Abstract
Although variation in the level of macrophage infiltration has been reported in uveal melanoma, little is known about the expression of other leucocyte markers. An immuno-histochemistry study of the levels of expression of macrophage and other leucocyte markers, in a series of 10 primary choroidal melanoma biopsies, was undertaken. Biopsies were either fixed immediately in formalin and embedded in paraffin wax or established as short-term cultures. Using single and double-labelling immunohistochemistry, cultured cells and paraffin sections were analysed for a range of melanoma (HMB45, Melan A, S100 and tyrosinase) and immune cell (CD68, CD163, CD45 and CD1a) markers. All samples expressed at least two known melanoma markers. Infiltrating macrophages were present in the majority of sections. When cultured specimens were studied by double-labelling immunofluorescence, uveal melanoma cells were seen to express macrophage markers or have cross-reactivity with related proteins. Expression of the leucocyte antigen CD45 was observed in three tumours but was not present in any cultured cells, whilst the expression of the dendritic cell marker CD1a was absent from all samples.
Keywords: dendritic cell, immunohistochemistry, leucocyte, macrophage, uveal melanoma
Tumour cells are able to escape immune attack, either through evading destruction or being recognized as immunologically normal tissue (Khong & Restifo 2002). As the tumour increases in size, however, secretion of soluble mediators by tumour cells and physiological properties of the tumour mass often attract circulating immune cells, stimulating their migration. Of such infiltrating cells, tumour-associated macrophages (TAMs) are a significant component of many tumours. Increasing evidence exists to suggest that TAMs produce a number of regulators responsible for promoting tumour growth, despite possessing the ability to destroy neoplastic cells, and for some tumour types, high levels of infiltration correlate with a worse prognosis (Coussens & Werb 2002).
Posterior uveal melanomas are aggressive tumours affecting the choroid and ciliary body. In common with many cancers (Leek et al. 1996; Ono et al. 1999; Torisu et al. 2000), the dominant infiltrating cells detected in uveal melanomas are macrophages, whilst only minimal levels of lymphocytes and other immune cell types have been described (de Waard-Siebinga et al. 1996; Collaborative Ocular Melanoma Study Group 1998; Anastassiou et al. 2001; Makitie et al. 2001). These tumours nevertheless typically are described as having low levels of TAM infiltration (Meecham et al. 1992; de Waard-Siebinga et al. 1996; Collaborative Ocular Melanoma Study Group 1998), and thus despite playing a role in the development of other malignancies, their precise involvement in uveal melanoma progression remains unclear. A more recent study, however, has provided conflicting evidence, describing moderate-to-high levels of macrophage infiltration in most uveal melanomas (Makitie et al. 2001). As the cytoplasm of uveal melanoma cells is reported to cross-react with specific antibody clones directed against the macrophage marker CD68, whilst others clones of anti-CD68 antibodies are reputedly less sensitive (Makitie et al. 2001), it is possible that over-or underestimations of macrophage infiltration may occur.
An alternative explanation could relate to the concept that malignant progression is often associated with tumour genotype and related phenotype dedifferentiation, whereby cells have been shown to express genes more commonly associated with other cell types. For uveal melanoma, expression of a range of genes has been identified in cell lines, and it has been proposed that cells potentially revert to a ‘pluripotent, embryonic-like genotype’ (Hendrix et al. 2003). More specifically, aggressive uveal melanomas (originating from the neuroectoderm) have been reported to express the CD34 antigen, a protein associated with cells derived from myeloid or lymphoid progenitors (Chen et al. 2002). Uveal melanoma cells might therefore similarly express markers linked with cell types such as macrophages, thus again leading to an overestimation of infiltration. In order to investigate these possibilities, this present study assessed paraffin-embedded sections from a series of choroidal melanomas, and the corresponding tumour cells in culture, for possible coexpression of a number of immune cell and melanoma-associated markers.
