Abstract
The purpose of this study was to determine the radiosensitivities of 31 human oesophageal squamous cell carcinoma cell lines with a colony-formation assay. A large variation in radiosensitivity existed among 31 cell lines. Such a large variation may partly explain the poor result of radiotherapy for this cancer. One cell line (KYSE190) demonstrated an unusual radiosensitivity. Ataxia-telangiectasia-mutated (ATM) gene in these cells had five missense mutations, and ATM protein was truncated or degraded. Inability to phosphorylate Chk2 in the irradiated KYSE190 cells suggests that the ATM protein in these cells had lost its function. The dysfunctional ATM protein may be a main cause of unusual radiosensitivity of KYSE190 cells. Because the donor of these cells was not diagnosed with ataxia telangiectasia, mutations in ATM gene might have occurred during the initiation and progression of cancer. Radiosensitive cancer developed in non-hereditary diseased patients must be a good target for radiotherapy.
Keywords: ATM, DNA-PKcs, DSBs repair, oesophageal squamous cell carcinoma, mutation, radiation sensitivity
Because about 90% of oesophageal squamous cell carcinoma (ESCC) would be diagnosed in late stage, radiotherapy is frequently applied to this disease. However, the overall therapeutic results are still poor. The 5-year survival rate after radiotherapy alone ranged from 6% to 11.6% (Newaishy et al. 1982; Harrison et al. 1988). Even though the stage I oesophageal cancer has been considered as a good target for radiation therapy, the 5-year overall survival ratio was only 62% (Shioyama et al. 2005). To develop a more effective radiotherapy protocol, we are planning to characterize the radiosensitive- or radioresistant-ESCC cells using the gene expression profiling. The aim of this study was to determine the radiation sensitivities of 31 human ESCC cell lines with a colony-formation assay. A large variation in radiosensitivity among 31 cell lines was found. Because the radiosensitivity of one cell line, KYSE190, was outside a cluster of radiation sensitivities of the other 30 cell lines, we also investigated the cause of unusual radiosensitivity of these cells. We considered that the repair of radiation-induced DNA double strand breaks (DSBs) was suppressed in the KYSE190 cells. Only one human cell line (M059J) has been reported as DNA-PKcs defective and radiosensitive (Allalunis-Turner et al. 1993; Lees-Miller et al. 1995). Ataxia-telangiectasia-mutated (ATM) protein plays a major role in the DSBs repair pathway (Bakkenist and Kastan 2003). Therefore, we analysed the sequences of DNA-PKcs and ATM genes and the products of both genes of KYSE190 cells. DSBs are the most deleterious and lethal form of DNA damage. DSBs are rejoined or repaired by two major pathways in mammalian cells: homologous recombination (HR) and non-homologous end joining (NHEJ). DNA-PK is a nuclear, serine/threonine protein kinase consisting of three subunits, DNA-dependent protein kinase catalytic subunit (DNA-PKcs), Ku70, and Ku80, that is involved in the NHEJ, V(D)J recombination, and modulation of transcription (Poltoratsky et al. 1995). It is well known that cells lacking DNA-PKcs activity are highly sensitive to DSB-inducing agents such as ionizing radiations (Sak et al. 2002). Carlomagno et al. (2000) compared the expression levels of nine DNA repair proteins (DNA-PKcs, Ku70, Ku80, XRCC4, LigaseIV, ATM, Rad1, Hus1, and Rad51) in human fibroblast cell lines with different radiosensitivities. All nine proteins were expressed at similar levels in all cell lines. Although small difference in the activities of the DNA-PKcs and XRCC4/ligaseIV complexes was found, the differences did not correlate with the cellular radiation sensitivity. Douglas et al. (2002) identified four phosphorylation sites in the DNA-PKcs protein (Thr-2609, Ser-2612, Thr-2638, and Thr-2647). These sites were autophosphorylated with their own DNA-PKcs. Chan et al. (2002) demonstrated that autophosphorylation of DNA-PKcs was required for the repair of DSBs.
Therefore, we investigated the phosphorylation of DNA-PKcs in irradiated KYSE190 cells using an anti-phosphorylated DNA-PKcs antibody that recognized the phosphorylation of Thr-2609. DNA-PKcs phosphorylation was not observed. No mutation was detected in the four-phosphorylation sites, except one base change in the FAT domain of the DNA-PKcs gene. However, non-truncated DNA-PKcs protein was produced in our KYSE190 cells. The expression of ATM protein was reduced. Five missense mutations were found in the ATM gene of KYSE190 cells. The phosphorylation of Chk2 was not observed in the irradiated KYSE190 cells. Because the phosphorylation of Chk2 is downregulated by ATM protein (Chaturvedi et al. 1999; Ahn et al. 2000), ATM in KYSE190 cells might lose its function. We consider that the high radiosensitivity of KYSE 190 cells was caused by the failure of delivery of a stress signal from ATM protein to DNA-PKcs. This cell line will be useful for investigating the mechanism of the NHEJ pathway, in particular, the association between ATM and DNA-PKcs proteins. We are considering that the occurrence of ATM mutation during the carcinogenesis is rare. Even if such a rare case is excluded, radiosensitive and radioresistant ESCCs are existed. It is an urgent necessity to establish the simple and precise methods to predict the cancer radiosensitivity before radiotherapy.
