Abstract
Using several techniques, we have assessed morphological characteristics of a malignant thymic tumour in SV12 transgenic (Tg) mice expressing SV40 T and t antigens under control of an L-PK promoter. We describe the development of a carcinoma originating from thymic hyperplasia and followed by the formation of a benign tumour composed chiefly of medullary epithelial cells expressing the transgene and of lymphocytes, a pathology very rarely reported in mice. Our study of the SV12 Tg mice represents the first description of a model of a pure malignant thymic tumour associated with extensive angiogenesis maintained in numerous descendants. The formation of a large tumoral neovascular network, observed here, has never been described in human and/or experimental thymic tumours. Tumoral transformation and angiogenesis are demonstrated by immunolabelling with antibodies against various cytokeratins (CKs) of different molecular weights, vascular endothelial cell markers and VEGF/receptor-2 (Flk-1) present on the neovascular endothelial cells. Different points raised by the originality of this model are discussed. These include the medullary nature of the cells expressing the SV40 transgene and their relationship with the tumoral development. The subset of different molecular weight CK components and their modifications are also considered, as well as the presence of type IV epithelial cells, progenitors of medullary epithelial cells. Finally, the cell signals involved in angiogenesis and the possible action of an angiogenic factor, probably secreted by the tumoral cells themselves, are discussed.
Keywords: carcinoma, electron microscopy, immunochemistry, neovascularization, precursor of thymic epithelial cells, thymic epithelial tumour, transgenic mice
The spontaneous development of malignant thymic neoplasms has been rarely reported in laboratory animals and never in mice (Murray et al. 1985; Naylor et al. 1988; Tamano et al. 1988; Frith et al. 1993). In rats, thymic hyperplasia and so-called thymomas and/or lymphomas have been described in various strains, but the distinction between these different entities is unclear, and carcinomas have been reported only occasionally (Naylor et al. 1988). Some thymomas have been described in rodents after induction by chemical carcinogens, radiation and/or virus injection but these tumours appear in fact to be lymphomas located in the thymus (Matsuyama et al. 1975; Hoot and Kettman 1989). For these reasons, practically all published descriptions and attempts at classification in animals have been, and are still, made with reference to human thymic neoplasms (Rosai and Levine 1976; Ho et al. 1994; Muller-Hermelink et al. 1994; Kornstein and Deblois 1995; Kuo and Chan 1998; Suster and Moran 1998,1999).
In models of targeted transgenic (Tg) mice using oncogenes, although animals may be expected to develop tumours in a particular organ, results are not always as planned (Hanahan 1986; Adams and Cory 1991). Thus, Tg mice harbouring the T antigen of simian virus 40 (SV40 Tag) develop various pathologies, often with neoplastic transformation (Van Dyke et al. 1987), and provide the opportunity to study the biological effects of gene expression under physiological conditions that cannot be reproduced in culture. It appears that a pure malignant tumour of the thymus, present in all mice harbouring the transgene in numerous generations and in the absence of tumours in other organs, is difficult to obtain in Tg mice. Using different constructions with fragments of SV40 Tag, various promoters/enhancers, thymic hyperplasia and/or a so-called thymoma (Brinster et al. 1984; Palmiter et al. 1985; Small et al. 1985; Botteri et al. 1987; Messing et al. 1988; Reynolds et al. 1988; Moll et al. 1992; Teitz et al. 1995) have been occasionally observed but were always associated with tumours in various other organs. Only one group, using SV40 Tag with its own promoter (Park et al. 1996; Lee et al. 1998), has reported the rapid development of a malignant thymic tumour in a few mice in several experimental series. The progressive development of a cortical thymoma arising from thymic hyperplasia and which transformed by 4 months into a carcinoma was reported. No angiogenesis was noted, and the mice died after a period of about 5 months.
Here we describe a new model which provides the opportunity to study a pure, reproducible tumour of the thymus. This tumour appears in the SV12 mouse line, obtained by transgenesis of SV40 T and t antigens with l-pyruvate kinase as the enhancer/promoter. In numerous series and generations, all mice expressing the transgene consistently develop thymic tumours (Nabarra et al. 2002).
This Tg mouse, named SV12 (see Materials and methods), first develops hyperplasia of both the thymic epithelium and the thymocytes, followed by a grossly visible medullary epithelial tumour, which progressively acquires malignant characteristics and is identified, in the final phase, as a carcinoma. Furthermore, we observe in SV12 Tg mice the development of extensive angiogenesis in association with the neoplastic transformation. Angiogenesis begins during the hyperplastic phase and largely develops during the malignant transformation. This is surprising because, in the published descriptions in human and in rare experimental thymic tumours, angiogenesis is rarely mentioned in contrast to observations in neoplastic processes in numerous other organs (Schor and Schor 1983; Hanahan and Folkman 1996; Hanahan 1997).
In a previous paper, we have described the first phases of modification of the microenvironment, which involve alterations in the number and phenotype of thymocytes. Indeed, the total number of thymocytes is increased, as well as the proportion of mature thymocytes and alterations of export function towards the periphery are detected (Nabarra et al. 2002).
Data presented here are based on different morphological strategies, used in human and in rare experimental diagnoses (Cooper et al. 1985; Kirchner and Muller-Hermelink 1989; Ward and Rehm 1990), and chosen to study tumoral transformation of epithelial tissues and angiogenesis. Thus, after different preliminary studies, cited in the next section, and considering the thymus as a complex tissue with several types of epithelial cells containing typical cytokeratin (CK) components of different molecular weights (Franke et al. 1981; Loning et al. 1982; Brekelmans and Van Ewijk 1990; Boyd et al. 1993), whose expression is modified in the tumoral process in relation with the stages of differentiation (Tseng et al. 1982; Debus et al. 1984; Ramaekers et al. 1985; Fukai et al. 1993), we have focused our study on the analysis of the CKs. Furthermore, most carcinomas originating from simple epithelial organs stain strongly with antibodies against one type of low-molecular-weight keratin, CK18 (Franke et al. 1981; Debus et al. 1984; Savino and Dardenne 1988; Fukai et al. 1993).
The characterization of the construction of neovessels appeared important in the description of this new experimental tumour model, as this process is often used as a criterion of carcinogenesis in tumours of various organs.
Angiogenesis is the formation of new vessels. This phenomenon is prominent during embryonic development and in the early postnatal period and rare in normal conditions in the adult mammal except in the female reproductive tract (Folkman and Klagsbrun 1987; Klagsbrun and D'Amore 1991; Senger et al. 1993). It is now agreed that the complex process of neoplastic transformation and the progressive growth of a solid tumour is associated with de novo formation of vessels by capillary sprouting, one of the mechanisms evoked during the initial stages of neoplasia and carcinogenesis (Schor and Schor 1983; Bouck 1992; Hanahan and Folkman 1996). We note that another process of neoformation of vessels, more recently described, is intussusceptive angiogenesis (Carmeliet 2000; Burri and Djonov 2002).
The growth of numerous solid tumours occurring in association with the recruitment of new blood vessels has been described in various organs, but not in the thymus (Rosai and Levine 1976; Schor and Schor 1983; Muller-Hermelink et al. 1994; Hanahan and Folkman 1996). According to these authors, the appearance of neovessels is thought to be initiated by local activation of genes encoding diffusible angiogenic factors. These secreted factors, in turn, initiate a cascade of responses that result in the growth of new capillaries directed towards the site emitting the angiogenic signals. It is strongly suggested that the tumour cells themselves are involved in this secretion.
Among several factors now known to induce the process of angiogenesis, vascular endothelial growth factor (VEGF) is specifically implicated in the tumoral process of neovascularization (Folkman and Klagsbrun 1987; Plate et al. 1992; Klagsbrun and Soker 1993; Senger et al. 1993; Ferrara 1995). This factor acts by binding with high affinity to several receptors identified on the endothelial cells. It appears that VEGF and its receptors (Ftl and Flk-1) are not present in normal adult mammal (except cyclically in the female reproductive tract). However, an important increase in levels of VEGF and its receptors have been observed in tumoral processes and/or carcinoma (Leung et al. 1989; Connolly et al. 1989; Risau et al. 1991; Jakeman et al. 1992; Neufeld et al. 1994; Dvorak et al. 1995).
We present here, for the first time, in addition to the identification of a carcinoma, our preliminary observations concerning the presence of a specific receptor Flk-1 (VEGF/R2), one of a group of factors known for their implication in angiogenesis related to tumoral progression (Millauer et al. 1993; Shalaby et al. 1997). We demonstrate this phenomenon by clear labelling of neoendothelial cells with a specific antibody against Flk1. However, a more detailed study should be performed in the future to characterize the exact role of the tumoral cells in the angiogenic process.
The specificity and originality of the tumours which develop in the SV12 model prompted us to report on the different morphological parameters of neoplastic transformation and the well-organized development of new blood vessels.
