Abstract
Worldwide, breast cancer is the second leading cause of cancer death among women and the third most common cancer. Although our understanding of the molecular basis of this fatal disease has improved, this malignancy remains elusive. Melatonin (Mel), retinoic acid (RA) and Nigella sativa (NS) are substances with anticancer effects. To date, our understanding of the mechanisms of therapeutic effects of these products in mammary cancer is still marginal. To look at the preventive and therapeutic values of these products, we carried out this investigation. An animal model formed of 80 rats was established. The animals were divided into eight groups of 10 animals each: (a) control group injected with the same vehicle used for treatments in the relevant dosages and routes; (b) carcinogen group injected with the known carcinogenic substance 7,12-di-methylbenz(a)anthracene (DMBA) that induces mammary carcinoma; (c) three prophylactic (Pro) groups (Mel-Pro, RA-Pro and NS-Pro) injected with test substances (Mel, RA and NS, respectively) 14 days before the intake of the carcinogenic substance DMBA and then continued until the end of the experiments; and (d) three treated (Tr) groups (Mel-Tr, RA-Tr and NS-Tr) injected with the vehicles after the intake of DMBA. In both the Pro and Tr groups, the drugs were daily administered for 3 months. The animals were killed, and their serum and tissues were evaluated for (a) markers of tumorigenicity [serum levels of total sialic acid (TSA) and lipid-bound sialic acid (LSA)], (b) markers of endocrine derangement (serum prolactin, estradiol and progesterone levels), (c) apoptotic changes [serum tumour necrosis factor (TNF)-α, tissue caspase-3 activity, percentage of DNA fragmentation and ultrastructural features of apoptosis] and (d) markers of oxidative stress (tissue levels of lipid peroxides and nitric oxide). Carcinoma was absent both in the control and in the NS-Pro groups. Mammary carcinoma occurred in DMBA and other Pro and Tr groups. The frequency of mammary carcinoma was high in the carcinogen DMBA group (60%), followed by the Tr (56%) and finally the Pro groups (33%). These tumours included papillary, comedo and cribriform carcinomas. As compared with the control group, the development of carcinoma in the carcinogen DMBA group was associated with increased levels of (a) markers of tumorigenicity (77.0 ± 3.3 vs. 209.0 ± 5.6 and P < 0.05 for TSA; 28.7 ± 1.7 vs. 41.8 ± 1.2 and P < 0.01 for LSA), (b) markers of endocrine derangement (2.5 ± 0.1 vs. 3.6 ± 0.3 and P < 0.05 for prolactin; 39.6 ± 1.3 vs. 24.8 ± 2.1 and P < 0.01 for progesterone and 31.0 ± 0.7 vs. 51.1 ± 3.4 and P < 0.01 for estradiol) and (c) markers of oxidative stress (2.3 ± 0.2 vs. 5.2 ± 0.7 and P < 0.01 for lipid peroxides and 4.4 ± 0.2 vs. 7.6 ± 0.8 and P < 0.01 for nitric oxide). Also, it was associated with decreased levels of markers of apoptotic activity (20.8 ± 1.1 vs. 13.4 ± 0.7 and P < 0.01 for caspase-3; 29.0 ± 1.7 vs. 20.9 ± 1.3 and P < 0.05 for percentage of DNA fragmentation; and 9.4 ± 0.8 vs. 52.1 ± 3.3 and P < 0.01 for TNF-α). When compared with the carcinogen DMBA group, the development of carcinoma in the Pro and Tr groups was associated with decreased levels of (a) markers of tumorigenicity, (b) markers of endocrine derangement and (c) markers of oxidative stress. Alternatively, carcinogenicity was associated with statistically significant (P < 0.01) increased levels of markers of apoptotic activity. To conclude, the administration of Mel, RA and NS reduced the carcinogenic effects of DMBA, suggesting a protective role. The possible underlying mechanisms of these effects await further investigations.
Keywords: breast, cancer, melatonin, Nigella, retinoic acid
Breast cancer is a major health problem in women in both developing and developed countries. One in 10 of all new cancers diagnosed worldwide each year is a cancer of the female breast. Also, it is the principal cause of death from cancer among women worldwide (Bray et al. 2004). The development of breast cancer is associated with (a) perturbations of the delicate balance between cell proliferation and apoptotic cell loss, (b) alterations in the oxidative stress and (c) endocrine derangement (Hussein & Ismael 2004). The latter includes increased prolactin level with induction of several apoptosis-suppressor genes, and increased oestrogen and decreased progesterone levels. In the breast, oestrogens are considered as complete carcinogens, with metabolic effects leading to the formation of reactive oxygen species. In this respect, oestrogen-mediated oxidative DNA damage in the mammary gland epithelium involves the induction of 8-oxo-2′-deoxyguanosine (Mobley & Brueggemeier 2004). Taken as a whole, the endocrine derangement that occurs in the human breast has an important impact on the development of breast cancer (Russo & Russo 1996; Gregoraszczuk et al. 2001; Peck et al. 2002; Portier 2002; Hussein & Ismael 2004).
In mammary carcinogenesis, apoptosis is mediated by several molecules such as tumour necrosis factor-alpha (TNF-α) and caspases. The former belongs to the death receptor gene superfamily. It is an inflammatory cytokine produced both by macrophages and lymphocytes. It exerts cytolytic or cytostatic activity against tumour cells. It is also responsible for a diverse range of signalling events within cells leading to necrosis or apoptosis. Moreover, caspase-3 (cysteinyl aspartate proteinase) is one of the cysteine proteases which plays a major role in the execution of apoptosis under oxidative stress conditions (Idriss & Naismith 2000; Kumar et al. 2000; Hussein et al. 2005). Oxidative stress results from the excessive production of reactive oxygen species that exhaust antioxidant defences. These stress conditions cause DNA damage, resulting in the production of mutated tumour-suppressor genes (Kang et al. 1997; Hussein et al. 2005). Also, oxidative stress has a role in the initiation and progression of breast cancer (Mobley & Brueggemeier 2004; Hussein et al. 2005).
Recent studies showed that some substances such as melatonin (Mel, N-acetyl-5-methoxy tryptamine), retinoic acid (RA) and Nigella sativa (NS) have potential protective effects in cancer (Hussein et al. 2005). Mel, a secretory product of the pineal gland, is a powerful antioxidant that not only scavenges the hydroxyl radical (Tan et al. 2002; Hussein et al. 2005), but also inhibits the production of nitric oxide (NO) by reducing NO synthase (Stasica et al. 1998). Mel can enter the nucleus where it protects DNA from oxidative damage, thereby decreasing the incidence of cancer (Reiter et al. 1999). Also, Mel can modulate the immune response and inhibit the development of hormone-dependent cancer (Maestroni & Conti 1989).
Retinoids are prototypical differentiation agents that play critical roles in the normal cellular functions and differentiation and as such they inhibit cell proliferation in breast cancer. These roles are achieved by different mechanisms such as (a) induction of differentiation and apoptosis, (b) modulation of oncogene expression and (c) modification of cell-membrane glycoprotein and glycolipids in a way that alters both cell-to-cell communication and cell adhesion (Miller 1998). NS is an annual herbaceous plant grown in Mediterranean countries. Its beneficial antineoplastic effects are related to its cytoprotective and antioxidant actions (Ali & Blunden 2003). Its administration inhibits the two-stage initiation/promotion both in squamous cutaneous tumorigenesis and colorectal carcinogenesis (Salim & Fukushima 2003; El-Metwally et al. 2005).
To date, our understanding of the possible protective effects of these substances (MEL, RA and NS) in the breast cancer is still incomplete. In this study, we hypothesized that these alterations include endocrine derangement, enhancement of apoptosis and inhibition of oxidative stress. An animal model formed of 80 rats was established. Mammary cancer was induced by intraperitoneal injection of the carcinogen. The animals were divided into four major groups: (a) control group, (b) carcinogen group, (c) prophylactic (Pro) group and (d) treatment (Tr) group. Mammary cancer was induced by the carcinogen DMBA, and the effects of these substances were examined by evaluation of (a) the serum levels of total sialic acid (TSA) and lipid-bound sialic acid (LSA) (markers of tumorigenicity), (b) the serum levels of prolactin, estradiol and progestrone (markers of endocrine derangement), (c) the serum and tissue levels of TNF-α, caspase-3 activities, percentage of DNA fragmentation as well as ultrastructural features of apoptosis (markers of apoptosis); and (d) lipid peroxidation and NOs (markers of oxidative stress). Our study clearly demonstrated that the administration of these substances reduced the carcinogenic effects of DMBA, suggesting a protective role.
