Abstract
The role of lymphocytes in the pathogenesis of lung fibrosis is not clear, but the weight of the evidence supports a pro-fibrotic effect for lymphocytes. The high-affinity interleukin-2 receptor (haIL-2R) is expressed on activated, but not quiescent, T lymphocytes. This selective expression of haIL-2R provides the basis for therapeutic strategies that target IL-2R-expressing cells. We hypothesized that elimination of activated lymphocytes by IL-2R-targeted chimeric proteins might ameliorate lung fibrosis. We investigated the effects of IL-2-Bax, a novel apoptosis-inducing IL-2R-targeted chimeric protein, on bleomycin-induced lung injury in mice. Treatment groups included (i) a single intratracheal instillation of bleomycin and twice-daily intraperitoneal injections of IL-2-Bax; (ii) intratracheal bleomycin and intraperitoneal IL-2-PE664Glu, an older-generation chimeric protein; (iii) intratracheal bleomycin/intraperitoneal PBS; (iv) intratracheal saline/intraperitoneal PBS. Lung injury was evaluated 14 days after intratracheal instillation by cell count in bronchoalveolar lavage (BAL) fluid, semi-quantitative and quantitative histomorphological measurements and by biochemical analysis of lung hydroxyproline. Bleomycin induced a BAL lymphocytosis that was significantly attenuated by IL-2-Bax and IL-2-PE664Glu. However, morphometric parameters and lung hydroxyproline were unaffected by the chimeric proteins. These results show that IL-2-Bax reduces the lymphocytic infiltration of the lungs in response to bleomycin, but this effect is not accompanied by a decrease in lung fibrosis.
Keywords: apoptosis, fibrosis, genetic engineering, immunotherapy, interstitial lung disease
Inflammation is a prominent feature of many disorders that may lead to lung fibrosis, such as desquamative interstitial pneumonia, non-specific interstitial pneumonia, radiation pneumonitis, drug-related lung injury and others (Katzenstein & Myers 1998).
Bleomycin-induced lung fibrosis in rodents is a commonly employed animal model of fibrotic lung disease (Snider et al. 1978; Hesterberg et al. 1981; Chandler et al. 1983; Lazo et al. 1990). Bleomycin-induced lung injury involves a complex interaction of many cell types, some resident in the lung, others migratory to the lung from the circulation. The role of lymphocytes in the pathogenesis of the pulmonary fibrotic response to bleomycin is somewhat obscure. T lymphocytes are capable of generating fibroblast chemotactic factors and factors that influence both fibroblast proliferation and collagen synthesis (Johnson & Ziff 1976; Hibbs et al. 1983; Postlethwaite et al. 1984). The inflammatory response to bleomycin instillation is demonstrated by means of analysis of BAL cells. An early increase in the percentage of neutrophils is followed by a more sustained BAL lymphocytosis (Izbicki et al. 2002b). In a study of parenchymal lung lymphocytes in bleomycin-treated rats, the normal CD4 : CD8 ratio of 1 : 1 was increased 14 days post-bleomycin to 2 : 1, reversing to 1 : 2 in the later stages of the disease process (Thrall & Barton 1984). Intriguingly, the increase in helper T cells occurs when collagen synthesis is most active, and the increase in suppressor T cells occurs when collagen synthesis has decreased, suggesting that helper T cells might enhance, and suppressor T cells suppress, collagen deposition in bleomycin lung injury.
A number of studies have attempted to define the role of lymphocytes in the pathogenesis of bleomycin lung fibrosis. Treatment of animals with anti-CD3 monoclonal antibody (Sharma et al. 1996), antilymphocyte globulin (Thrall et al. 1979) or with thymectomy and irradiation (Thrall et al. 1980) resulted in a decreased collagen deposition in bleomycin-exposed animals. However, SCID mice appear to develop lung injury of similar severity to wild-type animals (Zhu et al. 1996; Helene et al. 1999). Moreover, while one study of the response to bleomycin in athymic nude mice showed no difference in comparison with euthymic mice (Szapiel et al. 1979), two further studies demonstrated an attenuated response to bleomycin (Schrier et al. 1983) and peplomycin (Ekimoto et al. 1985), a bleomycin analogue, in nude mice. Thus, the pathogenetic role of lymphocytes in lung fibrosis is somewhat controversial, but there appears to be considerable evidence that supports a role for lymphocytes in the development of fibrosis. We, therefore, hypothesized that elimination of activated lymphocytes by interleukin-2 receptor (IL-2R)-targeted chimeric proteins might ameliorate lung fibrosis.
IL-2 and its receptor (IL-2R) regulate the magnitude and duration of the T-cell immune response. The IL-2R α-subunit, which confers high affinity for IL-2, is expressed on activated T cells, but not on normal resting cells. This selective expression of the high-affinity IL-2R provides the scientific basis for therapeutic strategies that target IL-2R-expressing cells (Waldmann 1993). One such strategy uses chimeric proteins, such as IL-2-based chimeras, in which IL-2 is responsible for the binding of the protein to activated lymphocytes. IL-2 is fused at the cDNA level to a cytotoxic protein, which comprises the cytotoxic component of the chimeric protein.
