Abstract
Interactions of tumour and stromal cells influence tumour cell proliferation and differentiation, stromal cell phenotypic transdifferentiation and secretion of extracellular matrix (ECM) components. In this study, we established a monolayer and a three-dimensional cell-to-cell interaction model between canine mammary stromal cells and human colonic carcinoma cell lines (Caco-2 and HT-29) to investigate mutual paracrine effects of tumour cells and stromal cells on (i) tumour cell differentiation, (ii) production of ECM components and (iii) phenotypic transdifferentiation of stromal cells. We showed that when Caco-2 or HT-29 cells are cultured in collagen gels, they form a few small solid cell clusters with no lumina, but when cocultured with stromal cells, the tumour cells formed glandular structures with central lumina. This fibroblast-induced organization and differentiation of Caco-2 cells (not HT-29 cells) appeared to be mediated by transforming growth factor-β (TGF-β). Culturing of stromal cells, Caco-2 cells or HT-29 cells alone in both monolayers and gels resulted in weak tenascin-C expression in stromal cells and HT-29 cells and no expression in the Caco-2 cells. Coculturing of stromal cells with tumour cells resulted in increased tenascin-C expression in the stromal cells and HT-29 cells and induced expression of tenascin-C in the Caco-2 cells. This induction and increased expression of tenascin-C appeared to be mediated by TGF-β. Culturing of stromal cells, Caco-2 cells or HT-29 cells alone on monolayers and in gels resulted in a weak expression of chondroitin sulfate (CS), chondroitin-6-sulfate (C-6-S) and versican in stromal cells and no expression in Caco-2 and HT-29 cells. Coculturing of stromal cells with tumour cells on monolayers and in gels resulted in increased CS, C-6-S and versican expression in stromal cells. This tumour cell-induced expression of CS, C-6-S and versican appeared to be mediated by TGF-β and platelet-derived growth factor (PDGF). Coculturing of Caco-2 and HT-29 and stromal cells promoted the transdifferentiation of stromal cells into myofibroblasts, and this appeared to be mediated by TGF-β. These results suggest that TGF-β and PDGF are part of a paracrine system involved in stromal–epithelial cell interaction important in stromal cell differentiation and ECM component production.
Keywords: colon carcinoma cells, cytokines, extracellular matrix, myofibroblasts, phenotypic transdifferentiation, stromal cells
Activation of the host stromal microenvironment is predicted to be a critical step in epithelial tumour growth and progression. However, the specific mechanisms of stromal activation by tumour cells are not known, and the extent to which the stroma regulates the biology of carcinogenesis is not fully understood. Tumour progression occurs within a microecosystem where cancer cells and stromal cells exchange proteinases and cytokines that promote growth directly through stimulation of proliferation and survival, as well as invasion through local proteolysis of the extracellular matrix (ECM). Evidence is accumulating that in addition to secretion of cytokines, tumour cells also influence stromal cells and vice versa through direct cell-to-cell contact (Zidar et al. 2002) and production of ECM components (Donjacour & Cunha 1991).
ECM proteins in the tumours may positively or negatively influence tumour progression. Tenascin-C, a large hexameric glycoprotein, is elevated in colorectal human tumours (Hanamura et al. 1997) and canine colorectal tumours (Mukaratirwa et al. 2003). The most prominent actions of tenascin-C include antiadhesion effects (Chiquet-Ehrismann et al. 1989) and inhibition of cell attachment (Orend & Chiquet-Ehrismann 2000), and these properties favour tumour cell motility and invasion. Chondroitin sulfate proteoglycans (CS-PGs) influence cell growth and differentiation (Caterson et al. 1990) and are elevated in malignant tumours (Hauptmann et al. 1995; Ricciardelli et al. 1997). Versican, a major CS-PG that is known to antagonize cell adhesion to pericellular matrix components (Wight 2002), is also elevated in some tumours (Ricciardelli et al. 1998, 2002; Touab et al. 2002), suggesting that increased deposition of versican in the stroma may contribute to progression of tumours.
Pathological conditions associated with tissue remodelling and fibrogenesis (wound healing and neoplastic lesions) are characterized by the appearance of stromal cells with ultrastructural features intermediate between those of typical fibroblasts and those of smooth muscle cells. These fibroblasts display phenotypic and functional features of smooth muscle cells and hence are named myofibroblasts Gabbiani et al. 1971, 1972; Gabbiani 1981). The function of myofibroblasts is still not clear. Because they are found in fibro-contractive diseases, e.g. in wound healing (Gabbiani 1981) and in stromal reaction to tumours (Schurch et al. 1981; Sappino et al. 1988; Mukaratirwa et al. 2003), it has been proposed that they are responsible for tissue retraction. Through their ability to secrete cytokines, chemokines, prostaglandins, growth factors and ECM components, myofibroblasts are thought to play critical roles in inflammation, growth, repair and neoplasia (Desmouliére et al. 1993). In canine colorectal cancer, myofibroblasts were found to be located at the invasion front (Mukaratirwa et al. 2003). These observations suggest that cytokines produced by invading tumour cells stimulate myofibroblast transformation. In canine colorectal tumours, there is colocalization of myofibroblasts and tenascin-C (Mukaratirwa et al. 2003), suggesting that myofibroblasts are responsible for tenascin secretion, or tenascin secretion and myofibroblast transformation are regulated in the same way or in a coordinated manner.