Materials and methods
Clinical material
A series of 10 primary untreated choroidal melanomas and, in addition, one liver metastasis were collected from theatre upon enucleation (Table 1). Primary uveal melanoma biopsies were either fixed in formalin for 24 h, processed to paraffin wax and sectioned (5 µm), or established as short-term cultures (STCs) as previously described (Baker et al. 2001). STCs were analysed within five passages of being established in culture. The liver biopsy (SOM 351 met) was not grown in culture and was fixed in formalin only, whilst samples from the primary tumour of SOM 351 were not available as the patient had received pervious stereotactic treatment. Two uveal melanoma cell lines were also included in the study (SOM 157d and SOM 196B). Ethical approval and informed patient consent were obtained prior to collection of tumour samples. STCs and cell lines were maintained by serial passage in RPMI-1640, supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml), Fungizone (5 µg/ml), epidermal growth factor (0.2 µg/ml), fetal calf serum (20%) and glucose (0.2%) at 37 °C in an atmosphere of 5% carbon dioxide/95% air. Owing to the constraints of culturing solid tumours, the study was restricted to 10 primary posterior uveal melanomas from which sufficient numbers of cells for all aspects of the study could be obtained. For each tumour, cultured cells and paraffin-embedded sections were initially analysed by single-labelling immunohistochemistry for expression of a panel of melanoma-associated antigens (Melan A, S100, HMB45 and tyrosinase) and macrophage (CD68 and CD163), dendritic cell (CD1a) and leucocyte (CD45) markers (Table 2). All primary mouse monoclonal antibodies were immunoglobulin G1 (IgG1) isotypes. An IgG1 isotype control antibody was therefore used as a negative control, and appropriate positive controls were included in all instances (breast carcinoma sections for macrophage and leucocyte markers and skin sections for the dendritic cell marker).
Table 1.
Histopathological data
| SOM | Age | Sex | Metastatic disease | Volume (mm3) | Pigmentation | Cell type | Status |
|---|---|---|---|---|---|---|---|
| 157d | 73 | Male | Yes | 3647 | Moderate | Epithelioid | Dead |
| 196B | 83 | Male | No | 885 | Moderate | Mixed | Alive |
| 256 | 84 | Female | No | 481 | High | Spindle B | Alive |
| 258 | 45 | Male | No | 568 | Low | Spindle B | Alive |
| 267 | 53 | Female | No | 1395 | Low | Spindle | Alive |
| 280 | 88 | Male | Yes | 2621 | Low | Mixed | Alive |
| 330 | 28 | Male | No | 1251 | Low | Spindle | Alive |
| 346 | 63 | Male | No | 862 | Low | Mixed | Alive |
| 349 | 66 | Female | No | 811 | Moderate | — | Alive |
| 350 | 48 | Male | No | 1485 | Low–moderate | Spindle B | Alive |
| 351 met | 52 | Male | Yes | — | Amelanotic | — | Alive |
Tumours volumes (mm3) were calculated using the following formula: (π/6) × length × height × width.
Table 2.
Details of the clones, dilutions and suppliers of the primary mouse IgG1 monoclonal antibodies used
| Primary monoclonal antibody | Clone | Dilution | Supplier |
|---|---|---|---|
| Melan A | A103 | 1:25 | Novocastra (Newcastle-upon-Tyne, UK) |
| S100 | S1/61/69 | 1:30 | Novocastra (Newcastle-upon-Tyne, UK) |
| HMB45 | HMB45 | 1:45 | Novocastra (Newcastle-upon-Tyne, UK) |
| Tyrosinase | T311 | 1:25 | Novocastra (Newcastle-upon-Tyne, UK) |
| CD1a | JPM30 | 1:20 | Novocastra (Newcastle-upon-Tyne, UK) |
| CD45 | X16/99 | 1:15 | Novocastra (Newcastle-upon-Tyne, UK) |
| CD68 | KP1 | 1:100 | Dako Corporation (Carpinteria, CA, USA) |
| CD68 | PG-M1 | 1:100 | Dako Corporation (Carpinteria, CA, USA) |
| CD163 | 10D6 | 1:50 | Novocastra (Newcastle-upon-Tyne, UK) |
Immunohistochemistry
Exploratory studies
Paraffin sections were initially de-waxed in xylene and dehydrated through graded alcohols. For antigen retrieval, sections were incubated in 0.1% trypsin in 0.1% calcium chloride at 37 °C for 10 min. One section from each tumour was also stained with haematoxylin and eosin. Alternatively, cultured cells were grown on sterile glass slides for 24 h, before washing in phosphate-buffered saline (PBS) and fixing in equal volumes of acetone and methanol for 10 min. Slides of STCs were then stored at −20 °C before use.
