Abstract
4-Nitroquinoline 1-oxide (4NQO)-induced rat tongue carcinogenesis is a useful model for studying oral squamous cell carcinoma. The aim of this study was to investigate the expression of bcl-2 and bax during tongue carcinogenesis induced by 4NQO. Male Wistar rats were distributed into three groups of 10 animals each and treated with 50 ppm 4NQO solution through their drinking water for 4, 12 or 20 weeks. Ten animals were used as negative control. Although no histological changes were induced in the epithelium after 4 weeks of carcinogen exposure, bcl-2 and bax were over-expressed (P < 0.01) in all layers of the ‘normal’ epithelium. The expression levels were the same in all layers of epithelium for both the antibodies used (bcl-2 or bax). In dysplastic lesions at 12 weeks following carcinogen administration, the levels of bcl-2 and bax expression did not increase when compared to negative control with the immunoreactivity for bcl-2 being restricted to the superficial layer of epithelium. In well-differentiated squamous cell carcinoma induced after 20 weeks of treatment with 4NQO, bcl-2 was expressed in some cells of tumour islands. On the other hand, immunostaining for bax was widely observed at the tumour nests. The labelling index for bcl-2 and bax showed an increase (P < 0.05) after only 4 weeks of 4NQO administration. In conclusion, our results suggest that abnormalities in the apoptosis pathways are associated with the development of persistent clones of mutated-epithelial cells in the oral mucosa. Bcl-2 and bax expression appears to be associated with a risk factor in the progression of oral cancer.
Keywords: 4-nitroquinoline 1-oxide, bax, bcl-2, oral cancer
Oral cancer is a common neoplasm worldwide, particularly in developing countries such as India, Vietnam and Brazil, where it constitutes up to 25% of all types of cancer (Magrath & Litvak 1993). Despite sophisticated surgical and radiotherapeutic therapies, patient survival has not improved significantly during the last decades (Xi & Grandis 2003). Tobacco and alcohol consumption is the most significant exogenous factors involved in tumourigenesis (Hahn et al. 2002). The most commonly used animal models in oral cancer research are the hamster buccal pouch using fat-soluble 7,12 dimethylbenz[a]anthracene (DMBA) and the rat tongue using water-soluble 4-nitroquinoline 1-oxide (4NQO). Considering that one of the most important routes of oral carcinogens is through liquid intake containing water-soluble carcinogens, we concluded that 4NQO is well suited for examining the role of xenobiotics in experimental oral carcinogenesis (Tanaka et al. 2002). Based on the multistep process of carcinogenesis characterized by the initiation, promotion and tumour progression, chronic administration of 4NQO in drinking water induces rat tongue cancer with morphological characteristics similar to that seen in man (Ohne et al. 1985; Nishimura 1999; Niwa et al. 2001; Okazaki et al. 2002; Vered et al. 2003).
It has been established that DNA damage and subsequent cell proliferation can promote tumour progression through multiple pathways. Apoptosis plays an important role in the control of cellular proliferation, in so far as the loss of apoptotic response in cells is one of the mechanisms involved into the transformation from benign to malignant phenotype (Arends & Wyllie 1991).
Bcl-2 and bax are two important effector genes during the apoptosis process. The bcl-2 proto-oncogene was originally discovered by analysis of the t(14;18) chromosomal translocation associated with human follicular B-cell lymphoma (Tsujimoto et al. 1985). The bcl-2 gene encodes a protein located in the nuclear membrane, on the inner surface of mitochondria, and the endoplasmic reticulum (Akao et al. 1994). It is the most important gene of the bcl-2 family and has been shown to be an inhibitor of apoptosis (Lu et al. 1996). Immunohistochemical overexpression of bcl-2 has been observed in carcinomas of the nasopharynx, lung, urinary bladder, colon, prostate, breast, thyroid and oral cavity (Lu et al. 1993; Ikegaki et al. 1994; Gazzaniga et al. 1995; Sinicrope et al. 1995; Krajewska et al. 1996; Ravi et al. 1996; Kapucuoglu et al. 1997; Manetto et al. 1997; Nylander et al. 1997; Singh et al. 1998). Bax, another member of the bcl-2 family, is considered to be a major effector of apoptosis (Oltvai et al. 1993). In normal and tumour tissues, the distribution of bax is inversely related to that of bcl-2 (Krajewski et al. 1994). Thus, the bcl-2/bax ratio controls the relative susceptibility of cells to lethal stimuli (Korsmeyer et al. 1993). In the present study, we used immunohistochemistry to investigate the expression of bcl-2 and bax during 4NQO-induced rat tongue multistep carcinogenesis.