Materials and methods
Transgenic mice
The SV12 Tg line, derived from a CBA strain, of H-2k background, was first generated at Cochin Hospital by the group of A. Khan and has since been maintained in our laboratory. The transgene contains T and t antigens from SV40 (a 2.7 kb fragment of simian virus) under the control of a pyruvate kinase promoter (fragment Cla I/EcoRV) and SV40 enhancer (fragment 270–95 nt). Newly integrated sequences were identified after hybridization of tail DNA with a probe for SV40 Tag. The lineage obtained is named SV12 because 12 copies are integrated in the genome. SV12 females are not fertile, and so the line is maintained by crossing heterozygous SV12 Tg males with CBA females.
This SV12 line is characterized by a rapidly growing thymic tumour: thymus weight is 1.6 g at 16 weeks (i.e. 10–30 times bigger than the normal thymus), whereas in age-matched controls it never exceeds 0.050 g The SV12 mice die around 5–6 months of age.
Twelve Tg mice of each of the following ages were studied: 3 weeks and 1, 1.5, 2, 2.5, 3, 3.5, 4 and 5 months. Few mice survived up to 6 months and so only four were available for study at this age. Non-Tg age-matched littermates were used as controls, as well as age-matched normal mice of the background strain.
Experimental design
This morphological characterization of malignant transformation and angiogenesis was carried out by light microscopy (histology and immunohistochemical identification of different antigens) and electron microscopy, techniques whose significant diagnostic power in the neoplastic process is well established.
In preliminary studies, an important panel of different antibodies, used in human and experimental pathology, was tested: antibodies against Ia, vimentin, CD5 and CD105 (both considered to be involved in epithelial carcinoma) and carcino-embryonic antigen (CEA). The results obtained with these various antibodies were difficult to interpret were not always reproducible, and we are not certain (for CEA) that the cross-reaction between human antibodies and mice antigens is always efficient.
We thus chose to study CKs of different molecular weights, present in abundance in the epithelial thymus and which provide clear-cut results in the definition of epithelial carcinoma (Espinoza and Azar 1982; Moll et al. 1983; Ramaekers et al. 1985). A panel of antibodies against total CK (40–65 kDa), against several CKs of low molecular weight (40–56 kDa, corresponding to a simple epithelium) and high molecular weight (57–67 kDa, corresponding to a more stratified epithelium) and, finally, an antibody specific for a limited subset of CKs (CK18, 45 kDa), which has been reported to be a marker of neoplasia (Ramaekers et al. 1985; Savino and Dardenne 1988; Fukai et al. 1993), was used. Furthermore, the use of an antibody against CK5/8, described recently as a marker of thymic epithelial precursors (Klug et al. 1998,2002), permits detection of the presence of this type of cell characterized in electron microscopy.
Regarding angiogenesis, the formation of neovessels from the pre-existing vascular network involves, in the case of a solid tumour, the activation of sprouting and proliferation of endothelial cells by triggering the receptors present on the surface of these cells. Thus, we studied vessels in their phase of construction by labelling with different classical antibodies specific for vascular endothelial cells: CD34 and CD31 antibodies (Fina et al. 1990; Schlingemann et al. 1990; Longacre and Rouse 1994; De Lisser et al. 1997) and with another important antibody raised against one of the VEGF receptors (VEGF/R2, Flk-1), expressed on vascular endothelial cells, and always present in tumours in increased levels (Millauer et al. 1993; Quinn et al. 1993; Shalaby et al. 1997).
Antibodies
Tumoral transformation was thus studied using the following primary antibodies.
For the study of oncogene localization.
MTS10 specific of epithelial medullary cells (Boyd et al. 1993) and the anti-SV40 T antigen (Pharmingen (Becton-Dickinson, Paris, France)) were used in double labelling.
For studies of keratins.
Antitotal keratin, 40–65.5 kDa (Novacastra-Tebu, Paris, France), antilow (56-52-45 kDa) and high (57–63 kDa) molecular weight keratins, antibody against a 45 kDa molecular weight keratin (CK18) (Immunotech, Marseilles, France); antibody against CKs 5 and 8: 58–52 kDa (Novacastra-Tebu).
For studies of angiogenesis.
Anti-CD31, anti-CD34 and the antibody against VEGF/receptor 2 (anti-Flk-1) (Pharmingen).
Apart from the revelation of antibodies against total keratins, which was done using RAM-FITC, all the other second steps used RAM-biotin and SAV-HRP (streptavidin horseradish peroxidase) (Immunotech) or TSA BiotinSystem (NEN Life-Sciences, Boston, MA, USA). Revelation of antibodies coupled with peroxidase was performed with 3-3′-diaminobenzidine as substrate (Fast DAB, Sigma, St Quentin Fallavier, France).
Morphological Techniques
Thymic tissues were removed and small fragments were immediately prepared for conventional histology, immunohistolabelling and electron microscopy according to standard techniques.
For histology.
Fragments were fixed in Bouin solution, dehydrated and embedded in paraffin. Four-micron sections were stained with haematoxylin–eosin and Masson's trichrome.
For electron microscopy.
Thymic fragments were fixed in 2% glutaraldehyde in cacodylate buffer, washed using the same buffer, postfixed in 2% osmium tetroxide, dehydrated and embedded in Epon. Ultrathin sections of 800Å were impregnated with uranyl acetate and lead citrate. Ten blocks per mouse were examined.
For immunolabelling.
Thymic fragments were frozen in 2-methyl butane (Merck, Fontenay sous Bois, France) prechilled by liquid nitrogen, fixed for 10 min in acetone and air-dried. Rehydration and washing of the sections were performed in PBS, pH 7.4, containing 5% newborn calf serum. The first step involved a 1-h incubation with specific antibodies, followed by a 1-h incubation with secondary antibodies labelled with fluorescein or peroxidase.
Controls were performed, firstly, by omitting the primary antibody on the sections and, secondly, by performing all steps on the thymus of littermates which did not express the transgene.
Results
In the thymus of SV12 Tg mice, progressive stages of morphological modifications are observed with age. The early phases, comprising hyperplasia and the initiation of tumoral development (postnatal to around 2 months), have been previously described in relation with lymphocyte maturation (Nabarra et al. 2002). After a brief summary of the morphological characteristics of this hyperplasia, which is rapidly followed by the development of a benign epithelial medullary tumour, we provide new results concerning the expression of CKs in these two early phases. Then, we describe the tumoral transformation with acquisition of signs of malignancy. The frank development of the malignant tumour occurs simultaneously with the formation of a network of neovessels.
Tumorigenesis
Hyperplasia and the early phase of tumoral development.
About 3–4 weeks after birth, hyperplasia concerns both the stromal cells and the thymocytes. The thymus appeared increased in size with a normal architecture. Examination of tissue sections showed increased numbers of hypertrophied stromal cells which were thus more visible by microscopy with numerous lymphocytes. Mitotic figures were also present in large numbers. The two parenchymal zones, the cortex and the medulla, exhibited normal organization during this time. Few neovessels appeared at the end of the hyperplastic phase (6 weeks).
Later on, at around 2 months of age, a considerable expansion of the medullary epithelial cells was observed. The normal thymic organization in two zones was totally disrupted by the extensive progression of epithelial proliferation, clearly identified as being medullary cells (by labelling with a specific antibody, MTS10), which had invaded the cortical areas and occupied almost all zones, reducing the cortex to an irregular band (Figure 1a). In double labelling, the transgene was visualized by SV40 Tag antibody in the nucleus of these MTS10-positive epithelial cells, scattered throughout the parenchyma (Figure 1b). The presence of clear, round, eosinophilic cells, infiltrating the stromal microenvironment, scattered in small groups in the stroma, was noted, as well as numerous mitoses (Figure 1c).
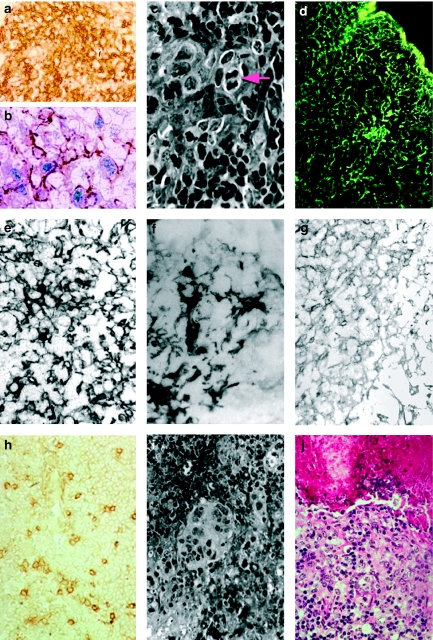
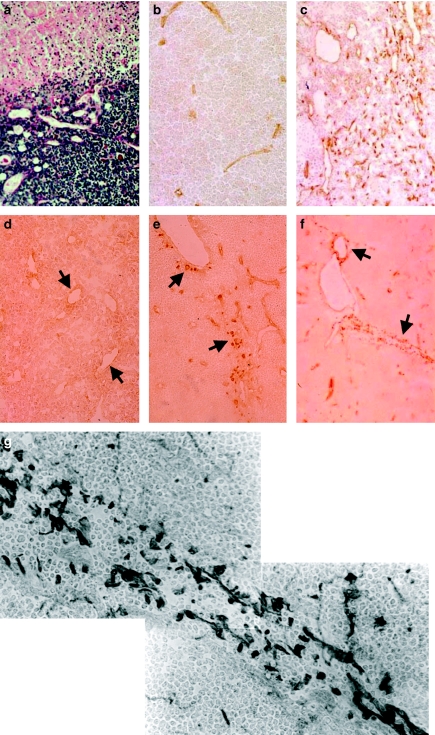
Figure 1.