Cells and paraffin sections were stained using an avidin-biotinylated peroxidase complex method (Vectastain ABC Elite Kit™, Vector Laboratories, Peterborough, UK) (Hsu et al. 1981). All samples were rinsed in PBS prior to incubation with the primary antibody (diluted in PBS) for 1 h at room temperature (Table 2). Slides were then thoroughly washed in PBS before detection with the immunoperoxidase technique and visualization with 3-amino-9-ethylcarbazole (AEC) (Vector Laboratories). Samples were counterstained with Gill's haematoxylin, mounted in aqueous mounting medium and examined using a light microscope. Staining intensity was assessed by two independent observers and scored as weak (+), medium (++) or strong (+++) as previously detailed (Baker et al. 2001). Two ocular pathologists (MAP and HSM) determined morphology and the percentage of immune cell infiltration.
Co-localization of expression of macrophage markers and melanoma cell markers
To investigate the possibility that melanoma cells themselves may express macrophage markers, sections and cultured cells from SOM 157d, 196B (cultured cells only), 280, 330, 346, 349 and 350 were fluorescently double labelled for both S100 and macrophage (CD68 and CD163) markers. Prior to incubation with the first monoclonal antibody, sections and cells were treated as detailed above. Samples were then incubated with either anti-S100 or an antimacrophage monoclonal antibody (CD68 or CD163) overnight at 4 °C (Table 2). Following further incubation with a biotinylated antimouse antibody (Vectastain ABC Elite Kit™), expression was detected with Texas red Avidin DCS (Vector Laboratories). Prior to incubation with the second monoclonal antibody, endogenous biotin and avidin was blocked using a Biotin/Avidin blocking kit (Vector Laboratories). Samples were subsequently treated with an antimacrophage or anti-S100 monoclonal antibody (dependent upon the first monoclonal antibody) for 1 h at room temperature, and expression was visualized using a fluorescein immunodetection system (Vector MOM™ Immuno-detection Kit, Vector Laboratories). Slides were mounted with an antifade mounting medium (Vectashield, Vector Laboratories) and observed under fluorescence. Negative control samples were included in all instances, in which either or both monoclonal antibodies were replaced with the IgG1 isotype control antibody. Breast carcinoma sections were again used as positive controls.
Results
Comparable ubiquitous levels of expression of the melanoma-associated antigens S100 and HMB45 were observed in a high proportion of the cells (90%) in the majority of samples, whilst the expression of Melan A and tyrosinase was less common (Table 3). SOM 351 met was the only exception, as strong HMB45 expression alone was apparent (Table 3). Consistent with previous reports (Facchetti et al. 1991; Falini et al. 1993; Makitie et al. 2001), clone KP1 (directed against intracytoplasmic CD68) strongly cross-reacted with the cytoplasm of most uveal melanoma cells included in the study and was thus excluded. Staining with clone PG-M1 (also directed against CD68) showed greater definition and was therefore used throughout to assess CD68 expression (Anastassiou et al. 2001; Makitie et al. 2001). These results were confirmed by analysing the expression of a further macrophage marker (CD163). This antigen is specific to a distinct stage of differentiation/activation of monocytes/macrophages (Backe et al. 1991) and, in this study, discretely stained a similar pattern of cells to clone PG-M1.
Table 3.
Results of staining uveal melanoma cultured cells and sections melanoma-associated antigens
| Level of melanoma-associated antigen staining | |||||
|---|---|---|---|---|---|
| SOM | Tissue | Melan A | S100 | Tyrosinase | HMB45 |
| 157d | Section | – | + | – | +++ |
| Cultured cells | – | + | – | – | |
| 196B | Section | – | +++ | + | +++ |
| Cultured cells | + | ++ | – | – | |
| 256 | Section | – | ++ | – | +++ |
| Cultured cells | + | ++ | – | +++ | |
| 258 | Section | – | ++ | – | +++ |
| Cultured cells | – | + | – | +++ | |
| 267 | Section | – | ++ | – | +++ |
| Cultured cells | – | + | ++ | + | |
| 280 | Section | – | ++ | – | +++ |
| Cultured cells | – | + | – | + | |
| 330 | Section | – | ++ | – | +++ |
| Cultured cells | – | + | – | + | |
| 346 | Section | – | + | – | ++ |
| Cultured cells | – | ++ | – | + | |
| 349 | Section | – | +/++ | – | +++ |
| Cultured cells | – | ++ | – | +/++ | |
| 350 | Section | – | ++/+++ | – | +++ |
| Cultured cells | – | ++ | – | + | |
| 351 | metSection | – | – | – | +++ |
Staining intensity was assessed as previously described (Baker et al. 2001) and was scored as weak (+), medium (++) or strong (+++). With the exception of SOM 351 met, expression of S100 and HMB45 was detected in 90% of melanoma cells in each sample. For SOM 351 met, expression of HMB45 alone was detected.