The aim of this study was to investigate the in vitro paracrine effects of tumour cells on stroma cells and vice versa on (i) tumour cell morphology and differentiation, (ii) production of ECM components [tenascin-C, CS, chondroitin-6-sulfate (C-6-S) and versican] and (iii) phenotypic differentiation of stromal cells to myofibroblasts.
Materials and methods
Cell lines
Caco-2 cells (American Type Cell Collection, HTB37, Rockville, MD, USA) and HT-29 cells (Lesuffleur et al. 1998) were maintained in Dulbecco's modified Eagle's medium (DMEM) (Imperial Laboratories, Salisbury, UK) supplemented with heat inactivated 10% fetal calf serum (FCS), 100 units/ml penicillin G sodium, 100 µg/ml streptomycin sulfate and non-essential amino acids (Sigma, St Louis, MO, USA). Both cell lines were incubated at 37 °C in an atmosphere of 5% CO2 and 95% air. For collection of tumour cell-conditioned medium, subconfluent monolayers of Caco-2 or HT-29 cells were washed two times with phosphate-buffered salt solution (PBS) and once with DMEM without FCS and subsequently cultured in DMEM without FCS for 24 h. The conditioned medium was stored at −70 °C until used in studies on canine stromal cell monolayers.
Isolation of primary stromal cells from normal canine mammary tissue
Primary cultures of stromal cells were isolated from normal non-lactating canine mammary gland using a technique based on that described by van Roozendaal et al. (1992) for the isolation of human breast fibroblasts. The resulting stromal cells showed 100% vimentin expression and no cytokeratin or desmin expression. Cells were used between passages 4 and 12. For stromal cell-conditioned medium, subconfluent monolayers of stromal cells were washed two times with PBS and once with DMEM without FCS and subsequently cultured in DMEM without FCS for 24 h. The conditioned medium was stored at −70 °C until used in studies on tumour cell monolayers.
Antibodies
The primary antibodies used were antivimentin diluted at 1:150 (Biogenex, San Ranon, CA, USA), undiluted antidesmin (RD 301 Euro Diagnostica B.V., Arnhem, The Netherlands), anti-α-smooth muscle actin (anti-α-SMA) diluted at 1:1200 (Biogenex), antitenascin diluted at 1:50 (Dako, Glostrup, Denmark), anti-CS diluted at 1:100 (Sigma), anti-C-6-S diluted at 1:1000 (Seikagaku, Kogyo, Tokyo, Japan), antiversican 2B1 diluted at 1:1000 (Seikagaku) and anti-TGF-β1 diluted at 25 µ/ml (R&D Systems/Biermann, Bad Nauheim, Germany).
Monolayer cell cultures and cocultures on glass slides
Cells trypsinized and diluted to 2 × 104 cells/ml for stromal cells and 1 × 104 cells/ml for colonic tumour cells (Caco-2 and HT-29) in DMEM with 10% heat-inactivated FCS were seeded on multichambered glass slides. For cocultures, mixtures of stromal cells (2 × 104 cells/ml) and Caco-2 or HT-29 cells (1 × 104 cells/ml) suspended in DMEM with 10% heat-inactivated FCS were seeded on multichambered glass slides. The cells were allowed to attach overnight, then washed with PBS and switched to experimental conditions. For single cultures of Caco-2 and HT-29 cells and cocultures with stromal cells, the cells were grown in DMEM with 10% FCS for 48 h, and then the media were replaced with DMEM without FCS supplemented with growth factors to be tested or stromal cell-conditioned media for 48 h. For single cultures of stromal cells, after 24 h growth in DMEM with 10% FCS, the media were replaced with DMEM without FCS, supplemented with growth factors to be tested, or Caco-2- or HT-29-conditioned media for 48 h. The following growth factors were used: transforming growth factor-β1 (TGF-β1; 13 ng/ml) (R&D Systems/Biermann), platelet-derived growth factor (PDGF-AB; 50 ng/ml) (Sigma), epidermal growth factor (EGF; 10 ng/ml) (Sigma), insulin-like growth factor-I (IGF-I; 10 ng/ml) (Sigma) and IGF-II (50 ng/ml) (Gibco, Gaithersburg, MD, USA). Assays were also performed in the presence of a neutralizing antibody against TGF-β1 (3 ng/ml) (R&D Systems). After 48 h, the slides were washed with PBS before the cells were fixed with ice-cold acetone for 10 min and stored at 4 °C. Each experimental condition was assayed in triplicate, and at least three independent experiments were performed (mean values were used).
Three-dimensional cell cultures and cocultures in collagen gels
Collagen was obtained from Wistar rat-tail tendons under sterile conditions. Tendons were dissolved in 0.1% acetic acid at 4 °C under stirring for 48 h. The solution was centrifuged (100 g, 10 min) to remove undissolved material. Collagen concentration was determined by freeze drying and weighing the residue of a known volume and adjusted to a concentration of 1.55 mg/ml in 0.1% acetic acid. Neutralized collagen was made by adding 1.7 volumes of collagen to 0.4 volumes of a 2:1 mixture of 10-fold concentrated DMEM (Imperial Laboratories) and 0.34 m NaOH.