Materials and methods
Cells and culture medium
All cell lines with a prefix of KYSE were established from the oesophageal cancer tissues excised from Japanese patients (Shimada et al. 1992, 2003). Originally, cells were maintained in Ham's F12/RPMI1640 supplemented with 2% foetal calf serum (FCS). A normal human-diploid fibroblast strain (NB1RGB) was obtained from Riken Cell Bank (http://www.brc.riken.jp/lab/cell/ Tsukuba, Japan). Because we intended to compare the radiation sensitivities of many cell lines, a single kind of medium, alpha MEM (Sigma, Tokyo, Japan) supplemented with l-glutamine, and 10% FCS was used in this study for maintenance and all experiments. A solution of 0.25% trypsin plus 0.02% ethylenediaminetetraacetic acid was used to harvest the cells.
X irradiation
Actively growing cells were harvested and suspended in growth medium. About 5 × 104 cells (1 ml) were put into a plastic test tube and irradiated with X-rays at room temperature (rt). The X-ray generator (Shimadzu, Kyoto, Japan) was operated at 200 kVp, 20 mA, 0.5 mm Cu plus 0.5 mm Al external filtration. The dose proximal to the cells was measured with a Victoreen condenser chamber (Victoreen Instruments, A-2340Moedling, Austria). The dose-rate was 1.0 Gy/min. Cells were exposed to doses of 0, 0.5, 1, 2, 4, 6, or 8 Gy.
Clonogenic assay for cell survival
Immediately after irradiation, appropriate numbers of cells were seeded in plastic culture dishes and cultured for 2 weeks in 95% air plus 5% CO2 at 37 °C. The colonies were fixed with ethanol and stained with 2% Giemsa. Colonies composed of 50 or more cells were scored, and plating efficiencies and percentage survivals were calculated (Ban et al. 1990).
Curve fitting and data analysis
The dose responses were analysed using a multi-target model
D is the dose in Gray and S is the surviving fraction at dose D. D0, the dose that causes the straight-line portion of the survival curve to decrease to 37%, is 1/e. Dq, the dose at shoulder part of dose-survival curve, was also calculated. The intercept, N, of each survival curve is estimated by least-squares regression analysis as the parameter of non-linear function (Ban et al. 1990). Dose-survival curves were computer-generated using the KALEIDAGRAPH program (Synergy Software, Reading, PA, USA).
Immunohistochemical staining of γH2AX and phosphorylated DNA-PKcs
Immunocytochemical procedures were carried out essentially as described by Rogakou et al. (1999) and Tomilin et al. (2001). Briefly, cells were cultured on cover glasses (Iwaki, Asahi Techno Glass, Tokyo, Japan; code no. 4925–040) placed in 6-well tissue culture plates (Falcon, Becton Dickinson Labware, Lincoln Park, NJ, USA). The cells were rinsed with phosphate buffered saline (PBS), treated with PBS supplemented with 0.2% Triton X-100 for 3.5 min at rt and then rinsed again with PBS. Cells were fixed for 10 min in ice-cold 4% paraformaldehyde solution in PBS. Cells were kept in 70% ethanol at 4 °C overnight, dehydrated in 99% ethanol and dried. Fixed cells were incubated in PBS for 15 min and in PBS containing 0.1% Triton X-100 for 25 min at rt, then, incubated in 8% BSA with 0.02% Tween 20 in PBS for 60 min at 37 °C. Then, the slides were incubated at 37 °C with a 200-fold dilution of mouse anti-phospho-H2AX antibody (Upstate, Waltham, MA, USA; catalogue number 07–164) or rabbit anti-phospho-DNA-PKcs antibody for 1.5 h at 37 °C. Anti-phospho-DNA-PKcs antibody (a gift from Dr R. Okayasu, International Space Radiation Laboratory, National Institute of Radiological Sciences) was prepared according to the information from Chan et al. (2002). Next, slides were washed ×4 in PBS supplemented with 0.2% Tween 20 for 15 min each and incubated with a 200-fold dilution of Cy2-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA; catalogue number 111-225-144) for 1.5 h at 37 °C. Finally, the slides were washed ×4 in PBS supplemented with 0.2% Tween 20 for 15 min each, and DNA was stained by incubating the slides with propidium iodide (25 µg/ml in PBS, for 15 min at room temperature).
Microscopy and image processing
An axioplan 2 microscope (Carl Zeiss, Germany) with appropriate filter sets for green (Cy2) and red (propidium iodide) fluorescence and a Plan-NEOFLOAR 100/1.30 objective lens was used. Images were captured in a computer using a digital camera (Hamamatsu Photonics, Japan; C4742-95 Digital Camera) and were then analysed using the isis (Meta Systems GmbH, Germany) and Adobe Photoshop programs (Adobe Systems, San Jose, CA, USA).
Sequencing of DNA-PKcs and ATM
Thirty-eight DNA fragments covering almost the entire coding region of DNA-PKcs were amplified by polymerase chain reaction (PCR). The primer sequence was designed with the aid of the Primer 3 program (S. Rozen and H.J. Skaletsky, code available at http://www.genome.wi.mit.edu/genome_software/other/primer3.html. Primer3, 1998).
Primer sets covering almost the entire coding region of ATM and PCR conditions were prepared according to the information from Sasaki et al. (1998). Primer sequences and PCR conditions for both genes are available on request from Sadayuki Ban or Takashi Imai.