Tumour sections
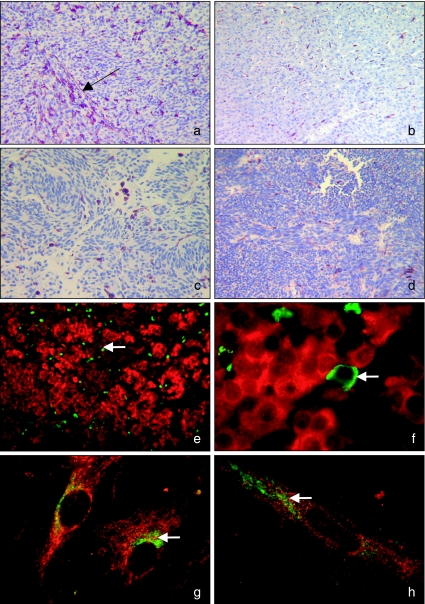
Pigmentation was observed in some tumours, but this did not obscure detection of the any immunopositive cells. Expression of the immature dendritic cell marker CD1a was absent from all sections, whilst the expression of the leucocyte antigen CD45 by a small number of infiltrating cells (<1–10% of the total cell number in the biopsy section), morphologically resembling lymphocytes, was observed in sections from three tumours (SOM 157d, 267 and 350) (Table 4) (Figure 1d). A small population of infiltrating macrophages (<1% of the total cell number in the biopsy section) was also identified in paraffin sections from the majority of melanomas (including SOM 351 met), staining strongly for both CD68 and CD163 (often in areas of high pigmentation) (Table 4 and Figure 1a–c). SOM 258 was the only tumour that did not contain cells staining positively with either macrophage marker (Table 4). When comparing staining patterns of anti-CD68 and anti-CD163 monoclonal antibodies, slight differences in numbers and strength of immunopositive cells were observed in five tumours (SOM 196B, 256, 330, 346 and 349), but as statistical analysis was not carried out, the significance cannot be determined and was most likely a result of inter-observer variation. In all cases, however, infiltration was still estimated at <1% of the total cell population. Patterns of immunopositive macrophages arranged along microvascular or extracellular matrix were also seen in some cases, similar to those observed by Makitie et al. (2001) (Figure 1a). As endothelial cells were not labelled in this study or matrix patterns stained, no association between these factors and macrophage infiltration could be made. In addition to the immunopositive infiltrating cells (Table 4), when observed under light microscope at ×100 magnification, tumour cells from six cases (SOM 157d, 280, 330, 346, 349 and 350) also had weak CD68 staining. This was not seen with positive controls (breast carcinoma sections) or with any of the other markers and, similar to clone KP1, could infer that the melanoma cells were cross-reacting with clone PG-M1.
Table 4.
Results of staining uveal melanoma sections for the macrophage markers CD68 and CD163, and the leucocyte antigen CD45, and approximate levels of infiltration as assessed by Dr h. S. Mudhar
| Intensity of staining | |||||
|---|---|---|---|---|---|
| SOM | Percentage of infiltrating cells | Cell type | CD45 | CD68 (PG-M1) | CD163 |
| 157d | <1 | Macrophages | +++ | +/++ | |
| 10 | Leucocytes | ++ | |||
| 196B | <1 | Macrophages | +++ | ++ | |
| 0 | Leucocytes | – | |||
| 256 | <1 | Macrophages | +++ | ++ | |
| 0 | Leucocytes | – | |||
| 258 | 0 | Macrophages | – | – | |
| 0 | Leucocytes | – | |||
| 267 | <1 | Macrophages | ++ | ++ | |
| <1 | Leucocytes | ++ | |||
| 280 | <1 | Macrophages | ++ | ++ | |
| 0 | Leucocytes | – | |||
| 330 | <1 | Macrophages | ++ | ++ | |
| 0 | Leucocytes | – | |||
| 346 | <1 | Macrophages | ++ | ++ | |
| 0 | Leucocytes | – | |||
| 349 | <1 | Macrophages | +++ | –/+ | |
| 0 | Leucocytes | – | |||
| 350 | <1 | Macrophages | +++ | +++ | |
| <1 | Leucocytes | +++ | |||
| 351 met | <1 | Macrophages | +++ | +++ | |
| 0 | Leucocytes | – | |||
Levels of infiltration are expressed as an approximate percentage of the total cell population. The anti-CD45 monoclonal antibody detects a range of leucocyte cell types, but morphologically immunopositive cells resembled lymphocytes. Cells expressing the dendritic cell marker CD1a were absent from all sections. Staining intensity was assessed as described for Table 3. Double-labelling immunofluorescence identified that infiltrating macrophages were S100-negative, whereas melanoma cells commonly expressed both S100 and HMB45.