Tumorigenesis by light microscopy. (a) Immunolabelling with MTS10 (antibody specific of the thymic medullary zone). Evidence of invasion of the cortical zone (capsule, at top left) by medullary cells. One-month-old SV12 transgenic (Tg) mouse, ×250. (b) Double immunolabelling of epithelial cells. The cells and their numerous cytoplasmic extensions stain brown with MTS10, and anti-SV40 Tag (blue) labels the nucleus. One-month-old SV12 Tg mouse, ×1000. (c) Histology showing the abundant, clear cytoplasm of epithelial cells with lightly stained nuclei surrounded by clusters of small lymphocytes with very dense nuclei. A mitotic figure is clearly visible (arrow). Two-month-old SV12 Tg mouse, Masson's Trichrome, ×1000. (d) Immunolabelling with an antibody against total keratin, showing the dense, complex cytokeratin (CK) network of the epithelial cells. Three-week-old SV12 Tg mouse, ×250. (e) Immunolabelling with an antibody against low-molecular-weight CKs. A dense network extending over the whole section is observed with a few isolated, scattered, more intensely stained cells. Two-month-old SV12 Tg mouse, ×400. (f) A lightly stained network of epithelial cells with scattered groups of intensely labelled cells is observed with the antibody against high-molecular-weight CKs. Two-month-old SV12 Tg mouse, ×400. (g) A background of a very lightly stained network with rare, isolated, moderately stained cells is observed with the antibody against CK18. Three-week-old SV12 Tg mouse, ×250. (h) Immunolabelling with an antibody against CK5/8, showing numerous dense isolated cells scattered on a lightly labelled network. 4.5-month-old SV12 Tg mouse, ×400. (i) Histology: at the beginning of malignant tumoral development, the clear cytoplasm of several cells lacking boundaries, forming a scyncitium with a multinucleus, surrounded by numerous lymphocytes with very dense nuclei and little cytoplasm, is observed. Two-month-old SV12 Tg mouse, Masson's Trichrome, ×250. (j) Histology: around a necrotic core containing lysed cells and pycnotic nuclei in an amorphous material (top), layers of clear cells are observed. Four-month-old SV12 Tg mouse, haematoxylin–eosin, ×400.
Cytokeratin immunohistolabelling in early phases.
The aspect of the total keratin network observed in the first month, in the hyperplasic phase, was similar in SV12 Tg mice, in littermate controls and in normal mice. Thus, we observed, in both cortex and medulla, an extended, regular and fine network (Figure 1d). The labelling with an antibody against several low molecular weight CKs revealed a large positive network with a few scattered, more strongly stained cells (Figure 1e). With an antibody against several high-molecular-weight CKs, a slightly positive network was observed containing groups of more densely labelled cells (Figure 1f). The labelling with antibodies against CK18 showed a discretely labelled network of epithelial background throughout the parenchyma and lightly labelled, scattered cells (Figure 1g).
With the antibody against CK5/8, we observed, on several different fields and sections, in controls as well as in the early phase of tumorigenesis, a background of a very lightly labelled network (not shown). From 2 months and during tumour development, CK5/8 labelling showed an increasing number of isolated, round positive cells, scattered throughout the parenchyma. This aspect was maintained over the subsequent months during carcinoma development (Figure 1h).
Tumoral development and malignancy.
In the subsequent phases, several types of lesions coexisted within the tumour mass displaying zones with clear-cut cortical and medullary areas and other zones exhibiting a process of tumoral modification. In the MTS10-positive cells forming the benign tumour (originating from the medullary area), malignant abnormalities representative of the neoplastic process were apparent, associated with the development and extension of the neovasculature.
Histological observations.
Many groups of large, clear stromal cells in small clusters were visible. Some of these clear cells with two or multiple round–oval nuclei were observed (Figure 1i). Several groups of these cells were organized in rounded formations or nodules with a necrotic zone (Figure 1j) and/or a vessel in the centre and sometimes surrounded by a fibrous lamina and fibroblasts (Figure 2a). Haemorrhagic areas and necrosis were also present in the largest tumours.
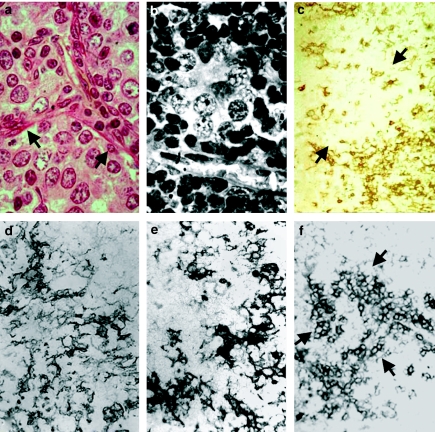
Figure 2.
Tumorigenesis by light microscopy. (a) Another area where clear dedifferentiated cells with atypical nuclei, possessing several nucleoli, are observed. Trabeculae of fibrous components and fibroblasts are present around these cellular nodules (arrow) as well as a vessel. Four-month-old SV12 transgenic (Tg) mouse, haematoxylin-eosin, ×1000. (b) Aspect of a vesicular nucleus with a proeminent nucleolus in cells with large, clear cytoplasm, surrounded by small lymphocytes with very dense nuclei. Four-month-old SV12 Tg mouse, Masson's Trichrome, ×1000. (c) Immunolabelling with an antibody against MTS10, showing a broken network of positive medullary cells around a large clear negative area (arrow) containing only a few scattered labelled cells. Four-month-old SV12 Tg mouse, ×250. (d) Immunolabelling with an antibody against total keratin: a variably dense, broken network with some clusters of more dense cells is observed. Four-month-old SV12 Tg mouse, ×400. (e) Labelling of low-molecular-weight CK: a similar aspect to that previously observed with total keratin is noted. Five-month-old SV12 Tg mouse, ×500. (f) Immunolabelling with antibody against CK18, showing very dense, positive cells isolated and/or in clusters (arrow) on a lightly labelled, broken network. 4.5-month-old SV12 Tg mouse, ×250.
Numerous atypical cellular characteristics were visible: tumour cells with a large, clear cytoplasm and indistinct borders, possessing a rounded nucleus with spots of chromatin scattered in the nucleoma, exhibiting a speckled or a vesicular aspect (Figure 2b). Lymphocytes were scattered throughout the layers of tumour cells or organized in variably sized clusters. Mitotic figures were always very numerous.
Labelling with MTS10 antibody showed that the network was less dense with large, rounded, unlabelled areas, which may correspond to dedifferentiated cells of tumoral zones, with a few scattered labelled cells (Figure 2c).
Cytokeratin immunohistolabelling.
Significant differences appeared in the phase of tumour formation. Indeed, we observed with the total CK antibody, a progressive loss of the keratin network, which appeared irregular and poorly labelled (Figure 2d). Labelling with antibodies against low-molecular-weight keratins showed a similar aspect of a patchy network, with numerous breaks and large unlabelled areas containing some scattered, strongly labelled cells (Figure 2e). With the antibody against high-molecular-weight keratins, a few scattered, small and lightly positive areas were present (not shown).
With CK18, an increased number of positive cells was observed, scattered or in small clusters, throughout the tumour (Figure 2f).
Thus, our studies of the CKs present in the thymic epithelial cells reveal modifications of their distribution and molecular weight during the different phases of tumoral transformation.
Electron microscopy.
At the ultrastructural level, we observed the presence, in the early phases and in all zones examined, of an increased number of the different types of epithelial medullary cells (type II and type III) (Nabarra and Andrianarison 1987). Furthermore, we noted the unusual presence of an increasing number of immature and/or poorly differentiated epithelial cells of type IV that we have previously identified as precursors of the medullary epithelial cells (Nabarra et al. 2001) and which appear in large numbers during the process of tumour formation (Figure 3a). Around 4–5 months, a large number of these cells displayed a degenerative aspect with the accumulation in their clear, more or less vacuolated cytoplasm of dense mitochondria and lipidic and myelinic inclusions (Figure 3b).