Materials and methods
Animals and experimental design
Forty male Wistar rats (8 weeks old) weighing approximately 250 g were obtained from Centro de Bioterismo (CEMIB), Universidade Estadual de Campinas, SP, Brazil. They were maintained under controlled temperature conditions (24 ± 2 °C), light-dark periods of 12 h and with free access to water and commercial diet (Nuvital, PR, Brazil). The animals were distributed into three groups of 10 and were treated with 50 ppm 4NQO (Sigma, St. Louis, MO, USA) solution through their drinking water for 4, 12 or 20 weeks. Ten animals were used as negative control. At the end of the experimental period, the rats were sacrificed with 0.4% sodium pentobarbital (1 ml/kg body weight, i.p.). The tongues were longitudinally cut into halves for histopathological examinations. The tissues were fixed in 10% buffered formalin (Merck, Darmstadt, Germany), embedded in paraffin blocks and stained with haematoxylin and eosin (H.E., Merck). The Animal Committee of Botucatu Medical School, UNESP, approved all experimental protocols.
Histopathological analysis
Histopathological evaluation was performed under light microscope. The tongue sections were analysed and graded as normal, hyperplasia, dysplasia and carcinoma by Kramer et al. (1978).
Immunohistochemistry
Serial sections of 4 µm were deparaffinized in xylene and rehydrated in graded ethanol, then pre-treated in a microwave (Eletrolux, SP, Brazil) with 10 mm citric acid buffer (pH = 6) for three cycles of 5 min each at 850 W for antigen retrieval. They were pre-incubated with 0.3% hydrogen peroxide in phosphate-buffered saline (PBS) for 5 min for inactivation of endogenous peroxidase and then blocked with 5% normal goat serum in PBS for 10 min. The specimens were then incubated with either anti-bcl-2 polyclonal antibody (N-19, Santa Cruz Biotechnology, CA, USA) at a concentration of 1:50 or anti-bax polyclonal antibody (P-19, Santa Cruz Biotechnology) at a concentration of 1:200. All incubations were carried out overnight at 4 °C within the refrigerator. This was followed by two washes in PBS for 10 min. The sections were then incubated with biotin-conjugated secondary antibody anti-mouse immunoglobulin G (IgG) for bcl-2 (Vector Laboratories, Burlingame, CA, USA) at a concentration of 1:200 or anti-rabbit IgG for bax (Vector Laboratories) at a concentration of 1:200 in PBS for 1 h. After that, the sections were washed twice with PBS followed by the application of preformed avidin biotin complex conjugated to peroxidase (Vector Laboratories) for 45 min. The bound complexes were visualized by the application of a 0.05% solution of 3-3′-diaminobenzidine solution and counterstained with haematoxylin. For control studies of the antibodies, the serial sections were treated with mouse IgG (Vector Laboratories) at a concentration of 1:200 or rabbit IgG (Vector Laboratories) at a concentration of 1:200 in place of the primary antibody.
Quantification of immunohistochemistry
Sections stained using immunohistochemistry were analysed for the percentages of immunopositive cells in areas diagnosed as normal, hyperplasia, dysplasia and carcinoma under the optical microscope. A total of 1000 epithelial cells were evaluated in 3-5 fields at ×400 magnification. These values were used as labelling indices. To determine the apoptotic ratio, bcl-2/bax expression ratio was calculated using the previously obtained individual percentages.