Figure 1.
Photographs of uveal melanoma sections and cultured cells to illustrate staining patterns taken under light (a–d) and fluorescent microscopy (e–h). Sections were stained with 3-amino-9-ethylcarbazole (AEC) (magenta) and counterstained with Gill's haematoxylin. Samples were double labelled with fluorescein (green) and Texas red (red).(a)SOM 157d paraffin section stained for CD68 (×100). Infiltrating CD68-positive cells can be seen arranged along an extracellular matrix channel (→).(b)SOM 351 met paraffin section stained for CD68 (×25).(c)SOM 350 paraffin section stained for CD163 (×160).(d)SOM 267 paraffin section stained for CD45 (×160).(e)SOM 350 paraffin section stained for CD68 (green and highlighted by the white arrow) and S100 (red) (×100).(f)SOM 350 paraffin section stained for CD68 (green and highlighted by the white arrow) and S100 (red) (×1000). Expression of CD68 was absent from S100-positive uveal melanoma cells.(g)SOM 280 cultured cells stained for CD68 (red) and S100 (green and highlighted by the white arrow) (×1000).(h)SOM 349 cultured cells stained for CD163 (red) and S100 (green and highlighted by the white arrow) (×1000). (g and h) Possible expression or cross-reactivity of anti-CD68 and anti-CD163 monoclonal antibodies by S100-positive uveal melanoma cells in culture.
To investigate further, sections showing some possible cross-reactivity (SOM 157d, 280, 330, 346, 349 and 350) were double labelled using immunofluorescence for CD68 and S100. Expression of both S100 and HMB45 was detected in each of these tumours (Table 3), but as both CD68 and HMB45 proteins are present in the cytoplasm, S100 was chosen as the confirmatory label of melanoma status because of its predominantly defined perinuclear staining pattern. As a control, experiments were also repeated with the macrophage markers CD163 and S100. In agreement with single-labelling studies, a small S100-negative infiltrating cellular population staining strongly with both macrophage markers could be seen (Figure 1e), with levels of infiltration comparable to those seen by light microscopy (Figure 1a, c). Specific staining of either CD68 or CD163, however, was absent from the S100-positive tumour cell population (Figure 1e, f). Negative control samples showed no evidence of background fluorescence.
Cultured tumour samples
As we have previously shown differences in integrin expression between uveal melanoma paraffin sections and cells in culture (Baker et al. 2001), the study was extended to investigate the expression of these immune cell markers by uveal melanoma STCs. With the exception of SOM 157d and SOM 196B, all cells were assessed within five passages of being established in culture. Single-labelling studies revealed an absence of expression of both the dendritic and leucocyte cell markers CD1a and CD45, respectively, from any uveal melanoma culture. For macrophage markers, however, cells derived from five tumours (SOM 196B, 280, 330, 349 and 350) stained positively for CD68, whilst three cases were CD163-positive (SOM 157d, 349 and 350). Using fluorescent double labelling, results were confirmed for both CD68 and CD163, whilst at the same time showing that cells were also positive for S100 with use of an anti-S100 monoclonal antibody (Figure 1g, h). Negative control samples similarly had no background staining.
Discussion
Cells of the immune system are frequently observed migrating into tumours, and evidence increasingly suggests that they are important regulators of tumour growth (Coussens & Werb 2002). For uveal melanomas, however, only low levels of infiltration have been typically described (Meecham et al. 1992; de Waard-Siebinga et al. 1996; Collaborative Ocular Melanoma Study Group 1998). In this present study, expression of the immature dendritic cell marker CD1a was absent from all uveal melanomas (Table 4) and is therefore not comparable with other malignancies in which expression of CD1a is reported (Coussens & Werb 2002). In contrast, infiltrating CD45-positive leucocytes were detected in three tumours morphologically resembling lymphocytes (Table 4 and Figure 1d), as previously described (Meecham et al. 1992; Ksander et al. 1998). However, as this receptor is widely expressed on a number of leucocyte types, detection of other cell types including monocytes (macrophage precursors) is also possible.
