Abstract
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in the United States and is projected to become the leading cause of mortality in the world. Atherosclerosis is the most important single factor contributing to this disease burden. In this study, we characterize relationships between endothelial dysfunction and vascular disease in an animal model of diet-induced, early-stage atherosclerotic vascular disease. We tested the hypothesis that hypercholesterolaemia induces vascular disease and impairs endothelium-dependent relaxation (EDR) in conduit arteries of adult male Yucatan pigs. Pigs were fed a normal fat (NF) or high fat cholesterol (HFC) diet for 20–24 weeks. Results indicate that, while the HFC diet did not alter EDR in femoral or brachial arteries, EDR was significantly decreased in both carotid and coronary arteries. Sudanophilic fatty streaks were significantly present in the abdominal aorta and common carotid artery. Histopathology revealed increased intima-media thickness (IMT) and foam cell accumulation in Stary Stage I–III lesions in the abdominal aorta, common carotid artery and femoral arteries. In the coronary arteries, the accumulation of foam cells in Stary Stage I and II lesions resulted in a trend for increased IMT. There was no evidence of vascular disease in the brachial arteries. These results indicate that early stages of CVD (Stary Stage I–III) precede decreases in EDR induced by HFC diet, because femoral arteries exhibited foam cell accumulation and an increased IMT but no change in endothelial function.
Keywords: aorta, atherosclerosis, carotid, coronary, endothelial dysfunction, femoral, hypercholesterolemia, porcine
Cardiovascular disease (CVD) continues to be the leading cause of morbidity and mortality in the United States and is projected to become the leading cause of mortality in the world over the next 20 years (Lopez & Murray 1998). Atherosclerosis is the most important single factor contributing to this disease burden (Libby 2002). Our understanding of the pathogenesis of CVD and atherosclerosis has increased dramatically through vascular biology and a growing appreciation of a role of inflammation in the pathogenesis of atherosclerosis (Libby 2002). Hypercholesterolaemia has been reported to cause decreased endothelium-dependent relaxation (EDR) of conduit arteries (endothelial dysfunction) in humans (Casino et al. 1993] Celermajer et al. 1994), monkeys (Heistad et al. 1984; Lopez et al. 1989) and pigs (Cohen et al. 1988; Komori et al. 1989; Shimokawa &, Vanhoutte 1989a, b; Best et al. 1999; Woodman et al. 2003). Blunted EDR is believed to be present early in the progression of coronary artery disease, even prior to evidence of lesions (Heistad et al. 1984; Lopez et al. 1989; McLenachan et al. 1991; Luscher et al. 1993; Dzau 1994; Nava et al. 1995; Ganz & Vita 2003; Verma et al. 2003). There is a growing body of evidence that disruption of the nitric oxide synthase (NOS) pathway (Meredith et al. 1993; Dzau 1994; Nava et al. 1995) and/or reduced availability of nitric oxide (NO) contribute to this dysfunction (Vita & Keaney 2002; Vita & Loscalzo 2002). Although brachial flow-mediated vasodilation is used as an index of endothelial dysfunction (Celermajer et al. 1994; Liang et al. 1998; Furamoto et al. 2002; Fathi et al. 2004; Jarvisalo et al. 2004), atherosclerosis develops less frequently in the brachial artery than the carotid and coronary arteries of humans (Sorensen et al. 1997; Bucciarelli et al. 2002). Because of their propensity to develop CVD, the carotid and femoral arteries have been proposed as superior to the brachial artery for the detection of increased intima-media thickness (IMT) in atherosclerosis using ultrasound measurements (Kosch et al. 2000; Bucciarelli et al. 2002; Jarvisalo et al. 2004).
Atherosclerosis is a progressive disease involving at least seven stages of pathogenesis (Stary et al. 1994; Stary 2000a). Although vascular disease generally progresses to advanced stages of atherosclerosis over decades, its progression can be slowed and/or reversed by a number of interventions (Stary et al. 1994; Stary 2000a). Perhaps because current evidence indicates that endothelial dysfunction is present throughout all stages of the development of atherosclerosis, it has been proposed that endothelial dysfunction plays a role in initiating atherosclerosis through alterations in expression of adhesion factors, inflammatory mediators and other atherogenic genes (Vita & Keaney 2002; Vita & Loscalzo 2002).
Based on these observations, a primary goal of the experiments reported herein was to evaluate the relationship between endothelial dysfunction and vascular disease in a number of arteries during early stages of disease development. We also evaluated the role of the NOS signalling pathway in these processes. The progressive nature of atherosclerosis also raises the question of whether similar mechanisms mediate the beneficial effects of therapeutic interventions at early and late stages of the vascular disease progression. It is important to improve therapeutic approaches for retarding the development of atherosclerosis in early stages of the disease, because available results indicate that vascular disease is present widely among adolescents as well as adults in our population (McGill et al. 2000; Stary 2000b; McGill & McMahan 2003). The recent Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study found atherosclerotic vascular disease to be prevalent in young adults (McGill et al. 2000; McGill & McMahan 2003).
During early stages in the pathogenesis of vascular disease, patients are asymptomatic, making it difficult to study the influence of interventions on these stages of progression. Therefore, the effectiveness of interventions on early development of vascular disease requires an animal model, because many of the measurements necessary for this analysis can not be ethically made on human subjects who are not symptomatic during the early stages of pathogenesis. We modified previous models of high fat and cholesterol (HFC) diet-induced atherosclerosis in pigs (Florentin et al. 1968; Gerrity et al. 1979; Gerrity 1981a,b; Reitman et al. 1982; Kim et al. 1985; Kim et al. 1987) to study early stage vascular disease (Stary Stage 1–3) (Stary et al. 1994; Stary 2000a,b; Thomas et al. 2002; Turk et al. 2003; Turk & Laughlin 2004). In addition to the goal of characterizing relationships between endothelial dysfunction and development of early-stage vascular disease in this animal model, we present and summarize data that allow consideration of the strengths and weaknesses of this animal model of early-stage vascular disease for use in evaluation of approaches to treat or prevent the development of vascular disease. In this report, we provide results characterizing the pathologic anatomy and pathophysiology of this animal model using measures of histopathology and endothelial vasomotor function of the major arteries. It appears that this HFC model will be useful to determine whether interventions retard the early stages of atherosclerosis and that this model can be used to improve understanding of mechanisms responsible for beneficial effects of therapeutic interventions.