Statistical methods
Statistical analysis was performed by Kruskal-Wallis nonparametric test using spss software pack (version 1.0). P value <0.05 was considered for statistical significance.
Results
Histopathological evaluation following 4NQO treatment
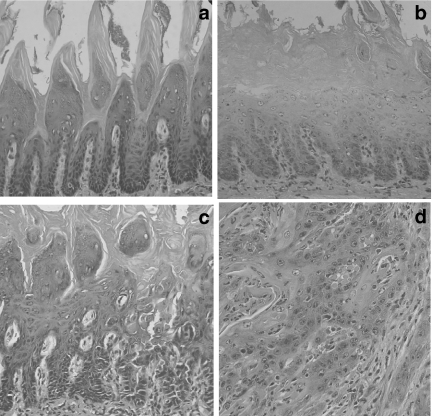
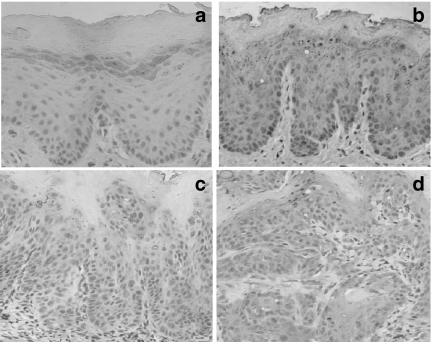
No histopathological changes in epithelial cells were observed in the control group (Figure 1a) nor after 4-week treatment with 4NQO. The primary histopathological change, i.e. hyperplasia and hyperkeratosis with the spinous cell layer gradually thickened, was evidenced after 12-week treatment (Figure 1b). In this period, epithelial dysplasia was also found in mild and moderate forms (Figure 1c). At 20 weeks, squamous cell carcinoma was found in the majority of animals. The histopathological grade of the carcinomas was usually squamous cell carcinoma of a well-differentiated type (Figure 1d). The tumours spread into the submucosa and underlying muscle layer, forming small nests with typical keratin pearl formation. In advanced cases, severe atypia was frequently found. The histopathological findings are summarized in Table 1.
Figure 1.
Photomicrographies showing the multistep process of rat tongue carcinogenesis: (a) no histopathological change (control); (b) hyperplasia and hyperkeratosis, (c) epithelial dysplasia and (d) squamous cell carcinoma of well-differentiated type. (Hematoxylin and Eosin stain; ×100 magnification).
Table 1.
Incidence of histopathological lesions in tongue of rats in the 4-nitroquinoline 1-oxide (4NQO)* model for oral carcinogenesis
| Lesions | |||||
|---|---|---|---|---|---|
| Groups (week) | Number of animals | Normal | Hyperplasia | Dysplasia | Carcinoma |
| 0 | 10 | 10 | 0 | 0 | 0 |
| Control | |||||
| 4 | 10 | 10 | 0 | 0 | 0 |
| 12 | 10 | 0 | 7 | 3 | 0 |
| 20 | 10 | 0 | 0 | 3 | 7 |
4NQO - 50 ppm by drinking water.
Immunohistochemistry
Immunohistochemical data for bcl-2 and bax are shown in Figures 2 and 3, respectively. Immunostaining for both the markers were detected in the cytoplasm with a granular pattern. In the normal epithelium, represented by the control group, immunostaining with anti-bcl-2 monoclonal antibody was weak and only identified in the basal and suprabasal cell layers (Figure 4a). In contrast, staining with anti-bax antibody was seen in the superficial layers of the epithelium (Figure 5a).
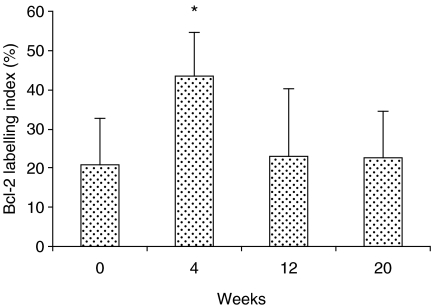
Figure 2.
