Abstract
Type II collagen-induced arthritis (CIA) is an animal model of rheumatoid arthritis that has been used extensively to address questions of disease pathogenesis and to validate novel therapeutic targets. Susceptibility to CIA is strongly associated with major histocompatibility complex class II genes, and the development of arthritis is accompanied by a robust T- and B-cell response to type II collagen. The main pathological features of CIA include proliferative synovitis with infiltration of inflammatory cells, pannus formation, cartilage degradation, erosion of bone and fibrosis. Pro-inflammatory cytokines, such as tumour necrosis factor α and interleukin-1β, are expressed in the arthritic joints in both murine CIA and human rheumatoid arthritis, and blockade of these molecules results in amelioration of disease. Hence, there is a great deal of interest in the development of small-molecular-weight inhibitors of pro-inflammatory cytokines. There is also interest in the development and testing of drugs with the capacity to modulate the immune pathways involved in driving the inflammatory response in arthritis. For these reasons, there is a need to monitor the effect of novel treatments on cytokine expression in vivo. In this review, we outline the various techniques used to detect cytokines in experimental arthritis and describe how these techniques have been used to quantify changes in cytokine expression following therapeutic intervention.
Keywords: autoimmunity, collagen-induced arthritis, cytokines, rheumatoid arthritis, TNF-α, type II collagen
Background to the model
Rheumatoid arthritis (RA) is a chronic and disabling disease affecting approximately 1% of the population on a worldwide basis. Much has been learnt in recent years about the cytokines and other mediators that drive the pathological processes in RA, and a number of studies have pointed to a major pathological role for tumour necrosis factor a (TNF-α) in the disease process (Feldmann et al. 2004). However, the underlying cause of RA is unknown and research aimed at understanding the aetiopathogenesis of the disease and improving treatment modalities remains a priority.
Animal models of arthritis offer an extremely important experimental tool for many different kinds of research in addition to the development and evaluation of novel therapeutic agents, including the identification of pro- and anti-inflammatory mediators, the analysis of genetic susceptibility factors and in the search for markers of disease progression (Williams 1998). Of the different animal models, collagen-induced arthritis (CIA) has come to be the most widely used model and occurs in rats, mice and primates following immunization with type II collagen in adjuvant. The pathological changes include synovitis with infiltration of polymorphonuclear and mononuclear cells, pannus formation, erosion of bone and cartilage and fibrosis. In mice, immunization with bovine, chicken or rat type II collagen usually leads to a relatively acute form of arthritis. In contrast, immunization with murine collagen results in a more chronic disease course (Holmdahl et al. 1986; Boissier et al. 1987; Malfait et al. 2001), and the chief determinant of chronicity in CIA is probably related to the extent to which the immune response is targeted at self collagen, as opposed to the collagen used for immunization.
The CIA model exhibits many clinical similarities to human RA (Holmdahl et al. 1989), and both the diseases exhibit similar patterns of synovitis, pannus formation, cartilage and bone erosion, fibrosis and loss of joint function (Trentham 1982). Another important similarity between RA and CIA is that susceptibility to both the diseases is strongly associated with genes encoding major histocompatibility complex (MHC) class II molecules, suggesting that CD4+ T cells are involved in the pathogenesis of both the forms of disease. Thus, mouse strains bearing MHC types I-Aq and I-Ar show the greatest susceptibility to CIA, and this is analogous to the situation in RA, in which particular subtypes of DR4 and DR1 are strongly associated with susceptibility to the disease.
In addition to the cellular arm of the immune response, it is also thought that humoural responses play a significant role in the pathogenesis of both human RA and murine CIA (Holmdahl et al. 1989), although in contrast to CIA, there is a lack of convincing data pointing to a role for type II collagen-specific autoantibodies in the pathogenesis of human RA.
Impact of TNF-α blockade on arthritis
One way of assessing the importance of a particular mediator in disease is to block its activity, and a number of studies have focused on the effect of TNF-α blockade during the induction phase of CIA. These studies showed that treatment of mice with monoclonal or polyclonal anti-TNF-α antibodies, or soluble TNF receptors, reduced the severity of arthritis when administered prior to the onset of clinical arthritis (Piguet et al. 1992; Thorbecke et al. 1992; Williams et al. 1992).
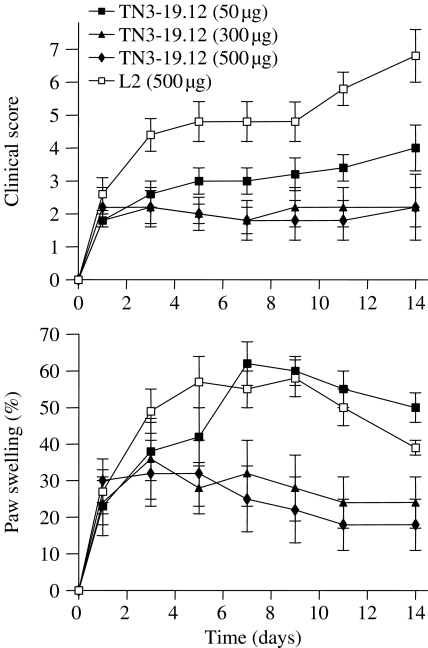
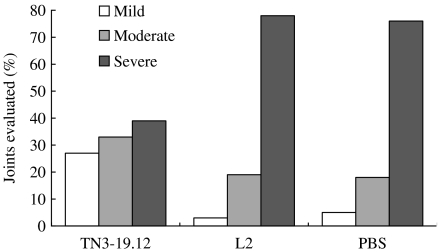
Subsequently, we assessed the effect of anti-TNF-α treatment in mice with established CIA (Williams et al. 1992). DBA/1 mice were immunized with type II collagen in complete Freund's adjuvant. The mice were inspected daily, and each mouse that exhibited clinical signs of arthritis was randomly assigned to one of the three treatment groups. The mice were then given twice-weekly intraperitoneal injections of TN3-19.12 (anti-TNF-α mAb), L2 (isotype control) or phosphate-buffered saline over a period of 14 days. The half-life of TN3-19.12 in mice had been previously estimated to be around 7 days (Sheehan et al. 1989). The doses of antibody used ranged from 50 to 500 µg per mouse. The results showed that there was a dose-dependent reduction in the severity of arthritis following treatment with anti-TNF-α mAb (Figure 1). At the end of the treatment period, arthritic paws were decalcified, sectioned and stained with haematoxylin and eosin. Individual joints were then graded according to the histopathological severity of arthritis. It was found that anti-TNF-α treatment reduced the histological severity of arthritis and protected joints from erosive changes (Figure 2).