Methods
Experimental design
Experimental animals.
Thirty-eight adult, male Yucatan miniature swine, 8–12 months of age, 25–40 kg body weight (Charles River, Maine and Sinclair Research Farm, Columbia, MO, USA) were used with protocols approved by the Animal Care and Use Committee at the University of Missouri. Pigs were housed in rooms maintained at 20–23 °C with a 12:12-h light-dark cycle. Half of the pigs were provided a normal fat (NF) diet (Purina Laboratory Mini-pig Chow; 8% of daily caloric intake derived from fat), and half of the pigs were fed a HFC diet (46% of their daily caloric intake from fat) consisting of pig chow supplemented with cholesterol (2.0%), coconut oil (17.1%), corn oil (2.3%) and sodium cholate (0.7%) (Dixon et al. 1999; Thomas et al. 2002; Woodman et al. 2003). Pigs were maintained on the HFC or NF diet for a period of 16-20 weeks. Plasma lipid data from a subset of the pigs used in this study have been reported previously (Thomas et al. 2002). In the animals included in the present study, the HFC diet-induced elevated plasma cholesterol; NF = 59 ± 2 mg/dl, HFC = 402 ± 26 mg/dl; high-density lipoprotein-C, NF = 31 ± 1 mg/dl, HFC = 108 ± 4 mg/dl and low-density lipoprotein-C, NF = 24 ± 3 mg/dl, HFC = 231 ± 22 mg/dl in HF pigs.
Pathologic anatomy
At the end of 20–24 weeks, pigs were anaesthetized with intramuscular atropine, ketamine and xylazine and intravenous pentobarbital, and the chest was opened to achieve euthanasia. Samples of the abdominal aorta, brachial, common carotid, coronary and femoral arteries were taken from the same location in all pigs and fixed in neutral-buffered 10% formalin for a minimum of 24 h using standard techniques (McGarry et al. 1985; Rao et al. 2000; Homma et al. 2001). Samples of abdominal aorta from the diaphragm to the iliac bifurcation and common carotid artery at the bifurcation from the brachiocephalic artery were opened longitudinally, pinned, stained with Sudan IV and photographed digitally. The area of positive staining was calculated as a percent of total intimal surface area (percent sudanophilia) of the sample utilizing ImagePro Plus (Gerrity et al. 1979; Dixon et al. 1999). Transverse slices of the abdominal aorta were taken at the level immediately proximal to the iliac bifurcation. Slices of the common carotid artery were taken from the most intense Sudanophilic portion of the sample in the pigs on the HFC diet, and a similar area from pigs fed the NF diet. Rings of the brachial and femoral arteries were sampled as described previously (Laughlin et al. 2003; Woodman et al. 2003). Samples of epicardial left anterior descending coronary artery (LAD) attached to the left ventricular myocardium were taken immediately distal to the proximal epicardial ring samples that were used for vascular ring preparations for assays of endothelial function below. Formalin-fixed samples were processed routinely through paraffin embedment. Five micron sections were stained with Verhoeff's method for elastin. Sections were examined using an Olympus B × 40 photomicroscope and photographed with a Spot Insight digital camera. The IMT was measured at the point of greatest thickness in sections from the standardized sample sites. Lesions were graded according to Stary Stage (Stary et al. 1994; Stary 2000a).
Vascular ring preparation for assay of endothelial function.
Segments of the carotid, brachial, femoral and LAD were removed (isolated from the same location in all pigs) and trimmed of connective tissue and fat. Axial length, inside diameter and outside diameter of each vascular ring was measured with a Filar calibrated micrometer eye piece. Vasomotor reactivity was examined with the rings stretched to the length that produced maximal active tension (Lmax) as described previously (Oltman et al. 1992; Oltman et al. 1995; McAllister & Laughlin 1997; Johnson et al. 2001).
Effects of HFC on contractile responses, endothelium-independent and endothelium-dependent responses were examined. Because there are reports that HFC diets produce impaired EDR in response to some but not all endothelium-dependent agonists (Komori et al. 1989; Shimokawa & Vanhoutte 1989a) while others report generalized endothelial dysfunction (Meredith et al. 1993; Dzau 1994; Nava et al. 1995), we determined whether HFC produced generalized or selective endothelial dysfunction in adult male pigs by examining responses to two endothelium-dependent agonists [bradykinin (BK) and acetylcholine (ACH)]. In several experiments, we also examined the relative contributions of NO, cyclooxygenase prostanoids (COX) and/or non-COX and non-NOS pathways, using procedures outlined in detail previously (Woodman et al. 2003,2004). In these experiments, four vascular rings were obtained from each pig: one untreated control, one treated with 0.3 mM NG-nitro-l-arginine methyl ester (l-NAME) to block NO release from NOS, one treated with 5 M indomethacin (INDO) to block COX and one treated with both blockers (l-NAME + INDO) to evaluate the role of endothelial-derived hyperpolarizing factor (EDHF) and/or other endothelium-derived mediators. All the four rings were precontracted with PGF2-α (30 M), and EDR was assessed using BK (10−11−10−6 M) and ACH (10-10−10-4 M).
Solutions and drugs for vasomotor response experiments.
Krebs bicarbonate buffer solution contained 131.5 mM NaCl, 5.0 mM KCL, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 2.5 mM CaCl2, 11.2 mM glucose, 20.8 mM NaHCO3, 0.003 mM propranolol and 0.025 mM EDTA. Solutions were aerated with 95% O2, 5% CO2 (pH 7.4) and maintained at 37 °C. All drugs and chemicals were purchased from Sigma Chemical (St Louis, MO, USA).
Statistical analysis.
All values are means ± SE. Differences between mean values of heart weight/body weight ratio, Sudanophilia and IMT were evaluated by Student's t or Mann–Whitney U tests using SigmaStat. Concentration-response curves for arterial rings were evaluated using mixed-factor repeated measures analysis of variance (anova). Data were analysed with each animal counted as one observation for comparisons between groups with respect to each vasoactive agent or combination of agents. Data for the contractile responses were analysed as developed tension (grams of tension). P values <0.05 were considered significant.