Bacterial toxins, such as Pseudomonas exotoxin (PE) or diphtheria toxin have been used as cytotoxic components of targeted chimeric proteins (Brinkmann & Pastan 1994). One such molecule is IL-2-PE40, a chimeric protein linking human IL-2 to a modified (truncated) form of PE, so that the toxin will no longer indiscriminately bind and kill normal cells but will, instead, selectively target and kill cells expressing the ligand identified by the binding component (Ogata et al. 1988; Lorberboum-Galski et al. 1988a; Lorberboum-Galski et al. 1988b). IL-2-PE40 effectively attenuates several experimental autoimmune conditions, such as adjuvant-induced arthritis (Case et al. 1989; Lorberboum-Galski et al. 1991), experimental autoimmune encephalomyelitis (Rose et al. 1991) and autoimmune uveoretinitis (Roberge et al. 1989), and prevents the rejection of heart and corneal allografts in rodents (Lorberboum-Galski et al. 1989; Herbort et al. 1991). IL-2-PE40 has also been showed to suppress the growth of a T-cell lymphoma in mice (Kozak et al. 1990). The effect of IL-2-PE40 has been showed to be specific in that IL-2-PE40Asp553, a mutant chimeric protein in which the enzymatic domain of PE is inactive, has no effect (Case et al. 1989; Lorberboum-Galski et al. 1989).
A disadvantage of this class of targeted chimeric toxins is that the bacterial toxins are recognized as foreign by the immune system of the recipient organism. In order to overcome this problem, a new generation of chimeric proteins exploiting human apoptosis-inducing proteins was developed. IL-2-Bax is the first prototype of this class. Bax is a pro-apoptotic member of the Bcl-2 protein family. IL-2-Bax specifically targets IL-2R-expressing cells and induces cell-specific, dose-dependent apoptosis (Aqeilan et al. 1999; Aqeilan et al. 2002). IL-2-BaxS184D, a mutant in which Bax is inactive, is taken up by IL-2R-expressing cells, such as IL-2-Bax, but does not activate the apoptotic pathway (Aqeilan et al. 2002). In this study, we investigated the effects of the novel apoptosis-inducing IL-2R-targeted chimeric protein IL-2-Bax on bleomycin-induced lung injury in mice.
Materials and methods
Animals
Male, 10 to 11-week-old C57Bl/6 mice were obtained from Harlan Sprague Dawley (Indianapolis, IN, USA). All procedures involving animals were approved by the institutional committee of animal care. Mice were housed in a specific pathogen-free environment in plastic cages on hardwood shavings, three to 10 animals per cage. A 12-h light\dark cycle was maintained, and mice had access to water and rodent laboratory chow ad libitum. Mice were acclimated to these conditions for 4–7 days before treatments commenced.
Construction and expression of the chimeric proteins
The plasmid pAY1 encoding the protein IL-2-Bax and the plasmid pHL823 encoding IL-2-PE664Glu were constructed as previously described (Aqeilan et al. 1999). The chimeric genes were expressed in Escherichia coli strain BL21. Subfractionation of the expressing cells revealed that both chimeric proteins were highly enriched in the insoluble fraction. The insoluble fraction was denatured and then renatured as described previously (Aqeilan et al. 1999). In brief, a pellet of expressing cells was suspended in 20 mm Tris–HCl pH 8.0, 1 mm EDTA containing 0.2 mg/ml of lysozyme, sonicated (three 30 s bursts) and centrifuged at 35,000 × g for 30 min. The supernatant (soluble fraction) was removed and kept for analysis. The pellet was denatured in extraction buffer A (6 m guanidine–HCl, 0.1 m Tris–HCl, pH 8.6, 1 mm EDTA, 0.05 m NaCl and 10 mm DTT) and stirred for 1 h. at 4 °C. The suspension was cleared by means of centrifugation at 35,000 × g for 15 min and the pellet was discarded. The protein solution was diluted 1 : 100 in refolding buffer (20 mm Tris–HCL, pH 8.0, 1 mm EDTA, 0.25 m NaCl and 5 mm dithiothreitol) and kept at 4 °C for 48 h. The partially purified chimeric proteins, enriched with IL-2-based chimeric proteins, were used in all our experiments.
Experimental design
The effect of IL-2-Bax on bleomycin-induced lung injury was assessed, and was compared to that of an older-generation chimeric protein, IL-2-PE664Glu. Animals were randomly divided into four experimental groups, as showed in Table 1. In addition to the two experimental groups, the Bleo/PBS and Sal/PBS groups served as positive and negative controls, respectively. In a preliminary study, administering IL-2-Bax and IL-2-PE664Glu to otherwise untreated mice did not have any effect on lung histology, thus obviating the necessity for Sal/IL-2-Bax and Sal/IL-2-PE664Glu groups.
Table 1. Experimental groups.
| Group | Intratracheal | Intraperitoneal | n |
|---|---|---|---|
| Bleo/IL-2-PE | Bleomycin | IL-2-PE664Glu | 6 |
| Bleo/IL-2-Bax | Bleomycin | IL-2-Bax | 8 |
| Bleo/PBS | Bleomycin | PBS | 8 |
| Sal/PBS | Saline | PBS | 9 |
Experimental groups for the central experiment (presented in Figures 2, 4) are listed with the treatment administered by intratracheal instillation (IT) and intraperitoneal injection, and the number of animals in each group (n). In addition to the two experimental groups (Bleo/IL-2-Bax and Bleo/IL-2-PE), the Bleo/PBS and Sal/PBS groups served as positive and negative controls, respectively.
Each animal received a single intratracheal instillation of bleomycin or control saline on day 0. Twice-daily intraperitoneal injections of the chimeric proteins or PBS were commenced 12–16 h later, and continued until the evening before killing, for a total of 13 days. IL-2-PE664Glu was administered at a dose of 15 µg/mouse/day (total protein amount) in two divided doses, each in 0.5 ml of PBS. IL-2-Bax was administered similarly at a dose of 100 µg/mouse/day for the first week, followed by 50 µg/mouse/day for the second week. The control groups received intraperitoneal injections of 0.5 ml of PBS twice daily. Mice were killed for study 14 days after intratracheal instillation.