This present study observed a low level of macrophage infiltration in uveal melanomas, which, in common with many other cancers (Leek et al. 1996; Ono et al. 1999; Torisu et al. 2000), are considered to be the major type of infiltrating cells observed (de Waard-Siebinga et al. 1996; Collaborative Ocular Melanoma Study Group 1998; Anastassiou et al. 2001; Makitie et al. 2001). Levels of infiltration and microvascular density have been independently considered as prognostic indicators in uveal melanoma (Foss et al. 1996; Makitie et al. 2001), and similar to breast carcinoma and cutaneous melanoma, macrophages accumulate in the proximity of endothelial cells (Leek et al. 1996; Torisu et al. 2000; Makitie et al. 2001; Clarijs et al. 2003). The use of macrophage infiltration as a prognostic indicator in uveal melanoma is nevertheless controversial, as wide variation in infiltrating numbers has been reported (de Waard-Siebinga et al. 1996; Collaborative Ocular Melanoma Study Group 1998; Anastassiou et al. 2001; Makitie et al. 2001). Consistent with the study of Makitie and coworkers (2001), clone KP1 detecting CD68 strongly cross-reacted with the cytoplasm of most uveal melanoma cells analysed in the study (both in tissue sections and in culture). In contrast, clone PG-M1 discretely labelled a population of infiltrating macrophages in tissue sections that commonly also expressed CD163 (Figure 1e). As the monoclonal antibody detecting CD163 (clone 10D6) is reported to be highly specific (Backe et al. 1991), our results would support its use, in conjunction with clone PG-M1, in future studies.
Although the number of infiltrating macrophages was low for all tumours, there was nevertheless slight variation in the level of CD68-positive and CD163-positive cells between tumours (Table 4 and Figure 1a–c,e), most likely due to small discrepancies between observers. In all cases, however, the numbers of macrophages detected were minimal, therefore differing from the study of Makitie and coworkers (2001) in which moderate-to-high numbers of macrophages were detected. Although no statistical correlation with prognosis was made, a small spindle cell tumour (SOM 258), associated with a better prognosis, contained no macrophage infiltrate. In comparison, macrophages were observed in larger melanomas (SOM 157d, 196B, 267, 280, 349 and 350) (Tables 1 and Tables 4), which, in agreement with previous studies (Makitie et al. 2001), could infer an association of infiltration with worse prognosis. However, as only a small number of choroidal tumours were assessed in this investigation, all of which were sufficiently large to require enucleation, samples were already biased towards those with a worse prognosis (Singh et al. 2001), and thus, no real conclusions may be drawn. Alternative explanations for differences between studies could relate to the scoring systems used for analysis or to the presence of heavy pigmentation obscuring the immunoreaction.
In contrast to tissue sections, uveal melanoma cells from the same tumours in culture showed specific staining patterns for these macrophage markers (Figure 1g, h), presumed as a response to the conditions in vitro. It is therefore of interest that the protein recognized by monoclonal antibodies detecting CD68 is a member of the lysosome-associated membrane protein (LAMP) family (Greaves & Gordon 2002). Although the function of the CD68 receptor is unknown, related LAMP proteins are involved in melanogenesis, located within the melanosome of cells of melanocytic origin (Salopek & Jimbow 1996). If these receptors, therefore, share any sequence homology with CD68, cross-reactivity of clone PG-M1 with LAMPs is a feasible explanation, which in turn could also be subject to upregulation upon culture.