Figure 1.
Effect of anti-tumour necrosis factor mAb (TN3-19.12) on clinical progression of established collagen-induced arthritis. Arrows indicate time of injection. L2 is an isotype control mAb. There were 10 mice per group. Top: Clinical score. The scoring system was based on the following criteria: 0 = normal, 1 = slight swelling and/or erythema, 2 = pronounced oedematous swelling, 3 = ankylosis. Each limb was graded, giving a maximum score of 12 per mouse. Bottom: Paw swelling. Expressed as the percentage increment in paw-width relative to the paw-width before the onset of arthritis. Adapted from Williams et al. (1992).
Figure 2.
Histopathological assessment of joints of arthritic DBA/1 mice treated with anti-tumour necrosis factor (TN3-19.12). L2 is an isotype control mAb. The scoring system was as follows. Mild, minimal synovitis, erosions limited to discrete foci, cartilage surface intact. Moderate, synovitis and erosions present but normal joint architecture intact. Severe, extensive erosions, joint architecture disrupted. Approximately 60 joints were examined per treatment group. Adapted from Williams et al. (1992).
Soluble TNF receptors are understood to play an important physiological role in regulating the activity of TNF-α, and it was subsequently shown that two soluble TNF receptor (TNFR) constructs were effective in established CIA. In the first study, a p75 TNFR-Fc fusion protein was found to reduce the severity of CIA whether given before or after the onset of the disease (Wooley et al. 1993). In another study, we showed that a p55 TNFR-immunoglobulin (-Ig) fusion protein was effective in reducing the clinical severity of established CIA (Williams et al. 1995). Furthermore, when the joints were examined by histology treatment with TNFR-Ig was found to have exerted a dose-dependent protective effect on joint erosion. The conclusion drawn from these studies was that TNF-α is involved in the pathogenesis of CIA. In addition, the findings provided support for the testing of anti-TNF-α antibody therapy in human RA.
A number of anti-TNF-α mAbs have now been shown in clinical trials to be effective in patients with severe RA, refractory to existing disease modifying drugs, including infliximab, a chimeric anti-TNF-α mAb (Elliott et al. 1993,1994a,b), Celltech's humanized anti-TNF-α, CDP571 (Rankin et al. 1995) and human anti-TNF-α, D2E7 (Kempeni 1999), have all been shown to be effective. In addition, various TNF receptor-based biologics have also been tested and shown to be effective, including dimeric p75 TNF receptor-Fc fusion protein, etanercept (Hasler 1996; Moreland et al. 1997; Weinblatt et al. 1999), p55 TNF receptor-Fc fusion protein, lenercept (Hasler 1996; Sander et al. 1996) and pegylated mononomeric p55 TNF-receptor (Edwards 1999).
The need to quantify cytokine expression in vivo
Despite the success of anti-TNF therapy in RA, TNF-α blockade has not been shown to provide a permanent cure for the disease, suggesting that the immunological processes driving TNF-α production remain intact despite treatment. For this reason, research continues into the development of novel forms of therapy that target both immune and inflammatory pathways, with the aim of producing a sustained reduction in disease activity. In addition, all of the TNF-α-blocking biologics used to date are relatively expensive to produce and need to be administered by injection, and there is great interest in the possibility of generating small-molecular-weight inhibitors of TNF-α activity that can be administered orally.
For these reasons, there is a need to be able to quantify changes in cytokine expression (in particular, at sites of disease activity), which occur when a new treatment is applied. It is important to bear in mind that in vitro studies using primary cells or cell lines treated with Toll-like receptor ligands, such as lipopolysaccharide, do not fully reproduce the situation in vivo, where the signalling pathways involved in driving cytokine expression are much more complex and less well-defined. For the remainder of this review, we will provide an overview of the different methodologies used for the detection of both pro- and anti-inflammatory cytokines in animal models of arthritis and summarize some of the changes observed as a result of therapeutic intervention.
Detection of cytokines in situ
The detection of cytokines in vivo allows detection of their precise location within specific areas of tissues. Two approaches that have been used successfully in the joints of collagen induced arthritic animals are immunohistochemistry with horseradish peroxidase (HRP)-labelled secondary antibodies and in situ hybridization with digoxyin-labelled riboprobes. By dissecting the synovium from joints, tissue can be sectioned fresh-frozen with ease, using a cryostat and processed as any soft tissue (Mattsson et al. 2000). In order to measure cytokines in whole joints, sectioning of bone needs to be performed, which is more difficult to perform on a cryostat as the bones often splinter. Reports have been made of detection of cytokines [interleukin-1β (IL-1β) and RANKL] in paraffin fixed, decalcified joints (Romas et al. 2002; Lubberts et al. 2004). Because of the difficulties in staining for cytokines in paraffin tissue, we have developed a method of fresh-frozen joint sectioning that preserves antigens and RNA integrity, detailed below (Marinova-Mutafchieva et al. 1997; Gabay et al. 2001).
Ankles are removed and snap-frozen in optimal cutting temperature using isopentane pre-chilled in liquid nitrogen. The frozen ankles are mounted for sagittal sectioning in a cryostat, and the block trimmed using a tungsten-carbide-tipped knife. Scotch tape is applied to the tissue block to ensure a flat section can be cut from the block without splintering. The tape can then be attached to a microscope slide and stained using immunohistochemistry. An alternative approach that we have taken is to use the tape-transfer system from Instrumedics (Hackensack, NJ, USA) in which an adhesive-coated tape is applied to the block and a section cut. The tape is then applied to an adhesive-coated slide. UV cross-linking is performed to bind the tissue to the slide, and the tape can then be removed. This results in a stronger bond formation and is thus useful for procedures involving harsher treatment of the tissue, such as in situ hybridization. The joints can then be stained using standard protocols, for example, fixing in 4% paraformaldehyde and using 0.1% saponin to permeabilize membranes and golgi to allow cytokine detection in vesicles.