Intratracheal instillation of animals
Mice were anaesthetized by means of intraperitoneal injection of 0.05–0.07 ml of 40 mg/ml of Ketalar (Parke-Davis, Pontypool, Gwent, UK) and 0.5 mg/ml of Droperidol (Janssen Pharmaceutica, Beerse, Belgium). The trachea was cannulated, and a dose of 0.08 mg of bleomycin (H. Lundbeck A/S, Copenhagen, Denmark) dissolved in 0.1 ml of sterile 0.9% saline solution or 0.1 ml of sterile 0.9% saline alone was slowly injected, as previously described in detail (Izbicki et al. 2002b).
Quantitative assessment of lung injury
Lung injury was assessed quantitatively as previously described (Kremer et al. 1999; Laxer et al. 1999; Lossos et al. 2000; Berkman et al. 2001; Segel et al. 2001; Lossos et al. 2002; Tzurel et al. 2002; Izbicki et al. 2002a; Izbicki et al. 2002b) by studies of bronchoalveolar lavage (BAL), semi-quantitative and quantitative morphological studies of lung injury and lung hydroxyproline measurements.
Bronchoalveolar lavage (BAL)
BAL was performed by means of injection and withdrawal of eight aliquots of 0.5 cc Ca++/Mg++ free PBS. Total cell count was performed using a haemocytometer. A differential cell count was performed on 200 cells by using cytospin slides stained with Diff-Quik (Baxter). Cells numbers for the different BAL cell types are the products (calculated for individual animals) of the total cell count and the percentages obtained from the differential count, expressed as cells/ml of BAL fluid.
Lung hydroxyproline content
Hydroxyproline content was measured as previously described (Izbicki et al. 2002b). In brief, after BAL and thoracotomy, the right main bronchus was ligated, and the lung was removed and dissected free of extraneous tissues. The lung was then hydrolysed in 6 N HCl for 24 h at 106 °C. An aliquot was analysed on an amino acid analyser (Beckman 6300 (Palo Alto, CA, USA)). Hydroxyproline results are expressed as nanomoles per lung.
Morphological examination
The left lung was initially fixed in situ by means of intratracheal infusion of 4% formalin/1% glutaraldehyde/0.1 m cacodylate buffer (pH 7.4) maintained at 25 cm hydrostatic pressure for 15 min. The trachea was then ligated and both the heart and the lung were removed en-block and immersed in fixative for an additional 24 h. Three 0.2-cm-thick transverse sections of the left lung were embedded in paraffin. Paraffin tissue blocks were cut to provide sections of 4–6 µm. The sections were stained with H&E and modified Masson's trichrome stains to be used for morphological assessment of the lung.
Semiquantitative morphological index (SMI)
Pathological changes in lung sections were graded semi-quantitatively, as previously reported (Lossos et al. 2000; Segel et al. 2001; Izbicki et al. 2002b), using the following grading scheme: 0, normal tissue; 1, a few foci of inflammation and epithelial hyperplasia usually limited to subpleural foci within just one to two sections; 2, greater involvement with several foci present in all lung sections; 3, extensive lesions in at least two of three sections; 4, extensive involvement in all lung sections, with only a few normal alveolar regions.
Quantitative image analysis
Morphological assessment was further refined by means of computer-assisted morphometry, using Optimas image analysis software (Optimas, Bothell, WA, USA), as we have previously described (Kremer et al. 1999; Laxer et al. 1999; Berkman et al. 2001; Izbicki et al. 2002a; Izbicki et al. 2002b).
Alveolar wall area fraction.
Alveolar wall thickness may increase due to either fibrosis or interstitial oedema. Alveolar wall area fraction was quantified by means of the image analysis program configured to determine, in sections of H&E-stained slides, the area fraction of alveolar wall tissue. Using a 10× objective lens, 10 randomly selected fields lacking visible blood vessels or airways were selected using a video camera and were displayed on a monitor. Each field of interest measured a constant 455 × 345 µm. The image analysis software was programmed to measure the area of all stained tissue and divide it by the field area, thereby calculating an alveolar wall area fraction value for 10 fields, which were averaged for each animal. Data have been presented as a percentage.
Fibrosis fraction.
The degree of fibrosis was quantified by analysing slides that were stained with a modified trichrome stain, to enhance the blue-stained collagen. The image analysis program was configured to detect areas of blue-stained collagen within each of 20 randomly selected high-power fields (135 × 95 µm) per slide. The fraction of blue-stained collagen areas for each field was averaged for each animal and is presented as a percentage.
Isolation of lung cells (LC)
Lungs were removed, minced with a razor blade and incubated at 37 °C for 90 min in RPMI-1640 containing 1 mg/ml collagenase (Sigma, St Louis, MO, USA). After enzyme treatment, lung tissue was finely minced and gently filtered through a nylon mesh. After two washes in Hanks Balanced Salt Solution, cells were resuspended in culture medium (RPMI-1640 supplemented with 10% FCS, 100 µ/ml of penicillin, 100 mg/ml of streptomycin, 25 mm Hepes buffer, 2 mm l-glutamine and 1 mm sodium pyruvate).
Flow cytometry for LC surface markers
LC were washed twice with FACS medium (0.02% NaN3, 2% FCS in PBS), fixed with 3.7% paraformaldehyde and washed again with FACS medium. Cells were stained with the following antibodies (all from Pharmingen BD, Temse, Belgium): anti-CD3 for all T cells, anti-CD4 for T-helper cells, anti-CD8 for T-cytotoxic and T-suppressor cells, anti-B220 for B lymphocytes and anti-NK1.1 for natural killer cells. Cells were then analysed by means of flow cytometry (FACScan with LYSYS II software for analysis, Beckton-Dickinson, San Jose, CA, USA).
