 of instead of a serine (A308S change) were the critical differences accounting for high affinity for bombesin (Fig. 4). Of these four critical differences the Q121R and R288H change had the most profound effect on determining both GRP and bombesin's affinity for the BB2 receptor (Akeson et al., 1997). Molecular modeling (Akeson et al., 1997) demonstrated the Q121, R288 and A308 all lie in a plane pointing inward toward the binding pocket. Furthermore, the critical Q121 lies in the same position in the BB2 receptor in TM3 as the highly conserved aspartate in biogenic amine receptors, which has been shown to be critical for their high affinity interaction, suggesting a similar interaction is critical for GRP high affinity. In a second study (Nakagawa et al., 2005) a modification of the above approach was used where amino acid differences from receptors with high affinity for GRP (BB2 receptor and frog BB4 receptor) were identified compared to the BB3, which has low affinity for GRP. Fourteen amino acid differences (Nakagawa et al., 2005) were found and each analyzed by site-directed mutagenesis with the results compared to the effects of the Q121R, P199S, R288H and A308S point mutations described above (Akeson et al., 1997). This study (Nakagawa et al., 2005) demonstrated that GRP's selectivity for the BB2 receptor was primarily determined by K101, Q121, A198, P199, S293, R288 and T297 of the BB2 receptor (Fig. 4). Molecular modeling of the BB2 receptor (Nakagawa et al., 2005) demonstrated the backbone substitution of eight of the 14 amino acids identified using this approach were facing inward to the binding pocket and were within 6 Å including the Q121, A193, S293 and R288 which were especially important for a GRP affinity. A phylogenetic analysis of the structures of the BB2 receptor from 21 species was performed and compared to other bombesin receptor members as well as other GPCR's (Baldwin et al., 2007). This analysis (Baldwin et al., 2007) demonstrated the sequence GVSVFTLTALS (125−136 in murine BB2 receptor) in the cytoplasmic side of TM3 is unique to the bombesin receptor family and retained by all members; the cysteines residues in positions C94, C114, C197, C277 and C317 in the murine BB2 are highly conserved in all BB2 receptors; and the important amino acids described for determining GRP affinity are generally well-conserved in all BB2 receptors.
BB2 receptor mutations are reported to occur in human colon and gastric cancer and a number of these have identified and characterized (Carroll et al., 1999a; Carroll et al., 2000b; Glover et al., 2003). In the human BB2 receptor P145Y, P198L, P200S and V316E mutations (equivalent to positions 146,199,210,317 in murine BB2 receptor, (Fig. 4) are found in colon and/or gastric cancers (Carroll et al., 1999a; Carroll et al., 2000b; Glover et al., 2003) and each resulted in no ligand binding of the expressed BB2 receptor, demonstrating these amino acids in the BB2 receptor are either essential for receptor expression and/ or binding.
A number of studies have attempted to examine the important amino acids in BB2 receptor mediating activation as well as the stimulation of various receptor modulatory processes (internalization, down-regulation and or desensitization) (Benya et al., 1994a; Tseng et al., 1995a; Donohue et al., 1999; Schumann et al., 2003). Because the BB2 receptor as well as the BB1 and BB3 receptors have a conserved aspartate residue at position 98 (D98) just at the extracellular border of TM 2 and a arginine residue (R309) at the top of TM7 (Fig. 4), the effect of these on receptor binding and activation was explored using site-directed mutagenesis, binding studies and an in situ reconstitution assay. The results (Donohue et al., 1999) demonstrated that these residues are not only important for high affinity binding, but they are critical for efficient coupling of the BB2 receptor to Gαq. The authors (Donohue et al., 1999) suggested that these results are consistent with the existence of a salt bridge interaction between these two polar and oppositely charge amino acids that maintains the proper BB2 receptor conformation necessary to interact with G proteins. The importance of the second and third intracellular domains (IC2, IC3) of the BB2 receptor for affinity, activation and internalization were examined by making BB2 recptor-/m3 muscarinic cholinergic receptor chimeras (Tseng et al., 1995a). Replacement of IC2 and/or IC3 domain alone or together in the BB2 receptor had minimal or no effect on receptor affinity or the occurrence of the high affinity receptor binding state, however the replacement of IC3, but not IC2, dramatically decreased the ability of the BB2 receptor to internalize bombesin or to activate the receptor and stimulate phospholipase A2 or C (Tseng et al., 1995a). It was proposed from these results that agonist activation of a similar conformational state is required for BB2 receptor G protein-coupling and internalization, but is not needed for generation of a high affinity binding state (Acs et al., 2000). The BB2 receptor, as well as other bombesin receptors and many GPCR's, have a retained DRY sequence at the beginning of the second intracellular domain and a conserved alanine in the distal third intracellular domain (Benya et al., 1994a) which have been shown in a number of GPCR's to be important for G-protein coupling and cell signaling (Benya et al., 1994a). Site-directed mutagenesis (Benya et al., 1994a; Benya et al., 1995a) was used to make a R139G and A263E mutant (Fig. 4) to explore the importance of these conserved residues for BB2 receptor affinity, cell signaling and activation of receptor modulatory processes (internalization, down-regulation, desensitization) (Benya et al., 1994a; Benya et al., 1995a). Both of these mutations decreased BB2 receptor affinity for bombesin by 9-fold, neither receptor could activate phospholipase C and the R139G, but not the A263E mutant, was uncoupled from G-proteins. Both mutant receptors demonstrated impaired internalization, however the impairment was much greater with the R139G mutant. These results demonstrated that BB2 receptor internalization occurs by both phospholipase-dependent and phospholipase-independent mechanisms and that both are dependent on G-protein coupling of the activated BB2 receptor. In contrast (Benya et al., 1995a), each of these mutant BB2 receptors demonstrated no bombesin-stimulated receptor down-regulation, whereas the wild type receptor underwent a > 75% decrease in receptor number when exposed to agonist. These results demonstrated that BB2 receptor internalization and down-regulation are at least partially mediated by different signaling mechanisms. In studies of the muscarinic cholinergic M3 receptor the central portion of the second intracellular domain (IC2) is important for G protein coupling and internalization (Moro et al., 1993; Moro et al., 1994). Results of a systematic analysis of this region of the BB2 receptor (amino acids 142−148, Fig. 4) are reported (Schumann et al., 2003). In this study (Schumann et al., 2003) each amino acid was mutated to an alanine either alone or in combination. The mutations had a minimal (<2 fold) to no effect on agonist receptor affinity, however 5 mutants showed decreased efficacy for activation of phospholipase C (Schumann et al., 2003). Two mutations the IM143,147AA and VM144,147AA showed markedly decreased abilities to activate phospholipase C. The IM double mutant had defective internalization, whereas the R145A mutant had enhanced internalization (Schumann et al., 2003). Both double mutants and 3 single mutants also had decreased down-regulation. Maximal changes in phospholipase C were significantly correlated with maximal down-regulation, but not with internalization. Therefore, amino acids within the IC2 of the BB2 receptor are important for activation of phospholipase C and support the proposal that internalization and down-regulation have a different dependence on phospholipase C activation and are largely independent processes (Schumann et al., 2003). Kinetic analysis of the effect of the R145A mutation on BB2 receptor binding and internalization support the conclusion that the R145 in the native receptor is having a restraining effect on internalization and its mutation deceased receptor recycling without altering the endocytotic rate (Schumann et al., 2003).
Residues in the cytoplasmic carboxyl terminus of the receptor are important for various receptor modulatory processes such as internalization or desensitization in numerous GPCR's (Benya et al., 1993; Tseng et al., 1995b). Two different approaches have been used with the BB2 receptor to investigate the importance of this region. In one study (Benya et al., 1993) serial truncation mutants of the BB2 receptor COOH terminus were constructed as well as site-directed mutation of PKC consensus sites, a potential palmitoylation site and of Ser/Thr residues. None of these mutations altered receptor affinity or altered the ability of the expressed mutant to activate phospholipase C. Longer truncations (at residue 358 or more proximal) resulted in increasing impairment of internalization, whereas the mutation of the potential palmitoylation site had no effect. Mutation of the distal PKC consensus site moderately reduced internalization (approximately 50%) whereas mutation of all Ser/Thr residues in the COOH tail almost completely inhibited internalization (Benya et al., 1993). These results (Benya et al., 1993) show that BB2 receptor internalization is dependent on residues in the COOH terminus and suggests that it is partially PKC-dependent, but completely dependent on the presence of at least some Ser or Thr residues in this region. A second approach used to examine the importance of the COOH terminus in BB2 receptor function was to make BB2 receptor/m3 muscarinic cholinergic receptor chimeras or BB2 receptor/CCKA receptor chimeras by substituting the COOH terminus of these receptors for that of the BB2 receptor (Tseng et al., 1995b). Each of the chimeric receptors demonstrated similar affinities to the wild type BB2 receptor for bombesin and similar potencies for activation by bombesin. Ligand internalization as well as receptor recycling by the chimeric BB2 receptors generally assumed the characteristics of the donor receptor (Tseng et al., 1995b). This study (Tseng et al., 1995b) demonstrated that carboxyl-terminal structures determine both the internalization of the ligand-receptor complex and the subsequent recycling. The BB2 receptor undergoes rapid down-regulation and desensitization in addition to internalization with agonist stimulation (Benya et al., 1994b; Benya et al., 1994d; Kroog et al., 1995a; Benya et al., 1995a). A number of studies have explored the receptor structural elements involved in stimulation of these receptor modulatory processes as well as the signaling cascades involved. The latter will be discussed in a later section on BB2 cell signaling mechanisms. In a number of GPCR's a conserved NPX(n)Y motif in the seventh transmembrane receptor domain (TM7) is important for mediating receptor internalization and/or resensitization (Slice et al., 1994). Mutation of T324 within this motif in the rat BB2 receptor did not effect receptor internalization or its resensitization (Slice et al., 1994) demonstrating that this motif is not universally involved in receptor internalization.
The importance of the COOH terminus of the BB2 receptor for mediating chronic desensitization or down-regulation was explored by using mutant BB2 receptors with increasing COOH terminal truncations, a distal PKC consensus mutation, a deletion of all COOH terminal Ser/Thr residues, or mutations that either prevent BB2 receptor activated phospholipase C activation (R139G, A263E) or G protein-coupling (R139G) (Benya et al., 1995a). Receptor mutants that did not activate phospholipase C did not show down-regulation or desensitization and removal of the distal PKC consensus sequence markedly attenuated both processes (Benya et al., 1995a). These results lead the authors to conclude that PKC activation was essential for chronic desensitization and down-regulation and provide no evidence for the involvement of second messenger-independent mechanisms driving these receptor modulatory processes.
IV.6.b. BB2 receptors antagonist binding
Numerous structure-function studies of primarily peptide antagonists demonstrated that the COOH terminal amino acid of GRP or bombesin was not required for high affinity interaction with the BB2 receptor, however it was required to activate the receptor (Coy et al., 1988; Heimbrook et al., 1989; Wang et al., 1990a; Wang et al., 1992). A number of results from these studies and molecular modeling studies supported the model proposed by Coy and collaborators in which the COOH terminus of GRP existed in a folded conformation, stabilized by hydrogen bonding, with the rest of the amino acid chains arranged as an antiparallel β-pleated sheet (Coy et al., 1988). Computer generated molecular modeling (Kull, Jr. et al., 1992) of the COOH terminus of various GRP/Bn pseuodopeptides and correlation with whether they behaved as an antagonist or partial agonists for the BB2 receptor, supported the Coy model (Coy et al., 1988). In detailed studies of [desMet14] bombesin amides and alkylamides (Wang et al., 1990a) the resultant antagonist activity could also be explained by the proposed model (Coy et al., 1988) with the loss of the COOH terminal carbonyl group disrupting hydrogen bonding and modifying the conformation from the active form. The effect of this disruption is similar to the introduction of pseudopeptide bonds, which were proposed to result in a conformation shift of the position 14-carboxamide groups in the receptor-bound peptide promoted by the increased rotational freedom and flexibility introduced (Coy et al., 1988; Wang et al., 1990a).
In contrast to agonists, only two studies have examined the BB2 receptor structural elements responsible for BB2 receptor high affinity or selectivity for antagonists(Maughfling et al., 1997; Tokita et al., 2001b)(Fig. 4). A chimeric approach using BB2/ BB1 receptor combinations was used to examine the region of the BB2 receptor responsible for the 500-fold selectivity of [D-Phe6]Bn6−13ethylamide for the human BB2 receptor over the human BB1 receptor (Maughfling et al., 1997). The region from the NH2 terminus to the end of TM2 and regions in the EC4 and TM7 were primarily responsible for this antagonist selectivity. Using BB2/BB1 receptor chimeras, site-directed mutagenesis and molecular modeling, the molecular basis was examined for the >3000-fold and >5000-fold selectivity of the two class 3 BB2 receptor antagonists JMV641 ([D-Phe6, statine13] Bn6−14) and JMV594, which contains a pseudopeptide bond that mimics the transition state analogue (Azay et al., 1996; Lamharzi et al., 1998). Both loss of affinity and gain of affinity chimera studies showed only differences in the fourth extracellular domain (EC4) contributed to the BB2 selectivity of these antagonists. Each of the 11 amino acid differences between BB2 and BB1 in EC4 was mutated one at a time. The important differences for determining each antagonist's selectivity was the presence of Thr297 in BB2 instead of a proline in the comparable position in the BB1 receptor, Phe302 in BB2 instead of a Met in the BB1 receptor and the presence of Ser305 instead of Thr in the BB1 receptor (Fig. 4). Receptor modeling showed that each of these three amino acids faced inward toward the binding pocket and each was within 5 Å of the putative binding pocket (Tokita et al., 2001b). These results suggest that both receptor-ligand cation- π interactions and hydrogen bonding are important for the high selectivity of these antagonists.
IV.7. BB2 receptors signaling, activation, and modulatory processes (internalization, down-regulation, desensitization)
The human BB2 receptor (Moody et al., 1986; Corjay et al., 1991; Williams and Schonbrunn, 1994; Benya et al., 1995b; Moody et al., 1996b), as well as the rat BB2 receptor (Deschodt-Lanckman et al., 1976; Matozaki et al., 1991; Garcia et al., 1997; Tapia et al., 2006), mouse (Huang et al., 1990; Garcia et al., 1997) guinea pig BB2 receptor (Jensen et al., 1978; Jensen et al., 1988a; Jensen et al., 1988b; Jensen, 1994; Garcia et al., 1997) and canine BB2 receptor (Seensalu et al., 1997) are coupled to phospholipase C resulting in breakdown of phosphoinositides, generation of diacylglycerol (DAG), stimulation of the mobilization of cellular calcium and PKC activation (Klein et al., 1979; Rozengurt, 1988; Jensen, 1994; Rozengurt, 1998a). BB2 receptor stimulation activates both phospholipase β1 and β3 and this is dependent on Gαq (MacKinnon et al., 2001). Activation of the BB2 receptor also results in activation of phospholipase D (Cook et al., 1991; Briscoe et al., 1994), phospholipase A2 (Currie et al., 1992; Nishino et al., 1998) and is reported to stimulate increased cyclic AMP in some tissues (Rozengurt and Sinnett-Smith, 1983; Bjoro et al., 1987; Millar and Rozengurt, 1988; Garcia et al., 1997). The increase in cyclic AMP in Swiss 3T3 cells was reduced by PKC down-regulation and inhibition of cyclooxygenase suggesting these pathways were involved (Rozengurt et al., 1987). However, a systematic study demonstrated the activation of BB2 receptors in normal pancreas from three species (rat, mouse, guinea pig) (Garcia et al., 1997) and the transfected human or mouse BB2 receptor did not stimulate an increase in cAMP (Benya et al., 1994b; Benya et al., 1995b). These results compared to those in a number of studies in the literature led the authors (Garcia et al., 1997) to propose that the BB2 receptor may be coupled differentially to different adenylate cylases in different tissues in the same species. Down-stream DAG leads to the activation of both classic and novel PKC's, which catalyze the phosphorylation of a number of membrane-bound and cytosolic proteins. Furthermore, specific protein kinase cascades are triggered including the Raf/MEK/ERK kinase cascade, activation of protein kinase D and rapamycin-sensitive p70s6k, which lead to increased expression of immediate early response genes (i.e. c-myc, c-jun, c-fos) leading to the regulation of the cell cycle and cell proliferation (Rozengurt, 1998a).
BB2 receptor stimulation also results in the activation of tyrosine kinases and tyrosine phosphorylation of a number of proteins including p125 focal adhesion kinase (FAK) and PYK2, paxillin, ERK kinase and P130CAS (Rozengurt, 1998a; Rozengurt, 1998b). Paxillin and P130CAS function as important adaptors with paxillin promoting protein-protein interactions and P130CAS interacting with Src, c-Crk, and with numerous proteins that have SH2 and SH3 binding domains (Turner, 1994; Harte et al., 1996). BB2 receptor stimulation of P125FAK tyrosine phosphorylation occurs largely independent of PKC activation (Sinnett-Smith et al., 1993), but is dependent on the small GTP binding protein Rho and the integrity of the actin cytoskeleton and focal adhesion plaques (Rozengurt, 1998a). In addition to BB2 receptor activation stimulating the formation of focal adhesion plaques via a rho dependent mechanism, it also stimulates actin proliferation resulting in membrane ruffling via rac proteins (Nobes et al., 1995) In contrast to P125FAK and paxillin tyrosine phosphorylation due to BB2 receptor stimulation, stimulation of ERK activation and tyrosine phosphorylation is not dependent on rho or the other factors listed above (Seufferlein et al., 1996a). GRP induced activation of ERK is dependent on PKC (Rozengurt, 1998b) and transactivation of the EGF receptor (MacKinnon et al., 2001; Lui et al., 2003; Thomas et al., 2005) which may be mediated by Gi proteins (MacKinnon et al., 2001). Recent studies provide evidence that BB2 receptor stimulation of tyrosine phosphorylation of P125FAK, paxillin and P130CAS occurs via an interaction with Gα12/13 and rho (Rozengurt, 1998a). BB2 receptor stimulation also leads to coupling to Gα12 to elicit c-Jun N-terminal kinase (JNK) activation (MacKinnon et al., 2001; Chan and Wong, 2005).
BB2 receptor stimulation results in a rapid activation of Src kinase family members (Rodriguez-Fernandez and Rozengurt, 1996; Vincent et al., 1999; Pace et al., 2006) which is not dependent on either PKC or mobilization of calcium, nor is it dependent on rho or the integrity of the cytoskeleton (Rodriguez-Fernandez and Rozengurt, 1996). Blockade of Src family kinases decreases BB2 receptor stimulated transactivation of the EGFR as well as MAP kinase stimulation (Vincent et al., 1999). The EGFR transactivation by BB2 receptor activation in head and neck squamous cancers is dependent on Src mediated cleavage and release of TGF-α and amphiregulin and is essential for invasion and growth of these cancers (Vincent et al., 1999).
Acute and chronic BB2 receptor stimulation results in an activation of a number of receptor modulatory processes (internalization, down-regulation, or desensitization) (Lee et al., 1980; Pandol et al., 1982; Millar and Rozengurt, 1990; Walsh et al., 1993; Briscoe et al., 1994; Benya et al., 1994b; Kroog et al., 1995a), and a number of studies have investigated the cell signaling processes involved. In cells containing human (Benya et al., 1995b), mouse (Zachary and Rozengurt, 1987; Brown et al., 1988; Wang et al., 1993; Benya et al., 1993; Benya et al., 1994d; Tsuda et al., 1997a; Acs et al., 2000) or rat BB2 receptors (Zhu et al., 1991), with agonist exposure the receptor-ligand complex is rapidly internalized (t0.5-5 min) with 80−85%, 70−90%, and 50% respectively of the bound ligand internalized. In epithelial cells transfected with the murine BB2 receptor, agonist ligand and receptor were internalized by 5 minutes into early endosomes, after 10 min both were in perinuclear vesicles and after 60 min the BB2 receptor had recycled back to the surface (Grady et al., 1995). In this study (Grady et al., 1995) and in others (Benya et al., 1994b; Benya et al., 1995a) there was a rapid down-regulation of cell surface receptors and the recovery was decreased by acidotropic agents, but not by inhibitors of new protein synthesis. The internalization of the BB2 receptor is partially dependent on phospholipase C activation (Benya et al., 1994a; Williams et al., 1998; Schumann et al., 2003) and requires clathrin-coated pits since it is inhibited by hyperosmolar sucrose as well as phenylarsine oxide (Grady et al., 1995). Acute desensitization of the BB2 receptor occurs within seconds to minutes of agonist exposure (Walsh et al., 1993; Briscoe et al., 1994) and is reported to occur with stimulated phospholipase D activity as well as stimulation of phosphoinositides breakdown (Briscoe et al., 1994; Williams et al., 1998) and for stimulation of changes in cytosolic calcium, with the latter shown to be homologous in nature (Walsh et al., 1993). In some tissues acute desensitization and down-regulation of the BB2 receptor is caused by hormones/neurotransmitters activating phospholipase C such as carbachol and cholecystokinin (Younes et al., 1989; Vinayek et al., 1990). Chronic BB2 receptor desensitization occurs after prolonged incubation with agonist (1−2 hours) and is homologous in nature (Lee et al., 1980; Benya et al., 1995a). Receptor structure-function studies reviewed above provide strong support for the conclusion that down-regulation and chronic desensitization are coupled processes being effected by similar receptor structural alterations and cellular signaling cascades and have a distinct mechanism from that causing internalization (Benya et al., 1994d; Benya et al., 1995a; Tsuda et al., 1997a; Schumann et al., 2003). The results of these studies provided no evidence for second messenger independent processes in mediating down-regulation or desensitization, whereas internalization is equally stimulated by second messenger-dependent and independent processes and the presence of the COOH terminal serines and threonines were essential for mediating these effects. In HIT-T15 cells BB2 receptor mediated desensitization was closely coupled to down-regulation (Swope and Schonbrunn, 1990).
Studies in the β-adrenergic receptor and a number of GPCR's demonstrate receptor phosphorylation, primarily by G protein-coupled receptor kinases (GRK) and subsequent binding of arrestins are critical for receptor internalization and deactivation during acute desensitization (Krupnick and Benovic, 1998; Ferguson, 2001; Premont and Gainetdinov, 2007). Studies demonstrate BB2 receptor activation results in rapid phosphorylation of the receptor (Kroog et al., 1995b; Williams et al., 1996; Kroog et al., 1999; Ally et al., 2003) as does stimulation of the BB2 receptor containing cells by the phorbol ester, TPA (Kroog et al., 1995b; Williams et al., 1996; Ally et al., 2003). However, agonist and TPA-induced BB2 receptor phosphorylation occur at different receptor sites (Williams et al., 1998). GRK's are serine-threonine kinases that preferentially phosphorylate agonist occupied, active conformation GPCR's and lead to uncoupling from G protein and endocytosis (Szekeres et al., 1998; Premont and Gainetdinov, 2007). Bn/GRP stimulates BB2 receptor phosphorylation at serine/threonine residues in the COOH terminus, but does not stimulate tyrosine phosphorylation in the BB2 receptor (Williams et al., 1996; Ally et al., 2003). With BB2 receptor activation arrestin translocation occurs to the plasma membrane (Ally et al., 2003) and requires an intact DRY sequence in the second intracellular domain of the BB2 receptor (Ally et al., 2003). BB2 receptor internalization has been proposed to play a key role in acute BB2 receptor desensitization (Swope and Schonbrunn, 1990) because the kinetics of each is identical. Furthermore, the kinetics of BB2 receptor phosphorylation correlate closely with both internalization and acute desensitization (Kroog et al., 1995b; Williams et al., 1996; Williams et al., 1998). Phosphorylation of the BB2 receptor after GRP/ Bn stimulation is reported in one study (Williams et al., 1996), but not another (Kroog et al., 1995b), to be mediated by both a PKC-dependent and PKC-independent process (likely a GRK family member).
Studies demonstrate that radiolabeled GRP/Bn is rapidly degraded by the BB2 receptor (Swope and Schonbrunn, 1987; Zachary and Rozengurt, 1987; Brown et al., 1988; Zhu et al., 1991; Wang et al., 1993; Williams et al., 1998). This degradation is best inhibited by the general inhibitor bacitracin or the thermolysin-like metalloproteinase inhibitor, phosphoramidon and to a less degree by leupeptin, bestatin>chymostatin>amastatin (Wang et al., 1993). The lysosomal proteinase inhibitor, choroquine, also inhibits degradation (Swope and Schonbrunn, 1987; Williams et al., 1998).
Activation of the BB2 receptor results in growth of both normal and neoplastic tissues (Moody et al., 2003a; Jensen and Moody, 2006). The cell signaling cascades involved have been studied extensively in both Swiss 3T3 cells and in numerous tumors cells. In 3T3 cells and a number of tumor cells (prostate, head and neck squamous cell cancer, nonsmall cell lung cancer cells) the activation of the BB2 receptor results in stimulation of phosphorylation of Akt (Liu et al., 2007) and extracellular regulated kinase (ERK) phosphorylation (Sakamoto et al., 1988; Rozengurt, 1998b; Koh et al., 1999; Vincent et al., 1999; Lui et al., 2003; Thomas et al., 2005) which has been shown in some cells to be dependent on the transactivation of the EGF receptor which is in turn depends on Src and changes in cytosolic calcium in some cases. Mitogenesis in 3T3 cells is dependent on BB2 receptor stimulated changes in cytosolic calcium; activation of PKC, PKD, ERK kinases, and release of arachidonic acid (Rozengurt, 1998b). BB2 receptor stimulation of ERK phosphorylation is dependent on Ras but not Rap1 in prostate tumor cells (Sakamoto et al., 1988). The transactivation of the EGF receptor by BB2 receptor activation is dependent on PKC and PKD activation in some cells (Seufferlein et al., 1996b; Rozengurt, 1998b; Sinnett-Smith et al., 2004; Sinnett-Smith et al., 2007). EGF receptor transactivation upon BB2 receptor stimulation as well as by a number of other GPCRs occurs via metalloproteinase-dependent cleavage and release of EGF-related peptides that then activate the receptor (Sakamoto et al., 1988; Vincent et al., 1999; Lui et al., 2003). The inhibition of either EGF receptor transactivation or ERK activation inhibited BB2 receptor-stimulated DNA synthesis in these tumor cells (Sakamoto et al., 1988). BB2 receptor activation stimulates the invasion and cell migration of tumor cells (Vincent et al., 1999; Thomas et al., 2005; Zheng et al., 2006). This stimulation occurs via Gα13 leading to activation of RhoA and Rho-associated coiled-coil forming protein kinase (ROCK) (Zheng et al., 2006). BB2 receptor activation promotes progression from the G1 to the S phase of the cell cycle by increasing the expression of cyclin D1 and E through the early growth response protein Egr-1, down-regulating the cyclin-dependent kinase inhibitor p27kip1 and hyperphosphorylating the retinoblastoma protein (Rb)(Mann et al., 1997; Rozengurt, 1998b; Xiao et al., 2005).
IV.8.BB2 receptor function in various tissues and in vivo
A major difficulty in assessing the effects of BB2 receptor activation in vivo and in a number of tissues in vitro is the fact they frequently possess both classes of bombesin receptors and bombesin, the agonist frequently used, has high affinity for both receptor subtypes. Recently a number of developments have contributed to solving this problem. Selective receptor antagonists for the BB2 receptor are described, studies on BB2 receptor knockout animals are being increasing performed, more selective BB2 receptor agonists such as GRP are being used and with the cloning of the mammalian bombesin receptors, it has become clear that some widely studied tissues such as Swiss 3T3 cells and pancreatic acinar cells only possess BB2 receptors.
Many effects of GRP are observed both in vivo and in vitro, but it remains unclear in many cases which are pharmacological or which are physiological. Studies support a role for BB2 receptor in numerous gastrointestinal functions, including regulation of gastric acid secretion via both stimulation of gastrin release from antral G cells and somatostatin release from D cells and stimulation of acid secretion (Schubert et al., 1991; Hildebrand et al., 2001; Schubert, 2002); regulation of gastrointestinal motility, especially gastric emptying, small intestinal transit and gallbladder emptying (Degen et al., 2001; Yegen, 2003); stimulation of pancreatic secretion (Niebergall-Roth and Singer, 2001; Nathan and Liddle, 2002), insulin release (Persson et al., 2002), colonic ion transport (Traynor and O'Grady, 1996); and stimulation of the secretion of a variety of hormones (gastrin, somatostatin, CCK, pancreatic polypeptide, enteroglucagon, pancreatic glucagon and gastric inhibitory polypeptide) (Modlin et al., 1981; Ghatei et al., 1982; Pettersson and Ahren, 1987; Bunnett, 1994). Activation of BB2 receptors have a number of immunologic effects including functioning as a chemoattractant in peritoneal macrophages, monocytes and lymphocytes (Ruff et al., 1985; Del Rio and De la Fuente, 1994), stimulating lymphocyte proliferation (Del Rio et al., 1994), and stimulating natural killer and antibody-dependent cellular cytotoxicity in leukocytes (De la Fuente et al., 1993a). BB2 receptors are reported to be important for fetal lung development including lung branching, cell proliferation and differentiation (Subramaniam et al., 2003) as well as a number of lung diseases, which will be discussed below. BB2 receptors are widely expressed in the CNS and in the spinal cord and numerous central effects have been described with their activation including: effects on satiety, regulation of circadian rhythm, thermoregulatory effects, grooming behaviors, modulation of stress, fear and anxiety response, memory and effects on gastrointestinal function such as acid secretion (Martinez and Tache, 2000; Yegen, 2003; Moody and Merali, 2004; Karatsoreos et al., 2006; Roesler et al., 2006a; Roesler et al., 2006b; Kallingal and Mintz, 2007; Presti-Torres et al., 2007). The satiety effect of BB2 receptors has been extensive studied (Gibbs et al., 1979; Gibbs and Smith, 1988; Flynn, 1997; Ladenheim and Knipp, 2007; Fekete et al., 2007). A recent study (Ladenheim and Knipp, 2007) shows the satiety effect of peripherally administered NMB, but not GRP, is inhibited by capsaicin pretreatment suggesting either the neural pathways involved in BB2 receptor mediated satiety are either by capsaicin-insensitive neurons or involve direct activation of BB2 receptors in the CNS (Ladenheim and Knipp, 2007). The importance of BB2 receptors in mediating the satiety effects of GRP was demonstrated by the ability of a specific BB2 receptor antagonist administered in the hindbrain of rats, to inhibit the satiety effects of peripherally administered GRP (Ladenheim et al., 1996a).