Information for the DNA-PKcs sequence was extracted in July 2003. GenBank accession numbers used in this study were as follows: DNA-PKcs, NM_006904; ATM, U33841
Western-blot analysis
Cells were lysed with PEB4 lysis buffer (0.5 m Tris/CL, 10% SDS, 5% 2-mercaptoethanol, 100 mm Na3VO4, 100 mm NaF). SDS-PAGE was performed on 2–15% gradient polyacrylamide gels [PAG Mini ‘DAIICHI’ 2/15 (13 W), Daiichi Pure Chemicals, Tokyo], and the separated ATM or DNA-PKcs protein was transferred onto Sequi-Blot PVDF Membranes (Bio-Rad, Hercules, CA, USA; catalogue number 162–0184) at 35 V for 16 h. The phosphorylated Chk2 protein was transferred onto membranes at 35 mA for 4 h. Membranes were blocked by incubation for 2 h with 3% ECLTM.blocking agent RPN2125 (Amersham Pharmacia Biotech, Piscataway, NJ, USA) in TBS containing 0.1% Tween 20 (TBS-T). The antigens were detected using goat anti-DNA-PKcs antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA; catalogue number E022), goat anti-ATM antibody (Novus Biological, Inc., Littleton, CO, USA; catalogue number NB100-270) or rabbit anti-phospho-Chk2 antibody (Cell Signalling Technology, Inc., Beverly, MA 01915, USA; catalogue number 2661), followed by incubation with the appropriate horseradish peroxide-conjugated secondary antibody. The signal was detected using the ECL plus Western Blotting Detection System RPN2132 (Amersham Biosciences, Bucks, UK) as described by the manufacturer. The marker used was the Precision Plus Protein Standards Dual Colour (Bio-Rad; catalogue number 161–0374).
Results
Radiation sensitivities of 31 ESCC cell lines
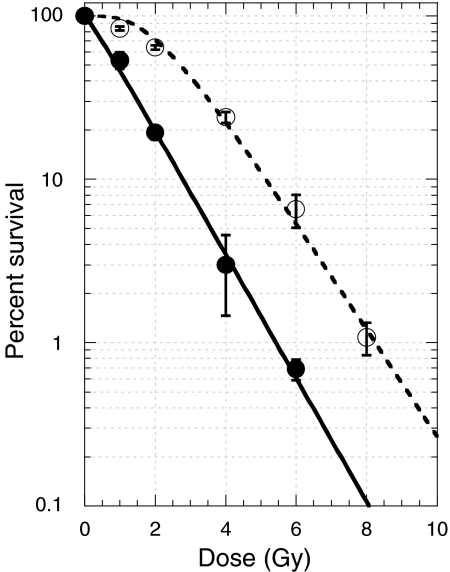
We investigated the radiation sensitivities of 31 human ESCC cell lines with a colony formation assay. Representative dose-survival responses of KYSE190 and KYSE70 cells are depicted in Figure 1. Data were plotted by the formula of S = 100[1 − (1 − e−D/D0)N] D0, D10, and Dq values were 1.1 Gy, 2.8 Gy, and 0.2 Gy for KYSE190 cells, and 1.3 Gy, 5.2 Gy, and 2.3 Gy for KYSE70 cells.
Figure 1.
X-ray dose–survival responses of KYSE190 cells (closed circles with solid line) and KYSE70 cells (open circles with dotted line). Actively growing cells were exposed to various doses of X-rays. Immediately after irradiation, appropriate number of cells were seeded and cultured for 2 weeks. Colonies composed of 50 or more cells were scored, and percentage survivals were calculated. Dose–survival curve of KYSE190 cells had a negligible shoulder, whereas that of KYSE70 cells had a shoulder.
The average X-ray survival curve values for 31 ESCC cell lines are given in Table 1.
Table 1.
Four survival parameters' value of 31 oesophageal cancer cell lines
| Cell lines | D0 (Gy) | D10 (Gy) | Dq (Gy) | N |
|---|---|---|---|---|
| KYSE-30 | 2.0 | 5.6 | 0.9 | 1.6 |
| KYSE-70 | 1.3 | 5.2 | 2.3 | 5.8 |
| KYSE-110 | 1.0 | 4.5 | 2.3 | 10.7 |
| KYSE-140 | 1.0 | 4.4 | 1.9 | 6.3 |
| KYSE-150 | 2.4 | 7.3 | 1.9 | 2.2 |
| KYSE-170 | 1.3 | 5.3 | 2.4 | 6.9 |
| KYSE-180 | 2.1 | 7.1 | 2.2 | 2.8 |
| KYSE-190 | 1.1 | 2.8 | 0.2 | 1.2 |
| KYSE-200 | 2.1 | 6.0 | 1.1 | 1.7 |
| KYSE-220 | 1.0 | 4.7 | 2.5 | 11.7 |
| KYSE-270 | 1.3 | 4.3 | 1.3 | 2.8 |
| KYSE-350 | 2.0 | 5.5 | 0.9 | 1.5 |
| KYSE-410 | 1.6 | 4.9 | 1.3 | 2.2 |
| KYSE-450 | 1.8 | 6.7 | 2.5 | 3.9 |
| KYSE-510 | 2.0 | 6.4 | 1.8 | 2.4 |
| KYSE-520 | 1.7 | 5.5 | 1.6 | 2.6 |
| KYSE-590 | 1.1 | 5.0 | 2.5 | 9.9 |
| KYSE-770 | 1.0 | 4.4 | 2.2 | 9.0 |
| KYSE-790 | 1.0 | 3.6 | 1.2 | 3.2 |
| KYSE-850 | 1.5 | 4.3 | 0.9 | 1.8 |
| KYSE-890 | 1.3 | 5.0 | 2.1 | 4.9 |
| KYSE-960 | 1.2 | 5.4 | 2.6 | 7.8 |
| KYSE-1170 | 1.0 | 4.5 | 2.2 | 9.1 |
| KYSE-1190 | 1.4 | 4.3 | 1.1 | 2.1 |
| KYSE-1240 | 1.5 | 5.6 | 2.2 | 4.2 |
| KYSE-1250 | 1.3 | 4.5 | 1.5 | 3.4 |
| KYSE-1260 | 1.1 | 5.2 | 2.7 | 12.3 |
| KYSE-1440 | 1.1 | 3.9 | 1.4 | 3.8 |
| KYSE-2270 | 1.2 | 4.8 | 2.1 | 5.7 |
| KYSE-2400 | 2.3 | 7.2 | 1.8 | 2.2 |
| KYSE-2650 | 1.6 | 4.8 | 1.2 | 2.1 |
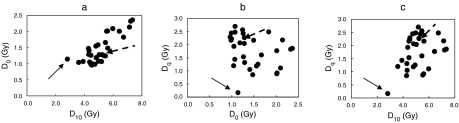
The correlations between three survival parameters (D0, D10, and Dq) of 31 cell lines of the KYSE series are summarized in Figure 2.