In vitro assay for the effect of IL-2-PE664Glu on LC extracted from bleomycin-treated mice
LC were extracted from the lungs of bleomycin-treated mice 6 days post-intratracheal instillation and were cultured. Recombinant IL-2 (PeproTech, London, UK) was added to the medium (10 U/ml), and cells were plated at 2 × 104 cells/200 µl/well in a 96-well tissue-culture plate. Cells were then incubated with 50, 100, 500, 1000 and 2000 ng (total protein/well) of IL-2-PE664Glu or with PBS (control) overnight. The cytotoxic activity of IL-2-PE664Glu was assessed by determining cell viability, using the trypan blue exclusion assay, and by measuring the inhibition of protein synthesis (3H-leucine incorporation) (Lorberboum-Galski et al. 1988b).
In vitro assay for the effect of IL-2-Bax on LC extracted from bleomycin-treated mice
LC (0.5 × 106 cells/10 ml) from mice killed 6 days post-intratracheal bleomycin were incubated with IL-2-Bax (5 µg/ml, total protein concentration) and were harvested after 24, 48 or 72 h. RNase (10 µl of 50 µg/ml) and propidium iodide (5 µg/ml) were added to the cell samples, which were then analysed by using flow cytometry for DNA content.
Statistical methods
BAL cell number, alveolar wall area, fibrosis fraction and hydroxyproline levels were analysed by using one-way anova. Post-test comparisons were performed by means of the Bonferroni test. Comparison of the non-parametric SMI scores was performed using the Kruskal–Wallis test, and post-test comparisons by the Dunn test. Comparisons of flow cytometry data (Bleo/IL-2-Bax vs. Bleo/PBS) were performed using the Mann–Whitney test. P-values of <0.05 were considered significant.
Results
IL-2-Bax and IL-2-PE664Glu effectively target cells from bleomycin-injured lungs
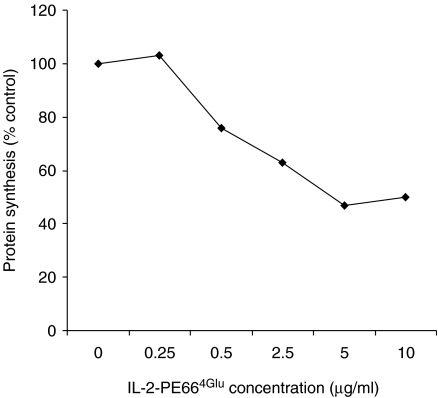
We initially studied the effect of IL-2-Bax and IL-2-PE664Glu on lung cells extracted from bleomycin-treated mice ex vivo in order to determine whether cells that are potential targets for IL-2-R-targeted chimeric toxins are present in the lungs of these mice. IL-2-PE664Glu caused inhibition of protein synthesis by lung cells in a dose-dependent manner (Figure 1). At the maximally effective concentration (5 µg/ml), protein synthesis decreased to 47% of the control level (median value of triplicate measurements).
Figure 1.
IL-2-PE664Glu dose-dependently inhibits protein synthesis in lung cells from bleomycin-treated mice. Cells were extracted from the lungs of mice 6 days after intratracheal instillation of bleomycin, and cultured ex vivo. The effect of different concentrations of IL-2-PE664Glu on protein synthesis (3H-leucine uptake) was measured. Median values of measurements done in triplicate are showed. The experiment was performed twice.
The apoptotic effect of IL-2-Bax on cultured lung cells extracted from bleomycin-treated mice was also demonstrated. After 24, 48 and 72 h in culture, the percentage of untreated cells that were apoptotic was 30, 41 and 45%, respectively. Treating the cells with IL-2-Bax consistently increased the apoptosis rate to 37, 50 and 65%, respectively (all values are medians of triplicate measurements).
IL-2-Bax reduces lymphocytic infiltration of the lung in response to bleomycin
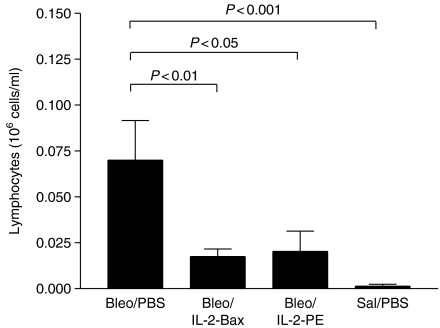
Bleomycin induced typical inflammatory changes as evident in the results of total and differential BAL cell counts (Table 2). At 14 days after intratracheal instillation of bleomycin or control saline, the total BAL cell count was increased more than five-fold in the Bleo/PBS group, compared to the Sal/PBS group (P < 0.001). The percentages of BAL lymphocytes and neutrophils were increased, and the percent of macrophages reciprocally decreased, in bleomycin-treated mice, compared to the saline group. Eosinophils represented <1% of BAL cells in all samples from all groups. In terms of absolute cell counts (cells/ml), there was a significant increase in the counts of macrophages (P < 0.001), as well as lymphocytes (P < 0.001) (Figure 2) and neutrophils (P < 0.05), in the bleomycin group.