Results
There were no differences between body weights of NF (44 ± 2 kg) and HFC pigs (45 ± 2 kg). Also, average heart weight to body weight ratios were similar between the groups (NF = 4.5 ± 0.1 g/kg and HFC = 4.4 ± 0.1 g/kg).
Pathologic anatomy
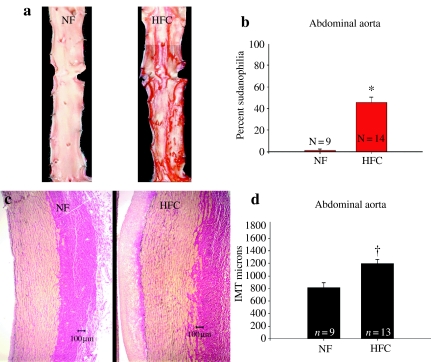
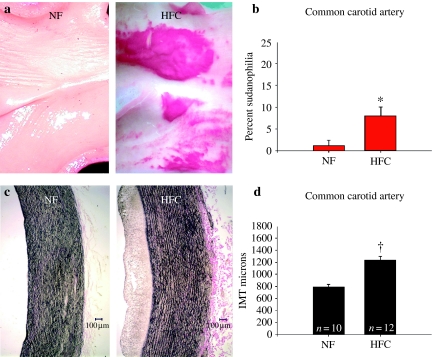
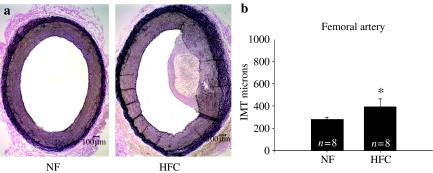
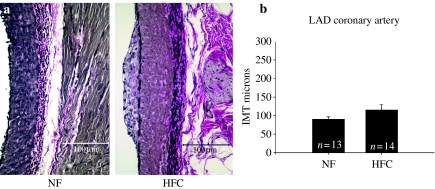
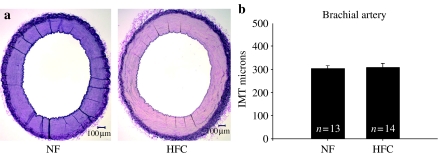
The HFC diet induced significant increase in the Sudanophilic area and the IMT of the abdominal aorta (P < 0.001) (Figure 1) and common carotid artery (P < 0.005) (Figure 2). There was significant increase in IMT of the femoral artery (P < 0.05) (Figure 3); however, IMT was not significantly different in the LAD (Figure 4) or brachial arteries (Figure 5). Foam cells and Stary Stage I-III lesions were present in all abdominal aortas and common carotid arteries and in three of eight femoral arteries and seven of 14 LADs from HFC pigs. Foam cells were not seen in brachial arteries.
Figure 1.
(a) Representative photographs of abdominal aorta stained with Sudan IV in pigs fed a normal fat (NF) and a high fat and cholesterol (HFC) diet. (b) The percent Sudanophilia was significantly greater in HFC than NF pigs (*P < 0.001). (c) Representative photographs of sections of abdominal aorta stained with Verhoeff's technique for elastin in pigs fed NF and HFC diet. (d) The aortic intima-media thickness (IMT) was significantly greater in HFC than NF pigs (†P < 0.001).
Figure 2.
(a) Representative photographs of common carotid artery stained with Sudan IV in pigs fed a normal fat (NF) and a high fat and cholesterol (HFC) diet. (b) The percent Sudanophilia was significantly greater in HFC than NF pigs (*P = 0.006). (c) Representative photographs of sections of common carotid artery stained with Verhoeff's technique for elastin in pigs fed NF and HFC diet. (d) The carotid intima-media thickness (IMT) was significantly greater in HFC than NF pigs (†P < 0.001).
Figure 3.
(a) Representative photographs of femoral artery stained with Verhoeff's technique for elastin in pigs normal fat (NF) and a high fat and cholesterol (HFC) diet. (b) The femoral intima-media thickness (IMT) was significantly greater in HFC than NF pigs (*P < 0.05).
Figure 4.
(a) Representative photographs of left anterior descending (LAD) branch of the coronary artery stained with Verhoeff's technique for elastin in pigs normal fat (NF) and a high fat and cholesterol (HFC) diet. (b) The LAD intima-media thickness (IMT) was not different in HFC and NF pigs (P = 0.145).
Figure 5.
(a) Representative photographs of brachial artery stained with Verhoeff's technique for elastin in pigs normal fat (NF) and a high fat and cholesterol (HFC) diet. (b) The brachial intima-media thickness (IMT) was not different in HFC and NF pigs (P = 0.86).
Characteristics of vascular rings used in vasomotor responsiveness experiments.
There were no differences between NF and HFC internal diameters, length of isolated segments, resting tension at Lmax and percent stretch at Lmax or PGF2-α-induced force in the four arteries examined in this study (Table 1). Thus, resting tension at Lmax and percent stretch to Lmax were not significantly altered by the HFC diet. Although the HFC carotid arteries tended to develop more tension, generally, arteries from HFC developed tension during preconstriction with 30 M PGF2-α that was similar to the tension produced by arteries from NF pigs.
Table 1. Characteristics of arteries from pigs on normal fat (NF) and high fat/cholesterol (HFC) diets.
| Variable | NF carotid | HFC carotid | NF coronary | HFC coronary | NF brachial | HFC brachial | NF femoral | HFC femoral |
|---|---|---|---|---|---|---|---|---|
| Internal diameter (mm) | 4.0 ± 0.2 | 4.1 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.2 |
| Length (cm) | 3.7 ± 0.2 | 3.4 ± 0.3 | 3.2 ± 0.2 | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.1 | 3.6 ± 0.4 | 3.5 ± 0.3 |
| Resting tension (g) | 23.8 ± 3.5 | 22.7 ± 5.1 | 5.6 ± 0.6 | 5.5 ± 1.0 | 5.3 ± 0.7 | 5.1 ± 1.0 | 12.8 ± 3.0 | 12.0 ± 2.0 |
| Percent stretch | 160 ± 5 | 154 ± 3 | 178 ± 3 | 178 ± 4 | 181 ± 3 | 183 ± 4 | 171 ± 2 | 172 ± 6 |
| PGF2-α-induced tension (g) | 17.8 ± 6.3 | 10.2 ± 2.5 | 12.2 ± 1.1 | 12.0 ± 1.1 | 21.2 ± 1.5 | 20.0 ± 1.4 | 33.5 ± 1.4 | 29.6 ± 1.4 |
| Number of animals | 8 | 7 | 12 | 11 | 12 | 11 | 7 | 6 |
Mean (SEM). There are no between group differences for any artery.