BB2 receptor knockout mice have been described and limited study results are available (Wada et al., 1997; Hampton et al., 1998). In the initial study performed with these mice (Hampton et al., 1998), no developmental abnormalities were seen, however bombesin failed to suppress glucose intake, whereas it caused a dose-dependent decrease in normal mice (Hampton et al., 1998). In a second study (Wada et al., 1997) the intracerebroventricular administration of GRP failed to cause hypothermia in the BB2 receptor knockout mice as observed in the wild type mice. Furthermore, the BB2 receptor knockout mice demonstrated abnormal behaviors and altered spontaneous activity during darkness (Wada et al., 1997). In a more detailed study of feeding behavior in these mice, neither GRP, NMB nor bombesin altered satiety in the knockout mice however the satiety response to cholecystokinin was present and in fact enhanced (Ladenheim et al., 2002). In a long term study (Ladenheim et al., 2002) BB2 receptor knockout mice ate more food than normal mice due to a defect in terminating meals and had greater weight gain supporting the conclusion that the BB2 receptor has important roles in satiety. These mice were used to study the effects of BB2 receptor activation on islet function (Persson et al., 2000; Persson et al., 2002). BB2 receptor knockout mice had impaired glucose tolerance, a defect in early insulin release (Persson et al., 2000) and the plasma GLP-1 response to gastric glucose administration was significantly reduced in the knockout mice suggesting BB2 receptor had an important role in normal GLP-1 release and insulin and glucose responses after glucose a glucose meal. In a second study (Persson et al., 2002) GRP was found to potentiate glucose-stimulated insulin release in wild type but not BB2 receptor knockout mice. This study (Persson et al., 2002) demonstrated that BB2 receptor activation contributes to insulin secretion induced by activation of autonomic nerves and that the deletion of the BB2 receptor is compensated for by increased cholinergic sensitivity (Persson et al., 2002). These results are consistent with earlier studies which demonstrated GRP potentiated glucose-induced insulin release by both a ganglionic and direct effect, but did not alter glucagon or pancreatic somatostatin release (Hermansen and Ahren, 1990; Gregersen and Ahren, 1996; Karlsson et al., 1998). BB2 receptor knockout mice were used to study possible behavioral effects of GRP (Shumyatsky et al., 2002). In one study the BB2 receptor was found in wild type, but not knockout mice, to be highly expressed in the lateral nucleus of the amydala, which is important in mediating fear responses. BB2 receptor knockout mice showed more persistent long-term fear responses (Shumyatsky et al., 2002) supporting other study results, which suggest the BB2 receptor, has an important role in memory and fear responses (Roesler et al., 2006a). Other behavior changes seen in BB2 receptor knockout mice include increased social investigatory behavior (Yamada et al., 2000b), preference for conspecific odors (Yamada et al., 2000b) and altered social preferences in females (Yamada et al., 2001). BB2 receptor knockout mice have also been used to investigate the role of this receptor in specific diseases, which will be discussed in the next section.
BB2 receptor activation has important growth effects on normal and neoplastic tissues (Moody et al., 1992; Jensen and Moody, 2006). BB2 receptor activation stimulates growth of normal endometrial stomal cells (Endo et al., 1991), bronchial epithelial cells (Willey et al., 1984; Siegfried et al., 1993), melanocytes (Terashi et al., 1998), chondrocytes (Hill and McDonald, 1992), normal enterocyte growth and turnover post small bowel resection (Chu et al., 1995; Sukhotnik et al., 2007) as well as normal development of the intestinal villus (Carroll et al., 2002) and normal fetal lung development (Emanuel et al., 1999; Shan et al., 2004). The effects of BB2 receptor activation on neoplastic growth have been extensively studied (Moody et al., 1992; Jensen et al., 2001; Patel et al., 2006). This widespread interest occurred after human small cell lung cancers were shown to possess high affinity BB2 receptors (Moody et al., 1985) and bombesin was shown to have an autocrine growth effect on these cells (Cuttitta et al., 1985). Subsequent studies demonstrated such an autocrine growth effect where the tumor cells not only possessed BB2 receptors, but also secreted bombesin-like peptides resulted in a growth stimulatory effect (Moody et al., 2003a; Jensen and Moody, 2006; Patel et al., 2006) in a large number of tumors cells including neuroblastomas (Kim et al., 2002), squamous head and neck tumors (Lango et al., 2002; Lui et al., 2003), pancreatic cancer cells (Wang et al., 1996; Murphy et al., 2001), colon cancer (Chave et al., 2000), prostate cancer (Plonowski et al., 2000), human glioblastoma cells (Sharif et al., 1997) and non-small cell lung cancer (Siegfried et al., 1999). Furthermore, many human cancers or the blood vessels in the cancers either overexpress or ectopically express BB2 receptors and the stimulation or inhibition of these receptors is reported to effect growth/differentiation (Jensen et al., 2001; Moody et al., 2003a; Heuser et al., 2005; Jensen and Moody, 2006; Patel et al., 2006; Fleischmann et al., 2007). The potential clinical importance of this will be discussed further in the next section. The role of the ectopic expression or overexpression in various cancers may be different with different tumors. Whereas, many of the studies referred to in the following paragraph emphasize the growth stimulatory effects of BB2 receptor on tumor cells, other studies especially in colon cancer support the conclusion that the ectopic expressing of BB2 receptor has a morphogenic effect rather than a mitogenic effect (Jensen et al., 2001). Whereas in normal colonic mucosal epithelial cells, the BB2 receptor is not found (Preston et al., 1995; Ferris et al., 1997; Carroll et al., 1999b), in 40−100% of colonic cancers (Carroll et al., 1999b) BB2 receptor is aberrantly expressed. BB2 receptor activation on some colonic cancer cells is reported to result in proliferation (Radulovic et al., 1991b; Frucht et al., 1992; Narayan et al., 1992). However, in detailed studies, although 62% of the tumors expressed both GRP and the BB2 receptor, their co-expression was equally frequent in early or late stage cancers and was rarely detected in metastases (Carroll et al., 1999b). However, GRP/ BB2 receptor expression was seen in all well-differentiated tumors, whereas poorly differentiated tumors never co-expressed GRP/ BB2 receptors (Carroll et al., 1999b). Furthermore, no difference in survival occurred in patients expressing or not expressing cancers with GRP/ BB2 receptor (Carroll et al., 1999b). In a study (Carroll et al., 2000a) of BB2 receptor knockout mice with colonic tumors induced by azoxymethane, larger tumors were better differentiated in wild type mice than BB2 receptor knockout mice. From these studies and others it was proposed that BB2 receptor activation in these cells is functioning primarily as a morphogenic or differentiating factor (Carroll et al., 1999b; Jensen et al., 2001). More recent studies show this morphogenic effect is mediated by activation of p125FAK, which inhibits invasion/metastases by enhancing cell attachment (Glover et al., 2004), most likely by up-regulating the expression of intracellular adhesion protein-1(ICAM-1) (Taglia et al., 2007). Subsequent studies showed that BB2 receptor mutations occurred frequently in poorly differentiated colonic tumor, resulting in the formation of inactive receptors, and the generation of these mutations correlated inversely with the differentiation of the tumor suggesting their production represents a new mechanism allowing for the differentiation of tumors (Carroll et al., 2000b; Glover et al., 2003).
A recent study (Ruginis et al., 2006) used a proteomic approach to identify proteins selectively up regulated in human colorectal cancer cells subsequent to BB2 receptor activation. This study took advantage of the fact that pre-confluent human colorectal cancer cells such as Caco-2 and HT-29 only secreted GRP and express BB2 receptors when they are pre-confluent and not when they are post-confluent (Glover et al., 2005). Total cellular proteins were isolated from pre-confluent, GRP and BB2 receptor expressing cells in the presence and absence of specific BB2 receptor antagonist [D-Phe6]bombesin6−13methyl ester, and from post-confluent cells not expressing GRP or BB2 receptors. Using 2D gel electrophoresis and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (Ruginis et al., 2006), at least six separate proteins are up-regulated subsequent to BB2 signaling when this receptor is aberrantly expressed in human colorectal cancer: gephyrin, heat shock protein 70, heterochromatin associated protein 1, ICAM-1, and THIL/acetyl co-A acetyltransferase (ACAT). These findings promise to further define the mechanism whereby aberrantly expressed BB2 receptors in human colorectal cancer promote tumor cell differentiation and improve patient outcome.
IV.9. BB2 receptor in diseases
BB2 receptor activation has been proposed to be important in the mediation of a number of human disorders including disorders of lung development, various pulmonary diseases, CNS disorders, and the growth/differentiation of human cancers. The tumor differentiation effects of BB2 receptor activation were discussed in the previous section and the growth effects and effects of BB2 receptor overexpression will be considered here. BB2 receptors are ectopically or over-expressed in a large number of tumors including 85−100% of small cell lung cancers, 74−78% of non-small cell lung cancer cells, 38−72% of breast cancer, 75% of pancreatic cancer cell lines and 10% of pancreatic cancers, 62−100% of prostate cancers, 100% of head/neck squamous cell cancers and 72−85% of neuroblastomas/glioblastomas (Jensen and Moody, 2006; Patel et al., 2006). Bn-like peptides are critical to the growth of some, but not all small cell lung cancers (Cuttitta et al., 1985), and as discussed above have been shown to have an autocrine growth effect on a large number of tumors as well as stimulating growth in a large number of these tumors (Jensen and Moody, 2006; Patel et al., 2006). In some tumors such as prostate cancer, BB2 receptor overexpression correlates with neoplastic transformation (Markwalder and Reubi, 1999). Bn peptides also function as proangiogenic factors in various tumors (Levine et al., 2003; Kanashiro et al., 2005; Martinez, 2005). The production of GRP-related peptides and/or overexpression of BB2 receptors by tumors are playing a potential role in a number of aspects of the treatment and management of these tumors. These aspects include functioning as targets for anti-tumor treatment, as prognostic factors, as targets to image the tumors and as targets to deliver cytotoxic treatment selectively to the tumor (Smith et al., 2003; Schally and Nagy, 2004; Jensen and Moody, 2006). Attempts to inhibit the autocrine growth effect of GRP-like peptides on tumor growth are reported in human and/or animal studies using monoclonal antibodies to GRP, antisense constructs, BB2 receptor antagonists or other inhibitors (Zhou et al., 2004). Infusion of the monoclonal antibody 2A11 directed against the biologically active COOH terminus of GRP is safe in humans (Chaudhry et al., 1999) and was given to 13 patients with small cell lung cancer (Kelley et al., 1997) One patient had complete remission and four patients had radiologically stable disease and further evaluation was recommended (Kelley et al., 1997). In a recent study (Schwartsmann et al., 2006) the BB2 receptor antagonist, RC-3095 was administer to 25 patients with different advanced malignancies. No side effects occurred and there were no tumor responses, however, maximal doses could not be reached by the methods used despite dose-escalation (Schwartsmann et al., 2006). EGF receptor transactivation upon BB2 receptor stimulation may also be a therapeutic site for intervention. Experimental studies demonstrate that activation of the BB2 receptor rescues tumor cells from the growth inhibiting effect of the EGF receptor inhibitor, gefitinib, by stimulating the release of amphiregulin and activation of the Akt pathway (Liu et al., 2007). When a BB2 receptor antagonist is combined with an EGF receptor inhibitor (erlotinib) there is marked enhanced antitumor activity (Zhang et al., 2007b), suggesting such an approach may be useful in some cancers such as head/neck tumors or lung tumors. In a number of studies plasma levels of GRP precursors such as pro-GRP or assessment of GRP expression in tumors provides prognostic information (Hamid et al., 1990; Okusaka et al., 1997; Sunaga et al., 1999; Shibayama et al., 2001; Yonemori et al., 2005).
Recent clinical and laboratory studies with somatostatin receptors demonstrate that many endocrine tumors over-express or ectopically express these receptors and that radiolabeled analogues of somatostatin can be used to both localize these tumors as well as for somatostatin receptor mediated cytotoxicity (Van Essen et al., 2007; Breeman et al., 2007). Somatostatin analogues coupled to 111Indium are now widely used to image neuroendocrine tumors and numerous studies demonstrate that they have greater sensitivity than conventional imaging modalities (computed tomography, magnetic resonance imaging, angiography, ultrasound) and that its routine use changes patient management in 20−47% of cases (Gibril et al., 1996; Gibril and Jensen, 2004; Breeman et al., 2007). Recent studies with somatostatin analogues coupled to 111Indium, 90Yttrium and 177 Lutetium show promising results for somatostatin receptor-mediated cytotoxicity in patients with advanced neuroendocrine tumors and have entered Phase 3 studies (Van Essen et al., 2007; Breeman et al., 2007; Forrer et al., 2007). Unfortunately many common tumors (colon, pancreas, head/neck, prostate, lung) may not overexpress somatostatin receptor, however they frequently overexpress Bn receptors, especially the BB2 receptor (Jensen et al., 2001; Reubi et al., 2002; Moody et al., 2003a; Heuser et al., 2005; Jensen and Moody, 2006; Patel et al., 2006; Fleischmann et al., 2007). This observation has led to considerable interest in the possibility of developing radiolabeled analogues of Bn that could be use for localization of the tumors containing Bn receptors or the development of radiolabeled Bn analogues or Bn analogues coupled to cytotoxic agents that could be used to treat tumors overexpressing Bn receptors through bombesin receptor-mediated cytotoxicity (Breeman et al., 2002; de Jong et al., 2003; Cornelio et al., 2007; de Visser et al., 2007a). Numerous radiolabeled [111Indium, 68Gallium, 177Lutetium,64Copper, 86Yttrium, 18F, 99mTc] GRP analogues with enhanced stability that bind with high affinity to BB2-receptors are reported, as well as there ability to image various human tumors in vivo using gamma detectors or PET imaging(Breeman et al., 2002; Smith et al., 2003; Nock et al., 2003; Smith et al., 2005; Zhang et al., 2006; Lantry et al., 2006; Johnson et al., 2006; Waser et al., 2007; Parry et al., 2007; Dimitrakopoulou-Strauss et al., 2007; Garrison et al., 2007; Prasanphanich et al., 2007; de Visser et al., 2007a; Zhang et al., 2007a). In some preliminary studies in humans, tumors were imaged in the majority of patients, and in some cases, tumors were detected using radiolabeled Bn analogues that were not seen on other commonly used imaging modalities (Scopinaro et al., 2004; De Vincentis et al., 2004; Scopinaro et al., 2005; Dimitrakopoulou-Strauss et al., 2007). At present no study has established the value of imaging using radiolabeled Bn analogues.
A number of Bn analogues coupled to radiolabeled compounds [177Lutetium] (Smith et al., 2005; Lantry et al., 2006; Johnson et al., 2006; Zhang et al., 2007a) as well as to cytotoxic agents (camptothecin-a topoisomerase inhibitor, doxorubicin analogues, paclitaxel) (Schally and Nagy, 1999; Breeman et al., 2002; Moody et al., 2004; Schally and Nagy, 2004; Engel et al., 2005; Buchholz et al., 2006; Safavy et al., 2006; Panigone and Nunn, 2006; Nanni et al., 2006; Moody et al., 2006b; Engel et al., 2007) have been described which retain high affinity for Bn receptors and are internalized by Bn-receptor-bearing tissues, for the possibility of delivering Bn-receptor mediated tumoral cytotoxicity. Many of these compounds have been shown to cause tumor cytotoxicity in animal studies and one study has provided evidence that it is due to specific interaction with the BB2-receptors overexpressed on the tumor (Moody et al., 2006b). At present it is unclear whether this approach will be effective in vivo in human tumors whether alone or in combination with other anti-tumor treatments. A recent study using a chemically identical active and inactive cytotoxic GRP analogue (i.e. camptothecin-L2-[D-Tyr6, β-Ala11, Phe13, Nle14] bombesin 6−14 or its D-Phe13 inactive form, where L2=[N-(N-methyl-amino ethyl)glycine carbamate]), demonstrated that specific tumor receptor interaction is important in mediating the tumor cytotoxicity of these compounds(Moody et al., 2006b). Various studies demonstrate that such an approach can inhibit the growth of pancreatic lung, prostate and gastric cancers (Schally and Nagy, 1999; Breeman et al., 2002; Moody et al., 2004; Schally and Nagy, 2004). At present the usefulness of GRP or BB2 receptor in management of human tumors in each of the areas discussed above is not established (Jensen and Moody, 2006).
BB2 receptor activation, GRP secretion, or abnormalities of either have been proposed to be important in a number of other diseases. In a recent study (Sun and Chen, 2007) evidence was presented that activation of the BB2-receptor in the dorsal spinal cord is important for mediating pruritus. GRPR knockout mice showed significantly decreased scratching behavior in response to pruritogenic stimuli, while other responses were normal. Furthermore, administration of a BB2 receptor antagonist into the spinal fluid inhibited scratching behavior in three different models of itching (Sun and Chen, 2007). The authors (Sun and Chen, 2007) point out the BB2 receptor may represent the first molecule identified that is dedicated to mediating the itch response in the spinal cord and may provide an important therapeutic target for the treatment of chronic pruritic conditions. Abnormalities of GRP, BB2 receptors and other bombesin-like peptides and/or their receptors are proposed to be important in normal lung development and mediating the lung injury in premature infants with bronchopulmonary dysplasia (Li et al., 1994; Sunday et al., 1998; Emanuel et al., 1999; Cullen et al., 2000; Ashour et al., 2006; Ganter and Pittet, 2006; Subramaniam et al., 2007). In one recent study (Ashour et al., 2006) GRP given to newborn mice induced features of human BPD including interstitial pulmonary fibrosis and alveolarization. In a hyperoxic baboon model of BPD (Subramaniam et al., 2007) the early overproduction of Bn-like peptides correlated with the development of BPD-like histological features and the blockage of GRP partially reversed these effects, leading the authors to suggest such an approach could have important implications for preventing BPD in premature infants. GRP has been shown to be protective to the small intestine in various injury models (Assimakopoulos et al., 2004; Kinoshita et al., 2005; Assimakopoulos et al., 2005a; Assimakopoulos et al., 2005b; Kimura et al., 2006b), enhance gut barrier function, prevent the atrophy of enteric ganglia caused by FK506 in small bowel (Assimakopoulos et al., 2005a; Higuchi et al., 2006; Kimura et al., 2006a; Kimura et al., 2006b) and in a recent study (Fujimura et al., 2007) to prevent the atrophy of Peyer's patches and dysfunction of M cell in rabbits receiving long-term parenteral nutrition. These studies suggest GRP agonists may have a potential therapeutic role in diseases causing this type of injury. Numerous studies in rodents provide evidence that GRP/ BB2-receptor activation is important for memory as well as a number of social behaviors (learning, grooming, stereotypy) (Roesler et al., 2006a; Roesler et al., 2006b). These results were supported by a recent study (Presti-Torres et al., 2007) in which the administration of BB2-receptor antagonists in neonatal rats resulted in marked impairment of memory, and social interaction. These changes have led one group (Roesler et al., 2006a) to propose that the BB2-receptor should be consider a therapeutic target in a subset of human CNS diseases, especially those involving memory, learning and fear. Specifically, in the CNS it has been proposed that alterations in either the GRP and/or BB2 receptor may be important in schizophrenia, Parkinson's disease, anxiety disorders, anorexia, bulimia, and mood disorders (Merali et al., 1999; Frank et al., 2001; Yegen, 2003; Moody and Merali, 2004; Merali et al., 2006; Roesler et al., 2006a).
V. BB3 receptor
V.1. Early studies of the BB3 receptor
Prior to the identification of the BB3 receptor when it was cloned in 1992 from guinea pig uterus (Gorbulev et al., 1992) no pharmacological or functional studies suggested its existence.
V.2. Cloned BB3 and receptor structure
The human BB3 receptor is a 399 amino acid protein (Fathi et al., 1993b) and it shows 95% amino acid identities with rhesus BB3 receptor (Sano et al., 2004), 80′% amino acid identity with the rat BB3 that shows 92% with the mouse, and 77% with the sheep BB3 receptor (Liu et al., 2002)(Table 2). The human BB3 receptor has 51% amino acid identities with the human BB2 and 47% with the human BB1 receptor (Fathi et al., 1993b). The human BB3 receptor has a predicted molecular weight of 44.4 kDa (Fathi et al., 1993b) and there are two potential N-linked glycosylation sites at Asn10 and Asn18 and a consensus site for potential PKC phosphorylation in the third cytoplasmic loop and carboxyl terminus (Fathi et al., 1993b; Whitley et al., 1999). A putative palmitoylation site existed at C347 and C348 (Fathi et al., 1993b; Whitley et al., 1999). Hydropathy plots yielded results consistent with a seven transmembrane structure typical for a G-protein coupled receptor (Fathi et al., 1993b). The BB3 receptor has been cloned from rat (Liu et al., 2002), mouse (Ohki-Hamazaki et al., 1997a), sheep (Whitley et al., 1999) and guinea pig (Gorbulev et al., 1992). In the chicken a receptor was cloned that has similarities to both the mammalian BB3 receptor and the frog BB4 receptor and has been termed the chBRS-3.5 receptor (Iwabuchi et al., 2003). No cross-linking studies have been performed on the mature BB3 receptor so the extent of glycosylation or type is unknown at present.
V.3. BB3 genomic organization
The human BB3 receptor gene is localized at human chromosome Xq25 and in the mouse on chromosome XA7.1−7.2 (Fathi et al., 1993b; Gorbulev et al., 1994; Weber et al., 1998). The human BB3 receptor gene (Fathi et al., 1993b; Gorbulev et al., 1994; Weber et al., 1998) contained two introns and three exons similar to the sheep (Whitley et al., 1999), rhesus (Sano et al., 2004), mouse (Ohki-Hamazaki et al., 1997a), and rat BB3 receptor gene (Liu et al., 2002). In the mouse BB3 receptor gene spanned more than 5kb with exon 1 of the BB3 gene separated from exon 2 by 1.6 kb and this in turn separated from exon 3 by 1.6 kb (Weber et al., 1998). In human, sheep, monkey, rat, mouse and guinea pig the exon/intron splice sites occurred at Arg145 in the second intracellular loop and at Ile263 in the third intracellular domain (Gorbulev et al., 1994; Weber et al., 1998; Sano et al., 2004).
V.4. BB3 receptor expression
Expression levels of the BB3 receptor mRNA has beenreported in the rat(Fathi et al., 1993b; Liu et al., 2002; Jennings et al., 2003), sheep (Whitley et al., 1999), mouse (Ohki-Hamazaki et al., 1997a), monkey (Sano et al., 2004) and guinea pig (Gorbulev et al., 1992). In the monkey where it was studied in detail, BB3 mRNA is found in the greatest amount in the CNS and in the testis (Sano et al., 2004). This high expression in the testis is not seen in the sheep (Weber et al., 2003) or mouse (Ohki-Hamazaki et al., 1997a) but is similar to the rat (Fathi et al., 1993b) where it was localized to the secondary spermatocytes and was not present in the sertoli cells or different maturation stages of the spermatogonia (Fathi et al., 1993b). Detectable levels were also found in the monkey pancreas, thyroid and ovary in peripheral tissues and it was either undetectable or in very low amounts in other tissues showing a very different distribution than the BB1 receptor or BB2 receptor (Fathi et al., 1993b; Sano et al., 2004).
In the CNS the BB3 receptor mRNA was expressed in a restricted distribution (Ohki-Hamazaki et al., 1997a; Liu et al., 2002; Jennings et al., 2003; Sano et al., 2004). In the rat and mouse (Ohki-Hamazaki et al., 1997a; Liu et al., 2002) the BB3 receptor was present in the highest amounts in the hypothalamic area notable the paraventricular, arcuate, striohypothalamic, dorsal hypothalamic and dorsomedial hypothalamic nuclei, a medial and lateral preoptic areas and lateral /posterior hypothalamic areas. In the rat expression was also detected in the medial habenula nucleus in one study (Liu et al., 2002) and a second study (Jennings et al., 2003) in the nucleus accumbens and the thalamus. In the monkey brain (Sano et al., 2004) PCR quantitation showed the BB3 receptor mRNA was present in highest amounts in the hypothalamus followed by the pituitary gland, amygdala, hippocampus and caudate nucleus.
Specific BB3 receptor antibodies have been used to localize the receptor in the tunica muscularis of the rat gastrointestinal tract (Porcher et al., 2005) and the rat CNS (Jennings et al., 2003). In the gastrointestinal tract tunica muscularis BB3 receptor immunoreactivity (IR) was observe in all regions studied (i.e. antrum, duodenum, ileum and colon) in nerves and nonneuronal cells but not in muscle cells (Porcher et al., 2005). It was detected in both myenteric and submucosal ganglia as well as in nerve fibers interconnecting myenteric ganglia (Porcher et al., 2005). BB3 receptor IR was observed in cell bodies and processes of c-kit interstitial cells of Cajal leading the authors to propose that the BB3 receptor was likely involved in the regulation of gastrointestinal motility through the enteric nervous system and possibly in the pacemaker function of the gastrointestinal smooth muscle (Porcher et al., 2005). In the CNS, particularly strong BB3 receptor IR was observed in the cerebral cortex, hippocampal formation, hypothalamus and thalamus (Jennings et al., 2003).
Using assessment of BB3 mRNA (Fathi et al., 1993b) and/or binding studies (Reubi et al., 2002), BB3 receptors have been shown to exist on a number of different human tumors (Fathi et al., 1993b; Reubi et al., 2002) including small cell and non-small cell lung cancers (Fathi et al., 1993b; Toi-Scott et al., 1996; Ryan et al., 1998b; Reubi et al., 2002), carcinoids (lung) (Fathi et al., 1993b; Reubi et al., 2002), renal cell cancers (Reubi et al., 2002), Ewing sarcomas (Reubi et al., 2002), pancreatic cancer (Schulz et al., 2006), pituitary tumors (Schulz et al., 2006), ovarian cancer (Sun et al., 2000b) and prostate cancer (Sun et al., 2000a; Schulz et al., 2006). BB3 receptors have also been shown to exist on normal bronchial epithelial cells (DeMichele et al., 1994; Tan et al., 2006), human islets (Fleischmann et al., 2000) and rat kidney cells (Dumesny et al., 2004).
V.5. BB3 pharmacology
V.5.a. BB3 receptor agonists
In the original studies describing the ability of GRP, neuromedin C or NMB to interact with the expressed cloned guinea pig BB3 receptor (Gorbulev et al., 1992) or GRP and NMB to activated the cloned human BB3 receptor expressed in Xenopus oocytes (Fathi et al., 1993b), it was clear that this receptor had low affinity for these peptides (Table 2). Similar results were later reported (Liu et al., 2002) with the rat BB3 receptor. A later study (Wu et al., 1996) demonstrated that human BB3 receptors expressed in Balb 3T3 cells had low affinity for all bombesin related peptides tested (i.e. ranatensin, litorin, NMB, GRP, bombesin and alytesin), but at concentrations >1 uM, each could activate the BB3 receptor and stimulate changes in cytosolic calcium. In 1997 Mantey and collaborators performed a detailed study of the ability of all naturally occurring bombesin related peptides and a number of novel synthetic analogues of bombesin to interact with the human BB3 receptor (Mantey et al., 1997). Because no cell lines existed with wild type BB3 receptors, to check that the correct pharmacology and cell signaling were being obtained, in this study (Mantey et al., 1997) human BB3 receptors were expressed in Balb 3T3 cells, which have been shown with transfected BB1 (Benya et al., 1992) and BB2 receptors (Benya et al., 1994b) to have similar characteristics to the wild type receptors, as well as overexpressing BB3 receptors in human nonsmall cell lung cancer cells, NCI-H1299. In this study (Mantey et al., 1997) none of the 15 naturally occurring bombesin-related peptides had a affinity >1 uM for the human BB3 receptor. Furthermore, none of the 26 synthetic bombesin analogues which functioned as BB1 or BB2 receptor agonists or antagonists had high affinity for the BB3 receptor including [D-Phe6 Bn 6−13 propylamide (Ki-2 uM) which had been reported in another study (Wu et al., 1996) assessing changes in cellular calcium to have a relatively high affinity of 84 nM for the human BB3 receptor. In this study (Mantey et al., 1997) one novel bombesin analogue, [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 was discovered which had high affinity (Ki-4 nM) and potency for activating the BB3 receptor and its Tyr6 analogue retained high affinity and could be radiolabeled to study the pharmacology and ligand receptor interaction in detail. Using this radioligand it was demonstrated (Mantey et al., 1997) that binding to the BB3 receptor fit a single site-binding model; it was rapid and temperature-dependent, with slow dissociation, supporting ligand internalization; and the binding affinities of all agonists and antagonists for the BB3 receptor could be determined for the first time and compared to BB1 receptor and BB2 receptor. These results demonstrated that the BB3 receptor has a unique pharmacology, not interacting with high affinity with any known naturally occurring bombesin peptide, supporting the conclusion that the natural ligand is either an undiscovered member of the bombesin family with significant structural differences or an unrelated peptide (Mantey et al., 1997). In a subsequent study (Ryan et al., 1996) two human lung cancer cell lines, NCIN417 and NCI-H720, were found to possess sufficient wild type BB3 receptors to allow assessment of the pharmacology of the native BB3 receptor using the 125I- [D-Tyr6, β-Ala11, Phe13, Nle14] bombesin 6−14 ligand described above. Similar pharmacology for all agonists and antagonists was found for the native BB3 receptor to that reported previously with the BB3 receptor transfected cell lines (Mantey et al., 1997) with only the agonist, [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 demonstrating high affinity (Ki-7.4 nM).
Subsequent studies demonstrate that the synthetic bombesin analogue [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14, in addition to having high affinity for the human BB3 receptor, also has a high affinity for the human BB1 receptor, the human BB2 receptor, the BB1 receptor and BB2 receptor from all species studied and the fBB4 (Mantey et al., 1997; Pradhan et al., 1998; Katsuno et al., 1999; Reubi et al., 2002; Iwabuchi et al., 2003) (Table 2). When the rat BB3 receptor was cloned (Liu et al., 2002) a surprising finding was that [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 had a low potency for this receptor (EC50 2 uM). In the chicken (Iwabuchi et al., 2003) a receptor that had high affinity for [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14, moderate affinity for bombesin and low affinity for GRP and NMB was found, which showed structural similarity to both mammalian BB3 receptor and the amphibian BB4 receptor, and thus was called ChBRS-3.5. A subsequent study demonstrated the monkey BB3 receptor (Sano et al., 2004) had a high potency for [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 (EC50-5.6 nM) similar to the human receptor. The molecular basis for the difference in affinity of [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 between human/monkey and rat BB3 receptors has been studied (Liu et al., 2002) and will be discussed in the section III.6.BB3 below.