Table 2. Total and differential bronchoalveolar lavage (BAL) cell counts.
| Bleo/PBS (n = 8) | Bleo/IL-2-Bax (n = 8) | Bleo/IL-2-PE (n = 6) | Sal/PBS (n = 9) | |
|---|---|---|---|---|
| Total (×106 cells/ml) | 0.21 ± 0.03* | 0.13 ± 0.02† | 0.11 ± 0.03† | 0.04 ± 0.004 |
| Lymphocytes (%) | 29 ± 7‡ | 13 ± 1† | 14 ± 4† | 0.5 ± 0.2 |
| Macrophages (%) | 63 ± 8‡ | 77 ± 2† | 79 ± 6† | 99 ± 0.3 |
| Neutrophils (%) | 7 ± 1.5‡ | 9 ± 1.5 | 9 ± 2.5 | 0.1 ± 0.1 |
Total and differential cell counts from the bronchoalveolar lavage (BAL) fluid are presented as mean ± SEM for each group. Differential counts are given as a percentage of the total cell count.
P < 0.001 compared to Sal/PBS.
P < 0.05 compared to Bleo/PBS.
P < 0.05 compared to Sal/PBS.
Figure 2.
IL-2-Bax and IL-2-PE664Glu attenuated the BAL lymphocytosis induced by bleomycin. Experimental groups and treatment protocols are detailed in the Materials and Methods section. Mice were studied 14 days after intratracheal instillation. Mean + SEM values (106 cells/ml) are showed for BAL lymphocyte counts, and were compared by means of anova/Bonferroni test.
The BAL lymphocytosis induced by bleomycin was attenuated in mice treated with IL-2-Bax or IL-2-PE664Glu– mean lymphocyte counts were approximately 25% that of the Bleo/PBS group (Figure 2). The total BAL cell counts were also reduced by 65% in the IL-2-Bax group and by 45% in the IL-2-PE664Glu group (Table 2) (P < 0.05). BAL macrophages and neutrophils in the Bleo/IL-2-Bax and Bleo/IL-2-PE groups were numerically decreased, compared to the Bleo/PBS group, but the differences did not achieve statistical significance.
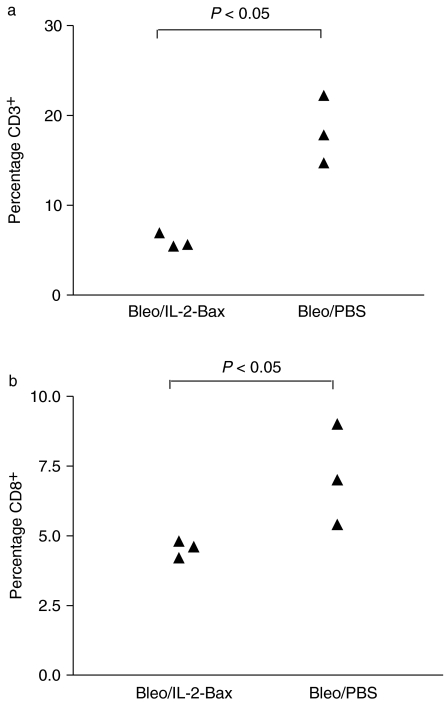
Flow cytometry of LC extracted 7 days post-intratracheal bleomycin showed a significant reduction of 69 and 34%, respectively, in the percentage of CD3+ and CD8+ cells in mice treated with IL-2-Bax, compared to LC extracted from mice treated with bleomycin alone (Figure 3). The percentage of CD4+, B220+ and NK1.1+ cells was unaffected by IL-2-Bax.
Figure 3.
Changes in lung cell (LC) subpopulations induced by IL-2-Bax. All animals received intratracheal bleomycin on day 0. Twice-daily injections of IL-2-Bax or PBS (three mice per group) were commenced 12–16 h later, and continued until the evening before killig, for a total of 6 days. LC were extracted on day 7. Percentages of (a) CD3+ and (b) CD8+ cells are showed, as detected by means of flow cytometry. Each point represents cells from a single animal. Comparison between the two groups was by the Mann–Whitney test.
Quantitative parameters of lung fibrosis
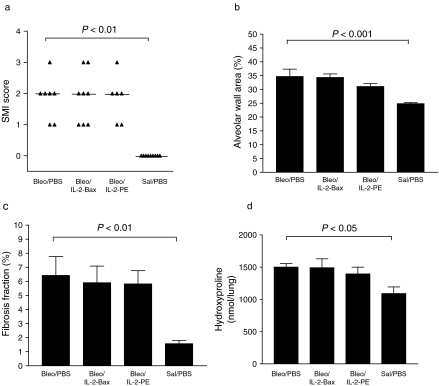
Bleomycin lung injury was reflected by an increased SMI score of lung pathology in all mice of the Bleo/PBS group, compared to the Sal/PBS group (P < 0.01)(Figure 4a). There was no significant difference in the SMI score between the Bleo/IL-2-Bax group, or the Bleo/IL-2-PE group, and the Bleo/PBS group.
Figure 4.
Morphological and biochemical measurements of lung injury. Experimental groups and treatment protocols are detailed in the Materials and methods section. Mice were studied 14 days after intratracheal instillation. (a) Semi-quantitative morphological index (SMI) – each point represents the score for a single animal; horizontal lines represent median scores; comparison between groups was by the Kruskal–Wallis test; (b) alveolar wall area (mean + SEM, comparison between groups by anova/Bonferroni test); (c) fibrosis fraction (mean + SEM, anova/Bonferroni test); (d) lung hydroxyproline (mean + SEM, anova/Bonferroni test). IL-2-Bax and IL-2-PE664Glu did not affect bleomycin -induced fibrosis, as assessed by these parameters.