Carotid artery relaxation responses: bradykinin.
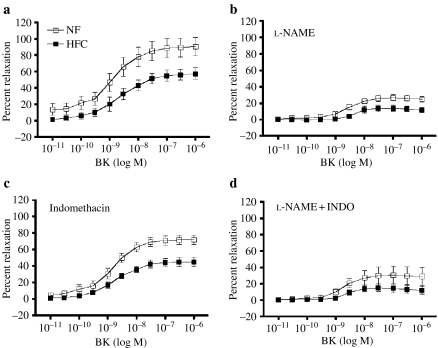
BK elicited a dose-dependent relaxation of carotid artery from both the groups of pigs, but the carotid arteries from HFC pigs exhibited less EDR (P = 0.0522) (Figure 6). Mixed-factor repeated measures anova revealed a significant diet BK dose interaction, indicating that HFC arteries exhibited less EDR at higher doses of BK (P = 0.0005). Following treatment with l-NAME, HFC arteries continued to exhibit less EDR. After treatment with l-NAME plus INDO, there was no difference between BK-induced dilation in NF and HFC carotid arteries. The majority of EDR appears to be mediated by NO release from NOS in both the groups, because the majority of EDR was blocked by l-NAME. The l-NAME plus INDO results suggest that the HFC diet attenuates the contribution of a non-NOS, non-COX mediator in BK-induced EDR of carotid arteries.
Figure 6.
Dose-dependent bradykinin (BK)-induced relaxation of carotid artery. Values are means ± SE. Normal fat (NF) (n = 8 pigs); high fat/cholesterol (HFC) (n = 6). Percent relaxation was calculated as percent reduction in force from PGF2-α (30 M)-induced tension. (a) BK-induced relaxation was impaired in HFC at intermediate and high doses of BK (P < 0.05). (b) Effects of NG-nitro-l-arginine methyl ester (l-NAME) on relaxation. (c) Effects of indomethacin (INDO) on relaxation. (d), Effects of l-NAME plus INDO on relaxation. Mixed-factor anova indicated that l-NAME significantly inhibited BK-induced relaxation.
Carotid artery relaxation responses: acetylcholine.
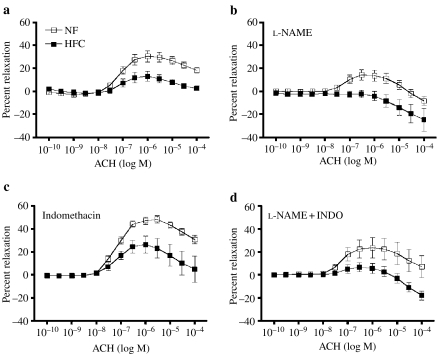
ACH elicited a dose-dependent relaxation of carotid artery from both the groups of pigs, but the carotid arteries from HFC pigs exhibited significantly less EDR (P = 0.0149) (Figure 7). ACH did not induce relaxation in HFC carotids treated with l-NAME, or l-NAME plus INDO, suggesting that HFC diet decreases the NOS- and COX-independent EDR. INDO treatment increased relaxation of both NF and HFC carotid arteries. Also, results following l-NAME treatment showing lack of EDF after l-NAME in HFC indicate that the majority of EDR in HFC carotids is mediated by NO release. The l-NAME sensitive EDR results also suggest that HFC diet decreased NO release from NOS, because the l-NAME-sensitive EDR was 8% in HFC and 16% in NF (Figure 7). Relaxations of carotid arteries to both ACH and BK were abolished in HFC and NF rings denuded of endothelium. Indeed, both ACH and BK produced further contraction in denuded carotid artery rings (data not shown).
Figure 7.
Dose-dependent acetylcholine (ACH)-induced relaxation of carotid artery. Values are means ± SE. Normal fat (NF) (n = 8 pigs); high fat/cholesterol (HFC) (n = 6). Percent relaxation was calculated as percent reduction in force from PGF2-α (30 M)-induced tension. ACH-induced relaxation was impaired in HFC at intermediate and high doses of ACH (P < 0.05). (a) ACH-induced relaxation was impaired in HFC at intermediate and high doses of BK (P = 0.0001). (b) Effects of NG-nitro-l-arginine methyl ester (l-NAME) on relaxation. (c) Effects of indomethacin (INDO) on relaxation. (d) Effects of l-NAME plus INDO on relaxation. Mixed-factor anova indicated that l-NAME significantly inhibited ACH-induced relaxation.
LAD relaxation responses.
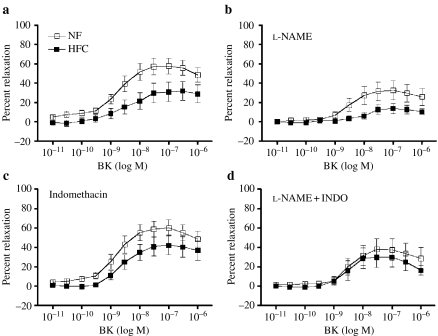
ACH only induced increases in developed force in LAD's from both the groups (data not shown) as reported previously for porcine coronary arteries (Oltman et al. 1992; Woodman et al. 2004). LADs from HFC pigs developed significantly more ACH-induced contraction (61 ± 11% increased force) than did NF LADs (36 ± 6%). BK elicited a dose-dependent relaxation of LAD rings from both NF and HFC groups (Figure 8), but the LADs from HFC pigs exhibited significantly less EDR (P < 0.05) (Figure 8). l-NAME treatment nearly abolished EDR in HFC LADs and decreased EDR in NF LAD. INDO treatment did not alter BK-induced responses of NF or HFC LADs, whereas following treatment with l-NAME plus INDO, there was no statistically significant difference between BK-induced dilation in NF LADs and BK-induced dilation in HFC LADs.