Figure 2.
Correlation of Dq, D0, and D10 values among 31 human oesophageal carcinoma cell lines. Arrows indicate the KYSE190 cell line, and broken arrows, KYSE70 cell line. The dose survival responses were analysed using a multitarget model, S = 100[1 − (1 − e−D/Do) N]. D0 is the dose that causes the straight-line portion of the survival curve to decrease to 37%, Dq, the dose at shoulder part of dose-survival curve, and D10, the dose that decrease the cell survival to 10%. Dq value of KYSE190 cells was close to zero.
There was a wide variation in each survival value of D0, Dq, or D10. In particular, one cell line (KYSE190) was set apart from a population of 30 cell lines. Dq and D10 values of KYSE190 cells were the smallest among those of 31 cell lines. Because the Dq value was close to zero, the repair activity of sublethal injuries may be suppressed in KYSE190 cells.
Radiation-induced DSBs were not efficiently rejoined in the radiosensitive KYSE190 cells
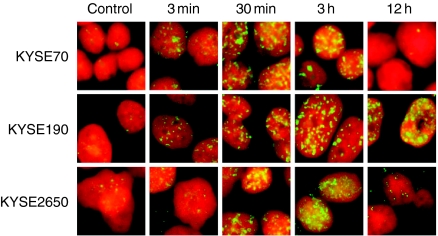
DSBs are considered to be one of the most serious forms of DNA damage. To confirm that a defect in the repair of DSBs is involved in the unusual radiosensitivity of KYSE190 cells, cells were exposed to X-rays at 0 Gy or 10 Gy and treated with the anti-γH2AX antibody at various times after X irradiation (Figure 3). An increase in the number of γH2AX foci appeared at 3 min after X irradiation. Numerous foci were observed at 0.5 h−3 h. At 12 h after irradiation, many foci remained in KYSE190 cells, although almost all foci had disappeared in KYSE70 and KYSE2650 cells. This suggested that the repair activity on DSBs was severely suppressed in KYSE190 cells.
Figure 3.
Formation and disappearance of the γH2AX foci at various times after irradiation. Three KYSE cell lines were exposed to 0 Gy or 10 Gy of X-rays, fixed at 3 min, 30 min, 3 h, and 12 h after X irradiation and treated with anti-γH2AX antibody. The presence of double strand breaks (DSBs) can be observed as γH2AX foci. Many γH2AX foci in irradiated KYSE190 cells remained at 12 h after irradiation, while almost all foci had disappeared in other cell lines. These mean that the X-ray-induced DSBs in KYSE190 cells were not repaired.
DNA-PKcs protein in KYSE190 cells was not phosphorylated after X-irradiation
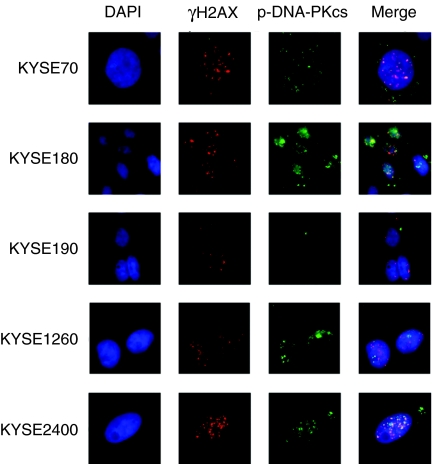
Cells were exposed to X-rays at 2 Gy and immunostained with the anti-γH2AX antibody or the anti-phospho-DNA-PKcs antibody (Figure 4). The formation of γH2AX foci suggested that the DSBs were induced in all KYSE cell lines studied. DNA-PKcs in KYSE70, 180, 1260, and 2400 cell lines were phosphorylated, but DNA-PKcs in KYSE190 cells was not phosphorylated.
Figure 4.