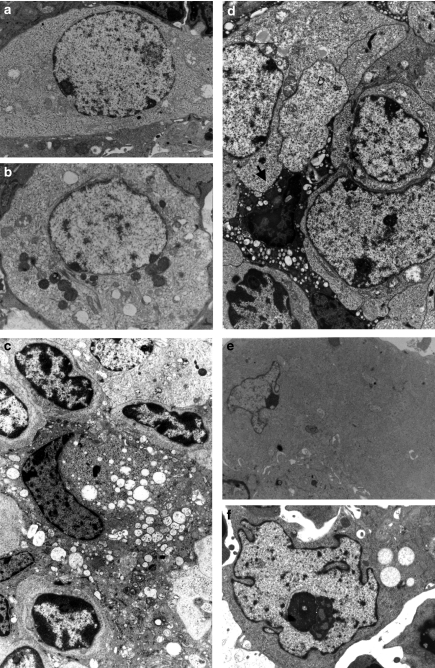
Figure 3.
Tumorigenesis by electron microscopy. All the sections are stained with uranyl acetate and lead citrate. (a) An epithelial cell of type IV with a nucleus exhibiting clear, coarse chromatin without dense clumps in an expanded, clear cytoplasm containing ribosomes but few other organelles. Lightly stained tonofilaments are present. Two-month-old SV12 transgenic (Tg) mouse, ×10,000. (b) A type IV epithelial cell, still poorly differentiated, at the beginning of the degenerescence phase with clear cytoplasm, vacuoles and dense inclusions. Four-month-old SV12 Tg mouse, ×10,000. (c) Type II medullary epithelial cells in a predegenerative stage, with a dense, highly vacuolated cytoplasm. Three-month-old SV12 Tg mouse, ××9500. (d) View of the tumoral stroma showing association of several dedifferentiated cells with a clear, smooth cytoplasm, a cell presenting a degenerative aspect with a very dense, vacuolized cytoplasm and a pycnotic nucleus (arrow). Four-month-old SV12 Tg mouse, ×12500. (e) A dedifferentiated tumoral cell with expanded, smooth cytoplasm lacking ribosomes, mitochondria and other cellular organelles. The transformed nucleus is irregular in shape with clear, coarse, immature chromatin and nucleoli. 4.5-month-old SV12 Tg mouse, ×9500. (f) Another tumoral cell with a moderately dense cytoplasm and an abnormal nucleus, irregular in shape, showing patchy chromatin and a very large nucleolus. 3.5-month-old SV12 Tg mouse, ×18,500.
During the different phases of tumoral development, various cellular modifications concerning the cytoplasm and the nucleus were visible. Thus, we observed cells with vacuolated cytoplasm and dilated profiles of rough endoplasmic reticulum (Figure 3c), cells showing an abnormal increase in the number of tonofilament bundles (not shown), cells exhibiting a degenerated aspect with a dense, vacuolated cytoplasm and a pycnotic nucleus (Figure 3d), and cells displaying a dedifferentiated aspect, especially in the later phases, with a smooth, clear, largely expanded cytoplasm, containing a modified nucleus and few or no organelles and showing sparse epithelial markers (tonofilaments and desmosomes) or the complete absence of these markers and no characteristics of the different types of normal epithelial thymic cells (Figure 3e).
The nuclear anomalies included a greatly increased nuclear size, often resulting in dysplasic, multilobed giant nuclei, and/or an irregular convoluted shape (Figures 3f and 4a). Other features included ring-shaped nuclei with cytoplasmic inclusions (not shown) or an elongated shape with invaginations containing rolled-up membranes (Figure 4b). The chromatin presented various aspects, being either distributed in small clumps of coarse material, irregularly scattered, or presenting a clear, smoother aspect (Figure 4b,c). Cases of nuclear membrane rupture, with chromatin appearing to be spilling out of the nucleus into the cytoplasm, were also visible. Furthermore, we often observed one or several nucleoli of increased size.
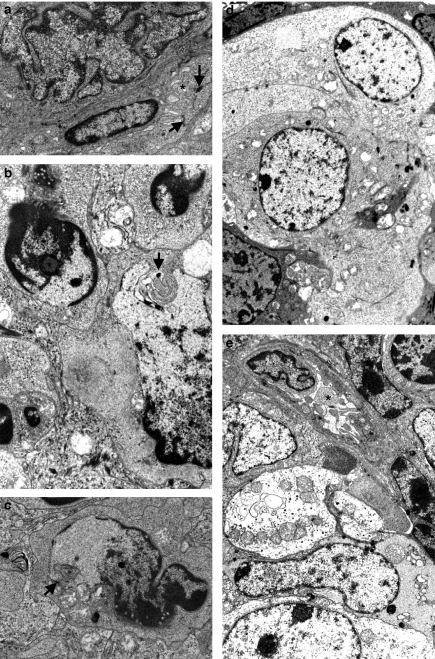
Figure 4.
Tumorigenesis by electron microscopy. (a) Another example of a large, atypical nucleus of highly irregular shape. A neovessel (star) along this epithelial cell is observed with a turgescent endothelial cell linked to another by visible tight junctions (arrow). 3.5-month-old SV12 transgenic (Tg) mouse, ×18,500. (b) Several cells with atypical nuclei. At right, irregular and elongated nucleus with, in an invagination, a rounded membranous inclusion (arrow). In all cells, the chromatin is abnormal, irregularly distributed with smooth and clear areas. 3.5 month-old SV12 Tg mouse, ×25,000. (c) Another nuclear aspect with areas lacking chromatin material and exhibiting membranous inclusions (arrow). Four-month-old SV12 Tg mouse, ×25,000. (d) Nodules with clear, poorly differentiated cells organized in a rounded formation, ×20,000 (e) Another nodule bordered by a neovessel (star) with turgescent endothelial cells. Four-month-old SV12 Tg mouse, ×20,000.
Concerning the structural organization of the tumour stroma, we observed round formations resulting from the association of differentiated and dedifferentiated epithelial cells with cells displaying either a smooth, clear cytoplasm or a vacuolated or degenerated dense cytoplasm with a prepycnotic or pycnotic dense nucleus (Figure 4d,e). Degenerescent cells, membranous fragments and granular, heterogeneous material formed necrotic areas in the centre of some of these round formations. Relatively decreased numbers of lymphocytes were observed, scattered throughout the lesions. A large number of mitotic figures concerning both epithelial cells and lymphocytes were present.
The electron microscopic studies provide evidence for the malignant transformation of an exclusive medullary epithelial tumour as well as the presence of an increasing number of thymic medullary epithelial precursor cells (type IV).
Angiogenesis
At the end of the hyperplastic phase, the development of neovessels began and during the subsequent phases, this vascular network was extended, appearing to be associated with the neoplastic process.
Histological observations of neovessel development.
In comparison to the normal and control thymus, at 1.5 months after birth, an increasing number of empty lumina, more or less elongated and irregular in size, was observed in the hyperplastic parenchyma and subsequently during the development of the clusters of clear cells and the formation of nodules (Figure 5a).
Figure 5.
Angiogenesis by light microscopy. (a) Aspect of development of a neovessel, appearing to be orientated towards the clear nodule. Four-month-old SV12 transgenic (Tg) mouse, Masson's Trichrome, ×400. (b) Immunolabelling with antibodies against vascular endothelial cells: control section showing very few elongated vessels stained by CD31 antibody. A similar aspect is observed with CD34. Three-month-old control mouse, ×400. (c) Aspect with the same antibody on a section of tumour present in a 3-month-old SV12 Tg mouse where increased numbers of positive cells are observed either in isolation or arranged in various elongated formations. Similar observations are made after labelling with the CD34 antibody. Three-month-old SV12 mouse, ××400. (d–g) Flk-1 (VEGF/R2) labelling. (d) Very light labelling with Flk-1 antibody on host vessel endothelial cells at the end of the hyperplasia stage. Five-week-old SV12 Tg mouse, ×250. (e) With the same antibody (against Flk-1 receptors), isolated positive cells adjacent to the vascular lumen (arrow) and some more remote scattered cells are observed. Two-month-old SV12 mouse, ×400. (f) Labelling with anti-Flk-1 showing positive cells either bordering host vessels (arrow), either isolated in the surrounding parenchyma or associated in a tubular-like formation (arrow). Three-month-old SV12 Tg mouse, ×400. (g) Higher magnification showing arrangement in a tubular formation of endothelial cells labelled with the antibody against Flk-1 (VEGF/R2 receptor). Labelled cells are either isolated in the surrounding parenchyma or arranged in a tubular formation around a virtual vascular lumen. Three-month-old SV12 Tg mouse, ×1000.