Figure 8.
Dose-dependent, bradykinin (BK)-induced relaxation of left anterior descending coronary arteries. Values are means ± SE. Normal fat (NF) (n = 12 pigs); high fat/cholesterol (HFC) (n = 11). Percent relaxation was calculated as percent reduction in force from PGF2-α (30 M)-induced tension. (a) BK-induced relaxation was impaired in HFC at intermediate and high doses of BK (P < 0.05). (b) Effects of NG-nitro-l-arginine methyl ester (l-NAME) on relaxation. (c) Effects of indomethacin (INDO) on relaxation. (d) Effects of l-NAME plus INDO on relaxation. Mixed-factor anova indicated that l-NAME significantly inhibited BK-induced relaxation.
Brachial artery relaxation responses: acetylcholine and bradykinin.
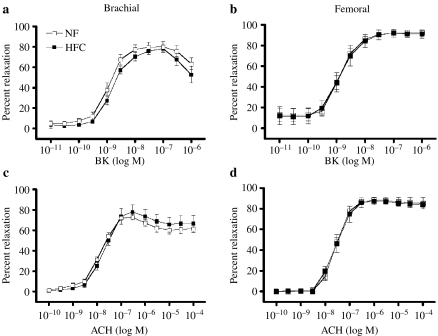
BK and ACH elicited similar dose-dependent relaxations of brachial arteries from both the groups of pigs (Figure 9). Responses of brachial arteries were also similar between NF and HFC following treatment with INDO, l-NAME and l-NAME plus INDO (data not shown).
Figure 9.
Dose-dependent acetylcholine (ACH) and bradykinin (BK)-induced relaxation of brachial and femoral artery. (a) BK elicited a similar dose-dependent relaxation of brachial arteries from both high fat/cholesterol (HFC) (n = 12) and normal fat (NF) (n = 11) diet groups. (b) BK elicited a similar dose-dependent relaxation of femoral arteries from both HFC (n = 6) and NF (n = 7) diet groups. (c) ACH elicited a similar dose-dependent relaxation of brachial arteries from both HFC (n = 11) and NF (n = 12) diet groups. (d) ACH elicited a similar dose-dependent relaxation of femoral arteries from both HFC (n = 6) and NF (n = 7) diet groups.
Femoral artery relaxation responses: acetylcholine and bradykinin.
BK and ACH elicited similar dose-dependent relaxations of femoral arteries from both the groups of pigs (Figure 9). Responses of femoral arteries were also similar between NF and HFC groups following treatment with INDO, l-NAME and l-NAME plus INDO (data not shown).
Sodium nitroprusside (SNP) responses.
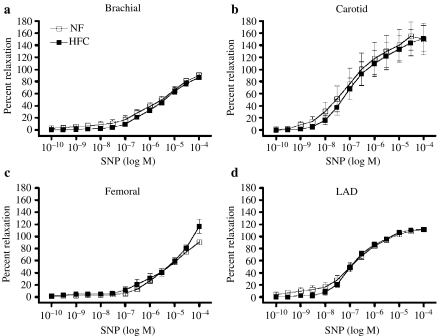
All the four arteries (carotids, LADs, brachial and femoral) from both the groups exhibited a dose-dependent relaxation in response to SNP (Figure 10). There were no differences between SNP responses of arteries from HFC and NF pigs (Figure 10).
Figure 10.
Dose-dependent sodium nitroprusside (SNP)-induced relaxation of carotid artery high fat/cholesterol (HFC) (n = 7) and normal fat (NF) (n = 8) diet groups (a). Coronary artery (left anterior descending) HFC (n = 12) and NF (n = 11) diet groups (b). Brachial artery HFC (n = 12) and NF (n = 11) diet groups (c). Femoral artery femoral arteries from both HFC (n = 6) and NF (n = 7) diet groups (d). There were no between group differences in the responses of any artery.
Discussion
The results presented in this report characterize relationships between arterial endothelial function and disease in a porcine model of vascular disease produced by feeding a high fat/high cholesterol diet to young adult male pigs for a period of 20–24 weeks (Thomas et al. 2002; Turk et al. 2003). Early-stage disease was present as reflected in Sudanophilic fatty streaks and accumulations of foam cells that were present in all of the aortas and carotid arteries of the HFC pigs. Also, in the HFC pigs, the IMT was significantly greater in the abdominal aortas, carotid arteries and femoral arteries. While IMT was not significantly greater in coronary arteries, seven of 14 coronary arteries examined from the HFC pigs contained foam cells in the arterial wall. Foam cells were also present in three of eight femoral arteries from HFC pigs whereas none of the 14 brachial arteries of HFC pigs contained foam cells. Woodman et al. (2003,2004) reported that foam cells (i.e. early lesions) are also seen in the coronary but not brachial arteries of female pigs on this HFC diet, and Thompson et al. (2004) reported foam cells and fatty streaks in coronary arteries of male pigs on this diet. Indeed, some of the pigs included in this study were a subset of pigs used in the study of Thompson et al. (2004). Foam cells and fatty streaks were not observed in any of the arteries of NF-diet pigs examined in this study. Thus, on the basis of these results, we conclude that this animal model of vascular disease exhibits vascular disease comparable to Stary Stage I-III (Stary et al. 1994; Stary 2000a), i.e. early in the progression of atherosclerosis. The lesions observed in the abdominal aorta, carotid and coronary arteries are similar to those reported in PDAY studies of early human atherosclerosis (McGarry et al. 1985; McGill et al. 2000; Rao et al. 2000; Stary 2000b; Homma et al. 2001).
We assessed endothelial function in the carotid, coronary, brachial and femoral arteries of HFC pigs and considered potential relationships of disease with endothelial function. Results indicate that impaired EDR to ACH and/or BK was present in the carotid and coronary arteries of the HFC pigs but not in the brachial or femoral arteries. Because foam cells were present and IMT increased in femoral arteries that exhibited normal EDR from HFC pigs, these results suggest that vascular lesion development occurs earlier than does development of blunted EDR. That is, there were early signs of vascular disease in the femoral arteries as reflected in foam cell accumulation, but endothelial function remained normal.