Cells dissolved in DMEM with 10% FCS were added (1 × 105 cells/ml for stromal cells and 5 × 104 cells/ml for Caco-2 and HT-29 cells) to neutralized collagen. For cocultures, mixtures of stromal cells (1 × 105 cells/ml) and Caco-2 and HT-29 cells (5 × 104 cells/ml) were added to neutralized collagen. Two millilitres of cell–collagen suspension was pipetted into each well of 12-well culture plates. Plates were brought to 37 °C to obtain gelation. After gelation, DMEM with 10% FCS was added on top of the gel. After growth for 48 h, the wells were subjected to different experimental conditions. For single cultures of Caco-2 and HT-29 cells, and cocultures, the cells were grown in DMEM with 10% FCS or 1% FCS for 14 days, and the media were replaced every 2–3 days. For single cultures of stromal cells, after 48 h growth in DMEM with 10% FCS, the media were replaced with DMEM with 1% FCS, supplemented with growth factors to be tested, or Caco-2- or HT-29-conditioned media. The following growth factors were used: TGF-β1 (3 ng/ml), PDGF-AB (50 ng/ml), EGF (10 ng/ml), IGF-I (10 ng/ml) and IGF-II (50 ng/ml). Assays were also performed in the presence of a neutralizing antibody against TGF-β1 (3 ng/ml). The cells were grown for 14 days, changing the experimental media every 2–3 days, and after 14 days, the gels were fixed in 4% buffered formaldehyde for 24 h. Each experimental condition was assayed in triplicate, and at least three independent experiments were performed (mean values were used).
Immunocytochemistry and immunohistochemistry
Immunolabelling of vimentin, desmin, α-SMA and ECM components (tenascin-C, CS, C-6-S and versican) was performed on cells grown on glass slides and on serial sections cut from collagen gels. The fixed cells on microscopic slides were washed three times with PBS containing 0.05% Tween 20. Serial sections from paraffin-embedded gels were cut and de-waxed. For labelling of antigens with antiversican and antitenascin-C, deparaffinized sections were pretreated with chondroitinase 0.2 U/ml avidin–biotin peroxidase complex (Seikagaku) in 0.2 m Tris–HCl buffer (pH 8.0) at 37 °C for 1 h and trypsin 0.1% in PBS (0.01 m, pH 7.4) containing CaCl2 0.1% at 37 °C for 10 min, respectively. Slides and sections were blocked with 1:20 normal horse serum for 20 min. Primary antibody incubations were carried out for 18 h at 4 °C, and secondary antibody incubations were carried out for 30 min at room temperature. After incubation with horse antimouse biotin and avidin–biotin peroxidase complex (Vector, Burlingame, CA, USA), the colour was developed in 3-3′-diaminobenzidine and 0.02% H2O2 in Tris buffer pH 7.8. The sections were counter-stained with 10% Mayer's haematoxylin. The intensity of ECM protein expression was evaluated for immunohistochemical reactivity by semiquantitative scoring in four grades (+++, intense; ++, moderate; +, weak and –, negative). Myofibroblasts transdifferentiation was evaluated by counting the number of stromal cells labelled with α-SMA and expressed as a percentage of the total number of cells. Each experimental condition was assayed in triplicate, and at least three independent experiments were performed (mean values were used).
Results
Morphology
When grown in monolayers, canine primary stromal cells presented themselves as large mesenchymal cells with many cytoplasmic extensions. When stromal cells were cocultured with Caco-2 and HT-29 cells on monolayers, the cells segregated after 48 h into distinct populations with stromal cells surrounding nests of Caco-2 cells or HT-29 cells (Figure 1a).
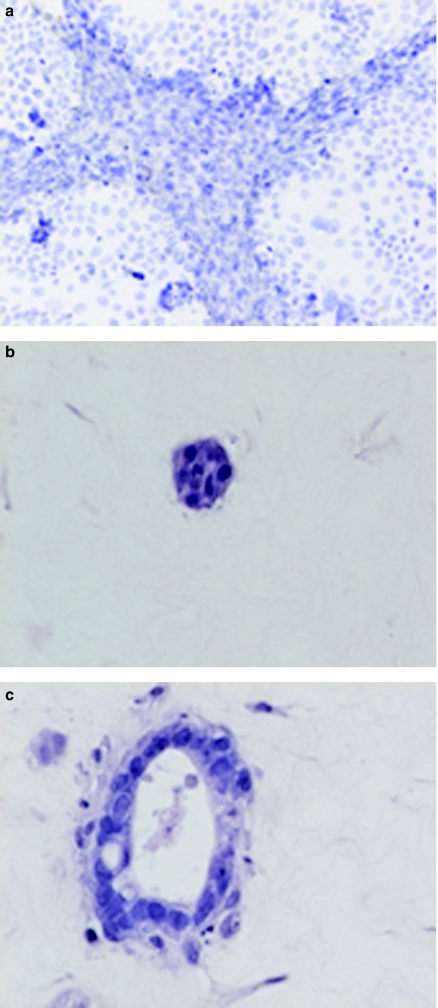
Figure 1.
Micrographs showing the morphology of cultured and cocultured tumour and stromal cells. (a) Caco-2 and stromal cell coculture cultured on monolayers; cells segregated after 48 h into distinct populations with stromal cells surrounding Caco-2 cells (haematoxylin ×400). (b) Caco-2 culture in gels; formation of small solid structures after 14 days [haematoxylin and eosin (H&E) ×400]. (c) Caco-2 and stromal cell coculture in gels; formation of glandular structures with lumina (H&E ×400).