Because of the lack of selectivity of the high affinity agonist,[D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 for the human BB3 receptor, there have been a number of groups that have attempted to develop more selective BB3 receptor ligands. Each of the different groups used [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 as the starting point to identify BB3 receptor selective agonists. In one study (Mantey et al., 2001) rational peptide design was used by substituting conformationally restricted amino acids into the prototype peptide, [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 or its D-Tyr6 analogue. A number of BB3 receptor selective agonists were identified with two peptides with either an (R) or (S)–amino-3-phenylpropionic acid substitution for β-Ala11 in the prototype ligand having the highest selectivity (i.e.17−19-fold) (Mantey et al., 2001). Molecular modeling demonstrated these two selective BB3 receptor ligands had a unique conformation of the position of the 11 β-amino acids, which likely accounted for their selectivity (Mantey et al., 2001). In a second study (Vincent et al., 1999) two strategies were used to attempt to develop a more selective BB3 receptor ligand: substitutions on the phenyl ring of Apa11 and the substitution of additional conformationally restricted amino acids into the position 11 of [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 or its D-Tyr6 analogue. One analogue, [D-Tyr6, Apa-4Cl11, Phe13, Nle14] bombesin 6−14 retained high affinity for BB3 receptor and was 227-fold selective for the BB3 receptor over the human BB2 receptor and 800-fold selective over the human BB1 receptor (Mantey et al., 2004). Using, [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6− or its D-Tyr6 analogue as the prototype, three studies (Weber et al., 2002; Weber et al., 2003; Boyle et al., 2005) reported shortened analogues with selectivity for BB3 receptor assessed by calcium or FLIPR calcium assays. A recent study has assessed the selectivity of four of the most selective of these shortened [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 analogues by binding assays as well as assessment of phospholipase C potencies (Mantey et al., 2006). Three analogues which were reported selective in calcium assays for the BB3 receptor; [H-D-Phe, Gln, D-Trp, NH (CH2) 2C6H5 and H-D-Phe, Gln, D-Trp, Phe-NH2, compounds 68 and 54 in (Weber et al., 2002), and 3-phenylpropionyl-Ala, D-Trp, NH (CH2) 2C6H5 compound 17d in (Weber et al., 2003)] were found (Mantey et al., 2006) in binding studies as well as potency for activation of phospholipase C to have affinities >5 uM for all three human bombesin receptor subtypes and therefore not to be useful. The novel compound Ac-Phe, Trp, Ala, His (tBzl), Nip, Gly, Arg-NH2 [compound 34 in (Boyle et al., 2005)] had a 14-fold higher affinity for BB3 receptor than BB1 receptor and >20 fold for BB2 receptor (Mantey et al., 2006), however it was less BB3 receptor selective than [D-Tyr6, Apa-4Cl11, Phe13, Nle14] bombesin 6−14 (i.e. >100 fold selectivity (Mantey et al., 2006)(Table 2).
V.5.b.BB3 receptor antagonists
No specific or potent antagonists of the BB3 receptor exist. In four studies (Ryan et al., 1996; Mantey et al., 1997; Ryan et al., 1998a; Ryan et al., 1999) none of the members of the different classes of potent BB2 receptor or BB1 receptor antagonists had an affinity <3 uM for the human BB3 receptor. In one study (Ryan et al., 1996) the D-amino acid substituted somatostatin analogue, D-Nal, Cys, Tyr, D-Trp, Lys, Val, Cys, Nal-NH2, had an affinity of 1 uM for the human BB3 receptor and was 30-fold more potent at inhibiting activation of the BB3 receptor than any other compound (Table 2). Unfortunately, this compound also functions as a BB1 receptor antagonist as well as a somatostatin and mu opioid receptor agonist (Orbuch et al., 1993; Ryan et al., 1999).
V.6. BB3 receptor structural basis of receptor binding/activation
V.6.a. BB3 receptor agonist binding/activation
At present because the natural ligand of the BB3 receptor is unknown there is minimal information available on the importance of amino acid residues in BB3 receptor activation or determining high affinity interaction. For the only ligand known with high affinity for the BB3 receptor, [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 (Ryan et al., 1996; Mantey et al., 1997; Ryan et al., 1998a) limited structure-function studies suggest that it is unlikely the deletion of the first five amino acids in [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14, the insertion of the D-Phe6 or the presence of either Phe13 or Nle14 moieties are determining the high affinity for the BB3 receptor, because other bombesin analogues with these substitutions do not have high affinity (Ryan et al., 1996; Mantey et al., 1997). These results suggest that the position 11 substitution (i.e. β-Ala11, Apa-4Cl11) in bombesin analogues is the key substitution for determining high affinity interaction with the BB3 receptor. At present the basis for the high affinity with these substitutions is unknown.
One study (Liu et al., 2002) investigated the molecular basis for the high affinity of [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 for the human BB3 receptor, but low affinity for the rat BB3 receptor. Using a chimeric receptor approach in which the individual extracellular loops of the rat BB3 receptor were replaced with the corresponding human sequences the important residues were localized to the 4th extracellular domain (1st=N-terminus) (Liu et al., 2002). Within this region using site-directed mutagenesis (Liu et al., 2002) the mutation of Y298E299S330 (rat) to S298Q299T300 (human) or of D306V307H308 (rat) to A306M307H308 (human) partially mimic the effect of switching the entire 4th extracellular domain. These results indicate that variations in the 4th extracellular domains of the rat and human BB3 receptor are responsible for the differences in affinity for [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 (Liu et al., 2002).
Whereas the is no information on the molecular basis of the selectivity of the various [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 analogues for the BB3 receptor, a number of studies have assessed the molecular basis for the low affinity of the human BB3 receptor for the natural occurring high affinity BB1 receptor and BB2 receptor agonists (GRP, bombesin, NMB). These studies have utilized an alignment of the receptor structures of the various bombesin receptors and identified key amino acid differences between the BB3 receptor which has low affinity for GRP, bombesin or NMB and the BB2 receptor, BB1 receptor or fBB4 receptors that have high affinity for these ligands (Akeson et al., 1997; Sainz et al., 1998; Nakagawa et al., 2005). The results of these studies are summarized above in either the BB1 receptor or BB2 receptor sections dealing with the structural basis of agonist binding.
No studies have investigated the structural basis for BB3 receptor activation.
V.6.b. BB3 receptor antagonist binding
No potent selective antagonists exist for the BB3 receptor.
V.7.BB3 receptor signaling, activation, and modulatory processes (internalization, down-regulation, desensitization)
The human BB3 receptor (Fathi et al., 1993b; Ryan et al., 1996; Wu et al., 1996; Ryan et al., 1998a), as well as the monkey (Sano et al., 2004) and rat BB3 receptor (Liu et al., 2002) is coupled to phospholipase C resulting in breakdown of phosphoinositides, mobilization of cellular calcium and presumed activation of protein kinase C.
BB3 receptor activation also results in the stimulation phospholipase D (Ryan et al., 1996), but does not activate adenylate cyclase (Ryan et al., 1996; Ryan et al., 1998a). BB3 receptor stimulation also results in activation of tyrosine kinases (Ryan et al., 1998a; Weber et al., 2001) stimulating tyrosine phosphorylation of p125FAK by a mechanism which is not dependent on either limb of the phospholipase C cascade (i.e. activation of PKC or mobilization of cellular calcium (Ryan et al., 1998a)). Activation of the BB3 receptor also stimulates MAP kinase activation resulting in rapid tyrosine phosphorylation of both a 42 and 44 kDa forms, which is inhibited by the MEK-1 inhibitor PD98059 (Weber et al., 2001). In BB3 receptor transfected NCI-1299 lung cancer cells, activation of the BB3 receptor by [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 resulted in stimulation of Elk-1 in a MEK-1 dependent manner as well as a 47-fold increase in c-fos mRNA (Weber et al., 2001). These results demonstrated that BB3 receptor activation causes increased nuclear proto-oncogene expression and upstream events including activation of MAP kinase and Ek-1 activation (Weber et al., 2001).
There have been no studies of BB3 receptor modulatory processes (internalization, down-regulation, or desensitization).
V.8.BB3 receptor function in various tissues and in vivo
At present the function of the BB3 receptor in normal physiology and pathologic conditions is largely unknown because the natural ligand is still not known. An important insight into possible BB3 receptor function was provided by studies of BB3 receptor knockout mice. In the initial study (Ohki-Hamazaki et al., 1997b) mice lacking the BB3 receptor developed mild obesity, associated with hypertension and impairment of glucose metabolism. These changes were associated with reduced metabolic rate, increased feeding behavior, a five-fold increase in serum leptin levels and hyperphagia (Ohki-Hamazaki et al., 1997b). These results suggested BB3 receptor might play an important role in the mechanisms responsible for energy balance and control of body weight. A number of studies have been performed subsequently on BB3 receptor knockout mice to attempt to establish the mechanism of these effects. BB3 receptor knockout mice were shown to have altered taste preference (Yamada et al., 1999) which was proposed to be due to the lack of BB3 receptor expression in the medial and central nuclei of the amydala and the hypothalamic nuclei which are known to be involved in taste perception (Yamada et al., 1999) and to be possibly a contributory factor to the obesity. BB3 receptors are present on pancreatic islets (Fleischmann et al., 2000) and BB3 receptor knockout mice have a 2.3 fold increase in plasma insulin levels (Matsumoto et al., 2003) (Table 2). One study (Matsumoto et al., 2003) concluded that the BB3 receptor contributes to regulation of plasma insulin concentration/secretion and that dysregulation in this contribution in these mice contributes to obesity (Matsumoto et al., 2003). In a second study (Nakamichi et al., 2004) it was concluded that the impaired glucose metabolism in BB3 receptor knockout mice is mainly due to impaired GLUT4 translocation in adipocytes.
IV.9. BB3 receptor in diseases
At present there are no diseases in which activation or alterations of the BB3 receptor have been shown to be involved. BB3 receptor activation has been proposed to be important in the mediation of a number of human disorders including disorders of lung development, various pulmonary diseases, CNS disorders, and the growth/differentiation of human cancers. The tumor differentiation effects of BB3 receptor activation were discussed in the previous section; the growth effects and effects of BB3 receptor overexpression will be considered here. In human cancer cells or cancers BB3 receptors are not only ectopically expressed in a large number of tumors, as reviewed above (Fathi et al., 1993b; Toi-Scott et al., 1996; Fathi et al., 1996; Sun et al., 2000b; Reubi et al., 2002; Schulz et al., 2006), but their activation alters lung cancer behavior by increasing MAP kinase activation, nuclear oncogene expression (Weber et al., 2001) and increasing adhesion of lung cancer tumor cells, which was proposed to contribute to increased tumor invasion and metastases by these tumors (Hou et al., 2006). In BB3 knockout mice (Maekawa et al., 2004) the hyperphagic response to melanin-concentrating hormone (MCH) is impaired, but not in BB2 receptor knockout mice. Furthermore, the levels of the MCH receptor and prepro-MCH mRNA's in the hypothalamus of BB3 receptor knockout mice were higher than controls, suggesting that up-regulation of the MCH-R and MCH occurs in the knockout mice that triggers hyperphagia and likely upsets the mechanism by which leptin decrease MCH-R and feeding (Maekawa et al., 2004). Studies of BB3 receptor knockout mice suggest that this receptor is important in various behavioral effects including the neural mechanisms that regulate social isolation (Yamada et al., 2000a) and are important in modulating emotion including forms of anxiety (Yamada et al., 2002a).
BB3 receptors as well as BB1 receptor and BB2 receptor are expressed in developing primate and murine fetal lungs (Emanuel et al., 1999; Shan et al., 2004). Studies (Tan et al., 2006; Tan et al., 2007) demonstrate that BB3 receptors are expressed in the airway in response to ozone injury and that wound repair and proliferation of bronchial epithelial cells is accelerated by BB3 receptor activation, suggesting it may mediate wound repair. The mechanism of lung ozone injury mediation of the up-regulation of BB3 receptors has been studied by examining proteins interacting with the BB3 receptor gene promoter region (Tan et al., 2007). AP-2 alpha and PPARalpha increased the ozone-inducible DNA binding on the BB3 receptor gene promoter suggesting they are specifically involved in the BB3 receptor up-regulation (Tan et al., 2007). BB3 receptors are expressed on small cell and nonsmall cell lung cancers (Fathi et al., 1993b; Toi-Scott et al., 1996; Ryan et al., 1998b; Reubi et al., 2002) as well as lung carcinoids (Fathi et al., 1993b; Reubi et al., 2002). In the small cell lung cancer cell line, NCI-N417, which is known to possess functional BB3 receptors (Ryan et al., 1998b), [D-Phe6, β-Ala11, Phe13, Nle14] bombesin 6−14 stimulated tumor cell adhesion, likely by stimulation of focal adhesion formation (Hou et al., 2006). It was proposed (Hou et al., 2006) that BB3 receptor activation in these cells may be important for their invasion and development of metastases.
Although the function of BB3 receptors in the gastrointestinal tract is largely unknown, specific BB3 receptor antibodies localized the receptor in the tunica muscularis of the rat gastrointestinal tract (Porcher et al., 2005). BB3 receptors were detected in both myenteric and submucosal ganglia as well as in nerve fibers interconnecting myenteric ganglia (Porcher et al., 2005). BB3 receptor IR was observed in cell bodies and processes of c-kit interstitial cells of Cajal leading the authors to propose that the BB3 receptor was likely involved in the regulation of gastrointestinal motility.
The above studies and those reviewed in the previous section suggest that BB3 receptor activation could be involved in human disorders of energy metabolism including obesity, glucose homeostasis, blood pressure control, lung injury, tumor growth, and possibility motility disorders, but all of these possibilities remain unproven at present.
One study screened 104 Japanese obese men for defects in the BB3 receptor gene but no mutations or polymorphisms were found (Hotta et al., 2000) suggesting BB3 receptor gene mutations are unlikely to be a major cause of obesity in humans.
VI. Therapeutic implications of Bn receptors
This area was partially covered under the sections dealing with disease for each of the three receptor classes, but a few important summary points will be made here. The principal therapeutic interests are in the BB2 receptors, to a lesser extent in the BB3 receptor and least in the BB1 receptor. In the case of the BB2 receptor the recent study (Sun and Chen, 2007) that provides evidence that activation of the BB2 receptor in the spinal cord may be an important pathway in mediating pruritic signals has profound clinical implications. Chronic itching is a very common problem (Yosipovitch et al., 2007): in a population survey of 18,770 adults in Norway (Dalgard et al., 2007a; Dalgard et al., 2007b) itching was the most common skin problem occurring in 7% and it is associated with poor general health. Often existing therapies provide limited relief and there are no general-purpose anti-pruritic drugs (Yosipovitch et al., 2007) therefore, identification of the BB2 receptor as possible central therapeutic target has significant therapeutic implications for this disorder. The tumoral growth effects and frequent overexpression or ectopic expression of all of the Bn receptors have important clinical implications. This is particularly true for the BB2 receptor, which is the most frequently overexpressed, and has been the most extensively investigated for its growth effects on different human tumors (Schulz et al., 2006; Lantry et al., 2006; Jensen and Moody, 2006; Patel et al., 2006; Engel et al., 2007; Cornelio et al., 2007). Studies demonstrating that GRP and NMB can have autocrine growth activity; in some tumors BB2 receptor activation results in stimulation of the EGF receptor; that continued stimulation through the BB2 receptor can counter the inhibitory effects of EGFR blockade on tumor growth and that the combination of a BB2 receptor blockade and EGF receptor inhibition can have profound inhibitory effects, on tumor growth, all have important therapeutic implications (Santiskulvong et al., 2001; Madarame et al., 2003; Xiao et al., 2003; Santiskulvong and Rozengurt, 2003; Santiskulvong et al., 2004; Thomas et al., 2005; Stangelberger et al., 2005; Jensen and Moody, 2006; Patel et al., 2006; Liu et al., 2007; Zhang et al., 2007b; de Visser et al., 2007b). As discussed in detail in the BB receptor disease sections the over-expression of particularly BB2 receptors, by many common tumors (breast, colon, head and neck squamous cancers, various CNS tumors, lung, prostate, ovary, and renal) has important therapeutic implications. This is particularly the case for the Bn family of receptors, because they are one of the classes of G protein-coupled receptors most frequently present on these tumors. Furthermore, in many cases existing therapies are inadequate with these tumors or the tumors frequently stop responding to current first-line treatments, and therefore new approaches are needed. These therapeutic implications include not only for the development of labeled Bn analogues for enhanced tumor imaging and staging (Breeman et al., 2002; Smith et al., 2003; Nock et al., 2003; Smith et al., 2005; Zhang et al., 2006; Lantry et al., 2006; Johnson et al., 2006; Waser et al., 2007; Parry et al., 2007; Dimitrakopoulou-Strauss et al., 2007; Garrison et al., 2007; Prasanphanich et al., 2007; de Visser et al., 2007a; Zhang et al., 2007a), but also for use for bombesin receptor-mediated cytotoxicity, either with radiolabeled compounds, as is being widely evaluated with somatostatin analogues in Phase 3 studies (Van Essen et al., 2007; Breeman et al., 2007; Forrer et al., 2007), or the use of Bn analogues coupled to other cytotoxic agents such as doxorubicin analogues, paclitaxel or camptothecin (Schally and Nagy, 1999; Breeman et al., 2002; Moody et al., 2004; Schally and Nagy, 2004; Engel et al., 2005; Buchholz et al., 2006; Safavy et al., 2006; Panigone and Nunn, 2006; Nanni et al., 2006; Moody et al., 2006b; Engel et al., 2007). The participation of BB3 receptors in energy balance and in glucose homeostasis as manifested by the BB3 receptor knockout animals developing obesity and diabetes (Ohki-Hamazaki et al., 1997a) has potential important clinical implications. At present there has been increased understanding of the mechanisms of these effects (see BB3 receptor diseases section: V.8 (Yamada et al., 1999; Matsumoto et al., 2003; Nakamichi et al., 2004), but possible progress in extending this to a clinical application is limited by the lack of identification of the natural ligand for this receptor.
Numerous other actions of each of the three Bn receptors have potential importance for therapeutic interventions, but at present either the understanding of their participation in normal and pathological conditions is insufficient to specifically target these receptors or the drugs to do this are not available. In the case of the BB1 receptor such areas include: involvement in thyroid function and alterations in thyroid disease (Ortiga-Carvalho et al., 2003; Pazos-Moura et al., 2003; Oliveira et al., 2006); behavior effects in mediating aspects of fear, anxiety, stress responses (Ohki-Hamazaki et al., 1999; Merali et al., 2002; Yamada et al., 2003; Merali et al., 2006; Bedard et al., 2007) and satiety effects (Merali et al., 1999; Ladenheim and Knipp, 2007). For the BB2 receptor such areas include: its role in motility with mediation of the descending peristaltic reflex (Grider, 2004); role in lung injury and development of lung diseases, particularly the neonatal lung disease, bronchopulmonary dysplasia, in which Bn-like peptides and the BB2 receptor were shown to play an important role in various animal models (Li et al., 1994; Sunday et al., 1998; Emanuel et al., 1999; Cullen et al., 2000; Ashour et al., 2006; Ganter and Pittet, 2006; Subramaniam et al., 2007); its role in sepsis and in small intestinal mucosal protection and prevention from injury (Assimakopoulos et al., 2004; Assimakopoulos et al., 2005a; Assimakopoulos et al., 2005b; Higuchi et al., 2006; Dal-Pizzol et al., 2006; Kimura et al., 2006a; Kimura et al., 2006b); satiety effects (Merali et al., 1999; Ladenheim and Knipp, 2007) and its CNS effects on memory, learning, various behaviors and response to stress (Merali et al., 1999; Yegen, 2003; Roesler et al., 2004; Moody and Merali, 2004; Merali et al., 2006; Luft et al., 2006; dos Santos Dantas et al., 2006; Roesler et al., 2006a; Roesler et al., 2006b; Presti-Torres et al., 2007). For the BB3 receptor such areas include: its possible role in lung development and responses to lung injury (Shan et al., 2004; Hou et al., 2006; Tan et al., 2006; Tan et al., 2007) and its possible role in regulation of aspects of gastrointestinal motility (Porcher et al., 2005).
VII. Unresolved nomenclature issues
The principal unresolved issue is the natural ligand of the BB3 receptor remains unknown and therefore its pharmacology and roles in normal physiology or pathological processes is unknown. Another unresolved issue is whether an equivalent receptor to the frog BB4 exists in human and mammals. Two studies have sought additional members of the bombesin receptor family and none were found in mammals (Fathi et al., 1993b; Sano et al., 2004). With human and mouse genome sequences now known, it is high unlikely that any other mammalian BB's will be found besides BB1, BB2, and BB3. An additional key issue unresolved at present is whether COOH terminal extended or precursor form or fragments of GRP or NMB have physiological or pathological effects that are not mediated by the three classes of mammalian receptors described in the current nomenclature. A number of recent studies (Patel et al., 2004; Dumesny et al., 2004; Dumesny et al., 2006; Patel et al., 2007a; Patel et al., 2007b) provide evidence that non-amidated precursor forms of GRP can stimulate proliferation of different tumors/tissues. COOH terminal precursor forms of GRP are reported to stimulate the proliferation and migration of the human colorectal carcinoma cell line DLD-1 (Patel et al., 2007a; Patel et al., 2007b) through a BB2 receptor independent mechanism and the growth of the prostate cancer cell line DU145 (Patel et al., 2007b). Furthermore, ProGRP immunoreactivity is reported in 90% of resected colorectal carcinomas and all endometrial, prostate and colon cancer cell lines tested, without any amidated forms present (Dumesny et al., 2006). Recombinant ProGRP stimulated proliferation of the colon cancer cell line DLD-1, activating MAPK, but did not stimulate phospholipase C activity nor bind to known bombesin receptors, suggesting it was stimulating the tumor growth through a novel receptor (Dumesny et al., 2006). At present no receptor has been isolated that mediates these actions, but they are not inhibited by BB2 receptor antagonists, raising the possibility they could be mediated by a novel receptor. A final key problem area unresolved at present, are the roles of the three described mammalian bombesin receptors in normal physiology and pathological conditions which are still largely unknown. This is due in large part to lack of specific antagonists for all subclasses of bombesin receptors, especially high affinity, selective nonpeptide receptor antagonists.
Acknowledgements
This research is partially supported by the Intramural Research Program of the NIDDK and NIDCD, NIH; NCI grant CA-094346 (R.V.B.) and a VA Merit review award (R.V.B.)
We thank Terry Moody, NCI for carefully proofreading the paper and for making thoughtful suggestions.
Abbreviations
- Bn
bombesin
- GRP
gastrin-releasing peptide
- NMB
neuromedin B
- CNS
central nervous system
- IR
immunoreactivity
- BRS-3
bombesin receptor subtype 3
- fBB4
frog bombesin receptor subtype 4
- COOH terminus
carboxyl terminus
- PKC
protein kinase C
- TM
transmembrane region
- GPCR
G protein-coupled receptor
- 5-HT
serotonin, 5-hydroxytryptamine
- NSCLC
nonsmall cell lung cancer cell
- CCK
cholecystokinin
- SP
substance P
- Cpa
chlorophenylalanine
- β—Ala
β—Alanine
- ψ bonds
pseudopeptide bonds
- DAG
diacylglycerol
- p125FAK
p125 focal adhesion kinase
- TPA
12-O-tetradecanoyl-phorbol-13-acetate
- EC1
extracellular domain 1
- IC1
intracellular domain 1
- PKD
protein kinase D
- ERK
extracellular regulated kinase
References
- Acs P, Beheshti M, Szallasi Z, Li L, Yuspa SH, Blumberg PM. Effect of a Tyrosine 155 to Phenylalanine Mutation of Protein Kinase Cδ on the Proliferative and Tumorigenic Properties of NIH 3T3 Fibroblasts. Carcinogenesis. 2000;21:887–891. doi: 10.1093/carcin/21.5.887. [DOI] [PubMed] [Google Scholar]