Compared to the early phase of hyperplasia, where endothelial labelling revealed few vascular sections (Figure 5b), from about 2 months of age, CD31 and CD34 labelling showed an increasing number of cells positive for endothelial markers either in an isolated position and/or arranged in an elongated vascular formation (Figure 5c).
This indicates an extensive proliferation of the endothelial cells, probably originating from the host vessels, scattered in the parenchyma, appearing to be orientated towards the tumoral areas.
Immunolabelling with an antibody against Flk-1 (VEGF/R2).
In the control, with the antibody against the Flk-1 receptor, the labelling was negative (not shown).
In the SV12 Tg, at the final stage of hyperplasia (about 5–6 weeks of age), very few, lightly positive cells bordering the pre-existent vascular lumina were observed (Figure 5d). Rapidly, from 2 months of age onwards, the presence of clearly positive cells, adjacent to the vascular lumen and/or isolated in the surrounded parenchyma were observed (Figure 5e). At the same time, in addition to scattered positive cells, several cell aggregates ressembling tubes in formation were observed. Thus, numerous micrographs showed a migrating column forming a tube as a precursor of elongated vascular walls. These tubes were more or less regularly arranged in the surrounding tissue, without cell–cell connections and/or basement membrane (Figure 5e,f, g). The isolated cells were scattered throughout the parenchyma and/or were situated adjacent to pre-existing vessels.
Electron microscopy.
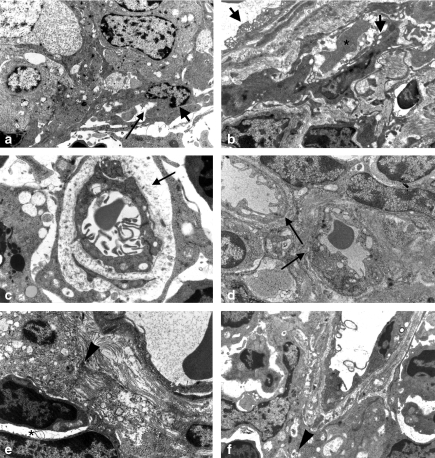
Firstly, we noted that, in ultrastructural examination, it is difficult to identify the endothelial cells in newly forming microvasculature because they possessed no clear and specific morphological characteristics. We propose an image of probable endothelial cells elongated along an empty area which could represent a future vascular lumen (Figure 6a). Numerous small vessel sections, with different morphological aspects according to the stages of their constitution, were then observed. Thus, in numerous neovessels, we observed turgescent endothelial cells, irregular in thickness, linked by dense junctions, sometimes showing apical, pycnotic microvesicles or irregular villosities, haphazardly arranged around a lumen, often containing red blood cells (Figures 4a and 6b,c). The basement membrane was either absent or in formation with light, granular, coarse material scattered along the basal zone of the cells (Figure 6c) or arranged in a narrow, variously dense band (Figure 6d). The sections of well-formed neovessels show turgescent endothelial cells joined by tight junctions and a basal lamina often incompletely formed. They were present in a modified interstitium with loose bundles of collagen and we noted, depending on the plane of section, the existence of areas where endothelial cells appeared to change shape, suggesting that they represent the advancing front of a vessel (Figure 6e).
Figure 6.
Angiogenesis by electron microscopy (a) Aspect of a modified group of cells (top half of picture) with, on its edge, a probable elongated endothelial cell lying along an empty area that could be a vascular lumen in formation (arrow). Three-month-old SV12 transgenic (Tg) mouse, ×8500. (b) A neovessel in formation with elongated turgescent cells (arrow), not joined by tight junctions, bordering a lumen containing a red blood cell (star). The basement membrane is not yet formed. Another, more organized vessel is present with a vacuolated endothelium (top left). The interstitium contains bundles of collagen fibres, which are more or less developed. 3.5-month-old SV12 Tg mouse, ×8000. (c) View of a new vessel with a well-opened lumen bordered by endothelial cells possessing elongated microvillosities and conspicuous tight junctions surrounded by a large zone of formation of the basement membrane containing granular, clear material (arrow). 3.5-month-old SV12 Tg mouse, ×9500. (d) Two vascular lumina showing different stages of neovessel formation (arrow). In the centre, an early stage where the endothelium is turgescent, with junctions beginning to appear between cells. 3.5-month-old SV12 Tg mouse, ×8000. (e) Aspect of a vessel showing an advancing endothelial front (arrow head) where the endothelial cells appear to be pointing towards an arrangement of two endothelial cells around a lumen (star). The extracellular matrix, with fibrous elements appears to loose. 3.5-month-old SV12 Tg mouse, ×10,500 (f) Another aspect of a vessel showing an advancing endothelial front (arrow head) with the insertion of a fragment of endothelial cytoplasm in layers of a poorly formed basement membrane. 3.5-month-old SV12 Tg mouse, ×10,500.
In other places, endothelial cells appeared to send out cell processes into the space between their basal pole and the basement membrane in formation (Figure 6f).
Discussion
This study provides insights into the formation of a pure malignant thymic epithelial neoplasm of a carcinoma type associated with extensive angiogenesis in a new model of SV40 Tg mice (named SV12 Tg mice). In this process of tumour development, different modifications in the thymic microenvironment can be identified, ranging from hyperplasia to carcinoma. Thymic tumours were present in all mice examined, which expressed the transgene.
Several original features appear important to discuss in this model, such as the exclusive proliferation of medullary epithelial cells, the sequential passage towards malignancy and the formation of an extensive neovasculature.
The first point of discussion concerns the almost exclusive proliferation of the MTS10-positive cells, corresponding to the epithelial cells present only in the medullary zone of the thymus. As reported previously (Nabarra et al. 2002) and in the present study, double labelling with specific antibodies showed that these medullary epithelial cells also express the transgene. This cellular expansion invaded all zones of the thymus, disrupting the architecture of the organ to form a tumour. This epithelial tumour progressively acquired characteristics of malignancy. Thus, the major effects of oncoproteins are proliferative, triggered by a direct action of SV40 Tag, since the cell population selected for proliferation was that which expressed the transgene (Lane and Crawford 1979; Hanahan 1986; Adams and Cory 1991). In certain cases, the induction of proliferation is not immediate and several days (variable according to the organ considered) are required before the abnormal proliferation begins. According to several authors, this implies dysregulation of the programme of cell proliferation by the nuclear insertion of the SV40 oncogene alongside the genes of key host proteins, p53 and pRb, which play a role in regulating normal cell proliferation and are considered as tumour suppressors (Ludlow 1993; Levine et al. 1994; Saenz-Robles et al. 1994). Indeed, this has been demonstrated by Sepulveda et al. (1989) in SV40 Tg mice with liver carcinoma and by Efrat et al. (1987) in a similar model with pancreatic tumours.
Other mechanisms concerning this proliferation cannot be excluded since the SV40 Tag initiates DNA synthesis and also transactivates the expression of viral and cell promoters, and the insertion might act directly on the production of cytokines which are involved in the proliferative process (Botteri et al. 1987; Galy et al. 1993). A third and more recent hypothesis, put forward by analogy with the process of thymocyte apoptosis (McCarthy et al. 1994) but not yet studied, could be the dysregulation of an eventual programmed epithelial cell death. This is difficult to verify without DNA studies, since morphological criteria of epithelial apoptosis have not been clearly defined.
In our study, we have identified the neoplastic transformation of a benign medullary epithelial tumour into a carcinoma by comparison with morphological descriptions of human thymic carcinoma (Rosai and Levine 1976; Truong et al. 1990; Kuo and Chan 1998; Suster and Moran 1998,1999). Thymic carcinoma is a rare epithelial neoplasm with obvious cytological abnormalities. This epithelial tumour exhibits clear-cut features of malignancy with pleiomorphic, undifferentiated cells showing hyperchromatic nuclei, prominent nucleoli and a high nuclear/cytoplasmic ratio. Cells organized in islets or foci surrounding a necrotic area, wide-spread haemorrhage and increased mitotic activity are very significant features of carcinoma development. Extensive neovascularization is also an important criterion in various carcinomas, but has been rarely mentioned in reports on thymic carcinoma. Furthermore, it is known that a certain number of tumours, including transitional cell carcinoma, appear at the morphological level to be composed of dedifferentiated and/or immature analogues of the normal cell type.