These results were confirmed by means of the computer-assisted quantitative image analysis. The alveolar wall area (Figure 4b) was increased about 1.5-fold in the Bleo/PBS group (P < 0.001). However, in the Bleo/IL-2-Bax and the Bleo/IL-2-PE groups, the alveolar wall area was not different from that of the Bleo/PBS group. Similarly, the fibrosis fraction (Figure 4c) was four-fold greater in the Bleo/PBS group than that in the Sal/PBS group (P < 0.01), and neither IL-2-Bax nor IL-2-PE664Glu had an effect.
Lung collagen, as assessed by means of hydroxyproline content (Figure 4d), was significantly higher in the Bleo/PBS group than that in the Sal/PBS group – 1501 ± 54 vs. 1091 ± 102 nmol/lung, respectively (P < 0.05). There was no significant difference between the Bleo/IL-2-Bax and the Bleo/IL-2-PE groups and the Bleo/PBS group.
Discussion
In this study, we investigated the use of immunotherapy for bleomycin lung injury by using chimeric proteins composed of a targeting component and a cytotoxic component, genetically engineered to be attached by peptide linkage.
The targeting component common to our chimeric proteins is human IL-2, which targets the IL-2R. The IL-2/IL-2R complex regulates the magnitude and duration of the T-cell immune response (Taniguchi & Minami 1993). The high-affinity IL-2R is not expressed on resting T or B cells, but is strongly induced on mature B (Arima et al. 1992) and T lymphocytes, following T-cell activation (Steinberger et al. 1997). Thus IL-2R-targeted chimeric toxins selectively kill activated lymphocytes, with little or no effect on quiescent cells (Ogata et al. 1988; Lorberboum-Galski et al. 1988a; Lorberboum-Galski et al. 1988b; Lorberboum-Galski et al. 1990; Steinberger et al. 1995; Aqeilan et al. 1999).
Our hypothesis was that elimination of activated lymphocytes by IL-2-Bax would attenuate bleomycin-induced lung injury. Our results show that IL-2-Bax is capable of eliminating lymphocytes in vivo– the lymphocytic infiltration of the lungs in response to bleomycin was significantly attenuated. However, the effect of IL-2-Bax on the inflammatory cells in the BAL fluid was not accompanied by a statistically significant effect on lung fibrosis as assessed by morphological and biochemical parameters. Of note, there was no substantial difference between the results obtained with IL-2-Bax and those achieved with IL-2-PE664Glu, a first-generation IL-2R-targeted chimeric toxin.
There are two possible explanations for our results. One is that lymphocytes do not contribute significantly to bleomycin-induced lung injury. The other possible explanation, consistent with the possibility that lymphocytes do play an important role in the pathogenesis of bleomycin lung injury, stems from the fact that although lymphocytic infiltration was reduced by IL-2-Bax, it was not abolished altogether. Thus, the remaining lymphocytes might be sufficient to activate the cascade of events leading to fibrosis. Moreover, we found that only CD8+ lymphocytes were significantly reduced by treatment with IL-2-Bax. As noted, there is circumstantial evidence that suppressor T cells might play a role in shutting off the fibrotic process (Thrall & Barton 1984). It has also been showed that IL-2-PE40, a first-generation IL-2R-targeted toxin, can induce IL-2 signal transduction and downstream cytokine production by T-helper cells, before exerting its cytotoxic effect (Volk et al. 1994).
The doses we used in these experiments were the maximum tolerable. It may be possible in future, with further refinement of the production of IL-2-Bax, to use higher doses and achieve complete elimination of the lymphocytic response. This would allow distinction between the two possibilities above.
Conclusions
The novel IL-2R-targeted chimeric toxin, IL-2-Bax, has a demonstrable biological effect in vivo, in that it reduces the lymphocytic infiltration of the lungs in response to intratracheal bleomycin. This effect on the inflammatory component of bleomycin-induced lung injury was not accompanied by a decrease in lung fibrosis, the endpoint of the injury cascade.
Acknowledgments
We thank Pazit Cohen and Anita Kol for expert technical assistance. This work was supported by the Israel Public Committee for Deposition of Estate Funds, the David Shainberg Fund and the Charlotte and Louis Kaitz Boston University School of Medicine/Hebrew University – Hadassah Medical School Program.