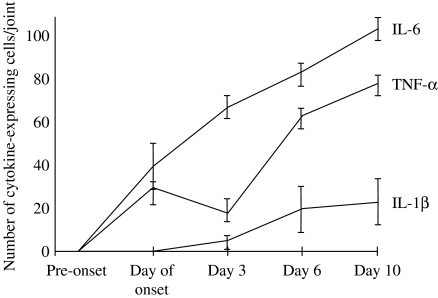
Immunohistochemistry and in situ hybridization in the joints of arthritic animals has been used to investigate the dynamics of cytokine expression in arthritis (Marinova-Mutafchieva et al. 1997). In this study, it was shown that TNF-α and IL-6 expression was increased in arthritic mice from onset, whilst IL-1β expression was detected in the joint from day three onwards (Figure 3). IL-1β expression was the greatest 10 days following arthritis onset and subsequently decreased (Gabay et al. 2001). IL-1Ra expression was also detected in arthritic joints, peaking 20 days post-onset and decreasing through to 60 days.
Figure 3.
Cytokine expression in the joints of DBA/1 mice with collagen-induced arthritis. Cytokines were detected by immunohistochemistry and calculated on the basis of the mean number of cells positive for tumour necrosis factor α (TNF-α), interleukin-1β (IL-1β) or IL-6. There were six mice per group. Adapted from Marinova-Mutafchieva et al. (1997).
CIA (induced with heterologous type II collagen) is generally regarded as a self-limiting disease that begins to subside around 10 days after onset of clinical arthritis. We carried out an immunohistochemical study to identify cytokines that may be involved in the spontaneous remission of arthritis. It was found that the expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) dominated in early CIA. In contrast, TGFβ1 and TGFβ2 predominated during the time of disease remission, raising the possibility that these cytokines are involved in regulating disease activity (unpublished data).
Expression of cytokines in joints has also been used as markers of inflammation in order to assess the effectiveness and mechanism of action of therapeutics (Ferrari-Lacraz et al. 2004; Lubberts et al. 2004). For example, an antagonistic mutant IL-15R/Fc fusion protein, which inhibited arthritis induction and reduced severity when administered therapeutically, was shown to decrease IL-1β and TNF-α expression in the synovium (Ferrari-Lacraz et al. 2004). In a separate study, therapeutic doses of a neutralizing anti-IL-17 antibody were shown to decrease IL-1β and RANKL expression in the joint (Lubberts et al. 2004). Most of the studies detailed above used complementary techniques to validate the immunohistochemical data. For example, through combining Western blot, ELISA, real-time polymerase chain reaction (RT-PCR), Rnase protection assay (RPA), immunohistochemistry and in situ hybridization, unequivocal information on cytokine expression can be gained.
Detection of cytokines in synovial membrane cell cultures
Cytokines secreted by cells cultured from the inflamed joint environment can be assayed in vitro using tissue culture techniques involving the isolation of primary synovial mononuclear cells followed by specific bioassays or enzyme-linked immunosorbent assays (ELISAs). Inflammatory cell recruitment from the surrounding vasculature is an important event prior to the setting up of a local inflammatory response in joints in RA. RA is widely believed to be an autoimmune disease involving uncontrolled cytokine release by inflammatory cells such as monocytes and macrophages (Feldmann et al. 1998). Several pro-inflammatory cytokines are involved in a complex feedback network involving cell-to-cell contact and utilizing both autocrine and paracrine pathways (Feldmann & Maini 1999). Predominantly, amongst inflammatory/immunomodulatory cells isolated from the joint milieu in RA are T lymphocytes, monocytes, synovial membrane-derived fibroblasts and macrophages. These cells secrete inflammatory cytokines, such as TNF-α, IL-6 and IL-1β, amongst a plethora of other cellular mediators and chemokines (Brennan et al. 1998).
Effective treatment therapies for arthritis could thus be potentially viewed in the light of their effectiveness at reducing the levels of bioactive inflammatory cytokine expression at the level of secretory output by freshly isolated inflammatory tissue from an arthritic joint. This can be achieved by isolating and culturing mononuclear cell mixtures in vitro from either biopsy material obtained from human RA patients (Brennan et al. 1989) or synovial tissue from arthritic knee joints of mice with CIA.
The method involves removal of knee joints from arthritic mice (around day 10 of arthritis) and excision of each synovial membrane using a dissecting microscope. Synovia from both knees is pooled and enzymatically digested with a mixture of collagenase A and DNAse type IV at 37 °C for about 30-60 min in the presence of polymyxin B to prevent endotoxin-mediated pro-inflammatory cytokine stimulation. The mixture of mononuclear cells obtained is then washed, counted and cultured in complete supplemented RPMI-1640 medium in the presence or absence of compounds being tested for their ability to inhibit cytokines. Alternatively, the same procedure may be followed for a cohort of arthritic mice subjected to therapeutic treatment or appropriate controls. Following a 24-h culture period, supernatants are harvested, aliquoted and stored frozen until assay of cytokines. Bioactive levels of TNF-α may then be assayed using a protocol previously outlined by Espevik and Nissen-Meyer, which utilizes a variant clone of the WEHI-164 cell line that is resistant to killing by TNF-αin vitro, other than in the presence of the mRNA transcription inhibitor actinomycin D (Espevik & Nissen-Meyer 1986). Bioactive levels of IL-6 may be assayed from culture supernatants by determining the degree of proliferation of an IL-6-dependent B9 murine hybridoma cell line (Helle et al. 1988).
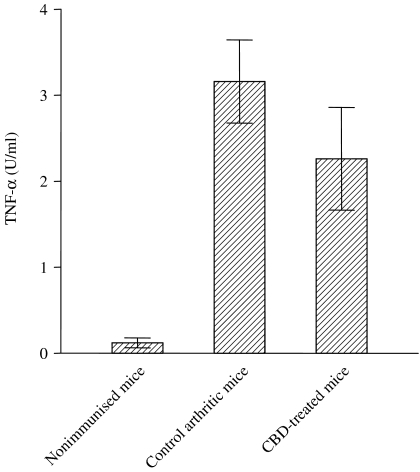
We have conducted several experiments to evaluate the efficacy of novel biological and compound anti-arthritic therapies in the CIA mouse model using cultured synoviocytes. For example, the therapeutic potential of cannabidiol (CBD), the major nonpsychoactive component isolated from the marijuana plant, Cannabis sativa was studied in CIA (Malfait et al. 2000). The anti-arthritic effect of CBD was partially attributed to the reduction observed in bioactive TNF-α output from knee synovial cells of CBD-treated mice when compared with controls (Figure 4).
Figure 4.
Bioactive tumour necrosis factor α (TNF-α) release from cultured synovial membrane cells from mice treated with cannabidiol or vehicle control. Synovial membrane cells were isolated by enzymatic digestion and cultured for 24 h. Bioactive TNF-α levels were determined by using the WEHI-164 cell line assay. Each bar represents the mean value ± SEM from five individual mice. Adapted from Malfait et al. (2000).