Bcl-2 labelling index in the negative control (zero) and those exposed to 4-nitroquinoline 1-oxide for 4, 12 and 20 weeks. Values were expressed as mean ± SD. *P < 0.01 when compared to negative control group.
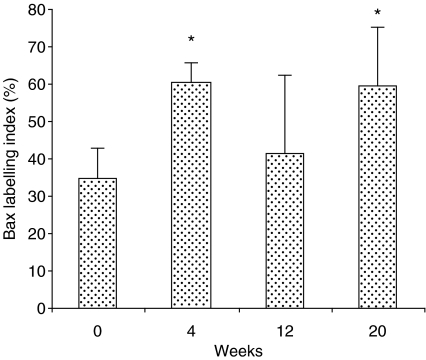
Figure 3.
Bax labelling index in the negative control (zero) and those exposed to 4-nitroquinoline 1-oxide for 4, 12 and 20 weeks. Values were expressed as mean ± SD. *P < 0.01 when compared to negative control group.
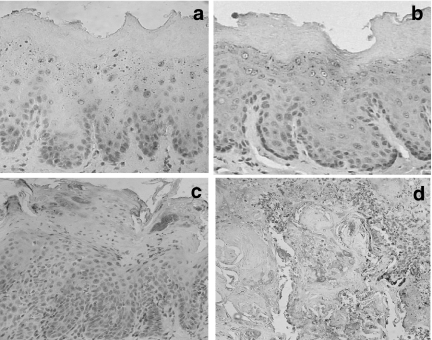
Figure 4.
Immunostaining for bcl-2: (a) rat control epithelium; (b) epithelium of the rat 4 weeks after the initiation of 4-nitroquinoline 1-oxide administration; (c) dysplastic lesion after 12 weeks of carcinogen administration and (d) squamous cell carcinoma of well-differentiated type (×400).
Figure 5.
Imunostaining for bax: (a) rat control epithelium; (b) epithelium of the rat 4 weeks after oral administration of 4NQO; (c) dysplastic lesion after 12 weeks following 4-nitroquinoline 1-oxide administration and (d) squamous cell carcinoma of well-differentiated type (×400).
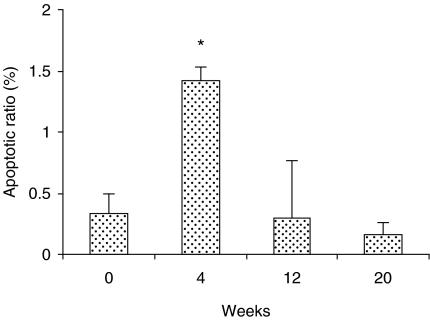
Although no histological changes were induced in the epithelium after 4 weeks of carcinogen exposure, bcl-2 and bax were over-expressed in all layers of the epithelium indistinctly, i.e. basal, prickle, granular and superficial layers (Figures 4b and 5b, respectively). The expression levels were the same in all layers of epithelium for both the antibodies used (bcl-2 or bax). Following 12 weeks of carcinogen administration, the rat tongues showed early proliferative changes of the epithelium characterized by hyperplasia and hyperkeratosis. However, there are no changes in the expression level of either bcl-2 or bax, but the distribution of bcl-2 is altered, being restricted to the superficial layers of the epithelium (Figure 4c), whereas the distribution of bax remains similar in both controls and animals following treatment (Figure 5c). In well-differentiated squamous cell carcinomas induced after 20 weeks of treatment with 4NQO, bcl-2 was expressed in some cells of tumour islands, and staining was sometimes absent (Figure 4d). On the other hand, immunostaining for bax was widely observed at the tumour nests (Figure 5d). In the negative immunohistochemical controls, no staining was seen for either antibody (Figure 6a,b). The labelling index for bcl-2 and bax is shown in Figure 7. Bcl-2 and bax ratio showed an increase (P < 0.05) after 4 weeks of 4NQO administration. With respect to 12 and 20 weeks, no statistically significant differences (P > 0.05) were found in labelling index for bcl-2 and bax.