Vascular disease appears to progress more rapidly in the aorta, carotid, coronary and femoral arteries in this model than in the brachial arteries. Similarly, in humans, increased IMT of the abdominal aorta and carotid artery has been reported to independently predict atherosclerosis, coronary artery disease, myocardial infarction and stroke (Ebrahim et al. 1999; Cerne & Kranjec 2002; Lacroix et al. 2003; van der Meer et al. 2004). Increased femoral artery IMT has been reported to be associated with CVD-risk factors and events (Cheng et al. 2002). The lack of lesions in the brachial arteries despite lesions in the carotid, coronary and femoral arteries in this pig model is similar to observations in humans with CVD (Sorensen et al. 1997; Bucciarelli et al. 2002). Current results indicate that this animal model will be useful in the determination of mechanisms whereby exercise and other interventions may allay progression of atherosclerotic vascular disease and in evaluation of the role of the endothelium in these processes (Thomas et al. 2002; Woodman et al. 2003; Thompson et al. 2004; Turk & Laughlin 2004).
Previous reports using this animal model have established that (1) coronary vascular smooth muscle calcium channel activity is attenuated by about 50% by HFC (Bowles et al. 2004), (2) voltage-gated calcium channel activity is attenuated by HFC diet because of deposition of free cholesterol in the sarcolemmal membrane of coronary smooth muscle (Wamhoff et al. 2002; Bowles et al. 2004), (3) HFC also alters the PKC isoform profile of coronary smooth muscle (Korzick et al. 2004), (4) endothelium-dependent dilation is blunted in the brachial artery (Woodman et al. 2003) and LAD of female pigs on this HFC diet (Woodman et al. 2004) and (5) hypercholesterolaemia correlates with inflammatory markers and lesion development (Turk et al. 2003). The present study was not designed to examine effects of HFC on smooth muscle function. Indeed, the observations that responses to SNP were not altered by HFC (Figure 10) and that PGF2-α-induced force was not different indicate that changes in smooth muscle function did not influence our evaluations of EDR in these arteries.
It is of interest that this HFC diet does not exert uniform effects on endothelial function in all systemic arteries and that within the arteries exhibiting decreased endothelial function, the mechanisms are not the same. Thus, endothelial function, as reflected in measurements of EDR, is impaired in carotid and coronary arteries of HFC pigs. In the carotid artery of HFC pigs, blockade of NOS activity nearly abolished EDR and substantially decreased EDR in NF carotids. The amount of EDR that was NOS dependent appeared to be decreased in HFC carotid arteries. Also, the fact that NF carotids continue to exhibit greater EDR after treatment with l-NAME and INDO suggests that HFC also decreased EDR of carotid artery by decreasing the release of non-NOS/non-COX-relaxing factors such as EDHF. Results from the coronary arteries of HFC pigs are similar relative to the effects of HFC on NO release, but there were no differences in the responses of HFC and NF LADs following treatment with l-NAME plus INDO. Thus, HFC does not appear to alter the role of non-NOS/non-COX-relaxing factors in LADs in this model. These results are interesting in that they suggest that the mechanisms whereby this model of HFC produces endothelial dysfunction were not the same in carotid and coronary arteries, even though both the arteries exhibited decreased EDR.
Importantly, endothelial function did not appear to be impaired in brachial or femoral arteries of HFC pigs. This was surprising given that Woodman et al. (2003) reported decreased EDR in response to ACH and BK in female HFC pig brachial arteries. Present results do not provide insight into why this HFC diet results in decreased EDR in brachial arteries of female pigs but not male pigs on the same diet. Similar interesting gender differences between the effects of exercise on brachial and femoral arteries of normal Yucatan pigs have been reported previously (Laughlin et al. 2001,2003). Future work will be required to determine the cause of this difference between female and male pigs on this HFC diet.
We conclude that this HFC diet-induced model of vascular disease produces early-stage vascular disease (Stary Stage 1-3) (Stary et al. 1994; Stary 2000a,b). Given that femoral arteries contained foam cells and an increased IMT but no change in endothelial function, the results indicate that early-stage disease development may precede the development of endothelial dysfunction. This model provides an important tool to evaluate the effectiveness of interventions in modifying or blocking the development of diet-induced vascular disease and the role of the vascular endothelium in these processes.
Acknowledgments
The authors gratefully acknowledge the expert technical assistance of Pam Thorne, Denize Holiman, Jennifer Casati and Tammy Strawn. This work was supported by National Heart, Lung and Blood Institute Grants HL-52490 and HL-36088 (to M. H. Laughlin).