Similarly, the therapeutic potential of salbutamol, a β2-adrenergic agonist was explored in CIA. Salbutamol has been reported to work through reduction in TNF-α and IL-12 levels by elevating cAMP, the secondary messenger in cells. Salbutamol reduced bioactive TNF-α levels in a dose-dependent manner from cultured knee synoviocytes of arthritic mice (Malfait et al. 1999).
In a study conducted by Butler et al., it was shown that the addition of anti-TNF-α antibody to synovial membrane cell cultures from arthritic mice reduced IL-12 levels (detected by an ELISA). However, blocking IL-12 release by the synovial membrane cells by the addition of anti-IL-12 neutralizing antibody had a modest insignificant reduction on bioactive TNF-α output (Butler et al. 1999). Thus, using the method of culturing synovial membrane cells from arthritic mice helped to dissect out the possible and partial regulation of IL-12 by TNF-α in the CIA model and thus shed new light on a possible synergistic therapeutic approach for the treatment of arthritis.
Furthermore, Butler et al. went on to demonstrate that estimating the levels of bioactive TNF-α, along with the estimation of other cytokines by ELISA such as IL-1β, IL-6 and IL-10, secreted predominantly by connective tissue type synovial cells, was an important underlying trigger to accelerated arthritis in genetically modified DBA/1 mice expressing the human TNF-α transgene (Butler et al. 1997).
It was also shown that continuous prophylactic administration by osmotic pump of the Th2 cytokine, IL-4, resulted in a delay in the onset of clinical signs of arthritis in collagen-primed mice (Horsfall et al. 1997). During the course of this prophylactic treatment of CIA with IL-4, it was reported that a dramatic decrease occurred in the output of TNF-α from cultured synoviocytes.
More recently, we investigated the therapeutic potential of P-selectin glycoprotein ligand (PSGL)-1 in established CIA. We sought to investigate a mechanism underlying the effect of a recombinant rPSGL-1 Ig fusion protein on the observed reduction of clinical signs of arthritis. It was shown that a partial nonsignificant reduction in bioactive output of TNF-α from cultured synoviocytes combined with reduced joint cellularity contributed to a possible mechanism of disease amelioration (Sumariwalla et al. 2004). These studies establish the value of cultured synovial membrane cells as a tool in our understanding of arthritis as well as to determine the efficacy of novel therapeutics.
Spleen and lymph node cell assays
It is generally accepted that T-cell activity within lymph nodes draining a particular site of inflammation provides important information regarding the magnitude and character of the immune response that is associated with, or driving, the inflammatory response. We used a lymph node cell (LNC) assay system to investigate the relationship between Th1/Th2 cytokines and arthritogenic response in CIA (Mauri et al. 1996). DBA/1 mice were immunized with type II collagen in adjuvant, and at different time points, the mice were killed and inguinal LNC was cultured in the absence or presence of type II collagen (50 µg/ml) at a cell density of 1 × 106/ml in 96-well plates in RPMI-1640 containing foetal calf serum (10% v/v), 2-mercaptoethanol (20 µm), l-glutamine (1% w/v), penicillin (100 U/ml) and streptomycin (100 µg/ml). Supernatants were collected after 24 h for determination of IL-1β, IL-4 and TNF and after 72 h for IL-10 and IFN-γ. All the cytokines were measured by a standard capture ELISA, except for TNF, which was measured by bioassay, using the WEHI-164 cell line (Espevik & Nissen-Meyer 1986).
The most striking finding to emerge from this study was the high level of IFN-γ production that was detected in LNC cultures from day 6 after immunization and which reached a peak around the time of disease onset (typically around 20–25 days after immunization). In contrast, although a transient increase in IL-10 production was detected 3 days after immunization, IL-10 was absent in LNC cultures from day 6 after immunization until day 5 after arthritis onset. It was also of interest that whilst IFN-γ production declined progressively after onset of CIA, relatively high levels of IL-10 production were observed from day 5 after arthritis onset and throughout the period of declining inflammation that occurs from around day 10 after arthritis onset (Mauri et al. 1996). These findings confirm the Th1-biased nature of CIA and suggest that IL-10 plays a role in suppressing disease activity during the period of spontaneous remission.
In a similar study, LNC from DBA/1 mice were harvested at different times after injection of type II collagen in Freund's complete adjuvant and checked by ELISA for the production of IFN-γ and IL-4. Collagen-specific T cells producing either IFN-γ or IL-4, or both, were detected before the onset of arthritis, and the number of IFN-γ-producing T cells reached a peak at 15 days post-immunization, whereas more IL-4-secreting cells were found at day 30, just before the onset of clinical arthritis (Doncarli et al. 1997). These findings show that the collagen-specific CD4+ T-cell response changes in vivo during the course of CIA from a dominant Th0/Th1 response towards a Th2 phenotype.
LNC assays offer the opportunity to determine the impact of different forms of therapy on T-cell cytokine production. For example, a variety of different cAMP-elevating agents have been tested for efficacy in CIA, including the PDE4 inhibitor rolipram (Nyman et al. 1997; Ross et al. 1997), the β2 adrenergic receptor agonist, salbutamol (Malfait et al. 1999) and the neuropeptides, vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP) (Abad et al. 2001; Delgado et al. 2001; Williams 2002). In addition to the ability of these cAMP-elevating agents to ameliorate arthritis, it was also reported that the suppression of CIA by rolipram, salbutamol, VIP and PACAP was accompanied by marked down-regulation of the Th1 response to type II collagen (Nyman et al. 1997; Ross et al. 1997; Malfait et al. 1999; Abad et al. 2001; Delgado et al. 2001; Williams 2002).
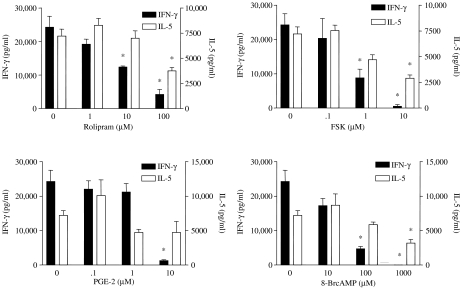
In order to analyse the effect of cAMP on the T-cell response in a more controlled system, we cultured spleen cells or LNC from type II collagen-immunized DBA/1 mice in the presence of collagen plus increasing concentrations of one of five different cAMP-elevating agents: rolipram, forskolin, PGE2, 8-bromo-cAMP or cholera toxin. Levels of IFN-γ, IL-4 and IL-5 were then measured in culture supernatants by ELISA. All of the cAMP-elevating agents markedly suppressed IFN-γ production in a dose-dependent manner (Ozegbe et al. 2004). IL-4 and IL-5 production was slightly increased at low concentrations of the cAMP-elevating agents and was slightly suppressed at the highest concentrations of cAMP-elevating agents (Figure 5).