All these morphological aspects are present in the SV12 thymus, with progressive acquisition of malignant characteristics. Other parameters are considered, in this study, to indicate tumoral transformation. Firstly, modifications of the cytoplasmic microfilament components, especially the keratins, are involved. During neoplastic development, a marked decrease in the density of the epithelial keratin network is observed. In some epithelial neoplasias, the abnormal regulation of cell division and differentiation might cause alterations in the pattern of keratin synthesis associated with a change in tissue topology, and the SV40 oncogene may interfere with the epithelial CK components, perhaps by acting with the tumour suppressors (Moroco et al. 1990). Indeed, it appears that epidermal cells in culture can lose their characteristics as a consequence of viral transformation (Hornis et al. 1984). The progressive loss of the CK network in SV12 Tg mice, as visualized by immunolabelling, may correspond to the presence of the dedifferentiated neoplastic cells observed in electron microscopy. Secondly, whereas the density of the network decreases, it appears that the epithelial tumour maintains the expression of a few types of keratin found in normal tissue. Indeed, the presence of rare CK18-positive cells in the normal thymic microenvironment has been described in some species, and in normal mice they are mainly present in the cortex and scattered in the medulla. In our model, CK18-positive cells were significantly increased in number in the late phases of tumorigenesis. It is probable that the small pool of positive cells in the normal thymus could be transformed into malignant cells under certain conditions, in relation with deactivation of tumour-suppressor proteins (Ledinko and Costantino 1990).
With reference to human thymic tumours, a further subdivision of thymic carcinomas is possible according to the type of keratin they express. A controversy exists as to whether tonofilaments are present in human thymic carcinoma. In epithelial tumours, a wide variability in the gain or loss of individual keratins during tumorigenesis has been described. According to Thomas et al. (1984), it is possible to identify two different types of carcinoma: the differentiated squamous carcinoma (with intracytoplasmic tonofilaments) and the poorly differentiated carcinoma (without tonofilaments). Likewise, using differentiation by the molecular weight of the keratins expressed, the squamous carcinoma cells show intense staining for high-molecular-weight keratins, whereas the simple basal carcinoma expresses more low-molecular-weight keratins. In view of these considerations, we can suggest that the tumour observed in our study represents a ‘simple basal poorly differentiated’ thymic carcinoma.
As a final point of discussion concerning results with CK labelling, we sought to correlate our observations, in SV12 Tg mice, of the increasing presence of both CK5/8-positive cells (visualized by immunohistolabelling in light microscopy) and type IV epithelial cells (visualized by electron microscopy). Type IV epithelial cells, rarely observed in normal thymus, because they are sparse in the thymic microenvironment and their maturation turnover appears to be rapid, correspond in our classification to precursors of the thymic medullary epithelial cells (Nabarra et al. 2001). Firstly, we note in the early phases of neoplastic development that this cell type proliferates largely. At the final stage of tumorigenesis, a morphological aspect of degeneration of a large number of type IV epithelial cells is observed, suggesting a phenomenon of blockade of this cell type in the early stages of maturation. Nevertheless, part of the pool must escape this process since, in the first phase, a considerable proliferation of medullary cells is observed with numerous epithelial cell mitoses. Secondly, we observe that the anti-CK5/8 antibody, considered as a marker of thymic epithelial precursor cells (Klug et al. 1998,2002), labels an increased number of cells in the SV12 tumour. By analogy, we suggest that these two cell types, the type IV epithelial cell and the CK5/8-positive cell, are identical. Furthermore, if we compare our results with those reported by Gill et al. (2002) and Bennett et al. (2002) which show that thymic epithelial cells positive for CK5/8 are also recognized by a new specific marker of thymic precursor cells (MTS 24) described previously (Blackburn et al. 1996; Bennett et al. 2002), we can propose that the type IV epithelial cell, the CK5/8-positive cell and the MTS24-positive cell all represent the same cell type, which appears to be the thymic epithelial precursor present in the medulla (manuscript in preparation).
In neoplasms, it is currently accepted that new blood vessels provide nourishment to the growing tumour, and the switch from an avascular neoplastic tissue to a vascularized one is a key event in the formation of a tumour nodule (Schor and Schor 1983; Folkman and Klagsbrun 1987; Klagsbrun and D'Amore 1991; Bouck 1992; Senger et al. 1993; Folkman 1995; Hanahan and Folkman 1996). Furthermore, for these authors, it is widely believed that progressively growing solid tumours produce a number of angiogenic factors that stimulate capillaries in the surrounding normal tissue to produce a new vascular network. Thus, establishment and remodelling of blood vessels is controlled by paracrine signals, subsequent to the release of angiogenic factors, mainly secreted by the tumour cells themselves or possibly indirectly by the host inflammatory cells (Berse et al. 1992). These signals trigger several functional responses in endothelial cells, mediated by different receptors carried by these cells involving proliferation, orientation and formation of neovessels. The angiogenic signal occurs very early in various models of tumour progression, often coinciding with the onset of precancerous lesions (Hanahan 1988; Folkman et al. 1989).
In our model, we show by labelling of endothelial cells that the angiogenic activity starts at the end of the phase of hyperplasia, when lightly labelled cells bordering pre-existing vessels were observed. The endothelial nature of these cells was demonstrated by positive immunostaining for CD31, CD34 and especially for Flk-1 receptor, which is highly specific for newly formed endothelial cells. This Flk-1 receptor, also known as VEGF/R2, is a VEGF-binding receptor, and thus its presence in SV12 thymic tumours strongly suggests the existence of this factor within the tumoral parenchyma, as already reported in numerous studies (Risau 1991; Breier et al. 1992; Senger et al. 1993). It has been shown that this factor and its receptors exist in the mouse at the foetal stage and during early postnatal development but is quasi-absent in the normal adult mouse except in the cycling female reproductive tract. Its concentration is greatly increased in neoplastic situations (Folkman and Klagsbrun 1987; Plate et al. 1992; Millauer et al. 1993; Quinn et al. 1993; Senger et al. 1993; Neufeld et al. 1994; Ferrara 1995).
VEGF expression appears to be exclusively observed in tumours where the sprouting mode of angiogenesis predominates (Burri and Djonov 2002). This factor acts directly on endothelial cells via several receptors such as VEGF/R2 or Flk-1. It is hypothesized that at the early stage of tumour pro- gression, the angiogenic switch is triggered by the induction of VEGF secreted by tumoral cells, leading to activation of sprouting and endothelial proliferation. It has been recently proposed that at the later stages of neoplasia, the two modes of angiogenesis (sprouting and intussusceptive) can coexist in the tumour centre (Burri and Djonov 2002).
Given that the Flk-1 receptor, specific for VEGF, is present on the newly formed endothelial cells, the question which arises concerns the secretion of VEGF by the thymic medullary epithelial cells modified by malignancy. At present, we do not even know whether normal adult thymic medullary epithelial cells are capable of secreting this factor. Indeed, the function and different secretion products of medullary and cortical thymic epithelial cells are poorly characterized because of the difficulty of isolating these different cell types to obtain pure populations for study in culture.
The first apparent response to an angiogenic stimulus from the tumour cells is the degradation of the basement membrane surrounding the pre-existing vessels, and the alteration of the extracellular matrix appears to be a necessary, or at least a facilitating, step in the angiogenic process (Form et al. 1986; Ingber and Folkman 1989). Then, the endothelial cells migrate out of the vessel towards the tumoral nodule, adopting a tubular arrangement.
The initiation of neovasculature is regulated by the balance between activities of inducers and inhibitors of angiogenesis present simultaneously in normal tissue, which determines the behaviour of endothelial cells in vivo (Bicknell and Harris 1991; Klagsbrun and D'Amore 1991; Bouck 1992; Cockerill et al. 1995; Rak et al. 1995)
In conclusion, the SV12 Tg line appears to be a good model to study two aspects of thymic tumour development: the formation of a malignant tumour originating from an extended hyperplasia of the medullary part of the microenvironment and the association of this process with the development of extensive angiogenesis. We observed the existence of a continuum in the spectrum of differentiation from hyperplasia towards thymic carcinoma characterized by the progressive acquisition of atypical cellular features. These different aspects support the notion that, at least in some cases, thymic carcinomas do not arise de novo but can originate from the malignant transformation and/or progressive loss of differentiation in a benign epithelial tumour.
Acknowledgments
We thank B. Chemini for printing photomicrographs, Martine Netter for the production and formatting of iconography, and Marie-Christine Launay for the preparation of the manuscript.