- Akeson M, Sainz E, Mantey SA, Jensen RT, Battey JF. Identification of Four Amino Acids in the Gastrin-Releasing Peptide C Receptor That Are Required for High Affinity Agonist Binding. J Biol Chem. 1997;272:17405–17409. doi: 10.1074/jbc.272.28.17405. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. BJP Guide to Receptors and Channels. Brit J Pharmacol. 2006;147:S6–S180. doi: 10.1038/sj.bjp.0706651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally RA, Ives KL, Traube E, Eltounsi I, Chen PW, Cahill PJ, Battey JF, Hellmich MR, Kroog GS. Agonistand Protein Kinase C-Induced Phosphorylation Have Similar Functional Consequences for Gastrin-Releasing Peptide Receptor Signaling Via Gq. Mol Pharmacol. 2003;64:890–904. doi: 10.1124/mol.64.4.890. [DOI] [PubMed] [Google Scholar]
- Alptekin N, Yagci RV, Ertan A, Jiang NY, Rice JC, Sbeiti M, Rossowski WJ, Coy DH. Comparison of Prolonged in Vivo Inhibitory Activity of Several Potent Bombesin (BN) Antagonists on BN-Stimulated Amylase Secretion in the Rat. Peptides. 1991;12:749–753. doi: 10.1016/0196-9781(91)90128-c. [DOI] [PubMed] [Google Scholar]
- Anonymous Bombesin-Like Peptides in Health and Disease. Nomenclature Meeting. Report and Recommendations. 14 October 1987. Ann N Y Acad Sci. 1988;547:1–2. [PubMed] [Google Scholar]
- Ashour K, Shan L, Lee JH, Schlicher W, Wada K, Wada E, Sunday ME. Bombesin Inhibits Alveolarization and Promotes Pulmonary Fibrosis in Newborn Mice. Am J Respir Crit Care Med. 2006;173:1377–1385. doi: 10.1164/rccm.200507-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood V, Brownhill V, Higginbottom M, Horwell DC, Hughes J, Lewthwaite RA, McKnight AT, Pinnock RD, Pritchard MC, Suman-Chauhan N, Webb C, Williams SC. PD 176252 - The First High Affinity Non-Peptide Gastrin-Releasing Peptide (BB2) Receptor Antagonist. Bioorg Med Chem. 1998;8:2589–2594. doi: 10.1016/s0960-894x(98)00462-4. [DOI] [PubMed] [Google Scholar]
- Assimakopoulos SF, Alexandris IH, Scopa CD, Mylonas PG, Thomopoulos KC, Georgiou CD, Nikolopoulou VN, Vagianos CE. Effect of Bombesin and Neurotensin on Gut Barrier Function in Partially Hepatectomized Rats. World J Gastroenterol. 2005a;11:6757–6764. doi: 10.3748/wjg.v11.i43.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimakopoulos SF, Scopa CD, Charonis A, Spiliopoulou I, Georgiou C, Nikolopoulou V, Vagianos CE. Experimental Obstructive Jaundice Disrupts Intestinal Mucosal Barrier by Altering Occludin Expression: Beneficial Effect of Bombesin and Neurotensin. J Am Coll Surg. 2004;198:748–757. doi: 10.1016/j.jamcollsurg.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Assimakopoulos SF, Scopa CD, Zervoudakis G, Mylonas PG, Georgiou C, Nikolopoulou V, Vagianos CE. Bombesin and Neurotensin Reduce Endotoxemia, Intestinal Oxidative Stress, and Apoptosis in Experimental Obstructive Jaundice. Ann Surg. 2005b;241:159–167. doi: 10.1097/01.sla.0000149306.35717.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azay J, Gagne D, Devin C, Llinares M, Fehrentz JA, Martinez J. JMV641: a Potent Bombesin Receptor Antagonist That Inhibits Swiss 3T3 Cell Proliferation. Regul Pept. 1996;65:91–97. doi: 10.1016/0167-0115(96)00077-8. [DOI] [PubMed] [Google Scholar]
- Azay J, Nagain C, Llinares M, Devin C, Fehrentz JA, Bernad N, Roze C, Martinez J. Comparative Study of in Vitro and in Vivo Activities of Bombesin Pseudopeptide Analogs Modified on the C-Terminal Dipeptide Fragment. Peptides. 1998;19:57–63. doi: 10.1016/s0196-9781(97)00275-1. [DOI] [PubMed] [Google Scholar]
- Bajo AM, Schally AV, Groot K, Szepeshazi K. Bombesin Antagonists Inhibit Proangiogenic Factors in Human Experimental Breast Cancers. Br J Cancer. 2004;90:245–252. doi: 10.1038/sj.bjc.6601404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin GS, Patel O, Shulkes A. Phylogenetic Analysis of the Sequences of Gastrin-Releasing Peptide and Its Receptors: Biological Implications. Regul Pept. 2007 doi: 10.1016/j.regpep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Battey J, Wada E. Two Distinct Receptors for Mammalian Bombesin-Like Peptides. Trends Neurosci. 1991;14(12):524–528. doi: 10.1016/0166-2236(91)90005-f. [DOI] [PubMed] [Google Scholar]
- Battey JF, Way JM, Corjay MH, Shapira H, Kusano K, Harkins R, Wu JM, Slattery T, Mann E, Feldman RI. Molecular Cloning of the Bombesin/Gastrin-Releasing Peptide Receptor From Swiss 3T3 Cells. Proc Natl Acad Sci USA. 1991;88:395–399. doi: 10.1073/pnas.88.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard T, Mountney C, Kent P, Anisman H, Merali Z. Role of Gastrin-Releasing Peptide and Neuromedin B in Anxiety and Fear-Related Behavior. Behav Brain Res. 2007;179:133–140. doi: 10.1016/j.bbr.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Benya RV, Akeson M, Mrozinski J, Jensen RT, Battey JF. Internalization of the Gastrin-Releasing Peptide Receptor Is Mediated by Phospholipase C-Dependent and -Independent Processes. Mol Pharmacol. 1994a;46:495–501. [PubMed] [Google Scholar]
- Benya RV, Fathi Z, Battey JF, Jensen RT. Serines and Threonines in the Gastrin-Releasing Peptide Receptor Carboxyl Terminus Mediate Internalization. J Biol Chem. 1993;268:20285–20290. [PubMed] [Google Scholar]
- Benya RV, Fathi Z, Pradhan T, Battey JF, Kusui T, Jensen RT. Gastrin-Releasing Peptide Receptor-Induced Internalization, Down-Regulation, Desensitization and Growth: Possible Role of CAMP. Mol Pharmacol. 1994b;46(2):235–245. [PubMed] [Google Scholar]
- Benya RV, Kusui T, Battey JF, Jensen RT. Chronic Desensitization and Down-Regulation of the Gastrin-Releasing Peptide Receptor Are Mediated by a Protein Kinase C-Dependent Mechanism. J Biol Chem. 1995a;270:3346–3352. doi: 10.1074/jbc.270.7.3346. [DOI] [PubMed] [Google Scholar]
- Benya RV, Kusui T, Battey JF, Jensen RT. Desensitizaton of Neuromedin B Receptors (NMB-R) on Native and NMB-R Transfected Cells Involves Down-Regulation and Internalization. J Biol Chem. 1994c;269:11721–11728. [PubMed] [Google Scholar]
- Benya RV, Kusui T, Fathi Z, Battey JF, Jensen RT. Glycosylation location and its functional significance in the gastrin-releasing peptide receptor (GRP-R). Digestive Diseases and Sciences. 1994d;39:1765. Ref Type: Abstract. [Google Scholar]
- Benya RV, Kusui T, Pradhan TK, Battey JF, Jensen RT. Expression and Characterization of Cloned Human Bombesin Receptors. Mol Pharmacol. 1995b;47:10–20. [PubMed] [Google Scholar]
- Benya RV, Wada E, Battey JF, Fathi Z, Wang LH, Mantey SA, Coy DH, Jensen RT. Neuromedin B Receptors Retain Functional Expression When Transfected into BALB 3T3 Fibroblasts: Analysis of Binding, Kinetics, Stoichiometry, Modulation by Guanine Nucleotide-Binding Proteins, and Signal Transduction and Comparison With Natively Expressed Receptors. Mol Pharmacol. 1992;42(6):1058–1068. [PubMed] [Google Scholar]
- Bitar KG, Coy DH. Identification and Initial Characterization of a Putative Neuromedin B-Type Receptor From Rat Urinary Bladder Membranes. Eur J Pharmacol. 1992;219:117–122. doi: 10.1016/0014-2999(92)90588-u. [DOI] [PubMed] [Google Scholar]
- Bjoro T, Torjesen PA, Ostberg BC, Sand O, Iversen JG, Gautvik KM, Haug E. Bombesin Stimulates Prolactin Secretion From Cultured Rat Pituitary Tumour Cells (GH4C1) Via Activation of Phospholipase C. Regul Pept. 1987;19:169–182. doi: 10.1016/0167-0115(87)90274-6. [DOI] [PubMed] [Google Scholar]
- Boden PR, Higginbottom M, Hill DR, Horwell DC, Hughes J, Rees DC, Roberts E, Singh L, Suman-Chauhan N, Woodruff GN. Cholecystokinin Dipeptoid Antagonists: Design, Synthesis, and Anxiolytic Profile of Some Novel CCK-A and CCK-B Selective and “Mixed” CCK-A/CCK-B Antagonists. J Med Chem. 1993;36:552–565. doi: 10.1021/jm00057a005. [DOI] [PubMed] [Google Scholar]
- Bouchard L, Drapeau V, Provencher V, Lemieux S, Chagnon Y, Rice T, Rao DC, Vohl MC, Tremblay A, Bouchard C, Perusse L. Neuromedin Beta: a Strong Candidate Gene Linking Eating Behaviors and Susceptibility to Obesity. Am J Clin Nutr. 2004;80:1478–1486. doi: 10.1093/ajcn/80.6.1478. [DOI] [PubMed] [Google Scholar]
- Boyle RG, Humphries J, Mitchell T, Showell GA, Apaya R, Iijima H, Shimada H, Arai T, Ueno H, Usui Y, Sakaki T, Wada E, Wada K. The Design of a New Potent and Selective Ligand for the Orphan Bombesin Receptor Subtype 3 (BRS3). J Pept Sci. 2005;11:136–141. doi: 10.1002/psc.599. [DOI] [PubMed] [Google Scholar]
- Boyle S, Guard S, Hodgson J, Horwell DC, Howson W, Hughes J, McKnight A, Martin K, Pritchard MC, Watling KJ. Rational Design of High Affiity Tachykinin NK2 Receptor Antagonists. Bioorg Med Chem. 1994;2:101–113. doi: 10.1016/s0968-0896(00)82006-4. [DOI] [PubMed] [Google Scholar]
- Breeman WA, de Jong M, Erion JL, Bugaj JE, Srinivasan A, Bernard BF, Kwekkeboom DJ, Visser TJ, Krenning EP. Preclinical Comparison of (111)In-Labeled DTPA-or DOTA-Bombesin Analogs for Receptor-Targeted Scintigraphy and Radionuclide Therapy. J Nucl Med. 2002;43:1650–1656. [PubMed] [Google Scholar]
- Breeman WA, Kwekkeboom DJ, de Blois E, de Jong M, Visser TJ, Krenning EP. Radiolabelled Regulatory Peptides for Imaging and Therapy. Anticancer Agents Med Chem. 2007;7:345–357. doi: 10.2174/187152007780618171. [DOI] [PubMed] [Google Scholar]
- Briscoe CP, Plevin R, Wakelam JO. Rapid Desensitization and Resensitization of Bombesin-Stimulated Phospholipase D Activity in Swiss 3T3 Cells. Biochem J. 1994;298:61–67. doi: 10.1042/bj2980061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccardo M, Erspamer GF, Melchiorri P, Negri L, DeCastiglione R. Relative Potency of Bombesin-Like Peptides. Br J Pharmacol. 1976;55:221–227. doi: 10.1111/j.1476-5381.1975.tb07631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KD, Laurie MS, Littlewood CJ, Blakeley DM, Corps AN. Characterization of the High-Affinity Receptors on Swiss 3T3 Cells Which Mediate the Binding, Internalization and Degradation of the Mitogenic Peptide Bombesin. Biochem J. 1988;252:227–235. doi: 10.1042/bj2520227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz S, Keller G, Schally AV, Halmos G, Hohla F, Heinrich E, Koester F, Baker B, Engel JB. Therapy of Ovarian Cancers With Targeted Cytotoxic Analogs of Bombesin, Somatostatin, and Luteinizing Hormone-Releasing Hormone and Their Combinations. Proc Natl Acad Sci U S A. 2006;103:10403–10407. doi: 10.1073/pnas.0602971103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnett N. Gastrin-releasing peptide. In: Walsh JH, Dockray GJ, editors. Gut Peptides. Raven Press, Ltd.; New York: 1994. pp. 423–445. [Google Scholar]
- Cai RZ, Radulovic S, Pinski J, Nagy A, Redding TW, Olsen DB, Schally AV. Pseudononapeptide Bombesin Antagonists Containing C-Terminal Trp or Tpi. Peptides. 1992;13:267–271. doi: 10.1016/0196-9781(92)90107-e. [DOI] [PubMed] [Google Scholar]
- Cai RZ, Reile H, Armatis P, Schally AV. Potent Bombesin Antagonists With C-Terminal Leu-Psi(CH2-N)-Tac-NH2 or Its Derivatives. Proc Natl Acad Sci U S A. 1994;91:12664–12668. doi: 10.1073/pnas.91.26.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camble R, Cotton R, Dutta AS, Garner A, Hayward CF, Moore VE, Scholes PB. N-Isobutyryl-His-Trp-Ala-Val-D-Ala-His-Leu-NHMe (ICI 216140). A Potent in Vivo Antagonist Analogue of Bombesin/Gastrin- Releasing Peptide (BN/GRP) Derived From the C-Terminal Sequence Lacking the Final Methionine Residue. Life Sci. 1989;45:1521–1527. doi: 10.1016/0024-3205(89)90417-7. [DOI] [PubMed] [Google Scholar]
- Carroll RE, Carroll R, Benya RV. Characterization of Gastrin-Releasing Peptide Receptors Aberrantly Expressed by Non-Antral Gastric Adenocarcinomas. Peptides. 1999a;20:229–237. doi: 10.1016/s0196-9781(98)00164-8. [DOI] [PubMed] [Google Scholar]
- Carroll RE, Matkowskyj K, Saunthararajah Y, Sekosan M, Battey JF, Benya RV. Contribution of Gastrin-Releasing Peptide and Its Receptor to Villus Development in the Murine and Human Gastrointestinal Tract. Mech Dev. 2002;113:121–130. doi: 10.1016/s0925-4773(02)00032-1. [DOI] [PubMed] [Google Scholar]
- Carroll RE, Matkowskyj KA, Chakrabarti S, McDonald TJ, Benya RV. Abberrant Expression of Gastrin-Releasing Peptide and Its Receptor by Well-Differentiated Colon Cancers in Humans. Am J Physiol. 1999b;276:G655–G665. doi: 10.1152/ajpgi.1999.276.3.G655. [DOI] [PubMed] [Google Scholar]
- Carroll RE, Matkowskyj KA, Tretiakova MS, Battey JF, Benya RV. Gastrin-Releasing Peptide Is a Mitogen and a Morphogen in Murine Colon Cancer. Cell Growth Differ. 2000a;11:385–393. [PubMed] [Google Scholar]
- Carroll RE, Ostrovskiy D, Lee S, Danilkovich A, Benya RV. Characterization of Gastrin-Releasing Peptide and Its Receptor Aberrantly Expressed by Human Colon Cancer Cell Lines. Mol Pharmacol. 2000b;58:601–607. doi: 10.1124/mol.58.3.601. [DOI] [PubMed] [Google Scholar]
- Chan AS, Wong YH. Gq-Mediated Activation of C-Jun N-Terminal Kinase by the Gastrin-Releasing Peptide-Preferring Bombesin Receptor Is Inhibited Upon Costimulation of the Gs-Coupled Dopamine D1 Receptor in COS-7 Cells. Mol Pharmacol. 2005;68:1354–1364. doi: 10.1124/mol.105.014548. [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Carrasquillo JA, Avis IL, Shuke N, Reynolds JC, Bartholomew R, Larson SM, Cuttitta F, Johnson BE, Mulshine JL. Phase I and Imaging Trial of a Monoclonal Antibody Directed Against Gastrin-Releasing Peptide in Patients With Lung Cancer. Clin Cancer Res. 1999;5:3385–3393. [PubMed] [Google Scholar]
- Chave HS, Gough AC, Palmer K, Preston SR, Primrose JN. Bombesin Family Receptor and Ligand Gene Expression in Human Colorectal Cancer and Normal Mucosa. Br J Cancer. 2000;82:124–130. doi: 10.1054/bjoc.1998.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KU, Evers BM, Ishizuka J, Townsend CM, Jr., Thompson JC. Role of Bombesin on Gut Mucosal Growth. Ann Surg. 1995;222:94–100. doi: 10.1097/00000658-199507000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SJ, Briscoe CP, Wakelam MJO. The Regulation of Phospholipase D Activity and Its Role in Sn-1,2-Diradylglycerol Formation in Bombesin- and Phorbol 12-Myristate 13-Acetate-Stimulated Swiss 3T3 Cells. Biochem J. 1991;280(Pt 2):431–438. doi: 10.1042/bj2800431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corjay MH, Dobrzanski DJ, Way JM, Viallet J, Shapira H, Worland P, Sausville EA, Battey JF. Two Distinct Bombesin Receptor Subtypes Are Expressed and Functional in Human Lung Carcinoma Cells. J Biol Chem. 1991;266:18771–18779. [PubMed] [Google Scholar]
- Cornelio D, Roesler R, Schwartsmann G. Gastrin-Releasing Peptide Receptor As a Molecular Target in Experimental Anticancer Therapy. Ann Oncol. 2007;18:1457–1466. doi: 10.1093/annonc/mdm058. [DOI] [PubMed] [Google Scholar]
- Coy D, Wang LH, Jiang NY, Jensen R. Short Chain Bombesin Pseudopeptides With Potent Bombesin Receptor Antagonistic Activity in Rat and Guinea Pig Pancreatic Acinar Cells. Europ J Pharmacol. 1990;190:31–38. doi: 10.1016/0014-2999(90)94109-b. [DOI] [PubMed] [Google Scholar]
- Coy DH, Jensen RT, Jiang NY, Lin JT, Bogden AE, Moreau JP. Systematic Development of Bombesin/Gastrin-Releasing Peptide Antagonists. Monogr Natl Cancer Inst #13. 1992a:133–139. [PubMed] [Google Scholar]
- Coy DH, Jiang NY, Kim SH, Moreau JP, Lin JT, Frucht H, Qian JM, Wang LW, Jensen RT. Covalently Cyclized Agonist and Antagonist Analogues of Bombesin and Related Peptides. J Biol Chem. 1991a;266(25):16441–16447. [PubMed] [Google Scholar]
- Coy DH, Jiang NY, Sasaki Y, Taylor J, Moreau JP, Wolfrey WT, Gardner JD, Jensen RT. Probing Peptide Backbone Function in Bombesin. A Reduced Peptide Bond Analogue With Potent and Specific Receptor Antagonist Activity. J Biol Chem. 1988;263(11):5056–5060. [PubMed] [Google Scholar]
- Coy DH, Mungan Z, Rossowski WJ, Cheng BL, Lin JT, Mrozinski JE, Jr., Jensen RT. Development of a Potent Bombesin Receptor Antagonist With Prolonged in Vivo Inhibitory Activity on Bombesin-Stimulated Amylase and Protein Release in the Rat. Peptides. 1992b;13:775–781. doi: 10.1016/0196-9781(92)90186-7. [DOI] [PubMed] [Google Scholar]
- Coy DH, Taylor JE, Jiang NY, Kim SH, Wang LH, Huang SC, Moreau JP, Gardner JD, Jensen RT. Short-Chain Pseudopeptide Bombesin Receptor Antagonists With Enhanced Binding Affinities for Pancreatic Acinar and Swiss 3T3 Cells Display Strong Antimitotic Activity. J Biol Chem. 1989;264:14691–14697. [PubMed] [Google Scholar]
- Coy DH, Wang LH, Jensen RT. Bombesin Agonists, Partial Agonists and Antagonists - Unexplained Biological Profiles Among Medicinally Interesting Antagonists. Actualities de Chimie Therapeutique. 1991b:157–165. dixhuitieme serie. [Google Scholar]
- Cullen A, Emanuel RL, Torday JS, Asokananthan N, Sikorski KA, Sunday ME. Bombesin-Like Peptide and Receptors in Lung Injury Models: Diverse Gene Expression, Similar Function. Peptides. 2000;21:1627–1638. doi: 10.1016/s0196-9781(00)00294-1. [DOI] [PubMed] [Google Scholar]
- Currie S, Smith GL, Crichton CA, Jackson CG, Hallam C, Wakelam MJ. Bombesin Stimulates the Rapid Activation of Phospholipase A2-Catalyzed Phosphatidylcholine Hydrolysis in Swiss 3T3 Cells. J Biol Chem. 1992;267:6056–6062. [PubMed] [Google Scholar]
- Cuttitta F, Carney DN, Mulshine J, Moody TW, Fedorko J, Fischler A, Minna JD. Bombesin-Like Peptides Can Function As Autocrine Growth Factors in Human Small-Cell Lung Cancer Cells. Nature. 1985;316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Dal-Pizzol F, Di Leone LP, Ritter C, Martins MR, Reinke A, Pens GD, Zanotto-Filho A, de Souza LF, Andrades M, Barbeiro DF, Bernard EA, Cammarota M, Bevilaqua LR, Soriano FG, Claudio J, Moreira F, Roesler R, Schwartsmann G. Gastrin-Releasing Peptide Receptor Antagonist Effects on an Animal Model of Sepsis. Am J Respir Crit Care Med. 2006;173:84–90. doi: 10.1164/rccm.200507-1118OC. [DOI] [PubMed] [Google Scholar]
- Dalgard F, Dawn AG, Yosipovitch G. Are Itch and Chronic Pain Associated in Adults? Results of a Large Population Survey in Norway. Dermatology. 2007a;214:305–309. doi: 10.1159/000100881. [DOI] [PubMed] [Google Scholar]
- Dalgard F, Holm JO, Svensson A, Kumar B, Sundby J. Self Reported Skin Morbidity and Ethnicity: a Population-Based Study in a Western Community. BMC Dermatol. 2007b;7:4. doi: 10.1186/1471-5945-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castiglione R, Gozzini L. Bombesin Receptor Antagonists. Crit Rev Oncol Hematol. 1996;24:117–151. doi: 10.1016/1040-8428(96)00220-x. [DOI] [PubMed] [Google Scholar]
- de Jong M, Kwekkeboom D, Valkema R, Krenning EP. Radiolabelled Peptides for Tumour Therapy: Current Status and Future Directions Plenary Lecture at the EANM 2002. Eur J Nucl Med Mol Imaging. 2003;30:463–469. doi: 10.1007/s00259-002-1107-8. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Del Rio M, Ferrandez MD, Hernanz A. Modulation of Phagocytic Function in Murine Peritoneal Macrophages by Bombesin, Gastrin-Releasing Peptide and Neuromedin C. Immunology. 1991;73:205–211. [PMC free article] [PubMed] [Google Scholar]
- De la Fuente M, Del Rio M, Hernanz A. Stimulation of Natural Killer and Antibody-Dependent Cellular Cytotoxicity Activities in Mouse Leukocytes by Bombesin, Gastrin-Releasing Peptide and Neuromedin C: Involvement of Cyclic AMP, Inositol 1,4,5-Trisphosphate and Protein Kinase C. Ann Oncol. 1993b;4:499–507. doi: 10.1016/0165-5728(93)90186-3. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Del Rio M, Hernanz A. Stimulation of Natural Killer and Antibody-Dependent Cellular Cytotoxicity Activities in Mouse Leukocytes by Bombesin, Gastrin-Releasing Peptide and Neuromedin C: Involvement of Cyclic AMP, Inositol 1,4,5-Trisphosphate and Protein Kinase C. J Neuroimmunol. 1993a;48:143–150. doi: 10.1016/0165-5728(93)90186-3. [DOI] [PubMed] [Google Scholar]
- De Vincentis G, Remediani S, Varvarigou AD, Di Santo G, Iori F, Laurenti C, Scopinaro F. Role of 99mTc-Bombesin Scan in Diagnosis and Staging of Prostate Cancer. Cancer Biother Radiopharm. 2004;19:81–84. doi: 10.1089/108497804773391711. [DOI] [PubMed] [Google Scholar]
- de Visser M, Bernard HF, Erion JL, Schmidt MA, Srinivasan A, Waser B, Reubi JC, Krenning EP, de Jong M. Novel (111)In-Labelled Bombesin Analogues for Molecular Imaging of Prostate Tumours. Eur J Nucl Med Mol Imaging. 2007a;34:1228–1238. doi: 10.1007/s00259-006-0356-3. [DOI] [PubMed] [Google Scholar]
- de Visser M, van Weerden WM, de Ridder CM, Reneman S, Melis M, Krenning EP, de Jong M. Androgen-Dependent Expression of the Gastrin-Releasing Peptide Receptor in Human Prostate Tumor Xenografts. J Nucl Med. 2007b;48:88–93. [PubMed] [Google Scholar]
- Degen LP, Peng F, Collet A, Rossi L, Letterer S, Serrano Y, Larsen F, Beglinger C, Hildebrand P. Blockade of GRP Receptors Inhibits Gastric Emptying and Gallbladder Contraction but Accelerates Small Intestinal Transit. Gastroenterology. 2001;120:361–368. doi: 10.1053/gast.2001.21174. [DOI] [PubMed] [Google Scholar]
- Del Rio M, De la Fuente M. Chemoattractant Capacity of Bombesin, Gastrin-Releasing Peptide and Neuromedin C Is Mediated Through PKC Activation in Murine Peritoneal Leukocytes. Regul Pept. 1994;49:185–193. doi: 10.1016/0167-0115(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Del Rio M, Hernanz A, De la Fuente M. Bombesin, Gastrin-Releasing Peptide, and Neuromedin C Modulate Murine Lymphocyte Proliferation Through Adherent Accessory Cells and Activate Protein Kinase C. Peptides. 1994;15:15–22. doi: 10.1016/0196-9781(94)90164-3. [DOI] [PubMed] [Google Scholar]
- DeMichele MA, Davis AL, Hunt JD, Landreneau RJ, Siegfried JM. Expression of MRNA for Three Bombesin Receptor Subtypes in Human Bronchial Epithelial Cells. Am J Respir Cell Mol Biol. 1994;11:66–74. doi: 10.1165/ajrcmb.11.1.8018339. [DOI] [PubMed] [Google Scholar]
- Deschodt-Lanckman M, Robberecht P, DeNeef P, Lammens M, Christophe J. In Vitro Action of Bombesin and Bombesin-Like Peptides on Amylase Secretion, Calcium Efflux and Adenylate Cyclase Activity in the Rat Pancreas: a Comparison With Other Secretagogues. J Clin Invest. 1976;58:891–898. doi: 10.1172/JCI108542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson KE, Uemara N, Sekar MC, McDaniel HB, Anderson W, Coy DH, Hirshowitz BI. Partial Agonist Activity of the Bombesin-Receptor Antagonist [Leu14-Psi-CH2-NH-Leu13]-Bombesin in Frog Peptic Cells. Biochem Biophys Res Commun. 1988;157:1154–1158. doi: 10.1016/s0006-291x(88)80994-x. [DOI] [PubMed] [Google Scholar]
- Dimitrakopoulou-Strauss A, Hohenberger P, Haberkorn U, Macke HR, Eisenhut M, Strauss LG. 68Ga-Labeled Bombesin Studies in Patients With Gastrointestinal Stromal Tumors: Comparison With 18FFDG. J Nucl Med. 2007;48:1245–1250. doi: 10.2967/jnumed.106.038091. [DOI] [PubMed] [Google Scholar]
- Djanani A, Kaneider NC, Sturn D, Wiedermann CJ. Agonist Function of the Neurokinin Receptor Antagonist, [D-Arg1,D-Phe5,D-Trp7,9,Leu11]Substance P, in Monocytes. Regul Pept. 2003;115:123–129. doi: 10.1016/s0167-0115(03)00148-4. [DOI] [PubMed] [Google Scholar]
- Dobrzanski D, Sharoni Y, Wada E, Battey J, Sausville E. Neuromedin-B Receptor Transfected BALB/3T3 Cells: Signal Transduction and Effects of Ectopic Receptor Expression on Cell Growth. Regul Pept. 1993;45:341–352. doi: 10.1016/0167-0115(93)90360-k. [DOI] [PubMed] [Google Scholar]
- Donohue RJ, Sainz E, Akeson M, Kroog GS, Mantey SA, Battey JF, Jensen RT, Northrup JK. An Aspartate Residue at the Extracellular Boundary of TMII, and an Arginine Residue at TMVII of the Gastrin-Releasing Peptide Receptor Interact to Facilitate Heterotrimeric G Protein Coupling. Biochemistry. 1999;38:9366–9372. doi: 10.1021/bi990544h. [DOI] [PubMed] [Google Scholar]
- dos Santos Dantas A, Luft T, Pegas Henriques JA, Schwartsmann G, Roesler R. Opposite Effects of Low and High Doses of the Gastrin-Releasing Peptide Receptor Antagonist RC-3095 on Memory Consolidation in the Hippocampus: Possible Involvement of the GABAergic System. Peptides. 2006;27:2307–2312. doi: 10.1016/j.peptides.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Dumesny C, Patel O, Lachal S, Giraud AS, Baldwin GS, Shulkes A. Synthesis, Expression and Biological Activity of the Prohormone for Gastrin Releasing Peptide (ProGRP). Endocrinology. 2006;147:502–509. doi: 10.1210/en.2005-0574. [DOI] [PubMed] [Google Scholar]
- Dumesny C, Whitley JC, Baldwin GS, Giraud AS, Shulkes A. Developmental Expression and Biological Activity of Gastrin-Releasing Peptide and Its Receptors in the Kidney. Am J Physiol Renal Physiol. 2004;287:F578–F585. doi: 10.1152/ajprenal.00416.2003. [DOI] [PubMed] [Google Scholar]
- Eden JM, Hall MD, Higginbottom M, Horwell DC, Howson W, Hughes J, Jordan RE, Lewthwaite RA, Martin K, McKnight AT, O'Toole JC, Pinnock RD, Pritchard MC, Suman-Chauhan N, Williams SC. PD 165929 -The First High Affinity Non-Peptide Neuromedin-B (NMB) Receptor Selective Antagonist. Bioorg Med Chem Lett. 1996;6:2617–2622. [Google Scholar]
- Emanuel RL, Torday JS, Mu Q, Asokananthan N, Sikorski KA, Sunday ME. Bombesin-Like Peptides and Receptors in Normal Fetal Baboon Lung: Roles in Lung Growth and Maturation. Am J Physiol. 1999;277:L1003–L1017. doi: 10.1152/ajplung.1999.277.5.L1003. [DOI] [PubMed] [Google Scholar]
- Endo T, Fukue H, Kanaya M, Mizunuma M, Fujii M, Yamamoto H, Tanaka S, Hashimoto M. Bombesin and Bradykinin Increase Inositol Phosphates and Cytosolic Free Ca2+ and Stimulate DNA Synthesis in Human Endometrial Stomal Cells. J Endocrinol. 1991;131:313–318. doi: 10.1677/joe.0.1310313. [DOI] [PubMed] [Google Scholar]
- Engel JB, Schally AV, Dietl J, Rieger L, Honig A. Targeted Therapy of Breast and Gynecological Cancers With Cytotoxic Analogues of Peptide Hormones. Mol Pharm. 2007 doi: 10.1021/mp0700514. [DOI] [PubMed] [Google Scholar]
- Engel JB, Schally AV, Halmos G, Baker B, Nagy A, Keller G. Targeted Cytotoxic Bombesin Analog AN-215 Effectively Inhibits Experimental Human Breast Cancers With a Low Induction of Multi-Drug Resistance Proteins. Endocr Relat Cancer. 2005;12:999–1009. doi: 10.1677/erc.1.01022. [DOI] [PubMed] [Google Scholar]
- Erspamer V. Discovery, Isolation and Characterization of Bombesin-Like Peptides. Ann N Y Acad Sci. 1988;547:3–9. doi: 10.1111/j.1749-6632.1988.tb23870.x. [DOI] [PubMed] [Google Scholar]
- Erspamer V, Erpamer GF, Inselvini M. Some Pharmacological Actions of Alytesin and Bombesin. J Pharm Pharmacol. 1970;22:875–876. doi: 10.1111/j.2042-7158.1970.tb08465.x. [DOI] [PubMed] [Google Scholar]
- Erspamer V, Erspamer GF, Inselvini M, Negri L. Occurrence of Bombesin and Alytesin in Extracts of the Skin of Three European Discoglossid Frogs and Pharmacological Actions of Bombesin on Extravascular Smooth Muscle. Br J Pharmacol. 1972;45:333–348. doi: 10.1111/j.1476-5381.1972.tb08087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V, Melchiorri P. Active Polypeptides of the Amphibian Skin and Their Synethetic Analogues. Pure Appl Chem. 1973;35:463–494. [Google Scholar]