Figure 5.
Inhibitory effect of four different cAMP-elevating agents on interferon γ and interleukin-5 production in vitro. Spleen cells from DBA/1 mice immunized with type II collagen were cultured in the presence of type II collagen (50 µg/ml) plus rolipram, forskolin, prostaglandin E2 or 8-bromo-cAMP. The agents were added 30 min prior to addition of collagen, and cytokines were measured by ELISA after 72 h. * denotes significant difference (P < 0.05) between treated group and untreated controls. Adapted from Ozegbe et al. (2004).
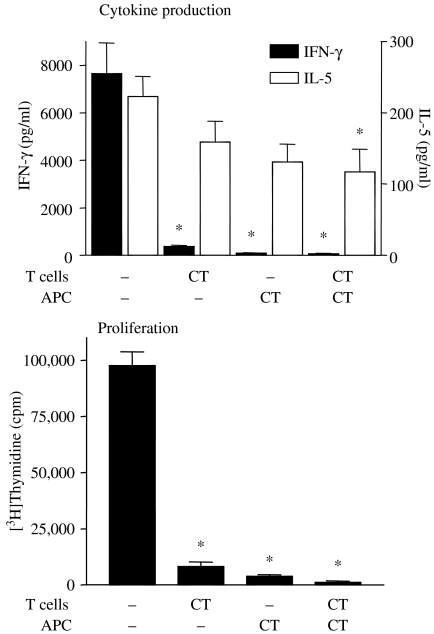
Further experiments were then performed to establish whether T cells were affected by cAMP-elevating agents directly or whether the immunomodulatory effects were mediated via antigen-presenting cells (APC). It was found that treating T cells with a cAMP-elevating agent (cholera toxin) for a brief period had the same effect as treating APC with cholera toxin, i.e. down-regulation of proliferation, down-regulation of IFN-γ production with little effect on IL-5 production (Figure 6). From this study, it was concluded that cAMP-elevating agents suppressed Th1 responses to a much greater extent than Th2 responses and that cAMP-elevating agents could directly influence the activity of T cells but, in addition, influenced the ability of APC to support Th1 responses (Ozegbe et al. 2004).
Figure 6.
Cholera toxin (CT) targets both T cells and APC. Lymph node cell from type II collagen-immunized DBA/1 mice were separated into CD4+ T cell and APC fractions and the two populations were treated separately with CT for 1 h at 37 °C, washed and then recombined. The recombined cells were cultured in the presence of type II collagen at 50 µg/ml for 72 h and interferon γ and interleukin-5 production were measured by ELISA. To measure proliferation, [3H]thymidine was added for the last 16 h. * denotes significant difference (P < 0.05) between CT-treated groups and controls. Adapted from Ozegbe et al. (2004).
The majority of studies of cytokine expression in LNC or spleen cell cultures have quantified cytokine production by ELISA. However, an alternative approach is to enumerate cytokine-producing cells by flow cytometry or ELISPOT. For example, as part of a study to investigate the potential role played by γ/δ T cells in arthritis, Corthay and colleagues used flow cytometry to compare the production of IL-2, IL-4, IFN-γ, IL-10 in vitro by LNC from wild-type mice (B10) and γ/δ T-cell-deficient mice stimulated with Concanavalin A or staphylococcal enterotoxin A and concluded that there were no differences in the percentage of cytokine-producing CD4+ and CD8+ T cells (Corthay et al. 1999). They used naïve animals for this experiment but later analysed the incidence of CIA in mice deficient for α/β or γ/δ T cells in both DBA/1 and B10.Q backgrounds and were able to demonstrate that aβ T cells, but not γδ T cells, were involved in the development of arthritis (Corthay et al. 1999).
Assays for the detection of cytokines produced by single cells, in particular the ELISPOT assay, have been used in a variety of disease models and the chief advantage of this assay is the high level of sensitivity, particularly when the proportion of antigen-specific T cells within the total T-cell population is low. Beech et al. describe the development of a sensitive ELISPOT for the detection of IL-2, IL-4 and IFN-γ in T cells stimulated in vitro with the appropriate antigen (Beech et al. 1997). A similar ELISPOT technique was used to analyse cytokine secreting (IL-2 and IL-4) cells in spleen and LN from mice with CIA. It was found that a high proportion of IL-2-producing cells developed after immunization (+12 days) that were reduced around the time of arthritis onset (+40 days), with a concomitant increase in IL-4-producing cells. It was concluded that the development of arthritis was associated with a shift from a Th1 phenotype to a Th2 phenotype (Okamoto et al. 2000). Similarly, the ELISPOT assay was used to enumerate cytokine-producing cells (IL-5, IFN-γ, TNF-α) in LNC from CIA-susceptible and -resistant Biozzi mice lines at different times after immunization with collagen. LNC from the susceptible line showed high numbers of IL-5-producing cells at all times which increased with time, transient IFN-γ production (day 10 only) and very low numbers of TNF-α-producing cells only detectable over background at day 10. Surprisingly, the resistant cell line had lower number of IL-5-producing cells at all times that decreased with time, and higher numbers of IFN-γ- and TNF-α-producing cells, peaking at day 10 (De Franco et al. 1995).
Measurement of cytokine mRNA
RT-PCR TaqMan® is a highly sensitive technique that can be applied to the reliable quantification of mRNA levels for different cytokines in rodent joints in animal models of arthritis. This technique allows the direct monitoring of amplicon accumulation during the PCR process using specific TaqMan® fluorogenic probes, providing fluorescence kinetics that accurately reflect the amounts of cytokine mRNA levels in biological samples (Overbergh et al. 2003).
Recently, Rioja and colleagues performed a comparative analysis between synovial mRNA and protein expression of IL-1β, TNF-α and IL-6 (Rioja et al. 2004). They studied the joints from CIA in DBA/1 mice and from the streptococcal cell wall (SCW)-induced arthritis model in Lewis rats. The study assessed the effect of prednisolone treatment on cytokine levels and described the kinetics of cytokine expression. In CIA, the synovial mRNA expression levels for IL-6 and IL-1β were found significantly reduced after daily prednisolone treatment (days 1-4, at doses of 1 and 9 mg/kg). However, TNF-α mRNA levels were only reduced at the higher dose of steroid tested. All the mRNA levels were normalized using ubiquitin as a housekeeping gene. Similar findings to mouse CIA were observed for both the kinetics and the prednisolone efficacy study in SCW-induced arthritis in rats.