Immunohistochemical analysis of the γH2AX and the phosphorylated DNA-PKcs. Five KYSE cell lines were exposed to 2 Gy of X-rays, fixed at 1 h after X irradiation and treated with DAPI, anti-γH2AX antibody or anti-phospho-DNA-PKcs antibody. DNA-PKcs in KYSE190 cells was not phosphorylated.
DNA-PKcs gene in KYSE190 cells had one mutation, but produced the non-truncated DNA-PKcs protein
Immunohistochemical staining assay demonstrated that the DNA-PKcs protein in the irradiated KYSE190 cells was not phosphorylated. As the first step, we suspected that the KYSE190 cells had mutations reducing the kinase activity of the DNA-PKcs.
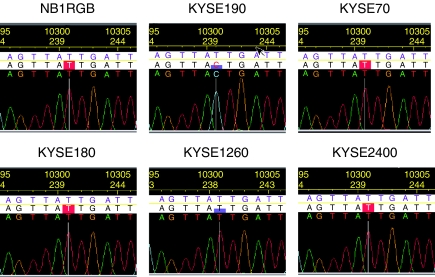
Sequences of almost all coding regions of DNA-PKcs gene were analysed in one normal fibroblast cell strain, NB1RGB, and 5 KYSE cell lines. Only one base change was found at 10301 in the FAT domain of KYSE190 cells, but not in other cell lines.
T at 10301 (10358 in NM_006904 revised on October 26, 2004) is the second base coding the isoleucine at 3434. The mutation from T to C converts isoleucine to threonine (Figure 5).
Figure 5.
A base change (T10301) observed in the FAT domain of the KYSE190 cell DNA-PKcs gene. Sequences of almost all coding regions were analysed in one normal fibroblast cell strain (NB1RGB) and 5 KYSE cell lines. The mutation from T at 10301 to C converts isoleucine to threonine.
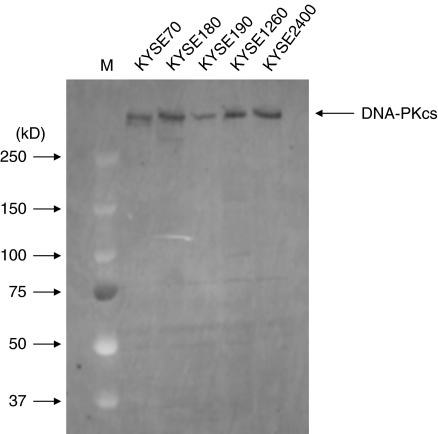
Figure 6 demonstrates that non-truncated DNA-PKcs protein was produced in KYSE190 cells. Truncated protein was also not observed.
Figure 6.
Western-blot analysis of the DNA-PKcs protein in 5 KYSE cell lines. KYSE190 cells produced normal DNA-PKcs protein.
ATM gene in KYSE190 cells had missense mutations and lost its function
Sequences of almost all coding regions of ATM gene were analysed (Table 2). Five missense and one silent mutations were found in the exons 12, 15, 29, 30, and 64.
Table 2.
Summary of Ataxia-telangiectasia-mutated mutations in KYSE190 cells
| Exon | Nucleotide | Amino acid change |
|---|---|---|
| 12 | G28774A | Ala554Thr |
| 15 | C33225G | Asn750Lys |
| 29 | C65890C/T | His1380His/Tyr |
| 30 | T66615A/T | Ser1455Ser/Arg |
| 64 | G142229A | Asp3003Asn |
| 64 | G142315A | No change |
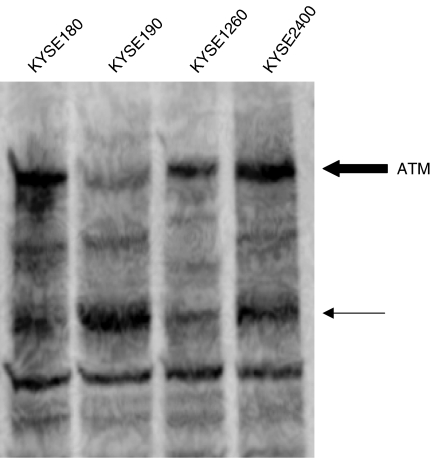
The expression of ATM protein (bold arrow in Figure 7) in KYSE 190 cells was reduced. The signal band marked with the thin arrow was stronger in KYSE190 cells than that in other cell lines. Missense mutations may cause the instability of ATM protein.
Figure 7.
Western-blot analysis of ATM protein in KYSE190 and three other KYSE cell lines. The expression of normal ATM protein (bold arrow) in KYSE190 cells was reduced. Thin arrow shows the presence of degraded or truncated protein.
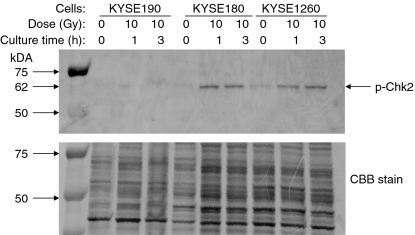
To determine whether the ATM protein in KYSE190 cells maintains its function, the phosphorylation of Chk2 protein was investigated (Figure 8). Three KYSE cell lines were exposed to X-rays at 0 Gy or10 Gy. Protein extracted at 1 h or 3 h after X irradiation was treated with the anti-phospho-Chk2 antibody. The phosphorylation of Chk2 was observed in KYSE180 and KYSE1260 cells at 1 h after X irradiation, but not in KYSE190 cells within 3 h after X irradiation. Failure in Chk2 phosphorylation after irradiation suggests that the ATM protein in KYSE190 cells has lost its function.