Materials and methods
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Assuit University, School of Medicine, Assuit, Egypt.
Rats and maintenance
Six-week-old female Sprague-Dawley rats were obtained from Assuit University Animal Facility, Faculty of Medicine, Assuit University, Assuit, Egypt. They were housed in an animal facility, with the room temperature maintained at 27 °C, relative humidity of 50–70% and an airflow rate of 15 exchanges per hour. Also, a time-controlled system provided 7/21 h (light/dark) cycles. This controlled system was used because (a) Mel secretion is high in the dark cycles and low in the light cycles and (b) to ensure that the secretion of Mel is comparable to that under the normal physiological states. All rats were given ad libitum access to Taklad rodent chow diet and water from sanitized bottle fitted with stopper and sipper tubes. These conditions were adopted following the methods of Vijayalaxmi et al. (1999); and Hussein et al. (2005).
Chemicals of the study
The carcinogen DMBA and the vehicles.
The chemicals (DMBA, Mel and RA) were purchased from Sigma Chemical Company (St Louis, MO, USA). NS was obtained from Pharco, Cairo, Egypt). The DMBA [7,12-di-methylbenz(a)anthracene] was used to induce mammary carcinoma in rats (1 g was dissolved in 25% dimethyl sulphoxide (DMSO) to make a total of 100 ml solution). Mel was prepared by dissolving 50 mg in 2 ml ethanol and diluted to 100 ml with distilled water to make 0.2% solution. NS oil cannot be taken orally. Also, it is not miscible with water, and hence it must be prepared in the form of emulsion. A total of 0.5 ml emulsion of NS was prepared as follows: 8 g of the NS oil was mixed with 50 ml distilled water. The mixture was emulsified with 2% polyethylene glycol 400 (0.01 mg/100 g body weight). Finally, distilled water was added to final volume of 100 ml water. Therefore, each 1 ml of this emulsion contained 80 mg NS oil. At the start of the experiments, several concentrations were tried and we found that 400 mg NS/100 g body weight was able to provide the maximal protective effects. The emulsion of NS was given orally. All-trans RA was prepared by dissolving 200 mg RA in 6.6 ml DMSO. Each 100 µg of the solution was diluted in 5 ml cotton oil (RA, 200 mg/100 g body weight). The RA solutions were used under yellow light.
Evaluation of markers of tumorigenicity.
The following reagents were used for evaluation of the serum levels of TSA: (a) resorcinol (10 ml of 2%, w/v, resorcinol in distilled water + 9.75 ml distilled water + 0.25 ml 0.1 m CuSO4 and complete to 100 ml with concentrated HCl); (b) butyl acetate/n-butanol, v/v, 85 : 15; and (c) standards formed of N-acetyl-neuraminic acid (NANA), 0.0, 12.5, 25, 37.5, 50, 100 and 200 ng/dl, in distilled water. Alternatively, for the evaluation of the serum levels of LSA, the following reagents were used: (a) resorcinol (10 ml of 2%, w/v, resorcinol in distilled water + 9.75 ml distilled water + 0.25 ml 0.1 m CuSO4 and complete to 100 ml with Conc./concentrated HCl); (b) butyl acetate/n-butanol, v/v, 85 : 15); (c) chloroform/methanol, 2 : 1, v/v; (d) phosphotungstic acid, 1 g/ml distilled water; and (e) standards formed of NANA, 0.0, 12.5, 25, 37.5, 50, 100 and 200 ng/dl, in distilled water.
Evaluation of hormonal levels.
Serum levels of estradiol, progesterone and prolactin were measured using enzyme-linked immunosorbent assays (ELISA) kits (following the manufacturer instructions).
Evaluation of apoptotic markers.
For the determination of rat TNF-α, ELISA kit was used (Catalogue no. KPC3011; Biosource International, Camarillo, CA, USA). The caspase-3 proteolytic activity was determined using a calorimetric assay kit (Caspase-3/CPP32, ApoTarget, Coloremetric Protease Assay kit, Catalogue no. KHZ0022; Biosource International). The reagents used for determination of percentage of DNA fragmentation included (a) 7.0 m perchloric acid; (b) 0.088 m diphenylamine in (98%, v/v; glacial acetic acid: 1.5%, v/v; sulphuric acid: 0.5%, v/v, of 1.6% acetaldehyde); and (c) standards formed of fragmented DNA dissolved in hot water to 0.0, 0.25, 0.5, 1, 5, 10 and 20 mg/ml.
Evaluation of oxidative stress markers.
For the evaluation of tissue lipid peroxides, the following reagents were used: (a) 0.375%, w/v, thiobarbituric acid (TBA) in (15%, w/v, trichloroacetic acid and 0.25N HCl solution in bi-distilled water; (b) 0.8 w/v, butylated hydroxytoluene (BHT) in hexane and (c) standards (1,1,3,3-tetraethoxypropane as malondialdehyde (MDA) precursor in bi-distilled water, 0.25, 0.5, 1.0, 2.0, 4.0, 8.0 and 16.0 µm/l).
Carcinogenic substance DMBA and the induction of mammary carcinoma
Several experiments were conducted using a total of 80 rats, 10 animals in each group. After a 7-day acclimatization period, a randomized block design based on the animal body weights was used to divide the rats into four major groups. The control group received the same vehicle corresponding to the dose and route of each chemical used and each treatment performed. The animals in the carcinogenic DMBA group were injected intraperitoneally with the DMBA to induce mammary carcinoma. The Pro group received the vehicles before the injection of the carcinogenic substance DMBA. The Pro group included three subgroups: (a) Mel-Pro subgroup, (b) RA-Pro subgroup and (c) NS-Pro subgroup. The Tr group received the corresponding chemical after the injection of the carcinogenic substance DMBA. The treatment group included three subgroups: (a) Mel-Tr subgroup; (b) RA-Tr subgroup and (c) NS-Tr subgroup.
In the DMBA group, each animal (age ranging from 50–60 days) was injected intraperitoneally with a single dose of 0.5 ml (10% DMSO) containing 10 mg DMBA. In the Pro group, the products were administered 14 days before the injection of DMBA and then continued until the end of the experiments. The 14-day time period before challenging the Pro groups was chosen to ensure sufficient time for the products to achieve their proper concentrations in the different tissues. In both the Pro and Tr groups, the drugs were daily administered for 3 months as follows: (a) Mel was injected subcutaneously in a dose of 250 µg Mel/100 g body weight in volume of 0.5 ml saline; (b) RA was given in an oral dose of 20 µg RA/100 g body weight in a volume of 0.5 ml cotton oil; and (c) NS was given orally in a dose of 400 mg NS/100 g body weight in 0.5 emulsion of NS extract. The time of Mel injection was adjusted to be 3 h before sunset. In the Tr group, the drugs were administered after the appearance of a palpable mass and continued for 3 months.
Specimens.
All the animals were killed at the end of the experiments. The blood samples were collected via retro-orbital puncture (from optic vein) in sterile tubes without anticoagulant. The blood was left to clot at room temperature, then centrifuged and the serum was separated for biochemical assays. The breast tissue samples were obtained, divided into three parts; one part was frozen at 70 °C for biochemical assays, second part was fixed in 10% phosphate-buffered formalin for routine histology and the third part was fixed in 5% glutaraldehyde for electron microscope studies.
Biochemical evaluations
Evaluation of serum estradiol, progesterone and prolactin levels.
Serum levels of estradiol, progesterone and prolactin were measured using enzyme-linked immunoassay kits (Catalogue no. 4006200, Catalogue no. 4014500 and Catalogue no. 730201, respectively, Biosource International) according to the method described by Abraham (1974).
Evaluation of the apoptotic changes.