- Falconieri Erspamer G, Severini C, Erspamer V, Melchiorri P, Delle Fave G, Nakajima T. Parallel Bioassay of 27 Bombesin-Like Peptides on 9 Smooth Muscle Preparations, Structure-Activity Relationships and Bombesin Receptor Subtypes. Regul Pept. 1988;21:1–11. doi: 10.1016/0167-0115(88)90085-7. [DOI] [PubMed] [Google Scholar]
- Fathi Z, Benya RV, Shapira H, Jensen RT, Battey JF. The Fifth Transmembrane Segment of the Neuromedin B Receptor Is Critical for High Affinity Neuromedin B Binding. J Biol Chem. 1993a;268(20):14622–14626. [PubMed] [Google Scholar]
- Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, Viallet J, Sausville EA, Battey JF. BRS-3: Novel Bombesin Receptor Subtype Selectively Expressed in Testis and Lung Carcinoma Cells. J Biol Chem. 1993b;268(8):5979–5984. [PubMed] [Google Scholar]
- Fathi Z, Way JW, Corjay MH, Viallet J, Sausville EA, Battey JF. Bombesin Receptor Structure and Expression in Human Lung Carcinoma Cell Lines. J Cell Biochem Suppl. 1996;24:237–246. doi: 10.1002/jcb.240630519. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Bagi EE, Toth K, Lenard L. Neuromedin C Microinjected into the Amygdala Inhibits Feeding. Brain Res Bull. 2007;71:386–392. doi: 10.1016/j.brainresbull.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Feldman RI, Wu JM, Jenson JC, Mann E. Purification and Characterization of the Bombesin/Gastrin-Releasing Peptide Receptor From Swiss 3T3 Cells. J Biol Chem. 1990;265:17364–17372. [PubMed] [Google Scholar]
- Ferguson SSG. Evolving Concepts in G Protein-Coupled Receptor Endocytosis: The Role in Receptor Desensitization and Signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Ferris HA, Carroll RE, Lorimer DL, Benya RV. Location and Characterization of the Human GRP Receptor Expressed by Gastrointestinal Epithelial Cells. Peptides. 1997;18:663–672. doi: 10.1016/s0196-9781(97)00127-7. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Laderach U, Friess H, Buechler MW, Reubi JC. Bombesin Receptors in Distinct Tissue Compartments of Human Pancreatic Diseases. Lab Invest. 2000;80:1807–1817. doi: 10.1038/labinvest.3780192. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Waser B, Gebbers JO, Reubi JC. Gastrin-Releasing Peptide Receptors in Normal and Neoplastic Human Uterus: Involvement of Multiple Tissue Compartments. J Clin Endocrinol Metab. 2005;90:4722–4729. doi: 10.1210/jc.2005-0964. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Waser B, Reubi JC. Overexpression of Gastrin-Releasing Peptide Receptors in Tumor-Associated Blood Vessels of Human Ovarian Neoplasms. Cell Oncol. 2007;29:421–433. doi: 10.1155/2007/798790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn FW. Bombesin Receptor Antagonists Block the Effects of Exogenous Bombesin but Not of Nutrients on Food Intake. Physiol Behav. 1997;62:791–798. doi: 10.1016/s0031-9384(97)00237-0. [DOI] [PubMed] [Google Scholar]
- Forrer F, Valkema R, Kwekkeboom DJ, de JM, Krenning EP. Neuroendocrine Tumors. Peptide Receptor Radionuclide Therapy. Best Pract Res Clin Endocrinol Metab. 2007;21:111–129. doi: 10.1016/j.beem.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Frank GK, Kaye WH, Ladenheim EE, McConaha C. Reduced Gastrin Releasing Peptide in Cerebrospinal Fluid After Recovery From Bulimia Nervosa. Appetite. 2001;37:9–14. doi: 10.1006/appe.2001.0407. [DOI] [PubMed] [Google Scholar]
- Frucht H, Gazdar AF, Park JA, Oie H, Jensen RT. Characterization of Functional Receptors for Gastrointestinal Hormones on Human Colon Cancer Cells. Cancer Res. 1992;52:1114–1122. [PubMed] [Google Scholar]
- Fujimura Y, Haruma K, Owen RL. Bombesin Prevents the Atrophy of Peyer's Patches and the Dysfunction of M Cells in Rabbits Receiving Long-Term Parenteral Nutrition. JPEN J Parenter Enteral Nutr. 2007;31:75–85. doi: 10.1177/014860710703100275. [DOI] [PubMed] [Google Scholar]
- Ganter MT, Pittet JF. Bombesin-Like Peptides: Modulators of Inflammation in Acute Lung Injury? Am J Respir Crit Care Med. 2006;173:1–2. doi: 10.1164/rccm.2510002. [DOI] [PubMed] [Google Scholar]
- Garcia LJ, Pradhan TK, Weber HC, Moody TW, Jensen RT. The Gastrin-Releasing Peptide Receptor Is Differentially Coupled to Adenylate Cyclase and Phospholipase C in Different Tissues. Biochim Biophys Acta. 1997;1356:343–354. doi: 10.1016/s0167-4889(97)00007-4. [DOI] [PubMed] [Google Scholar]
- Garrison JC, Rold TL, Sieckman GL, Figueroa SD, Volkert WA, Jurisson SS, Hoffman TJ. In Vivo Evaluation and Small-Animal PET/CT of a Prostate Cancer Mouse Model Using 64Cu Bombesin Analogs: Side-by-Side Comparison of the CB-TE2A and DOTA Chelation Systems. J Nucl Med. 2007;48:1327–1337. doi: 10.2967/jnumed.107.039487. [DOI] [PubMed] [Google Scholar]
- Ghatei MA, Jung RT, Stevenson JC, Hillyard CJ, Adrian TE, Lee YC, Christofides ND, Sarson DL, Mashiter K, MacIntyre I, Bloom SR. Bombesin Action on Gut Hormones and Calcium in Man. J Clin Endocrinol Metab. 1982;54:980–985. doi: 10.1210/jcem-54-5-980. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Fauser DJ, Rowe EA, Rolls BJ, Rolls ET, Maddison SP. Bombesin Suppresses Feeding in Rats. Nature. 1979;282:208–210. doi: 10.1038/282208a0. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Smith GP. The Actions of Bombesin-Like Peptides on Food Intake. Ann N Y Acad Sci. 1988;547:210–216. doi: 10.1111/j.1749-6632.1988.tb23889.x. [DOI] [PubMed] [Google Scholar]
- Gibril F, Jensen RT. Diagnostic Uses of Radiolabelled Somatostatin-Receptor Analogues in Gastroenteropancreatic Endocrine Tumors. Dig Liver Dis. 2004;36:S106–S120. doi: 10.1016/j.dld.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Gibril F, Reynolds JC, Doppman JL, Chen CC, Venzon DJ, Termanini B, Weber HC, Stewart CA, Jensen RT. Somatostatin Receptor Scintigraphy: Its Sensitivity Compared With That of Other Imaging Methods in Detecting Primary and Metastatic Gastrinomas: a Prospective Study. Ann Intern Med. 1996;125:26–34. doi: 10.7326/0003-4819-125-1-199607010-00005. [DOI] [PubMed] [Google Scholar]
- Giraud AS, Soll AH, Cuttitta F, Walsh JH. Bombesin Stimulation of Gastrin Release From Canine Gastrin Cells in Primary Culture. Am J Physiol. 1987;252:G413–G420. doi: 10.1152/ajpgi.1987.252.3.G413. [DOI] [PubMed] [Google Scholar]
- Glover S, Delaney M, Dematte C, Kornberg L, Frascp M, Tan-Son-Tay R, Benya RV. Phosphorylation of Focal Adhesion Kinase Tyrosine 397 Critically Mediates Gastrin-Releasing Peptide's Morphogenic Properties. J Cell Physiol. 2004;199:77–88. doi: 10.1002/jcp.10456. [DOI] [PubMed] [Google Scholar]
- Glover S, Nathaniel R, Shakir L, Perrault C, Anderson RK, Tran-Son-Tay R, Benya RV. Transient Upregulation of GRP and Its Receptor Critically Regulate Colon Cancer Cell Motility During Remodeling. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1274–G1282. doi: 10.1152/ajpgi.00108.2004. [DOI] [PubMed] [Google Scholar]
- Glover SC, Tretiakova MS, Carroll RE, Benya RV. Increased Frequency of Gastrin-Releasing Peptide Receptor Gene Mutations During Colon-Adenocarcinoma Progression. Mol Carcinog. 2003;37:5–15. doi: 10.1002/mc.10117. [DOI] [PubMed] [Google Scholar]
- Gorbulev V, Akhundova A, Buchner H, Fahrenholz F. Molecular Cloning of a New Bombesin Receptor Subtype Expressed in Uterus During Pregnancy. Eur J Biochem. 1992;208(2):405–410. doi: 10.1111/j.1432-1033.1992.tb17201.x. [DOI] [PubMed] [Google Scholar]
- Gorbulev V, Akhundova A, Grzeschik KH, Fahrenholz F. Organization and Chromosomal Localization of the Gene for the Human Bombesin Receptor Subtype Expressed in Pregnant Uterus. FEBS Lett. 1994;340(3):260–264. doi: 10.1016/0014-5793(94)80150-9. [DOI] [PubMed] [Google Scholar]
- Grady EF, Slice LW, Brant WO, Walsh JH, Payan DG, Bunnett NW. Direct Observation of Endocytosis of Gastrin Releasing Peptide and Its Receptor. J Biol Chem. 1995;270:4603–4611. doi: 10.1074/jbc.270.9.4603. [DOI] [PubMed] [Google Scholar]
- Greeley GH, Jr., Spannagel A, Trowbridge J, Thompson JC. Effect of Bombesin and Gastrin-Releasing Peptide on the Release of Gastric Inhibitory Polypeptide and Insulin in Rats. Proc Soc Exp Biol Med. 1986;182:540–542. doi: 10.3181/00379727-182-42377. [DOI] [PubMed] [Google Scholar]
- Gregersen S, Ahren B. Studies on the Mechanisms by Which Gastrin Releasing Peptide Potentiates Glucose-Induced Insulin Secretion From Mouse Islets. Pancreas. 1996;12:48–57. doi: 10.1097/00006676-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Grider JR. Gastrin-Releasing Peptide Is a Modulatory Neurotransmitter of the Descending Phase of the Peristaltic Reflex. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1109–G1115. doi: 10.1152/ajpgi.00080.2004. [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-Pdb Viewer: an Environment for Comparative Protein Modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Gugger M, Reubi JC. Gastrin-Releasing Peptide Receptors in Non-Neoplastic and Neoplastic Human Breast. Am J Pathol. 1999;155:2067–2076. doi: 10.1016/S0002-9440(10)65525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffar BM, Hocart SJ, Coy DH, Chiang HC, Mantey S, Jensen RT. Reduced Peptide Bond Pseudopeptide Analogues of Secretin: a New Class of Secretin Receptor Antagonists. J Biol Chem. 1991;266(1):316–322. [PubMed] [Google Scholar]
- Hamid QA, Corrin B, Dewar A, Hoefler H, Sheppard MN. Expression of Gastrin-Releasing Peptide (Human Bombesin) Gene in Large Cell Undifferentiated Carcinoma of the Lung. J Pathol. 1990;161:145–151. doi: 10.1002/path.1711610209. [DOI] [PubMed] [Google Scholar]
- Hampton LL, Ladenheim EE, Akeson M, Way JM, Weber HC, Sutliff VE, Jensen RT, Wine LJ, Arnheiter H, Battey JF. Loss of Bombesin-Induced Feeding Suppression in Gastrin-Releasing Peptide Receptor-Deficient Mice. Proc Natl Acad Sci USA. 1998;95:3188–3192. doi: 10.1073/pnas.95.6.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte MT, Hildebrand JD, Burnham MR, Bouton AH, Parsons JT. P130cas, a Substrate Associated With VSrc and V-Crk, Localizes to Focal Adhesions and Binds to Focal Adhesion Kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- Heimbrook DC, Boyer ME, Garsky VM, Balishin NL, Kiefer DM, Oliff A, Riemen MW. Minimal Ligand Analysis of Gastrin-Releasing Peptide Receptor. Receptor Binding and Mitogenesis. J Biol Chem. 1988;263:7016–7019. [PubMed] [Google Scholar]
- Heimbrook DC, Saari WS, Balishin NL, Fisher TW, Friedman A, Kiefer DM, Rotberg NS, Wallen JW, Oliff A. Gastrin Releasing Peptide Antagonists With Improved Potency and Stability. J Med Chem. 1991;34:2102–2107. doi: 10.1021/jm00111a027. [DOI] [PubMed] [Google Scholar]
- Heimbrook DC, Saari WS, Balishin NL, Friedman A, Moore KS, Reiman MW, Kiefer DM, Rotberg NS, Wallen JW, Oliff A. Carboxyl-Terminal Modification of a Gastrin Releasing Peptide Derivative Generates Potent Antagonists. J Biol Chem. 1989;264:11258–11262. [PubMed] [Google Scholar]
- Heinz-Erian P, Coy DH, Tamura M, Jones SW, Gardner JD, Jensen RT. [D-Phe12]Bombesin Analogues: a New Class of Bombesin Receptor Antagonists. Am J Physiol. 1987;252:G439–G442. doi: 10.1152/ajpgi.1987.252.3.G439. [DOI] [PubMed] [Google Scholar]
- Hermansen K, Ahren B. Gastrin Releasing Peptide Stimulates the Secretion of Insulin, but Not That of Glucagon or Somatostatin, From the Isolated Perfused Dog Pancreas. Acta Physiol Scand. 1990;138:175–179. doi: 10.1111/j.1748-1716.1990.tb08830.x. [DOI] [PubMed] [Google Scholar]
- Herold CL, Behm DJ, Buckley PT, Foley JJ, Wixted WE, Sarau HM, Douglas SA. The Neuromedin B Receptor Antagonist, BIM-23127, Is a Potent Antagonist at Human and Rat Urotensin-II Receptors. Br J Pharmacol. 2003;139:203–207. doi: 10.1038/sj.bjp.0705251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser M, Schlott T, Schally AV, Kahler E, Schliephake R, Laabs SO, Hemmerlein B. Expression of Gastrin Releasing Peptide Receptor in Renal Cell Carcinomas: a Potential Function for the Regulation of Neoangiogenesis and Microvascular Perfusion. J Urol. 2005;173:2154–2159. doi: 10.1097/01.ju.0000158135.26893.bc. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Kimura O, Furukawa T, Kinoshita H, Chujo S, Iwai N. FK506 Induces the Atrophy of Enteric Ganglia in Small Bowel Transplantation, Which Can Be Prevented by the Neuropeptide Bombesin. Transplant Proc. 2006;38:1823–1824. doi: 10.1016/j.transproceed.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Hildebrand P, Lehmann FS, Ketterer S, Christ AD, Stingelin T, Beltinger J, Gibbons AH, Coy DH, Calam J, Larsen F, Beglinger C. Regulation of Gastric Function by Endogenous Gastrin Releasing Peptide in Humans: Studies With a Specific Gastrin Releasing Peptide Receptor Antagonist. Gut. 2001;49:23–28. doi: 10.1136/gut.49.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DJ, McDonald TJ. Mitogenic Action of Gastrin-Releasing Polypeptide on Isolated Epiphyeal Growth Plate Chondrocytes From the Ovine Fetus. Endocrinology. 1992;130:2811–2819. doi: 10.1210/endo.130.5.1572294. [DOI] [PubMed] [Google Scholar]
- Hoggard N, Bashir S, Cruickshank M, Miller JD, Speakman JR. Expression of Neuromedin B in Adipose Tissue and Its Regulation by Changes in Energy Balance. J Mol Endocrinol. 2007;39:199–210. doi: 10.1677/JME-07-0071. [DOI] [PubMed] [Google Scholar]
- Horwell DC. The 'Peptoid' Approach to the Design on Non-Peptide, Small Molecule Agonists and Antagonists of Neuropeptides. Trends Biotechnol. 1995;13:132–134. doi: 10.1016/s0167-7799(00)88923-4. [DOI] [PubMed] [Google Scholar]
- Horwell DC, Howson W, Rees DC. Peptoid Design. Drug Des Discov. 1994;12:63–75. [PubMed] [Google Scholar]
- Hotta K, Matsukawa Y, Nishida M, Kotani K, Takahashi M, Kuriyama H, Nakamura T, Wada K, Yamashita S, Funahashi T, Matsuzawa Y. Mutation in Bombesin Receptor Subtype-3 Gene Is Not a Major Cause of Obesity in the Japanese. Horm Metab Res. 2000;32:33–34. doi: 10.1055/s-2007-978582. [DOI] [PubMed] [Google Scholar]
- Hou W, Tsuda T, Jensen RT. Neuromedin B Activates Phospholipase D Through Both PKC-Dependent and PKC-Independent Mechanisms. Biochem Biophys Acta (Lipids and Lipid Metab ) 1998;1391:337–350. doi: 10.1016/s0005-2760(98)00014-9. [DOI] [PubMed] [Google Scholar]
- Hou X, Wei L, Harada A, Tatamoto K. Activation of Bombesin Receptor Subtype-3 Stimulates Adhesion of Lung Cancer Cells. Lung Cancer. 2006;54:143–148. doi: 10.1016/j.lungcan.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Houben H, Denef C. Effect of the Bombesin Receptor Blockers [Leu13, Psi CH2NH-Leu14]Bombesin and N-Pivaloyl GRP(20−25) Alkylamide (L 686,095−001C002) on Basal and Neuromedin C-Stimulated PRL and GH Release in Pituitary Cell Aggregates. Peptides. 1991;12:371–374. doi: 10.1016/0196-9781(91)90028-n. [DOI] [PubMed] [Google Scholar]
- Huang SC, Yu DH, Wank SA, Gardner JD, Jensen RT. Characterization of Bombesin Receptors on Mouse Pancreatic Acini by Chemical Cross-Linking. Peptides. 1990;11(6):1143–1150. doi: 10.1016/0196-9781(90)90144-t. [DOI] [PubMed] [Google Scholar]
- Iwabuchi M, Ui-Tei K, Yamada K, Matsuda Y, Sakai Y, Tanaka K, Ohki-Hamazaki H. Molecular Cloning and Characterization of Avian Bombesin-Like Peptide Receptors: New Tools for Investigating Molecular Basis for Ligand Selectivity. Br J Pharmacol. 2003;139:555–566. doi: 10.1038/sj.bjp.0705282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarpe MB, Knail C, Mitchell FM, Buhl AM, Duzic E, Johnson GL. [D-Argl, D-Phe5,Trp7,9,Leu11) Substance P Acts As a Biased Agonist Toward Neuropeptide and Chemokine Receptors. J Biol Chem. 1998;273:3097–3104. doi: 10.1074/jbc.273.5.3097. [DOI] [PubMed] [Google Scholar]
- Jennings CA, Harrison DC, Maycox PR, Crook B, Smart D, Hervieu GJ. The Distribution of the Orphan Bombesin Receptor Subtype-3 in the Rat CNS. Neuroscience. 2003;120:309–324. doi: 10.1016/s0306-4522(03)00260-4. [DOI] [PubMed] [Google Scholar]
- Jensen JA, Carroll RE, Benya RV. The Case for Gastrin-Releasing Peptide Acting As a Morphogen When It and Its Receptor Are Aberrantly Expressed in Cancer. Peptides. 2001;22:689–699. doi: 10.1016/s0196-9781(01)00380-1. [DOI] [PubMed] [Google Scholar]
- Jensen RT. Gastrin-releasing peptide. In: Johnson LJ, editor. Encyclopedia of Gastroenterology. Academic Press Elsevier Science Publishing Co.; San Diego: 2003. pp. 179–185. [Google Scholar]
- Jensen RT. Receptors on pancreatic acinar cells. In: Johnson LR, Jacobson ED, Christensen J, Alpers DH, Walsh JH, editors. Physiology of the Gastrointestinal Tract, Third Edition. Raven Press; New York: 1994. pp. 1377–1446. [Google Scholar]
- Jensen RT, Coy DH. Progress in the Development of Potent Bombesin Receptor Antagonists. Trends Pharmacol Sci. 1991;12(1):13–19. doi: 10.1016/0165-6147(91)90483-9. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Coy DH, Saeed ZA, Heinz-Erian P, Mantey S, Gardner JD. Interaction of Bombesin and Related Peptides With Receptors on Pancreatic Acini. Ann N Y Acad Sci. 1988a;547:138–149. doi: 10.1111/j.1749-6632.1988.tb23882.x. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Gardner JD. Identification and Characterization of Receptors for Secretagogues on Pancreatic Acinar Cells. Fed Proc. 1981;40:2486–2496. [PubMed] [Google Scholar]
- Jensen RT, Heinz-Erian P, Mantey S, Jones SW, Gardner JD. Characterization of the Ability of Various Substance P Antagonists to Inhibit Action of Bombesin. Am J Physiol. 1988b;254:G883–G890. doi: 10.1152/ajpgi.1988.254.6.G883. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Jones SW, Folkers K, Gardner JD. A Synthetic Peptide That Is a Bombesin Receptor Antagonist. Nature. 1984;309:61–63. doi: 10.1038/309061a0. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Moody T, Pert C, Rivier JE, Gardner JD. Interaction of Bombesin and Litorin With Specific Membrane Receptors on Pancreatic Acinar Cells. Proc Natl Acad Sci (USA) 1978;75:6139–6143. doi: 10.1073/pnas.75.12.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RT, Moody TW. Bombesin-related peptides and neurotensin: effects on cancer growth/proliferation and cellular signaling in cancer. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Elsevier; Amsterdam: 2006. pp. 429–434. [Google Scholar]
- Jensen RT, Mrozinski JE, Jr., Coy DH. Bombesin Receptor Antagonists: Different Classes and Cellular Basis of Action. Recent Results Cancer Res. 1993;129:87–113. doi: 10.1007/978-3-642-84956-5_8. [DOI] [PubMed] [Google Scholar]
- Jian X, Sainz E, Clark WA, Jensen RT, Battey JF, Northrup JK. The Bombesin Receptor Subtypes Have Distinct G Protein Specificities. J Biol Chem. 1999;274:11573–11581. doi: 10.1074/jbc.274.17.11573. [DOI] [PubMed] [Google Scholar]
- Johnson CV, Shelton T, Smith CJ, Ma L, Perry MC, Volkert WA, Hoffman TJ. Evaluation of Combined (177)Lu-DOTA-8-AOC-BBN (7−14)NH(2) GRP Receptor-Targeted Radiotherapy and Chemotherapy in PC-3 Human Prostate Tumor Cell Xenografted SCID Mice. Cancer Biother Radiopharm. 2006;21:155–166. doi: 10.1089/cbr.2006.21.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PM, Withers DJ, Ghatei MA, Bloom SR. Evidence for Neuromedin-B Synthesis in the Rat Anterior Pituitary Gland. Endocrinology. 1992;130:1829–1836. doi: 10.1210/endo.130.4.1547712. [DOI] [PubMed] [Google Scholar]
- Jungwirth A, Schally AV, Halmos G, Groot K, Szepeshazi K, Pinski J, Armatis P. Inhibition of the Growth of Caki-I Human Renal Adenocarcinoma in Vivo by Luteinizing Hormone-Releasing Hormone Antagonist Cetrorelix, Somatostatin Analog RC-160, and Bombesin Antagonist RC-3940-II. Cancer. 1998;82:909–917. doi: 10.1002/(sici)1097-0142(19980301)82:5<909::aid-cncr16>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kallingal GJ, Mintz EM. Gastrin Releasing Peptide and Neuropeptide Y Exert Opposing Actions on Circadian Phase. Neurosci Lett. 2007;422:59–63. doi: 10.1016/j.neulet.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamichi S, Wada E, Aoki S, Sekiguchi M, Kimura I, Wada K. Immunohistochemical Localization of Gastrin-Releasing Peptide Receptor in the Mouse Brain. Brain Res. 2005;1032:162–170. doi: 10.1016/j.brainres.2004.10.068. [DOI] [PubMed] [Google Scholar]
- Kanashiro CA, Schally AV, Cai RZ, Halmos G. Antagonists of Bombesin/Gastrin-Releasing Peptide Decrease the Expression of Angiogenic and Anti-Apoptotic Factors in Human Glioblastoma. Anti-Cancer Drugs. 2005;16:159–165. doi: 10.1097/00001813-200502000-00007. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Romeo RD, McEwen BS, Silver R. Diurnal Regulation of the Gastrin-Releasing Peptide Receptor in the Mouse Circadian Clock. Eur J Neurosci. 2006;23:1047–1053. doi: 10.1111/j.1460-9568.2006.04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S, Sundler F, Ahren B. Insulin Secretion by Gastrin-Releasing Peptide in Mice: Ganglionic Versus Direct Islet Effect. Am J Physiol. 1998;274:E124–E129. doi: 10.1152/ajpendo.1998.274.1.E124. [DOI] [PubMed] [Google Scholar]
- Katsuno T, Pradhan TK, Ryan RR, Mantey SA, Hou W, Donohue PJ, Akeson MA, Spindel ER, Battey JF, Coy DH, Jensen RT. Pharmacology and Cell Biology of the Bombesin Receptor Subtype 4 (BB4-R). Biochemistry. 1999;38:7307–7320. doi: 10.1021/bi990204w. [DOI] [PubMed] [Google Scholar]
- Kawai K, Chiba Y, Mukai H, Munekata E, Yamashita K. Effects of Neuromedin B, Gastrin-Releasing Peptide-10 and Their Fragment Peptides on Secretion of Gastrointestinal and Pancreatic Hormones in Dogs. Acta Endocrinol (Copenh) 1988;117:205–213. doi: 10.1530/acta.0.1170205. [DOI] [PubMed] [Google Scholar]
- Kelley MJ, Linnoila RI, Avis IL, Georgiadis MS, Cuttitta F, Mulshine JL, Johnson BE. Antitumor Activity of a Monoclonal Antibody Directed Against Gastrin-Releasing Peptide in Patients With Small Cell Lung Cancer. Chest. 1997;112:256–261. doi: 10.1378/chest.112.1.256. [DOI] [PubMed] [Google Scholar]
- Kilgore WR, Mantyh PW, Mantyh CR, McVey DC, Vigna SR. Bombesin/GRP-Preferring and Neuromedin B-Preferring Receptors in the Rat Urogenital System. Neuropeptides. 1993;24:43–52. doi: 10.1016/0143-4179(93)90039-d. [DOI] [PubMed] [Google Scholar]
- Kim CM, Dion SB, Benovic JL. Mechanism of Beta-Adrenergic Receptor Kinase Activation by G Proteins. J Biol Chem. 1993;268:15412–15418. [PubMed] [Google Scholar]
- Kim S, Kelly DR, Hellmich MR, Evers BM, Chung DH. Gastrin-Releasing Peptide Is a Growth Factor for Human Neuroblastomas. Ann Surg. 2002;235:621–629. doi: 10.1097/00000658-200205000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura O, Higuchi K, Furukawa T, Kinoshita H, Chujo S, Go S, Iwai N. Neuroendocrine-Immune Modulation May Be Useful for Allograft-Specific Immunosuppression in Small Bowel Transplantation. Transplant Proc. 2006a;38:1825–1826. doi: 10.1016/j.transproceed.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Kimura O, Kinoshita H, Furukawa T, Higuchi K, Chujo S, Iwai N. Prevention of Warm Ischemic Injury by Neuropeptide Bombesin in Small Bowel Transplantation. Transplant Proc. 2006b;38:1794–1795. doi: 10.1016/j.transproceed.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Kimura O, Furukawa T, Higuchi K, Chujo S, Iwai N. Preoperative Bombesin Administration Can Protect the Rat Small Bowel Allograft From Ischemic Reperfusion Injury. J Pediatr Surg. 2005;40:1877–1880. doi: 10.1016/j.jpedsurg.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Walsh CA, Gibbs J, Smith GP, Leban J, McDermed J. A Novel Bombesin Receptor Antagonist Selectively Blocks the Satiety Action of Peripherally Administered Bombesin. Pharmacol Biochem Behav. 1994;48:809–811. doi: 10.1016/0091-3057(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Klein WL, Nathanson N, Nirenberg M. Muscarinic Acetylcholine Receptor Regulation by Accelerated Rate of Receptor Loss. Biochem Biophys Res Commun. 1979;90:506–512. doi: 10.1016/0006-291x(79)91264-6. [DOI] [PubMed] [Google Scholar]
- Knuhtsen S, Holst JJ, Schwartz TW, Jensen SL, Nielsen OV. The Effect of Gastrin-Releasing Peptide on the Endocrine Pancreas. Regul Pept. 1987;17:269–276. doi: 10.1016/0167-0115(87)90284-9. [DOI] [PubMed] [Google Scholar]
- Koh SW, Leyton J, Moody TW. Bombesin Activates MAP Kinase in Non-Small Cell Lung Cancer Cells. Peptides. 1999;20:121–126. doi: 10.1016/s0196-9781(98)00144-2. [DOI] [PubMed] [Google Scholar]
- Kris RM, Hazan R, Villines J, Moody TW, Schlessinger J. Identification of the Bombesin Receptor on Murine and Human Cells by Cross-Linking Experiments. J Biol Chem. 1987;262:11215–11220. [PubMed] [Google Scholar]
- Kroog GS, Jensen RT, Battey JF. Mammalian Bombesin Receptors. Med Res Rev. 1995a;15(5):389–417. doi: 10.1002/med.2610150502. [DOI] [PubMed] [Google Scholar]
- Kroog GS, Jian X, Chen L, Northup JK, Battey JF. Phosphorylation Uncouples the Gastrin-Releasing Peptide Receptor From Gq. J Biol Chem. 1999;274:36700–36706. doi: 10.1074/jbc.274.51.36700. [DOI] [PubMed] [Google Scholar]
- Kroog GS, Sainz E, Worland PJ, Akeson MA, Benya RV, Jensen RT, Battey JF. The Gastrin Releasing Peptide Receptor Is Rapidly Phosphorylated by a Kinase Other Than Protein Kinase C After Exposure to Agonist. J Biol Chem. 1995b;270:8217–8224. doi: 10.1074/jbc.270.14.8217. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The Role of Receptor Kinases and Arrestins in G Protein-Coupled Receptor Regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Kull FC, Jr., Leban JJ, Landavazo A, Stewart KD, Stockstill B, McDermed JD. Conveyance of Partial Agonism/Antagonism to Bombesin/Gastrin- Releasing Peptide Analogues on Swiss 3T3 Cells by a Carboxyl-Terminal Leucine Insertion. J Biol Chem. 1992;267:21132–21138. [PubMed] [Google Scholar]
- Kusui T, Benya RV, Battey JF, Jensen RT. Glycosylation of Bombesin Receptors: Characterization, Effect on Binding and G-Protein Coupling. Biochemistry. 1994;33:12968–12980. doi: 10.1021/bi00248a005. [DOI] [PubMed] [Google Scholar]
- Kusui T, Hellmich MR, Wang LH, Evans RL, Benya RV, Battey JF, Jensen RT. Characterization of Gastrin-Releasing Peptide Receptor Expressed in Sf9 Insect Cells by Baculovirus. Biochemistry. 1995;34:8061–8075. doi: 10.1021/bi00025a012. [DOI] [PubMed] [Google Scholar]
- Lach EB, Broad S, Rozengurt E. Mitogenic Signaling by Transfected Neuromedin B Receptors in Rat-1 Cells. Cell Growth Differ. 1995;6(11):1427–1435. [PubMed] [Google Scholar]