Another detailed study examined the potential of targeting the IL-15R on the prevention and treatment of CIA in mice (Ferrari-Lacraz et al. 2004). The antagonistic IL-15 mutant/Fcγ2a fusion protein (CRB-15) that targets the IL-15R was administered after initiation of CIA onset for 14 consecutive days (5 µg/day). TaqMan RT-PCR analysis was used to quantify synovial IL-1β, IL-6, IL-17 and TNF-α expression profiles; the data were controlled against the housekeeping gene cyclophylin. A significant linear correlation between disease severity in individual paws and all cytokine expression levels in both the CRB-15 and control treatment groups was detected. At the same time, treatment with CRB-15 resulted in a reduced expression of all the inflammatory cytokines studied. The effect on TNF-α was particularly striking, because treatment with CRB-15 strongly reduced expression of this cytokine in both the disease induction and the established disease models.
Yamanishi and colleagues demonstrated that p53 tumour suppressor protein plays a protective role in inflammatory synovitis: absence of p53 in knockout mice increased severity and joint destruction compared with wild-type mice in the CIA model (Yamanishi et al. 2002). Accordingly, quantitative RT-PCR was utilized to clarify gene expression of IL-6, as well as proinflammatory biomarkers MMP3 (stromelysin-1) and MMP13 (collagenase-3) in ankle joints of p53 knockout and p53 wild-type mice with two passively induced experimental arthritis models; these included anti-type II collagen Ab-induced arthritis and K/BxN serum-induced arthritis (Simelyte et al. submitted for publication). Similar levels of all the genes studied were observed between the p53 knockout and wild-type mice; for normalization of RNA content, a control gene hypoxanthine-guanine phosphoribosyl transferase (HPRT) was used.
In summary, quantifying multiple cytokines by the RT-PCR is a powerful and highly sensitive technique that also provides possibility to overcome the limitation of very small biological tissue samples that are available from rodent joints. In order to investigate the mechanism of action of any potential anti-arthritic compounds, the optimal time point when the production of each specific cytokine of interest and inflammation are both maximal should be selected.
The RPA is another method that facilitates the simultaneous analysis of the genes studied from small amounts of tissue and is a good method to explore the cytokine expression and the clinical efficacy of novel anti-rheumatic drugs on cytokine expression. In addition, the use of total murine paw RNA has been shown to accurately reflect synovial RNA changes of cytokine levels in CIA (Thornton et al. 1999).
For example, a number of techniques were employed to study the patterns of production of IL-1 receptor antagonist (IL-1Ra) isoforms and of IL-1β in dissected synovial tissues during the course of CIA (Gabay et al. 2001). RPA showed that the IL-1β : IL-1Ra mRNA ratio was increased in inflamed joints of mice through day 14 of arthritis. However, a reverse pattern was present later, from day 20 throughout day 60 of CIA, suggesting that production of IL-1Ra isoforms is stimulated in arthritic mice joints during CIA.
A study by Marty et al. investigated the effect of anti-coagulants on the course of mouse CIA (Marty et al. 2001). They used the RPA and found that synovial mRNA levels of IL-1α, IL-1β, IL-Ra, IL-12, and macrophage migration inhibitory factor (MIF) were up-regulated in arthritic mice compared with nonarthritic mice; mRNA levels of all the cytokines analysed were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Further, thrombin inhibitor polyethyleneglycol-hirudin (PEG-hirudin) was administered for 16 days (1 mg/kg/day), either starting 20 days after the first immunization with heterologous CII or at the onset of clinical signs of arthritis. The results of the RPA revealed that there was a significant down-modulation of the synovial proinflammatory IL-1β and IL-12p35 cytokine mRNAs (P < 0.05 and P < 0.01, respectively) in the mice that received PEG-hirudin, compared with that in the untreated controls, confirming that this drug can both prevent the onset of CIA and ameliorate established arthritis.
Ma et al. determined the effects of gene transfer of viral IL-10 (vIL-10) on autoimmune arthritis and reported that intravenous or intra-articular injections of Avenue (vIL-10), a replication-deficient adenovirus encoding vIL-10, was associated with delayed synovial expression of proinflammatory cytokines IL-2 and IL-1β mRNA in response to CII immunization as determined by RPA (Ma et al. 1998).
The RPA was also applied to determine the effects of 3-week treatment with murine IL-18-binding protein (mIL−18-bp) on the local production of cytokines (Banda et al. 2003). Two doses of the mIL−18-bp were administered at the time of the booster injection of CII and the steady state levels of IFN-γ, TNF-α and IL-1β mRNA were measured in isolated joints of mice with CIA and expressed as the ratio to GAPDH mRNA. Again, compared with the control mice, the levels of cytokine expression were decreased in mice treated with both doses of mIL−18-bp (0.5 and 3 mg/kg).
Conclusions and future directions
It is clear that many useful techniques exist for the detection and quantification of cytokines in animal models of arthritis and all of them have the potential to increase our knowledge and understanding not only of disease processes but also of how drugs affect these processes. One potential criticism of the methodologies described in this review is that they involve making assumptions about which cytokines will be affected by treatment. Thus, we tend to assay only those cytokines that we expect to see changes in, and in this way, we may miss unexpected changes. The use of new multiplex methods of cytokine measurement will undoubtedly expand our knowledge of cytokine expression in arthritis. In addition, developments in genomic and proteomic array technologies will enable much more comprehensive and nonhypothesis-driven studies of disease and the response to therapy.