Stromal cells grown inside collagen gel proliferated fast, assuming a spindle shape, sending thin cytoplasmic processes into the collagen. The morphology of Caco-2 and HT-29 cells in collagen gels differed from morphology on monolayers. While on monolayers, Caco-2 and HT-29 cells displayed a typical, cuboidal morphology, in collagen gels, they became rounded and remained quiescent, even at high concentration of 5 × 106 cells/ml. After 14 days, few cells proliferated forming solid aggregates (Figure 1b), while the rest died. When Caco-2 or HT-29 cells were cocultured with stromal cells, or stromal cell-conditioned media, there was no tumour cell death, and both Caco-2 and HT-29 cells formed glandular structures with lumina (Figure 1c). This organization and differentiation of Caco-2 cells, but not HT-29 cells, could be partially induced by TGF-β (3 ng/ml).
Deposition of extracellular matrix component
Tenascin-C.
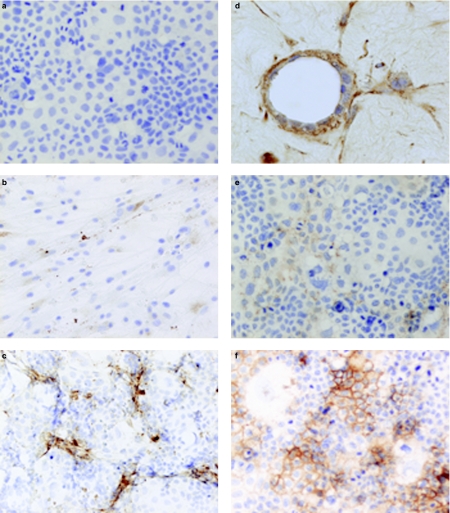
In both monolayers and three-dimensional cell cultures in gels, there was no tenascin-C immunoreactivity in Caco-2 cells (Figure 2a), and there was a weak cytoplasmic reactivity in HT-29 and stromal cells (Figure 2b). Coculturing of Caco-2 or HT-29 and stromal cells on monolayers or gels resulted in a strong tenascin-C immunoreactivity in the stromal cells and granular tenascin immunoreactivity in the tumour cells (Figure 2c, d). Culturing of stromal cells with Caco-2- or HT-29-conditioned media resulted in an increase in weak to moderate tenascin-C immunoreactivity in the stromal cells, and this response was partially blocked by a TGF-β1-neutralizing antibody. Culturing Caco-2 or HT-29 cells with stromal cell-conditioned media induced a weak tenascin-C expression in Caco-2 cells (Figure 2e) and an increased weak to strong tenascin-C expression in HT-29 cells, and this response was partially blocked by a TGF-β1-neutralizing antibody. Addition of TGF-β1 to the media in both monolayers and three-dimensional cell cultures resulted in a strong expression of tenascin-C in stromal cells, induced strong tenascin-C expression in Caco-2 cells (Figure 2f) and an increased weak to strong tenascin-C expression in HT-29 cells. Addition of PDGF-AB, basic fibroblast growth factor (bFGF), IGF-I and IGF-II to cell cultures did not affect tenascin-C expression (Table 1).
Figure 2.
Micrographs showing tenascin-C expression in cultured and cocultured tumour and stromal cells. (a) Caco-2 cell culture on monolayers; no tenascin-C immunoreactivity [avidin–biotin peroxidase complex (ABC), haematoxylin counter-stain]. (b) Stromal cell culture on monolayers; weak tenascin-C immunoreactivity (ABC, haematoxylin counter-stain ×200). (c) Caco-2 and stromal cell coculture on monolayers; strong tenascin immunoreactivity in stromal cells and Caco-2 cells (ABC, haematoxylin counter-stain ×200). (d) Caco-2 and stromal cell coculture in gels; strong immunoreactivity in Caco-2 cells and stromal cells (ABC, haematoxylin counter-stain ×200). (e) Caco-2 cell culture on monolayers, addition of stromal cell-conditioned media; induction of a weak tenascin-C immunoreactivity (ABC, haematoxylin counter-stain ×200). (f) Caco-2 cell culture on monolayers, addition of transforming growth factor (TGF)-β; induction of a strong tenascin-C immunoreactivity (ABC, haematoxylin counter-stain ×200).
Table 1.