References
- Aqeilan R, Kedar R, Ben-Yehudah A, Lorberboum-Galski H. Mechanism of action of IL2-Bax: an apoptosis-inducing chimeric protein targeted against interleukin-2 receptor expressing cells. Biochem J. 2002;30:332–338. doi: 10.1042/BJ20020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan R, Yarkoni S, Lorberboum-Galski H. Interleukin 2-Bax: a novel prototype of human chimeric proteins for targeted therapy. FEBS Lett. 1999;457:271–276. doi: 10.1016/s0014-5793(99)01050-9. [DOI] [PubMed] [Google Scholar]
- Arima N, Kamio M, Imada K, et al. Pseudo-high affinity interleukin 2 (IL-2) receptor lacks the third component that is essential for functional IL-2 binding and signaling. J Exp Med. 1992;176:1265–1272. doi: 10.1084/jem.176.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman N, Kremer S, Or R, et al. Human recombinant interferon-alpha2a and interferon-alphaA/D have different effects on bleomycin-induced lung injury. Respiration. 2001;68:169–177. doi: 10.1159/000050488. [DOI] [PubMed] [Google Scholar]
- Brinkmann U, Pastan I. Immunotoxins against cancer. Biochim Biophys Acta. 1994;1198:27–45. doi: 10.1016/0304-419x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Case JP, Lorberboum-Galski H, Lafyatis R, FitzGerald D, Wilder RL, Pastan I. Chimeric cytotoxin IL2-PE40 delays and mitigates adjuvant-induced arthritis in rats. Proc Natl Acad Sci USA. 1989;86:287–291. doi: 10.1073/pnas.86.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DB, Hyde DM, Giri SN. Morphometric estimates of infiltrative cellular changes during the development of bleomycin-induced pulmonary fibrosis in hamsters. Am J Pathol. 1983;112:170–177. [PMC free article] [PubMed] [Google Scholar]
- Ekimoto H, Aikawa M, Ohnuki T, et al. Immunological involvement in pulmonary fibrosis induced by peplomycin. J Antibiot. 1985;38:94–98. doi: 10.7164/antibiotics.38.94. (Tokyo) [DOI] [PubMed] [Google Scholar]
- Helene M, Lake-Bullock V, Zhu J, Hao H, Cohen DA, Kaplan AM. T cell independence of bleomycin-induced pulmonary fibrosis. J Leukoc Biol. 1999;65:187–195. doi: 10.1002/jlb.65.2.187. [DOI] [PubMed] [Google Scholar]
- Herbort CP, de Smet MD, Roberge FG, et al. Treatment of corneal allograft rejection with the cytotoxin IL-2-PE40. Transplantation. 1991;52:470–474. doi: 10.1097/00007890-199109000-00015. [DOI] [PubMed] [Google Scholar]
- Hesterberg TW, Gerriets JE, Reiser KM, Jackson AC, Cross CE, Last JA. Bleomycin-induced pulmonary fibrosis: correlation of biochemical, physiological, and histological changes. Toxicol Appl Pharmacol. 1981;60:360–367. doi: 10.1016/0041-008x(91)90239-b. [DOI] [PubMed] [Google Scholar]
- Hibbs MS, Postlethwaite AE, Mainardi CL, Seyer JM, Kang AH. Alterations in collagen production in mixed mononuclear leukocyte-fibroblast cultures. J Exp Med. 1983;157:47–59. doi: 10.1084/jem.157.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izbicki G, Or R, Christensen TG, et al. Bleomycin-induced lung fibrosis in IL-4-overexpressing and knockout mice. Am J Physiol Lung Cell Mol Physiol. 2002a;283:L1110–L1116. doi: 10.1152/ajplung.00107.2002. [DOI] [PubMed] [Google Scholar]
- Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol. 2002b;83:111–119. doi: 10.1046/j.1365-2613.2002.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Ziff M. Lymphokine stimulation of collagen accumulation. J Clin Invest. 1976;58:240–252. doi: 10.1172/JCI108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- Kozak RW, Lorberboum-Galski H, Jones L, et al. IL-2-PE40 prevents the development of tumors in mice injected with IL-2 receptor expressing EL4 transfectant tumor cells. J Immunol. 1990;145:2766–2771. [PubMed] [Google Scholar]
- Kremer S, Breuer R, Lossos IS, et al. Effect of immunomodulators on bleomycin-induced lung injury. Respiration. 1999;66:455–462. doi: 10.1159/000029410. [DOI] [PubMed] [Google Scholar]
- Laxer U, Lossos IS, Gillis S, et al. The effect of enoxaparin on bleomycin-induced lung injury in mice. Exp Lung Res. 1999;25:531–541. doi: 10.1080/019021499270114. [DOI] [PubMed] [Google Scholar]
- Lazo JS, Hoyt DG, Sebti SM, Pitt BR. Bleomycin: a pharmacologic tool in the study of the pathogenesis of interstitial pulmonary fibrosis. Pharmacol Ther. 1990;47:347–358. doi: 10.1016/0163-7258(90)90061-6. [DOI] [PubMed] [Google Scholar]
- Lorberboum-Galski H, Barrett LV, Kirkman RL, et al. Cardiac allograft survival in mice treated with IL-2-PE40. Proc Natl Acad Sci USA. 1989;86:1008–1012. doi: 10.1073/pnas.86.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberboum-Galski H, FitzGerald D, Chaudhary V, Adhya S, Pastan I. Cytotoxic activity of an interleukin 2-pseudomonas exotoxin chimeric protein produced in Escherichia coli. Proc Natl Acad Sci USA. 1988a;85:1922–1926. doi: 10.1073/pnas.85.6.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberboum-Galski H, Garsia RJ, Gately M, et al. IL2-PE664Glu, a new chimeric protein cytotoxic to human-activated T lymphocytes. J Biol Chem. 1990;265:16311–16317. [PubMed] [Google Scholar]
- Lorberboum-Galski H, Kozak RW, Waldmann TA, Bailon P, FitzGerald DJ, Pastan I. Interleukin 2 (IL2) PE40 is cytotoxic to cells displaying either the p55 or p70 subunit of the IL2 receptor. J Biol Chem. 1988b;263:18650–18656. [PubMed] [Google Scholar]