References
- Abad C, Martinez C, Leceta J, Gomariz RP, Delgado M. Pituitary adenylate cyclase-activating polypeptide inhibits collagen-induced arthritis: an experimental immunomodulatory therapy. J Immunol. 2001;167:3182–3189. doi: 10.4049/jimmunol.167.6.3182. [DOI] [PubMed] [Google Scholar]
- Banda NK, Vondracek A, Kraus D, Dinarello CA, Kim SH, Bendele A, Senaldi G, Arend WP. Mechanisms of inhibition of collagen-induced arthritis by murine IL-18 binding protein. J Immunol. 2003;170:2100–2105. doi: 10.4049/jimmunol.170.4.2100. [DOI] [PubMed] [Google Scholar]
- Beech JT, Bainbridge T, Thompson SJ. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J Immunol Methods. 1997;205:163–168. doi: 10.1016/s0022-1759(97)00072-0. [DOI] [PubMed] [Google Scholar]
- Boissier MC, Feng XZ, Carlioz A, Roudier R, Fournier C. Experimental autoimmune arthritis in mice. I. Homologous type II collagen is responsible for self-perpetuating chronic polyarthritis. Ann Rheum Dis. 1987;46:691–700. doi: 10.1136/ard.46.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FM, Chantry D, Jackson A, Maini R, Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;2:244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Brennan FM, Maini RN, Feldmann M. Role of pro-inflammatory cytokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20:133–147. doi: 10.1007/BF00832003. [DOI] [PubMed] [Google Scholar]
- Butler DM, Malfait AM, Maini RN, Brennan FM, Feldmann M. Anti-IL-12 and anti-TNF antibodies synergistically suppress the progression of murine collagen-induced arthritis. Eur J Immunol. 1999;29:2205–2212. doi: 10.1002/(SICI)1521-4141(199907)29:07<2205::AID-IMMU2205>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Butler DM, Malfait AM, Mason LJ, et al. DBA/1 mice expressing the human TNF-alpha transgene develop a severe, erosive arthritis: characterization of the cytokine cascade and cellular composition. J Immunol. 1997;159:2867–2876. [PubMed] [Google Scholar]
- Corthay A, Johansson A, Vestberg M, Holmdahl R. Collagen-induced arthritis development requires alpha beta T cells but not gamma delta T cells: studies with T cell-deficient (TCR mutant) mice. Int Immunol. 1999;11:1065–1073. doi: 10.1093/intimm/11.7.1065. [DOI] [PubMed] [Google Scholar]
- De Franco M, Gille-Perramant MF, Mevel JC, Couderc J. T helper subset involvement in two high antibody responder lines of mice (Biozzi mice): HI (susceptible) and HII (resistant) to collagen-induced arthritis. Eur J Immunol. 1995;25:132–136. doi: 10.1002/eji.1830250123. [DOI] [PubMed] [Google Scholar]
- Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- Doncarli A, Stasiuk LM, Fournier C, Abehsira-Amar O. Conversion in vivo from an early dominant Th0/Th1 response to a Th2 phenotype during the development of collagen-induced arthritis. Eur J Immunol. 1997;27:1451–1458. doi: 10.1002/eji.1830270623. [DOI] [PubMed] [Google Scholar]
- Edwards CKI. PEGylated recombinant human soluble tumour necrosis factor receptor type I (r-Hu-sTNF-RI): novel high affinity TNF receptor designed for chronic inflammatory diseases. Ann Rheum Dis. 1999;58:173–181. doi: 10.1136/ard.58.2008.i73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumour necrosis factor a. Arthritis Rheum. 1993;36:1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, et al. Treatment with a chimaeric monoclonal antibody to tumour necrosis factor α suppresses disease activity in rheumatoid arthritis: results of a multi-centre, randomised, double blind trial. Lancet. 1994a;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, et al. Repeated therapy with a monoclonal antibody to tumour necrosis factor a in patients with rheumatoid arthritis. Lancet. 1994b;344:1125–1127. doi: 10.1016/s0140-6736(94)90632-7. [DOI] [PubMed] [Google Scholar]
- Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Maini R. Cytokines in autoimmune disorders. Int Rev Immunol. 1998;17:217–228. doi: 10.3109/08830189809084493. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Williams RO, Woody JN, Maini RN. The transfer of a laboratory based hypothesis to a clinically useful therapy: the development of anti-TNF therapy of rheumatoid arthritis. Baillieres Best Pract Res Clin Rheumatol. 2004;18:59–80. doi: 10.1016/j.berh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Maini RN. The role of cytokines in the pathogenesis of rheumatoid arthritis. Rheumatology. 1999;38(Suppl. 2):3–7. (Oxford) [PubMed] [Google Scholar]
- Ferrari-Lacraz S, Zanelli E, Neuberg M, et al. Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J Immunol. 2004;173:5818–5826. doi: 10.4049/jimmunol.173.9.5818. [DOI] [PubMed] [Google Scholar]
- Gabay C, Marinova-Mutafchieva L, Williams RO, et al. Increased production of intracellular interleukin-1 receptor antagonist (icIL-1Ra1) in the synovium in collagen-induced arthritis: a possible role in the resolution of arthritis. Arthritis Rheum. 2001;44:451–462. doi: 10.1002/1529-0131(200102)44:2<451::AID-ANR64>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hasler F, Van De Putte L, Baudin M, et al. Chronic TNF neutralization (up to 1 year) by lenercept (TNFR 55 IgG1, Ro 45-2081) in patients with rheumatoid arthritis: Results from open label extension of a double blind single-dose phase I study. Arthritis Rheum. 1996;39:5243. [Google Scholar]
- Helle M, Boeije L, Aarden LA. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988;18:1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Holmdahl R, Andersson ME, Goldschmidt TJ, Jansson L, Karlsson M, Malmström VMOJ. Collagen induced arthritis as an experimental model for rheumatoid arthritis. Immunogenetics, pathogenesis and autoimmunity. APMIS. 1989;97:575–584. doi: 10.1111/j.1699-0463.1989.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Holmdahl R, Jansson L, Larsson E, Rubin K, Klareskog L. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986;29:106–113. doi: 10.1002/art.1780290114. [DOI] [PubMed] [Google Scholar]
- Horsfall AC, Butler DM, Marinova L, et al. Suppression of collagen-induced arthritis by continuous administration of IL-4. J Immunol. 1997;159:5687–5696. [PubMed] [Google Scholar]
- Kempeni J. Preliminary results of early clinical trials with the fully human anti-TNF monoclonal antibody D2E7. Ann Rheum Dis. 1999;58(Suppl. 1):I70–I72. doi: 10.1136/ard.58.2008.i70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubberts E, Koenders MI, Oppers-Walgreen B, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- Ma Y, Thornton S, Duwel LE, et al. Inhibition of collagen-induced arthritis in mice by viral IL-10 gene transfer. J Immunol. 1998;161:1516–1524. [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait AM, Malik AS, Marinova-Mutafchieva L, Butler DM, Maini RN, Feldmann M. The beta2-adrenergic agonist salbutamol is a potent suppressor of established collagen-induced arthritis: mechanisms of action. J Immunol. 1999;162:6278–6283. [PubMed] [Google Scholar]