The following parameters were examined to evaluate apoptosis: serum TNF-α, caspase-3 activity, percentage of DNA fragmentation and ultrastructural features of apoptosis. The serum TNF-α level was measured using BioSource Enzyme Linked Immunoassay kit (Catalogue no. KPC301, Biosource International) as described by Stepaniak et al. (1995). The caspase-3 was determined using Aoptarget caspase-3 protease, calorimetric assay kit (Catalogue no. KH20022, Biosource Europe, Brussels, Belgium). The caspase-3 proteolytic activity was determined using a modified procedure described by Kim et al. (2000). Briefly, cytosolic extracts were prepared by homogenization of mammary specimens (50 mg) in lysis buffer. Subsequently, the homogenates were centrifuged at 13,000 × g for 15 min at 4 °C. The supernatant was used to determine caspase-3 activity. A 50 µl of reaction buffer was added to 50 µl of each sample (cytosol extract) and then 50 µl of diluting buffer was added. A total of 10 µl of DEVD-pNA substrate was added to each tube and incubated at 37 °C for 2 h. The samples were kept in the dark during incubation. The protein concentrations were assayed using the methods of Kurita-Ochiai et al. (1999). The samples were read at 405 nm in a spectrophotometer (Schemazu type, Tokyo, Japan) using regular cuvette. The substrate DEVD-pNA was composed of the chromophore p-nitroanilide and a synthetic tetra peptide, DEVD (Asp-Glue-Val-Asp).
The percentage of DNA fragmentation was determined by following the methods of Kurita-Ochiai et al. (1999). The substrate paranitroanillide and the chemicals for DNA fragmentation were obtained from Sigma. Briefly, tissue samples were lysed by homogenization in 0.2% Triton X-100 (2 ml), 10 mm Tris (10 ml), 1 mm EDTA (1 ml), pH 8.0, incubated on ice for a few minutes, homogenized again and centrifuged at 14,000 × g for 15 min. The supernatant, pellet and the homogenates were used for fragmented, intact and total DNA assays, respectively. Perchloric acid was added to the sample to make 0.5 m, i.e. one part of the stock 70% (7 m) acid : 13 parts of sample (20 µl perchloric acid + 260 µl sample), mixed well and left for 10 min. A total of 300 µl reagent, 150 µl sample/standard/distilled water blank was incubated for 48 h. Optical density (OD575 nm) was measured against reagent blank, and sample content of fragmented DNA was calculated from the standard curve. For tissues, the ratio of DNA in supernatant and pellet of the total was calculated.
Evaluation of markers of tumorigenicity in mammary carcinoma.
Sialic acid, a family of acetylated derivatives of neuraminic acid, is found either as TSA or LSA. Its level rapidly increases in breast cancer (Sonmez et al. 1999; Raval et al. 2003). TSA and LSA, in tissues, were determined according to the method described by Plucinsky et al. (1986). For determination of TSA, 20 µl serum/standard + 980 µl distilled water was mixed and put on ice for 20 min + 1.0 ml resorcinol reagent, mixed and boiled in water bath for 15 min, cooled on ice for 10 min. Then, 2.0 ml butyl acetate/n-butanol was added and vortexed for 1 min to extract the chromogen. Centrifugation was done at 3000 rpm for 10 min. Finally, the OD of supernatant was determined at 580 nm against water blank. For determination of LSA, 50 µl serum/standard + 150 µl H202 (36%) on ice was vortexed and put on ice + 3 ml cold chloroform/methanol. Then, 0.5 ml cold water was added, vortexed and centrifuged at 3000 rpm for 5 min. One millilitre of supernatant was transferred in a new tube and 50 µl phosphotungstic acid was added, vortexed and incubated for 5 min at 25 °C and then centrifuged at 3000 rpm for 5 min. The pellet was dissolved in 1.0 ml distilled water, and 1.0 ml resorcinol reagent was added, mixed and boiled in water bath for 15 min, cooled on ice for 10 min, and 2 ml butyl acetate/n-butanol was added and vortexed for 1 min to extract the chromogen. Centrifugation was done at 3000 rpm for 10 min. Finally, the OD of supernatant was read at 580 nm against water blank.
Lipid peroxidation.
The procedure was based on the formation of the lipid peroxidation product MDA which reacts with thiobarbituric acid to generate a coloured product. The latter can be determined colorimetrically as thiobarbituric acid-reactive substances (Satoh 1978). Lipid peroxide levels in the tissues were measured according to the method described by Satoh (1978). Equal volume (200 µl) of the TBA reagent and samples or standards was mixed with 10 µl BHT solution (butylated hydroxytoluene), incubated at 100 °C for 15 min and left to cool. Centrifugation at 3000 rpm for 10 min was done. Absorbance at 535 nm of the supernatant was recorded against distilled water blank. Calculations were done using the following equation: lipid peroxides (nmol/ml) = absorbance × 6 × 106/1.56 × 105. Then, by dividing the previous results by milligram protein concentration in tissues to give lipid peroxides (nmol/mg protein).
NO measurement.
NO levels in the tissues were measured by the evaluation of total nitrate and nitrites using a method described by van Bezooijen et al. (1998). Briefly, 260 µl tissue homogenate was mixed with 26 µl ZnSO4 (30%, w/v in H2O), incubated for 15 min at room temperature and then centrifuged (10,000 rpm) for 15 min. Cadmium was activated twice with 2% HCl (diluted to 37% solution, 54 ml + 946 ml H2O, v/v) for 30 min under continuous shaking. The activated cadmium (90 µl) was washed extensively (10 times) with distilled water with shaking. Cadmium was prepared by soaking zinc rods in 20% cadmium sulphate, w/v in H2O, overnight, then the formed cadmium precipitate was isolated, washed, activated and washed again with H2O as above and was stored under H2O. Nitrate-to-nitrite reduction was done by mixing 200 µl tissue supernatant with 80 µl of a mixture of three parts of NH4Cl (2.6%, w/v in H2O), one part of sodium borate (borax, 2.1%, w/v in H2O) and activated cadmium. The mixture was incubated for 30 min at room temperature and centrifuged at 10,000 rpm for 10 min.
Morphological analysis
Histopathological measurements.
Morphological examination of the tissue specimens was done following the method of Hussein et al. (2005). Mammary gland tissues obtained from the animals were examined for pathological changes. For each animal, a part of the tissue was fixed in 10% phosphate-buffered formalin for 24 h. After fixation, specimens were dehydrated and embedded in paraffin. The blocks were sectioned at a 5 µm thickness and stained with haematoxylin and eosin for routine histological evaluation (light microscopy).
Transmission electron microscopy.
Some tissue fragments were fixed in 5% glutaraldehyde in 0.1 m sodium cacodylate buffer at 4 °C and pH 7.2 for 24 h, washed in 0.1 m buffer, postfixed in osmium tetroxide in 0.2 m buffer for 1 h. The specimens were dehydrated in 70, 90 and 100% ethanol and then embedded in labelled capsules with freshly prepared resin and left to polymerize at 60 °C for 48 h. Several resin-semithin sections were cut at about 1 μm using glass knives and an ultramicrotome, then stained with 1% toluidine blue in 1% borax solution for 1 min at 80 °C. The stain was rinsed off with distilled water, and the sections were dried and examined. Selective areas from the trimmed blocks were cut by using a diamond knife, with the ultramicrotome set to cut at around 50–70 nm using heat advances. The sections were picked up onto 300-mesh copper grids stained with previously prepared methanolic uranyl acetate and examined by transmission electron microscopy. Some of the examined fields were photographed (Hussein et al. 2003- a; Hussein et al. 2005).
Statistical analysis
Statistical analysis was performed using multivariate anova test (analysis of variance). The results were presented as mean ± standard deviation (SD). P-value < 0.05 was considered statistically significant.
Results
The examination of the animals (carcinogen DMBA, Pro and Tr groups) revealed the development of mammary carcinomas featuring the presence of both morphological and biochemical alterations as described:
Histopathological studies
Initial examination revealed the development of variable-sized (0.5–2.5 cm) single or multiple masses involving the mammary gland tissues of some animals in the DMBA, Pro and Tr groups. The tumours were usually seen in the inguinal regions (anatomical sites of the mammary glands in rats). Total dimensions of the lesions were measured to the nearest millimetre. The cut surface of some tumours showed areas of haemorrhage and necrosis with occasional cystic changes. Also, some of these tumours were associated with ulceration of the overlying skin. The frequency of carcinomas was high in the DMBA group followed by the Tr group and finally the Pro-one (6, 5, 6, 1, 4 and 5 animals belonging to DMBA, Mel-Pro, Mel-Tr, RA-Pro, RA-Tr and NS-Tr groups, respectively). Carcinomas were absent in both the control and NS-Pro groups.