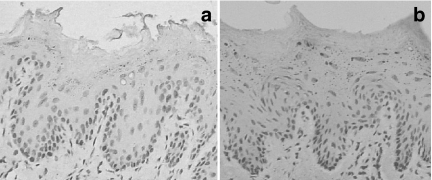
Figure 6.
Negative immunohistochemical controls shown to be unstained. (a) bcl-2 and (b) bax. (×400).
Figure 7.
Apoptotic index depicted by bcl-2/bax ratio in the negative control and those rats treated with 4-nitroquinoline 1-oxide. Values were expressed as mean ± SD. *P < 0.05 when compared to control (zero).
Discussion
The aim of chemical carcinogenesis models is to provide further understanding concerning the multistep process of carcinogenesis in order to investigate noxious activities caused by environmental agents as well as to develop strategies for early diagnostic and/or cancer prevention. Several medium-term duration assay systems for oral carcinogenesis offer particular promise. One of these models is DMBA-induced carcinoma produced in the hamster cheek pouch. Because of its peculiar anatomic site and histology, some authors have questioned the suitability of the hamster cheek pouch for studies related to oral tumours (Nauta et al. 1995). In this regard, the Wistar rat is a good alternative because of its ease of handling and availability. Furthermore, 4NQO-induced rat tongue tumours are more realistic, because they reveal histological and biochemical characteristics similar to human tumours (Ohne et al. 1985; Nishimura 1999; Niwa et al. 2001; Okazaki et al. 2002; Vered et al. 2003). Thus, in this study, 4NQO was used to induce tongue carcinogenesis in Wistar rats. Our results clearly demonstrated that 4NQO in drinking water induced histopathological changes in tongue mucosa along a time-course experiment from normal epithelium, hyperplasia and hyperkeratosis, premalignant dysplasia, and carcinoma in situ to invasive squamous cell carcinoma.
In this study, we evaluated the expressivity of bcl-2 and bax following 4NQO administration in order to determine whether the deregulation of apoptosis may be associated with oral tumourigenesis phase by phase. In normal oral epithelia, represented by the control group, bcl-2 protein presence was restricted to the basal and suprabasal layers. This suggests that bcl-2 participates in the control of the terminal differentiation of keratinocytes by protecting their stem cells from apoptosis (Harada et al. 1998). On the other hand, our results showed abnormal expression of bcl-2 in ‘normal’ oral mucosa 4 weeks following the initiation of 4NQO administration. These findings are in agreement with other studies which have also demonstrated an up-regulation of bcl-2 in oral mucosa cells 4 weeks after 4NQO administration in rats (Nishimura 1999).
In humans, only a small portion of initial lesions develops oral carcinomas. Therefore, the challenge is to identify which lesions have a real malignant potential. It is conceivable that, during the multistep progression of cancer, accumulated genetic damage results in the loss of the ability to appropriately regulate cell proliferation, so that maintenance of the equilibrium between cell proliferation and cell deaths depends upon prompt apoptotic elimination of damaged and abnormally proliferating cells (Polverini & Nor 1999). In this study, bcl-2 was expressed in the superficial layer of epithelium in oral epithelial dysplasias. These results concur with other studies investigating pre-cancerous lesions in the colorectal, oral cavity, stomach and oesophagus (Lauwers et al. 1994; Sinicrope et al. 1995; Nishimura 1999; Okazaki et al. 2002). However, others have reported a sporadic bcl-2 expression or lack of expression for bcl-2 in oral dysplasias (Schoelch et al. 1999; McAlinden et al. 2000; Loro et al. 2002). On the basis of our results, we postulated that bcl-2 over-expression also appears to be associated with the progression of oral cancer. Oral cancer and, in particular, squamous cell carcinoma has been repeatedly linked to dysregulation of apoptosis (Gastman 2001). Regarding the oral squamous cell carcinomas 4NQO-induced herein, the expressivity of bcl-2 was weak. Some studies have addressed an over-expression of bcl-2 in poorly differentiated carcinomas (Jordan et al. 1996; Chen et al. 2000). In this study, the oral squamous cell carcinomas were of a well-differentiated type. This might explain the discrepancies between these findings.