- Ladenheim EE, Hampton LL, Whitney AC, White WO, Battey JF, Moran TH. Disruptions in Feeding and Body Weight Control in Gastrin-Releasing Peptide Receptor Deficient Mice. J Endocrinol. 2002;174:273–281. doi: 10.1677/joe.0.1740273. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Jensen RT, Mantey SA, McHugh PR, Moran TH. Distinct Distributions of Bombesin Receptor Subtypes in the Rat Central Nervous System. Brain Res. 1992;593:168–178. doi: 10.1016/0006-8993(92)91305-x. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Jensen RT, Mantey SA, McHugh PR, Moran TH. Receptor Heterogeneity for Bombesin-Like Peptides in the Rat Central Nervous System. Brain Res. 1990;537:233–240. doi: 10.1016/0006-8993(90)90363-g. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Jensen RT, Mantey SA, Taylor JE, Coy DH, Moran TH. Bombesin Receptor Antagonists Differentiate Receptor Subtypes in Rat Brain. Eur J Pharmacol. 1993a;235(1):121–125. doi: 10.1016/0014-2999(93)90830-b. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Jensen RT, Moran TM. Receptors for Bombesin-Like Peptides in the Rat Central Nervous System. Methods Neurosci. 1993b;11:283–293. [Google Scholar]
- Ladenheim EE, Knipp S. Capsaicin Treatment Differentially Affects Feeding Suppression by Bombesin-Like Peptides. Physiol Behav. 2007;91:36–41. doi: 10.1016/j.physbeh.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim EE, Moore KA, Salorio CF, Mantey SA, Taylor JE, Coy DH, Jensen RT, Moran TH. Characterization of Bombesin Binding Sites in Rat Stomach. Europ J Pharmacol. 1997a;319:245–251. doi: 10.1016/s0014-2999(96)00854-0. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Taylor JE, Coy DH, Carrigan TS, Wohn A, Moran TH. Caudal Hindbrain Neuromedin B-Preferring Receptors Participate in the Control of Food Intake. Am J Physiol. 1997b;272:R433–R437. doi: 10.1152/ajpregu.1997.272.1.R433. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Taylor JE, Coy DH, Moore KA, Moran TH. Hindbrain GRP Receptor Blockade Antagonizes Feeding Suppression by Peripherally Administered GRP. Am J Physiol. 1996a;271:R180–R184. doi: 10.1152/ajpregu.1996.271.1.R180. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Taylor JE, Coy DH, Moran TH. Blockade of Feeding Inhibition by Neuromedin B Using a Selective Receptor Antagonist. Eur J Pharmacol. 1994;271:R7–R9. doi: 10.1016/0014-2999(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Wirth KE, Moran TH. Receptor Subtype Mediation of Feeding Suppression by Bombesin-Like Peptides. Pharmacol Biochem Behav. 1996b;54:705–711. doi: 10.1016/0091-3057(96)00023-8. [DOI] [PubMed] [Google Scholar]
- Lamharzi N, Schally AV, Koppan M, Groot K. Growth Hormone-Releasing Hormone Antagonist MZ-5−156 Inhibits Growth of DU-145 Human Androgen-Independent Prostate Carcinoma in Nude Mice and Suppresses the Levels and MRNA Expression of Insulin-Like Growth Factor II in Tumors. Proc Natl Acad Sci (USA) 1998;95:8864–8868. doi: 10.1073/pnas.95.15.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon S, Sethi T, Ritchie A, Muir M, Smyth J, Rozengurt E. Broad Spectrum Neuropeptide Antagonists Inhibit the Growth of Small Cell Lung Cancer in Vivo. Cancer Res. 1992;52:4554–4557. [PubMed] [Google Scholar]
- Lango MN, Dyer KF, Lui VW, Gooding WE, Gubish C, Siegfried JM, Grandis JR. Gastrin-Releasing Peptide Receptor-Mediated Autocrine Growth in Squamous Cell Carcinoma of the Head and Neck. J Natl Cancer Inst. 2002;94:375–383. doi: 10.1093/jnci/94.5.375. [DOI] [PubMed] [Google Scholar]
- Lantry LE, Cappelletti E, Maddalena ME, Fox JS, Feng W, Chen J, Thomas R, Eaton SM, Bogdan NJ, Arunachalam T, Reubi JC, Raju N, Metcalfe EC, Lattuada L, Linder KE, Swenson RE, Tweedle MF, Nunn AD. 177Lu-AMBA: Synthesis and Characterization of a Selective 177Lu-Labeled GRP-R Agonist for Systemic Radiotherapy of Prostate Cancer. J Nucl Med. 2006;47:1144–1152. [PubMed] [Google Scholar]
- Lebacq-Verheyden AM, Trepel J, Sausville EA, Battey JF. Peptide Growth Factors and Their Receptors. Handb Exp Pharmacol. 1990;95:71–124. [Google Scholar]
- Leban JJ, Kull FC, Jr., Landavazo A, Stockstill B, McDermed JD. Development of Potent Gastrin-Releasing Peptide Antagonists Having a D-Pro-Psi (CH2NH)-Phe-NH2 C Terminus. Proc Natl Acad Sci USA. 1993;90:1922–1926. doi: 10.1073/pnas.90.5.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, Jensen RT, Gardner JD. Bombesin-Induced Desensitization of Enzyme Secretion in Dispersed Acini From the Guinea Pig Pancreas. Am J Physiol. 1980;238:G213–G218. doi: 10.1152/ajpgi.1980.238.3.G213. [DOI] [PubMed] [Google Scholar]
- Levine L, Lucci JA3, Pazdrak B, Cheng JZ, Guo YS, Townsend CM, Jr., Hellmich HL. Bombesin Stimulates Nuclear Factor Kappa B Activation and Expression of Proangiogenic Factors in Prostate Cancer Cells. Cancer Res. 2003;63:3495–3502. [PubMed] [Google Scholar]
- Li K, Nagalla SR, Spindel ER. A Rhesus Monkey Model to Characterize the Role of Gastrin-Releasing Peptide (GRP) in Lung Development. Evidence for Stimulation of Airway Growth. J Clin Invest. 1994;94:1605–1615. doi: 10.1172/JCI117502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JT, Coy DH, Mantey SA, Jensen RT. Comparison of the Peptide Structural Requirements for High Affinity Interaction With Bombesin Receptors. Eur J Pharmacol. 1996;294:55–69. doi: 10.1016/0014-2999(95)00510-2. [DOI] [PubMed] [Google Scholar]
- Lin JT, Coy DH, Mantey SA, Jensen RT. Peptide Structural Requirements for Antagonism Differ Between the Two Mammalian Bombesin Receptor Subtypes. J Pharmacol Exp Ther. 1995;275:285–295. [PubMed] [Google Scholar]
- Lin Y, Jian X, Lin Z, Kroog GS, Mantey S, Jensen RT, Battey J, Northup J. Two Amino Acids in the Sixth Transmembrane Segment of the Mouse Gastrin-Releasing Peptide Receptor Are Important for Receptor Activation. JPET. 2000;294:1053–1062. [PubMed] [Google Scholar]
- Liu J, Lao ZJ, Zhang J, Schaeffer MT, Jiang MM, Guan XM, Van der Ploeg LH, Fong TM. Molecular Basis of the Pharmacological Difference Between Rat and Human Bombesin Receptor Subtype-3 (BRS-3). Biochemistry. 2002;41:8954–8960. doi: 10.1021/bi0202777. [DOI] [PubMed] [Google Scholar]
- Liu X, Carlisle DL, Swick MC, Gaither-Davis A, Grandis JR, Siegfried JM. Gastrin-Releasing Peptide Activates Akt Through the Epidermal Growth Factor Receptor Pathway and Abrogates the Effect of Gefitinib. Exp Cell Res. 2007;313:1361–1372. doi: 10.1016/j.yexcr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Llinares M, Devin C, Chaloin O, Azay J, Noel-Artis AM, Bernad N, Fehrentz JA, Martinez J. Syntheses and Biological Activities of Potent Bombesin Receptor Antagonists. J Pept Res. 1999;53:275–283. doi: 10.1034/j.1399-3011.1999.00028.x. [DOI] [PubMed] [Google Scholar]
- Luft T, Flores DG, Vianna MR, Schwartsmann G, Roesler R, Izquierdo I. A Role for Hippocampal Gastrin-Releasing Peptide Receptors in Extinction of Aversive Memory. Neuroreport. 2006;17:935–939. doi: 10.1097/01.wnr.0000221832.33717.48. [DOI] [PubMed] [Google Scholar]
- Lui VW, Thomas SM, Wentzel AM, Siegfried JM, Li JY, Grandis JR. Mitogenic Effects of Gastrin-Releasing Peptide in Head and Neck Squamous Cancer Cells Are Mediated by Activation of the Epidermal Growth Factor Receptor. Oncogene. 2003;22:6183–6193. doi: 10.1038/sj.onc.1206720. [DOI] [PubMed] [Google Scholar]
- MacKinnon AC, Waters C, Jodrell D, Haslett C, Sethi T. Bombesin and Substance P Analogues Differentially Regulate G-Protein Coupling to the Bombesin Receptor. Direct Evidence for Biased Agonism. J Biol Chem. 2001;276:28083–28091. doi: 10.1074/jbc.M009772200. [DOI] [PubMed] [Google Scholar]
- Madarame J, Higashiyama S, Kiyota H, Madachi A, Toki F, Shimomura T, Tani N, Oishi Y, Matsuura N. Transactivation of Epidermal Growth Factor Receptor After Heparin-Binding Epidermal Growth Factor-Like Growth Factor Shedding in the Migration of Prostate Cancer Cells Promoted by Bombesin. Prostate. 2003;57:187–195. doi: 10.1002/pros.10295. [DOI] [PubMed] [Google Scholar]
- Maekawa F, Quah HM, Tanaka K, Ohki-Hamazaki H. Leptin Resistance and Enhancement of Feeding Facilitation by Melanin-Concentrating Hormone in Mice Lacking Bombesin Receptor Subtype-3. Diabetes. 2004;53:570–576. doi: 10.2337/diabetes.53.3.570. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Coy DH, Giuliani S. Effect of [D-Phe6] Bombesin (6−13) Methylester, a Bombesin Receptor Antagonist, Towards Bombesin-Induced Contractions in the Guinea-Pig and Rat Isolated Urinary Bladder. J Auton Pharmacol. 1992;12:215–222. doi: 10.1111/j.1474-8673.1992.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Makarenkova VP, Shurin GV, Tourkova IL, Balkir L, Pirtskhalaishvili G, Perez L, Gerein V, Siegfried JM, Shurin MR. Lung Cancer-Derived Bombesin-Like Peptides Down-Regulate the Generation and Function of Human Dendritic Cells. J Neuroimmunol. 2003;145:55–67. doi: 10.1016/j.jneuroim.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Malendowicz LK, Macchi C, Nussdorfer GG, Nowak M. The Role of Neuromedin B in the Regulation of Rat Pituitary-Adrenocortical Function. Histol Histopathol. 1996;11:895–897. [PubMed] [Google Scholar]
- Mann DJ, Higgins T, Jones NC, Rozengurt E. Differential Control of Cyclins D1 and D3 and the Cdk Inhibitor P27Kip1 by Diverse Signalling Pathways in Swiss 3T3 Cells. Oncogene. 1997;14:1759–1766. doi: 10.1038/sj.onc.1201134. [DOI] [PubMed] [Google Scholar]
- Mantey S, Frucht H, Coy DH, Jensen RT. Characterization of Bombesin Receptors Using a Novel, Potent, Radiolabeled Antagonist That Distinguishes Bombesin Receptor Subtypes. Mol Pharmacol. 1993;45:762–774. [PubMed] [Google Scholar]
- Mantey SA, Coy DH, Entsuah LK, Jensen RT. Development of Bombesin Analogs With Conformationally Restricted Amino Acid Substitutions With Enhanced Selectivity for the Orphan Receptor Human Bombesin Receptor Subtype 3. J Pharmacol Exp Ther. 2004;310:1161–1170. doi: 10.1124/jpet.104.066761. [DOI] [PubMed] [Google Scholar]
- Mantey SA, Coy DH, Pradhan TK, Igarashi H, Rizo IM, Shen L, Hou W, Hocart SJ, Jensen RT. Rational Design of a Peptide Agonist That Interacts Selectively With the Orphan Receptor, Bombesin Receptor Subtype 3. J Biol Chem. 2001;276:9219–9229. doi: 10.1074/jbc.M008737200. [DOI] [PubMed] [Google Scholar]
- Mantey SA, Gonzalez N, Schumann M, Pradhan TK, Shen L, Coy DH, Jensen RT. Identification of Bombesin Receptor Subtype-Specific Ligands: Effect of N-Methyl Scanning, Truncation, Substitution, and Evaluation of Putative Reported Selective Ligands. J Pharmacol Exp Ther. 2006;319:980–989. doi: 10.1124/jpet.106.107011. [DOI] [PubMed] [Google Scholar]
- Mantey SA, Weber HC, Sainz E, Akeson M, Ryan RR, Pradhan TK, Searles RP, Spindel ER, Battey JF, Coy DH, Jensen RT. Discovery of a High Affinity Radioligand for the Human Orphan Receptor, Bombesin Receptor Subtype 3, Which Demonstrates It Has a Unique Pharmacology Compared to Other Mammalian Bombesin Receptors. J Biol Chem. 1997;272(41):26062–26071. doi: 10.1074/jbc.272.41.26062. [DOI] [PubMed] [Google Scholar]
- Marki W, Brown M, Rivier JE. Bombesin Analogs: Effects on Thermoregulation and Glucose Metabolism. Peptides. 1981;2:169–177. doi: 10.1016/0196-9781(81)90027-9. [DOI] [PubMed] [Google Scholar]
- Markwalder R, Reubi J-C. Gastrin-Releasing Peptide Receptors in the Human Prostate: Relation to Neoplastic Transformation. Cancer Res. 1999;59:1152–1159. [PubMed] [Google Scholar]
- Martinez A. A New Family of Angiogenic Factors. Cancer Lett. 2005 doi: 10.1016/j.canlet.2005.04.008. (in press) [DOI] [PubMed] [Google Scholar]
- Martinez J, Bali JP, Rodriquez M, Castro B, Magous R, Laur J, Ligon MF. Synthesis and Biologic Activity of Some Pseudo-Peptide Analogues of Tetragastrin: the Importance of the Peptide Backbone. J Med Chem. 1985;28:1874–1879. doi: 10.1021/jm00150a020. [DOI] [PubMed] [Google Scholar]
- Martinez V, Tache Y. Bombesin and the Brain-Gut Axis. Peptides. 2000;21:1617–1625. doi: 10.1016/s0196-9781(00)00293-x. [DOI] [PubMed] [Google Scholar]
- Maslen GL, Boyd Y. Comparative Mapping of the Grpr Locus on the X Chromosomes of Man and Mouse. Genomics. 1993;17:106–109. doi: 10.1006/geno.1993.1290. [DOI] [PubMed] [Google Scholar]
- Matozaki T, Zhu WY, Tsunoda Y, Goke B, Williams JA. Intracellular Mediators of Bombesin Action on Rat Pancreatic Acinar Cells. Am J Physiol. 1991;260:G858–G864. doi: 10.1152/ajpgi.1991.260.6.G858. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Yamada K, Wada E, Hasegawa T, Usui Y, Wada K. Bombesin Receptor Subtype-3 Modulates Plasma Insulin Concentration. Peptides. 2003;24:83–90. doi: 10.1016/s0196-9781(02)00279-6. [DOI] [PubMed] [Google Scholar]
- Matusiak D, Glover S, Nathaniel R, Matkowskyj K, Yang J, Benya RV. Neuromedin B and Its Receptor Are Mitogens in Both Normal and Malignant Epithelial Cells Lining the Colon. Am J Physiol Gastrointest Liver Physiol. 2005;288:G718–G728. doi: 10.1152/ajpgi.00156.2004. [DOI] [PubMed] [Google Scholar]
- Maughfling EJ, Boden P, Hall MD. Construction of Chimeric Human Bombesin Receptors to Identify Neuromedin B and Gastrin-Releasing Peptide Receptor Binding Sites. Biochem Soc Trans. 1997;25:455S. doi: 10.1042/bst025455s. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Erspamer GF, Piccinelli D. Relative Potencies of Porcine Bombesin-Like Heptocosapeptide (PB-27), Amphibian Bombesin (B-14) and Litorin, and Bombesin C-Terminal Nonapeptide (B-9) on in Vitro and in Vivo Smooth Muscle Preparations. J Pharm Pharmacol. 1982;34:120–121. doi: 10.1111/j.2042-7158.1982.tb04200.x. [DOI] [PubMed] [Google Scholar]
- McDonald TJ, Ghatei MA, Bloom SR, Adrian TE, Mochizuki T, Yanaihara C, Yanaihara N. Dose-Response Comparisons of Canine Plasma Gastroenteropancreatic Hormone Responses to Bombesin and the Porcine Gastrin-Releasing Peptide (GRP). Regul Pept. 1983;5:125–137. doi: 10.1016/0167-0115(83)90120-9. [DOI] [PubMed] [Google Scholar]
- McDonald TJ, Jornvall H, Nilsson G, Vagne M, Ghatei M, Bloom SR, Mutt V. Characterization of a Gastrin-Releasing Peptide From Porcine Non- Antral Gastric Tissue. Biochem Biophys Res Commun. 1979;90:227–233. doi: 10.1016/0006-291x(79)91614-0. [DOI] [PubMed] [Google Scholar]
- Merali Z, Bedard T, Andrews N, Davis B, McKnight AT, Gonzalez MI, Pritchard M, Kent P, Anisman H. Bombesin Receptors As a Novel Anti-Anxiety Therapeutic Target: BB1 Receptor Actions on Anxiety Through Alterations of Serotonin Activity. J Neurosci. 2006;26:10387–10396. doi: 10.1523/JNEUROSCI.1219-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Kent P, Anisman H. Role of Bombesin-Related Peptides in the Mediation or Integration of the Stress Response. Cell Mol Life Sci. 2002;59:272–287. doi: 10.1007/s00018-002-8422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Anisman H. Role of Bombesin-Related Peptides in the Control of Food Intake. Neuropeptides. 1999;33:376–386. doi: 10.1054/npep.1999.0054. [DOI] [PubMed] [Google Scholar]
- Metz DC, Patto RJ, Mrozinski JE, Jr., Jensen RT, Turner RJ, Gardner JD. Thapsigargin Defines the Roles of Cellular Calcium in Secretagogue-Stimulated Enzyme Secretion From Pancreatic Acini. J Biol Chem. 1992;267:20620–20629. [PubMed] [Google Scholar]
- Mihara S, Hara M, Nakamura M, Sakurawi K, Tokura K, Fujimoto M, Fukai T, Nomura T. Non-Peptide Bombesin Receptor Antagonists, Kuwanon G and H, Isolated From Mulberry. Biochem Biophys Res Commun. 1995;213:594–599. doi: 10.1006/bbrc.1995.2173. [DOI] [PubMed] [Google Scholar]
- Millar JB, Rozengurt E. Bombesin Enhancement of CAMP Accumulation in Swiss 3T3 Cells: Evidence of a Dual Mechanism of Action. J Cell Physiol. 1988;137(2):214–222. doi: 10.1002/jcp.1041370203. [DOI] [PubMed] [Google Scholar]
- Millar JB, Rozengurt E. Chronic Desensitization to Bombesin by Progressive Down- Regulation of Bombesin Receptors in Swiss 3T3 Cells. J Biol Chem. 1990;265:12052–12058. [PubMed] [Google Scholar]
- Milusheva EA, Kortezova NI, Mizhorkova ZN, Papasova M, Coy DH, Balint A, Vizi ES, Varga G. Role of Different Bombesin Receptor Subtypes Mediating Contractile Activity in Cat Upper Gastrointestinal Tract. Peptides. 1998;19:549–556. doi: 10.1016/s0196-9781(97)00467-1. [DOI] [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Matsuo H. Neuromedin B: a Novel Bombesin-Like Peptide Identified in Porcine Spinal Cord. Biochem Biophys Res Commun. 1983;114:541–548. doi: 10.1016/0006-291x(83)90814-8. [DOI] [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Matsuo H. Neuromedin C: a Bombesin-Like Peptide Identified in Porcine Spinal Cord. Biochem Biophys Res Commun. 1984b;119:14–20. doi: 10.1016/0006-291x(84)91611-5. [DOI] [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Matsuo H. Neuromedin B Is a Major Bombesin-Like Peptide in Rat Brain: Regional Distribution of Neuromedin B and Neuromedin C in Rat Brain, Pituitary and Spinal Cord. Biochem Biophys Res Comm. 1984a;124:925–932. doi: 10.1016/0006-291x(84)91046-5. [DOI] [PubMed] [Google Scholar]
- Minamino N, Sudoh T, Kangawa K, Matsuo H. Neuromedin B-32 and B-30: Two “Big” Neuromedin B Identified in Porcine Brain and Spinal Cord. Biochem Biophys Res Commun. 1985;130:685–691. doi: 10.1016/0006-291x(85)90471-1. [DOI] [PubMed] [Google Scholar]
- Modlin IM, Lamers CB, Walsh JH. Stimulation of Canine Pancreatic Polypeptide, Gastrin, and Gastric Acid Secretion by Ranatensin, Litorin, Bombesin Nonapeptide and Substance P. Regul Pept. 1981;1:279–288. doi: 10.1016/0167-0115(81)90051-3. [DOI] [PubMed] [Google Scholar]
- Moody TW, Carney DN, Cuttitta F, Quattrocchi K, Minna JD. High Affinity Receptors for Bombesin/GRP-Like Peptides on Human Small Cell Lung Cancer. Life Sci. 1985;37:105–113. doi: 10.1016/0024-3205(85)90413-8. [DOI] [PubMed] [Google Scholar]
- Moody TW, Chan D, Fahrenkrug J, Jensen RT. Neuropeptides As Autocrine Growth Factors in Cancer Cells. Curr Pharm Des. 2003a;9:495–509. doi: 10.2174/1381612033391621. [DOI] [PubMed] [Google Scholar]
- Moody TW, Fagarasan M, Zia F. Neuromedin B Stimulates Arachidonic Acid Release, C-Fos Gene Expression, and the Growth of C6 Glioma Cells. Peptides. 1995a;16:1133–1140. doi: 10.1016/0196-9781(95)00085-x. [DOI] [PubMed] [Google Scholar]
- Moody TW, Getz R, O'Donohue TL, Rosenstein JM. Localization of Receptors for Bombesin-Like Peptides in the Rat Brain. Ann N Y Acad Sci. 1988;547:114–130. doi: 10.1111/j.1749-6632.1988.tb23880.x. [DOI] [PubMed] [Google Scholar]
- Moody TW, Jensen RT. Bombesin/GRP and vasoactive intestinal peptide/PACAP as growth factors. In: LeRoith D, Bondy C, editors. Growth Factors and Cytokines in Health and Disease. JAI Press, Inc.; Greenwich, CT: 1996. pp. 491–535. [Google Scholar]
- Moody TW, Jensen RT, Garcia L, Leyton J. Nonpeptide Neuromedin B Receptor Antagonists Inhibit the Proliferation of C6 Cells. Eur J Pharmacol. 2000;409:133–142. doi: 10.1016/s0014-2999(00)00828-1. [DOI] [PubMed] [Google Scholar]
- Moody TW, Korman LY, O'Donohue TL. Neuromedin B-Like Peptides in Rat Brain: Biochemical Characterization, Mechanism of Release and Localization in Synaptosomes. Peptides. 1986;7:815–820. doi: 10.1016/0196-9781(86)90100-2. [DOI] [PubMed] [Google Scholar]
- Moody TW, Leyton J, Garcia-Marin L, Jensen RT. Nonpeptide Gastrin Releasing Peptide Receptor Antagonists Inhibit the Proliferation of Lung Cancer Cells. Eur J Pharmacol. 2003b;474:21–29. doi: 10.1016/s0014-2999(03)01996-4. [DOI] [PubMed] [Google Scholar]
- Moody TW, Mantey SA, Pradhan TK, Schumann M, Nakagawa T, Martinez A, Fuselier J, Coy DH, Jensen RT. Development of High Affinity Camptothecin-Bombesin Conjugates That Have Targeted Cytotoxicity for Bombesin Receptor-Containing Tumor Cells. J Biol Chem. 2004;279:23580–23589. doi: 10.1074/jbc.M401938200. [DOI] [PubMed] [Google Scholar]
- Moody TW, Merali Z. Bombesin-Like Peptides and Associated Receptors Within the Brain: Distribution and Behavioral Implications. Peptides. 2004;25:511–520. doi: 10.1016/j.peptides.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Moody TW, Nakagawa T, Kang Y, Jakowlew S, Chan D, Jensen RT. Bombesin/Gastrin-Releasing Peptide Receptor Antagonists Increase the Ability of Histone Deacetylase Inhibitors to Reduce Lung Cancer Proliferation. J Mol Neurosci. 2006a;28:231–238. doi: 10.1385/JMN:28:3:231. [DOI] [PubMed] [Google Scholar]
- Moody TW, Pert CB, Rivier J, Brown MR. Bombesin: Specific Binding to Rat Brain Membranes. Proc Natl Acad Sci USA. 1978;75:5372–5376. doi: 10.1073/pnas.75.11.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TW, Staley J, Zia F, Coy DH, Jensen RT. Neuromedin B Binds With High Affinity, Elevates Cytosolic Calcium and Stimulates the Growth of Small Cell Lung Cancer Cell Lines. J Pharmacol Exp Ther. 1992;263:311–317. [PubMed] [Google Scholar]
- Moody TW, Sun LC, Mantey SA, Pradhan T, Mackey LV, Gonzales N, Fuselier JA, Coy DH, Jensen RT. In Vitro and in Vivo Antitumor Effects of Cytotoxic Camptothecin-Bombesin Conjugates Are Mediated by Specific Interaction With Cellular Bombesin Receptors. J Pharmacol Exp Ther. 2006b;318:1265–1272. doi: 10.1124/jpet.106.104141. [DOI] [PubMed] [Google Scholar]
- Moody TW, Venugopal R, Hu V, Gozes Y, McDermed J, Leban JJ. BW 1023U90: a New GRP Receptor Antagonist for Small-Cell Lung Cancer Cells. Peptides. 1996a;17:1337–1343. doi: 10.1016/s0196-9781(96)00195-7. [DOI] [PubMed] [Google Scholar]
- Moody TW, Venugopal R, Zia F, Patierno S, Leban JJ, McDermed J. BW2258U89: a GRP Receptor Antagonist Which Inhibits Small Cell Lung Cancer Growth. Life Sci. 1995b;56:521–529. doi: 10.1016/0024-3205(94)00481-7. [DOI] [PubMed] [Google Scholar]
- Moody TW, Zia F, Venugopal R, Fagarasan M, Oie H, Hu V. GRP Receptors Are Present in Non Small Cell Lung Cancer Cells. J Cell Biochem Suppl. 1996b;24:247–256. doi: 10.1002/jcb.240630520. [DOI] [PubMed] [Google Scholar]
- Moro O, Lameh J, Hogger P, Sadee W. Hydrophobic Amino Acid in the I2 Loop Plays a Key Role in Receptor-G Protein Coupling. J Biol Chem. 1993;268:22273–22276. [PubMed] [Google Scholar]
- Moro O, Shockley MS, Lameh J, Sadee W. Overlapping Multi-Site Domains of the Muscarinic Cholinergic Hm1 Receptor Involved in Signal Transduction and Sequestration. J Biol Chem. 1994;269:6651–6655. [PubMed] [Google Scholar]
- Murphy LO, Abdel-Wahab YH, Wang QJ, Knezetic JA, Permnert J, Larsson J, Hollingsworth AM, Adrian TE. Receptors and Ligands for Autocrine Growth Pathways Are Up-Regulated When Pancreatic Cancer Cells Are Adapted to Serum-Free Culture. Pancreas. 2001;22:293–298. doi: 10.1097/00006676-200104000-00011. [DOI] [PubMed] [Google Scholar]
- Nagalla SR, Barry BJ, Creswick KC, Eden P, Taylor JT, Spindel ER. Cloning of a Receptor for Amphibian [Phe13]Bombesin Distinct From the Receptor for Gastrin-Releasing Peptide: Identification of a Fourth Bombesin Receptor Subtype (BB4). Proc Natl Acad Sci USA. 1995;92:6205–6209. doi: 10.1073/pnas.92.13.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalla SR, Barry BJ, Falick AM, Gibson BW, Taylor JE, Dong JZ, Spindel ER. There Are Three Distinct Forms of Bombesin. Identification of [Leu13]Bombesin, [Phe13]Bombesin, and [Ser3, Arg10, Phe13]Bombesin in the Frog Bombina Orientalis. J Biol Chem. 1996;271:7731–7737. doi: 10.1074/jbc.271.13.7731. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Hocart SJ, Schumann M, Tapia JA, Mantey SA, Coy DH, Tokita K, Katsuno T, Jensen RT. Identification of Key Amino Acids in the Gastrin-Releasing Peptide Receptor (GRPR) Responsible for High Affinity Binding of Gastrin-Releasing Peptide (GRP). Biochem Pharmacol. 2005;69:579–593. doi: 10.1016/j.bcp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Nakamichi Y, Wada E, Aoki K, Ohara-Imaizumi M, Kikuta T, Nishiwaki C, Matsushima S, Watanabe T, Wada K, Nagamatsu S. Functions of Pancreatic Beta Cells and Adipocytes in Bombesin Receptor Subtype-3-Deficient Mice. Biochem Biophys Res Commun. 2004;318:698–703. doi: 10.1016/j.bbrc.2004.04.081. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Oda M, Kaneko K, Akaiwa Y, Tsukada N, Komatsu H, Tsuchiya M. Autoradiographic Demonstration of Gastrin-Releasing Peptide-Binding Sites in the Rat Gastric Mucosa. Gastroenterology. 1988;94:968–976. doi: 10.1016/0016-5085(88)90555-0. [DOI] [PubMed] [Google Scholar]
- Nanni C, Rubello D, Fanti S. 18F-DOPA PET/CT and Neuroendocrine Tumours. Eur J Nucl Med Mol Imaging. 2006;33:509–513. doi: 10.1007/s00259-006-0079-5. [DOI] [PubMed] [Google Scholar]
- Narayan S, Spindel ER, Rubin NH, Singh P. A Potent Bombesin Receptor Antagonist Inhibits Bombesin-Stimulated Growth of Mouse Colon Cancer Cells in Vitro: Absence of Autocrine Effects. Cell Growth Differ. 1992;3:111–118. [PubMed] [Google Scholar]
- Nathan JD, Liddle RA. Neurohormonal Control of Pancreatic Exocrine Secretion. Curr Opin Gastroenterol. 2002;18:536–544. doi: 10.1097/00001574-200209000-00003. [DOI] [PubMed] [Google Scholar]
- Niebergall-Roth E, Singer MV. Central and Peripheral Neural Control of Pancreatic Exocrine Secretion. J Physiol Pharmacol. 2001;52:523–538. [PubMed] [Google Scholar]
- Nishino H, Tsunoda Y, Owyang C. Mammalian Bombesin Receptors Are Coupled to Multiple Signal Transduction Pathways in Pancreatic Acini. Am J Physiol. 1998;274:G525–G534. doi: 10.1152/ajpgi.1998.274.3.G525. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the Small GTP-Binding Proteins Rho and Rac by Growth Factor Receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Nock B, Nikolopoulou A, Chiotellis E, Loudos G, Maintas D, Reubi JC, Maina T. [99mTc]Demobesin 1, a Novel Potent Bombesin Analogue for GRP Receptor-Targeted Tumour Imaging. Eur J Nucl Med Mol Imaging. 2003;30:247–258. doi: 10.1007/s00259-002-1040-x. [DOI] [PubMed] [Google Scholar]
- Oeffner F, Bornholdt D, Ziegler A, Hinney A, Gorg T, Gerber G, Goldschmidt HP, Siegfried W, Wright A, Hebebrand J, Grzeschik KH. Significant Association Between a Silent Polymorphism in the Neuromedin B Gene and Body Weight in German Children and Adolescents. Acta Diabetol. 2000;37:93–101. doi: 10.1007/s005920070026. [DOI] [PubMed] [Google Scholar]
- Ohki-Hamazaki H. Neuromedin B. Prog Neurobiol. 2000;62:297–312. doi: 10.1016/s0301-0082(00)00004-6. [DOI] [PubMed] [Google Scholar]
- Ohki-Hamazaki H, Iwabuchi M, Maekawa F. Development and Function of Bombesin-Like Peptides and Their Receptors. Int J Dev Biol. 2005;49:293–300. doi: 10.1387/ijdb.041954ho. [DOI] [PubMed] [Google Scholar]
- Ohki-Hamazaki H, Sakai Y, Kamata K, Ogura H, Okuyama S, Watase K, Yamada K, Wada K. Functional Properties of Two Bombesin-Like Peptide Receptors Revealed by the Analysis of Mice Lacking Neuromedin B Receptor. J Neurosci. 1999;19:948–954. doi: 10.1523/JNEUROSCI.19-03-00948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki-Hamazaki H, Wada E, Matsui K, Wada K. Cloning and Expression of the Neuromedin B Receptor and the Third Subtype of Bombesin Receptor Genes in the Mouse. Brain Res. 1997a;762:165–172. doi: 10.1016/s0006-8993(97)00380-6. [DOI] [PubMed] [Google Scholar]