Histologically, several types of mammary carcinomas were identified including comedo, papillary and cribriform carcinomas. In the papillary carcinoma, uniformly malignant ductal epithelial cells (pleomorphism, hyperchromatism and nucleomegally) were seen growing in layers lining fibrovascular connective tissue cores. The latter was infiltrated by lymphocytes and histiocytes, i.e. histological features of regression. In the cribriform carcinoma, the tumour was formed of solid sheets of malignant ductal epithelial cells interrupted by variable-sized rounded lumina. Infiltration of the surrounding stroma by strands of the malignant cells, histiocytes and lymphocytes was also seen. Occasionally, the tumour cells infiltrated the overlying dermis, adjacent fibroconnective tissue and underlying skeletal muscles. The comedo carcinoma was formed of distended ductal structure lined by multilayered epithelium and centrally located necrotic debris. The malignant cells were present at the periphery of the glandular lumina. The surrounding stromal reaction was formed of admixture of lymphocytes, histiocytes and fibroblasts (signs of tumour regression) (Figures 1–4).
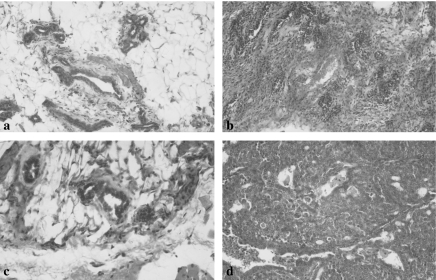
Figure 1.
Histological changes in the control (a), and both Nigella sativa (NS) prophylactic (Pro) (b, c) and treated (d) groups. (a) The mammary gland tissues in the control group is formed of admixture of fibrofatty tissues and ductal structures. (b, c) Absence of malignant changes in the mammary glands of the NS-Pro groups with the presence of variable fibrosis and inflammatory cell infiltrate. (d) The development of carcinoma in NS treated groups was evident by the presence of uniformly malignant ductal epithelial cells growing in vague cribriform pattern (×200).
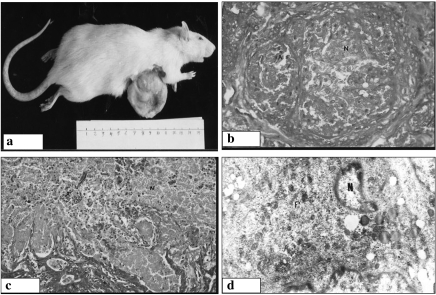
Figure 4.
Histological and ultrastructural features of mammary carcinoma in the retinoic acid (RA) prophylactic (Pro) (a, b) and melatonin (Mel) treated (c, d) groups. (a) Malignant cells with rounded-to-oval nuclei, inconspicuous nucleoli and cytoplasm arranged in solid pattern with extensive necrotic areas (×200). (b) Malignant ductal epithelial cell featuring the presence of relatively regular nuclear contour, slightly increased euchromatin mass, chromatin clumps and the formation of apoptotic bodies (×14,000). (c) Malignant cells with rounded-to-oval nuclei, inconspicuous nucleoli and cytoplasm arranged in both tubular and solid patterns with extensive areas of necrosis (×200). (d) Malignant cell with irregular nuclear membrane, increased euchromatin mass, cytoplasmic vacuolization and the formation of a large apoptotic body (×14,000).
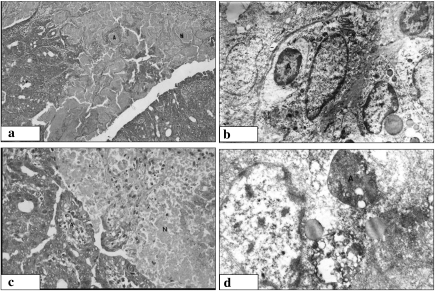
Figure 2.
Gross (a), histological (b) and ultrastructural (c, d) features of mammary carcinoma in the carcinogen DMBA group. (a) Ulcerating, roundedto- oval tumour mass with extensive areas of haemorrhage and necrosis. (b) Malignant cells with rounded-to-oval nuclei, inconspicuous nucleoli and cytoplasm arranged in solid pattern with frequent papillary formations (·200). (c) Malignant ductal epithelial cell with ultrastructural features of malignancy including irregularity of the nuclear contour, abundance of the euchromatin, formation of both marginal chromatin masses and nuclear pseudopockets. (d) Hypertrophied rough endoplasmic reticulum (·14,000).
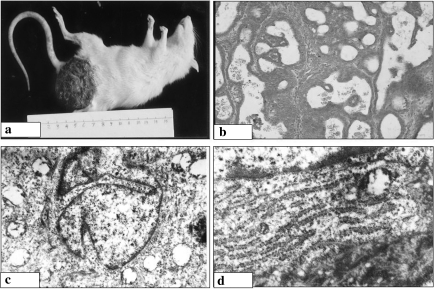
Figure 3.
Gross (a), histological (b, c) and ultrastructural (d) features of mammary carcinoma in the melatonin (Mel)-prophylactic (Pro) group. (a) Well circumscribed, rounded tumour mass without areas of haemorrhage or necrosis. (b) Malignant cells with rounded-to-oval nuclei, inconspicuous nucleoli and cytoplasm arranged in comedo pattern with extensive central necrosis (·200). (c) Malignant cells with rounded-to-oval nuclei, inconspicuous nucleoli and cytoplasm displaying ductal pattern with extensive areas of necrosis (·200). (d) Malignant ductal epithelial cell featuring the presence of half-moon nucleus, relatively regular nuclear contour, increased euchromatin mass, chromatin margination and formation of apoptotic bodies (·14,000).
Ultrastructural changes.
As compared to control group, examination of the tumours of the DMBA, Pro and Tr groups revealed ultrastructural features of malignancy including: (a) the nuclei were pleomorphic with irregular contour and increased euchromatin, formation of marginal chromatin masses and nuclear pseudopockets; and (b) the cells have less cytoplasmic area and the cytoplasmic organelles, especially the rough endoplasmic reticulum was hypertrophied, forming localized aggregates. Further, ultrastructural evaluation of the tumour cells revealed the presence of more frequent apoptotic changes in the Pro and Tr groups than in the carcinogen DMBA group. These changes included reduction in the cytoplasmic and nuclear volume, extensive cytoplasmic vacuolization, condensation of the nuclear chromatin and the formation of numerous apoptotic bodies (Figures 1–4).
Biochemical alterations
The biochemical alterations included changes in the levels of markers of tumorigenicity, endocrine derangement, oxidative stress and apoptotic activity.
Decreased serum levels of the markers of tumorigenicity in the Pro and treated groups.
When the markers of tumorigenicity were examined in the control and DMBA groups, there was a statistically significant increase in the TSA and LSA levels, respectively, in the latter (77.0 ± 3.3 vs. 209.0 ± 5.6 and 28.7 ± 1.7 vs. 41.8 ± 1.2, P < 0.01). Alternatively, as compared to DMBA group, there was a statistically significant decrease in the TSA and LSA levels in the Pro (209.0 ± 5.6 vs. 111.3 ± 4.7 vs. 115.3 ± 5.0 vs. 159.8 ± 6.3, P < 0.01 for TSA and 41.8 ± 1.2 vs. 27.5 ± 1.2 vs. 29.7 ± 2.0 vs. 34.5 ± 2.3, P < 0.05 for LSA) and Tr (209.0 ± 5.6 vs. 130.0 ± 4.7 vs. 126.0 ± 5.0 vs. 162.3 ± 4.2, P < 0.01 for TSA and 41.8 ± 1.2 vs. 27.0 ± 1.3 vs. 25.8 ± 1.5 vs. 39.8 ± 2.0, P < 0.05 for LSA) groups. There were no statistically significant differences between the Pro and Tr groups. The results are summarized in Table 1.
Table 1.