- Malfait AM, Williams RO, Malik AS, Maini RN, Feldmann M. Chronic relapsing homologous collagen-induced arthritis in DBA/1 mice as a model for testing disease-modifying and remission-inducing therapies. Arthritis Rheum. 2001;44:1215–1224. doi: 10.1002/1529-0131(200105)44:5<1215::AID-ANR206>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Marinova-Mutafchieva L, Williams RO, Mason LJ, Mauri C, Feldmann M, Maini RN. Dynamics of proinflammatory cytokine expression in the joints of mice with collagen-induced arthritis (CIA) Clin Exp Immunol. 1997;107:507–512. doi: 10.1046/j.1365-2249.1997.2901181.x. [DOI] [PubMed] [Google Scholar]
- Marty I, Peclat V, Kirdaite G, Salvi R, So A, Busso N. Amelioration of collagen-induced arthritis by thrombin inhibition. J Clin Invest. 2001;107:631–640. doi: 10.1172/JCI11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson L, Larsson P, Erlandsson-Harris H, Klareskog L, Harris RA. Parasite-mediated down-regulation of collagen-induced arthritis (CIA) in DA rats. Clin Exp Immunol. 2000;122:477–483. doi: 10.1046/j.1365-2249.2000.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri C, Williams RO, Walmsley M, Feldmann M. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur J Immunol. 1996;26:1511–1518. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- Nyman U, Mussener A, Larsson E, Lorentzen J, Klareskog L. Amelioration of collagen II-induced arthritis in rats by the type IV phosphodiesterase inhibitor Rolipram. Clin Exp Immunol. 1997;108:415–419. doi: 10.1046/j.1365-2249.1997.3931291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Gotoh Y, Tokui H, Mizuno A, Kobayashi Y, Nishida M. Characterization of the cytokine network at a single cell level in mice with collagen-induced arthritis using a dual color ELISPOT assay. J Interferon Cytokine Res. 2000;20:55–61. doi: 10.1089/107999000312739. [DOI] [PubMed] [Google Scholar]
- Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech. 2003;14:33–43. [PMC free article] [PubMed] [Google Scholar]
- Ozegbe P, Foey AD, Ahmed S, Williams RO. Impact of cAMP on the T cell response to type II collagen. Immunology. 2004;111:35–40. doi: 10.1111/j.1365-2567.2003.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet PF, Grau GE, Vesin C, Loetscher H, Gentz R, Lesslauer W. Evolution of collagen arthritis in mice is arrested by treatment with anti-tumour necrosis factor (TNF) antibody or a recombinant soluble TNF receptor. Immunology. 1992;77:510–514. [PMC free article] [PubMed] [Google Scholar]
- Rankin ECC, Choy EHS, Kassimos D, et al. The therapeutic effects of an engineered human anti-tumour necrosis factor alpha antibody (CDP571) in rheumatoid arthritis. Br J Rheumatol. 1995;34:334–342. doi: 10.1093/rheumatology/34.4.334. [DOI] [PubMed] [Google Scholar]
- Rioja I, Bush KA, Buckton JB, Dickson MC, Life PF. Joint cytokine quantification in two rodent arthritis models: kinetics of expression, correlation of mRNA and protein levels and response to prednisolone treatment. Clin Exp Immunol. 2004;137:65–73. doi: 10.1111/j.1365-2249.2004.02499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romas E, Sims NA, Hards DK, et al. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am J Pathol. 2002;161:1419–1427. doi: 10.1016/S0002-9440(10)64417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Williams RO, Mason LJ, et al. Suppression of TNF-α expression, inhibition of Th1 activity, and amelioration of collagen-induced arthritis by rolipram. J Immunol. 1997;159:6253–6259. [PubMed] [Google Scholar]
- Sander O, Rau R, Van Riel P, et al. Neutralization of TNF by Lenercept (TNFR55-IgG1, Ro 45-2081) in patients with rheumatoid arthritis treated for 3 months: results of a European phase II trial. Arthritis Rheum. 1996;39:5242. [Google Scholar]
- Sheehan KC, Ruddle NH, Schreiber RD. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989;142:3884–3893. [PubMed] [Google Scholar]
- Sumariwalla PF, Malfait AM, Feldmann M. P-selectin glycoprotein ligand 1 therapy ameliorates established collagen-induced arthritis in DBA/1 mice partly through the suppression of tumour necrosis factor. Clin Exp Immunol. 2004;136:67–75. doi: 10.1111/j.1365-2249.2004.02421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorbecke GJ, Shah R, Leu CH, Kuruvilla AP, Hardison AM, Palladino MA. Involvement of endogenous tumor necrosis factor α and transforming growth factor ß during induction of collagen type II arthritis in mice. Proc Natl Acad Sci USA. 1992;89:7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S, Duwel LE, Boivin GP, Ma Y, Hirsch R. Association of the course of collagen-induced arthritis with distinct patterns of cytokine and chemokine messenger RNA expression. Arthritis Rheum. 1999;42:1109–1118. doi: 10.1002/1529-0131(199906)42:6<1109::AID-ANR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Trentham DE. Collagen arthritis as a relevant model for rheumatoid arthritis: evidence pro and con. Arthritis Rheum. 1982;25:911–916. doi: 10.1002/art.1780250801. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- Williams RO. Rodent models of arthritis: relevance for human disease. Clin Exp Immunol. 1998;114:330–332. doi: 10.1046/j.1365-2249.1998.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RO. Therapeutic effect of vasoactive intestinal peptide in collagen-induced arthritis. Arthritis Rheum. 2002;46:271–273. doi: 10.1002/1529-0131(200201)46:1<271::AID-ART10039>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RO, Ghrayeb J, Feldmann M, Maini RN. Successful therapy of collagen-induced arthritis with TNF receptor-IgG fusion protein and combination with anti-CD4. Immunology. 1995;84:433–439. [PMC free article] [PubMed] [Google Scholar]
- Wooley PH, Dutcher J, Widmer MB, Gillis S. Influence of a recombinant human soluble tumour necrosis factor receptor Fc fusion protein on type II collagen-induced arthritis in mice. J Immunol. 1993;151:6602–6607. [PubMed] [Google Scholar]
- Yamanishi Y, Boyle DL, Pinkoski MJ, et al. Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am J Pathol. 2002;160:123–130. doi: 10.1016/S0002-9440(10)64356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]