Effect of coculturing, tumour cell-conditioned media and growth factors on extracellular matrix (ECM) component production on microscopic slides and in three-dimensional gels
| ECM components | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CS | C-6-S | Tenascin-C | Versican | ||||||
| Cells | Culture conditions | M | G | M | G | M | G | M | G |
| HT-29 | 10% FCS | − | − | − | − | + | + | − | − |
| NFCS or 1% FCS | − | − | − | − | + | + | − | − | |
| S-CM | − | − | − | − | +++ | ++ | − | − | |
| TGF-β | − | − | − | − | +++ | +++ | − | − | |
| Caco-2 | 10% FCS | − | − | − | − | − | − | − | − |
| NFCS or 1% FCS | − | − | − | − | − | − | − | − | |
| S-CM | − | − | − | − | ++ | ++ | − | − | |
| TGF-β | − | − | − | − | +++ | +++ | − | − | |
| Stromal cells | 10% FCS | + | + | + | + | + | + | + | + |
| NFCS or 1% FCS | + | + | + | + | + | + | + | + | |
| Caco-2-CM | +++ | ++ | +++ | ++ | +++ | ++ | +++ | ++ | |
| Caco-2-CM + anti-TGF-β | + | + | + | + | + | + | + | + | |
| HT-29-CM | ++ | ++ | ++ | ++ | + | ++ | ++ | +++ | |
| HT-29-CM + anti-TGF-β | + | + | + | + | + | + | + | ++ | |
| TGF-β | +++ | +++ | ++ | ++ | +++ | +++ | +++ | ++ | |
| PDGF | +++ | +++ | +++ | +++ | + | + | +++ | +++ | |
| bEGF | + | + | + | + | + | + | + | + | |
| IGF-I | + | + | + | + | + | + | + | + | |
| IGF-II | + | + | + | + | + | + | + | + | |
| Caco-2 and stromal cells | NFCS or 1% FCS | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ |
| HT-29 and stromal cells | NFCS or 1% FCS | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
FCS, fetal calf serum; NFCS, no fetal calf serum; S-CM, stromal cell-conditioned media; Caco-2-CM, Caco-2 cell-conditioned media; HT-29-CM, HT-29-conditioned media; TGF, transforming growth factor; PDGF, platelet-derived growth factor; bEGF, basic epidermal growth factor; IGF, insulin-like growth factor; M, monolayers on plastic; G, three-dimensional gels; +++, strong reaction; ++, moderate reaction; +, weak reaction; –, negative reaction.
Chondroitin sulfate, chondoitin-6-sulfate and versican.
In both monolayers and three-dimensional cultures, there was no immunoreactivity of CS, C-6-S and versican in Caco-2 and HT-29 cells. There was a weak CS and C-6-S immunoreactivity and no versican immunoreactivity in stromal cells. Coculturing of Caco-2 or HT-29 cells and stromal cells on monolayer and gels resulted in strong expression of CS, C-6-S and versican in and around stromal cells. Culturing of stromal cells with Caco-2- or HT-29-conditioned media resulted in a strong CS, C-6-S and versican immunoreactivity. Addition of TGF-β1 or PDGF-AB to the media resulted in a strong immunolabelling of CS, C-6-S and versican in stromal cells. Addition of stromal cell-conditioned media or TGF-β1, PDGF-AB, bFGF or IGF-II to tumour cells did not induce CS, C-6-S and versican expression in Caco-2 or HT-29 cells (Table 1).
Myofibroblast transdifferentiation
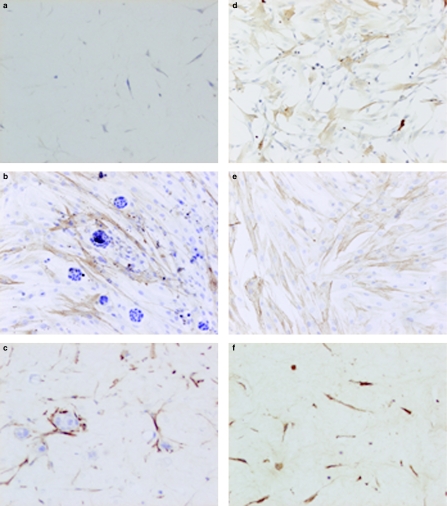
When stromal cells were grown in monolayer cell cultures with media containing 10% FCS for 48 h, about 20% of the cells were positive for α-SMA. Growing the stromal cells in serum-free media for 48 h resulted in a decrease in the number of cells (5%) expressing α-SMA, suggesting that FCS contains a factor which induces myofibroblast transdifferentiation. In three-dimensional cell cultures, growing stromal cells in media with 10% FCS for 14 days resulted in about 25% of the cells expressing α-SMA, and culturing of the stromal cells in media containing 1% FCS resulted in a decrease in the number of cells (>5%) expressing α-SMA (Figure 3a). Coculturing stromal cells with Caco-2 or HT-29 cells on monolayers or in gels resulted in about 90% of the cells expressing α-SMA (Figures 3b, c). The cytoskeleton in the stromal cell was much more brightly stained, and many individual actin filaments were discernable. Culturing stromal cells with Caco-2- or HT-29-conditioned media in monolayers (Figure 3d) and on gels resulted in an increase in the number of stromal cells expressing α-SMA. As expected, treating the stromal cells with TGF-β on monolayers (Figure 3e) and in gels (Figure 3f) resulted in about 90% of the cells expressing α-SMA. Treating the cells with PDGF in both monolayers and on gels resulted in a decrease in the numbers of cells expressing α-SMA. Addition of EGF, IGF-I and IGF-II did not affect α-SMA expression (Table 2).
Figure 3.
Micrographs showing α-smooth muscle actin (α-SMA) expression in cultured and cocultured tumour and stromal cells. (a) Stromal cells cultured in gels in medium with 1% fetal calf serum; less than 5% of the cells are positive for α-SMA (ABC, haematoxylin counter-stain ×200). (b) Coculture of stromal cells with HT-29 cells on monolayers; more than 90% of the cells expressing α-SMA (ABC, haematoxylin counter-stain ×200). (c) Coculture of stromal cells with Caco-2 in gels; more than 90% of the cells expressing α-SMA (ABC, haematoxylin counter-stain ×200). (d) Stromal cells cultured on monolayers with HT-29-conditioned media in monolayers. Increase in the number of stromal cells expressing α-SMA (ABC, haematoxylin counter-stain ×200). (e) Stromal cells cultured on monolayers, addition of TGF-β; more than 90% of the cells expressing α-SMA (ABC, haematoxylin counter-stain ×200). (f) Stromal cells cultured in gels, addition of TGF-β; more than 90% of the cells expressing α-SMA (ABC, haematoxylin counter-stain ×200).