References
- Adams JM, Cory S. Transgenic models of tumor development. Science. 1991;254:1161–1167. doi: 10.1126/science.1957168. [DOI] [PubMed] [Google Scholar]
- Bennett AR, Farley A, Blair NF, Gordon J, Sharp L, Blackburn CC. Identification and characterization of thymic epithelial progenitor cells. Immunity. 2002;16:803–814. doi: 10.1016/s1074-7613(02)00321-7. [DOI] [PubMed] [Google Scholar]
- Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages and tumors. MolBiolCell. 1992;3:211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R, Harris AL. Novel growth regulatory factors and tumor angiogenesis. EurJCancer. 1991;27:781–785. doi: 10.1016/0277-5379(91)90189-k. [DOI] [PubMed] [Google Scholar]
- Blackburn CC, Augustine CL, Li R, et al. The nu gene acts cell-autonomously and is required for differentiation of thymic epithelial progenitors. ProcNatlAcadSciUSA. 1996;93:5742–5746. doi: 10.1073/pnas.93.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteri FM, Van der Putten H, Wong DF, Sauvage CA, Evans RM. Unexpected thymic hyperplasia in transgenic mice harboring a neuronal promoter fused with simian virus 40 large T antigen. MolCell Biol. 1987;7:3178–3184. doi: 10.1128/mcb.7.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N. Angiogenesis: a mechanism by which oncogenes and tumor supressor genes regulate tumorigenesis. Cancer TreatRes. 1992;63:359–371. doi: 10.1007/978-1-4615-3088-6_19. [DOI] [PubMed] [Google Scholar]
- Boyd RL, Tucek CL, Godfrey DI, et al. The thymic microenvironment. ImmunolToday. 1993;14:445–459. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Brekelmans P, Van Ewijk W. Phenotypic characterization of murine thymic micro-environment. Semin Immunol. 1990;2:13–24. [PubMed] [Google Scholar]
- Brinster RL, Chen HY, Messing A, Van Dyke T, Levine AJ, Palmiter RD. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984;37:367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri P, Djonov V. Intussuceptive angiogenesis, the alternative to capillary sprouting. MolAspects Med. 2002;23:S1–S27. doi: 10.1016/s0098-2997(02)00096-1. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. NatMed. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Cockerill GW, Gamble JR, Vadas MA. Angiogenesis models and modulators. Int. Rev. Cytol. 1995;159:113–160. doi: 10.1016/s0074-7696(08)62106-3. [DOI] [PubMed] [Google Scholar]
- Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J. Clin. Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D, Schermer A, Sun TT. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications and limitations. LabInvest. 1985;52:243–256. [PubMed] [Google Scholar]
- De Lisser HM, Christofidou-Solomidou M, Strieter RM, et al. Involvement of endothelial PECAM/CD31 in angiogenesis. Am. J. Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- Debus E, Moll R, Francke WW, Weber K, Osborn M. Immunohistochemical distinction of human carcinomas by cytokeratin typing with monoclonal antibodies. Am. J. Pathol. 1984;114:121–130. [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular, hyperpermeability and angiogenesis. Am. J. Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Efrat S, Baekkeskov S, Lane D, Hanahan D. Coordinate expression of the endogenous p53 gene in beta cells of transgenic mice expressing hybrid insulin-SV40 T antigen genes. EMBO J. 1987;6:2699–2704. doi: 10.1002/j.1460-2075.1987.tb02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza CG, Azar HA. Immunohistochemical localization of keratin-type proteins in epithelial neoplasms. Am. J. ClinPathol. 1982;78:500–507. doi: 10.1093/ajcp/78.4.500. [DOI] [PubMed] [Google Scholar]
- Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer ResTreat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- Fina L, Molgaard HV, Robertson D, et al. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other diseases. NatMed. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;359:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Form DM, Pratt BM, Madri JA. Endothelial cell proliferation during angiogenesis. In vitro modulation of basement membrane components. LabInvest. 1986;55:521–530. [PubMed] [Google Scholar]
- Franke WW, Schiller DL, Moll R, et al. Diversity of cytokeratins: differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J. Mol. Biol. 1981;153:933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Frith CH, Ward JM, Chandra M. The morphology, immunochemistry and incidence of hematopoietic neoplasm in mice and rats. ToxicolPathol. 1993;21:206–218. doi: 10.1177/019262339302100213. [DOI] [PubMed] [Google Scholar]
- Fukai I, Masaoka A, Hashimoto T, Yamakawa Y, Mizuno T, Tanamura O. Cytokeratins in normal thymus and thymic epithelial tumors. Cancer. 1993;71:99–105. doi: 10.1002/1097-0142(19930101)71:1<99::aid-cncr2820710116>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Galy AH, de Waal Malefyt R, Barcena A, Mohan-Peterson S, Spits H. Untransfected and SV40-transfected fetal and post-natal human thymic stroma cells. Thymus. 1993;22:13–33. [PubMed] [Google Scholar]
- Gill J, Malin M, Hollander GA, Boyd R. Generation of a complete thymic micro-environment by MTS24+ thymic epithelial cells. Nat Immunol. 2002;3:635–642. doi: 10.1038/ni812. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Oncogenes and Growth Control. Berlin: Springer Verlag; 1986. Oncogenesis in transgenic mice; pp. 349–363. [Google Scholar]
- Hanahan D. Dissecting multistep tumorigenesis in transgenic mice. Annu. Rev. Genet. 1988;22:479–519. doi: 10.1146/annurev.ge.22.120188.002403. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:335–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Ho FC, Fu KH, Lam SY, Chiu SW, Chan AC, Muller-Hermelink HK. Evaluation of a histogenetic classification for thymic epithelial tumors. Histopathology. 1994;25:21–29. doi: 10.1111/j.1365-2559.1994.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Hoot GP, Kettman JR. Primary polyoma virus-induced murine thymic epithelial tumors. Am. J. Pathol. 1989;135:679–695. [PMC free article] [PubMed] [Google Scholar]
- Hornis TS, Steinberg ML, Defendi V, Sun TT. Simple epithelial nature of simian virus-40-transformed human epidermal keratinocytes. Cancer Res. 1984;44:5797–5804. [PubMed] [Google Scholar]
- Ingber DE, Folkman J. How does extracellular matrix control capillary morphogenesis. Cell. 1989;58:803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Winer J, Bennett GL, Altar CA, Ferrara N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissue. J. Clin. Invest. 1992;89:244–253. doi: 10.1172/JCI115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner T, Muller-Hermelink HK. New approaches to the diagnosis of thymic epithelial tumors. Prog. Surg. Pathol. 1989;10:167–189. [Google Scholar]
- Klagsbrun M, D'Amore PA. Regulation of angiogenesis. Annu. Rev. Physiol. 1991;53:217–239. doi: 10.1146/annurev.ph.53.030191.001245. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M, Soker S. VEGF/VPF: the angiogenesis factor found? Current Biol. 1993;10:699–702. doi: 10.1016/0960-9822(93)90073-w. [DOI] [PubMed] [Google Scholar]
- Klug DB, Carter C, Crouch D, Roop C, Gimenez-Conti CJ, Richie ER. Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. ProcNatlAcadSciUSA. 1998;95:11822–11824. doi: 10.1073/pnas.95.20.11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug DB, Carter C, Gimenez-Conti IB, Richie ER. Cutting edge: Thymocyte-independent and thymocyte-dependent phases of epithelial patterning in the fetal thymus. JImmunol. 2002;169:2842–2845. doi: 10.4049/jimmunol.169.6.2842. [DOI] [PubMed] [Google Scholar]
- Kornstein M, Deblois G. Tumors of the thymic epithelial cells. In: Kornstein) M, editor. Pathology of the Thymus and Mediastinum. Philadelphia: W. Saunders.; 1995. pp. 67–113. [Google Scholar]
- Kuo TT, Chan JK. Thymic carcinoma arising in thymoma is associated with alterations in immunohistochemical profile. Am. J. SurgPathol. 1998;22:1474–1481. doi: 10.1097/00000478-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278:261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Ledinko N, Costantino RL. Modulation of p53 gene expression of cytokeratin 18 in retinoid-mediated invasion suppressed lung carcinoma cells. Anticancer Res. 1990;10:1335–1339. [PubMed] [Google Scholar]
- Lee SS, Park WY, Chi JG, et al. Thymic epithelial tumor progression in an SV40 T transgenic mouse model. Virchows Arch. 1998;432:33–42. doi: 10.1007/s004280050131. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Perry ME, Chang A, et al. The role of the p53 tumor-suppressor gene in tumorigenesis. BrJCancer. 1994;69:409–416. doi: 10.1038/bjc.1994.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longacre T, Rouse R. CD31: a new marker for vascular neoplasia. Adv. Anat. Pathol. 1994;1:16–20. [Google Scholar]