Expression of the scavenger receptor cysteine-rich macrophage marker CD163 was nevertheless also detected in cultures derived from two tumours (SOM 349 and SOM 350) which were similarly CD68-positive (Figure 1g, h), whilst there is no evidence to date of anti-CD163 monoclonal antibody cross-reactivity with related proteins. Although the specificity of CD163 encourages its use in future studies, its expression by cultured uveal melanomas could suggest that cross-reactivity with LAMPs is not a feature, and instead cultured uveal melanomas share expression of antigens previously only associated with macrophages. Despite reports of similarities in the expression of antigens by macrophages and melanocytes in melanomas (Pernick et al. 1999) and although it is possible that this phenomenon contributes to the pattern of expression in vivo, it is unlikely to have affected the staining of specimens subsequently cultured. In addition, although investigations of dendritic tumours have indicated that whilst being negative for dendritic cell markers (CD1a), these tumours can express S100 and CD68 (Pileri et al. 2002), as the majority of cultured uveal melanomas studied expressed both S100 and HMB45 – this would confirm their melanoma origin. Furthermore, as degenerative behaviour by uveal melanomas has been reported (Chen et al. 2002; Hendrix et al. 2003), expression of CD163 and CD68, seen here in response to alterations in the microenvironment produced by growth in culture, could provide further evidence of such degeneration.
The effect of macrophage gene upregulation may also be specific to environmental conditions created by culturing, as analysis of tumour sections taken from SOM 351 met (HMB45-positive melanoma cells resected from the hepatic not orbital environment) did not show evidence of CD163 or CD68 upregulation by melanoma cells. Because other metastatic samples, or the corresponding SOM 351 met cells in culture, were not available for study, no true conclusions can therefore be drawn and further studies with additional metastatic lesions are required to assess differences in expression patterns between primary and metastatic tumours. As the metastatic lesion was derived from a patient who had received prior stereotactic treatment, it is possible that the immune response would differ, a situation that could generally arise as a response to metastasizing tumours. Until further metastatic tumours are available for study, these alterations in the immune response cannot be studied.
It is, however, clear that changes in the microenvironment of tumours nevertheless have a significant effect on behaviour on tumour and host cells (Fidler 2002), and the pluripotent genotype and related phenotype of uveal melanoma cells could allow them to rapidly adapt to environmental conditions (Hendrix et al. 2003). Certainly, recent work by Busund et al. (2002) has suggested that spontaneous tumour cell-macrophage fusion in highly aggressive sarcoma cells correlates with an increase in macrophage traits of tumour cells. These observations were accompanied by an increase in microvascular density (Busund et al. 2002), implying that expression of macrophage genes by tumour cells confers a growth advantage. As a small number of cultures were, nevertheless, studied here, further independent expression studies are required to confirm these hypotheses.
In conclusion, double-labelling immunofluorescence for macrophage and melanoma markers would suggest that uveal melanoma cells do not normally express CD68 or CD163. Variation in levels of macrophage infiltration between studies may therefore relate to individual tumours or methods of assessment used. In contrast, cultured uveal melanomas stained positively for macrophage markers, due either to possible expression of these or related antigens, or to cross-reactivity with elements related to melanogenesis, both presumably as a response to the cultural environment.
Acknowledgments
We thank Yorkshire Cancer Research for providing funding for this project and Ms Hannah Browne for assistance with preliminary work.
References
- Anastassiou G, Coupland SE, Stang A, Boeloeni R, Schilling H, Bornfeld N. Expression of Fas and Fas ligand in uveal melanoma, biological implication and prognostic value. J. Pathol. 2001;194:466–472. doi: 10.1002/path.926. [DOI] [PubMed] [Google Scholar]
- Backe E, Schwarting R, Gerdes J, Ernst M, Stein H. Ber-MAC3, new monoclonal antibody that defines human monocyte/macrophage differentiation antigen. J. Clin. Pathol. 1991;44:936–945. doi: 10.1136/jcp.44.11.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Elshaw S, Mathewman G, et al. Expression of integrins, degradative enzymes and their inhibitors in uveal melanoma, differences between in vitro and in vivo expression. Melanoma Res. 2001;11(3):265–273. doi: 10.1097/00008390-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Busund LT, Killie MK, Bartnes K, Seljelid R. Spontaneously formed tumorigenic hybrids of Meth A sarcoma and macrophages grow faster and are better vascularized than the parental tumor. Int. J. Cancer. 2002;100(4):407–413. doi: 10.1002/ijc.10502. [DOI] [PubMed] [Google Scholar]