Figure 8.
Western-blot analysis of the phosphorylation of Chk2 protein. Three KYSE cell lines were exposed to 0 Gy or 10 Gy of X-rays and cultured for 1 h or 3 h. The antigens were detected with the anti-phospho-Chk2 antibody. The sample loading was monitored by Coomassie Brilliant Blue R-250 (CBB) staining. Unphosphorylation of Chk2 after irradiation suggests that the ATM protein in KYSE190 cells has lost its function.
Discussion
Radiosensitivities of 31 human ESCC cell lines were investigated. There was a large variation in radiosensitivity among 31 cell lines (Table 1 and Figure 2). In particular, only the KYSE190 cell line had a negligible shoulder in its dose–survival curve (Figure 1). It is well known that the dose-survival curve after exposure to high-linear energy transfer (LET) radiation has no shoulder, and that non-repairable DSBs are the main lesions induced by exposure to high-LET radiation (Ritter et al. 1977; Weber and Flentje 1993; Stenerlow et al. 1996). Information from the high-LET radiation study suggested that the radiation-induced DSBs have not been repaired in cells having a negligible shoulder in their dose-survival curve. Thus, we considered that the repair of X-ray-induced DSBs was suppressed in KYSE190 cells. If the KYSE190 cells are defective in the repair of DSBs, these cells may be useful as a material to investigate the mechanism of DSB repair pathway.
When cells are exposed to ionizing radiation, DSBs are induced (Ward 1985; Goodhead 1989). Immediately after DSBs occur in living cells, histone H2AX at the site of the DSB is phosphorylated by ATM protein (Bakkenist and Kastan 2003). This phosphorylated histone H2AX is called as γH2AX. During appropriate periods of incubation, some DSBs are repaired, but others are not. When the DSBs are repaired, the γH2AX at the site of the repaired DSB is dephosphorylated (Paul et al. 2000; Redon et al. 2002). When irradiated cells are stained immunohistochemically with an anti-γH2AX antibody, the presence of DSBs can be observed as γH2AX foci under a fluorescence microscope. While almost all γH2AX foci had disappeared at 12 h after irradiation in KYSE70 and KYSE2650 cells, the γH2AX foci in irradiated KYSE190 cells remained (Figure 3). That is, the repair activity of X-ray-induced DSBs was severely suppressed in KYSE190 cells. It is well known that the DSBs induced in mammalian cells are repaired by two repair pathways. One is NHEJ, and the other is HR. Almost all DSBs in mammalian cells are known to be repaired by the NHEJ. DNA-PK is a serine-threonine protein kinase consisting of three subunits DNA-PKcs, Ku70, and Ku80 that plays a major role in NHEJ. Okayasu et al. (2000) demonstrated that the expression level of DNA-PKcs protein was significantly reduced in cells from radiosensitive BALB/c mice. DNA-PKcs is autophosphorylated at four sites (2609Thr, 2612Ser, 2638Thr, and 2647Thr) after exposure to radiation. Sak et al. (2002) demonstrated inhibited repairing of DSBs and enhanced radiosensitivity in cells whose DNA-PKcs kinase activity was knock-downed with anti-sense RNAs. These results demonstrated that DNA-PKcs is one of the main proteins responsible to the cellular radiation sensitivity. When cells are exposed to external stresses such as X-rays or UV light, the ATM monomer is phosphorylated with the kinase of the other monomer (Bakkenist and Kastan 2003). After the phosphorylated, ATM monomer binds to DNA-PKcs protein, the DNA-PKcs is autophosphorylated with its own DNA-PKcs kinase. Immediately after DSBs are induced, Ku70 and Ku80 protein bind to the ends of DSBs and form a complex with the DNA-PKcs protein (Lieber et al. 2003). Ku70, Ku80, and DNA-PKcs are phosphorylated by DNA-PKcs (Lieber et al. 2003). These results suggest that the phosphorylation of DNA-PKcs is an important step in the NHEJ pathway (Chan et al. 2002).
Therefore, we investigated the phosphorylation level of DNA-PKcs of KYSE190 and other cells with an immunohistochemical assay using an antibody to phosphorylated DNA-PKcs. DNA-PKcs protein has four phosphorylation sites, as shown here. In this study, we used an antibody that recognizes the phosphorylated threonine at 2609. However, absolutely no signal of DNA-PKcs phosphorylation was found in KYSE190 cells (Figure 4). Thus, we concluded that the NHEJ pathway in KYSE190 cells was suppressed by a defect of autophosphorylation of DNA-PKcs.