References
- Best JM, Lerman LO, Romero JC, Richarson D, Holmes DR, Lermer A. Coronary endothelial function is preserved with chronic endothelin receptor antagonism in experimental hypercholesterolemia in vitro. Arterioscler Thromb Vasc Biol. 1999;19:2769–2775. doi: 10.1161/01.atv.19.11.2769. [DOI] [PubMed] [Google Scholar]
- Bowles DK, Heaps CL, Turk JR, Maddali KK, Price EM. Hypercholesterolemia inhibits L-type calcium current in coronary macro-, not microcirculation. J Appl Physiol. 2004;96:2240–2248. doi: 10.1152/japplphysiol.01229.2003. [DOI] [PubMed] [Google Scholar]
- Bucciarelli P, Sramek A, Reiber JH, Rosendaal FR. Arterial intima-media thickness and its relationship with cardiovascular disease and atherosclerosis: a possible contribution of medium-sized arteries. Thromb Haemost. 2002;88:961–966. [PubMed] [Google Scholar]
- Casino PR, Kilcoyne RN, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Cerne A, Kranjec I. Atherosclerotic burden in coronary and peripheral arteries in patients with first clinical manifestation of coronary artery disease. Heart Vessels. 2002;16:217–226. doi: 10.1007/s003800200028. [DOI] [PubMed] [Google Scholar]
- Cheng KS, Mikhailidis DP, Hamilton G, Seifalian AM. A review of the carotid and femoral intima-media thickness as an indicator of the presence of peripheral vascular disease and cardiovascular risk factors. Cardiovasc Res. 2002;54:528–538. doi: 10.1016/s0008-6363(01)00551-x. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Zitnay KM, Haudenschild CC, Cunningham LD. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ Res. 1988;63:903–910. doi: 10.1161/01.res.63.5.903. [DOI] [PubMed] [Google Scholar]
- Dixon JL, Stoops JD, Parker JL, Laughlin MH, Weisman GA, Sturek M. Dyslipidemia and vascular dysfunction in diabetic pigs fed an atherogenic diet. Arterioscler Thromb Vasc Biol. 1999;19:2981–2992. doi: 10.1161/01.atv.19.12.2981. [DOI] [PubMed] [Google Scholar]
- Dzau VJ. Pathobiology of atherosclerosis and plaque complications. Am Heart J. 1994;128:1300–1304. doi: 10.1016/0002-8703(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Ebrahim S, Papacosta O, Whincup P, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- Fathi R, Haluska B, Isbel N, Short L, Marwick TH. The relative importance of vascular structure and function in predicting cardiovascular events. J Am Coll Cardiol. 2004;43:616–623. doi: 10.1016/j.jacc.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Florentin RA, Nam SC, Daoud AS, et al. Dietary-induced atherosclerosis in miniature swine. Exp Mol Pathol. 1968;8:263–301. doi: 10.1016/s0014-4800(68)80001-2. [DOI] [PubMed] [Google Scholar]
- Furumoto T, Fujii S, Salto N, et al. Relationships between brachial artery flow mediated dilation and carotid artery intima-media thickness in patients with suspected coronary artery disease. Jpn Heart J. 2002;43:117–125. doi: 10.1536/jhj.43.117. [DOI] [PubMed] [Google Scholar]
- Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. 2003;108:2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- Gerrity RG. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981a;103:191–200. [PMC free article] [PubMed] [Google Scholar]
- Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981b;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- Gerrity RG, Naito HK, Richardson M, Schwartz CJ. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979;95:775–792. [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, Armstrong ML, Marcus ML, Piegors DJ, Mark AL. Augmented responses to vasoconstrictor stimuli in hypercholesterolemic and atheroclerotic monkeys. Circ Res. 1984;54:711–718. doi: 10.1161/01.res.54.6.711. [DOI] [PubMed] [Google Scholar]
- Homma S, Ishii T, Malcom GT, et al. Histopathological modifications of early atherosclerotic lesions by risk factors – findings in PDAY subjects. Atherosclerosis. 2001;156:389–399. doi: 10.1016/s0021-9150(00)00669-9. [DOI] [PubMed] [Google Scholar]
- Jarvisalo MJ, Lehtimaki T, Raitakari OT. Determinants of arterial nitrate-mediated dilatation in children: role of oxidized low-density lipoprotein, endothelial function, and carotid intima-media thickness. Circulation. 2004;109:2885–2889. doi: 10.1161/01.CIR.0000129304.98566.D8. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Rush JW, Turk JR, Price EM, Laughlin MH. Short-term exercise training increases ACh-induced relaxation and eNOS protein in porcine pulmonary arteries. J Appl Physiol. 2001;90:1102–1110. doi: 10.1152/jappl.2001.90.3.1102. [DOI] [PubMed] [Google Scholar]
- Kim DN, Imai H, Schmee J, Lee KT, Thomas WA. Intimal cell mass-derived atherosclerotic lesions in the abdominal aorta of hyperlipidemic swine. Part 1. Cell of origin, cell divisions and cell losses in first 90 days on diet. Atherosclerosis. 1985;56:169–188. doi: 10.1016/0021-9150(85)90017-6. [DOI] [PubMed] [Google Scholar]
- Kim DN, Schmee J, Lee KT, Thomas WA. Atherosclerotic lesions in the coronary arteries of hyperlipidemic swine. Part 1. Cell increases, divisions, losses and cells of origin in first 90 days on diet. Atherosclerosis. 1987;64:231–242. doi: 10.1016/0021-9150(87)90251-6. [DOI] [PubMed] [Google Scholar]
- Komori K, Shimokawa H, Vanhoutte PM. Hypercholesterolemia impairs endothelium-dependent relaxations to aggregating platelets in porcine iliac arteries. J Vasc Surg. 1989;10:318–325. [PubMed] [Google Scholar]
- Korzick DH, Laughlin MH, Bowles DK. Alterations in PKC signaling underlie enhanced myogenic tone in exercise-trained porcine coronary resistance arteries. J Appl Physiol. 2004;96:1425–1432. doi: 10.1152/japplphysiol.01077.2003. [DOI] [PubMed] [Google Scholar]
- Kosch M, Hausberg M, Vormbrock K, Kisters K, Rahn KH, Barenbrock M. Studies on flow-mediated vasodilation and intima-media thickness of the brachial artery in patients with primary hyperparathyroidism. Am J Hypertens. 2000;13:759–764. doi: 10.1016/s0895-7061(00)00248-x. [DOI] [PubMed] [Google Scholar]
- Lacroix P, Aboyans V, Espaliat E, Cornu E, Virot P, Laskar M. Carotid intima-media thickness as predictor of secondary events after coronary angioplasty. Int Angiol. 2003;22:279–283. [PubMed] [Google Scholar]
- Laughlin MH, Schrage WG, McAllister RM, Garverick HA, Jones AW. Interaction of gender and exercise training: vasomotor reactivity of porcine skeletal muscle arteries. J Appl Physiol. 2001;90:216–227. doi: 10.1152/jappl.2001.90.1.216. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Welshons WV, Sturek M, et al. Gender, exercise training, and eNOS expression in porcine skeletal muscle arteries. J Appl Physiol. 2003;95:250–264. doi: 10.1152/japplphysiol.00061.2003. [DOI] [PubMed] [Google Scholar]