Bax interacts with bcl-2 in its regulation of apoptosis by the formation of bcl-2/bax heterodimers (Oltvai et al. 1993). In the present study, the expression of bax increased after 4 weeks of 4NQO chronic administration. This increase was unexpected and was regarded as a result of homeostatic pathways against the abnormal expression of bcl-2. In well-differentiated cell carcinomas, there was a general increase of bax expression at the tumour nests. Some authors have pointed out very weak or absent immunostaining for bax in the poorly differentiated oral carcinomas, suggesting therefore that bax may play a role in the differentiation of carcinoma cells (Jordan et al. 1996; Chen et al. 2000). Our results are consistent with the above reports. Considering that the bcl-2/bax ratio reflects the regulation of apoptosis more clearly than the detection of either bcl-2 or bax separately (Korsmeyer et al. 1993), we were able to calculate the bcl-2/bax ratio here. The labelling index for bcl-2 and bax showed an increase after only 4 weeks of 4NQO administration. This finding suggests a preferential expression of bcl-2, which is thought to protect cells from apoptosis and render them susceptible to mutations and tumour progression (Jordan et al. 1996; Polverini & Nor 1999).
In conclusion, our results suggest that abnormalities in the apoptosis pathways are associated with the development of persistent clones of mutated-epithelial cells in the oral mucosa. Bcl-2 and bax expression appears to be associated with a risk factor in the progression of oral cancer. However, this suggestion should be verified by further investigation.
Acknowledgments
The authors are thankful to Paulo Roberto Cardoso and Maria Luiza Falaguera Ardanaz for their technical assistance. The authors are also grateful to Prof Luis Fernando Barbisan for his suggestions. This work was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant number: 141501/2002-2), FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, Grant number: 02/09454-0) and TOXICAN (Núcleo de Avaliação Toxicogenética e Cancerígena). This study was part of the PhD thesis of Daniel A. Ribeiro.
References
- Akao Y, Otsuki Y, Kataoka S, Ito Y, Tsujimoto Y. Multiple subcellular localization of bcl-2: detection in nuclear outer membrane, endoplasmic reticulum membrane, and mitochondrial membranes. Cancer Res. 1994;54:2468–2471. [PubMed] [Google Scholar]
- Arends MJ, Wyllie AH. Apoptosis: mechanisms and roles in pathology. Int. Rev. ExpPathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Chen Y, Kayano T, Takagi M. Dysregulated expression of bcl-2 and bax in oral carcinomas: evidence of post-transcriptional control. J. Oral Pathol. Med. 2000;29:63–69. doi: 10.1034/j.1600-0714.2000.290203.x. [DOI] [PubMed] [Google Scholar]
- Gastman BR. Apoptosis and predisposition to oral cancer. Head Neck. 2001;23:409–425. doi: 10.1002/hed.1052. [DOI] [PubMed] [Google Scholar]
- Gazzaniga P, Gallucci M, Gradilone A, et al. Detection of BCL-2 RNA in low grade tumours of the urinary bladder. EurJCancer. 1995;31A:2119–2120. doi: 10.1016/0959-8049(95)00404-1. [DOI] [PubMed] [Google Scholar]
- Hahn M, Hagedorn G, Kuhlisch E, Schackert HK, Edkelt U. Genetic polymorphisms of drug-metabolizing enzymes and susceptibility to oral cavity cancer. Oral Oncol. 2002;38:486–490. doi: 10.1016/s1368-8375(01)00086-0. [DOI] [PubMed] [Google Scholar]