- Ohki-Hamazaki H, Watase K, Yamamoto K, Ogura H, Yamano M, Yamada K, Maeno H, Imaki J, Kikuyama S, Wada E, Wada K. Mice Lacking Bombesin Receptor Subtype-3 Develop Metabolic Defects and Obesity. Nature. 1997b;390(6656):165–169. doi: 10.1038/36568. [DOI] [PubMed] [Google Scholar]
- Okusaka T, Eguchi K, Kasai T, Kurata T, Yamamoto N, Ohe Y, Tamura T, Shinkai T, Saijo N. Serum Levels of Pro-Gastrin-Releasing Peptide for Follow-Up of Patients With Small Cell Lung Cancer. Clin Cancer Res. 1997;3:123–127. [PubMed] [Google Scholar]
- Oliveira KJ, Ortiga-Carvalho TM, Cabanelas A, Veiga MA, Aoki K, Ohki-Hamazaki H, Wada K, Wada E, Pazos-Moura CC. Disruption of Neuromedin B Receptor Gene Results in Dysregulation of the Pituitary-Thyroid Axis. J Mol Endocrinol. 2006;36:73–80. doi: 10.1677/jme.1.01892. [DOI] [PubMed] [Google Scholar]
- Orbuch M, Taylor JE, Coy DH, Mrozinski JE, Jr., Mantey SA, Battey JF, Moreau JP, Jensen RT. Discovery of a Novel Class of Neuromedin B Receptor Antagonists: Substituted Somatostatin Analogues. Mol Pharmacol. 1993;44(4):841–850. [PubMed] [Google Scholar]
- Ortiga-Carvalho TM, Curty FH, Nascimento-Saba CC, Moura EG, Polak J. Pituitary Neuromedin B Content in Experimental Fasting and Diabetes Mellitus and Correlation With Thyrotropin Secretion. Metabolism. 1997;46:149–153. doi: 10.1016/s0026-0495(97)90293-6. [DOI] [PubMed] [Google Scholar]
- Ortiga-Carvalho TM, Oliveira KJ, Morales MM, Martins VP, Pazos-Moura CC. Thyrotropin Secretagogues Reduce Rat Pituitary Neuromedin B, a Local Thyrotropin Release Inhibitor. Exp Biol Med (Maywood ) 2003;228:1083–1088. doi: 10.1177/153537020322800916. [DOI] [PubMed] [Google Scholar]
- Pace A, Tapia JA, Garcia-Marin LJ, Jensen RT. The Src Family Kinase, Lyn, Is Activated in Pancreatic Acinar Cells by Gastrointestinal Hormones/Neurotransmitters and Growth Factors Which Stimulate Its Association With Numerous Other Signaling Molecules. Biochim Biophys Acta. 2006;1763:356–365. doi: 10.1016/j.bbamcr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Pandol SJ, Jensen RT, Gardner JD. Mechanism of [Tyr4]Bombesin-Induced Desensitization in Dispersed Acini From Guinea Pig Pancreas. J Biol Chem. 1982;257:12024–12029. [PubMed] [Google Scholar]
- Panigone S, Nunn AD. Lutetium-177-Labeled Gastrin Releasing Peptide Receptor Binding Analogs: a Novel Approach to Radionuclide Therapy. Q J Nucl Med Mol Imaging. 2006;50:310–321. [PubMed] [Google Scholar]
- Panula P, Nieminen O, Falkenberg M, Auvinen S. Localization and Development of Bombesin/GRP-Like Immunoreactivity in the Rat Central Nervous System. Ann N Y Acad Sci. 1988;547:54–69. doi: 10.1111/j.1749-6632.1988.tb23875.x. [DOI] [PubMed] [Google Scholar]
- Panula P, Yang HY, Costa E. Neuronal Location of the Bombesin-Like Immunoreactivity in the Central Nervous System of the Rat. Regul Pept. 1982;4:275–283. doi: 10.1016/0167-0115(82)90120-3. [DOI] [PubMed] [Google Scholar]
- Parkman HP, Vozzelli MA, Pagano AP, Cowan A. Pharmacological Analysis of Receptors for Bombesin-Related Peptides on Guinea Pig Gallbladder Smooth Muscle. Regul Pept. 1994;52:173–180. doi: 10.1016/0167-0115(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Parry JJ, Andrews R, Rogers BE. MicroPET Imaging of Breast Cancer Using Radiolabeled Bombesin Analogs Targeting the Gastrin-Releasing Peptide Receptor. Breast Cancer Res Treat. 2007;18:110–117. doi: 10.1007/s10549-006-9287-8. [DOI] [PubMed] [Google Scholar]
- Patel KV, Schrey MP. Modulation of Inositol Lipid Hydrolysis in Human Breast Cancer Cells by Two Classes of Bombesin Antagonist. J Mol Endocrinol. 1991;6:71–78. doi: 10.1677/jme.0.0060071. [DOI] [PubMed] [Google Scholar]
- Patel O, Dumesny C, Giraud AS, Baldwin GS, Shulkes A. Stimulation of Proliferation and Migration of a Colorectal Cancer Cell Line by Amidated and Glycine-Extended Gastrin-Releasing Peptide Via the Same Receptor. Biochem Pharmacol. 2004;68:2129–2142. doi: 10.1016/j.bcp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Patel O, Dumesny C, Shulkes A, Baldwin GS. Recombinant C-Terminal Fragments of the Gastrin-Releasing Peptide Precursor Are Bioactive. Cancer Lett. 2007a;254:87–93. doi: 10.1016/j.canlet.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Patel O, Dumesny C, Shulkes A, Baldwin GS. C-Terminal Fragments of the Gastrin-Releasing Peptide Precursor Stimulate Cell Proliferation Via a Novel Receptor. Endocrinology. 2007b;148:1330–1339. doi: 10.1210/en.2006-0466. [DOI] [PubMed] [Google Scholar]
- Patel O, Shulkes A, Baldwin GS. Gastrin-Releasing Peptide and Cancer. Biochim Biophys Acta. 2006;1766:23–41. doi: 10.1016/j.bbcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Pazos-Moura CC, Moura EG, Rettori V, Polak J, McCann SM. Role of Neuromedin B in the in Vitro Thyrotropin Release in Response to Thyrotropin-Releasing Hormone From Anterior Pituitaries of Eu-,Hypo-, and Hyperthyroid Rats. Proc Soc Exp Biol Med. 1996;211:353–358. doi: 10.3181/00379727-211-43980. [DOI] [PubMed] [Google Scholar]
- Pazos-Moura CC, Ortiga-Carvalho TM, Gaspar de ME. The Autocrine/Paracrine Regulation of Thyrotropin Secretion. Thyroid. 2003;13:167–175. doi: 10.1089/105072503321319477. [DOI] [PubMed] [Google Scholar]
- Penman E, Wass JA, Butler MG, Penny ES, Price J, Wu P, Rees LH. Distribution and Characterisation of Immunoreactive Somatostatin in Human Gastrointestinal Tract. Regul Pept. 1983;7:53–65. doi: 10.1016/0167-0115(83)90281-1. [DOI] [PubMed] [Google Scholar]
- Persson K, Gingerich RL, Nayak S, Wada K, Wada E, Ahren B. Reduced GLP-1 and Insulin Responses and Glucose Intolerance After Gastric Glucose in GRP Receptor-Deleted Mice. Am J Physiol Endocrinol Metab. 2000;279:E956–E962. doi: 10.1152/ajpendo.2000.279.5.E956. [DOI] [PubMed] [Google Scholar]
- Persson K, Pacini G, Sundler F, Ahren B. Islet Function Phenotype in Gastrin-Releasing Peptide Receptor Gene-Deficient Mice. Endocrinology. 2002;143:3717–3726. doi: 10.1210/en.2002-220371. [DOI] [PubMed] [Google Scholar]
- Pettersson M, Ahren B. Gastrin Releasing Peptide (GRP): Effects on Basal and Stimulated Insulin and Glucagon Secretion in the Mouse. Peptides. 1987;8:55–60. doi: 10.1016/0196-9781(87)90165-3. [DOI] [PubMed] [Google Scholar]
- Pinnock RD, Reynolds T, Woodruff GN. Different Types of Bombesin Receptors on Neurons in the Dorsal Raphe Nucleus and the Rostal Hypothalamus in Rat Brain Slices in Vitro. Brain Res. 1994;653:119–124. doi: 10.1016/0006-8993(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Plaisancie P, Barcelo A, Moro F, Claustre J, Chayvialle JA, Cuber JC. Effects of Neurotransmitters, Gut Hormones, and Inflammatory Mediators on Mucus Discharge in Rat Colon. Am J Physiol. 1998;275:G1073–G1084. doi: 10.1152/ajpgi.1998.275.5.G1073. [DOI] [PubMed] [Google Scholar]
- Plonowski A, Nagy A, Schally AV, Sun B, Groot K, Halmos G. In Vivo Inhibition of PC-3 Human Androgen-Independent Prostate Cancer by a Targeted Cytotoxic Bombesin Analogue, AN-215. Int J Cancer. 2000;88:652–657. doi: 10.1002/1097-0215(20001115)88:4<652::aid-ijc21>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Porcher C, Juhem A, Peinnequin A, Bonaz B. Bombesin Receptor Subtype-3 Is Expressed by the Enteric Nervous System and by Interstitial Cells of Cajal in the Rat Gastrointestinal Tract. Cell Tissue Res. 2005;320:21–31. doi: 10.1007/s00441-004-1032-1. [DOI] [PubMed] [Google Scholar]
- Pradhan TK, Katsuno T, Taylor JE, Kim SH, Ryan RR, Mantey SA, Donohue PJ, Weber HC, Sainz E, Battey JF, Coy DH, Jensen RT. Identification of a Unique Ligand Which Has High Affinity for All Four Bombesin Receptor Subtypes. Eur J Pharmacol. 1998;343:275–287. doi: 10.1016/s0014-2999(97)01527-6. [DOI] [PubMed] [Google Scholar]
- Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC, Hoffman TJ, Sieckman GL, Figueroa SD, Smith CJ. [64Cu-NOTA-8-Aoc-BBN(7−14)NH2] Targeting Vector for Positron-Emission Tomography Imaging of Gastrin-Releasing Peptide Receptor-Expressing Tissues. Proc Natl Acad Sci U S A. 2007;104:12462–12467. doi: 10.1073/pnas.0705347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. Physiological Roles of G Protein-Coupled Receptor Kinases and Arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- Presti-Torres J, de Lima MN, Scalco FS, Caldana F, Garcia VA, Guimaraes MR, Schwartsmann G, Roesler R, Schroder N. Impairments of Social Behavior and Memory After Neonatal Gastrin-Releasing Peptide Receptor Blockade in Rats: Implications for an Animal Model of Neurodevelopmental Disorders. Neuropharmacology. 2007;52:724–732. doi: 10.1016/j.neuropharm.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Preston SR, Woodhouse LF, Jones-Blackett S, Miller GV, Primrose JN. High-Affinity Binding Sites for Gastrin-Releasing Peptide on Human Colorectal Cancer Tissue but Not Uninvolved Mucosa. Br J Cancer. 1995;71:1087–1089. doi: 10.1038/bjc.1995.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian JM, Coy DH, Jiang NY, Gardner JD, Jensen RT. Reduced Peptide Bond Pseudopeptide Analogues of Substance P: A New Class of Substance P Receptor Antagonists With Enhanced Specificity. J Biol Chem. 1989;264:16667–16671. [PubMed] [Google Scholar]
- Qin Y, Ertl T, Cai RZ, Horvath JE, Groot K, Schally AV. Antagonists of Bombesin/Gastrin-Releasing Peptide Inhibit Growth of SW-1990 Human Pancreatic Adenocarcinoma and Production of Cyclic AMP. Int J Cancer. 1995;63:257–262. doi: 10.1002/ijc.2910630219. [DOI] [PubMed] [Google Scholar]
- Qin Y, Halmos G, Cai RZ, Szoke B, Ertl T, Schally AV. Bombesin Antagonists Inhibit in Vitro and in Vivo Growth of Human Gastric Cancer and Binding of Bombesin to Its Receptors. J Cancer Res Clin Oncol. 1994;120:519–528. doi: 10.1007/BF01221028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic S, Cai RZ, Serfozo P, Groot K, Redding TW, Pinski J, Schally AV. Biological Effects and Receptor Binding Affinities of New Pseudononapeptide Bombesin/GRP Receptor Antagonists With N-Terminal DTrp or D-Tpi. Int J Pept Protein Res. 1991a;38:593–600. doi: 10.1111/j.1399-3011.1991.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Radulovic S, Miller G, Schally AV. Inhibition of Growth of HT-29 Human Colon Cancer Xenografts in Nude Mice by Treatment With Bombesin/Gastrin Releasing Peptide Antagonist (RC-3095). Cancer Res. 1991b;51:6006–6009. [PubMed] [Google Scholar]
- Regoli D, Dion S, Rhaleb NE, Drapeau G, Rouissi N, Orleans-Juste P. Receptors for Neurokinins, Tachykinins, and Bombesin: a Pharmacological Study. Ann N Y Acad Sci. 1988;547:158–173. doi: 10.1111/j.1749-6632.1988.tb23884.x. [DOI] [PubMed] [Google Scholar]
- Reile H, Armatis PE, Schally AV. Characterization of High-Affinity Receptors for Bombesin/Gastrin Releasing Peptide on the Human Prostate Cancer Cell Lines PC-3 and DU-145: Internalization of Receptor Bound 125I-(Tyr4) Bombesin by Tumor Cells. The Prostate. 1994;25:29–38. doi: 10.1002/pros.2990250105. [DOI] [PubMed] [Google Scholar]
- Rettori V, Pazos-Moura CC, Moura EG, Polak J, McCann SM. Role of Neuromedin B in Control of the Release of Thyrotropin in Hypothyroid and Hyperthyroid Rats. Proc Natl Acad Sci USA. 1992;89:3035–3039. doi: 10.1073/pnas.89.7.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi JC, Wenger S, Schumuckli-Maurer J, Schaer JC, Gugger M. Bombesin Receptor Subtypes in Human Cancers: Detection With the Universal Radoligand (125)I-[D-TYR(6), Beta-ALA(11),PHE(13), NLE(14)] Bombesin(6−14). Clin Cancer Res. 2002;8:1139–1146. [PubMed] [Google Scholar]
- Rivier JE, Brown MR. Bombesin, Bombesin analogues and Related Peptides: Effects on Thermo Regulation. Biochemistry. 1978;17:1766–1771. doi: 10.1021/bi00602a030. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fernandez JL, Rozengurt E. Bombesin, Bradykinin, Vasopressin, and Phorbol Esters Rapidly and Transiently Activate Src Family Tyrosine Kinases in Swiss 3T3 Cells. Dissociation From Tyrosine Phosphorylation of P125 Focal Adhesion Kinase. J Biol Chem. 1996;271:27895–27901. doi: 10.1074/jbc.271.44.27895. [DOI] [PubMed] [Google Scholar]
- Rodriquez M, Bali JP, Magous R, Castro B, Martinez J. Synthesis of Pseudo-Peptide Analogues of the C-Terminal Tetrapeptide of Gastrin and Evaluation of Their Biological Activity on Acid Secretion. Int J Pept Protein Res. 1986;27:293–299. doi: 10.1111/j.1399-3011.1986.tb01823.x. [DOI] [PubMed] [Google Scholar]
- Roesler R, Henriques JA, Schwartsmann G. Gastrin-Releasing Peptide Receptor As a Molecular Target for Psychiatric and Neurological Disorders. CNS Neurol Disord Drug Targets. 2006a;5:197–204. doi: 10.2174/187152706776359673. [DOI] [PubMed] [Google Scholar]
- Roesler R, Lessa D, Venturella R, Vianna MR, Luft T, Henriques JA, Izquierdo I, Schwartsmann G. Bombesin/Gastrin-Releasing Peptide Receptors in the Basolateral Amygdala Regulate Memory Consolidation. Eur J Neurosci. 2004;19:1041–1045. doi: 10.1111/j.0953-816x.2004.03175.x. [DOI] [PubMed] [Google Scholar]
- Roesler R, Luft T, Oliveira SH, Farias CB, Almeida VR, Quevedo J, Dal PF, Schroder N, Izquierdo I, Schwartsmann G. Molecular Mechanisms Mediating Gastrin-Releasing Peptide Receptor Modulation of Memory Consolidation in the Hippocampus. Neuropharmacology. 2006b;51:350–357. doi: 10.1016/j.neuropharm.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Signal Transduction Pathways in the Mitogenic Response to G Protein-Coupled Neuropeptide Receptor Agonists. J Cell Physiol. 1998b;177:507–517. doi: 10.1002/(SICI)1097-4652(199812)177:4<507::AID-JCP2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Bombesin-Induction of Cell Proliferation in 3T3 Cells. Specific Receptors and Early Signaling Events. Ann N Y Acad Sci. 1988;547:277–292. doi: 10.1111/j.1749-6632.1988.tb23896.x. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. V. Gastrointestinal Peptide Signaling Through Tyrosine Phosphorylation of Focal Adhesion Proteins. Am J Physiol. 1998a;275:G177–G182. doi: 10.1152/ajpgi.1998.275.2.G177. [DOI] [PubMed] [Google Scholar]
- Rozengurt E, Murray M, Zachary I, Collins M. Protein Kinase C Activation Enhances CAMP Accumulation in Swiss 3T3 Cells: Inhibition by Pertussis Toxin. Proc Natl Acad Sci U S A. 1987;84:2282–2286. doi: 10.1073/pnas.84.8.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E, Sinnett-Smith J. Bombesin Stimulation of DNA Synthesis and Cell Division in Cultures of Swiss 3T3 Cells. Proc Natl Acad Sci U S A. 1983;80:2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff M, Schiffmann E, Terranova V, Pert CB. Neuropeptides Are Chemoattractants for Human Tumor Cells and Monocytes: a Possible Mechanism for Metastasis. Clin Immunol Immunopathol. 1985;37:387–396. doi: 10.1016/0090-1229(85)90108-4. [DOI] [PubMed] [Google Scholar]
- Ruginis T, Taglia L, Matusiak D, Lee BS, Benya RV. Consequence of Gastrin-Releasing Peptide Receptor Cancers: Detection With the Universal Radoligand (125)I-[D-TYR(6), Activation in a Human Colon Cancer Cell Line: a Proteomic Approach. J Proteome Res. 2006;5:1460–1468. doi: 10.1021/pr060005g. [DOI] [PubMed] [Google Scholar]
- Ryan RR, Katsuno T, Mantey SA, Pradhan TP, Weber HC, Battey JF, Jensen RT. Comparative Pharmacology of a Nonpeptoid Neuromedin B Antagonist PD 168368. J Exp Pharmacol Ther. 1999;290:1202–1211. [PubMed] [Google Scholar]
- Ryan RR, Taylor JE, Daniel JL, Cowan A. Pharmacological Profiles of Two Bombesin Analogues in Cells Transfected With Human Neuromedin B Receptors. Eur J Pharmacol. 1996;306(1−3):307–314. doi: 10.1016/0014-2999(96)00223-3. [DOI] [PubMed] [Google Scholar]
- Ryan RR, Weber HC, Hou W, Sainz E, Mantey SA, Battey JF, Coy DH, Jensen RT. Ability of Various Bombesin Receptor Agonists and Antagonists to Alter Intracellular Signaling of the Human Orphan Receptor BRS-3. J Biol Chem. 1998a;273:13613–13624. doi: 10.1074/jbc.273.22.13613. [DOI] [PubMed] [Google Scholar]
- Ryan RR, Weber HC, Mantey SA, Hou W, Hilburger ME, Pradhan TK, Coy DH, Jensen RT. Pharmacology and Intracellular Signaling Mechanisms of the Native Human Orphan Receptor BRS-3 in Lung Cancer Cells. J Pharmacol Exp Ther. 1998b;287:366–380. [PubMed] [Google Scholar]
- Saeed ZA, Huang SC, Coy DH, Jiang NY, Heinz-Erian P, Mantey SA, Gardner JD, Jensen RT. Effect of Substitutions in Position 12 of Bombesin on Antagonist Activity. Peptides. 1989;10(3):597–603. doi: 10.1016/0196-9781(89)90149-6. [DOI] [PubMed] [Google Scholar]
- Safavy A, Raisch KP, Matusiak D, Bhatnagar S, Helson L. Single-Drug Multiligand Conjugates: Synthesis and Preliminary Cytotoxicity Evaluation of a Paclitaxel-Dipeptide “Scorpion” Molecule. Bioconjug Chem. 2006;17:565–570. doi: 10.1021/bc050224c. [DOI] [PubMed] [Google Scholar]
- Sainz E, Akeson M, Mantey SA, Jensen RT, Battey JF. Four Amino Acid Residues Are Critical for High Affinity Binding of Neuromedin B to the Neuromedin B Receptor. J Biol Chem. 1998;273:15927–15932. doi: 10.1074/jbc.273.26.15927. [DOI] [PubMed] [Google Scholar]
- Sakamoto C, Nagao M, Matozaki T, Nishizaki H, Konda Y, Baba S. Somatostatin Receptors on Rat Cerebrocortical Membranes. Structural Characterization of Somatostatin-14 and Somatostatin- 28 Receptors and Comparison With Pancreatic Type Receptors. J Biol Chem. 1988;263:14441–14445. [PubMed] [Google Scholar]
- Sano H, Feighner SD, Hreniuk DL, Iwaasa H, Sailer AW, Pan J, Reitman ML, Kanatani A, Howard AD, Tan CP. Characterization of the Bombesin-Like Peptide Receptor Family in Primates. Genomics. 2004;84:139–146. doi: 10.1016/j.ygeno.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Santiskulvong C, Rozengurt E. Galardin (GM 6001), a Broad-Spectrum Matrix Metalloproteinase Inhibitor, Blocks Bombesin- and LPA-Induced EGF Receptor Transactivation and DNA Synthesis in Rat-1 Cells. Exp Cell Res. 2003;290:437–446. doi: 10.1016/s0014-4827(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Santiskulvong C, Sinnett-Smith J, Rozengurt E. EGF Receptor Function Is Required in Late G(1) for Cell Cycle Progression Induced by Bombesin and Bradykinin. Am J Physiol Cell Physiol. 2001;281:C886–C898. doi: 10.1152/ajpcell.2001.281.3.C886. [DOI] [PubMed] [Google Scholar]
- Santiskulvong C, Sinnett-Smith J, Rozengurt E. Insulin Reduces the Requirement for EGFR Transactivation in Bombesin-Induced DNA Synthesis. Biochem Biophys Res Commun. 2004;318:826–832. doi: 10.1016/j.bbrc.2004.04.100. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Murphy WA, Heiman ML, Lance VA, Coy DH. Solid-Phase Synthesis and Biological Properties of Psi [CH2NH] Pseudopeptide Analogues of a Highly Potent Somatostatin Octapeptide. J Med Chem. 1987;30:1162–1166. doi: 10.1021/jm00390a008. [DOI] [PubMed] [Google Scholar]
- Schally AV, Comaru-Schally AM, Plonowski A, Nagy A, Halmos G, Rekasi Z. Peptide Analogs in the Therapy of Prostate Cancer. Prostate. 2000;45:158–166. doi: 10.1002/1097-0045(20001001)45:2<158::aid-pros10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Schally AV, Nagy A. Cancer Chemotherapy Based on Targeting of Cytotoxic Peptide Conjugates to Their Receptors on Tumors. Eur J Endocrinol. 1999;141:1–14. doi: 10.1530/eje.0.1410001. [DOI] [PubMed] [Google Scholar]
- Schally AV, Nagy A. Chemotherapy Targeted to Cancers Through Tumoral Hormone Receptors. Trends Endocrinol Metab. 2004;15:300–310. doi: 10.1016/j.tem.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Schubert ML. Gastric Secretion. Curr Opin Gastroenterol. 2002;18:639–649. doi: 10.1097/00001574-200211000-00002. [DOI] [PubMed] [Google Scholar]
- Schubert ML, Hightower J, Coy DH, Makhlouf GM. Regulation of Acid Secretion by Bombesin/GRP Neurons of the Gastric Fundus. Am J Physiol. 1991;260:G156–G160. doi: 10.1152/ajpgi.1991.260.1.G156. [DOI] [PubMed] [Google Scholar]
- Schulz S, Rocken C, Schulz S. Immunohistochemical Detection of Bombesin Receptor Subtypes GRP-R and BRS-3 in Human Tumors Using Novel Antipeptide Antibodies. Virchows Arch. 2006;449:421–427. doi: 10.1007/s00428-006-0265-7. [DOI] [PubMed] [Google Scholar]
- Schumann M, Nakagawa T, Mantey SA, Tokita K, Venzon DJ, Hocart SJ, Benya RV, Jensen RT. Importance of Amino Acids of the Central Portion of the Second Intracellular Loop of the Gastrin-Releasing Peptide Receptor for Phospholipase C Activation, Internalization, and Chronic Down-Regulation. J Pharmacol Exp Ther. 2003;307:597–607. doi: 10.1124/jpet.103.055087. [DOI] [PubMed] [Google Scholar]
- Schwartsmann G, DiLeone LP, Horowitz M, Schunemann D, Cancella A, Pereira AS, Richter M, Souza F, da Rocha AB, Souza FH, Pohlmann P, De Nucci G. A Phase I Trial of the Bombesin/Gastrin-Releasing Peptide (BN/GRP) Antagonist RC3095 in Patients With Advanced Solid Malignancies. Invest New Drugs. 2006;24:403–412. doi: 10.1007/s10637-006-6886-5. [DOI] [PubMed] [Google Scholar]
- Scopinaro F, De Vincentis G, Corazziari E, Massa R, Osti M, Pallotta N, Covotta A, Remediani S, Paolo MD, Monteleone F, Varvarigou A. Detection of Colon Cancer With 99mTc-Labeled Bombesin Derivative (99mTc-Leu13-BN1). Cancer Biother Radiopharm. 2004;19:245–252. doi: 10.1089/108497804323072020. [DOI] [PubMed] [Google Scholar]
- Scopinaro F, Di Santo GP, Tofani A, Massari R, Trotta C, Ragone M, Archimandritis S, Varvarigou AD. Fast Cancer Uptake of 99mTc-Labelled Bombesin (99mTc BN1). In Vivo. 2005;19:1071–1076. [PubMed] [Google Scholar]
- Scott N, Millward E, Cartwright EJ, Preston SR, Coletta PL. Gastrin Releasing Peptide and Gastrin Releasing Peptide Receptor Expression in Gastrointestinal Carcinoid Tumours. J Clin Pathol. 2004;57:189–192. doi: 10.1136/jcp.2003.10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seensalu R, Avedian D, Barbuti R, Song M, Slice L, Walsh JH. Bombesin-Induced Gastrin Release From Canine G Cells Is Stimulated by Ca2+ but Not by Protein Kinase C, and Is Enhanced by Disruption of Rho/Cytoskeletal Pathways. J Clin Invest. 1997;100:1037–1046. doi: 10.1172/JCI119614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufferlein T, Withers DJ, Mann D, Rozengurt E. Dissociation of Mitogen-Activated Protein Kinase Activation From P125 Focal Adhesion Kinase Tyrosine Phosphorylation in Swiss 3T3 Cells Stimulated by Bombesin, Lysophosphatidic Acid, and Platelet-Derived Growth Factor. Mol Biol Cell. 1996a;7:1865–1875. doi: 10.1091/mbc.7.12.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufferlein T, Withers DJ, Rozengurt E. Reduced Requirement of Mitogen-Activated Protein Kinase (MAPK) Activity for Entry into the S Phase of the Cell Cycle in Swiss 3T3 Fibroblasts Stimulated by Bombesin and Insulin. J Biol Chem. 1996b;271:21471–21477. doi: 10.1074/jbc.271.35.21471. [DOI] [PubMed] [Google Scholar]
- Severi C, Jensen RT, Erspamer V, D'Arpino L, Coy DH, Torsoli A, Delle Fave G. Different Receptors Mediate the Action of Bombesin-Related Peptides on Gastric Smooth Muscle Cells. Am J Physiol. 1991;260:G683–G690. doi: 10.1152/ajpgi.1991.260.5.G683. [DOI] [PubMed] [Google Scholar]
- Shan L, Emanuel RL, Dewald D, Torday JS, Asokanathan N, Wada K, Wada E, Sunday ME. Bombesin-Like Peptide Receptor Gene Expression, Regulation, and Function in Fetal Murine Lung. Am J Physiol Lung Cell Mol Physiol. 2004;286:L165–L173. doi: 10.1152/ajplung.00436.2002. [DOI] [PubMed] [Google Scholar]
- Shapira H, Wada E, Jensen RT, Battey JF. Distinguishing Bombesin Receptors. Methods Neurosci. 1993;13:220–237. [Google Scholar]
- Shapira H, Way J, Lipinsky D, Oron Y, Battey JF. Neuromedin B Receptor, Expressed in Xenopus Laevis Oocyts, Selectively Couples to G Alpha q and Not G Alpha 11. FEBS Lett. 1994;348:89–92. doi: 10.1016/0014-5793(94)00570-2. [DOI] [PubMed] [Google Scholar]
- Sharif TR, Luo W, Sharif M. Functional Expression of Bombesin Receptor in Most Adult and Pediatric Human Glioblastoma Cell Lines; Role in Mitogenesis and in Stimulating the Mitogen-Activated Protein Kinase Pathway. Mol Cell Endocrinol. 1997;130:119–130. doi: 10.1016/s0303-7207(97)00080-4. [DOI] [PubMed] [Google Scholar]
- Shibayama T, Ueoka H, Nishii K, Kiura K, Tabata M, Miyatake K, Kitajima T, Harada M. Complementary Roles of Pro-Gastrin-Releasing Peptide (ProGRP) and Neuron Specific Enolase (NSE) in Diagnosis and Prognosis of Small-Cell Lung Cancer (SCLC). Lung Cancer. 2001;32:61–69. doi: 10.1016/s0169-5002(00)00205-1. [DOI] [PubMed] [Google Scholar]
- Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Identification of a Signaling Network in Lateral Nucleus of Amygdala Important for Inhibiting Memory Specifically Related to Learned Fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- Siegfried JM, DeMichele MA, Hunt JD, Davis AG, Vohra KP, Pilewski JM. Expression of MRNA for Gastrin-Releasing Peptide Receptor by Human Bronchial Epithelial Cells. Association With Prolonged Tobacco Exposure and Responsiveness to Bombesin-Like Peptides. Am J Respir Crit Care Med. 1997;156:358–366. doi: 10.1164/ajrccm.156.2.9608047. [DOI] [PubMed] [Google Scholar]
- Siegfried JM, Guentert PJ, Gaither AL. Effects of Bombesin and Gastrin-Releasing Peptide on Human Bronchial Epithelial Cells From a Series of Donors: Individual Variation and Modulation by Bombesin Analogs. Anat Rec. 1993;236:241–247. doi: 10.1002/ar.1092360129. [DOI] [PubMed] [Google Scholar]
- Siegfried JM, Krishnamachary N, Davis AG, Gubish C, Hunt JD, Shriver SP. Evidence for Autocrine Actions of Neuromedin B and Gastrin-Releasing Peptide in Non-Small Cell Lung Cancer. Pulm Pharmacol Ther. 1999;12:291–302. doi: 10.1006/pupt.1999.0210. [DOI] [PubMed] [Google Scholar]
- Singh P, Guo YS, Kull FC, Leban JJ. A Novel Bombesin Receptor Antagonist (2258U89), Potently Inhibits Bombesin Evoked Release of Gastrointestinal Hormones From Rats and Dogs, in Vitro and in Vivo. Regul Pept. 1992;40:75–86. doi: 10.1016/0167-0115(92)90085-9. [DOI] [PubMed] [Google Scholar]
- Sinnett-Smith J, Santiskulvong C, Duque J, Rozengurt E. [D-Arg(1),D-Trp(5,7,9),Leu(11)]Substance P Inhibits Bombesin-Induced Mitogenic Signal Transduction Mediated by Both G(q) and G(12) in Swiss 3T3cells. J Biol Chem. 2000;275:30644–30652. doi: 10.1074/jbc.M003702200. [DOI] [PubMed] [Google Scholar]
- Sinnett-Smith J, Zachary I, Rozengurt E. Characterization of a Bombesin Receptor on Swiss Mouse 3T3 Cells by Affinity Cross-Linking. J Cell Biochem. 1988;38:237–249. doi: 10.1002/jcb.240380403. [DOI] [PubMed] [Google Scholar]