- Lorberboum-Galski H, Lafyatis R, Case JP, FitzGerald D, Wilder RL, Pastan I. Administration of IL-2-PE40 via osmotic pumps prevents adjuvant induced arthritis in rats. Improved therapeutic index of IL-2-PE40 administered by continuous infusion. Int J Immunopharmacol. 1991;13:305–315. doi: 10.1016/0192-0561(91)90112-k. [DOI] [PubMed] [Google Scholar]
- Lossos IS, Izbicki G, Or R, Goldstein RH, Breuer R. The effect of suramin on bleomycin-induced lung injury. Life Sci. 2000;67:2873–2881. doi: 10.1016/s0024-3205(00)00865-1. [DOI] [PubMed] [Google Scholar]
- Lossos IS, Or R, Ginzburg V, Christensen TG, Mashriki Y, Breuer R. Cyclosporin A upmodulates bleomycin-induced pulmonary fibrosis in BALB/c mice. Respiration. 2002;69:344–349. doi: 10.1159/000063273. [DOI] [PubMed] [Google Scholar]
- Ogata M, Lorberboum-Galski H, FitzGerald D, Pastan I. IL-2-PE40 is cytotoxic for activated T lymphocytes expressing IL-2 receptors. J Immunol. 1988;141:4224–4228. [PubMed] [Google Scholar]
- Postlethwaite AE, Smith GN, Mainardi CL, Seyer JM, Kang AH. Lymphocyte modulation of fibroblast function in vitro: stimulation and inhibition of collagen production by different effector molecules. J Immunol. 1984;132:2470–2477. [PubMed] [Google Scholar]
- Roberge FG, Lorberboum-Galski H, Le Hoang P, et al. Selective immunosuppression of activated T cells with the chimeric toxin IL-2-PE40. Inhibition of experimental autoimmune uveoretinitis. J Immunol. 1989;143:3498–3502. [PubMed] [Google Scholar]
- Rose JW, Lorberboum-Galski H, Fitzgerald D, et al. Chimeric cytotoxin IL2-PE40 inhibits relapsing experimental allergic encephalomyelitis. J Neuroimmunol. 1991;32:209–217. doi: 10.1016/0165-5728(91)90190-i. [DOI] [PubMed] [Google Scholar]
- Schrier DJ, Phan SH, McGarry BM. The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis. A biochemical evaluation. Am Rev Respir Dis. 1983;127:614–617. doi: 10.1164/arrd.1983.127.5.614. [DOI] [PubMed] [Google Scholar]
- Segel MJ, Or R, Tzurel A, et al. All-trans-retinoic acid (ATRA) is of no benefit in bleomycin-induced lung injury. Pulm Pharmacol Ther. 2001;14:403–407. doi: 10.1006/pupt.2001.0300. [DOI] [PubMed] [Google Scholar]
- Sharma SK, MacLean JA, Pinto C, Kradin RL. The effect of an anti-CD3 monoclonal antibody on bleomycin-induced lymphokine production and lung injury. Am J Respir Crit Care Med. 1996;154:193–200. doi: 10.1164/ajrccm.154.1.8680680. [DOI] [PubMed] [Google Scholar]
- Snider GL, Celli BR, Goldstein RH, O'Brien JJ, Lucey EC. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin. Lung volumes, volume–pressure relations, carbon monoxide uptake, and arterial blood gas studied. Am Rev Respir Dis. 1978;117:289–297. doi: 10.1164/arrd.1978.117.2.289. [DOI] [PubMed] [Google Scholar]
- Steinberger I, Ben-Bassat H, Hochberg E, Lorberboum-Galski H. Interleukin-2 (IL-2) receptor alpha, beta and gamma subunit expression as a function of B-cell lineage ontogeny: the use of IL-2-PE66 (4Glu) to characterize internalization via IL-2 receptor subunits. Scand J Immunol. 1997;46:129–136. doi: 10.1046/j.1365-3083.1997.d01-101.x. [DOI] [PubMed] [Google Scholar]
- Steinberger I, Brenner T, Lorberboum-Galski H. Interleukin-2 pseudomonas exotoxin chimeric protein is cytotoxic to B cell cultures derived from myasthenia gravis patients. J Neurol Sci. 1995;133:183–191. doi: 10.1016/0022-510x(95)00221-m. [DOI] [PubMed] [Google Scholar]
- Szapiel SV, Elson NA, Fulmer JD, Hunninghake GW, Crystal RG. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis. 1979;120:893–899. doi: 10.1164/arrd.1979.120.4.893. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993;73:5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- Thrall RS, Barton RW. A comparison of lymphocyte populations in lung tissue and in bronchoalveolar lavage fluid of rats at various times during the development of bleomycin-induced pulmonary fibrosis. Am Rev Respir Dis. 1984;129:279–283. [PubMed] [Google Scholar]
- Thrall RS, Lovely EJI, Baron RW, McCormick JR, Phan SH, Ward PA. The effect of T cell depletion on the development of bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis. 1980;121(s):99. [Google Scholar]
- Thrall RS, McCormick JR, Jack RM, Phan SH, Ward PA. The effect of antilymphocyte globulin on the development of bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis. 1979;119(s):83. [Google Scholar]
- Tzurel A, Segel MJ, Or R, Goldstein RH, Breuer R. Halofuginone does not reduce fibrosis in bleomycin-induced lung injury. Life Sci. 2002;71:1599–1606. doi: 10.1016/s0024-3205(02)01902-1. [DOI] [PubMed] [Google Scholar]
- Volk HD, Muller S, Yarkoni S, Diamantstein T, Lorberboum-Galski H. Mechanisms of dichotomous action of IL-2-pseudomonas exotoxin 40 (IL-2-PE40) on cell-mediated and humoral immune response. J Immunol. 1994;153:2497–2505. [PubMed] [Google Scholar]
- Waldmann TA. The IL-2/IL-2 receptor system: a target for rational immune intervention. Immunol Today. 1993;14:264–270. doi: 10.1016/0167-5699(93)90043-K. [DOI] [PubMed] [Google Scholar]
- Zhu J, Cohen DA, Goud SN, Kaplan AM. Contribution of T lymphocytes to the development of bleomycin-induced pulmonary fibrosis. Ann N Y Acad Sci. 1996;796:194–202. doi: 10.1111/j.1749-6632.1996.tb32581.x. [DOI] [PubMed] [Google Scholar]