- Liang YL, Teede H, Kotsopoulos D, et al. Non-invasive measurements of arterial structure and function: repeatability, interrelationships and trial sample size. Clin Sci. 1998;95:669–679. doi: 10.1042/cs0950669. (Lond) [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Lopez JAG, Armstrong ML, Peigors DJ, Heistad DD. Effect of early and advanced atherosclerosis on vascular responses to serotonin, thromboxane A2, and ADP. Circulation. 1989;79:698–705. doi: 10.1161/01.cir.79.3.698. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Murray CC. The global burden of disease, 1990-2020. Nat Med. 1998;4:1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med. 1993;44:395–418. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- van der Meer IM, Bots ML, Hofman A, Del Sol AI, Van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation. 2004;109:1089–1094. doi: 10.1161/01.CIR.0000120708.59903.1B. [DOI] [PubMed] [Google Scholar]
- McAllister RM, Laughlin MH. Short-term exercise training alters responses of porcine femoral and brachial arteries. J Appl Physiol. 1997;82:1438–1444. doi: 10.1152/jappl.1997.82.5.1438. [DOI] [PubMed] [Google Scholar]
- McGarry P, Solberg LA, Guzman MA, Strong JP. Cerebral atherosclerosis in New Orleans. Comparisons of lesions by age, sex, and race. Lab Invest. 1985;52:533–539. [PubMed] [Google Scholar]
- McGill HC, Jr, McMahan CA. Starting earlier to prevent heart disease. JAMA. 2003;290:2320–2322. doi: 10.1001/jama.290.17.2320. [DOI] [PubMed] [Google Scholar]
- McGill HC, Jr, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000;72(Suppl.):1307S–1315S. doi: 10.1093/ajcn/72.5.1307s. [DOI] [PubMed] [Google Scholar]
- McLenachan JM, Williams JK, Fish RD, Ganz P, Selwyn AP. Loss of flow-mediated endothelium-dependent dilation occurs early in the development of atherosclerosis. Circulation. 1991;84:1273–1278. doi: 10.1161/01.cir.84.3.1273. [DOI] [PubMed] [Google Scholar]
- Meredith IT, Yeung AC, Weidinger FF, et al. Role of impaired endothelium-dependent vasodilation in ischemic manifestations of coronary artery diseases. Circulation. 1993;87:56–66. [Google Scholar]
- Nava E, Noll G, Luscher TF. Nitric oxide in cardiovascular diseases. Ann Med. 1995;27:343–351. doi: 10.3109/07853899509002587. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Parker JL, Adams HR, Laughlin MH. Effects of exercise training on vasomotor reactivity of porcine coronary arteries. Am J Physiol Heart Circ Physiol. 1992;263:H372–H382. doi: 10.1152/ajpheart.1992.263.2.H372. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Parker JL, Laughlin MH. Endothelium-dependent vasodilation of proximal coronary arteries from exercise-trained pigs. J Appl Physiol. 1995;79:33–40. doi: 10.1152/jappl.1995.79.1.33. [DOI] [PubMed] [Google Scholar]
- Rao RN, Falls DG, Gerrity RG, Sethuraman SN, Thiruvaiyaru DS. Intimal thickness and layering, and smooth muscle cell phenotypes in aorta of youth. Pathobiology. 2000;68:18–28. doi: 10.1159/000028111. [DOI] [PubMed] [Google Scholar]
- Reitman JS, Mahley RW, Fry DL. Yucatan miniature swine as a model for diet-induced atherosclerosis. Atherosclerosis. 1982;43:119–132. doi: 10.1016/0021-9150(82)90104-6. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Vanhoutte PM. Impaired endothelium-dependent relaxation to aggregating platelets and related vasoactive substances in porcine coronary arteries in hypercholesterolemia and atherosclerosis. Circ Res. 1989a;64:900–914. doi: 10.1161/01.res.64.5.900. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Vanhoutte PM. Hypercholesterolemia causes generalized impairment of endothelium-dependent relaxation to aggregating platelets in porcine arteries. J Am Coll Cardiol. 1989b. pp. 1402–1408. [DOI] [PubMed]
- Sorensen KE, Kristensen IB, Celermajer DS. Atherosclerosis in the human brachial artery. J Am Coll Cardiol. 1997;29:318–322. doi: 10.1016/s0735-1097(96)00474-3. [DOI] [PubMed] [Google Scholar]
- Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000a;20:1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- Stary HC. Lipid and macrophage accumulations in arteries of children and the development of atherosclerosis. Am J Clin Nutr. 2000b;72(Suppl. 5):1297S–1306S. doi: 10.1093/ajcn/72.5.1297s. [DOI] [PubMed] [Google Scholar]
- Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- Thomas TR, Pellechia J, Rector RS, Sun GY, Sturek MS, Laughlin MH. Exercise training does not reduce hyperlipidemia in pigs fed a high-fat diet. Metabolism. 2002;51:1587–1595. doi: 10.1053/meta.2002.36313. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Henderson KK, Woodman CR, et al. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol. 2004;96:1114–1126. doi: 10.1152/japplphysiol.00768.2003. [DOI] [PubMed] [Google Scholar]
- Turk JR, Carroll JA, Laughlin MH, et al. C-reactive protein correlates with macrophage accumulation in coronary arteries of hypercholesterolemic pigs. J Appl Physiol. 2003;95:1301–1304. doi: 10.1152/japplphysiol.00342.2003. [DOI] [PubMed] [Google Scholar]
- Turk JR, Laughlin MH. Physical activity and atherosclerosis: which animal model?. Invited review. Can J Appl Physiol. 2004;29:657–683. doi: 10.1139/h04-042. [DOI] [PubMed] [Google Scholar]
- Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- Vita JA, Loscalzo J. Shouldering the risk factor burden: infection, atherosclerosis, and the vascular endothelium. Circulation. 2002;106:164–166. doi: 10.1161/01.cir.0000023452.26135.34. [DOI] [PubMed] [Google Scholar]
- Wamhoff BR, Bowles DK, Dietz NJ, Hu Q, Sturek M. Exercise training attenuates coronary smooth muscle phenotypic modulation and nuclear Ca2+ signaling. Am J Physiol Heart Circ Physiol. 2002;283:H2397–H2410. doi: 10.1152/ajpheart.00371.2001. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Turk JR, Rush JWE, Laughlin MH. Exercise attenuates the effects of hypercholesterolemia on endothelium-dependent relaxation in coronary arteries from adult female pigs. J Appl Physiol. 2004;96:1105–1113. doi: 10.1152/japplphysiol.00767.2003. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Turk JR, Williams DP, Laughlin MH. Exercise training preserves endothelium-dependent relaxation in brachial arteries from hyperlipidemic pigs. J Appl Physiol. 2003;94:2017–2026. doi: 10.1152/japplphysiol.01025.2002. [DOI] [PubMed] [Google Scholar]