We found a base change of T10301C in KYSE190 cells (Figure 5), which suggested the replacement of Ile with Thr at the residue 3434. DNA sequencing and the western-blot analysis demonstrated that the DNA-PKcs protein in KYSE190 cells was not truncated (Figure 6), but had an amino acid change of I3434T. The CHAFAS program (http://www.fasta.bioch.virginia.edu/fasta_www/chafas.htm) predicts that an I3434T mutation may add one turn in the secondary structure of DNA-PKcs protein. If the I3434T mutation causes a change in the secondary structure of DNA-PKcs protein, the mutated protein may be not able to associate with ATM, Ku70, and Ku86 proteins. Hartley et al. (1995) and Poltoratsky et al. (1995) also independently reported the amino acid sequence of DNA-PKcs. Comparing both sequences revealed different amino acid residues at four positions (3434, 3660, 3817, and 3862). Although Hartley et al. (1995) used the HeLa S3 cell cDNA library, Poltoratsky et al. (1995) analysed a human foetal lung cell cDNA library. The single nucleotide polymorphism (SNP) information extracted in February 2005 from the NCBI Single Nucleotide Polymorphism database (http://www.ncbi.nih.gov/projects/SNP) suggested that the T10301C of DNA-PKcs was a genomic SNP (refSNP ID: rs7830743). Average allele frequency of T is 0.857 and that of C is 0.147. The T10301C may be a silent SNP. The ATM gene in KYSE190 cells had five missense mutations in five coding regions (Table 2). Those mutations may cause the degradation of the ATM protein. The decreased expression of normal ATM protein may severely reduce the stress signal to DNA-PKcs. As mentioned above, the phosphorylated ATM monomer associates with DNA-PKcs. On the otherhand, the ATM monomer phosphorylates the Chk1 (Gatei et al. 2003), Chk2 (Chaturvedi et al. 1999; Ahn et al. 2000) and 53 bp proteins (Tibbetts et al. 1999). The phosphorylation of these proteins is an essential step in cell cycle control and apoptosis. It is well known that the phosphorylated DNA-PK complex does not contribute to these aspects of cell cycle checkpoint and apoptosis (Jimenez et al. 1999; Stiff et al. 2004). To determine whether the ATM protein in KYSE190 had a biological function, we investigated the phosphorylation of Chk2 protein in the irradiated KYSE190 cells. Phosphorylation was not observed in KYSE190 cells (Figure 8). The lack of phosphorylation of Chk2 after irradiation suggests that the ATM protein in KYSE190 cells has lost its function. We can conclude that the reduction of normal ATM protein may be the main cause of the deficiency in the phosphorylation of DNA-PKcs in the radiosensitive KYSE190 cells. DNA-PK of ATM-proficient cells phosphorylates and activates c-Abl in vitro (Kharbanda et al. 1997). Shangary et al. (2000) demonstrated that DNA-PK of ATM-deficient cells phosphorylated and activated p53, but not c-Abl in vitro. They concluded that ATM was the principal regulator of c-Abl activity. Our results suggest a similar conclusion that the stress signal from ATM may regulate the autophosphorylation of DNA-PKcs.
We can conclude that the reduction of normal ATM protein may be the main cause of the unusual radiosensitivity of KYSE190 cells. KYSE190 cells were established from a well-differentiated squamous cell carcinoma developed at the lower intrathoracic oesophagus of 69-year-old Japanese female (Shimada et al. 1992). She was not diagnosed as AT. Shimada et al. (1992) observed that the growing potential of KYSE190 cells was already very poor at early passages. Poor growth may be resulted from the mutated ATM gene. Thus, we are considering that the ATM mutation occurred in the process of initiation and progression of carcinogenesis. Cancer tissues bearing the ATM mutation may be rare. Even if such a rare case in excluded, our data suggest that the radiosensitive ESCC does exist. Such the radiosensitive cancer must be a good target for radiotherapy. Therefore, the characterization of radiosensitive- or radioresistant-ESCC cells using the gene expression profiling is an urgent necessity of establishing the assay to predict the ESCC tissues' radiation sensitivity. Thirty-one ESCC cell lines classified according to their radiation sensitivities provides a fundamental material required for this necessity.
On the other hand, we believe that the KYSE190 cells can provide another useful tool to further investigate the mechanism of the DSBs repair, particularly in relation to the association with ATM protein.
Acknowledgments
The authors are grateful for the cooperation of Drs Ryuichi Okayasu of National Institute of Radiological Sciences, Masaaki Yamazaki and Hiroyuki Tashiro of Fujiya Co.Ltd, and Issei Imoto of Tokyo Medical and Dental University. The authors are also grateful to Akiko Sakurai and Atsushi Umetsu for their technical helps. The authors sincerely thank Dr Syuhei Noda and Mr Atsushi Okabe for technical suggestions and Ms. Nobuko Ogiu for secretarial help.