Table 2.
Effect of coculturing, tumour cell-conditioned media and growth factors on myofibroblast differentiation of stromal cells in collagen gels
| % of stromal cells positive for α-smooth muscle actin | ||
|---|---|---|
| Culture conditions | Monolayers | Gels |
| 10% FCS | 20 ± 2.6 | 24 ± 1.0 |
| NFCS or 1% FCS | 6 ± 0.2 | 15 ± 0.8 |
| Coculture with Caco-2 cells | 96 ± 4.2 | 91 ± 3.1 |
| Coculture with HT-29 cells | 94 ± 3.8 | 90 ± 3.2 |
| Caco-2-CM | 53 ± 1.7 | 74 ± 1.3 |
| HT-29-CM | 60 ± 0.8 | 70 ± 1.3 |
| TGF-β | 90 ± 0.6 | 100 ± 0.8 |
| PDGF | 1 ± 0.1 | 1 ± 0.1 |
| bEGF | 5 ± 0.5 | 15 ± 0.8 |
| IGF-I | 5 ± 0.4 | 15 ± 0.6 |
| IGF-II | 5 ± 0.3 | 15 ± 0.5 |
FCS, fetal calf serum; NFCS, no fetal calf serum; S-CM, stromal cell-conditioned media; Caco-2-CM, Caco-2 cell-conditioned media; HT-29-CM, HT-29-conditioned media; TGF, transforming growth factor; PDGF, platelet-derived growth factor; bEGF, basic epidermal growth factor; IGF, insulin-like growth factor. Each experimental condition was assayed in triplicate, and three independent experiments were performed and the mean values are shown above.
Discussion
Signals transmitted from stromal cells to tumour cells or vice versa can occur through direct cell–cell contact, in the form of growth factors or through secretion of ECM molecules, and this constitute the basis of reciprocal mesenchymal–epithelial interaction. In this study, we used two models of stromal–tumour cell coculture systems to investigate the regulation of cell differentiation and ECM component production.
Our study using a three-dimensional coculture system revealed that stromal cells reduce tumour cell death and promote cell differentiation. Previous experiments (Olumi et al. 1998) have shown that death of cultured tumour cells is fivefold less when they are cocultured with normal human prostatic fibroblasts in comparison to when cultured alone, and proliferation of the tumour cells was similar when cocultured with normal prostatic fibroblasts or when grown alone. These data suggest that stromal cells promote tumour progression through attenuation of cell death rather than increased proliferation. The ability of normal fibroblasts to induce differentiation of tumour cell lines such as of a human colon carcinoma cell line HT-29 (Halttunen et al. 1996) has been demonstrated in our study as well. Furthermore, our results showed that this stromal-induced organization of and differentiation Caco-2 cells is partially mediated by TGF-β.
In previous studies where HT-29 cells were grown in a three-dimensional culture system with human embryonic lung fibroblasts or skin fibroblasts (Halttunen et al. 1996), the HT-29 failed to form glandular structures. This is in contrast to the present study, where canine mammary stromal cells induced HT-29 cell differentiation. This observed difference may result from variations in the establishment of reciprocal interactions between epithelial cells and mesenchymal cells.
Of interest in our investigation was the regulation of the synthesis of the ECM proteins: tenascin-C, CS-PGs and versican, because these proteins are expressed in large quantities in a variety of malignant tumours and have prognostic value in some tumours (Koukoulis et al. 1991; Hauptmann et al. 1995; Ricciardelli et al. 1997, 1998, 2002; Karvinen et al. 2003). In this experiment, both epithelial cells and stromal cells were able to produce tenascin-C. This accorded with previous experiments (Ishihara et al. 1995; Vollmer et al. 1997). In vivo, the quantitative and qualitative contribution to tenascin-C accumulation by epithelial tumour cells and stromal cells remains to be investigated. In this study, stromal cells or stromal cell-conditioned media were able to induce tenascin-C secretion by tumour cells and vice versa, suggesting that paracrine interaction between tumour and stromal cell is important for tenascin-C synthesis. This suggests that the modulation of stromal cells by tumour cells and vice versa to secrete tenascin-C was due to some soluble factors in the conditioned media. Culturing of stromal or tumour cells with various growth factors showed that TGF-β was responsible for the increase and induction of tenascin-C secretion. This is in accordance with previous reports (Vollmer et al. 1997; Wilson et al. 1999; Tuxhorn et al. 2002).
In this study, coculturing of stromal cells and tumour cells increased the production of CS, C-6-S and versican by stromal cells. Their increased production was mediated by TGF-β and PDGF. PDGF and TGF-β have been shown to increase the net synthesis of versican by stimulating transcription and translation and increase the glycosaminoglycan chain length (Schonherr et al. 1991).