- Sinnett-Smith J, Zachary I, Valverde AM, Rozengurt E. Bombesin Stimulation of P125 Focal Adhesion Kinase Tyrosine Phosphorylation. Role of Protein Kinase C, Ca2+ Mobilization, and the Actin Cytoskeleton. J Biol Chem. 1993;268(19):14261–14268. [PubMed] [Google Scholar]
- Sinnett-Smith J, Zhukova E, Hsieh N, Jiang X, Rozengurt E. Protein Kinase D Potentiates DNA Synthesis Induced by Gq-Coupled Receptors by Increasing the Duration of ERK Signaling in Swiss 3T3 Cells. J Biol Chem. 2004;279:16883–16893. doi: 10.1074/jbc.M313225200. [DOI] [PubMed] [Google Scholar]
- Sinnett-Smith J, Zhukova E, Rey O, Rozengurt E. Protein Kinase D2 Potentiates MEK/ERK/RSK Signaling, C-Fos Accumulation and DNA Synthesis Induced by Bombesin in Swiss 3T3 Cells. J Cell Physiol. 2007;211:781–790. doi: 10.1002/jcp.20984. [DOI] [PubMed] [Google Scholar]
- Slice LW, Wong HC, Sternini C, Grady EF, Bunnett NW, Walsh JH. The Conserved NPXnY Motif Present in the Gastrin-Releasing Peptide Receptor Is Not a General Sequestration Sequence. J Biol Chem. 1994;269:21755–21762. [PubMed] [Google Scholar]
- Smith CJ, Volkert WA, Hoffman TJ. Radiolabeled Peptide Conjugates for Targeting of the Bombesin Receptor Superfamily Subtypes. Nucl Med Biol. 2005;32:733–740. doi: 10.1016/j.nucmedbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Volkert WA, Hoffman TJ. Gastrin Releasing Peptide (GRP) Receptor Targeted Radiopharmaceuticals: a Concise Update. Nucl Med Biol. 2003;30:861–868. doi: 10.1016/s0969-8051(03)00116-1. [DOI] [PubMed] [Google Scholar]
- Spindel ER. Amphibian Bombesin-like Peptides. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Elsevier; Amsterda: 2006. pp. 277–281. [Google Scholar]
- Spindel ER, Giladi E, Brehm P, Goodman RH, Segerson TP. Cloning and Functional Characterization of a Complementary DNA Encoding the Murine Fibroblast Bombesin/Gastrin-Releasing Peptide Receptor. Mol Endocrinol. 1990;4:1956–1963. doi: 10.1210/mend-4-12-1956. [DOI] [PubMed] [Google Scholar]
- Spindel ER, Giladi E, Segerson TP, Nagalla S. Bombesin-Like Peptides: of Ligands and Receptors. Recent Prog Horm Res. 1993;48:365–391. doi: 10.1016/b978-0-12-571148-7.50017-8. [DOI] [PubMed] [Google Scholar]
- Staley J, Coy DH, Jensen RT, Moody TW. Solubilization and Purification of Bombesin/Gastrin Releasing Peptide Receptors From Human Cell Lines. J Mol Neurosci. 1993;4:29–40. doi: 10.1007/BF02736688. [DOI] [PubMed] [Google Scholar]
- Stangelberger A, Schally AV, Varga JL, Hammann BD, Groot K, Halmos G, Cai RZ, Zarandi M. Antagonists of Growth Hormone Releasing Hormone (GHRH) and of Bombesin/Gastrin Releasing Peptide (BN/GRP) Suppress the Expression of VEGF, BFGF, and Receptors of the EGF/HER Family in PC-3 and DU-145 Human Androgen-Independent Prostate Cancers. Prostate. 2005;64:303–315. doi: 10.1002/pros.20262. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Gibbs J, Coy DH, Smith GP. Fourth Ventricular Injection of the Bombesin Receptor Antagonist [D-Phe6]Bombesin(6−13)Methyl Ester, but Not BW2258U89, Increases Food Intake in Rats. Pharmacol Biochem Behav. 1995;50:463–471. doi: 10.1016/0091-3057(94)00319-e. [DOI] [PubMed] [Google Scholar]
- Subramaniam M, Bausch C, Twomey A, Andreeva S, Yoder BA, Chang L, Crapo JD, Pierce RA, Cuttitta F, Sunday ME. Bombesin-Like Peptides Modulate Alveolarization and Angiogenesis in Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200611-1734OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M, Sugiyama K, Coy DH, Kong Y, Miller YE, Weller PF, Wada K, Wada E, Sunday ME. Bombesin-Like Peptides and Mast Cell Responses: Relevance to Bronchopulmonary Dysplasia? Am J Respir Crit Care Med. 2003;168:601–611. doi: 10.1164/rccm.200212-1434OC. [DOI] [PubMed] [Google Scholar]
- Sukhotnik I, Slijper N, Karry R, Shaoul R, Coran AG, Lurie M, Shiloni E, Mogilner JG. Bombesin Stimulates Enterocyte Turnover Following Massive Small Bowel Resection in a Rat. Pediatr Surg Int. 2007;23:397–404. doi: 10.1007/s00383-006-1862-x. [DOI] [PubMed] [Google Scholar]
- Sun B, Halmos G, Schally AV, Wang X, Martinez M. Presence of Receptors for Bombesin/Gastrin-Releasing Peptide and MRNA for Three Receptor Subtypes in Human Prostate Cancers. Prostate. 2000a;42:295–303. doi: 10.1002/(sici)1097-0045(20000301)42:4<295::aid-pros7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Sun B, Schally AV, Halmos G. The Presence of Receptors for Bombesin/GRP and MRNA for Three Receptor Subtypes in Human Ovarian Epithelial Cancers. Regul Pept. 2000b;90:77–84. doi: 10.1016/s0167-0115(00)00114-2. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A Gastrin-Releasing Peptide Receptor Mediates the Itch Sensation in the Spinal Cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sunaga N, Tsuchiya S, Minato K, Watanabe S, Fueki N, Hoshino H, Makimoto T, Ishihara S, Saito R, Mori M. Serum Pro-Gastrin-Releasing Peptide Is a Useful Marker for Treatment Monitoring and Survival in Small-Cell Lung Cancer. Oncology. 1999;57:143–148. doi: 10.1159/000012022. [DOI] [PubMed] [Google Scholar]
- Sunday ME, Kaplan LM, Motoyama E, Chin WW, Spindel ER. Gastrin-Releasing Peptide (Mammalian Bombesin) Gene Expression in Health and Disease. Lab Invest. 1988;59:5–24. [PubMed] [Google Scholar]
- Sunday ME, Yoder BA, Cuttitta F, Haley KJ, Emanuel RL. Bombesin-Like Peptide Mediates Lung Injury in a Baboon Model of Bronchopulmonary Dysplasia. J Clin Invest. 1998;102:584–594. doi: 10.1172/JCI2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope SL, Schonbrunn A. Characterization of Ligand Binding and Processing by Bombesin Receptors in an Insulin-Secreting Cell Line. Biochem J. 1987;247:731–738. doi: 10.1042/bj2470731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope SL, Schonbrunn A. Desensitization of Islet Cells to Bombesin Involves Both Down- Modulation and Inhibition of Receptor Function. Mol Pharmacol. 1990;37:758–766. [PubMed] [Google Scholar]
- Szekeres PG, Koenig JA, Edwardson JM. The Relationship Between Agonist Intrinsic Activity and the Rate of Endocytosis of Muscarinic Receptors in a Human Neuroblastoma Cell Line. Mol Pharmacol. 1998;53:759–765. doi: 10.1124/mol.53.4.759. [DOI] [PubMed] [Google Scholar]
- Tache Y, Melchiorri P, Negri L. Bombesin-Like Peptides in Health and Disease. Ann N Y Acad Sci. 1988;547:1–541. doi: 10.1111/j.1749-6632.1988.tb23907.x. [DOI] [PubMed] [Google Scholar]
- Taglia LN, Matusiak DP, Matkowskyj KA, Benya RV. Gastrin-Releasing Peptide Mediates Its Morphogenic Properties in Human Colon Cancer by Up-Regulating Intracellular Adhesion Protein-1 (ICAM-1) Via Focal Adhesion Kinase. Am J Physiol Gastrointest Liver Physiol. 2007;92:G182–G190. doi: 10.1152/ajpgi.00201.2006. [DOI] [PubMed] [Google Scholar]
- Tan YR, Qi MM, Qin XQ, Xiang Y, Li X, Wang Y, Qu F, Liu HJ, Zhang JS. Wound Repair and Proliferation of Bronchial Epithelial Cells Enhanced by Bombesin Receptor Subtype 3 Activation. Peptides. 2006;27:1852–1858. doi: 10.1016/j.peptides.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Tan YR, Qin XQ, Xiang Y, Yang T, Qu F, Wang Y, Liu HJ, Weber HC. PPARalpha and AP-2alpha Regulate Bombesin Receptor Subtype 3 Expression in Ozone-Stressed Bronchial Epithelial Cells. Biochem J. 2007 doi: 10.1042/BJ20061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia JA, Jensen RT, Garcia-Marin LJ. Rottlerin Inhibits Stimulated Enzymatic Secretion and Several Intracellular Signaling Transduction Pathways in Pancreatic Acinar Cells by a Non-PKC-Delta-Dependent Mechanism. Biochim Biophys Acta. 2006;1763:25–38. doi: 10.1016/j.bbamcr.2005.10.007. [DOI] [PubMed] [Google Scholar]
- ter Beek WP, Muller ES, Van Hogezand RA, Biemond I, Lamers CB. Gastrin Releasing Peptide Receptor Expression Is Decreased in Patients With Crohn's Disease but Not in Ulcerative Colitis. J Clin Pathol. 2004;57:1047–1051. doi: 10.1136/jcp.2003.014993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashi H, Itami S, Tadokoro T, Takeyama M, Katagiri K, Takayasu S. Growth Stimulation of Normal Melanocytes and Nevocellular Nevus Cells by Gastrin Releasing Peptide (GRP). J Dermatol Sci. 1998;17:93–100. doi: 10.1016/s0923-1811(97)00079-0. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Grandis JR, Wentzel AL, Gooding WE, Lui VW, Siegfried JM. Gastrin-Releasing Peptide Receptor Mediates Activation of the Epidermal Growth Factor Receptor in Lung Cancer Cells. Neoplasia. 2005;7:426–431. doi: 10.1593/neo.04454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toi-Scott M, Jones CLA, Kane MA. Clinical Correlates of Bombesin-Like Peptide Receptor Subtype Expression in Human Lung Cancer Cells. Lung Cancer. 1996;15:341–354. doi: 10.1016/0169-5002(95)00597-8. [DOI] [PubMed] [Google Scholar]
- Tokita K, Hocart SJ, Coy DH, Jensen RT. Molecular Basis of the Selectivity of the Gastrin-Releasing Peptide Receptor for Gastrin-Releasing Peptide. Mol Pharmacol. 2002;61:1435–1443. doi: 10.1124/mol.61.6.1435. [DOI] [PubMed] [Google Scholar]
- Tokita K, Hocart SJ, Katsuno T, Mantey SA, Coy DH, Jensen RT. Tyrosine 220 in the Fifth Transmembrane Domain of the Neuromedin B Receptor Is Critical for the High Selectivity of the Peptoid Antagonist PD168368. J Biol Chem. 2001a;276:495–504. doi: 10.1074/jbc.M006059200. [DOI] [PubMed] [Google Scholar]
- Tokita K, Katsuno T, Hocart SJ, Coy DH, Llinares M, Martinez J, Jensen RT. Molecular Basis for Selectivity of High Affinity Peptide Antagonists for the Gastrin-Releasing Peptide Receptor. J Biol Chem. 2001b;276:36652–36663. doi: 10.1074/jbc.M104566200. [DOI] [PubMed] [Google Scholar]
- Tran TA, Mattern RH, Afargan M, Amitay O, Ziv O, Morgan BA, Taylor JE, Hoyer D, Goodman M. Design, Synthesis, and Biological Activities of Potent and Selective Somatostatin Analogues Incorporating Novel Peptoid Residues. J Med Chem. 1998;41:2679–2685. doi: 10.1021/jm970393l. [DOI] [PubMed] [Google Scholar]
- Traynor TR, O'Grady SM. Regulation of Colonic Ion Transport by GRP. I. GRP Stimulates Transepithelial K and Na Secretion. Am J Physiol. 1996;270:C848–C858. doi: 10.1152/ajpcell.1996.270.3.C848. [DOI] [PubMed] [Google Scholar]
- Trepel JB, Moyer JD, Cuttitta F, Frucht H, Coy DH, Natale RB, Mulshine JL, Jensen RT, Sausville EA. A Novel Bombesin Receptor Antagonist Inhibits Autocrine Signals in a Small Cell Lung Carcinoma Cell Line. Biochem Biophys Res Commun. 1988;156:1383–1389. doi: 10.1016/s0006-291x(88)80785-x. [DOI] [PubMed] [Google Scholar]
- Tseng MJ, Coon S, Stuenkel E, Struk V, Logsdon CD. Influence of Second and Third Cytoplasmic Loops on Binding, Internalization, and Coupling of Chimeric Bombesin/M3 Muscarinic Receptors. J Biol Chem. 1995a;270:17884–17891. doi: 10.1074/jbc.270.30.17884. [DOI] [PubMed] [Google Scholar]
- Tseng MJ, Detjen K, Struk V, Logsdon CD. Carboxyl-Terminal Domains Determine Internalization and Recycling Characteristics of Bombesin Receptor Chimeras. J Biol Chem. 1995b;270:18858–18864. doi: 10.1074/jbc.270.32.18858. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Kusui T, Hou W, Benya RV, Akeson MA, Kroog GS, Battey JF, Jensen RT. Effect of Gastrin-Releasing Peptide Receptor Number on Receptor Affinity, Coupling, Degradation and Receptor Modulation. Mol Pharmacol. 1997a;51(5):721–732. doi: 10.1124/mol.51.5.721. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Kusui T, Jensen RT. Neuromedin B Receptor Activation Causes Tyrosine Phosphorylation of P125FAK by a Phospholipase C Independent Mechanism Which Requires P21rho and Integrity of the Acini Cytoskeleton. Biochemistry. 1997b;36(51):16328–16337. doi: 10.1021/bi971448o. [DOI] [PubMed] [Google Scholar]
- Turner CE. Paxillin: a Cytoskeletal Target for Tyrosine Kinases. BioEssays. 1994;16:47–52. doi: 10.1002/bies.950160107. [DOI] [PubMed] [Google Scholar]
- Van Essen M, Krenning EP, de Jong M, Valkema R, Kwekkeboom DJ. Peptide Receptor Radionuclide Therapy With Radiolabelled Somatostatin Analogues in Patients With Somatostatin Receptor Positive Tumours. Acta Oncol. 2007;46:723–734. doi: 10.1080/02841860701441848. [DOI] [PubMed] [Google Scholar]
- van Tol EA, Verspaget HW, Hansen BE, Lamers CB. Neuroenteric Peptides Affect Natural Killer Activity by Intestinal Lamina Propria Mononuclear Cells. J Neuroimmunol. 1993;42:139–145. doi: 10.1016/0165-5728(93)90003-h. [DOI] [PubMed] [Google Scholar]
- Varga G, Reidelberger RD, Liehr RM, Bussjueger LJ, Coy DH, Solomon TE. Effects of Potent Bombesin Antagonist on Exocrine Pancreatic Secretion in the Rat. Peptides. 1991;12:493–497. doi: 10.1016/0196-9781(91)90090-c. [DOI] [PubMed] [Google Scholar]
- Vigne P, Feolde E, Van Renterghem C, Breittmayer JP, Frelin C. Properties and Functions of a Neuromedin-B-Preferring Bombesin Receptor in Brain Microvascular Endothelial Cells. Eur J Biochem. 1997;272:R433–R437. [Google Scholar]
- Vinayek R, Murakami M, Sharp CM, Jensen RT, Gardner JD. Carbachol Desensitizes Pancreatic Enzyme Secretion by Down- Regulation of Receptors. Am J Physiol. 1990;258:G107–G121. doi: 10.1152/ajpgi.1990.258.1.G107. [DOI] [PubMed] [Google Scholar]
- Vincent JP, Mazella J, Kitabgi P. Neurotensin and Neurotensin Receptors. Trends Pharmacol Sci. 1999;20:302–309. doi: 10.1016/s0165-6147(99)01357-7. [DOI] [PubMed] [Google Scholar]
- von Schrenck T, Heinz-Erian P, Moran T, Mantey SA, Gardner JD, Jensen RT. Neuromedin B Receptor in Esophagus: Evidence for Subtypes of Bombesin Receptors. Am J Physiol. 1989;256:G747–G758. doi: 10.1152/ajpgi.1989.256.4.G747. [DOI] [PubMed] [Google Scholar]
- von Schrenck T, Wang LH, Coy DH, Villanueva ML, Mantey S, Jensen RT. Potent Bombesin Receptor Antagonists Distinguish Receptor Subtypes. Am J Physiol. 1990;259:G468–G473. doi: 10.1152/ajpgi.1990.259.3.G468. [DOI] [PubMed] [Google Scholar]
- Wada E, Watase K, Yamada K, Ogura H, Yamano M, Inomata Y, Eguchi J, Yamamoto K, Sunday ME, Maeno H, Mikoshiba K, Ohki-Hamazaki H, Wada K. Generation and Characterization of Mice Lacking Gastrin-Releasing Peptide Receptor. Biochem Biophys Res Commun. 1997;239:28–33. doi: 10.1006/bbrc.1997.7418. [DOI] [PubMed] [Google Scholar]
- Wada E, Way J, Lebacq-Verheyden AM, Battey JF. Neuromedin B and Gastrin-Releasing Peptide MRNAs Are Differentially Distributed in the Rat Nervous System. J Neurosci. 1990;10:2917–2930. doi: 10.1523/JNEUROSCI.10-09-02917.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Way J, Shapira H, Kusano K, Lebacq-Verheyden AM, Coy D, Jensen R, Battey J. CDNA Cloning, Characterization, and Brain Region-Specific Expression of a Neuromedin B-Preferring Bombesin Receptor. Neuron. 1991;6(3):421–430. doi: 10.1016/0896-6273(91)90250-4. [DOI] [PubMed] [Google Scholar]
- Walsh JH, Bouzyk M, Rozengurt E. Homologous Desensitization of Bombesin-Induced Increases in Intracellular Ca2+ in Quiescent Swiss 3T3 Cells Involves a Protein Kinase C-Independent Mechanism. J Cell Physiol. 1993;156:333–340. doi: 10.1002/jcp.1041560216. [DOI] [PubMed] [Google Scholar]
- Wang LH, Battey JF, Wada E, Lin JT, Mantey S, Coy DH, Jensen RT. Activation of Neuromedin B-Preferring Bombesin Receptors on Rat Glioblastoma C-6 Cells Increases Cellular Ca2+ and Phosphoinositides. Biochem J. 1992;286:641–648. doi: 10.1042/bj2860641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Coy DH, Taylor JE, Jiang NY, Kim SH, Moreau JP, Huang SC, Mantey SA, Frucht H, Jensen RT. Desmethionine Alkylamide Bombesin Analogues: a New Class of Bombesin Receptor Antagonists With a Potent Antisecretory Activity in Pancreatic Acini and Antimitotic Activity in Swiss 3T3 Cells. Biochemistry. 1990a;29(3):616–622. doi: 10.1021/bi00455a004. [DOI] [PubMed] [Google Scholar]
- Wang LH, Coy DH, Taylor JE, Jiang NY, Moreau JP, Huang SC, Frucht H, Haffar BM, Jensen RT. Des-Met Carboxyl-Terminally Modified Analogues of Bombesin Function As Potent Bombesin Receptor Antagonists, Partial Agonists, or Agonists. J Biol Chem. 1990b;265(26):15695–15703. [PubMed] [Google Scholar]
- Wang LH, Mantey SA, Lin JT, Frucht H, Jensen RT. Ligand Binding, Internalization, Degradation and Regulation by Guanine Nucleotides of Bombesin Receptor Subtypes: A Comparative Study. Biochim Biophys Acta. 1993;1175:232–242. doi: 10.1016/0167-4889(93)90028-n. [DOI] [PubMed] [Google Scholar]
- Wang QJ, Knezetic JA, Schally AV, Pour PM, Adrian TE. Bombesin May Stimulate Proliferation of Human Pancreatic Cancer Cells Through an Autocrine Pathway. Int J Cancer. 1996;68:528–534. doi: 10.1002/(SICI)1097-0215(19961115)68:4<528::AID-IJC20>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Waser B, Eltschinger V, Linder K, Nunn A, Reubi JC. Selective in Vitro Targeting of GRP and NMB Receptors in Human Tumours With the New Bombesin Tracer (177)Lu-AMBA. Eur J Nucl Med Mol Imaging. 2007;34:95–100. doi: 10.1007/s00259-006-0229-9. [DOI] [PubMed] [Google Scholar]
- Watling KJ. The Sigma-RBI Handbook of Receptor Classification and Signal Transduction. Sigma-RBI; Natick. MA: 2007. [Google Scholar]
- Weber D, Berger C, Eickelmann P, Antel J, Kessler H. Design of Selective Peptidomimetic Agonists for the Human Orphan Receptor BRS-3. J Med Chem. 2003;46:1918–1930. doi: 10.1021/jm0210921. [DOI] [PubMed] [Google Scholar]
- Weber D, Berger C, Heinrich T, Eickelmann P, Antel J, Kessler H. Systematic Optimization of a Lead-Structure Identities for a Selective Short Peptide Agonist for the Human Orphan Receptor BRS-3. J Pept Sci. 2002;8:461–475. doi: 10.1002/psc.407. [DOI] [PubMed] [Google Scholar]
- Weber HC, Hampton LL, Jensen RT, Battey JF. Structure and Chromosomal Localization of the Mouse Bombesin Receptor Subtype 3 Gene. Gene. 1998;211:125–131. doi: 10.1016/s0378-1119(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Weber HC, Jensen RT, Battey JF. Molecular Organization of the Mouse Gastrin-Releasing Peptide Receptor Gene and Its Promoter. Gene. 2000;224:137–149. doi: 10.1016/s0378-1119(99)00563-6. [DOI] [PubMed] [Google Scholar]
- Weber HC, Walters J, Leyton J, Casibang M, Purdom S, Jensen RT, Coy DH, Ellis C, Clark G, Moody TW. A Bombesin Receptor Subtype-3 Peptide Increases Nuclear Oncogene Expression in a MEK-1 Dependent Manner in Human Lung Cancer Cells. Europ J Pharmacol. 2001;412:13–20. doi: 10.1016/s0014-2999(00)00941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert N, Li YY, Schick RR, Coy DH, Classen M, Schusdziarra V. Role of Vagal Fibers and Bombesin/Gastrin-Releasing Peptide-Neurons in Distention-Induced Gastrin Release in Rats. Regul Pept. 1997;69:33–40. doi: 10.1016/s0167-0115(97)02127-7. [DOI] [PubMed] [Google Scholar]
- Whitley JC, Moore C, Giraud AS, Shulkes A. Molecular Cloning, Genomic Organization and Selective Expression of Bombesin Receptor Subtype 3 in the Sheep Hypothalamus and Pituitary. J Mol Endocrinol. 1999;23:107–116. doi: 10.1677/jme.0.0230107. [DOI] [PubMed] [Google Scholar]
- Willey JC, Lechner JF, Harris CC. Bombesin and C-Terminal Tetradecapeptide of Gastrin-Releasing Peptide Are Growth Factors for Normal Human Bronchial Epithelial Cells. Exp Cell Res. 1984;153:245–248. doi: 10.1016/0014-4827(84)90466-x. [DOI] [PubMed] [Google Scholar]
- Williams BY, Dion SB, Schonbrunn A. Role of Receptor and Protein Kinase C Activation in the Internalization of the Gastrin-Releasing Peptide Receptor. Mol Pharmacol. 1998;54:889–898. doi: 10.1124/mol.54.5.889. [DOI] [PubMed] [Google Scholar]
- Williams BY, Schonbrunn A. Bombesin Receptors in a Human Duodenal Tumor Cell Line: Binding Properties and Function. Cancer Res. 1994;54(3):818–824. [PubMed] [Google Scholar]
- Williams BY, Wang Y, Schonbrunn A. Agonist Binding and Protein Kinase C Activation Stimulate Phosphorylation of the Gastrin-Releasing Peptide Receptor at Distinct Sites. Mol Pharmacol. 1996;50:716–727. [PubMed] [Google Scholar]
- Woll PJ, Rozengurt E. Bombesin and Bombesin Antagonists: Studies in Swiss 3T3 Cells and Human Small Cell Lung Cancer. Br J Cancer. 1988a;57:579–586. doi: 10.1038/bjc.1988.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll PJ, Rozengurt E. [D-Arg1-,D-Phe5,DTrp7,9,Leu11]-Substance P: a Potent Bombesin Antagonist in Murine Swiss 3T3 Cells Inhibits the Growth of Human Small-Cell Lung Cancer Cells in Vitro. Proc Natl Acad Sci USA. 1988b;85:1859–1863. doi: 10.1073/pnas.85.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JM, Hoang DO, Feldman RI. Differential Activation of Human Gastrin-Releasing Peptide Receptor-Mediated Responses by Bombesin Analogs. Mol Pharmacol. 1995;47(4):871–881. [PubMed] [Google Scholar]
- Wu JM, Nitecki DE, Biancalana S, Feldman RI. Discovery of High Affinity Bombesin Receptor Subtype 3 Agonists. Mol Pharmacol. 1996;50(5):1355–1363. [PubMed] [Google Scholar]
- Xiao D, Chinnappan D, Pestell R, Albanese C, Weber HC. Bombesin Regulates Cyclin D1 Expression Through the Early Growth Response Protein Egr-1 in Prostate Cancer Cells. Cancer Res. 2005;65:9934–9942. doi: 10.1158/0008-5472.CAN-05-1830. [DOI] [PubMed] [Google Scholar]
- Xiao D, Qu X, Weber HC. Activation of Extracellular Signal-Regulated Kinase Mediates Bombesin-Induced Mitogenic Responses in Prostate Cancer Cells. Cell Signal. 2003;15:945–953. doi: 10.1016/s0898-6568(03)00059-7. [DOI] [PubMed] [Google Scholar]
- Xiao D, Wang J, Hampton LL, Weber HC. The Human Gastrin-Releasing Peptide Receptor Gene Structure, Its Tissue Expression and Promoter. Gene. 2001;264:95–103. doi: 10.1016/s0378-1119(00)00596-5. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ohki-Hamazaki H, Wada K. Differential Effects of Social Isolation Upon Body Weight, Food Consumption, and Responsiveness to Novel and Social Environment in Bombesin Receptor Subtype-3 (BRS-3) Deficient Mice. Physiol Behav. 2000a;68:555–561. doi: 10.1016/s0031-9384(99)00214-0. [DOI] [PubMed] [Google Scholar]
- Yamada K, Santo-Yamada Y, Wada E, Wada K. Role of Bombesin (BN)-Like Peptides/Receptors in Emotional Behavior by Comparison of Three Strains of BN-Like Peptide Receptor Knockout Mice. Mol Psychiatry. 2002a;7:113–7. doi: 10.1038/sj.mp.4000974. [DOI] [PubMed] [Google Scholar]
- Yamada K, Santo-Yamada Y, Wada K. Stress-Induced Impairment of Inhibitory Avoidance Learning in Female Neuromedin B Receptor-Deficient Mice. Physiol Behav. 2003;78:303–309. doi: 10.1016/s0031-9384(02)00979-4. [DOI] [PubMed] [Google Scholar]
- Yamada K, Santo-Yamada Y, Wada K. Restraint Stress Impaired Maternal Behavior in Female Mice Lacking the Neuromedin B Receptor (NMB-R) Gene. Neurosci Lett. 2002b;330:163–166. doi: 10.1016/s0304-3940(02)00771-1. [DOI] [PubMed] [Google Scholar]
- Yamada K, Wada E, Imaki J, Ohki-Hamazaki H, Wada K. Hyperresponsiveness to Palatable and Aversive Taste Stimuli in Genetically Obese (Bombesin Receptor Subtype-3-Deficient) Mice. Physiol Behav. 1999;66:863–867. doi: 10.1016/s0031-9384(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Yamada K, Wada E, Wada K. Male Mice Lacking the Gastrin-Releasing Peptide Receptor (GRP-R) Display Elevated Preference for Conspecific Odors and Increased Social Investigatory Behaviors. Brain Res. 2000b;870:20–26. doi: 10.1016/s0006-8993(00)02395-7. [DOI] [PubMed] [Google Scholar]
- Yamada K, Wada E, Wada K. Female Gastrin-Releasing Peptide Receptor (GRP-R)-Deficient Mice Exhibit Altered Social Preference for Male Conspecifics: Implications for GRP/GRP-R Modulation of GABAergic Function. Brain Res. 2001;894:281–287. doi: 10.1016/s0006-8993(01)02032-7. [DOI] [PubMed] [Google Scholar]
- Yamano M, Ogura H, Okuyama S, Ohki-Hamazaki H. Modulation of 5-HT System in Mice With a Targeted Disruption of Neuromedin B Receptor. J Neurosci Res. 2002;68:59–64. doi: 10.1002/jnr.10194. [DOI] [PubMed] [Google Scholar]
- Yegen BC. Bombesin-Like Peptides: Candidates As Diagnostic and Therapeutic Tools. Curr Pharm Des. 2003;9:1013–1022. doi: 10.2174/1381612033455134. [DOI] [PubMed] [Google Scholar]
- Yonemori K, Sumi M, Fujimoto N, Ito Y, Imai A, Kagami Y, Ikeda H. Pro-Gastrin-Releasing Peptide As a Factor Predicting the Incidence of Brain Metastasis in Patients With Small Cell Lung Carcinoma With Limited Disease Receiving Prophylactic Cranial Irradiation. Cancer. 2005;104:811–816. doi: 10.1002/cncr.21238. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Carstens E, McGlone F. Chronic Itch and Chronic Pain: Analogous Mechanisms. Pain. 2007;131:4–7. doi: 10.1016/j.pain.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Younes M, Wank SA, Vinayek R, Jensen RT, Gardner JD. Regulation of Bombesin Receptors on Pancreatic Acini by Cholecystokinin. Am J Physiol. 1989;256:G291–G298. doi: 10.1152/ajpgi.1989.256.2.G291. [DOI] [PubMed] [Google Scholar]
- Zachary I, Rozengurt E. Internalization and Degradation of Peptides of the Bombesin Family in Swiss 3T3 Cells Occurs With Ligand-Induced Receptor Down Regulation. EMBO J. 1987;6:2233–2239. doi: 10.1002/j.1460-2075.1987.tb02495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schuhmacher J, Waser B, Wild D, Eisenhut M, Reubi JC, Maecke HR. DOTA-PESIN, a DOTA-Conjugated Bombesin Derivative Designed for the Imaging and Targeted Radionuclide Treatment of Bombesin Receptor-Positive Tumours. Eur J Nucl Med Mol Imaging. 2007a;34:1198–1208. doi: 10.1007/s00259-006-0347-4. [DOI] [PubMed] [Google Scholar]
- Zhang L, Mantey S, Jensen RT, Gardner JD. An Analogue of Substance P With Broad Receptor Antagonist Activity. Biochim Biophys Acta. 1988;972:37–44. doi: 10.1016/0167-4889(88)90100-0. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Bhola NE, Lui VW, Siwak DR, Thomas SM, Gubish CT, Siegfried JM, Mills GB, Shin D, Grandis JR. Antitumor Mechanisms of Combined Gastrin-Releasing Peptide Receptor and Epidermal Growth Factor Receptor Targeting in Head and Neck Cancer. Mol Cancer Ther. 2007b;6:1414–1424. doi: 10.1158/1535-7163.MCT-06-0678. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cai W, Cao F, Schreibmann E, Wu Y, Wu JC, Xing L, Chen X. 18F-Labeled Bombesin Analogs for Targeting GRP Receptor-Expressing Prostate Cancer. J Nucl Med. 2006;47:492–501. [PubMed] [Google Scholar]
- Zheng R, Iwase A, Shen R, Goodman OB, Jr., Sugimoto N, Takuwa Y, Lerner DJ, Nanus DM. Neuropeptide-Stimulated Cell Migration in Prostate Cancer Cells Is Mediated by RhoA Kinase Signaling and Inhibited by Neutral Endopeptidase. Oncogene. 2006;25:5942–5952. doi: 10.1038/sj.onc.1209586. [DOI] [PubMed] [Google Scholar]
- Zhou J, Chen J, Mokotoff M, Ball ED. Targeting Gastrin-Releasing Peptide Receptors for Cancer Treatment. Anti-Cancer Drugs. 2004;15:921–927. doi: 10.1097/00001813-200411000-00001. [DOI] [PubMed] [Google Scholar]
- Zhu WY, Goke B, Williams JA. Binding, Internalization and Processing of Bombesin by Rat Pancreatic Acini. Am J Physiol. 1991;261:G57–G64. doi: 10.1152/ajpgi.1991.261.1.G57. [DOI] [PubMed] [Google Scholar]