- Chen Z, Maniotis AJ, Majumdar D, Pe'er J, Folberg R. Uveal melanoma cell staining for CD34 and assessment of tumour vascularity. Invest. Ophthalmol. Vis. Sci. 2002;43(8):2533–2539. [PubMed] [Google Scholar]
- Clarijs R, Schalkwijk L, Ruiter DJ, De Waal RM. EMAP-II expression is associated with macrophage accumulation in primary uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2003;44(5):1801–1806. doi: 10.1167/iovs.02-0624. [DOI] [PubMed] [Google Scholar]
- Collaborative Ocular Melanoma Study Group. Histopathologic characteristics of uveal melanomas enucleated from the Collaborative Ocular Melanoma Study. COMS Report, 6. Am. J. Ophthalmol. 1998;125(6):745–766. doi: 10.1016/s0002-9394(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchetti F, Bertalot G, Grigolato PG. KP1 (CD68) staining for malignant melanomas. Histopathology. 1991;19(2):141–145. doi: 10.1111/j.1365-2559.1991.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Falini B, Flenghi L, Pileri S, et al. PG-M1, a new mAb directed against a fixative resistant epitope on the macrophage-restricted form of the CD68 molecule. Am. J. Pathol. 1993;142(5):1359–1372. [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2000;70(9–10):498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- Foss AJ, Alexander RA, Jefferies LW, Hungerford JL, Harris AL, Lightman S. Microvessel count predicts survival in uveal melanoma. Cancer Res. 1996;56(13):2900–2903. [PubMed] [Google Scholar]
- Greaves DR, Gordon S. Macrophage-specific gene expression, current paradigms and future challenges. Int. J. Hematol. 2002;76:6–15. doi: 10.1007/BF02982713. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Molecular plasticity of human melanoma cells. Oncogene. 2003;22(20):3070–3075. doi: 10.1038/sj.onc.1206447. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. The use of anti-avidin antibody-peroxidase complex in immunoperoxidase techniques. Am. J. Clin. Pathol. 1981;75:816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- Khong TH, Restifo NP. Natural selection of tumour variants in the generation of ‘tumor escape’ phenotypes. Nat. Immunol. 2002;3(11):999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksander BR, Geer DC, Chen PW, Salgaller ML, Rubsamen P, Murray TG. Uveal melanomas contain antigenically specific and non-specific infiltrating lymphocytes. Curr. Eye Res. 1998;17(2):165–173. doi: 10.1076/ceyr.17.2.165.5607. [DOI] [PubMed] [Google Scholar]
- Leek RD, Lewis CE, Whitehouse R, Greenhall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- Makitie T, Summanen P, Tarkkanen A, Kivela T. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2001;42(7):1414–1421. [PubMed] [Google Scholar]
- Meecham WJ, Char DH, Kaleta-Michaels S. Infiltrating lymphocytes and antigen expression in uveal melanoma. Ophthalmic Res. 1992;24(1):20–26. doi: 10.1159/000267140. [DOI] [PubMed] [Google Scholar]
- Ono M, Torisu H, Fukushi J, Nishie A, Kuwano M. Biological implications of macrophage infiltration in human tumor angiogenesis. Cancer Chemother Pharmacol. 1999;43(Suppl.):S69–S71. doi: 10.1007/s002800051101. [DOI] [PubMed] [Google Scholar]
- Pernick NL, Dasilva M, Gangi MD, Crissman J, Adsay V. “Histiocytic markers” in melanoma. Mod Pathol. 1999;12(11):1072–1077. [PubMed] [Google Scholar]
- Pileri SA, Grogan TM, Harris NL, et al. Tumours of histiocytes and accessory dendritic cells, an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology. 2002;41(1):1–29. doi: 10.1046/j.1365-2559.2002.01418.x. [DOI] [PubMed] [Google Scholar]
- Salopek TG, Jimbow K. Induction of melanogenesis during the various melanoma growth phases and the role of tyrosinase, lysosome-associated membrane proteins, and p90 calnexin in the melanogenesis cascade. J. Invest Dermatol. Symp. Proc. 1996;1:195–202. [PubMed] [Google Scholar]
- Singh AD, Shields CL, Shields JA. Prognostic factors in uveal melanoma. Melanoma Res. 2001;11(3):255–263. doi: 10.1097/00008390-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Torisu H, Ono M, Kiryu H, et al. Macrophage infiltration correlates with tumour stage and angiogenesis in human malignant melanoma, possible involvement of TNFα and IL-1α. Int. J. Cancer. 2000;85:182–188. [PubMed] [Google Scholar]
- De Waard-Siebinga I, Hilders C, Hansen B, van Delft J, Jager M. HLA expression and tumour-infiltrating immune cells in uveal melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 1996;234:34–42. doi: 10.1007/BF00186516. [DOI] [PubMed] [Google Scholar]