Myofibroblasts are the main stromal cells in human (Sappino et al. 1989) and canine (Mukaratirwa et al. 2003) colorectal tumours and are responsible for the synthesis of ECM proteins (Powell et al. 1999). We therefore investigated whether coculturing of stromal cells and tumour cells has an effect on the phenotypic transdifferentiation of stromal cells to myofibroblasts. We showed that coculturing of stromal cells with tumour cells resulted in transdifferentiation of stromal cells to myofibroblasts (Figure 3) and that TGF-β enhanced and PDGF reduced stromal transdifferentiation to myofibroblasts. Similar results have been observed in a variety of stromal cells from rats (Desmouléire et al. 1993), rabbits (Nakamura et al. 2002) and humans (Valenti et al. 2001; Kinner et al. 2002). Myofibroblasts may stimulate tumour progression by stimulating the growth of a cancer cell population (Atula et al. 1997; Nakamura et al. 1997), sustaining blood vessel formation and lymphangiogenesis (Orimo et al. 2001), attenuating cancer cell death (Olumi et al. 1998) and stimulating invasion and metastasis by activating proteolysis (Basset et al. 1990; Singer et al. 1997).
Combining our results on phenotypic modulation of stromal cells by tumour cells and the modulation of ECM components' secretion, we conclude that TGF-β is part of a paracrine system regulating stromal–epithelial cell interaction. The first step in this pathway might be induction of myofibroblast transdifferentiation of stromal cells. The second step might be the induction of myofibroblasts to secrete ECM proteins, which in turn regulate tumour cell differentiation, proliferation and invasion. The cellular mechanisms of how TGF-β mediates myofibroblast transdifferetiation and ECM production are as yet undefined. The partial blockage of myofibroblast differentiation and ECM production by anti-TGF antibodies in our study suggest that TGF-β pathways involved are not direct and the processes involved appear to be regulated by a complex system with other molecules involved, or there are other cytokines involved in these processes.
References
- Atula S, Grenman R, Syrjanen S. Fibroblasts can modulate the phenotype of malignant epithelial cells in vitro. Exp Cell Res. 1997;235:180–187. doi: 10.1006/excr.1997.3676. [DOI] [PubMed] [Google Scholar]
- Basset P, Bellocq JP, Wolf C, et al. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature (Lond) 1990;348:699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Caterson B, Mahmoodian F, Sorrell JM, et al. Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci. 1990;97:411–417. doi: 10.1242/jcs.97.3.411. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Kalla P, Pearson CA. Participation of tenascin and transforming growth factor-beta in reciprocal epithelial–mesenchymal interactions of MCF7 cells and fibroblasts. Cancer Res. 1989;49:4322–4325. [PubMed] [Google Scholar]
- Desmouliére A, Geino ZA, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR. Stromal regulation of epithelial function. Cancer Treat Res. 1991;53:335–364. doi: 10.1007/978-1-4615-3940-7_16. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast: a key cell for wound healing and fibrocontractive diseases. Prog Clin Biol Res. 1981;54:183–194. [PubMed] [Google Scholar]
- Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR, Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972;135:719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Halttunen T, Marttinen A, Rantala I, Kainulainen H, Maki M. Fibroblasts and transforming growth factor beta induce organization and differentiation of T84 human epithelial cells. Gastroenterology. 1996;111:1252–1262. doi: 10.1053/gast.1996.v111.pm8898639. [DOI] [PubMed] [Google Scholar]
- Hanamura N, Yoshida T, Matsumoto E, Kawarada Y, Sakakura T. Expression of fibronection and tenascin-C mRNA by myofibroblast, vascular cells and epithelial cells in human colon adenomas and carcinomas. Int J Cancer. 1997;73:10–15. doi: 10.1002/(sici)1097-0215(19970926)73:1<10::aid-ijc2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Hauptmann S, Zardi L, Siri A, et al. Extracellular matrix proteins in colorectal carcinomas. Expression of tenascin and fibronectin isoforms. Lab Invest. 1995;73:172–182. [PubMed] [Google Scholar]
- Ishihara A, Yoshida T, Tamaki H, Sakakura T. Tenascin expression in cancer cells and stroma of human breast cancer and its prognostic significance. Clin Cancer Res. 1995;1:1035–1041. [PubMed] [Google Scholar]
- Karvinen S, Kosma VM, Tammi MI, Tammi R. Hyaluronan, CD44 and versican in epidermal keratinocyte tumours. Br J Dermatol. 2003;148:86–94. doi: 10.1046/j.1365-2133.2003.05028.x. [DOI] [PubMed] [Google Scholar]
- Kinner B, Zaleskas JM, Spector M. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res. 2002;278:72–83. doi: 10.1006/excr.2002.5561. [DOI] [PubMed] [Google Scholar]
- Koukoulis GK, Gould VE, Bhattacharyya A, Gould JE, Howeedy AA, Virtanen I. Tenascin in normal, reactive, hyperplastic, and neoplastic tissues: biologic and pathologic implications. Hum Pathol. 1991;22:636–642. doi: 10.1016/0046-8177(91)90285-w. [DOI] [PubMed] [Google Scholar]