- Harada H, Mitsuyasu T, Seta Y, Maruoka Y, Toyoshima K, Yasumoto S. Overexpression of bcl-2 protein inhibits terminal differentiation of oral keratinocytes in vitro. J. Oral Pathol. Med. 1998;27:11–17. doi: 10.1111/j.1600-0714.1998.tb02084.x. [DOI] [PubMed] [Google Scholar]
- Ikegaki N, Katsumata M, Minna J, Tsujimoto Y. Expression of bcl-2 in small cell lung carcinoma cells. Cancer Res. 1994;54:6–8. [PubMed] [Google Scholar]
- Jordan RC.K, Catzavelos GC, Barrett AW, Speight PM. Differential expression of bcl-2 and bax in squamous cell carcinomas of the oral cavity. Oral Oncol. 1996;32B:394–400. doi: 10.1016/s0964-1955(96)00033-4. [DOI] [PubMed] [Google Scholar]
- Kapucuoglu N, Losi L, Eusebi V. Immunohistochemical localization of Bcl-2 and Bax proteins in in situ and invasive duct breast carcinomas. Virchows Arch. 1997;430:17–22. doi: 10.1007/BF01008011. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- Krajewska M, Fenoglio-Preiser CM, Krajewski S, et al. Immunohistochemical analysis of Bcl-2 family proteins in adenocarcinomas of the stomach. Am. J. Pathol. 1996;149:1449–1457. [PMC free article] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am. J. Pathol. 1994;145:1323–1336. [PMC free article] [PubMed] [Google Scholar]
- Kramer IR, Lucas RB, Pindborg JJ. Definition of leukoplakia and related lesions: an aid to studies on oral precancer. Oral Surg. Oral Med. Oral Pathol. 1978;46:518–539. [PubMed] [Google Scholar]
- Lauwers GY, Scott GV, Hendricks J. Immunohistochemical evidence of aberrant bcl-2 protein expression in gastric epithelial dysplasia. Cancer. 1994;73:2900–2904. doi: 10.1002/1097-0142(19940615)73:12<2900::aid-cncr2820731205>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Loro LL, Johannessen AC, Vintermyr OK. Decreased expression of bcl-2 in moderate and severe oral dysplasias. Oral Oncol. 2002;38:691–698. doi: 10.1016/s1368-8375(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Lu QL, Abel P, Foster CS, Lalani EN. Bcl-2: role in epithelial differentiation and oncogenesis. HumPathol. 1996;27:102–110. doi: 10.1016/s0046-8177(96)90362-7. [DOI] [PubMed] [Google Scholar]
- Lu QL, Elia G, Lucas S, Thomas JA. Bcl-2 proto-oncogene expression in Epstein-Barr-virus-associated nasopharyngeal carcinoma. IntJCancer. 1993;53:29–35. doi: 10.1002/ijc.2910530107. [DOI] [PubMed] [Google Scholar]
- Magrath I, Litvak J. Cancer in developing countries: opportunity and challenge. J. Natl. Cancer Inst. 1993;85:862–874. doi: 10.1093/jnci/85.11.862. [DOI] [PubMed] [Google Scholar]
- Manetto V, Lorenzini R, Cordon-Cardo C, et al. Bcl-2 and Bax expression in thyroid tumours. An immunohistochemical and western blot analysis. Virchows Arch. 1997;430:125–130. doi: 10.1007/BF01008033. [DOI] [PubMed] [Google Scholar]
- McAlinden RL, Maxwell P, Napier S, et al. Bcl-2 expression in sequential biopsies of potentially malignant oral mucosal lesions assessed by immunocytochemistry. Oral Dis. 2000;6:318–326. doi: 10.1111/j.1601-0825.2000.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Nauta JM, Roodenberg JL.N, Nikkels PG.J, Witjes MJ.H, Vermey A. Comparison of epithelial dysplasia - the 4NQO rat palate model and human oral mucosa. Int. J. Oral Maxillofac Surg. 1995;24:53–58. doi: 10.1016/s0901-5027(05)80857-4. [DOI] [PubMed] [Google Scholar]