Serum levels of total sialic acid and lipid-bound sialic acid
| Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Control | DMBA | Pro-Mel | Pro-RA | Pro-NS | Tr-Mel | Tr-RA | Tr-NS |
| Total sialic acid (ng/dl) | 77.0 ± 3.3 | 209.0 ± 5.6** | 111.3 ± 4.7** | 115.3 ± 5.0** | 159.8 ± 6.3** | 130.0 ± 4.7** | 126.0 ± 5.0** | 162.3 ± 4.2** |
| Lipid bound sialic acid (ng/dl) | 28.7 ± 1.7 | 41.8 ± 1.2** | 27.5 ± 1.2 | 29.7 ± 2.0 | 34.5 ± 2.3* | 27.0 ± 1.3 | 25.8 ± 1.5 | 39.8 ± 2.0** |
DMBA, 7,12-di-methylbenz(a)anthracene; Mel, melatonin; NS, Nigella sativa; Pro, prophylactic; RA, retinoic acid; Tr, treated. Values represent mean ± standard error of mean.
P < 0.05.
P < 0.01.
Decreased serum levels of markers of endocrine derangement in the Pro and treated groups.
As compared to the control group, there was a statistically significant increase in the prolactin and estradiol levels in DMBA group (2.5 ± 0.1 vs. 3.6 ± 0.3 and 31.0 ± 0.7 vs. 51.1 ± 3.4, P < 0.01). Alternatively, as compared to DMBA group, there was a statistically significant decrease in the prolactin and estradiol levels in the Pro (3.6 ± 0.3 vs. 2.4 ± 0.1 vs. 2.9 ± 0.1 vs. 3.0 ± 0.2 for prolactin and 51.1 ± 3.4 vs. 25.9 ± 1.2 vs. 30.0 ± 1.9 vs. 45.1 ± 2.5, P < 0.05 for estradiol) and Tr groups (3.6 ± 0.3 vs. 2.8 ± 0.2 vs. 2.8 ± 0.1 vs. 3.2 ± 0.2 for prolactin and 51.1 ± 3.4 vs. 28.5 ± 1.6 vs. 31.9 ± 1.8 vs. 44.5 ± 2.4, P < 0.05 for estradiol). When compared to control group, there was a statistically significant decrease in the serum progesterone level in DMBA (39.6 ± 1.3 vs. 24.8 ± 2.1, P < 0.05). There were no statistically significant differences between the progesterone levels in DMBA and in both Pro (13.5 ± 1.7 vs. 22.5 ± 2.6 vs. 29.2 ± 4.0) and Tr (23.4 ± 2.6 vs. 23.6 ± 3.0 vs. 25.5 ± 3.6) groups. There were no statistically significant differences between the Pro and Tr groups. The results are summarized in Table 2.
Table 2.
Serum levels of prolactin, estradiol and progesterone
| Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Control | DMBA | Pro-Mel | Pro-RA | Pro-NS | Tr-Mel | Tr-RA | Tr-NS |
| Prolactin (ng/ml) | 2.5 ± 0.1 | 3.6 ± 0.3 | 2.4 ± 0.1 | 2.9 ± 0.1 | 3.0 ± 0.2 | 2.8 ± 0.2 | 2.8 ± 0.1 | 3.2 ± 0.2* |
| Estradio (pg/ml) | 31.0 ± 0.7 | 51.1 ± 3.4** | 25.9 ± 1.2 | 30.0 ± 1.9 | 45.1 ± 2.5** | 28.5 ± 1.6 | 31.9 ± 1.8 | 44.5 ± 2.4** |
| Progesteron (ng/ml) | 39.6 ± 1.3 | 24.8 ± 2.1** | 13.5 ± 1.7** | 22.5 ± 2.6** | 29.2 ± 4.0 | 23.4 ± 2.6** | 23.6 ± 3.0** | 25.5 ± 3.6** |
DMBA, 7,12-di-methylbenz(a)anthracene; Mel, melatonin; NS, Nigella sativa; Pro, prophylactic; RA, retinoic acid; Tr, treated. Values represent mean ± standard error of mean.
P < 0.05.
P < 0.01.
Increased levels of caspase-3, percentage of DNA fragmentation and TNF-α in the Pro and treated groups.
When the markers of apoptotic activity were compared among the control, Pro and treated groups, there was a statistically significant decrease in the levels of caspase-3 and percentage of DNA fragmentation except for TNF-α in the control and in the DMBA groups (20.7 ± 1.1 vs. 13.4 ± 0.7, 29.0 ± 1.7 vs. 20.9 ± 1.3 and 9.4 ± 0.8 vs. 52.1 ± 3.3, P < 0.01). Alternatively, as compared to DMBA group, there was a statistically significant increase in the levels of caspase-3, percentage of DNA fragmentation and TNF-α in both the Pro (13.4 ± 0.7 vs. 35.2 ± 2.6 vs. 36.1 ± 1.8 vs. 22.5 ± 1.5, P < 0.01 for caspase-3, 20.9 ± 1.3 vs. 40.2 ± 3.0 vs. 42.1 ± 2.5 vs. 27.1 ± 0.9, P < 0.01 for DNA fragmentation and 52.1 ± 3.3 vs. 126.8 ± 11.3 vs. 128.4 ± 12.0 vs. 86.7 ± 4.8, P < 0.01 for TNF-α) and Tr (13.4 ± 0.7 vs. 35.7 ± 3.1 vs. 38.4 ± 4.1 vs. 22.1 ± 1.3, P < 0.01 for caspase-3; 20.9 ± 1.3 vs. 37.1 ± 1.7 vs. 34.0 ± 2.0 vs. 26.3 ± 0.8, P < 0.05 for percentage of DNA fragmentation and 52.1 ± 3.3 vs. 110.3 ± 10.8 vs. 122.9 ± 11.4 vs. 82.9 ± 8.6, P < 0.01 for TNF-α) groups. There were no statistically significant differences between the Pro and Tr groups. The results are summarized in Table 3.
Table 3.
Tissue caspase-3 activity, percentage of DNA and serum TNF-α
| Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Control | DMBA | Pro-Mel | Pro-RA | Pro-NS | Tr-Mel | Tr-RA | Tr-NS |
| Caspase-3 (pNA/min/mg protein) | 20.8 ± 1.1 | 13.4 ± 0.7* | 35.2 ± 2.6** | 36.1 ± 1.8** | 22.5 ± 1.5 | 35.7 ± 3.1** | 38.4 ± 4.1** | 22.1 ± 1.3 |
| DNA fragmentation (%) | 29.0 ± 1.7 | 20.9 ± 1.3* | 40.2 ± 3.0** | 42.1 ± 2.5** | 27.1 ± 0.9 | 37.1 ± 1.7* | 34.0 ± 2.0 | 26.3 ± 0.8 |
| TNF-α (pg/ml) | 9.4 ± 0.8 | 52.1 ± 3.3** | 126.8 ± 11.3* | 128.4 ± 12.0** | 86.7 ± 4.8** | 110.3 ± 10.8** | 122.9 ± 11.4** | 82.9 ± 8.6** |
DMBA, 7,12-di-methylbenz(a)anthracene; Mel, melatonin; NS, Nigella sativa; Pro, prophylactic; RA, retinoic acid; TNF, tumour necrosis factor; Tr, treated. Values represent mean ± standard error of mean.
P < 0.05.
P < 0.01.
Decreased tissue levels of the markers of oxidative stress in the Pro and treated groups.
As compared to the control group, there was a statistically significant increase in the tissue levels of lipid peroxides and NO in DMBA group (2.3 ± 0.1 vs. 5.2 ± 0.7 and 4.4 ± 0.2 vs. 7.6 ± 0.8, P < 0.01). As compared to DMBA group, there was a statistically significant decrease in the tissue levels of lipid peroxides and NO in the Pro (5.2 ± 0.7 vs. 2.1 ± 0.2 vs. 2.8 ± 0.6 vs. 3.6 ± 0.4, P < 0.01 for lipid peroxides and 7.6 ± 0.8 vs. 5.0 ± 0.3 vs. 5.4 ± 0.4 vs. 4.2 ± 0.3, P < 0.01 for NO) and Tr (5.2 ± 0.7 vs. 2.6 ± 0.2 vs. 2.9 ± 0.1 vs. 4.1 ± 0.2, P < 0.01 for lipid peroxides and 7.6 ± 0.8 vs. 4.3 ± 0.2 vs. 4.6 ± 0.2 vs. 3.7 ± 0.2, P < 0.01 for NO) groups. There were no statistically significant differences between the Pro and Tr groups. The results are summarized in Table 4.
Table 4.