- Loning T, Viac J, Caselitz J, Thivolet J, Otto HF, Seifert G. Comparative investigation of keratin filaments in normal tissues and tumours of skin, oral mucosa, salivary glands and thymus. Pathol. Res. Pract. 1982;175:256–265. doi: 10.1016/S0344-0338(82)80112-X. [DOI] [PubMed] [Google Scholar]
- Ludlow JW. Interaction between SV40 large-tumor antigen and the growth suppressor proteins pRb and p53. FASEB J. 1993;5:866–871. doi: 10.1096/fasebj.7.10.8344486. [DOI] [PubMed] [Google Scholar]
- Matsuyama M, Suzuki H, Yamada S, Ito M, Nagayo T. Ultrastructure of spontaneous and urethan-induced thymomas in Buffalo rats. Cancer Res. 1975;35:2771–2779. [PubMed] [Google Scholar]
- McCarthy SA, Symonds HS, Van Dyke T. Regulation of apoptosis in transgenic mice by simian virus 40 T antigen-mediated inactivation of p53. ProcNatlAcadSciUSA. 1994;91:3979–3983. doi: 10.1073/pnas.91.9.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing A, Pinkert CA, Palmiter RD, Brinster RL. Developmental study of SV40 large T antigen expression in transgenic mice with choroid plexus neoplasia. Oncogene Res. 1988;3:87–97. [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnurch H, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Moll J, Eibel H, Botteri F, Sansig G, Regnier C, van der Putten H. Transgenes encoding mutant simian virus 40 large T antigens unmask phenotypic and functional constraints in thymic epithelial cells. Oncogene. 1992;7:2175–2187. [PubMed] [Google Scholar]
- Moll R, Krepler R, Franke WW. Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation. 1983;23:256–269. doi: 10.1111/j.1432-0436.1982.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Moroco JR, Solt DB, Polverini PJ. Sequential loss of suppressor genes for three specific functions during in vivo carcinogenesis. LabInvest. 1990;63:298–306. [PubMed] [Google Scholar]
- Muller-Hermelink H, Marx A, Kirchner T. Advances in the diagnosis and classification of thymic epithelial tumors. Rec. Adv. Histopathol. 1994;16:49–72. [Google Scholar]
- Murray AB, Schaffer E, Nussel M, LuZ A. Incidence, morphology and ultrastructure of spontaneous thymoma, the most common neoplasm in W/Nhg rats. JNatlCancer Inst. 1985;75:369–379. [PubMed] [Google Scholar]
- Nabarra B, Andrianarison I. Ultrastructural studies of thymic reticulum. I. Epithelial components. Thymus. 1987;9:95–121. [PubMed] [Google Scholar]
- Nabarra B, Mulotte M, Casanova M, Godard C, London J. Ultrastructural study of the FVB/N mouse thymus: presence of an immature epithelial cell in the medulla and premature involution. Dev. Comp. Immunol. 2001;25:231–243. doi: 10.1016/s0145-305x(00)00054-9. (Erratum in Dev. Comp. Immunol. 25, 539–543). [DOI] [PubMed] [Google Scholar]
- Nabarra B, Martinon C, Godard C, et al. Early steps of a thymic tumor in SV40 transgenic mice: hyperplasia of medullary epithelial cells and increased mature thymocyte number disturb thymic export. DevImmunol. 2002;9:223–231. doi: 10.1080/10446670310001593532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor DC, Krinke GJ, Ruefenacht HJ. Primary tumors of the thymus in the rat. J. Comp. Pathol. 1988;99:187–203. doi: 10.1016/0021-9975(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Tessler S, Gitay-Goren H, Cohen T, Levi BZ. Vascular endothelial growth factor and its receptors. Prog. Growth Fact. Res. 1994;5:89–97. doi: 10.1016/0955-2235(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Palmiter R, Chen HY, Messing A, Brinster RL. SV40 enhancer and large T antigen are instrumental in development of choroid plexus tumors in transgenic mice. Nature. 1985;316:457–460. doi: 10.1038/316457a0. [DOI] [PubMed] [Google Scholar]
- Park WY, Kim JI, Shim EH, et al. Development of thymic carcinoma in transgenic mice expressing SV40 T antigen. Cancer Lett. 1996;107:293–300. doi: 10.1016/0304-3835(96)04413-8. [DOI] [PubMed] [Google Scholar]
- Plate KH, Breir G, Weich HA, Risau W. Vascular endothelial growth is a potential tumor angiogenesis factor in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT. Fetal liver kinase-1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. ProcNatlAcadSciUSA. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak J, Filmus J, Finkenzeller G, Grugel S, Marme D, Kerbel RS. Oncogenes as inductors of tumor angiogenesis. Cancer Metastasis Rev. 1995;14:263–277. doi: 10.1007/BF00690598. [DOI] [PubMed] [Google Scholar]
- Ramaekers F, Huysmans A, Moesker O, Schaart O, Herman C, Vooijs P. Cytokeratin expression during neoplastic progression of human transitional cell carcinomas as detetected by monoclonal and polyclonal antibodies. LabInvest. 1985;52:31–38. [PubMed] [Google Scholar]
- Reynolds RK, Hoekzema GS, Vogel J, Hinrichs SH, Jay G. Multiple endocrine neoplasia induced by the promiscuous expression of a viral oncogen. ProcNatlAcadSciUSA. 1988;85:3135–3139. doi: 10.1073/pnas.85.9.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Vasculogenesis, angiogenesis and endothelial cell differentiation during embryonic development. In: Feinberg R, Sherer G, Auerbach R, editors. The Development of Vascular System. Basel: Karger; 1991. pp. 58–68. [Google Scholar]
- Rosai J, Levine G. Atlas of Tumor Pathology. Washington, DC: : Armed Forces Institute of Pathology.; 1976. Tumors of the thymus. [Google Scholar]
- Saenz-Robles MT, Symonds H, Chen J, Van Dyke T. Induction versus progression of brain tumor development: differential functions for pRb- and p53-targeting domains of simian virus 40 T antigen. MolCell Biol. 1994;14:2686–2698. doi: 10.1128/mcb.14.4.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W, Dardenne M. Immunohistochemical studies on a human thymic epithelial cell subset defined by the anti-cytokeratin 18 monoclonal antibody. Cell Tissue Res. 1988;254:225–231. doi: 10.1007/BF00220038. [DOI] [PubMed] [Google Scholar]
- Schlingemann RO, Rietveld FJ, de Waal RM, et al. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. LabInvest. 1990;62:690–696. [PubMed] [Google Scholar]
- Schor AM, Schor SL. Tumor angiogenesis. JPathol. 1983;141:385–413. doi: 10.1002/path.1711410315. [DOI] [PubMed] [Google Scholar]
- Senger DR, Van de Water L, Brown LF. Vascular permeability factor (VPF/VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12:303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- Sepulveda AR, Finegold MJ, Smith B, et al. Development of a transgenic mouse system for the analysis of stages in liver carcinoma genesis using tissue-specific expression of SV40 large T-antigen controlled by regulatory elements of the human alpha-1-antitrypsin gene. Cancer Res. 1989;49:6108–6117. [PubMed] [Google Scholar]
- Shalaby F, Ho J, Standford WL, et al. A requirement for Flk-1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Small JA, Blair DG, Showalter SD, Scangos GA. Analysis of a transgenic mouse containing SV40 and v-myc sequence. Mol. Cell. Biol. 1985;5:642–648. doi: 10.1128/mcb.5.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suster S, Moran CA. Thymic carcinoma: spectrum of differentiation and histologic types. Pathology. 1998;30:111–122. doi: 10.1080/00313029800169056. [DOI] [PubMed] [Google Scholar]
- Suster S, Moran CA. Primary thymic epithelial neoplasms: spectrum of differentiation and histological features. Semin. Diagn. Pathol. 1999;16:2–17. [PubMed] [Google Scholar]
- Tamano S, Hagiwara A, Shibata MA, Kurata Y, Fukushima S, Ito N. Spontaneous tumors in aging (C57BL/6N × C3H/HeN) F1 (B6C3F1) mice. ToxicolPathol. 1988;16:321–326. doi: 10.1177/019262338801600302. [DOI] [PubMed] [Google Scholar]
- Teitz T, Chang JC, Kan YW, Yen TS. Thymic epithelial neoplasm in transgenic mice expressing SV40 T antigen under the control of an erythroid-specific enhancer. JPathol. 1995;177:309–315. doi: 10.1002/path.1711770314. [DOI] [PubMed] [Google Scholar]
- Thomas P, Said JW, Nash G, Banks-Schlegel S. Profiles of keratin proteins in basal and squamous cell carcinomas in the skin. An immunohistochemical study. LabInvest. 1984;50:36–41. [PubMed] [Google Scholar]
- Truong LD, Mody DR, Cagle PT, Jackson-York GL, Schwartz MR, Wheeler TM. Thymic carcinoma. A clinicopathologic study of 13 cases. Am. J. SurgPathol. 1990;14:151–166. doi: 10.1097/00000478-199002000-00007. [DOI] [PubMed] [Google Scholar]
- Tseng SC, Jarvinen MJ, Nelson WG, Huang JW, Woodcock-Mitchell J, Sun TT. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982;30:361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Van Dyke TA, Finlay C, Miller D, Marks J, Lozano G, Levine AJ. Relationship between simian virus 40 large tumor antigen expression and tumor formation in transgenic mice. JVirol. 1987;61:2029–2032. doi: 10.1128/jvi.61.6.2029-2032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Rehm S. Applications of immunohistochemistry in rodent tumor pathology. ExpPathol. 1990;40:301–312. doi: 10.1016/s0232-1513(11)80317-8. [DOI] [PubMed] [Google Scholar]