- Nishimura A. Changes in Bcl-2 and Bax expression in rat tongue during 4-nitroquinoline 1-oxide-induced carcinogenesis. J. Dent. Res. 1999;78:1264–1269. doi: 10.1177/00220345990780061101. [DOI] [PubMed] [Google Scholar]
- Niwa S, Ueno S, Shirasu R. Alteration of pRB expression in the development of rat tongue carcinoma induced by 4-nitroquinoline 1-oxide. Oral Oncol. 2001;37:579–585. doi: 10.1016/s1368-8375(00)00141-x. [DOI] [PubMed] [Google Scholar]
- Nylander K, Schildt EB, Eriksson M, Roos G. PCNA, Ki-67, p53, bcl-2 and prognosis in intraoral squamous cell carcinoma of the head and neck. AnalCell Pathol. 1997;14:101–110. doi: 10.1155/1997/591251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohne M, Satoh T, Yamada S, Takai H. Experimental tongue carcinoma of rats induced by oral administration of 4-nitroquinoline 1-oxide (4NQO) in drinking water. Oral Surg. Oral Med. Oral Pathol. 1985;59:600–607. doi: 10.1016/0030-4220(85)90189-6. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Tanaka Y, Tonogi M, Yamane G. Investigation of environmental factors for diagnosing malignant potential in oral epithelial dysplasia. Oral Oncol. 2002;38:562–573. doi: 10.1016/s1368-8375(01)00119-1. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Polverini PJ, Nor JE. Apoptosis and predisposition to oral cancer. Crit. Rev. Oral BiolMed. 1999;10:139–152. doi: 10.1177/10454411990100020201. [DOI] [PubMed] [Google Scholar]
- Ravi D, Nalinakumari KR, Rajaram RS, Nair MK, Pillai MR. Expression of programmed cell death regulatory p53 and bcl-2 proteins in oral lesions. Cancer Lett. 1996;105:139–146. doi: 10.1016/0304-3835(96)04258-9. [DOI] [PubMed] [Google Scholar]
- Schoelch ML, Le QT, Silverman S, Jr, et al. Apoptosis-associated proteins and the development of oral squamous cell carcinoma. Oral Oncol. 1999;35:77–85. doi: 10.1016/s1368-8375(98)00065-7. [DOI] [PubMed] [Google Scholar]
- Singh BB, Chandler FW, Jr, Whitaker SB, Forbes-Nelson AE. Immunohistochemical evaluation of bcl-2 oncoprotein in oral dysplasia and carcinoma. Oral Surg. Oral Med. Oral PatholOral RadiolEndod. 1998;85:692–698. doi: 10.1016/s1079-2104(98)90037-3. [DOI] [PubMed] [Google Scholar]
- Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. Bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237–241. [PubMed] [Google Scholar]
- Tanaka T, Kohno H, Sakata K, et al. Modifying effects of dietary capsaicin and rotenone on 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. Carcinogenesis. 2002;23:1361–1367. doi: 10.1093/carcin/23.8.1361. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Jaffe E, Cossman J, Gorham J, Nowell PC, Croce CM. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985;315:340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- Vered M, Daniel N, Hirshberg A, Dayan D. Histomorphologic and morphometric changes in minor salivary glands of the rat tongue during 4-nitroquinoline 1-oxide-induced carcinogenesis. Oral Oncol. 2003;39:491–496. doi: 10.1016/s1368-8375(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Xi S, Grandis JR. Gene therapy for the treatment of oral squamous cell carcinoma. J. Dent. Res. 2003;82:11–16. doi: 10.1177/154405910308200104. [DOI] [PubMed] [Google Scholar]