Lipid peroxides and nitric oxide levels
| Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Control | DMBA | Pro-Mel | Pro-RA | Pro-NS | Tr-Mel | Tr-RA | Tr-NS |
| Lipid peroxides (nmol/mg protein) | 2.3 ± 0.1 | 5.2 ± 0.7** | 2.1 ± 0.2 | 2.8 ± 0.5 | 3.6 ± 0.4 | 2.6 ± 0.2 | 2.9 ± 0.1 | 4.1 ± 0.2* |
| Nitric oxide (nmol/mg protein) | 4.4 ± 0.2 | 7.6 ± 0.8** | 5.0 ± 0.3 | 5.4 ± 0.4 | 4.2 ± 0.3 | 4.3 ± 0.2 | 4.6 ± 0.2 | 3.7 ± 0.2 |
DMBA, 7,12-di-methylbenz(a)anthracene; Mel, melatonin; NS, Nigella sativa; Pro, prophylactic; RA, retinoic acid; TNF, tumour necrosis factor; Tr, treated. Values represent mean ± standard error of mean.
P < 0.05.
P < 0.01.
Discussion
Our knowledge of the mechanisms of therapeutic effects of Mel, RA and NS in mammary cancer is still incomplete. In this investigation, we hypothesized that in mammary carcinogenesis, the administration of Mel, RA and NS is associated with biochemical and morphological alterations including endocrine derangement, enhancement of apoptosis and inhibition of oxidative stress. To characterize these alterations and to test our hypothesis, we carried out this investigation. To accomplish our goals, we established an animal model formed of control, DMBA-injected, Pro and Tr groups. Our study clearly demonstrated several observations. First, administration of DMBA (carcinogen) was associated with development of mammary carcinoma, elevated levels of markers of tumorigenicity, endocrine derangement, oxidative stress and decreased apoptotic activity. Second, the administration of Mel, RA and NS was associated with decrease in the frequency of mammary carcinoma in rats, i.e. possible Pro effects. Third, administration of these products was associated with decreased levels of TSA and LSA, prolactin, estradiol, markers of oxidative stress and enhanced apoptotic activity, i.e. possible therapeutic effects. Finally, mammary carcinoma was absent in the NS-Pro group.
The administration of carcinogen DMBA was associated with the development of mammary carcinomas, elevated levels of markers of tumorigenicity, endocrine derangement, oxidative stress and decreased apoptotic activity. Sialic acid, a family of acetylated derivatives of neuraminic acid, is widely distributed in mammals in two forms, TSA and LSA. Sialic acid occurs as a terminal component at the non-reducing end of carbohydrate chains of glycoproteins and glycolipids. The increase in the serum levels of TSA and LSA after the injection of DMBA is not only in agreement with previous reports (Patel et al. 1990; Raval et al. 1997; Raval et al. 2003) but also suggests their possible diagnostic values in breast cancer. This increase may be explained by enhanced activity of sialidase enzyme in breast cancer (Sonmez et al. 1999).
Our finding of an increased serum levels of prolactin and estradiol in DMBA group is supported by the findings of Li et al. (2002). In this regard, the contribution of prolactin to the pathogenesis and progression of breast cancer is increasingly appreciated. The possible roles of prolactin in mammary carcinogenesis include the induction of several apoptosis-suppressor genes (Krishnan et al. 2001), stimulation of DNA synthesis and ability to increase oestrogen receptors sensitivity. Interestingly, while the intact prolactin molecule is responsible for lactogenesis, its cleavage product (16 kDa fragment) has mitogenic effects, i.e. it stimulates uncontrollable cell division in the epithelial tissues of the mammary gland (Mertani et al. 1998).
The increase in the serum estradiol level is not only in agreement with previous reports (Peck et al. 2002; Vondracek et al. 2002) but also suggests a linkage to mammary carcinogenesis. The underlying carcinogenic effects of oestrogen include stimulation of DNA synthesis, promotion of cell division and induction of synthesis of various peptide growth factors with direct mitogenic influence on mammary tissues. The decrease in the serum progesterone level in DMBA-treated rats not only agrees with other reports (Cheeseman et al. 1988) but also supports its cytoprotective effects against mammary dysplasia (Gregoraszczuk et al. 2001). The decrease in the tissue levels of caspase-3 activity and percentage of DNA fragmentation in the DMBA group as compared to the control group is in line with previous reports (Huigsloot et al. 2001). This reduction may be due to overexpression of caspase-3 inhibitors and survivin in tumour cells (Krajewski et al. 1999). A hypothesis, to be tested, that this decrease may reflect downregulation of death receptors (cell-surface receptors and death domains) and/or mitochondrial pathways (Bcl-2 family of proteins and cytochrome c) of apoptosis (Hussein et al. 2003a, b).
The increased levels of TNF-α in DMBA group, as compared to the control group, agrees with the findings of other studies (Ardizzoia et al. 1992; Bower et al. 2002). This high level may be due to increased production by the tumour-infiltrating lymphocytes and/or by the tumour cells (Lind et al. 1993; Kopreski et al. 1996). The high levels of lipid peroxides and NO in the DMBA group agree with that of previous reports (Jadeski et al. 2002; Jang & Kim 2002; Kumaraguruparan et al. 2002). In support, breast cancer tissues are known to have an enhanced activity of antioxidant enzymes, reflecting increased oxygen-free radicals in these malignant cells. These radicals can promote tumour growth and metastasis by enhancing invasive, angiogenic and migratory capacities of tumour cells (Hussein et al. 2005).
Ultrastructurally, mammary carcinoma, in our series, featured the presence of nucleomegally, more convoluted nuclear borders, dispersed chromatin. These features are indicative of atypical ultrastructural changes. The increased irregularity of the nuclear membrane may be due to the presence of intracytoplasmic filaments. These irregularities provide more areas of contact between the nucleus and the cytoplasm, i.e. nucleocytoplasmic exchange that in turn enhances the metabolic activity of these malignant cells. Also, the presence of relatively more euchromatin in the neoplastic cells suggests their enhanced metabolic activity (Hussein et al. 2003b,2005).
The administration of Mel, RA and NS not only decreased the frequency of mammary carcinoma in rats but also associated with decreased levels of markers of tumorigenicity, endocrine derangement, oxidative stress and increased apoptotic activity. The ability of Mel, RA and NS to decrease the frequency of mammary carcinomas in rats not only agrees with previous reports but also supports their possible use as anticancer drugs or adjuvant chemotherapeutics (Cohen et al. 1978; Miller 1998; Badary & Gamal El-Din 2001). These effects may be due to their ability to (a) act as an antioxidant, scavenging the hydroxyl radical and inhibiting the production of NO by reducing NO synthase (Mel and NS) (Stasica et al. 1998; Tan et al. 2002; Mahmood et al. 2003; Hussein et al. 2005). Of note, Mel enters the nucleus protecting DNA from oxidative damage (Reiter et al. 1999; Hussein et al. 2005). The Pro role of Mel in our series is in agreement with other authors. In support, (a) subcutaneous injection of Mel can inhibit the development of DMBA-induced mammary tumours in rats (Tamarkin et al. 1981) and (b) pinealectomy or calcification of the pineal gland can enhance the development of DMBA-induced mammary tumours (Cohen et al. 1978).
The decreased level of prolactin, estradiol, lipid peroxides following Mel, NS and RA administration agrees with previous studies (Kothari 1987; Blask et al. 1991; Abou-Issa et al. 1993; Teixeira et al. 2003). This finding may be explained by (a) the inhibitory effect of these substances on the release of pituitary hormones, particularly, prolactin (Mel); (b) inhibition of the expression of prolactin receptor (RA) (Widschwendter et al. 1999); (c) inhibition of oestrogen-receptor activity (RA); and (d) antioxidant activity and interference with the DNA synthesis coupled with enhancement of detoxification processes (Mel, RA and NS) (Ali et al. 2003).
It is possible that the antiproliferative effect of Mel may be dependent on the presence of a complex interaction with hormones such as estradiol and prolactin. Interestingly, the presence of apoptosis in the tumour treated with Mel, RA and NS was associated with increased levels of TNF-α. In support, TNF-α can induce apoptosis in tumour cell lines in vitro (Wright et al. 1992). Therefore, it is conceivable that some of the apoptosis observed in tumours in vivo may be attributable to the release of this cytokine by infiltrating macrophages and tumour cells. TNF-α causes arrest of the cell cycle in the transition from G1 to S phase in the cells of mammary carcinoma (Pagliacci et al. 1993) and induces apoptosis in tumour cells. The RA can increase soluble receptors for TNF-α, in tumour cells (Aderka 1996) and thus induces apoptotic cell death in part by activating caspase-3/poly(ADP-ribose) polymerase one-effector pathway.