- Lesuffleur T, Violette S, Vasile-Pandrea I, et al. Resistance to high concentrations of methotrexate and 5-fluorouracil of differentiated HT-29 colon-cancer cells is restricted to cells of enterocytic phenotype. Int J Cancer. 1998;76:383–392. doi: 10.1002/(sici)1097-0215(19980504)76:3<383::aid-ijc16>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mukaratirwa S, de Witte E, van Ederen AM, Nederbragt H. Tenascin expression in relation to stromal tumour cells in canine gastrointestinal epithelial tumours. J Comp Pathol. 2003;129:137–146. doi: 10.1016/s0021-9975(03)00021-5. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kurosaka D, Yoshino M, Oshima T, Kurosaka H. Injured corneal epithelial cells promote myodifferentiation of corneal fibroblasts. Invest Ophthalmol Vis Sci. 2002;43:2603–2608. [PubMed] [Google Scholar]
- Nakamura T, Matsumoto K, Kiritoshi A, Tano Y, Nakamura T. Induction of hepatocyte growth factor in fibroblasts by tumor-derived factors affects invasive growth of tumor cells: in vitro analysis of tumor–stromal interactions. Cancer Res. 1997;57:3305–3313. [PubMed] [Google Scholar]
- Olumi AF, Dazin P, Tlsty TD. A novel coculture technique demonstrates that normal human prostatic fibroblasts contribute to tumor formation of LNCaP cells by retarding cell death. Cancer Res. 1998;58:4525–4530. [PubMed] [Google Scholar]
- Orend G, Chiquet-Ehrismann R. Adhesion modulation by antiadhesive molecules of the extracellular matrix. Exp Cell Res. 2000;261:104–110. doi: 10.1006/excr.2000.5041. [DOI] [PubMed] [Google Scholar]
- Orimo A, Tomioka Y, Shimizu Y, et al. Cancer-associated myofibroblasts possess various factors to promote endometrial tumor progression. ClinCancer Res. 2001;7:3097–3105. [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- Ricciardelli C, Brooks JH, Suwiwat S, et al. Regulation of stromal versican expression by breast cancer cells and importance to relapse-free survival in patients with node-negative primary breast cancer. Clin Cancer Res. 2002;8:1054–1060. [PubMed] [Google Scholar]
- Ricciardelli C, Mayne K, Sykes PJ, et al. Elevated levels of versican but not decorin predict disease progression in early-stage prostate cancer. Clin Cancer Res. 1998;4:963–971. [PubMed] [Google Scholar]
- Ricciardelli C, Mayne K, Sykes PJ, et al. Elevated stromal chondroitin sulfate glycosaminoglycan predicts progression in early-stage prostate cancer. Clin Cancer Res. 1997;3:983–992. [PubMed] [Google Scholar]
- van Roozendaal CE, van Ooijen B, Klijn JGM, et al. Stromal influences on breast cancer cell growth. Br J Cancer. 1992;65:77–81. doi: 10.1038/bjc.1992.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino AP, Dietrich PY, Skalli O, Widgren S, Gabbiani G. Colonic pericryptal fibroblasts. Differentiation pattern in embryogenesis and phenotypic modulation in epithelial proliferative lesions. Virchows Arch A Pathol Anat Histopathol. 1989;415:551–557. doi: 10.1007/BF00718649. [DOI] [PubMed] [Google Scholar]
- Sappino AP, Skalli O, Jackson B, Schurch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988;41:707–712. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- Schonherr E, Jarvelainen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor-beta 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991;266:17640–17647. [PubMed] [Google Scholar]
- Schurch W, Seemayer TA, Lagace R. Stromal myofibroblasts in primary invasive and metastatic carcinomas. A combined immunological, light and electron microscopic study. Virchows Arch A Pathol Anat Histol. 1981;391:125–139. doi: 10.1007/BF00437591. [DOI] [PubMed] [Google Scholar]
- Singer CF, Rasmussen A, Lippman ME, Cullen KJ. Coexpression of stromelysin-3 and insulin-like growth factor II in tumors of ectodermal, mesodermal, and endodermal origin: indicator of a fetal cell phenotype. J Clin Endocrinol Metab. 1997;82:1917–1922. doi: 10.1210/jcem.82.6.4023. [DOI] [PubMed] [Google Scholar]
- Touab M, Villena J, Barranco C, Arumi-Uria M, Bassols A. Versican is differentially expressed in human melanoma and may play a role in tumor development. Am J Pathol. 2002;160:549–557. doi: 10.1016/S0002-9440(10)64874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- Valenti MT, Azzarello G, Balducci E, et al. Conditioned medium from MCF-7 cell line induces myofibroblast differentiation, decreased cell proliferation, and increased apoptosis in cultured normal fibroblasts but not in fibroblasts from malignant breast tissue. Histochem J. 2001;33:499–509. doi: 10.1023/a:1014927305775. [DOI] [PubMed] [Google Scholar]
- Vollmer G, Tan MI, Wunsche W, Frank K. Expression of tenascin-C by human endometrial adenocarcinoma and stroma cells: heterogeneity of splice variants and induction by TGF-beta. Biochem Cell Biol. 1997;75:759–679. [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Wilson KE, Bartlett JM, Miller EP, et al. Regulation and function of the extracellular matrix protein tenascin-C in ovarian cancer cell lines. Br J Cancer. 1999;80:685–692. doi: 10.1038/sj.bjc.6690410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidar N, Gale N, Kambic V, Fischinger J. Proliferation of myofibroblasts in the stroma of epithelial hyperplastic lesions and squamous carcinoma of the larynx. Oncology. 2002;62:381–385. doi: 10.1159/000065071. [DOI] [PubMed] [Google Scholar]