References
- Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60:5934–5936. [PubMed] [Google Scholar]
- Allalunis-Turner MJ, Barron GM, Day RD, Dobler KD, Mirzayans R. Isolation of two cell lines from a human malignant glioma specimen differing in sensitivity to radiation and chemotherapeutic drugs. Radiat Res. 1993;134:349–354. [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Ban S, Setlow RB, Bender MA, et al. Radiosensitivity of skin fibroblasts from atomic bomb survivors with and without breast cancer. Cancer Res. 1990;50:4050–4055. [PubMed] [Google Scholar]
- Carlomagno F, Burnet NG, Turesson I, et al. Comparison of DNA repair protein expression and activities between human fibroblast cell lines with different radiosensitivities. Int J Cancer. 2000;85:845–849. doi: 10.1002/(sici)1097-0215(20000315)85:6<845::aid-ijc18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Chan DW, Chen BP-C, Prithivirajsingh S, et al. Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes Dev. 2002;16:2333–2338. doi: 10.1101/gad.1015202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P, Eng WK, Zhu Y, et al. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- Douglas P, Sapkota GP, Morrice N, et al. Identification of in vitro and in vivo phosphorylation sites in the catalytic subunit of the DNA-dependent protein kinase. Biochem J. 2002;368:243–251. doi: 10.1042/BJ20020973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatei M, Sloper K, Sorensen C, et al. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J Biol Chem. 2003;278:14806–14811. doi: 10.1074/jbc.M210862200. [DOI] [PubMed] [Google Scholar]
- Goodhead DT. The initial physical damage produced by ionizing radiations. Int J Radiat Biol. 1989;56:623–634. doi: 10.1080/09553008914551841. [DOI] [PubMed] [Google Scholar]
- Harrison LB, Fogel TD, Picone JR, Fischer DB, Weissberg JB. Radiation therapy for squamous cell carcinoma of the esophagus. J Surg Oncol. 1988;37:40–43. doi: 10.1002/jso.2930370112. [DOI] [PubMed] [Google Scholar]
- Hartley KO, Gell D, Smith GCM, et al. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell. 1995;82:849–856. doi: 10.1016/0092-8674(95)90482-4. [DOI] [PubMed] [Google Scholar]
- Jimenez GS, Bryntesson F, Torres-Arzayus MI, et al. DNA-dependent protein kinase is not required for the p53-dependent response to DNA damage. Nature. 1999;400:81–83. doi: 10.1038/21913. [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Pandey P, Jin S, et al. Functional interaction between DNA-PK and c-Abl in response to DNA damage. Nature. 1997;386:732–735. doi: 10.1038/386732a0. [DOI] [PubMed] [Google Scholar]
- Lees-Miller SP, Godbout R, Chan DW, et al. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science. 1995;267:1183–1185. doi: 10.1126/science.7855602. [DOI] [PubMed] [Google Scholar]
- Lieber MR, Ma Y, Pannicke U, et al. Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol. 2003;4:712–720. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- Newaishy GA, Read GA, Duncan W, Kerr GR. Results of radical radiotherapy of squamous cell carcinoma of the oesophagus. Clin Radiol. 1982;33:347–352. doi: 10.1016/s0009-9260(82)80288-2. [DOI] [PubMed] [Google Scholar]
- Okayasu R, Suetomi K, Yu Y, et al. A deficiency in DNA repair and DNA-PKcs expression in the radiosensitive BALB/c mouse. Cancer Res. 2000;60:4342–4345. [PubMed] [Google Scholar]
- Paul TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Poltoratsky VP, Shi X, York JD, Lieber MP, Carter TH. Human DNA-activated protein kinase (DNA-PK) homologous to phosphatidylinositol kinases. J Immunol. 1995;155:4529–4533. [PubMed] [Google Scholar]
- Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- Ritter MA, Cleaver JE, Tobias CA. High-LET radiations induce a large proportion of non-rejoining DNA breaks. Nature. 1977;266:653–655. doi: 10.1038/266653a0. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sak A, Stuschke M, Wurm R, et al. Selective inactivation of DNA-dependent protein kinase with antisense oligodeoxynucleotides: consequences for the rejoining of radiation-induced DNA double-strand breaks and radiosensitivity of human cancer cell lines. Cancer Res. 2002;62:6621–6624. [PubMed] [Google Scholar]
- Sasaki T, Tian H, Kukita Y, et al. ATM mutations in patients with ataxia telangiectasia screened by a hierarchical strategy. Hum Mutat. 1998;12:186–195. doi: 10.1002/(SICI)1098-1004(1998)12:3<186::AID-HUMU6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Shangary S, Brown KD, Adamson AW, et al. Regulation of DNA-dependent protein kinase activity by ionizing radiation-activated abl kinase is an ATM-dependent process. J Biol Chem. 2000;275:30163–30168. doi: 10.1074/jbc.M004302200. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 1992;69:277–284. doi: 10.1002/1097-0142(19920115)69:2<277::aid-cncr2820690202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Maeda M, Watanabe G, et al. Cell culture in esophageal squamous cell carcinoma and the association with molecular markers. Clin Cancer Res. 2003;9:243–249. [PubMed] [Google Scholar]
- Shioyama Y, Nakamura K, Sasaki T, et al. Clinical results of radiation therapy for stage I esophageal cancer: a single institutional experience. Am J Clin Oncol. 2005;28:75–80. doi: 10.1097/01.coc.0000139021.91718.ee. [DOI] [PubMed] [Google Scholar]
- Stenerlow B, Blomquist E, Grusell E, Hartman T, Carlsson J. Rejoining of DNA double-strand breaks induced by accelerated nitrogen ions. Int J Radiat Biol. 1996;70:413–420. doi: 10.1080/095530096144888. [DOI] [PubMed] [Google Scholar]
- Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- Tibbetts RS, Brumbaugh KM, Williams JM, et al. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomilin NV, Solovjeva LV, Svetlova MP, et al. Visualization of focal nuclear sites of DNA repair synthesis induced by bleomycin in human cells. Radiat Res. 2001;156:347–354. doi: 10.1667/0033-7587(2001)156[0347:vofnso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ward JF. Biochemistry of DNA lesions. Radiat Res. 1985;104(Suppl.):S103–S111. [PubMed] [Google Scholar]
- Weber KJ, Flentje M. Lethality of heavy ion-induced DNA double-strand breaks in mammalian cells. Int J Radiat Biol. 1993;64:169–178. doi: 10.1080/09553009314551261. [DOI] [PubMed] [Google Scholar]