Mammary carcinoma was absent in the NS-Pro group. In our series, mammary carcinoma was absent in the NS-Pro group. This interesting finding is in support of observation by other groups (Badary & Gamal El-Din 2001). In this respect, Badary & Gamal El-Din (2001) found that thymoquinon, the main active constituents of NS, reduces both the tumour incidence and burden in fibrosarcoma induced by 20-methyl cholanthrene. The possible modes of action of thymoquinon include its (a) antioxidant activity, (b) interference with the DNA synthesis and (c) enhancement of the detoxification processes. Moreover, the aqueous extract of NS seeds was found to have a strong inhibitory effect on NO production by murine macrophages (Mahmood et al. 2003).
Taken as a whole, our investigation reported the biochemical and morphological changes associated with the administration of Mel, RA and NS. It demonstrated their ability to (a) minimize the frequency of DMBA-induced carcinomas, (b) decrease the deleterious endocrine derangement, (c) increase the apoptotic tumour cell loss and (d) decrease the level of detrimental oxidative stress. These findings suggest possible Pro and therapeutic implications in mammary carcinogenesis. The clinical ramifications of these findings mandate further investigations.
References
- Abou-Issa H, Dwivedi C, Curley RW, et al. Basis for the anti-tumor and chemopreventive activities of glucarate and the glucarate: retinoid combination. Anticancer Res. 1993;13:395–399. [PubMed] [Google Scholar]
- Abraham GE. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J. Clin. EndocrinolMetab. 1974;39:340–346. doi: 10.1210/jcem-39-2-340. [DOI] [PubMed] [Google Scholar]
- Aderka D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor Rev. 1996;7:231–240. doi: 10.1016/s1359-6101(96)00026-3. [DOI] [PubMed] [Google Scholar]
- Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. PhytotherRes. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- Ardizzoia A, Lissoni P, Brivio F, et al. Tumor necrosis factor in solid tumors: increased blood levels in the metastatic disease. J. Biol. RegulHomeostAgents. 1992;6:103–107. [PubMed] [Google Scholar]
- Badary OA, Gamal El-Din AM. Inhibitory effects of thymoquinone against 20-methylcholanthrene-induced fibrosarcoma tumorigenesis. Cancer DetectPrev. 2001;25:362–368. [PubMed] [Google Scholar]
- van Bezooijen RL, Que I, Ederveen AG, Kloosterboer HJ, Papapoulos SE, Lowik CW. Plasma nitrate + nitrite levels are regulated by ovarian steroids but do not correlate with trabecular bone mineral density in rats. JEndocrinol. 1998;159:27–34. doi: 10.1677/joe.0.1590027. [DOI] [PubMed] [Google Scholar]
- Blask DE, Pelletier DB, Hill SM, et al. Pineal melatonin inhibition of tumor promotion in the N-nitroso-N-methylurea model of mammary carcinogenesis: potential involvement of antiestrogenic mechanisms in vivo. J. Cancer Res. ClinOncol. 1991;117:526–532. doi: 10.1007/BF01613283. [DOI] [PubMed] [Google Scholar]
- Bower JE, GanZ PA, AziZ N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. PsychosomMed. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6:229–239. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman KH, Emery S, Maddix SP, Slater TF, Burton GW, Ingold KU. Studies on lipid peroxidation in normal and tumour tissues. The Yoshida rat liver tumour. BiochemJ. 1988;250:247–252. doi: 10.1042/bj2500247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Lippman M, Chabner B. Role of pineal gland in aetiology and treatment of breast cancer. Lancet. 1978;2:814–816. doi: 10.1016/s0140-6736(78)92591-6. [DOI] [PubMed] [Google Scholar]
- El-Metwally TH, Hussein MR, Pour PM, Kuszynski CA, Adrian TE. High concentrations of retinoids induce differentiation and late apoptosis in pancreatic cancer cells in vitro. Cancer BiolTher. 2005;4:82–91. doi: 10.4161/cbt.4.5.1762. [DOI] [PubMed] [Google Scholar]
- Gregoraszczuk EL, Milewicz T, Kolodziejczyk J, et al. Progesterone-induced secretion of growth hormone, insulin-like growth factor I and prolactin by human breast cancer explants. GynecolEndocrinol. 2001;15:251–258. doi: 10.1080/gye.15.4.251.258. [DOI] [PubMed] [Google Scholar]
- Huigsloot M, Tijdens IB, Mulder GJ, van de Water B. Differential regulation of phosphatidylserine externalization and DNA fragmentation by caspases in anticancer drug-induced apoptosis of rat mammary adenocarcinoma MTLn3 cells. BiochemPharmacol. 2001;62:1087–1097. doi: 10.1016/s0006-2952(01)00755-9. [DOI] [PubMed] [Google Scholar]
- Hussein MR, Abu-Dief EE, Abd El-Reheem MH, Abd-Elrahman A. Ultrastructural evaluation of the radioprotective effects of melatonin against X-ray-induced skin damage in Albino rats. Int. J. ExpPathol. 2005;86:45–55. doi: 10.1111/j.0959-9673.2005.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein MR, Hassan M, Wood GS. Morphological changes and apoptosis in radial growth phase melanoma cell lines following ultraviolet-B irradiation. Am. J. Dermatopathol. 2003a;25:466–472. doi: 10.1097/00000372-200312000-00003. [DOI] [PubMed] [Google Scholar]
- Hussein MR, Haemel AK, Wood GS. Apoptosis and melanoma: molecular mechanisms. JPathol. 2003b;199:275–288. doi: 10.1002/path.1300. [DOI] [PubMed] [Google Scholar]
- Hussein MR, Ismael HH. Alterations of p53, Bcl-2, and hMSH2 protein expression in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas in the upper Egypt. Cancer BiolTher. 2004;3:983–988. doi: 10.4161/cbt.3.10.1136. [DOI] [PubMed] [Google Scholar]
- Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s) Microsc. Res. Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Jadeski LC, Chakraborty C, Lala PK. Role of nitric oxide in tumour progression with special reference to a murine breast cancer model. Can. J. PhysiolPharmacol. 2002;80:125–135. doi: 10.1139/y02-007. [DOI] [PubMed] [Google Scholar]
- Jang TJ, Kim DK. Inducible nitric oxide synthase expression of tumor and stromal cells is associated with the progression of 7,12-dimethylbenz[a]anthracene-induced rat mammary tumors. Cancer Lett. 2002;182:121–126. doi: 10.1016/s0304-3835(02)00091-5. [DOI] [PubMed] [Google Scholar]
- Kang SM, Maeda K, Onoda N, et al. Combined analysis of p53 and vascular endothelial growth factor expression in colorectal carcinoma for determination of tumor vascularity and liver metastasis. Int. J. Cancer. 1997;74:502–507. doi: 10.1002/(sici)1097-0215(19971021)74:5<502::aid-ijc4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kim HS, Hausman DB, Compton MM, et al. Induction of apoptosis by all-trans-retinoic acid and C2-ceramide treatment in rat stromal-vascular cultures. Biochem. Biophys. ResCommun. 2000;270:76–80. doi: 10.1006/bbrc.2000.2373. [DOI] [PubMed] [Google Scholar]
- Kopreski MS, Lipton A, Harvey HA, Kumar R. Growth inhibition of breast cancer cell lines by combinations of anti-P185HER2 monoclonal antibody and cytokines. Anticancer Res. 1996;16:433–436. [PubMed] [Google Scholar]
- Kothari LS. Influence of chronic melatonin on 9,10-dimethyl-1,2-benzanthracene-induced mammary tumors in female Holtzman rats exposed to continuous light. Oncology. 1987;44:64–66. doi: 10.1159/000226445. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Turner BC, et al. Prognostic significance of apoptosis regulators in breast cancer. EndocrRelatCancer. 1999;6:29–40. doi: 10.1677/erc.0.0060029. [DOI] [PubMed] [Google Scholar]
- Krishnan N, Buckley DJ, Zhang M, Reed JC, Buckley AR. Prolactin-Stimulated X-linked inhibitor of apoptosis protein expression during S phase cell cycle progression in rat Nb2 lymphoma cells. Endocrine. 2001;15:177–186. doi: 10.1385/ENDO:15:2:177. [DOI] [PubMed] [Google Scholar]
- Kumar R, Vadlamudi RK, Adam L. Apoptosis in mammary gland and cancer. EndocrRelatCancer. 2000;7:257–269. doi: 10.1677/erc.0.0070257. [DOI] [PubMed] [Google Scholar]
- Kumaraguruparan R, Subapriya R, Viswanathan P, Nagini S. Tissue lipid peroxidation and antioxidant status in patients with adenocarcinoma of the breast. Clin. Chim. Acta. 2002;325:165–170. doi: 10.1016/s0009-8981(02)00292-9. [DOI] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Fukushima K, Ochiai K. Lipopolysaccharide stimulates butyric acid-induced apoptosis in human peripheral blood mononuclear cells. InfectImmun. 1999;67:22–29. doi: 10.1128/iai.67.1.22-29.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SA, Weroha SJ, Tawfik O, Li JJ. Prevention of solely estrogen-induced mammary tumors in female aci rats by tamoxifen: evidence for estrogen receptor mediation. JEndocrinol. 2002;175:297–305. doi: 10.1677/joe.0.1750297. [DOI] [PubMed] [Google Scholar]
- Lind DS, Tuttle TM, Bethke KP, Frank JL, McCrady CW, Bear HD. Expansion and tumour specific cytokine secretion of bryostatin-activated T-cells from cryopreserved axillary lymph nodes of breast cancer patients. SurgOncol. 1993;2:273–282. doi: 10.1016/s0960-7404(06)80002-2. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ, Conti A. Beta-endorphin and dynorphin mimic the circadian immunoenhancing and anti-stress effects of melatonin. Int. J. Immunopharmacol. 1989;11:333–340. doi: 10.1016/0192-0561(89)90078-7. [DOI] [PubMed] [Google Scholar]
- Mahmood MS, Gilani AH, Khwaja A, Rashid A, Ashfaq MK. The in vitro effect of aqueous extract of Nigella sativa seeds on nitric oxide production. PhytotherRes. 2003;17:921–924. doi: 10.1002/ptr.1251. [DOI] [PubMed] [Google Scholar]
- Mertani HC, Garcia-Caballero T, Lambert A, et al. Cellular expression of growth hormone and prolactin receptors in human breast disorders. IntJCancer. 1998;79:202–211. doi: 10.1002/(sici)1097-0215(19980417)79:2<202::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Miller WH., Jr The emerging role of retinoids and retinoic acid metabolism blocking agents in the treatment of cancer. Cancer. 1998;83:1471–1482. doi: 10.1002/(sici)1097-0142(19981015)83:8<1471::aid-cncr1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mobley JA, Brueggemeier RW. Estrogen receptor-mediated regulation of oxidative stress and DNA damage in breast cancer. Carcinogenesis. 2004;25:3–9. doi: 10.1093/carcin/bgg175. [DOI] [PubMed] [Google Scholar]
- Pagliacci MC, Fumi G, Migliorati G, Grignani F, Riccardi C, Nicoletti I. Cytostatic and cytotoxic effects of tumor necrosis factor alpha on MCF-7 human breast tumor cells are differently inhibited by glucocorticoid hormones. Lymphokine Cytokine Res. 1993;12:439–447. [PubMed] [Google Scholar]
- Patel PS, Baxi BR, Adhvaryu SG, Balar DB. Evaluation of serum sialic acid, heat stable alkaline phosphatase and fucose as markers of breast carcinoma. Anticancer Res. 1990;10:1071–1074. [PubMed] [Google Scholar]
- Peck JD, Hulka BS, Poole C, Savitz DA, Baird D, Richardson BE. Steroid hormone levels during pregnancy and incidence of maternal breast cancer. Cancer EpidemiolBiomarkers Prev. 2002;11:361–368. [PubMed] [Google Scholar]
- Plucinsky MC, Riley WM, Prorok JJ, Alhadeff JA. Total and lipid-associated serum sialic acid levels in cancer patients with different primary sites and differing degrees of metastatic involvement. Cancer. 1986;58:2680–2685. doi: 10.1002/1097-0142(19861215)58:12<2680::aid-cncr2820581222>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Portier CJ. Endocrine dismodulation and cancer. Neuro. Endocrinol. Lett. 2002;23(Suppl. 2):43–47. [PubMed] [Google Scholar]
- Raval GN, Parekh LJ, Patel MM, et al. Role of sialic acid and alkaline DNase in breast cancer. IntJBiolMarkers. 1997;12:61–67. doi: 10.1177/172460089701200204. [DOI] [PubMed] [Google Scholar]
- Raval GN, Patel DD, Parekh LJ, Patel JB, Shah MH, Patel PS. Evaluation of serum sialic acid, sialyltransferase and sialoproteins in oral cavity cancer. OralDis. 2003;9:119–128. doi: 10.1034/j.1601-0825.2003.01795.x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Cabrera J, et al. The oxidant/antioxidant network: role of melatonin. Biol. Signals. Recept. 1999;8:56–63. doi: 10.1159/000014569. [DOI] [PubMed] [Google Scholar]
- Russo J, Russo IH. Experimentally induced mammary tumors in rats. Breast. Cancer Res. Treat. 1996;39:7–20. doi: 10.1007/BF01806074. [DOI] [PubMed] [Google Scholar]
- Salim EI, Fukushima S. Chemopreventive potential of volatile oil from black cumin (Nigella sativa L.) seeds against rat colon carcinogenesis. NutrCancer. 2003;45:195–202. doi: 10.1207/S15327914NC4502_09. [DOI] [PubMed] [Google Scholar]
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- Sonmez H, Suer S, Gungor Z, Baloglu H, Kokoglu E. Tissue and serum sialidase levels in breast cancer. Cancer Lett. 1999;136:75–78. doi: 10.1016/s0304-3835(98)00295-x. [DOI] [PubMed] [Google Scholar]
- Stasica P, Ulanski P, Rosiak JM. Melatonin as a hydroxyl radical scavenger. J. Pineal. Res. 1998;25:65–66. doi: 10.1111/j.1600-079x.1998.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Stepaniak JA, Gould KE, Sun D, Swanborg RH. A comparative study of experimental autoimmune encephalomyelitis in Lewis and DA rats. JImmunol. 1995;155:2762–2769. [PubMed] [Google Scholar]
- Tamarkin L, Cohen M, Roselle D, Reichert C, Lippman M, Chabner B. Melatonin inhibition and pinealectomy enhancement of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in the rat. Cancer Res. 1981;41:4432–4436. [PubMed] [Google Scholar]
- Tan DX, Reiter RJ, Manchester LC, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. MedChem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- Teixeira A, Morfim MP, de Cordova CA, Charao CC, de Lima VR, Creczynski-Pasa TB. Melatonin protects against pro-oxidant enzymes and reduces lipid peroxidation in distinct membranes induced by the hydroxyl and ascorbyl radicals and by peroxynitrite. J. Pineal. Res. 2003;35:262–268. doi: 10.1034/j.1600-079x.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi, Meltz ML, Reiter RJ, Herman TS, Kumar KS. Melatonin and protection from whole-body irradiation: survival studies in mice. MutatRes. 1999;425:21–27. doi: 10.1016/s0027-5107(98)00246-2. [DOI] [PubMed] [Google Scholar]
- Vondracek J, Kozubik A, Machala M. Modulation of estrogen receptor-dependent reporter construct activation and G0/G1-S-phase transition by polycyclic aromatic hydrocarbons in human breast carcinoma MCF-7 cells. ToxicolSci. 2002;70:193–201. doi: 10.1093/toxsci/70.2.193. [DOI] [PubMed] [Google Scholar]
- Widschwendter M, Widschwendter A, Welte T, et al. Retinoic acid modulates prolactin receptor expression and prolactin-induced STAT-5 activation in breast cancer cells in vitro. BrJCancer. 1999;79:204–210. doi: 10.1038/sj.bjc.6690034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SC, Tam AW, Kumar P. Selection of tumor cell variants for resistance to tumor necrosis factor also induces a form of pleiotropic drug resistance. Cancer ImmunolImmunother. 1992;34:399–406. doi: 10.1007/BF01741751. [DOI] [PMC free article] [PubMed] [Google Scholar]